Abstract
Serial evaluation of blood lactate, including lactate clearance, may have greater value over single measurement at the time of presentation. The rationale of the current study was to evaluate the use of lactate clearance after 6 hours of admission to pediatric intensive care unit (PICU) as a predictor of mortality in critically ill children. A prospective observational study was conducted in a nine-bed PICU of a tertiary care teaching hospital over a period of 6 months. Lactate levels were measured in arterial blood samples of 76 patients at the time of admission and 6 hours later. According to calculated lactate clearance, patients were divided into group A (lactate clearance more than 0) which included 71% of patients and group B (lactate clearance ≤0) which included 29% of patients. Lactate level at admission was a poor predictor of mortality (area under receiver operating characteristic curve [AUC] = 0.519, p = 0.789). Lactate clearance after 6 hours of admission was a significant predictor of mortality (AUC = 0.766, p < 0.001). Using Kaplan–Meier survival curve, overall survival was significantly better among group A ( p < 0.001). Using multivariate logistic regression model, lactate clearance after 6 hours (odds ratio = 0.98, 95% confidence interval [CI]: 0.96–0.99) and The Pediatric Index of Mortality 2 (PIM2) score (odds ratio = 4.7, 95% CI: 1.85–12.28) had independent prognostic significance with regard to mortality ( p = 0.030, 0.001 respectively). We conclude that lactate clearance after 6 hours of admission can predict mortality in critically ill children.
Keywords: critical illness, kinetics, lactic acid, intensive care units, pediatric
Introduction
The concentrations of arterial lactate represent the balance between lactate production and clearance. Hyperlactatemia occurs when production exceeds clearance which has been confirmed to be associated with worse clinical outcomes in critically ill patients. 1 In critically ill patients, failure of oxygen delivery to meet oxygen demand results in tissue hypoxia and increases anaerobic metabolism. Elevated serum lactate levels reflect the anaerobic metabolism related to cellular hypoxia and are thought to be an important marker of impaired tissue perfusion. 2 Unresolved global tissue hypoxia, as indicated by inadequate lactate clearance, is associated with multiorgan dysfunction and increased mortality during the early phase of resuscitation. 2 3 Lactic acidosis can also occur with no clinical evidence of poor tissue perfusion or oxygenation, it is present due to an underlying etiology such as inborn error/s of metabolism, drug intoxication, renal or hepatic impairment. However, in many of these cases, occult tissue hypoperfusion is now recognized to accompany the primary etiology. 4
Serial evaluation of blood lactate, including lactate clearance, may have greater value over single measurement at the time of presentation. 5 Lactate clearance has been suggested as a parameter to evaluate the effectiveness of resuscitation. 5 6 Although the relationship between lactate clearance and the mortality of critically ill children is theoretically well proven by biochemical evidences, only few clinical studies have tried to prove this relation clinically. 3 7
The rationale of the current study was to evaluate the role of lactate clearance, after 6 hours of admission, in prediction of mortality in critically ill children.
Methods
This prospective observational study included 76 patients admitted to pediatric intensive care unit (PICU) belonging to a tertiary care hospital from March 1, 2017, to September 30, 2017. An approval of the study by the University Ethical Committee and an informed consent from the patients' parents were obtained.
Consecutive patients aged 1 month to 16 years admitted to PICU were eligible. Children having inborn error/s of metabolism or malignancy were excluded. All patients received echocardiography-guided management of shock as per our PICU protocol/s. Detailed echocardiographic findings using two-dimensional (B mode), M mode, pulsed Doppler were obtained. Therapy was adjusted according to the echocardiographic findings. First, to guide fluid therapy, when inferior vena cava (IVC) was collapsed or >50% respirophasic variation, fluid boluses were continued and when IVC was normal or full with minimal respirophasic variation, fluid boluses were discontinued or slowed. Second, when myocardial dysfunction was present, inotropes were started. Ascending and descending titration of inotropes was guided by the echocardiography findings. Echocardiography was done at time of admission and serially before and after changes in treatments and then daily, till patient is discharged or deceased.
Protocol of the Study
Lactate levels were measured in arterial blood samples collected from the patients using GEM premier 3500 (United States, serial no.13073215) blood gas and electrolytes analyzer. Blood samples were collected at the time of admission to the PICU and 6 hours later. Lactate clearance was calculated using the following equation: 7
Lactate clearance = [(arterial lactate level on admission – arterial lactate level after 6 hours of admission) × 100 / arterial lactate level on admission].
Patients were divided according to lactate clearance after 6 hours of admission into two groups. Group A in which lactate clearance was more than zero (improving lactate) and group B in which lactate clearance was less than or equal to zero (worsening lactate).
Data Collection
Routine investigations were performed including complete blood count, C-reactive protein, and renal and liver function tests. Pediatric Index of Mortality 2 (PIM 2) 8 score was calculated for rating the severity of medical illness for children. Because PIM2 describes how ill the child was at the time of providing intensive care, PIM2 was calculated from the information collected at the time a child was admitted to PICU. Items included are as follows: systolic blood pressure, pupillary reactions to bright light, partial oxygen pressure (PaO 2 ), fractional inspired oxygen (FIO 2 ) at the time of PaO 2 (if oxygen via endo-tracheal tube or head box), base excess in arterial or capillary blood, mechanical ventilation at any time during the first hour in PICU, either admission to PICU was elective, recovery from surgery or a procedure was the main reason for PICU admission, cardiac bypass, and whether it was high- or low-risk diagnosis. Length of PICU stays, as well as the in-hospital mortality, was recorded.
Sample Size
A sample size of 70 patients was the minimum required to detect an area under the receiver operating characteristics (ROC) curve (AUC) of 0.65, relative to a null value of 0.5, as statistically significant with 80% power and at a significance of 0.05. The sample size was calculated using Medcalc Software version 14.
Statistical Analysis
Data were collected, revised, coded, and fed to statistical software SPSS-IBM version 20. To test for differences at percentages; exact tests were used while Mann–Whitney test and independent samples t -test were used for comparing median and means, respectively. The AUC was carried using MedCalc Software version 14. Youden's index was used to determine the best cut-off value. All variables were candidate for univariate analysis to investigate the significant factors associated with mortality. Significant variables revealed by univariate analysis were subjected to a multivariate logistic regression model to identify independent risk factors of mortality. Kaplan–Meier Survival curve for overall survival among groups was used. A statistical significant value of less than 0.05 was adopted.
Results
Total PICU admissions were 118 during the study period, 35.6% ( n = 42) were excluded as per prespecified exclusion criteria. The 76 enrolled patients were divided according to their lactate clearance after 6 hours of admission. Group A (improving lactate) included 54 patients (71%) while group B (worsening lactate) included 22 patients (29%).
There were no statistically significant differences between the studied groups as regard to sex, age, or diseases affecting the patients, except that patients having sepsis were significantly more distributed among group B ( p = 0.041). Mean PIM2 score and risk of mortality were significantly higher in group B patients, while there was no significant difference as regard to PICU length of stay ( p = 0.657). Mortality was markedly higher among group B (86.4%) compared with that of group A (9.3%; Table 1 ). Using Kaplan–Meier survival curve, overall survival was significantly better among group A ( p < 0.001; Fig. 1 ). Table 2 shows the laboratory findings of both groups while Table 3 records arterial blood gases and lactate levels.
Table 1. Comparison between groups as regard demographic data, diagnosis and fate.
| Group A ( n = 54) | Group B ( n = 22) | p -Value | |
|---|---|---|---|
| Sex n (%) | b 0.428 | ||
| Males | 29 (53.7) | 14 (63.6) | |
| Females | 25 (46.3) | 8 (36.4) | |
| Age (mo) n (%) | c 0.116 | ||
| 1–6 | 22 (40.7) | 12 (54.5) | |
| > 6 12 | 13 (24.1) | 3 (13.6) | |
| > 12–24 | 5 (9.3) | 3 (13.6) | |
| > 24–36 | 8 (14.2) | 1 (4.5) | |
| > 36–48 | 5 (9.3) | 0 | |
| > 48–60 | 1 (1.9) | 3 (13.6) | |
| Diagnosis n (%) | |||
| Pulmonary diseases | 19 (35.4) | 4 (18.2) | b 0.14 |
| Neurological diseases | 8 (14.8) | 7 (31.8) | b 0.11 |
| Gastrointestinal diseases | 8 (14.8) | 3 (13.6) | b 1.00 |
| Renal diseases | 5 (9.3) | 3 (13.6) | b 0.68 |
| Cardiovascular diseases | 4 (7.1) | 2 (9.1) | b 1.00 |
| Sepsis and septic shock | 3 (5.6) | 5 (22.7) | b 0.04 e |
| Diabetic ketoacidosis | 4 (7.4) | 0 | b 0.37 |
| Others | 3 (5.6) | 0 | b 0.43 |
| PIM2 score a | 7.4 (0.2–80.5) | 44.55 (4.1–86.5) | d 0.001 e |
| Length of PICU stay (d) a | 4 (1–45) | 3.5 (1–98) | d 0.657 |
| Mortality rate n (%) |
5 (9.3) | 19 (86.4) | b ≤0.001 e |
Abbreviation: PICU, pediatric intensive care unit; PIM2, Pediatric Index of Mortality 2.
Note: Group A: lactate clearance at 6 hours >0; group B: lactate clearance at 6 hours ≤0.
Median (minimum–maximum).
p -Value of Fisher's exact test.
p -Value of the Mont Carlo test.
p -Value of Mann–Whitney test.
Statistically significant at p ≤ 0.05.
Fig. 1.
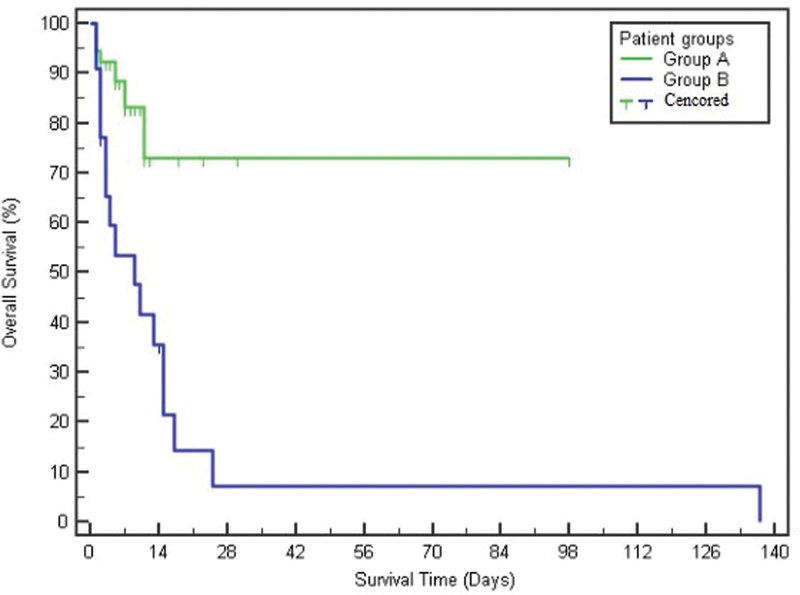
Kaplan–Meier survival curve of the studied groups. Group A: the improving lactate group (lactate clearance at 6 hours >0). Group B: the worsening lactate group (lactate clearance at 6 hours ≤0).
Table 2. Comparison between groups as regard to laboratory findings.
| Laboratory findings | Group A ( n = 54) | Group B ( n = 22) | p -Value |
|---|---|---|---|
| White blood cells (×10 3 /µL) | 386.50 (0.41–711) | 365 (12–692) | c 0.292 |
| Alanine aminotransferase (U/L) a | 0.31 (9–665) | 28 (13–692) | c 0.614 |
| Aspartate aminotransferase (U/L) a | 48.5 (10–844) | 45 (18–756) | c 0.722 |
| Serum albumin (g/dL) b | 3.06 (0.78) | 2.59 (0.94) | d 0.026 e |
| Total bilirubin (mg/dL) a | 0.34 (0.1–4.6) | 0.32 (0.1–5.1) | c 0.836 |
| Direct bilirubin (mg/dL) b | 0.1 (0–4) | 0.1 (0–1.76) | c 0.676 |
| Prothrombin time (s) a | 12.8 (9.7–56.8) | 15.1 (11.3–41.4) | c 0.125 |
| Partial thromboplastin time (s) a | 33.2 (20–120) | 37.45 (16–120) | cp = 0.488 |
| International normalized ratio a | 1.18 (1–5.1) | 1.34 (1–4) | c 0.184 |
| Blood urea nitrogen (mg/dL) a | 12.0 (3–92) | 13.5 (1–130) | c 0.859 |
| Serum creatinine (mg/dL) a | 0.47 (0.16–6.9) | 0.44 (0.04–3.8) | c 0.709 |
| C-reactive protein (mg/mL) a | 5 (1.1–263) | 12.4 (1.4–243) | c 0.146 |
| Serum sodium (mmol/L) b | 140.4 (12.2) | 144.1 (16.2) | d 0.286 |
| Serum potassium (mmol/L) b | 4.06 (0.88) | 3.43 (0.92) | d 0.007 e |
| Serum calcium (mg/dL) b | 8.7 (0.88) | 7.64 (1.79) | d 0.013 e |
| Serum phosphorus (mg/dL) a | 4.6 (2.1–10.6) | 5.5 (1.8–10.4) | c 0.136 |
| Alkaline phosphatase (U/L) a | 169.5 (28–534) | 156.5 (64–276) | c 0.689 |
Note: Group A: lactate clearance at 6 hours >0; group B: lactate clearance at 6 hours ≤0.
Median (minimum–maximum).
Mean (standard deviation).
p -Value of Mann–Whitney test.
p -Value for Student's t -test.
Statistically significant at p ≤ 0.05.
Table 3. Admission arterial blood gases and lactate levels.
| Group A ( n = 54) | Group B ( n = 22) | p -Value | |
|---|---|---|---|
| Arterial pH a | 7.31 (0.13) | 7.26 (0.18) | b 0.136 |
| Arterial O 2 sat % a | 84.69 (14.76) | 74.73 (19.12) | b 0.017 d |
| Arterial PaO 2 (mm Hg) a | 83 (24–214) | 60.5 (29–115) | c 0.001 d |
| Arterial PaCO 2 (mm Hg) a | 35 (12–69) | 40 (18–101) | c 0.16 |
| Arterial HCO 3 (mmol/L) a | 19.45 (8.07) | 20.44 (10.35) | b 0.67 |
| Admission lactate (mmol/L) a | 1.6 (0.5–19) | 1.2 (0.2–3.6) | c 0.010 d |
| Lactate after 6 hour (mmol/L) a | 0.9 (0.3–11.5) | 1.95 (0.5–15) | c 0.001 d |
| Admission lactate/pyruvate ratio a | 24 (8–32.2) | 19 (4–61) | c 0.025 d |
| Lactate/pyruvate ratio after 6 hours a | 15 (4–23) | 33.5 (9–22.9) | c 0.001 d |
Abbreviations: PaO 2 , partial oxygen pressure; PaCO 2 , partial carbon dioxide pressure; HCO 3 , bicarbonate.
Note: Group A: lactate clearance at 6 hours >0; group B: lactate clearance at 6 hours ≤0.
Median (minimum-–maximum).
p -Value for Student's t -test.
p -Value of Mann–Whitney test.
Statistically significant at p ≤ 0.05.
PIM2 score was a significant predictor of mortality (AUC = 0.935, p ≤ 0.001) followed by lactate clearance after 6 hours of admission (AUC = 0.766, p ≤ 0.001). However, lactate level on admission could not predict mortality (AUC = 0.519, p = 0.789; Table 4 ; Fig. 2 ).
Table 4. Agreement (sensitivity and specificity) of lactate on admission, lactate clearance at 6 hours and PIM2 as predictors of mortality.
| AUC | p -Value | Cut-off | Sensitivity | Specificity | PPV | NPV | |
|---|---|---|---|---|---|---|---|
| Serum lactate on admission | 0.519 | 0.789 | >1.3 | 62.5 | 51.9 | 37.5 | 75.0 |
| Lactate clearance after 6 hours from admission | 0.766 | <0.001 a | ≤0 | 70.8 | 90.4 | 77.3 | 87.0 |
| PIM2 score | 0.935 | <0.001 a | > − 0.92 | 91.7 | 94.2 | 88.0 | 96.1 |
Abbreviations: AUC, area under the receiver operating characteristic curve; NPV, negative predictive value; PIM2, Pediatric Index of Mortality 2; PPV, positive predictive value.
Statistically significant at p < 0.05.
Fig. 2.
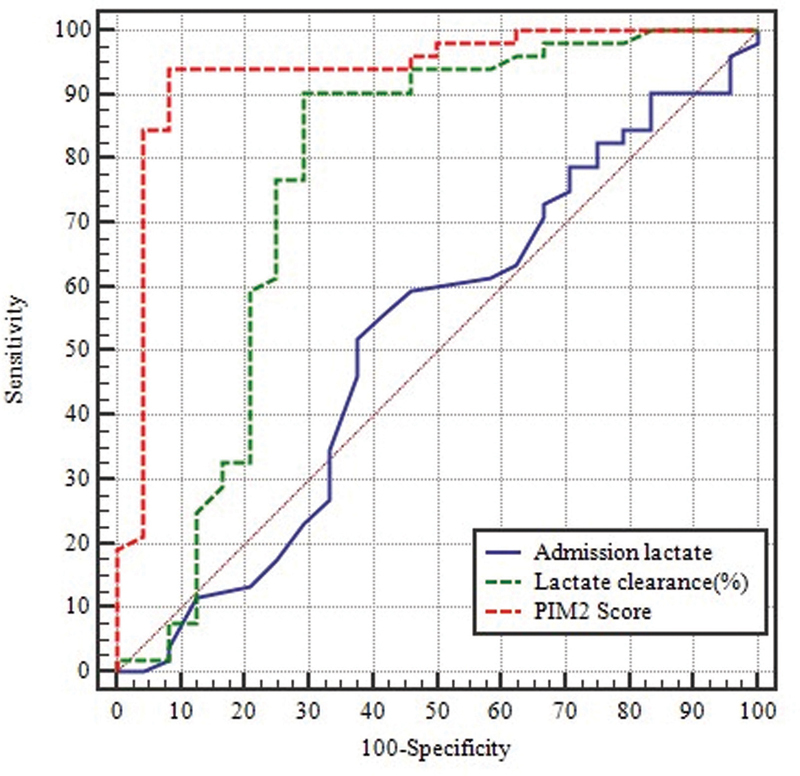
Receiver operating characteristics (ROC) curve for lactate on admission, lactate clearance at 6 hours from admission and Pediatric Index of Mortality 2 score.
Univariate analysis revealed that serum aspartate transaminase, albumin, calcium, phosphorus, PaO 2 , initial lactate, lactate clearance after 6 hours of admission, and PIM2 score were significantly affecting mortality. Using multivariate logistic regression model, only lactate clearance after 6 hours (odds ratio = 0.98, 95% confidence interval [CI]: 0.96–0.99) and PIM2 score (odds ratio = 4.7, 95% CI: 1.85–12.28) had independent prognostic significance as regards mortality ( p = 0.030 and 0.001, respectively).
Discussion
A significant reduction in mortality was seen in lactate-guided resuscitation compared with that in resuscitation without lactate monitoring in different trials. 9 10 11 Lactate clearance has been studied as early as 2 hours of admission and up to 2 days of admission. 12 13 Nowadays, it is easy and feasible to measure lactate level in a practical way with the point of care blood gas and electrolytes analyzers present in PICUs. In the current study, lactate clearance after the golden 6 hours of admission was studied and patients were classified into improving or worsening lactate groups. Early risk stratification and prognostication in critically ill patients are crucial because high-risk patients may benefit from earlier clinical interventions, whereas low-risk patients may benefit from avoiding unnecessary procedures.
The current study showed that lactate clearance after 6 hours of admission was a significant predictor of mortality after PIM2 score. Lactate clearance of ≤0% after 6 hours of admission predicted mortality with sensitivity of 70.8% and specificity of 90.4%. Similarly, Kumar and Kumar 14 reported that AUC of lactate clearance after 6 hours of admission was 0.823 and AUC of PIM2 score was 0.906. Munde et al 7 reported that lactate clearance <30% at 6 hours predicted mortality with sensitivity of 75% and specificity of 97% which was similar to the current study. Marty et al, 15 in a population of severe sepsis or septic shock patients, proved that lactate clearance after 6 hours of admission predicted mortality with sensitivity (63.46%) and specificity (56.1%). In the present study, serum lactate on admission was a poor predictor of mortality (AUC = 0.519). These results were comparable to Choudhary et al 16 who demonstrated that serum lactate on admission was a fair predictor of mortality (AUC = 0.697) compared with lactate clearance (AUC = 0.755). Other different studies concluded that lactate levels on admission did not have sensitivity or specificity as a predictor of death and serial lactate sampling rather than isolated measurement was fundamental in the prediction of outcome in critically ill children. 17 18
Multivariate logistic regression analysis showed that lactate clearance after 6 hours and PIM2 score had independent prognostic significance as predictors of mortality in our study. These results were parallel to that of Nguyen et al. 2 Donnino et al 19 proved that high lactate clearance at 12 hours remained predictive of survival in their multivariable analysis. On the other hand, Marty et al 15 and Wang et al 20 found that lactate clearance after 6 hours was not significantly related to mortality in their multivariate analysis.
Overall survival probability using Kaplan–Meier survival curve was significantly better in the group of improving lactate. In accordance to the study by Nguyen et al, 2 we also revealed significant lower mortality in patients with higher lactate clearance compared with the low clearance group.
The current study showed that patients with sepsis were statistically and significantly more distributed in the worsening lactate group. These results were comparable with a study by Marik and Bellomo 1 who concluded that failing to decrease blood lactate levels after the initiation of treatment of sepsis is an ominous sign. The cause of an elevated serum lactate in patients with sepsis can be multifactorial. In sepsis, lactate is overproduced and underutilized because of impaired mitochondrial oxidation, mainly through anaerobic glycolysis. Also, sepsis is accompanied by a hypermetabolic state, with enhanced glycolysis and hyperlactatemia even without an evidence of tissue hypoxia. Other possible causes may include sepsis-induced impairment of pyruvate-dehydrogenase enzyme activity, increased lactate production via catecholamine-driven pathways, and decreased lactate clearance due to hepatic dysfunction. 21 22 23 24
Limitations and Strengths
Limitations of the current study include being a single-center study and that lactate levels were measured after admission at PICU, therefore preintensive care managements might influence their lactate levels. Yet, this study can be considered as one of the few studies addressing this domain in PICU population.
Conclusion
Although PIM2 score still outperforms lactate, lactate clearance at 6 hours of admission is reliable and feasible method for prediction of mortality in critically ill children admitted to PICU.
Footnotes
Conflict of Interest None declared.
References
- 1.Marik P, Bellomo R. Lactate clearance as a target of therapy in sepsis: a flawed paradigm. OA Crit Care. 2013;1(01):3. [Google Scholar]
- 2.Nguyen H B, Rivers E P, Knoblich B P. Early lactate clearance is associated with improved outcome in severe sepsis and septic shock. Crit Care Med. 2004;32(08):1637–1642. doi: 10.1097/01.ccm.0000132904.35713.a7. [DOI] [PubMed] [Google Scholar]
- 3.Kim Y A, Ha E-J, Jhang W K, Park S J. Early blood lactate area as a prognostic marker in pediatric septic shock. Intensive Care Med. 2013;39(10):1818–1823. doi: 10.1007/s00134-013-2959-z. [DOI] [PubMed] [Google Scholar]
- 4.Kraut J A, Madias N E. Lactic acidosis. N Engl J Med. 2014;371(24):2309–2319. doi: 10.1056/NEJMra1309483. [DOI] [PubMed] [Google Scholar]
- 5.Kushimoto S, Akaishi S, Sato T. Lactate, a useful marker for disease mortality and severity but an unreliable marker of tissue hypoxia/hypoperfusion in critically ill patients. Acute Med Surg. 2016;3(04):293–297. doi: 10.1002/ams2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rhodes A, Evans L E, Alhazzani W. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(03):304–377. doi: 10.1007/s00134-017-4683-6. [DOI] [PubMed] [Google Scholar]
- 7.Munde A, Kumar N, Beri R S, Puliyel J M. Lactate clearance as a marker of mortality in pediatric intensive care unit. Indian Pediatr. 2014;51(07):565–567. doi: 10.1007/s13312-014-0448-2. [DOI] [PubMed] [Google Scholar]
- 8.Paediatric Index of Mortality (PIM) Study Group . Slater A, Shann F, Pearson G. PIM2: a revised version of the Paediatric Index of Mortality. Intensive Care Med. 2003;29(02):278–285. doi: 10.1007/s00134-002-1601-2. [DOI] [PubMed] [Google Scholar]
- 9.LACTATE study group . Jansen T C, van Bommel J, Schoonderbeek F J. Early lactate-guided therapy in intensive care unit patients: a multicenter, open-label, randomized controlled trial. Am J Respir Crit Care Med. 2010;182(06):752–761. doi: 10.1164/rccm.200912-1918OC. [DOI] [PubMed] [Google Scholar]
- 10.Emergency Medicine Shock Research Network (EMShockNet) Investigators . Jones A E, Shapiro N I, Trzeciak S, Arnold R C, Claremont H A, Kline J A. Lactate clearance vs central venous oxygen saturation as goals of early sepsis therapy: a randomized clinical trial. JAMA. 2010;303(08):739–746. doi: 10.1001/jama.2010.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lyu X, Xu Q, Cai G, Yan J, Yan M. [Efficacies of fluid resuscitation as guided by lactate clearance rate and central venous oxygen saturation in patients with septic shock] (in Chinese) Zhonghua Yi Xue Za Zhi. 2015;95(07):496–500. [PubMed] [Google Scholar]
- 12.Scott S, Antonaglia V, Guiotto G, Paladino F, Schiraldi F. Two-hour lactate clearance predicts negative outcome in patients with cardiorespiratory insufficiency. Crit Care Res Pract. 2010;2010:917053. doi: 10.1155/2010/917053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chertoff J, Chisum M, Simmons L, King B, Walker M, Lascano J. Prognostic utility of plasma lactate measured between 24 and 48 h after initiation of early goal-directed therapy in the management of sepsis, severe sepsis, and septic shock. J Intensive Care. 2016;4(01):13. doi: 10.1186/s40560-016-0142-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumar R, Kumar N. Validation of lactate clearance at 6 h for mortality prediction in critically ill children. Indian J Crit Care Med. 2016;20(10):570–574. doi: 10.4103/0972-5229.192040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marty P, Roquilly A, Vallée F. Lactate clearance for death prediction in severe sepsis or septic shock patients during the first 24 hours in Intensive Care Unit: an observational study. Ann Intensive Care. 2013;3(01):3. doi: 10.1186/2110-5820-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choudhary R, Sitaraman S, Choudhary A. Lactate clearance as the predictor of outcome in pediatric septic shock. J Emerg Trauma Shock. 2017;10(02):55–59. doi: 10.4103/JETS.JETS_103_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vişneci E F, Cander B, Gül M, Dündar Z D, Dur A, Girişgin A S. Prognostic value of red cell distribution width in critically ill patients and comparison with intensive care unit scoring systems. Journal of Academic Emergency Medicine. 2017;16(01):2–7. [Google Scholar]
- 18.Umashankar M, Karthikeyan M. Serum lactate levels as a predictor of outcome in pediatric septic shock. IOSR J Dent Med Sci. 2016;15(08):24–27. [Google Scholar]
- 19.Donnino M W, Miller J, Goyal N. Effective lactate clearance is associated with improved outcome in post-cardiac arrest patients. Resuscitation. 2007;75(02):229–234. doi: 10.1016/j.resuscitation.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 20.Wang B, Chen G, Cao Y, Xue J, Li J, Wu Y. Correlation of lactate/albumin ratio level to organ failure and mortality in severe sepsis and septic shock. J Crit Care. 2015;30(02):271–275. doi: 10.1016/j.jcrc.2014.10.030. [DOI] [PubMed] [Google Scholar]
- 21.Tunney P, Chinnan N K. Serum Lactate in intensive care: practical points and pitfalls. Inflammation. 2016;6:5–8. [Google Scholar]
- 22.Nuzzo E, Berg K M, Andersen L W. Pyruvate dehydrogenase activity is decreased in the peripheral blood mononuclear cells of patients with sepsis. A prospective observational trial. Ann Am Thorac Soc. 2015;12(11):1662–1666. doi: 10.1513/AnnalsATS.201505-267BC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sterling S A, Puskarich M A, Jones A E. The effect of liver disease on lactate normalization in severe sepsis and septic shock: a cohort study. Clin Exp Emerg Med. 2015;2(04):197–202. doi: 10.15441/ceem.15.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ismail F, Mackay W G, Kerry A, Staines H, Rooney K D. The accuracy and timeliness of a Point Of Care lactate measurement in patients with Sepsis. Scand J Trauma Resusc Emerg Med. 2015;23(01):68. doi: 10.1186/s13049-015-0151-x. [DOI] [PMC free article] [PubMed] [Google Scholar]


