Abstract
Heart failure (HF) is a leading cause of morbidity and mortality worldwide. Patients with HF exhibit a loss of junctophilin-2 (JPH2), a structural protein critical in forming junctional membrane complexes in which excitation-contraction takes place. Several mechanisms have been proposed to mediate the loss of JPH2, one being cleavage by the calcium-dependent protease calpain. The downstream mechanisms underlying HF progression after JPH2 cleavage are presently poorly understood. In this study, we used Labcas to bioinformatically predict putative calpain cleavage sites on JPH2. We identified a cleavage site that produces a novel C-terminal JPH2 peptide (JPH2-CTP) using several domain-specific antibodies. Western blotting revealed elevated JPH2-CTP levels in hearts of patients and mice with HF, corresponding to increased levels of calpain-2. Moreover, immunocytochemistry demonstrated nuclear localization of JPH2-CTP within ventricular myocytes isolated from a murine model of pressure overload-induced HF as well as rat ventricular myocytes treated with isoproterenol. Nuclear localization of JPH2-CTP and cellular remodeling were abrogated by genetic mutation of the nuclear localization sequence within JPH2-CTP.
Conclusion
Taken together, our studies identified a novel C-terminal fragment of JPH2 (JPH2-CTP) generated by calpain-2 mediated cleavage which localizes within the cardiomyocyte nucleus during HF. Blocking nuclear localization of JPH2-CTP protects cardiomyocytes from isoproterenol-induced hypertrophy in vitro. Future in vivo studies of the nuclear role of JPH2-CTP may reveal a causal association with adverse remodeling during HF and establish CTP as a therapeutic target.
Keywords: Calpain, Heart failure, Junctophilin-2, Nuclear translocation
Introduction
Heart failure (HF) results from inefficient cardiac contraction and relaxation, which fails to match the body’s metabolic demands. HF is a major public health concern, affecting over 23 million people worldwide with a median mortality rate of approximately five years [5]. HF disproportionately affects minorities and the elderly and is the leading cause of hospitalization of those over 65 in the United States. Moreover, HF poses a substantial financial burden to the health-care system [5]. Currently available therapeutic approaches to treat HF patients are limited due to unfavorable side effects and limited ability to rescue cardiac contractility in HF with reduced ejection fraction [2]. Understanding the underlying mechanism of HF will be required to achieve the long-term goal of reducing mortality and improving patients’ quality of life.
HF leads to adverse remodeling in the myocardium at multiple levels [13]. At the molecular level, HF progression is marked by disturbances in intracellular calcium (Ca2+) handling within cardiomyocytes[6, 9, 20]. Cardiomyocyte Ca2+ handling is coordinated by the dyad-spanning protein junctophilin-2 (JPH2), the cardiac isoform of the junctophilin family of proteins, which facilitates excitation-contraction coupling in excitable cells. JPH2 maintains junctional membrane complexes by approximating plasmalemmal and intracellular ion channels within cardiomyocyte dyads [17, 18]. Additionally, JPH2 directly binds to and regulates several Ca2+ channels including the ryanodine receptor type 2 (RyR2) and L-type Ca2+ channel [3]. Previous studies have demonstrated that loss of JPH2 promotes increased Ca2+ release through “leaky” RyR2 channels, indicating that JPH2 maintains normal Ca2+-induced Ca2+-release by directly stabilizing the RyR2 channel. In addition, JPH2 helps maintain the appropriate dyadic distance between the plasma membrane invaginations known as transverse tubules and the sarcoplasmic reticulum (SR) [22, 27]. Several studies have revealed a loss of transverse tubule structures and junctional membrane complexes during HF, which has been attributed to a loss of JPH2 levels in HF [27, 31, 38]. Moreover, over-expression of JPH2 in transgenic mice and JPH2 re-expression via adeno-associated virus in a murine model of pressure overload-induced HF prevented decompensated HF by restoring transverse tubule regularity and stabilizing RyR2, establishing JPH2 as a potential HF therapy [28].
The mechanisms by which JPH2 levels are reduced in HF are incompletely understood. Three overarching hypotheses have been examined as a mechanism underlying JPH2 loss including reduced translation mediated by micro RNAs[34], mechanical redistribution by microtubules[37], and cleavage by proteases such as Ca2+-sensitive proteolytic enzyme calpain (CAPN)[11, 12, 33] and matrix metalloproteinase-2 (MMP-2) [8]. Recent studies revealed that overexpression of CAPN1 can cause cleavage of JPH2 into multiple N- and C-terminal fragments [11, 33]. Moreover, calpastatin, a physiological inhibitor of CAPN, was shown to have a protective role in murine models of ischemic HF [36] whereas calpain inhibition by pharmacological inhibitor MDL-28170 protects cardiac function in pressure-overload-induced HF, ischemic HF and stress induced HF in mice [30]. Another recent study revealed JPH2 degradation in ischemia due to MMP-2 mediated cleavage [8]. These studies are important because they suggest that proteases such as CAPN and MMP-2 could potentially cleave recombinant JPH2 overexpressed by means of gene therapy, thus limiting the therapeutic effects of such an approach [27]. Recently, Guo et al.[12] reported that a large N-terminal JPH2 peptide is formed as a result of CAPN1 activation. This peptide can translocate into the nucleus where it exerts a protective effect on cardiomyocytes through Mef2 gene repression. However, the role of CAPN2 mediated proteolysis of JPH2 in HF remains unknown. Given that CAPN2 is activated under higher concentrations of intracellular Ca2+, which are often seen during HF progression, we hypothesize that JPH2 is also cleaved by CAPN2 during adverse remodeling associated with heart failure.
Methods
For full details refer to the methods in Online Resource 1.
Cloning
N-terminal or C-terminally HA-tagged JPH2 was amplified with KOD Xtreme Hot Start polymerase and cloned into a pcDNA3.1+ vector as previously described [25]. See Online Resource 1, Supplementary Table 1 for details.
Cell culture and transfection
HEK293T, H9C2, and neonatal rat ventricular myocytes (NRVMs) were cultured and transfected according to established protocols [25].
Human samples
Left ventricular samples were obtained from patients undergoing heart transplantation for end-stage heart failure (HF) after obtaining informed consent. Non-failing (NF) human hearts (which could not be used for transplantation) were obtained from the ‘International Institute for the Advancement of Medicine’ (IIAM) or the Gift of Hope. Human samples used in this study are listed in Online Resource 1, Supplementary Table 2. All studies were performed with IRB approval from Baylor College of Medicine and Texas Heart Institute (H31918), or the human studies committees at the University of Alabama, Birmingham [26].
Animal studies
Studies including transverse aortic constriction (TAC) and echocardiography were performed on 2-month old male and female C57Bl6/J mice (Online Resource 1, Supplementary Table 3) as described [28], according to protocols approved by the Institutional Animal Care and Use Committee of Baylor College of Medicine conforming to the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health (NIH Publication No. 85–23, revised 1996).
Western blot
Western blots were performed according to previously described protocols [25, 28]. See Online Resource 1 for details.
Immunocytochemistry
JPH2 localization in TAC isolated cardiomyocytes and H9C2, HEK293T, and NRVM cell cultures were visualized by immunostaining and confocal microscopy according to previously established protocols with minor adjustments [28]. Refer to Online Resource 1 for more detail.
Statistics
Results are expressed as mean ± standard error of the mean (SEM). The Shapiro-Wilk test was performed to determine whether data were normally distributed. The unpaired T-test, Mann-Whitney test, one-way ANOVA, or nonparametric Kruskal Wallis test were used for comparisons between groups, as specified in figure legends. A P-value of less than 0.05 was considered statistically significant.
Results
JPH2 is cleaved at its C-terminus
Full-length JPH2 is a 696-bp protein consisting of six ‘membrane occupation and recognition nexus’ (MORN) domains at its N-terminus which mediates attachment to the plasma membrane, followed by a dyad-spanning alpha-helical region, a divergent region, and a transmembrane domain that inserts into the SR at its C-terminus (Fig. 1A). While previous studies focused on N-terminal peptides cleaved from JPH2 following calpain-mediated proteolysis,[11, 12] we set out to identify potential C-terminal cleavage peptides. First, we identified three different JPH2 antibodies that recognize epitopes consisting of amino acids (aa) 458–475 (Ab1), 568–580 (Ab2), and 431–680 (Ab3), respectively (Fig. 1A; Online Resource 1, Supplementary Table 4). Lysates from HEK293 cells expressing mouse JPH2 cDNA or empty pcDNA3.1+ vector control were exposed to CaCl2 (2 mM) for 2 hours at 37 °C to activate endogenous calpains. Immunoblotting revealed that all 3 antibodies identified full-length JPH2 (JPH2-FL) (Fig. 1B). In contrast, only Ab2 and Ab3 detected a distinct 25-kDa C-terminal fragment (JPH2-CTP). To confirm that JPH2-CTP is comprised of the C-terminus of JPH2, the JPH2 sequence was fused with an N-terminal (HA-JPH2) or C-terminal (JPH2-HA) HA-tag for identification on immunoblots. Following expression of HA-JPH2, JPH2-HA, and empty vector control in HEK293 cells, lysates were exposed to 2 mM Ca2+ as before. Immunoblotting using HA antibody detected the 25-kDa cleavage peptide only when the HA-tag was fused to the C-terminus, thus confirming that JPH2-CTP is comprised of the C-terminus of JPH2 (Fig. 1C). Taken together these results indicate that JPH2 is most likely cleaved near its C-terminus resulting a novel 25-kDa peptide that is different from the C terminal peptide shown in previous Calpain/JPH2 studies[11].
Fig. 1. JPH2 is cleaved at its C-terminus.
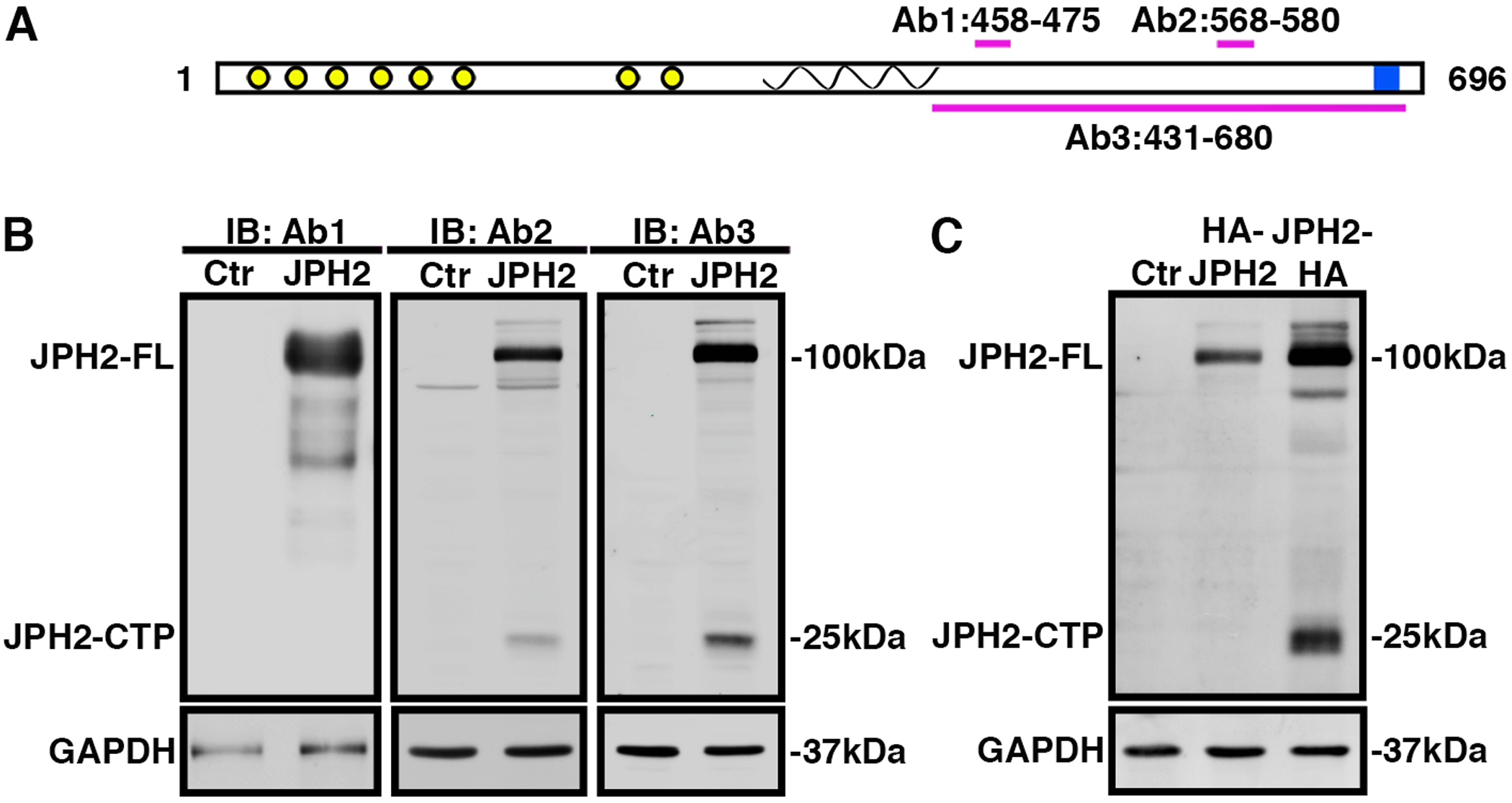
A) Cartoon of JPH2 protein with N-terminal MORN domains (yellow), α-helix (spiral), and C-terminal transmembrane domain (blue). Antibody epitopes are labeled Ab1, Ab2 and Ab3. B) Western blots using three different JPH2 antibodies revealing expression of full-length (FL) JPH2 and a 25-kDa C-terminal peptide in lysates of HEK293 cells expressing either JPH2 or pcDNA3.1 vector control (Ctr). C) Western blots using HA antibody revealing expression of JPH2-FL and/or JPH2-CTP in lysates of HEK293 cells that were not transfected (Ctr; lane 1), expressing HA-JPH2 (lane 2), or JPH2-HA (lane 3), respectively. All the experiments were performed at least 3 times to validate reproducibility.
JPH2 amino acids G482 and T483 are required for the formation of JPH2-CTP
Previous studies identified three CAPN cleavage sites in JPH2 (V155, L201, R565) with R565 being the most predominant among them [11]. We ran the Labcas algorithm to identify unbiased potential calpain cleavage sites on JPH2. Our initial prediction indicated 65 possible calpain cleavage sites. To narrow the list of putative calpain cleavage sites, we filtered these initial predictions based on antibody detection (Ab2 positive, Ab1 negative), since results from Fig. 1B demonstrate that JPH2 cleaved product was detected by Ab2 but not Ab1. Therefore, any predicted calpain sites N-terminal of amino acid 464 were excluded. HA antibody also detected the C-terminally HA-tagged JPH2 ~25 kDa band (Fig. 1C), suggesting that the JPH2 fragment includes the JPH2 C-terminal end. Therefore, Expasy was used to predict the molecular weights of fragments from the cleavage site to the C-terminal end of JPH2 and predicted molecular weights under 20 kDa were excluded based on expected size (~25 kDa). These filters resulted in a list of 13 cleavage sites, which we then ordered by cleavage probability rank (Online Resource 1, Supplementary Table 5 and Supplementary Fig. 1A–B). The top four ranked sites include G482, L500, A481 and E467 (Fig. 2A; Online Resource 1, Supplementary Table 5). Based on these result and predictions, the top predicted cleavage site is G482, which would result in the production of a C-terminal peptide including amino acids T483-T696 with a molecular weight of ~24kDa. The C terminal R565 described in previous study[11] is detected by the algorithm. The probability score of R565 (0.008908) is almost half of G482 (0.017001). Taken together, we inferred that 479SPAG/TPPQ486 is the predicted cleavage motif for CTP formation due to cleavage after G482.
Fig. 2. JPH2 amino acids G482 and T483 are required for the formation of JPH2-CTP.
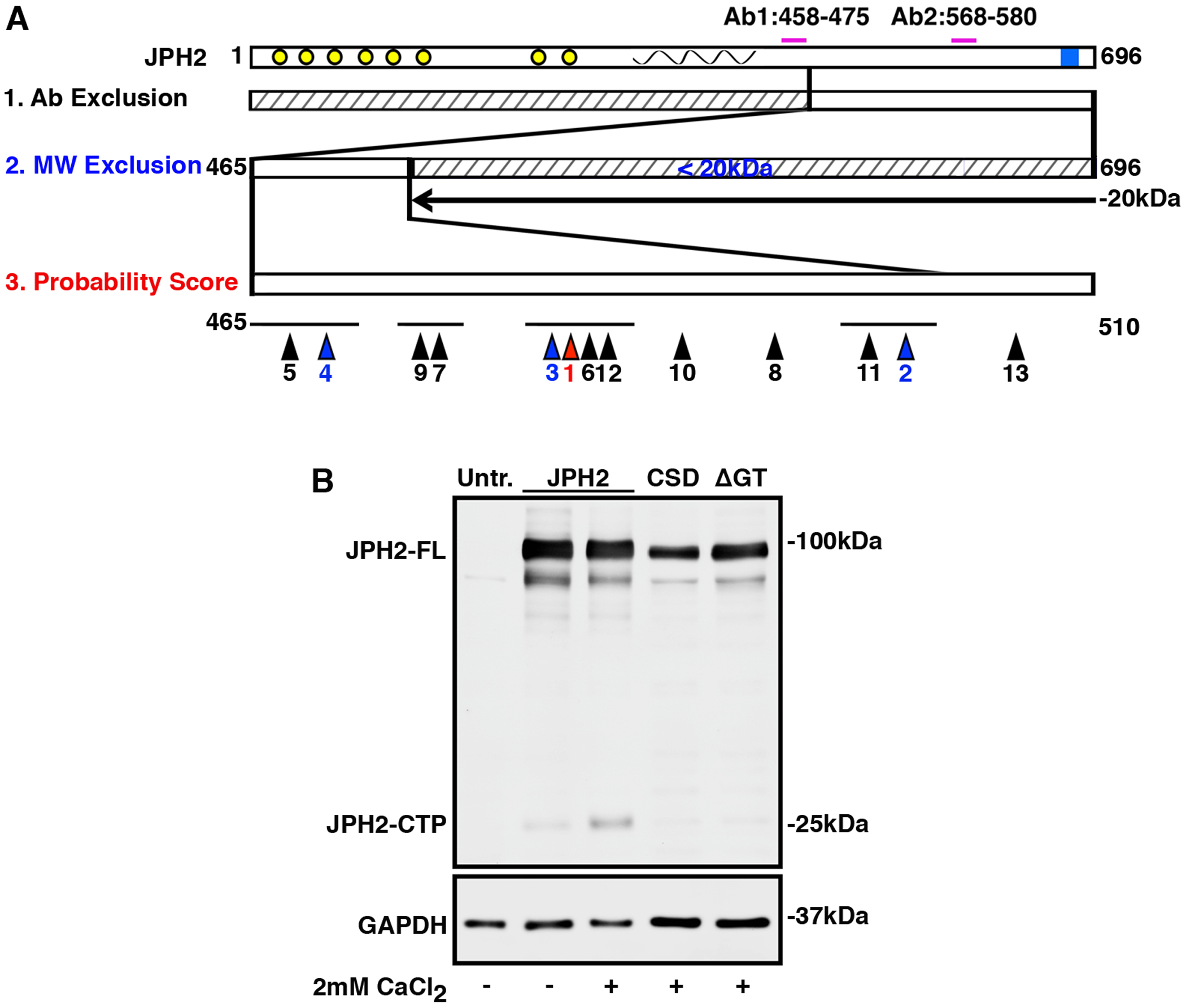
A) JPH2 cartoon showing full length protein with the antibody epitopes (top). Since JPH2-CTP is not detected by Ab1, the domain N-terminal of Ab1 is excluded (see 1). Based on western blotting, the molecular weight of JPH2-CTP is estimated to exceed 20kDa, hence the C-terminal domain is excluded (see 2). The domain spanning amino acids 465 to 510 (see 3) is predicted to contain the calpain cleavage site. Bioinformatic predictions of possible cleavage sites are shown, with the relative rankings below the sites marked by arrow heads. The site with the highest predicted score is colored red, the 2nd-4th highest prediction scores are colored blue. B) Western blots showing levels of full length JPH2 (JPH2-FL) and JPH2-CTP in lysates of HEK293 cells expressing wild-type JPH2, JPH2-CSD, and JPH2-ΔGT (in which residues 482G-483T were removed). All the experiments were performed at least 3 times to validate reproducibility.
In order to test this hypothesis, we constructed two cleavage site mutants, 1) JPH2-CSD deleting the complete cleavage motif 479SPAG/TPPQ486 and 2) JPH2-Δ482GT483 deleting 482GT483 only. Deleting the complete motif would prevent JPH2 cleavage at secondary cleavage sites in close proximity like A481, T483 or P484 (3rd, 6th and 12th predicted cleavage sites, respectively; Online Resource 1, Supplementary Table 5). We expressed C-terminally HA-tagged JPH2 cleavage site mutants in HEK293 cells and exposed cell lysates to 2mM CaCl2. Western blotting with anti-JPH2 antibody Ab2 revealed that deletion of either the complete motif or only the 2 amino acids surrounding the predicted cleavage site diminished JPH2-CTP formation. This suggests that 479SPAG↓TPPQ486 with 482GT483 at its core is the calpain cleavage site that produces the JPH2-CTP (Fig. 2B).
Activated CAPN2 is required for JPH2-CTP formation
Based on previous reports showing CAPN-mediated JPH2 proteolysis and our observation that Ca2+ increased JPH2-CTP formation at a predicted CAPN-cleavage site (Fig. 2B), we investigated which CAPN isoform(s) is/are responsible for generating JPH2-CTP. We transfected JPH2 in HEK cells grown on separate wells as biological repeats (N=6). We collected cell lysates from separate wells after 48 hrs and exogenous treatment with 2mM CaCl2 individually resulted in a significant increase in JPH2-CTP formation (2.67 ± 0.47 arbitrary units (AU) in CaCl2 treated compared to 1.16 ± 0.11AU in untreated; P=0.0073) and decreased full length (FL)-JPH2 (0.59 ± 0.05 in CaCl2-treated compared to 0.96 ± 0.04 AU in untreated; P=0.0006). By contrast, 2.5 μM calpastatin treatment blocked this Ca2+-induced JPH2 cleavage (1.46 ± 0.14 AU in CaCl2 + calpastatin-treated compared to 2.67 ± 0.47 AU in CaCl2-only group; P=0.03). Moreover, calpastatin was able to rescue JPH2-FL protein levels (0.95 ± 0.06 AU in the CaCl2 + calpastatin-treated group compared to 0.59 ± 0.05 AU in CaCl2-treated group; P=0.0008) (Online Resource 1, Supplementary Fig. 2A–C), suggesting that calpain is required for the formation of the CTP.
However, since calpastatin is a nonselective inhibitor of CAPNs [32], we also used shRNA-mediated reduction of specific CAPN isoforms to determine which isoform was responsible for JPH2 cleavage at the predicted site. The most active forms of CAPN in cardiac tissue are CAPN1 and CAPN2 while CAPN4 serves as the small subunit for both these CAPNs [23]. Differentiated H9C2 rat ventricular myocytes grown in separate plates (N=6) were infected with lentiviral vectors expressing shRNAs against Capn1 (shCapn1), or Capn2 (shCapn2), or scrambled shRNA control (shScr). The specificity of these shRNAs was confirmed by measuring Capn mRNA levels relative to the L7 housekeeping gene. shCapn1 transfected cells had decreased levels of Capn1 (0.34 ± 0.01 AU) but not Capn2 (0.84±0.06 AU) compared to shScr controls (1.04±0.03, P=0.0005); whereas, shCapn2-transfected cells had decreased Capn2 (0.25 ± 0.003 AU) but not Capn1 transcripts (0.81 ± 0.02 AU) compared to shScr controls (0.99 ± 0.03 AU, P=0.0002, Fig. 3A). These results validated the specificity the isoform specific Capn shRNAs used.
Fig. 3. Activated calpain-2 is required for JPH2-CTP formation.
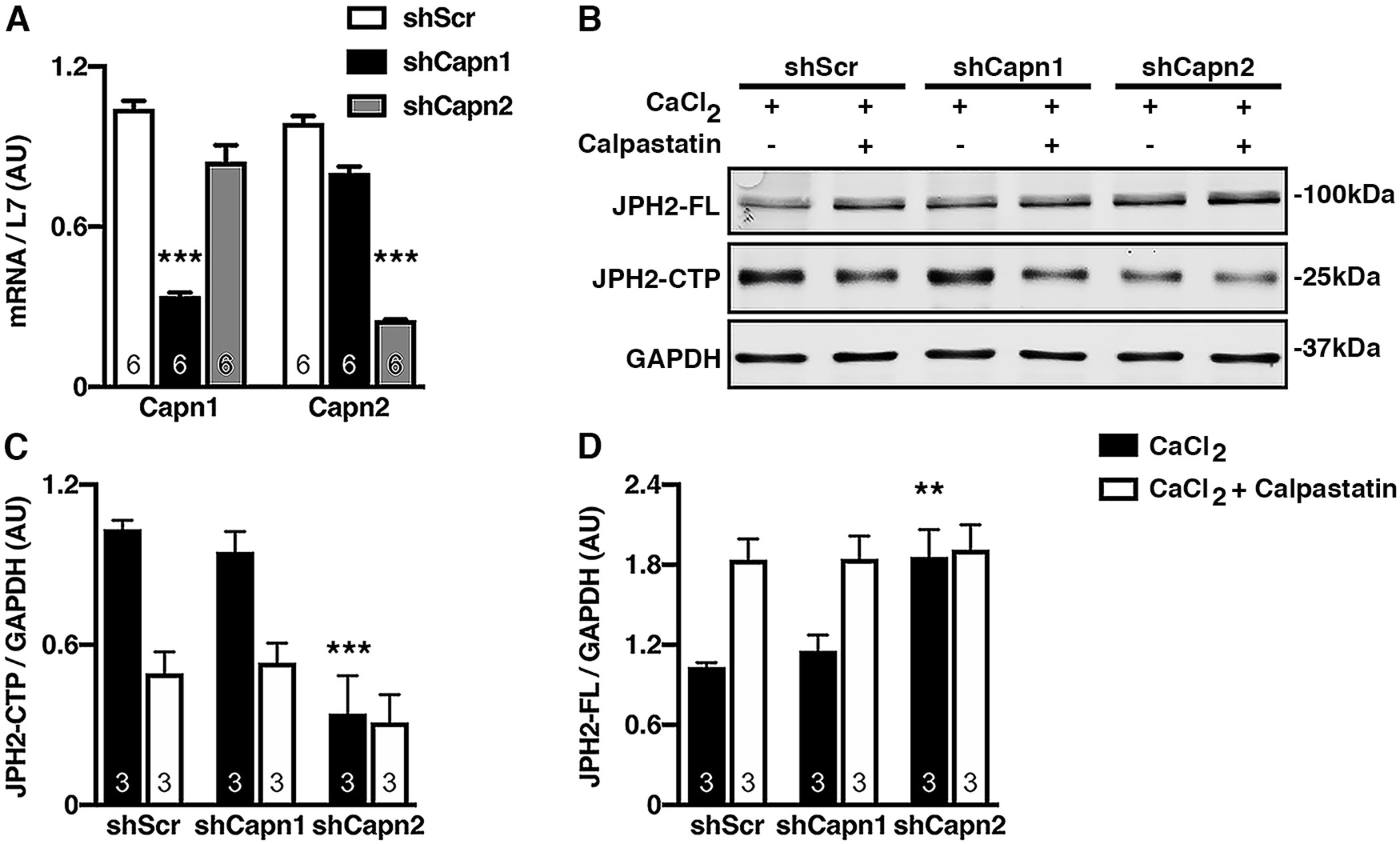
A) Quantification of qPCR measurements of Capn1 and Capn2 mRNA levels relative to L7 in H9C2 cells infected with lentiviral vectors containing shRNA against Capn1 (shCapn1), Capn2 (shCapn2), or a scrambled control (shScr). B) Representative western blot with JPH2 antibody (Ab2) showing expression of full-length JPH2 (JPH2-FL) and JPH2-CTP in H9C2 cells following Capn1 or Capn2 inhibition by shRNA in presence of 2mM CaCl2 plus or minus calpastatin. GAPDH was used as a loading control. Western blot quantification of JPH2-CTP (C) and JPH2-FL (D) normalized to GAPDH. Data were analyzed using the nonparametric Kruskal-Wallis test (*P<0.05; **P<0.01; ***P<0.001).
To determine whether CAPN1 or CAPN2 was responsible for the JPH2 C-terminal cleavage, H9C2 cells were treated with shCapn1, shCapn2, or shScr in individual wells (N=3), and these lysates were exposed to 2mM CaCl2 with or without calpastatin. shCapn1 treated cells retained the formation of JPH2-CTP; whereas, shCapn2 knockdown and calpastatin inhibited the formation of the JPH2-CTP (0.34 ± 0.14 AU compared to shScr, 1.03 ± 0.03 AU, P=0.0005; and shCapn1, 0.95 ± 0.08 AU, P=0.0014; Fig. 3B,C). Moreover, shCapn2 rescued JPH2-FL protein levels (1.86 ± 0.20 AU compared to shScr, 1.10 ± 0.06 AU, P=0.0126; and shCapn1, 1.15 ± 0.12 AU, P=0.0196 Fig. 3B,D). We did a separate western blot with the Ca2+ untreated H9C2 lysate alongside Ca2+ and shRNA treated lysates. Our results (Online Resource 1, Supplementary Fig. 3) further validated CAPN2 mediated generation of CTP as shCapn2 abrogated CTP to basal level as Ca2+ untreated controls. These results suggested Ca2+-activated CAPN2 is required to generate JPH2-CTP in physiological conditions. Although our data predicts CAPN2 to cleave predominantly at G483 to generate JPH2-CTP and previous studies showed that CAPN1 cleaves JPH2 to generate the 75kDa N terminal fragment (JPH2-NTP), both CAPN1 and CAPN2 could generate NTP and CTP in non-physiological conditions when we incubated recombinant JPH2 with either recombinant CAPN1 or CAPN2 (Online Resource 1, Supplementary Fig. 4). Coomassie staining, JPH2 N term and C term antibody blotting shows that both calpain-1 and calpain-2 cleave JPH2 to generate JPH2-NTP and CTP.
JPH2 C-terminal cleavage is increased in HF
Prior studies reported increased JPH2 cleavage of CAPN1 induced JPH2 peptides in mice with HF [33]. To assess the physiological significance of CTP, we investigated its abundance during HF by measuring JPH2-CTP with western blot detected by JPH2 antibody Ab2. Interestingly, CTP was significantly increased almost four-fold in human HF ventricular lysates (N=6) compared to non-failing (NF) (N=8) controls (3.65 ± 1.34 AU in HF compared to 1 ± 0.14 AU in NF, P=0.0007); whereas, JPH2-FL is decreased significantly in HF samples (0.75 ± 0.11 AU in HF compared to 1 ± 0.03 AU in NF, P=0.0127) (Fig. 4A–C). Further, we subjected 3-month-old Bl6/J mice to transverse aortic constriction (TAC) to model pressure-overload induced HF. Echocardiography to assess cardiac function 8-weeks post TAC revealed reduced EF in TAC mice (27.1 ± 0.7%) compared to sham controls (73.3 ± 0.8%, P<0.0001; Online Resource 1, Supplementary Table 3). Western blotting from ventricular lysates with antibody Ab2 revealed significantly decreased levels (0.26 ± 0.02 AU compared to 0.41 ± 0.03 AU in controls, P=0.0004, N=11) of full-length (FL) JPH2 but increased levels (0.28 ± 0.06 AU compared to 0.1±0.01 AU, P=0.0006, N=11) of JPH2-CTP in TAC mice, compared to sham controls (Fig. 4D–F). GAPDH was used as a loading control. Overall, these results demonstrate elevated JPH2-CTP in both human and murine HF, suggesting that this particular JPH2-CTP plays a potential role in HF progression.
Fig. 4. JPH2 C-terminal cleavage is increased in HF.
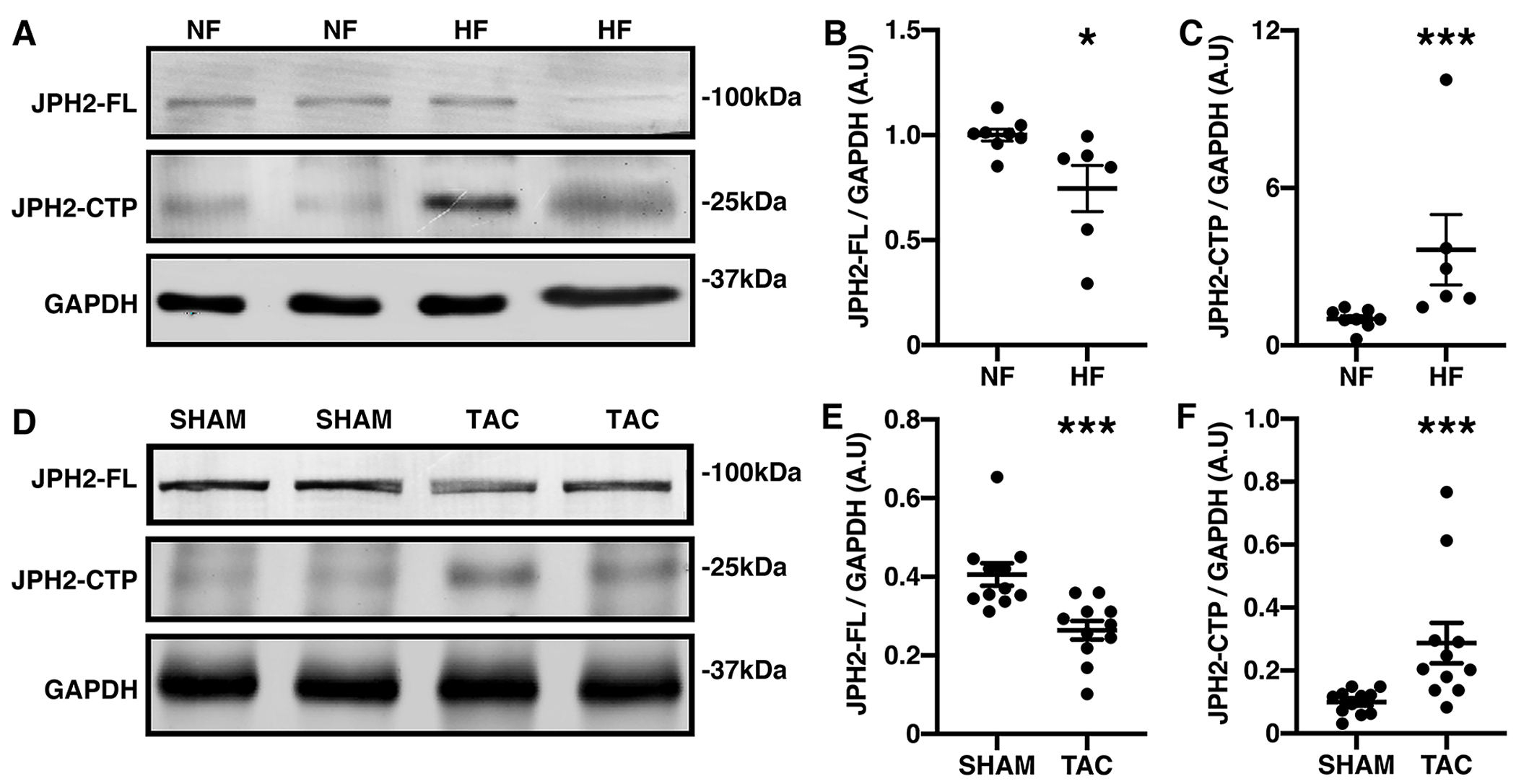
A-B) Representative western blots using JPH2-Ab2 of nonfailing (NF) and heart failure (HF) human heart lysates (A) and quantification (B-C) showing unaltered full-length (FL) JPH2 but increased C-terminal peptide (CTP) expression normalized to GAPDH loading control. D-F) Representative western blots and quantification of JPH2-FL (E) and CTP (F) in sham and TAC mice relative to GAPDH from mice heart left ventricles after transverse aortic constriction (TAC) compared to sham controls. Data were analyzed using Mann-Whitney test (*P<0.05; **P<0.01; ***P<0.001). All the experiments were performed at least 3 times to validate reproducibility.
In order to determine Calpain expressions in these human HF and TAC induced mouse HF samples, we measured both CAPN2 expression at protein level from these samples. Prior studies showed that in HF, CAPN1 promotes JPH2 R565 cleavage, creating a 75kDa cleaved N-terminal fragment [12]. So, we also measured CAPN1 protein levels by western blot from these aforementioned samples (Online Resource 1, Supplementary Fig. 5). Western blot with CAPN isoform-specific antibodies (Online Resource 1, Supplementary Fig. 5A–C) revealed a significant increase in protein expression of CAPN2 only in human HF samples (2.661±0.56 vs 1.00±0.229 a.u. in HF vs NF, P=0.034, N=4) whereas increase in CAPN1 expression was not significant (1.682±0.379 vs 1.00±0.172 a.u. in HF vs NF, P=0.11, N=4) in these samples. We further assessed the correlation between calpain isoforms and JPH2-FL and CTP from these human samples (N=8(NF + HF)). Our results (Online Resource 1, Supplementary Fig. 6A–D) interestingly revealed a strong positive correlation between CAPN2 and JPH2-CTP (R=0.8611, P=0.006) whereas positive correlation between CAPN1 and JPH2-CTP was not significant (R=0.3147, P=0.448) suggesting calpain-2 as responsible to generate CTP. JPH2-FL had significant negative correlation with both CAPN2 (R=−0.8805, P=0.004) and CAPN1 (R=−0.8936, P=0.003) further validating previous calpain-1 mediated JPH2 degradation studies and our current findings with calpain-2. Moreover, western blot from TAC induced HF samples (Online Resource 1, Supplementary Fig. 5D–F) further revealed that CAPN2 protein expression was also significantly increased in TAC-induced HF (1.54±0.15 vs 1.00±0.10 a.u. in TAC vs SHAM, P=0.001, N=8); whereas, CAPN1 was not significantly changed (1.23±0.14 vs 1.00±0.13 a.u. in HF vs NF, P=0.28, N=8). Overall, these results further validate calpain-2 mediated JPH2-CTP formation.
JPH2-CTP localizes to the nucleus via an NLS
Normally, JPH2 is a junctional membrane complex protein, which tethers the plasma membranes and the SR at cardiomyocyte dyads through its affinity for the plasma membrane due to its N-terminal membrane occupation and recognition nexus (MORN) domains and C-terminal transmembrane domain insertion in the SR. However, previous studies have shown that during HF a protective N-terminal peptide translocates to the nucleus diffusely [12]. In order to understand the more about the role of the new JPH2-CTP, we constructed a dual-tagged Myc-JPH2-HA plasmid to observe its subcellular localization after the activation of calpain with CaCl2 (Fig. 5A). Anti-Myc and -HA antibodies demonstrated a punctate nuclear localization of the JPH2 C-terminus (HA) but not the N-terminus (Myc) after CaCl2 addition (Fig. 5B). Nuclear JPH2 was only detected with HA antibody suggesting this might be the JPH2-CTP, while JPH2-FL (merged HA and Myc staining) was observed in the extranuclear space.
Fig. 5. Nuclear localization of JPH2-CTP requires the nuclear localization signal (NLS).
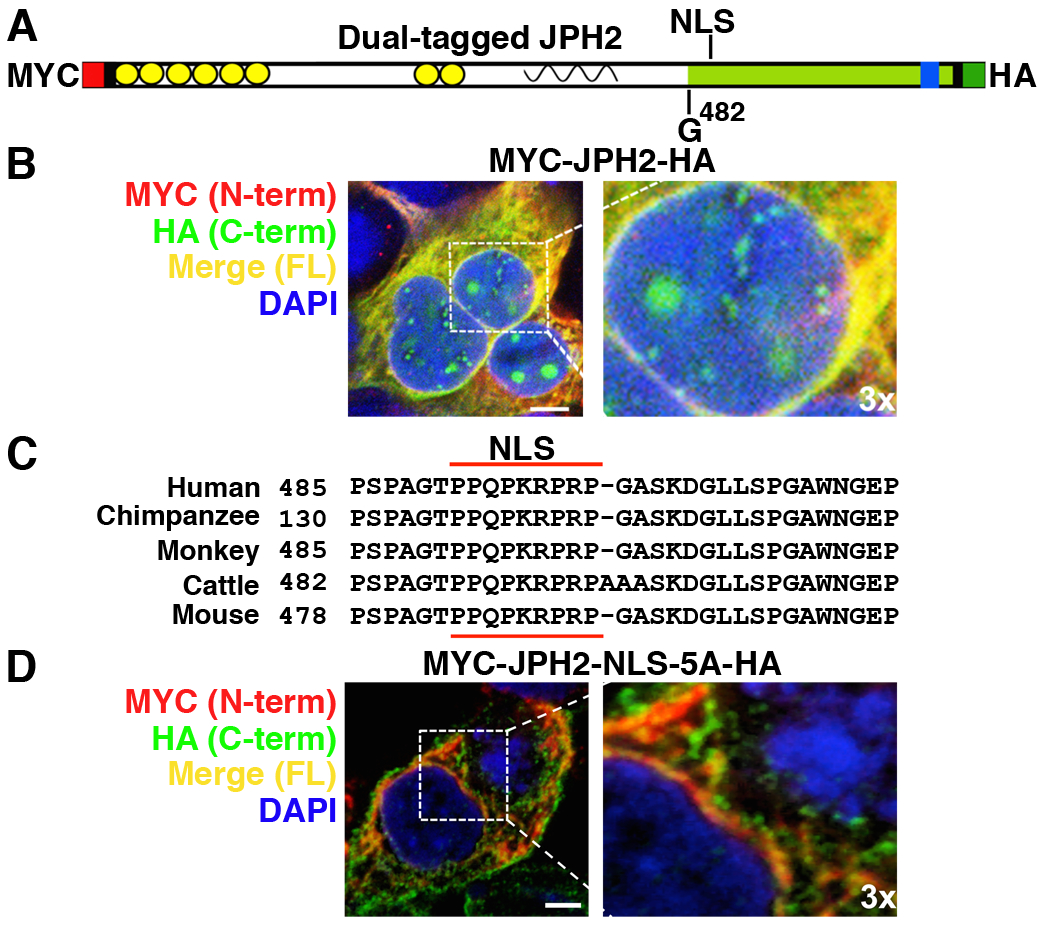
A) Cartoon of JPH2 showing location of the NLS, the G482 cleavage site, and the N-terminal MYC and C-terminal HA tags. B) Confocal images of HEK293 cells expressing dual-tagged JPH2 stained with MYC (red), HA (green) antibodies, and DAPI (blue). Green staining shows that JPH2-CTP translocated into the nucleus. C) Alignment of a putative NLS in JPH2 across species. D) Confocal images of HEK293 cells expressing dual-tagged JPH2-NLS-5A (mutated NLS peptide) stained with MYC (N-terminus, red) HA (C-terminus, green), and DAPI (nucleus, blue) antibodies. Lack of green staining shows that the NLS is required for nuclear translocation of JPH2-CTP. Dotted white box indicates magnified region (3x zoom); scale bar is 10μm. All the experiments were performed at least 3 times to validate reproducibility.
Since JPH2-CTP localized to the nucleus, we analyzed the JPH2 sequence to determine whether the JPH2-CTP contained a nuclear localization sequence (NLS). NLS-mapper identified a consensus NLS downstream of the CAPN2 cleavage site in the JPH2 sequence (T484-PPQKRPR-P492), which was conserved among different species (Fig. 5C). To validate the predicted JPH2 monopartite NLS, we developed a dual-tagged JPH2 NLS mutant construct (Myc-JPH2-NLS-5A-HA) by alanine mutagenesis of the proline-arginine rich domain of the NLS (K488-RPR-P492 to A488-AAA-A492). While HEK293 cells transfected with wildtype dual-tagged JPH2 demonstrated HA-stained punctate nuclear staining after CaCl2 addition, mutagenesis of the NLS abolished nuclear localization of JPH2 (Fig. 5D; Online Resource 1, Supplementary Fig. 7A). These results suggest that this monopartite NLS is required for nuclear localization of the JPH2-CTP. NLS-dependent punctate nuclear localization of C-terminal JPH2 occurs in other cell types like C2C12 myocytes (Online Resource 1, Supplementary Fig. 7B). To track full length JPH2 and its cleaved fragments localization in live cells, we cloned JPH2 and NLS mutant tagged with eGFP at its C terminal. HEK cells transfected with JPH2-eGFP showed punctate nuclear GFP staining following exposure to 2mM CaCl2, while eGFP controls did not localize to the nucleus (Online Resource 1, Supplementary Fig. 8). Overall, these results demonstrate that CTP translocation to nucleus after Ca2+-induced calpain cleavage depends on the NLS.
Nuclear localization of JPH2-CTP is increased in HF
Since we observed elevated JPH2-CTP formation during HF (Fig. 4), we investigated whether nuclear localization of CTP is also increased. To assess the subcellular localization of JPH2 in mouse hearts, we immune-stained isolated cardiomyocytes from both TAC-induced HF mice and sham controls with JPH2 Ab2, which recognizes both FL-JPH2 and JPH2-CTP, or Ab1 which recognizes FL-JPH2 but not JPH2-CTP. Confocal microscopy revealed increased nuclear JPH2 in TAC compared to sham controls (Fig. 6A). In alignment with our prior findings, Ab2 detected nuclear and non-nuclear JPH2 while Ab1 detected only JPH2 localized to dyads and the periphery of the nucleus (Fig. 6B). To better quantify the nuclear localization of JPH2-CTP in HF, the number of cardiomyocytes with a minimum of one nuclear puncta staining positive for JPH2 were compared between sham and TAC mice. Assessment of cardiomyocytes with JPH2-positive nuclei indicated an increase in the percent of cardiomyocytes positive for nuclear puncta after TAC-induced HF (73.50 ± 1.55% in TAC compared to 31.25 ± 5.54% in sham controls; P=0.029, N=4; Fig. 6E). Moreover, the number of puncta within each JPH2-positive nucleus was significantly increased in TAC cardiomyocytes relative to sham controls. (6.77 ± 0.72 puncta in TAC cardiomyocyte nuclei compared to 1.30 ± 0.32 puncta in sham control nuclei, N=number of nuclei, 23 in Sham and 17 in TAC, P<0.0001; Fig. 6F). Zoomed images revealed nuclear JPH2 in areas lacking heterochromatin (stained with DAPI; Fig. 6C–D). Taken together our findings indicate that nuclear localization of CTP is increased in a mouse model of HF.
Fig. 6. JPH2-CTP translocates into the nucleus in myocytes from mice subjected to TAC.
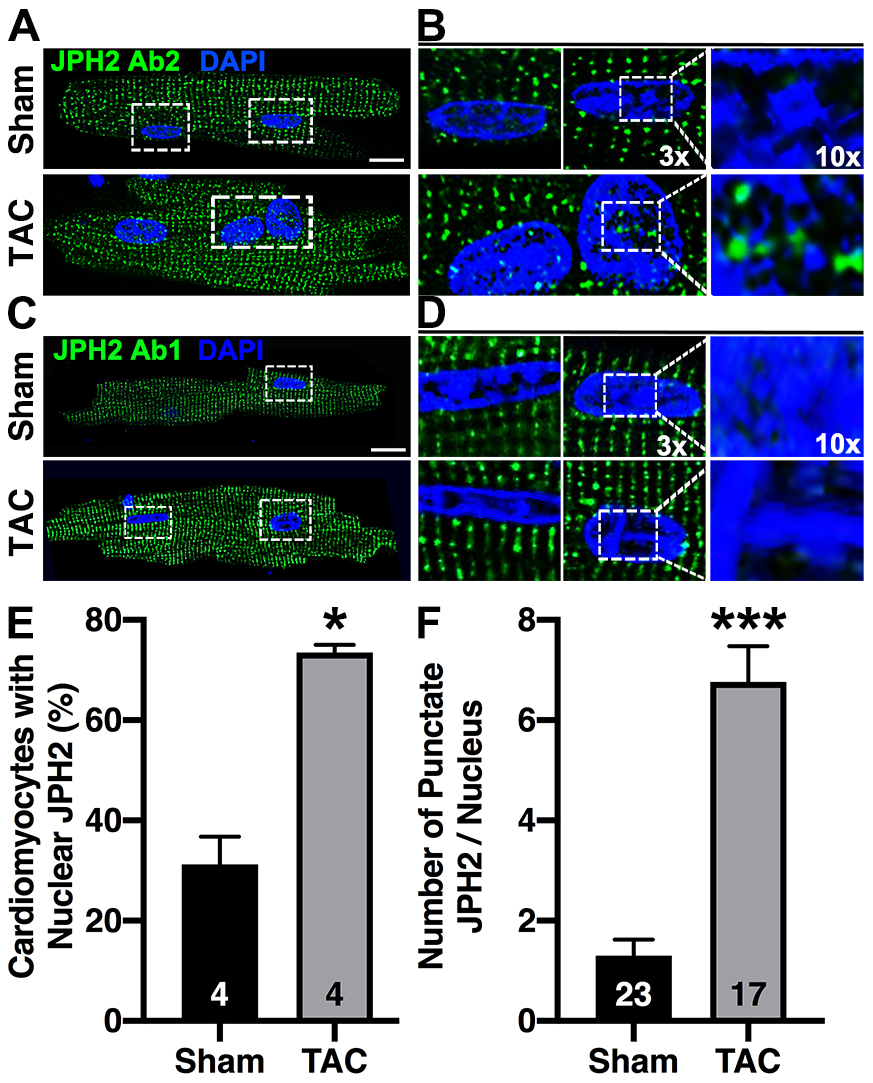
Immunofluorescence of fixed ventricular cardiomyocytes isolated from mice subjected to TAC or a sham procedure following staining with A) JPH2 C-terminal antibody (Ab2); 3x and 10x zoom images are shown on the right (B). C) Staining with JPH2 Ab1 which is N-terminal to the CTP; 3x and 10x zoom images on the right (D). White dotted box indicates zoom region. Scale bar = 10μm. E) Quantification of the percent of cardiomyocytes with positive nuclear staining after TAC, N = number of mice. F) Quantification of average number of JPH2 puncta per nuclei after TAC-induced HF, N = number of nuclei. Data were analyzed using Mann-Whitney test (*P<0.05; **P<0.01; ***P<0.001). All the experiments were performed at least 3 times to validate reproducibility.
JPH2 nuclear localization is critical for isoproterenol-induced hypertrophy
We found that nuclear transport of CAPN-cleaved JPH2-CTP was significantly increased in HF; however, the association of nuclear JPH2-CTP in the context of HF was still inconclusive. To assess the involvement of JPH2-CTP in cardiomyocyte remodeling, we first developed an in vitro cardiomyocyte hypertrophy model to assess if isoproterenol (ISO), which mimics the excessive β-adrenergic stimulation in HF and increases intracellular Ca2+ concentrations, could induce this CAPN-mediated JPH2 cleavage. Calpains have been shown to mediate isoproterenol mediated hypertrophy [1]. To test our hypothesis, we treated isolated cardiomyocytes from 2-month old mice (N=4) with saline or ISO. Immunoblotting with JPH2 Ab2 demonstrated that β-adrenergic stimulation reduced the amount of FL-JPH2 (0.25±0.11 in ISO vs. 0.56±0.04 in Ctr; P=0.029) and increased JPH2-CTP formation (0.47±0.05 in ISO vs. 0.13±0.02 in Ctr; P=0.03) in ISO-treated samples compared to saline treated controls (Online Resource 1, Supplementary Fig. 9A–C). Our results indicate that our model of cellular stress activated JPH2-CTP formation.
Next, to test the association of the nuclear localization of JPH2-CTP to cardiomyocyte modeling, we infected neonatal rat ventricular cardiomyocytes (NRVMs) with adenoviral vectors overexpressing JPH2-HA or JPH2-NLS-5A-HA (N=3 NRVM isolations). To assess whether nuclear localization of the CAPN-cleaved CTP plays a role in cardiomyocyte remodeling in our in vitro model, infected NRVM cells were exposed to 10μM of ISO to induce cellular hypertrophy, one of the primary signs of maladaptive cardiac remodeling. After 24 hrs of ISO exposure, these NRVMs were co-stained with anti-α-actinin and anti-HA antibodies. Anti-HA staining clearly showed increased nuclear JPH2-CTP after ISO exposure in JPH2-HA infected cells, which was abolished in JPH2-NLS-5A-HA infected cardiomyocytes (Fig. 7A). Moreover, ISO treatment promoted cellular hypertrophy quantified by cell surface area in JPH2-HA infected cells (2319.4±666.7μm2 in ISO vs. 1347.2±361μm2 in Ctr; P<0.05, Fig. 7B). JPH2-NLS-5A-HA abrogated ISO-induced hypertrophy in NRVMs (1152.8±263.9μm2 in ISO vs. 1166.7±208.3μm2 in Ctr, Fig. 7B) indicating that JPH2-CTP nuclear localization is required for ISO-mediated in vitro cardiomyocyte hypertrophy. Taken together, these data suggest that nuclear CTP is required for cardiomyocyte remodeling. However, further studies with genetically engineered mouse models abrogating JPH2-CTP formation and nuclear localization are warranted to assess whether nuclear CTP-induced remodeling is detrimental or compensatory.
Fig. 7. JPH2 nuclear localization is critical for isoproterenol-induced hypertrophy.
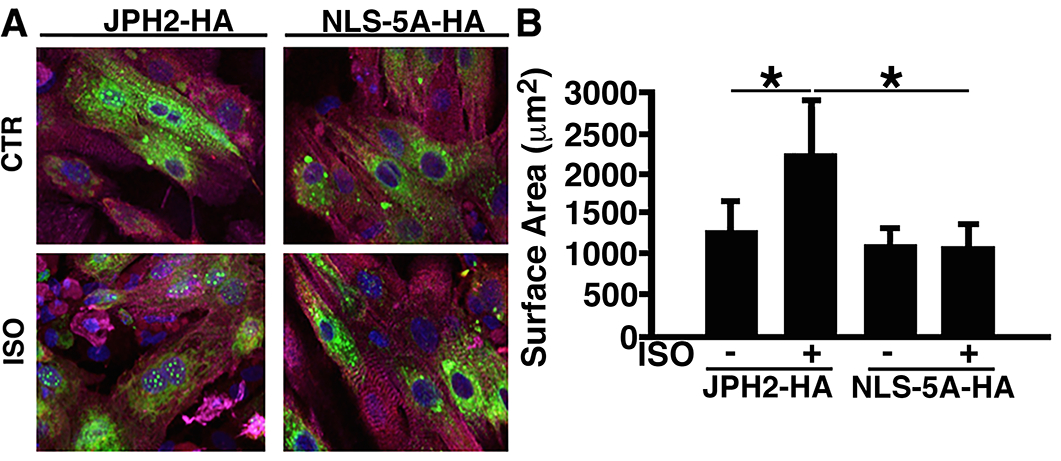
A) Representative immunofluorescence images of neonatal rat ventricular myocytes infected with adenovirus overexpressing JPH2-HA or JPH2-NLS-5A-HA, following treatment with isoproterenol (ISO) or buffer control (Ctr). Fixed cells were stained with HA (green, located at JPH2 C-terminus), counterstained with α-actinin (magenta) and DAPI (blue) B) Quantification of cell surface area. Scale bar =10 μm. Data were analyzed using one-way analysis of variance (ANOVA) (*P<0.05).
Discussion
In this study, we identified a calpain-cleaved JPH2 C-terminal fragment (JPH2-CTP), the levels of which are increased in HF. First, we found that JPH2 is cleaved in its C-terminal domain by CAPN2 at the 482GT483 site forming a 25kDa JPH2-CTP. Second, we found that the calpain-2 isoform is responsible for the cleavage of JPH2-CTP. Third, in patients with HF and mice with pressure overload-induced HF, JPH2-CTP levels are elevated. Fourth, we found that JPH2-CTP translocates into discrete areas within the nucleus due to a nuclear localization sequence within this peptide. Nuclear JPH2-CTP was also observed in cardiomyocytes isolated with mice subjected to transverse aortic constriction and myocytes treated with isoproterenol. Finally, mutagenesis of the NLS within JPH2-CTP prevented hypertrophic cardiomyocyte remodeling in ISO induced neonatal rat cardiomyocytes in vitro model, suggesting potential association of JPH2-CTP in stress-induced cardiomyocyte remodeling.
Calpains as a therapeutic target in heart failure
CAPNs are Ca2+-activated proteolytic enzymes that have been identified as potential therapeutic targets in HF. CAPN1 and CAPN2 are the most active CAPN isoforms within the heart. Prior studies showed that both CAPN1 and CAPN2 are localized to the Z-disk [16], which aligns with the cardiomyocyte dyads where JPH2 is typically found. CAPN1 (activated by μM Ca2+ concentration) and CAPN2 (activated by mM Ca2+ concentration) are referred as μ-CAPN m-CAPN, respectively, based on their Ca2+ requirements [24]. Prior studies have demonstrated that both TAC and I/R injury-induced Ca2+ overload in rodent cardiomyocytes leads to CAPN-mediated proteolytic cleavage of several SR proteins promoting contractile dysfunction [19]. A number of studies established inhibition of CAPN activity as a therapeutic strategy for HF [10, 21]. However, these studies have taken a broad approach of indiscriminate CAPN inhibition to prevent downstream effects of pressure overload. Moreover, they did not look at long-term effects of CAPN inhibition [21, 23]. Additionally, multiple studies have demonstrated the paradoxical effects of CAPN1 and CAPN2 in heart disease pathogenesis [29]. For instance, CAPN activation has an anti-hypertrophic response through the eNOS/AKT signaling pathway [4]. Moreover, both CAPN1 and CAPN2 regulate a number of essential cardiac proteins such as spectrin, desmin, PKCα, annexin 1, titin, calcineurin and cardiac troponin [19, 24]. Taken together, these studies suggest that CAPN has an important role in cardiac physiology. Considering the complex nature of CAPN’s role in cell signaling, broad CAPN inhibition may have unforeseen side effects. As Calpain has both positive and negative effect on cardiac function, it will be important to distinguish between physiological and deleterious effects of CAPN activation to design Calpain targeted therapeutic approach in HF.
Differential calpain cleavage of Junctophlin-2
One important protein that is cleaved by CAPN is JPH2. In normal cardiomyocytes, JPH2 maintains a critical inter-dyadic distance through its N-terminal interaction with the plasmalemma via membrane occupation and recognition nexus (MORN) domains, a dyad-spanning alpha helix, and a transmembrane domain that anchors its C-terminus in the SR. Previous studies found that JPH2 is cleaved by CAPN1 to form multiple N-terminal and C-terminal fragments [12]. Our study identifies a novel CAPN-cleaved C-terminal fragment that spans from 483T to the end of JPH2. In contrast to previously identified CAPN1-cleaved fragments, our data suggest that the CAPN2 isoform cleaves JPH2 at the 482GT483 site since shCapn2 but not shCapn1 abolished JPH2-CTP formation. Both recombinant calpain-1 and calpain-2 cleaves JPH2 to generate NTP (amino acids 1–565) and CTP (amino acids 483–696) in vitro. Therefore, although we cannot rule out that the JPH2 482GT483 site may be cleaved by CAPN1 under different physiological conditions, our results show that the CAPN2 isoform is responsible for cleavage of JPH2-CTP. CAPN1 is activated by μM amounts of Ca2+ seen in the dyad during normal physiological Ca2+-induced Ca2+ release, and CAPN2 is activated by mM Ca2+ seen in pathological conditions. Therefore, CAPN2 and its cleavage of JPH2 at the 482GT483 site may have a more important role in pathological rather than physiological or compensatory conditions. This difference in how CAPN1 and CAPN2 proteolyze JPH2 could be exploited as a therapeutic strategy to maintain physiological but not pathological calpain activity in HF.
Junctophilin-2 nuclear localization
This study also complements and expands upon the newly identified role of JPH2 in the nucleus. Guo et al.[12] identified a long N-terminal JPH2 fragment that was cleaved by CAPN at the 565RT566 site, which included a monopartite NLS The authors of this study found diffuse nuclear localization of this N-terminal peptide, where it was targeted to the chromatin by its alpha helix, where it bound and repressed Mef2 transcription [12]. It was proposed that nuclear localization of this N-terminal peptide acts as a compensatory mechanism opposing the detrimental effects of full-length JPH2 loss in HF. On the other hand, our studies demonstrate that CAPN2-mediated cleavage of JPH2 at the 482GT483 site led to inclusion of the monopartite NLS with the JPH2-CTP instead of the N-terminal fragment. This led to punctate as opposed to diffuse nuclear localization of the C-terminal fragment within cardiomyocyte nuclei. We investigated if CTP acts as a transcription factor similar to NTP. We utilized the UAS-Gal4 tool to analyze CTP mediated transcriptional regulation. Our results from these experiments suggests that JPH2-CTP does not act as a transcriptional factor as similar to JPH2-NTP (Online Resource 1, Supplementary Fig. 10) In cardiomyocyte nuclei isolated from TAC hearts, JPH2-CTP localized within discrete aggregates in the nuclei, which appear to be separate from the densely stained blue chromatin. It is possible that these aggregates are part of a nuclear subcompartment in the nucleoplasm itself or that they are located within nuclear “tunnels” bound to the nuclear membrane [14].
While further studies are needed to determine the specific role of JPH2-CTP in the nucleus, our present study demonstrates that blocking nuclear localization of the JPH2-CTP prevent ISO-induced cellular hypertrophy in NRVMs. Mutation of the monopartite NLS within JPH2 decreased nuclear localization of JPH2-CTP and abrogated ISO-mediated hypertrophy indicating that JPH2-CTP may play an opposing role to the N-terminal peptide in the nucleus. This finding differs from what was found in mice missing the monopartite NLS in the paper by Guo et al.[12] In this paper, the authors claim that mice missing the JPH2 NLS were protected after TAC. However, cardiac function in these mice was only assessed for a very brief time after TAC, which was not sufficient to determine whether loss of the NLS was indeed detrimental. Whether or not the nuclear localization of JPH2 is beneficial or harmful might depend on whether the N-terminal or the C-terminal fragment gains the NLS and translocate to the nucleus. Previous studies with Bid [15] and PARP-1 [7] showed differential cellular functions of cleaved peptides from the same protein, further suggesting that similarly in-depth mechanistic studies with JPH2-NTP and CTP in the cardiomyocyte nucleus may reveal if these fragments cooperate or compete with each other during adverse remodeling associated with HF.
Therapeutic implications of differential Junctophilin-2 cleavage and nuclear localization
The cleavage of JPH2 into different peptides based on the CAPN isoform involved, and their subsequent nuclear translocation may be important for designating the timing of JPH2 gene therapy. Adeno-associated virus delivery given prior to acute decompensation in a mouse TAC model halted HF progression in mice [28]. One downside to JPH2 gene therapy is the possibility that CAPNs could theoretically decrease the amount of therapeutic full-length JPH2 over time, although such a decrease was not seen after seven weeks in TAC mice receiving JPH2 gene therapy[28]. Our current study helps to inform the timing of this gene therapy since CAPN2 may be more active in later stages of HF [35]. Although CAPN1 activation in the early stages of HF could lead JPH2 cleavage at the 565RT566 site leading to N-terminal nuclear localization which may be protective, CAPN2 activation in end-stage HF could enhance JPH2-CTP nuclear localization, which may cause detrimental remodeling. Thus, a 482GT483 site cleavage resistant JPH2 may be beneficial in cases in which CAPN2 activity is increased. While further studies are needed to determine whether nuclear localization of the JPH2-CTP promotes decompensated cardiac remodeling, understanding the differences in JPH2 cleavage by each CAPN isoform will help provide a more specific therapeutic window for JPH2 gene therapy. Moreover, as JPH2 cleavage is in upstream of the signaling cascade, blocking nuclear translocation of CTP wouldn’t prevent calpain-2 mediated JPH2 cleavage at G482/T483 and it would further accumulate CTP in cytosol which can be potentially harmful. Therefore, it would be more efficient to target JPH2 cleavage site compared to the NLS as it is also shared between different cleaved JPH2 fragments (NTP, CTP) with paradoxical functions.
Conclusions
Taken together, we discovered a novel C-terminal fragment of JPH2 cleaved by CAPN2, which translocates to the nucleus during HF. Several groups have studied targeting CAPN to prevent development of HF; however, broad CAPN blockade may be detrimental due to the complex role of CAPN in cellular signaling. In contrast, our work has focused on understanding the pathological effects of CAPN on one of its critical cardiac targets, JPH2. Since our findings indicate that blocking nuclear localization of JPH2-CTP abrogates ISO-induced cellular hypertrophy in our in vitro model, preventing JPH2-CTP formation and its nuclear localization by blocking the CAPN2 cleavage site 482GT483 on JPH2 will reveal the nuclear role of JPH2-CTP associated to HF and may represent a more strategic therapeutic approach.
Supplementary Material
Acknowledgements
Echocardiograms were performed at the Baylor College of Medicine Phenotyping Core.
Funding
This work was supported by the National Institutes of Health (R01-HL089598, R01-HL091947, R01-HL117641, and T32HL07676); Dutch Heart Foundation (NHS) 2012T094 and the American Heart Association (18POST34080154); and by the Deutsche Forschungsgemeinschaft through SFB 1002-S02, SFB 1190-P03, and IRTG-RP2.
Abbreviations
- Ca2+
Calcium
- CAPN
Calpain
- CTP
C-terminal peptide
- HF
Heart failure
- ISO
Isoproterenol
- JPH2
Junctophillin-2
- NLS
Nuclear localization signal
- RyR2
Ryanodine receptor type 2
- SR
Sarcoplasmic reticulum
- TAC
Transverse aortic constriction
Footnotes
Compliance with ethical standards
Conflict of interest XHTW is a founding partner of Elex Biotech, a start-up company that develops drug molecules that target ryanodine receptors for the treatment of cardiac arrhythmia disorders.
Reference
- 1.Aluja D, Inserte J, Penela P, Ramos P, Ribas C, Iniguez MA, Mayor F Jr., Garcia-Dorado D (2019) Calpains mediate isoproterenol-induced hypertrophy through modulation of GRK2. Basic Res Cardiol 114:21. doi: 10.1007/s00395-019-0730-5. [DOI] [PubMed] [Google Scholar]
- 2.Asrar ul Haq M, Wong C, Mutha V, Anavekar N, Lim K, Barlis P, Hare DL (2014) Therapeutic interventions for heart failure with preserved ejection fraction: A summary of current evidence. World J Cardiol 6:67–76. doi: 10.4330/wjc.v6.i2.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beavers DL, Wang W, Ather S, Voigt N, Garbino A, Dixit SS, Landstrom AP, Li N, Wang Q, Olivotto I, Dobrev D, Ackerman MJ, Wehrens XH (2013) Mutation E169K in junctophilin-2 causes atrial fibrillation due to impaired RyR2 stabilization. J Am Coll Cardiol 62:2010–2019. doi: 10.1016/j.jacc.2013.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhuiyan MS, Shioda N, Fukunaga K (2009) Chronic beta-AR activation-induced calpain activation and impaired eNOS-Akt signaling mediates cardiac injury in ovariectomized female rats. Expert Opin Ther Targets 13:275–286. doi: 10.1517/14728220902721312. [DOI] [PubMed] [Google Scholar]
- 5.Blair JE, Huffman M, Shah SJ (2013) Heart failure in North America. Curr Cardiol Rev 9:128–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brixius K, Savvidou-Zaroti P, Mehlhorn U, Bloch W, Kranias EG, Schwinger RH (2002) Increased Ca2+-sensitivity of myofibrillar tension in heart failure and its functional implication. Basic Res Cardiol 97 Suppl 1:I111–117. doi: 10.1007/s003950200039. [DOI] [PubMed] [Google Scholar]
- 7.Castri P, Lee YJ, Ponzio T, Maric D, Spatz M, Bembry J, Hallenbeck J (2014) Poly(ADP-ribose) polymerase-1 and its cleavage products differentially modulate cellular protection through NF-kappaB-dependent signaling. Biochim Biophys Acta 1843:640–651. doi: 10.1016/j.bbamcr.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chan BYH, Roczkowsky A, Cho WJ, Poirier M, Lee TYT, Mahmud Z, Schulz R (2019) Junctophilin-2 is a target of matrix metalloproteinase-2 in myocardial ischemia-reperfusion injury. Basic Res Cardiol 114:42. doi: 10.1007/s00395-019-0749-7. [DOI] [PubMed] [Google Scholar]
- 9.Connell P, Word TA, Wehrens XHT (2020) Targeting pathological leak of ryanodine receptors: preclinical progress and the potential impact on treatments for cardiac arrhythmias and heart failure. Expert Opin Ther Targets 24:25–36. doi: 10.1080/14728222.2020.1708326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greyson CR, Schwartz GG, Lu L, Ye S, Helmke S, Xu Y, Ahmad H (2008) Calpain inhibition attenuates right ventricular contractile dysfunction after acute pressure overload. J Mol Cell Cardiol 44:59–68. doi: 10.1016/j.yjmcc.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo A, Hall D, Zhang C, Peng T, Miller JD, Kutschke W, Grueter CE, Johnson FL, Lin RZ, Song LS (2015) Molecular Determinants of Calpain-dependent Cleavage of Junctophilin-2 Protein in Cardiomyocytes. J Biol Chem 290:17946–17955. doi: 10.1074/jbc.M115.652396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guo A, Wang Y, Chen B, Wang Y, Yuan J, Zhang L, Hall D, Wu J, Shi Y, Zhu Q, Chen C, Thiel WH, Zhan X, Weiss RM, Zhan F, Musselman CA, Pufall M, Zhu W, Au KF, Hong J, Anderson ME, Grueter CE, Song LS (2018) E-C coupling structural protein junctophilin-2 encodes a stress-adaptive transcription regulator. Science 362. doi: 10.1126/science.aan3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heusch G, Libby P, Gersh B, Yellon D, Bohm M, Lopaschuk G, Opie L (2014) Cardiovascular remodelling in coronary artery disease and heart failure. Lancet 383:1933–1943. doi: 10.1016/S0140-6736(14)60107-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ibarra C, Vicencio JM, Estrada M, Lin Y, Rocco P, Rebellato P, Munoz JP, Garcia-Prieto J, Quest AF, Chiong M, Davidson SM, Bulatovic I, Grinnemo KH, Larsson O, Szabadkai G, Uhlen P, Jaimovich E, Lavandero S (2013) Local control of nuclear calcium signaling in cardiac myocytes by perinuclear microdomains of sarcolemmal insulin-like growth factor 1 receptors. Circ Res 112:236–245. doi: 10.1161/CIRCRESAHA.112.273839. [DOI] [PubMed] [Google Scholar]
- 15.Kudla G, Montessuit S, Eskes R, Berrier C, Martinou JC, Ghazi A, Antonsson B (2000) The destabilization of lipid membranes induced by the C-terminal fragment of caspase 8-cleaved bid is inhibited by the N-terminal fragment. J Biol Chem 275:22713–22718. doi: 10.1074/jbc.M003807200. [DOI] [PubMed] [Google Scholar]
- 16.Kumamoto T, Kleese WC, Cong JY, Goll DE, Pierce PR, Allen RE (1992) Localization of the Ca(2+)-dependent proteinases and their inhibitor in normal, fasted, and denervated rat skeletal muscle. Anat Rec 232:60–77. doi: 10.1002/ar.1092320108. [DOI] [PubMed] [Google Scholar]
- 17.Landstrom AP, Beavers DL, Wehrens XH (2014) The junctophilin family of proteins: from bench to bedside. Trends Mol Med 20:353–362. doi: 10.1016/j.molmed.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Landstrom AP, Weisleder N, Batalden KB, Bos JM, Tester DJ, Ommen SR, Wehrens XH, Claycomb WC, Ko JK, Hwang M, Pan Z, Ma J, Ackerman MJ (2007) Mutations in JPH2-encoded junctophilin-2 associated with hypertrophic cardiomyopathy in humans. J Mol Cell Cardiol 42:1026–1035. doi: 10.1016/j.yjmcc.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Letavernier E, Zafrani L, Perez J, Letavernier B, Haymann JP, Baud L (2012) The role of calpains in myocardial remodelling and heart failure. Cardiovasc Res 96:38–45. doi: 10.1093/cvr/cvs099. [DOI] [PubMed] [Google Scholar]
- 20.Lindner M, Bohle T, Beuckelmann DJ (2002) Ca2+-handling in heart failure--a review focusing on Ca2+ sparks. Basic Res Cardiol 97 Suppl 1:I79–82. doi: 10.1007/s003950200034. [DOI] [PubMed] [Google Scholar]
- 21.Mani SK, Shiraishi H, Balasubramanian S, Yamane K, Chellaiah M, Cooper G, Banik N, Zile MR, Kuppuswamy D (2008) In vivo administration of calpeptin attenuates calpain activation and cardiomyocyte loss in pressure-overloaded feline myocardium. Am J Physiol Heart Circ Physiol 295:H314–326. doi: 10.1152/ajpheart.00085.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Munro ML, Jayasinghe ID, Wang Q, Quick A, Wang W, Baddeley D, Wehrens XH, Soeller C (2016) Junctophilin-2 in the nanoscale organisation and functional signalling of ryanodine receptor clusters in cardiomyocytes. J Cell Sci 129:4388–4398. doi: 10.1242/jcs.196873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patterson C, Portbury AL, Schisler JC, Willis MS (2011) Tear me down: role of calpain in the development of cardiac ventricular hypertrophy. Circ Res 109:453–462. doi: 10.1161/CIRCRESAHA.110.239749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Potz BA, Abid MR, Sellke FW (2016) Role of Calpain in Pathogenesis of Human Disease Processes. J Nat Sci 2. [PMC free article] [PubMed] [Google Scholar]
- 25.Quick AP, Wang Q, Philippen LE, Barreto-Torres G, Chiang DY, Beavers D, Wang G, Khalid M, Reynolds JO, Campbell HM, Showell J, McCauley MD, Scholten A, Wehrens XH (2017) SPEG (Striated Muscle Preferentially Expressed Protein Kinase) Is Essential for Cardiac Function by Regulating Junctional Membrane Complex Activity. Circ Res 120:110–119. doi: 10.1161/CIRCRESAHA.116.309977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Respress JL, van Oort RJ, Li N, Rolim N, Dixit SS, deAlmeida A, Voigt N, Lawrence WS, Skapura DG, Skardal K, Wisloff U, Wieland T, Ai X, Pogwizd SM, Dobrev D, Wehrens XH (2012) Role of RyR2 phosphorylation at S2814 during heart failure progression. Circ Res 110:1474–1483. doi: 10.1161/CIRCRESAHA.112.268094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reynolds JO, Chiang DY, Wang W, Beavers DL, Dixit SS, Skapura DG, Landstrom AP, Song LS, Ackerman MJ, Wehrens XH (2013) Junctophilin-2 is necessary for T-tubule maturation during mouse heart development. Cardiovasc Res 100:44–53. doi: 10.1093/cvr/cvt133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reynolds JO, Quick AP, Wang Q, Beavers DL, Philippen LE, Showell J, Barreto-Torres G, Thuerauf DJ, Doroudgar S, Glembotski CC, Wehrens XH (2016) Junctophilin-2 gene therapy rescues heart failure by normalizing RyR2-mediated Ca2+ release. Int J Cardiol 225:371–380. doi: 10.1016/j.ijcard.2016.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y, Briz V, Chishti A, Bi X, Baudry M (2013) Distinct roles for mu-calpain and m-calpain in synaptic NMDAR-mediated neuroprotection and extrasynaptic NMDAR-mediated neurodegeneration. J Neurosci 33:18880–18892. doi: 10.1523/JNEUROSCI.3293-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Y, Chen B, Huang CK, Guo A, Wu J, Zhang X, Chen R, Chen C, Kutschke W, Weiss RM, Boudreau RL, Margulies KB, Hong J, Song LS (2018) Targeting Calpain for Heart Failure Therapy: Implications From Multiple Murine Models. JACC Basic Transl Sci 3:503–517. doi: 10.1016/j.jacbts.2018.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wei S, Guo A, Chen B, Kutschke W, Xie YP, Zimmerman K, Weiss RM, Anderson ME, Cheng H, Song LS (2010) T-tubule remodeling during transition from hypertrophy to heart failure. Circ Res 107:520–531. doi: 10.1161/CIRCRESAHA.109.212324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wendt A, Thompson VF, Goll DE (2004) Interaction of calpastatin with calpain: a review. Biol Chem 385:465–472. doi: 10.1515/BC.2004.054. [DOI] [PubMed] [Google Scholar]
- 33.Wu CY, Chen B, Jiang YP, Jia Z, Martin DW, Liu S, Entcheva E, Song LS, Lin RZ (2014) Calpain-dependent cleavage of junctophilin-2 and T-tubule remodeling in a mouse model of reversible heart failure. J Am Heart Assoc 3:e000527. doi: 10.1161/JAHA.113.000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu M, Wu HD, Li RC, Zhang HB, Wang M, Tao J, Feng XH, Guo YB, Li SF, Lai ST, Zhou P, Li LL, Yang HQ, Luo GZ, Bai Y, Xi JJ, Gao W, Han QD, Zhang YY, Wang XJ, Meng X, Wang SQ (2012) Mir-24 regulates junctophilin-2 expression in cardiomyocytes. Circ Res 111:837–841. doi: 10.1161/CIRCRESAHA.112.277418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang D, Ma S, Tan Y, Li D, Tang B, Zhang X, Sun M, Yang Y (2010) Increased expression of calpain and elevated activity of calcineurin in the myocardium of patients with congestive heart failure. Int J Mol Med 26:159–164. doi: 10.3892/ijmm_00000448. [DOI] [PubMed] [Google Scholar]
- 36.Ye T, Wang Q, Zhang Y, Song X, Yang D, Li D, Li D, Su L, Yang Y, Ma S (2015) Over-expression of calpastatin inhibits calpain activation and attenuates post-infarction myocardial remodeling. PLoS One 10:e0120178. doi: 10.1371/journal.pone.0120178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang C, Chen B, Guo A, Zhu Y, Miller JD, Gao S, Yuan C, Kutschke W, Zimmerman K, Weiss RM, Wehrens XH, Hong J, Johnson FL, Santana LF, Anderson ME, Song LS (2014) Microtubule-Mediated Defects in Junctophilin-2 Trafficking Contribute to Myocyte T-Tubule Remodeling and Ca2+ Handling Dysfunction in Heart Failure. Circulation. doi: 10.1161/CIRCULATIONAHA.113.008452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang HB, Li RC, Xu M, Xu SM, Lai YS, Wu HD, Xie XJ, Gao W, Ye H, Zhang YY, Meng X, Wang SQ (2013) Ultrastructural uncoupling between T-tubules and sarcoplasmic reticulum in human heart failure. Cardiovasc Res 98:269–276. doi: 10.1093/cvr/cvt030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


