Abstract
Objectives
The only approved vaccine, Bacillus Calmette Guérin (BCG) used in global tuberculosis (TB) immunization programmes has been very effective in childhood TB but not in adult pulmonary and latent TB. Moreover, the emergence of multi-drug resistance-TB cases demands either to increase efficiency of BCG or replace it with the one with improved efficacy.
Results
A novel combination of two most effective secreted protein antigens specific for Mycobacterium tuberculosis (Mtb), ESAT-6 and MPT-64 (but not present in BCG strains) fused with a cholera toxin B subunit (CTB) and tagged with 6xHis was expressed for the first time in Escherichia coli as well as in transgenic cucumber plants developed using Agrobacterium tumefaciens-mediated transformation. The recombinant fusion protein (His6x.CTB-ESAT6-MPT64) expressed in E. coli was purified by a single-step affinity chromatography and used to produce polyclonal antibodies in rabbit. The transgenic cucumber lines were confirmed by polymerase chain reaction (PCR), Southern blot hybridization, reverse transcriptase PCR (RT-PCR), real-time PCR (qRT-PCR) and expression of recombinant fusion protein by western blot analysis and its quantification by enzyme-linked immunosorbent assay (ELISA). A maximum value of the fusion protein, 478 ng.g−1 (0.030% of the total soluble protein) was obtained in a transgenic cucumber line. Rabbit immunized orally showed a significant increase in serum IgG levels against the fusion protein as compared to the non-immunized rabbit.
Conclusions
Stable expression of Mtb antigens with CTB in edible cucumber plants (whose fruits are eaten raw) in sufficient amount possibly would facilitate development of a safe, affordable and orally delivered self-adjuvanted, novel dual antigen based subunit vaccine against TB.
Graphical Abstract
Keywords: Cucumber, Edible vaccine, ESAT-6, MPT-64, Subunit vaccine, Tuberculosis
Introduction
Tuberculosis (TB) is one of the three top-most infectious killer diseases (AIDS, TB and malaria) in the world that has been called as ‘a universal pandemic disease’ by the World Health Organization (WHO) in 2020 (Ghebreyesus 2020; Mane 2022). Globally, it has affected 10 million peoples and has caused 1.3 million deaths among Human immunodeficiency virus (HIV)-negative people in 2020 (Global Tuberculosis Report 2021, WHO). It affects people of any age group but it occurs at a higher rate in people with weak immune or immuno-compromised systems, for example, HIV-infected people, are at high risk. Every second individual in the world is infected with TB bacilli and out of ten lately infected people one will become infectious in its lifetime (WHO 2022). TB infection in healthy people is usually asymptomatic and it is divided into 2 stages: asymptomatic latent TB infection or TB disease. Individuals infected with Mtb without symptoms and disease are at latent stage, while people with symptoms like cough lasting for more than 3 weeks, weight loss, night sweat and fever usually have TB disease. Various types of treatments, therapies and drugs are used to combat TB but currently world is facing major problems of drug resistance-TB, multidrug resistant-TB and extensively drug resistant-TB which are lethal, and treatments for them are very complicated and expensive (Joshi et al. 2021). Vaccination is effective to control tuberculosis. The only WHO-approved vaccine, Bacillus Calmette-Guérin (BCG) developed in 1921 is a live attenuated strain of M. bovis which is administered via intra-dermal route to protect infants against extra-pulmonary TB but it is not effective against pulmonary TB for adolescent, adults and immuno-compromised humans (Zhang et al. 2012; Kaufmann 2021). It is, therefore, imperative either to increase efficiency of BCG or replace it with the one with improved efficacy (Kaufmann 2021). The non-replicating subunit vaccines being safe, specific and with a minimum reactogenicity are preferred over conventional (live attenuated) vaccines (Wang et al. 2019). Plant-made subunit vaccines based on the expression of bacterial/viral protein antigens in plants have been shown to safe, efficacious, easy to produce and scalable, apt to store and transport, and affordable (Saba et al. 2019a). Mtb being a respiratory infectious agent, oral administration of vaccine antigen expressed in transgenic plants is very effective to develop immune responses at a significantly reduced cost (Permyakova et al. 2015; Saba et al. 2019b). Further, the plant cells can bio-encapsulate subunit vaccine to protect it from the acidic and enzymatic environments of the stomach and gastrointestinal tract. Moreover, an effective transmucosal carrier such as cholera toxin B subunit (CTB) is fused with antigen(s) for their delivery from the intestine to the immune or circulatory system. CTB is a pentameric peptide that binds to GM1 ganglioside receptor present in the cell membrane of gut epithelium for subsequent translocation of antigen across mucosal lining and accessible to the dendritic cells to boost mucosal immune response (Kwon et al. 2013; Wallis et al. 2019). In the present research, a novel combination of two most effective secreted protein antigens specific for Mtb that are not present in BCG strains (Nemes et al. 2019), ESAT-6 (6 kDa early secreted antigen target) and MPT-64, fused with a transmucosal carrier, CTB have been simultaneously expressed for the first time in E. coli and transgenic cucumber (Cucumis sativus) plants developed using Agrobacterium-assisted nuclear stable transformation. The transgenic plants were authenticated by PCR and Southern blot hybridization, RT-PCR and qRT-PCR, expression and quantification of fusion antigens protein by western blot and ELISA, and their immunogenicity on an animal model, rabbit. The dual Mtb-specific antigens, ESAT-6 and MPT-64 in combination with a mucosal adjuvant, CTB expressed in transgenic cucumber plants could be an effective strategy to induce higher protective immune responses than the vaccines with individual antigen alone or boost BCG with non-BCG antigens specific to Mtb for the long-term prevention of TB worldwide.
Materials and methods
Vector designing
Gene sequences of CTB (Gene ID = AY307389.1), Mtb antigen proteins, ESAT-6 (Gene ID = 886,209) and MPT-64 (Gene ID = 885,925) were retrieved in FASTA format from nucleotide database of the National Centre for Biotechnological Information (NCBI) (http://www.ncbi.nlm.nih.gov/). All the three gene sequences were joined with each other (CTB.ESAT-6.MPT-64) using the hinge region and tagged with polyhistidine (6 × His) affinity tag at the 5’ end. This recombinant gene cassette (E) was synthesized by the Biobasic Company, Canada. It was cloned into E. coli -and plant-expression vectors.
Cloning, expression and purification of recombinant protein (His6x.CTB.ESAT-6.MPT-64) from E. coli and production of polyclonal antibodies in rabbit.
The protocols for the cloning of gene cassette, His6x.CTB.ESAT-6.MPT-64 downstream of T7 promoter into pET28a bacterial expression vector and its expression in E. coli BL-21(DE3) cells to produce recombinant fusion protein for the induction of polyclonal antibodies in rabbit and their confirmation as described by Yadav et al. (2021) were followed with slight modifications.
Cloning and transformation of recombinant gene cassette into plant expression vector
The gene cassette, His6x.CTB.ESAT-6.MPT-64 was cloned downstream of CaMV35S promoter into 5′ BamHI and 3′ SacI restriction sites by replacing the GUS gene of the binary vector, pBI121 to yield pBI121.His6x.CTB.ESAT-6.MPT-64. The resultant recombinant binary vector was transformed into a disarmed A. tumefaciens strain EHA105 using freeze thaw method. The binary vector also contains a plant selectable marker gene, neomycin phosphotransferase (nptII) under the command of nos (nopaline synthase) promoter and terminator. nptII provides resistance to transformants for their selection on kanamycin containing medium. A. tumefaciens strain EHA105 carrying a binary vector, pBI121 with a gene cassette His6x.CTB.ESAT-6.MPT-64 under the regulation of Cauliflower mosaic virus 35S promoter (CaMV35S) and a nopaline synthase (nos) terminator was utilized for the plant transformation study. T-DNA region of the binary vector, pBI121.His6x.CTB.ESAT-6.MPT-64 is shown in Fig. 1. The cloning of E-cassette (His6x.CTB.ESAT-6.MPT-64) into the binary vector, pBI121 was confirmed by the restriction endo-nuclease digestion and PCR amplification.
Fig. 1.
T-DNA of the binary vector, pBI121-CTB.ESAT6.MPT64 used for transformation. LB-left border of T-DNA, CaMV35S P- Cauliflower Mosaic Virus 35S promoter, nos T-nopaline synthase poly A terminator, nptII- neomycin phosphotransferase, nosP-nopaline synthase promoter, CTB.ESAT-6.MPT-64 (E)-E gene cassette consists of cholera toxin B subunit (CTB) fused with antigens specific for M. tuberculosis, ESAT-6 (6 kDa early secreted antigen target) and MPT-64, RB-right border of T-DNA
Genetic transformation, regeneration, and selection of transgenic cucumber plants
Cucumber seeds of Green long S-82 variety were procured from a local market at New Delhi (India). Cotyledonary explants of cucumber var. Green long S-82 were transformed using A. tumefaciens EHA105 (pBI121-His6x.CTB.ESAT-6.MPT-64) as per the protocol described by Wang et al. (2015) with some changes. A PCR positive A. tumefaciens EHA105 colony was cultured in LB broth with 50 mg kanamycin l−1 and 10 mg rifampicin l−1 on an orbital shaker at 120 rpm for overnight at 27°C until OD600 reached to 0.8. The bacterial culture was centrifuged at 6,000 rpm for 10 min to pellet the bacteria. Pellets were then dissolved in the liquid Murashige and Skoog (MS) medium (Murashige and Skoog 1962) containing 1 µM 6-benzylaminopurine (BAP), 100 µM acetosyringone (ACS), 2 mg zeatin l−1, 0.5 g MES buffer l−1, and 6 mg L-cystein l−1 at pH 5.3.
Mature cucumber seeds were washed with 70% ethanol for 60 s and surface sterilized with 0.1% HgCl2 for 3–5 min. The seeds were then washed with autoclaved water for 3–4 times and were grown in vitro on solidified MS medium with sucrose. The cotyledonary explants were excised from 5 days old seedlings and dipped into Agrobacterium-suspension (MS + BAP + acetosyringone + MES + zeatin + L-cystein) for sonication (at 40 kHz) in a bath-type ultrasonic sonicator for 10–15 s and vacuum infiltrated for 45–50 s (at 0.78 atm) and ultimately inoculated in a fresh Agrobacterium-suspension for 20 min on orbital shaker (80 rpm) at 22°C. These Agrobacterium-infected explants were co-cultured onto fresh co-cultivation medium (CCM) containing 100 mM acetosyringone adjusted to pH (5.4) for 2 days under dark conditions. After co-cultivation, explants were washed five times with autoclaved water and finally washed in 200 mg cefotaxime l−1 containing water. The explants were dried on sterilized tissue paper and cultured on the selection medium (MS with 8.0 µM BAP, 250 mg cefotaxime l−1 and 80 mg kanamycin l−1) for regeneration and selection of transformed shoots. All explants were maintained at 16 h cool-white fluorescent lights (80 mEm−2 s−2) at 25 ± 2°C in a culture room. The shoots regenerated on shoot selection medium were rooted on the solid MS medium containing 20 mg kanamycin l−1 and 250 mg cefotaxime l−1. Rooted-shoots (T0) were then transferred to the earthen pots containing soil. The pots were shifted to the green house for flowering and maturation of fruits/seeds.
Genomic DNA extraction and PCR analysis
The cetyl trimethyl ammonium bromide (CTAB) method (Rogers and Bendich 1989) was used for the isolation of total genomic DNA from immature leaves of wild (control) and presumed transgenic plants. Extracted genomic DNA was then purified according to the scheme illustrated by Sambrook et al. (1989) and quantified using nanodrop spectrophotometer. Regenerated transformed plants were analysed for PCR amplification of nptII and E-cassette genes using gene-specific primers (Table 1). PCR reaction was carried out in 15 μl reaction mixture, containing 7.5 μl ready to use master mix, 0.3 μl of both primers (forward and reverse), 4.9 μl of nuclease-free water, and 2 μl of ~ 100 ng plant genomic DNA in a thermal cycler (Applied Biosystems) as per the conditions described in Table 1. Genomic DNA from the wild type (control) plants was taken as a negative control and the binary vector plasmid DNA was taken as a positive control. The PCR reaction products were separated on 1% agarose gel via electrophoresis. Further, all PCR-positive plants were checked for the residual Agrobacterium contamination using virA gene-specific primers and conditions as described in Table 1.
Table 1.
Primer sequences and PCR conditions used for the amplification of CTB.ESAT-6.MPT-64 (E) cassette, nptII, virA and β-actin genes with their amplicon sizes
| S No |
Gene name | Primer sequence | PCR conditions Denaturation–Annealing–Extension |
Amplicon size |
|---|---|---|---|---|
| 1 | CTB.ESAT-6.MPT-64 (E) cassette |
Forward primer 5′GAATTCGGATCCATGACACCTCAAAA3′ Reverse primer 5′CTCGAGGAACGTGGGACCAATACCTG3′ |
(94°C − 30 s)–(56°C − 45 s)–(72°C − 1 min) | 1.344 kb |
| 2 | nptII |
Forward primer 5′CCACCATGATATTCGGCAAC3′ Reverse primer 5′GTGGAGAGGCTATTCGGCTA3′ |
(94°C − 1 min)–(56°C − 1 min)–(72°C − 1 min) | 540 bp |
| 3 | virA |
Forward primer 5′AATTCACCCACGCGGCAGGATTTTAAGACAG3′ Reverse primer 5′AGCTTTGGTACGAGAGACTATTTCGCGTAG3′ |
(94°C − 30 s)–(55°C − 45 s)–(72°C − 1 min) | 1.00 kb |
| 4 | β-actin |
Forward primer 5′GCGGTCGAACAACTGGTATT3′ Reverse primer 5′AGTCCCCTTCACCGACTCTT3′ |
(94°C − 30 s)–(52°C − 45 s)–(72°C − 1 min) | 250 bp |
Southern blot analysis
The PCR positive T0 cucumber plants were analyzed for insertion of transgene and its copy number into the plant genome using the Southern blot hybridization. Twelve microgram of purified DNA isolated from the wild (control) and transformed plants were digested overnight with HindIII restriction endonuclease enzyme. The digested products were electrophorically separated on the 0.8% agarose gel. The gel was then subjected to depurination, denaturation, neutralization, and capillary transfer onto the Hybond N+ (positively-charged nylon) membrane. A purified 1.3 kb long amplicon of E-cassette (His6x.CTB.ESAT-6.MPT-64) was used to prepare a biotin-labeled DNA probe using ‘Biotin DNA labeling kit’. The blot was subjected to pre-hybridization, hybridization, and detection as per the instructions given in the manual of biotin-labeled DNA detection kit (Thermo Fisher Scientific).
Total RNA isolation and reverse transcriptase (RT)-PCR analysis
RNA from the leaves of wild (control) and transgenic cucumber plants was isolated using RiboZol™ RNA Extraction Reagent (Amresco) and resolved on 1% denaturing agarose gel. One microgram of isolated RNA was used for the cDNA synthesis via reverse transcriptase cDNA synthesis kit (Thermo Fischer Scientific). One microliter of cDNA was used as template for the polymerase chain reaction using primers specific to E-cassette as per the conditions described in Table 1. The resultant amplified products were resolved on 1% agarose gel using electrophoresis.
Real time PCR (qRNA) analysis
The expression levels of E-cassette (His6x.CTB.ESAT-6.MPT-64) in transgenic cucumber plants were determined by qRT-PCR using the One Step SYBR PrimeScript RT-PCR kit on a CFX96 Touch™ Real-Time PCR Detection System (Bio-Rad, USA). In real-time PCR, cDNA synthesized by reverse transcriptase-PCR, was taken as a template for the amplification of β-actin, a housekeeping gene, as a reference gene and E-cassette as a query. A 20 µl of total volume of a PCR reaction for qRT-PCR contains cDNA (100 ng), ready to use SYBR Green master mix (10 µL), primers (1 µM), and water. The primer sequences and PCR conditions used for both the genes (β-actin and E-cassette) are presented in Table 1.
Protein extraction and quantification
One gram fresh leaves of wild (control) and transgenic cucumber plants were used for the extraction of total soluble protein using extraction buffer, 50 mM phosphate buffer (pH 7.4). The leaf samples were grounded in pre-chilled mortar and pestle in liquid nitrogen and mixed with protein extraction buffer which was centrifuged at 20,000*g for 10 min. The supernatant was collected and used to estimate protein by the Bradford’s method (Bradford 1976).
Western blot analysis
The total isolated proteins (25 µg) heated in boiling water for 10 min were resolved on 10% sodium dodecyl sulphate-polyacrylamide gel (SDS-PAGE) by electrophoresis followed by their transfer onto a Hybond PVDF (Polyvinylidene fluoride) membrane using the transfer buffer (Tris 1.5 g, glycine 7.2 g and methanol 100 ml and final volume was made to 500 ml with autoclaved water). The membrane was initially blocked with phosphate-buffered saline (PBS) with 0.5% Tween-20 and 5% BSA at room temperature for 1 h to prevent non-specific protein interaction. The blot was then incubated with polyclonal anti-rCTB.ESAT-6.MPT-64 primary antibodies (developed in rabbit in the present study) (diluted in 1:1000 ratios with wash buffer) at 4°C for one hour. The blot was washed with buffer and incubated with alkaline phosphatase (AP)-conjugated goat anti-rabbit IgG as secondary antibodies (diluted in 1:1000 ratios in with wash buffer) (Sigma Aldrich USA, A0418). Incubation of blot was followed by washing with 1 × phosphate-buffered saline with Tween-20 (PBST) and the protein bands were detected after adding premixed substrate BCIP (5-bromo-4-chloro-3-indolyl phosphate)/NBT (nitro blue tetrazolium) in dark.
Enzyme-linked immunosorbent assay
ELISA was performed to quantify the fusion protein (CTB.ESAT-6.MPT-64) expressed in transgenic cucumber plants using a linear curve with a quantification range 2.5–160 ng of recombinant E-cassette protein expressed and purified from E. coli BL21 (DE3) strain by Ni-NTA-Agarose beads. ELISA plate wells were coated with protein isolated from wild (control) and transgenic plants for overnight at 4°C. The plate was then washed with a washing solution, PBST. Thereafter, wells were blocked with a blocking solution (PBST-BSA) for 1 h at 37°C. Primary antibodies against the antigen rCTB.ESAT-6.MPT-64 (E-cassette) were diluted in PBS (1:1,000 dilution ratios) and poured into the antigen-coated wells for an incubation period of 1 h. The coated wells were then washed with PBST and incubated for 1 h at 37°C with AP-conjugated secondary antibodies developed in goat and diluted in (1:3,000) ratios. After incubation, the coated wells were washed three times with PBST. For detection, 100 µL of pNPP (p-nitrophenyl phosphate) substrate was added to the wells. The enzyme-substrate reaction was blocked by adding 0.1 ml of stop solution in each well and absorbance was recorded at 405 nm on an ELISA plate reader.
Immunogenicity assay of cucumber expressed recombinant fusion protein (His6x.CTB.ESAT-6.MPT-64) in rabbit
Healthy thirteen-week-old white New Zealand female rabbits maintained at the Central Animal House, M. D. University (MDU), Rohtak (India) were used for the immunogenicity assay as per the approval and guidelines of the Institutional Animal Ethics Committee, MDU. The rabbits were divided into two groups: Group 1 (treatment group) and Group 2 (control group) fed orally on transgenic and the wild-type cucumber fruits, respectively. The group 1 rabbits were orally immunized by feeding 3 g transgenic plant material on 0- (first feeding), 7- (second feeding) and 14-days (third feeding) and serum was collected intravenously on 21 day. Changes in immunoglobulin G (IgG) levels in the serum of non-immunized (control) and immunized rabbits were determined by enzyme-linked immunosorbent assay (ELISA). Ninety six-wells of ELISA plate were coated with 1 µg antigen overnight at 4°C. Coated plate was washed with washing solution, PBST for 1 h. The wells were then blocked with a blocking solution (PBST-BSA) at 37°C for 1 h. Sera samples against the antigen were diluted in PBS (1:250 dilution ratios) and incubated with antigen coated wells for 1 h. The wells were then washed three times with PBST and incubated with an AP-conjugated secondary antibody developed in goat against rabbit IgG diluted in (1:3,000) ratio for 1 h at 37°C. After incubation, the coated wells were washed three times with PBST and substrate pNPP (0.1 ml) was added in each well. The enzyme-substrate reaction was blocked by adding 0.1 ml of stop solution in each well and absorbance was taken at 405 nm on an ELISA plate reader.
Results
Cloning, expression and purification of recombinant protein (His6x.CTB.ESAT-6.MPT-64) from E. coli and production of polyclonal antibodies.
The genes cassette, His6x.CTB.ESAT-6.MPT-64 (size 1.34 kb) was cloned into BamHI and SacI restriction enzyme sites of pET28a bacterial expression vector (5.37 kb) to yield a recombinant vector pET28a-His6x.CTB.ESAT-6.MPT-64 of 6.71 kb size on agarose gel electrophoresis (Fig. 2). This vector was transformed into the E. coli BL-21(DE3) cells and the PCR analysis of recombinant bacterial colonies showed amplification of 1.34 kb band (Fig. 3i). These bacterial colonies were induced by IPTG to express recombinant protein which was isolated and purified to homogeneity in a single step using Ni2+-NTA chromatography. The molecular weight of the protein estimated by the online protein molecular weight calculator ExPASY tool was 48.1 kDa. The SDS-PAGE analysis of cellular protein of bacterial culture induced by IPTG also confirmed increase in a major protein band of molecular weight 48.1 kDa with increase in induction time (Figs. 3ii, iii). The recombinant purified protein on introduction in rabbit induced anti-recombinant fusion protein polyclonal antibodies whose specificity to fusion protein was confirmed by western blot (Fig. 3iv).
Fig. 2.

Enzymatic digestion of pET28a-His6x.CTB.ESAT-6.MPT-64, Lane M = 1.0 kb DNA marker, Lane SD = Single digestion of pET28a-His6x.CTB.ESAT-6.MPT-64 with BamHI enzyme resulted into 6.71 kb long fragment, Lane DD = Double digestion of pET28a-His6x.CTB.ESAT-6.MPT-64 plasmid with BamHI and SacI enzyme showed two bands of sizes, 5.36 kb (vector) and 1.34 kb (insert)
Fig. 3.

i Colony PCR of E. coli BL21 (DE3) bacterial cells transformed with pET28a-His6x.CTB.ESAT-6.MPT-64. Lane M-1.0 kb DNA marker, Lanes 1–3 showed amplification of 1.34 kb size band corresponding to recombinant gene cassette, ii SDS-PAGE protein analysis of IPTG induced PCR positive BL21 (DE3) cells. Lane 1-protein form non-induced BL21 (DE3) bacterial cells, Lane 2-protein after 2 h of IPTG induction, Lane 3-protein after 6 h of IPTG induction. Lanes 2 and 3 showed increase in expression of recombinant protein of 48.1 kDa band size, iii SDS-PAGE analysis of purified His-tagged recombinant protein. Lane M-protein markers, Lane 1-purified protein with band size of 48.1 kDa, iv Western blot analysis of recombinant fusion protein specificity to Anti-rCTB.ESAT-6.MPT-64 antibodies developed in rabbit
The allergenicity of the recombinant protein was evaluated by online bioinformatics tool Allergen FP v.1.0. The analysis showed that the protein produced from the designed construct was non-allergen (https://ddg-pharmfac.net/AllergenFP/feedback.py) and safe to human.
Development of T0 cucumber plant with Mtb antigens
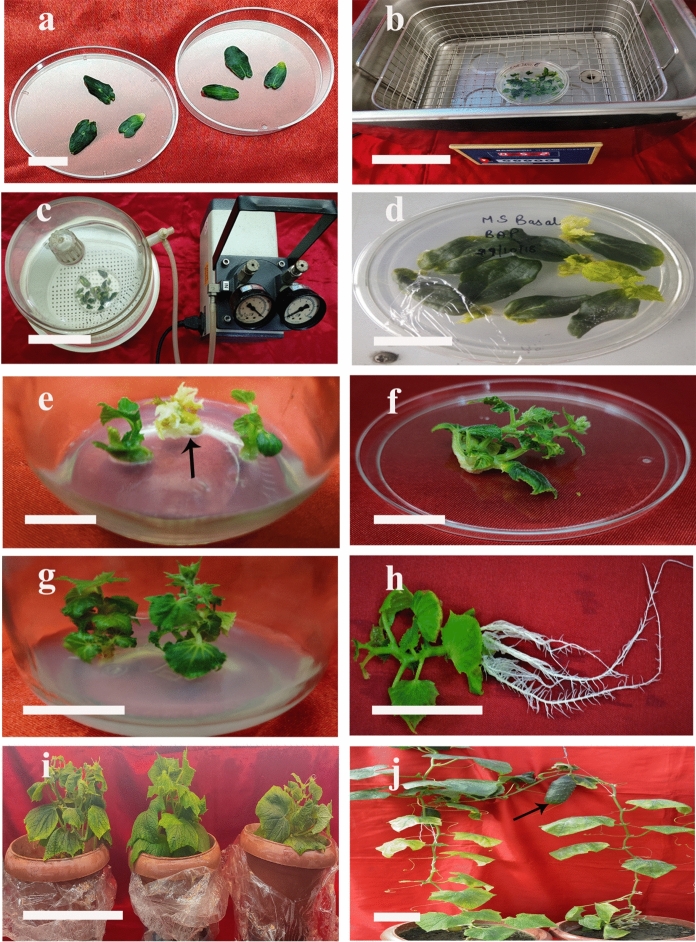
In three different experiments, overall 142 cotyledonary explants excised from 5 days old seedlings were sonicated and vacuum infiltrated with Agrobacterium (carrying pBI121 vector with gene cassette containing His6x.CTB.ESAT-6.MPT-64 genes) suspension (Figs. 4a–d). Agrobacterium-infected explants co-cultured in the presence of 6 mg L-cystein l−1 and 100 -µM acetosyringone on shoot selection medium with 80 mg kanamycin l−1 regenerated 55 healthy green presumptive transformed shoots (Figs. 4f–g) while non-transformed shoots turned colourless (Fig. 4e). Of 55 healthy shoots, 32 on rooting medium containing 20 mg kanamycin l−1 developed roots (Fig. 4 h). These rooted shoots were set in pots filled with soil for further growth and flowering (Table 2). Transgenic plants were similar to seed-raised plants in morphological characters (Fig. 4i) and they grew normally and produced female flowers which were artificially pollinated with the pollens from male flowers of the same plant. The pollinated female flowers developed into fruits (Fig. 4j) which were collected when their epidermis turned yellow.
Fig. 4.
a–j Genetic transformation of cucumber (Cucumis sativus) cv. Green long using Agrobacterium tumefaciens EHA105 (pBI121.CTB.ESAT-6.MPT-64). a cotyledonary explants excised from five-day-old seedlings, (bar = 1.5 cm), b sonication of explants dipped in Agrobacterium suspension in a bath type ultrasonic sonicator, (bar = 8.75 cm), c vacuum infiltration of cotyledonary explants with bacterial suspension in a vacuum chamber, (bar = 11.5 cm), d Agrobacterium infected cotyledonary explants cultured on the shoot selection medium, (bar = 1.8 cm), e transformed explants regenerating green putative transformed and albino (non-transformed, marked by arrow) shoots on the selection medium, (bar = 2.2), f multiple shoots regenerated from explants on the shoot selection medium, (bar = 2.5 cm), g regenerated shoots growing on the shoot selection medium, (bar = 3.5 cm), h a rooted putative transformed shoot (bar = 4.6 cm), i putative T0 transgenic plants established in soil (bar = 18 cm), j Mature fertile T0 plants with fruits (marked by arrow) (bar = 14 cm)
Table 2.
Genetic transformation of cucumber (Cucumis sativa) variety Green long S-82 using cotyledonary explants and Agrobacterium tumefaciens EHA105 (pBI121.CTB.ESAT-6.MPT64)
| Expt No | Number of explants infected with Agrobacterium suspension | No. of shoots regenerated on selection medium with 80 mg kanamycin l−1a |
No. of shoots rooted in presence of 20 mg kanamycin l−1b |
No. of transgenic plants established in soil | No. of plants + ve for E -cassette (CTB.ESAT-6.MPT-64) and nptII genes by PCR | Transformation efficiency (%) |
|---|---|---|---|---|---|---|
| 1 | 58 | 25 | 10 | 10 | 2 | 3.4 |
| 2 | 52 | 18 | 12 | 12 | 2 | 3.8 |
| 3 | 32 | 12 | 10 | 10 | 1 | 3.1 |
| Total | 142 | 55 | 32 | 32 | 5 | 3.5c |
aShoot selection medium: MS + 1.0 μM BAP + 250 mg cefotaxime.l−1 + 80 mg kanamycin.l−1
bRooting medium: MS + 2.0 μM IBA + 250 mg cefotaxime.l−1 + 20 mg kanamycin.l−1
cAverage
Molecular confirmation of T0 transgenic lines
Of the transformed plants survived in soil, 5 plants showed amplification of DNA fragments of 1.34 kb (Fig. 5a) and 540 bp (Fig. 5b) using primers specific to E-cassette and nptII, correspondingly, indicating the presence of E (His6x.CTB.ESAT-6.MPT-64) cassette and nptII genes in transformed plants. However, non-transformed (wild, control) plants did not show any band on the gel. An average transformation efficiency of 3.5% was derived from the number of transgenic plants positive for nptII and E-cassette genes divided by the total number of explants infected with Agrobacterium. The transgenic plants were further evaluated to confirm the absence of residual Agrobacterium contamination by PCR using virA gene specific primers. PCR results revealed that transgenic plants did not show any amplification with virA gene primers indicating the absence of Agrobacterium in transgenic plants (Fig. 5c).
Fig. 5.
Molecular analysis of T0 generation plants, a–b PCR analysis of putative T0 (Lanes 1 to 5) and non-transformed (Lane NT) plants using the primers specific to E-cassette CTB.ESAT-6.MPT-64 (a) and nptII (b) genes for the amplification of 1.34 kb and 540 bp bands corresponding to the coding regions of CTB.ESAT-6.MPT-64 and nptII genes, respectively. Lane P = positive (plasmid) control and, Lane M = 1 kb DNA marker. c PCR analysis of putative T0 (Lanes 1 to 5) and non-transformed (Lane N) plants using the primers specific to virA gene showed no amplification of band corresponding to the size of 1.0 kb of virA region, d Reverse transcriptase-PCR analysis of the mRNA transcript levels of gene, CTB.ESAT-6.MPT-64 in T0 (Lanes 1–5) and non-transformed (control, Lane NT) plants, Lane-M-1.0 kb DNA marker, e Southern blot analysis of five PCR positive T0 (Lanes 1 to 5) and non-transformed (control, Lane NT) plants, Genomic DNA of T0 and non-transformed (control) plants and pBI121.CTB.ESAT-6.MPT-64 digested with HindIII (positive control size of 14.20 kb, Lane P) and probed with a 1.34 kb biotin labelled CTB.ESAT-6.MPT-64 probe, Lane M = marker, f Western blot analysis of T0 (Lanes 1 to 5) and non-transformed (control, lane-NT) plants for the expression of the fusion protein, CTB.ESAT-6.MPT-64 of 48.1 kDa, Lane M-protein ladder
All the transgenic plants positive for transgenes by PCR were analyzed for the integration of transgene(s) and their copy numbers by the Southern blot analysis. The T-DNA of pBI121.His6x.CTB.ESAT-6.MPT-64 contains a single unique HindIII restriction site which just cuts T-DNA single time into a right side fragment of 3.14 kb containing E-cassette (His6x.CTB.ESAT-6.MPT-64) and a left side fragment of 2.5 kb with nptII gene cassette (Fig. 1). HindIII restriction endonuclease digested genomic DNA blot of transgenic and wild lines on hybridization with the E-cassette (1.34 kb long fragment) probe showed hybridization signals greater than the 3.14 kb junction fragments representing incorporation of E-cassette genes into the genome of cucumber plants. However, no probe hybridization signals were observed in wild forms (non-transgenic lines control) (Fig. 5e). Each transgenic line showed different patterns of hybridization signals on the blot membrane highlighting their origin as the independent transformation events. The number of hybridization signal in the form of bands obtained in T0 transgenic lines point out the copy number of E-cassette integrated into their genome. Three transgenic lines (T01, T04 and T05) showed a single copy insertion, while T02 and T03 lines showed two and three copies of inserts in their genomes, respectively (Fig. 5e).
The transcript expression levels of transgenes in both PCR and Southern positive transgenic plants was studied by subjecting the total isolated RNA to RT-PCR to synthesize cDNA using enzyme, reverse transcriptase and cDNA was amplified by PCR using E-cassette specific primers. RT-PCR revealed amplification of an expected size fragment of 1.34 kb equivalent to the E-cassette gene in transformed plants indicating the absence of gene silencing. The wild (control, non-transformed) plants did not show such amplification (Fig. 5d). The possibility of Agrobacterium contamination was also ruled out by the amplification of transgenes from the plant RNA template in RT-PCR.
The qRT-PCR was also used to estimate the transcript expression levels of E-cassette genes in all the 5 transgenic plants using a housekeeping gene, β-actin as a reference (Fig. 6). The expression level of E-cassette varied considerably in the transgenic and wild type plants. The Cq values of E-cassette gene of transgenic lines were presented in the Fig. 6. The Cq value differed considerably among the transgenic lines and an inverse correlation was noticed between Cq value and transgene expression level. The transgenic line T03 showed the lowest Cq value indicating the highest expression of E-cassette transcripts in this line due to the more copy number of transgenes as revealed by the Southern blot.
Fig. 6.
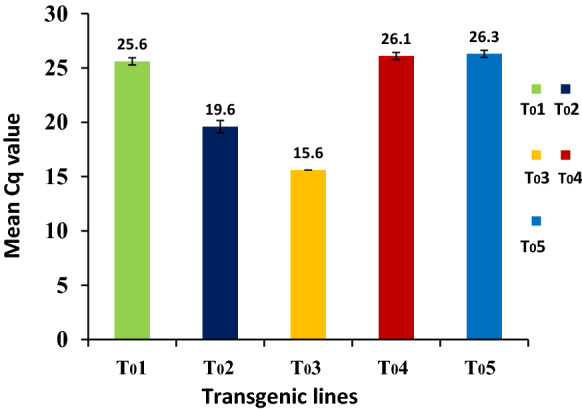
Real-time PCR analysis of five transgenic cucumber lines (T01 to T05) for the expression of CTB.ESAT-6.MPT-64 (E) cassette gene using β-actin gene, a housekeeping gene, as a reference. X-axis represents the different transgenic events and Y-axis represents the mean Cq value. The Cq mean value of the target gene is significantly different among T0 lines at P < 0.05 significance level
Confirmation of fusion protein expression by western blot and quantification analysis by ELISA
The total soluble proteins from the fruits of PCR and Southern hybridization confirmed plants were extracted and analyzed under reducing conditions of SDS-PAGE and western blotting using rabbit anti rHis6x.CTB.ESAT-6.MPT-64 polyclonal antibodies, for the expression of fused recombinant protein of E-cassette genes. The western blot showed the presence of fused recombinant proteins with single band of expected size ~ 48.1 kDa corresponding to the monomer form of ESAT-6, MPT-64 and CTB fused proteins with his-tag. However, in the wild type (control) plants no such band was observed (Fig. 5 f). A linear standard curve was plotted between the mean OD values and the amount, 2.5–160 ng of recombinant E-cassette (ESAT-6, MPT-64 and CTB) protein antigen expressed and purified from E. coli, and coated onto the ELISA plate wells for the quantification of E-cassette (ESAT-6, MPT-64 and CTB) protein expressed in cucumber transgenic plants. In T03 transgenic plant line, the maximum accumulation of E-cassette protein up to 478 ng g−1, i. e., 0.030% (v/v) of total soluble protein was estimated (Fig. 7).
Fig. 7.

Quantification of the fusion protein, CTB.ESAT-6.MPT-64 in transgenic (T01 to T05) and non-transgenic (NT) cucumber lines by ELISA method. Anti-rCTB.ESAT-6.MPT-64 specific primary antibodies were used to analyze the total soluble protein of transformed cucumber lines. The limit of detection (LOD) was 0.115628. X-axis represents the different transgenic events and Y-axis represents the amount of protein (ng/g) in transgenic lines. Graph Pad Prism 9.5.0 was used for statistical analysis. Significance levels in different transgenic lines are: *P < 0.05, **P < 0.01, ***P < 0.001, ns-non significant
Immunogenecity of fusion E-cassette protein in laboratory animal after oral immunization
The immunological response of recombinant E-cassette protein expressed in transgenic plants was assessed by feeding their fruits to white New Zealand female rabbits. Orally immunised rabbit with transgenic plants showed a significant increase in serum IgG antibodies specific to the recombinant E-cassette protein compared with rabbit fed with wild (non-transgenic) type plants. The mean OD values of orally vaccinated animal were forty times higher than those of non-vaccinated ones (Fig. 8).
Fig. 8.

Immunization of rabbits by oral feeding of transgenic cucumber T0 lines on 0, 7 and 14 days (Group 1, treated) and wild-type (non-transgenic) cucumber (Group 2 (control). Increase in serum IgG concentration in group 1 rabbits on 21 day of immunization was statistical significant at P < 0.05 as shown by an asterisk (*)
Discussion
Vaccination is one of the most effective ways to control infectious diseases like TB. The only solitary authorized vaccine BCG against TB is useful only in children but not against adult pulmonary TB that necessitates development of an affordable vaccine on a large scale and efficacious at all age groups. Expression of recombinant protein in E. coli is an expedient and time-saving approach but may be contaminated with endotoxins. In this study, recombinant antigens fusion protein expressed and purified from E. coli was effective in producing polyclonal antibodies in rabbit whose specificity to fusion protein was settled by western blot analysis (Fig. 3iv). However, plant-based production of bacterial/viral antigens (subunit vaccines) in edible plant parts have emerged advantageous over fermenters based microbial (bacterial and yeast) and animal cells due to their safety, high scalability, easy to produce and low-cost. Moreover, oral delivery of antigens targeting respiratory mucosa, the site of invasion of Mtb, is beneficial even at the low expression of target antigen in transgenic plants in inducing specific immune responses (Uvarova et al. 2013; Saba et al. 2019a, b). Until now the plant-based vaccines against TB have expressed a single antigen at a time in plants and very few studies have expressed more than one antigen. Highly immunogenic early secretory antigenic proteins specific to Mtb like ESAT-6, CFP 10, MPT64, Ag85A, Ag85B, Mtb72F and LipY are attractive antigens for vaccine against TB. M. tuberculosis secretes a 6 kDa early secretory antigenic target (ESAT-6) proteins into the host via ESX-1 system that plays role in Mtb virulence and pathogenesis (Jha et al. 2020). Mtb genomic region RD1 carries the coded information for the secretion of ESAT-6 but this region is absent in BCG vaccine strain (Lewis et al. 2003). ESAT-6 interacts with a small 13 kDa host protein, beta-2-microglobulin (β2M) causing suppression of major histo-compatibility complex 1 (MHC-I) antigen presentation (Sreejit et al. 2014). ESAT-6 deposes the immune response by inducing the granuloma formation through activation and differentiation of macrophages inside the host body. This tricky behaviour leads to persistent infection (Refai et al. 2018). MPT-64, a 24 kDa secretory protein, is a major antigen specific for Mtb only and is absent in BCG strains (Hasegawa et al. 2002; Kumar et al. 2011). MPT-64 is often used as a candidate protein for diagnosis of pediatric extrapulmonary tuberculosis and pulmonary TB (PTB), and in vaccines (Xiao et al. 2019; Sakashita et al. 2020; Cao et al. 2021). Some of the Mtb antigens have been previously expressed in plants using nuclear (Floss et al. 2010; Uvarova et al. 2013, Saba et al. 2019b) and chloroplast transformations (Lakshmi et al. 2013; Módolo et al. 2018; Saba et al. 2019a). However, chloroplast transformation has been perfected only in a few plant species. Most of the attempts for plant-based TB vaccine have been made using nuclear transformation and that too with model plant species that are unsuitable for human use. Only two edible crops like carrot and broccoli have been used for TB vaccine production (Permyakova et al. 2015; Saba et al. 2019b). The present study was carried out on an important budget-friendly vegetable crop, cucumber which is grown world over for its immature fruits that are eaten raw. The fruits have nutritional values due to their high water (95%) content, vitamins (B, C, and folate), minerals (potassium, sodium, magnesium etc.), polyphenol (lignans), fibers and no fat (low calorie) and are feasible to freeze-dried (Sharma et al. 2020). Although genetic transformation of cucumber has been reported earlier by several groups, it is still tricky to achieve at high frequency and to carry out functional analysis using transgenic plants (Hu et al. 2017; Zhang et al. 2017; Nanasato and Tabei 2020). In the present work, transgenic cucumber lines simultaneously expressing dual Mtb antigens (that are absent in BCG) fused with CTB (gut mucosal adjuvant) have been developed for the first time using a high yielding commercially valuable pure line than an F1 hybrid. The Southern blot, qRT-PCR and ELISA analyses revealed nuclear integration, copy number, expression and quantification of antigen genes/proteins in five transgenic cucumber lines (Figs. 5, 6 and 7). Transformation frequency ranging between 1.1 and 1.7% was obtained earlier using nptII/kanamycin selection system (Chee 1990; Vengadesan et al. 2005; Rajagopalan and Perl-Treves 2005; Hu et al. 2017). With the same selection system, the genetic transformation efficiency of an Indian cucumber cultivar in this work has been improved to 3.5% (Table 2) by wounding the most widely used explants for transformation, cotyledons with sonicator prior to Agrobacterium vacuum infiltration to enhance the Agrobacterium access to the deeper cell layers (Nanasato et al. 2013; Hu et al. 2017; Nanasato and Tabei 2020). However, the vacuum infiltration in Chinese and Japanese cucumber cultivars induced a much higher transformation frequency with Agrobacterium-suspension and kanamycin as a selective agent (Zhang et al. 2017; Nanasato and Tabei 2020). This indicates that the genetic transformation efficiency depends on the plant genotype. The expression of the fusion recombinant CTB.ESAT-6.MPT-64 protein in transgenic cucumber is 0.030% of the total soluble protein which is similar to the previously expressed ESAT 6 antigen in edible plants using nuclear transformation (Uvarova et al. 2013, Permyakova et al. 2015) but lower than those reported in broccoli (Saba et al. 2019b). The difference in expression may be due to the difference in plant species, environment conditions and regulatory elements present in expression cassette. The protein expressions among transgenic lines differ due to the insertion of transgene at different locations in the plant genome and their copy number. These transgenic lines on oral administration in rabbits induced the humoral and cell-mediated immune responses. Statistical analysis of sera showed significantly higher (P < 0.05) production of IgG in rabbits fed with transgenic cucumber as compared to those fed on wild (control) type (Fig. 8). Data from the previous experiments on mice indicates that the ESAT-6 protein is highly immunogenic and can induce immune responses (Saba et al. 2019b). ESAT-6 in combination of MPT-64 elicits immune responses against different targets and is highly specific for strong delayed-type hypersensitivity (DTH) responses. Oral delivery of plant-based subunit vaccine as raw biomass has huge benefits like safety, cost-effectiveness, easy to transport, long storage, and no purification, cold chain and sterile injections. Such edible vaccine is highly beneficial for developing countries where the incidences and transmission of TB are high. The main problems of oral immunization like the degradation and denaturation of recombinant protein in harsh environment of the digestive tract have been overcome by the encapsulation of vaccine candidate inside the plant cell and its absorption through the gut epithelium via the transmucosal carrier, CTB. This study has facilitated the development of cucumber as self-adjuvanted edible vaccine for the simultaneous expression of two Mtb-specific antigenic proteins fused with CTB to induce humoral and cell-mediated immune responses against TB.
Acknowledgements
PKJ and JY are grateful to the UGC, New Delhi for the award of the BSR Faculty Fellowship and Senior Research Fellowship, respectively. SP is thankful to the CSIR, New Delhi for the award of Junior Research Fellowship.
Author contributions
JY, SP: Investigation, methodology, Collection, analysis and interpretation of data, writing-original draft. DC: Project administration, resources, writing-review and editing. RJ: Conceptualization, supervision, writing-Review and editing. PKJ: Conceptualization, study design, writing-Review and editing.
Funding
No specific funding has been received for this work.
Data availability
The data generated or analyzed during the current study have been incorporated in this manuscript.
Declarations
Conflict of interest
The authors declare no conflicting interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Bradford MM. A rapid and sensitive method for the quantisation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cao XJ, Li YP, Wang JY, et al. MPT64 assays for the rapid detection of Mycobacterium tuberculosis. BMC Infect Dis. 2021;21:336. doi: 10.1186/s12879-021-06022-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee PP. Transformation of Cucumis sativus tissue by Agrobacterium tumefaciens and the regeneration of transformed plants. Plant Cell Rep. 1990;9:245–248. doi: 10.1007/BF00232293. [DOI] [PubMed] [Google Scholar]
- Floss DM, Mockey M, Zanello G, Brosson D, Diogon M, Frutos R, Bruel T, Rodrigues V, Garzon E, Chevaleyre C. Expression and immunogenicity of the mycobacterial Ag85B/ESAT-6 antigens produced in transgenic plants by elastin-like peptide fusion strategy. Biomed Res Int. 2010 doi: 10.1155/2010/274346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghebreyesus TA (2020) WHO Director-General’s opening remarks at the media briefing on COVID-19 - 11 March 2020. Geneva: World Health Organization
- Global Tuberculosis Report (2021) WHO, https://ddg-pharmfac.net/AllergenFP/feedback.py
- Hasegawa N, Miura T, Ishii K, Yamaguchi K, Lindner TH, Merritt S, Matthews JD, Siddiqi SH. New simple and rapid test for culture confirmation of Mycobacterium tuberculosis complex: a multicenter study. J Clin Microbiol. 2002;40:908–912. doi: 10.1128/JCM.40.3.908-912.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu B, Li D, Liu X, Qi J, Gao D, Zhao S, Huang S, Sun J, Yang L. Engineering non-transgenic gynoecious cucumber using an improved transformation protocol and optimized CRISPR/Cas9 system. Mol Plant. 2017;10:1575–1578. doi: 10.1016/j.molp.2017.09.005. [DOI] [PubMed] [Google Scholar]
- Jha V, Pal R, Kumar D, Mukhopadhyay S. ESAT-6 protein of Mycobacterium tuberculosis increases holotransferrin-mediated iron uptake in macrophages by down regulating surface hemochromatosis protein HFE. J Immunol (Baltimore, Md.:1950) 2020;205:3095–3106. doi: 10.4049/jimmunol.1801357. [DOI] [PubMed] [Google Scholar]
- Joshi H, Kandari D, Bhatnagar R. Insights into the molecular determinants involved in Mycobacterium tuberculosis persistence and their therapeutic implications. Virulence. 2021;12:2721–2749. doi: 10.1080/21505594.2021.1990660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann SHE. Vaccine development against tuberculosis over the last 140 years: Failure as part of success. Front Microbiol. 2021;12:750124. doi: 10.3389/fmicb.2021.750124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar VG, Urs TA, Ranganath RR. MPT 64 antigen detection for rapid confirmation of M. tuberculosis isolates. BMC Res Notes. 2011;4:79. doi: 10.1186/1756-0500-4-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon KC, Verma D, Singh ND, Herzog R, Daniell H. Oral delivery of human biopharmaceuticals, autoantigens and vaccine antigens bioencapsulated in plant cells. Adv Drug Deliv Rev. 2013;65:782–799. doi: 10.1016/j.addr.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshmi PS, Verma D, Yang X, Lloyd B, Daniell H. Low cost tuberculosis vaccine antigens in capsules: expression in chloroplasts, bio-encapsulation, stability and functional evaluation in vitro. PLoS One. 2013;8:e54708. doi: 10.1371/journal.pone.0054708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis KN, Liao R, Guinn KM, Hickey MJ, Smith S, Behr MA, Sherman DR. Deletion of RD1 from Mycobacterium tuberculosis mimics Bacilli Calmette-Guérin attenuation. J Infect Dis. 2003;187:117–123. doi: 10.1086/345862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mane SS. Tuberculosis: the silent pandemic. Curr Sci. 2022;123:835–836. [Google Scholar]
- Módolo DG, Horn CS, Soares JSM, Yunes JA, Lima LM, de Sousa SM, Menossi M. Transgenic Nicotiana tabacum seeds expressing the Mycobacterium tuberculosis alanine and proline-rich antigen. AMB Express. 2018;8:178. doi: 10.1186/s13568-018-0708-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue culture. Plant Physiol. 1962;15:473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- Nanasato Y, Tabei Y. A method of transformation and current progress in transgenic research on cucumbers and Cucurbita species. Plant Biotechnol. 2020;37:141–146. doi: 10.5511/plantbiotechnology.20.0225a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanasato Y, Konagaya K, Okuzaki A, Tsuda M, Tabei Y. Improvement of Agrobacterium-mediated transformation of cucumber (Cucumis sativus L.) by combination of vacuum infiltration and co-cultivation on filter paper wicks. Plant Biotechnol Rep. 2013;7:267–276. doi: 10.1007/s11816-012-0260-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemes E, Abrahams D, Scriba TJ, Ratangee F, Keyser A, Makhethe L, et al. Diagnostic accuracy of early secretory antigenic target-6–free interferon-gamma release assay compared to quantiFERON-TB gold in-tube. Clin Infect Dis. 2019 doi: 10.1093/cid/ciz034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Permyakova NV, Zagorskaya AA, Belavin PA, Uvarova EA, Nosareva OV, Nesterov AE. Transgenic carrot expressing fusion protein comprising M. tuberculosis antigens induces immune response in mice. Biomed Res Int. 2015 doi: 10.1155/2015/417565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagoplan PA, Perl-Treves R. Improved cucumber transformation by a modified explant dissection and selection protocol. Hortic Sci. 2005;40:431–435. [Google Scholar]
- Refai A, Gritli S, Barbouche MR, Essafi M. Mycobacterium tuberculosis virulent factor ESAT-6 drives macrophage differentiation toward the pro-inflammatory M1 phenotype and subsequently switches it to the anti-inflammatory M2 phenotype. Front Cell Infect Microbiol. 2018;8:327. doi: 10.3389/fcimb.2018.00327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers SO, Bendich AJ. Extraction of DNA from plant tissues Plant Mol Biol manual. Dordrecht: Kluwer Academic Publishers Springer; 1989. pp. 73–83. [Google Scholar]
- Saba K, Gottschamel J, Younus I, Syed T, Gull K, Lossl AG, Mirza B, Waheed MT. Chloroplast-based inducible expression of ESAT-6 antigen for development of a plant-based vaccine against tuberculosis. J Biotechnol. 2019;305:1–10. doi: 10.1016/j.jbiotec.2019.08.016. [DOI] [PubMed] [Google Scholar]
- Saba K, Sameeullah M, Asghar A, Gottschamel J, Latif S, Lossl AG, Mirza B, Mirza O, Waheed MT. Expression of ESAT-6 antigen from Mycobacterium tuberculosis in broccoli: an edible plant. Biotech Appl Biochem. 2019;67:148–157. doi: 10.1002/bab.1867. [DOI] [PubMed] [Google Scholar]
- Sakashita K, Takeuchi R, Takeda K, Takamori M, Ito K, et al. Ultrasensitive enzyme-linked immunosorbent assay for the detection of MPT64 secretory antigen to evaluate Mycobacterium tuberculosis viability in sputum. Int J Infect Dis. 2020;96:244–256. doi: 10.1016/j.ijid.2020.04.059. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. New York: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- Sharma V, Sharma L, Sandhu KS. Cucumber (Cucumis sativus L.) In: Nayik GA, Gull A, editors. Antioxidants in vegetables and nuts - Properties and health benefits. Singapore: Springer; 2020. pp. 333–340. [Google Scholar]
- Sreejit G, Ahmed A, Parveen N, Jha V, Valluri VL. The ESAT-6 protein of Mycobacterium tuberculosis interacts with beta-2-microglobulin (β2M) affecting antigen presentation function of macrophage. PLOS Pathog. 2014;10:e1004446. doi: 10.1371/journal.ppat.1004446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uvarova EA, Belavin PA, Permyakova NV, Zagorskaya AA, Nosareva OV, Kakimzhanova AA, Deineko EV. Oral immunogenicity of plant-made Mycobacterium tuberculosis, ESAT6 and CFP10. Biomed Res Int. 2013 doi: 10.1155/2013/316304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vengadesan G, Anand RP, Selvaraj N, Perl-Treves R, Ganapthi A. Transfer and expression of nptII and bar Genes in cucumber (Cucumber sativus L.) In Vitro Cell Dev Biol. 2005;41:17–21. doi: 10.1079/IVP2004602. [DOI] [Google Scholar]
- Wallis J, Shenton DP, Carlisle RC. Novel approaches for the design, delivery and administration of vaccine technologies. Clin Exp Immunol. 2019;196:189–204. doi: 10.1111/cei.13287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SL, Ku SS, Ye XG, et al. Current status of genetic transformation technology developed in cucumber (Cucumis sativus L.) J Integr Agric. 2015;14:469–482. doi: 10.1016/S2095-3119(14)60899-6. [DOI] [Google Scholar]
- Wang N, Qiu C, Chen M, Liu T, Wang T. Covering aluminum oxide nanoparticles with biocompatible materials to efficiently deliver subunit vaccines. Vaccines. 2019;7:52. doi: 10.3390/vaccines7020052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (2022) Tuberculosis-key facts, file:///C:/Users/Sony/Desktop/Jyoti-Sep,%202022–2/Tuberculosis.html
- Xiao T, Jiang Y, Li G, Pang H, Zhao L, Zhao X, et al. Polymorphism of MPT64 and PstS1 in Mycobacterium tuberculosis is not likely to affect relative immune reaction in human. Medicine (Baltimore) 2019;98:e18073. doi: 10.1097/MD.0000000000018073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav H, Malik K, Parmar S, Kumar S, Jaiwal PK. Generation of polyclonal antibodies against recombinant Agrobacterium tumefaciens decaprenyl diphosphate synthase produced in Escherichia coli. J Plant Biochem Biotech. 2021;30:487–495. doi: 10.1007/s13562-020-00635-z. [DOI] [Google Scholar]
- Zhang Y, Chen S, Liu JY, Yuanlei Hu, Hong C. Oral immunogenicity of potato-derived antigens to Mycobacterium tuberculosis in mice. Acta Biochim Biophys Sin. 2012;44:823–830. doi: 10.1093/abbs/gms068. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Li X, Ma S, Shan N, Zhang X, Sui X. A protocol for Agrobacterium- mediated transformation of cucumber (Cucumis sativus L.) from cotyledon explants. Protoc Exch. 2017 doi: 10.1038/protex.2017.107. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data generated or analyzed during the current study have been incorporated in this manuscript.