ABSTRACT
Loss of FGF signaling leads to defects in salivary gland branching, but the mechanisms underlying this phenotype remain largely unknown. We disrupted expression of Fgfr1 and Fgfr2 in salivary gland epithelial cells and found that both receptors function coordinately in regulating branching. Strikingly, branching morphogenesis in double knockouts is restored by Fgfr1 and Fgfr2 (Fgfr1/2) knock-in alleles incapable of engaging canonical RTK signaling, suggesting that additional FGF-dependent mechanisms play a role in salivary gland branching. Fgfr1/2 conditional null mutants showed defective cell-cell and cell-matrix adhesion, both of which have been shown to play instructive roles in salivary gland branching. Loss of FGF signaling led to disordered cell-basement membrane interactions in vivo as well as in organ culture. This was partially restored upon introducing Fgfr1/2 wild-type or signaling alleles that are incapable of eliciting canonical intracellular signaling. Together, our results identify non-canonical FGF signaling mechanisms that regulate branching morphogenesis through cell-adhesion processes.
Keywords: FGF, Cell signaling, Branching morphogenesis, Salivary gland, Basement membrane
Highlighted Article: FGFR1 and FGFR2 receptors function coordinately in salivary gland epithelial tissue to regulate branching morphogenesis by impinging on cell adhesion processes independently of FGF canonical signaling.
INTRODUCTION
The submandibular and the sublingual glands (SMG and SLG) regulate most of the saliva production in humans and have been widely studied in the context of branching morphogenesis (Larsen et al., 2010; Patel et al., 2006). In the mouse embryo, a stratified epithelial bud encased within a basement membrane (BM) starts to branch at embryonic (E) day 13. Progressive branching continues through E18.5 and requires instructive signals from the stromal mesenchyme to direct bud expansion followed by recurrent clefting, a feature shared with other branching organs (Costantini and Kopan, 2010; Larsen et al., 2010; Moskwa et al., 2022; Wang et al., 2017). However, ex vivo experiments suggest the salivary gland epithelium has an intrinsic branching potential, provided that appropriate instructive signals are available (Ewald et al., 2008; Nogawa and Takahashi, 1991; Takahashi and Nogawa, 1991).
FGF signaling has been implicated in multiple steps of salivary gland branching. Our current understanding implicates FGF signaling primarily in mesenchymal-epithelial interactions (Chatzeli et al., 2017; Makarenkova et al., 2009; Sakakura et al., 1976; Steinberg et al., 2005; Wei et al., 2007). Fgf10−/− mutants exhibit branching defects in multiple exocrine organs (Hoffman et al., 2002; Jaskoll et al., 2005; Mailleux et al., 2002; Makarenkova et al., 2000; May et al., 2016, 2019; Ohuchi et al., 2000). Fgf8 hypomorphic mutants also exhibit SMG hypoplasia (Jaskoll et al., 2004). Although SMG development remains unaffected in Fgf7−/− mutants, ex vivo culture experiments have shown that FGF7 can potentially regulate SMG branching (Guo et al., 1996; Steinberg et al., 2005). The FGF receptors (FGFRs) FGFR1 and FGFR2 were thought to function in mesenchymal or epithelial contexts, respectively; however, they can be co-expressed and recent reports have shown they can have combinatorial functions (Ornitz and Itoh, 2022; Ray et al., 2020). Loss of the Fgfr2IIIb (Fgfr2tm1.1Dsn) epithelial isoform leads to acute defects in SMG development (De Moerlooze et al., 2000). More subtle effects in SMG branching have been observed by inhibiting Fgfr1 expression ex vivo (Hoffman et al., 2002). In humans, haploinsufficiency of FGF10 and FGFR2 has also been implicated in autosomal dominant salivary and lacrimal gland aplasia (ALSG, OMIM 180920 and OMIM 602115; LADD, OMIM 149730) (Entesarian et al., 2007, 2005; Milunsky et al., 2006; Nie et al., 2006; Shams et al., 2007). Previous studies have shown that FGF signaling has an important function in the maintenance of epithelial progenitors in the SMG and is crucial for branching (Chatzeli et al., 2017).
FGF activation engages canonical intracellular pathways, including ERK1/2, PI3K, PLCγ, JAK-STAT, JNK and p38 (Brewer et al., 2016). Inhibitor studies have implicated ERK1/2 and PI3K in SMG branching downstream of the FGF pathway and other receptor tyrosine kinases (RTKs) (Kashimata et al., 2000; Larsen et al., 2003). However, FGFRs can also engage non-canonical functions (Clark and Soriano, 2022; Ray et al., 2020). Previous studies from our laboratory have revealed that Fgfr1 and Fgfr2 alleles lacking all canonical RTK signaling still retain extensive kinase-dependent biological activity (Brewer et al., 2015; Ray et al., 2020). FGFRs can interact through their extracellular domain with cell-adhesion molecules (Doherty and Walsh, 1996; Francavilla et al., 2007; Latko et al., 2019; Nguyen and Mege, 2016; Sanchez-Heras et al., 2006; Williams et al., 1994) or heparan sulfate proteoglycans (HSPGs), which regulate BM dynamics during SMG development (Makarenkova et al., 2009). Laminin and collagen IV act as indispensable BM components in the salivary gland (Miner and Yurchenco, 2004). Lama5null mutants develop BM defects and attenuated SMG branching (Kadoya et al., 1995; Kadoya and Yamashina, 1993; Rebustini et al., 2007). Laminin in the BM is a crucial substrate for integrin signaling and ex vivo culture experiments have shown that attenuation of integrin signaling function can lead to defects in salivary gland branching (Kadoya et al., 1995; Kadoya and Yamashina, 1993; Kashimata and Gresik, 1997; Wang et al., 2017). Likewise, collagen IV has been implicated in the same process by explant culture experiments in which collagenase treatment led to defects in branching (Wang et al., 2021).
It is unknown exactly how FGFs and downstream signaling pathways regulate salivary gland epithelial branching. Previous studies using ex vivo culture experiments have not fully delineated FGF signaling functions in the stromal mesenchyme versus the salivary gland epithelium. Here, we explore epithelial-specific FGF signaling roles during salivary gland branching. Using conditional deletion of Fgfr1 and Fgfr2 in the salivary gland epithelial tissue, we find that both receptors function coordinately to regulate branching morphogenesis. Surprisingly, we found that these receptors regulate this process not through canonical RTK signaling, but by impinging on cell matrix interactions that regulate branching morphogenesis. Our studies highlight previously unreported functions for FGF signaling during salivary gland development.
RESULTS
Spatial distribution of Fgfr1 and Fgfr2
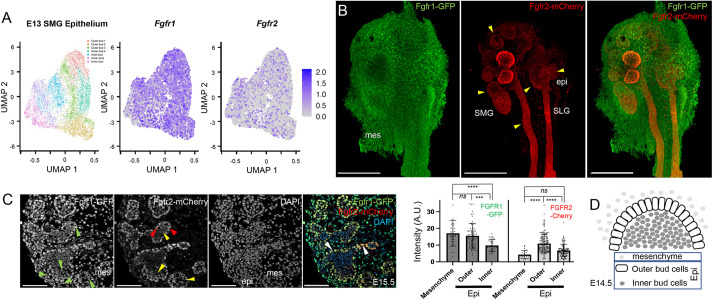
Previous research has shown that FGF signaling plays a crucial role during submandibular gland (SMG) branching. To analyze FGF receptor expression levels and heterogeneity, we mined a previously published E13 mouse SMG scRNA-Seq dataset (GSE159780) (Wang et al., 2021). Based on selective marker expression followed by epithelial cell re-analysis, we identified the seven subpopulations originally visualized by the authors using graph-based dimensional Uniform Manifold Approximation and Projection (UMAP) reductions (Fig. 1A). This analysis showed that, among the four FGF receptors, Fgfr1 and Fgfr2 were predominantly expressed in the SMG epithelial cells, with both receptors showing significant heterogeneity in terms of expression levels (Fig. 1A). Fgfr3 and Fgfr4 were expressed at significantly lower levels and were not further considered in the course of this study (Fig. S1A,B).
Fig. 1.
Expression of Fgfr1 and Fgfr2 in salivary gland. (A) Scatterplot of a single-cell transcriptome from E13 mouse salivary gland epithelium in the GSE159780 dataset. UMAP embedding and color coding represent seven clusters originally identified (Wang et al., 2021). Each dot represents a single cell. Scatterplots in UMAP embedding were also color coded by expression levels for Fgfr1 and Fgfr2. Fgfr1 was more broadly expressed than Fgfr2. (B) GFP and mCherry immunofluorescence shows Fgfr1-GFP (Fgfr1) and Fgfr2-mCherry (Fgfr2) expression from reporter alleles on wholemounts. Broad Fgfr1 expression was observed in epithelial cells (epi) and the stromal mesenchyme (mes) at E13.5. Fgfr2 expression was predominantly restricted to epithelial cells (yellow arrowheads) both in the submandibular gland (SMG) and sublingual gland (SLG). Scale bars: 200 µm. (C) The expression of Fgfr1-GFP and Fgfr2-mCherry was analyzed on sections at E15.5. Broad Fgfr1 expression (green arrowheads) was observed in the mesenchyme, peripheral (outer bud) and core (inner bud) epithelial cells. Strong Fgfr2 expression (red arrowheads) was restricted in the outer bud cells in the SMG. A low and heterogenous Fgfr2 expression was observed in inner bud cells (yellow arrowheads). Numerous peripheral and core epithelial cells co-expressed both Fgfr1 and Fgfr2 (white arrowheads). Scale bars: 100 µm. Integrated fluorescence intensity for Fgfr1 and Fgfr2 were quantified for stromal cells (mesenchyme), peripheral epithelial cells (outer) and inner core epithelial cells (inner) at E15.5 for 80-120 cells in each domain. The bar graph represents the mean integrated fluorescence (±s.d.) from Fgfr1-GFP and Fgfr2-mCherry. ***P<0.001, ****P<0.0001; ns, P<0.05 (one-way ANOVA using Bonferroni multiple comparisons test, two tailed). (D) Schematic showing relative location of stromal and/or mesenchymal cells (mesenchyme), and epithelial (Epi), outer bud and inner bud cells.
We next used fluorescent Fgfr1-GFP and Fgfr2-mCherry reporter alleles (Molotkov et al., 2017) to analyze spatial domains of expression in the salivary glands. In these alleles, expression of Fgfr1 and Fgfr2 is unperturbed, and the fluorescent proteins are inserted downstream of a T2A self-cleaving peptide to avoid any associated phenotypes that may be due to alterations in Fgfr gene levels. At E14.5, when several rounds of branching have occurred and the acinar program has initiated, Fgfr1 was broadly expressed in the SMG and SLG epithelial cells, as well as in mesenchyme/stroma. In contrast, Fgfr2 expression was restricted to epithelial cells (Fig. 1B). The outer bud epithelial cells, which make strong cell-basement membrane interactions and play important roles in remodeling the BM during branching, expressed higher levels of both Fgfr1 and Fgfr2. Outer bud cells subsequently lose cell-basement membrane contacts during epithelial expansion and populate the inner core (Wang et al., 2021). We further analyzed expression of Fgfr1-GFP and Fgfr2-mCherry on sections at E15.5. Broad Fgfr1 expression was observed at this stage in both outer and inner bud epithelial and stromal (mesenchymal) cells. Strong Fgfr2 expression was found primarily in the outer bud epithelial cell population, similar to Fgfr1. The majority of inner bud cells co-expressed Fgfr1 and Fgfr2 at generally lower and heterogeneous levels (Fig. 1C,D). Together, our analysis showed that both FGF receptors are dynamically expressed during SMG development, with high levels of both receptors in outer bud epithelial cells and heterogenous expression in inner core epithelial cells.
Fgfr1 and Fgfr2 regulate SMG branching
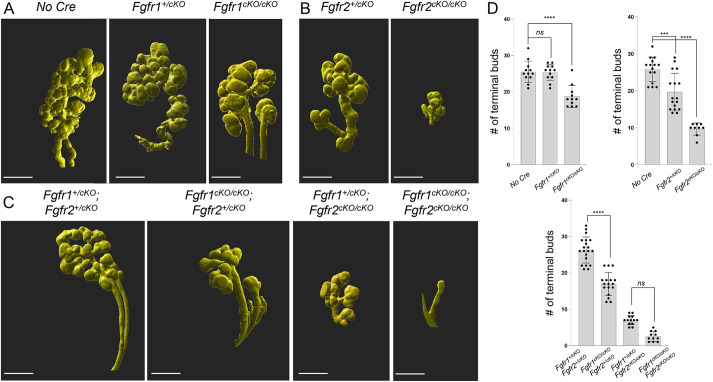
Salivary glands progressively branch between E13 and E18.5. To further understand the role of Fgfr1 and Fgfr2 in epithelial cells during branching morphogenesis, we combined a K14Cre driver that is active in the SMG (Lombaert et al., 2013) with conditional null alleles for Fgfr1 and Fgfr2 (henceforth referred to as cKO) to attenuate FGF signaling in the salivary gland epithelium and ductal progenitors (Fig. S2A,B). Tissues were harvested at E14.5, followed by immunostaining of epithelial cells for E-cadherin (ECAD) in whole mounts, and the number of terminal buds were counted for conditional mutants of Fgfr1 (Fig. 2A,D), Fgfr2 (Fig. 2B,D) and compound (Fgfr1/2) double mutants (Fig. 2C,D), as well as Cre-negative controls from ECAD 3D rendered images. Fgfr1+/cKO mutants did not develop branching defects. However, a 43% reduction in the number of terminal buds was observed in Fgfr1cKO/cKO mutants (Fig. 2A,D). The SLG remained unaffected in these mutants. Interestingly, Fgfr2+/cKO mutants developed a slight reduction in terminal bud numbers at E14.5. Fgfr2cKO/cKO mutants exhibited a large reduction in the number of SMG terminal buds and also did not develop a SLG (Fig. 2). This suggests the two receptors may regulate specific aspects of branching. Interestingly, the conditional Fgfr2cKO/cKO mutant phenotype was less severe than Fgfr2IIIbnull mutants, which survive through mid-gestation but do not develop any salivary glands and die at birth (De Moerlooze et al., 2000). Because all epithelial lineages in the salivary gland originate from Krt14+ lineages (May et al., 2018), Fgfr2 might have earlier functions in the oral ectoderm or in progenitor maintenance. Finally, we observed that Fgfr1cKO/cKO; Fgfr2cKO/cKO double conditional mutants showed the most severe phenotype, with only one to four terminal buds (Fig. 2C,D), indicating that both receptors have combinatorial functions during epithelial branching. The fact that both receptors are co-expressed throughout the SMG cells (Fig. 1C,D) also suggests that they might have overlapping roles. The presence of a single wild-type Fgfr1 or Fgfr2 allele could partially rescue branching defects in Fgfr1cKO/cKO; Fgfr2cKO/cKO compound conditional mutants. Fgfr1+/cKO; Fgfr2cKO/cKO compound conditional mutants rescued terminal branching defects by 19.2%. A more robust rescue (∼71%) in terminal branching was observed in Fgfr1cKO/cKO; Fgfr2+/cKO mutants, again supporting a more extensive role for Fgfr2 in branching morphogenesis.
Fig. 2.
Branching defects in Fgfr1 and Fgfr2 conditional mutants. Conditional depletion of (A) Fgfr1, (B) Fgfr2 or (C) compound Fgfr1/2 using K14Cre resulted in branching defects at E14.5 in developing salivary glands. Whole-mount E-cadherin immunofluorescence followed by 3D rendering of high-resolution images was used to visualize these defects. The number of terminal buds across various Fgfr1 and Fgfr2 conditional mutants was analyzed. Severe defects in submandibular gland (SMG) branching were observed in compound Fgfr1cKO/cKO; Fgfr2cKO/cKO mutants where the number of buds varied between 1 and 5. Conditional Fgfr2cKO/cKO mutants (n=17), Fgfr1+/cKO; Fgfr2cKO/cKO mutants (n=20) and Fgfr1cKO/cKO; Fgfr2cKO/cKO mutants (n=14) did not develop a sublingual gland. Scale bars: 200 µm. (D) Bar graphs show the number of terminal buds in the SMG for various conditional Fgfr1 and Fgfr2 mutants. Data are expressed as mean (±s.d.), ***P<0.001, ****P<0.0001; ns, P<0.05 (one-way ANOVA using Bonferroni multiple comparisons test, two tailed).
Canonical FGF signaling does not affect SMG branching
We sought to understand whether branching defects in various Fgfr1/2 compound mutants result from changes in cell proliferation or survival: two processes known to be regulated by FGF signaling. We assessed cell proliferation by EdU incorporation across various Fgfr1/2 conditional mutants at E14.5 when defects in branching already appeared. Control or compound Fgfr1/2 mutants showed similar levels of EdU incorporation in both the epithelium and the stromal mesenchyme (Fig. S3A). Likewise, we found little cell death and no increase in single or compound conditional Fgfr1/2cKO/cKO mutants at this stage by TUNEL staining (Fig. S3B). However, defects in proliferation or cell death may be present at earlier stages, as Fgfr1+/cKO; Fgfr2+/cKO controls showed many more K14 lineage GFP+ cells than mutants. Alternatively, it is possible that loss of FGF receptors affects the recruitment of epithelial progenitors from the oral ectoderm to the salivary glands.
FGF receptors can engage multiple signaling pathways, including ERK1/2, PI3K/Akt, PLCγ and STATs (Brewer et al., 2016). Previous pharmacological inhibitors studies in ex vivo explant cultures have implicated ERK1/2 activation during epithelial branching (Kashimata et al., 2000). To further explore the contribution of ERK1/2 activation downstream of FGF receptors, we used a conditional constitutively active Braf allele (BrafCA) to induce the RAF-MEK-ERK pathway in the K14Cre lineage independently of Fgfr1/2 expression. We observed significant defects in branching in BrafCA mutants in a Fgfr1cKO/cKO; Fgfr2cKO/cKO null background. RAF-MEK-ERK activation in Fgfr1cKO/cKO; Fgfr2cKO/cKO; BrafCA mutants (Fig. S3C) could not alleviate branching defects observed upon elimination of Fgfr1 and Fgfr2 (Fig. 3A). These observations suggest that that ERK1/2 activation is not sufficient to rescue defects caused by loss of FGF signaling.
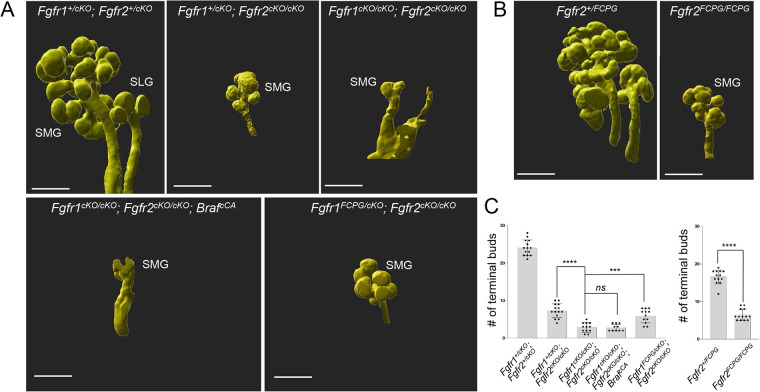
Fig. 3.
Branching in signaling mutants. (A) Whole-mount E-cadherin immunofluorescence followed by 3D rendering was used to analyze changes in the number of terminal buds at E14.5 across various Fgfr1/2 signaling mutants. Fgfr1cKO/cKO; Fgfr2cKO/cKO; BrafCA mutants (n=11) did not rescue branching defects in the submandibular gland (SMG). The sublingual gland (SLG) did not develop in these mutants. Compared with severe branching defects observed in Fgfr1cKO/cKO; Fgfr2cKO/cKO mutants (n=17), Fgfr1FCPG/cKO; Fgfr2cKO/cKO mutants (n=12) exhibited a partial rescue of branching defects in the SMG. The SLG did not develop in Fgfr1FCPG/cKO; Fgfr2cKO/cKO mutants. A representative image is shown for each genotype. Scale bars: 200 µm. (B) 3D rendering was used to analyze SMG defects in Fgfr2+/FCPG and Fgfr2FCPG/FCPG mutants at E14.5. Fgfr2FCPG/FCPG mutants (n=13) did not develop SLGs and showed reduced terminal bud numbers in the SMG. Scale bars: 200 µm. (C) The mean terminal bud number (±s.d.) in the SMG for various Fgfr1 and Fgfr2 signaling mutants at E14.5 are represented in bar graphs. Fgfr1cKO/cKO; Fgfr2cKO/cKO mutants (n=17) showed a 94.5% reduction in terminal bud numbers. A similar level of reduction was observed upon ectopic activation of BRAF in Fgfr1cKO/cKO; Fgfr2cKO/cKO; BrafcCA mutants (n=11). Fgfr1FCPG/cKO; Fgfr2cKO/cKO mutants (n=12) rescued branching defects by 19.1%. A 61% reduction in terminal bud number was observed in Fgfr2FCPG/FCPG mutants (n=12) compared with controls. Data are mean (±s.d.), ***P<0.001, ****P<0.0001; ns, P<0.05 (one-way ANOVA using Bonferroni multiple comparisons test, two tailed, or a two-tailed Student's t-test).
As ERK1/2 activation did not rescue epithelial branching defects in Fgfr1/2 compound mutants, we wanted to explore whether other FGF signaling functions mediate this process. To interrogate signaling mechanisms downstream of FGF signaling in vivo, we previously generated mice with knock-in point mutations at the Fgfr1 and Fgfr2 loci that prevent binding of effectors to the receptors. The most severe alleles, Fgfr1FCPG and Fgfr2FCPG, removed all canonical intracellular signaling downstream of the receptors, including ERK1/2, PI3K, PLC γ and STAT signaling, but failed to recapitulate null mutant phenotypes (Brewer et al., 2016, 2015; Ray et al., 2020). To investigate the role of canonical FGF intracellular signaling activation during branching, we analyzed Fgfr1FCPG/cKO; Fgfr2cKO/cKO and Fgfr2FCPG/FCPG mutants.
Whole-mount ECAD immunostaining followed by 3D rendering of images was used to determine changes in terminal number of buds in compound signaling mutants at E14.5. Fgfr1cKO/cKO; Fgfr2cKO/cKO mutants showed pronounced branching defects, as described earlier. Fgfr1FCPG/cKO; Fgfr2cKO/cKO mutants showed a partial rescue (19.1% compared with Fgfr1cKO/cKO; Fgfr2cKO/cKO compound conditional mutants) in terminal bud formation, to nearly the same extent as a wild-type Fgfr1 allele. Fgfr1FCPG allele did not affect defects observed in SLG development. Morphologically, branching defects in Fgfr1FCPG/cKO; Fgfr2cKO/cKO compound conditional mutants phenocopied Fgfr1+/cKO; Fgfr2cKO/cKO mutants (Fig. 3A,C). Thus, both Fgfr1FCPG/cKO; Fgfr2cKO/cKO and Fgfr1+/cKO; Fgfr2cKO/cKO compound conditional mutants showed similar rescue of branching defects, as determined by terminal end bud number upon loss of FGFR1/2 functions.
Likewise, Fgfr2FCPG/FCPG mutants exhibited partial rescue of terminal bud formation in contrast to Fgfr2IIIb mutants that show agenesis of the salivary glands (Fig. 3B,C) (Celli et al., 1998; De Moerlooze et al., 2000). Interestingly, defects in SLG formation were still not rescued in Fgfr2FCPG/FCPG mutants (Fig. 3B). We found that the SLG never formed in Fgfr2FCPG/FCPG adult mice, but that the SMG was similarly sized to controls, suggesting that branching morphogenesis catches up after E14.5. We were unable to analyze conditional mutants in combination with the Fgfr2FCPG allele due to loxP sites carried over from the sequential introduction of the FCPG mutations. Taken together, these results indicate that both Fgfr1FCPG and Fgfr2FCPG alleles can significantly rescue Fgfr1−/− and Fgfr2−/− mutant branching morphogenesis defects and that both receptors must function in part through other mechanisms than canonical signaling in salivary glands.
BM defects in Fgfr1/2 mutants
The BM surrounding the salivary gland has been shown to play crucial roles in instructing epithelial remodeling and SMG branching, in part governed by the strength of outer bud cell-matrix adhesion (Wang et al., 2021). As FGFRs have functions in regulating cell adhesion beyond their classical roles in canonical signaling (Ray et al., 2020), we turned our attention to interactions between outer bud cells and the BM, which are crucial for SMG branching morphogenesis. We investigated the dynamics of epithelial branching in the SMG between E13.5 and E15.5, when epithelial buds are most significantly remodeled. Branching has been shown to occur by proliferation of the terminal buds and recurrent clefting events during outgrowth. We carried out a time course analysis using ECAD whole-mount immunostaining to label the epithelial sheet and measure the average curvature as development progresses. This analysis provided a quantitative perspective regarding the extent of epithelial remodeling (Fig. 4A,A′).
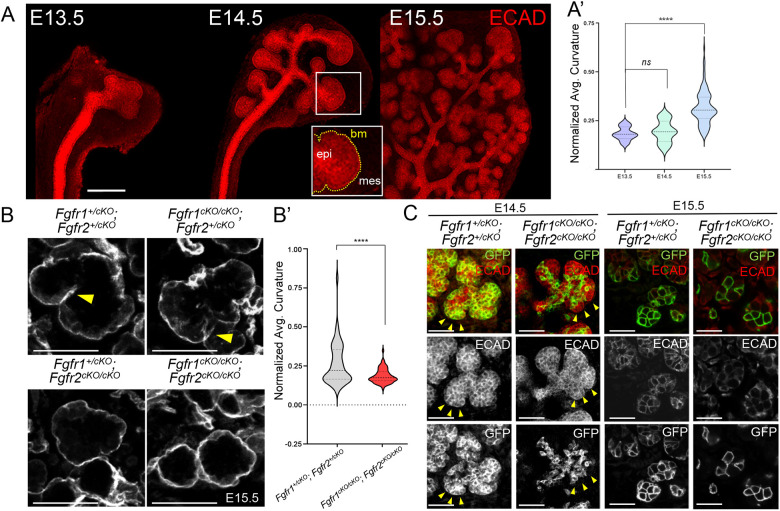
Fig. 4.
Defects in the basement membrane in Fgfr1/2 mutants. (A) E-cadherin immunofluorescence was used to analyze submandibular gland (SMG) epithelial branching between E13.5 and E15.5 in control SMGs (n=6). The epithelial surface (epi) sheet is enclosed by a basement membrane (bm) that undergoes dramatic folding within the mesenchyme (mes), due to recurrent clefting and outgrowth. The median of average curvature of the epithelial surface is represented as violin plots (A′) from E13.5 to E15.5. The horizontal darker dashed bar represents the median. The lighter bars represent the first and third quartiles. Scale bar: 200 µm. (B) The basement membrane (BM) of the SMG was labeled using laminin immunofluorescence in Fgfr1/2 compound mutant sections at E15.5 to estimate the extent of branching. Numerous invaginations (yellow arrowheads) that represent independent clefting events, were detected in control Fgfr1+/cKO (n=14) and Fgfr2+/cKO and Fgfr1cKO/cKO; Fgfr2+/cKO mutant (n=9) SMGs. A significant reduction in clefting was observed in Fgfr1+/cKO; Fgfr2cKO/cKO mutants and Fgfr1cKO/cKO; Fgfr2cKO/cKO compound mutant SMGs. Median average curvature for the laminin surface is shown as violin plots for controls and Fgfr1cKO/cKO; Fgfr2cKO/cKO mutants (B′). Scale bars: 50 µm. (C) Compound Fgfr1/2 mutant SMGs were analyzed at E14.5 and E15.5. GFP+ Fgfr1cKO/cKO; Fgfr2cKO/cKO (n=3 at E14.5; n=4 at E15.5) K14 lineage cells were restricted to the inner ECAD+ epithelial domain compared with controls (n=7 at E14.5; n=12 at E15.5) at E14.5. Very few GFP+ cells could be detected in the peripheral border region (yellow arrowheads) in Fgfr1cKO/cKO; Fgfr2cKO/cKO mutants at E14.5. Scale bars: 50 µm. ****P<0.0001; ns, P<0.05 (A′, one-way ANOVA using Bonferroni multiple comparisons test, two tailed; B′, a two-tailed Student's t-test).
During branching, as the outer bud cells invaginate as a surface sheet to facilitate clefting, the BM is remodeled and the average curvature of the BM increases. We analyzed changes in BM curvature in compound Fgfr1/2 mutants on a ROSA26mTmG background by immunostaining for laminin on sections at E15.5. We observed that control compound Fgfr1+/cKO; Fgfr2+/cKO mutants developed extensive invaginations by E15.5 (Fig. 4B, yellow arrowhead). Fgfr1cKO/cKO; Fgfr2+/cKO mutants also developed profound invaginations; however, they were more subtle in nature compared with controls. Fgfr1+/cKO; Fgfr2cKO/cKO mutants showed fewer clefting events along with reduced changes in BM curvature and smoother margins (Fig. 4B). The most severe defects in BM curvatures were observed in Fgfr1cKO/cKO; Fgfr2cKO/cKO compound mutants, as indicated by the smooth BM, and rarely showed sporadic clefting events compared with Fgfr1+/cKO; Fgfr2+/cKO compound heterozygotes. This is consistent with the significant reduction in average basement membrane curvature observed in compound Fgfr1cKO/cKO; Fgfr2cKO/cKO null mutants (Fig. 4B,B′). Fgfr1cKO/cKO; Fgfr2cKO/cKO compound mutants consistently showed defects in cell BM interactions. At E14.5, GFP+ Fgfr1cKO/cKO; Fgfr2cKO/cKO mutant cells showed a striking failure to populate the outer margins of the epithelial buds, which were instead composed of ECAD+; GFP− cells abutting the BM; these defects were exacerbated by E15.5 (Fig. 4C).
Budding morphogenesis has been shown to be driven by strong cell-matrix adhesion, but is also concomitant with weak cell-cell adhesion at the periphery and strong cell-cell adhesion of inner epithelial cells (Wang et al., 2021). We observed that although K14Cre labeled GFP+ cells populated salivary gland acini extensively and exhibited strong cell-cell adhesion marked by ECAD staining in control Fgfr1+/cKO; Fgfr2+/cKO, far fewer cells were seen in Fgfr1cKO/cKO; Fgfr2cKO/cKO acini. Remarkably, these cells showed reduced ECAD accumulation at the cell-cell junctions, suggesting weak cell-cell adhesion (Fig. S4A,B). Together, these results indicate that loss of FGF signaling diminishes cell-cell adhesion within the SMG. Interestingly, Fgfr1FCPG/cKO; Fgfr2cKO/cKO mutants maintain robust ECAD accumulation at the cell-cell junctions that is comparable with Fgfr1+/cKO; Fgfr2+/cKO controls (Fig. S4C), indicating that cell-adhesion regulation is controlled by non-canonical FGF signaling.
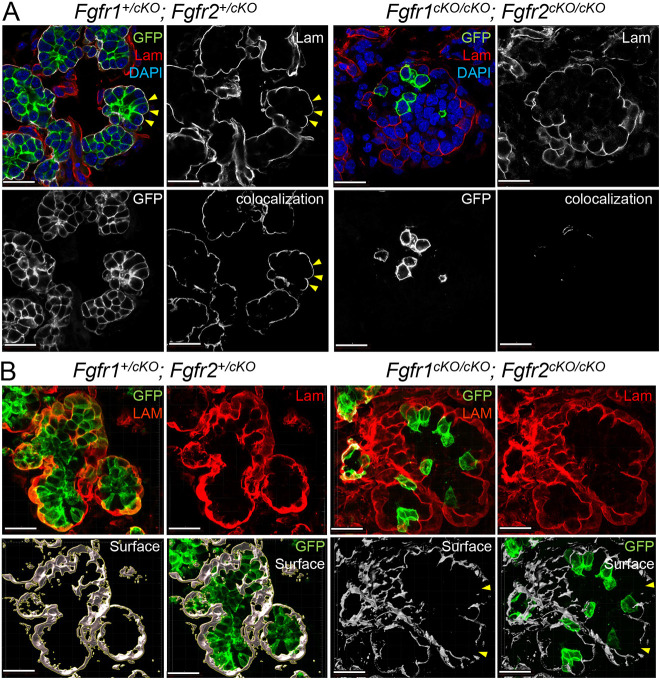
The basement membrane that surrounds the epithelial end buds becomes remodeled during branching morphogenesis and is perforated at areas of local expansion to allow further growth (Harunaga et al., 2014). We examined BM integrity in Fgfr1/2 conditional null mutants on sections. In control Fgfr1+/cKO; Fgfr2+/cKO mutants, the basal edges of GFP+ cells made extensive contacts with the BM. These interactions were largely compromised in Fgfr1cKO/cKO; Fgfr2cKO/cKO mutants (Fig. 5A). Fgfr1cKO/cKO; Fgfr2cKO/cKO mutants also showed major disintegration and discontinuity of the BM specifically around the developing acini (Fig. 5B, yellow arrowheads). We further analyzed cell BM interactions in Fgfr1cKO/cKO; Fgfr2cKO/cKO mutants using high-resolution confocal microscopy of 50 µm sections stained for the BM protein laminin. Controls showed extensive colocalization of laminin and membrane GFP; however, this was diminished in Fgfr1cKO/cKO; Fgfr2cKO/cKO mutants (Fig. S5A). Laminin staining showed large areas of discontinuity in Fgfr1cKO/cKO; Fgfr2cKO/cKO mutant tissues, but not controls (Fig. S5B, white arrowheads). Therefore, a qualitative examination revealed that, in Fgfr1cKO/cKO; Fgfr2cKO/cKO mutants, the BM appeared disintegrated in certain domains, with large gaps, variable thickness and minimal interaction with GFP+ cells. These results indicate that Fgfr1/2 plays an important role in cell-BM interaction and in maintaining BM integrity.
Fig. 5.
Cell-basement membrane interactions in Fgfr1/2 compound mutants. (A) Cell-basement membrane (BM) interactions in the submandibular gland (SMG) were analyzed for compound Fgfr1/2 compound mutants (n=24) at E15.5 by generating 3D colocalization maps. The BM was marked by laminin (Lam). K14 lineage epithelial cells expressed GFP. High-resolution confocal imaging followed by 3D rendering was used to detect colocalization between GFP of laminin and colocalization maps were generated. Control cells formed robust cell-BM contacts (yellow arrowheads). Several GFP+, Fgfr1cKO/cKO; Fgfr2cKO/cKO cells did not interact with the BM. Scale bars: 20 µm. (B) The integrity of the BM in compound Fgfr1/2 mutants was analyzed. 3D surfaces around laminin were generated from high-resolution 3D rendered confocal images. Compound Fgfr1cKO/cKO; Fgfr2cKO/cKO mutants showed discontinuity along the laminin surfaces (yellow arrowheads). Scale bars: 20 µm.
FGF-dependent integrin functions during branching morphogenesis
Our analysis suggests that altered SMG branching in compound Fgfr1/2 mutants might arise from defects in cell-BM interactions. The integrins ITGA6 and ITGB1 have been shown to interact with laminin during SMG remodeling. In organ culture, anti-ITGA6 antibodies inhibit SMG branching (Kadoya et al., 1995; Kadoya and Yamashina, 1993). We further mined the published sc-RNA seq data for E13 mouse SMG (GSE159780; Wang et al., 2021), and found Itga6, Itga9 and Itgb1 to be strongly expressed in the outer bud epithelial cells (Fig. S6A). Using immunofluorescence, we found no difference in the expression levels of ITGA1, ITGA5 and ITGB1 in SMGs from E15.5 Fgfr1+/cKO; Fgfr2+/cKO, Fgfr1FCPG/cKO; Fgfr2cKO/cKO, Fgfr2FCPG/FCPG and Fgfr1cKO/cKO; Fgfr2cKO/cKO embryos (Fig. S6B). As integrins positively regulate branching, we wanted to investigate whether ITGB1 can cooperate with or attenuate FGF signaling function during branching.
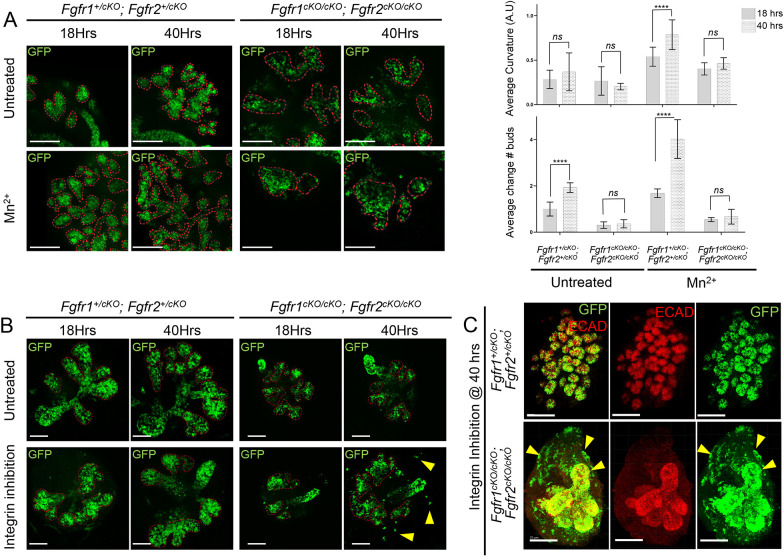
To investigate whether integrin signaling activation could rescue branching defects in Fgfr1/2 conditional mutants, we collected Fgfr1/2 mutant SMGs at E14.5 and cultured them in media supplemented with Mn2+, which is known to lock ITGB1 in a ligand bound active conformation and ectopically activate integrin signaling (Bazzoni et al., 1995). We observed robust integrin signaling activation upon Mn2+ treatment within 8 h of culture (Fig. S6C). Changes in branching were analyzed at 18 h and 40 h (Fig. 6A, Fig. S6C). Compared with untreated culture conditions, we found that integrin activation resulted in a robust increase in the curvature index and bud number in Fgfr1+/cKO; Fgfr2+/cKO controls at 40 h. However, Fgfr1cKO/cKO; Fgfr2cKO/cKO mutants did not show a significant increase in branching, suggesting that activation of integrin signaling is not sufficient to induce ectopic branching upon loss of both FGF receptors.
Fig. 6.
Integrin signaling cooperates with FGFR function during submandibular gland branching. (A) Submandibular gland (SMG) explants from various Fgfr1 and Fgfr2 compound mutants (n=18; E14.5) were cultured for 40 h in presence of Mn2+ (integrin signaling activation) and compared with untreated controls. Mutants were analyzed in more than 20 separate experiments. Tissues were subjected to immunofluorescence on eight separate occasions, and data were compiled from six separate experiments. Explants were imaged during branching at 18 h and 40 h of culture. The curvature of the epithelial surface is indicated by dashed red lines. Median average curvatures (error bar represent the 95% confidence interval, CI) and average change in bud numbers (error bar represent s.d.) were measured for each genotype and are shown in the bar graphs. Robust branching was observed upon integrin signaling activation in all mutants except for Fgfr1cKO/cKO; Fgfr2cKO/cKO. Scale bars: 100 µm. ****P<0.0001; ns, P<0.05 (two-way ANOVA using Bonferroni multiple comparisons test, two tailed). (B) SMG explants (n=14; E14.5) were treated with an integrin β1-blocking antibody from various Fgfr1 and Fgfr2 compound mutants. Mutants were analyzed in more than 20 separate experiments. Tissues were subjected to immunofluorescence on eight separate occasions, and data were compiled from six separate experiments. Epithelial branching was assessed at 18 h and 40 h during culture (median average curvature is plotted as bar graphs in Fig. S6). Neither control Fgfr1+/cKO; Fgfr2+/cKO nor Fgfr1cKO/cKO; Fgfr2cKO/cKO compound mutant explants showed robust changes in branching upon integrin signaling inhibition. However, several GFP+ double mutant cells extruded from the explants (yellow arrowheads). Scale bars: 100 µm. (C) Upon integrin signaling inhibition for 40 h, explants were immunostained for ECAD to mark epithelial cells. Compared with Fgfr1+/cKO; Fgfr2+/cKO controls, several GFP+ cells in Fgfr1cKO/cKO; Fgfr2cKO/cKO explants extruded out of the ECAD+ epithelial domain into the surrounding mesenchyme (yellow arrowheads). Scale bars: 200 µm.
Next, we used a blocking antibody to inhibit integrin signaling in culture. We cultured control Fgfr1+/cKO; Fgfr2+/cKO and Fgfr1cKO/cKO; Fgfr2cKO/cKO salivary glands in the presence and absence of a blocking antibody, and analyzed branching at 18 h and 40 h. Branching remained unaffected in both controls and mutants upon integrin signaling inhibition (Fig. 6B, Fig. S6E). However, we observed extensive extrusion of GFP+ cells into the surrounding mesenchyme in Fgfr1cKO/cKO; Fgfr2cKO/cKO mutants at the 40 h endpoint, exclusively in the presence of the integrin-blocking antibody (Fig. 6B,C, yellow arrowhead). We further analyzed these mutants on sections. In control Fgfr1+/cKO; Fgfr2+/cKO explants, we observed extensive remodeling of the BM in both the untreated and integrin-blocking conditions, and GFP+ cells made extensive contacts with the BM in both conditions. Fgfr1cKO/cKO; Fgfr2cKO/cKO compound mutants did not show overt defects in the BM in the untreated condition, indicating that the culture conditions do not fully recapitulate the in vivo situation, but upon integrin inhibition the BM showed drastically reduced laminin levels and GFP+ cells often had an abnormal shape, appearing more elongated than round or cuboidal, as in the untreated condition (Fig. S6E). These results suggest that integrin inhibition further exacerbates Fgfr1/2 mutant defects.
FGF receptor signaling mutants rescue branching defects upon integrin function activation
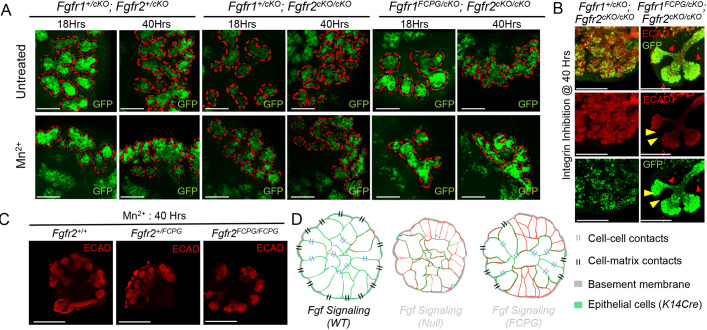
We earlier observed that integrin signaling activation could not rescue branching defects in Fgfr1/2 compound null mutants (Fig. 6A). To determine whether canonical Fgfr1 and Fgfr2 signaling plays a role during branching upon integrin activation, we cultured E14.5 SMGs from Fgfr1+/cKO; Fgfr2+/cKO controls, and Fgfr1+/cKO; Fgfr2cKO/cKO and Fgfr1FCPG/cKO; Fgfr2cKO/cKO mutants in media supplemented with Mn2+ or in the presence of integrin-blocking antibodies. Changes in branching were analyzed at 18 h and 40 h of culture. At both time points, Fgfr1FCPG/cKO; Fgfr2cKO/cKO mutants showed a similar extent of branching to Fgfr1+/cKO; Fgfr2cKO/cKO mutants, quantified by average curvature analysis and number of buds (Fig. 7A,B; Fig. S7A). These results suggest that FGFR1 canonical signaling is not required for branching upon integrin signaling activation. We further evaluated changes in branching at 40 h upon Mn2+ treatment in Fgfr2FCPG/FCPG mutants. Compared with Fgfr2+/+ and Fgfr2+/FCPG mutants, Fgfr2FCPG/FCPG mutants showed only a mild reduction in overall curvature (Fig. 7C, Fig. S7B). These results suggest that both Fgfr1FCPG and Fgfr2FCPG signaling alleles can partially rescue branching defects during SMG development.
Fig. 7.
Integrin signaling activation rescues branching defects in Fgfr1 and Fgfr2 signaling mutants. (A) Submandibular gland (SMG) explants (E14.5) from Fgfr1 signaling mutants (n=6) were cultured for 40 h in the presence of Mn2+ to activate integrin signaling. These experiments were carried out as four separate sets, according to the availability of mutants. Tissues were imaged at 18 h, by removing them from the incubator for less than 1 h, and at 40 h. Representative images for respective genotypes are shown. Epithelial surfaces are indicated by dotted red lines. At 40 h, an increase in overall branching was observed across all mutants upon integrin signaling activation. Similar levels of branching were observed for all the mutants analyzed. Scale bars: 50 µm. (B) Fgfr1+/cKO; Fgfr2cKO/cKO (n=4) and Fgfr1FCPG/cKO; Fgfr2cKO/cKO mutant (n=5) SMG explants (E14.5) were cultured with a blocking antibody to inhibit integrin β1 signaling. These experiments were carried out as four separate sets, according to the availability of mutants. Tissues were analyzed 40 h post-treatment. ECAD immunostaining was used to mark epithelial cells (yellow arrowheads). Only rarely were GFP+ cells observed outside epithelial population (red arrowheads). Scale bars: 200 µm. (C) Integrin signaling activation in Fgfr2 signaling mutants [at E14.5; Fgfr2+/+ (n=12), Fgfr2+/FCPG (n=9), Fgfr2FCPG/FCPG (n=3)] was carried out in culture for 40 h. ECAD immunostaining was then used to mark the epithelial tissue. These experiments were carried out as three separate sets, according to the availability of mutants. Representative images for respective genotype are shown (median average curvature values were plotted as a bar graph for respective genotypes in Fig. S7). Overall, the extent of branching remained unchanged across all genotypes. Scale bars: 200 µm. (D) FGF signaling plays a crucial role during SMG branching. Upon loss of FGF signaling, salivary gland epithelial cells fail to maintain strong cell-cell and cell-basement membrane interactions, resulting in defects in epithelial branching and reduction in the number of acini. Both branching and cell-cell contacts are partially restored in Fgfr1FCPG or Fgfr2FCPG mutants, indicating that non-canonical FGF signaling is important for maintaining strong cell-cell and cell-basement membrane interactions.
DISCUSSION
Growth factors expressed in the salivary gland mesenchyme are known to signal to the epithelium to regulate epithelial morphogenesis, and previous genetic studies have shown that the FGF pathway is paramount in this process (Hoffman et al., 2002; Min et al., 1998; Ohuchi et al., 2000). Accordingly, we observed that Fgfr1 and Fgfr2 were highly expressed in the outer layer of the epithelial buds. To determine epithelial-specific FGFR functions, we conditionally deleted Fgfr1 and/or Fgfr2 using a K14Cre driver. SMGs showed clear branching defects when either Fgfr1 or Fgfr2 activity was lost, with a more pronounced defect with loss of Fgfr2. Compound Fgfr1/2 null mutants developed the most severe defects, indicating that both receptors function coordinately during epithelial branching. However, the contribution of canonical intracellular signaling downstream of FGFs remained to be assessed. In this work, we tie FGF signaling to the ability of epithelial cells to adhere to the matrix and to each other, and show how FGF driven cell-matrix interactions regulate epithelial branching.
As RTKs, FGF receptors classically activate multiple signal transduction cascades upon receptor dimerization, including ERK1/2, PI3K, PLCγ, JNK, JAK/STAT and p38 (Brewer et al., 2016; Lanner and Rossant, 2010). ERK1/2 has widely been considered a hallmark of the downstream FGF signaling pathway (Brewer et al., 2016; Lanner and Rossant, 2010). However, ligand-independent RAF-MEK-ERK activation, using a conditional constitutively active BrafCA allele, could not alleviate branching defects in compound Fgfr1/2 null mutants. Conversely, Fgfr1FCPG and Fgfr2FCPG mutants, which broadly eliminate classic RTK signaling outputs (Ray et al., 2020), partially alleviated branching defects. In the compound Fgfr1/2 null mutant background, restoration of a single Fgfr1FCPG allele could reconstitute terminal branching to a similar extent to a wild-type Fgfr1 allele. Likewise, Fgfr2FCPG/FCPG mutants rescued ∼50% of the SMG terminal branching defects, although they did not rescue SLG development. These results indicate that FGFs exert their functions in salivary glands, at least in part, through mechanisms beyond canonical RTK signaling.
Cell-cell and cell-matrix adhesion have been shown to play instructive roles in epithelial morphogenesis. In the salivary gland, the surface epithelial sheet establishes strong cell matrix adhesion to the BM, and inward folding of cells promotes budding and accompanying clefting (Wang et al., 2021). We investigated cell adhesion in mutant salivary glands and organ culture, as FGFRs have been shown to interact with integrins and cadherins through their extracellular domain, which has not been altered in our most severe Fgfr1FCPG and Fgfr2FCPG signaling alleles (Endo et al., 2012; Geiger and Yamada, 2011; McQuade et al., 2006; Moser et al., 2009; Rapraeger et al., 1991; Yayon et al., 1991). Moreover, we have previously observed that Fgfr1−/− and/or Fgfr2−/− primary neural crest cells failed to spread on fibronectin-coated dishes in culture and to form extensive cell-cell contacts in vivo, but that both processes were restored in Fgfr1 and/or Fgfr2 signaling mutant backgrounds (Ray et al., 2020). Remarkably, we found similar behaviors with SMG epithelial cells, as Fgfr1/2 null mutant acinar cells had compromised adhesion to the BM and failed to exhibit extensive cell-cell contacts. As in previous work, we observed that adhesion defects were rescued to a large extent by reintroducing Fgfr1FCPG and Fgfr2FCPG alleles to double mutant backgrounds, indicating that FGF-mediated regulation of cell adhesion operates by pathways that are independent of canonical RTK signaling (Fig. 7D).
Our fluorescent reporters showed that both Fgfr1 and Fgfr2 are expressed more highly in the outer cells, although considerable heterogeneity in H2B-GFP and H2B-mCherry levels was detected. As it has been suggested that outer cells that move into the interior retain low cell-cell adhesion compared with the bulk of interior cells, and that interior cells then move back to the periphery by competitive adhesion (Wang et al., 2021), FGFR1 and FGFR2 with their role in cell adhesion may play a crucial role in this process. It would be interesting in the future to document protein levels of these receptors using epitope-tagged alleles or antibodies suitable for sub-cellular analysis. How FGF signaling works with cell-adhesion pathways remains to be determined (Clark and Soriano, 2022; Ferguson et al., 2021). The fact that loss of FGFR affects cell-matrix adhesion, as shown here and in previous work (Ray et al., 2020), suggests that FGF signaling operates at some level upstream of cell-matrix adhesion pathways to regulate their activity. We observed that Fgfr1cKO/cKO; Fgfr2cKO/cKO mutant cells failed to populate the outer margins of the epithelial buds. Outer bud epithelial cells, which significantly co-express both Fgfr1 and Fgfr2, normally show accumulation of integrin β1. In addition, the integrity of the BM was affected by FGF signaling, as compound Fgfr1/2 null mutants develop ruptures of the BM in vivo, with some cells extruding into the surrounding mesenchyme. Inhibiting integrin activation in culture led to a similar phenotype. It has been suggested that interactions with integrins might seed laminin polymerization to initiate BM assembly (Yurchenco and Patton, 2009). Therefore, reduced integrin activation might lead to a reduction in BM formation and thus be responsible for the BM ruptures. Conversely, activating integrin signaling in an ex vivo culture model resulted in a significant increase in SMG branching, but was unable to rescue the extensive defects in conditional Fgfr1/2 null mutant cultures, which still exhibited defects in the BM. Under similar conditions, reintroducing a single Fgfr1FCPG mutant allele, and to a lesser extent a similar Fgfr2FCPG copy, could rescue Fgfr1/2 null mutant defects.
Alternatively, it is possible that Fgfr1cKO/cKO; Fgfr2cKO/cKO mutant cells can rupture the basement membrane, similar to metastatic cancer cells or C. elegans anchor cells, due to weakened integrin signaling but also to possibly improper cadherin switching, where FGF has been shown to play a crucial role (Sun et al., 1999; Ciruna and Rossant, 2001; Nieto et al., 2016). The escape of these cells might also explain why we find fewer K14Cre lineage GFP+ cells in mutant salivary glands, as we failed to observe a defect in cell proliferation or cell death at E14.5 despite the known roles of FGF signaling in mediating these processes. The effect that we have observed upon loss of FGF signaling on ECAD expression ties into previous investigations, as FGF signaling has been shown to affect E-cadherin localization in multiple contexts, including the mural trophectoderm (Kurowski et al., 2019), Drosophila mesoderm (Sun and Stathopoulos, 2018) and zebrafish cardiomyocytes (Rasouli et al., 2018). Taken together, our observations suggest that non-canonical FGF signaling plays a significant role in regulating cell-cell and cell-matrix interactions. Further investigations will shed light into how these interactions come into play to remodel the BM and promote branching of the epithelial tissue during salivary gland branching morphogenesis.
MATERIALS AND METHODS
Mouse strains
All animal experiments were approved by the Institutional Animal Care and Use Committee at the Icahn School of Medicine at Mount Sinai. Fgfr1Lox (Fgfr1tm5.1Sor), Fgfr2Lox (Fgfr2tm1.1Sor), Fgfr1FCPG (Fgfr1tm10.1Sor), Fgfr2FCPG (Fgfr2tm8.1Sor), Fgfr1-GFP (Fgfr1tm12.1Sor) and Fgfr2-mCherry (Fgfr2tm2.1Sor) have been previously described (Hoch and Soriano, 2006; Molotkov et al., 2017; Brewer et al., 2015; Ray et al., 2020) and are available from the Jackson Laboratory or the Mutant Mouse Resource and Research Center (MMRRC). (Cg)-Braftm1Mmcm/J, a conditional constitutive active Braf allele is referred in the text as BrafCA, has been described previously (Dankort et al., 2007). Tg(KRT14-cre)Smr, Gt(ROSA)26Sortm1Sor and Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo are referred to in the text as K14-Cre, ROSA26LacZ and ROSA26mT/mG, respectively (Andl et al., 2004; Muzumdar et al., 2007; Soriano, 1999). All lines were maintained on a 129S4 co-isogenic background, except for BrafCA, which was used in crosses after four generations of backcross to 129S4. Successful mating for all timed pregnancies was assessed by the presence of a vaginal plug, which was designated embryonic day (E) 0.5.
Whole-mount immunostaining
The extent of branching in the developing salivary glands was analyzed on whole mounts. Intact salivary glands (stromal mesenchyme and epithelium) were harvested for wild-type or different Fgfr1/2 conditional/signaling mutants in 1×PBS. Tissues were washed and fixed overnight in 4% paraformaldehyde in PBS (4%PFA/PBS) at 4°C. Fixed tissues were washed three times in PBS followed by permeabilization in 1×PBT (PBS supplemented with 0.5% Triton X-100) for the following 2 days with intermittent changes of fresh PBT. 5% BSA in PBT was used for blocking before antibody incubations for another 2 days at 4°C. This was followed by incubation with an appropriate dilution of primary antibodies/blocking solution for whole-mount immunostaining for 5 days. Salivary glands were washed in PBT multiple times for the next 2 days before further incubations with appropriate secondary antibody in PBT overnight, followed by counterstaining with DAPI. After washing, tissues were gradually dehydrated to 100% methanol in a gradient of increasing methanol concentration followed by clearing by multiple changes of (1:3) benzyl alcohol: benzyl benzoate (BABB). Whole-mount imaging was carried out on a Leica TCS SP8 confocal microscope. Imaris 9.8.0 (Oxford Instruments) was used to import z-stack image sequences and compiled to create 3D images. The number of terminal buds was counted for wild-type or different Fgfr1/2 conditional mutants from 3D rendered images across XY rotational axes using Imaris 9.8.0.
Tissue processing for sections
Salivary glands were harvested at E14.5, E15.5 and E18.5 for various Fgfr1/2 conditional mutants in 1×PBS. Tissues were fixed in 4% PFA/PBS overnight followed by washes in 1×PBS. Fixed tissues were next incubated in increasing sucrose concentrations gradients (10-30%) in PBS and left in 30% sucrose overnight and finally embedded in OCT. 10 µm frozen sections were cut using a cryostat (Leica) and stored at −20°C for analysis. Before immunofluorescence, slides were washed three times for 5 mins each in 1×PBT, before blocking in 5% BSA/PBT for 1 h. Tissues were then incubated with primary antibody at appropriate dilutions.
For LacZ staining, tissues were fixed in 2% formaldehyde and 0.2% glutaraldehyde in PBS for 10 mins followed by OCT embedding .10 µm sections were generated. During detection, tissues were rehydrated by washing in 1×PBS. Tissues were next washed in 5 mM potassium ferricyanide, 5 mM potassium ferrocyanide, 2 mM MgCl2, 0.01% sodium deoxycholate and 0.02% Nonidet P-40 (NP-40) in PBS, before adding 1 mg/ml X-Gal in the same solution to detect lacZ activity.
For immunofluorescence, primary antibody incubations were carried out overnight at 4°C. Next, slides were washed three times for 5 min each in PBT before incubating with appropriate secondary antibodies for 5 h at room temperature. The primary antibodies and dilutions used were: GFP (1:500; AvesLab, GFP-1020), mCHERRY (1:100, Abcam, ab167453), ECAD (1:200, CST, 3195S), laminin (1:200, MilliporeSigma, L9393), integrinβ1 (1:100; Abcam, ab30394) and activated integrinβ1 (1:100, MilliporeSigma, MAB2079-AF647, direct immunofluorescence). All secondary antibodies were used at 1:5000 dilutions as follows: Alexa Fluor 488 AffiniPure donkey anti-chicken IgY (IgG) (H+L) (Jackson ImmunoResearch Laboratories; 703-545-155); donkey anti-rabbit IgG (H+L) (Alexa Fluor 488, Alexa Fluor 546 and Alexa Fluor 647; Thermo Fisher Scientific, A-21206, A-10040 and A-31573, respectively); and donkey anti-mouse IgG (H+L) (Alexa Fluor 488, Alexa Fluor 546 and Alexa Fluor 647; Thermo Fisher Scientific, A-21202, A-10036 and A-31571, respectively). DAPI (1 µg/ml: Life Technologies, D1306) was used for nuclei staining together with secondary antibodies. Samples from different genotypes were processed on slides so that the treatment remained the same. Upon processing, samples were analyzed in parallel to allow comparison across genotypes.
Cell proliferation assay
14.5 dpc pregnant females were injected intraperitoneally with 100 mg/kg body weight of EdU. Salivary glands were harvested from embryos 1 h after EdU injection. Fixed tissues were processed and 10 µm sections were generated. EdU detection was carried out as per manufacturer's instruction using the Click-iT EdU Cell Proliferation Kit (Thermo Fisher; C10340) on sections.
TUNEL assay
Sections generated from E14.5 salivary glands were rehydrated in PBS, followed by post-fixation in 4% PFA for 10 min. Tissues were next washed in 1×PBS to remove traces of PFA followed by washes in PBT (PBS+0.1% Triton X-100). Next, tissues were blocked in 5% BSA/PBT. The TUNEL In Situ Cell Death Detection Kit TMR red (MilliporeSigma, 12156792910) was used to detect cell death.
Curvature analysis
Salivary gland epithelial tissues were labeled in wholemount followed by tissue clearing and confocal microscopy imaging. Optical sections were generated. Curvature features were extracted from tissue sections immunostained for laminin that mark the BM. The curvature of the peripheral epithelial cell layer at the epithelial boundaries or from the BM marked by laminin, were analyzed using the Kappa plug-in in the FIJI implementation of ImageJ (Mary and Brouhard, 2019 preprint). Similarly, curvature features were extracted from explant cultures, either from the images collected at different timepoints or from ECAD immunostained fixed tissues. Closed B-Spline curves were traced, and the average curvature was estimated for SMGs. The data (median with 95% CI) were plotted as a bar graph or violin plots. Cell-BM interaction maps were generated using Imaris 9.8.0 (Oxford Instrument). 3D maps were generated using COLOC function to give a visual estimate of cell-BM interactions.
Isolation and culture of salivary glands
Mouse salivary glands were isolated at E14.5 by decapitating embryos with fine scissors. In a fresh plate containing PBS, a scalpel was used to slice in between the lower and the upper jaw to separate the mandible and tongue from the rest of the head. The salivary glands, sandwiched between the base of the tongue and the lower jaw, were next transferred to a fresh plate containing PBS on a dissection scope. The tissue was positioned with the tongue facing the top. A pair of forceps was used to gently lift the tongue and subsequently two salivary glands on either side were removed and transferred to a fresh plate with DMEM/F-12 (Thermo Fisher, 11039047) medium before culture.
Isolated salivary glands were cultured in Ibidi 8-well µ-slides. Upon coating with Corning GFR Matrigel (1:5 dilution; Thermo Fisher CB-40230), salivary glands were laid out while avoiding drying and overlaid with organ culture medium containing DMEM/F-12 supplemented with 150 μg/ml vitamin C (MilliporeSigma, A7506), 2XITS and 1×PenStrep (100 units/ml penicillin, 100 μg/ml streptomycin; Thermo Fisher, 15140163). Tissues were incubated at 37°C in 5% CO2 for 40 h.
Organ culture medium was supplemented with 50 μM MnCl2 to enhance integrin-mediated cell-matrix adhesion signaling functions. To block β1-integrin functions, we used either rat monoclonal (mAb13) β1-integrin blocking antibodies at 100 μg/ml (MilliporeSigma; # MABT821) or mouse monoclonal (P5D2) β1-integrin blocking antibodies at 50 μg/ml (Abcam; ab24693).
Statistical analysis
For pairwise comparisons, an unpaired two-tailed Student's t-test was used to obtain P values. For comparisons of multiple samples, we used one-way or two-way ANOVA with a two-tailed Bonferroni multiple comparisons test. The error bars represent standard deviations of means for all the graphs except for the average curvature analysis. P-values are: P>0.05 (not significant, ns), *P<0.05, **P<0.01, ***P<0.001 and ****P<0.0001).
For all graphs where average curvatures were analyzed, median average curvatures are either represented as violin plots or as bar graphs. The error bars represent 95% confidence intervals.
Supplementary Material
Acknowledgements
We thank our laboratory colleagues, Rob Krauss, Ali May and Checco Ramirez for helpful discussions and critical comments on the manuscript. We very much appreciate the help of James Clark with statistical analysis. We thank Yeifei Sun for help on analyzing scRNA seq data, and the Microscopy and Advanced Imaging Core for assistance and suggestions on data analysis.
Footnotes
Author contributions
Conceptualization: A.T.R., P.S.; Methodology: A.T.R., P.S.; Software: A.T.R.; Validation: A.T.R.; Formal analysis: A.T.R., P.S.; Investigation: A.T.R., P.S.; Resources: A.T.R.; Data curation: A.T.R.; Writing - original draft: A.T.R., P.S.; Writing - review & editing: A.T.R., P.S.; Visualization: A.T.R.; Supervision: P.S.; Project administration: A.T.R., P.S.; Funding acquisition: P.S.
Funding
This work was supported by the National Institute of Dental and Craniofacial Research (RO1 grant DE022778 to P.S.). Deposited in PMC for release after 12 months.
Data availability
All relevant data can be found within the article and its supplementary information.
Peer review history
The peer review history is available online at https://journals.biologists.com/dev/lookup/doi/10.1242/dev.201293.reviewer-comments.pdf.
References
- Andl, T., Ahn, K., Kairo, A., Chu, E. Y., Wine-Lee, L., Reddy, S. T., Croft, N. J., Cebra-Thomas, J. A., Metzger, D., Chambon, P.et al. (2004). Epithelial Bmpr1a regulates differentiation and proliferation in postnatal hair follicles and is essential for tooth development. Development 131, 2257-2268. 10.1242/dev.01125 [DOI] [PubMed] [Google Scholar]
- Bazzoni, G., Shih, D. T., Buck, C. A. and Hemler, M. E. (1995). Monoclonal antibody 9EG7 defines a novel β1 integrin epitope induced by soluble ligand and manganese, but inhibited by calcium. J. Biol. Chem. 270, 25570-25577. 10.1074/jbc.270.43.25570 [DOI] [PubMed] [Google Scholar]
- Brewer, J. R., Molotkov, A., Mazot, P., Hoch, R. V. and Soriano, P. (2015). Fgfr1 regulates development through the combinatorial use of signaling proteins. Genes Dev. 29, 1863-1874. 10.1101/gad.264994.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer, J. R., Mazot, P. and Soriano, P. (2016). Genetic insights into the mechanisms of Fgf signaling. Genes Dev. 30, 751-771. 10.1101/gad.277137.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celli, G., Larochelle, W. J., Mackem, S., Sharp, R. and Merlino, G. (1998). Soluble dominant-negative receptor uncovers essential roles for fibroblast growth factors in multi-organ induction and patterning. EMBO J. 17, 1642-1655. 10.1093/emboj/17.6.1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatzeli, L., Gaete, M. and Tucker, A. S. (2017). Fgf10 and Sox9 are essential for the establishment of distal progenitor cells during mouse salivary gland development. Development 144, 2294-2305. 10.1242/dev.146019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciruna, B. and Rossant, J. (2001). FGF signaling regulates mesoderm cell fate specification and morphogenetic movement at the primitive streak. Dev. Cell 1, 37-49. 10.1016/s1534-5807(01)00017-x [DOI] [PubMed] [Google Scholar]
- Clark, J. F. and Soriano, P. M. (2022). Pulling back the curtain: The hidden functions of receptor tyrosine kinases in development. Curr. Top. Dev. Biol. 149, 123-152. 10.1016/bs.ctdb.2021.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costantini, F. and Kopan, R. (2010). Patterning a complex organ: branching morphogenesis and nephron segmentation in kidney development. Dev. Cell 18, 698-712. 10.1016/j.devcel.2010.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dankort, D., Filenova, E., Collado, M., Serrano, M., Jones, K. and Mcmahon, M. (2007). A new mouse model to explore the initiation, progression, and therapy of BRAFV600E-induced lung tumors. Genes Dev. 21, 379-384. 10.1101/gad.1516407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Moerlooze, L., Spencer-Dene, B., Revest, J. M., Hajihosseini, M., Rosewell, I. and Dickson, C. (2000). An important role for the IIIb isoform of fibroblast growth factor receptor 2 (FGFR2) in mesenchymal-epithelial signalling during mouse organogenesis. Development 127, 483-492. 10.1242/dev.127.3.483 [DOI] [PubMed] [Google Scholar]
- Doherty, P. and Walsh, F. S. (1996). CAM-FGF receptor interactions: a model for axonal growth. Mol. Cell. Neurosci. 8, 99-111. 10.1006/mcne.1996.0049 [DOI] [PubMed] [Google Scholar]
- Endo, Y., Ishiwata-Endo, H. and Yamada, K. M. (2012). Extracellular matrix protein anosmin promotes neural crest formation and regulates FGF, BMP, and WNT activities. Dev. Cell 23, 305-316. 10.1016/j.devcel.2012.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entesarian, M., Matsson, H., Klar, J., Bergendal, B., Olson, L., Arakaki, R., Hayashi, Y., Ohuchi, H., Falahat, B., Bolstad, A. I.et al. (2005). Mutations in the gene encoding fibroblast growth factor 10 are associated with aplasia of lacrimal and salivary glands. Nat. Genet. 37, 125-128. 10.1038/ng1507 [DOI] [PubMed] [Google Scholar]
- Entesarian, M., Dahlqvist, J., Shashi, V., Stanley, C. S., Falahat, B., Reardon, W. and Dahl, N. (2007). FGF10 missense mutations in aplasia of lacrimal and salivary glands (ALSG). Eur. J. Hum. Genet. 15, 379-382. 10.1038/sj.ejhg.5201762 [DOI] [PubMed] [Google Scholar]
- Ewald, A. J., Brenot, A., Duong, M., Chan, B. S. and Werb, Z. (2008). Collective epithelial migration and cell rearrangements drive mammary branching morphogenesis. Dev. Cell 14, 570-581. 10.1016/j.devcel.2008.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson, H. R., Smith, M. P. and Francavilla, C. (2021). Fibroblast growth factor receptors (FGFRs) and noncanonical partners in cancer signaling. Cells 10, 1201. 10.3390/cells10051201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francavilla, C., Loeffler, S., Piccini, D., Kren, A., Christofori, G. and Cavallaro, U. (2007). Neural cell adhesion molecule regulates the cellular response to fibroblast growth factor. J. Cell Sci. 120, 4388-4394. 10.1242/jcs.010744 [DOI] [PubMed] [Google Scholar]
- Geiger, B. and Yamada, K. M. (2011). Molecular architecture and function of matrix adhesions. Cold Spring Harb. Perspect. Biol. 3, a005033. 10.1101/cshperspect.a005033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo, L., Degenstein, L. and Fuchs, E. (1996). Keratinocyte growth factor is required for hair development but not for wound healing. Genes Dev. 10, 165-175. 10.1101/gad.10.2.165 [DOI] [PubMed] [Google Scholar]
- Harunaga, J. S., Doyle, A. D. and Yamada, K. M. (2014). Local and global dynamics of the basement membrane during branching morphogenesis require protease activity and actomyosin contractility. Dev. Biol. 394, 197-205. 10.1016/j.ydbio.2014.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch, R. V. and Soriano, P. (2006). Context-specific requirements for Fgfr1 signaling through Frs2 and Frs3 during mouse development. Development 133, 663-673. 10.1242/dev.02242 [DOI] [PubMed] [Google Scholar]
- Hoffman, M. P., Kidder, B. L., Steinberg, Z. L., Lakhani, S., Ho, S., Kleinman, H. K. and Larsen, M. (2002). Gene expression profiles of mouse submandibular gland development: FGFR1 regulates branching morphogenesis in vitro through BMP- and FGF-dependent mechanisms. Development 129, 5767-5778. 10.1242/dev.00172 [DOI] [PubMed] [Google Scholar]
- Jaskoll, T., Witcher, D., Toreno, L., Bringas, P., Moon, A. M. and Melnick, M. (2004). FGF8 dose-dependent regulation of embryonic submandibular salivary gland morphogenesis. Dev. Biol. 268, 457-469. 10.1016/j.ydbio.2004.01.004 [DOI] [PubMed] [Google Scholar]
- Jaskoll, T., Abichaker, G., Witcher, D., Sala, F. G., Bellusci, S., Hajihosseini, M. K. and Melnick, M. (2005). FGF10/FGFR2b signaling plays essential roles during in vivo embryonic submandibular salivary gland morphogenesis. BMC Dev. Biol. 5, 11. 10.1186/1471-213X-5-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadoya, Y. and Yamashina, S. (1993). Distribution of alpha 6 integrin subunit in developing mouse submandibular gland. J. Histochem. Cytochem. 41, 1707-1714. 10.1177/41.11.8409377 [DOI] [PubMed] [Google Scholar]
- Kadoya, Y., Kadoya, K., Durbeej, M., Holmvall, K., Sorokin, L. and Ekblom, P. (1995). Antibodies against domain E3 of laminin-1 and integrin alpha 6 subunit perturb branching epithelial morphogenesis of submandibular gland, but by different modes. J. Cell Biol. 129, 521-534. 10.1083/jcb.129.2.521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashimata, M. and Gresik, E. W. (1997). Epidermal growth factor system is a physiological regulator of development of the mouse fetal submandibular gland and regulates expression of the alpha6-integrin subunit. Dev. Dyn. 208, 149-161. [DOI] [PubMed] [Google Scholar]
- Kashimata, M., Sayeed, S., Ka, A., Onetti-Muda, A., Sakagami, H., Faraggiana, T. and Gresik, E. W. (2000). The ERK-1/2 signaling pathway is involved in the stimulation of branching morphogenesis of fetal mouse submandibular glands by EGF. Dev. Biol. 220, 183-196. 10.1006/dbio.2000.9639 [DOI] [PubMed] [Google Scholar]
- Kurowski, A., Molotkov, A. and Soriano, P. (2019). FGFR1 regulates trophectoderm development and facilitates blastocyst implantation. Dev. Biol. 446, 94-101. 10.1016/j.ydbio.2018.12.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanner, F. and Rossant, J. (2010). The role of FGF/Erk signaling in pluripotent cells. Development 137, 3351-3360. 10.1242/dev.050146 [DOI] [PubMed] [Google Scholar]
- Larsen, M., Hoffman, M. P., Sakai, T., Neibaur, J. C., Mitchell, J. M. and Yamada, K. M. (2003). Role of PI 3-kinase and PIP3 in submandibular gland branching morphogenesis. Dev. Biol. 255, 178-191. 10.1016/S0012-1606(02)00047-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen, M., Yamada, K. M. and Musselmann, K. (2010). Systems analysis of salivary gland development and disease. Wiley Interdiscip. Rev. Syst. Biol. Med. 2, 670-682. 10.1002/wsbm.94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latko, M., Czyrek, A., Porebska, N., Kucinska, M., Otlewski, J., Zakrzewska, M. and Opalinski, L. (2019). Cross-talk between fibroblast growth factor receptors and other cell surface proteins. Cells 8, 455. 10.3390/cells8050455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lombaert, I. M., Abrams, S. R., Li, L., Eswarakumar, V. P., Sethi, A. J., Witt, R. L. and Hoffman, M. P. (2013). Combined KIT and FGFR2b signaling regulates epithelial progenitor expansion during organogenesis. Stem Cell Rep. 1, 604-619. 10.1016/j.stemcr.2013.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailleux, A. A., Spencer-Dene, B., Dillon, C., Ndiaye, D., Savona-Baron, C., Itoh, N., Kato, S., Dickson, C., Thiery, J. P. and Bellusci, S. (2002). Role of FGF10/FGFR2b signaling during mammary gland development in the mouse embryo. Development 129, 53-60. 10.1242/dev.129.1.53 [DOI] [PubMed] [Google Scholar]
- Makarenkova, H. P., Ito, M., Govindarajan, V., Faber, S. C., Sun, L., Mcmahon, G., Overbeek, P. A. and Lang, R. A. (2000). FGF10 is an inducer and Pax6 a competence factor for lacrimal gland development. Development 127, 2563-2572. 10.1242/dev.127.12.2563 [DOI] [PubMed] [Google Scholar]
- Makarenkova, H. P., Hoffman, M. P., Beenken, A., Eliseenkova, A. V., Meech, R., Tsau, C., Patel, V. N., Lang, R. A. and Mohammadi, M. (2009). Differential interactions of FGFs with heparan sulfate control gradient formation and branching morphogenesis. Sci. Signal. 2, ra55. 10.1126/scisignal.2000304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mary, H. and Brouhard, G. J. (2019). Kappa (K): analysis of curvature in biological image data using B-splines. bioRxiv 10.1101/852772 [DOI] [Google Scholar]
- May, A. J., Headon, D., Rice, D. P., Noble, A. and Tucker, A. S. (2016). FGF and EDA pathways control initiation and branching of distinct subsets of developing nasal glands. Dev. Biol. 419, 348-356. 10.1016/j.ydbio.2016.08.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- May, A. J., Cruz-Pacheco, N., Emmerson, E., Gaylord, E. A., Seidel, K., Nathan, S., Muench, M. O., Klein, O. D. and Knox, S. M. (2018). Diverse progenitor cells preserve salivary gland ductal architecture after radiation-induced damage. Development 145, dev166363. 10.1242/dev.166363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- May, A. J., Teshima, T. H. N., Noble, A. and Tucker, A. S. (2019). FGF10 is an essential regulator of tracheal submucosal gland morphogenesis. Dev. Biol. 451, 158-166. 10.1016/j.ydbio.2019.03.017 [DOI] [PubMed] [Google Scholar]
- Mcquade, K. J., Beauvais, D. M., Burbach, B. J. and Rapraeger, A. C. (2006). Syndecan-1 regulates alphavbeta5 integrin activity in B82L fibroblasts. J. Cell Sci. 119, 2445-2456. 10.1242/jcs.02970 [DOI] [PubMed] [Google Scholar]
- Milunsky, J. M., Zhao, G., Maher, T. A., Colby, R. and Everman, D. B. (2006). LADD syndrome is caused by FGF10 mutations. Clin. Genet. 69, 349-354. 10.1111/j.1399-0004.2006.00597.x [DOI] [PubMed] [Google Scholar]
- Min, H., Danilenko, D. M., Scully, S. A., Bolon, B., Ring, B. D., Tarpley, J. E., Derose, M. and Simonet, W. S. (1998). Fgf-10 is required for both limb and lung development and exhibits striking functional similarity to Drosophila branchless. Genes Dev. 12, 3156-3161. 10.1101/gad.12.20.3156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner, J. H. and Yurchenco, P. D. (2004). Laminin functions in tissue morphogenesis. Annu. Rev. Cell Dev. Biol. 20, 255-284. 10.1146/annurev.cellbio.20.010403.094555 [DOI] [PubMed] [Google Scholar]
- Molotkov, A., Mazot, P., Brewer, J. R., Cinalli, R. M. and Soriano, P. (2017). Distinct requirements for FGFR1 and FGFR2 in primitive endoderm development and exit from pluripotency. Dev. Cell 41, 511-526.e4. 10.1016/j.devcel.2017.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser, M., Legate, K. R., Zent, R. and Fassler, R. (2009). The tail of integrins, talin, and kindlins. Science 324, 895-899. 10.1126/science.1163865 [DOI] [PubMed] [Google Scholar]
- Moskwa, N., Mahmood, A., Nelson, D. A., Altrieth, A. L., Forni, P. E. and Larsen, M. (2022). Single-cell RNA sequencing reveals PDFGRalpha+ stromal cell subpopulations that promote proacinar cell differentiation in embryonic salivary gland organoids. Development 149, dev200167. 10.1242/dev.200167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzumdar, M. D., Tasic, B., Miyamichi, K., Li, L. and Luo, L. (2007). A global double-fluorescent Cre reporter mouse. Genesis 45, 593-605. 10.1002/dvg.20335 [DOI] [PubMed] [Google Scholar]
- Nguyen, T. and Mege, R. M. (2016). N-Cadherin and Fibroblast Growth Factor Receptors crosstalk in the control of developmental and cancer cell migrations. Eur. J. Cell Biol. 95, 415-426. 10.1016/j.ejcb.2016.05.002 [DOI] [PubMed] [Google Scholar]
- Nie, X., Luukko, K. and Kettunen, P. (2006). FGF signalling in craniofacial development and developmental disorders. Oral Dis. 12, 102-111. 10.1111/j.1601-0825.2005.01176.x [DOI] [PubMed] [Google Scholar]
- Nieto, M. A., Huang, R. Y., Jackson, R. A. and Thiery, J. P. (2016). Emt: 2016. Cell 166, 21-45. 10.1016/j.cell.2016.06.028 [DOI] [PubMed] [Google Scholar]
- Nogawa, H. and Takahashi, Y. (1991). Substitution for mesenchyme by basement-membrane-like substratum and epidermal growth factor in inducing branching morphogenesis of mouse salivary epithelium. Development 112, 855-861. 10.1242/dev.112.3.855 [DOI] [PubMed] [Google Scholar]
- Ohuchi, H., Hori, Y., Yamasaki, M., Harada, H., Sekine, K., Kato, S. and Itoh, N. (2000). FGF10 acts as a major ligand for FGF receptor 2 IIIb in mouse multi-organ development. Biochem. Biophys. Res. Commun. 277, 643-649. 10.1006/bbrc.2000.3721 [DOI] [PubMed] [Google Scholar]
- Ornitz, D. M. and Itoh, N. (2022). New developments in the biology of fibroblast growth factors. WIREs Mech. Dis. 14, e1549. 10.1002/wsbm.1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel, V. N., Rebustini, I. T. and Hoffman, M. P. (2006). Salivary gland branching morphogenesis. Differentiation 74, 349-364. 10.1111/j.1432-0436.2006.00088.x [DOI] [PubMed] [Google Scholar]
- Rapraeger, A. C., Krufka, A. and Olwin, B. B. (1991). Requirement of heparan sulfate for bFGF-mediated fibroblast growth and myoblast differentiation. Science 252, 1705-1708. 10.1126/science.1646484 [DOI] [PubMed] [Google Scholar]
- Rasouli, S. J., El-Brolosy, M., Tsedeke, A. T., Bensimon-Brito, A., Ghanbari, P., Maischein, H. M., Kuenne, C. and Stainier, D. Y. (2018). The flow responsive transcription factor Klf2 is required for myocardial wall integrity by modulating Fgf signaling. eLife 7, e38889. 10.7554/eLife.38889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray, A. T., Mazot, P., Brewer, J. R., Catela, C., Dinsmore, C. J. and Soriano, P. (2020). FGF signaling regulates development by processes beyond canonical pathways. Genes Dev. 34, 1735-1752. 10.1101/gad.342956.120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebustini, I. T., Patel, V. N., Stewart, J. S., Layvey, A., Georges-Labouesse, E., Miner, J. H. and Hoffman, M. P. (2007). Laminin alpha5 is necessary for submandibular gland epithelial morphogenesis and influences FGFR expression through beta1 integrin signaling. Dev. Biol. 308, 15-29. 10.1016/j.ydbio.2007.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakura, T., Nishizuka, Y. and Dawe, C. J. (1976). Mesenchyme-dependent morphogenesis and epithelium-specific cytodifferentiation in mouse mammary gland. Science 194, 1439-1441. 10.1126/science.827022 [DOI] [PubMed] [Google Scholar]
- Sanchez-Heras, E., Howell, F. V., Williams, G. and Doherty, P. (2006). The fibroblast growth factor receptor acid box is essential for interactions with N-cadherin and all of the major isoforms of neural cell adhesion molecule. J. Biol. Chem. 281, 35208-35216. 10.1074/jbc.M608655200 [DOI] [PubMed] [Google Scholar]
- Shams, I., Rohmann, E., Eswarakumar, V. P., Lew, E. D., Yuzawa, S., Wollnik, B., Schlessinger, J. and Lax, I. (2007). Lacrimo-auriculo-dento-digital syndrome is caused by reduced activity of the fibroblast growth factor 10 (FGF10)-FGF receptor 2 signaling pathway. Mol. Cell. Biol. 27, 6903-6912. 10.1128/MCB.00544-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano, P. (1999). Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 21, 70-71. 10.1038/5007 [DOI] [PubMed] [Google Scholar]
- Steinberg, Z., Myers, C., Heim, V. M., Lathrop, C. A., Rebustini, I. T., Stewart, J. S., Larsen, M. and Hoffman, M. P. (2005). FGFR2b signaling regulates ex vivo submandibular gland epithelial cell proliferation and branching morphogenesis. Development 132, 1223-1234. 10.1242/dev.01690 [DOI] [PubMed] [Google Scholar]
- Sun, X., Meyers, E. N., Lewandoski, M. and Martin, G. R. (1999). Targeted disruption of Fgf8 causes failure of cell migration in the gastrulating mouse embryo. Genes Dev. 13, 1834-1846. 10.1101/gad.13.14.1834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, Y. and Nogawa, H. (1991). Branching morphogenesis of mouse salivary epithelium in basement membrane-like substratum separated from mesenchyme by the membrane filter. Development 111, 327-335. 10.1242/dev.111.2.327 [DOI] [PubMed] [Google Scholar]
- Wang, S., Sekiguchi, R., Daley, W. P. and Yamada, K. M. (2017). Patterned cell and matrix dynamics in branching morphogenesis. J. Cell Biol. 216, 559-570. 10.1083/jcb.201610048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, S., Matsumoto, K., Lish, S. R., Cartagena-Rivera, A. X. and Yamada, K. M. (2021). Budding epithelial morphogenesis driven by cell-matrix versus cell-cell adhesion. Cell 184, 3702-3716.e30. 10.1016/j.cell.2021.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei, C., Larsen, M., Hoffman, M. P. and Yamada, K. M. (2007). Self-organization and branching morphogenesis of primary salivary epithelial cells. Tissue Eng. 13, 721-735. 10.1089/ten.2006.0123 [DOI] [PubMed] [Google Scholar]
- Williams, E. J., Furness, J., Walsh, F. S. and Doherty, P. (1994). Activation of the FGF receptor underlies neurite outgrowth stimulated by L1, N-CAM, and N-cadherin. Neuron 13, 583-594. 10.1016/0896-6273(94)90027-2 [DOI] [PubMed] [Google Scholar]
- Yayon, A., Klagsbrun, M., Esko, J. D., Leder, P. and Ornitz, D. M. (1991). Cell surface, heparin-like molecules are required for binding of basic fibroblast growth factor to its high affinity receptor. Cell 64, 841-848. 10.1016/0092-8674(91)90512-W [DOI] [PubMed] [Google Scholar]
- Yurchenco, P. D. and Patton, B. L. (2009). Developmental and pathogenic mechanisms of basement membrane assembly. Curr. Pharm. Des. 15, 1277-1294. 10.2174/138161209787846766 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.