Abstract
Background
It has been shown that circular RNAs (circRNAs) are involved in the pathogenesis of non-small cell lung cancer (NSCLC). However, the molecular mechanisms of circRNAs in tumor malignant progression and tyrosine kinase inhibitors (TKI) resistance remain undefined. Hereby, we explored the mechanisms by which circRBM33 promotes NSCLC progression and epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKI) resistance.
Methods
Transcriptome sequencing (RNA-seq) was carried out to obtain the circRBM33 we investigated. Quantitative reverse transcriptase-polymerase chain reaction was performed to detect the expression of circRBM33. Cell counting kit-8 assay was performed to detect cell proliferation as well as flow cytometry to detect cell cycle and apoptosis. Transwell assay was performed to detect cell migration and invasion. In vivo tumourigenesis assays were performed to further validate the function of circRBM33. The transcriptome was sequenced after RNA-pulldown and knockdown of cirRBM33 to identify the proteins bound by cirRBM33 and the downstream mechanisms involved in the regulation of cirRBM33.
Results
The sequencing results revealed that cirRBM33 was highly expressed in the cell line of osimertinib resistant H1975. In vitro functional validation demonstrated that knockdown of circRBM33 inhibited H1975 proliferation, migration and invasion, changed cell cycle and promoted apoptosis. In vivo, knockdown of circRBM33 inhibited tumour growth. Mass spectrometry results and sequencing analysis of knockdown circRBM33 suggest that circRBM33 may mediate resistance to osimertinib in H1975-OR cells through regulate the DNMT1/interleukin-6 (IL-6) axis.
Conclusions
CircRBM33 is upregulated in NSCLC and that knockdown of circRBM33 inhibits the progression of NSCLC. CircRBM33 may combine with DNMT1, and regulate the resistance of H1975 osimertinib-resistant cells to osimertinib that mediated by IL6. CircRBM33 is a promising diagnostic and prognostic marker to provide effective treatment strategies for NSCLC patients.
Keywords: Non-small-cell lung cancer (NSCLC), circular RNAs (circRNAs), circRBM33, osimertinib, drug-resistance
Highlight box.
Key findings
• CircRBM33 can promote NSCLC progression and resistance to the EGFR-TKI osimertinib.
What is known and what is new?
• Knockdown of circRBM33 can inhibit cell proliferation, cycle, migration and invasion and promote apoptosis Report here about what does this manuscript adds.
• CircRBM33 may regulate IL-6 by interacting with DNMT1, thereby promoting the resistance of H1975-OR to osimertinib.
What is the implication, and what should change now?
• CircRBM33 may become a biomarker for the diagnosis and treatment of Non-small-cell lung cancer.
Introduction
Non-small cell lung cancer (NSCLC), the most common histological subtype of lung cancer, accounts for around 85% of the current incidence of lung cancer (1). In recent years, despite constant improvements in diagnostic and treatment techniques, the mortality rate of NSCLC patients remains high (2,3). Epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKI), mainly including erlotinib, afatinib, gefitinib and osimertinib, are often used as priority drugs in the treatment of patients with EGFR-mutated NSCLC (4). As a third-generation EGFR-TKI, osimertinib is currently recommended as first-line and adjuvant therapy for metastatic advanced NSCLC with sensitive EGFR mutations (19del and L858R) in domestic and foreign guidelines. However, drug resistance will inevitably develop within one year, which greatly limits the clinical application of osimertinib (5,6). In order to address or at least alleviate resistance to osimertinib in NSCLC patients, several studies have elucidated the underlying mechanisms of NSCLC development, nevertheless, the exact pathogenesis of NSCLC and resistance to osimertinib remains unclear.
Circular RNAs (circRNAs) belong to the family of non-coding RNA (ncRNA) characterised by a specific single-stranded closed loop that lacks 5′ caps and 3′ polyadenylated tails. CircRNAs have been found to be specifically expressed in different diseases and tumours, suggesting that circRNAs have multiple functional roles in biology and pathogenesis (7,8). According to recent studies, one of the most important functions of circRNAs is their ability to act as microRNA (miRNA) sponges, which mainly bind through miRNA-specific sequences and act as competitive endogenous RNAs (ceRNAs) to mediate the regulatory effects of miRNAs on downstream target mRNAs. The regulatory mechanism of ceRNAs is also the most studied so far. Secondly, some studies have found that some circRNAs have transcriptional and post-transcriptional regulatory functions, by regulating the expression of parental genes or downstream target genes. Similar to their role as miRNA sponges, some circRNAs have functions that can bind to RNA binding proteins (RBPs) and recruit or repress RBPs. In addition, several studies have found that circRNAs are able to use themselves as templates for protein translation (9-11). CircRNAs act as miRNA sponges, receiving and digesting miRNAs to control malignant cells chemoresistance (12). Additionally, it has been shown that circ-PAN3 activates the AMP activated protein kinase/mammalian target of rapamycin (AMPK/mTOR) pathway in acute myeloid lymphoma cells to enhance treatment resistance (13). A growing number of studies have shown that circRNAs are expressed differently in various cancers, such as breast, lung, oral squamous cell and gastric cancers (14-16). Further studies have shown that circRNAs are involved in a variety of biological processes in cancers, including proliferation, migration, apoptosis and the cell cycle, which in turn are involved in tumourigenesis and resistance to treatment (17).
DNA methyltransferase 1 (DNMT1), which encodes an enzyme that transfers methyl to the cytosine nucleotides of genomic DNA. This protein plays an important function in maintaining DNA methylation patterns after replication. Methylation of DNA is an important component of epigenetic gene regulation in mammals. Normal maintenance of methylation is beneficial for development, while abnormal methylation patterns are often closely associated with the development of diseases (18,19). Many studies have shown that aberrant expression and variation of DNMT1 is closely associated with tumour development, metastasis and drug resistance (20-22). Liu et al. found that SOX2 directly counteracted DNMT1 expression, thereby altering the methylation pattern, which in turn feedback inhibited FOXO3a expression, and that inhibition of DNMT1 activity could modulate the methylation pattern in breast cancer (23). Jiang et al. ARID2 expression was found to be negatively correlated with metastasis of hepatocellular carcinoma (HCC) and positively correlated with the prognosis of HCC patients, demonstrating that ARID2 inhibits tumor growth by recruiting DNMT1 to the promoter of SNAIL, elevating DNA methylation and inhibiting snail transcription to metastasis of HCC cells (24). In the present study, we found that circRBM33 was able to bind to DNMT1 by mass spectrometry and combined with transcriptome sequencing analysis revealed that DNMT1 may target IL-6, which is closely associated with the development of H1975 osimertinib-resistant (H1975-OR) cells and resistance to osimertinib.
CircRBM33 (circbase ID: hsa_circ_0001772) is one of the member molecules of circRNAs. Recent studies have shown that CircRBM33 is involved in the progression of gastric and cervical cancer, and its expression is closely related to the proliferation, invasion, metastasis and apoptosis of tumor cells (25,26). In the present study, we found that circRBM33 was highly expressed in the H1975 osimertinib-resistant cells. CircRBM33was derived from a reverse spliced. Knockdown of circRBM33 inhibited the proliferation, migration, invasion and promoted apoptosis of H1975-OR cells. We further showed by transcriptome sequencing and mass spectrometry that circRBM33 may mediate osimertinib resistance in H1975-OR cells through modulation of the DNMT1/IL-6 axis. It provides a new idea for the study of osimertinib resistance mechanism, in order to make more patients receiving osimertinib treatment to obtain better therapeutic effect and longer survival benefit. We present the following article in accordance with the ARRIVE reporting checklist (available at https://atm.amegroups.com/article/view/10.21037/atm-22-6346/rc).
Methods
RNA-seq
The total RNA was extracted from H1975-OR cells and wild-type H1975 cells using Trizol reagent (Takara, Japan) in three replicates per sample. Next, rRNA was removed using the RiboMinus Eukaryote kit (Novozymes, Nanjing, China). mRNA was then added to Fragmentation Buffer to make short fragments, and cDNA was synthesized using the fragmented mRNA as a template and polymerase chain reaction (PCR) amplification was performed. The entire library was prepared and the constructed library was sequenced using Illumina HiSeq2000 (Illumina, San Diego, USA).
Cell line and culture
NSCLC H1975 cells (containing EGFR L858R and T790M locus mutations) derived from non-smokers were purchased from Shanghai Cell Bank (Shanghai, China). Roswell park memorial institute (RPMI)- 1640 medium with 10% fetal bovine serum (FBS; Gbico) and antibiotics (100 µg/mL penicillin and 100 µg/mL streptomycin) was used during cell culture. And the cells were maintained in a 5% CO2 incubator at 37 ℃.
The establishment of osimertinib-resistant H1975 cells
H1975 cells in good condition were exposed to 0.03 µM osimertinib. 72 hours later, the cells were switched to fresh drug-free medium for culture. Until the surviving cells recovered and showed a normal exponential growth rate, the cells were replaced with one higher osimertinib for 72 h. The procedure was repeated throughout by gradual increases in concentration from 0.03 to 1.5 µM at progressively higher osimertinib concentrations. Approximately 5 months later, cells developed stable resistance to osimertinib. The eventually surviving cells were harvested and propagated in medium containing 2 µM osimertinib. The neo-established osimertinib-resistant cells were named H1975-OR cells. During the establishment of H1975-OR cells, parental H1975 cells were always cultured in drug-free medium. In this study cells of the 20th generation were used.
Vectors transfection
The human circRBM33 knockdown plasmid and control vector were designed and constructed by Reebok Biotech (Guangzhou, China). Short harpin RNA (shRNA) for circRBM33 was purchased from Sangon Biotech (Shanghai, China). Lentiviruses were used to package the circRBM33 shRNA. The lentiviral vector DNAs, comprising sh-PLVX and sh-circRBM33-shRNA (sh-circRBM33), were subsequently transfected into NSCLC cells. The cells were transfected, then cultured at 32 ℃ while the supernatant was collected. Then, sh-circRBM33 and sh-PLVX supernatants were filtered into particles. Finally, in accordance with the manufacturer’s procedure, lentiviral particles were introduced into NSCLC cells. Here are the specifics: sh-circRBM33 has the following sense and antisense mutations: sense: GATCCGTAGATGAATTTACATGAACTTTTCAAGAGAAAGTTCATGTAAATTCATCT-ATTTTTTG; antisense: AATTCAAAAAATAGATGAATTTACATGAACTTTCTCTTGAAAAGTTC-ATGTAAATTCATCTACG.
Quantitative reverse transcriptase-polymerase chain reaction (qRT-PCR)
Total RNA was extracted from cell and tissue samples using TRIzol Reagent (Life Technologies, Carlsbad, CA, USA) according to the manufacturer’s instructions. After spectrophotometric quantification, 1 µg of total RNA in a final volume of 20 µL was used for reverse transcription (RT) with a Script cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA) following the manufacturer’s instructions. According to the manufacturer’s protocol, total cDNA was then used for qRT-PCR with the TaqMan Gene Expression Assay (Thermo Fisher Scientific, Rockford, IL, USA) in a StepOne Plus Real-time PCR System (Thermo Fisher Scientific). The expression of human GAPDH genes was used as a control to calibrate the original concentration of tissue or cell mRNA, respectively. Target gene expression was calculated using the 2-ΔΔCT method. Each quantitative PCR assay was performed in triplicate and independently repeated three times. The primer information for the exhibition is as follows: circRBM33, forward: 5'-ATGTGGAAGAGCCAGAGGAG-3', reverse: 5'-GCCAGATAGCAAATCTTCTCCA-3'; GAPDH: forward: 5′-AGATCCCTCCAAAATCAAGTGG -3′, reverse: 5′-GGCAGAGATGATGACCCTTTT-3′.
Cell Counting Kit-8 (CCK-8) assay
The CCK-8 assay (Abcam, Shanghai, China) was used to detect sh-cirRBM33 and sh-PLVX H1975-OR cells proliferation. Cells were inoculated in 96-well plates at 2.0×103 cells/well. 100 µL of the same RPMI-1640 medium was added to each well of cells and 10 µM osimertinib was added. Subsequently, at 0, 24, 48, 72 and 96 hours, 10 µL of CCK-8 reagent and 100 µL of medium mix were added to protect from light. After incubation for 2 hours in a 37 ℃ incubator, the absorbance of each well at 450 nm was measured using an enzyme marker. Five replicate wells were set up for evaluation of each sample in this study and the measurements were repeated three times independently.
Apoptosis and cell cycle progression analysis by flow cytometry
First, cultured sh-circRBM33 and sh-PLVX H1975-OR cells were collected and cells were resuspended in phosphate buffered saline (PBS). After stopping digestion with the addition of 4 mL of full culture media, single cell suspension was created. The single cell suspensions were put into flow tubes, twice rinsed with PBS, then centrifuged for five minutes at 1,000 rpm. Then, the cells were resuspended with binding buffer, and a certain amount of cells were incubated with apoptosis detection reagent (Beyoncé Biologicals, Shanghai, China) in a tube for 15 min at room temperature and protected from light. Finally, apoptosis was detected on the flow cytometer machine. For cell cycle assay, cells were collected, fixed in 80% pre-cooled ethanol for about 18 hours at 4 ℃ in a refrigerator. The following day, cells were resuspended with propidine iodide staining solution and incubated at 37 ℃ for 30 minutes protected from light. Ensure that the cycle assay is completed by flow cytometry within 1 hour and the results are analysed for data using ModFit software.
Transwell assay
Transwell assay was applied to evaluate the migration capacity of sh-circRBM33 and sh-PLVX H1975-OR cells. Sh-circRBM33 and sh-PLVX H1975-OR cells were grown in serum-free media and counted. A culture medium containing 10% FBS (500 µL) was added to the lower layer of each compartment after 1×104 cells had been equally distributed into the upper layer of each compartment to stimulate cell migration to the opposite side. The cells were fixed in 4% methanol for 30 minutes, stained with 0.1% crystalline violet for 20 minutes, and then washed with PBS after being incubated for 24 hours at 37 ℃ in 5% CO2.
RNA pull-down assay
For RNA pull-down experiment, 1×107 cells were collected and resuspended in ice-cold PBS and washed. A total of 400 µL Dilution buffer (Thermo Scientific) was added for full lysis, together with a cocktail of protease inhibitors, phosphatase inhibitors and RNase inhibitors (Invitrogen). Then, 150 µL of Pierce Nucleic-Acid Compatible Streptavidin Magnetic Beads (Thermo Scientific) were taken, the beads were washed twice with wash buffer and the stock solution was removed with RNA capture buffer. Beads were incubated for 15–30 minutes with labeled circRBM33 biotin-RNA. The beads were washed five times with wash buffer and the proteins were eluted using 50 µL Biotin Elution buffer (Thermo Scientific). Finally, the retrieved proteins were used for mass spectrometry or western blot analysis.
Animal studies
BALB/c male nude mice (Vitalriver, Beijing, China) aged 4 weeks were divided into two groups of six each, both kept in a sterile environment for the experiment. Then, sh-circRBM33 and sh-PLVX H1975-OR cells were collected and their density was adjusted to 2×107/mL using RPMI-1640 medium. Next, the 100 µL cells were injected into the nude mice individually. The volume of the tumours was observed and measured regularly (every 3 days). All mice weighed 17.1–19.8 g during the experiment. After 31 days, the nude mice were sacrificed and we removed the tumor surgically. This study was reviewed and approved by the University of Science and Technology of China Animal Experimentation Ethics Committee [No. 2020-N(A)-116], in compliance with the University of Science and Technology guidelines for the care and use of animals.
Statistics
Images and statistical analyses were processed by GraphPad Prism 8 software. Data represent the mean ± standard deviation (SD). The significance of differences between groups was assessed by an unpaired two-tailed Student’s t-test. P<0.05 was considered statistically significant.
Results
CircRBM33 is highly expressed in the H1975-OR cells than H1975 cells
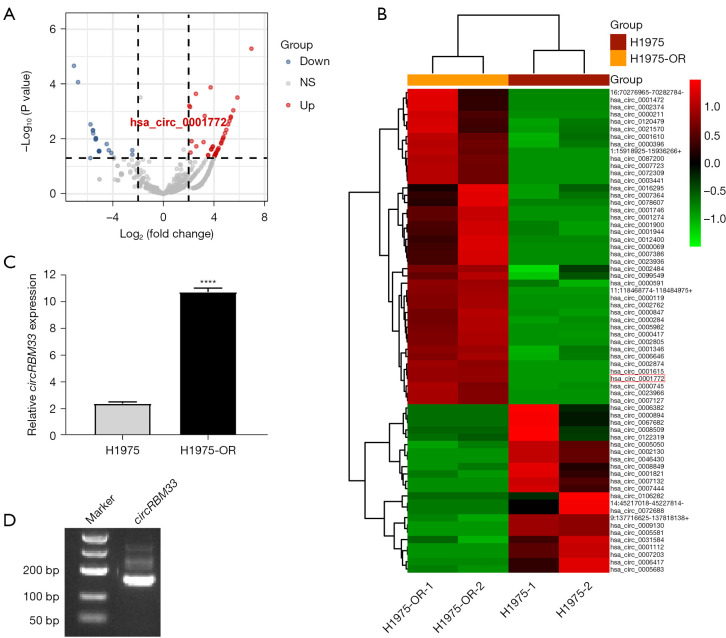
To investigate the role and mechanism of circRNAs in promoting EGFR-TKIs resistance in NSCLC, we extracted total RNA from NSCLC cell line H1975 and H1975-OR cells for transcriptome sequencing respectively. By processing and analysing the sequencing result, we mapped the volcanoe. The result showed that there were 43 differential circRNAs up-regulated and 23 down-regulated in H1975-OR cells (Figure 1A). In addition, we provide a heat map to give an overview of the expression of these different circRNAs in all samples (Figure 1B). Based in our previous pre-experiments with multiple candidate molecules, we found that circRBM33 had the most obvious results. Since circRBM33 has been reported to play a certain regulatory role in breast, gastric and cervical cancer, but not in lung cancer, so we chose circRBM33 as the preferred target circRNA. And there are no studies reporting the role and mechanism of hsa_circ_0001772 in NSCLC resistance to EGFR-TKIs. Therefore, we selected hsa_circ_0001772 for our follow-up study. Hsa_circ_0001772, also known as circRBM33 is encoded by position Chr7:15562867-155680908 of gene RBM33. qRT-PCR result confirmed that circRBM33 expression in H1975-OR cells significantly higher than that of H1975 cells (Figure 1C). PCR amplification by the primer designed for both ends of the circRBM33 junction site. A band of circRBM33 was shown in the gel electrophoresis result, indicating that circRBM33 was able to be expressed in a loop in H1975-OR cells (Figure 1D). Taken together, we found that circRBM33 is highly expressed in H1975-OR cells and it may be closely associated with the malignant progression of H1975-OR cells.
Figure 1.
CircRBM33 is highly expressed in H1975-OR cells than H1975 cells. (A) Volcano plot showing all differential circRNAs sequenced in the transcriptome, where blue indicates down-regulated circRNAs and red indicates up-regulated circRNAs. (B) Heatmap showing the expression of all differential circRNAs in each sequenced sample. (C) qRT-PCR result found that circRBM33 was significantly higher in H1975-OR cells than in H1975 cells. (D) Gel electrophoresis result showed the band of circRBM33, indicating that circRBM33 can be expressed in a loop. ****, P<0.0001; NS, no significant; H1975-OR, H1975 osimertinib-resistant; circRNA, circular RNA; qRT-PCR, quantitative reverse transcriptase-polymerase chain reaction.
Knockdown of circRBM33 reduces H1975-OR cells resistance to osimertinib, inhibits migration, invasion, cell cycle progression and induces apoptosis
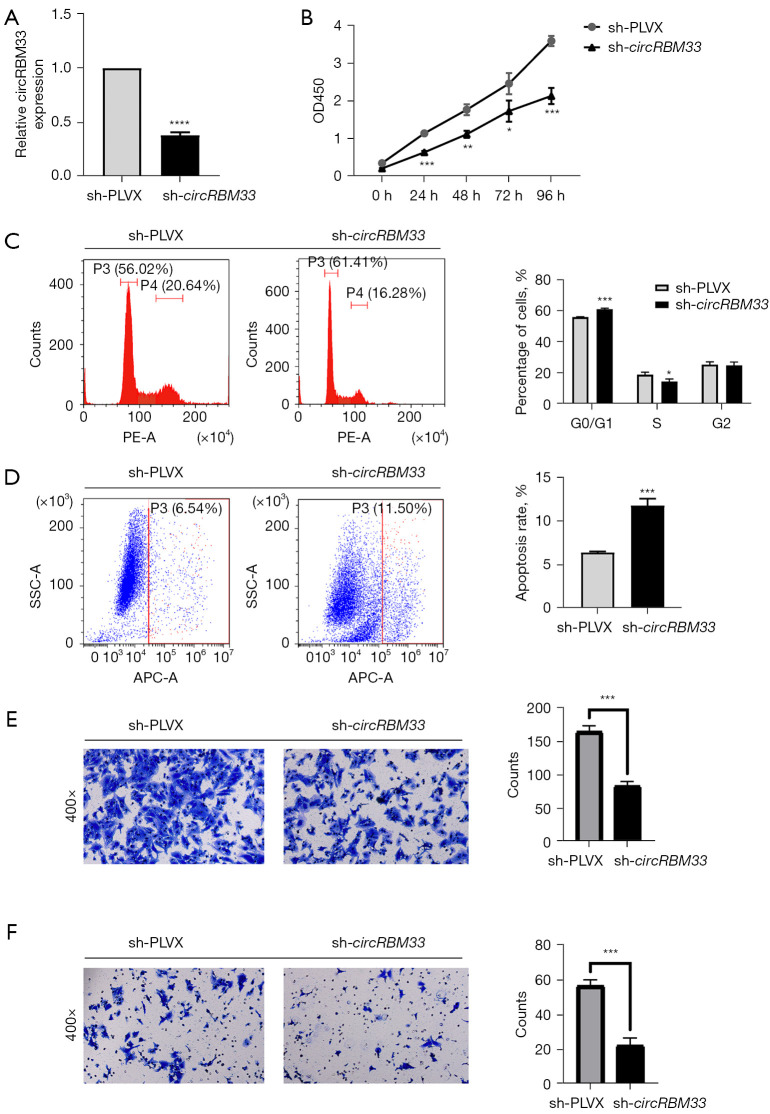
To investigate the effect of circRBM33 on the progression of H1975-OR cells, we transfected H1975-OR cells with circRBM33 knock-down plasmid, resulting in a robust reduction in circRBM33 levels (Figure 2A). CCK8 assay showed that knockdown of circRBM33 was able to result in significantly smaller OD450 values in sh-circRBM33 cells than sh-PLVX cells when treated with 10µM of osimertinib (Figure 2B). It indicated that knockdown of circRBM33 significantly reduced the resistance of H1975-OR cells to osimertinib. Flow cytometric assays revealed that knockdown of circRBM33 increased the G0/S1 phase and decreased the S/M phase of H1975-OR cells compared to controls (Figure 2C). At the same time, knockdown of circRBM33 greatly promoted apoptosis (Figure 2D). In addition, Transwell assay showed that knockdown of circRBM33 significantly inhibited the invasion and migration ability of H1975-OR cells compared to control (Figure 2E,2F). Taken together, knockdown of circRBM33 reduced H1975-OR cells resistance to osimertinib and inhibited H1975-OR cells adverse progression.
Figure 2.
Knockdown of circRBM33 reduced H1975-OR cells resistance to osimertinib, inhibited migration, invasion, change the cell cycle and promoted apoptosis. (A) qRT-PCR results demonstrating the knockdown efficiency of circRBM33 after H1975-OR transfection with sh-circRBM33 plasmid. (B) OD450 values of sh-circRBM33 and sh-PLVX groups were measured by CCK-8 at 0, 24, 48, 72, and 96 h using osimertinib concentration of 10 µM, respectively. (C,D) Detection of apoptosis and cycle of sh-circRBM33 and sh-PLVX cells using an attrition cytometry assay. (E,F) Migration and invasion of sh-circRBM33 and sh-PLVX were detected by transwell assay after crystal violet staining. Magnification, 400×. *, P<0.05; **, P<0.01; ***, P<0.001; ****, P<0.0001; H1975-OR, H1975 osimertinib-resistant; qRT-PCR, quantitative reverse transcriptase-polymerase chain reaction; CCK-8, cell counting kit-8.
Knockdown of circRBM33 inhibits tumour growth in vivo
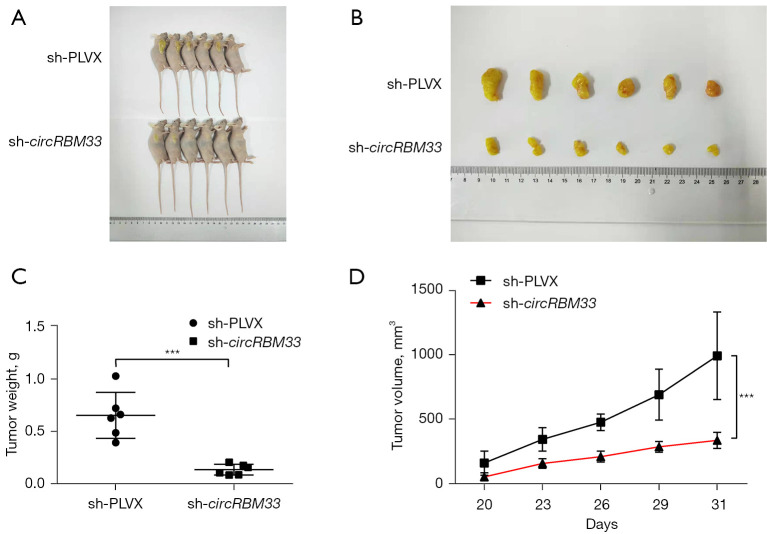
To further validate the function of circRBM33 in H1975-OR cells, we performed in vivo tumorigenic assays in nude mice. We divided the nude mice into sh-circRBM33 experimental group and sh-PLVX control group. Thirty-one days later, after sacrificing the mice, we harvested the tumours. It was found that knockdown of circRBM33 significantly inhibited tumour growth in vivo (Figure 3A). The weight, size and volume of the tumours in the experimental group were significantly smaller compared to the control group (Figure 3B-3D). Taken together, circRBM33 was able to promote tumour growth in vivo and may act as a pro-oncogene in H1975-OR cells.
Figure 3.
Knockdown of circRBM33 inhibited tumor growth in vivo. Nude mice were divided into sh-circRBM33 experimental group and sh-PLVX control group for in vivo tumorigenesis experiments (n=6). (A-D) The results of tumorigenesis, tumor weight and volume in the sh-circRBM33 and sh-PLVX groups, respectively, are shown. ***, P<0.001.
CircRBM33 may mediate resistance to osimertinib in H1975-OR cells through regulate the DNMT1/IL-6 axis
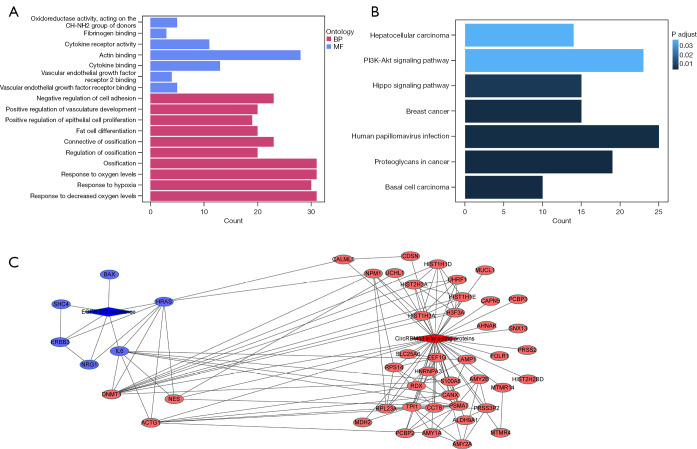
To explore the mechanism by which circRBM33 regulates drug resistance in H1975-OR cells, we performed RNA pulldown assay. Mass spectrometry result was analyzed to obtain circRBM33-bound proteins, and the top 20 differential proteins are shown in Table 1. In addition, after knocking down circRBM33 in H1975-OR cells, we extracted RNA for transcriptome sequencing. After processing the sequencing results, we performed Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis of the differential genes. GO and KEGG enrichment results demonstrate the relationship between differential genes and positive regulation of epithelial cells proliferation, response to oxygen levels, response to oxygen levels and phosphatidylinositol-3-kinase-AKT (PI3K-AKT) signaling pathway (Figure 4A,4B), all of which are closely related to tumor development. Afterwards, we visualized the network analysis of circRBM33 binding-related proteins and key genes in the EGFR-TKI resistance pathway (Figure 4C), which demonstrated that DNMT1 interacts with IL-6. Together with the above, circRBM33 may mediate resistance to osimertinib in H1975-OR cells through regulate the DNMT1/IL-6 axis.
Table 1. The top 20 differential proteins of the mass spectrometry result.
| Accession | Symbol ID | Description | -10lgP |
|---|---|---|---|
| P63261 | ACTG1 | Actin cytoplasmic 2 | 141.3 |
| P07478 | PRSS2 | Trypsin-2 | 66.11 |
| Q8NHM4 | PRSS3P2 | Putative trypsin-6 | 66.11 |
| Q71DI3 | HIST2H3C | Histone H3.2 | 64.1 |
| P68431 | HIST1H3A | Histone H3.1 | 64.1 |
| P84243 | H3F3A | Histone H3.3 | 64.1 |
| P26358 | DNMT1 | DNA (cytosine-5)-methyltransferase 1 | 57.56 |
| Q9NZT1 | CALML5 | Calmodulin-like protein 5 | 53.64 |
| P15328 | FOLR1 | Folate receptor alpha | 47 |
| P12236 | SLC25A6 | ADP/ATP translocase 3 | 43.89 |
| P09936 | UCHL1 | Ubiquitin carboxyl-terminal hydrolase isozyme L1 | 38.42 |
| P51991 | HNRNPA3 | Heterogeneous nuclear ribonucleoprotein A3 | 38.05 |
| P16402 | HIST1H1D | Histone H1.3 | 36.77 |
| P10412 | HIST1H1E | Histone H1.4 | 36.77 |
| Q96T88 | UHRF1 | E3 ubiquitin-protein ligase UHRF1 | 36.59 |
| P11279 | LAMP1 | Lysosome-associated membrane glycoprotein 1 | 35.95 |
| P04745 | AMY1A | Alpha-amylase 1 | 33.9 |
| P04746 | AMY2A | Pancreatic alpha-amylase | 33.9 |
| P19961 | AMY2B | Alpha-amylase 2B | 33.9 |
| P60174 | TPI1 | Triosephosphate isomerase | 33.32 |
Figure 4.
CircRBM33 may regulate resistance to osimertinib in H1975-OR cells through DNMT1/IL-6 axis. Sh-circRBM33 and sh-PLVX cells were transcriptome sequenced and all differentially expressed genes were obtained after analysis, respectively. (A,B) Showing the results of GO and KEGG enrichment of all differentially expressed genes. (C) The red part shows the proteins that interacted with circRBM33 in the mass spectrometry result, and the blue part shows the key genes associated with EGFR-TKI resistance obtained by RNA-seq after knocking down circRBM33 in H1975-OR cells. The link between the two parts of the results is shown by the Cytoscape visualization network. H1975-OR, H1975 osimertinib-resistant; GO, Gene Ontology; BP, biological process; MF, molecular function; KEGG, Kyoto Encyclopedia of Genes and Genomes; EGFR-TKI, epidermal growth factor receptor tyrosine kinase inhibitors.
Discussion
As one of the most aggressive malignancies, NSCLC has a poor prognosis, a high recurrence rate and the 5-year survival rate of patients remains low (1,27). EGFR-TKIs are a good option for the treatment of NSCLC patients, who show a good response rate (up to 75%) in the early stages of treatment, however, as treatment progresses, patients always acquire resistance to EGFR-TKIs and develop metastases, which severely limits the clinical benefit of EGFR-TKIs (28-30). Therefore, more therapeutic measures need to be urgently explored to overcome or at least alleviate resistance to EGFR-TKIs in NSCLC patients. It has been shown that circRNAs are involved in the proliferation, migration, invasion and differentiation of a variety of human tumour cells (31-33). In addition, some circRNAs have been found to play a role in chemoresistance of tumours. For example, Zhao et al. identified a novel circRNA CDR1as/miR-641/HOXA9 pathway regulating DDP chemoresistance in NSCLC. Knockdown of circRNA CDR1as increased the sensitivity of DDP-resistant NSCLC cells and reversed this by downregulating miR-641 or upregulating HOXA9. Consistently, overexpression of circRNA CDR1as increased the resistance of DDP-sensitive NSCLC cells by regulating the miR-641/HOXA9 axis (34). Recently, Hong et al. found that circ-CPA4 promoted NSCLC cell growth by upregulating PD-L1 through interaction with let-7 miRNA. And high expression of circ-CPA4 promoted metastasis, epithelial-mesenchymal transition (EMT) and resistance to cisplatin in NSCLC cells (32).
In the present study, we obtained H1975-OR cells differentially expressed circRNAs by transcriptomic profiling and found that circRBM33 was highly expressed in H1975-OR cells. And high expression of circRBM33 was associated with poor H1975-OR cells progression. Currently, some studies have reported that circRBM33 regulated the development of gastric cancer through the miR-149/IL-6 signaling axis, and that knockdown of circRBM33 significantly inhibited the proliferation, migration and invasion of gastric cancer cells and promotes apoptosis (25). Another study found that circRBM33 acted as a sponge for miR-758-3p to regulate PUM2 expression, thereby promoting proliferation, migration, EMT and glucose uptake in cervical cancer (26). However the function and role of circRBM33 in NSCLC resistance to EGFR-TKIs has not been explored. We found that in vitro validation showed that knockdown of circRBM33 reduced H1975-OR cells resistance to osimertinib, and that knockdown of circRBM33 inhibited cells proliferation, cycling, migration, invasion and promoted apoptosis. Further in vivo tumorigenic assay demonstrated that knockdown of circRBM33 significantly inhibited tumor growth. These results suggest that circRBM33 may act as a carcinogenic factor that promotes the development of H1975-OR cells and mediates the resistance of H1975-OR cells to the third-generation EGFR-TKI osimertinib.
As a special class of ncRNA, circRNA is highly conserved, heterogeneous and tissue-specific due to its own unique structure. Previous studies have well demonstrated that circRNAs have important functions and roles in tumour development (7,8). It is able to act as a sponge for miRNAs, thereby regulating the overexpression or repression of downstream target genes; it is also able to directly target target proteins, further regulating the expression of target genes of target proteins and subsequently participating in various biological and physiological processes. Wang et al. showed that circCCDC66 was able to act as a sponge for miR-33a-5p to target and regulate KPNA4, which in turn promoted cells proliferation, migration and invasion, while inhibiting apoptosis and accelerating the progression of NSCLC (35). Li et al. showed that circ_0072083 was highly expressed in NSCLC, and it directly acted on miR-545-3p to regulate the expression of downstream CBLL1, thereby promoting NSCLC cells colony formation, cells cycle and metastasis, while inhibiting DDP-stimulated apoptosis in NSCLC cells (36). Mechanistically, we carried out bioinformatic analysis of the results of RNA-pulldown and transcriptome sequencing analysis. The results revealed that circRBM33 may drive resistance to osimertinib in H1975-OR cells by interacting with DNMT1, thereby regulating IL-6.
Conclusions
In summary, we demonstrated that knockdown of circRBM33 reduced H1975-OR cells resistance to osimertinib, promoted migration and invasion, and inhibited apoptosis in H1975-OR cells. By bioinformatics analysis, we establish a new circRBM33/DNMT1/IL-6 signaling axis in NSCLC. circRBM33 may become a biomarker for the diagnosis and treatment of NSCLC.
Supplementary
The article’s supplementary files as
Acknowledgments
Funding: This study was supported by the Natural Foundation of Anhui Province (No. 1908085MH260), and Anhui Province Cancer Bioimmunotherapy Clinical Medical Research Center (No. 202101B10202005), and on the construction project of provincial key medical and health specialties (No. 2021sjlczdzk).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was reviewed and approved by the University of Science and Technology of China Animal Experimentation Ethics Committee [No. 2020-N(A)-116], in compliance with the University of Science and Technology guidelines for the care and use of animals.
Footnotes
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://atm.amegroups.com/article/view/10.21037/atm-22-6346/rc
Data Sharing Statement: Available at https://atm.amegroups.com/article/view/10.21037/atm-22-6346/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://atm.amegroups.com/article/view/10.21037/atm-22-6346/coif). The authors have no conflicts of interest to declare.
References
- 1.Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature 2018;553:446-54. 10.1038/nature25183 [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin 2016;66:115-32. 10.3322/caac.21338 [DOI] [PubMed] [Google Scholar]
- 3.Reck M, Popat S, Reinmuth N, et al. Metastatic non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2014;25 Suppl 3:iii27-39. 10.1093/annonc/mdu199 [DOI] [PubMed] [Google Scholar]
- 4.Gettinger S, Hellmann MD, Chow LQM, et al. Nivolumab Plus Erlotinib in Patients With EGFR-Mutant Advanced NSCLC. J Thorac Oncol 2018;13:1363-72. 10.1016/j.jtho.2018.05.015 [DOI] [PubMed] [Google Scholar]
- 5.Greillier L, Gauvrit M, Paillaud E, et al. Targeted Therapy for Older Patients with Non-Small Cell Lung Cancer: Systematic Review and Guidelines from the French Society of Geriatric Oncology (SoFOG) and the French-Language Society of Pulmonology (SPLF)/French-Language Oncology Group (GOLF). Cancers (Basel) 2022. [DOI] [PMC free article] [PubMed]
- 6.Zhao Y, Liu J, Cai X, et al. Efficacy and safety of first line treatments for patients with advanced epidermal growth factor receptor mutated, non-small cell lung cancer: systematic review and network meta-analysis. BMJ 2019;367:l5460. 10.1136/bmj.l5460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kristensen LS, Andersen MS, Stagsted LVW, et al. The biogenesis, biology and characterization of circular RNAs. Nat Rev Genet 2019;20:675-91. 10.1038/s41576-019-0158-7 [DOI] [PubMed] [Google Scholar]
- 8.Goodall GJ, Wickramasinghe VO. RNA in cancer. Nat Rev Cancer 2021;21:22-36. 10.1038/s41568-020-00306-0 [DOI] [PubMed] [Google Scholar]
- 9.Chen LL. The expanding regulatory mechanisms and cellular functions of circular RNAs. Nat Rev Mol Cell Biol 2020;21:475-90. 10.1038/s41580-020-0243-y [DOI] [PubMed] [Google Scholar]
- 10.Chen LL. The biogenesis and emerging roles of circular RNAs. Nat Rev Mol Cell Biol 2016;17:205-11. 10.1038/nrm.2015.32 [DOI] [PubMed] [Google Scholar]
- 11.Zhang Z, Yang T, Xiao J. Circular RNAs: Promising Biomarkers for Human Diseases. EBioMedicine 2018;34:267-74. 10.1016/j.ebiom.2018.07.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang X, Li Z, Zhang Q, et al. Circular RNA AKT3 upregulates PIK3R1 to enhance cisplatin resistance in gastric cancer via miR-198 suppression. Mol Cancer 2019;18:71. 10.1186/s12943-019-0969-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shang J, Chen WM, Liu S, et al. CircPAN3 contributes to drug resistance in acute myeloid leukemia through regulation of autophagy. Leuk Res 2019;85:106198. 10.1016/j.leukres.2019.106198 [DOI] [PubMed] [Google Scholar]
- 14.Zheng X, Huang M, Xing L, et al. The circRNA circSEPT9 mediated by E2F1 and EIF4A3 facilitates the carcinogenesis and development of triple-negative breast cancer. Mol Cancer 2020;19:73. 10.1186/s12943-020-01183-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li R, Jiang J, Shi H, et al. CircRNA: a rising star in gastric cancer. Cell Mol Life Sci 2020;77:1661-80. 10.1007/s00018-019-03345-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li C, Zhang L, Meng G, et al. Circular RNAs: pivotal molecular regulators and novel diagnostic and prognostic biomarkers in non-small cell lung cancer. J Cancer Res Clin Oncol 2019;145:2875-89. 10.1007/s00432-019-03045-4 [DOI] [PubMed] [Google Scholar]
- 17.Wang Y, Liu J, Ma J, et al. Exosomal circRNAs: biogenesis, effect and application in human diseases. Mol Cancer 2019;18:116. 10.1186/s12943-019-1041-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lyko F. The DNA methyltransferase family: a versatile toolkit for epigenetic regulation. Nat Rev Genet 2018;19:81-92. 10.1038/nrg.2017.80 [DOI] [PubMed] [Google Scholar]
- 19.Svedruzic ZM. Dnmt1: Structure and Function. In: Cheng XD, Blumenthal RM. editors. Modifications of nuclear DNA and its regulatory proteins. Academic Press; 2011:221-54. [Google Scholar]
- 20.Chen N, Zhao G, Yan X, et al. A novel FLI1 exonic circular RNA promotes metastasis in breast cancer by coordinately regulating TET1 and DNMT1. Genome Biol 2018;19:218. 10.1186/s13059-018-1594-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luo Y, Xie C, Brocker CN, et al. Intestinal PPARα Protects Against Colon Carcinogenesis via Regulation of Methyltransferases DNMT1 and PRMT6. Gastroenterology 2019;157:744-59.e4. 10.1053/j.gastro.2019.05.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu J, Liu Y, Meng L, et al. Targeting the PD-L1/DNMT1 axis in acquired resistance to sorafenib in human hepatocellular carcinoma. Oncol Rep 2017;38:899-907. 10.3892/or.2017.5722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu H, Song Y, Qiu H, et al. Downregulation of FOXO3a by DNMT1 promotes breast cancer stem cell properties and tumorigenesis. Cell Death Differ 2020;27:966-83. 10.1038/s41418-019-0389-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang H, Cao HJ, Ma N, et al. Chromatin remodeling factor ARID2 suppresses hepatocellular carcinoma metastasis via DNMT1-Snail axis. Proc Natl Acad Sci U S A 2020;117:4770-80. 10.1073/pnas.1914937117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang N, Lu K, Qu H, et al. CircRBM33 regulates IL-6 to promote gastric cancer progression through targeting miR-149. Biomed Pharmacother 2020;125:109876. 10.1016/j.biopha.2020.109876 [DOI] [PubMed] [Google Scholar]
- 26.Ding Y, Yuan X, Gu W. Circular RNA RBM33 contributes to cervical cancer progression via modulation of the miR-758-3p/PUM2 axis. J Mol Histol 2021;52:173-85. 10.1007/s10735-020-09933-1 [DOI] [PubMed] [Google Scholar]
- 27.Chen Z, Fillmore CM, Hammerman PS, et al. Non-small-cell lung cancers: a heterogeneous set of diseases. Nat Rev Cancer 2014;14:535-46. 10.1038/nrc3775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu SG, Shih JY. Management of acquired resistance to EGFR TKI-targeted therapy in advanced non-small cell lung cancer. Mol Cancer 2018;17:38. 10.1186/s12943-018-0777-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tan CS, Kumarakulasinghe NB, Huang YQ, et al. Third generation EGFR TKIs: current data and future directions. Mol Cancer 2018;17:29. 10.1186/s12943-018-0778-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kobayashi S, Canepa HM, Bailey AS, et al. Compound EGFR mutations and response to EGFR tyrosine kinase inhibitors. J Thorac Oncol 2013;8:45-51. 10.1097/JTO.0b013e3182781e35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xie M, Yu T, Jing X, et al. Exosomal circSHKBP1 promotes gastric cancer progression via regulating the miR-582-3p/HUR/VEGF axis and suppressing HSP90 degradation. Mol Cancer 2020;19:112. 10.1186/s12943-020-01208-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hong W, Xue M, Jiang J, et al. Circular RNA circ-CPA4/ let-7 miRNA/PD-L1 axis regulates cell growth, stemness, drug resistance and immune evasion in non-small cell lung cancer (NSCLC). J Exp Clin Cancer Res 2020;39:149. 10.1186/s13046-020-01648-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Du WW, Yang W, Li X, et al. The Circular RNA circSKA3 Binds Integrin β1 to Induce Invadopodium Formation Enhancing Breast Cancer Invasion. Mol Ther 2020;28:1287-98. 10.1016/j.ymthe.2020.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao Y, Zheng R, Chen J, et al. CircRNA CDR1as/miR-641/HOXA9 pathway regulated stemness contributes to cisplatin resistance in non-small cell lung cancer (NSCLC). Cancer Cell Int 2020;20:289. 10.1186/s12935-020-01390-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang Y, Zhao W, Zhang S. STAT3-induced upregulation of circCCDC66 facilitates the progression of non-small cell lung cancer by targeting miR-33a-5p/KPNA4 axis. Biomed Pharmacother 2020;126:110019. 10.1016/j.biopha.2020.110019 [DOI] [PubMed] [Google Scholar]
- 36.Li H, Liu F, Qin W. Circ_0072083 interference enhances growth-inhibiting effects of cisplatin in non-small-cell lung cancer cells via miR-545-3p/CBLL1 axis. Cancer Cell Int 2020;20:78. 10.1186/s12935-020-1162-x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The article’s supplementary files as