Abstract
Aims
To determine the spectral dynamics of early spontaneous polymorphic ventricular tachycardia and ventricular fibrillation (PVT/VF) in humans.
Methods and results
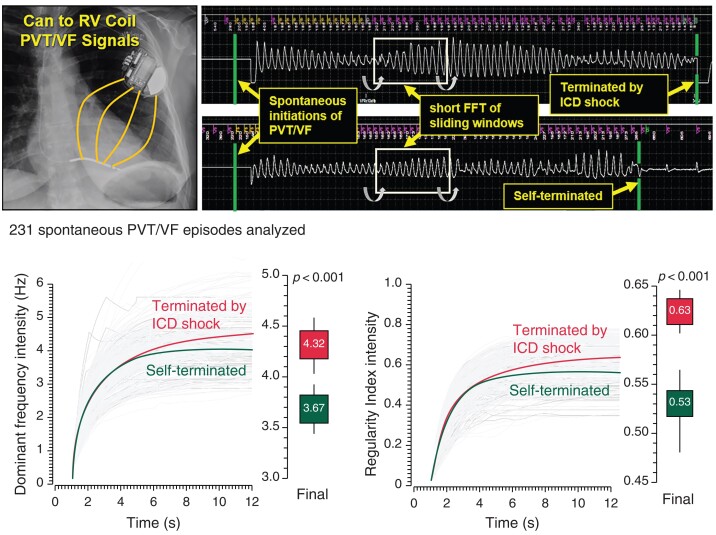
Fifty-eight self-terminated and 173 shock-terminated episodes of spontaneously initiated PVT/VF recorded by Medtronic implanted cardiac defibrillators (ICDs) in 87 patients with various cardiac pathologies were analyzed by short fast Fourier transform of shifting segments to determine the dynamics of dominant frequency (DF) and regularity index (RI). The progression in the intensity of DF and RI accumulations further quantified the time course of spectral characteristics of the episodes. Episodes of self-terminated PVT/VF lasted 8.6 s [95% confidence interval (CI): 8.1–9.1] and shock-terminated lasted 13.9 s (13.6–14.3) (P < 0.001). Recordings from patients with primarily electrical pathologies displayed higher DF and RI values than those from patients with primarily structural pathologies (P < 0.05) independently of ventricular function or antiarrhythmic drug therapy. Regardless of the underlying pathology, the average DF and RI intensities were lower in self-terminated than shock-terminated episodes [DF: 3.67 (4.04–4.58) vs. 4.32 (3.46–3.93) Hz, P < 0.001; RI: 0.53 (0.48–0.56) vs. 0.63 (0.60–0.65), P < 0.001]. In a multivariate analysis controlled by the type of pathology and clinical variables, regularity remained an independent predictor of self-termination [hazard ratio: 0.954 (0.928–0.980)]. Receiver operating characteristic (ROC) curve analysis of DF and RI intensities demonstrated increased predictability for self-termination in time with 95% CI above the 0.5 cut-off limit at about t = 8.6 s and t = 6.95 s, respectively.
Conclusion
Consistent with the notion that fast organized sources maintain PVT/VF in humans, reduction of frequency and regularity correlates with early self-termination. Our findings might help generate ICD methods aiming to reduce inappropriate shock deliveries.
Keywords: Polymorphic ventricular tachycardia • Ventricular fibrillation • Implanted cardiac defibrillators • Arrhythmogenic substrate • Spectral analysis • Dominant frequency • Regularity index
Graphical Abstract
Graphical Abstract.
What’s new?
Characterizing the dynamics of spectral properties of short human spontaneous polymorphic ventricular tachycardia and ventricular fibrillation (PVT/VF) in implanted cardiac defibrillator (ICD) recordings shows decreasing frequency and regularity prior to self-termination before shocks for a wide range of cardiac pathologies.
The maintenance or increment, but not decrease, of spectral regularity during the PVT/VF episodes that do not self-terminate is consistent with periodic sources driving the arrhythmias.
Analysis of the temporal trend in PVT/VF spectral regularity may allow prediction of self-termination and may help improvements in ICDs to limit inappropriate shock therapies.
Introduction
Ventricular fibrillation (VF) is characterized by highly irregular ECG complexes, evoking the notion of fractionated waves of ventricular excitation propagating randomly in space and time. During the last few decades, however, evidence has accumulated indicating that human VF can be governed by deterministic patterns with identifiable self-organized re-entrant drivers that activate the ventricles at exceedingly high frequencies.1 Polymorphic ventricular tachycardia (PVT) is more organized than VF, with continuous waxing and waning of the complexes around the ECG axis but could be maintained also by deterministic re-entrant mechanisms and is frequently preceding VF.
Episodes of PVT/VF may presents as syncope, are an index for increased risk of death in most cardiomyopathies and primary electrical disorders, as well as an implantation of cardiac defibrillators (ICDs). For both PVT and VF, the manner by which the process turns self-sustained, leading to sudden cardiac death, remains elusive2 and better characterization of that process could potentially lead to better risk stratification and improved therapies. We focus here on early PVT/VF episodes spontaneously initiated and recorded by ICDs in out-of-hospital patients with various underlying cardiac pathologies. The study tests the hypothesis that the dynamics of the spectral features of the self-terminated arrhythmias are distinct from longer lasting arrhythmias terminated by the ICD shock. Our results suggest that characterization of spectral dynamics of ICD signals can provide clues about organization involved in early PVT/VF maintenance and could be a potential approach for improved algorithms for ICD shock delivery.
Methods
Patients and data
We show the results of a multicenter substudy conducted with data derived from a patient cohort available as part of the UMBRELLA study (Incidence of Arrhythmias in Spanish Population with a Medtronic Implantable Cardiac Defibrillator Implant; clinicaltrials.gov ID: NCT01561144; See supplementary material for details). The respective ethical institutional review committees approved the study, and all subjects gave informed consent.
Data episodes and polymorphic ventricular tachycardia and ventricular fibrillation definitions
We analyzed all episodes recorded on patients’ ICDs during a 4-year-follow-up period. Voltage time-series were recorded between the ICD can and the right ventricle (RV) coil at a 128 Hz sampling rate and exported for off-line analysis. An ‘episode Review Committee’ (see Supplementary material for details) screened PVT/VF based on the morphology of the exported signals with continuous activations and changing configuration of the complexes. All spontaneously initiated PVT/VF episodes were included in further analysis and all induced episodes, either at the time of implantation or during electrophysiology studies, were excluded.
Processing of polymorphic ventricular tachycardia and ventricular fibrillation signals
Exported signals were processed in several steps and algorithms (see Supplementary material for details). Initially, the beginning and end times of each PVT/VF episode were determined on the exported signal based on the first ventricular ectopy marked by the ICD and the last ventricular ectopy prior to sinus rhythm or ICD shock, respectively. Thereafter, power spectra periodograms were generated for each episode based on short Fast Fourier Transformation (SFFT) performed on windowed and zero-padded 4096-point long signals of moving 2 s long segments sliding at 50 ms strides. The sliding segments covered times exclusively between the starting detection and termination. We used the power spectrum of each of the sliding segment to determine the instantaneous dominant frequency (DF) and regularity index (RI). We defined DF as the frequency of highest power in the power spectrum. We defined RI as the ratio of the power at the DF and adjacent frequencies (±0.75 Hz band) to the sum of the power in the 1–20 Hz band. The DF and RI of each particular segment in the episode were then considered sequentially for their temporal characterization. As demonstrated in testing simulations provided in the Supplemental material (see Supplementary material online, Figures S1–S3), the SFFT procedure on the 2 s long moving windows has the resolution of depicting temporal evolution of DFs and RIs in signals modulated by frequencies and amplitudes.
For additional temporal characterizations, we defined cumulative intensity metrics of the DF and RI time courses, starting 1 s after the initiation of the detected arrhythmias to avoid inconsistent initial markings (see Supplementary material for details), as follows:
where t is the time (expressed in seconds after initiation and parametrized by s) at which the intensity is evaluated and f(s) denotes the DF or RI calculated at segments of 2 s duration shifted sequentially by Δt = 0.05 s. To compare PVT/VF episodes of various durations, the time-series of all spectral parameters were truncated at 12 s or extended to 12 s beyond their self-termination moment with constant values corresponding to those calculated at the moment of self-termination.
Statistical analysis
Continuous variables are reported as mean and 95% confidence interval (95% CI) or standard deviation (SD) where indicated (See Supplemental material for details). Models introduced DF and RI as time-dependent variables for χ2, Student’s t-test and ANOVA for compassions. A proportional hazard Cox regression model was used to study the influence of different variables on the self-termination event. For this purpose, shock-terminated episodes were censored for analysis at the time of the shock. Univariate and multivariate models consider DFs and RIs as time-dependent variables for the entire duration of each episode and for all the episodes. We tested if frequency and regularity changes of 0.1 and 0.01 Hz, respectively, predict self-termination of PVT/VF episodes without and with adjustment of the models to control variables. We used the area under the receiver operating characteristic (ROC) curve to measure the predictive value of the DF and RI intensities (defined above) for self-termination of PVT/VF episodes. All comparisons accounted for uneven data replications in patients and resampling methods have been used to make inferences when dealing with dependent data structures. Specifically, the general Bootstrap Algorithm was used to contrast the intra- and inter-cluster similarity or difference between groups (see also Supplemental material).
Results
Myocardial characteristics and the spectral properties of polymorphic ventricular tachycardia and ventricular fibrillation episodes
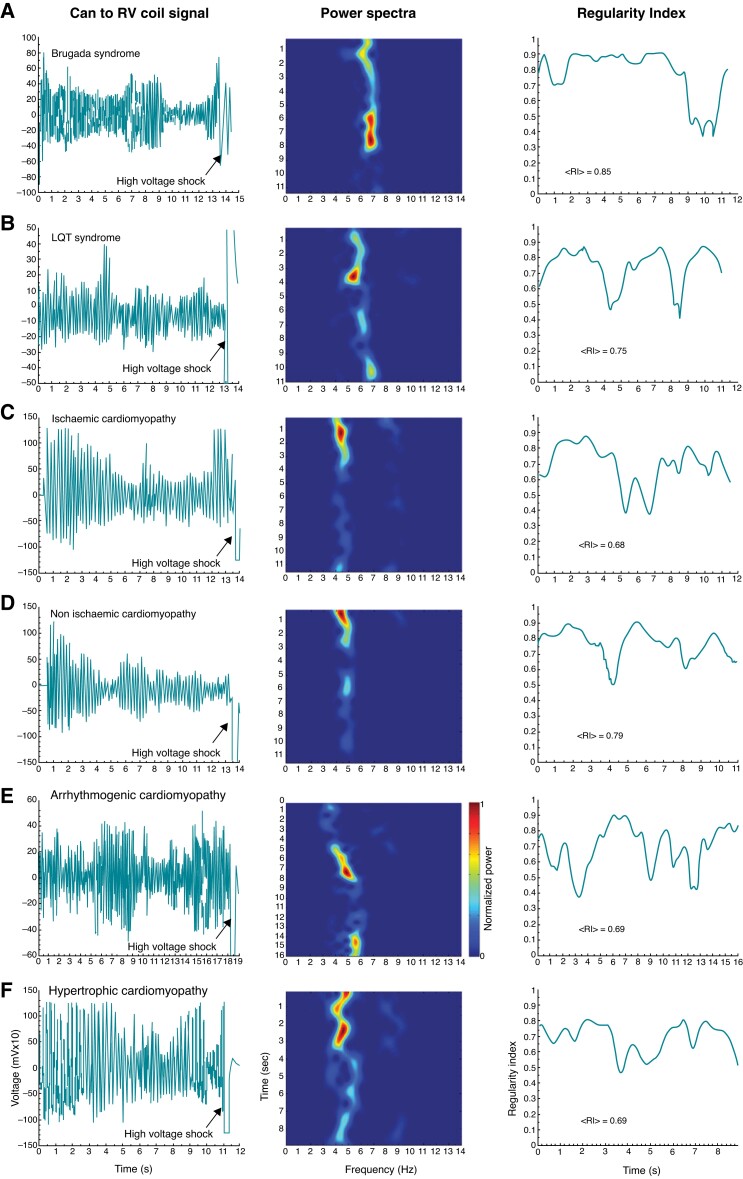
We studied 231 spontaneous PVT/VF episodes recorded by ICDs, of which 58 self-terminated and 173 terminated by shock (from 40 and 74 patients, respectively). The episodes were collected from 87 patients; five patients provided 21, 19, 17, 14 and 13 episodes each (36% of all episodes), whereas 51 patients provided a single episode each (22% of all episodes). The other 31 patients provided variable numbers of episodes ranging between 2 and 8 (42% of episodes). The episodes of the self-terminating group lasted on average 8.6 s (95% CI: 8.1–9.1 s) and were highly significantly shorter than the episodes terminated by shock, which lasted on average 13.9 s (95% CI: 13.6–14.3 s; P < 0.001). The clinical characteristics of patients are shown in Table 1. We included episodes from patients with nine different cardiac pathologies, including mostly electrical (e.g. Brugada syndrome, BrS, and Long QT syndrome, LQTS) and mostly structural and variably scarred [e.g. ischaemic cardiomyopathy (ICM) and hypertrophic cardiomyopathy (HCM), respectively]. As expected, we found significant differences in functional and structural parameters among the disorders, as well as different comorbidities. In contrast, the ICD programing settings for tachycardia detection and the characteristics of leads and generators were similar across the different pathologies (see Supplementary Results and Tables S1, S2, S3, and S4 for details).
Table 1.
Cardiac pathologies and clinical characteristics of patients
| Pathology (N) | Secondary prevention (N;%) | Age at 1st VF (years) | Male sex (N;%) | HYT (N:%) | Heart failure (N:%) | CKD (N:%) | LVEF (%) | QRS width (ms) |
|---|---|---|---|---|---|---|---|---|
| BrS (6) | 4 (67) | 49 ± 11 | 6 (100) | 0 (0) | 0 (0) | 2 (33) | 60 ± 5 | 88 ± 11 |
| LQTS (1) | 1 (100) | 49 | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 60 | 90 |
| ICM (34) | 19 (56) | 67 ± 9 | 27 (79) | 16 (47) | 29 (85) | 5 (15) | 30 ± 7 | 109 ± 23 |
| Non-ICM (22) | 10 (46) | 61 ± 14 | 16 (73) | 11 (50) | 16 (73) | 0 (0) | 31 ± 7 | 114 ± 27 |
| ACM (3) | 2 (67) | 48 ± 20 | 2 (67) | 1 (33) | 1 (33) | 0 (0) | 60 ± 5 | 80 ± 20 |
| HCM (6) | 2 (33) | 40 ± 19 | 5 (83) | 1 (20) | 4 (67) | 0 (0) | 57 ± 6 | 97 ± 15 |
| CCM (3) | 3 (100) | 57 ± 22 | 0 (0) | 0 (0) | 2 (67) | 0 (0) | 55 ± 9 | 117 ± 46 |
| VCM (4) | 3 (75) | 67 ± 9 | 3 (75) | 3 (75) | 3 (75) | 1 (25) | 30 ± 10 | 123 ± 53 |
| Idiopathic VF (8) | 7 (88) | 46 ± 18 | 6 (75) | 2 (25) | 2 (25) | 0 (0) | 60 ± 5 | 87 ± 10 |
| P-value | 0.301 | >0.001 | 0.001 | 0.038 | 0.001 | 0.207 | 0.000 | 0.069 |
BrS, Brugada syndrome; LQTS, long QT syndrome; ICM, ischaemic cardiomyopathy; Non-ICM, non-ischaemic cardiomyopathy; ACM, arrhythmogenic cardiomyopathy; HCM, hypertrophic cardiomyopathy; CCM, congenital cardiomyopathy; VCM, valvular cardiomyopathy; CKD, chronic kidney disease; N, number of patients; NA, not applicable; VF, ventricular fibrillation. P-value: Comparisons between cardiac pathologies.
For initial comparative analysis among pathologies, only episodes terminated by the ICD shock were considered as their duration was longer and more uniform than the self-terminating episodes. Figure 1 illustrates the time courses of voltage recordings of PVT/VF episodes terminated by shock, along with their corresponding power spectra and regularity indices time courses, for 6 representative patients with different electrical or structural heart diseases. In all cases, a non-uniform progression of the episodes’ parameters is seen before shock termination. The power spectra show relatively narrow power bands with varying peak values between 4 and 7 Hz and variable frequency distribution of power across time and pathologies. As shown by the peak power spectral bands for the sample cases in Figure 1 (see also Table 2), primary electrical disorders (Panels A and B: BrS and LQTS) displayed higher mean DF values than structural heart diseases (Panels C–F: non-ischaemic, ICM, arrhythmogenic cardiomyopathy, and HCM). Quantifiable differences in PVT/VF dynamics were reflected also in the regularity of the episodes, in which BrS displayed the highest mean RI during the initial and subsequent 5 s after the onset of the PVT/VF episode (see Table 2). Altogether, the data for PVT/VF episodes terminated by shock indicate that in addition to being a function of time, the mean frequency and regularity depend on the electrophysiological substrate associated with the specific pathology.
Figure 1.
Time course of voltage recordings and spectral parameters of PVT/VF episodes terminated by shock in various cardiac diseases. Panels A–F illustrate representative PVT/VF dynamics for each specified cardiac disease. Left, voltage recorded between the ICD can and the RV coil. Middle: Periodogram for moving 2 s long windows across the voltage time course. Y axis: time of starting of each moving window, X axis: frequency. Colour scale: Normalized power from blue (minimum) to red (maximum) in each patient. Right: RI for moving 2 s long windows across the voltage time course. ICD, implantable cardioverter defibrillator; PVT/VF, polymorphic ventricular tachycardia/ventricular fibrillation; RI, regularity index; mean RI in the first 10 s. RV, right ventricle.
Table 2.
Comparisons between spectral parameters of PVT/VF episodes terminated by shocks in different pathologies
| Pathology (N;n) | 0–5 s | 5–10 s | ||
|---|---|---|---|---|
| DF (Hz) | RI | DF (Hz) | RI | |
| BrS (6;15) | 5.5 (5–5.9) | 0.79 (0.76–0.81) | 5.7 (5.1–6.2) | 0.81 (0.79–0.84) |
| LQTS (1;10) | 5.5 (NA) | 0.71 (NA) | 5.9 (NA) | 0.74 (NA) |
| ICM (28;44) | 4.3 (4.1–4.5) | 0.65 (0.62–0.69) | 4.6 (4.3–4.9) | 0.67 (0.64–0.71) |
| Non-ICM (19;45) | 4.8 (4.5–5) | 0.72 (0.63–0.77) | 5.1 (4.7–5.3) | 0.74 (0.67–0.78) |
| ACM (2;2) | 4.1 (–2.5–10.7) | 0.6 (0.22–0.98) | 4.5 (3.5–5.4) | 0.65 (–0.36–1.67) |
| HCM (6;8) | 3.9 (3.8–4.1) | 0.72 (0.66–0.77) | 4.2 (3.7–4.4) | 0.69 (0.62–0.75) |
| CCM (2;19) | 3.7 (–7.3–16.1) | 0.62 (0.49–0.76) | 3.7 (–9.1–18.2) | 0.63 (0.54–0.70) |
| VCM (4;10) | 4.5 (4.2–5.4) | 0.63 (0.58–0.67) | 4.9 (4.3–5.9) | 0.62 (0.55–0.71) |
| Idiopathic VF (7;9) | 4.9 (4.5–5.6) | 0.72 (0.56–0.75) | 5.1 (4.6–5.9) | 0.69 (0.54–0.73) |
| P-value | 0.0005 | 0.0191 | 0.001 | 0.0057 |
Mean and 95% CI for DF and RI values over the corresponding time periods. BrS, Brugada syndrome; LQTS, long QT syndrome; ICM, ischaemic cardiomyopathy; Non-ICM, non-ischaemic cardiomyopathy; ACM, arrhythmogenic cardiomyopathy; HCM, hypertrophic cardiomyopathy; CCM, congenital cardiomyopathy; VCM, valvular cardiomyopathy; PVT/VF, polymorphic ventricular tachycardia/ventricular fibrillation; 95% CI, 95% confidence interval; DF, dominant frequency; N, number of patients; n, number of episodes; NA, not applicable; RI, regularity index; P values obtained for comparisons between pathologies based on 10 000 iterations in the general bootstrap algorithm.
In a subset of 149 PVT/VF episodes (57 patients) from which information was available on antiarrhythmic drug intake during the time of PVT/VF, episodes in the presence of the Class III antiarrhythmic drug amiodarone displayed lower DF than episodes without the drug. However, there was no significant difference in the magnitude of the RI between those episodes occurring under the effect of amiodarone vs. those which not (Table 3). Further statistical analysis using generalized linear model for the type of pathology, LVEF, QRS width, and presence of class III antiarrhythmic drug at the time of the PVT/VF confirmed the type of pathology as an independent predictor of the magnitude of DF and RI (statistical significancy of type of pathology as predicting variable was found to be P = 0.001, 0.002, <0.001, and <0.001 for DF 0–5 s, RI 0–5 s, DF 5–10 s, and RI 5–10 s, respectively).
Table 3.
Effect of amiodarone on PVT/VF dynamics
| Amiodarone | 0–5 s | 5–10 s | ||
|---|---|---|---|---|
| DF (Hz) | RI | DF (Hz) | RI | |
| Without | 4.8 (4.7–4.9) | 0.68 (0.66–0.70) | 5 (4.9–5.2) | 0.71 (0.69–0.73) |
| With | 4.1 (3.9–4.2) | 0.69 (0.67–0.73) | 4.2 (4.1–4.3) | 0.69 (0.66–0.73) |
| P-value | <0.001 | 0.402 | <0.001 | 0.460 |
Mean and 95% CI for DF and RI values over the corresponding time periods. PVT/VF, polymorphic ventricular tachycardia/ventricular fibrillation; DF, dominant frequency; RI, regularity index. P-values based on univariate analysis.
Distinct spectral dynamics for self-terminating vs. shock-terminated polymorphic ventricular tachycardia and ventricular fibrillation episodes
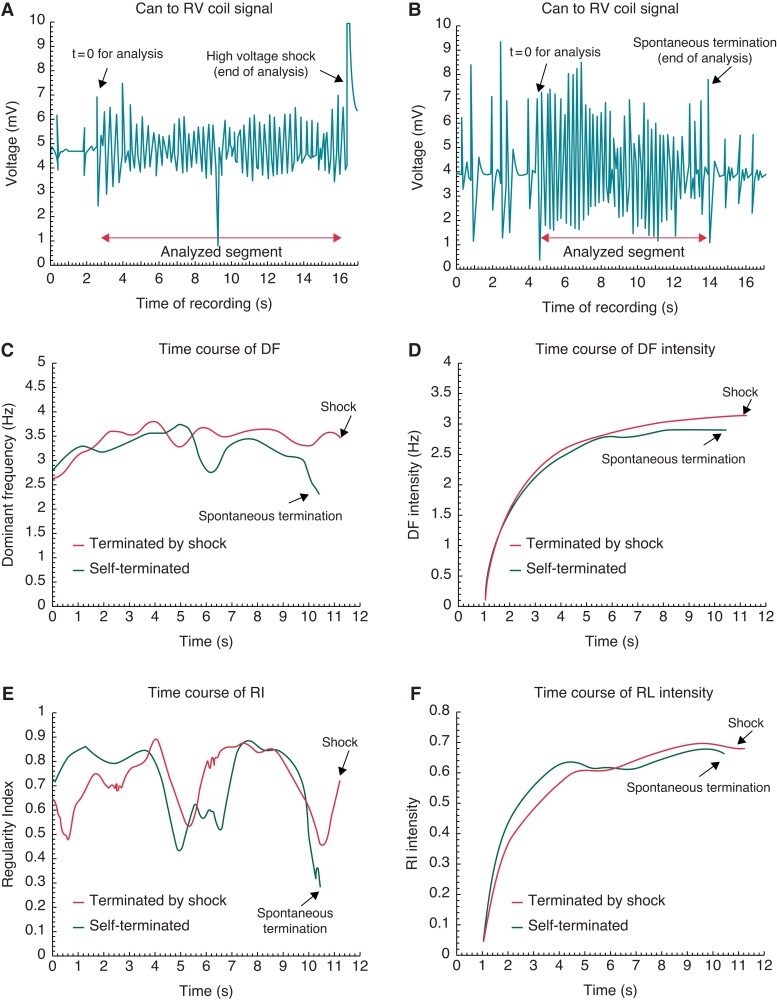
Next, we determined whether the DF and RI time courses are different in self-terminating vs. shock-terminated PVT/VF episodes. Figure 2 shows two different episodes from a patient with ICM. One episode terminated by a shock after 13.3 s (Panel A) and the other self-terminated after 12.5 s (Panel B). Panel C shows that in both cases DF varied greatly over time, but increased gradually during the first 5 s. Thereafter, DF stabilized before the episode was terminated by a shock but decreased substantially for the self-terminated episode. The trend of decreased DF toward the self-termination moment coincides with a prolongation of the inter-beat intervals on the ICD signal toward the end of the PVT/VF episode as shown in Panel B. In panel D, the time course of the DF intensities is the cumulative and time-averaged progression of the DFs shown in panel C (see Methods). Thus, the DF intensity (Panel D) shows smoother progressive time course than the DFs in Panel C. The initial increase was similar for both episodes, but later diverged with slower increase for the self-terminating episode (black trace) than the shock-terminated episode (red trace). Panel E of Figure 2 illustrates further the complex dynamics of the RI of the episodes presented in panels A and B. The RI time courses display non-monotonic variations during both episodes, however notably the RI in the self-terminated episode (black) is lowest at the final moment, coinciding with an amplitude increase of the last few beats in Panel B and a reduction in the DF in panel C (see Supplementary material online, Figures S2 and S3). The RI intensity curves at the final moment of the two episodes are almost indistinguishable, but the self-terminated episode displays a higher RI intensity level at an earlier stage. The data presented in Panels D and F suggest that the intensity curve values during the late stage (>5 s) relative to the early stage (<5 s) may be associated with self-terminating PVT/VF episodes. Figure S4 displays a similar analysis in a BrS patient and shows a behaviour close to the patient displayed in Figure 2, which suggests a possible common spectral markers for self-termination of the PVT/VF episodes prior to the electric shock between patients with structural (ICM) and electrical (BrS) cardiac pathologies.
Figure 2.
Representative examples of shock-terminated (A) and self-terminated PVT/VF (B) in a patient with ischaemic cardiomyopathy. (C) Time course of the DFs of the two analyzed segments in panels A (red) and B (green). (D) The time course of the intensity (See Methods) of the DFs in Panel C. (E) Time course of the RIs of the two analyzed segments in Panels A (red) and B (green). (F) The time course of the intensity (See Methods) of the RIs in panel E. DF, dominant frequency; RI, regularity index; RV, right ventricle.
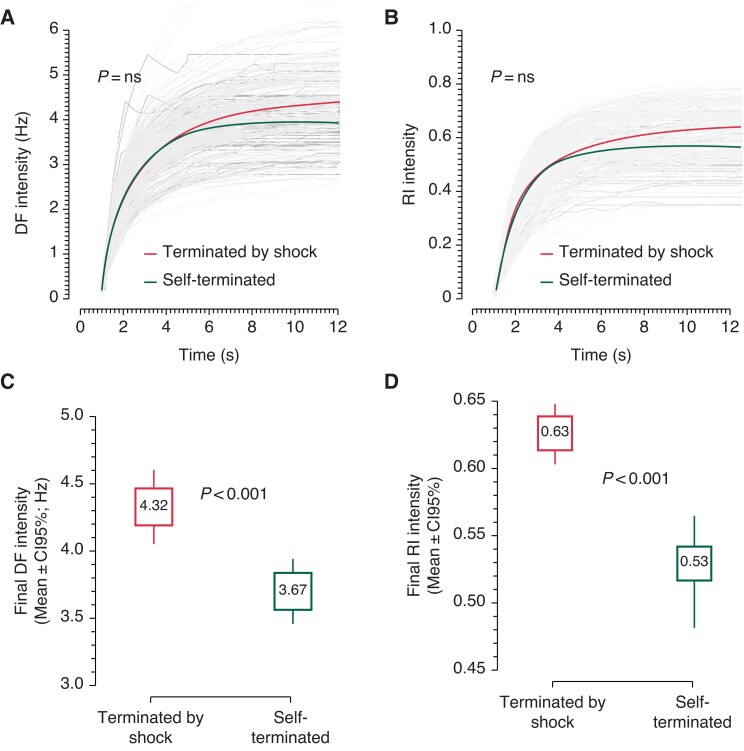
Figure S5 and Figure 3 show cumulative data from patients with a variety of pathologies. Panels A and B in Figure S5 show that the time courses of the mean DF and mean RI gradually diverge from similar initial values with lower values in the self-terminating group than the shock-terminated group toward the end of the episodes. The data in Figure 3 further demonstrate the temporal cumulative spectral dynamics of divergence of the DF and RI intensities. The individual and average intensities of both DF (Panel A) and RI (Panel B) are plotted from 1 s post-PVT/VF detection until the last moment of all self-terminating episodes. The curves demonstrate attenuations in their rate of rise, with the average intensity of self-terminating episodes (black) showing greater attenuations and stabilizations compared with the average intensity of the shock-terminated episodes (red). While the full-time courses of the intensities for the shock-terminated (red) and self-terminated (black) episodes do not significantly differ, their increased divergence over time leads us to surmise that the final portions of the time course will differ between the two groups. Indeed, the graphs in Panels C and D of Figure 3 demonstrate that the very last value of DF intensity is higher for the shock-terminated than for the self-terminating episodes [4.32 (4.04–4.58) vs. 3.67 (3.46–3.93) Hz, P < 0.001]. Similarly, the final RI intensity is higher for the shock-terminated than the self-terminating episodes [0.63 (0.60–0.65) vs. 0.53 (0.48–0.56), P < 0.001].
Figure 3.
Cumulative time course analysis of the Intensity of DF and RI during PVT/VF episodes. Panel A and B show time courses of DF and RI intensity for all episodes (grey) with mean time courses for episodes terminated by shock (red) and self-terminated episodes (green). Panels C and D are box plots of the final DF and RI intensity values, respectively, for all shock-terminated episodes (left) and self-terminated episodes (right). DF, dominant frequency; PVT/VF, polymorphic ventricular tachycardia/ventricular fibrillation; RI, regularity index.
Regularity relates to polymorphic ventricular tachycardia and ventricular fibrillation sustainability regardless of the pathophysiological substrate
We analyzed the progression of DF and RI values with time in controlled models in an attempt to quantify their relationship with the PVT/VF self-termination (see Statistical Methods). In Table 4, hazard ratios based on univariate analysis confirms that RI is related to self-termination while DF is at the limit of statistical significance. Therefore, in the multivariate analysis only RI behaves as an independent predictor of PVT/VF self-termination. The latter remains valid even if the analysis is controlled by the pathophysiology (model adjusted by pathophysiology), or by a summary of clinical variables (fully adjusted model) including age at the first episode, sex, type of pathophysiology, existence of prior documented congestive heart failure, width of the QRS and LVEF. The latter allows the inference that loss of regularity is a feature that relates to self-termination of PVT/VF regardless of the aetiology (i.e. cardiomyopathies vs. primary electrical disorders) or the functional and structural abnormalities affecting the myocardium.
Table 4.
Hazard ratios of frequency and regularity to self-termination
| Model | Variable | HR (95% CI)a | ||
|---|---|---|---|---|
| Unadjusted model | Adjusted by pathology | Fully adjusted | ||
| Univariate | DF | 0.969 (0.844–1.065) | 0.971 (0.842–1.070) | 0.966 (0.841–1.063) |
| RI | 0.952 (0.927–0.977) | 0.952 (0.926–0.978) | 0.953 (0.927–0.979) | |
| Multivariate | DF | 0.986 (0.861–1.082) | 0.985 (0.859–1.049) | 0.983 (0.857–1.047) |
| RI | 0.953 (0.928–0.978) | 0.953 (0.927–0.979) | 0.954 (0.928–0.980) | |
Univariate and multivariate models introduce DF and RI as time-dependent variables and report corresponding hazard ratios (HRs) for self-termination of PVT/VF episodes. The time variables DFs and RIs are for the entire duration of each episode and for all the episodes. The univariate (DF or IR) and multivariate (DF and RI) models analyze the HR for 0.1 Hz DF and 0.01 RI changes. Models are tested unadjusted, adjusted by type of pathology, or fully adjusted by type of pathology and controlling variables of age at the first PVT/VF episode, sex, existence of prior documented congestive heart failure, width of the QRS and LV ejection fraction at the time of the ICD implantation. HR, hazard ratio; 95% CI, 95% confidence interval; DF, dominant frequency; RI, regularity index. a95% CI based on 10 000 iterations of the general bootstrap algorithm.
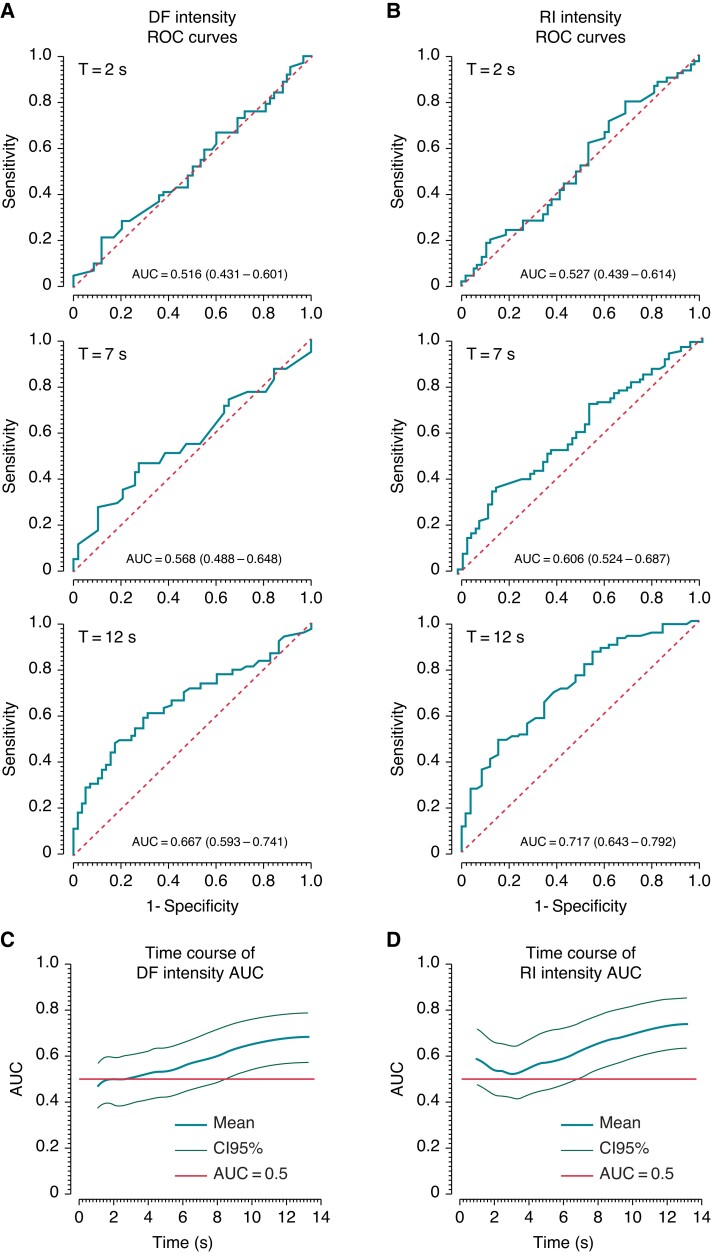
Thereafter, we analyzed the ability to predict the fate of the episodes using ROC on the DF and RI intensity curves, prior to the last moment of the episodes which was already analyzed in Figure 3 and has shown a difference in the final intensity values. In Figure 4, Panels A and B display sample ROC curves for DF and RI intensities, respectively, evaluated at 2 (top), 7 (middle), and 12 s(bottom) from the beginning of the analyzed episodes. Area under the curve (AUC) is seen to progressively increase in time for both DF and RI intensities. Panels C and D plot the continuous time dependency of the AUC values for DF and RI intensities, respectively, with 95% CI limits (grey traces). The plots show that, for both DF and RI, the average AUC increases above the 0.5 value after about 3 s. The 95% CI remains above the 0.5 value at about t = 8.6 and t = 6.95 s for the DF and RI intensities, respectively, marking the times after which the distinction between self-terminating and shock-terminated episodes becomes significant.
Figure 4.
Self-termination predictive value of the DF and RI intensity. Panels A and B display sample ROC curves for DF and RI intensities, respectively, at 2 (top), 7 (middle), and 12 s(bottom) from the beginning of the analyzed episodes. AUC is seen to progressively increase from 2 to 12 s for both DF and RI intensities. Panels C and D plot the time course of the AUC values for DF and RI intensity, respectively, with 95% confidence interval limits (light green traces). Red horizontal lines illustrate AUC = 0.5 levels. AUC, area under the curve; DF, dominant frequency; RI, regularity index; ROC, receiver operating characteristic.
Discussion
The main finding of our study is that the temporal progressions of the frequency and regularity of ICD signals during spontaneous human PVT/VF onset display a distinct pattern before self-termination. A multivariate analysis finds that while there is a gradual increase in frequency and regularity of all recorded episodes, the final frequency and regularity are lower before self-termination than before shock termination. Despite differences in the magnitude of the RI, the distinct time course conducting to self-termination is found in a number of pathologies ranging from cardiac electrical diseases with no apparent structural abnormalities (e.g. BrS and LQTS) to mostly structural heart diseases with variably scarred ventricles (e.g. ischaemic and hypertrophic cardiomyopathies). To our knowledge, our study is the first to investigate dynamic spectral properties of spontaneous PVT/VF episodes with early self-termination and various conditions of the patients.
Comparing our findings in spontaneous PVT/VF episodes with previous studies who analyzed the stationary spectral properties of induced episodes of VF recorded by ICDs reveals similarities and disparities. Panfilov et al. analyzed signals from ICD leads in the RV and found DFs to be reduced by Class III agents but being similar between patients with underlying ischaemic heart disease when compared with dilated cardiomyopathy.3 The agents effects on DFs are consistent with our finding of reduced DFs in the presence of amiodarone (Table 3). However, the similar DFs in the different cardiac pathologies in the study by Panfilov is inconsistent with our finding (Table 2). In another study, Sánchez Muñoz et al. analyzed spectral behaviour over the earliest 3 s duration of induced human VF onsets recorded form ICDs and showed that DF of self-terminated VF was lower than the DF observed in shock-terminated episodes, but the regularity did not differ between the two groups.4 The finding of lower DFs in self-terminating than shock-terminated episodes is consistent with our finding, but the unaffected regularity is inconsistent (Figures 3 and Figure S5). The different timings or algorithms for the DFs and regularity as well as different lead configurations between studies may explain the differences, but the spontaneous vs. induced origin of the episodes between the studies may also play a role in the differences as it was found that the degree of organization is different between induced and spontaneous VF episodes.5,6
Sustainability of human polymorphic ventricular tachycardia and ventricular fibrillation
The mechanisms that maintain ventricular tachycardia and fibrillation are not yet fully understood. Studies have provided evidence for deterministic and hierarchical frequency and phase organization in tachycardia and fibrillation with discrete driving patterns of activation.1,2 Using frequency-phase domain analysis across the standard precordial leads, we recently demonstrated gradients in frequency and phases suggesting high-frequency driving sources localized at pathological substrate areas with higher frequency in VF vs. ventricular tachycardia.1 However, whether the highly periodic inputs drive the arrhythmias or whether they represent epiphenomena embedded in a multiple-wavelet self-sustained system remain controversial. More recently, Panitchob et. al. studied an animal model of long duration VF with detailed mapping of the endocardium (basket catheters) and mid-myocardium using plunged needles.7 They observed chaotic patterns of activation primarily during initial VF, which became more regular as VF persisted and tended to stabilize, consistent with our finding that self-termination of spontaneous PVT/VF is associated with a reduction of regularity. Our observation of dynamic DF and RI decrease before self-termination is consistent with clinical observations by Cismaru et al. who reported on progressive lengthening of inter-beat intervals characterizing self-termination of VF induced in BrS patients during electrophysiological studies.8 Others have demonstrated that induced VF in humans evolves to exhibit more stable activation in the left ventricle, suggesting that there is a trend toward increased organization in sustained fibrillation.9 In addition, quantitative analysis of fibrillatory signals on the ECG has recently pointed to periodic sources as a better explanation for VF dynamics than other proposed mechanisms.10 Altogether, the data presented here highlight organized high-rate activity as an important phenomenon linked to maintenance of human VF.
Medications may however affect the characteristics of underlying mechanisms discussed above. In some of our patients, PVT/VF episodes may have occurred under beta-blocker treatment which could have favour VF to be driven by a single source.11 Nevertheless, a previous study on induced VF recorded by ICDs did not find an effect of beta-blockers on DFs3 and the fact that we observed a similar behaviour during VF in patients not taking such drugs (i.e. BrS) suggests the beta-blocker treatment did not influence our conclusions. Consistent with that study, Krummen et al.2 also found that beta-blockers and ACEI/ARB medications were used by similar number of patients in which induced VF necessitated termination by shock and those in which VF self-terminated. Amiodarone on the other hand was found to impact DFs in our study consistent with other studies.3 In optical mapping of VF in rabbits, amiodarone caused prolongation of VT cycle length and destabilization of rotors.12 Consistently, our data also display that the DF of the PVT/VF is reduced in the presence of amiodarone, but the regularity in our patients cohort remained unaffected suggesting a preserved variation in frequencies variability which requires further investigation.
Implications for implantable defibrillator therapy
Mapping studies have shown that maintenance of VF in humans and animals may be due to high-frequency periodic re-entrant drivers whose stability depends on the underlying pathophysiologic substrate13–16 and could determine whether the VF will sustain or self-terminate.2 In addition to their stability, the activation rate of the re-entrant sources would also determine whether the arrhythmia may manifest as a PVT or as a VF.17 The characteristics of the power spectrum of fibrillatory signals in the frequency domain has also been linked to the ability of the arrhythmia to sustain; a controlled study with exercised animals demonstrated that a wider spectral distribution of the power of ventricular signals (i.e. less regular signals) was associated with the inability to sustain VF.18 Thus a combination of regular periodicity and activation rate metrics such as the DF and RI used in this study might provide analytical framework to track the stability of PVT/VF episodes.
From a therapeutic standpoint, monitoring RI intensity to predict self-termination might help avoid inappropriate shock delivery from the defibrillators. In fact, some studies found that inappropriate shocks on non-sustained rhythms account for 8% of fast ventricular rhythms such as PVT/VF19 and those inappropriate shocks are thought to increase morbidity and mortality.20 Inappropriate electrical shocks have been shown also to be associated with premature battery depletion of the ICD and to balance the ICD utility and longevity several studies have addressed the performance of prolonged detection time and/or shorter cycle thresholds for triggering the shock.20 Our results on the distinct time courses of frequency and regularity during PVT/VF onset offer a rationale for possibly developing algorithms for earlier and more reliable detection of self-terminated PVT/VF than currently available detections. More specifically, Figure 4 shows that the AUCs of DF and RI intensities for detection of self-terminated PVT/VF is a continuously varying function of time. The 95% CI of AUCs for detection of those episodes reach the 0.5 values at and before the average self-termination moment of 8.6 s, respectively. Thus, it is possible that those or other new metrics based on the dynamic spectral characteristic of the early PVT/VF onset be developed to either increase robustness or speed of existing algorithms based on discrete and pre-programed time zones, rates, and delays.20 Such potentially faster and more reliable detection should allow the abortion of a defibrillation shock, thus minimizing battery depletion, and reducing patient´s discomfort and risk.
Limitations
Our study has the following limitations. First, the differentiation between polymorphic VT and VF from the ICD signals is challenging. Thus, we cannot ascertain that the time course of DF and RI as described here relate to one or the other arrhythmia. However, from a risk perspective the two arrhythmias are typically set to trigger a shock. Second, the signals analyzed here were derived exclusively from Medtronic ICDs with far-field recordings between the can and the RV coil and may depend on the technical features of the Medtronic ICDs (e.g. the PVT/VF detection algorithm and the recording initiation). Therefore, our results cannot be extrapolated to ICDs from other manufacturers with different recording techniques. And finally, our results depend on the parameters of our processing algorithms, including filtering, blanking initial periods, moving window size and zero-padded signal length.
Conclusions and clinical implications
We demonstrated that frequency and regularity of activity in spontaneous early human PVT/VF episodes recorded by ICDs varied in time. Compared with episodes terminated by shock, the shorter self-terminated episodes were characterized by more stable frequencies and decreased regularity toward termination. Our findings have two implications: First, our data are consistent with the notion that organized fast sources maintain PVT/VF in humans; and second, monitoring the time course of frequency and regularity in PVT/VF onset may help to limit inappropriate shock delivery from the defibrillators.
Supplementary material
Supplementary material is available at Europace online.
Supplementary Material
Contributor Information
David Calvo, Arrhythmia Unit, Hospital Universitario Central de Asturias, Avd. Roma, s/n; 33011, Oviedo, Spain; Instituto de Investigación Sanitaria del Principado de Asturias, Oviedo, Spain.
Lucia Salinas, Center for Arrhythmia Research, University of Michigan, Ann Arbor, USA.
Pablo Martínez-Camblor, Department of Biomedical Data Science, Geisel Medical School, Hanover, NH, USA.
Daniel García-Iglesias, Arrhythmia Unit, Hospital Universitario Central de Asturias, Avd. Roma, s/n; 33011, Oviedo, Spain; Instituto de Investigación Sanitaria del Principado de Asturias, Oviedo, Spain.
Javier Alzueta, Arrhythmia Unit, Hospital Virgen de la Victoria, Málaga, Spain.
Anibal Rodríguez, Arrhythmia Unit, Hospital Universitario de Canarias, Canarias, Spain.
Rafael Romero, Arrhythmia Unit, Hospital Universitario Ntra Señora de la Candelaria, Canarias, Spain.
Xavier Viñolas, Arrhythmia Unit, Hospital Sant Pau, Barcelona, Spain.
Ignacio Fernández-Lozano, Arrhythmia Unit, Hospital Puerta de Hierro-Majadahonda, Madrid, Spain.
Ignasi Anguera, Arrhythmia Unit, Hospital Bellvitge, Barcelona, Spain.
Julián Villacastín, Arrhythmia Unit, Hospital Clínico San Carlos, Madrid, Spain.
Andrés Bodegas, Arrhythmia Unit, Hospital de Cruces, Bilbao, Spain.
Adolfo Fontenla, Arrhythmia Unit, Hospital 12 de Octubre, Madrid, Spain.
José Jalife, Center for Arrhythmia Research, University of Michigan, Ann Arbor, USA; Cardiac Arrhythmia Laboratory, Myocardial Pathophysiology Area, Centro Nacional de Investigaciones Cardiovasculares (CNIC), Madrid, Spain; CIBER de Enfermedades Cardiovasculares (CIBERCV), Madrid, Spain.
Omer Berenfeld, Center for Arrhythmia Research, University of Michigan, Ann Arbor, USA.
Funding
Supported in part by grants from the Instituto de Salud Carlos III, Spain (PI18/01268) and the Heart Rhythm Society of the Spanish Society of Cardiology (Calvo); National Institutes of Health R01-HL122352 (Jalife), R01-HL118304 and R21-HL153694 (Berenfeld).
Data availability
Data are available on reasonable request to the authors.
References
- 1. Calvo D, Atienza F, Saiz J, Martínez L, Ávila P, Rubín Jet al. Ventricular tachycardia and early fibrillation in patients with Brugada syndrome and ischemic cardiomyopathy show predictable frequency-phase properties on the precordial ECG consistent with the respective arrhythmogenic substrate. Circ Arrhythm Electrophysiol 2015;8:1133–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Krummen DE, Hayase J, Morris DJ, Ho J, Smetak MR, Clopton Pet al. Rotor stability separates sustained ventricular fibrillation from self-terminating episodes in humans. J Am Coll Cardiol 2014;63:2712–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Panfilov I, Lever NA, Smaill BH, Larsen PD. Ventricular fibrillation frequency from implanted cardioverter defibrillator devices. Europace 2009;11:1052–6. [DOI] [PubMed] [Google Scholar]
- 4. Sánchez Muñoz JJ, Rojo Alvarez JL, García Alberola A, Requena Carrión J, Everss E, Ortiz Met al. Spectral analysis of sustained and non-sustained ventricular fibrillation in patients with an implantable cardioverter-defibrillator. Rev Esp Cardiol 2009;62:690–3. [DOI] [PubMed] [Google Scholar]
- 5. Sánchez-Muñoz JJ, Rojo-Alvarez JL, García-Alberola A, Everss E, Alonso-Atienza F, Ortiz Met al. Spectral analysis of intracardiac electrograms during induced and spontaneous ventricular fibrillation in humans. Europace 2009;11:328–31. [DOI] [PubMed] [Google Scholar]
- 6. Lever NA, Newall EG, Larsen PD. Differences in the characteristics of induced and spontaneous episodes of ventricular fibrillation. Europace 2007;9:1054–8. [DOI] [PubMed] [Google Scholar]
- 7. Panitchob N, Li L, Huang J, Ranjan R, Ideker RE, Dosdall DJ. Endocardial activation drives activation Patterns during long-duration ventricular fibrillation and defibrillation. Circ Arrhythm Electrophysiol 2017;10:e005562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cismaru G, Brembilla-Perrot B, Pauriah M, Zinzius PY, Sellal JM, Schwartz Jet al. Cycle length characteristics differentiating non-sustained from self-terminating ventricular fibrillation in Brugada syndrome. Europace 2013;15:1313–8. [DOI] [PubMed] [Google Scholar]
- 9. Vidmar D, Krummen DE, Hayase J, Narayan SM, Ho G, Rappel W-J. Spatiotemporal Progression of Early Human Ventricular Fibrillation. JACC Clin Electrophysiol 2017;3:1437–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ho G, Villongco CT, Yousefian O, Bradshaw A, Nguyen A, Faiwiszewski Yet al. Rotors exhibit greater surface ECG variation during ventricular fibrillation than focal sources due to wavebreak, secondary rotors, and meander. J Cardiovasc Electrophysiol 2017;28:1158–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pak H-N, Oh Y-S, Liu Y-B, Wu T-J, Karagueuzian HS, Lin S-Fet al. Catheter ablation of ventricular fibrillation in rabbit ventricles treated with beta-blockers. Circulation 2003;108:3149–56. [DOI] [PubMed] [Google Scholar]
- 12. Nakagawa H, Honjo H, Ishiguro YS, Yamazaki M, Okuno Y, Harada Met al. Acute amiodarone promotes drift and early termination of spiral wave re-entry. Heart Vessels 2010;25:338–47. [DOI] [PubMed] [Google Scholar]
- 13. Zaitsev AV, Guha PK, Sarmast F, Kolli A, Berenfeld O, Pertsov AMet al. Wavebreak formation during ventricular fibrillation in the isolated, regionally ischemic pig heart. Circ. Res 2003;92:546–53. [DOI] [PubMed] [Google Scholar]
- 14. Chow AWC, Segal OR, Davies DW, Peters NS. Mechanism of pacing-induced ventricular fibrillation in the infarcted human heart. Circulation 2004;110:1725–30. [DOI] [PubMed] [Google Scholar]
- 15. Nair K, Umapathy K, Farid T, Masse S, Mueller E, Sivanandan RVet al. Intramural activation during early human ventricular fibrillation. Circ Arrhythm Electrophysiol 2011;4:692–703. [DOI] [PubMed] [Google Scholar]
- 16. Bourgeois EB, Reeves HD, Walcott GP, Rogers JM. Panoramic optical mapping shows wavebreak at a consistent anatomical site at the onset of ventricular fibrillation. Cardiovasc. Res 2012;93:272–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Samie FH, Mandapati R, Gray RA, Watanabe Y, Zuur C, Beaumont Jet al. A mechanism of transition from ventricular fibrillation to tachycardia : effect of calcium channel blockade on the dynamics of rotating waves. Circ Res 2000;86:684–91. [DOI] [PubMed] [Google Scholar]
- 18. Dor-Haim H, Berenfeld O, Horowitz M, Lotan C, Swissa M. Reduced ventricular arrhythmogeneity and increased electrical complexity in normal exercised rats. PLoS One 2013;8:e66658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chen J, Johnson G, Hellkamp AS, et al. Rapid-rate nonsustained ventricular tachycardia found on implantable cardioverter-defibrillator interrogation: relationship to outcomes in the SCD-HeFT (Sudden Cardiac Death in Heart Failure Trial). J Am Coll Cardiol 2013;61:2161–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moss AJ, Schuger C, Beck CA, Brown MW, Cannom DS, Daubert JPet al. Reduction in inappropriate therapy and mortality through ICD programming. N Engl J Med 2012;367:2275–83. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available on reasonable request to the authors.