Abstract
To understand the current state of prediabetes burden and treatment in the US, we examined recent trends in prediabetes prevalence, testing, and access to preventive resources. We estimated 13.5 percent prevalence of diagnosed prediabetes in the overall US adult population, using national survey data. Although prediabetes prevalence increased by 4.8 percentage points from 2010 to 2020, access to preventive resources remained low. The most effective intervention for diabetes prevention, known as the National Diabetes Prevention Program, remained woefully undersupplied and underused. There are only 2,098 National Diabetes Prevention Program–recognized providers nationally, and only 3 percent of adults with prediabetes have participated in the program. We suggest three actions to augment prevention efforts: increase payment for prevention interventions to avoid supply distortions, improve data integration and patient follow-up, and extend coverage and broaden access for preventive interventions. These actions, which would require policy-level changes, could lower the barriers to prevention.
Preventing type 2 diabetes is a priority, as ninety-six million US adults now live with prediabetes,1 a condition marked by slightly elevated blood sugar that is considered a risk factor for type 2 diabetes (hereafter referred to as diabetes).Wide heterogeneity in risk for incident diabetes exists within the large population of people with prediabetes; the condition (hemoglobin A1c level ranging from ≥5.7 percent to <6.5 percent) is associated with a 2–10 percent annualized risk of developing diabetes.2 Even with a risk progression as low as 2 percent per year, the burden amounts to nearly a 20 percent risk over the course of ten years for a given individual—and a daunting population-level burden, with more than one in three US adults affected.1 Further, people with prediabetes have increased mortality and cardiovascular disease risk3 even if they do not develop diabetes.
Although some people have criticized the use of a prediabetes diagnosis as the medicalization of a risk factor,4 national care guidelines uniformly endorse screening and managing prediabetes.5 The evidence supporting the efficacy and effectiveness of a variety of preventive approaches is robust. Lifestyle modifications focusing on diet and exercise remain the first-line approach recommended by the American Diabetes Association (ADA)5 and the United States Preventive Services Task Force.6
Healthy People 2030 contains two key objectives related to diabetes prevention. The first is reducing the proportion of adults who are unaware that they have prediabetes from an estimated 38.0 percent in 2013–16 to 33.2 percent by 2030.7 The second, currently with no attached metrics, is increasing the proportion of eligible people completing the Centers for Disease Control and Prevention (CDC)-recognized lifestyle modification program that is now referred to as the National Diabetes Prevention Program.8 Meta-analyses show that weight loss via this program of at least 5 percent reduces the overall risk for diabetes by half.9,10 Although the National Diabetes Prevention Program has been considered the gold standard for diabetes prevention for more than two decades, its reach and engagement remain limited.11
Compounding the urgency for action is the fact that diabetes disproportionately affects vulnerable populations. Non-Hispanic Black and Hispanic adults and adults with lower socioeconomic status have higher rates of diabetes and related complications than White adults and adults with higher socioeconomic status.12 Hence, any efforts to prevent diabetes should explicitly tackle these disparities. If they are ignored, they can snowball into the need for costlier secondary and tertiary care.
To better understand current gaps in prediabetes care, we aimed to estimate the magnitude of the problem (that is, prediabetes prevalence rates, testing rates, and access to preventive care by sociodemographic characteristics) and to identify major gaps in prevention as well as opportunities to address such gaps.
Study Data And Methods
DATA SOURCES
To examine current prediabetes prevalence rates, testing, and access to preventive care by sociodemographic characteristics in the US, we used data from two nationally representative surveys with complementary information: the Behavioral Risk Factor Surveillance System (BRFSS), 2010–20, and the National Health Interview Survey (NHIS), 2016–17.We used these two sources of national data because they provide complementary information. From the BRFSS we obtained data on prediabetes, glucose testing, access, and cost barriers by state, insurance status, race, sex, and age. From the NHIS we obtained data on National Diabetes Prevention Program referrals and participation, as well as national-level information on diabetes risk and, in particular, family history of diabetes, which is not available in the BRFSS.
Using the BRFSS, we classified a prediabetes diagnosis as answering “Yes” or “Yes, during pregnancy” to the question, “Have you ever been told by a doctor or other health professional that you have prediabetes or borderline diabetes?” Using the NHIS, we classified prediabetes diagnosis as answering affirmatively to the question, “Other than during pregnancy, have you ever been told by a physician or other health professional that you have borderline diabetes or pre-diabetes?” For those at high risk for diabetes without a known prediabetes diagnosis, we used the ADA diabetes risk score (ranging from 0 to 11) computed from seven questions related to age, sex, race, ethnicity, family history of diabetes, body mass index (BMI), history of gestational diabetes, diagnosed hypertension, and lack of physical activity. For details of the score, see online appendix table A-2.13 Following current ADA and CDC recommendations, a score greater than 5 is considered high risk.14
STATISTICAL ANALYSIS
To estimate the mean and 95% confidence intervals of diagnosed and undiagnosed prediabetes prevalence, referrals and participation in the National Diabetes Prevention Program, BMI, testing rates, access, and affordability among people with prediabetes, we used the Stata 17.0 svy command to account for the sampling weight design in both the BRFSS and NHIS. To assess whether associations between the metrics of interest and patient characteristics (race, sex, and insurance status) were statistically significant, we used two-sided tests with p< 0:05 indicating significance.
LIMITATIONS
We acknowledge some limitations. Our estimates were based on data from the BRFSS, which focuses on the US adult population; hence, we did not discuss prediabetes prevalence or management in children or adolescents. Also, we used data based on self-reports from the NHIS in 2016–17 to illustrate gaps in referrals and access to preventive resources; these are subject to recall bias and might not reflect more recent trends.
Study Results
PREDIABETES TESTING AND PREVALENCE
In 2020 the estimated prevalence of diagnosed prediabetes in the overall population was 13.5 percent (95% confidence interval: 13.1, 13.8) (exhibit 1; 95% CI not shown), which was a 4.8-percentage-point increase since 2010. The diagnosed prevalence was highest among people with high (>25 kg/m2) BMI levels, as shown in appendix figure A-1, panel A.13 However, 19.8 percent of those diagnosed with prediabetes had a BMI of less than 25, as shown in appendix figure A-1, panel B.13 Diagnosed prediabetes prevalence was approximately 5 percentage points higher among women than men in 2020 (women, 15.9 percent [95% CI: 15.4, 16.4]; men, 10.8 percent [95% CI: 9.6, 12.1]) (exhibit 1).Women were also more likely to be tested than men, by 4.3 percentage points (95% CI: 5.4, 3.2) (appendix table A-1).13 Appendix table A-1 shows that the difference in testing across sexes was more pronounced among non-Hispanic Black and Hispanic adults than among White adults.13 Testing peaks after age sixty-five, when patients gain Medicare coverage. See appendix figure A-2 for a graphical representation of testing prevalence by age group.13
Exhibit 1.
Diagnosed prediabetes in the US, by race, ethnicity, and sex, 2010–20
| Percent diagnosed | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|||||||||||
| 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | 2020 | |
| Asiana | |||||||||||
| Female | 15.03% | 16.52% | 19.21% | 17.70% | 17.13% | 31.03% | 22.74% | 19.05% | 19.80% | 20.46% | 23.83% |
| Male | 5.18 | 10.10 | 3.66 | 9.79 | 12.06 | 10.44 | 15.47 | 11.39 | 14.14 | 11.50 | 12.79 |
| Hispanic | |||||||||||
| Female | 17.01 | 22.00 | 14.65 | 16.92 | 17.56 | 27.12 | 20.07 | 18.15 | 19.11 | 18.93 | 18.65 |
| Male | 5.80 | 8.83 | 5.61 | 6.97 | 6.92 | 9.36 | 9.93 | 8.94 | 12.01 | 10.55 | 11.70 |
| Blacka | |||||||||||
| Female | 10.08 | 13.31 | 11.58 | 12.92 | 13.07 | 14.62 | 15.52 | 15.24 | 16.48 | 17.62 | 17.56 |
| Male | 6.00 | 8.53 | 6.51 | 7.77 | 8.42 | 7.58 | 10.09 | 9.92 | 10.95 | 9.72 | 12.13 |
| Whitea | |||||||||||
| Female | 10.31 | 13.92 | 10.81 | 12.08 | 11.39 | 15.16 | 13.77 | 12.63 | 13.93 | 14.39 | 14.12 |
| Male | 5.97 | 7.75 | 7.06 | 7.46 | 7.58 | 7.88 | 8.87 | 8.64 | 9.56 | 9.78 | 10.18 |
| Total | 8.63 | 11.75 | 9.36 | 10.36 | 10.27 | 12.79 | 12.69 | 11.65 | 12.97 | 13.18 | 13.47 |
| Female | 11.05 | 15.13 | 11.58 | 12.97 | 12.56 | 16.96 | 15.54 | 14.09 | 15.37 | 16.04 | 15.90 |
| Male | 6.03 | 8.03 | 6.94 | 7.51 | 7.79 | 8.18 | 9.59 | 9.02 | 10.35 | 10.03 | 10.85 |
SOURCE Centers for Disease Control and Prevention, US Diabetes Surveillance System and Behavioral Risk Factor Surveillance System. NOTE We classified a prediabetes diagnosis as a respondent answering “Yes” or “Yes during pregnancy” to the question “Have you ever been told by a doctor or other health professional that you have prediabetes or borderline diabetes?”
Non-Hispanic.
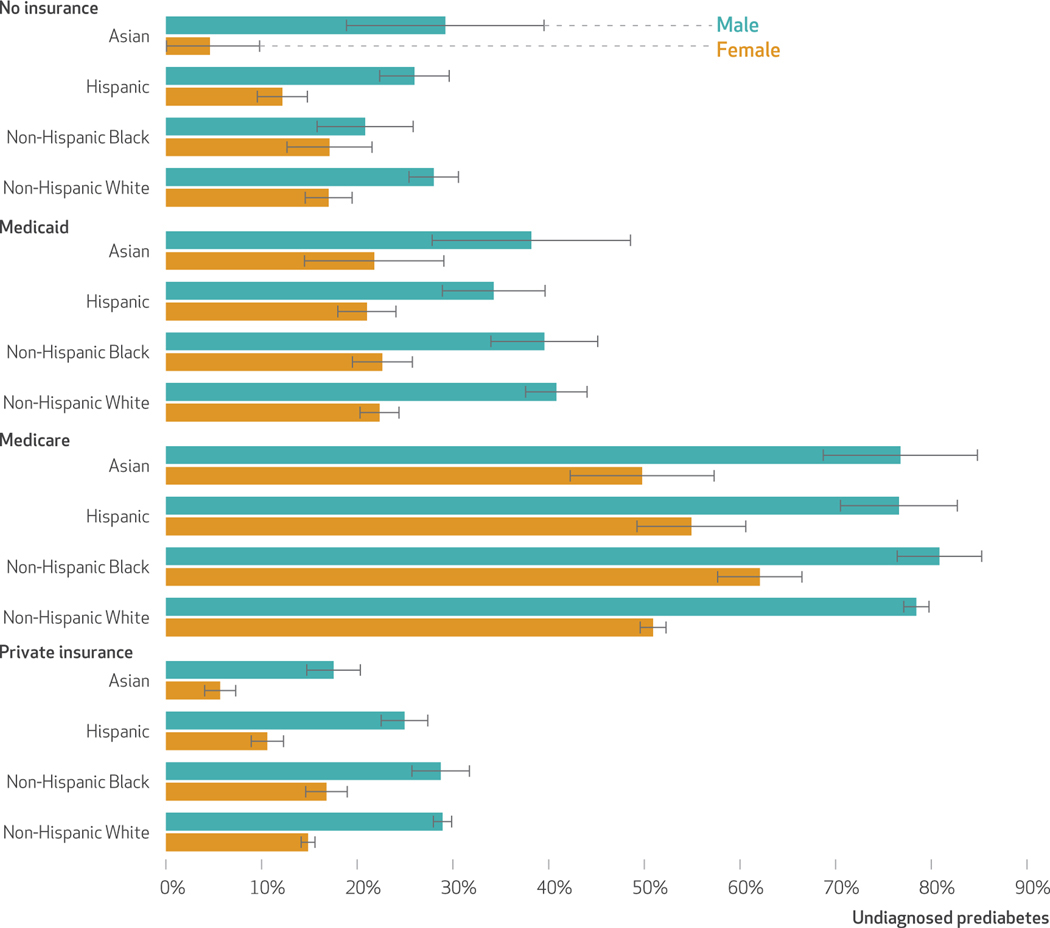
Using the ADA algorithm to determine risk,14 we found that 30 percent of the population is potentially undiagnosed with prediabetes. The undiagnosed rate in women is 21.2 percent (95% CI: 20.6, 21.9), and in men it is 35.8 percent (95% CI: 34.8, 36.7) (data not shown). Exhibit 2 reports the prevalence of undiagnosed prediabetes among US adults by insurance status in 2016–17. Both female and male Asian and Hispanic adults were less likely to be un-diagnosed than non-Hispanic White adults if they had private insurance. Overall, Asian adults were 14 percent less likely and Hispanic adults 9 percent less likely than Non-Hispanic White adults to be undiagnosed (data not shown). Medicaid beneficiaries were more likely to be undiagnosed than people with no insurance coverage (7.1 percentage points; 95% CI: 5.3, 8.8). Medicare beneficiaries had the highest prevalence of undiagnosed prediabetes across all race, ethnicity, and sex combinations (exhibit 2), despite their higher testing rates. This is in part a construct of the ADA risk algorithm that gives three points for being older than age sixty, classifying virtually all Medicare beneficiaries at risk (that is, score ≥5).
ExhiBit 2.
Undiagnosed prediabetes in the US, by race, ethnicity, sex, and insurance status, 2016–17
SOURCE National Health Interview Survey. NOTES Undiagnosed prediabetes was defined as adults with an elevated American Diabetes Association risk score (>5) but with no self-reported diagnosis of prediabetes. Total mean values of this measure of undiagnosed prediabetes by insurance category are as follows: no insurance, 21.0%; Medicaid, 28.1%; Medicare, 62.7%; and private, 20.6%.
PROGRAM REFERRALS, AVAILABILITY, AND ENGAGEMENT
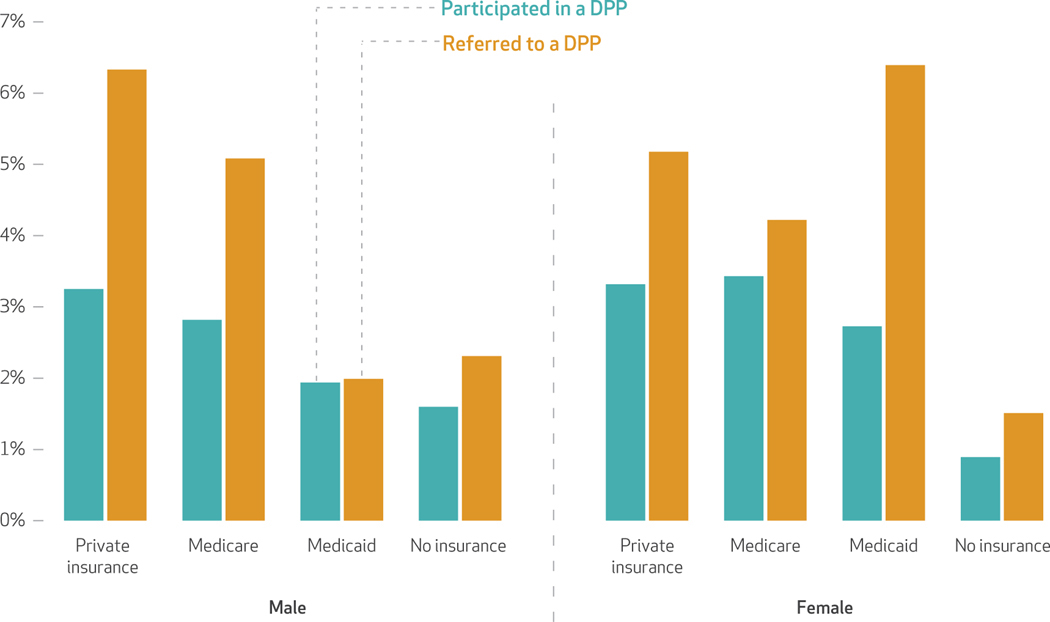
The National Diabetes Prevention Program is an intensive twelve-month, group-based, lifestyle intervention focused on modest weight loss (5–7 percent) through healthy eating and increased physical activity. It is considered the first-line approach for diabetes prevention, but program referral and engagement remain low. We found that only 5 percent of patients diagnosed with prediabetes were referred by a health care professional to a diabetes prevention program (data not shown). The proportion of patients who have ever participated in the National Diabetes Prevention Program is even lower, at 3 percent (data not shown). Men are more likely to be referred than women (5.3 percent versus 4.7 percent) but less likely to participate when referred (55 percent of men versus 65 percent of women participated once they were referred; data not shown). Exhibit 3 examines referrals and participation in a diabetes prevention program by insurance source in 2016–17. Referral and participation rates were low, but some differences are apparent by sex. For example, among Medicaid enrollees, women were substantially more likely to be referred than men (6.4 percent versus 2.0 percent) (exhibit 3).
Exhibit 3.
Referrals to and participation in diabetes prevention programs (DPPs) in the US, by sex and insurance status, 2016–17
SOURCE National Health Interview Survey. NOTES National Health Interview Survey questions pertaining to participation in and referral to type 42 diabetes prevention programs: Have you EVER participated in this type (DPP) of year-long program to prevent type 2 diabetes? Has a doctor or other health care professional ever referred you to such a program to prevent type 2 diabetes?
BROADER BARRIERS TO DIABETES PREVENTION
Lack of access and affordability hamper people’s engagement in prevention. Appendix figure A-313shows access and affordability and no marked improvements in the ability of people with prediabetes to find a personal doctor and to obtain the care and medications they needed between 2014 and 2020. Approximately 20 percent of Medicaid beneficiaries did not have a primary care provider, approximately 11 percent could not afford to see a doctor, and 14 percent could not afford medication when needed. In contrast, approximately 7 percent of Medicare beneficiaries reported not having a primary care provider, 5 percent could not afford to see a doctor, and 8 percent could not afford medication. Approximately 18 percent of people covered by private insurance did not have a primary care provider, 7 percent could not afford to see a doctor, and 7 percent could not afford medications when needed.
The National Diabetes Prevention Program is currently the only covered benefit for prediabetes, but it remains underused and undersupplied. As of February 2022 there were 2,098 National Diabetes Prevention Program suppliers, but approximately 40 percent had full CDC recognition status, which is achieved when program milestones such as mean participant weight loss of 5 percent are met. This translates to roughly 1 supplier per 10,000 cases of diagnosed pre-diabetes. Approximately 50 percent of National Diabetes Prevention Program providers are open to the public (that is, are not membership or employer based), and about 25 percent offer distance learning. Appendix table A-313 summarizes this information across states and lists the number of organizations participating in the National Diabetes Prevention Program, the proportion of CDC-accredited organizations, and the proportion of organizations currently offering distance learning.
Although Medicare began covering the National Diabetes Prevention Program in 2018, there are only 940 Medicare sites across the country and no sites in Nevada, Rhode Island, South Dakota, or Vermont. Appendix table A-4 lists the number of Medicare Diabetes Prevention Program providers by state.13 Only fourteen states currently include the program as a Medic-aid benefit. Appendix table A-5 provides an overview of Medicaid Diabetes Prevention Program eligibility criteria and reimbursement across states.13
Discussion
Although prediabetes prevalence increased by percentage points between 2010 and 2020, access to and coverage of diabetes preventive resources remain limited. The existing gaps in preventive services represent opportunities to improve diabetes prevention in the US. We comment on three specific actions that can advance this effort: increase payment for preventive interventions to avoid supply distortions, improve data integration and patient follow-up to increase testing, and extend coverage and broaden access for preventive interventions to reach a larger share of the population at risk. Below we provide details on each action.
PAYING FOR PREVENTION
Gaps between the cost to deliver programs and the amount reimbursed for delivery are disincentives that reduce the supply of preventive services. Based on the originally proposed Medicare reimbursement rates15 and existing estimates of the impact of the Medicare Diabetes Prevention Program on total health care spending,16 the return on investment from a Medicare perspective was estimated to be $2.2 per $1 for the first year and $3 per $1 over the course of three years. Studies have shown that the cost of delivering the National Diabetes Prevention Program far outweighed Medicare reimbursement amounts, especially in large urban health systems serving diverse populations.17 Starting in 2022, reimbursement for participants who meet all performance benchmarks for the twelve-month program is scheduled to increase 56 percent, from $450 to $705 per person.18 As the Medicare Diabetes Prevention Program is structured as a pay-for-performance intervention, reimbursement for participants who meet all attendance benchmarks but do not meet the 5 percent weight loss benchmark will increase 124 percent, from $203 to $455.18 It is still too early to know whether the proposed change in payment will increase supply.
It is also unclear whether pay-for-performance is having a detrimental impact on outreach. Non-Hispanic White adults are most likely to achieve the mean 5 percent weight loss goal linked to Medicare Diabetes Prevention Program pay-for-performance reimbursement.19 The CDC’s National Diabetes Prevention Program data showed that between 2012 and 2016 only 13.8 percent of participants were Black and 10.0 percent were Hispanic.19 Although the NHIS data do not show discrimination in referrals, pay-for-performance might lead providers to offer the National Diabetes Prevention Program in predominantly affluent White neighborhoods, leading to higher White participation and increasing the gap in diabetes prevalence between non-Hispanic White adults and other groups.
IMPROVING DATA INTEGRATION AND PATIENT FOLLOW-UP
Fragmentation in the US health care system frequently leads to missed opportunities to connect high-risk people to effective preventive interventions. Tremendous variation in electronic medical record systems contributes to missed opportunities for more standardized, widespread prediabetes screening and management in routine health care settings. Several institutions have developed innovative and effective methods to increase prediabetes awareness and screening and to facilitate physician referrals to diabetes prevention programs, using information and tools available in their electronic medical record systems.20–22 However, these innovations are difficult to disseminate outside the institution that developed them because of well-established barriers to the interoperability of electronic medical record systems.20 Such barriers contribute, in part, to persistent low rates of prediabetes awareness and physician referrals to diabetes prevention programs at a population level.23,24
There is also a disconnect in two additional areas: the coverage of preventive services across a patient’s lifetime, and the gap between recommendations and covered benefits. Universal screening for gestational diabetes during pregnancy, for example, affords a unique opportunity to identify women at risk of developing type 2 diabetes.25 Not surprisingly, we found that more women were tested for type 2 diabetes than men. A study published in 2017 found that approximately 70–85 percent of women who were diagnosed with hyperglycemia and received medical nutrition therapy alone achieved adequate sugar control during pregnancy.26 Yet women with a history of gestational diabetes usually do not receive medical nutrition therapy after delivery because of a lack of coverage,27 despite evidence that up to 50 percent of women with a history of gestational diabetes develop type 2 diabetes within five to ten years of the index pregnancy.28 Policy-level changes are needed to address this missed opportunity for prevention.
EXTENDING COVERAGE AND BROADENING ACCESS
Preventive interventions for diabetes exist at both the individual level and the population level. Here we describe these two types of interventions and the challenges inherent in covering and deploying them.
INDIVIDUAL LEVEL: Individual-level interventions focus on the actions of people with diabetes, coverage of interventions, and providers that help people obtain them. Approaches such as these require testing, referrals, risk awareness, availability of suppliers, coverage, participation, and adherence.
One limitation of the current National Diabetes Prevention Program rollout strategy is low use, with just 3 percent of people with prediabetes having ever participated in this program based on our NHIS analysis. Low use of the program may partly be the result of restrictive and complex eligibility (that is, differential payer coverage of the program as a benefit, requiring overweight and obese BMI levels despite the existence of prediabetes in other BMI categories, and restricting participation to people older than age eighteen). The age limit is incongruous with the onset of prediabetes at increasingly younger ages. The United States Preventive Services Task Force took promising steps by lowering the recommended age of prediabetes screening from forty in 2015 to thirty-five in 2021. Still, recommendations do not translate into full coverage benefits for the National Diabetes Prevention Program or consistent reimbursement criteria across payer groups. To date, uniform coverage benefits are consistently provided only to people ages sixty-five and older, via the Medicare Diabetes Prevention Program. In addition, people must have a BMI of at least 25 kg/m2 (at least 23 kg/m2 if Asian American) to participate. However, not all people with prediabetes are overweight or obese, which makes them ineligible for program participation. Moreover, the Medicare Diabetes Prevention Program is also a once-per-lifetime benefit. The Medicaid Diabetes Prevention Program is available only in a handful of states, and eligibility criteria and covered benefit status under private insurance are not mandated and are at the discretion of insurance providers.
Although National Diabetes Prevention Program eligibility should be less restrictive, engaging fully in a year-long program such as this requires not only close proximity to an accredited provider but also the luxury of time and the access to affordable nutritious food. Pharmacological interventions and shorter diet and exercise programs can widen access. Among pharmacological options available, metformin is a leading candidate for diabetes prevention because of its effectiveness and long-term safety,29,30 even though it is less effective than the National Diabetes Prevention Program in head-to-head trials. National care guidelines endorse metformin for diabetes prevention because of evidence that it can significantly reduce the risk for incident diabetes, particularly for adults younger than age sixty, those with a BMI of at least 35 kg/m2, those with fasting plasma glucose levels at least 110 mg/dL, and women with a history of gestational diabetes.31 The ADA has recommended metformin for diabetes prevention since 2008, but it is currently in formularies only for patients with type 2 diabetes, as it does not have a Food and Drug Administration indication for diabetes prevention. Studies show that most patients are not offered this option,32,33 despite metformin being an inexpensive medication that has been shown to be cost saving for diabetes prevention.34
Although studies examining medical nutrition therapy and behavioral counseling for diabetes prevention are more limited in number and quality than studies examining the National Diabetes Prevention Program, both the ADA35 and the Academy of Nutrition and Dietetics36 have recommended medical nutrition therapy for diabetes prevention. The therapy is reimbursed by most payers for the treatment of gestational diabetes and for diabetes self-management and training, but not for any activities related to pre-diabetes. Medicare covers three hours of medical nutrition therapy in the year of referral and up to two hours for subsequent years, but only for people with diabetes and kidney disease diagnoses, not for prediabetes. This allowance is far lower than the twelve contacts over the course of six to eighteen months recommended by the United States Preventive Services Task Force for behavioral counseling interventions.37 In medical nutrition therapy, registered dietitians provide nutrition diagnosis, therapy, and counseling. At this time, even in states that offer nutrition benefits, registered dietitian nutritionists are not always recognized as providers, making it impossible to bill for medical nutrition therapy.
The options available to patients are likely dependent on where they live and what insurance coverage they have, rather than their needs. Effective prevention strategies such as the National Diabetes Prevention Program, metformin, and medical nutrition therapy should be prescribed and reimbursed, as these might help reach a larger share of the prediabetes population. Expanded descriptions of these interventions are in appendix table A-6.13
POPULATION LEVEL: Another way to increase coverage and access to preventive resources is through population-level interventions. These are top-down approaches that target economic incentives and the regulatory38 and physical environments needed to reduce risk. Examples of population-level interventions are taxes on sugar-sweetened beverages, public infrastructure (for example, bike lanes and supermarkets), safety-net programs (for example, the Supplemental Nutrition Assistance Program), and mandating restaurants to provide nutrition information. On the last point, morethan 50 percent of US restaurants do not provide nutrition information, and 92 percent of US restaurants serve meals that exceed typical energy requirements.39
The evidence base in support of population-level interventions is still growing; particularly promising is the evidence on the efficacy of soda taxes. Taxes are revenue generating, and if it is redistributed among the communities taxed, a sugar-sweetened-beverage tax might not be regressive. Although we are not aware of studies on soda taxes for which the outcome is diabetes prevention, a tax of 1 cent per ounce has been shown to lower purchases of sugar-sweetened beverages by fifty-three ounces per month and caloric intake by twenty-one calories per day per household, or five calories per household member.37 This translates to a reduction of 0.5 pounds in about three years per household member (using Hall’s rule of thumb).41 The 0.5-pound weight loss estimate is promising when compared with the population-level impact of the National Diabetes Prevention Program. Finland offered a diabetes prevention program (FIN-D2D) to the entire population and achieved a distribution shift in body weight of 1.3 pounds on an intent-to-treat basis.42
Conclusion
In this study we identified and measured gaps in the detection and prevention of prediabetes in the US. We found that the prevalence of pre-diabetes has increased by almost 5 percentage points in the past decade and that an estimated 30 percent of adults with prediabetes are undiagnosed. We also identified gaps in the use and availability of preventive resources. The most studied and effective diabetes prevention program in the US—the National Diabetes Prevention Program—not only has low rates of use (3 percent of eligible people have participated) but also is severely undersupplied (there are only 2,098 program providers almost a decade after Congress authorized the CDC to establish and lead the program).
If the two prediabetes-specific goals in Healthy People 2030 (reducing the proportion of people unaware of their prediabetes status and increasing diabetes prevention program use) are to be achieved, they need to be made specific, measurable, attainable, relevant, and time based—qualities collectively known as SMART. Although these goals are already specific and time based, they have measurement challenges. Monitoring testing rates nationally and longitudinally is more specific and less prone to error than estimating the proportion of adults who are not aware of their prediabetes status.
A 4.8 percent increase in testing in a decade is attainable even without making prediabetes testing (or health care coverage) universal. However, there is currently no benchmark to track progress toward the National Diabetes Prevention Program utilization goal because at the time the goal was set, no reliable baseline data existed. To overcome this, the program questionnaire should be reintroduced in the NHIS at regular intervals, and a specific and attainable goal for participation—possibly at 50 percent—should be set.
The underuse and undersupply of the National Diabetes Prevention Program highlight the importance of addressing provider incentives by using a consistent payment mechanism and broadening program options and health care coverage for prevention. The latter can be achieved by addressing gaps in reimbursement and coverage so that programs are not time limited or do not have narrow eligibility criteria.
Although data integration and patient follow-up could improve testing rates, people might be also more motivated to seek testing when actionable and affordable treatment options are available to them. Once a person has been diagnosed with prediabetes, access should not be limited by their age, weight, or pregnancy status. Coverage should follow Community Preventive Services Task Force recommendations and not be dependent on location or payer type. Achieving this will require mandates to align recommendations to benefits and population-level health interventions. These opportunities must be capitalized on, and the chances of halting prediabetes for all Americans must be improved.
Supplementary Material
Acknowledgments
Rosette Chakkalakal reports receiving support from the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health (NIH); Centers for Disease Control and Prevention (CDC); and Tennessee Department of Health. Tannaz Moin reports receiving support from the NIH, CDC, and Department of Veterans Affairs. Karla Galaviz reports receiving support from the National Heart, Lung, and Blood Institute, NIH (Grant No. 1K01HL149479–01). Findings, opinions, and recommendations expressed in this paper are those of the authors and do not reflect the views of funding agencies. The authors are grateful for valuable comments from the anonymous referees and helpful editorial feedback from Jonathan Bor. Thanks are also owed to Srujana Sai Illa for her excellent assistance in data collection and fact finding.
Contributor Information
Maria L. Alva, Georgetown University, Washington, D.C.
Rosette Chakkalakal, Emory University, Atlanta, Georgia..
Tannaz Moin, University of California Los Angeles, Irvine, California..
Karla Galaviz, Indiana University, Bloomington, Indiana..
NOTES
- 1.Centers for Disease Control and Prevention. Prediabetes—your chance to prevent type 2 diabetes [Internet]. Atlanta (GA): CDC; 2021 Dec 21 [cited 2022 May 25]. Available from: https://www.cdc.gov/diabetes/basics/prediabetes.html [Google Scholar]
- 2.Tabák AG, Herder C, Rathmann W, Brunner EJ, Kivimäki M. Prediabetes: a high-risk state for diabetes development. Lancet. 2012; 379(9833):2279–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ali MK, Bullard KM, Saydah S, Imperatore G, Gregg EW. Cardio-vascular and renal burdens of pre-diabetes in the USA: analysis of data from serial cross-sectional surveys, 1988–2014. Lancet Diabetes Endocrinol. 2018;6(5):392–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Piller C. Dubious diagnosis. Science. 2019;363(6431):1026–31. [DOI] [PubMed] [Google Scholar]
- 5.American Diabetes Association. Introduction: standards of medical care in diabetes—2022. Diabetes Care. 2022;45(Suppl 1):S1–2. [DOI] [PubMed] [Google Scholar]
- 6.Davidson KW, Barry MJ, Mangione CM, Cabana M, Caughey AB, Davis EM, et al. Screening for prediabetes and type 2 diabetes: US Preventive Services Task Force recommendation statement. JAMA. 2021;326(8): 736–43. [DOI] [PubMed] [Google Scholar]
- 7.Healthy People 2030. Reduce the proportion of adults who don’t know they have prediabetes—D-02 [Internet]. Washington (DC): Department of Health and Human Services, Office of Disease Prevention and Health Promotion; [cited 2022 May 25]. Available from: https://health.gov/healthypeople/objectives-and-data/browse-objectives/diabetes/reduce-proportion-adults-who-dont-know-they-have-prediabetes-d-02 [Google Scholar]
- 8.Healthy People 2030. Increase the proportion of eligible people completing CDC-recognized type 2 diabetes prevention programs—D-D01 [Internet]. Washington (DC): Department of Health and Human Services, Office of Disease Prevention and Health Promotion; [cited 2022 May 25]. Available from: https://health.gov/healthypeople/objectives-and-data/browse-objectives/diabetes/increase-proportion-eligible-people-completing-cdc-recognized-type-2-diabetes-prevention-programs-d-d01 [Google Scholar]
- 9.Uusitupa M, Khan TA, Viguiliouk E, Kahleova H, Rivellese AA, Hermansen K, et al. Prevention of type 2 diabetes by lifestyle changes: a systematic review and meta-analysis. Nutrients. 2019;11(11):e2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glechner A, Keuchel L, Affengruber L, Titscher V, Sommer I, Matyas N, et al. Effects of lifestyle changes on adults with prediabetes: a systematic review and meta-analysis. Prim Care Diabetes. 2018;12(5):393–408. [DOI] [PubMed] [Google Scholar]
- 11.Ali MK, McKeever Bullard K, Imperatore G, Benoit SR, Rolka DB, Albright AL, et al. Reach and use of diabetes prevention services in the United States, 2016–2017. JAMA Netw Open. 2019;2(5):e193160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saydah S, Lochner K. Socioeconomic status and risk of diabetes-related mortality in the U.S. Public Health Rep. 2010;125(3):377–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.To access the appendix, click on the Details tab of the article online.
- 14.Bang H, Edwards AM, Bomback AS, Ballantyne CM, Brillon D, Callahan MA, et al. Development and validation of a patient self-assessment score for diabetes risk. Ann Intern Med. 2009;151(11):775–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Medicare and Medicaid Services. Medicare program; revisions to payment policies under the physician fee schedule and other revisions to Part B for CY 2017; Medicare Advantage bid pricing data release; Medicare Advantage and Part D medical loss ratio data release; Medicare Advantage provider network requirements; expansion of Medicare Diabetes Prevention Program Model; Medicare Shared Savings Program requirements. Fed Regist. 2016;81(220): 80482. [PubMed] [Google Scholar]
- 16.Alva ML, Hoerger TJ, Jeyaraman R, Amico P, Rojas-Smith L. Impact of the YMCA of the USA diabetes prevention program on Medicare spending and utilization. Health Aff (Millwood). 2017;36(3):417–24. [DOI] [PubMed] [Google Scholar]
- 17.Parsons AS, Raman V, Starr B, Zezza M, Rehm CD. Medicare under-payment for Diabetes Prevention Program: implications for DPP suppliers. Am J Manag Care. 2018; 24(10):475–8. [PubMed] [Google Scholar]
- 18.Centers for Medicare and Medicaid Services. Medicare program; CY 2022 payment policies under the physician fee schedule and other changes to Part B payment policies; Medicare Shared Savings Program requirements; provider enrollment regulation updates; and provider and supplier prepayment and post-payment medical review requirements. Fed Regist. 2021;86(221): 64996–6031. [Google Scholar]
- 19.Ely EK, Gruss SM, Luman ET, Gregg EW, Ali MK, Nhim K, et al. A national effort to prevent type 2 diabetes: participant-level evaluation of CDC’s National Diabetes Prevention Program. Diabetes Care. 2017; 40(10):1331–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holliday CS, Williams J, Salcedo V, Kandula NR. Clinical identification and referral of adults with prediabetes to a diabetes prevention program. Prev Chronic Dis. 2019;16: E82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kirley K, Khan T, Aquino G, Brown A, Meier S, Chambers N, et al. Using a certified electronic health record technology platform to screen, test, and refer patients with prediabetes. JAMIA Open [serial on the Internet]. 2021 Nov 30 [cited 2022 May 25]. Available from: https://academic.oup.com/jamiaopen/article/4/4/ooab101/6446863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rehm CD, Marquez ME, Spurrell-Huss E, Hollingsworth N, Parsons AS. Lessons from launching the diabetes prevention program in a large integrated health care delivery system: a case study. Popul Health Manag. 2017;20(4):262–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tseng E, Greer RC, O’Rourke P, Yeh H-C, McGuire MM, Clark JM, et al. Survey of primary care providers’ knowledge of screening for, diagnosing, and managing prediabetes. J Gen Intern Med. 2017;32(11): 1172–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hulbert LR, Zhang X, Ng BP, Nhim K, Khan T, Cannon MJ. Health care providers’ knowledge, attitudes, and practices and the association with referrals to the National Diabetes Prevention Program lifestyle change program. Am J Health Promot. 2022;36(2):236–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moyer VA US Preventive Services Task Force. Screening for gestational diabetes mellitus: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(6):414–20. [DOI] [PubMed] [Google Scholar]
- 26.Brown J, Alwan NA, West J, Brown S, McKinlay CJ, Farrar D, et al. Lifestyle interventions for the treatment of women with gestational diabetes. Cochrane Database Syst Rev. 2017; 5(5):CD011970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.National Conference of State Legislatures. Diabetes state mandates and insulin copayment caps [Internet]. Washington (DC): NCSL; 2022 May 23 [cited 2022 Jun 3]. Available from: https://www.ncsl.org/research/health/diabetes-health-coverage-state-laws-and-programs.aspx [Google Scholar]
- 28.Kim C, Newton KM, Knopp RH. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care. 2002;25(10): 1862–8. [DOI] [PubMed] [Google Scholar]
- 29.Sheng Z, Cao J-Y, Pang Y-C, Xu H-C, Chen J-W, Yuan J-H, et al. Effects of lifestyle modification and anti-diabetic medicine on prediabetes progress: a systematic review and meta-analysis. Front Endocrinol (Lausanne). 2019. Jul 12. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Madsen KS, Chi Y, Metzendorf M-I, Richter B, Hemmingsen B. Metformin for prevention or delay of type 2 diabetes mellitus and its associated complications in persons at increased risk for the development of type 2 diabetes mellitus. Cochrane Database Syst Rev. 2019;12(12): CD008558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moin T, Schmittdiel JA, Flory JH, Yeh J, Karter AJ, Kruge LE, et al. Review of metformin use for type 2 diabetes prevention. Am J Prev Med. 2018;55(4):565–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Moin T, Li J, Duru OK, Ettner S, Turk N, Keckhafer A, et al. Metformin prescription for insured adults with prediabetes from 2010 to 2012: a retrospective cohort study. Ann Intern Med. 2015;162(8):542–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turbow S, Hollberg J, Ali M. Electronic health record interoperability: how did we get here and how do we move forward? JAMA Health Forum. 2021;2(3):e210253. [DOI] [PubMed] [Google Scholar]
- 34.Diabetes Prevention Program Research Group. The 10-year cost-effectiveness of lifestyle intervention or metformin for diabetes prevention: an intent-to-treat analysis of the DPP/DPPOS. Diabetes Care. 2012;35(4):723–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bantle JP, Wylie-Rosett J, Albright AL, Apovian CM, Clark NG, Franz MJ, et al. Nutrition recommendations and interventions for diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2008;31(Suppl 1):S61–78. [DOI] [PubMed] [Google Scholar]
- 36.Briggs Early K, Stanley K. Position of the Academy of Nutrition and Dietetics: the role of medical nutrition therapy and registered dietitian nutritionists in the prevention and treatment of prediabetes and type 2 diabetes. J Acad Nutr Diet. 2018; 118(2):343–53. [DOI] [PubMed] [Google Scholar]
- 37.Krist AH, Davidson KW, Mangione CM, Barry MJ, Cabana M, Caughey AB, et al. Behavioral counseling interventions to promote a healthy diet and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: US Preventive Services Task Force recommendation statement. JAMA. 2020;324(20):2069–75. [DOI] [PubMed] [Google Scholar]
- 38.Alva ML. A review of the impacts of different approaches for diabetes prevention and a framework for making investment decisions. Int J Environ Res Public Health. 2018; 15(3):e522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Urban LE, Weber JL, Heyman MB, Schichtl RL, Verstraete S, Lowery NS, et al. Energy contents of frequently ordered restaurant meals and comparison with human energy requirements and U.S. Department of Agriculture database information: a multisite randomized study. J Acad Nutr Diet. 2016;116(4):590–8.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cawley J, Frisvold D, Jones D. The impact of sugar-sweetened beverage taxes on purchases: evidence from four city-level taxes in the U.S. [Internet]. Cambridge (MA): National Bureau of Economic Research; 2019 Oct [cited 2022 May 25]. (NBER Working Paper No. 26393). Available from: https://www.nber.org/papers/w26393 [Google Scholar]
- 41.Hall KD, Sacks G, Chandramohan D, Chow CC, Wang YC, Gortmaker SL, et al. Quantification of the effect of energy imbalance on bodyweight. Lancet. 2011;378(9793):826–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Salopuro TM, Saaristo T, Oksa H, Puolijoki H, Vanhala M, Ebeling T, et al. Population-level effects of the National Diabetes Prevention Programme (FIN-D2D) on the body weight, the waist circumference, and the prevalence of obesity. BMC Public Health. 2011;11(1):350. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.