Abstract
Objective
The aim of our study was to assess the close direct relationship between the development of neurological manifestations and SARS-CoV-2 infection in paediatric patients, by a systematic review of the literature.
Methods
PubMed and Google Scholar were searched for publications from the start of the pandemic up to 31 December 2022. We included articles that described nervous system manifestations in patients aged 0–18 years with RT-PCR nasopharyngeal swab positivity for SARS-CoV-2.
Results
The initial search produced 3456 records, of which 37, involving a total of 48 paediatric patients, were included in our systematic review. The neurological complications were heterogeneous, due at least in part to the different age groups considered. In the literature, encephalitis was the most common diagnosis (20.83%). Clinical presentation, diagnostic support, treatment and outcome were analysed both in the literature and in our experience.
Conclusions
Neurological impairment can occur with the first clinical presentation of COVID-19, even in previously healthy children and with mild forms of the disease. The clinical presentations are heterogeneous in the different age groups. In most patients the outcome is good and neurological manifestations subside after discharge.
Keywords: SARS-CoV-2, Children, Neurological complications, Clinical presentations, Diagnostics, Outcome
1. Introduction
The pandemic relating to Severe Acute Respiratory Syndrome coronavirus 2 (SARS-CoV-2) and the associated coronavirus disease 2019 (COVID-19) still represents an important challenge for physicians and researchers worldwide. While SARS-CoV-2 presents primarily as a respiratory disease, typically characterised by fever, cough and fatigue [1], injury to other organ systems, including the nervous system, is well documented [2]. Although more than two years have passed since the discovery of this novel virus, the available literature data on the presentation of COVID-19 in children are still scanty. The primary objective of our study was to assess the close direct relationship between the development of neurological manifestations (MeSH) and SARS-CoV-2 infection (MeSH) as confirmed by nasopharyngeal swab positivity, in paediatric patients (MeSH). The secondary objective was to specifically define the clinical picture of these children, considering when and how neurological symptoms present, progress and resolve in relation to infection. We also focused on the diagnostic investigations carried out to establish the diagnosis, and on treatment and outcomes after discharge to analyse the prognosis of these patients.
1.1. Evidence on SARS-CoV-2-induced neurological impairment
On the basis of the evidence generated by a great many studies, we now know that SARS-CoV-2 can be responsible for central and peripheral nervous system damage through several mechanisms. The interaction between cellular receptors and the spike protein of the virus and the subsequent activation of intracellular pathways are the key elements of SARS-CoV-2 pathogenesis [3]. It was already known that other SARS coronaviruses utilise the human angiotensin converting enzyme 2 (hACE2) receptor of the host cell for cell entry [4]. Although SARS-CoV-2 uses the same mechanism, its ACE2 receptor binding affinity is 10–20 times greater, due to structural differences in the receptor binding domain of the spike protein [5,6]. ACE2 is not the only receptor exploited for cell entry by SARS-CoV-2, which requires other receptor bindings, some of which are known, such as CD147, while others are as yet unknown [5,7]. These receptors are also expressed in the circumventricular organs of the CNS, glial cells, and neurons: due to this mechanism, SARS-CoV-2 can potentially invade the nervous system, resulting in neurological manifestations [7,8]. This explains how the virus could directly invade the nervous system; however, it has been shown that COVID-19-induced neurological impairment is also caused by indirect effects of infection, including immune response and cytokine storms [9]. We can therefore consider two different mechanisms underlying nervous system damage [10]: 1) haematogenic (infection of endothelial cells or leukocytes) or trans-neuronal (through the olfactory tract or other cranial nerves) with dissemination to the central nervous system through viral neurotropism; 2) abnormal immune response, responsible for indirect secondary neurological damage. The subsequent neurological manifestations depend on these mechanisms. The former is responsible for the most common neurological symptoms of COVID-19 (hypogeusia, hyposmia, headache, dizziness, vertigo). The latter may cause more serious complications: diseases such as Guillain-Barré Syndrome (GBS), encephalitis and myelitis if the abnormal immune reaction prevails; states of hypercoagulability and acute cerebrovascular events if a cytokine storm develops. These two mechanisms should not be considered as separate, since the virus can enter the nervous system directly and subsequently cause microglial activation resulting in the production of pro-inflammatory cytokines. This is the case in seizures secondary to SAR-CoV-2, when a substantial virus-induced inflammatory cascade leads to neuronal necrosis in the central nervous system, specifically in various parts of the hippocampus [11].
2. Methods
2.1. Systematic review
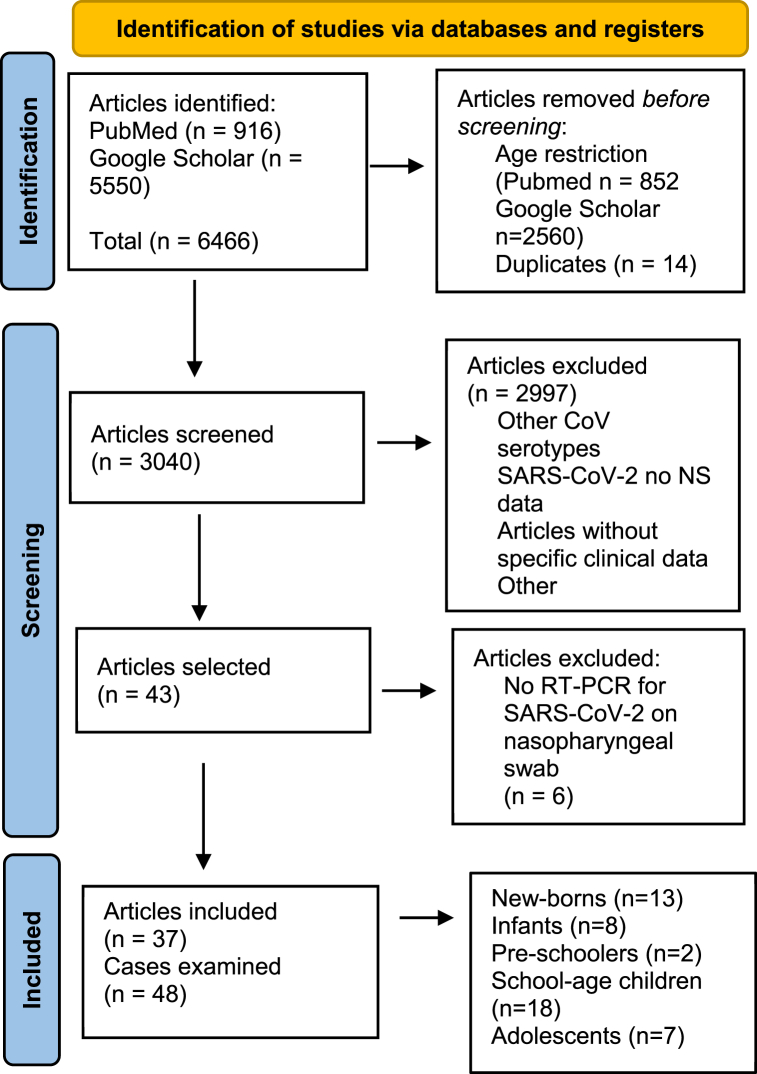
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed while conducting the study. We searched PubMed Central and Google Scholar using the keywords “Coronavirus”, “COVID-19”, “SARS-COV-2”, “brain”, “central nervous system”, “nervous system”, “neurological”. To restrict the search to the paediatric age, in PubMed we added the age filter “Child: birth-18 years”, whereas in Google Scholar we added the keyword “children”. The search was restricted to English language manuscripts alone, with no time limit. The last literature search was carried out on 31 March 2022. Duplicates were removed after review. All full texts were read by the same authors and data were extracted and discussed within the group to assess for quality indicators and reliability. Articles were included if they discussed a nervous system manifestation of SARS-COV-2 infection in patients aged 0–18 years. The infection had to have been confirmed by RT-PCR detection of the virus on a nasopharyngeal swab. The screened articles included case reports, case series and letters to the editor. We excluded all the studies in which it was not possible to collect specific clinical data on individual patients and studies describing other coronavirus serotypes. A flow diagram of the search strategy applied is provided in Fig. 1.
Fig. 1.
PRISMA 2020 flow diagram for new systematic reviews which included searches of databases and registers only. From Page et al. (1). For more information, visit http://www.prisma-statement.org/. 1. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021 Mar 29; n71.
2.2. Quality assessment
All included studies were assessed using QUADAS-2 (Quality Assessment of Diagnostic Accuracy Studies), a tool for analysing the quality of primary diagnostic accuracy studies included in systematic reviews that focuses on the risk of bias and applicability to the study.
The risk of bias judgement is based on the predefined signalling questions with regard to the four domains: patient selection, index test, reference standard and flow and timing.
The applicability judgement is based on the extent to which bias in any domain is likely to affect the question under review.
The risk of bias and applicability concerns were rated as ‘‘low’, ‘‘high’’ or ‘‘unclear’‘.
3. Results
3.1. Systematic review
We included a total of 37 articles involving 48 paediatric patients. The male:female ratio was 30 (62.5%) to 17 (35.4%); in one case gender was not specified [12]. The age of the patients ranged from 1 day to 17 years. Classifying the children by age group, the articles examined included 13 new-borns (<28 d), 5 infants (4w-24 m), 2 pre-schoolers (3 y-5y), 20 school-age children (6-14 y) and 7 adolescents (>15 y).
Just 4/48 patients had a history of one or more medical conditions and just 2 had a prior history of neurological disorders.
A total of 10 children (20.83%) presented non-specific neurological signs that were insufficient to establish a definite diagnosis. In the remaining cases (79.17%), the neurological impairment was further characterised by means of neurological examinations and instrumental diagnostic support. Encephalitis was the most common diagnosis (20.83%), followed by seizures (10.42%), Guillain-Barré Syndrome (GBS) (10.42%), cerebrovascular involvement (10.42%), ADEM (8.33%) and encephalopathy (8.33%). In the remaining cases, the neurological signs were secondary to central nervous system lesions (4.17%) and peripheral neuropathy (4.17%).
For 10/48 children (20.83%), neurological signs represented the first clinical presentation of COVID-19, without any prior respiratory symptoms or fever. Neurological symptoms presented on average 5 days after the onset of infection.
Table 1, Table 2, Table 3, Table 4, summarise the diagnostic results for the systematic review, classified by age group.
Table 1.
Systematic review results relating to neurological manifestations in new-borns with COVID-19.
| Author | PN | Age/gender | Diagnosis | Initial NS | NE | Fever before NS | Brain US | MRI-CT | CSF analysis |
|---|---|---|---|---|---|---|---|---|---|
| Chacòn-Aguilar R | 1 | 26 d/M | Febrile seizures | Paroxysmal episodes | A | yes | U | NP | NP |
| Lorenz N | 1 | 1 d/F | Encephalitis | Lethargy and inconsolable crying | NA | yes | U | NP | U |
| Vivanti A | 1 | 3 d/M | Non-specific neurological diagnosis | Poor feeding, hypertonia, and opisthotonos | NA | no | U | A | U |
| Martin P | 1 | 9 d/M | Encephalitis | Seizures | NA | no | NP | A | A |
| Fragoso D | 1 | 2 d/M | Encephalitis | Seizures | A | no | NP | A | NP |
| Yildiz H | 1 | 4 d/M | Encephalopathy | Atypical right limb movements | N | no | A | A | U |
| Yan K | 3 | <1 m/M | Non-specific neurological diagnosis | Non-specific neurological signs | A | no | NP | U | NP |
| Yan K | 2 | <1 m/M | Non-specific neurological diagnosis | Non-specific neurological signs | A | yes | NP | U | NP |
| Alvarado – Socarras J | 1 | 21 d/M | Non-specific neurological diagnosis | Drowsiness, weak suckling and hypotonia | NA | yes | U | NP | NP |
| Brum A | 1 | 17 d/M | Ischemic brain lesions | Seizures | A | yes | NP | A | NP |
PN: Patient Number; NE: Neurological examination; NS: neurological signs; US: Ultrasound; CSF: Cerebrospinal Fluid; U: unremarkable; NP: Not performed; NA: Not available; A: abnormal.
Table 2.
Systematic review results relating to neurological manifestations in infants with COVID-19.
| Author | PN | Age/gender | Diagnosis | Initial NS | NE | Fever before NS | LD | EEG | MRI-CT | CSF analysis | SD on CSF | Treatment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dugue R | 1 | 6w/M | Seizures | Seizures | N | yes | A | A | N | NP | N | NA |
| Nathan N | 4 | <3 m/M | Non-specific neurological diagnosis | Axial hypotonia and moaning sounds | NA | yes | NA | NA | NA | N | N | NA |
| McLendon L | 1 | 17 m/F | ADEM | Fatigue, progressively worsening weakness, and unsteady gait | A | yes | A | NP | A | A | NP | IVIG; steroids |
| Vraka K | 1 | 13 m/F | ADEM | Altered consciousness and seizures | A | yes | NA | A | A | A | N | Steroids |
| Garcia-Howard M | 1 | 3 m/F | Seizures | Seizures | A | yes | A | N | N | N | NP | Levetiracetam |
PN: Patient Number; NE: Neurological examination; NS: neurological signs; RS: Respiratory support; LD: Laboratory data; EEG: Electroencephalography; EMG: Electromyography; NCS: Nerve Conduction Study; CSF: Cerebrospinal Fluid; SD: SARS-CoV-2 Detection; NP: Not performed; NA: Not available; A: abnormal; N: normal; ADEM: Acute Disseminated Encephalomyelitis; IVIG: Intravenous immunoglobulins.
Table 3.
Systematic review results relating to neurological manifestations in pre-schoolers and school-age children with COVID-19.
| Author | PN | Age/gender | Diagnosis | Initial NS | NE | Fever before NS | RS | LD | EEG | NCS | EMG | MRI-CT | CSF analysis | SD on CSF | Treatment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| McAbee G | 1 | 11 y/M | Encephalitis | Status epilepticus | NA | yes | no | N | NP | NA | NA | N | A | NP | NA |
| Bhatta S | 1 | 11 y/M | Seizures | Generalised tonic-clonic seizure | N | yes | no | N | NP | NA | NA | N | NA | N | Levetiracetam |
| Enner S | 1 | 14 y/F | Encephalopathy | Generalised tonic-clonic seizure and apnoeic episodes | N | yes | yes | N | A | NA | NA | N | N | N | NA |
| Abdel -Mannan O | 1 | 9 y/M | Encephalitis | Confusion; ataxia; dysarthria; headache | NA | yes | yes | A | A | NA | NP | A | A | NP | NA |
| Abdel – Mannan O | 1 | 8 y/M | Encephalitis | Confusion and agitation; meningism; headache | NA | yes | yes | N | A | NP | A | A | A | NP | NA |
| Vraka K | 1 | 10 y/F | Encephalitis | Vomiting and lethargy | NA | yes | yes | N | N | NA | NA | A | A | N | NA |
| Khair A | 1 | 8 y/M | Convulsive status epilepticus | Seizure | NA | no | no | N | A | NA | NA | NP | N | NP | Lorazepam; Levetiracetam |
| Khalifa M | 1 | 11 y/M | GBS | Unsteady gait; inability to walk; tingling sensation in legs | A | yes | no | A | NP | A | NP | A | A | NP | IVIG |
| Goel N | 1 | 3 y/M | GBS | Progressive weakness of the distal lower extremities | A | yes | no | N | NP | A | NP | NP | NP | NP | IVIG |
| Manzo M | 1 | 6 y/M | ADEM | Generalised tonic-clonic seizure | NA | no | no | N | A | NP | NP | A | A | NP | Steroids |
| Maria A | 1 | 12 y/F | ADEM | Motor weakness and legs numbness | A | yes | yes | A | NP | NP | NP | A | A | N | NA |
| Roussel A | 1 | 6 y/? | Cranial polyneuropathy | Bilateral facial palsy, left trigeminal hypoesthesia, and voice changes | A | yes | yes | N | NP | NP | NP | A | N | N | IVIG, Remdesivir, Tocilizumab |
| Theophanous C | 1 | 6 y/M | Bell’s palsy | Right sided facial droop | A | no | no | A | NP | NP | NP | NP | NP | NP | IVIG |
| Ippolito Bastidas H | 1 | 13 y/F | Cerebral Venous Sinus Thrombosis | Impaired consciousness and intense headache | A | yes | no | A | NP | NP | NP | A | NP | NP | Anticoagulant |
| Gulko E | 1 | 13 y/F | Focal cerebral Arteriopathy of childhood-inflammatory type | Headache, speech difficulties and weakness of extremities | A | yes | no | N | NP | NP | NP | A | N | N | NA |
| Mirzaee S | 1 | 12 y/M | Focal cerebral Arteriopathy of childhood | Generalised seizures, right-sided hemiparesis and dysarthria | NA | no | no | A | NP | NP | NP | A | A | P | NA |
| Savic D | 1 | 13 y/F | Pseudoaneurysm of the left middle cerebral artery | Right side weakness | A | no | no | N | NP | NP | NP | A | NP | NP | NA |
| Poisson K.E. | 1 | 8 y/F | Cerebral Vasculitis | Left hemiparesis | A | yes | no | A | NP | NP | NP | A | NA | NA | Steroids, plasmapheresis, cyclophosphamide |
| Arslan G. | 1 | 5 y/F | PRES | Encephalopathy, vision loss and generalised tonic-clonic seizures | N | yes | yes | A | NP | NP | NP | A | NP | NP | Levetiracetam |
| Korkmazer B. | 1 | 10 y/M | PRES | Seizures | yes | yes | A | NP | NP | NP | A | NP | NP | Levetiracetam and phenytoin |
PN: Patient Number; NE: Neurological examination; NS: neurological signs; RS: Respiratory support; LD: Laboratory data; EEG: Electroencephalography; EMG: Electromyography; NCS: Nerve Conduction Study; CSF: Cerebrospinal Fluid; SD: SARS-CoV-2 Detection; NP: Not performed; NA: Not available; A: abnormal; N: normal; GBS: Guillain-Barré Syndrome; ADEM: Acute Disseminated Encephalomyelitis; PRES: Posterior reversible encephalopathy syndrome; IVIG: Intravenous immunoglobulin.
Table 4.
Systematic review results relating to neurological manifestations in adolescents with COVID-19.
| Author | PN | Age/gender | Diagnosis | Initial NS | NE | Fever before NS | RS | LD | EEG | NCS | EMG | MRI-CT | CSF analysis | SD on CSF | Treatment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bhavsar S | 1 | 16 y/F | Encephalitis | Generalised weakness and drowsiness | N | yes | yes | N | A | NP | NP | NA | A | N | NA |
| Abdel -Mannan O | 1 | 15 y/F | Encephalitis | Global flaccid weakness | A | yes | yes | N | A | NP | A | A | NP | NP | NA |
| Abdel -Mannan O | 1 | 15 y/F | Encephalitis | Global proximal weakness | A | yes | yes | N | NP | NP | A | A | NP | NP | NA |
| Maghrabi Y | 1 | 15 y/M | Acute Haemorrhagic Myelitis | Rapidly progressive lower limb weakness; urinary and bowel incontinence | A | yes | yes | A | NP | NP | NP | A | A | N | Steroids and IVIG |
| Terencio B | 1 | 16 y/F | AIDP - variant of GBS | Progressive symmetric ascending quadriparesis | A | no | no | N | NP | A | A | A | NP | NP | IVIG |
| Frank C | 1 | 15 y/M | AMAN – variant of GBS | Emetic episodes, weakness and ascending pain starting in the lower limbs | A | yes | no | N | N | NP | A | NP | N | N | IVIG |
| Araùjo N | 1 | 17 y/F | GBS | Severe low back pain; weakness of extremities with loss of ambulation | A | yes | no | N | NP | NP | A | A | A | P | IVIG |
PN: Patient Number; NE: Neurological examination; NS: neurological signs; RS: Respiratory support; LD: Laboratory data; EEG: Electroencephalography; EMG: Electromyography; NCS: Nerve Conduction Study; CSF: Cerebrospinal Fluid; SD: SARS-CoV-2 Detection; NP: Not performed; NA: Not available; A: abnormal; N: normal; AIDP: Acute Inflammatory Demyelinating Polyneuropathy; AMAN: Acute Motor Axonal Neuropathy; GBS: Guillain-Barré Syndrome; IVIG: Intravenous immunoglobulin.
3.1.1. Clinical features
Of the 13 new-borns, 8 presented non-specific neurological symptoms [[13], [14], [15]] characterised by poor feeding, hypertonia, opisthotonos, drowsiness, weak suckling and hypotonia. Three new-borns with encephalitis [[16], [17], [18]] and ischaemic brain lesions [19] presented generalised tonic-clonic seizures, and another new-born with encephalitis presented lethargy and inconsolable crying [20]. The clinical onset of the encephalopathy [18] was characterised by atypical right limb movements.
In the infant group, just one patient presented non-specific neurological symptoms [21], such us axial hypotonia and moaning sounds. Two infants with seizures showed a different clinical presentation: García-Howard M et al. [22] described a girl with two focal motor seizures with impaired consciousness and awareness; Dugue at al [23] reported a child with brief episodes of sustained upward gaze associated with bilateral leg stiffening after a nappy change. ADEM [24,25] also had a different clinical onset in two female infants: the first presented fatigue, progressively worsening weakness and unsteady gait; the second presented an altered state of consciousness and seizures.
The pre-schooler and school-age group was the largest in our review and the neurological diagnoses were heterogenous, including: encephalitis [[25], [26], [27]], GBS [28,29], ADEM [30,31], encephalopathy [32], posterior reversible encephalopathy syndrome (PRES) [33,34], seizures [35], convulsive status epilepticus [36], cranial polyneuropathy [12], Bell’s palsy [37], cerebral venous sinus thrombosis [38], focal cerebral arteriopathy of the childhood inflammatory type [39,40], pseudoaneurysm of the left middle cerebral artery [41] and cerebral vasculitis [42]. Seven children in this age group presented generalised tonic-clonic seizures as the first neurological sign of COVID-19, including children with PRES, focal cerebral arteriopathy of the childhood inflammatory type and ADEM. Children with GBS experienced unsteady gait, inability to walk and progressive weakness of the distal lower extremities.
As far as the 7 adolescents are concerned, 3 presented Guillain-Barré Syndrome [43], although two cases had different variants: acute inflammatory demyelinating polyneuropathy (AIDP) [44] and acute motor axonal neuropathy (AMAN) [45]. The other neurological diagnoses in the adolescent group were encephalitis [27,46] and acute haemorrhagic myelitis [47]. Amongst the adolescents, global or progressive ascending lower limb weakness was the most common initial neurological sign; however, in each case, it was associated with at least one other sign: drowsiness [46]; weak reflexes [27], urinary and bowel incontinence [44,47], loss of ambulation [43], pain in the lower limbs [45].
3.1.2. Diagnostic support
New-born and infant diagnoses were based on clinical examinations and instrumental neuroimaging. Brain US was used for new-borns alone. The only abnormal finding regarded the 4-day-old male with encephalopathy, in whom the ultrasound showed altered echogenicity in the periventricular and deep white matter. This alteration was confirmed by brain and spinal MRI: Yildiz et al. [18] were the first to report symmetrical restricted diffusion involving different brain regions associated with SARS-CoV-2 in a new-born with afebrile seizures. For the remaining new-borns and infants, in encephalitis cases the MRI primarily showed signal alterations in the periventricular and subcortical white matter, corpus callosum, internal capsule, optic radiation and posterior thalami.
As regards the pre-schooler and school-age patients, in all cases in which neuroimaging was performed it confirmed the clinical suspicion, apart from three cases in which the results were unremarkable [26,32,35]. For just one child with GBS, the MRI revealed enhancement of the cauda equina nerve roots in the postcontrast images [28]. The MRI of both children with ADEM, on the other hand, showed extensive bilateral and symmetrical restricted diffusion involving the subcortical and deep white matter and subtentorial cerebral parenchymal lesions with corpus callosum involvement [30,31]. For the 8-year-old girl with cerebral vasculitis, the MRI showed a right frontal lobe-enhancing lesion with vasogenic oedema; however, diagnostic certainty was only achieved with the brain biopsy, which showed perivascular histiocytic and T cell infiltrates with infarct-like necrosis [42]. In the children with focal cerebral arteriopathy of childhood, the MRI features combined with the clinical presentation were sufficient to formulate a diagnosis: the MRI depicted focal irregular narrowing and banding of the left middle cerebral artery with slightly reduced distal flow and concentric contrast enhancement at the site of stenosis [39,40]. For this age group, CSF analysis was important for establishing a diagnosis: white blood cell count elevation was confirmed in all the diagnoses of encephalitis [[25], [26], [27]]; as regards GBS, in just one case it revealed a high protein value (316.7 mg/dL) [28]; in the 6-year-old boy with ADEM it showed oligoclonal bands and expression of intrathecal synthesis [31].
MRI and CT neuroimaging were also performed in adolescents to confirm the clinical diagnoses, apart from in the case described by Bhavsar et al. [46] in which it was not specified. Again, the white matter was the most damaged. In the case of the adolescent patient with acute haemorrhagic myelitis, the T1-weighted images showed a hyperintense signal from the second cervical segment to the conus medullaris consistent with early subacute haemorrhage, associated with extensive spinal oedema [47]. Nerve conduction studies (NCS) were carried out in an adolescent with GBS in order to analyse his lower extremity weakness, revealing an absence of compound muscle action potential in both the upper and lower extremities [44]. Electromyography (EMG) was performed in 5/7 adolescents, revealing in all cases differing levels of myopathic changes. CSF analysis showed typical albuminocytological dissociation in the only case of GBS in which it was performed [43].
Of all the children studied, the SARS-CoV-2 RT-PCR test on CSF was positive for just 2 patients: a 12-year-old boy with focal cerebral arteriopathy of childhood [40] and a 17-year-old girl with GBS [43].
EEG was performed between episodes in a total of 11 patients: the abnormalities depicted were non-specific and never considered decisive for diagnosis.
3.1.3. Treatment
No specific treatments for neurological complications of COVID-19 were required or specified in the articles for the new-borns group. High-dose steroids were used to treat the cases of ADEM; IVIG therapy was required in just one case, due to a deterioration in the patient’s clinical condition characterised by increased lethargy and autonomic instability [24]. A combination of steroids and IVIG was preferred for the treatment of acute haemorrhagic myelitis [47]. Seizures were treated with levetiracetam, whereas an initial dose of lorazepam was administered to the child with convulsive status epilepticus. PRES was also treated with Levetiracetam, with addition of phenytoin in the case described by Korkmazer et al. [33]. A combination of steroids, cyclophosphamide and plasmapheresis was the complex treatment choice for the cerebral vasculitis case.
3.1.4. Outcome
After discharge, outcomes were better in the new-born and infant groups than in the preschooler, school-age and adolescent groups, since the neurological complications did not persist in the former two groups after the acute phase of infection. Patients in the latter three groups all showed clinical improvements after admission to hospital; however, some substantial changes in neurological functions persisted in the follow-up period for a total of 8 patients. In the case of the 16-year-old girl with acute inflammatory demyelinating polyneuropathy (AIDP), a variant of GBS, Terencio et al. [44] specified that, two months after discharge, she had a steppage gait but was able to walk unaided and her Guillain-Barré Syndrome Disability Score was 3 on discharge. The same score was allocated on discharge to the other adolescent patients with GBS [43,45]. The Medical Research Council (MRC) Scale was used to monitor muscle strength. In the case of the 12-year-old girl with ADEM, Maria et al. [30] reported that on discharge her strength had improved from grade zero in all four limbs to grade three in the left upper limb, grade three in the lower limbs, and grade two in the right upper limb, in addition to incomplete sphincter control. The patient with focal cerebral arteriopathy of childhood was still hemiparetic at home [40]. All seizures were limited to the acute phase of infection and did not occur in the follow-up period in any case. In one case the neurological complications of COVID-19 were fatal: for this previously healthy 8-year-old girl with cerebral vasculitis the weekly MRI studies showed progression of the lesion, which had extended into corpus callosum despite all the interventions carried out, and her neurological examination continued to deteriorate until her death 93 days after onset.
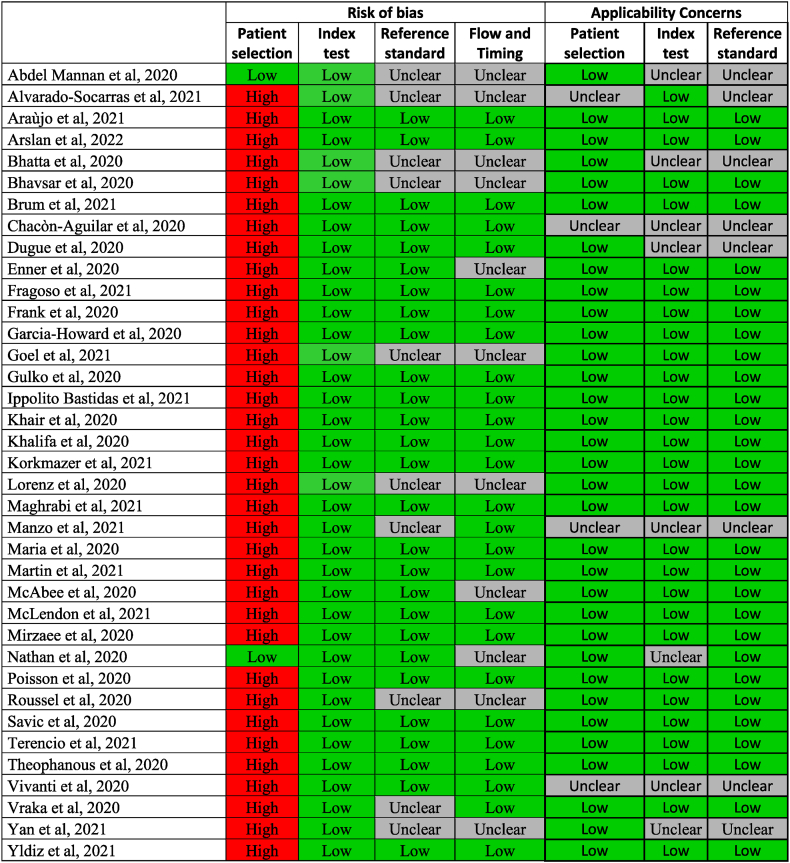
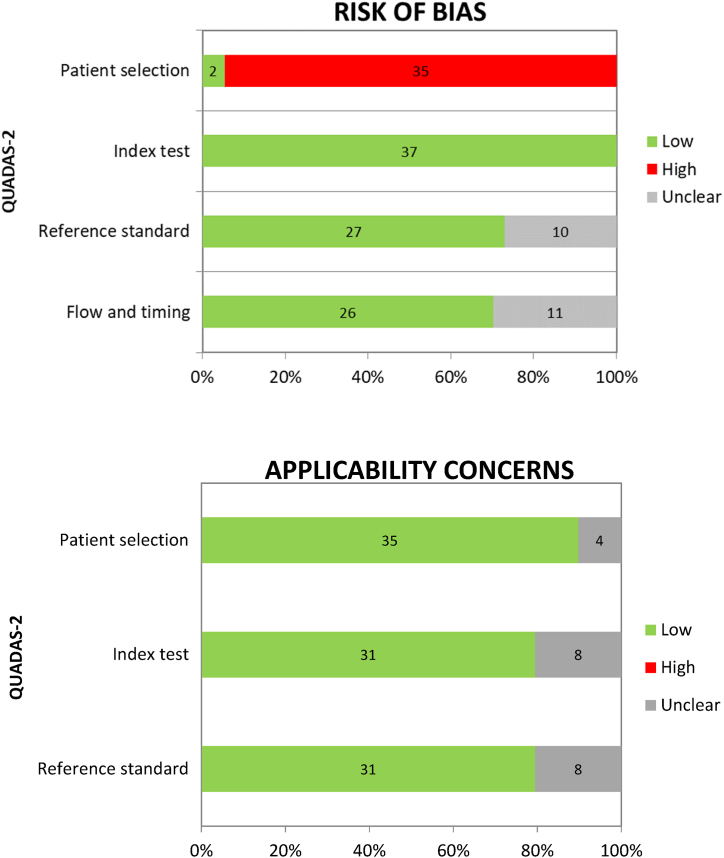
3.2. Quality assessment
The results of the quality assessment using the QUADAS-2 tool are summarised in Fig. 2, and the percentage of studies with risk of bias and applicability concerns are shown in the chart in Fig. 3. The risk of bias was generally low for “index test”, “reference standard” and “flow and timing”.
Fig. 2.
Quality assessment of included studies using the QUADAS-2 tool.
Fig. 3.
Percentage of studies with risk of bias and applicability concerns in the different domains of the QUADAS-2 tool.
Unfortunately, there was a high risk of bias for patient selection, since all but two of the studies selected were case reports. In these cases, as the patients with neurological manifestations were selected amongst others hospitalised for COVID-19, we decided to consider them as having a low risk. Despite being aware of this limitation, it should be pointed out that in the case of COVID-19, especially for paediatric patients, studies with a lower risk of bias are still lacking in literature. We considered the international guidelines defined for the same neurological manifestations occurring in absence of COVID-19 as the reference standard. For this reason, 10/37 studies were classified as “unclear” when the reference standard was not followed completely. As regards “flow and timing”, 11/37 studies were defined as having an intermediate risk because the patient’s follow-up observation period was not specified.
The risk of applicability concerns was generally low for all three domains considered. Overall, 4/37 studies for “patient selection”, 8/37 for “index test” and 8/37 for “reference standard” deviated slightly from our systematic review question and consequently an intermediate risk was considered.
4. Discussion
To the best of our knowledge, this will be the first systematic review available in literature to include such a high number of solely paediatric patients with neurological complications of SARS-CoV-2 infection, as confirmed by RT-PCR on nasopharyngeal swabs, with a well-defined clinical presentation. This study may help physicians to identify how these neurological complications can appear, for suspecting the presence of COVID-19 infection beyond. We believe that the greater insight obtained through the literature review will, above all, improve our clinical practice.
Our experience results are generally in line with the literature data, although in the literature we found a greater heterogeneity of diagnoses and clinical presentations.
It is true that in most cases neurological complications follow respiratory involvement and the onset of fever, which will already have aroused suspicions of a viral infection. However, it is not uncommon for neurological signs to occur as the first clinical manifestation of COVID-19 disease in children: 10/48 children (20.83%) had not presented fever or respiratory symptoms before the onset of neurological signs.
Various studies on the neurological complications of COVID-19, both in adults and children, all specify that nervous system involvement is more frequent in the presence of severe illness [48,49]. The Centers for Disease Control and Prevention (CDC) add that children with underlying medical conditions and infants (age <1 year) may be at increased risk of severe forms of COVID-19 [50,51]. Furthermore, on the basis of international data from the CDC, we decided to consider respiratory failure and MIS-C evolution as determining factors of severe forms of COVID-19 in children. In accordance with earlier data available in the literature [52,53], we would like to consider high serum IL-6 values as predictive of severe forms of COVID-19; however, we decided not to include it in the results as this data was not reported in most of the articles included in the review. Considering our results, 11 patients (22.92%) needed respiratory support and 4 patients (8.33%) presented MIS-C evolution. Severe forms of disease were observed only in children aged >2 years. Previous or concomitant diseases apart from COVID-19 in the patient’s history were also more common in children aged >2 years. Overall, 4/48 patients had a positive medical history and just 2 of them had a prior history of neurological disorders.
We noticed a particular aspect in line with previously-published studies [54,55]: the role of the infection in inducing neurological manifestations is not related to new diagnoses alone, since it can be the cause of flare-ups of previously controlled conditions or a triggering factor for previously unknown illnesses. As a matter of fact, Khair et al. [36] described a case in which a child with epilepsy that had been completely controlled for more than 3 years with antiepileptics developed evidence of infection with recurring seizures through to the onset of convulsive status epilepticus.
There were no significant differences in the diagnostic process used to define the neurological manifestations from that suggested by the guidelines for the same diseases before SARS-CoV-2 infection. What was decisive, but also more complex to define, was the clinical picture, which has always been the guiding element for any subsequent investigation required. Brain and spinal MRI was found to be the key instrumental investigation for establishing a definitive diagnosis: it was performed in 34 children and showed significant alterations in 25 of them. CSF analysis was the second most important investigation, and revealed abnormalities in a total of 14 children. As regards SARS-CoV-2 RT-PCR tests on CSF, our results did not show it to be of relevance to better defining the various aspects of the neurological complications of COVID-19 in children; however, this is in line with other data available in the literature, particularly for adult patients [56]. Just 2 patients tested positive and both presented central nervous system symptoms, without significant changes compared to other patients who tested negative with similar clinical presentations [40,43].
5. Limitations
The first limitation of our study is the limited number of reports providing complete information regarding neurological complications of SARS-CoV-2 infection in paediatric patients. The number of studies in literature has certainly increased since the beginning of the pandemic. Nevertheless, we think there is still much that can and must be done to define many aspects of this infection that are still unknown, especially in paediatric patients. Another important limitation regards the quality assessment of the articles included, since most of them are case reports, which entails a high risk of bias.
Most of the studies reported COVID-19 cases referring to the first year of the pandemic, when the infection was uncommon in patients under 18 years of age and physicians’ attention was intrigued by diverse and atypical manifestations of this novel viral disease. Consequently, on the one hand, the incidence of these atypical manifestations of COVID-19 may be overestimated due to the greater attention they attracted, and on the other, it may be underestimated due to delays in reporting this infection in the paediatric age. This consideration may suggest that the real incidence of these manifestations in all children with COVID-19 is not yet definable.
We considered only reports in which the infection was confirmed by RT-PCR detection of the virus on nasopharyngeal swabs; however, all the articles refer to different variants of the virus. As a result, it is impossible to establish how the incidence of neurological manifestations has changed in the most recent phase of the pandemic, during which the greater diffusion of the Omicron variant resulted in a higher incidence of infection in the paediatric age, as reported by the CDC. Whether the vaccine campaign in patients aged >5 years will lead to changes in the incidence of neurological complications remains to be seen.
6. Conclusions
Neurological complications can occur during SARS-CoV-2 infection in children, even in mild forms of infection and in previously healthy children. Clinical presentations are heterogenous, due at least in part to the different age groups considered. It is not possible to expect a specific neurological picture associated with COVID-19, even within the context of the same diagnosis. Although neurological signs may be the first clinical manifestation of infection, SARS-CoV-2 infection should be suspected in all children presenting such symptoms. In most patients, the outcome is good and neurological signs subside after discharge. We hope our study will provide the stepping-stone for further research on this topic, as greater knowledge regarding the heterogenous clinical presentation of this infection in children will improve our approach in the acute setting and during the follow-up period.
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of interest's statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Contributor Information
Raffaele Falsaperla, Email: raffaelefalsaperla@hotmail.com.
Chiara Gulisano, Email: chiaragulisano1@gmail.com.
References
- 1.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mao L., Jin H., Wang M., et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in wuhan, China. JAMA Neurol. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shang J., Wan Y., Luo C., et al. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. USA. 2020;117(21):11727–11734. doi: 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamming I., Cooper M., Haagmans B., et al. The emerging role of ACE2 in physiology and disease. J. Pathol. 2007;212(1):1–11. doi: 10.1002/path.2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baig A.M., Khaleeq A., Ali U., Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host–virus interaction, and proposed neurotropic mechanisms. ACS Chem. Neurosci. 2020;11(7):995–998. doi: 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- 6.Campos DM. de O., Fulco U.L., Oliveira C.B.S., Oliveira J.I.N. SARS‐CoV‐2 virus infection: targets and antiviral pharmacological strategies. J. Evid. Base Med. 2020;13(4):255–260. doi: 10.1111/jebm.12414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qiao J., Li W., Bao J., et al. The expression of SARS-CoV-2 receptor ACE2 and CD147, and protease TMPRSS2 in human and mouse brain cells and mouse brain tissues. Biochem. Biophys. Res. Commun. 2020;533(4):867–871. doi: 10.1016/j.bbrc.2020.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alquisiras-Burgos I., Peralta-Arrieta I., Alonso-Palomares L.A., Zacapala-Gómez A.E., Salmerón-Bárcenas E.G., Aguilera P. Neurological complications associated with the blood-brain barrier damage induced by the inflammatory response during SARS-CoV-2 infection. Mol. Neurobiol. 2021;58(2):520–535. doi: 10.1007/s12035-020-02134-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lima M., Siokas V., Aloizou A.M., et al. Unraveling the possible routes of SARS-COV-2 invasion into the central nervous system. Curr. Treat. Options Neurol. 2020;22(11):37. doi: 10.1007/s11940-020-00647-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mahboubi Mehrabani M., Karvandi M.S., Maafi P., Doroudian M. Neurological complications associated with Covid‐19; molecular mechanisms and therapeutic approaches. Rev. Med. Virol. 2022 doi: 10.1002/rmv.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nikbakht F., Mohammadkhanizadeh A., Mohammadi E. How does the COVID-19 cause seizure and epilepsy in patients? The potential mechanisms. Multiple Sclerosis Rel. Disord. 2020;46 doi: 10.1016/j.msard.2020.102535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roussel A., Germanaud D., Bouchoucha Y., et al. Cranial polyneuropathy as the first manifestation of a severe COVID-19 in a child. Pediatr. Blood Cancer. 2021;68(3) doi: 10.1002/pbc.28707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vivanti A.J., Vauloup-Fellous C., Prevot S., et al. Transplacental transmission of SARS-CoV-2 infection. Nat. Commun. 2020;11(1) doi: 10.1038/s41467-020-17436-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yan K., Xiao F.F., Jiang Y.W., et al. Effects of SARS-CoV-2 infection on neuroimaging and neurobehavior in neonates. World J. Pediatrics. 2021;17(2):171–179. doi: 10.1007/s12519-021-00423-2. [DOI] [PubMed] [Google Scholar]
- 15.Alvarado-Socarras J.L., Theurel-Martin D., Cruz-Hernandez M., Rodriguez-Morales A.J. Community-acquired neonatal SARS-CoV-2 infection associated with neurological symptoms in Colombia. J. Trop. Pediatr. 2021;67(1) doi: 10.1093/tropej/fmab022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin P.J., Felker M., Radhakrishnan R. MR imaging findings in a neonate with COVID -19-Associated encephalitis. Pediatr. Neurol. 2021;119:48–49. doi: 10.1016/j.pediatrneurol.2021.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fragoso D.C., Marx C., Dutra B.G., et al. COVID-19 as a cause of acute neonatal encephalitis and cerebral cytotoxic Edema. Pediatr. Infect. Dis. J. 2021;40(7):e270–e271. doi: 10.1097/INF.0000000000003145. [DOI] [PubMed] [Google Scholar]
- 18.Yildiz H., Yarci E., Bozdemir S.E., et al. COVID-19-Associated cerebral white matter injury in a newborn infant with afebrile seizure. Pediatr. Infect. Dis. J. 2021;40(7):e268–e269. doi: 10.1097/INF.0000000000003143. [DOI] [PubMed] [Google Scholar]
- 19.Brum A.C., Glasman M.P., de Luca M.C., et al. Ischemic lesions in the brain of a neonate with SARS-CoV-2 infection. Pediatr. Infect. Dis. J. 2021:E340–E343. doi: 10.1097/INF.0000000000003244. [DOI] [PubMed] [Google Scholar]
- 20.Lorenz N., Treptow A., Schmidt S., et al. Neonatal early-onset infection with SARS-CoV-2 in a newborn presenting with encephalitic symptoms. Pediatr. Infect. Dis. J. 2020;39(8):e212. doi: 10.1097/INF.0000000000002735. [DOI] [PubMed] [Google Scholar]
- 21.Nathan N., Prevost B., Corvol H. Atypical presentation of COVID-19 in young infants. Lancet. 2020;395(10235):1481. doi: 10.1016/S0140-6736(20)30980-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.García-Howard M., Herranz-Aguirre M., Moreno-Galarraga L., et al. Case report: benign infantile seizures temporally associated with COVID-19. Front. Pediatrics. 2020;8 doi: 10.3389/fped.2020.00507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dugue R., Cay-Martínez K.C., Thakur K.T., et al. Neurologic manifestations in an infant with COVID-19. Neurology. 2020;94(24):1100–1102. doi: 10.1212/WNL.0000000000009653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McLendon L.A., Rao C.K., da Hora C.C., Islamovic F., Galan F.N. Post-COVID-19 acute disseminated encephalomyelitis in a 17-month-old. Pediatrics. 2021;147(6) doi: 10.1542/peds.2020-049678. [DOI] [PubMed] [Google Scholar]
- 25.Vraka K., Ram D., West S., et al. Two paediatric patients with encephalopathy and concurrent COVID-19 infection: two sides of the same coin? Case Rep. Neurol. Med. 2021;2021:1–4. doi: 10.1155/2021/6658000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McAbee G.N., Brosgol Y., Pavlakis S., Agha R., Gaffoor M. Encephalitis associated with COVID-19 infection in an 11-year-old child. Pediatr. Neurol. 2020;109:94. doi: 10.1016/j.pediatrneurol.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abdel-Mannan O., Eyre M., Löbel U., et al. Neurologic and radiographic findings associated with COVID-19 infection in children. JAMA Neurol. 2020;77(11):1440. doi: 10.1001/jamaneurol.2020.2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khalifa M., Zakaria F., Ragab Y., et al. Guillain-Barré syndrome associated with severe acute respiratory syndrome coronavirus 2 detection and coronavirus disease 2019 in a child. J. Pediatric Infect. Dis. Soc. 2020;9(4):510–513. doi: 10.1093/JPIDS/PIAA086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goel N., Singh A. 2021. Post COVID-19 Guillain Barre Syndrome in Immunocompromised Children-A Report of Two Cases and Review of Literature. [DOI] [Google Scholar]
- 30.Maria A, Henriques-Souza M, Cláudia A, et al. Acute Disseminated Encephalomyelitis in a COVID-19 Pediatric Patient. doi:10.1007/s00234-020-02571-0/Published.
- 31.Manzo ML, Galati C, Gallo C, et al. ADEM Post-sars-CoV-2 Infection in a Pediatric Patient with Fisher-Evans Syndrome. doi:10.1007/s10072-021-05311-1/Published. [DOI] [PMC free article] [PubMed]
- 32.Enner S., Hormozdyaran S., Varughese R., et al. Central apnea in an adolescent with COVID-19. Pediatr. Neurol. 2020;110:87–88. doi: 10.1016/j.pediatrneurol.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Korkmazer B., Ozogul M., Hikmat E., et al. Posterior reversible encephalopathy syndrome in a pediatric COVID-19 patient. Pediatr. Infect. Dis. J. 2021:E240–E242. doi: 10.1097/INF.0000000000003130. [DOI] [PubMed] [Google Scholar]
- 34.Arslan G., Besci T., Karaca Ö., Aylan Gelen S. Posterior reversible encephalopathy syndrome related to COVID-19 in a child. Pediatr. Int. 2022;64(1) doi: 10.1111/ped.14908. [DOI] [PubMed] [Google Scholar]
- 35.Bhatta S., Sayed A., Ranabhat B., Bhatta R.K., Acharya Y. New-onset seizure as the only presentation in a child with COVID-19. Cureus. 2020 doi: 10.7759/cureus.8820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khair A.M., Husain S., Kaur G., Falchek S. Convulsive status epilepticus in a child with controlled epilepsy and concurrent COVID-19 infection: a case report and a quick review. Clin. Med. Insights Case Rep. 2020;13 doi: 10.1177/1179547620984126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Theophanous C., Santoro J.D., Itani R. Bell’s palsy in a pediatric patient with hyper IgM syndrome and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Brain Dev. 2021;43(2):357–359. doi: 10.1016/j.braindev.2020.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ippolito Bastidas H., Márquez-Pérez T., García-Salido A., et al. Cerebral venous sinus thrombosis in a pediatric patient with COVID-19. Neurology: Clin. Pract. 2021;11(2):e208–e210. doi: 10.1212/cpj.0000000000000899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gulko E., Overby P., Ali S., Mehta H., Al-Mufti F., Gomes W. Vessel wall enhancement and focal cerebral arteriopathy in a pediatric patient with acute infarct and COVID-19 infection. Am. J. Neuroradiol. 2020;41(12):2348–2350. doi: 10.3174/ajnr.A6778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mirzaee S.M.M., Gonçalves F.G., Mohammadifard M., Tavakoli S.M., Vossough A. Focal cerebral arteriopathy in a pediatric patient with COVID-19. Radiology. 2020;297(2):E274–E275. doi: 10.1148/radiol.2020202197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Savić D., Alsheikh T.M., Alhaj A.K., et al. Ruptured cerebral pseudoaneurysm in an adolescent as an early onset of COVID-19 infection: case report. Acta Neurochir. 2020;162(11):2725–2729. doi: 10.1007/s00701-020-04510-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Poisson K.E., Zygmunt A., Leino D., et al. Lethal pediatric cerebral vasculitis triggered by severe acute respiratory syndrome coronavirus 2. Pediatr. Neurol. 2022;127:1–5. doi: 10.1016/j.pediatrneurol.2021.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Araújo N.M., Ferreira L.C., Dantas D.P., et al. First report of SARS-COV-2 detection in cerebrospinal fluid in a child with Guillain-Barré syndrome. Pediatr. Infect. Dis. J. 2021;40(7):E274–E276. doi: 10.1097/INF.0000000000003146. [DOI] [PubMed] [Google Scholar]
- 44.Terencio B.B., Patiño R.F., Dominic R., Jamora G. Guillain-Barré Syndrome in a Pediatric Patient with COVID-19: A Case Report and Review of Literature. Acta Med Philipp [Internet] 2022 Sep.29;56(17) [cited 2023Apr.21] [Google Scholar]
- 45.Frank C.H.M., Almeida T.V.R., Marques E.A., et al. Guillain-barre syndrome associated with SARS-CoV-2 infection in a pediatric patient. J. Trop. Pediatr. 2021;67(3) doi: 10.1093/tropej/fmaa044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bhavsar S.M., Agarwal S., Lewis R., et al. COVID-19 infection associated with encephalitis in an adolescent. Neurology: Clin. Pract. 2021;11(2):e189–e192. doi: 10.1212/cpj.0000000000000911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Maghrabi Y., Baeesa S.S. Acute hemorrhagic myelitis in an adolescent with COVID-19: a case report and review of literature. Cureus. 2021 doi: 10.7759/cureus.20553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Panda P.K., Sharawat I.K., Panda P., Natarajan V., Bhakat R., Dawman L. Neurological complications of SARS-CoV-2 infection in children: a systematic review and meta-analysis. J. Trop. Pediatr. 2021;67(3) doi: 10.1093/tropej/fmaa070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ahmad I., Rathore F.A. Neurological manifestations and complications of COVID-19: a literature review. J. Clin. Neurosci. 2020;77:8–12. doi: 10.1016/j.jocn.2020.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shekerdemian L.S., Mahmood N.R., Wolfe K.K., et al. Characteristics and outcomes of children with coronavirus disease 2019 (COVID-19) infection admitted to US and Canadian pediatric intensive care units. JAMA Pediatr. 2020;174(9):868. doi: 10.1001/jamapediatrics.2020.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dong Y., Mo X., Hu Y., et al. Epidemiology of COVID-19 among children in China. Pediatrics. 2020;145(6) doi: 10.1542/peds.2020-0702. [DOI] [PubMed] [Google Scholar]
- 52.Leisman D.E., Ronner L., Pinotti R., et al. Cytokine elevation in severe and critical COVID-19: a rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir. Med. 2020;8(12):1233–1244. doi: 10.1016/S2213-2600(20)30404-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Broman N., Rantasärkkä K., Feuth T., et al. IL-6 and other biomarkers as predictors of severity in COVID-19. Ann. Med. 2021;53(1):410–412. doi: 10.1080/07853890.2020.1840621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kurd M., Hashavya S., Benenson S., Gilboa T. Seizures as the main presenting manifestation of acute SARS-CoV-2 infection in children. Seizure. 2021;92:89–93. doi: 10.1016/j.seizure.2021.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brisca G., Siri L., Olcese C., Brunenghi B.M., Pirlo D., Mancardi M.M. Is SARS-CoV-2 infection a risk for potentiation of epileptic seizures in children with pre-existing epilepsy? Pediatr. Neurol. 2021;116:31. doi: 10.1016/j.pediatrneurol.2020.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lewis A., Frontera J., Placantonakis D.G., et al. Cerebrospinal fluid in COVID-19: a systematic review of the literature. J. Neurol. Sci. 2021:421. doi: 10.1016/j.jns.2021.117316. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.