Abstract
Acute COVID-19 infection is followed by prolonged symptoms in approximately one in ten cases: known as Long COVID. The disease affects ~65 million individuals worldwide. Many pathophysiological processes appear to underlie Long COVID, including viral factors (persistence, reactivation, and bacteriophagic action of SARS CoV-2); host factors (chronic inflammation, metabolic and endocrine dysregulation, immune dysregulation, and autoimmunity); and downstream impacts (tissue damage from the initial infection, tissue hypoxia, host dysbiosis, and autonomic nervous system dysfunction). These mechanisms culminate in the long-term persistence of the disorder characterized by a thrombotic endothelialitis, endothelial inflammation, hyperactivated platelets, and fibrinaloid microclots. These abnormalities of blood vessels and coagulation affect every organ system and represent a unifying pathway for the various symptoms of Long COVID.
Keywords: long COVID, clotting pathology, cardiovascular outcomes, endothelialitis
Introductory overview and classification of Long COVID
Coronavirus disease (COVID-19) is an airborne infectious disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1]. It has had a disastrous effect on the world’s demographics, resulting in more than 6.5 million deaths worldwide [2]. It represents the worst global health crisis since the influenza pandemic of 1918 [2]. Since the WHO declared a global pandemic, COVID-19 has overwhelmed and crippled healthcare systems around the world.
Most people infected with the virus will experience a mild to moderate illness and recover without specialized treatment [3]. Common symptoms of acute COVID-19 include profound fatigue, breathlessness, cough, chest pain, palpitations, headache, joint pain, myalgia, weakness, insomnia, pins and needles, hair loss, impaired balance, neurocognitive issues including memory and concentration problems, anxiety, depression, and loss of taste and smell [4]. However, some sufferers have experienced more serious complications, such as coagulopathy, thromboembolism, multiorgan failure, septic shock, and death [3].
While symptoms usually only last for up to 2–3 weeks, ~10% of patients experience continued or new symptoms beyond the acute phase; this condition is known as Long COVID/post-acute sequelae of COVID-19 (PASC) [5]. Hereafter, we refer to the illness by the patient-defined term ‘Long COVID’ [6]. In this condition, there may be persistence of symptoms of acute COVID, or the appearance of new symptoms unrelated to the acute illness. In some cases, the ‘acute’ phase may have been almost asymptomatic. Depending upon the duration of symptoms, Long COVID can be divided into two stages. ‘post-acute COVID’ is the term used to describe symptoms that extend beyond 3 weeks and up to 12 weeks, and ‘chronic COVID’ is where symptoms extend beyond 12 weeks [4,7]. Persistence of symptoms has now been observed for up to 3 years [8,9].
Here, we shed light on the various pathophysiological factors that contribute to Long COVID and highlight the resultant blood abnormalities. The first three sections examine the prevalence and manifestations, risk factors and predictors, and subtypes of Long COVID. We also briefly discuss the effects of vaccination in the context of Long COVID. Following this, we discuss the pathophysiological factors that can contribute to Long COVID and the symptoms that ensue. This section is divided into three parts: viral factors, host factors, and downstream impacts. To provide a comprehensive picture of how these factors contribute to abnormal coagulation, the next section outlines the interplay between systemic inflammation and coagulation. We summarize findings that point to a failed fibrinolytic system [1,8,10., 11., 12.]. Finally, we discuss in detail the abnormalities of coagulation observed in Long COVID.
Prevalence and manifestations of Long COVID
Long COVID has resulted in a major global health and economic burden, with at least 65 million individuals around the world having Long COVID [13]. In the USA, economists have estimated that Long COVID will incur cumulative future costs of more than US$4 trillion [14].
Approximately 87% of people who recover from COVID-19 and are discharged from hospital exhibit at least one symptom of Long COVID after 60 days, 32% have one or two symptoms, whereas 55% have three or more [15,16]. The most common symptoms are fatigue (53.1%), shortness of breath (43.4%), joint pain (27.3%), and chest pain 21.7% [15]. Other less prevalent symptoms include cough, skin rashes, palpitations, headache, and ‘pins and needles’ sensations. Research has shown that the most common Long COVID symptoms are fatigue, cognitive dysfunction (‘brain fog’), shortness of breath, as well as joint and muscle pains [8]. Unsurprisingly for a postviral illness, most individuals with Long COVID exhibit postexertional symptom exacerbation (PESE) [13,17]; in addition, 50% of people with Long COVID meet the criteria for myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) [17., 18., 19.].
Many patients struggle to do basic daily activities and experience a wide range of secondary mental health issues. In a large global survey of 3762 patients with Long COVID across 56 countries, approximately half were unable to work full-time 6 months post infection, primarily due to fatigue, postexertional malaise, and cognitive dysfunction [20].
Children of all ages can also develop Long COVID. Surveys demonstrate that they experience the full range of symptoms seen in adults [21., 22., 23.]. Notable manifestations in children include brain hypometabolism, hepatic involvement, ME/CFS, and pulmonary abnormalities [21,24,25].
Risk factors and predictors of Long COVID
Various risk factors influence the precise presentation of Long COVID in a particular patient. The number and type of symptoms during the initial infection are strong predictors of Long COVID. Those who experience more than five symptoms during the first week of infection are more likely to develop Long COVID, irrespective of age or gender [26., 27., 28.]. Specific symptoms during the first week that are predictive of Long COVID include fatigue, headache, shortness of breath, and muscle pains [27,28]. Other risk factors for Long COVID include age (47% risk in those 50 years and above), and comorbidities such as hypertension, dyslipidemia, cardiovascular disease, and metabolic and endocrine dysregulation [e.g., body mass index (BMI) >30, and type 2 diabetes mellitus (T2DM)] [8,29,30]. Women have greater prevalence, lower mortality, lower levels of inflammation, higher lymphocyte counts, and faster antibody responses compared with men [31]. This suggests that male sex is an independent risk factor for COVID-19 infection and death, but not for Long COVID itself. Long COVID also appears to be more prevalent among disadvantaged ethnic and socioeconomic groupsi [32,33].
Subtypes of Long COVID: implications for treatment
Although millions of people are living with symptoms of Long COVID, what remains striking is the heterogeneity of the clinical syndrome [34]. This is due, in part, to the fact that the term encompasses many different clinical conditions with distinct pathobiological processes at play. Over time, the groups that advocated for the creation of the terms ‘Long COVID’ (patient created), ‘Long Haul COVID’ (patient created), ‘Post-Acute Sequelae of COVID-19’ (National Institutes of Health and Centers for Disease Control and Prevention) and ‘Post-COVID-19 Condition’ (WHO) have reached a level of tacit agreement that these terms are, in fact, describing the same broad clinical presentation: namely, any person who has survived an acute SARS-CoV-2 infection but is still experiencing persistent symptoms months after their acute symptoms have resolved. We continue to use the name ‘Long COVID’ in this paper because it is the most widely used patient-derived term, although we acknowledge that these other terms broadly describe the same disease.
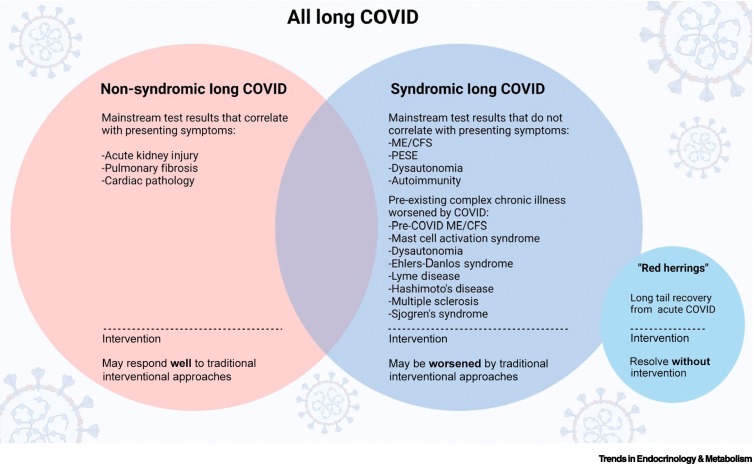
One of the first distinctions that clinicians and researchers evaluating patients with Long COVID must establish is whether the symptoms can be linked to organ dysfunction that is evident from mainstream clinical investigations. For instance, consider a hypothetical patient hospitalized for a severe COVID-19 pneumonia. This patient may continue to experience shortness of breath, chest pain, and exertional intolerance for months after the initial infection, meeting criteria for a diagnosis of Long COVID. If these impairments are accompanied by clear changes in pulmonary function testing and pulmonary fibrosis on imaging, then it is possible that this patient will have a positive response to traditional pulmonary rehabilitation efforts. By contrast, consider a second hypothetical patient who was infected with SARS-CoV-2, but experienced less-severe illness that did not require hospitalization. Following resolution of the acute COVID-19 symptoms, this patient goes on to develop extreme fatigue, shortness of breath, chest pain, and exertional intolerance. Although these postacute symptoms are severe and the patient meets criteria for a diagnosis of Long COVID, most of their mainstream clinical investigations return normal (or near normal) and do not correlate with symptom severity. In these cases, as we have learned from other postviral illnesses, such as ME/CFS, interventions including pulmonary rehabilitation are likely to significantly worsen symptoms due to PESE [17,35., 36., 37.]. Therefore, there is a critical need to create diagnostic criteria and biomarkers that can assist in differentiating the different endotypes of Long COVID to ensure that precision medicine approaches can be applied to each endotype. Here, we propose a naming convention that seeks to better identify those with ‘Syndromic’ or ‘Non-Syndromic’ Long COVID, acknowledging that some patients may exhibit elements of both and must be managed accordingly.
In addition to identifying different endotypes of Long COVID, care must also be taken to avoid a misdiagnosis of Long COVID when a patient is simply experiencing a long-tail recovery from COVID-19 (Figure 1 ). The initial clinical case definition offered by the Centers for Disease Control and Prevention (CDC) in the USA stated that a Long COVID diagnosis should be considered if symptoms are persisting beyond 1 month of the initial infectionii , iii. However, the WHO clinical case definition differs by recommending consideration of the diagnosis if symptoms persist beyond 3 months of the initial infection [38]. Although cases of prolonged recovery to COVID-19 can closely resemble a diagnosis of Long COVID, given time the former will gradually and spontaneously recover. This is not the pattern for Long COVID.
Figure 1.
Long COVID subtypes, including Non-Syndromic Long COVID, Syndromic Long COVID, and ‘Red herrings’.
Patients with Non-Syndromic Long COVID may have had a severe acute infection that resulted in hospitalization and caused significant organ damage and dysfunction. This subtype may respond well to traditional interventional approaches, such as pulmonary rehabilitation. By contrast, patients with Syndromic Long COVID might have had a mild acute infection that did not require hospitalization, but develop extreme fatigue, shortness of breath, chest pain, and exertional intolerance. This subtype tends to not respond well to conventional treatments. Within this subtype, certain pre-existing chronic illnesses, such as myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS), may be worsened by the acute COVID. Furthermore, ‘Red herrings’ is the long tail recovery from acute COVID, where symptoms gradually resolve within a few months without any intervention. Abbreviation: PESE: Post-Exertional Symptom Exacerbation.
Finally, the interaction of COVID-19 with pre-existing complex chronic illness results in a potentially distinct set of endotypes of Long COVID. For instance, emerging evidence suggests that individuals with pre-existing immune issues and chronic viral infections and reactivations are more susceptible to Long COVID [39,40]. However, what remains unclear is whether these individuals are experiencing an infection-triggered worsening of their pre-existing diagnosis and pathophysiology, or the same condition as the rest of the population with Syndromic Long COVID. Thus, the interaction of Long COVID with pre-existing complex chronic illness is an area that requires active investigation so that all patients with a Long COVID diagnosis can access evidence-based, precision medicine approaches.
Vaccination and Long COVID
Studies looking at the impact of vaccination on the likelihood of developing Long COVID have yielded conflicting results. A recent systematic review suggested that, overall, vaccination is associated with a reduced risk of Long COVID, with two doses conferring greater protection than one [41]. However, there are also studies showing no reduction in the risk of Long COVID following vaccination [42]. Research looking at the impact of vaccination on existing Long COVID has also yielded varying results, with improvement, no change, and worsening all being reported [41,43]. These inconsistent results could partly be due to heterogeneity of study design, inclusion criteria, and the definition used of Long COVID [13].
An additional consideration is the varying impact of vaccination on the risk of developing Long COVID following different variants. As an example, double-vaccinated individuals with Omicron BA.1 were half as likely to develop Long COVID compared with double-vaccinated individuals with Delta [44]. We still do not fully understand the interplay between multiple infections with different variants and multiple, sometimes different, vaccines administered to an individual patient.
The benefits of vaccination far outweigh any pitfalls. However, similar to any other vaccine, indeed any other drug, COVID-19 vaccination can, in some cases, result in significant adverse effects (vaccine injury) [45]ii. The presentation can be very similar to Long COVIDiv. As with virus-induced Long COVID, the mechanisms involved are not fully defined; coagulopathy may be one potential explanation, because the spike protein has been shown to bind to fibrinogen in vitro, giving rise to anomalous fibrin microclots [10]. However, there may be other factors at play that are yet to be defined. Unfortunately, there is hesitancy among patients and researchers to acknowledge and openly discuss vaccine injury, due to fear of being labeled ‘anti-vax’iv. Patients with vaccine injury should be able to access medical care without fear of being stigmatized, and vaccine injury should be researched like any other disease. This will facilitate the development of better vaccines and help us identify those at higher risk of an adverse event.
Pathophysiology of Long COVID
Viral factors
Viral persistence
Viruses (and indeed bacteria [46]) can be present in a chronically lytic and/or latent form in the host after the initial phase of infection [47., 48., 49., 50., 51., 52.]. Persistent infections are characterized as those in which the microbe and/or its fragments are not cleared from the host following primary infection [51]. Certain viruses, such as varicella-zoster virus (VZV), HIV, hepatitis C virus (HCV), Epstein–Barr virus (EBV), and human papillomavirus (HPV), are notorious for remaining in the body and causing pathology [51].
There is growing evidence that, in some patients with Long COVID, SARS-CoV-2 may persist in tissue reservoirs after acute infection [47., 48., 49., 50., 51.,53]. These hidden viral reservoirs may trigger repeated immune responses that contribute to persistent symptoms. Months after infection, viral mRNA and spike protein from SARS-CoV-2 have been detected in the digestive system and urinary tract of patients with Long COVID [54] through immunofluorescence and PCR analysis of intestinal biopsies. Other studies have also detected SARS-CoV-2 in multiple organs and in feces, indicating prolonged viral shedding [55., 56., 57.].
The main virulence factor of SARS-Cov-2, the spike protein, is a key element for viral attachment to target cells through angiotensin-converting 2 enzyme (ACE-2) surface receptors [58], TMPRSS-1 receptors, and extracellular vimentin [10,59]. Spike proteins are class I viral fusion proteins that are present as protruding homotrimers on the viral surface; they facilitate virus entry into target host cells [60,61]. An isolated spike protein is between 180 and 200 kDa in size and contains an extracellular N terminus, a transmembrane domain fixed in the membrane of the virus, and a short intracellular C-terminal segment [60,62]. The S1 subunit permits receptor binding to the host cell [63], whereas the S2 subunit enables viral fusion and entry [64]. However, receptor binding alone does not solely explain the cell-mediated pathologies present in the disease [65].
Spike protein can be shed by the host cell itself via extracellular vesicles (EVs) and spread via the circulatory system to distant tissues and organs [64]. EVs are bilayer lipid membrane-bound structures released from host cells, such as neutrophils, monocytes, lymphocytes, platelets, epithelial cells, and endothelial cells (ECs), under physiological and pathological conditions [64]. These vesicles may contain biologically active compounds, such as mRNA, miRNAs, DNA, lipids, and assorted proteins. The main function of these EVs is to transport cargo to neighboring or distant cells to support homeostasis [66]. Extracellular vesicles also share certain resemblances with viruses, such as small size, biogenesis mechanism, and cell entry mechanism [67].
Once the spike protein has attached to the host cell and replication is complete, SARS-CoV-2 buds in the endoplasmic reticulum–Golgi intermediate compartment (ERGIC) or in the Golgi apparatus (Figure 2). Finally, SARS-CoV-2 can exit the cell via a biosynthetic secretory pathway [68,69]. In Long COVID, SARS-CoV-2 may hide in these EVs and reattack various tissues and organs through the circulatory system. This may contribute to the symptoms of Long COVID [67].
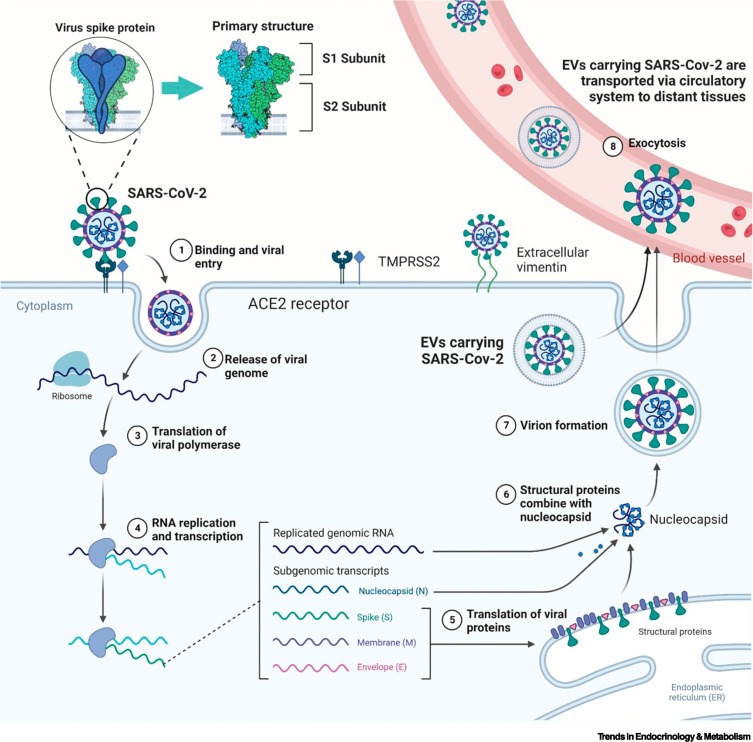
Figure 2.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): receptor binding, entry into host cell, replication, and transport to other tissues
(1) SARS-CoV-2 enters host cells through angiotensin converting enzyme 2 (ACE2) and transmembrane protease serine 2 (TMPRSS2) receptors, as well as extracellular vimentin. The S1 subunit permits receptor binding and S2 facilitates entry and fusion. (2–4) Once inside the host cell, viral replication occurs. (5–8) SARS-CoV-2 buds in the endoplasmic reticulum–Golgi intermediate compartment (ERGIC) or Golgi apparatus and exits the cell via a biosynthetic secretory pathway. SARS-CoV-2 can also hide in extracellular vesicles (EVs) and reattack various tissues and organs through the circulatory system. Figure created with BioRender (https://biorender.com/).
Reactivation of latent viruses
It is well understood that, as with dormant bacteria [46,70], humans can harbor dormant viruses that persist in a latent form without causing pathology [49]. These dormant viruses may reactivate under conditions of stress or immunosuppression [20,49,71]. If the immune response is weakened, challenged, or dysregulated, these dormant viruses may become reactivated and alter human gene expression, protein production, and immune regulation [49]. This sequence of events allows the virus to infect new body sites and drive new chronic symptoms. This has also been demonstrated during and after COVID-19 infection, where previously dormant viruses, such as EBV, herpes simplex virus, and HCV, are reactivated in various organs [49,72,73]. These viruses have been tied to the development of several chronic conditions, such as Alzheimer’s disease, cancer, rheumatoid arthritis, and type 1 diabetes mellitus (T1DM), and may be one explanation for why some patients develop chronic conditions after COVID-19 infection [49].
Bacteriophage-like actions of SARS-Cov-2
Previously, it was assumed that SARS-CoV-2 can only replicate and survive in mammalian eukaryotic cells. However, it has recently been suggested that SARS-CoV-2 can infect and replicate in gut bacteria, indicating that the virus could act in a sense as a bacteriophage [74]. This could result in a particular and potent type of viral persistence, and may also partly explain the gut dysbiosis seen in patients with Long COVID, because the bacteriophagic action of SARS-Cov-2 can directly promote the replication of certain bacteria, causing an imbalance in the gut microbiota [74]. This may further contribute to the chronic inflammation, endothelial dysfunction, and hypercoagulation seen in Long COVID.
Host factors
Chronic inflammation and immune dysregulation
In response to the virus in acute COVID-19, the immune system stimulates polyclonal T cell activation and the release of different inflammatory molecules, such as cytokines, interleukins, and chemokines [75,76]. This event is known as a cytokine storm [75,77], and is a distinct immunopathological feature of COVID-19. As the cytokine storm intensifies, high levels of inflammatory molecules, such as serum amyloid A (SAA), von Willebrand factor (VWF), interleukin (IL)-6, IL-8, IL-10, and tumor necrosis factor alpha (TNF-α) are increased drastically [75].
It has also been shown that severe COVID-19 causes B cell and T cell lymphocyte deficiency, otherwise known as lymphopenia [78]. This can in turn cause hyperinflammation [78,79], because lymphocytes participate in the resolution of inflammation after infection [80,81]. Depleted T cell and B cell numbers are also strongly associated with persistent SARS-CoV-2 shedding, which may further contribute to the chronic immune activation in Long COVID [82].
Autoimmunity
Bacterial and viral infections have been identified as a key environmental trigger in the pathophysiology of autoimmune diseases. Different mechanisms for the generation of autoimmunity following infections have been proposed. These may include epitope spreading, bystander activation, molecular mimicry, and activation of antigen-presenting cells [83]. For instance, T1DM has been associated with coxsackievirus [84] and enteroviruses [85]. HCV has been postulated to be associated with systemic lupus erythematosus [56,86], while molecular mimicry of Proteus spp. antigens is closely involved in the pathology of rheumatoid arthritis [87,88].
Evolving data suggest that autoimmunity contributes to the pathophysiology of SARS-CoV-2 infection, both during the acute illness and in Long COVID. For instance, similar to pre-eclampsia [89], antiphospholipid autoantibodies have been detected in 52% of serum samples of hospitalized patients; this directly correlates with neutrophil hyperactivity and more severe clinical outcomes [90]. Numerous studies have also identified autoantibodies against interferons, neutrophils, and cyclic citrullinated peptides [90., 91., 92., 93.].
Functional autoantibodies targeting G-protein-coupled receptors (GPCR-AAbs) have also been demonstrated in the sera of patients with COVID-19; these are associated with disease severity [94., 95., 96.]. In a pilot study, the neutralization of GPCR-AAbs improved capillary impairment and fatigue after COVID-19 infection [97].
Mast cell activation
The hyperinflammatory responses in acute COVID-19 infection and Long COVID have been hypothesized to be facilitated, in part, by mast cell activation [98]. Mast cell activation can escalate into mast cell activation syndrome (MCAS), which causes repeated severe allergic symptoms affecting several body systems. Unregulated release of chemical mediators produces a multitude of symptoms, including food allergies, urticaria, gastrointestinal upset, shortness of breath, and wheezing, all of which are reported in Long COVID [99]. The proposed mechanisms whereby MCAS is triggered in Long COVID include dysregulation of genes by SARS-CoV-2, resulting in the loss of genetic regulation of mast cells, as well as development of autoantibodies which react with immunoglobulin receptors on mast cells [100,101].
Melatonin deficiency
Melatonin is a sleep hormone with several attributes that help combat viral infection. It has been shown that melatonin effectively subdues an overactive innate immune response, thereby downregulating inflammation [102,103]. It also endorses the adaptive immune reaction, resulting in enhanced antibody formation, thereby inhibiting the entrance of viruses into cells and limiting their replication [102]. This has also been demonstrated in acute SARS-CoV-19 infection, where patients with higher levels of melatonin had lower mortality [102]. It could be argued that if a patient has melatonin deficiency, they may be at increased risk of developing Long COVID. Based on the results of previous studies of melatonin in other viral infections, as well as its role in a variety of chronic inflammatory diseases [104], it could be used to help prevent and treat COVID-19 and Long COVID [105].
Connective tissue abnormalities
As mentioned previously, various autoantibodies have been found in the systemic circulation of patients with Long COVID. In fact, connective tissue disorders, such as arthritis, lupus, and myositis, have been reported after COVID-19 [106]. These may be precipitated by autoantibodies attacking connective tissue and muscle [106]. In those with Ehlers–Danlos syndrome (EDS) and hypermobility spectrum disorder (HSD), the high levels of inflammation present in Long COVID may result in increased connective tissue laxity, which, if left unmitigated, could cause visceroptosis [106]. This may manifest, for example, with an ME/CFS-like picture due to cranio-cervical instability.
Downstream impacts
Tissue damage due to initial COVID-19 infection
Given that SARS-CoV-2 is airborne, it was anticipated at the beginning of the pandemic (and found) that lung injury would be common in patients. However, an unforeseen complication of the virus was the multiorgan impairment that it caused [107]. Structural brain and metabolic abnormalities were reported among survivors of COVID-19, which were directly associated with ongoing neurological symptoms, such as memory loss, anosmia, and fatigue [107,108]. Cardiac abnormalities and myocardial inflammation were observed in 78% of participants who were discharged from hospital, indicating cardiac injury [109]. Other radiological abnormalities were observed in the lungs, liver, pancreas, kidneys, and spleen persisting for a minimum of 2–3 months after hospital discharge [107,110., 111., 112.].
While the initial acute infection can cause severe tissue and organ damage, this does not fully explain the diverse abnormalities seen in Long COVID. There is mounting evidence that the organ involvement seen in COVID-19 occurs due to spread of the virus by the oro-systemic route [113]. Virus contained in the mouth may spread to different organs through the blood. The distribution of the illness in the organs lends weight to this argument. For example, abnormalities in the lungs are predominantly seen in the peripheral and basal regions, reflecting the areas that are most perfused but least aerated [113]. The most aerated areas (the apices) are spared, which is against spread by inhalation from the upper airway. It is likely that, once the virus has found its way into the organs, it stimulates microvascular in situ thrombosis, leading to the multiple clinical features and radiological appearances of acute COVID [114]. Continuation of such a process may contribute to ongoing organ involvement in Long COVID.
Tissue hypoxia
Autopsy results of lungs from individuals deceased from COVID-19 have shown significant pulmonary vascular changes, extensive endothelial damage, and thrombosis [115]. The extent of pulmonary vascular shunting in patients with COVID-19 is strongly correlated with poor oxygenation of blood passing through the lungs [116]. The resultant systemic arterial hypoxemia can cause tissue hypoxia throughout the body [117,118]. Under these hypoxic conditions, immune cells may be triggered to produce inflammatory cytokines, which may further intensify capillary dysfunction. Not only does hypoxia in the lung indirectly cause tissue hypoxia across the entire body, but SARS-CoV-2 may also bind to ECs across various tissue types, altering cell morphology and inducing ECs to undergo apoptosis. For instance, in the heart, endothelial dysfunction is associated with EC swelling in small arterioles, capillaries, and venules, as well as scattered necrosis of individual myocytes [119]. In the brain, infection of the microvascular endothelium in the subcortical white matter is associated with microscopic ischemic and hemorrhagic lesions [120].
Due to the chronic inflammatory milieu in Long COVID, neutrophils may cause capillary obstruction, which can stall blood flow. This happens because neutrophils are larger than erythrocytes and the average capillary diameter; thus, when excessively activated, they can obstruct capillary flow [121]. It has been demonstrated that neutrophil adhesion in brain capillaries may impair brain function and can produce a substantial decline in cerebral blood flow in animal models [122]. In Long COVID, neutrophil degranulation is upregulated, suggesting continued inflammatory responses and immune dysregulation even after acute COVID [123]. Therefore, as seen in acute COVID, the adhesion of hyperactivated neutrophils to capillaries within the lungs, brain, heart, and other organs may contribute to the symptoms seen in Long COVID [124].
Patients with Long COVID may present with fibrin amyloid microclots that promote tissue hypoxia and impaired oxygen exchange [1,8,12,125]. These microclots are resistant to fibrinolysis and can block capillaries, thereby causing tissue hypoxia. If the oxygen supplied to aerobic tissue is restricted, and then rapidly restored (‘reperfusion’), it may cause severe tissue damage. This is known as ischemia–reperfusion injury [125]. This process involves the production of reactive oxygen species (ROS), which increase oxidative stress [126,127]. Oxidative stress refers to an imbalance between the rate of production of ROS and reactive nitrogen species and their elimination via antioxidants [125]. Oxidative stress is known to promote the production of inflammatory cytokines, and vice versa, producing a vicious cycle [125,128,129]. If this persists and is not treated appropriately, tissue hypoxia may linger for months and contribute to the multitude of symptoms seen in Long COVID [1,8,12,125].
Since a previous review [125] covered this in some detail for both acute and Long COVID, we review only briefly some of the evidence for tissue hypoxia, which includes low venous saturation [130], heart rate variability, markers of oxidative stress continent on hypoxia, lactate accumulation [131,132], biomarkers of ischemia–reperfusion injury, poor oxygen transfer [112], as well of the benefits of therapies designed to alleviate tissue hypoxia [125]. Overall, we would regard the breadth and extent of such evidence as both mechanistically integrated and compelling.
SARS-CoV-2 interactions with the host microbiome
Several studies suggest that COVID-19 promotes microbiome/virome dysbiosis that could result in persistent symptoms. One study revealed that the microbiome in the bronchoalveolar fluid of patients with COVID-19 showed increased pathogenic bacteria and higher levels of oral and upper respiratory commensal bacteria compared with healthy controls [133]. Furthermore, the gut microbiome of patients with COVID-19 is characterized by the augmentation of opportunistic pathogens and the reduction of beneficial species [134].
Microbiome/virome dysbiosis can disturb the homeostasis and functioning of host signaling pathways in a way that may facilitate chronic disease development [135]. Dysbiosis is accompanied by inflammation that can instigate dysfunction and breakdown of gut epithelial linings [136]. In turn, this can cause increased epithelial permeability that allows pathogens to translocate into the blood, where their presence can contribute to a range of systemic inflammatory processes. This can result in endothelial damage and hypercoagulation [135].
Autonomic nervous system dysfunction
Symptoms consistent with dysautonomia are commonly reported by patients with Long COVID. Although the rate of formal diagnosis of autonomic dysfunction varies widely [137., 138., 139., 140.], it is a significant cause of symptom burden for patients with Long COVID and consensus guidelines for management have been developed [37]. Patients with Long COVID display high rates of symptoms consistent with dysautonomia, such as orthostatic intolerance, fatigue, palpitations, cognitive impairment, nausea, and temperature dysregulation [34,141]. Data on heart rate variability (HRV), which is a measure of parasympathetic nervous system health, have demonstrated that patients with Long COVID have a lower HRV compared with matched controls [142].
There are multiple potential causes for dysautonomia in Long COVID:
-
•
Relative hypovolemia due to failure of peripheral vasoconstriction is a feature of both postural orthostatic tachycardia syndrome (POTS) and orthostatic hypotension (OH) [143,144]; this causes reduced stroke volume and cardiac output, resulting in impaired tissue oxygen supply. This can result in a compensatory sympathetic overdrive and tachycardia.
-
•
Cerebral hypoperfusion: a recent series of nine patients with Long COVID demonstrated that orthostatic intolerance, cerebral hypoperfusion (as measured by transcranial Doppler ultrasound) and dysautonomia [as measured by the Quantitative Scale for Grading of Cardiovascular Autonomic Reflex Tests and Small Fibers from Skin Biopsies (QASAT) score] were present in all patients regardless of whether they met criteria for POTS or OH [145]. This is also consistent with studies of impaired cerebral perfusion in ME/CFS [146., 147., 148.]. Therefore, a reliance on criteria for POTS and OH is likely to miss many cases of dysautonomia in Long COVID.
-
•
Small fiber neuropathy (SFN), defined as ‘preferential damage to unmyelinated or thinly myelinated group C or A nerve fibers’ [149], has been documented in Long COVID and is a recognized cause of dysautonomia in the condition [150., 151., 152.]. We suggest that SFN in Long COVID results from autoantibodies [153] (which have previously been associated with POTS and OH) [154., 155., 156., 157., 158.] or from ischemia of small fibers due to microclots.
-
•
Damage due to direct infection or inflammation: SARS-CoV-2 is known to infect and produce its RNA and spike proteins widely in both the peripheral and central nervous system [159]. Damage associated with past infection, or persistent infection of the vagus or trigeminal nerves, may be a driver of dysautonomia symptoms [160,161].
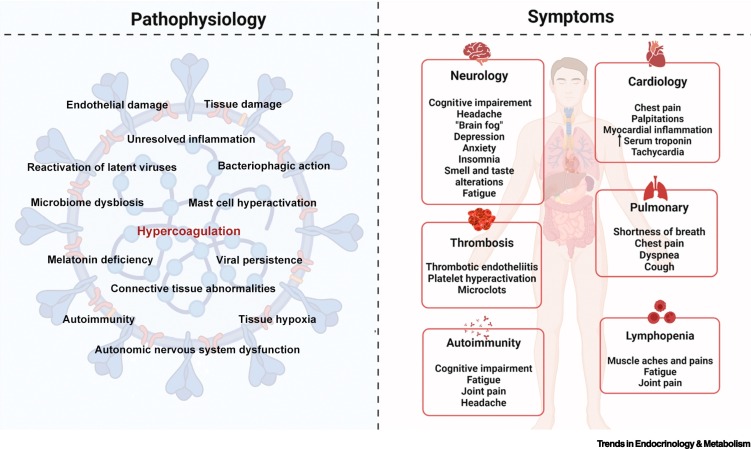
Figure 3 illustrates the complex interactions between the pathophysiology described in the previous sections and the symptoms that patients with Long COVID exhibit.
Figure 3.
Possible pathophysiological factors of Long COVID and resulting symptom manifestations.
Figure created with BioRender (https://biorender.com/).
Interaction between systemic inflammation and coagulation
The processes of systemic inflammation and hypercoagulation are interdependent, as demonstrated by the interactive crosstalk between these systems. Under normal physiological conditions, these systems function as protective mechanisms and are closely regulated. Dysregulation may occur within these systems as a result of chronic systemic inflammation and thrombotic complications. When a pathogen invades the host, or if tissue injury occurs, an inflammatory response is elicited to eliminate the insult and promote healing and tissue repair. However, if the inflammatory process is not properly resolved, acute inflammation may transition to a chronic state. The coagulation cascade can be activated due to the consequent increase in proinflammatory cytokines.
An important molecule in the context of inflammation-induced coagulation is tissue factor (TF) [162]. The tenase complex, comprising TF and factor VIIa, activates the extrinsic coagulation pathway, resulting in the generation of thrombin. Toward the end of the coagulation cascade, thrombin catalyzes the conversion of soluble fibrinogen to insoluble fibrin. Proinflammatory cytokines can upregulate the expression of TF [163]. In addition, chronic systemic inflammation promotes the suppression of certain anticoagulant mechanisms, including the antithrombin and protein C pathways [164,165]. Likewise, fibrinolytic activity may be suppressed due to a rise in PAI-1 levels [166., 167., 168.] fueled by proinflammatory molecules [165]. In summary, chronic systemic inflammation may alter the hemostatic balance to a prothrombotic state [169].
Dysregulated coagulation may modify and prolong the inflammatory response. Coagulation proteases may bind to protease-activated receptors on the activated endothelium and induce the synthesis and expression of cell adhesion molecules [170,171]. These molecules have a critical role in the extravasation of leukocytes to sites of inflammation. Moreover, activated coagulation factors can also provoke an inflammatory response by interacting with immune cells to induce the production of inflammatory cytokines [172,173].
Platelets also have a role in regulating and facilitating inflammation. Inflammatory molecules can bind to specific receptors on platelets and activate them to release their granular content, which comprises procoagulant and proinflammatory molecules. Platelets are also implicated in the recruitment of leukocytes and the regulation of vascular permeability [174].
Abnormalities of coagulation in Long COVID
Fibrinaloid microclots
The processes of normal blood clotting are well established. The terminal steps involve the self-assembly of fibrinogen molecules that have been cleaved by thrombin into long fibers [175,176]. It has been established that blood sometimes clots into anomalous forms, previously referred to as ‘dense matted deposits’; these anomalous microclots are resistant to fibrinolysis (proteolysis) [177]. It was subsequently recognized that this anomalous form was in fact amyloid in character [178]. These fibrinaloid microclots or fibrin(ogen) aggregates have also been reported in the plasma of patients with T2DM [179], ME/CFS [180], acute COVID-19 [1,181,182], and Long COVID [1,8,10., 11., 12.]. These microclots, along with hyperactivated platelets, are likely to be contributing to the thrombotic and systemic inflammatory manifestations of these diseases.
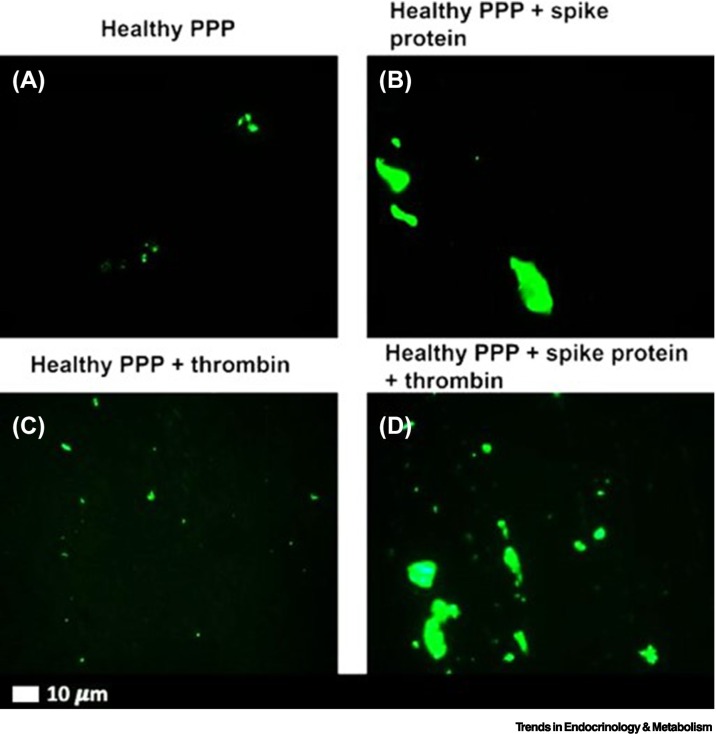
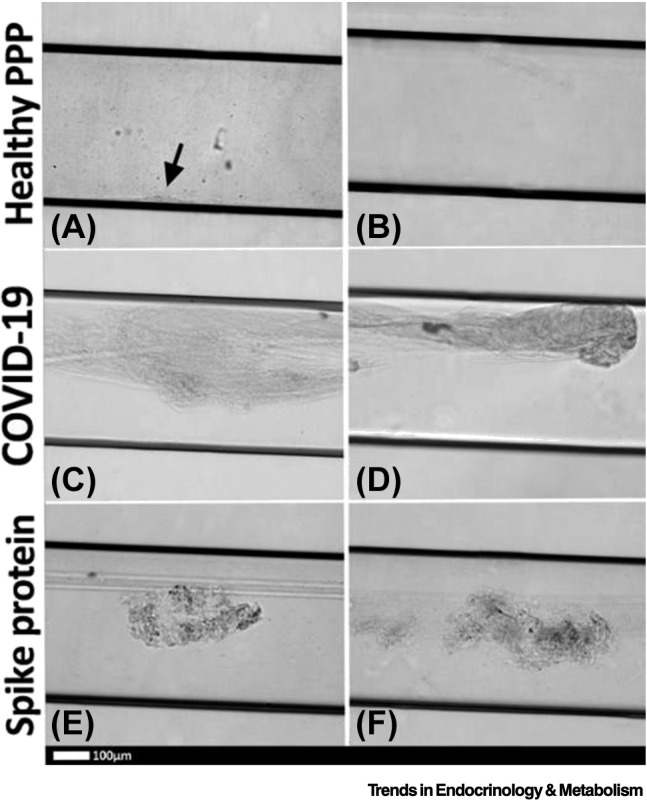
It has also been demonstrated that the isolated SARS-CoV-2 spike protein S1 subunit is a proinflammatory inflammagen [10]. SARS-CoV-2 can directly interact with platelets and fibrin(ogen) to induce changes in fibrin(ogen) and hypercoagulability [10]. This suggests that spike protein has direct pathological effects without being taken up by cells [10]. Figure 4 shows representative micrographs of purified fluorescent fibrinogen with added thrombin after exposure to spike protein. Here, we can see dense fibrin clots forming (green, fluorescent signal) in the presence of spike protein. In platelet-poor plasma (PPP) with and without thrombin, the green fluorescent Thioflavin T (ThT) signal indicates areas of amyloid deposit formation. Microfluidics demonstrate that spike protein can generate a disorderly clotted mass when added to PPP (Figure 5) [10]. The microclots cover most of the channel and often protrude into the center of the flow channel, thereby disrupting flow [10]. Aside from the fact that the SARS-CoV-2 S1 spike protein can directly induce the formation of fibrinaloid microclots in healthy PPP samples, these microclots have also been observed in patients with acute COVID-19 or Long COVID. The microclots are very resistant to digestion protocols [177,178]. Amyloid protein structures are known to be naturally recalcitrant to proteolysis [178]. However, by way of contrast, microclots in T2DM (and the few that may be present in plasma samples of healthy participants), degrade with standard protein digestion protocols [1].
Figure 4.
Representative fluorescence micrographs of platelet-poor plasma (PPP) from healthy individuals after addition of Thioflavin T (green fluorescent signal).
(A) PPP smear. (B) PPP with spike protein. (C) PPP with thrombin to create extensive fibrin clot. (D) PPP exposed to spike protein followed by addition of thrombin. Reproduced from [10].
Figure 5.
Representative micrographs of platelet-poor plasma (PPP) microclots in microfluidic chambers coated with thrombin.
(A) Healthy PPP clot, with small clot formation (arrow), with (B) no clot formed in the healthy PPP sample. (C,D) Examples of clots from Coronavirus 2019 (COVID-19) PPP samples and (E,F) healthy PPP clot with spike protein. Reproduced from [10].
Numerous inflammatory molecules trapped inside Long COVID microclots have been identified by proteomics. These include alpha 2-antiplasmin (α2AP), VWF, platelet factor 4 (PF4), serum amyloid A (SAA), various fibrinogen chains, as well as numerous antibodies [1,8,12].
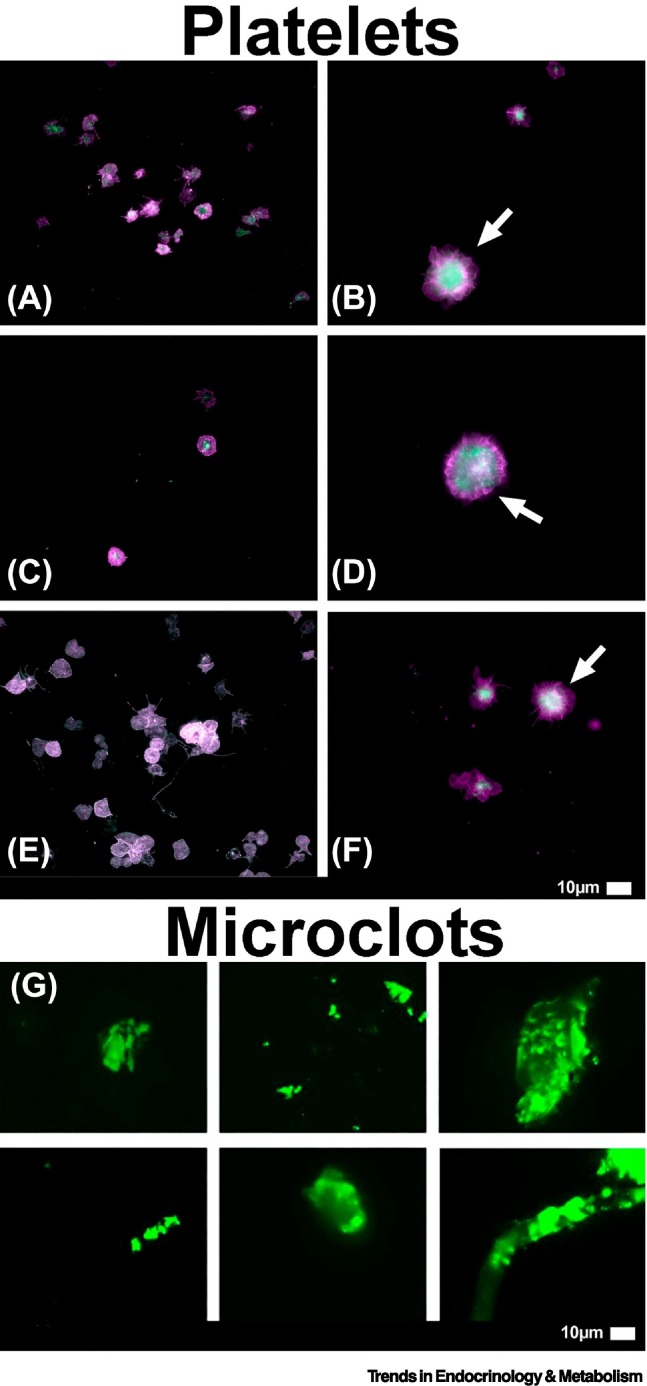
If the coagulopathy during the acute phase of the disease is not adequately treated, the resulting impaired oxygen exchange and tissue hypoxia may linger for months [116]. This may explain the multiorgan manifestations of Long COVID. [1,8,12]. Figure 6 illustrates the presence of fibrinaloid microclots and hyperactivated platelets in patients with Long COVID.
Figure 6.
Representative micrographs of samples from patients with Long COVID showing (A–F) hyperactivated platelets [8] and (G) large fibrinaloid microclots [1].
Platelets are fundamental to blood clotting and directly interact with red blood cells and the vascular endothelium. When platelets are hyperactivated, as displayed by the arrows, this promotes blood clotting and the attachment of microclots to the endothelium. The fluorescent marker, Thioflavin T, binds to misfolded protein and causes the microclots to fluoresce green. These large microclots (>10 μm) may cause severe pathology and block capillaries. Reproduced from [1,8].
Dysregulated coagulation due to endothelial damage and dysfunction
Recent research has shown that endothelial inflammation, otherwise known as endothelialitis, in COVID-19 may have long-term consequences for vascular function [18]. Endothelial damage and dysfunction are observed in Long COVID and have been shown to be strong correlates of the disease [183]. Certain EC biomarkers, such as VWF and Factor VIII, are significantly elevated in patients recovering from COVID-19. Eight months after mild-to-moderate COVID-19 infection, other endothelial markers, such as ET-1 and angiopoietin-2, were dysregulated in patients with Long COVID [18].
In acute COVID-19, it has already been established that endothelial dysfunction and impairment of the microcirculation are present [184,185]. SARS-CoV-2 directly impairs vascular homeostasis by infecting ECs via the ACE-2 receptor [185]. The Ang II hyper-reactivity that this provokes leads to a flare of inflammation and progression of fibrosis [186]. Upregulated inflammatory mediators within the endothelial matrix also indirectly contribute to endothelialitis and EC injury [187].
ECs have also been found to undergo apoptosis several months after initial COVID-19 infection [102]. This directly impairs signaling between intercellular connexin channels and upstream vascular smooth muscle cells. In addition, the capillary endothelium is protected by a glycocalyx matrix that acts as a fluid barrier; elevated inflammatory mediators in Long COVID may cause shedding of this glycocalyx, causing profound changes in microvascular resistance and capillary hemodynamics [102].
The vascular endothelium serves as a central interface between inflammation and coagulation, with a crucial role in the regulation of two systems. Under normal physiological conditions, the intact endothelium has anti-inflammatory properties and expresses anticoagulant proteins [188,189]. However, in Long COVID, it is hypothesized that cryptic SARS-Cov-2 reservoirs and various inflammatory molecules bind to receptors on ECs [190]. This may cause the endothelium to become activated and to upregulate the expression of cell adhesion molecules, such as ICAM-1, VCAM-1, selectins (E-selectin and P-selectin), inflammatory mediators, and procoagulant factors, while weakening the expression of anticoagulant factors [10]. Cytokines can also directly stimulate TF and the formation of neutrophil extracellular traps (NETs) to induce thrombosis [191,192]. Additionally, the inflamed endothelium promotes adhesion and hyperactivation of platelets [193]. SARS-Cov-2 can also directly bind to platelets via the ACE2 and TMPRSS2 receptors to promote platelet activation, adhesion, and aggregation, thereby causing increased coagulation [194]. Moreover, platelets can also become hyperactivated due to upregulation of inflammatory and adhesion molecules.
Autopsy studies have revealed the extensive effects of SARS-CoV-2 infection on ECs., including endothelialitis, endothelial damage, capillary inflammation, and thrombosis [183]. Endothelial damage may increase cell permeability and leukocyte adhesion while inhibiting anticoagulant properties. Previous studies showed that antithrombin III, tissue factor pathway inhibitor, and activated protein C (APC) are significantly decreased in the context of endothelial damage, promoting coagulation [195,196]. Furthermore, damaged ECs become procoagulant when exposed to TF, exposed phosphatidylserine (PS), increased VWF, and factor VIII. ECs can also fuel the expression of chemokines on their surface, promoting neutrophil recruitment that may contribute to thrombosis [197].
Dysregulated coagulation due to viral persistence
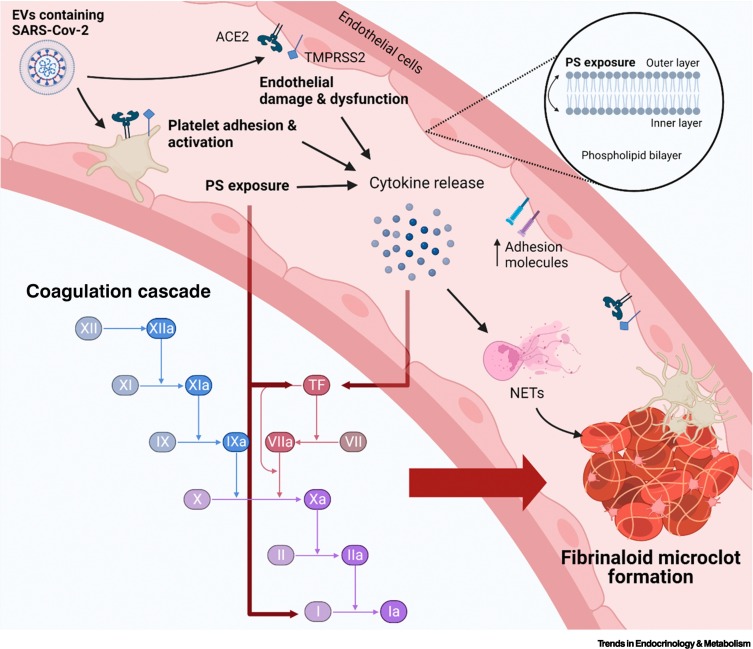
As previously mentioned, SARS-CoV-2 may hide in EVs to reattack various tissues through the circulatory system. Apart from their important function as transporters, EVs also have a vital part in inflammation, coagulation, and immune regulation. It has been demonstrated that extracellular vesicle-TF (EV-TF) activity is significantly upregulated in patients hospitalized with COVID-19. Furthermore, TF-positive EVs are released into the circulation, which may lead to thrombosis [198]. In addition, previous studies have demonstrated that PS exposure in the outer membrane of the cell membrane due to viral infection may also promote activation of coagulation [199]. PS is a membranous phospholipid normally found in the inner membrane of a double cell membrane. When vascular ECs and circulating blood cells are damaged, the lipid distribution is altered in the membrane and PS moves from the inner to the outer membrane. When PS is exposed in the outer cell membrane, it creates a catalytic surface for clotting factors, which facilitate the conversion of prothrombin to thrombin [200]. In addition, PS externalization in patients with COVID-19 is strongly associated with increased D-dimer levels and patients with thrombosis have significantly higher PS externalization compared with patients without thrombosis [201]. This further implies that EVs may carry SARS-CoV-2 to distant tissues, reinjuring the vascular endothelium. Figure 7 illustrates how EVs may promote endothelial damage and platelet hyperactivation and subsequently cause abnormal coagulation.
Figure 7.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) may bind to endothelial cells and platelets via the angiotensin converting enzyme 2 (ACE2)/transmembrane protease serine 2 (TMPRSS2) receptors to promote endothelial dysfunction and platelet hyperactivation, thereby promoting the activation of the coagulation cascade and formation of fibrinaloid microclots.
In Long COVID, SARS-CoV-2 may hide in extracellular vesicles (EVs) and reattack various tissues and organs through the circulatory system. When SARS-Cov-2 binds to endothelial cells and platelets via the ACE2/TMPRSS2 receptors, it causes endothelial dysfunction and platelet hyperactivation. This may lead to phosphatidylserine (PS) exposure in the outer cell membrane. PS exposure can directly promote certain factors within the coagulation cascade, such as tissue factor (TF). TF is also a complex with FVIIa, and provides binding sites for procoagulant complexes, leading to the formation of thrombin. Thrombin is the final step in the coagulation cascade, which promotes conversion of fibrin to fibrinogen, causing blood coagulation. Furthermore, endothelial damage and PS exposure also causes endothelial cells and platelets to further produce various cytokines, resulting in chronic systemic inflammation. Cytokines can also directly stimulate TF within the coagulation cascade as well as the formation of neutrophil extracellular traps (NETs) to induce thrombosis. SARS-CoV-2 persistence promoting chronic inflammation in Long COVID may be a mechanism that stimulates endothelial cells, platelets, and other inflammatory cells. This promotes the upregulation of procoagulant factors within the coagulation cascade and, ultimately, the formation of fibrinaloid microclots that are resistant to fibrinolysis. Figure created with BioRender (https://biorender.com/).
Dysregulated coagulation due to autoimmunity
Autoantibodies that promote thrombosis have recently been recognized as an important factor in Long COVID [202]. Antiphospholipid (APL) antibodies, in particular, promote thrombosis by stimulating neutrophils to release NETs and by activating ECs and platelets [73,202]. It was previously also shown that APL antibodies can directly cause endothelial damage by binding to receptors on ECs [203]. When APL antibodies bind to these receptors on the endothelial wall, they inhibit nitric oxide synthase (NOS) production, thereby decreasing the production of NO [204]. NO is known for its anti-inflammatory and vasodilatory properties. The reduction in NO production may contribute to endothelial damage. Endothelial injury promotes the release of inflammatory cytokines and recruitment of neutrophils via increased neutrophil adhesion in the setting of decreased NO and complement activation. This ultimately leads to impaired vascular integrity and increased platelet aggregation, causing hypercoagulation [205]. Therefore, it is not surprising that markers of endothelial activation/damage, such as VWF, have been shown to correlate with disease severity in Long COVID [206].
Dysregulated coagulation due to chronic hypoxia and persistent inflammation
Hypoxia is a condition in which sufficient oxygen is not available to tissues to support satisfactory homeostasis [207]. This can result from insufficient oxygen delivery to the tissues and organs (due to low blood supply or low oxygen content within the blood), and increased tissue oxygen demand. The response and tolerance to hypoxia is variable; while some tissues/organs can tolerate some forms of hypoxia for a longer period, other tissues may be seriously damaged.
Chronic hypoxia within tissue may also promote hypercoagulation. It may trigger transcription factor early growth response-1, upregulate TF expression, and promote the expression of PAI-1, thereby increasing coagulation [208]. Chronic hypoxia can also promote apoptosis of ECs. This reduces endothelial anticoagulant properties, vascular permeability, leukocyte adhesion, and microparticle production [208].
Chronic inflammation in Long COVID can also stimulate ECs, platelets, and inflammatory cells to produce inflammatory cytokines and procoagulant factors [8]. This chain of events can damage the protective function of the vascular endothelium, consequently causing abnormal coagulation [208].
Priorities
In this review, we have described the multiple pathophysiological factors at play in Long COVID and their interactions with the coagulation system and endothelium. It is now clear that widespread endothelial inflammation is a key feature of COVID-19 disease. In fact, we would argue that, while there may be many mechanisms at play in the pathogenesis of Long COVID, a dominant pathological process driving symptom burden is a thrombotic endothelialitis. This induces a systemic prothrombotic state with the formation of anomalous circulating fibrinaloid microclots and hyperactivated platelets, driven by elevated levels of procoagulant inflammatory molecules, which interact with each other as well as with platelets and the endothelium.
Several studies have demonstrated a significant increase in the incidence of thromboembolic phenomena after COVID-19 infection [209., 210., 211.]. A study of the electronic health records of 48 million adults across England and Wales revealed that, in the week following acute COVID-19, the adjusted hazard ratios for first arterial thrombosis and first venous thrombosis were 28.7 and 33.2 times, respectively that of those with no COVID-19 diagnosis. While the risk diminished over time, it remained elevated up to 49 weeks after diagnosis [211]. An analysis of 11.7 million US Veterans’ health records, including over 153 000 who had acute COVID-19 infection, revealed a significantly increased risk of adverse cardiovascular outcomes, including thrombosis-related diseases, such as myocardial infarction, acute coronary syndrome, pulmonary embolism, and stroke at 1 year [212]. The hazard ratio remained elevated in ‘mild’ cases and in younger patients with no underlying health issues.
Despite the extensive body of research into the condition, there are currently no validated evidence-based treatments for Long COVID. There clearly needs to be further research into the pathophysiological mechanisms; however, given the scale and debilitating nature of the condition, this should not delay good-quality trials of candidate treatments. High caliber research can (and should) be accelerated; this was demonstrated during the first year of the pandemic with trials, such as RECOVERY, which identified effective treatments for acute COVID-19 [213]. A recent comprehensive review of Long COVID identified several research priorities, including the need to build on existing knowledge from similar conditions, such as ME/CFS; recognition of the limitations of laboratory testing for COVID-19 with a move to clinically defined inclusion and exclusion criteria for studies; appropriate representation of disadvantaged groups; and patient engagement at all stages of research, beginning with study design [13].
There needs to be a multipronged treatment agenda for Long COVID treatment trials, incorporating drugs targeting all potential mechanisms, including viral persistence, autoimmunity, immune dysregulation, and gut dysbiosis. However, given the pathophysiology of the disease and the clearly increased risk of thrombotic events, attention needs to be paid to the endothelialitis and coagulation abnormalities as a priority. There are promising pilot data for improvement in symptoms and endothelial function with the drug sulodexide [214]. Initial observational experience with triple anticoagulant therapy to address the anomalous clotting is also encouraging [8]. A pilot study of the drug Pycnogenol® demonstrated improvements in endothelial function, microcirculation, inflammatory molecules, oxidative stress, and symptoms [215]. For any of these drugs to be adopted widely, these results need to be replicated further in randomized controlled trials. Finally, heparin-induced extracorporeal lipoprotein (HELP) apheresis has been proven to reduce hypercoagulability and inflammatory molecules in cardiovascular disease; while there is potential for its use in Long COVID [216], the treatment is expensive and not widely accessible, and so far there are no objective data of its efficacy in Long COVID.
In parallel with treatment trials, it is necessary to develop validated, accessible methods for detecting and tracking endothelialitis, microclots, and platelet hyperactivation to aid diagnostics and monitor response to treatment (see Outstanding questions). The currently available techniques are expensive and not readily available in clinical settings [1,183,217].
Outstanding questions.
How can we accelerate clinically defined inclusion and exclusion criteria for Long COVID studies?
How can we ensure appropriate representation of disadvantaged groups with Long COVID, and patient engagement at all stages of research, beginning with study design?
How can we accelerate diagnosis and clinical trials to test treatment regimens? Despite the extensive body of research into the condition, there are currently no validated evidence-based treatments for Long COVID.
How can we build on existing knowledge from similar conditions, such as ME/CFS?
Alt-text: Outstanding questions
The ongoing search for definitive treatment options does not mean that nothing can be done to help patients in the meantime. There is plenty that clinicians can offer. In the first instance, care must be taken to ‘do no harm’ by determining whether the patient has the ‘syndromic’ or ‘non-syndromic’ subtype of Long COVID, so that individuals with the former are not offered treatments, such as graded exercise therapy (GET) or cognitive behavioral therapy (CBT). These treatments have been shown to be harmful and/or ineffective and several guidelines explicitly advise against them [36,218]. Once the patient has been subtyped, symptomatic treatment should be offered depending on the presentation [13], such as antihistamines for MCAS [219], and cardiac rate-limiting medication for dysautonomia [37,220]. The response to therapy should be reviewed on a regular basis, with treatments titrated/stopped/substituted as appropriate.
Concluding remarks
Long COVID is a multisystem disease with debilitating symptoms, which has had a profound impact on society and the global economy. There are several potential pathophysiological mechanisms, some of which may be causative and others likely to be epiphenomena. These include viral factors, host factors, and downstream impacts. These mechanisms interact with the vascular endothelium to induce a persistent thrombotic endothelialitis with systemic hypercoagulability. This may promote the formation of fibrinaloid microclots, platelet hyperactivation, and endothelial dysfunction, which can lead to various clotting pathologies. Currently, there are no definitive treatments for the condition. There is a pressing need for randomized placebo-controlled trials of therapeutics targeting the dysregulated coagulation and endothelialitis, especially given the increased long-term risk of thromboembolic outcomes (see Outstanding questions).
Acknowledgments
Acknowledgments
D.B.K. thanks the Novo Nordisk Foundation for funding (grant NNF20CC0035580). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. E.P. thanks the National Research Foundation of South Africa (grant number 142142) and South African Medical Research Council [self-initiated research (SIR) grant]. The content and findings reported and illustrated are the sole deduction, view and responsibility of the researchers and do not reflect the official position and sentiments of the funders.
Author contributions
S.T. and D.P., writing of paper; M.A.K., writing and editing of paper; D.B.K., A.W., editing of paper; E.P., study leader, student supervisor, funding, and editing of paper.
Declaration of interests
None are declared by the authors.
Resources
iwww.cdc.gov/nchs/covid19/pulse/long-covid.htmiiwww.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.htmliiiwww.covid.gov/longcovidivwww.science.org/content/article/rare-cases-coronavirus-vaccines-may-cause-long-covid-symptomsReferences
- 1.Pretorius E., et al. Persistent clotting protein pathology in Long COVID/Post-Acute Sequelae of COVID-19 (PASC) is accompanied by increased levels of antiplasmin. Cardiovasc. Diabetol. 2021;20:172. doi: 10.1186/s12933-021-01359-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cascella M., et al. StatPearls Publishing; 2022. Features, Evaluation, and Treatment of Coronavirus (COVID-19) [PubMed] [Google Scholar]
- 3.Wiersinga W.J., et al. Pathophysiology, transmission, diagnosis, and treatment of Coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;324:782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- 4.Raveendran A.V. Long COVID-19: challenges in the diagnosis and proposed diagnostic criteria. Diabetes Metab. Syndr. 2021;15:145–146. doi: 10.1016/j.dsx.2020.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ballering A.V., et al. Persistence of somatic symptoms after COVID-19 in the Netherlands: an observational cohort study. Lancet. 2022;400:452–461. doi: 10.1016/S0140-6736(22)01214-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Callard F., Perego E. How and why patients made Long Covid. Soc. Sci. Med. 2021;268 doi: 10.1016/j.socscimed.2020.113426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Greenhalgh T., et al. Management of post-acute covid-19 in primary care. BMJ. 2020;370 doi: 10.1136/bmj.m3026. [DOI] [PubMed] [Google Scholar]
- 8.Pretorius E., et al. Prevalence of symptoms, comorbidities, fibrin amyloid microclots and platelet pathology in individuals with Long COVID/Post-Acute Sequelae of COVID-19 (PASC) Cardiovasc. Diabetol. 2022;21:148. doi: 10.1186/s12933-022-01579-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seessle J., et al. Persistent symptoms in adult patients 1 year after Coronavirus disease 2019 (COVID-19): a prospective cohort study. Clin. Infect. Dis. 2022;74:1191–1198. doi: 10.1093/cid/ciab611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grobbelaar L.M., et al. SARS-CoV-2 spike protein S1 induces fibrin(ogen) resistant to fibrinolysis: implications for microclot formation in COVID-19. Biosci. Rep. 2021;41 doi: 10.1042/BSR20210611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kell D.B., et al. A central role for amyloid fibrin microclots in long COVID/PASC: origins and therapeutic implications. Biochem. J. 2022;479:537–559. doi: 10.1042/BCJ20220016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kruger A., et al. Proteomics of fibrin amyloid microclots in long COVID/post-acute sequelae of COVID-19 (PASC) shows many entrapped pro-inflammatory molecules that may also contribute to a failed fibrinolytic system. Cardiovasc. Diabetol. 2022;21:190. doi: 10.1186/s12933-022-01623-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davis H.E., et al. Long COVID: major findings, mechanisms and recommendations. Nat. Rev. Microbiol. 2023;21:133–146. doi: 10.1038/s41579-022-00846-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cutler D.M., Summers L.H. The COVID-19 pandemic and the $16 trillion virus. JAMA. 2020;324:1495–1496. doi: 10.1001/jama.2020.19759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carfì A., et al. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324:603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang C., et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Twomey R., et al. Chronic fatigue and postexertional malaise in people living with long COVID: an observational study. Phys. Ther. 2022;102 doi: 10.1093/ptj/pzac005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haffke M., et al. Endothelial dysfunction and altered endothelial biomarkers in patients with post-COVID-19 syndrome and chronic fatigue syndrome (ME/CFS) J. Transl. Med. 2022;20:138. doi: 10.1186/s12967-022-03346-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mancini D.M., et al. Use of cardiopulmonary stress testing for patients with unexplained dyspnea post-Coronavirus disease. JACC Heart Fail. 2021;9:927–937. doi: 10.1016/j.jchf.2021.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Komaroff A.L., Bateman L. Will COVID-19 lead to myalgic encephalomyelitis/chronic fatigue syndrome? Front. Med. (Lausanne) 2020;7 doi: 10.3389/fmed.2020.606824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morrow A.K., et al. Long-term COVID 19 sequelae in adolescents: the overlap with orthostatic intolerance and ME/CFS. Curr. Pediatr. Rep. 2022;10:31–44. doi: 10.1007/s40124-022-00261-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sorensen A.I.V., et al. A nationwide questionnaire study of post-acute symptoms and health problems after SARS-CoV-2 infection in Denmark. Nat. Commun. 2022;13:4213. doi: 10.1038/s41467-022-31897-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berg S.K., et al. Long COVID symptoms in SARS-CoV-2-positive children aged 0–14 years and matched controls in Denmark (LongCOVIDKidsDK): a national, cross-sectional study. Lancet Child Adolesc. Health. 2022;6:614–623. doi: 10.1016/S2352-4642(22)00154-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morand A., et al. Similar patterns of [(18)F]-FDG brain PET hypometabolism in paediatric and adult patients with long COVID: a paediatric case series. Eur. J. Nucl. Med. Mol. Imaging. 2022;49:913–920. doi: 10.1007/s00259-021-05528-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cooper S., et al. Long COVID-19 liver manifestation in children. J. Pediatr. Gastroenterol. Nutr. 2022;75:244–251. doi: 10.1097/MPG.0000000000003521. [DOI] [PubMed] [Google Scholar]
- 26.Menni C., et al. Real-time tracking of self-reported symptoms to predict potential COVID-19. Nat. Med. 2020;26:1037–1040. doi: 10.1038/s41591-020-0916-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Asadi-Pooya A.A., et al. Risk factors associated with Long COVID syndrome: a retrospective study. Iran J. Med. Sci. 2021;46:428–436. doi: 10.30476/ijms.2021.92080.2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sudre C.H., et al. Attributes and predictors of long COVID. Nat. Med. 2021;27:626–631. doi: 10.1038/s41591-021-01292-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raveendran A.V., et al. Long COVID: an overview. Diabetes Metab. Syndr. 2021;15:869–875. doi: 10.1016/j.dsx.2021.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tenforde M.W., et al. Symptom duration and risk factors for delayed return to usual health among outpatients with COVID-19 in a multistate health care systems network - United States, March-June 2020. MMWR Morb. Mortal. Wkly Rep. 2020;69:993–998. doi: 10.15585/mmwr.mm6930e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang B., et al. Sex-based clinical and immunological differences in COVID-19. BMC Infect. Dis. 2021;21:647. doi: 10.1186/s12879-021-06313-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ziauddeen N., et al. Characteristics and impact of Long Covid: findings from an online survey. PLoS ONE. 2022;17 doi: 10.1371/journal.pone.0264331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williamson A.E., et al. Short-term and long-term impacts of COVID-19 on economic vulnerability: a population-based longitudinal study (COVIDENCE UK) BMJ Open. 2022;12 doi: 10.1136/bmjopen-2022-065083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davis H.E., et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. 2021;38 doi: 10.1016/j.eclinm.2021.101019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stussman B., et al. Characterization of post-exertional malaise in patients with myalgic encephalomyelitis/chronic fatigue syndrome. Front. Neurol. 2020;11:1025. doi: 10.3389/fneur.2020.01025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wright J., et al. The relationship between physical activity and Long COVID: a cross-sectional study. Int. J. Environ. Res. Public Health. 2022;19:5093. doi: 10.3390/ijerph19095093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blitshteyn S., et al. Multi-disciplinary collaborative consensus guidance statement on the assessment and treatment of autonomic dysfunction in patients with post-acute sequelae of SARS-CoV-2 infection (PASC) PM R. 2022;14:1270–1291. doi: 10.1002/pmrj.12894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soriano J.B., et al. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect. Dis. 2022;22:e102–e107. doi: 10.1016/S1473-3099(21)00703-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Peluso M.J., et al. Chronic viral coinfections differentially affect the likelihood of developing long COVID. J. Clin. Invest. 2023;133 doi: 10.1172/JCI163669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Su Y., et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell. 2022;185:881–895. doi: 10.1016/j.cell.2022.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Notarte K.I., et al. Impact of COVID-19 vaccination on the risk of developing long-COVID and on existing long-COVID symptoms: a systematic review. eClinicalMedicine. 2022;53 doi: 10.1016/j.eclinm.2022.101624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taquet M., et al. Six-month sequelae of post-vaccination SARS-CoV-2 infection: a retrospective cohort study of 10,024 breakthrough infections. Brain Behav. Immun. 2022;103:154–162. doi: 10.1016/j.bbi.2022.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tsuchida T., et al. Relationship between changes in symptoms and antibody titers after a single vaccination in patients with Long COVID. J. Med. Virol. 2022;94:3416–3420. doi: 10.1002/jmv.27689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ayoubkhani D., Bosworth M. Office for National Statistics; 2022. Self-Reported Long COVID after Infection with the Omicron Variant in the UK. [Google Scholar]
- 45.Frontera J.A., et al. Neurological events reported after COVID-19 vaccines: an analysis of VAERS. Ann. Neurol. 2022;91:756–771. doi: 10.1002/ana.26339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Potgieter M., et al. The dormant blood microbiome in chronic, inflammatory diseases. FEMS Microbiol. Rev. 2015;39:567–591. doi: 10.1093/femsre/fuv013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jacobs J.J.L. Persistent SARS-2 infections contribute to long COVID-19. Med. Hypotheses. 2021;149 doi: 10.1016/j.mehy.2021.110538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Griffin D.A.-O. Why does viral RNA sometimes persist after recovery from acute infections? PLoS Biol. 2022;20 doi: 10.1371/journal.pbio.3001687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Proal A.D., VanElzakker M.B. Long COVID or post-acute sequelae of COVID-19 (PASC): an overview of biological factors that may contribute to persistent symptoms. Front. Microbiol. 2021;12 doi: 10.3389/fmicb.2021.698169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mantovani A., et al. Long Covid: where we stand and challenges ahead. Cell Death Differ. 2022;29:1891–1900. doi: 10.1038/s41418-022-01052-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Desimmie B.A., et al. Insights into SARS-CoV-2 persistence and its relevance. Viruses. 2021;13:1025. doi: 10.3390/v13061025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rouse B.T., Sehrawat S. Immunity and immunopathology to viruses: what decides the outcome? Nat. Rev. Immunol. 2010;10:514–526. doi: 10.1038/nri2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pinho J.R.R., et al. Long term persistence of coronavirus SARS-CoV-2 infection. Einstein (Sao Paulo) 2021;19 doi: 10.31744/einstein_journal/2021RC6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Marx V. Scientists set out to connect the dots on long COVID. Nat. Methods. 2021;18:449–453. doi: 10.1038/s41592-021-01145-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hoang V.T., et al. Recurrence of positive SARS-CoV-2 in patients recovered from COVID-19. J. Med. Virol. 2020;92:2366–2367. doi: 10.1002/jmv.26056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kalkeri R., et al. SARS-CoV-2 shedding from asymptomatic patients: contribution of potential extrapulmonary tissue reservoirs. Am. J. Trop. Med. Hyg. 2020;103:18–21. doi: 10.4269/ajtmh.20-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xing Y.H., et al. Prolonged viral shedding in feces of pediatric patients with Coronavirus disease 2019. J. Microbiol. Immunol. Infect. 2020;53:473–480. doi: 10.1016/j.jmii.2020.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bergmann C.C., Silverman R.H. COVID-19: coronavirus replication, pathogenesis, and therapeutic strategies. Cleve. Clin. J. Med. 2020;87:321–327. doi: 10.3949/ccjm.87a.20047. [DOI] [PubMed] [Google Scholar]
- 59.Suprewicz Ł., et al. Extracellular vimentin as a target against SARS-CoV-2 host cell invasion. Small. 2022;18 doi: 10.1002/smll.202105640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kawase M., et al. Biochemical analysis of coronavirus spike glycoprotein conformational intermediates during membrane fusion. J. Virol. 2019;93 doi: 10.1128/JVI.00785-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Walls A.C., et al. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell. 2020;181:281–292. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang C., et al. Protein structure and sequence reanalysis of 2019-nCoV genome refutes snakes as its intermediate host and the unique similarity between its spike protein insertions and HIV-1. J. Proteome Res. 2020;19:1351–1360. doi: 10.1021/acs.jproteome.0c00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Watanabe Y., et al. Site-specific glycan analysis of the SARS-CoV-2 spike. Science. 2020;369:330–333. doi: 10.1126/science.abb9983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Flores-Alanis A., et al. The receptor binding domain of SARS-CoV-2 spike protein is the result of an ancestral recombination between the bat-CoV RaTG13 and the pangolin-CoV MP789. BMC Res. Notes. 2020;13:398. doi: 10.1186/s13104-020-05242-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Meyer B., et al. Characterising proteolysis during SARS-CoV-2 infection identifies viral cleavage sites and cellular targets with therapeutic potential. Nat. Commun. 2021;12:5553. doi: 10.1038/s41467-021-25796-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Karn V., et al. Extracellular vesicle-based therapy for COVID-19: promises, challenges and future prospects. Biomedicines. 2021;9:1373. doi: 10.3390/biomedicines9101373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Borowiec B.M., et al. Small extracellular vesicles and COVID19-using the ‘Trojan Horse’ to tackle the giant. Cells. 2021;10:3383. doi: 10.3390/cells10123383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Barberis E., et al. Circulating exosomes are strongly involved in SARS-CoV-2 infection. Front. Mol. Biosci. 2021;8 doi: 10.3389/fmolb.2021.632290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Eymieux S., et al. Secretory vesicles are the principal means of SARS-CoV-2 egress. Cells. 2021;10:2047. doi: 10.3390/cells10082047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kell D.B., Pretorius E. No effects without causes: the Iron Dysregulation and Dormant Microbes hypothesis for chronic, inflammatory diseases. Biol. Rev. Camb. Philos. Soc. 2018;93:1518–1557. doi: 10.1111/brv.12407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rebman A.W., Aucott J.N. Post-treatment Lyme Disease as a model for persistent symptoms in Lyme Disease. Front. Med. (Lausanne) 2020;7:57. doi: 10.3389/fmed.2020.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kumata R., et al. A tissue level atlas of the healthy human virome. BMC Biol. 2020;18:55. doi: 10.1186/s12915-020-00785-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen C., et al. Risk surveillance and mitigation: autoantibodies as triggers and inhibitors of severe reactions to SARS-CoV-2 infection. Mol. Med. 2021;27:160. doi: 10.1186/s10020-021-00422-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brogna C., et al. Could SARS-CoV-2 have bacteriophage behavior or induce the activity of other bacteriophages? Vaccines (Basel) 2022;10:708. doi: 10.3390/vaccines10050708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Patterson B.K., et al. Immune-based prediction of COVID-19 severity and chronicity decoded using machine learning. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.700782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Scaglioni V., Soriano E.R. Are superantigens the cause of cytokine storm and viral sepsis in severe COVID-19? Observations and hypothesis. Scand. J. Immunol. 2020;92 doi: 10.1111/sji.12944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kell D.B., Pretorius E. To what extent are the terminal stages of sepsis, septic shock, systemic inflammatory response syndrome, and multiple organ dysfunction syndrome actually driven by a prion/amyloid form of fibrin? Semin. Thromb. Hemost. 2017;44:224–238. doi: 10.1055/s-0037-1604108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tavakolpour S., et al. Lymphopenia during the COVID-19 infection: what it shows and what can be learned. Immunol. Lett. 2020;225:31–32. doi: 10.1016/j.imlet.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fathi N., Rezaei N. Lymphopenia in COVID-19: therapeutic opportunities. Cell Biol. Int. 2020;44:1792–1797. doi: 10.1002/cbin.11403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cheng Y., et al. Dynamic changes of lymphocyte counts in adult patients with severe pandemic H1N1 influenza A. J. Infect. Public Health. 2019;12:878–883. doi: 10.1016/j.jiph.2019.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kong M., et al. Higher level of neutrophil-to-lymphocyte is associated with severe COVID-19. Epidemiol. Infect. 2020;148 doi: 10.1017/S0950268820001557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hu F., et al. A compromised specific humoral immune response against the SARS-CoV-2 receptor-binding domain is related to viral persistence and periodic shedding in the gastrointestinal tract. Cell. Mol. Immunol. 2020;17:1119–1125. doi: 10.1038/s41423-020-00550-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ercolini A.M., Miller S.D. The role of infections in autoimmune disease. Clin. Exp. Immunol. 2009;155:1–15. doi: 10.1111/j.1365-2249.2008.03834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Eizirik D.L., Op de Beeck A. Coxsackievirus and Type 1 diabetes mellitus: the wolf's footprints. Trends Endocrinol. Metab. 2018;29:137–139. doi: 10.1016/j.tem.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 85.Hober D., et al. Immunology in the clinic review series; focus on type 1 diabetes and viruses: role of antibodies enhancing the infection with Coxsackievirus-B in the pathogenesis of type 1 diabetes. Clin. Exp. Immunol. 2012;168:47–51. doi: 10.1111/j.1365-2249.2011.04559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Stolzel U., et al. Autoimmunity and HCV infection in porphyria cutanea tarda: a controlled study. Cell Mol. Biol. (Noisy-le-grand) 2002;48:43–47. [PubMed] [Google Scholar]
- 87.Ebringer A., et al. Rheumatoid arthritis, Proteus, anti-CCP antibodies and Karl Popper. Autoimmun. Rev. 2010;9:216–223. doi: 10.1016/j.autrev.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 88.Pretorius E., et al. Major involvement of bacterial components in rheumatoid arthritis and its accompanying oxidative stress, systemic inflammation and hypercoagulability. Exp. Biol. Med. (Maywood) 2017;242:355–373. doi: 10.1177/1535370216681549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kell D.B., Kenny L.C. A dormant microbial component in the development of preeclampsia. Front. Med. (Lausanne) 2016;3:60. doi: 10.3389/fmed.2016.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhou Y., et al. Clinical and autoimmune characteristics of severe and critical cases of COVID-19. Clin. Transl. Sci. 2020;13:1077–1086. doi: 10.1111/cts.12805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bastard P., et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370 doi: 10.1126/science.abd4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Vlachoyiannopoulos P.G., et al. Autoantibodies related to systemic autoimmune rheumatic diseases in severely ill patients with COVID-19. Ann. Rheum. Dis. 2020;79:1661–1663. doi: 10.1136/annrheumdis-2020-218009. [DOI] [PubMed] [Google Scholar]
- 93.Gao Z.W., et al. Autoantibodies in COVID-19: frequency and function. Autoimmun. Rev. 2021;20 doi: 10.1016/j.autrev.2021.102754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wallukat G., et al. Functional autoantibodies against G-protein coupled receptors in patients with persistent Long-COVID-19 symptoms. J. Transl. Autoimmun. 2021;4 doi: 10.1016/j.jtauto.2021.100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cabral-Marques O., et al. Autoantibodies targeting GPCRs and RAS-related molecules associate with COVID-19 severity. Nat. Commun. 2022;13:1220. doi: 10.1038/s41467-022-28905-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Szewczykowski C., et al. Long COVID: association of functional autoantibodies against G-protein-coupled receptors with an impaired retinal microcirculation. Int. J. Mol. Sci. 2022;23:7209. doi: 10.3390/ijms23137209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hohberger B., et al. Case report: neutralization of autoantibodies targeting G-protein-coupled receptors improves capillary impairment and fatigue symptoms after COVID-19 infection. Front. Med. (Lausanne) 2021;8 doi: 10.3389/fmed.2021.754667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kritas S.K., et al. Mast cells contribute to coronavirus-induced inflammation: new anti-inflammatory strategy. J. Biol. Regul. Homeost. Agents. 2020;34:9–14. doi: 10.23812/20-Editorial-Kritas. [DOI] [PubMed] [Google Scholar]
- 99.Afrin L.B., et al. Mast cell activation disease: An underappreciated cause of neurologic and psychiatric symptoms and diseases. Brain Behav. Immun. 2015;50:314–321. doi: 10.1016/j.bbi.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 100.Maxwell A.J., et al. Identification of key signaling pathways induced by SARS-CoV2 that underlie thrombosis and vascular injury in COVID-19 patients. J. Leukoc. Biol. 2021;109:35–47. doi: 10.1002/JLB.4COVR0920-552RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Vojdani A., et al. Reaction of human monoclonal antibodies to SARS-CoV-2 proteins with tissue antigens: implications for autoimmune diseases. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.617089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jarrott B., et al. ‘LONG COVID’-a hypothesis for understanding the biological basis and pharmacological treatment strategy. Pharmacol. Res. Perspect. 2022;10 doi: 10.1002/prp2.911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tan D.X., Reiter R.J. Mechanisms and clinical evidence to support melatonin's use in severe COVID-19 patients to lower mortality. Life Sci. 2022;294 doi: 10.1016/j.lfs.2022.120368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kell D.B. Iron behaving badly: inappropriate iron chelation as a major contributor to the aetiology of vascular and other progressive inflammatory and degenerative diseases. BMC Med. Genet. 2009;2:2. doi: 10.1186/1755-8794-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cross K.M., et al. Melatonin for the early treatment of COVID-19: a narrative review of current evidence and possible efficacy. Endocr. Pract. 2021;27:850–855. doi: 10.1016/j.eprac.2021.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gavrilova N., et al. New clinical phenotype of the post-covid syndrome: fibromyalgia and joint hypermobility condition. Pathophysiology. 2022;29:24–29. doi: 10.3390/pathophysiology29010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yong S.J. Long COVID or post-COVID-19 syndrome: putative pathophysiology, risk factors, and treatments. Infect. Dis. (Lond) 2021;53:737–754. doi: 10.1080/23744235.2021.1924397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jarrahi A., et al. Neurological consequences of COVID-19: what have we learned and where do we go from here? J. Neuroinflammation. 2020;17:286. doi: 10.1186/s12974-020-01957-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Helms J., et al. Cardiac injury in COVID-19. Intensive Care Med. 2022;48:111–113. doi: 10.1007/s00134-021-06555-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhang C., et al. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol. Hepatol. 2020;5:428–430. doi: 10.1016/S2468-1253(20)30057-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Heiss R., et al. Pulmonary dysfunction after pediatric COVID-19. Radiology. 2022;306 doi: 10.1148/radiol.221250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Grist J.T., et al. Lung abnormalities detected with hyperpolarized (129)Xe MRI in patients with Long COVID. Radiology. 2022;305:709–717. doi: 10.1148/radiol.220069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lloyd-Jones G., et al. The COVID-19 pathway: a proposed oral-vascular-pulmonary route of SARS-CoV-2 infection and the importance of oral healthcare measures. J. Oral Med. Dental Res. 2021;2:1–25. [Google Scholar]
- 114.van Haren F.M.P., et al. Nebulised heparin as a treatment for COVID-19: scientific rationale and a call for randomised evidence. Crit. Care. 2020;24:454. doi: 10.1186/s13054-020-03148-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wichmann D., et al. Autopsy findings and venous thromboembolism in patients with COVID-19: a prospective cohort study. Ann. Intern. Med. 2020;173:268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ostergaard L. SARS CoV-2 related microvascular damage and symptoms during and after COVID-19: Consequences of capillary transit-time changes, tissue hypoxia and inflammation. Physiol. Rep. 2021;9 doi: 10.14814/phy2.14726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang C., et al. Long COVID: the nature of thrombotic sequelae determines the necessity of early anticoagulation. Front. Cell. Infect. Microbiol. 2022;12 doi: 10.3389/fcimb.2022.861703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Moasefi N., et al. How could perfluorocarbon affect cytokine storm and angiogenesis in coronavirus disease 2019 (COVID-19): role of hypoxia-inducible factor 1alpha. Inflamm. Res. 2021;70:749–752. doi: 10.1007/s00011-021-01469-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Fox S.E., et al. Unexpected features of cardiac pathology in COVID-19 infection. Circulation. 2020;142:1123–1125. doi: 10.1161/CIRCULATIONAHA.120.049465. [DOI] [PubMed] [Google Scholar]
- 120.Paniz-Mondolfi A., et al. Central nervous system involvement by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) J. Med. Virol. 2020;92:699–702. doi: 10.1002/jmv.25915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Harris A.G., Skalak T.C. Effects of leukocyte activation on capillary hemodynamics in skeletal muscle. Am. J. Phys. 1993;264:H909–H916. doi: 10.1152/ajpheart.1993.264.3.H909. [DOI] [PubMed] [Google Scholar]
- 122.Cruz Hernandez J.C., et al. Neutrophil adhesion in brain capillaries reduces cortical blood flow and impairs memory function in Alzheimer's disease mouse models. Nat. Neurosci. 2019;22:413–420. doi: 10.1038/s41593-018-0329-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ryan F.J., et al. Long-term perturbation of the peripheral immune system months after SARS-CoV-2 infection. BMC Med. 2022;20:26. doi: 10.1186/s12916-021-02228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang D., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kell D.B., Pretorius E. The potential role of ischaemia-reperfusion injury in chronic, relapsing diseases such as rheumatoid arthritis, Long COVID, and ME/CFS: evidence, mechanisms, and therapeutic implications. Biomed. J. 2022;479:1653–1708. doi: 10.1042/BCJ20220154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chouchani E.T., et al. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature. 2014;515:431–435. doi: 10.1038/nature13909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Granger D.N., Kvietys P.R. Reperfusion injury and reactive oxygen species: The evolution of a concept. Redox Biol. 2015;6:524–551. doi: 10.1016/j.redox.2015.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jaeschke H., Woolbright B.L. Current strategies to minimize hepatic ischemia-reperfusion injury by targeting reactive oxygen species. Transplant. Rev. (Orlando) 2012;26:103–114. doi: 10.1016/j.trre.2011.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ren G., et al. Inflammatory mechanisms in myocardial infarction. Curr. Drug Targets Inflamm. Allergy. 2003;2:242–256. doi: 10.2174/1568010033484098. [DOI] [PubMed] [Google Scholar]
- 130.Oliynyk O.V., et al. Oxygen metabolism markers as predictors of mortality in severe COVID-19. Int. J. Infect. Dis. 2021;103:452–456. doi: 10.1016/j.ijid.2020.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Santinelli L., et al. Oral bacteriotherapy reduces the occurrence of chronic fatigue in COVID-19 patients. Front. Nutr. 2021;8 doi: 10.3389/fnut.2021.756177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nalbandian A., et al. Post-acute COVID-19 syndrome. Nat. Med. 2021;27:601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Shen Z., et al. Genomic diversity of severe acute respiratory syndrome-coronavirus 2 in patients with Coronavirus disease 2019. Clin. Infect. Dis. 2020;71:713–720. doi: 10.1093/cid/ciaa203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zuo T., et al. Alterations in gut microbiota of patients with COVID-19 during time of hospitalization. Gastroenterology. 2020;159:944–955. doi: 10.1053/j.gastro.2020.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Proal A.D., et al. Microbe-microbe and host-microbe interactions drive microbiome dysbiosis and inflammatory processes. Discov. Med. 2017;23:51–60. [PubMed] [Google Scholar]
- 136.Shin W., Kim H.J. Intestinal barrier dysfunction orchestrates the onset of inflammatory host-microbiome cross-talk in a human gut inflammation-on-a-chip. Proc. Natl. Acad. Sci. U. S. A. 2018;115:E10539–E10547. doi: 10.1073/pnas.1810819115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Blitshteyn S., Whitelaw S. Postural orthostatic tachycardia syndrome (POTS) and other autonomic disorders after COVID-19 infection: a case series of 20 patients. Immunol. Res. 2021;69:205–211. doi: 10.1007/s12026-021-09185-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bisaccia G., et al. Post-acute sequelae of COVID-19 and cardiovascular autonomic dysfunction: what do we know? J. Cardiovasc. Dev. Dis. 2021;8:156. doi: 10.3390/jcdd8110156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Aranyo J., et al. Inappropriate sinus tachycardia in post-COVID-19 syndrome. Sci. Rep. 2022;12:298. doi: 10.1038/s41598-021-03831-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Ladlow P., et al. Dysautonomia following COVID-19 is not associated with subjective limitations or symptoms but is associated with objective functional limitations. Heart Rhythm. 2022;19:613–620. doi: 10.1016/j.hrthm.2021.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lopez-Leon S., et al. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci. Rep. 2021;11:16144. doi: 10.1038/s41598-021-95565-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Barizien N., et al. Clinical characterization of dysautonomia in long COVID-19 patients. Sci. Rep. 2021;11:1–7. doi: 10.1038/s41598-021-93546-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Vernino S., et al. Postural orthostatic tachycardia syndrome (POTS): State of the science and clinical care from a 2019 National Institutes of Health Expert Consensus Meeting - Part 1. Auton. Neurosci. 2021;235 doi: 10.1016/j.autneu.2021.102828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Stewart J.M., Montgomery L.D. Regional blood volume and peripheral blood flow in postural tachycardia syndrome. Am. J. Physiol. Heart Circ. Physiol. 2004;287:H1319–H1327. doi: 10.1152/ajpheart.00086.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Novak P., et al. Multisystem Involvement in post-acute sequelae of Coronavirus disease 19. Ann. Neurol. 2022;91:367–379. doi: 10.1002/ana.26286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Campen C., et al. Cerebral blood flow is reduced in ME/CFS during head-up tilt testing even in the absence of hypotension or tachycardia: a quantitative, controlled study using Doppler echography. Clin. Neurophysiol. Pract. 2020;5:50–58. doi: 10.1016/j.cnp.2020.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Campen C., et al. Reductions in cerebral blood flow can be provoked by sitting in severe myalgic encephalomyelitis/chronic fatigue syndrome patients. Healthcare (Basel) 2020;8:394. doi: 10.3390/healthcare8040394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.van Campen C.M.C., et al. Cerebral blood flow is reduced in severe myalgic encephalomyelitis/chronic fatigue syndrome patients during mild orthostatic stress testing: an exploratory study at 20 degrees of head-up tilt testing. Healthcare. 2020;8:169. doi: 10.3390/healthcare8020169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Louapre C., et al. Clinical characteristics and outcomes in patients with Coronavirus disease 2019 and multiple sclerosis. JAMA Neurol. 2020;77:1079–1088. doi: 10.1001/jamaneurol.2020.2581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Novak P. Post COVID-19 syndrome associated with orthostatic cerebral hypoperfusion syndrome, small fiber neuropathy and benefit of immunotherapy: a case report. eNeurologicalSci. 2020;21 doi: 10.1016/j.ensci.2020.100276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Oaklander A.L., et al. Peripheral neuropathy evaluations of patients with prolonged Long COVID. Neurol Neuroimmunol. Neuroinflamm. 2022;9 doi: 10.1212/NXI.0000000000001146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Abrams R.M.C., et al. Small fiber neuropathy associated with SARS-CoV-2 infection. Muscle Nerve. 2022;65:440–443. doi: 10.1002/mus.27458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Guilmot A., et al. Immune-mediated neurological syndromes in SARS-CoV-2-infected patients. J. Neurol. 2021;268:751–757. doi: 10.1007/s00415-020-10108-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ruzieh M., et al. The role of autoantibodies in the syndromes of orthostatic intolerance: a systematic review. Scand. Cardiovasc. J. 2017;51:243–247. doi: 10.1080/14017431.2017.1355068. [DOI] [PubMed] [Google Scholar]
- 155.Li H., et al. Agonistic autoantibodies as vasodilators in orthostatic hypotension: a new mechanism. Hypertension. 2012;59:402–408. doi: 10.1161/HYPERTENSIONAHA.111.184937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Li H., et al. Autoimmune basis for postural tachycardia syndrome. J. Am. Heart Assoc. 2014;3 doi: 10.1161/JAHA.113.000755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Yu X., et al. Autoantibody activation of beta-adrenergic and muscarinic receptors contributes to an ‘autoimmune’ orthostatic hypotension. J. Am. Soc. Hypertens. 2012;6:40–47. doi: 10.1016/j.jash.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Fedorowski A., et al. Antiadrenergic autoimmunity in postural tachycardia syndrome. Ep Europace. 2017;19:1211–1219. doi: 10.1093/europace/euw154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Stein S.R., et al. SARS-CoV-2 infection and persistence in the human body and brain at autopsy. Nature. 2022;612:758–763. doi: 10.1038/s41586-022-05542-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Moyano A.J., et al. Vagus nerve neuropathy related to SARS COV-2 infection. IDCases. 2021;26 doi: 10.1016/j.idcr.2021.e01242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sarubbo F., et al. Neurological consequences of COVID-19 and brain related pathogenic mechanisms: a new challenge for neuroscience. Brain Behav. Immun. Health. 2022;19 doi: 10.1016/j.bbih.2021.100399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Esmon C.T. The interactions between inflammation and coagulation. Br. J. Haematol. 2005;131:417–430. doi: 10.1111/j.1365-2141.2005.05753.x. [DOI] [PubMed] [Google Scholar]
- 163.Szotowski B., et al. Procoagulant soluble tissue factor is released from endothelial cells in response to inflammatory cytokines. Circ. Res. 2005;96:1233–1239. doi: 10.1161/01.RES.0000171805.24799.fa. [DOI] [PubMed] [Google Scholar]
- 164.Esmon C.T. The impact of the inflammatory response on coagulation. Thromb. Res. 2004;114:321–327. doi: 10.1016/j.thromres.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 165.Levi M., van der Poll T. Two-way interactions between inflammation and coagulation. Trends Cardiovasc. Med. 2005;15:254–259. doi: 10.1016/j.tcm.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 166.Zuo Y., et al. Plasma tissue plasminogen activator and plasminogen activator inhibitor-1 in hospitalized COVID-19 patients. Sci. Rep. 2021;11:1580. doi: 10.1038/s41598-020-80010-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Fricke-Galindo I., et al. SERPINE1 rs6092 variant is related to plasma coagulation proteins in patients with severe COVID-19 from a tertiary care hospital. Biology (Basel) 2022;11:595. doi: 10.3390/biology11040595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Khan S.S. The central role of PAI-1 in COVID-19: thrombosis and beyond. Am. J. Respir. Cell Mol. Biol. 2021;65:238–240. doi: 10.1165/rcmb.2021-0208ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Visser M.J.E., et al. Thrombosis in Psoriasis: Cutaneous Cytokine Production as a Potential Driving Force of Haemostatic Dysregulation and Subsequent Cardiovascular Risk. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.688861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Camerer E., et al. Tissue factor- and factor X-dependent activation of protease-activated receptor 2 by factor VIIa. Proc. Natl. Acad. Sci. U. S. A. 2000;97:5255–5260. doi: 10.1073/pnas.97.10.5255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Camerer E., et al. Genetic evidence that protease-activated receptors mediate factor Xa signaling in endothelial cells. J. Biol. Chem. 2002;277:16081–16087. doi: 10.1074/jbc.M108555200. [DOI] [PubMed] [Google Scholar]
- 172.de Jonge E., et al. Activation of coagulation by administration of recombinant factor VIIa elicits interleukin 6 (IL-6) and IL-8 release in healthy human subjects. Clin. Diagn. Lab. Immunol. 2003;10:495–497. doi: 10.1128/CDLI.10.3.495-497.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Vorlova S., et al. Coagulation factor XII induces pro-inflammatory cytokine responses in macrophages and promotes atherosclerosis in mice. Thromb. Haemost. 2017;117:176–187. doi: 10.1160/TH16-06-0466. [DOI] [PubMed] [Google Scholar]
- 174.Gros A., et al. Platelets in inflammation: regulation of leukocyte activities and vascular repair. Front. Immunol. 2014;5:678. doi: 10.3389/fimmu.2014.00678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Mullin J.L., et al. Recombinant fibrinogen studies reveal that thrombin specificity dictates order of fibrinopeptide release. J. Biol. Chem. 2000;275:25239–25246. doi: 10.1074/jbc.M004142200. [DOI] [PubMed] [Google Scholar]
- 176.Riedel T., et al. Fibrinopeptides A and B release in the process of surface fibrin formation. Blood. 2011;117:1700–1706. doi: 10.1182/blood-2010-08-300301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kell D.B., Pretorius E. The simultaneous occurrence of both hypercoagulability and hypofibrinolysis in blood and serum during systemic inflammation, and the roles of iron and fibrin(ogen) Integr. Biol. 2015;7:24–52. doi: 10.1039/c4ib00173g. [DOI] [PubMed] [Google Scholar]
- 178.Kell D.B., Pretorius E. Proteins behaving badly. Substoichiometric molecular control and amplification of the initiation and nature of amyloid fibril formation: lessons from and for blood clotting. Prog. Biophys. Mol. Biol. 2017;123:16–41. doi: 10.1016/j.pbiomolbio.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 179.Pretorius E., et al. Substantial fibrin amyloidogenesis in type 2 diabetes assessed using amyloid-selective fluorescent stains. Cardiovasc. Diabetol. 2017;16:141. doi: 10.1186/s12933-017-0624-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Nunes J.M., et al. The occurrence of hyperactivated platelets and fibrinaloid microclots in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) Pharmaceuticals (Basel) 2022;15:931. doi: 10.3390/ph15080931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Laubscher, G.J. et al. TEG(®), microclot and platelet mapping for guiding early management of severe COVID-19 coagulopathy. J. Clin. Med. 10, 5381 [DOI] [PMC free article] [PubMed]
- 182.Pretorius E., et al. Prevalence of readily detected amyloid blood clots in 'unclotted' type 2 diabetes mellitus and COVID-19 plasma: a preliminary report. Cardiovasc. Diabetol. 2020;19:193. doi: 10.1186/s12933-020-01165-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Charfeddine S., et al. Long COVID 19 syndrome: is it related to microcirculation and endothelial dysfunction? Insights from TUN-EndCOV study. Front. Cardiovasc. Med. 2021;8 doi: 10.3389/fcvm.2021.745758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Huertas A., et al. Endothelial cell dysfunction: a major player in SARS-CoV-2 infection (COVID-19)? Eur. Respir. J. 2020;56 doi: 10.1183/13993003.01634-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Varga Z., et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417–1418. doi: 10.1016/S0140-6736(20)30937-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Perico L., et al. Immunity, endothelial injury and complement-induced coagulopathy in COVID-19. Nat. Rev. Nephrol. 2021;17:46–64. doi: 10.1038/s41581-020-00357-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Teuwen L.A., et al. COVID-19: the vasculature unleashed. Nat. Rev. Immunol. 2020;20:389–391. doi: 10.1038/s41577-020-0343-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Abeyama K., et al. The N-terminal domain of thrombomodulin sequesters high-mobility group-B1 protein, a novel antiinflammatory mechanism. J. Clin. Invest. 2005;115:1267–1274. doi: 10.1172/JCI22782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Iwaki T., et al. A cardioprotective role for the endothelial protein C receptor in lipopolysaccharide-induced endotoxemia in the mouse. Blood. 2005;105:2364–2371. doi: 10.1182/blood-2004-06-2456. [DOI] [PubMed] [Google Scholar]
- 190.Ahamed J., Laurence J. Long COVID endotheliopathy: hypothesized mechanisms and potential therapeutic approaches. J. Clin. Invest. 2022;132 doi: 10.1172/JCI161167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.MacDonald M.E., et al. Lymphatic coagulation and neutrophil extracellular traps in lung-draining lymph nodes of COVID-19 decedents. Blood Adv. 2022;6:6249–6262. doi: 10.1182/bloodadvances.2022007798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Pisareva E., et al. Persistence of neutrophil extracellular traps and anticardiolipin auto-antibodies in post-acute phase COVID-19 patients. J. Med. Virol. 2023;95 doi: 10.1002/jmv.28209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Wagner D.D., Frenette P.S. The vessel wall and its interactions. Blood. 2008;111:5271–5281. doi: 10.1182/blood-2008-01-078204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Zhang S., et al. SARS-CoV-2 binds platelet ACE2 to enhance thrombosis in COVID-19. J. Hematol. Oncol. 2020;13:120. doi: 10.1186/s13045-020-00954-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Yau J.W., et al. Endothelial cell control of thrombosis. BMC Cardiovasc. Disord. 2015;15:130. doi: 10.1186/s12872-015-0124-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Mast A.E. Tissue factor pathway inhibitor: multiple anticoagulant activities for a single protein. Arterioscler. Thromb. Vasc. Biol. 2016;36:9–14. doi: 10.1161/ATVBAHA.115.305996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Speyer C.L., Ward P.A. Role of endothelial chemokines and their receptors during inflammation. J. Investig. Surg. 2011;24:18–27. doi: 10.3109/08941939.2010.521232. [DOI] [PubMed] [Google Scholar]
- 198.Rosell A., et al. Patients with COVID-19 have elevated levels of circulating extracellular vesicle tissue factor activity that is associated with severity and mortality-brief report. Arterioscler. Thromb. Vasc. Biol. 2021;41:878–882. doi: 10.1161/ATVBAHA.120.315547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Arganaraz G.A., et al. Phosphatidylserine inside out: a possible underlying mechanism in the inflammation and coagulation abnormalities of COVID-19. Cell Commun. Signal. 2020;18:190. doi: 10.1186/s12964-020-00687-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Lind S.E. Phosphatidylserine is an overlooked mediator of COVID-19 thromboinflammation. Heliyon. 2021;7 doi: 10.1016/j.heliyon.2021.e06033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Althaus K., et al. Antibody-induced procoagulant platelets in severe COVID-19 infection. Blood. 2021;137:1061–1071. doi: 10.1182/blood.2020008762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Knight J.S., et al. The intersection of COVID-19 and autoimmunity. J. Clin. Invest. 2021;131 doi: 10.1172/JCI154886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Ruiz-Arguelles G.J., et al. Natural anticoagulants in systemic lupus erythematosus. Deficiency of protein S bound to C4bp associates with recent history of venous thromboses, antiphospholipid antibodies, and the antiphospholipid syndrome. J. Rheumatol. 1991;18:552–558. [PubMed] [Google Scholar]
- 204.Mineo C. Inhibition of nitric oxide and antiphospholipid antibody-mediated thrombosis. Curr. Rheumatol. Rep. 2013;15:324. doi: 10.1007/s11926-013-0324-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Krone K.A., et al. Impaired fibrinolysis in the antiphospholipid syndrome. Curr. Rheumatol. Rep. 2010;12:53–57. doi: 10.1007/s11926-009-0075-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Mei Z.W., et al. Role of von Willebrand Factor in COVID-19 Associated Coagulopathy. J. Appl. Lab. Med. 2021;6:1305–1315. doi: 10.1093/jalm/jfab042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Eltzschig H.K., Carmeliet P. Hypoxia and inflammation. N. Engl. J. Med. 2011;364:656–665. doi: 10.1056/NEJMra0910283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Deng F., et al. Endothelial microvesicles in hypoxic hypoxia diseases. J. Cell. Mol. Med. 2018;22:3708–3718. doi: 10.1111/jcmm.13671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Katsoularis I., et al. Risks of deep vein thrombosis, pulmonary embolism, and bleeding after covid-19: nationwide self-controlled cases series and matched cohort study. BMJ. 2022;377 doi: 10.1136/bmj-2021-069590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Jiménez-Rodríguez B.M., et al. On the single and multiple associations of COVID-19 post-acute sequelae: 6-month prospective cohort study. Sci. Rep. 2022;12:3402. doi: 10.1038/s41598-022-07433-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Knight R., et al. Association of COVID-19 with major arterial and venous thrombotic diseases: a population-wide cohort study of 48 million adults in England and Wales. Circulation. 2022;146:892–906. doi: 10.1161/CIRCULATIONAHA.122.060785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Xie Y., et al. Long-term cardiovascular outcomes of COVID-19. Nat. Med. 2022;28:583–590. doi: 10.1038/s41591-022-01689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Horby P., et al. Dexamethasone in hospitalized patients with Covid-19. N. Engl. J. Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Charfeddine S., et al. Sulodexide in the treatment of patients with long COVID 19 symptoms and endothelial dysfunction: the results of TUN-EndCOV study. Arch. Cardiovasc. Dis. Suppl. 2022;14:127. [Google Scholar]
- 215.Belcaro G., et al. Preventive effects of Pycnogenol® on cardiovascular risk factors (including endothelial function) and microcirculation in subjects recovering from coronavirus disease 2019 (COVID-19) Minerva Med. 2022;113:300–308. doi: 10.23736/S0026-4806.21.07650-3. [DOI] [PubMed] [Google Scholar]
- 216.Jaeger B.R., et al. The potential of heparin-induced extracorporeal LDL/fibrinogen precipitation (H.E.L.P.)-apheresis for patients with severe acute or chronic COVID-19. Front. Cardiovasc. Med. 2022;9 doi: 10.3389/fcvm.2022.1007636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Moerland M., et al. Evaluation of the EndoPAT as a tool to assess endothelial function. Int. J. Vasc. Med. 2012;2012 doi: 10.1155/2012/904141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Heerdt P.M., et al. Impaired systemic oxygen extraction long after mild COVID-19: potential perioperative implications. Br. J. Anaesth. 2022;128:e246–e249. doi: 10.1016/j.bja.2021.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Glynne P., et al. Long COVID following mild SARS-CoV-2 infection: characteristic T cell alterations and response to antihistamines. J. Investig. Med. 2022;70:61–67. doi: 10.1136/jim-2021-002051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Dani M., et al. Autonomic dysfunction in 'long COVID': rationale, physiology and management strategies. Clin. Med. (Lond) 2021;21:e63–e67. doi: 10.7861/clinmed.2020-0896. [DOI] [PMC free article] [PubMed] [Google Scholar]