Abstract
This study aimed to characterize aging-induced tendinopathy in mouse Achilles tendon and also to assess the treatment effects of metformin (Met) on aging tendon. We showed that compared to young tendon, aging tendon was in an inflammatory and senescent state as shown by increased expression of inflammatory disulfide HMGB1 (dsHMGB1), inflammatory macrophage marker CD68, and senescent cell markers SA-β-gal, p53 and p16. Moreover, aging tendon was degenerated marked by accumulation of proteoglycans and lipids in its interior. However, treatment of aging tendon by intraperitoneal (IP) injection of Met, a specific inhibitor of HMGB1, reduced dsHMGB1 levels, decreased the expression of CD68, SA-β-gal, CCN1 and p16 in vitro and in vivo. Furthermore, Met treatment also increased the number of NS, SSEA-1, and CD73 positive stem cells in culture and improved the tendon structure in aging mouse. These findings of this study indicate that Met exerts anti-inflammatory and anti-senescent effects on aging tendon.
Keywords: Aging, tendon, inflammation, degeneration, senescence, HMGB1, metformin
1. INTRODUCTION
The US population is aging; it is estimated that by 2030, one in five Americans will be 65 or over 1. Aging is known to be a key risk factor in the development of tendinopathy 2. Lower extremity tendon degeneration such as Achilles tendinopathy is more prevalent in aged population 3; 4. Aging alters tendon tissues with gross changes in structure, composition, and mechanical properties and is known to induce tendinopathy due to reduction in regenerative capacity and loss of stem cell function 3; 5. However, current treatment methods for tendinopathy regardless of age are largely palliative that involve short term symptomatic relief, such as with NSAIDS and corticosteroid injections, which may cause serious side effects 6. Thus, there is an urgent need to improve clinical treatments for the “aging-induced tendinopathy” using safe and effective treatment options.
Metformin (Met) is an FDA approved hypoglycemic drug used for the treatment of diabetes. But Met as an “anti-aging” drug is also tested in the TAME (targeting aging with metformin) trials in aging-related morbidities such as cancer, stroke, and heart disease 7. Met has been shown to suppress pro-inflammatory cytokines and senescence-associated secretary proteins (SASPs) in cell lines and model organisms 8; 9. Senescence-associated-β-galactosidase (SA-β-gal) activity is the major biomarker identified in aging cells and tissues 10. Cyclin-dependent kinase inhibitor p16 and its regulator p53 are often expressed in senescent cells 11. Also, matricellular protein CCN1 can induce cellular senescence 12. Senescent cells may stimulate age-related inflammation and tissue degeneration by secreting pro-inflammatory cytokines 13, and a high mobility group box1 (HMGB1) can be one such a potent inflammatory mediator that is secreted to extracellular matrix (ECM) by senescent cells 14; 15.
Extracellular HMGB1 has both chemotactic and inflammatory functions depending on the redox state of its cysteine residues 16. Fully reduced HMGB1 (frHMGB1) promotes cell recruitment, including leukocytes and stem cells and as a result, can enhance regeneration of injured tissues; however, partially oxidized disulfide HMGB1 (dsHMGB1) activates leukocytes and triggers the release of proinflammatory cytokines, and thus it is detrimental to tissues 16. We have shown that HMGB1, likely its disulfide form, is translocated into cytoplasm and then released to ECM in mouse Achilles tendon and initiate an inflammatory cascade in response to long-term intensive treadmill running (ITR) 17.
In this study, considering that Met is an FDA-approved drug and an HMGB1 inhibitor 18 with anti-aging effects 9; 19, we used Met as a repurposed drug to test its effect on Achilles tendons in aging mice. We report that Met injection was able to reduce tendon inflammation and senescence, improve Achilles tendon degenerative changes by blocking HMGB1 translocation, and increase stem cell numbers in aging mouse tendon.
2. METHODS
2.1. Animals
All animal studies were performed according to the relevant guidelines and regulations. The protocol for animal use was approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Pittsburgh (IACUC protocol # 18083391). A total of 130 C57BL/6J female mice (Jackson Laboratory (Bar Harbor, ME)) were used.
2.2. Histochemical staining on structural and degenerative changes in mouse tendon
Achilles tendon tissue samples obtained from young (2.5 to 4.5 months old) and aging (14 to 19 months old) mice were fixed with 4% paraformaldehyde overnight at 4°C and were cut into 5 μm thick tissue sections. The tissue sections were examined for structural and degenerative changes with hematoxylin and eosin (H&E), Alcian Blue, Oil Red O, and Masson’s trichrome staining according to the standard protocols. The senescent cells in mouse tendon tissues were also examined on the fixed tissue sections with SA-β-gal staining kit (Cat. # 9860, Cell Signaling Technology, Danvers, MT) according to the manufacturer’s protocol. The stained tissue sections were examined under light microscope (Nikon eclipse, TE2000-U).
2.3. Picro Sirius red staining and polarized light microscopy of mouse tendon
The tissue sections of young and aging mouse Achilles tendons were cut into 5 μm thick tissue sections and stained with Picro Sirius red kit (Cat. #ab150681, Abcam, Cambridge, MA) according to the manufacturer’s protocol. Such stained tendon tissue sections were examined under polarized light microscope (Nikon).
2.4. Quantitative real-time RT-PCR (qRT-PCR)
Total RNA was extracted from 50 mg tendon tissues and qRT-PCR was carried out using QIAGEN QuantiTect SYBR Green PCR Kit (Qiagen, Valencia, CA) following our published protocol 20. The same mouse specific primers were used for PPARγ (adipocyte-related gene marker), SOX-9 (chondrocyte-related gene marker), Runx2 (osteocyte-related gene marker)’ and Collagen I (tenocyte-related gene marker) as described in our previous study 20. For collagen type II (chondrocyte-related gene marker) forward 5’-CAG GTG AAC CTG GAC GAG AG-3’ and reverse 5’-ACC ACG ATC TCC CTT GAC TC-3’ primers were used 21. For collagen III (scar tissue marker) forward 5’-TGC CCA CAG CCT TCT ACA CCT-3’ and reverse 5’-CCA GCTGGG CCT TTG ATA CCT-3’ primers were used 22. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as an internal control using the same primers as before 20. All primers were obtained from Invitrogen (Grand Island, NY). The PCR reaction conditions were essentially as described previously 20. The formula 2−ΔΔCT, where ΔΔCT= (CTtarget – CTGAPDH)aging – (CTtarget – CTGAPDH)young, was used to calculate the relative gene expression levels in tendon tissues. CT represents the cycle threshold of each RNA sample. At least three parallel tests were performed to determine standard deviation (SD) of the ΔCT.
2.5. Immunohistochemical analysis on mouse tendon
Mouse Achilles tendon tissues were fixed according to our published protocol 23. The sections were then incubated either with rabbit anti-mouse HMGB1 antibody (1:350, Cat. # ab18256; Abcam), or with rabbit anti-beta galactosidase (β-gal) antibody (1:350. Cat. #PA5–102503; ThermoFisher Scientific), or with rabbit anti-mouse CD68 antibody (1:500, Cat. # 125212; Abcam) at 4°C overnight. The next morning, the tissue sections were washed 3 times with PBS and incubated at room temperature for 2 hrs with Cy3-conjugated goat anti-rabbit IgG antibody (1:500, Cat. # AP132C; Millipore). Finally, all cells were stained with 4, 6-diamidino-2-phenylindole (DAPI) (1μg/ml, Sigma, St. Louis, MO). The results of stained tendon tissue sections were determined under a fluorescent microscope (Nikon, Eclipse TE2000U).
2.6. Tendon cell isolation and culture
Tendon cells were isolated from the Achilles tendons of young (2.5 months) and aging (18 months) mice according to our published protocol 24. The isolated cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Fisher Scientific, Hampton, NH). The cells in passages 1–3 were used for the experiments.
2.7. Immunostaining of HMGB1 and stem cell markers in mouse tendon cells
Achilles tendon cells isolated from young and aging mice at passage 1 were seeded into 12-well plates at the density of 3 × 104 cells/well and cultured for 5 days. After removing the medium, the cells were washed twice with PBS, and fixed with 4% paraformaldehyde-PBS solution for 30 min, then treated with 0.1% Triton X-100 in PBS for 30 min and washed with PBS for three times. The treated cells were incubated either with rabbit anti-mouse HMGB1 antibody (1:350, Cat. # Ab79823; Abcam), or with goat anti-NS (1:350, Cat. #GT15050, Neuromics, Edina, MN) overnight at 4°C. After incubation, the cells were washed with PBS for three times, and incubated with goat anti-rabbit secondary antibody conjugated with Cy3 for 1 hr at room temperature for HMGB1 testing (1:500, Cat. # AP132C; Millipore, Billerica, MA), and Cy3-conjugated donkey anti-goat IgG antibody for 1 hr at room temperature to detect nucleostemin (NS) (1:500, Cat. #AC180C; Millipore, Billerica, MA). For stage-specific embryonic antigen-1 (SSEA-1) staining, the fixed cells were incubated with 2% mouse serum at room temperature for 30 min, and then incubated with FITC-conjugated mouse anti-SSEA-1 antibody at room temperature for 3 hrs (1:250, Cat. #MAB4301X; Chemicon International, Temecula, CA). Finally, the cells were counterstained with DAPI, and the stained cells were analyzed under a fluorescent microscope (Nikon, Eclipse TE2000U).
2.8. Assessment of the effects of blocking HMGB1 by Met on aging mouse tendon cells
Achilles tendon cells isolated from aging mice (18 months old) at passage 2 were seeded into 12-well plates at the density of 3.5 × 104 cell/well, and cultured for 24 hrs. Next day, the medium was changed by adding various concentrations of Met (0–1 mg/ml) into each well, and further cultured for 5 days. Then, the medium was removed, the cells were washed twice with PBS. The washed cells were used either for immunostaining or Western blot analysis.
For immunostaining, the washed cells were stained either with SA-β-gal staining kit (Cat. # 9860, Cell Signaling Technology, Danvers, MT), or with rabbit anti-mouse HMGB1, or with goat anti-NS or with rabbit anti-CD73 antibody (1:500, Cat. #13160; Cell Signaling Technology) overnight at 4°C. Next morning, the cells were washed with PBS for 3 times, and incubated either with Cy3-conjugated goat anti-rabbit secondary antibody to detect HMGB1 or CD73, or with Cy3-conjugated donkey anti-goat IgG antibody at room temperature for 1 hr to detect NS. Finally, the cells were counterstained with DAPI, and the stained cells were analyzed under a fluorescent microscope (Nikon, Eclipse TE2000U).
2.9. Assessment of the effects of blocking HMGB1 by Met in aging mouse tendon
C57BL/6J mice (female, 19 months old) were divided into two groups with 6 mice per group. The mice in Group-1 received daily intraperitoneal (IP) injection of Met (50 mg/kg body weight/day, 100 μl of 10 mg/ml metformin/mouse/day) for 8 weeks 23; 25. The mice in Group-2 received daily IP injection of 0.9% saline (100 μl/mouse/day) for 8 weeks. After treatments, mice in both groups were sacrificed, and the Achilles tendons were harvested. For histological analysis, three Achilles tendons were used, whereas nine Achilles tendons from each group were used for Western blot analysis.
2.10. Western blot for analyzing HMGB1, CD68, p53, p16, and CCN1
Aging mouse Achilles tendon cells cultured with various concentrations of Met for 5 days described above were used to determine HMGB1 expression using Western blot. After removing the medium, the cells were washed with PBS, then 100 μl of RIPA buffer were added into each well of 12-well plate to extract cellular protein using standard procedures provided by the manufacturer (Sigma, St. Louis, MO, United States). On the other hand, mouse Achilles tendon tissues were first weighed and cut into small pieces. Then the tissue lysates were prepared with RIPA buffer. The protein concentrations in each sample (cells and tissues) were measured using a BCA Protein Assay Kit (Thermo Fisher Scientific, Pittsburgh, PA) to ensure equal loading. Western blot analysis was performed as described previously 17. The following primary antibodies were used: monoclonal rabbit anti-HMGB1 antibody (1:1000, Cat. # ab79823, Abcam), rabbit anti-CD68 antibody (1:500, ab283654 Abcam), rabbit anti-p53 antibody (1:1000, Cat. # sc-6243, Santa Cruz Biotechnology), rabbit anti-CCN1 antibody (1:500, Cat. # ABC102, Millipore Sigma), and rabbit anti-p16 antibody (1:500, Cat. # ab211542, Abcam). Following overnight incubation, the membranes were incubated with the corresponding secondary antibodies (1:15,000, LI-COR Biosciences) for 1 hr at room temperature. Following another three washes with TBS-T buffer, and the visualization of the protein bands on the blots was realized with LiCoR Odyssey imager (LI-COR Biosciences, Lincoln, NE). Finally, the membrane was incubated with anti-β-actin Alexa Fluor 680-conjugated antibody (1:1000, Cat. # sc-47778, Santa Cruz Biotechnology) at room temperature for 1 hr, and semi-quantification of the protein bands was done using the software provided by LiCoR Odyssey imager.
2.11. Semiquantification of positively stained tendon tissue sections and tendon cells
For semi-quantification of staining results on tendon tissue sections, three random images were taken from each tissue section under a microscope (Nikon Eclipse, TE2000-U). Using three sections from three mice for each group, a total of 9 images consisting of about 1000 cells were used for each group. The positively stained areas in tissue sections, which were manually identified by examining the images taken, were processed using SPOT imaging software (Diagnostic Instruments). The percentage of positive staining was calculated by dividing the positively stained area by total area viewed under the microscope and multiplying by 100. The values were averaged to represent the percentage positive staining in each group.
Similarly, to quantify staining results of cell markers (SA-β-gal, HMGB1, NS, SSEA-1, CD68, and CD73), three random images were taken from each well using the same equipment and software as described above. Three wells were used for each group, with a total of nine images (about 1000–1500 cells) being analyzed. The percentage of positive staining in each image was estimated by dividing the number of positively stained cells by the total number of cells counterstained with DAPI in the microscopic field and multiplying by 100. The final percentage of positive staining was obtained by averaging the values from all nine images.
2.12. Statistical analysis
GraphPad Prism (version 7.03) was used for data analysis. Statistical analysis was performed using one-way ANOVA, followed by Fisher’s PLSD test. P < 0.05 between two groups was considered to be significantly different.
3. RESULTS
3.1. Aging tendon exhibits degenerative changes
No degenerative changes were present in young tendon, which was minimally stained for proteoglycans (PG), and the cells displayed an elongated shape (Fig. 1A, B). In contrast, aging tendon had degenerative changes as evidenced by robust positive staining for PG with cell morphology changed to a round shape (Fig. 1C, D). The number of round-shaped cells in aging tendon were significantly higher (11.5 times) compared to young tendon (Fig. 1E). Also, while young tendon contained no lipids (Fig. 1F), aging tendon had robust presence of lipids (red areas in Fig. 1G). Semi-quantification confirmed the observations showing a 5.6-fold increase in lipids in aging tendon compared to young tendon (Fig. 1H). The results also show that young tendon was formed by dense collagen fibers and stained with red under bright light microscopy (Fig. 1I), however, the aging tendon was formed by loose collagen fibers and stained with yellow under bright light microscopy (Fig. 1K). Moreover, young tendon contained high levels of collagen type I stained with red color (Fig. 1J). However, high levels of collagen type III were found in aging tendon (green in Fig. 1L). Semi-quantification showed 62% of the collagen III in aging tendon (Fig. 1M), but only 5.7% in young tendon (Fig. 1M).
Fig. 1. Aging tendon exhibits degenerative changes.
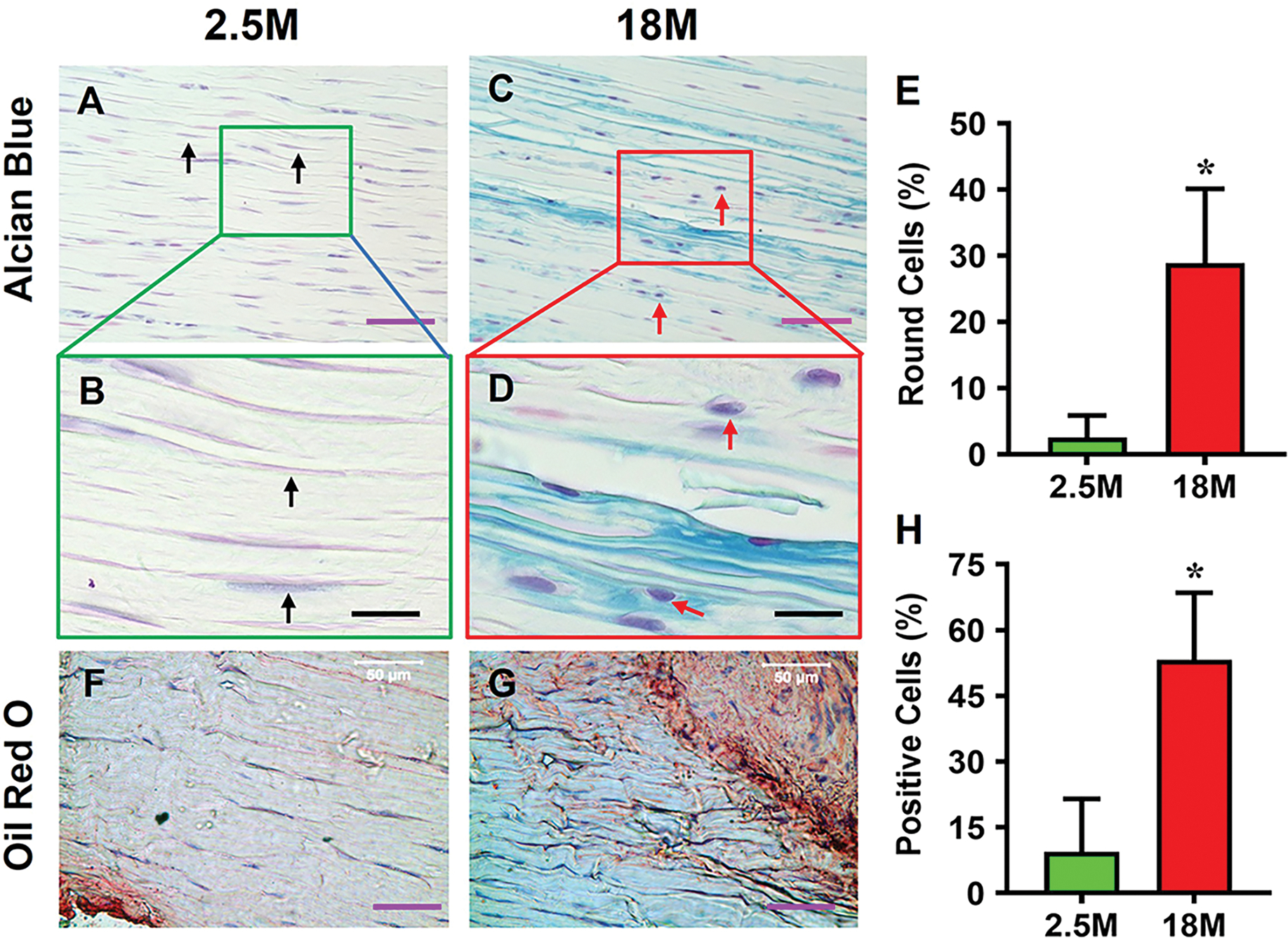
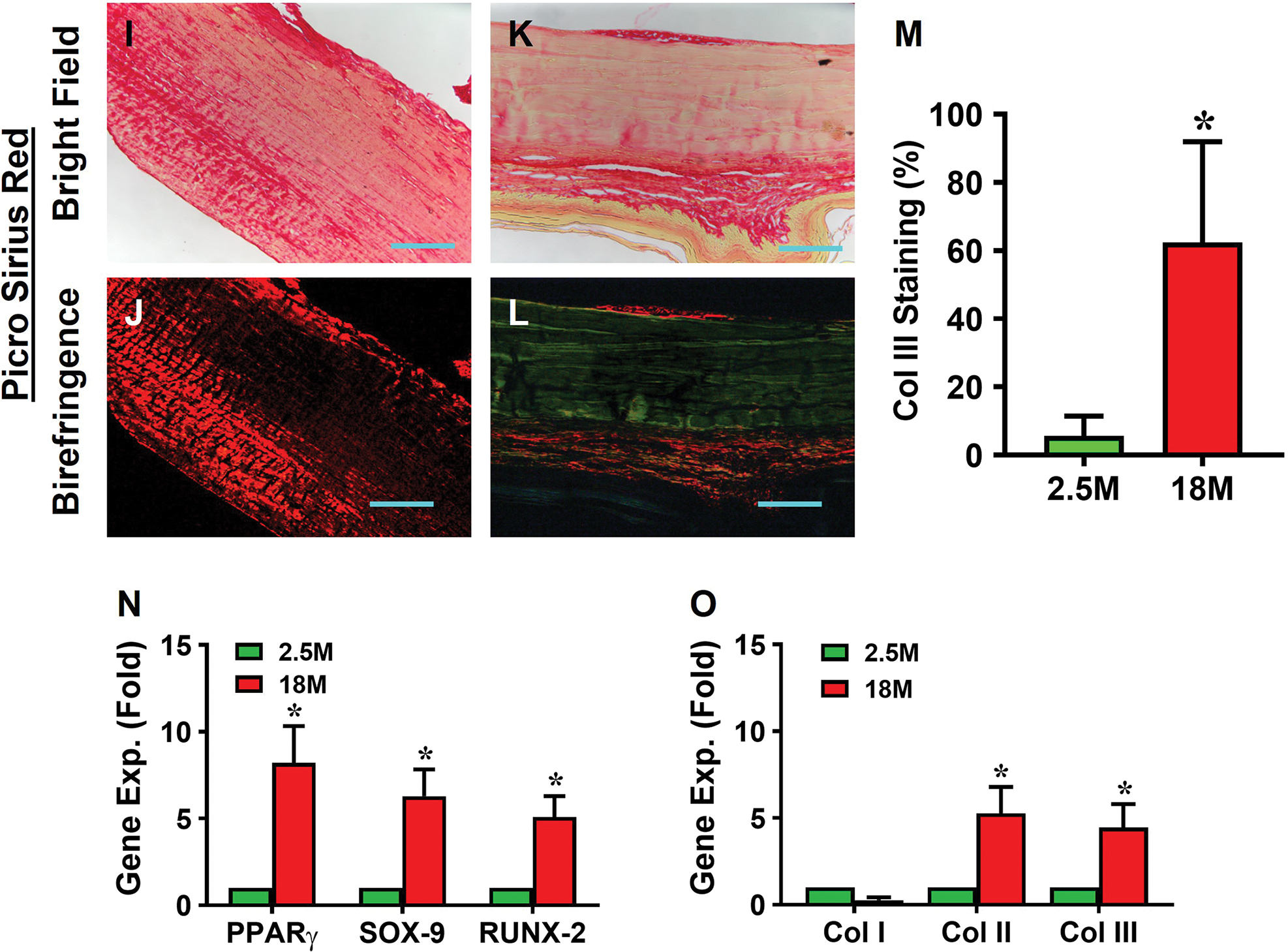
Histochemical staining for proteoglycans (PG) by Alcian blue shows minimal staining for PG in young tendon, and the cells exhibit an elongated morphology (A, B, black arrows). In contrast, aging tendon shows robust presence of PG along with round shaped cells (C, D, red arrows). Semi-quantification shows a significantly higher number of round cells in aging tendon compared to young tendon, with more than 28% round cells in aging tendon vs 2% cells in young tendon I. Similarly, young tendon does not show the presence of lipids by Oil Red O staining (F), while aging tendon has extensive lipid staining (G, red area). Aging tendon has significantly greater lipid staining compared to young tendon, with 53% staining in aging vs 9% in young tendon as shown by semi-quantification (H). Picro Sirius Red staining shows that young tendon under light microscope is formed by strong collagen fibers (red in I), while aging tendon under light microscope is formed by loose collagen fibers (yellow in K). Polarized light microscopy results indicate that the thick collagen fibers in young tendon are formed by collagen type I (red/yellow in J), while the loose collagen fibers in aging tendon are formed by collagen type III (green in L). Semi-quantification shows 62% of collagen fibers in aging tendon are collagen III, but 5.7% of collagen fibers in young tendon are collagen III (M). Gene analysis by qRT-PCR shows significantly decreased expression of collagen I (Col I) for tendon-related gene marker, and increased expression of non-tenocyte-related genes, PPARγ for adipocytes, SOX-9 and collagen II (Col II) for chondrocytes, Runx-2 for osteocytes, and collagen III (Col III) for scar tissue in aging tendon compared to those in young tendon (N, O). Purple bars: 50 μm; Black bars: 12.5 μm; Blue bars: 100 μm. *p < 0.05 (aging compared to young).
These results were further corroborated by expression of non-tenocyte-related genes including PPARγ, SOX-9, and Runx-2; specifically, the expression levels of PPARγ, SOX-9, and Runx-2 genes in aging tendon were 8.2, 6.3, and 5.1 times higher than their counterparts in the young tendon (Fig. 1N). The expression levels of collagen I in young tendon were 3.7 times higher than in the aging tendon (Fig. 1O). The gene levels of collagen II and collagen III in aging tendons were 5.3 and 4.5 times higher than young tendons (Fig. 1O).
3.2. Aging tendon exhibits senescence-associated secretory phenotype (SASP)
Aging mouse tendon expressed SASP. Senescent marker, SA-β-gal, was not detected in young tendon either by histochemical staining (Fig. 2A–D) or by immunostaining (Fig. 2F–I); however, senescent cells accumulated within aging mouse tendon as shown by the robust expression of SA-β-gal (Fig. 2C, D; H, I) by the two staining methods. Compared to young tendon, aging tendon showed significantly higher SA-β-gal staining (53% vs. 5%, Fig. 2E; 66% vs. 7%, Fig. 2J). Also, in young tendon, CD68, an inflammatory macrophage marker, was minimally stained (Fig. 2K, L), but it was extensively present in aging tendon (Fig. 2M, N, brown). Indeed, as shown by semi-quantification, more than 56% of the cells in aging tendon, as opposed to 7.5% in young tendon, were positively stained for CD68 (Fig. 2O). Furthermore, additional senescent markers, p53 and p16, were expressed at a much higher level in aging tendon compared to young tendon; in fact, p16 expression was nearly absent in young tendon but highly expressed in aging tendon (Fig. 2P).
Fig. 2. Senescent cells are present in aging tendon.
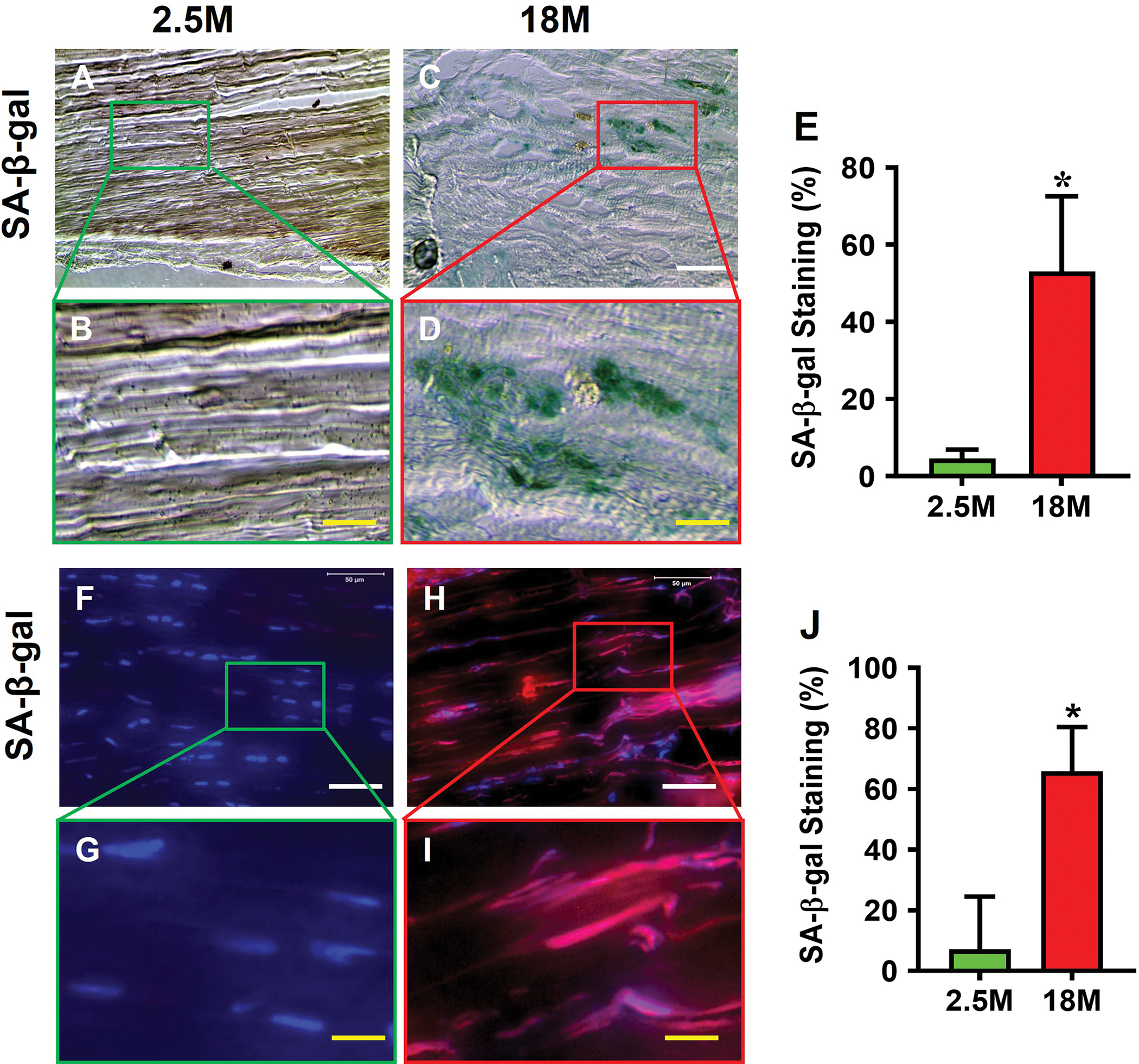
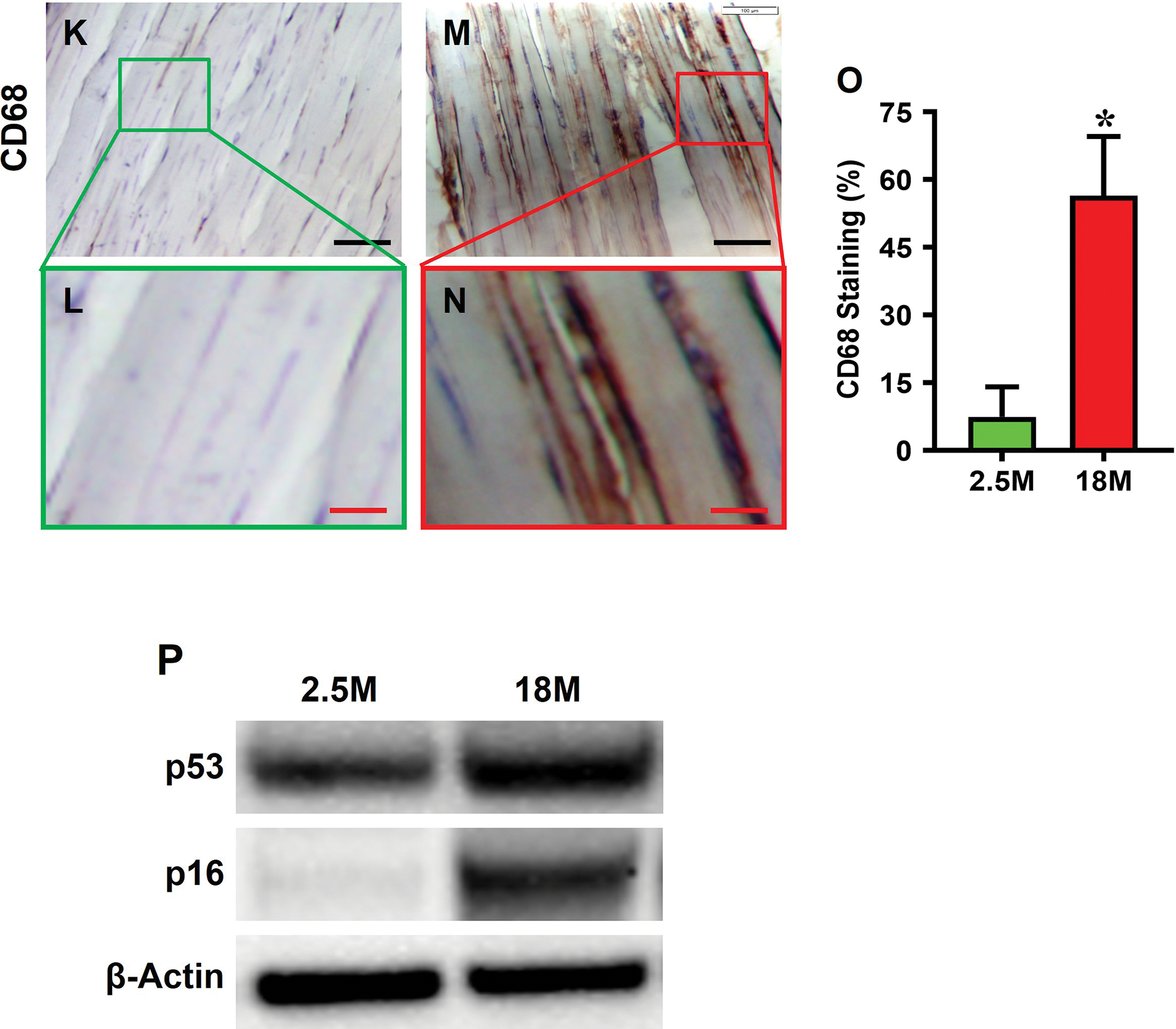
Histochemical (top panel) and immunostaining (bottom panel) show that few cells are positively stained with SA-β-gal in young tendon (A, B, F, G), but abundant staining is evident in aging tendon (green in C, D and red in H, I), which are confirmed by semi-quantification (E, J). Immunostaining for CD68 on young tendon tissue section shows minimal staining (K, L), but aging tendon tissue section exhibits abundant positive staining (M, N, brown), which are confirmed by semi-quantification showing 56.5% positive staining in aging vs 7.5% in young tendon (O). Western blot results show that the expression of senescent cell markers p53 and p16 are greatly increased in aging tendon compared to young tendon, which shows nearly no expression of p16 (P). Black bars: 100 μm; White bars: 50 μm; Red bars: 25 μm; Yellow bars: 12.5 μm; *p < 0.01 (aging compared to young).
3.3. HMGB1 is translocated from the nucleus to cytoplasm and extracellular matrix in aging tendon
HMGB1 was predominantly within the nuclei in young tendon tissue (red fluorescence in Fig. 3A, B), but in aging tendon tissue, HMGB1 was translocated from nuclei to the cytoplasm (Fig. 3C, D). Specifically, 91% of the cells in young tendon had HMGB1 within their cell nuclei; however, 15% of the cells in aging tendon had HMGB1 within the nuclei (Fig. 3E). Similarly, cells isolated from young tendon showed the presence of HMGB1 within their nuclei (Fig. 3F, G). But HMGB1 was translocated to the cytoplasm in aging tendon cells (Fig. 3H, I). Semi-quantification showed that 93% of the cells isolated from young tendon contained HMGB1 in the nuclei, but only 33% of the cells isolated in aging tendon had HMGB1 in the nuclei (Fig. 3J).
Fig. 3. HMGB1 is translocated from the nucleus to cytoplasm and extracellular matrix in aging tendon.
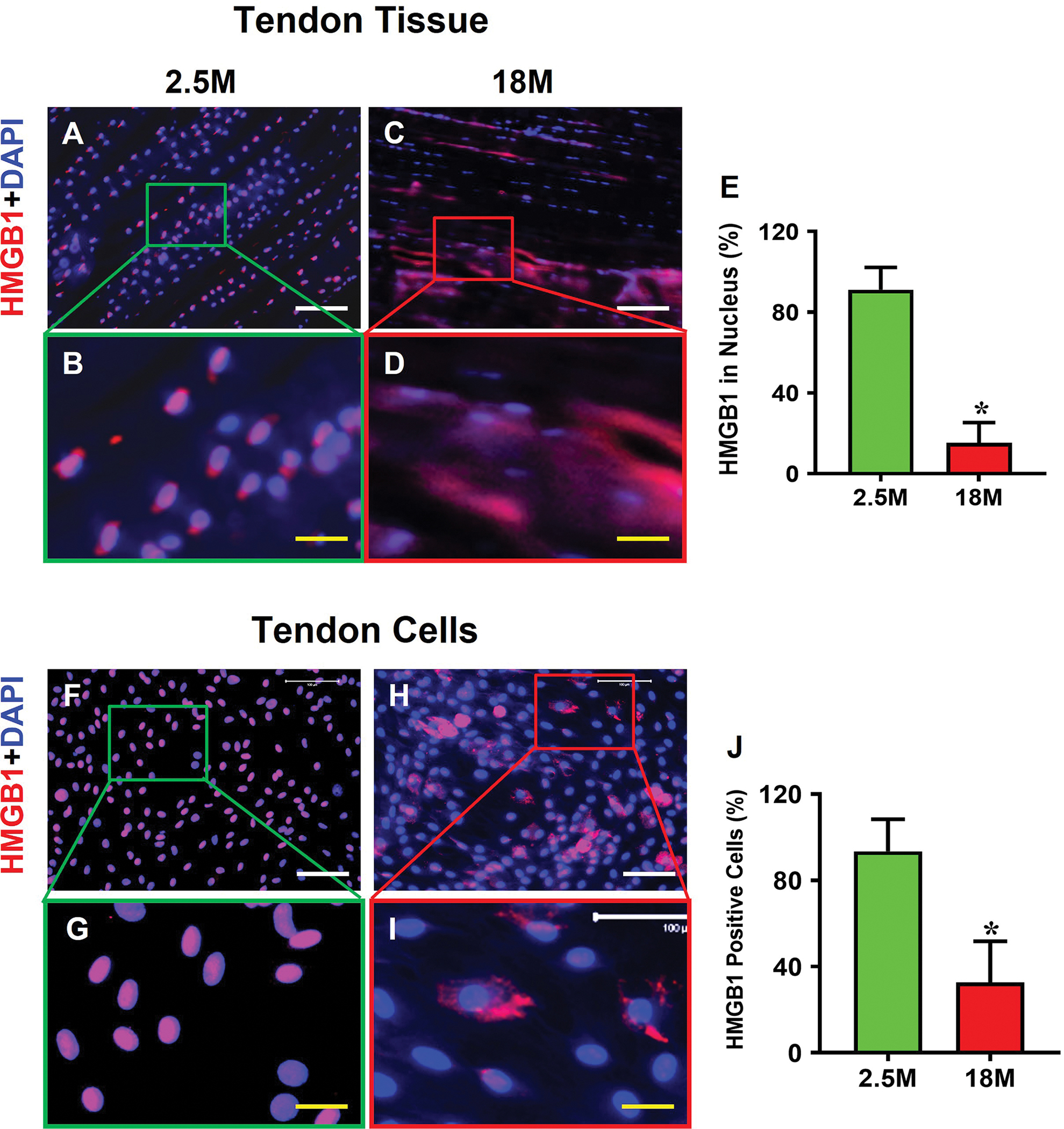
Immunofluorescence analysis on mouse tendon tissue sections shows that HMGB1 is present within the cell nuclei in young tendon (A, B). In contrast, HMGB1 in aging tendon is translocated to cytoplasm (C, D). Semi-quantification results of tendon tissue sections indicate that 91% of the cells in young tendon have HMGB1 within the nuclei, but only 15% of the cells in aging tendon have HMGB1 in the cell nuclei (E). Similarly, isolated cells cultured from young tendon harbor HMGB1 within the cell nuclei (F, G), whereas the aging cells show HMGB1 staining in the cytoplasm (H, I). Semi-quantification confirms the results showing that 93% of the cells in young tendon cells have HMGB1 in the nuclei with 33% in aging tendon cell nuclei (J). White bars: 100 μm; Yellow bars: 25 μm, *p < 0.01 (aging compared to young).
3.4. Aging changes cell morphology, population doubling time (PDT), and stem cell number
The cells isolated from young tendon showed a cobblestone-like morphology (Fig. 4A, B), but the cells isolated from aging tendon had a pancake-like morphology (Fig. 4C, D). The young tendon cells proliferated 2.6 times faster than that of aging tendon cells as measured by PDT (Fig. 4E). Immunostaining results showed that more than 97% of young tendon cells were stem cells as evidenced by NS staining (Fig. 4F, G, J), but less than 27% of aging tendon cells were negatively stained with NS (Fig. 4H, I, J). Similarly, more than 69% of the young tendon cells (Fig. 4K, L, O) and less than 24% of the aging tendon cells were positively stained with SSEA-1 (Fig. 4M, N, O).
Fig. 4. Cell morphology, proliferation, and stem cell marker expression in young vs aging tendon.
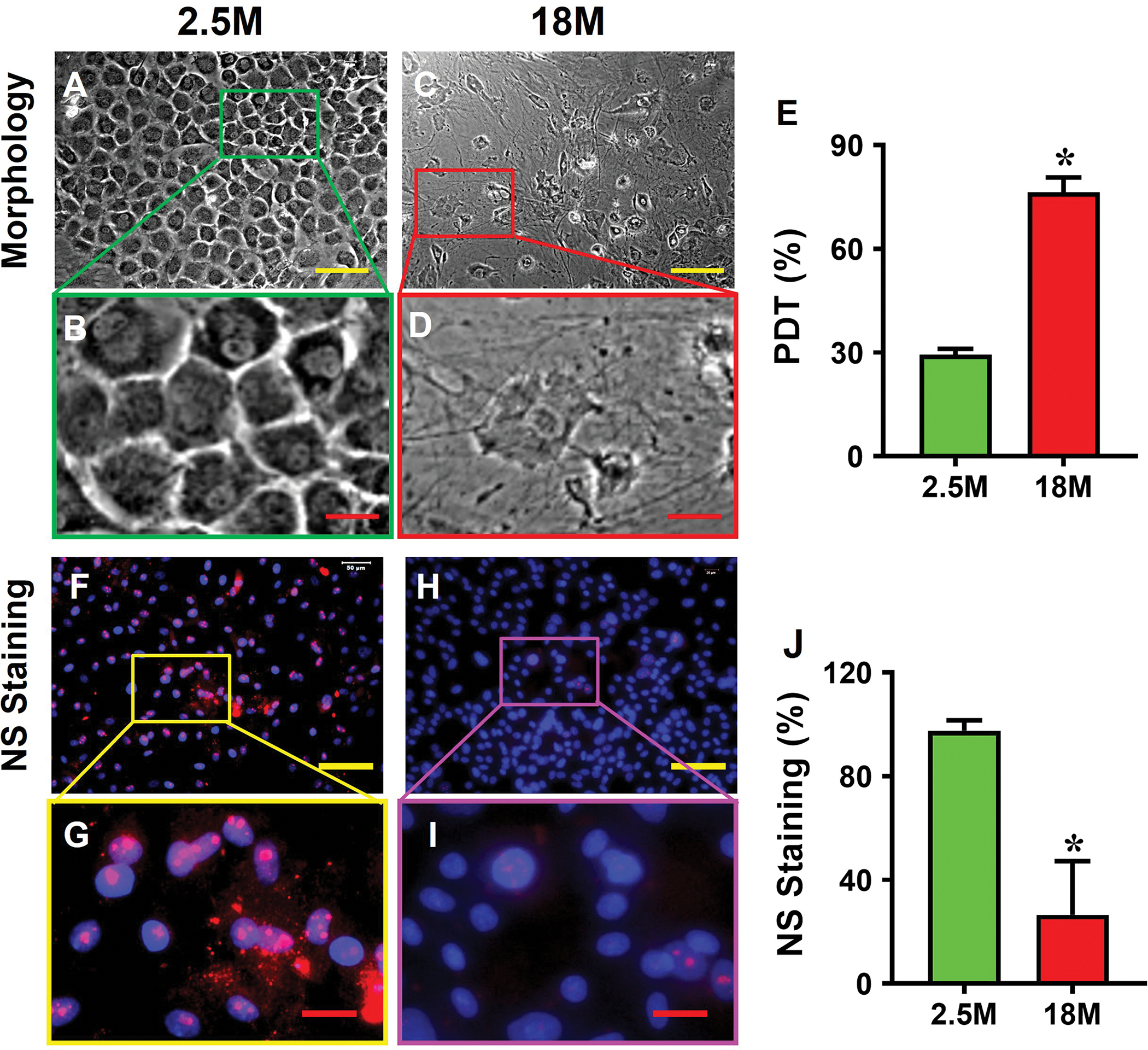
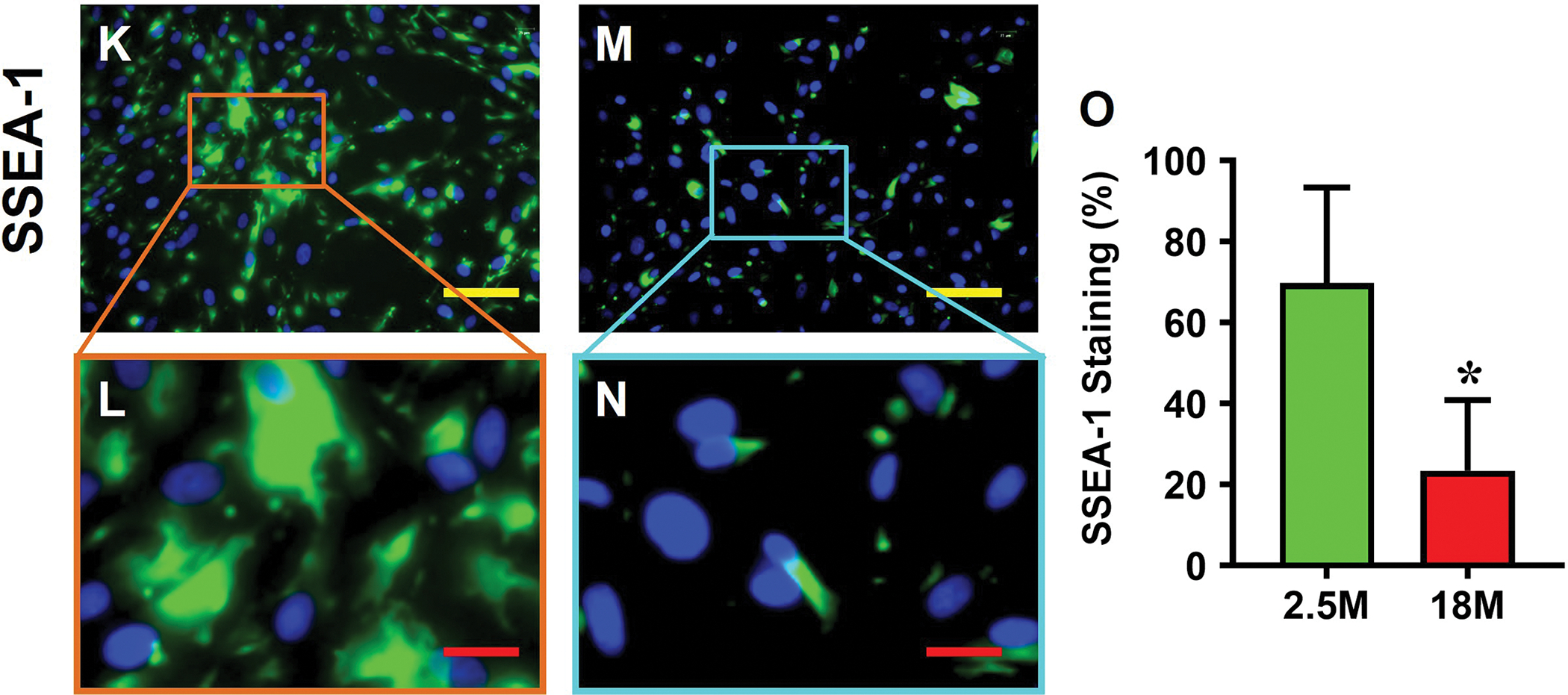
Young tendon cells are cobble-stone-like in shape (A, B), but the cells isolated from aging tendon are pancake-like in shape (C, D). PDT results show that the cells isolated from young tendon grow much faster than the cells isolated from aging tendon (E). Immunostaining for NS shows that more than 90% of the cells isolated from young mouse tendon tissues are positively stained with NS (F, G), but less than 27% of the cells isolated from aging tendon tissues are positively stained with NS (red in H, I). Similarly, more than 69% of the cells isolated from young tendon tissues are positively stained with SSEA-1 (green in K, L), but less than 24% of the aging tendon cells express SSEA-1 (green in M, N). These results are further confirmed by semi-quantification (J, O). Yellow bars: 100 μm; Red bars: 25 μm, *p < 0.01 (aging compared to young).
3.5. Met inhibits cell senescence and HMGB1 translocation in aging tendon
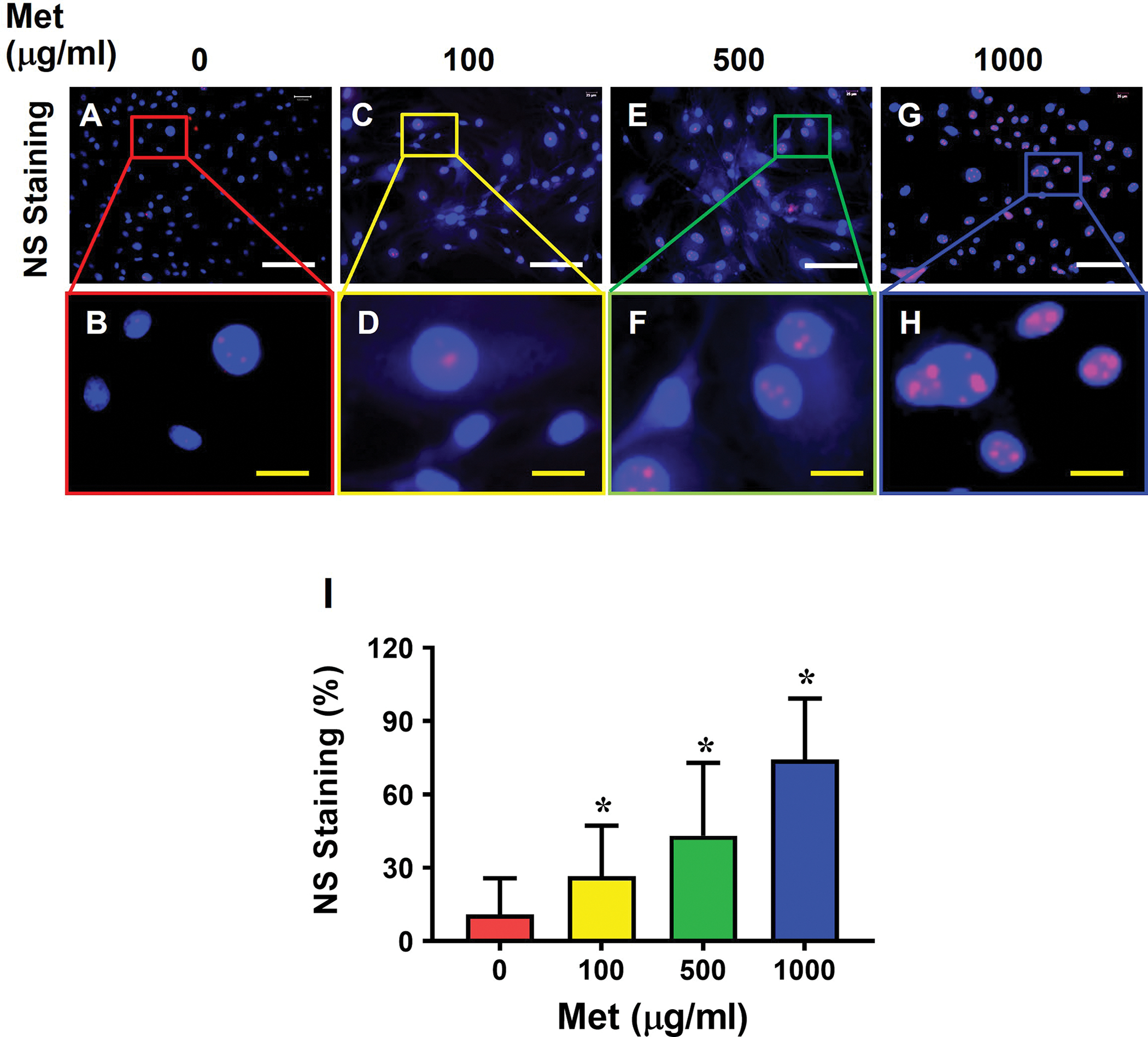
Most cells isolated from aging mouse tendon were positively stained for SA-β-gal (Fig. 5A, B). However, Met treatment decreased the number of the SA-β-gal positively stained cells in a Met concentration-dependent manner (Fig. 5C–H), which was confirmed by semi-quantification (Fig. 5I).
Fig. 5. Met treatment decreases the number of SA-β-gal positive cells from aging tendon.
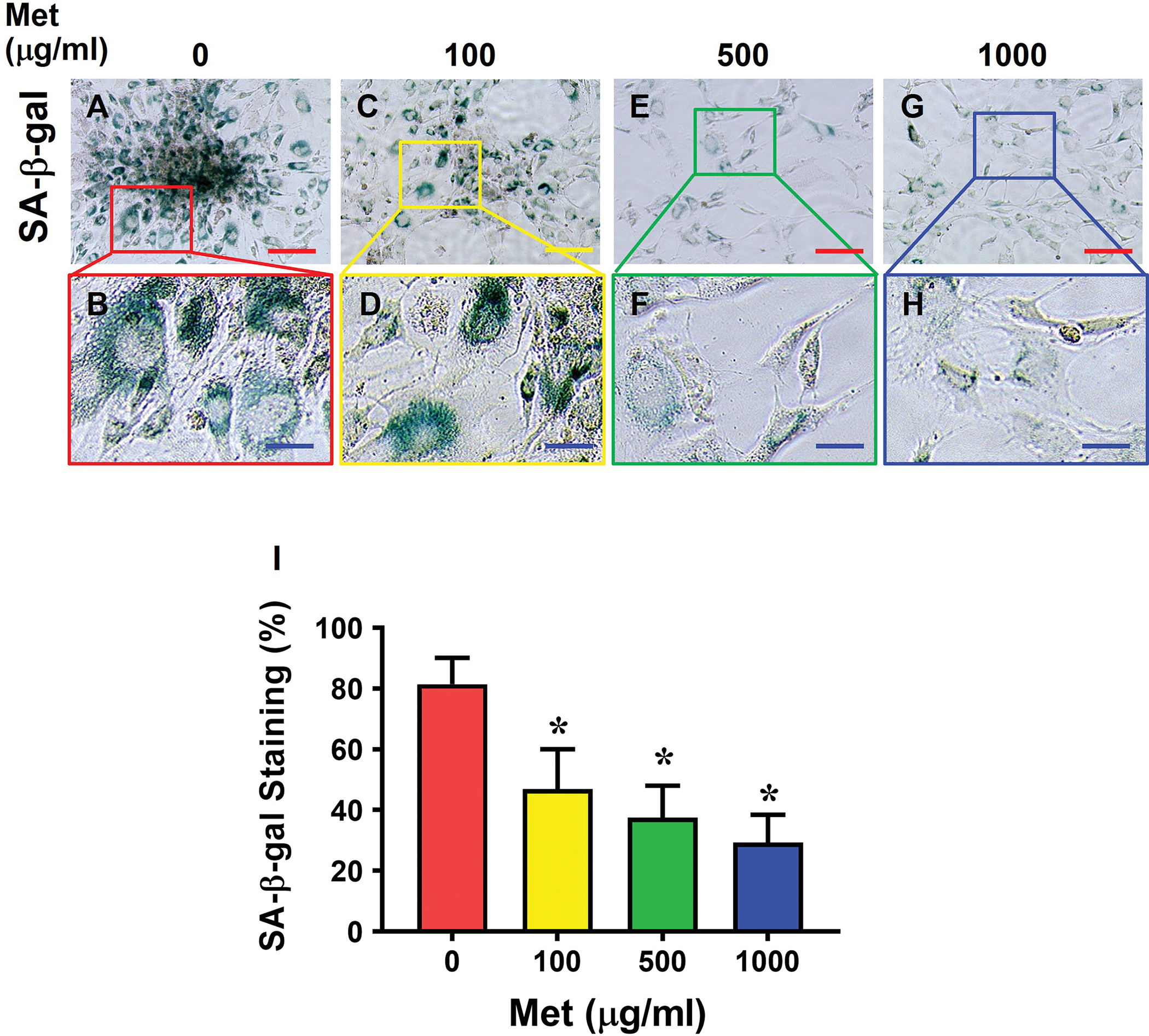
SA-β-gal staining is evident in control cells without Met treatment (A-B), but it decreases markedly in a Met concentration-dependent manner (C-H). Semi-quantification confirms the results (I). Red bars: 100 μm; Blue bars: 25 μm, *p < 0.01 (treatment groups compared to without Met).
Moreover, Met treatment also blocked the HMGB1 translocation in aging tendon cells in a concentration-dependent manner (Fig. 6A–I). Specifically, while most of HMGB1 was present in cytoplasm in aging tendon cells (Fig. 6A, B), Met treatment inhibited HMGB1 translocation (Fig. 6C–H). Also, nearly 75% of the cells had HMGB1 in the nuclei when treated at 1000 μg/ml Met (Fig. 6I). Moreover, Met treatment decreased dsHMGB1 levels and increased frHMGB1 levels in the cytoplasm (Fig, 6J). Additionally, Met treatment also enhanced stem cell numbers as the expression of stem cell markers NS (Fig. 7A–I) and CD73 (Fig. 7J–R) increased with the increase of the Met concentrations in the aging tendon cells.
Fig. 6. Met treatment inhibits HMGB1 translocation from the nuclei of the cells from aging tendon to the cytoplasm.
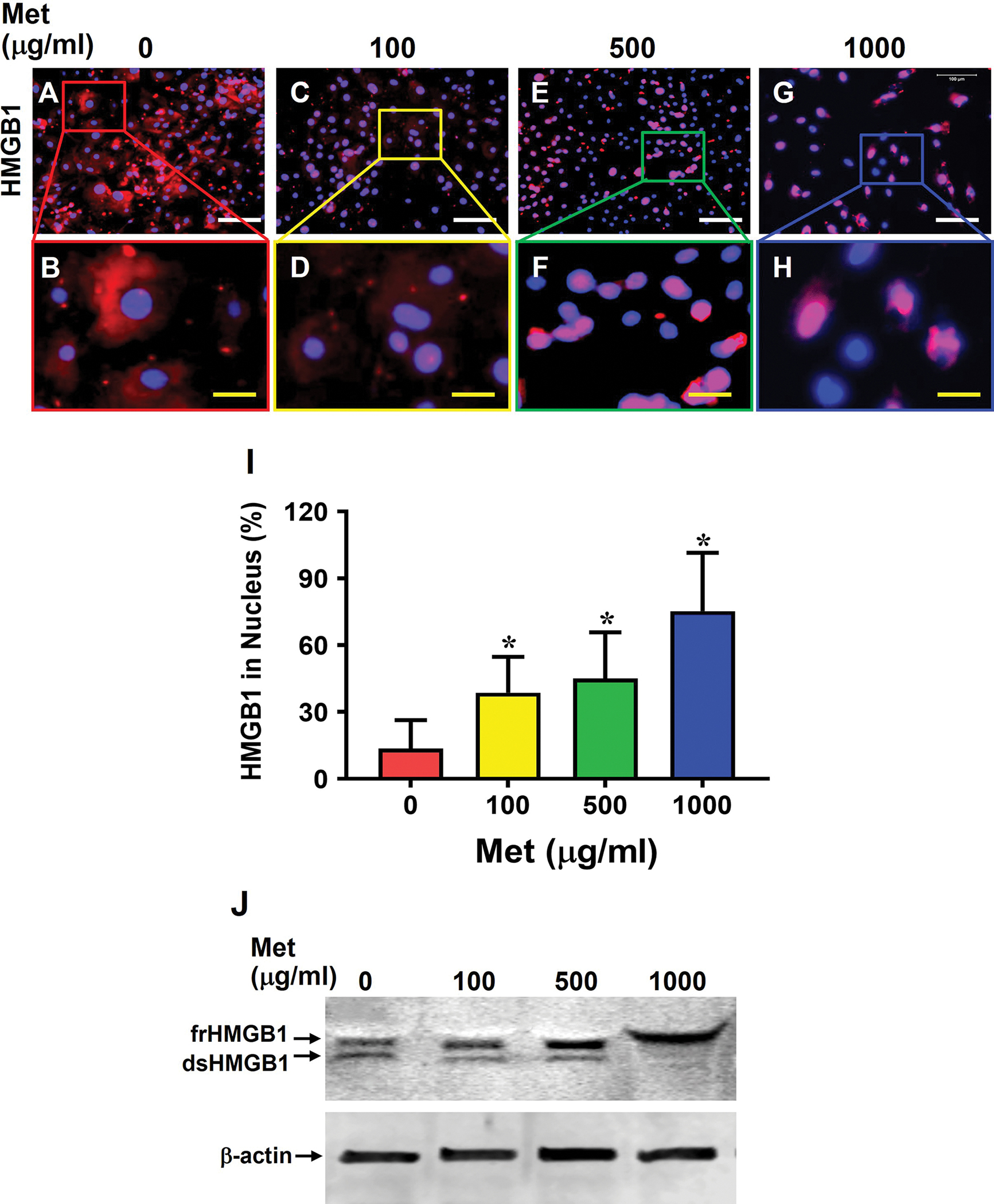
Most of HMGB1 in aging tendon cells without Met treatment (0) are in cytoplasm (A, B), but Met treatment inhibits HMGB1 translocation in a concentration-dependent manner, with the difference between 100 and 500 μg/ml Met not being significantly different(C-H). Semi-quantification confirms the results (I). Western blot result further shows that both frHMGB1 and dsHMGB1 exist in aging tendon cells, but Met treatment decreases dsHMGB1 levels and increases frHMGB1 levels in aging tendon cells in an apparent concentration-dependent manner (J). White bars: 100 μm; Yellow bars: 25 μm, *p < 0.01 (treatment groups compared to without Met).
Fig. 7. Met treatment increases stem cell numbers in aging tendon.
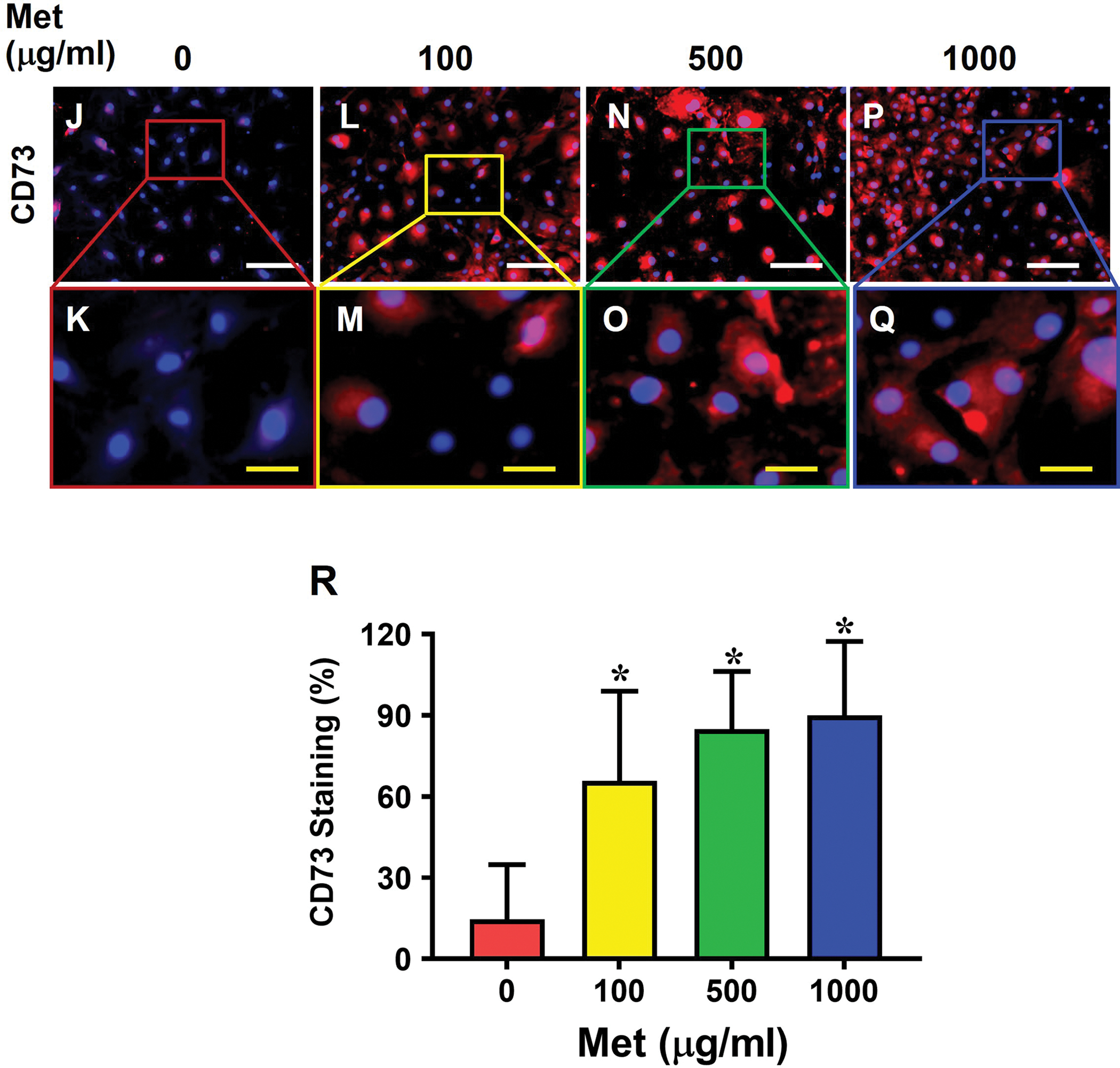
Cells from aging tendon without Met treatment show few NS positive cells (A, B), or CD73 positive cells (J, K), but the number of NS positively stained cells (C-H, I) increases in a Met concentration-dependent manner. However, the number of CD73 positively stained cells has no significant difference between 500 and 1000 μg/ml Met concentrations (N-Q, R). Semi-quantification confirms the results (I, R). White bars: 100 μm; Yellow bars: 20 μm, *p < 0.01 (treatment groups compared to without Met).
3.6. Met reduces the expression of inflammatory and senescent markers in aging tendon
The protein expression levels of both redox forms of HMGB1, or frHMGB1 and dsHMGB1, were markedly higher in 19 months old mouse tendon (19M) compared to 4 months old mouse tendon (4M) (Fig. 8A). There was a slight expression of frHMGB1 but almost no expression of dsHMGB1 in young tendon, while both were significantly higher in aging tendon. Met injection eliminated dsHMGB1 in aging tendon matrix completely and reduced frHMGB1 level close to that in young tendon. Similarly, the protein levels of inflammatory macrophage marker CD68, senescent cell marker CCN1 and p16 were increased in aging mouse tendon, while Met injection decreased their expression levels. Finally, Met injection significantly decreased the levels of both frHMGB1 and dsHMGB1 in aging tendons as determined by semi-quantification (Fig. 8B).
Fig. 8. IP injection of Met decreases HMGB1 levels and reduce inflammation and cellular senescence in aging tendon.
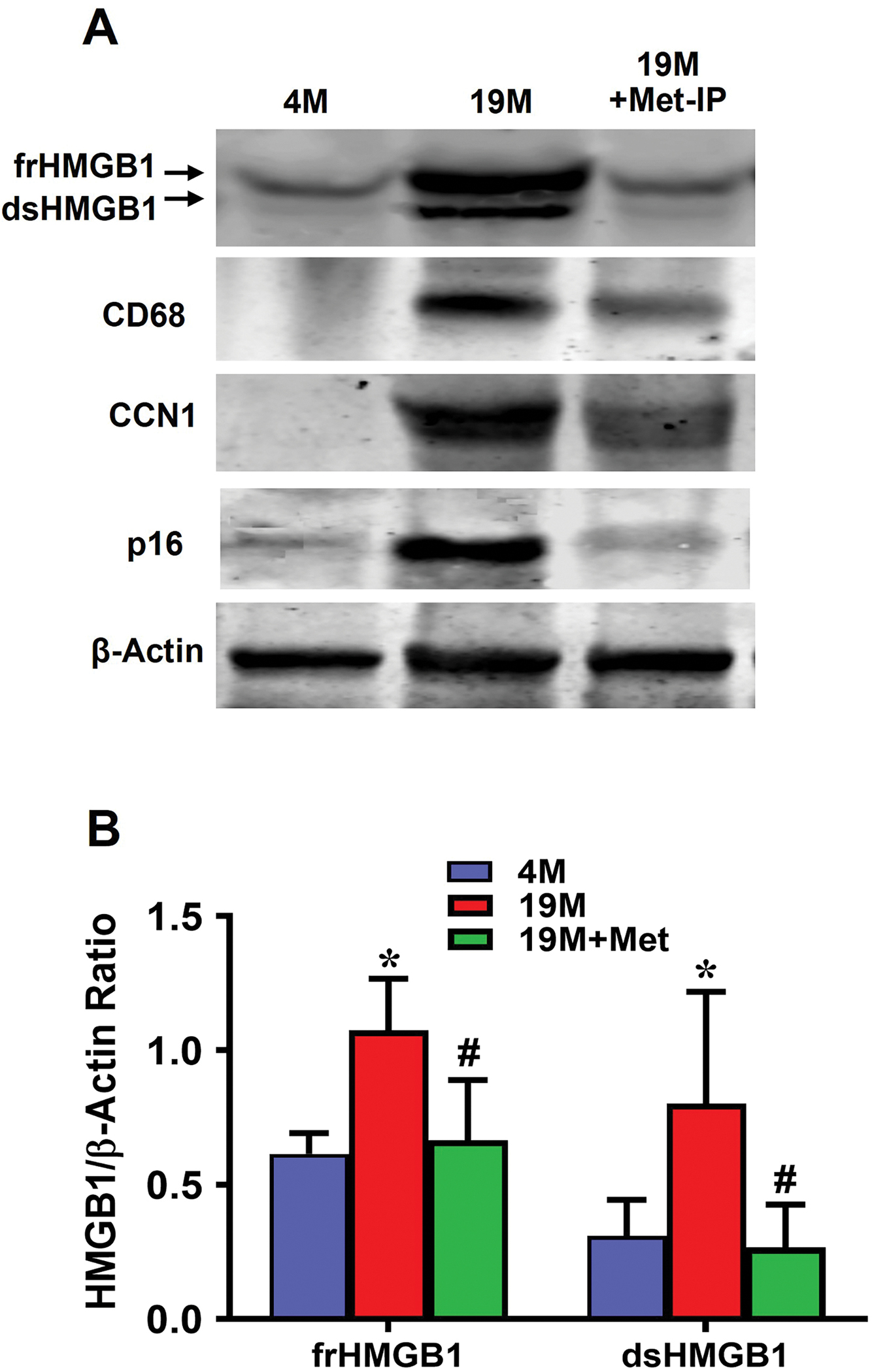
Western blot results show that both fr- and ds-HMGB1 are expressed in the extracellular matrix of aging mouse tendon, but Met treatment nearly eliminates dsHMGB1, reduces frHMGB1 to the level close to that of young tendon, decreases the expression of inflammatory macrophage marker CD68, and senescent markers CCN1 and p16 expression in aging tendon (A). Semi-quantification of the Western blot shows the increased levels of both frHMGB1 and dsHMGB1in aging tendon, but Met treatment decreases both frHMGB1 and dsHMGB1, with dsHMGB1 nearly absent (B). *p < 0.01 (19M compared to 4M); #p < 0.01 (19M+Met compared to 19M).
3.7. Met decreases degenerative changes in aging tendon
The cells in 4M mouse tendon had elongated shape, and the collagen fibers were well organized (Fig. 9A, B). However, many cells in 19M aging tendon were round shaped, and the collagen fibers were disorganized (Fig. 9C, D). Met injection decreased round cell numbers and increased elongated cells in aging tendon (Fig. 9E, F). The young tendon was formed by dense collagen fibers stained with all red by Masson trichrome staining (Fig. 9G, H); however, in aging tendon, blue staining was interspersed in the red staining areas with some round cells and surrounding collagen fibers being stained blue indicating degenerative changes (Fig. 9I, J). But, Met injection improved the structure of aging tendon as shown by less blue staining as compared to aging tendon (Fig. 9K, L).
Fig. 9. IP injection of Met decreases degenerative changes in aging tendon.
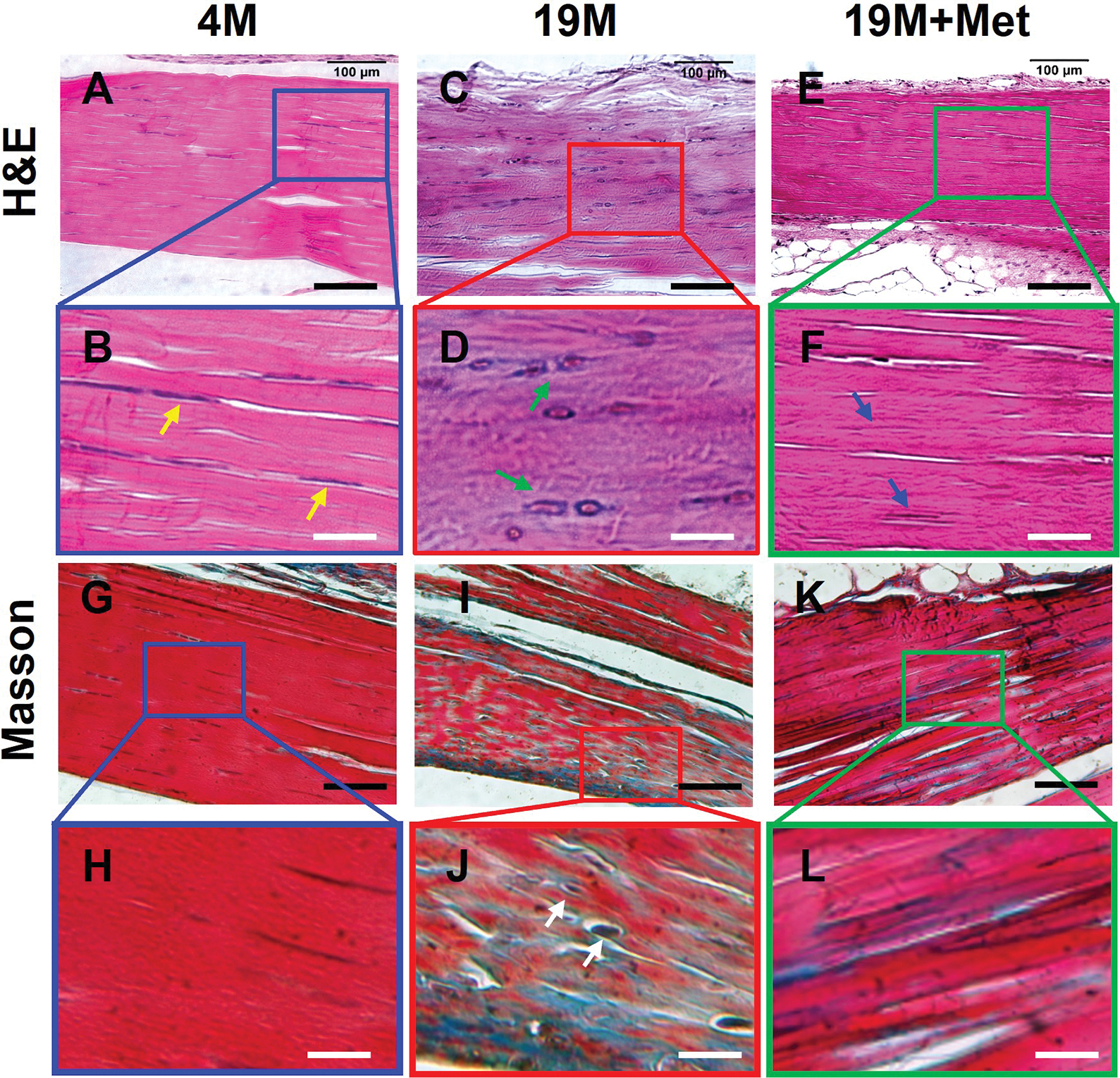
H&E staining on young mouse (4M) tendon tissue section shows that the cells are elongated in shape (B, yellow arrows), whereas cells in aging tendon (19M), are round shaped (D, green arrows). However, Met injection decreases the number of round shape cells in aging tendons (F, blue arrows). Masson trichrome staining on young tendon tissue sections shows that it is formed by dense collagen fibers all stained red (G, H), but the aging tendon has some blue staining interspersed across the tendon section, indicating loose collagen fibers with tendon cells (J, white arrows). However, IP injection of Met for 8 weeks decreases the presence of loose collagen fibers in aging tendon (K, L). Black bars: 100 μm; White bars: 25 μm.
4. DISCUSSION
This study showed that aging mouse Achilles tendon underwent inflammation and degeneration and contained senescent cells with fewer stem cells compared to normal young tendon. Met treatment reduced inflammatory marker protein expression of CD68 in vitro, decreased the expression of senescent cell markers SA-β-gal, CCN1 and p16, increased expression of stem cell markers NS, SSEA-1, and CD73, and finally reduced tendon degenerative changes in aging mouse Achilles tendon. In particular, Met reduced the expression of the pro-inflammatory dsHMGB1, which was present with an elevated level in aging tendon, but not in young tendon. Thus, these findings suggest that Met is anti-inflammatory and anti-senescent.
As shown by this study, unlike young tendon, aging tendon lost matrix integrity with accumulation of proteoglycans and lipids, decreased the levels of collagen I and increased the levels of collagen III, and elevated expression of non-tenocyte related genes that are markers of chondrocytes, adipocytes, and osteocytes. These results are in general consistent with previous findings showing that pathological tendons lose matrix integrity with increased production of proteoglycans and glycosaminoglycans 26; 27. Also, aging causes alterations in tendon cell morphology; Achilles tendon fibroblasts from young mice displayed normal elongated shape whereas cells had a round shape in aging mice 28. Our results agree with this finding.
This study showed that aging mice tendons contain senescent cells, marked by the high expression of senescent cell markers SA-β-gal, p53, and p16, and high-level expression of inflammatory macrophage marker, CD68. Previous studies demonstrated chronic inflammation and cellular senescence are pervasive features in aging that aged tendon stem/progenitor cells (TSCs) displayed cellular senescence, and the expression of p21 and p16 was higher in human aged/degenerated Achilles TSCs 29–31. Increase in the expression p16 was linked to the impairment of number and/or function of adult stem cells and abolishing p16 function enhanced regenerative potential of stem cells 32.
Our results also show that unlike young tendon, which retains HMGB1 in nuclei, HMGB1 in aging tendon was translocated to cytoplasm in culture and in tendon tissue. This finding is consistent with previous studies showing that nuclear HMGB1 is released to the extracellular milieu in senescent human and mouse fibroblasts in culture and in vivo 15. The translocated HMGB1 is in the oxidized state, i.e., dsHMGB1 that can initiate and sustain chronic inflammation 16. Therefore, inhibition of dsHMGB1 can reduce chronic inflammation 33, which is a feature of Achilles tendinopathy in symptomatic patients 34. We speculate that in aging mouse tendon, dsHMGB1 is responsible for the observed pathological changes such as inflammation and senescence. The senescent cells in aging tendons may secrete this inflammatory form of HMGB1, which may be responsible for inflammation in aging tendons, marked by inflammatory macrophages infiltration in this study. Recent studies also support the pathogenic role of HMGB1, presumably dsHMGB1, in tendinopathy by showing that HMGB1 is upregulated in tendinopathy patients, and the upregulation of HMGB1 is associated with inflammatory responses and ECM disorganization in rat rotator cuff tendon injury model 35–37. However, these studies did not perform further analysis to identify the distinct isoforms of HMGB1 (namely, frHMGB1 and dsHMGB1). Our findings in this study demonstrated that Met can reduce the amount of dsHMGB1 in aging tendon cells in vitro (Fig. 6) and in aging mouse tendon in vivo (Fig. 8). Our previous study showed that IP injection of Met into young mice prevents the tendinopathy development due to mechanical overloading/overuse by ITR 23. Others showed that Met decreases inflammatory response in rabbit stem/progenitor cells 38, protects against apoptosis and senescence in nucleus pulposus cells, and ameliorates disc degeneration in rat intervertebral disc degeneration (IDD) 39–42. Thus, dsHMGB1 may serve as a biomarker for tendon aging, and Met may be an effective therapeutic to target dsHMGB1 to reduce inflammation and senescence in aging-induced tendinopathy. Further research is however needed to confirm the inhibitory effect of Met on dsHMGB1 and its molecular mechanisms.
In this study, we used Masson trichrome (MT) staining to detect whether aging tendon is susceptive to disorganization and degenerative changes. This method is primarily used for distinguishing collagen fibers that are stained blue 43; 44. But our results showed that normal, young tendon was stained all red but in aging tendon, some blue staining was interspersed in red area. However, these results agree with our own and others’ previous studies, where normal tendon appeared uniformly red and degenerative pathological tendon stained completely blue by MT staining 45; 46. In MT staining, the dye penetration is associated with the tensional state of collagen fibers and hence the penetration of blue dye is easier in loose tendon tissue (or degenerated tendon) compared to normal dense tendon tissue. MT staining is recommended as a precise and quick method to distinguish between healthy and lesioned tendon 45.
In summary, aging tendon is marked by inflammation and degeneration, and cell senescence. Moreover, pro-inflammatory dsHMGB1 is released from tendon cells to ECM in aging tendon. Met treatment inhibits dsHMGB1 release, decreases tendon inflammation and the number of senescent cells, enhances the presence of stem cells in aging tendon, and finally improves the tendon structure compromised by aging. This study suggests that dsHMGB1 may serve as a biomarker of tendon aging and a therapeutic target by administration of Met to treat the aging-induced tendinopathy.
Clinical significance:
Metformin may be used as a therapeutic to aging-induced tendinopathy in clinics
ACKNOWLEDGENENTS:
This work was supported in part by the NIH AR070340, and Pittsburgh Foundation Awards (AD2021–120108, and AD2021–120112). The authors declare do no conflict of interests. We thank Dr. Bhavani P. Thampatty for her assistance in the preparation of the manuscript.
REFERENCES
- 1.Colby SL, Ortman JM. 2015. Projections of the Size and Composition of the U.S. Population: 2014 to 2060. In: Commerce editor; pp. 1–13. [Google Scholar]
- 2.Dean BJF, Dakin SG, Millar NL, et al. 2017. Review: Emerging concepts in the pathogenesis of tendinopathy. Surgeon 15:349–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou B, Zhou Y, Tang K. 2014. An overview of structure, mechanical properties, and treatment for age-related tendinopathy. J Nutr Health Aging 18:441–448. [DOI] [PubMed] [Google Scholar]
- 4.Albers IS, Zwerver J, Diercks RL, et al. 2016. Incidence and prevalence of lower extremity tendinopathy in a Dutch general practice population: a cross sectional study. BMC Musculoskelet Disord 17:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lui PPY, Wong CM. 2019. Biology of Tendon Stem Cells and Tendon in Aging. Front Genet 10:1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lipman K, Wang C, Ting K, et al. 2018. Tendinopathy: injury, repair, and current exploration. Drug Des Devel Ther 12:591–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barzilai N, Crandall JP, Kritchevsky SB, et al. 2016. Metformin as a Tool to Target Aging. Cell metabolism 23:1060–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cameron AR, Morrison VL, Levin D, et al. 2016. Anti-Inflammatory Effects of Metformin Irrespective of Diabetes Status. Circ Res 119:652–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kulkarni AS, Gubbi S, Barzilai N. 2020. Benefits of Metformin in Attenuating the Hallmarks of Aging. Cell metabolism 32:15–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dimri GP, Lee X, Basile G, et al. 1995. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A 92:9363–9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Campisi J 2013. Aging, cellular senescence, and cancer. Annu Rev Physiol 75:685–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jun JI, Lau LF. 2010. The matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing. Nat Cell Biol 12:676–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Freund A, Orjalo AV, Desprez PY, et al. 2010. Inflammatory networks during cellular senescence: causes and consequences. Trends Mol Med 16:238–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim DE, Davalos AR. 2019. Alarmin Detection in Senescent Cells. Methods Mol Biol 1896:71–81. [DOI] [PubMed] [Google Scholar]
- 15.Davalos AR, Kawahara M, Malhotra GK, et al. 2013. p53-dependent release of Alarmin HMGB1 is a central mediator of senescent phenotypes. J Cell Biol 201:613–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Venereau E, Casalgrandi M, Schiraldi M, et al. 2012. Mutually exclusive redox forms of HMGB1 promote cell recruitment or proinflammatory cytokine release. The Journal of experimental medicine 209:1519–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao G, Zhang J, Nie D, et al. 2019. HMGB1 mediates the development of tendinopathy due to mechanical overloading. PloS one 14:e0222369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Horiuchi T, Sakata N, Narumi Y, et al. 2017. Metformin directly binds the alarmin HMGB1 and inhibits its proinflammatory activity. The Journal of biological chemistry 292:8436–8446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohammed I, Hollenberg MD, Ding H, et al. 2021. A Critical Review of the Evidence That Metformin Is a Putative Anti-Aging Drug That Enhances Healthspan and Extends Lifespan. Front Endocrinol (Lausanne) 12:718942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang J, Wang JH. 2013. The effects of mechanical loading on tendons--an in vivo and in vitro model study. PloS one 8:e71740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Perrier-Groult E, Pasdeloup M, Malbouyres M, et al. 2013. Control of collagen production in mouse chondrocytes by using a combination of bone morphogenetic protein-2 and small interfering RNA targeting Col1a1 for hydrogel-based tissue-engineered cartilage. Tissue Eng Part C Methods 19:652–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Min LJ, Cui TX, Yahata Y, et al. 2004. Regulation of collagen synthesis in mouse skin fibroblasts by distinct angiotensin II receptor subtypes. Endocrinology 145:253–260. [DOI] [PubMed] [Google Scholar]
- 23.Zhang J, Li F, Nie D, et al. 2020. Effect of Metformin on Development of Tendinopathy Due to Mechanical Overloading in an Animal Model. Foot & ankle international 41:1455–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang J, Pan T, Liu Y, et al. 2010. Mouse treadmill running enhances tendons by expanding the pool of tendon stem cells (TSCs) and TSC-related cellular production of collagen. Journal of orthopaedic research : official publication of the Orthopaedic Research Society 28:1178–1183. [DOI] [PubMed] [Google Scholar]
- 25.Kisfalvi K, Moro A, Sinnett-Smith J, et al. 2013. Metformin inhibits the growth of human pancreatic cancer xenografts. Pancreas 42:781–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Riley GP. 2005. Gene expression and matrix turnover in overused and damaged tendons. Scand J Med Sci Sports 15:241–251. [DOI] [PubMed] [Google Scholar]
- 27.Xu Y, Murrell GA. 2008. The basic science of tendinopathy. Clin Orthop Relat Res 466:1528–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arnesen SM, Lawson MA. 2006. Age-related changes in focal adhesions lead to altered cell behavior in tendon fibroblasts. Mech Ageing Dev 127:726–732. [DOI] [PubMed] [Google Scholar]
- 29.Kohler J, Popov C, Klotz B, et al. 2013. Uncovering the cellular and molecular changes in tendon stem/progenitor cells attributed to tendon aging and degeneration. Aging Cell 12:988–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Han W, Wang B, Liu J, et al. 2017. The p16/miR-217/EGR1 pathway modulates age-related tenogenic differentiation in tendon stem/progenitor cells. Acta Biochim Biophys Sin (Shanghai) 49:1015–1021. [DOI] [PubMed] [Google Scholar]
- 31.Hu C, Zhang Y, Tang K, et al. 2017. Downregulation of CITED2 contributes to TGFbeta-mediated senescence of tendon-derived stem cells. Cell Tissue Res 368:93–104. [DOI] [PubMed] [Google Scholar]
- 32.Janzen V, Forkert R, Fleming HE, et al. 2006. Stem-cell ageing modified by the cyclin-dependent kinase inhibitor p16INK4a. Nature 443:421–426. [DOI] [PubMed] [Google Scholar]
- 33.Yang H, Wang H, Andersson U. 2020. Targeting Inflammation Driven by HMGB1. Frontiers in immunology 11:484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dakin SG, Newton J, Martinez FO, et al. 2018. Chronic inflammation is a feature of Achilles tendinopathy and rupture. Br J Sports Med 52:359–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akbar M, Gilchrist DS, Kitson SM, et al. 2017. Targeting danger molecules in tendinopathy: the HMGB1/TLR4 axis. RMD Open 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mosca MJ, Carr AJ, Snelling SJB, et al. 2017. Differential expression of alarmins-S100A9, IL-33, HMGB1 and HIF-1alpha in supraspinatus tendinopathy before and after treatment. BMJ Open Sport Exerc Med 3:e000225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thankam FG, Roesch ZK, Dilisio MF, et al. 2018. Association of Inflammatory Responses and ECM Disorganization with HMGB1 Upregulation and NLRP3 Inflammasome Activation in the Injured Rotator Cuff Tendon. Sci Rep 8:8918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Han Y, Yuan F, Deng C, et al. 2019. Metformin decreases LPS-induced inflammatory response in rabbit annulus fibrosus stem/progenitor cells by blocking HMGB1 release. Aging (Albany NY) 11:10252–10265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuang Y, Hu B, Feng G, et al. 2020. Metformin prevents against oxidative stress-induced senescence in human periodontal ligament cells. Biogerontology 21:13–27. [DOI] [PubMed] [Google Scholar]
- 40.Le Pelletier L, Mantecon M, Gorwood J, et al. 2021. Metformin alleviates stress-induced cellular senescence of aging human adipose stromal cells and the ensuing adipocyte dysfunction. Elife 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fang J, Yang J, Wu X, et al. 2018. Metformin alleviates human cellular aging by upregulating the endoplasmic reticulum glutathione peroxidase 7. Aging Cell 17:e12765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen D, Xia D, Pan Z, et al. 2016. Metformin protects against apoptosis and senescence in nucleus pulposus cells and ameliorates disc degeneration in vivo. Cell Death Dis 7:e2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kinoshita H, Umezawa T, Omine Y, et al. 2013. Distribution of elastic fibers in the head and neck: a histological study using late-stage human fetuses. Anat Cell Biol 46:39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kwon SY, Chung JW, Park HJ, et al. 2014. Silk and collagen scaffolds for tendon reconstruction. Proc Inst Mech Eng H 228:388–396. [DOI] [PubMed] [Google Scholar]
- 45.Martinello T, Pascoli F, Caporale G, et al. 2015. Might the Masson trichrome stain be considered a useful method for categorizing experimental tendon lesions? Histol Histopathol 30:963–969. [DOI] [PubMed] [Google Scholar]
- 46.Nie D, Zhou Y, Wang W, et al. 2021. Mechanical Overloading Induced-Activation of mTOR Signaling in Tendon Stem/Progenitor Cells Contributes to Tendinopathy Development. Front Cell Dev Biol 9:687856. [DOI] [PMC free article] [PubMed] [Google Scholar]


