Abstract
Advancement in our understanding of immune cell recognition and emerging cellular engineering technologies during the last decades made active manipulation of the T cell response possible. Synthetic immunology is providing us with an expanding set of composite receptor molecules capable to reprogram immune cell function in a predefined fashion. Since the first prototypes in the late 1980s, the design of chimeric antigen receptors (CARs; T-bodies, immunoreceptors), has followed a clear line of stepwise improvements from antigen-redirected targeting to designed “living factories” delivering transgenic products on demand. Building on basic research and creative clinical exploration, CAR T cell therapy has been achieving spectacular success in the treatment of hematologic malignancies, now beginning to improve the outcome of cancer patients. In this study, we briefly review the history of CARs and outline how the progress in the basic understanding of T cell recognition and of cell engineering technologies made novel therapies possible.
Keywords: T-body, immunoreceptor, CAR, chimeric antigen receptor, TCR, T cell receptor, adoptive cell therapy
Introduction
Historically, treatment regimens in oncology are based on three pillars, namely, surgery, chemotherapy, and radiotherapy. Advancement in the basic understanding of the molecular processes involved in T cell recognition and the application of synthetic immunology technologies made it possible to reprogram patients' own immune cells to recognize and combat cancer. These developments during the last decades are providing the basis of rationally designed immunotherapy that is now recognized as the fourth pillar in cancer therapy.
The recent breakthrough in cancer treatment with designed immune cells is a result of a long development in biomedical research and in cellular engineering technologies reaching back to the early times of cellular immunology. It is more than a century ago when Paul Ehrlich presented the hypothesis that the immune system has the power to selectively attack and control cancer.1 Only modern molecular immunology, genetic engineering, and cell manufacturing technologies provided us with tools to test for the hypothesis and to establish immuno-oncology in clinical practice. Within this development, it is just three decades ago that the first synthetic immune receptors were genetically engineered to engraft predefined T cell specificity and to redirect immune effector functions. Among these, chimeric antigen receptor (CAR)-redirected T cells were crowned with success when entering clinical exploration, becoming the first commercial gene transfer therapy being approved by the U.S. Food and Drug Administration (FDA) in August 2017. The FDA Commissioner at that time, Scott Gottlieb, noted the significance of the approval stating that the CAR T cells represent a “milestone in the development of a whole new scientific paradigm for the treatment of serious diseases.”2 While still away from being perfect, CAR T cell therapy provides hope for patients whose cancer became resistant to traditional therapies.
In this study, we briefly outline the major steps in the continuous evolution of the CAR design from the perspective of synthetic immunology and focus on redirecting engineered T cells against cancer. The review aims to indicate how the progress in both the understanding of molecular processes in T cell recognition and immune cell engineering technologies made novel therapies possible that are redirecting patient's own immune cells to attack cancer.
The Basis of Adoptive Cell Therapy: T Cells are Capable to Control Cancer
Since the discovery of T cells originating from the thymus by Miller in the early 1960s3 and the discovery of their lytic activities, cytotoxic T lymphocytes (CTLs) were recognized as major effectors that initiate and mediate an antigen-specific lytic immune response against infected cells and cancer (Fig. 1).4 On the other hand, natural killer (NK) cells, first described by Kiessling et al. (Karolinska Institute, Stockholm) in 1975,5–8 were identified to kill target cells in an antigen-independent fashion, which laid the basis to understand the innate immune response in the control of cancer.
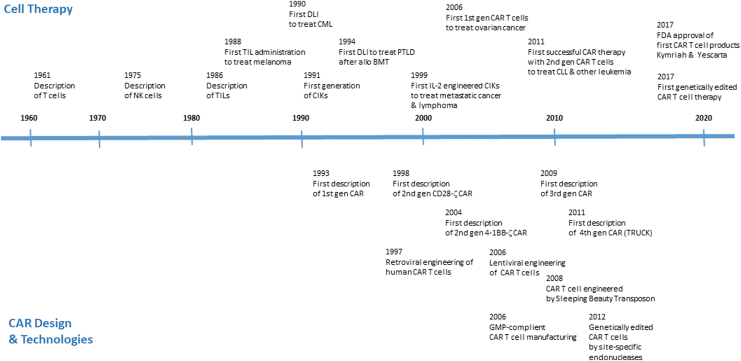
Figure 1.
Overview of major steps in the development of adoptive T cell therapy.
Aiming at using the lytic power of the cellular immune response, Rosenberg and his team at the NCI (Surgical Branch, NIH, Bethesda, MD) established technologies in the early 1980s to generate ex vivo cytotoxic cells in the presence of interleukin (IL)-2, so-called lymphokine-activated killer (LAK) cells, an enriched cell product with lytic activities.9 LAK cells attracted interest due to their ability to kill cancer cells in vitro that are resistant to NK cell-mediated lysis. These efforts resulted in 1985 in the first safe administration of LAK cells together with IL-2 to patients with metastatic cancer.10 In subsequent trials, however, the benefit of LAK cells proved small in comparison to IL-2 administration11,12 making research in more antigen-specific cytotoxic immune cells necessary.
In contrast to NK cells, T cells recognize targets in an antigen-specific fashion and, once activated, can eliminate cancer cells by various means, including perforin/granzymes, Fas ligand, and cytokines like interferon (IFN)-γ and tumor necrosis factor (TNF)-α; however, their activation requires presentation of the respective antigen by professional antigen-presenting cells (APCs). In this situation, melanoma became a tumor of particular interest to study due to its high T cell immunogenicity.13 An additional advantage of studying melanoma at that time was that patient-specific tumor lines could be established from surgical specimens and infiltrating immune cells, in particular tumor infiltrating lymphocytes (TILs), could be isolated and propagated in vitro.14 These T cells recognizing autologous cancer cells appeared to be much more prevalent in melanoma patients for reasons not understood at the time. Melanoma cells frequently undergo high mutation rates displaying mutated peptides as altered self-antigens on the cell surface, where they can be recognized by T cells.15,16
Moreover, there is an intense communication network between innate and adoptive immunity within the tumor lesion, in that also cancer cells finally participate to escape destruction. These observations made clear that cancer patients' immune system is actively experiencing tumor cells,17,18 mostly for long periods of time, and accumulating T cells within the tumor correlate with prognosis.19
By putting these observations together, tumor resident T cells were disputed to transfer antitumor immunity. It was until 1986, when the hypothesis was experimentally evaluated by Rosenberg et al. (NCI, NIH) by isolating TILs from melanoma lesions and amplifying the cells in the presence of recombinant IL-2 for several weeks.14 The ex vivo amplified TILs recognize melanoma-associated antigens and lyse autologous tumor cells in an in vitro short-term assay.20 A milestone toward specific T cell therapy of cancer was made in 1991 when van der Bruggen et al. in Boon et al.'s team (Ludwig Institute for Cancer Research, Brussels, Belgium) identified the antigen recognized by a tumor-reactive T cell clone from a melanoma patient.21 Many other tissue differentiation antigens shared by melanomas and melanocytes were subsequently cloned by a similar procedure.22,23
Built on these laboratory observations, amplified autologous TILs were applied together with IL-2 to melanoma patients producing substantial tumor regression, in some cases for several months and years. The pioneering work first established that systemic infusion of tumor-isolated, ex vivo amplified, and activated autologous T cells can induce an antitumor response.24 Together with the stepwise refinement of the clinical protocol, including the introduction of transient nonmyeloablative lymphodepletion before T cell transfer, called host preconditioning (reviewed in reference25), the Rosenberg team paved the way for clinical application of adoptive T cell therapy in general and specifically for the treatment of melanoma. The TIL approach, unfortunately, was not applicable to all melanoma patients and not to most other malignancies, indicating the need to engraft tumor specificity to naive T cells from patients' peripheral blood that has the advantage that these cells can more easily be obtained and ex vivo amplified for adoptive transfer.
Two years after Rosenberg's report on a TIL trial, Kolb et al. (Technische Universität München, München, Germany) demonstrated therapeutic efficacy of allogeneic donor lymphocyte infusion (DLI) in combination with IFN-α for the treatment of recurrent chronic myeloid leukemia26 in patients after allogeneic stem cell transplantation (allo-SCT). In 1994, O'Reilly achieved by DLI regression of Epstein-Barr virus (EBV)-associated posttransplant lymphoproliferative disease after allo-SCT.27 The pioneering work by these groups together laid the basis of the concept that adoptive transfer of ex vivo amplified T cells is capable to control cancer.
The elucidation of the heterodimeric structure of the T cell receptor complex (TCR) for antigen recognition28–30 and the development of efficient gene transfer technologies paved the way to genetically engineer T cells with antigen specificity. In a significant step forward toward antigen-specific T cell therapy, human T cells were equipped in vitro by gene transfer technologies with transgenic TCR αβ chains gaining human leukocyte antigen (HLA)-restricted specificity for a cancer-associated antigen.31,32 In that time, research was much facilitated by more efficient technologies in transferring vectors to peripheral blood T cells. First applied to treat a melanoma patient, ex vivo TCR-engineered T cells showed remarkable efficacy and tumor selectivity after adoptive transfer.33
These research activities together draw the concept that TILs harbor specificity for the cancer they are isolated from and that the cancer specificity can be transferred to naive T cells by engineering with the respective CAR. Adoptive transfer of such engineered T cells conveys profound anticancer activities. However, several hurdles from the cancer cell site were arising, in particular the frequent downregulation in HLA presentation and/or deficiencies in antigen processing by cancer cells making them invisible to T cells.34 Within the following two decades, recombinant TCRs evolved toward synthetic immune receptors by combining antibody and T cell immune modules, which finally accelerated basic immune cell research to rapidly progress to clinical exploration.
Redirecting T Cells by Synthetic Immune Receptors: The First T-Bodies/Immunoreceptors/Cars
The T cell specificity is defined by the TCR αβ chains, however, the activation is initiated by associated signaling domains, in particular the CD3ζ chain. The molecular cloning of the individual chains of the CD3 complex35 was a major milestone in the stepwise evolution of synthetic immune receptors. Three teams, the Weiss, Seed, and Klausner team, independently drew the concept to covalently link the signaling CD3ζ chain with the extracellular part of CD8, CD4, or the IL-2 receptor CD25, respectively, to obtain a chimeric receptor with both a targeting extracellular part and a signaling intracellular moiety. Expressed by engineered Jurkat T leukemic cells or cytotoxic T cells,36–38 crosslinking the synthetic receptors by target engagement induced T cell activation indicated by induced Ca2+ influx and other activation markers. This was a pioneering step since their work established that antigen-driven crosslinking of CD3ζ is sufficient to initiate a functional response in T cells.
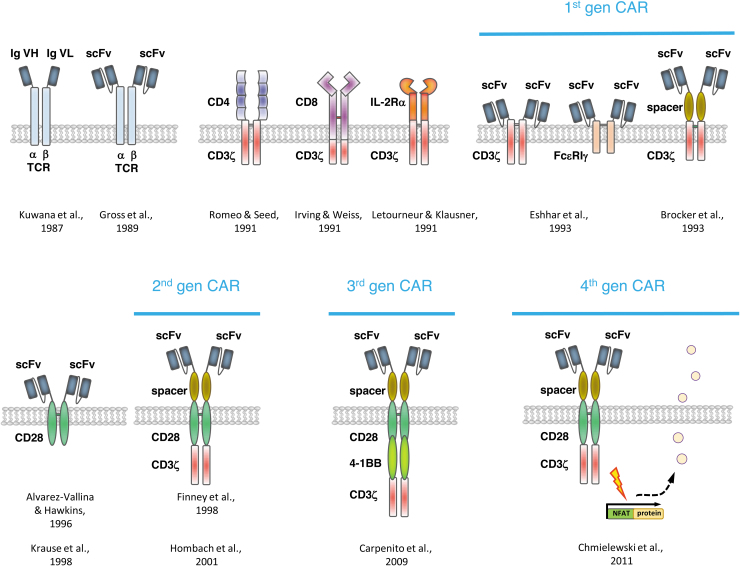
On the other hand, it remained unresolved how to engraft predefined antigen specificity to a T cell activating complex. Since antibodies are prototypes par excellence to provide antigen specificity, the concept was drawn to covalently link antibody-derived binding domains to the TCR constant regions as first reported by Kuwana et al. (Fig. 2).39 This was a significant step forward in redirecting T cells toward defined targets since the major histocompatibility complex (MHC) restriction of the TCR was overcome by an antibody.
Figure 2.
A brief summary of some major steps in the development of CARs. CAR, chimeric antigen receptor.
It was in the late 1980s when the Eshhar's group (Weizmann Institute of Science, Rehovot, Israel) coupled a single-chain fragment of variable region (scFv) antibody specific for the hapten anti-trinitrophenyl to the α- or β-chain of a TCR (Fig. 2).40 Such hybrid molecules formed heterodimers with the TCR chains to recruit the T cell activation signaling machinery. To form a functional antibody, the variable region of the immunoglobulin heavy chain was covalently linked by a short flexible peptide to the variable region of the light chain; the scFv antibody format is still commonly used as antigen-sensing domain of recombinant immune receptors. The Eshhar's group also linked the scFv to the extracellular end of the CD3ζ chain or the high-affinity immunoglobulin E receptor γ chain (FcɛRIγ) to produce T cell activation. Indeed, such antibody/TCR chimera induced IL-2 release and cytolytic activity toward target cells in an MHC-independent fashion.41,42 The same research team redirected CTL hybridoma cells to cancer cells by a recombinant receptor that binds by the linked scFv antibody to the epidermal growth factor receptor (Her2/neu, ErbB2) overexpressed by a variety of cancers.43
Simultaneously, Brocker et al. presented a chimeric receptor composed of a single-chain antibody-binding site that was connected over a short spacer to CD3ζ.44 Following the same construction scheme Moritz et al. reported in 1994 a chimeric receptor ErbB2 and composed of a scFv, a hinge region as a spacer, and the CD3ζ.45 All these examples demonstrated that antigen specificity can be redirected toward a defined antigen by a chimeric receptor consisting of an antibody for targeting and the TCR invariant chain CD3ζ or the γ-chain of the IgE receptor for intracellular activation. Redirected recognition of target cells could not only be achieved in T cell hybridomas but also in peripheral blood T cells of humans and mice.46–48 These chimeric receptors were named by the Eshhar's group “T-body” as a hybrid of an antibody and a TCR, subsequently by our and other groups “immunoreceptors” and are now known as “chimeric antigen receptors”, precisely nowadays CARs of first generation.
The fundamental advantage of the strategy is that engineering with a synthetic receptor ex vivo creates expandable antigen-specific human T cells circumventing the limitations of active immunization to prime T cells in vivo. Another crucial property is that the CAR not only provides MHC-independent targeting but also initiates T cell activation. This is based on the discovery of the CD4 and CD8-p56(lck) complexes and leads to the understanding how receptors that lack intrinsic signaling control the T cell response to antigens by association to nonreceptor kinases (reviewed by reference49). Taken together, the concept of a downstream protein tyrosine phosphorylation cascade initiated by the TCR ζ-chain or the immunoglobulin receptor γ-chain was applied to design the first hybrid receptors that convert binding to defined target into cellular activation.
Because such chimeric receptors bind their cognate target independently of MHC presentation, these scFv-containing receptors can basically also recognize nonclassical T cell targets like carbohydrates and lipids. As early as in 1997, our group demonstrated CAR-redirected T cell targeting toward the carbohydrate TAG72,50 a cancer-associated antigen highly expressed by gastrointestinal carcinoma cells; other examples of nonprotein targeting followed with time.51
A major step toward exploring applicability of CAR T cells for therapeutic application was made in 1999 by Sadelain's group (Memorial Sloan Kettering Cancer Center [MSKCC], New York, NY) by redirecting patients' T cells toward cancer cells through such chimeric receptor in vitro.52 These CAR T cells mediated substantial cytotoxicity, however, showed only poor capacities to expand upon repetitive antigen engagement. Subsequently, our group at Cologne University (Cologne, Germany) demonstrated that patient's T cells engineered with an anti-CD30 CAR can recognize and eliminate autologous T lymphoma cells isolated from patient's biopsy.53
One of the first demonstrations of in vivo efficacy of engineered CAR T cells was provided by Haynes et al.54 who showed that adoptive transfer of genetically retargeted mouse T cells eradicated transplanted colon carcinomas in mice. However, early transgenic mouse models also revealed that such ζ-chain CARs could not sufficiently activate resting T cells44,55 and CAR T cells could only modestly control tumor growth, produced low amounts of IFN-γ, and rapidly entered anergy.55,56
In summary, the first prototype CARs of “first generation” evolved in three substantial stages,
-
(i)
the CD3ζ chain, which lacks extracellular domain, was linked to CD8, CD4, or CD25 to allow signal transmission and T cell activation upon crosslinking36–38;
-
(ii)
linking an scFv in the extracellular part to CD3ζ or FcɛRI γ41,44 made redirecting engineered cells with any predefined specificity possible;
-
(iii)
by retroviral transduction procedures, the fusion receptors were engrafted on the surface of human naive blood T cells paving the way to adoptive cell therapy.57,58
The short-lived CAR-driven T cell activation and insufficient antitumor activity stimulated research toward the next step in the evolution of CARs, the “second generation” CAR, to provide sufficient and lasting activation to the engineered T cell.
A Major Step Toward Clinical Efficacy: Combining Costimulation with the Primary TCR Signal in ine Chimeric Receptor Improves Car T Cell Antitumor Response
TCR signaling controls T cell differentiation59 and is physiologically amplified and modulated by a series of concomitant costimulatory signals. TCR engagement of MHC-loaded antigen presented by dendritic cells along with a costimulatory signal induces clonal expansion and differentiation into effector and memory T cells. According to the “two-signal” hypothesis,60 the primary TCR signal (signal-1) together with costimulation (signal-2) is required for full activation of naive T cells, to drive amplification, to prevent unresponsiveness (“anergy”), some forms of activation-induced cell death (AICD), and exhaustion. While signal-1 and signal-2 are sufficient for inducing CD8+ T cells to proliferate und to produce cytokines, a third signal, signal-3 according to Lafferty's concept,61 is required to execute cytotoxic effector functions.62
Upon engagement of antigen through the TCR αβ chains, the prototype costimulatory receptor CD28 is recruited to the immunological synapse to stabilize the TCR microcluster and enhance TCR signaling. This process results in increased cytokine production, cell cycle progression and amplification, differentiation and survival, and in altered epigenetic structure and cellular metabolism. Other costimulatory receptors, such as 4-1BB (CD137), CD27, OX40, and ICOS, are also involved, each addressing a different, however overlapping panel of functions.63 Based on the understanding of the relevance of costimulation in mediating sufficient T cell activation, it was disputed how to provide signal-2 in addition to primary T cell signaling in the absence of APCs. The prototype CD3ζ CAR, as it was in use in the late 1990s, was by itself not capable to provide costimulatory help.
From the historical perspective, cytokine gene transfer was one of the first strategies to enhance T cell function by providing additional help. In particular, engineering with the IL-2 cDNA allowed robust amplification of T cells in the long term.64,65 The development was dropped, however, since IL-2- or TNF-α-modified TILs did not show clinical benefit over nonmodified TILs.66 Evidence reported by the Sadelain group52 indicated that IFN-γ and IL-2 release upon redirected activation of patients' T cells was increased when the targeted cancer cells express CD80, the ligand for CD28 on T cells. To provide a costimulatory signal for robust signaling, Alvarez-Vallina and Hawkins67 and subsequently the Sadelain group51 linked an extracellular scFv for antigen engagement to the transmembrane and signaling domain of CD28 resulting in a scFv-CD28 receptor (Fig. 2). Such chimeric costimulatory receptor induced IL-2 release and prevented AICD in engineered T cells. In summary, the scFv-CD28 receptor demonstrated that costimulation can be provided to engineered T cells in an antigen-triggered fashion in the absence of CD80 on the tumor cell and in the absence of APCs.
Consequently, several groups tried to combine antigen-dependent CD3ζ signaling by the CAR and concomitant CD28 costimulation within one receptor molecule to augment T cell activation.
It was Finney et al.68 who demonstrated that integrating the CD28 endodomain into the signaling chain of the prototype ζ-chain CAR in a membrane proximal position resulted in a CD28-CD3ζ dual signaling receptor capable of inducing IL-2 release in engineered Jurkat cells. Our group first demonstrated in 2001 that human blood T cells can fully be activated by a CD28-ζ CAR independently of CD80/CD86 on cancer cells indicating CAR-autonomous signaling through both CD3ζ and CD28.69,70 The demonstrated full CAR-driven activation of primary blood T cells was a crucial step toward clinical application; T cell hybridoma or leukemic cells used before are not adequate in this respect due to their neoplastic transformation.
Both CD28 and CD3ζ signaling were simultaneously required to provide a receptor-triggered, MHC-independent fashion in human T cells,69,70 once more sustaining the concept to integrate CD28 and TCR CD3ζ-chain into one dual-signaling receptor. Shortly afterward, the Sadelain group also showed the benefit of integrating CD28 signaling domain into the CD3ζ receptor to provide full T cell activation.51,71 This dual-signaling CAR was capable of redirecting patient's blood T cells toward autologous cancer cells as shown by our group using carcinoembryonic antigen-specific CAR T cells redirected against autologous colon cancer cells isolated from a biopsy72; other examples for leukemia and solid cancer followed from other groups.73,74
The CAR with integrated costimulatory and primary signaling domain is now defined as CAR of “second generation” (Fig. 2); any other costimulatory domain, each with some different impact on T cell function, can be added to the primary signaling domain, including 4-1BB (CD137) as reported in 2004,75 CD27, OX40, or ICOS.76–79 Basically, the second-generation CARs provide the T cell the adequate signals required to bypass anergy and apoptosis, thereby extending CAR T cell function toward more potent antitumor activities, increased cytokine production, and improved amplification and persistence compared with first-generation CARs.75,76,80,81 The benefit in extending T cell performance became obvious when metastases in mice were more efficiently reduced by CD28-ζ CAR T cells compared with the previously used ζ CAR T cells of first generation.81 In a parallel development based on the rationale that lymphocyte-specific protein tyrosine kinase (Lck) promotes CD3ζ immunoreceptor tyrosine-based activation motif phosphorylation, the src-family kinase Lck signaling domain was integrated into the ζ-chain CAR, also resulting in augmented T cell activation.82 This type of CAR has not been studied intensively in the following years, however, nicely demonstrates the modularity and flexibility of CAR design.
Given the biological differences in costimulation by the various receptors, comparative analyses of the CD28-ζ CAR versus 4-1BB-ζ CAR expectedly confirmed that the individual costimulatory domains differently impact a number of functional properties, including the metabolism,83 T cell memory development,83,84 and antigen-independent tonic signaling.85 CD28-ζ CARs induce a strong and rapid T cell activation while 4-1BB-ζ CARs gradually induce the T cell response that persists over a longer period. Moreover, the activation-induced cytokine profile of CAR T cells depends on CAR-provided costimulation; for example, IL-2 and IL-10 are released predominantly upon CD28-ζ and less by 4-1BB-ζ CAR signaling.86 Debulking larger tumor mass is more rapidly performed by CD28-ζ CAR T cells than 4-1BB-ζ CAR T cells, however, the latter compensate the lower cytolytic activity by prolonged activities in time.87,88 It is also becoming clear that the sensitivity to tumor-driven repression is different between the second-generation CARs; CD28-ζ CAR T cells are resistant to transforming growth factor (TGF)-β repression while 4-1BB-ζ CAR T cells are efficiently repressed, which is crucial when targeting solid cancer lesions with high TGF-β load.89
While each costimulatory signal triggers a distinct, although overlapping pattern of T cell responses, for adoptive cell therapy the combination of two costimulatory signals was hypothesized to be beneficial.90,91 First, the group by Carl H. June (University of Pennsylvania, Philadelphia, PA) showed in 2009 that CAR T cells with both CD28 and 4-1BB costimulation successfully controlled large established tumors, which was not the case for CARs providing one costimulatory signal only.92 The observations were confirmed by the Sadelain group91 demonstrating a combined activation of the phosphoinositide 3-kinase/protein kinase B (PKB, AKT) pathway through combined CD28-4-1BB-ζ CAR signaling. Engineered CAR T cells showed increased Bcl-xL, decreased apoptosis in vitro, and augmented tumor elimination in a mouse tumor model. The CAR takes benefit of the cooperativity of CD28 and 4-1BB in augmenting antitumor activity by inducing lytic effector molecules and polarizing toward a type-1 cytokine response. This type of CARs with two costimulatory domains in any combination was established as “third generation” CARs (Fig. 2).
Later, other combinations of costimulatory signals came to interest in specific situations, including the combination CD28-OX4093 which showed superior activity in T cells in advanced stages of development.94 The CD28-OX40 CAR was shown to repress CD28-induced IL-10 release that compromises T cell activity.95 ICOS together with CD28 or 4-1BB costimulation increased the CAR T cell persistence in vivo; MyD88/CD40 costimulation improved in vivo amplification.96 Expanding research in third-generation CARs over the following years, however, revealed that the combination of two costimuli is not in every cell type potent to augment the antitumor efficacy95; current research is focusing back on second-generation CAR T cells, particularly since, in the meantime, these CARs have produced spectacular success in the treatment of hematologic malignancies.
CARs of Second Generation are Taking the Front Seat in Clinical Exploration
Primarily developed to understand TCR-mediated activation, chimeric receptor-redirected T cells rapidly developed toward clinical application bringing hope to control tumors in the long term. First-generation CARs were explored in the early trials targeting ovarian carcinoma in 2006,97 metastatic renal cell carcinoma,98 and neuroblastoma99; however, CAR T cells insufficiently persisted in a functional state to mediate lasting antitumor efficacy.97,100 It is rather the loss of function than the lack of persistence of first-generation CAR T cells that basically contributes to the treatment failure since even a decade after application CAR T cells could be molecularly detected in patients treated for HIV infection.101
For further clinical exploration, CARs of second generation targeting CD19 came into focus to treat B cell malignancies. Using CD19 as target has several advantages; first, CD19 is a B lineage-associated antigen expressed by most B cell leukemia and lymphoma, second, hematologic malignancies are more easily accessible than solid cancer cells, and third, CD19 CAR T cell-induced aplasia of healthy B cells can be clinically managed.
The first successful treatment of a patient with chronic lymphocytic leukemia with CD19-specific CAR T cells was reported in 2011 by June's team at the Abramson Family Cancer Research Institute at the University of Pennsylvania.102 In trials simultaneously performed at the National Cancer Institute (NIH/NCI) and Memorial Sloan-Kettering Cancer Center (MSKCC), both using CD28-ζ CAR T cells, and at the Abramson Center/University of Pennsylvania, using 4-1BB-ζ CAR T cells, all achieved spectacular treatment results of relapsed/refractory B cell malignancies. At the Abramson Center/UPenn and MSKCC, anti-CD19 CAR T cells produced complete remissions in 60–93% of B cell acute lymphocytic leukemia (B-ALL) and 50–93% overall responses in the treatment of B cell non-Hodgkin's lymphoma.103–108 In contrast to first-generation CARs, second-generation CAR T cells amplified in vivo and persisted for prolonged time in the peripheral blood upon application to lymphoma patients.109 While the first treatment responses were very similar, the respective CARs differ in their costimulatory domains with some differences in the response kinetics and pharmacology.110
All trials followed the same clinical regimen primarily developed in the late 1980s by the Rosenberg team at the NCI, comprising leukapheresis to obtain patient's T cells, ex vivo engineering of T cells with a CAR-encoding retro- or lentivirus, amplification of T cells to clinically relevant numbers, and patient “preconditioning” by nonmyeloablative lymphodepletion before CAR T cell infusion. However, due to a number of variables during the entire process on the clinical as well as on the manufacturing side, there is currently no consensus protocol available, which makes comparisons between clinical trial data difficult. Apart thereof, all trials achieved dramatic complete remissions of the targeted disease, some are long lasting. Based on these and more upcoming spectacular results, the journal “Science” proclaimed the CAR T cell therapy together with the checkpoint blockade as “breakthrough of the year 2013.”111
As early as in 2014, several CAR T cell products targeting CD19 received U.S. FDA “breakthrough designation” like Memorial Sloan Kettering Cancer Center CD28-ζ CAR for adult B-ALL,112–114 University of Pennsylvania 4-1BB-ζ CAR for pediatric ALL,115 and National Cancer Institute/Kite CD28-ζ CAR for diffuse large B cell lymphoma (DLBCL)116 (NCT00924326). In 2015 Kite's CD28-ζ CAR (NCT02348216) and in 2016 Juno's 4-1BB-ζ CAR (NCT02631044) followed for the treatment of DLBCL.117
Based on this and other data, in August 2017 U.S. FDA approved Novartis' 4-1BB-ζ CAR-based product tisagenlecleucel (CTL-019; Kymriah®) for pediatric B-ALL118–121 and in May 2018 for DLBLCL. CD28-ζ CAR-based Kite Pharma/Gilead axicabtagene ciloleucel (axi-cel; Yescarta®) was approved in October 2017,122–124 followed by CD28-ζ CAR-based brexucabtagene autoleucel (KTE-X19; Tecartus®)125–127 in 2020, and in February 2021 Juno Therapeutics' 4-1BB-ζ CAR-based lisocabtagene maraleucel (liso-cel; Breyanzi®).128 These CAR T cell products are the best clinically studied CARs to date and are being applied for the treatment of B cell malignancies in the standard-of-care setting. In March 2021, U.S. FDA approved Celgene/BMs' anti-BCMA CAR Abecma® (idecabtagene vicleucel) for the treatment of multiple myeloma. Taken together, the emerging field of CAR T cells targeting CD19 has become a paradigm for evaluating CAR T cell therapy (reviewed by reference76).
With the first trials, it also became clear that CAR T cell administration is often associated with severe systemic toxicities that require intensive care, including the cytokine release syndrome (CRS) or the poorly understood immune effector cell-associated neurotoxicity syndrome (ICANS), which are both usually transient and reversible. Nowadays, clinical scoring and handling protocols of side effects are becoming more established making clinical management more standardized. Research is currently aiming to improve safety in this respect by fine-tuning the CAR or T cell function to reduce the risk for CRS, for instance by disrupting the granulocyte/macrophage colony-stimulating factor locus129 and by cytokine sequestering by a nonsignaling IL-6 receptor.130
The numbers of active trials are steadily increasing in the United States, China,131 Europe,132 and other countries establishing CAR T cell therapy in the forefront of treatment modalities. However, evaluating clinical data in a comparative fashion is complex since a broad diversity in the manufacturing processes and in clinical protocols exists. To address the situation, the CARAMBA consortium set up a first consensus procedure for a trial with 10 partners from 6 EU countries targeting SLAMF7-specific CAR T cells, manufactured by transposon-mediated gene transfer, to treat patients with multiple myeloma (NCT 04499339).
Manufacturing the respective cell products for clinical application was first performed at academic centers, and then taken by commercial suppliers; however, CAR T cell manufacturing for clinical trials remains a bottleneck. Technically, T cells are engineered from the patient's leukapheresis product by a viral vector and amplified in a local or centralized good manufacturing procedure (GMP)-certified facility before given back to the patient.133 While γ-retroviral and lentiviral vectors are used from the beginning, now nonviral vector systems like sleeping beauty transposon and, first in 2017, CRISPR/Cas genome editing134 are also used. The alternatives to viral gene transfer technologies are steadily increasing, including extrachromosomal nonintegrating S/MART vectors, as recently reported for CAR T cell engineering in vitro.135 The manufacturing process was initially, and still is in the majority of trials, done by a manual process136; the entire procedure currently requires 12–15 days; modifications to shorten the process down to 10 days and less are explored in pilot trials.
In parallel to the stepwise optimization of CARs, biotech companies started to develop automated, supervised, and closed manufacturing systems aiming for local use in the hospital to produce the cell product in the near vicinity of the patient. Such decentralized manufacturing spots, however, still require tremendous efforts in investments with respect to the clean room facility, training the manufacturing team, setup of quality control, and other mechanisms to allow GMP-compliant manufacturing of the CAR T cell product.137 Despite these hurdles, the number of decentralized manufacturing spots is continuously growing establishing a new biotech infrastructure on the hospital campus. This is a fundamentally new development beyond the classical hospital pharmacy with respect to producing an individualized living drug for a specific patient by genetic engineering and biotechnological manufacturing.
There are a number of variables that affect the quality of the final CAR T cell product, and not all are identified yet. Among these variables are the ratio of CD4+/CD8+ T cells, the expression level of the respective CAR, the exhaustion and terminal differentiation level of the engineered T cells, and others.138 Research on the preclinical as well as clinical side is aiming to identify and standardize crucial parameters to deliver a potent cell product in a robust process and in due time. It is currently under investigation whether a specific T cell subset such as memory stem cells, a specific polarization of T cells before and/or after genetic engineering, and during expansion will provide clinical benefit. The complexity of CAR design becomes exemplarily obvious by the fact that different anti-BCMA CARs with similar affinity drive T cell activation differently139; targeting the same epitope of CD19 with similar affinity, however, by a structurally different CAR, results in different degrees of T cell activation.140
Along with the success of the first clinical trials in the first decade, established companies as well as a number of young companies started to develop their own CAR products. Companies are running target discovery platforms to identify new and more suitable targets for CAR T cell therapies of particular malignancies; the field is now more than rapidly growing worldwide. While successful in the treatment of hematologic malignancies, the development of CAR T cell therapies for treating solid tumors is recognized to require more sophisticated strategies to overcome the broad panel of tumor-defense mechanisms, including the physical barrier to enter the tumor tissue, the immunosuppressive stroma, the antigen loss or downregulation, and others.141,142
Trucks: Car T Cells Become “Living Factories”
While B cell malignancies are being successfully treated, solid tumors so far resisted CAR T cell treatment that is thought to be due to the specific tumor stroma, including physical barriers in penetrating the tumor tissue, active immune repression, and hostile environment within the tumor and others.143 To address the situation, researchers are aiming to use the CAR T cells not only as targeted “living drugs” but also to produce a transgenic therapeutic protein to sustain the CAR T cell attack. Such “fourth-generation” CAR T cells, or TRUCKs (“T cells redirected for universal cytokine-mediated killing”) as described by our group, produce a therapeutic protein, mostly a cytokine, under control of a CAR-inducible promoter (Fig. 2). The protein is only produced and released when the CAR T cell is engaging the cognate target, thereby delivering the “payload” at the targeted tumor site and only there.144,145 In tissues without CAR-recognized target, no payload protein is released. CAR engagement of antigen triggers a downstream signaling cascade that drives by binding to a nuclear factor of activated T cells responsible enhancer element a minimal promoter for synthesis of the transgenic protein, going far beyond cancer cell targeting. The concept is universal, a number of proteins can basically be delivered; for instance, the proinflammatory IL-12, induced and constitutively expressed,146–148 acts in trans for attracting and activating innate cells in the targeted tumor lesion, repolarizes tumor-resident macrophages and resist inhibitory elements in the tumor tissue, including Treg cells and myeloid-derived suppressor cells.149
Combining the CAR T cell attack with an innate immune response may represent a strategy to prevent tumor relapse by remaining cancer cells that downregulated the target antigen and are invisible to CAR T cells. CAR T cells targeting vascular endothelial growth factor receptor-2 and releasing inducible transgenic IL-12 produce augmented tumor regression.150 TRUCKs with inducible IL-12 and targeting Epstein/Barr nuclear antigen (EBNA)-3C responded even more effectively; released IL-12 recruited additional immune cells, which are generally missing in proximity of lymphoproliferation in immunocompromised posttransplantation lymphoproliferative disorder patients demonstrating a strategy how healthy immune cells can be mobilized to control EBV-associated lymphoproliferation.151 These and other examples show a further development of CAR T cells from “living drugs” toward “living factories” that produce therapeutic proteins with several advantages: (i) the therapeutic protein is deposited in the CAR-targeted lesion, which is otherwise not accessible, (ii) inducible release avoids systemic toxicity of the therapeutic protein while accumulating in the targeted tissue, and (iii) the protein is continuously released achieving locally high levels as long as the TRUCK cell is activated.
In a first-in-man trial, researchers modified TILs, which are thought to be tumor specific without the need to be redirected by a CAR, with inducible IL-12; such IL-12 TILs were highly efficacious in the treatment of melanoma since a therapeutic dose was achieved with 50- to 100-fold fewer cell numbers than commonly with unmodified TILs.152 However, IL-12 TIL therapy was accompanied by severe systemic toxicity, most likely to leakage of the vector in highly activated TILs.
Like cytokines, TRUCK cells were engineered releasing an antibody to block PD1,153 a proteolytic enzyme to degrade the tumor matrix,154 or the costimulatory ligand CD40L to prolong T cell activation,155,156 or the combination of CCL19 and IL-7 to recruit endogenous immune cells and antitumor memory157; the list of transgenic payloads is continuously growing.158,159
A significant further development was made by enhancing T cell intrinsic functions by the inducible release of an immune response modifier. We first demonstrated the feasibility of the strategy by engineering a TRUCK with inducible release of IL-18 that alters the expression of two key transcription factors, T-bet (Tbx21) and forkhead box protein-O1 (FoxO1), in CAR T cells. IL-18 was identified to tip the balance toward improved granzyme-mediated killing capacities.160,161 IL-18 augmented IFN-γ secretion and proliferation of T cells activated by the endogenous TCR159 and CAR.160,161 TRUCKs with inducible IL-18 reinforce their own killing capacity when engaging their cognate target, thereby capable to control larger tumors than the corresponding CAR T cells.162 Another example of an action in cis is the release of IL-15 to polarize CAR T cell function.163 The path for using “living factories” for therapeutic purposes is opened; other possible applications for cancer therapy and beyond will likely be envisaged in the near future.
During three decades of development, CAR T cell engineering has steadily followed an evolution to improve T cell potency and sustainability after adoptive transfer producing an increasing number of CAR generations and strategies to use engineered cells as factories. The evolution is clearly driven by the modularity of the major components of a CAR; the impact of each individual domain on CAR T cell performance is nowadays recognized more important than previously anticipated. Research in this field is becoming much diverse and produced different CAR candidates with different advantages; for details, I refer to a recent review.164 Two developments are currently triggering the next steps in CAR evolution: spiking a “universal” CAR with different targeting domains and engineering logic gating CARs as discussed below.
Cars Evolve Toward Universal Adaptor Receptors And Logic Gating Devices Controlling Cellular Functions in Space and Time
Multiple receptors with different binding partners can be used to transmit activating or inhibiting signals to differentially program T cell activation or inhibition.165 While the approach shows the power of synthetic receptor combinations, however, there is an increasing complexity in the multicomponent systems that require optimization of each component with respect to multiple parameters questioning a robust therapeutic use. To address the shift of targetable antigens within a progressing tumor, CARs are understood as transmembrane signaling molecules with tunable specificity by noncovalently spiking with an adaptor protein that confers the targeting specificity; adding different adaptor proteins allows targeting multiple antigens by the same CAR. The strategy has the advantage of flexibility in specificity to address the risk of antigen escape by administering different targeting antibodies to the patient. In addition, the concepts allow an ON/OFF switch by adding or withdrawing the biotinylated antibody mitigating toxicity. As a proof-of-concept, Urbanska et al.166 designed a CAR with a biotin-binding domain to allow binding of any biotinylated antibody for target recognition. Based thereon, several other adapter-protein-spiked CARs were developed in the following years.167–174 Some of these “uniCARs” are in clinical trials175 to explore the benefits in targeting antigen-heterogeneous tumors.
Recent developments understand conventional CARs to produce single-input, single-output signaling, which is thought not to be sufficient to address the complexity of antigen pattern for specific cancer cell targeting. To solve the situation, “AND” and “OR” Boolean logic gating is currently discussed and translated into a CAR design.
“OR” gating CARs harbor two binding domains, for example, an anti-CD19 and anti-CD20 scFv, both covalently linked together in a specific order.176 Binding of either cognate antigen is sufficient to induce CAR-redirected T cell activation. Such CD20-CD19 or CD19-CD22 dual specific CAR T cells and other combinations are currently in clinical exploration177 with the aim to prevent tumor relapse by cancer cell variants that lost one of the targeted antigens.
In comparison, “AND” gating CARs turned out to be technically more challenging.178 The primary CD3ζ signal was separated from the costimulatory signal, each CAR providing only one signal and each CAR recognizing a different antigen on cancer cells; full T cell activation is only provided upon simultaneous engagement of both targets.179,180 Some leakage in signaling of the CAR with signal-1, however, weakens logic gating of such dual CAR T cells. In a further development, Ho et al.181 proposed to deliver upon engagement of one antigen, a conditionally active molecule that becomes cytotoxic only upon recognition of the second antigen.
As an alternative strategy, two groups simultaneously proposed to take advantage of the synthetic Notch receptor (synNotch) system to achieve combinatorial antigen recognition and to control T cell recognition of complex targets.182,183 The CAR-like synNotch receptor induces the transcriptional activation of an authentic CAR through proteolysis mediated by the transmembrane core domain; the authentic CAR recognizes a second antigen on the cancer cell for driving T cell activation. The strategy has the advantage to cause less off-tumor toxicities compared with a conventional CAR.184 The synNotch receptor harbors an scFv for target recognition, as well as Gal4-VP64 or TetR-VP64, which are transcriptional effector domains required to induce CAR expression. This allows T cell activation only when both the synNotch CAR and the authentic CAR engage their respective targets. The synNotch system is a strategy to integrate multiple cellular inputs into predefined signaling cascades to trigger specific cellular functions in space and time. This will open new fields how to control cellular functions dependent on the environmental tissue. As a result of this development, the discussion arises how to make CAR T cells not only targeting defined tissues but also sensitizing and modulating the tissue they are penetrating.
Toward Environment Sensing Car T Cells
During the last years, identification of the hallmarks of the tumor microenvironment (reviewed by references185,186) have driven research toward a CAR design that allows modulating the tumor stroma. The strategies include (i) enabling CAR T cells to home to and to infiltrate the tumor tissue, (ii) disrupting the suppressive signaling axes in the tumor, (iii) inducing autocrine stimulation to augment CAR T cell amplification and persistence, and (iv) inducing an endogenous immune response.
To home to the tumor site, CAR T cells were guided by chemokine receptors, like CCR8, CXCR6, and others.187–189 It has not only improved homing to the tumor tissue but also attacking the tumor stroma that augments antitumor efficacy. As a result of these analyses, the concept is drawn that successful eradication of advanced tumors requires CAR T cells that target the stroma in addition to targeting the specific cancer cells.190,191 The issue is still underestimated and requires to envision tumors not only as accumulated cancer cells but as an organ orchestrating cancer cells within a connective and immunosuppressive tissue.
Once infiltrated into the tumor tissue, CAR T cells need to stay activated in the hostile environment facing a number of inhibitory molecules, including TGF-β, B7H1, mucin, and prostaglandin E2 (PGE2), a bioactive lipid often upregulated in tumors that activates protein kinase A (PKA).192 To counteract repression, several new CAR strategies are being exploited during the last years pointing to a more advanced functional remodeling the T cell response. To counteract PGE2, CAR T cells were engineered to express a peptide inhibitor of PKA translocation to the immune synapse that improves CAR T cell activation and augments the antitumor attack.193 CAR T cells that express catalase are capable of metabolizing suppressive H2O2 in the tumor, thereby also augmenting the efficacy of their antitumor attack.194
Antibody mediated blockade of immune checkpoints PD1, PD-L1, CTLA-4 and others have revolutionized treatment modalities in the last years195 and are currently translated to CAR T cell therapy by combined treatment in trials, for example, CD19-CD22 CAR T cells together with pembrolizumab (anti-PD1 antibody) (NCT03287817), and CD19 CAR T cells together with utomimulab (anti-CD137 antibody) (NCT03704298) are currently explored. Alternatively, the so-called switch receptors are coexpressed that comprise a suppressive factor-binding domain and an intracellular activating domain, thereby converting engagement of a negative factor into positive T cell activation. A first example is a chimeric receptor binding IL-4 through an IL-4 receptor-α chain and transmitting a positive signal through the IL-7 receptor α-chain or the IL-2 receptor β-chain, thereby augmenting the T cell antitumor activity.196,197 Others are engaging CTLA-4 or PD-1 while transmitting CD28 costimulation.198–201 A switch receptor composed of a binding domain for T cell immunoreceptor with Ig and ITIM domain (TIGIT) protein and a CD28 signaling domain augments cytokine release and T cell activation.202 To modify CAR T cell activity, “armored” CARs with transgenic expression of an additional costimulatory protein, for example, 4-1BB-L, were designed by Stephan et al.90; the CD19 CAR with 4-1BB-L is now explored in a clinical trial (NCT03085173).
Addressing the crucial role of inhibitory receptors, several groups are currently following the strategy to genetically edit the inhibitory receptors in engineered T cells. CRISPR-Cas9-mediated disruption of PD-1 augments killing efficacy of CD19 CAR T cells toward PD-L1 tumors in a mouse model.203 As a proof of concept, a recent trial demonstrated feasibility and safety of CRISPR-depleted PD-1 and the endogenous TCR αβ in NY-ESO-1 TCR-engineered T cells.204 This is a step forward toward genetically edited T cells that are fine tuned for redirected targeting. One application is making allogeneic CAR T cells clinically applicable by targeting the TCR α-locus and the β2 microglobulin locus and to make them resistant to repression by targeting PD-1 or Fas locus.205–207 In continuation of the technology, a pooled knockin screen using CRISPR-Cas9 integration into the TCR α-locus was applied to test for transgenes improving performance of CAR T cells against solid cancer; a newly identified prototype that addresses the issue was a TGF-β engaging 4-1BB signaling switch receptor.208
Given the central role in T cell repression, attempts to counteract TGF-β were early undertaken during CAR development; application of TGF-β blocking antibodies augment antitumor activity.209,210 T cells equipped with a mutant TGF-β receptor gained superior activity and survival in the presence of TGF-β.211–213 Coexpressed with the CAR, a recombinant dominant-negative (DN) receptor sequesters TGF-β from the environment to protect the CAR T cell from repression.214 A clinical trial currently explores the benefit of TGF-β DN receptor while targeting prostate cancer (NCT 03089203). In a further development, TGF-β-targeting CARs and TGF-β switch receptors215,216 provide a stimulatory signal to the CAR T cell in contrast to the DN-TGF-β receptor. Costimulation through CD28 counteracts suppression by TGF-β,217 whereas 4-1BB costimulation does not. TGF-β resistance upon CD28 CAR T cell stimulation is due to released IL-2 and IL-2 receptor signaling. To provide IL-2 receptor signaling in the absence of IL-2, a synthetic receptor was designed that binds IL-7 and provides IL-2 receptor signaling to overcome TGF-β repression.89
There are also examples for an emerging concept to sensitize the tumor tissue before a T cell attack. Early studies interfered with the apoptotic pathway of lymphoma or melanoma cells to make them susceptible to CAR T cells.218,219 A recent strategy is based on pretreating tumors with senescence-inducing drugs prior application of CAR T cells that recognize urokinase-type plasminogen activator receptor broadly expressed during senescence to target and ablate cancer cells that were turned into senescence.220
TCR-Like Cars and CAR-Like TCRs: The Same Immune Receptor at The End?
There are two challenges when redirecting T cells by a transgenic TCR, the mispairing with the endogenous TCR and the rate-limiting competition with the CD3 signaling complex and downstream kinases. While the latter also apply to CARs, modifications of the TCR chains prevented mispairing of the transgenic with the endogenous TCR; adding a signaling domain to the intracellular chains of the TCR could overcome both limitations to a certain extent,221 ending up in a hybrid receptor similar to first-generation CARs.
In contrast to TCRs, the weakness of prototype CARs is still that intracellular proteins cannot be targeted. However, some groups demonstrated that intracellular proteins presented by HLA/peptide complexes can basically be targeted by CAR T cells using a peptide/MHC-specific antibody for binding; this was as exemplarily shown for targeting NY-ESO1.222 To specifically target EBV-infected B cells, a TCR-like CAR was recently reported that recognizes an EBNA-3C-derived peptide in HLA-B*35 in a TCR-like fashion.151 Such TCR-like CAR T cells may have extended capacities compared with classical CARs or TCRs in that intracellular targets can be recognized without major competition with the native TCR or CD3. This may become of major interest in the near future when targeting HLA-presented proteins with tumor-specific mutations being explored on a more systematic and broader basis.
We Just Started to Learn
Looking at the history of cancer therapy, it is rare that a single agent achieves remission of advanced tumors in the long term. Although extraordinarily efficacious in the treatment of hematologic malignancies, CAR T cell therapy will become one pillar in the orchestra of treatment modalities in oncology. During the last three decades, much research has been done on the CAR design to improve antitumor efficacy leading to a growing family of CAR generations. By multilayered approaches, the critical needs of T cells as “living drugs” are being identified and stepwise addressed. Basic research has followed a clear line of improvements in vitro, in mouse models and subsequently translated the CAR T cell strategy in clinical exploration. The success of the second-generation CAR in clinical trials justifies to continue our efforts in making CAR T cell therapy efficacious in targeting solid cancer and in exploring CAR T cells for the treatment of various other diseases.
Currently, much effort is invested to optimize the individual CAR domains on a case-to-case basis with the aim to obtain clinically efficacious CAR T cell products. A growing number of factors are being identified that affect CAR activity, including the targeted antigen, its epitope, its density, and mobility on the cell membrane, the CAR-binding affinity and CAR spacer length, the clustering on T cell surface, and the cell interacting avidity, among others. Consensus on a “one-for-all” prototype CAR design is not in sight, and probably will not be achievable; currently thorough optimization still needs to be performed for each CAR on an empiric basis to obtain a synergistically complemented, modularly calibrated, and clinically efficacious CAR T cell product. Despite extensive safety measures and ongoing optimization in the molecular design, CAR T cell therapy remains experimental with the risk of unpredictable side effects; careful clinical exploration is and will be needed to make such powerful immunotherapy robust and safe.
There was a long way from early recombinant TCR and T-body engineering toward the first commercial approval of a form of gene transfer therapy by the U.S. FDA and European EMA. The success of the anti-CD19 CAR T cell therapy so far clearly indicates the power of immune cell therapy to control leukemia/lymphoma transforming the clinical management of these diseases. CAR T cell therapy demonstrates greater incremental effectiveness and similar cost-effectiveness compared with prior U.S. FDA-approved pharmacological innovations.223 However, great challenges in translating CAR T cell therapy to clinical application remain, including the paucity of preclinical models to evaluate safety and efficacy.
Adoptive cell therapy is now becoming a pillar in immuno-oncology: TILs with or without checkpoint blockers for the therapy of tumors with high mutational burden, TCR, or CAR engineered T cells for tumors with low mutational burden or targetable tumor-related antigen. Efforts are made to engineer T cells, NK cells, and TILs with CARs with greater precision. The field is rapidly moving forward; multiple novel receptors are making preclinical impact, and many biotech and pharma companies are preparing for the next generation of clinical trials.
There are still many nonunderstood hurdles that hamper translating the therapy in particular to solid cancer. There is consensus that CAR T cells require more fine tuning and the immune and tissue environment needs additionally to be addressed. With respect to engineering, recent advancements in genetic editing and in designing novel functional circuits enable us to augment T cell fitness and to design “intelligent” CAR T cells. Recognizing redirected T cells as “living factories,” we can produce a therapeutic protein on demand and in a predefined tissue, which opens new concepts to fight cancer. Technical considerations remain with respect to manufacturing practicability, clinical trial approaches, cell quality and persistence, and patient management. Recent advances also fuel hope that synthetic immunology, genetic engineering and editing, and cell manufacturing will broaden CAR-redirected cell therapy to the relevant T cell subset and to other immune cell types, and to develop new applications beyond oncology, including autoimmunity and infectious diseases.
Acknowledgments
The author likes to thank all members, present and past, of his team and all collaborators around the world during nearly three decades for their enthusiasm and spirit, their suggestions and discussions to move the experimental idea toward clinical reality. Thanks also to research agencies, foundations, and sponsors who made this work possible through their generous grants. The authors apologize to all colleagues whose contributions and clinical trials are not mentioned in this brief review due to space limitations.
Author Disclosure
H.A. is an inventor of patents related to CAR T cell technologies.
Funding Information
No funding was received for writing this review.
References
- 1. The collected papers of Paul Ehrlich. Proc R Soc Med 1957;50:210. [Google Scholar]
- 2. FDA approves CAR-T cell therapy to treat adults with certain types of large B-cell lymphoma. 2017. https://www.fda.gov/news-events/press-announcements (last accessed August 2, 2021).
- 3. Miller JF. Immunological function of the thymus. Lancet Lond Engl 1961;2:748–749. [DOI] [PubMed] [Google Scholar]
- 4. Farhood B, Najafi M, Mortezaee K. CD8+ cytotoxic T lymphocytes in cancer immunotherapy: a review. J Cell Physiol 2019;234:8509–8521. [DOI] [PubMed] [Google Scholar]
- 5. Kiessling R, Klein E, Wigzell H. “Natural” killer cells in the mouse. I. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Specificity and distribution according to genotype. Eur J Immunol 1975;5:112–117. [DOI] [PubMed] [Google Scholar]
- 6. Kiessling R, Klein E, Pross H, et al. “Natural” killer cells in the mouse. II. Cytotoxic cells with specificity for mouse Moloney leukemia cells. Characteristics of the killer cell. Eur J Immunol 1975;5:117–121. [DOI] [PubMed] [Google Scholar]
- 7. Herberman RB, Nunn ME, Lavrin DH. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic acid allogeneic tumors. I. Distribution of reactivity and specificity. Int J Cancer 1975;16:216–229. [DOI] [PubMed] [Google Scholar]
- 8. Herberman RB, Nunn ME, Holden HT, et al. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic and allogeneic tumors. II. Characterization of effector cells. Int J Cancer 1975;16:230–239. [DOI] [PubMed] [Google Scholar]
- 9. Grimm EA, Mazumder A, Zhang HZ, et al. Lymphokine-activated killer cell phenomenon. Lysis of natural killer-resistant fresh solid tumor cells by interleukin 2-activated autologous human peripheral blood lymphocytes. J Exp Med 1982;155:1823–1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rosenberg S. Lymphokine-activated killer cells: a new approach to immunotherapy of cancer. J Natl Cancer Inst 1985;75:595–603. [PubMed] [Google Scholar]
- 11. Law TM, Motzer RJ, Mazumdar M, et al. Phase III randomized trial of interleukin-2 with or without lymphokine-activated killer cells in the treatment of patients with advanced renal cell carcinoma. Cancer 1995;76:824–832. [DOI] [PubMed] [Google Scholar]
- 12. Margolin KA, Aronson FR, Sznol M, et al. Phase II trial of high-dose interleukin-2 and lymphokine-activated killer cells in Hodgkin's disease and non-Hodgkin's lymphoma. J Immunother (1991) 1991;10:214–220. [DOI] [PubMed] [Google Scholar]
- 13. Lotze MT, Custer MC, Bolton ES, et al. Mechanisms of immunologic antitumor therapy: lessons from the laboratory and clinical applications. Hum Immunol 1990;28:198–207. [DOI] [PubMed] [Google Scholar]
- 14. Rosenberg SA, Spiess P, Lafreniere R. A new approach to the adoptive immunotherapy of cancer with tumor-infiltrating lymphocytes. Science 1986;233:1318–1321. [DOI] [PubMed] [Google Scholar]
- 15. Kawakami Y, Nishimura MI, Restifo NP, et al. T-cell recognition of human melanoma antigens. J Immunother Emphas Tumor Immunol 1993;14:88–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kawakami Y, Robbins PF, Wang RF, et al. The use of melanosomal proteins in the immunotherapy of melanoma. J Immunother 1997 1998;21:237–246. [DOI] [PubMed] [Google Scholar]
- 17. Maeurer MJ, Lotze MT. Tumor recognition by the cellular immune system: new aspects of tumor immunology. Int Rev Immunol 1997;14:97–132. [DOI] [PubMed] [Google Scholar]
- 18. Urban JL, Schreiber H. Tumor antigens. Annu Rev Immunol 1992;10:617–644. [DOI] [PubMed] [Google Scholar]
- 19. Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006;313:1960–1964. [DOI] [PubMed] [Google Scholar]
- 20. Topalian SL, Solomon D, Rosenberg SA. Tumor-specific cytolysis by lymphocytes infiltrating human melanomas. J Immunol 1989;142:3714–3725. [PubMed] [Google Scholar]
- 21. van der Bruggen P, Traversari C, Chomez P, et al. A gene encoding an antigen recognized by cytolytic T lymphocytes on a human melanoma. Science 1991;254:1643–1647. [DOI] [PubMed] [Google Scholar]
- 22. Boon T, Cerottini JC, Van den Eynde B, et al. Tumor antigens recognized by T lymphocytes. Annu Rev Immunol 1994;12:337–365. [DOI] [PubMed] [Google Scholar]
- 23. Kawakami Y, Eliyahu S, Delgado CH, et al. Cloning of the gene coding for a shared human melanoma antigen recognized by autologous T cells infiltrating into tumor. Proc Natl Acad Sci U S A 1994;91:3515–3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rosenberg SA, Packard BS, Aebersold PM, et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N Engl J Med 1988;319:1676–1680. [DOI] [PubMed] [Google Scholar]
- 25. Gattinoni L, Powell DJ, Rosenberg SA, et al. Adoptive immunotherapy for cancer: building on success. Nat Rev Immunol 2006;6:383–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kolb HJ, Mittermüller J, Clemm C, et al. Donor leukocyte transfusions for treatment of recurrent chronic myelogenous leukemia in marrow transplant patients. Blood 1990;76:2462–2465. [PubMed] [Google Scholar]
- 27. Papadopoulos EB, Ladanyi M, Emanuel D, et al. Infusions of donor leukocytes to treat Epstein-Barr virus-associated lymphoproliferative disorders after allogeneic bone marrow transplantation. N Engl J Med 1994;330:1185–1191. [DOI] [PubMed] [Google Scholar]
- 28. Hedrick SM, Cohen DI, Nielsen EA, et al. Isolation of cDNA clones encoding T cell-specific membrane-associated proteins. Nature 1984;308:149–153. [DOI] [PubMed] [Google Scholar]
- 29. Hedrick SM, Nielsen EA, Kavaler J, et al. Sequence relationships between putative T-cell receptor polypeptides and immunoglobulins. Nature 1984;308:153–158. [DOI] [PubMed] [Google Scholar]
- 30. Yanagi Y, Yoshikai Y, Leggett K, et al. A human T cell-specific cDNA clone encodes a protein having extensive homology to immunoglobulin chains. Nature 1984;308:145–149. [DOI] [PubMed] [Google Scholar]
- 31. Clay TM, Custer MC, Sachs J, et al. Efficient transfer of a tumor antigen-reactive TCR to human peripheral blood lymphocytes confers anti-tumor reactivity. J Immunol 1999;163:507–513. [PubMed] [Google Scholar]
- 32. Stanislawski T, Voss RH, Lotz C, et al. Circumventing tolerance to a human MDM2-derived tumor antigen by TCR gene transfer. Nat Immunol 2001;2:962–970. [DOI] [PubMed] [Google Scholar]
- 33. Morgan RA, Dudley ME, Wunderlich JR, et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science 2006;314:126–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Seliger B, Ferrone S. HLA class I antigen processing machinery defects in cancer cells-frequency, functional significance, and clinical relevance with special emphasis on their role in T cell-based immunotherapy of malignant disease. Methods Mol Biol 2020;2055:325–350. [DOI] [PubMed] [Google Scholar]
- 35. Weissman AM, Baniyash M, Hou D, et al. Molecular cloning of the zeta chain of the T cell antigen receptor. Science 1988;239:1018–1021. [DOI] [PubMed] [Google Scholar]
- 36. Irving BA, Weiss A. The cytoplasmic domain of the T cell receptor zeta chain is sufficient to couple to receptor-associated signal transduction pathways. Cell 1991;64:891–901. [DOI] [PubMed] [Google Scholar]
- 37. Romeo C, Seed B. Cellular immunity to HIV activated by CD4 fused to T cell or Fc receptor polypeptides. Cell 1991;64:1037–1046. [DOI] [PubMed] [Google Scholar]
- 38. Letourneur F, Klausner RD. T-cell and basophil activation through the cytoplasmic tail of T-cell-receptor zeta family proteins. Proc Natl Acad Sci U S A 1991;88:8905–8909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kuwana Y, Asakura Y, Utsunomiya N, et al. Expression of chimeric receptor composed of immunoglobulin-derived V regions and T-cell receptor-derived C regions. Biochem Biophys Res Commun 1987;149:960–968. [DOI] [PubMed] [Google Scholar]
- 40. Gross G, Gorochov G, Waks T, et al. Generation of effector T cells expressing chimeric T cell receptor with antibody type-specificity. Transplant Proc 1989;21:127–130. [PubMed] [Google Scholar]
- 41. Eshhar Z, Waks T, Gross G, et al. Specific activation and targeting of cytotoxic lymphocytes through chimeric single chains consisting of antibody-binding domains and the gamma or zeta subunits of the immunoglobulin and T-cell receptors. Proc Natl Acad Sci U S A 1993;90:720–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Gross G, Waks T, Eshhar Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc Natl Acad Sci U S A 1989;86:10024–10028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Stancovski I, Schindler DG, Waks T, et al. Targeting of T lymphocytes to Neu/HER2-expressing cells using chimeric single chain Fv receptors. J Immunol 1993;151:6577–6582. [PubMed] [Google Scholar]
- 44. Brocker T, Peter A, Traunecker A, et al. New simplified molecular design for functional T cell receptor. Eur J Immunol 1993;23:1435–1439. [DOI] [PubMed] [Google Scholar]
- 45. Moritz D, Wels W, Mattern J, et al. Cytotoxic T lymphocytes with a grafted recognition specificity for ERBB2-expressing tumor cells. Proc Natl Acad Sci U S A 1994;91:4318–4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Altenschmidt U, Klundt E, Groner B. Adoptive transfer of in vitro-targeted, activated T lymphocytes results in total tumor regression. J Immunol 1997;159:5509–5515. [PubMed] [Google Scholar]
- 47. Jensen M, Tan G, Forman S, et al. CD20 is a molecular target for scFvFc:zeta receptor redirected T cells: implications for cellular immunotherapy of CD20+ malignancy. Biol Blood Marrow Transplant 1998;4:75–83. [DOI] [PubMed] [Google Scholar]
- 48. Hombach A, Heuser C, Sircar R, et al. An anti-CD30 chimeric receptor that mediates CD3-zeta-independent T-cell activation against Hodgkin's lymphoma cells in the presence of soluble CD30. Cancer Res 1998;58:1116–1119. [PubMed] [Google Scholar]
- 49. Rudd CE. How the discovery of the CD4/CD8-p56lck complexes changed immunology and immunotherapy. Front Cell Dev Biol 2021;9:626095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Hombach A, Heuser C, Sircar R, et al. T cell targeting of TAG72+ tumor cells by a chimeric receptor with antibody-like specificity for a carbohydrate epitope. Gastroenterology 1997;113:1163–1170. [DOI] [PubMed] [Google Scholar]
- 51. Krause A, Guo HF, Latouche JB, et al. Antigen-dependent CD28 signaling selectively enhances survival and proliferation in genetically modified activated human primary T lymphocytes. J Exp Med 1998;188:619–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gong MC, Latouche JB, Krause A, et al. Cancer patient T cells genetically targeted to prostate-specific membrane antigen specifically lyse prostate cancer cells and release cytokines in response to prostate-specific membrane antigen. Neoplasia 1999;1:123–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hombach A, Muche JM, Gerken M, et al. T cells engrafted with a recombinant anti-CD30 receptor target autologous CD30 + cutaneous lymphoma cells. Gene Ther 2001;8:891–895. [DOI] [PubMed] [Google Scholar]
- 54. Haynes NM, Snook MB, Trapani JA, et al. Redirecting mouse CTL against colon carcinoma: superior signaling efficacy of single-chain variable domain chimeras containing TCR-ζ vs FcɛRI-γ. J Immunol 2001;166:182–187. [DOI] [PubMed] [Google Scholar]
- 55. Brocker T, Karjalainen K. Signals through T cell receptor-zeta chain alone are insufficient to prime resting T lymphocytes. J Exp Med 1995;181:1653–1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Brocker T. Chimeric Fv-zeta or Fv-epsilon receptors are not sufficient to induce activation or cytokine production in peripheral T cells. Blood 2000;96:1999–2001. [PubMed] [Google Scholar]
- 57. Gallardo HF, Tan C, Ory D, et al. Recombinant retroviruses pseudotyped with the vesicular stomatitis virus G glycoprotein mediate both stable gene transfer and pseudotransduction in human peripheral blood lymphocytes. Blood 1997;90:952–957. [PubMed] [Google Scholar]
- 58. Hombach A, Heuser C, Gerken M, et al. T cell activation by recombinant FcepsilonRI gamma-chain immune receptors: an extracellular spacer domain impairs antigen-dependent T cell activation but not antigen recognition. Gene Ther 2000;7:1067–1075. [DOI] [PubMed] [Google Scholar]
- 59. Nakayama T, Yamashita M. The TCR-mediated signaling pathways that control the direction of helper T cell differentiation. Semin Immunol 2010;22:303–309. [DOI] [PubMed] [Google Scholar]
- 60. Lafferty KJ, Cunningham AJ. A new analysis of allogeneic interactions. Aust J Exp Biol Med Sci 1975;53:27–42. [DOI] [PubMed] [Google Scholar]
- 61. Gill RG, Coulombe M, Lafferty KJ. Pancreatic islet allograft immunity and tolerance: the two-signal hypothesis revisited. Immunol Rev 1996;149:75–96. [DOI] [PubMed] [Google Scholar]
- 62. Thomas R. Signal 3 and its role in autoimmunity. Arthritis Res Ther 2004;6:26–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Croft M. Costimulation of T cells by OX40, 4-1BB, and CD27. Cytokine Growth Factor Rev 2003;14:265–273. [DOI] [PubMed] [Google Scholar]
- 64. Yamada G, Kitamura Y, Sonoda H, et al. Retroviral expression of the human IL-2 gene in a murine T cell line results in cell growth autonomy and tumorigenicity. EMBO J 1987;6:2705–2709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Karasuyama H, Tohyama N, Tada T. Autocrine growth and tumorigenicity of interleukin 2-dependent helper T cells transfected with IL-2 gene. J Exp Med 1989;169:13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Hwu P, Yannelli J, Kriegler M, et al. Functional and molecular characterization of tumor-infiltrating lymphocytes transduced with tumor necrosis factor-alpha cDNA for the gene therapy of cancer in humans. J Immunol 1993;150:4104–4115. [PubMed] [Google Scholar]
- 67. Alvarez-Vallina L, Hawkins RE. Antigen-specific targeting of CD28-mediated T cell co-stimulation using chimeric single-chain antibody variable fragment-CD28 receptors. Eur J Immunol 1996;26:2304–2309. [DOI] [PubMed] [Google Scholar]
- 68. Finney HM, Lawson AD, Bebbington CR, et al. Chimeric receptors providing both primary and costimulatory signaling in T cells from a single gene product. J Immunol 1998;161:2791–2797. [PubMed] [Google Scholar]
- 69. Hombach A, Wieczarkowiecz A, Marquardt T, et al. Tumor-specific T cell activation by recombinant immunoreceptors: CD3ζ signaling and CD28 costimulation are simultaneously required for efficient IL-2 secretion and can be integrated into one combined CD28/CD3ζ signaling receptor molecule. J Immunol 2001;167:6123–6131. [DOI] [PubMed] [Google Scholar]
- 70. Hombach A, Sent D, Schneider C, et al. T-cell activation by recombinant receptors: CD28 costimulation is required for interleukin 2 secretion and receptor-mediated T-cell proliferation but does not affect receptor-mediated target cell lysis. Cancer Res 2001;61:1976–1982. [PubMed] [Google Scholar]
- 71. Maher J, Brentjens RJ, Gunset G, et al. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRζ/CD28 receptor. Nat Biotechnol 2002;20:70–75. [DOI] [PubMed] [Google Scholar]
- 72. Hombach A, Schlimper C, Sievers E, et al. A recombinant anti-carcinoembryonic antigen immunoreceptor with combined CD3ζ-CD28 signalling targets T cells from colorectal cancer patients against their tumour cells. Gut 2006;55:1156–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Koehler P, Schmidt P, Hombach AA, et al. Engineered T cells for the adoptive therapy of B-cell chronic lymphocytic leukaemia. Adv Hematol 2012;2012:595060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Hollyman D, Stefanski J, Przybylowski M, et al. Manufacturing validation of biologically functional T cells targeted to CD19 antigen for autologous adoptive cell therapy. J Immunother 2009;32:169–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Imai C, Mihara K, Andreansky M, et al. Chimeric receptors with 4-1BB signaling capacity provoke potent cytotoxicity against acute lymphoblastic leukemia. Leukemia 2004;18:676–684. [DOI] [PubMed] [Google Scholar]
- 76. van der Stegen SJC, Hamieh M, Sadelain M. The pharmacology of second-generation chimeric antigen receptors. Nat Rev Drug Discov 2015;14:499–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Weinkove R, George P, Dasyam N, et al. Selecting costimulatory domains for chimeric antigen receptors: functional and clinical considerations. Clin Transl Immunol 2019;8:e1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Hombach AA, Holzinger A, Abken H. The weal and woe of costimulation in the adoptive therapy of cancer with chimeric antigen receptor (CAR)-redirected T cells. Curr Mol Med 2013;13:1079–1088. [DOI] [PubMed] [Google Scholar]
- 79. Abken H, Hombach A, Heuser C, et al. Tuning tumor-specific T-cell activation: a matter of costimulation? Trends Immunol 2002;23:240–245. [DOI] [PubMed] [Google Scholar]
- 80. Sadelain M, Brentjens R, Rivière I. The promise and potential pitfalls of chimeric antigen receptors. Curr Opin Immunol 2009;21:215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Haynes NM, Trapani JA, Teng MWL, et al. Single-chain antigen recognition receptors that costimulate potent rejection of established experimental tumors. Blood 2002;100:3155–3163. [DOI] [PubMed] [Google Scholar]
- 82. Geiger TL, Nguyen P, Leitenberg D, et al. Integrated src kinase and costimulatory activity enhances signal transduction through single-chain chimeric receptors in T lymphocytes. Blood 2001;98:2364–2371. [DOI] [PubMed] [Google Scholar]
- 83. Kawalekar OU, O'Connor RS, Fraietta JA, et al. Distinct signaling of coreceptors regulates specific metabolism pathways and impacts memory development in CAR T cells. Immunity 2016;44:380–390. [DOI] [PubMed] [Google Scholar]
- 84. Kalos M, Levine BL, Porter DL, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med 2011;3:95ra73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Long AH, Haso WM, Shern JF, et al. 4-1BB Costimulation ameliorates T cell exhaustion induced by tonic signaling of chimeric antigen receptors. Nat Med 2015;21:581–590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Hombach A, Abken H. Costimulation tunes tumor-specific activation of redirected T cells in adoptive immunotherapy. Cancer Immunol Immunother 2007;56:731–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Zhao Z, Condomines M, van der Stegen SJC, et al. Structural design of engineered costimulation determines tumor rejection kinetics and persistence of CAR T cells. Cancer Cell 2015;28:415–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Abate-Daga D, Davila ML. CAR models: next-generation CAR modifications for enhanced T-cell function. Mol Ther Oncolytics 2016;3:16014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Golumba-Nagy V, Kuehle J, Hombach AA, et al. CD28-ζ CAR T cells resist TGF-β repression through IL-2 signaling, which can be mimicked by an engineered IL-7 autocrine loop. Mol Ther 2018;26:2218–2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Stephan MT, Ponomarev V, Brentjens RJ, et al. T cell-encoded CD80 and 4-1BBL induce auto- and transcostimulation, resulting in potent tumor rejection. Nat Med 2007;13:1440–1449. [DOI] [PubMed] [Google Scholar]
- 91. Zhong X-S, Matsushita M, Plotkin J, et al. Chimeric antigen receptors combining 4-1BB and CD28 signaling domains augment PI3kinase/AKT/Bcl-XL activation and CD8+ T cell-mediated tumor eradication. Mol Ther 2010;18:413–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Carpenito C, Milone MC, Hassan R, et al. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc Natl Acad Sci U S A 2009;106:3360–3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Hombach AA, Abken H. Costimulation by chimeric antigen receptors revisited the T cell antitumor response benefits from combined CD28-OX40 signalling. Int J Cancer 2011;129:2935–2944. [DOI] [PubMed] [Google Scholar]
- 94. Hombach AA, Chmielewski M, Rappl G, et al. Adoptive immunotherapy with redirected T cells produces CCR7- cells that are trapped in the periphery and benefit from combined CD28-OX40 costimulation. Hum Gene Ther 2013;24:259–269. [DOI] [PubMed] [Google Scholar]
- 95. Hombach AA, Rappl G, Abken H. Arming cytokine-induced killer cells with chimeric antigen receptors: CD28 outperforms combined CD28–OX40 “Super-stimulation.” Mol Ther 2013;21:2268–2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Collinson-Pautz MR, Chang W-C, Lu A, et al. Constitutively active MyD88/CD40 costimulation enhances expansion and efficacy of chimeric antigen receptor T cells targeting hematological malignancies. Leukemia 2019;33:2195–2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Kershaw MH, Westwood JA, Parker LL, et al. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin Cancer Res 2006;12:6106–6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Lamers CH, Sleijfer S, van Steenbergen S, et al. Treatment of metastatic renal cell carcinoma with CAIX CAR-engineered T cells: clinical evaluation and management of on-target toxicity. Mol Ther 2013;21:904–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Louis CU, Savoldo B, Dotti G, et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood 2011;118:6050–6056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Park JR, Digiusto DL, Slovak M, et al. Adoptive transfer of chimeric antigen receptor re-directed cytolytic T lymphocyte clones in patients with neuroblastoma. Mol Ther 2007;15:825–833. [DOI] [PubMed] [Google Scholar]
- 101. Scholler J, Brady TL, Binder-Scholl G, et al. Decade-long safety and function of retroviral-modified chimeric antigen receptor T cells. Sci Transl Med 2012;4:132ra53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Porter DL, Levine BL, Kalos M, et al. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med 2011;365:725–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Curran KJ, Margossian SP, Kernan NA, et al. Toxicity and response after CD19-specific CAR T-cell therapy in pediatric/young adult relapsed/refractory B-ALL. Blood 2019;134:2361–2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Maude SL, Laetsch TW, Buechner J, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med 2018;378:439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Lee DW, Kochenderfer JN, Stetler-Stevenson M, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet Lond Engl 2015;385:517–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Schuster SJ, Svoboda J, Chong EA, et al. Chimeric antigen receptor T cells in refractory B-cell lymphomas. N Engl J Med 2017;377:2545–2554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Locke FL, Ghobadi A, Jacobson CA, et al. Long-term safety and activity of axicabtagene ciloleucel in refractory large B-cell lymphoma (ZUMA-1): a single-arm, multicentre, phase 1–2 trial. Lancet Oncol 2019;20:31–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Cerrano M, Ruella M, Perales M-A, et al. The advent of CAR T-cell therapy for lymphoproliferative neoplasms: integrating research into clinical practice. Front Immunol 2020;11:888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Savoldo B, Ramos CA, Liu E, et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J Clin Invest 2011;121:1822–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Cappell KM, Kochenderfer JN. A comparison of chimeric antigen receptors containing CD28 versus 4-1BB costimulatory domains. Nat Rev Clin Oncol 2021 July 6 [Epub ahead of print]; DOI: 10.1038/s41571-021-00530-z. [DOI] [PubMed] [Google Scholar]
- 111. Couzin-Frankel J. Breakthrough of the year 2013. Cancer immunotherapy. Science 2013;342:1432–1433. [DOI] [PubMed] [Google Scholar]
- 112. Davila ML, Riviere I, Wang X, et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med 2014;6:224ra25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Brentjens RJ, Rivière I, Park JH, et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood 2011;118:4817–4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Brentjens RJ, Davila ML, Riviere I, et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci Transl Med 2013;5:177ra38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 2014;371:1507–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Kochenderfer JN, Dudley ME, Kassim SH, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol 2015;33:540–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Turtle CJ, Hanafi L-A, Berger C, et al. Immunotherapy of non-Hodgkin's lymphoma with a defined ratio of CD8+ and CD4+ CD19-specific chimeric antigen receptor-modified T cells. Sci Transl Med 2016;8:355ra116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Schuster SJ, Bishop MR, Tam CS, et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N Engl J Med 2019;380:45–56. [DOI] [PubMed] [Google Scholar]
- 119. Center for Biologics Evaluation and Research. KYMRIAH (tisagenlecleucel). FDA. 2021. https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/kymriah-tisagenlecleucel (last accessed July 14, 2021).
- 120. Ali S, Kjeken R, Niederlaender C, et al. The European Medicines Agency Review of Kymriah (Tisagenlecleucel) for the treatment of acute lymphoblastic leukemia and diffuse large B-cell lymphoma. Oncologist 2020;25:e321–e327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. O'Leary MC, Lu X, Huang Y, et al. FDA approval summary: tisagenlecleucel for treatment of patients with relapsed or refractory B-cell precursor acute lymphoblastic leukemia. Clin Cancer Res 2019;25:1142–1146. [DOI] [PubMed] [Google Scholar]
- 122. Bouchkouj N, Kasamon YL, de Claro RA, et al. FDA approval summary: axicabtagene ciloleucel for relapsed or refractory large B-cell Lymphoma. Clin Cancer Res 2019;25:1702–1708. [DOI] [PubMed] [Google Scholar]
- 123. European Medicines Agency. YESCARTA: axicabtagene ciloleucel. https://www.ema.europa.eu/ (last accessed July 14, 2021).
- 124. U.S. Food & Drug Administration. YESCARTA (axicabtagene ciloleucel). https://www.fda.gov/ (last accessed July 14, 2021).
- 125. Gilead Sciences, Inc., U.S. FDA Approves Kite's Tecartus™, the First and Only CAR T treatment for relapsed or refractory mantle cell lymphoma. https://www.gilead.com/news-and-press (last accessed July 14, 2021).
- 126. U.S. Food & Drug Administration. TECARTUS (brexucabtagene autoleucel). https://www.fda.gov/ (last accessed July 14, 2021).
- 127. U.S. Food and Drug Administration. Approved Cellular and Gene Therapy Products. Silver Spring, MD: U.S. Food and Drug Administration. [Google Scholar]
- 128. Abramson JS, Palomba ML, Gordon LI, et al. Lisocabtagene maraleucel for patients with relapsed or refractory large B-cell lymphomas (TRANSCEND NHL 001): a multicentre seamless design study. Lancet Lond Engl 2020;396:839–852. [DOI] [PubMed] [Google Scholar]
- 129. Sterner RM, Sakemura R, Cox MJ, et al. GM-CSF inhibition reduces cytokine release syndrome and neuroinflammation but enhances CAR-T cell function in xenografts. Blood 2019;133:697–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Tan AHJ, Vinanica N, Campana D. Chimeric antigen receptor-T cells with cytokine neutralizing capacity. Blood Adv 2020;4:1419–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Wei J, Guo Y, Wang Y, et al. Clinical development of CAR T cell therapy in China: 2020 update. Cell Mol Immunol 2021;18:792–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Pinte L, Cunningham A, Trébéden-Negre H, et al. Global perspective on the development of genetically modified immune cells for cancer therapy. Front Immunol 2020;11:608485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Levin AG, Rivière I, Eshhar Z, et al. CAR T cells: building on the CD19 paradigm. Eur J Immunol 2021 July 1 [Epub ahead of print]; DOI: 10.1002/eji.202049064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Eyquem J, Mansilla-Soto J, Giavridis T, et al. Targeting a CAR to the TRAC locus with CRISPR/Cas9 enhances tumour rejection. Nature 2017;543:113–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Bozza M, Roia AD, Correia MP, et al. A nonviral, nonintegrating DNA nanovector platform for the safe, rapid, and persistent manufacture of recombinant T cells. Sci Adv 2021;7:eabf1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Stock S, Schmitt M, Sellner L. Optimizing manufacturing protocols of chimeric antigen receptor T cells for improved anticancer immunotherapy. Int J Mol Sci 2019;20:E6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Ortiz de Landazuri I, Egri N, Muñoz-Sánchez G, et al. Manufacturing and management of CAR T-cell therapy in “COVID-19's Time”: central versus point of care proposals. Front Immunol 2020;11:573179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Levine BL, Miskin J, Wonnacott K, et al. Global manufacturing of CAR T cell therapy. Mol Ther Methods Clin Dev 2016;4:92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Smith EL, Staehr M, Masakayan R, et al. Development and evaluation of an optimal human single-chain variable fragment-derived BCMA-targeted CAR T cell vector. Mol Ther 2018;26:1447–1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Sommermeyer D, Hill T, Shamah SM, et al. Fully human CD19-specific chimeric antigen receptors for T-cell therapy. Leukemia 2017;31:2191–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Mardiana S, Solomon BJ, Darcy PK, et al. Supercharging adoptive T cell therapy to overcome solid tumor-induced immunosuppression. Sci Transl Med 2019;11:eaaw2293. [DOI] [PubMed] [Google Scholar]
- 142. Abken H. Driving CARs on the highway to solid cancer: some considerations on the adoptive therapy with CAR T cells. Hum Gene Ther 2017;28:1047–1060. [DOI] [PubMed] [Google Scholar]
- 143. Balta E, Wabnitz GH, Samstag Y. Hijacked immune cells in the tumor microenvironment: molecular mechanisms of immunosuppression and cues to improve T cell-based immunotherapy of solid tumors. Int J Mol Sci 2021;22:5736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Chmielewski M, Hombach AA, Abken H. Of CARs and TRUCKs: chimeric antigen receptor (CAR) T cells engineered with an inducible cytokine to modulate the tumor stroma. Immunol Rev 2014;257:83–90. [DOI] [PubMed] [Google Scholar]
- 145. Chmielewski M, Abken H. TRUCKs: the fourth generation of CARs. Expert Opin Biol Ther 2015;15:1145–1154. [DOI] [PubMed] [Google Scholar]
- 146. Chmielewski M, Kopecky C, Hombach AA, et al. IL-12 release by engineered T cells expressing chimeric antigen receptors can effectively Muster an antigen-independent macrophage response on tumor cells that have shut down tumor antigen expression. Cancer Res 2011;71:5697–5706. [DOI] [PubMed] [Google Scholar]
- 147. Koneru M, Purdon TJ, Spriggs D, et al. IL-12 secreting tumor-targeted chimeric antigen receptor T cells eradicate ovarian tumors in vivo. Oncoimmunology 2015;4:e994446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. You F, Jiang L, Zhang B, et al. Phase 1 clinical trial demonstrated that MUC1 positive metastatic seminal vesicle cancer can be effectively eradicated by modified anti-MUC1 chimeric antigen receptor transduced T cells. Sci China Life Sci 2016;59:386–397. [DOI] [PubMed] [Google Scholar]
- 149. Pegram HJ, Lee JC, Hayman EG, et al. Tumor-targeted T cells modified to secrete IL-12 eradicate systemic tumors without need for prior conditioning. Blood 2012;119:4133–4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Chinnasamy D, Yu Z, Kerkar SP, et al. Local delivery of interleukin-12 using T cells targeting VEGF receptor-2 eradicates multiple vascularized tumors in mice. Clin Cancer Res 2012;18:1672–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Dragon AC, Zimmermann K, Nerreter T, et al. CAR-T cells and TRUCKs that recognize an EBNA-3C-derived epitope presented on HLA-B*35 control Epstein-Barr virus-associated lymphoproliferation. J Immunother Cancer 2020;8:e000736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Zhang L, Morgan RA, Beane JD, et al. Tumor-infiltrating lymphocytes genetically engineered with an inducible gene encoding interleukin-12 for the immunotherapy of metastatic melanoma. Clin Cancer Res 2015;21:2278–2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Rafiq S, Yeku OO, Jackson HJ, et al. Targeted delivery of a PD-1-blocking scFv by CAR-T cells enhances anti-tumor efficacy in vivo. Nat Biotechnol 2018;36:847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Caruana I, Savoldo B, Hoyos V, et al. Heparanase promotes tumor infiltration and antitumor activity of CAR-redirected T lymphocytes. Nat Med 2015;21:524–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Curran KJ, Seinstra BA, Nikhamin Y, et al. Enhancing antitumor efficacy of chimeric antigen receptor T cells through constitutive CD40L expression. Mol Ther 2015;23:769–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Kuhn NF, Purdon TJ, van Leeuwen DG, et al. CD40 ligand-modified chimeric antigen receptor T cells enhance antitumor function by eliciting an endogenous antitumor response. Cancer Cell 2019;35:473–488.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Adachi K, Kano Y, Nagai T, et al. IL-7 and CCL19 expression in CAR-T cells improves immune cell infiltration and CAR-T cell survival in the tumor. Nat Biotechnol 2018;36:346–351. [DOI] [PubMed] [Google Scholar]
- 158. Hoyos V, Savoldo B, Quintarelli C, et al. Engineering CD19-specific T lymphocytes with interleukin-15 and a suicide gene to enhance their anti-lymphoma/leukemia effects and safety. Leukemia 2010;24:1160–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Hu B, Ren J, Luo Y, et al. Augmentation of antitumor immunity by human and mouse CAR T cells secreting IL-18. Cell Rep 2017;20:3025–3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Chmielewski M, Abken H. CAR T cells releasing IL-18 convert to T-Bethigh FoxO1low effectors that exhibit augmented activity against advanced solid tumors. Cell Rep 2017;21:3205–3219. [DOI] [PubMed] [Google Scholar]
- 161. Kunert A, Chmielewski M, Wijers R, et al. Intra-tumoral production of IL18, but not IL12, by TCR-engineered T cells is non-toxic and counteracts immune evasion of solid tumors. Oncoimmunology 2017;7:e1378842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Chmielewski M, Abken H. TRUCKs with IL-18 payload: toward shaping the immune landscape for a more efficacious CAR T-cell therapy of solid cancer. Adv Cell Gene Ther 2018;1:e7. [Google Scholar]
- 163. Chen Y, Sun C, Landoni E, et al. Eradication of neuroblastoma by T cells redirected with an optimized GD2-specific chimeric antigen receptor and interleukin-15. Clin Cancer Res 2019;25:2915–2924. [DOI] [PubMed] [Google Scholar]
- 164. Holzinger A, Abken H. Chimeric antigen receptor (CAR) redirected T cells. In: Rüker F, Wozniak-Knopp G, eds. Introduction to Antibody Engineering. Cham: Springer International Publishing, 2021;251–302. [Google Scholar]
- 165. Cho JH, Collins JJ, Wong WW. Universal chimeric antigen receptors for multiplexed and logical control of T cell responses. Cell 2018;173:1426–1438.e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Urbanska K, Lanitis E, Poussin M, et al. A universal strategy for adoptive immunotherapy of cancer through use of a novel T-cell antigen receptor. Cancer Res 2012;72:1844–1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Bachmann M. The UniCAR system: a modular CAR T cell approach to improve the safety of CAR T cells. Immunol Lett 2019;211:13–22. [DOI] [PubMed] [Google Scholar]
- 168. Cartellieri M, Feldmann A, Koristka S, et al. Switching CAR T cells on and off: a novel modular platform for retargeting of T cells to AML blasts. Blood Cancer J 2016;6:e458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Herzig E, Kim KC, Packard TA, et al. Attacking latent HIV with convertible CAR-T cells, a highly adaptable killing platform. Cell 2019;179:880–894.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Lee YG, Marks I, Srinivasarao M, et al. Use of a single CAR T cell and several bispecific adapters facilitates eradication of multiple antigenically different solid tumors. Cancer Res 2019;79:387–396. [DOI] [PubMed] [Google Scholar]
- 171. Qi J, Tsuji K, Hymel D, et al. Chemically programmable and switchable CAR-T therapy. Angew Chem Int Ed Engl 2020;59:12178–12185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Raj D, Yang M-H, Rodgers D, et al. Switchable CAR-T cells mediate remission in metastatic pancreatic ductal adenocarcinoma. Gut 2019;68:1052–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Rodgers DT, Mazagova M, Hampton EN, et al. Switch-mediated activation and retargeting of CAR-T cells for B-cell malignancies. Proc Natl Acad Sci U S A 2016;113:E459–E468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Seitz CM, Kieble V, Illi C, et al. Combinatorial targeting of multiple shared antigens by adapter-CAR-T cells (aCAR-Ts) allows target cell discrimination and specific lysis based on differential expression profiles. Blood 2018;132:4543. [Google Scholar]
- 175. Wermke M, Kraus S, Ehninger A, et al. Proof of concept for a rapidly switchable universal CAR-T platform with UniCAR-T-CD123 in relapsed/refractory AML. Blood 2021;137:3145–3148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Tong C, Zhang Y, Liu Y, et al. Optimized tandem CD19/CD20 CAR-engineered T cells in refractory/relapsed B-cell lymphoma. Blood 2020;136:1632–1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Shah NN, Johnson BD, Schneider D, et al. Bispecific anti-CD20, anti-CD19 CAR T cells for relapsed B cell malignancies: a phase 1 dose escalation and expansion trial. Nat Med 2020;26:1569–1575. [DOI] [PubMed] [Google Scholar]
- 178. Hong M, Clubb JD, Chen YY. Engineering CAR-T cells for next-generation cancer therapy. Cancer Cell 2020;38:473–488. [DOI] [PubMed] [Google Scholar]
- 179. Kloss CC, Condomines M, Cartellieri M, et al. Combinatorial antigen recognition with balanced signaling promotes selective tumor eradication by engineered T cells. Nat Biotechnol 2013;31:71–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Wilkie S, van Schalkwyk MCI, Hobbs S, et al. Dual targeting of ErbB2 and MUC1 in breast cancer using chimeric antigen receptors engineered to provide complementary signaling. J Clin Immunol 2012;32:1059–1070. [DOI] [PubMed] [Google Scholar]
- 181. Ho P, Ede C, Chen YY. Modularly constructed synthetic granzyme B molecule enables interrogation of intracellular proteases for targeted cytotoxicity. ACS Synth Biol 2017;6:1484–1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Morsut L, Roybal KT, Xiong X, et al. Engineering customized cell sensing and response behaviors using synthetic Notch receptors. Cell 2016;164:780–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Roybal KT, Rupp LJ, Morsut L, et al. Precision tumor recognition by T cells with combinatorial antigen-sensing circuits. Cell 2016;164:770–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Srivastava S, Salter AI, Liggitt D, et al. Logic-gated ROR1 chimeric antigen receptor expression rescues T cell-mediated toxicity to normal tissues and enables selective tumor targeting. Cancer Cell 2019;35:489–503.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Whiteside T. The tumor microenvironment and its role in promoting tumor growth. Oncogene 2008;27:5904–5912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Gajewski TF, Schreiber H, Fu Y-X. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol 2013;14:1014–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Kobold S, Steffen J, Chaloupka M, et al. Selective bispecific T cell recruiting antibody and antitumor activity of adoptive T cell transfer. J Natl Cancer Inst 2015;107:364. [DOI] [PubMed] [Google Scholar]
- 188. Cadilha BL, Benmebarek M-R, Dorman K, et al. Combined tumor-directed recruitment and protection from immune suppression enable CAR T cell efficacy in solid tumors. Sci Adv 2021;7:eabi5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Lesch S, Blumenberg V, Stoiber S, et al. T cells armed with C-X-C chemokine receptor type 6 enhance adoptive cell therapy for pancreatic tumours. Nat Biomed Eng 2021 June 3 [Epub ahead of print]; DOI: 10.1038/s41551-021-00737-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Textor A, Listopad JJ, Wührmann LL, et al. Efficacy of CAR T-cell therapy in large tumors relies upon stromal targeting by IFNγ. Cancer Res 2014;74:6796–6805. [DOI] [PubMed] [Google Scholar]
- 191. Bunse M, Pfeilschifter J, Bluhm J, et al. CXCR5 CAR-T cells simultaneously target B cell non-Hodgkin's lymphoma and tumor-supportive follicular T helper cells. Nat Commun 2021;12:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Buchbinder EI, Desai A. CTLA-4 and PD-1 pathways: similarities, differences, and implications of their inhibition. Am J Clin Oncol 2016;39:98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Newick K, O'Brien S, Sun J, et al. Augmentation of CAR T-cell trafficking and antitumor efficacy by blocking protein kinase A localization. Cancer Immunol Res 2016;4:541–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Ligtenberg MA, Mougiakakos D, Mukhopadhyay M, et al. Coexpressed catalase protects chimeric antigen receptor-redirected T cells as well as bystander cells from oxidative stress-induced loss of antitumor activity. J Immunol 2016;196:759–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Wei SC, Duffy CR, Allison JP. Fundamental mechanisms of immune checkpoint blockade therapy. Cancer Discov 2018;8:1069–1086. [DOI] [PubMed] [Google Scholar]
- 196. Wilkie S, Burbridge SE, Chiapero-Stanke L, et al. Selective expansion of chimeric antigen receptor-targeted T-cells with potent effector function using interleukin-4. J Biol Chem 2010;285:25538–25544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Leen AM, Sukumaran S, Watanabe N, et al. Reversal of tumor immune inhibition using a chimeric cytokine receptor. Mol Ther 2014;22:1211–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Shin JH, Park HB, Oh YM, et al. Positive conversion of negative signaling of CTLA4 potentiates antitumor efficacy of adoptive T-cell therapy in murine tumor models. Blood 2012;119:5678–5687. [DOI] [PubMed] [Google Scholar]
- 199. Liu X, Ranganathan R, Jiang S, et al. A chimeric switch-receptor targeting PD1 augments the efficacy of second-generation CAR T cells in advanced solid tumors. Cancer Res 2016;76:1578–1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Chmielewski M, Abken H. Release brakes: chimeric antigen receptor T cells with PD-1 switch receptor. Transl Cancer Res 2016 June 5 [Epub ahead of print]; DOI: 10.21037/8157. [DOI] [Google Scholar]
- 201. Kobold S, Grassmann S, Chaloupka M, et al. Impact of a new fusion receptor on PD-1-mediated immunosuppression in adoptive T cell therapy. J Natl Cancer Inst 2015;107:djv146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Hoogi S, Eisenberg V, Mayer S, et al. A TIGIT-based chimeric co-stimulatory switch receptor improves T-cell anti-tumor function. J Immunother Cancer 2019;7:243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Rupp LJ, Schumann K, Roybal KT, et al. CRISPR/Cas9-mediated PD-1 disruption enhances anti-tumor efficacy of human chimeric antigen receptor T cells. Sci Rep 2017;7:737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Stadtmauer EA, Fraietta JA, Davis MM, et al. CRISPR-engineered T cells in patients with refractory cancer. Science 2020;367:eaba7365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Liu X, Zhang Y, Cheng C, et al. CRISPR-Cas9-mediated multiplex gene editing in CAR-T cells. Cell Res 2017;27:154–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Ren J, Liu X, Fang C, et al. Multiplex genome editing to generate universal CAR T cells resistant to PD1 inhibition. Clin Cancer Res 2017;23:2255–2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Ren J, Zhang X, Liu X, et al. A versatile system for rapid multiplex genome-edited CAR T cell generation. Oncotarget 2017;8:17002–17011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Roth TL, Li PJ, Blaeschke F, et al. Pooled knockin targeting for genome engineering of cellular immunotherapies. Cell 2020;181:728–744.e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Maeda H, Shiraishi A. TGF-beta contributes to the shift toward Th2-type responses through direct and IL-10-mediated pathways in tumor-bearing mice. J Immunol 1996;156:73–78. [PubMed] [Google Scholar]
- 210. Gorelik L, Flavell RA. Immune-mediated eradication of tumors through the blockade of TGF- signaling in T cells. Nature Med 2001;7:1118–1122. [DOI] [PubMed] [Google Scholar]
- 211. Bollard CM, Rössig C, Calonge MJ, et al. Adapting a transforming growth factor beta-related tumor protection strategy to enhance antitumor immunity. Blood 2002;99:3179–3187. [DOI] [PubMed] [Google Scholar]
- 212. Wieser R, Attisano L, Wrana JL, et al. Signaling activity of transforming growth factor beta type II receptors lacking specific domains in the cytoplasmic region. Mol Cell Biol 1993;13:7239–7247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Cárcamo J, Zentella A, Massagué J. Disruption of transforming growth factor beta signaling by a mutation that prevents transphosphorylation within the receptor complex. Mol Cell Biol 1995;15:1573–1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Kloss CC, Lee J, Zhang A, et al. Dominant-negative TGF-β receptor enhances PSMA-targeted human CAR T cell proliferation and augments prostate cancer eradication. Mol Ther 2018;26:1855–1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Chang ZL, Lorenzini MH, Chen X, et al. Rewiring T-cell responses to soluble factors with chimeric antigen receptors. Nat Chem Biol 2018;14:317–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Hou AJ, Chang ZL, Lorenzini MH, et al. TGF-β-responsive CAR-T cells promote anti-tumor immune function. Bioeng Transl Med 2018;3:75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Koehler H, Kofler D, Hombach A, et al. CD28 costimulation overcomes transforming growth factor-beta-mediated repression of proliferation of redirected human CD4+ and CD8+ T cells in an antitumor cell attack. Cancer Res 2007;67:2265–2273. [DOI] [PubMed] [Google Scholar]
- 218. Kashkar H, Seeger J-M, Hombach A, et al. XIAP targeting sensitizes Hodgkin lymphoma cells for cytolytic T-cell attack. Blood 2006;108:3434–3440. [DOI] [PubMed] [Google Scholar]
- 219. Seeger JM, Schmidt P, Brinkmann K, et al. The proteasome inhibitor bortezomib sensitizes melanoma cells toward adoptive CTL attack. Cancer Res 2010;70:1825–1834. [DOI] [PubMed] [Google Scholar]
- 220. Amor C, Feucht J, Leibold J, et al. Senolytic CAR T cells reverse senescence-associated pathologies. Nature 2020;583:127–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Sebestyén Z, Schooten E, Sals T, et al. Human TCR that incorporate CD3zeta induce highly preferred pairing between TCRalpha and beta chains following gene transfer. J Immunol 2008;180:7736–7746. [DOI] [PubMed] [Google Scholar]
- 222. Stewart-Jones G, Wadle A, Hombach A, et al. Rational development of high-affinity T-cell receptor-like antibodies. Proc Natl Acad Sci U S A 2009;106:5784–5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Baumgardner JR, Brauer MS, Zhang J, et al. CAR-T therapy and historical trends in effectiveness and cost–effectiveness of oncology treatments. J Comp Eff Res 2020;9:327–340. [DOI] [PubMed] [Google Scholar]