Abstract
In the central nervous system, nitric oxide (NO), a free gas with multitudinous bioactivities, is mainly produced from the oxidation of L-arginine by neuronal nitric oxide synthase (nNOS). In the past 20 years, the studies in our group and other laboratories have suggested a significant involvement of nNOS in a variety of neurological and neuropsychiatric disorders. In particular, the interactions between the PDZ domain of nNOS and its adaptor proteins, including post-synaptic density 95, the carboxy-terminal PDZ ligand of nNOS, and the serotonin transporter, significantly influence the subcellular localization and functions of nNOS in the brain. The nNOS-mediated protein-protein interactions provide new attractive targets and guide the discovery of therapeutic drugs for neurological and neuropsychiatric disorders. Here, we summarize the work on the roles of nNOS and its association with multiple adaptor proteins on neurological and neuropsychiatric disorders.
Keywords: nNOS, nNOS-PSD95, nNOS-CAPON, nNOS-SERT, Neurological and neuropsychiatric disorder
Introduction
Three genetically different isoforms of nitric oxide synthase (NOS), namely neuronal NOS (nNOS or NOS-Ι), inducible NOS (iNOS or NOS-II), and endothelial NOS (eNOS or NOS-III), account for nitric oxide (NO) production [1]. nNOS, a Ca2+-dependent constitutive synthase, is mainly expressed in neurons and is strictly regulated by N-methyl-D-aspartate receptor (NMDAR)-mediated changes in the concentration of intracellular Ca2+ [2]. In the central nervous system (CNS), nNOS functions by producing NO and peroxynitrite [1–4]. Different from its isoenzymes iNOS and eNOS, the nitrogen terminal of nNOS contains PDZ (spot-synchronous density protein, discs-large, ZO-1) domains, an additional N-terminal extension mostly involved in specific subcellular targeting [5] (Fig. 1). Proteins with PDZ domains, such as postsynaptic density-95 (PSD-95), can directly bind to nNOS via PDZ/PDZ interaction [1, 2]. The docking of the last 3 residues at the C-terminus of the peptide ligand into a binding pocket constituted by the αB helix and βB fold of the PDZ domain is the canonical PDZ/PDZ interaction [6]. However, the core PDZ domain of nNOS is not itself the binding partner of PSD-95-type PDZ domains [7, 8]. In other words, the interaction between nNOS and PSD-95 is not a canonical PDZ/PDZ interaction. nNOS PDZ has a peptide-binding groove between a β sheet and an α helix [6]. Apart from nNOS-PSD-95 coupling, the PDZ domain of nNOS also binds to NOS1AP (nNOS adaptor protein, previously termed CAPON: C-terminal PDZ-domain ligand of neuronal NOS) in neurons via peptide/PDZ interaction [9]. Although the C-terminal sequence of CAPON is not completely consistent with the Asp/Glu-X-Val motif, an optimal nNOS PDZ binding sequence [10], it binds to the same pocket of the nNOS PDZ domain [11]. Serotonergic signaling is critically regulated by the reuptake of 5-HT via the serotonin transporter (SERT). Recently, it has been demonstrated that the C-terminal sequence of SERT is another nNOS PDZ binding motif, and the physical interaction between nNOS and SERT controls the cell surface localization and activity of SERT [3]. Moreover, nNOS also interacts with clathrin assembly lymphoid leukemia, Ca2+/calmodulin-dependent protein kinase II alpha, Disks large homolog 4, DLG2, 6-phosphofructokinase (muscle type), syntrophin, and dynein light chain [2]. In the past 20 years, our group has investigated the roles of nNOS and its coupling proteins in stroke, major depressive disorder (MDD), generalized anxiety disorder (GAD), post-traumatic stress disorder (PTSD), Alzheimer's disease (AD), and chronic pathological pain, and revealed several new therapeutic targets and discovered several innovative candidate drugs [12–17], among which, ZL006-05 is going through a phase II clinical trial (sFDA, Registration No: CTR20221109, http://www.chinadrugtrials.org.cn/clinicaltrails.searchlistdetail.dhtml).
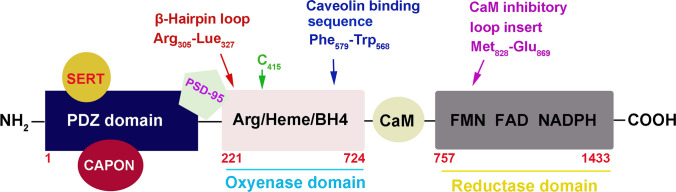
Fig. 1.
Domain structure of human nNOS. PDZ domains, oxygenase, and reductase are denoted by solid boxes in different colors, and the amino-acid residue number is shown at the start/end of each domain. Sequence locations of the N-terminal hairpin loop, heme-binding cysteine (C415), the caveolin binding consensus sequence, and the putative CaM autoinhibitory loop are noted. The structural organization of iNOS and eNOS are similar to nNOS except that they do not contain a PDZ domain and the iNOS reductase domain lacks the CaM autoinhibitory loop. The possible binding sites of SERT, CAPON, and PSD-95 with nNOS PDZ are indicated.
nNOS and stroke
Stroke is a major public health problem leading to high rates of death and disability in adults. Among strokes, ischemic stroke accounts for >70% [18]. After a stroke, iNOS is expressed in the brain of rodents and humans and participates in the deleterious effects of inflammation [19]. Animal studies suggest the existence of the ischemic penumbra, a brain region that is destined to die with time due to reduced cerebral blood flow [20]. The time-dependent enlargement of infarct volume after stroke suggests that more and more ischemic penumbra is recruited into infarction with elapsing time. More recently, penumbral identification imaging studies have verified the progression of the ischemic core at the expense of the penumbra [21]. Thus, the salvage of ischemic penumbra by pharmacological agents or physical means is a key therapeutic strategy for ischemic stroke. However, the precise pathophysiological mechanisms underlying the recruitment of the ischemic penumbra into infarction remain to be clarified. Our study suggested that cerebral ischemia-induced iNOS expression is involved in the recruitment of penumbra into infarction after stroke in mice [22], and curcumin, the active ingredient of the Curcuma longa plant, can prevent cerebral ischemic injury by inhibiting the expression of iNOS in rats [23]. Therefore, inhibition of iNOS expression or activity may stop or slow the recruitment of the penumbra into infarction and may offer a valuable therapeutic strategy to selectively target the delayed phase of the damage. At the same time, however, we found that the ischemia-induced iNOS expression is necessary for neurogenesis [22, 24], implicating it in brain repair after stroke. The double faces of iNOS make the prospect of iNOS inhibitors in the treatment of stroke dim. The use of nNOS inhibitors or nNOS knockout (KO) ameliorates cerebral ischemic damage in mice [25, 26]. Moreover, nNOS inhibition not only has a very significant protective effect against stroke [25] but also promotes neurogenesis [27, 28]. After revealing that the activation of cAMP response element binding protein (CREB) is necessary for stroke-induced neurogenesis [29], we demonstrated that nNOS-derived NO inhibits neurogenesis by down-regulating CREB phosphorylation in the normal [30] and ischemic hippocampus of rodents [31]. These studies suggest that nNOS is an attractive target for stroke treatment.
nNOS and neuropsychiatric disorders
In addition to stroke, nNOS also plays a key role in neuropsychiatric disorders, such as MDD and GAD. Neuropsychiatric disorders are complex, heterogeneous disorders and rank among the top causes of disease burden and disability worldwide [32, 33]. Increasing data suggest that life stress leads to enhancement of nNOS expression and activity in brain regions including the cortex, hippocampus, amygdala, hypothalamus, dorsal raphe nucleus, striatum, basal ganglia, and locus coeruleus, and selective nNOS inhibitors have antidepressant-like and anxiolytic effects [4, 34]. Because nNOS-derived NO exerts a negative control on the neurogenesis in the adult hippocampus [27, 28, 30] and hippocampal neurogenesis is necessary for the behavioral effects of antidepressants [35], we investigated whether nNOS regulates depressive behaviors by affecting neurogenesis. We found that nNOS KO or using an nNOS inhibitor in mice reverses the chronic mild stress (CMS)-induced behavioral despair and hippocampal neurogenesis impairment, and disrupting hippocampal neurogenesis abolishes the antidepressant effect of nNOS inhibition, implicating nNOS-mediated neurogenesis impairment in MDD [36]. Moreover, our study indicated that nNOS-derived NO downregulates hippocampal glucocorticoid receptor expression through both soluble guanylate cyclase/cGMP and peroxynitrite ONOO(-)/extracellular signal-regulated kinase (ERK) signal pathways, and thereby activates the hypothalamic-pituitary-adrenal axis and leads to depressive behaviors in mice [37].
The serotonergic system has long been implicated in the pathogenesis of MDD and GAD. Among the various 5-hydroxytryptamine (5-HT) receptor subtypes, the 5-HT1A receptor (5-HT1AR) has been predominantly implicated in the modulation of anxiety-related behaviors [38]. We found that hippocampal nNOS is a negative regulator of the anxiolytic effects of 5-HT1AR agonists and selective serotonin reuptake inhibitors (SSRIs) [39]. A female preponderance of MDD and GAD is universal and substantial, however, the mechanisms underlying the female preponderance in affective disorders are poorly understood. We revealed a molecular mechanism underlying the sex difference in affective behaviors. The basal NO level in the hippocampus of female mice is substantially lower than that in the male hippocampus. CMS leads to excessive NO production in the male hippocampus because of the upregulation of nNOS by glucocorticoid release, whereas it causes a NO shortage in the female hippocampus because of the downregulation of nNOS by decreased estrogen. Importantly, the sex gap in affective behaviors disappears when eliminating the difference in hippocampal NO levels between males and females [40]. However, the mechanism underlying the sex difference in affective behaviors is complex and influenced by various factors. Differences in immune function, the gene co-expression networks, and amygdala activation patterns and connectivity may also be possible causes of sex differences in MDD [41–45]. Although NO produced by nNOS in the hippocampus is involved in sex differences in emotional behavior, the actual reason for the lack of NO in the female hippocampus remains to be further explored. Moreover, we found that excitatory neurons in the posterior subregion of the paraventricular thalamic nucleus (pPVT) drive chronic pain-induced anxiety behaviors through the activation of nNOS-expressing neurons in the ventromedial prefrontal cortex (vmPFC), resulting in NO-mediated AMPAR trafficking in vmPFC pyramidal neurons, suggesting that nNOS is involved in chronic pain-induced anxiety behaviors [46]. More recently, we found that nNOS in the nucleus accumbens of mice specifically mediates susceptibility to social defeat stress through cyclin-dependent kinase 5, which may explain how the brain transduces social stress exposure into depressive symptoms [47].
Long-term potentiation (LTP) contributes to certain forms of learning and memory [48]. Inhibition of nNOS can cause a major loss of LTP, particularly of late LTP in the hippocampus [49]. Moreover, nNOS is involved in the LTP in the stratum radiatum [50]. nNOS KO mice have major deficits in the acquisition of contextual fear conditioning, social behavior, nocturnal motor coordination, and remote spatial memory [51–53], and display a large increase in aggressive behavior and excessive, inappropriate sexual behavior [54]. These findings diminish the hope for nNOS inhibitors in treating stroke and neuropsychiatric disorders.
nNOS-PSD-95 interaction and neurological diseases
Because directly inhibiting nNOS may cause neurological and affective problems, we turn our attention to how to indirectly interfere with nNOS after stroke. Overstimulation of NMDARs leads to cerebral ischemic damage, which depends on NMDAR-mediated Ca2+ influx and the association of nNOS with PSD-95, a scaffolding protein at excitatory synapses [55, 56]. Because of the nNOS-PSD-95 interaction, a large amount of NO is produced within minutes after ischemic stroke, resulting in a cascade of excitotoxicity reactions [1, 2, 57]. Brain nNOS is distributed mainly in the cytosol and is targeted to membranes by binding to PSD-95 [1]. We thus hypothesized that stroke may induce the translocation of nNOS from the cytosol to the membrane via the nNOS-PSD-95 interaction, and the drugs dissociating nNOS-PSD-95 may hinder the nNOS translocation, and thereby prevent stroke damage. The work of Zhou et al. in our lab tested the hypothesis. We found that ischemia-induced NMDAR overactivation provokes nNOS translocation from cytosol to the membrane through the nNOS-PSD-95 interaction, that the translocation is necessary for NMDAR-dependent neuronal death, and that disrupting the nNOS-PSD-95 interaction prevents cerebral ischemic injury in mice and rats [58]. The key structural basis of nNOS-PSD-95 association is an intra-nNOS salt bridge between Asp62 of the PDZ domain and Arg121 of the β-finger domain, and residues Leu107 to Phe111 on the β-finger of nNOS contribute to conformational changes induced by their binding to PSD-95 [8]. Based on this, we designed and developed the small molecule nNOS-PSD-95 inhibitor ZL006. This drug can prevent ischemic stroke damage without affecting NMDARs function and the catalytic activity of nNOS, avoiding major side-effects of NMDARs antagonists and nNOS inhibitors [58]. This finding opens up the possibility that dissociating nNOS-PSD-95 with small-molecule drugs and brings hope for clinical therapeutics for stroke. To develop an nNOS-PSD-95 inhibitor with high affinity, a cyclic nNOS β-hairpin mimetic peptide with natural and unnatural amino-acids was recently designed [59]; this may serve as a template for the development of strong anti-stroke drugs. Moreover, using a humanized ischemic stroke model in three-dimensional cerebral organoids derived from human pluripotent stem cells, Miao's group demonstrated the beneficial effect of ZL006 [60]. Interestingly, a nanocarrier based on liposome conjugated with T7 peptide and stroke homing peptide allows the drug to easily penetrate the blood-brain barrier to increase the distribution in the brain and thereby enhance the anti-stroke effect of ZL006 [61, 62]. These findings strengthen the clinical translation potential of ZL006.
The mammalian brain has the ability to rewire itself to restore lost functions. We investigated the role of nNOS-PSD-95 association in brain repair after stroke and found that nNOS derived from neurons and neural stem cells bidirectionally regulates neurogenesis through CREB signaling [63]. The nNOS-PSD-95 association negatively controls neurogenesis and neuroregeneration via upregulating histone deacetylase 2 (HDAC2), and blocking nNOS-PSD-95 during the repair phase improves the stroke outcome by promoting regenerative repair in rats [64]. In addition, we found that ZL006 positively regulates the fate of transplanted NSCs and benefits the functional outcome after stroke in rats [65]. A narrow therapeutic window limits current therapies for stroke patients. Our studies showed that ischemia-induced HDAC2 upregulation after stroke causes secondary functional loss by reducing the survival and neuroplasticity of peri-infarct neurons as well as augmenting neuroinflammation and inhibiting HDAC2 can promote functional recovery from stroke in rodents, opening a new time window for its treatment [66, 67]. We also showed that the ischemia-induced nNOS-PSD-95 association in the peri-infarct area of mice lasts at least until day 7 after stroke and the association causes excessive tonic gamma-aminobutyric acid (GABA) inhibition due to GABA release from reversed GABA transporter-3/4 (GAT-3/4) in reactive astrocytes. Treatment with ZL006 after stroke inhibits astrocyte activation and GABA production, prevents the reversal of GAT-3/4, and consequently promotes functional recovery from stroke [68]. In addition, a study has shown that ZL006 can reverse the astrocyte impairment induced by the astrocytic toxin l-alpha amino adipic acid and the consequent loss of neuronal complexity and synapse loss [69]. Therefore, nNOS-PSD-95 regulates functional and structural plasticity via not only directly targeting neurons but also indirectly targeting astrocytes.
In the acute phase of stroke, the surface expression of GABA type A receptors (GABAARs) is substantially decreased due to their rapid nanoscale rearrangement, which contributes to the lethal excitotoxicity, and ischemic neuronal death can be attenuated by positive allosteric modulation of GABAARs [70–73]. The functions of GABAARs are negatively regulated by nNOS-derived NO [13, 74]. We thus developed the dual-target anti-stroke drug ZL006-05 that simultaneously disrupts nNOS-PSD-95 and potentiates α2-containing GABAARs. We showed that the administration of ZL006-05 in the acute phase of stroke attenuates transient and permanent ischemic injury, and significantly ameliorates long-term functional impairments. ZL006-05 has a treatment window of 12 h after ischemia, crosses the blood-brain barrier and distributes into the brain rapidly, and has a high safety profile in toxicokinetics and long-term toxicological studies. Now, the drug is going through a phase II clinical trial (Registration No: CTR20221109, http://www.chinadrugtrials.org.cn/clinicaltrails.searchlistdetail.dhtml). It not only prevents ischemic brain damage, but ZL006 also benefits the outcome of traumatic brain injury (TBI). It has been reported that ZL006 treatment after TBI significantly reduces brain lesion volume, improves somatosensory, motor, and memory deficits, and attenuates cognitive impairment via inhibiting neuronal apoptosis and p38 MAPK signaling [75].
Neuropathic pain can result from various disorders, including postherpetic neuralgia, painful diabetic polyneuropathy, spinal cord injury, stroke, and cancer, which are usually severe and persistent. Overactivation of NMDARs in the spinal dorsal horn in the setting of injury represents a key mechanism of neuropathic pain [76]. Although short-term use of NMDAR antagonists has antinociceptive efficacy in animal pain models and clinical practice by reducing central sensitization, we found that chronically blocking NMDARs aggravates central sensitization and produces analgesic tolerance mainly due to the diminished inhibitory GABAergic synaptic transmission [77]. It has been reported that dissociation of nNOS-PSD-95 by ZL006 has an antinociceptive effect [78] and suppresses inflammation-evoked neuronal activation at the level of the spinal dorsal horn [79]. However, our study indicated that chronically dissociating nNOS from PSD-95 leads to the dysfunction of GABAARs at the spinal level and analgesic tolerance through the NO-mediated brain-derived neurotrophic factor (BDNF)-K-Cl cotransporter 2 pathway in rodents [77]. GABAARs in the brain and spinal cord are heteropentameric ion channels composed of two α, two β, and one γ2 subunit. There are 4 α subunits in the mammalian CNS, α1, α2, α3, and α5, in which, α1-containing GABAARs mediate benzodiazepines (BZDs)-associated side-effects, whereas α2- and α3-containing GABAARs are critical components of spinal pain control [80]. Based on the finding that (+)-borneol, a bicyclic monoterpene alcohol present in the essential oils of numerous medicinal plants, selectively potentiates α2- and α3-containing GABAARs and prevents the analgesic tolerance caused by the chronic use of the nNOS-PSD-95 blocker ZL006, we designed dual-target compounds that simultaneously dissociate nNOS-PSD-95 and potentiate α2 and/or α3 GABAARs. Among these compounds, we found that ZL006-05 blocks nNOS-PSD-95 interaction and selectively potentiates α2-containing GABAARs, a specific subtype for spinal pain control. ZL006-05 reduces segmental spinal nerve ligation (SNL)-induced mechanical hyperalgesia and thermal pain, inhibits Freund's adjuvant-induced inflammatory pain, and more importantly, it does not produce analgesic tolerance and unwanted side-effects in rats [13]. Metastatic bone pain and chemotherapy-induced peripheral neuropathic pain are the most common clinical symptoms in cancer patients. Interestingly, Tao's group found that ZL006-05 dose-dependently alleviates the bone cancer pain induced by inoculating prostate tumor cells and the peripheral neuropathic pain induced by intraperitoneal injection of paclitaxel, without analgesic tolerance and changes in basal/acute pain and locomotor function [81], suggesting a new candidate for the management of cancer pain and chemotherapy-induced peripheral neuropathic pain.
To further unleash its therapeutic potential, we prepared a ZL006-incorporated P407-based thermoresponsive injectable hydrogel and found that a single subcutaneous injection of the hydrogel has a prolonged and stable analgesic action in mice with SNL [82]. Hemorrhages within the thalamus often lead to thalamic pain, a pain syndrome without effective therapeutics. Using an animal model of thalamic pain prepared by microinjecting type IV collagenase into unilateral ventral posterior medial/lateral nuclei of the thalamus, a study has shown that ZL006 alleviates pain hypersensitivity in mice, including mechanical allodynia, heat hyperalgesia, and cold allodynia, in a dose-dependent manner [83]. Collectively, nNOS-PSD-95 plays a significant role in mediating neuropathological, inflammatory, and bone cancer pain and thalamic pain. Thus, nNOS-PSD-95 may be a promising therapeutic target in the clinical management of chronic pain.
NMDAR-mediated excitotoxicity is widely accepted to be a common mechanism for neuronal loss in neurodegenerative diseases. Amyloid beta (Aβ)-induced neurotoxicity and oxidative stress are critical for the pathogenesis of AD. It has been reported that the nNOS-PSD-95 inhibitor ZL006 reduces Aβ1-42-induced neuronal damage and oxidative stress through activating Akt/Nrf2/heme oxygenase-1 signaling pathways [84]. Sirtuin-3 (SIRT3), an NAD+-dependent protein deacetylase located in mitochondria, regulates mitochondrial functions, maintains cellular antioxidant status, and plays a role in Parkinson’s disease (PD) [85, 86]. In an in vitro PD model, ZL006 attenuates MPP+-induced neuronal injury through Sirt3-mediated inhibition of mitochondrial dysfunction [87], suggesting a novel class of therapeutics for PD. Moreover, ZL006 protects spinal cord neurons against ischemia through Sirt3-mediated inhibition of mitochondrial dysfunction [88].
nNOS-PSD-95 interaction and neuropsychiatric disorders
Exposure to stress stimulation increases c-FOS immunoreactivity in the lateral septum, paraventricular nucleus of the hypothalamus, periaqueductal grey, dentate gyrus (DG), and ventral CA1 of the hippocampus. Disruption of nNOS-PSD-95 by ZL006 reduces the stress-induced c-FOS immunoreactivity in the dorsal DG and ventral CA1 [89], implicating hippocampal nNOS-PSD-95 in the modulation of stress-related behaviors. It has been reported that the nNOS-PSD-95 inhibitors ZL006 and IC87201 possess antidepressant-like behavioral properties without effect on locomotor activity, and more interestingly, different from various traditional drugs, they have more immediate activity [90].
Social isolation (SI) was common during the COVID-19 pandemic and may enhance attack behavior in humans, but lacks effective clinical management for the behavior so far. It is known that nNOS inhibition can cause aggressive behavior [54]. A recent study showed that the nNOS-PSD-95 pathway mediates the SI-induced escalation of attack behavior and the dissociation of nNOS-PSD-95 by ZL006 mitigates SI-induced escalated attack behaviors in mice [91], suggesting a promising therapeutic strategy for treating aggressive behaviors.
Opioids are widely used in the clinic as analgesics with strong effects, but their application is restricted by addiction. Opioid abuse is a dramatic challenge for the whole of society because of the high relapse rate. Overactivation of NMDARs is implicated in altered forms of neural plasticity in opioid addiction [92, 93]. Oliva et al. evaluated the impact of a PSD95-nNOS inhibitor on the rewarding effects of morphine and found that inhibition of PSD95-nNOS decreases morphine reward and relapse-like behavior, highlighting a novel therapeutic for the treatment of opioid addiction [94].
Normal fear learning and memory allow animals to predict and avoid physical dangers but these mechanisms can lead to symptoms of syndromes, such as PTSD, a neuropsychiatric affective disorder that can result in excessive fear and anxiety after exposure to or witnessing traumatic events [95]. Fear conditioning causes a robust increase in nNOS-PSD95 binding in the amygdala, the inhibition of nNOS-PSD95 by systemic and direct intra-amygdala infusion of ZL006 attenuates conditioned fear memory, and more interestingly, unlike NMDAR antagonists, the administration of ZL006 does not affect locomotion, social interaction, object recognition memory, and spatial memory [96] as well as source memory, a key feature of episodic memory in humans [97]. Fear renewal is defined as the return of the conditioned fear responses after extinction. It has been shown that S-nitrosylation of GluA1 in the lateral amygdala is required for fear renewal and ZL006 inhibits fear renewal by reducing the S-nitrosylation [98]. Moreover, we have shown that PSD-95-nNOS coupling can regulate contextual fear extinction in the dorsal CA3 of mice [15]. Therefore, these findings highlight PSD95-nNOS interaction as a novel target for PTSD.
Pain consists of sensory and affective components. Although the sensory component of pain has been clearly explained at the molecular and neural circuit levels, the neuronal mechanism underlying the effective component of pain is still unknown. It has been reported that activation of glutamatergic transmission and subsequent nNOS-PSD-95 interaction within the ventral part of the bed nucleus of the stria terminalis mediates the negative affective component of pain, and ZL006 dose-dependently suppressed formalin-induced conditioned place aversion [99]. Chronic pain patients often have GAD, and some of them suffer from anxiety even after analgesic administration. We have shown that administration of ZL006 significantly reduces the S-nitrosylation of AMPAR-interacting proteins in the vmPFC, resulting in anxiolytic-like effects in anxious mice after ibuprofen treatment [100].
nNOS-CAPON interaction and neuropsychiatric disorders
Anxiety disorders are among the most common psychiatric illnesses, with core features including excessive fear and anxiety or avoidance of perceived threats that are persistent and impairing [101]. Anxiety disorders generally start before or in early adulthood and cause significant suffering and disability. Among children and adolescents, the lifetime prevalence of anxiety disorders is estimated to be between 15% and 20% [102].
SSRIs and BZDs are the most commonly prescribed anxiolytics. However, the severe side-effects of BZDs and the slow onset of SSRIs render their use problematic [103]. CAPON, which has a phosphotyrosine-binding domain (PTB) at its N-terminal and a PDZ ligand motif at its C-terminal, mediates the interactions with dexamethasone-induced ras protein 1 (Dexras1), scribble, and synapsin via PTB and binds to the nNOS PDZ via a peptide/PDZ interaction [104]. The work of Zhu et al. in our lab indicated that the nNOS-CAPON association plays a critical role in emotional regulation and can serve as a target for developing new anxiolytic agents [17]. We found that augmenting nNOS-CAPON interaction in the hippocampus gives rise to anxiogenic-like behaviors, whereas dissociating CAPON from nNOS in the hippocampus produces anxiolytic-like effects in mice. Animals subjected to CMS display a substantially increased nNOS-CAPON interaction in the hippocampus and a consequent anxiogenic-like phenotype. Uncoupling nNOS-CAPON reverses the CMS-induced anxiogenic-like behaviors. Dexras1-ERK signaling accounts for the behavioral effects of the nNOS-CAPON association [17]. By analyzing how the C-terminal sequence of CAPON binds to the pocket of the nNOS PDZ domain and the chemical conformation of Val0 of the C-terminal peptide of CAPON affects the nNOS-CAPON interaction, we designed and synthesized a series of compounds that are derived from the condensation of dicarboxylic acids with D- or L-valine methyl ester. Among them, ZLc-002 is a potent nNOS-CAPON blocker and it uncouples nNOS and CAPON but not nNOS and PSD-95 and produces significant anxiolytic-like effects in a rapid-onset manner. Moreover, our follow-up study found that NF-κB in the hippocampus mediates anxiogenic behaviors probably via regulating the association of nNOS-CAPON-Dexras1 signaling in mice [105], and the nNOS-CAPON inhibitor ZLc-002 produces anxiolytic effects by promoting synaptogenesis in CMS-induced animal models of anxiety [106]. Moreover, fluoxetine, a classical SSRI, activates postsynaptic 5-HT1ARs, and in turn, disrupts the nNOS-CAPON interaction in the DG, thereby modifying anxiety behaviors in mice [107].
PTSD is a neuropsychiatric affective disorder that can result in excessive fear and anxiety after exposure to or witnessing traumatic events. Exposure therapy based on extinction learning is the first-line treatment for PTSD. However, fear extinction is relatively easy to learn but difficult to remember; extinguished fear relapses under a number of circumstances [108]. The consolidation of fear extinction memory is a time-dependent process by which recently learned safe memory is transformed into long-term memory and is crucial for preventing the return of fear memory. The consolidation of fear extinction requires gene expression in the mPFC and amygdala, whereas the consolidation of fear memory does it in the hippocampus and amygdala [109]. We showed that the nNOS-CAPON blocker can prevent the return of extinguished fear extinction. Extinction learning-induced association of CAPON with nNOS in the infralimbic (IL) subregion of the mPFC negatively regulates extinction memory and dissociating nNOS-CAPON can prevent the return of extinguished fear in mice, suggesting a new target for treating PTSD [16]. NMDAR activation and ERK signaling upregulation in the IL have been shown to be critical for the consolidation of extinction memory [110, 111]. It is known that the nNOS-CAPON association causes ERK dysfunction through Dexras1 nitrosylation [17]. We found that NMDAR activation can induce nNOS-CAPON association after extinction learning, resulting in ERK dysfunction and Dexras1 nitrosylation in the IL of mice [16]. Thus, the role of NMDAR activation in the IL on the consolidation of fear extinction memory is contradictory: while NMDAR activation mediates the consolidation of extinction memory, it diminishes the consolidation of extinction memory via leading to ERK dysfunction, which may explain why fear extinction is difficult to remember.
The negative role of nNOS-CAPON in extinction consolidation also works in drug abuse. We found that the nNOS-CAPON association in the dorsal hippocampus blocks the consolidation of morphine-conditioned place preference (CPP) extinction. Morphine CPP extinction training increases nNOS-CAPON interaction, and blocking the morphine-induced nNOS-CAPON association during and after extinction training prevents the reinstatement and spontaneous recovery of morphine CPP through ERK2-mediated neuroplasticity and extinction memory consolidation in mice, revealing a target to prevent the reinstatement of drug abuse [112].
A growing number of studies have demonstrated that ketamine has a rapid and sustained antidepressant action. Recently, it has been reported that ketamine may improve depressive behavior by reducing nNOS expression and enhancing CAPON and Dexras1 expression in rats [113]. CAPON is colocalized with spinophilin in the dorsolateral PFC of MDD patients and interacts with spinophilin in the human brain. There are increased CAPON and spinophilin levels and decreased synapsin in the dorsolateral PFC of MDD patients and the PFC of mice exposed to chronic unpredictable mild stress [114]. Moreover, fluoxetine inhibits depressive behaviors in mice by reducing nNOS-CAPON interaction in the DG [107]. Together, these studies suggest that the nNOS-CAPON interaction may involve the modulation of depressive behaviors.
Schizophrenia involves morphological brain changes, including changes in synaptic plasticity and altered dendritic development. Overexpressing CAPON in the hippocampus markedly increases nNOS-PSD-95 interaction, reduces dendritic spine density, changes dendritic spine morphology at CA1 synapses, causes impairment in social memory, and decreases spatial working memory in mice [115]. In cultured neurons, overexpressing CAPON causes excessive growth of filopodia-like protrusions, a neuropathological feature of schizophrenia [116]. Thus, the nNOS-CAPON interaction may be involved in changes in brain morphology reported in schizophrenia.
nNOS-CAPON interaction and neurological diseases
Excepting the negative implication of the nNOS-CAPON association in emotional disorders, it is also involved in the pathophysiology of neurological diseases. We have shown that increased nNOS-CAPON association contributes to excitotoxicity and abnormal dendritic spine development in cultured neurons and models of AD. Dissociating CAPON from nNOS reduces memory defects in 4-month-old APP/PS1 mice and ameliorates dendritic injuries both in vivo and in vitro. S-nitrosylation of Dexras1 and downregulation of ERK-CREB-BDNF signaling might be downstream of NOS-CAPON [14]. Excepting extracellular deposits of Aβ, the major component of senile plaques, neurofibrillary tangles composed of hyperphosphorylated tau protein are another neuropathological hallmarks of AD. Aβ pathology leads to the accumulation of CAPON protein and thereby induces tau pathology and neuronal cell death, revealing a novel mediator that links Aβ, tau, and neurodegeneration [9]. The gradual accumulation of CAPON over long periods could enhance the CAPON-nNOS interaction, therefore disrupting nNOS-CAPON could be a novel approach for the treatment of AD and related diseases. Moreover, neuroinflammation plays a critical role in the pathogenesis of AD [117]. It has been reported that CAPON plays a proactive role in the process of inflammation in the hippocampus and cerebral cortex by transferring from the cytoplasm to the nucleus, and through the NMDAR-nNOS signal pathway, implicating nNOS-CAPON in neuroinflammation [118].
An excitotoxic stimulus can induce nNOS-CAPON interaction in neurons. Uncoupling nNOS-CAPON inhibits the excitotoxic activation of p38MAPK and subsequent neuronal death [119]. The nNOS-CAPON inhibitor N-cyclohexylethyl-AD(OMe)AV, designed by targeting the PDZ of nNOS, has a neuroprotective effect on ischemic stroke in rats [120]. Interestingly, it has been reported that the CAPON PDZ ligand motif does not bind to nNOS, and in contrast, full-length CAPON forms an unusually stable interaction with nNOS. By mapping the discrepancy between full-length CAPON and its C-terminal PDZ motif, the authors showed that the ExF motif, a novel internal region, is sufficient and necessary for binding to nNOS, and the C-terminal PDZ motif promotes the stability of the nNOS-CAPON complex. Peptides comprising both the PDZ ligand motif and the ExF motif or only comprising the ExF motif are able to inhibit the NMDA-evoked activation of p38 excitotoxic pathways, providing a distinct pharmacological target in nNOS-CAPON-mediated excitotoxicity [121]. Moreover, we found that the nNOS-CAPON interaction affects stroke recovery. Ischemia increases nNOS-CAPON association in the peri-infarct area of mice in the subacute phase of stroke. Dissociating nNOS-CAPON in the delayed period reverses the stroke-induced impairment of motor function by enhancing functional and structural neuroplasticity, offering a target for functional restoration after stroke [122].
As downstream signaling of NMDAR activation, nNOS-CAPON is also involved in the regulation of chronic pain. TAT-GESV, a selective peptide inhibitor of nNOS-CAPON, attenuates mechanistically distinct forms of neuropathic pain without the unwanted motor ataxic effects of NMDAR antagonists in mice, possibly via p38 MAPK-mediated downstream effects [123]. Using ZLc002, a small molecular drug we developed, it has been reported that dissociating CAPON from nNOS suppresses formalin-evoked inflammatory pain in rats and mechanical and cold allodynia in a mouse model of paclitaxel-induced neuropathic pain. More interestingly, the administration of ZLc002 also enhances the inhibitory effect of paclitaxel on breast or ovarian tumor cell line viability [124]. In addition, CAPON is involved in peripheral nerve regeneration through the regulation of nNOS activity, the nNOS-CAPON interaction occurs in Schwann cells, and sciatic nerve injury induces nNOS-CAPON interaction in rats [125, 126], implicating the nNOS-CAPON interaction in peripheral nerve injury.
nNOS-SERT interaction and MDD
MDD is one of the most common mental disorders and current treatments have notable limitations. The SERT is presently the primary target for antidepressants [127]. However, the therapeutic effects of SSRIs can take several weeks to emerge [128]. Ketamine and its derivatives have rapid action but produce unwanted side-effects, including abuse potential, cognitive impairment, psychotomimetic/dissociative symptoms, and neurotoxicity [129, 130]. Sun et al. in our lab investigated the role of SERT-nNOS coupling in the dorsal raphe nucleus (DRN) and found that the DRN SERT-nNOS interaction is implicated in the modulation of depressive behaviors and dissociating SERT from nNOS in the DRN produces a fast-onset antidepressant effect, revealing a fast-onset antidepressant target [12]. By inhibiting 5-HT reuptake, SSRIs elevate 5-HT levels in the synaptic cleft and thereby increase the activation of postsynaptic 5-HT1ARs in the cortex, hippocampus, and other brain regions [131]. The serotonergic neurons in the DRN project to the cortex and limbic system and are a major source of 5-HT in the brain [132]. Unlike postsynaptic 5-HT1ARs, the activation of somatodendritic 5-HT1ARauto in the DRN has an autoinhibitory function, suppressing serotonergic signaling [133]. Desensitization of 5-HT1ARauto breaks the signaling balance in favor of postsynaptic 5-HT1ARs activation after weeks of SSRIs treatment, which accounts for the delayed onset of SSRI action. In the recent study, we sought to separate these opposing effects of 5-HT1ARs. In the serotonergic neurons of the DRN, there is physical interaction between nNOS and SERT controlling the cell surface localization of the SERT [3]. We found that CMS exposure increases the nNOS-SERT interaction, blockade of which enhances SERT translocation to the cell surface selectively in DRN neurons, reduces extracellular 5-HT levels and 5-HT1ARauto activity, and exhibits fast-onset antidepressant effects in mice. Using optogenetic stimulation and pharmacogenetic studies, we demonstrated that the rapid antidepressant effect depends on the increased firing of serotonergic DRN neurons and DRN-mPFC serotonergic circuit activity [12].
The SERT is a natural ligand of the nNOS PDZ domain and binds to the pocket constituted by the αB helix and βB fold of this domain through its C-terminal peptides [3]. The binding site on the nNOS PDZ domain is a shallow and long groove containing the binding pocket of the conserved sequence GLGF (Gly21, Leu22, Gly23, Phe24) [11]. By analyzing the chemical mechanism of binding the C-tail of SERT to the PDZ of nNOS in the DRN, the group of Prof. Li TY designed a common chemical structure and synthesized a series of compounds. The group of Prof. Zhou QG assessed the effects of a series of compounds on the SERT-nNOS interaction, using cultured 293T cells transfected with plasmids encoding nNOS and SERT, and showed that the small molecule ZZL-7 is a potent blocker of SERT-nNOS, and more importantly, intraperitoneal or intragastric administration of ZZL-7 produces a fast-onset antidepressant effect without abnormal behaviors, including locomotor activity, memory, aggressive behavior, addiction, or abnormal brain waves [12]. ZZL-7 may serve as a novel approach to selectively promote serotonergic signaling to create rapidly acting antidepressant activity.
Conclusions and perspectives
Although we found that nNOS in the CNS is implicated in ischemia-induced brain injury, the modulation of MDD and GAD, and chronic pain-induced anxiety, the development of drugs targeting nNOS is limited, because directly inhibiting nNOS enzymatic activity can result in side-effects like impairment of memory formation and aggressive behaviors. nNOS-mediated protein-protein interactions, including the nNOS-PSD-95, nNOS-CAPON, and nNOS-SERT interactions, are involved in the development of neurological and neuropsychiatric disorders. Based on the chemical mechanism of binding the coupling proteins to the nNOS PDZ domain, we designed and developed small molecule inhibitors of protein-protein interactions, such as ZL006, ZLc002, and ZZL-7. Our and other follow-up studies have demonstrated that ZL006 and ZLc002 are effective for the treatment of neurological and neuropsychiatric disorders, including stroke, MDD, GAD, PTSD, AD, PD, chronic pain, drug addiction, and other disorders, without unwanted side-effects (Fig. 2). Given that the nNOS-PSD-95 association and GABAARs dysfunction are deeply involved in ischemic brain damage, and the functions of GABAAR are negatively regulated by nNOS-derived NO [13, 58, 74], we developed the dual-target anti-stroke drug ZL006-05 that blocks nNOS-PSD-95 and selectively potentiates ɑ2-containing GABAARs (Fig. 3). In the field of MDD treatment, rapid-onset antidepressants are urgently needed. Although it has been extensively demonstrated that ketamine has rapid and robust effectiveness in treatment-resistant MDD, its clinical use has notable limitations as it is a scheduled agent with abuse potential. We found that dissociating nNOS-SERT in the DRN can serve as a new, rapidly-acting treatment for MDD, and the small molecular compound ZZL-7 that disrupts nNOS-SERT creates a fast-onset antidepressant effect (Fig. 4). In our study, ZLc002 was designed based on the binding of the PDZ ligand motif to the nNOS PDZ. However, the ExF motif but not the PDZ ligand motif is sufficient and necessary for the nNOS-CAPON interaction. Thus, the design concept of small molecule inhibitors of nNOS-CAPON interaction needs to be updated. Based on the above research progress on nNOS and its protein-protein interactions, we believe that the research and development of drugs targeting nNOS-mediated protein-protein interactions will bring hope for the clinical therapy of neurological and neuropsychiatric disorders. However, it is worth emphasizing that excitation-inhibition balance is the basic condition for the CNS to normally work. Excitation and inhibition are usually interdependent. Interference with excitatory or inhibitory targets alone may lead to excitation/inhibition imbalance and thereby cause unwanted side-effects. Therefore, double-target drugs based on excitation-inhibition balance will be the future trend of drug development for neurological and neuropsychiatric diseases.
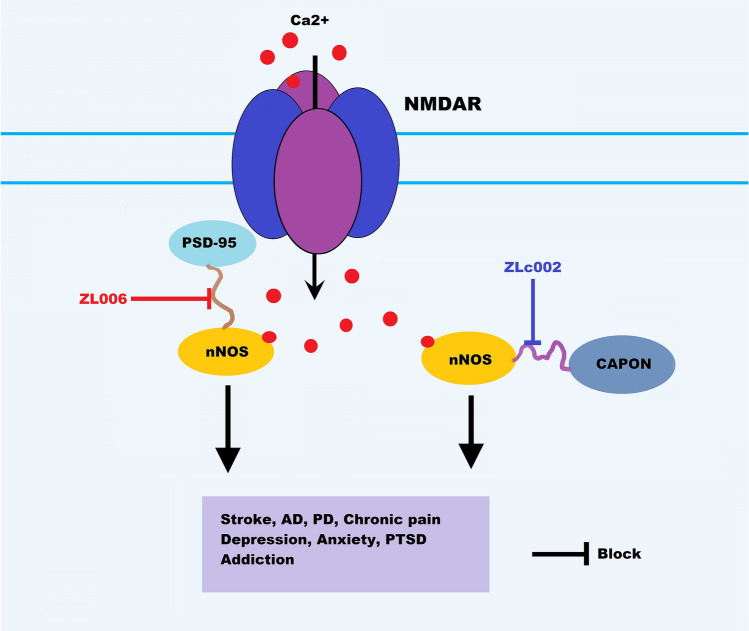
Fig. 2.
Implications of NMDAR activation-induced nNOS-PSD-95 and nNOS-CAPON associations in neurological and neuropsychiatric disorders. ZL006 disrupts the nNOS-PSD-95 interaction and ZLc002 uncouples nNOS and CAPON and shows significant beneficial effects on stroke, PD, AD, chronic pain, MDD, generalized anxiety disorder, PTSD, and addiction. NMDAR, N-methyl-D-aspartate receptor; PD, Parkinson's disease; AD, Alzheimer's disease; PTSD, post-traumatic stress disorder.
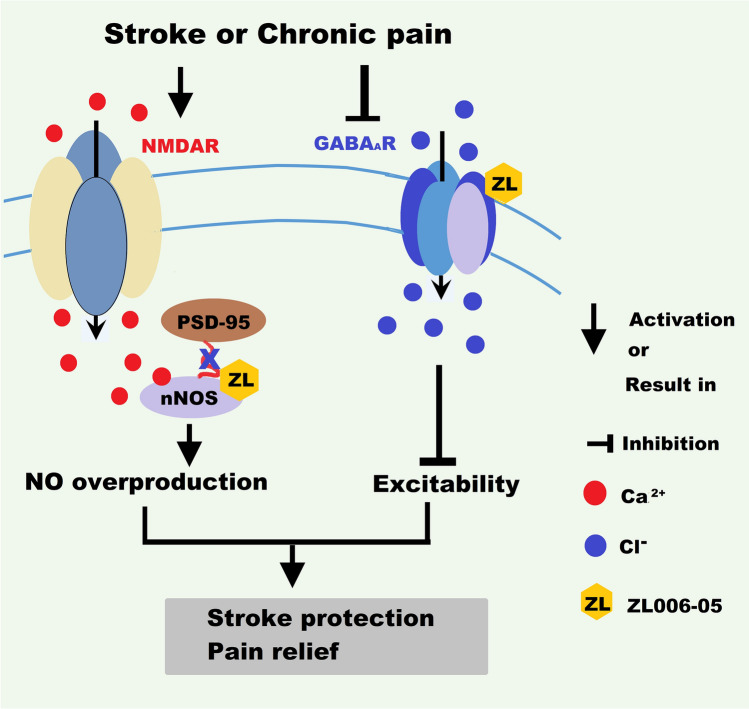
Fig. 3.
Dual-target anti-stroke drug ZL006-05. ZL006-05 blocks the nNOS-PSD-95 interaction while selectively potentiating ɑ2-containing GABAARs and thereby inhibits NO overproduction and reduces excitability, consequently preventing stroke damage and relieving chronic pain. GABAARs, GABA type A receptors; NMDAR, N-methyl-D-aspartate receptor.
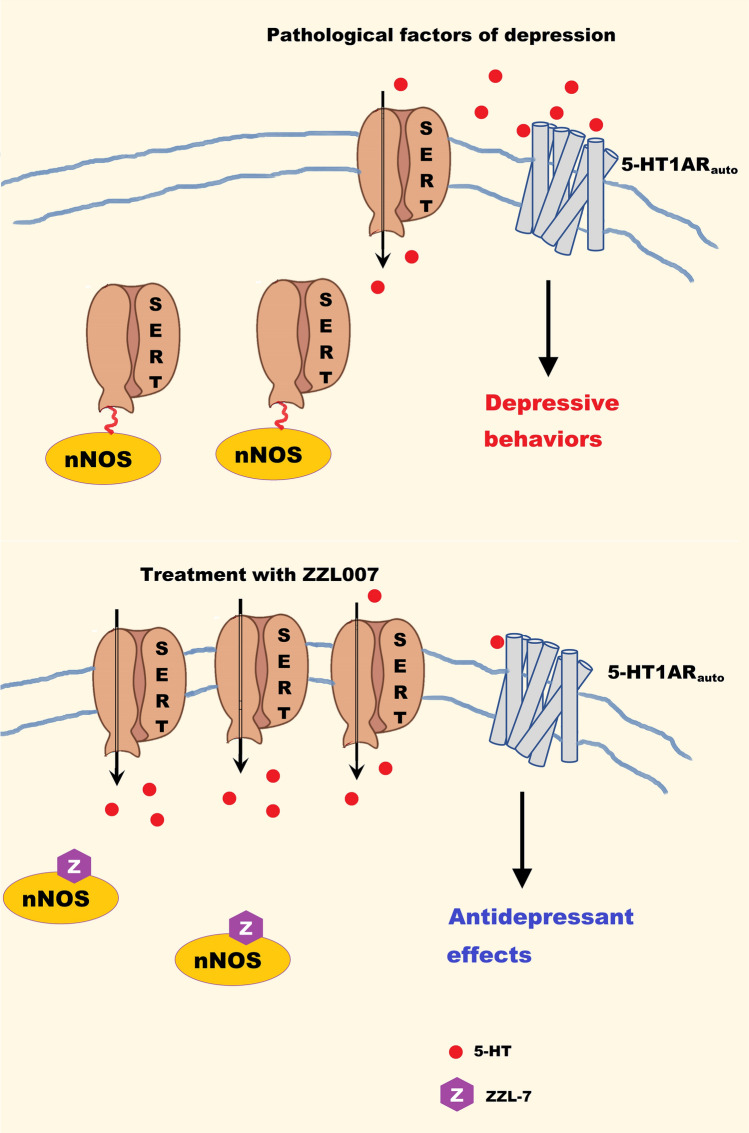
Fig. 4.
The nNOS-SERT inhibitor ZZL007 has a rapid-onset antidepressant effect. Pathological factors of MDD induce the nNOS-SERT interaction in the dorsal raphe nucleus and thereby lead to the internalization of SERT. The decreased cell surface SERT causes overactivation of 5-HT1ARauto and depressive behaviors. ZZL-7 blocks the nNOS-SERT interaction and facilitates the membrane localization of SERT, thereby increasing 5-HT reuptake and reducing 5-HT1ARauto activity, consequently producing a rapid antidepressant effect. SERT, serotonin transporter; 5-HT1ARauto, 5-HT1A autoreceptor.
Limitation
NO is an important signaling molecule that is involved in synaptic functional plasticity. NO modulates several important neuronal processes, including glutamate and GABA release [134], the mobility of synaptic vesicles [135], the intrinsic excitability of target neurons [136], the expression of synaptic proteins [137], modification of BDNF-TrkB signaling [138], and both LTP and long-term depression (LTD) [139]. NO can either facilitate or suppress synaptic plasticity, depending on the brain area, concentration, and cellular environment [134, 140]. The production of NO by neurons is caused by NMDAR-mediated activation of nNOS. Thus, the nNOS-PSD-95 interaction, as a downstream event of NMDAR activation, may have a significant impact on synaptic plasticity. However, the effect of nNOS-PSD-95 coupling and blocking this coupling on LTP and LTD has rarely been studied. Although our research shows that the nNOS-PSD-95 blocker ZL006 has no effect on spatial memory [58], its impact on learning and memory cannot be ruled out, because the paradigms of learning and memory are diverse. Particularly, chronic psychological stress can facilitate LTD and impair LTP; this alteration is critical for the development of MDD, as it leads to particular biased learned cognition and behavioral patterns [141].
Acknowledgements
We thank students for studying nNOS and it-mediated protein-protein interactions in our lab: Xin-Jian Zhu, Qi-Gang Zhou, Li Zhou, Chun-Xia Luo, Fei Li, Jing Zhang, Yao Hu, Li-Juan Zhu, Yu-Hui Lin, Ying Tang, Jun Li, Huan-Yu Ni, Cheng-Yun Cai, Cheng Qin, Hai-Ying Liang, Chun-Yu Yin, and Nan Sun, among others. We thank Prof. Ting-You Li for his long-term cooperation with our group; he has carried out a lot of fruitful work on drug design and chemical synthesis. This review was supported by grants from the National Natural Science Foundation of China (82090042).
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Zhou L, Zhu DY. Neuronal nitric oxide synthase: Structure, subcellular localization, regulation, and clinical implications. Nitric Oxide. 2009;20:223–230. doi: 10.1016/j.niox.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 2.Luo CX, Zhu DY. Research progress on neurobiology of neuronal nitric oxide synthase. Neurosci Bull. 2011;27:23–35. doi: 10.1007/s12264-011-1038-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chanrion B, Mannoury la Cour C, Bertaso F, Lerner-Natoli M, Freissmuth M, Millan MJ, et al. Physical interaction between the serotonin transporter and neuronal nitric oxide synthase underlies reciprocal modulation of their activity. Proc Natl Acad Sci U S A 2007, 104: 8119–8124. [DOI] [PMC free article] [PubMed]
- 4.Zhou QG, Zhu XH, Nemes AD, Zhu DY. Neuronal nitric oxide synthase and affective disorders. IBRO Rep. 2018;5:116–132. doi: 10.1016/j.ibror.2018.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Merino-Gracia J, Costas-Insua C, Canales MÁ, Rodríguez-Crespo I. Insights into the c-terminal peptide binding specificity of the pdz domain of neuronal nitric-oxide synthase: Characterization of the interaction with the tight junction protein claudin-3. J Biol Chem. 2016;291:11581–11595. doi: 10.1074/jbc.M116.724427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doyle DA, Lee A, Lewis J, Kim E, Sheng M, MacKinnon R. Crystal structures of a complexed and peptide-free membrane protein-binding domain: Molecular basis of peptide recognition by PDZ. Cell. 1996;85:1067–1076. doi: 10.1016/S0092-8674(00)81307-0. [DOI] [PubMed] [Google Scholar]
- 7.Hillier BJ, Christopherson KS, Prehoda KE, Bredt DS, Lim WA. Unexpected modes of PDZ domain scaffolding revealed by structure of nNOS-syntrophin complex. Science. 1999;284:812–815. doi: 10.1126/science.284.5415.812. [DOI] [PubMed] [Google Scholar]
- 8.Tochio H, Mok YK, Zhang Q, Kan HM, Bredt DS, Zhang M. Formation of nNOS/PSD-95 PDZ dimer requires a preformed beta-finger structure from the nNOS PDZ domain. J Mol Biol. 2000;303:359–370. doi: 10.1006/jmbi.2000.4148. [DOI] [PubMed] [Google Scholar]
- 9.Hashimoto S, Matsuba Y, Kamano N, Mihira N, Sahara N, Takano J, et al. Tau binding protein CAPON induces tau aggregation and neurodegeneration. Nat Commun. 2019;10:2394. doi: 10.1038/s41467-019-10278-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stricker NL, Christopherson KS, Yi BA, Schatz PJ, Raab RW, Dawes G, et al. PDZ domain of neuronal nitric oxide synthase recognizes novel C-terminal peptide sequences. Nat Biotechnol. 1997;15:336–342. doi: 10.1038/nbt0497-336. [DOI] [PubMed] [Google Scholar]
- 11.Tochio H, Zhang Q, Mandal P, Li M, Zhang M. Solution structure of the extended neuronal nitric oxide synthase PDZ domain complexed with an associated peptide. Nat Struct Biol. 1999;6:417–421. doi: 10.1038/8216. [DOI] [PubMed] [Google Scholar]
- 12.Sun N, Qin YJ, Xu C, Xia T, Du ZW, Zheng LP, et al. Design of fast-onset antidepressant by dissociating SERT from nNOS in the DRN. Science. 2022;378:390–398. doi: 10.1126/science.abo3566. [DOI] [PubMed] [Google Scholar]
- 13.Li J, Zhang L, Xu C, Shen YY, Lin YH, Zhang Y, et al. A pain killer without analgesic tolerance designed by co-targeting PSD-95-nNOS interaction and α2-containning GABAARs. Theranostics. 2021;11:5970–5985. doi: 10.7150/thno.58364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, Zhu Z, Liang HY, Zhang L, Zhou QG, Ni HY, et al. nNOS-CAPON interaction mediates amyloid-β-induced neurotoxicity, especially in the early stages. Aging Cell. 2018;17:e12754. doi: 10.1111/acel.12754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cai CY, Chen C, Zhou Y, Han Z, Qin C, Cao B, et al. PSD-95-nNOS coupling regulates contextual fear extinction in the dorsal CA3. Sci Rep. 2018;8:12775. doi: 10.1038/s41598-018-30899-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qin C, Bian XL, Wu HY, Xian JY, Lin YH, Cai CY, et al. Prevention of the return of extinguished fear by disrupting the interaction of neuronal nitric oxide synthase with its carboxy-terminal PDZ ligand. Mol Psychiatry. 2021;26:6506–6519. doi: 10.1038/s41380-021-01118-w. [DOI] [PubMed] [Google Scholar]
- 17.Zhu LJ, Li TY, Luo CX, Jiang N, Chang L, Lin YH, et al. CAPON-nNOS coupling can serve as a target for developing new anxiolytics. Nat Med. 2014;20:1050–1054. doi: 10.1038/nm.3644. [DOI] [PubMed] [Google Scholar]
- 18.Mao R, Zong N, Hu Y, Chen Y, Xu Y. Neuronal death mechanisms and therapeutic strategy in ischemic stroke. Neurosci Bull. 2022;38:1229–1247. doi: 10.1007/s12264-022-00859-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.del Zoppo G, Ginis I, Hallenbeck JM, Iadecola C, Wang X, Feuerstein GZ. Inflammation and stroke: Putative role for cytokines, adhesion molecules and iNOS in brain response to ischemia. Brain Pathol. 2000;10:95–112. doi: 10.1111/j.1750-3639.2000.tb00247.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hossmann KA. Viability thresholds and the penumbra of focal ischemia. Ann Neurol. 1994;36:557–565. doi: 10.1002/ana.410360404. [DOI] [PubMed] [Google Scholar]
- 21.Ermine CM, Bivard A, Parsons MW, Baron JC. The ischemic penumbra: From concept to reality. Int J Stroke. 2021;16:497–509. doi: 10.1177/1747493020975229. [DOI] [PubMed] [Google Scholar]
- 22.Zhu DY, Deng Q, Yao HH, Wang DC, Deng Y, Liu GQ. Inducible nitric oxide synthase expression in the ischemic core and penumbra after transient focal cerebral ischemia in mice. Life Sci. 2002;71:1985–1996. doi: 10.1016/S0024-3205(02)01970-7. [DOI] [PubMed] [Google Scholar]
- 23.Jiang J, Wang W, Sun YJ, Hu M, Li F, Zhu DY. Neuroprotective effect of curcumin on focal cerebral ischemic rats by preventing blood-brain barrier damage. Eur J Pharmacol. 2007;561:54–62. doi: 10.1016/j.ejphar.2006.12.028. [DOI] [PubMed] [Google Scholar]
- 24.Luo CX, Zhu XJ, Zhang AX, Wang W, Yang XM, Liu SH, et al. Blockade of L-type voltage-gated Ca channel inhibits ischemia-induced neurogenesis by down-regulating iNOS expression in adult mouse. J Neurochem. 2005;94:1077–1086. doi: 10.1111/j.1471-4159.2005.03262.x. [DOI] [PubMed] [Google Scholar]
- 25.Huang Z, Huang PL, Panahian N, Dalkara T, Fishman MC, Moskowitz MA. Effects of cerebral ischemia in mice deficient in neuronal nitric oxide synthase. Science. 1994;265:1883–1885. doi: 10.1126/science.7522345. [DOI] [PubMed] [Google Scholar]
- 26.Samdani AF, Dawson TM, Dawson VL. Nitric oxide synthase in models of focal ischemia. Stroke. 1997;28:1283–1288. doi: 10.1161/01.STR.28.6.1283. [DOI] [PubMed] [Google Scholar]
- 27.Packer MA, Stasiv Y, Benraiss A, Chmielnicki E, Grinberg A, Westphal H, et al. Nitric oxide negatively regulates mammalian adult neurogenesis. Proc Natl Acad Sci U S A. 2003;100:9566–9571. doi: 10.1073/pnas.1633579100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moreno-López B, Romero-Grimaldi C, Noval JA, Murillo-Carretero M, Matarredona ER, Estrada C. Nitric oxide is a physiological inhibitor of neurogenesis in the adult mouse subventricular zone and olfactory bulb. J Neurosci. 2004;24:85–95. doi: 10.1523/JNEUROSCI.1574-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu DY, Lau L, Liu SH, Wei JS, Lu YM. Activation of cAMP-response-element-binding protein (CREB) after focal cerebral ischemia stimulates neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci U S A. 2004;101:9453–9457. doi: 10.1073/pnas.0401063101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu XJ, Hua Y, Jiang J, Zhou QG, Luo CX, Han X, et al. Neuronal nitric oxide synthase-derived nitric oxide inhibits neurogenesis in the adult dentate gyrus by down-regulating cyclic AMP response element binding protein phosphorylation. Neuroscience. 2006;141:827–836. doi: 10.1016/j.neuroscience.2006.04.032. [DOI] [PubMed] [Google Scholar]
- 31.Luo CX, Zhu XJ, Zhou QG, Wang B, Wang W, Cai HH, et al. Reduced neuronal nitric oxide synthase is involved in ischemia-induced hippocampal neurogenesis by up-regulating inducible nitric oxide synthase expression. J Neurochem. 2007;103:1872–1882. doi: 10.1111/j.1471-4159.2007.04915.x. [DOI] [PubMed] [Google Scholar]
- 32.Wang M, Zhang L, Gage FH. Modeling neuropsychiatric disorders using human induced pluripotent stem cells. Protein Cell. 2020;11:45–59. doi: 10.1007/s13238-019-0638-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.GBD Mental Disorders Collaborators, Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet Psychiatry. 2019;2022(9):137–150. doi: 10.1016/S2215-0366(21)00395-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shi HJ, Wang S, Wang XP, Zhang RX, Zhu LJ. Hippocampus: Molecular, cellular, and circuit features in anxiety. Neurosci Bull 2023: 1–18. [DOI] [PMC free article] [PubMed]
- 35.Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 36.Zhou QG, Hu Y, Hua Y, Hu M, Luo CX, Han X, et al. Neuronal nitric oxide synthase contributes to chronic stress-induced depression by suppressing hippocampal neurogenesis. J Neurochem. 2007;103:1843–1854. doi: 10.1111/j.1471-4159.2007.04914.x. [DOI] [PubMed] [Google Scholar]
- 37.Zhou QG, Zhu LJ, Chen C, Wu HY, Luo CX, Chang L, et al. Hippocampal neuronal nitric oxide synthase mediates the stress-related depressive behaviors of glucocorticoids by downregulating glucocorticoid receptor. J Neurosci. 2011;31:7579–7590. doi: 10.1523/JNEUROSCI.0004-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gross C, Zhuang X, Stark K, Ramboz S, Oosting R, Kirby L, et al. Serotonin1A receptor acts during development to establish normal anxiety-like behaviour in the adult. Nature. 2002;416:396–400. doi: 10.1038/416396a. [DOI] [PubMed] [Google Scholar]
- 39.Zhang J, Huang XY, Ye ML, Luo CX, Wu HY, Hu Y, et al. Neuronal nitric oxide synthase alteration accounts for the role of 5-HT1A receptor in modulating anxiety-related behaviors. J Neurosci. 2010;30:2433–2441. doi: 10.1523/JNEUROSCI.5880-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu Y, Wu DL, Luo CX, Zhu LJ, Zhang J, Wu HY, et al. Hippocampal nitric oxide contributes to sex difference in affective behaviors. Proc Natl Acad Sci U S A. 2012;109:14224–14229. doi: 10.1073/pnas.1207461109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berkiks I, Garcia-Segura LM, Nassiri A, Mesfioui A, Ouichou A, Boulbaroud S, et al. The sex differences of the behavior response to early Life immune stimulation: Microglia and astrocytes involvement. Physiol Behav. 2019;199:386–394. doi: 10.1016/j.physbeh.2018.11.037. [DOI] [PubMed] [Google Scholar]
- 42.Berkiks I, Mesfioui A, Ouichou A, Nakache R, Ajonijebu DC, El Hessni A. Affective behavior shows sex differences in mid-adulthood rats following postnatal immune stimulation. Neuroscience. 2019;421:69–81. doi: 10.1016/j.neuroscience.2019.09.014. [DOI] [PubMed] [Google Scholar]
- 43.Lenz KM, McCarthy MM. A starring role for microglia in brain sex differences. Neuroscientist. 2015;21:306–321. doi: 10.1177/1073858414536468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stevens JS, Hamann S. Sex differences in brain activation to emotional stimuli: A meta-analysis of neuroimaging studies. Neuropsychologia. 2012;50:1578–1593. doi: 10.1016/j.neuropsychologia.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 45.Labonté B, Engmann O, Purushothaman I, Menard C, Wang J, Tan C, et al. Sex-specific transcriptional signatures in human depression. Nat Med. 2017;23:1102–1111. doi: 10.1038/nm.4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liang HY, Chen ZJ, Xiao H, Lin YH, Hu YY, Chang L, et al. nNOS-expressing neurons in the vmPFC transform pPVT-derived chronic pain signals into anxiety behaviors. Nat Commun. 2020;11:2501. doi: 10.1038/s41467-020-16198-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yin CY, Huang SY, Gao L, Lin YH, Chang L, Wu HY, et al. Neuronal nitric oxide synthase in nucleus accumbens specifically mediates susceptibility to social defeat stress through cyclin-dependent kinase 5. J Neurosci. 2021;41:2523–2539. doi: 10.1523/JNEUROSCI.0422-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.O'dell TJ, Huang PL, Dawson TM, Dinerman JL, Snyder SH, Kandel ER, et al. Endothelial NOS and the blockade of LTP by NOS inhibitors in mice lacking neuronal NOS. Science 1994, 265: 542–546. [DOI] [PubMed]
- 49.Hopper RA, Garthwaite J. Tonic and phasic nitric oxide signals in hippocampal long-term potentiation. J Neurosci. 2006;26:11513–11521. doi: 10.1523/JNEUROSCI.2259-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Son H, Hawkins RD, Martin K, Kiebler M, Huang PL, Fishman MC, et al. Long-term potentiation is reduced in mice that are doubly mutant in endothelial and neuronal nitric oxide synthase. Cell. 1996;87:1015–1023. doi: 10.1016/S0092-8674(00)81796-1. [DOI] [PubMed] [Google Scholar]
- 51.Kriegsfeld LJ, Eliasson MJ, Demas GE, Blackshaw S, Dawson TM, Nelson RJ, et al. Nocturnal motor coordination deficits in neuronal nitric oxide synthase knock-out mice. Neuroscience. 1999;89:311–315. doi: 10.1016/S0306-4522(98)00614-9. [DOI] [PubMed] [Google Scholar]
- 52.Tanda K, Nishi A, Matsuo N, Nakanishi K, Yamasaki N, Sugimoto T, et al. Abnormal social behavior, hyperactivity, impaired remote spatial memory, and increased D1-mediated dopaminergic signaling in neuronal nitric oxide synthase knockout mice. Mol Brain. 2009;2:19. doi: 10.1186/1756-6606-2-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kelley JB, Balda MA, Anderson KL, Itzhak Y. Impairments in fear conditioning in mice lacking the nNOS gene. Learn Mem. 2009;16:371–378. doi: 10.1101/lm.1329209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nelson RJ, Demas GE, Huang PL, Fishman MC, Dawson VL, Dawson TM, et al. Behavioural abnormalities in male mice lacking neuronal nitric oxide synthase. Nature. 1995;378:383–386. doi: 10.1038/378383a0. [DOI] [PubMed] [Google Scholar]
- 55.Sattler R, Xiong Z, Lu WY, Hafner M, MacDonald JF, Tymianski M. Specific coupling of NMDA receptor activation to nitric oxide neurotoxicity by PSD-95 protein. Science. 1999;284:1845–1848. doi: 10.1126/science.284.5421.1845. [DOI] [PubMed] [Google Scholar]
- 56.Aarts M, Liu Y, Liu L, Besshoh S, Arundine M, Gurd JW, et al. Treatment of ischemic brain damage by perturbing NMDA receptor-PSD-95 protein interactions. Science. 2002;298:846–850. doi: 10.1126/science.1072873. [DOI] [PubMed] [Google Scholar]
- 57.Liu H, Li J, Zhao F, Wang H, Qu Y, Mu D. Nitric oxide synthase in hypoxic or ischemic brain injury. Rev Neurosci. 2015;26:105–117. doi: 10.1515/revneuro-2014-0041. [DOI] [PubMed] [Google Scholar]
- 58.Zhou L, Li F, Xu HB, Luo CX, Wu HY, Zhu MM, et al. Treatment of cerebral ischemia by disrupting ischemia-induced interaction of nNOS with PSD-95. Nat Med. 2010;16:1439–1443. doi: 10.1038/nm.2245. [DOI] [PubMed] [Google Scholar]
- 59.Balboa JR, Essig DJ, Ma S, Karer N, Clemmensen LS, Pedersen SW, et al. Development of a potent cyclic peptide inhibitor of the nNOS/PSD-95 interaction. J Med Chem. 2023;66:976–990. doi: 10.1021/acs.jmedchem.2c01803. [DOI] [PubMed] [Google Scholar]
- 60.Wang SN, Wang Z, Wang XY, Zhang XP, Xu TY, Miao CY. Humanized cerebral organoids-based ischemic stroke model for discovering of potential anti-stroke agents. Acta Pharmacol Sin. 2023;44:513–523. doi: 10.1038/s41401-022-00986-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang Z, Zhao Y, Jiang Y, Lv W, Wu L, Wang B, et al. Enhanced anti-ischemic stroke of ZL006 by T7-conjugated PEGylated liposomes drug delivery system. Sci Rep. 2015;5:12651. doi: 10.1038/srep12651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhao Y, Jiang Y, Lv W, Wang Z, Lv L, Wang B, et al. Dual targeted nanocarrier for brain ischemic stroke treatment. J Control Release. 2016;233:64–71. doi: 10.1016/j.jconrel.2016.04.038. [DOI] [PubMed] [Google Scholar]
- 63.Luo CX, Jin X, Cao CC, Zhu MM, Wang B, Chang L, et al. Bidirectional regulation of neurogenesis by neuronal nitric oxide synthase derived from neurons and neural stem cells. Stem Cells. 2010;28:2041–2052. doi: 10.1002/stem.522. [DOI] [PubMed] [Google Scholar]
- 64.Luo CX, Lin YH, Qian XD, Tang Y, Zhou HH, Jin X, et al. Interaction of nNOS with PSD-95 negatively controls regenerative repair after stroke. J Neurosci. 2014;34:13535–13548. doi: 10.1523/JNEUROSCI.1305-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang DL, Qian XD, Lin YH, Tian BB, Liang HY, Chang L, et al. ZL006 promotes migration and differentiation of transplanted neural stem cells in male rats after stroke. J Neurosci Res. 2017;95:2409–2419. doi: 10.1002/jnr.24068. [DOI] [PubMed] [Google Scholar]
- 66.Lin YH, Dong J, Tang Y, Ni HY, Zhang Y, Su P, et al. Opening a new time window for treatment of stroke by targeting HDAC2. J Neurosci. 2017;37:6712–6728. doi: 10.1523/JNEUROSCI.0341-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tang Y, Lin YH, Ni HY, Dong J, Yuan HJ, Zhang Y, et al. Inhibiting histone deacetylase 2 (HDAC2) promotes functional recovery from stroke. J Am Heart Assoc. 2017;6:e007236. doi: 10.1161/JAHA.117.007236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lin YH, Liang HY, Xu K, Ni HY, Dong J, Xiao H, et al. Dissociation of nNOS from PSD-95 promotes functional recovery after cerebral ischaemia in mice through reducing excessive tonic GABA release from reactive astrocytes. J Pathol. 2018;244:176–188. doi: 10.1002/path.4999. [DOI] [PubMed] [Google Scholar]
- 69.David J, O'Toole E, O'Reilly K, Thuery G, Assmann N, Finlay D, et al. Inhibitors of the NMDA-nitric oxide signaling pathway protect against neuronal atrophy and synapse loss provoked by l-alpha aminoadipic acid-treated astrocytes. Neuroscience. 2018;392:38–56. doi: 10.1016/j.neuroscience.2018.09.023. [DOI] [PubMed] [Google Scholar]
- 70.Costa JT, Mele M, Baptista MS, Gomes JR, Ruscher K, Nobre RJ, et al. Gephyrin cleavage in in vitro brain ischemia decreases GABAA receptor clustering and contributes to neuronal death. Mol Neurobiol. 2016;53:3513–3527. doi: 10.1007/s12035-015-9283-2. [DOI] [PubMed] [Google Scholar]
- 71.Mele M, Aspromonte MC, Duarte CB. Downregulation of GABAA receptor recycling mediated by HAP1 contributes to neuronal death in in vitro brain ischemia. Mol Neurobiol. 2017;54:45–57. doi: 10.1007/s12035-015-9661-9. [DOI] [PubMed] [Google Scholar]
- 72.Garcia JD, Gookin SE, Crosby KC, Schwartz SL, Tiemeier E, Kennedy MJ, et al. Stepwise disassembly of GABAergic synapses during pathogenic excitotoxicity. Cell Rep. 2021;37:110142. doi: 10.1016/j.celrep.2021.110142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lamtahri R, Hazime M, Gowing EK, Nagaraja RY, Maucotel J, Alasoadura M, et al. The gliopeptide ODN, a ligand for the benzodiazepine site of GABAA receptors, boosts functional recovery after stroke. J Neurosci. 2021;41:7148–7159. doi: 10.1523/JNEUROSCI.2255-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dejanovic B, Schwarz G. Neuronal nitric oxide synthase-dependent S-nitrosylation of gephyrin regulates gephyrin clustering at GABAergic synapses. J Neurosci. 2014;34:7763–7768. doi: 10.1523/JNEUROSCI.0531-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Qu W, Liu NK, Wu X, Wang Y, Xia Y, Sun Y, et al. Disrupting nNOS-PSD95 interaction improves neurological and cognitive recoveries after traumatic brain injury. Cereb Cortex. 2020;30:3859–3871. doi: 10.1093/cercor/bhaa002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139:267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Li J, Zhang L, Xu C, Lin YH, Zhang Y, Wu HY, et al. Prolonged use of NMDAR antagonist develops analgesic tolerance in neuropathic pain via nitric oxide reduction-induced GABAergic disinhibition. Neurotherapeutics. 2020;17:1016–1030. doi: 10.1007/s13311-020-00883-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee WH, Xu Z, Ashpole NM, Hudmon A, Kulkarni PM, Thakur GA, et al. Small molecule inhibitors of PSD95-nNOS protein-protein interactions as novel analgesics. Neuropharmacology. 2015;97:464–475. doi: 10.1016/j.neuropharm.2015.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Carey LM, Lee WH, Gutierrez T, Kulkarni PM, Thakur GA, Lai YY, et al. Small molecule inhibitors of PSD95-nNOS protein-protein interactions suppress formalin-evoked Fos protein expression and nociceptive behavior in rats. Neuroscience. 2017;349:303–317. doi: 10.1016/j.neuroscience.2017.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ralvenius WT, Benke D, Acuña MA, Rudolph U, Zeilhofer HU. Analgesia and unwanted benzodiazepine effects in point-mutated mice expressing only one benzodiazepine-sensitive GABAA receptor subtype. Nat Commun. 2015;6:6803. doi: 10.1038/ncomms7803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wei W, Liu W, Du S, Govindarajalu G, Irungu A, Bekker A, et al. A compound mitigates cancer pain and chemotherapy-induced neuropathic pain by dually targeting nNOS-PSD-95 interaction and GABAA receptor. Neurotherapeutics. 2021;18:2436–2448. doi: 10.1007/s13311-021-01158-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zeng J, Su P, Li F, Yun Y, Liang H, Qu K, et al. An injectable hydrogel for treatment of chronic neuropathic pain. Macromol Biosci. 2022;22:e2100529. doi: 10.1002/mabi.202100529. [DOI] [PubMed] [Google Scholar]
- 83.Cai W, Wu S, Pan Z, Xiao J, Li F, Cao J, et al. Disrupting interaction of PSD-95 with nNOS attenuates hemorrhage-induced thalamic pain. Neuropharmacology. 2018;141:238–248. doi: 10.1016/j.neuropharm.2018.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tao WY, Yu LJ, Jiang S, Cao X, Chen J, Bao XY, et al. Neuroprotective effects of ZL006 in Aβ1-42-treated neuronal cells. Neural Regen Res. 2020;15:2296–2305. doi: 10.4103/1673-5374.285006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hirschey MD, Shimazu T, Goetzman E, Jing E, Schwer B, Lombard DB, et al. SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature. 2010;464:121–125. doi: 10.1038/nature08778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shen Y, Wu Q, Shi J, Zhou S. Regulation of SIRT3 on mitochondrial functions and oxidative stress in Parkinson’s disease. Biomed Pharmacother. 2020;132:110928. doi: 10.1016/j.biopha.2020.110928. [DOI] [PubMed] [Google Scholar]
- 87.Hu W, Guan LS, Dang XB, Ren PY, Zhang YL. Small-molecule inhibitors at the PSD-95/nNOS interface attenuate MPP+-induced neuronal injury through Sirt3 mediated inhibition of mitochondrial dysfunction. Neurochem Int. 2014;79:57–64. doi: 10.1016/j.neuint.2014.10.005. [DOI] [PubMed] [Google Scholar]
- 88.Liu SG, Wang YM, Zhang YJ, He XJ, Ma T, Song W, et al. ZL006 protects spinal cord neurons against ischemia-induced oxidative stress through AMPK-PGC-1α-Sirt3 pathway. Neurochem Int. 2017;108:230–237. doi: 10.1016/j.neuint.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 89.Sherwin E, Lennon A, Harkin A. Regional specific modulation of stress-induced neuronal activation associated with the PSD95/NOS interaction inhibitor ZL006 in the wistar Kyoto rat. Int J Neuropsychopharmacol. 2017;20:833–843. doi: 10.1093/ijnp/pyx053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Doucet MV, Levine H, Dev KK, Harkin A. Small-molecule inhibitors at the PSD-95/nNOS interface have antidepressant-like properties in mice. Neuropsychopharmacology. 2013;38:1575–1584. doi: 10.1038/npp.2013.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yang L, Cui J, Zeng L, Lu W. Targeting PSD95/nNOS by ZL006 alleviates social isolation-induced heightened attack behavior in mice. Psychopharmacology. 2022;239:267–276. doi: 10.1007/s00213-021-06000-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Trujillo KA. The neurobiology of opiate tolerance, dependence and sensitization: Mechanisms of NMDA receptor-dependent synaptic plasticity. Neurotox Res. 2002;4:373–391. doi: 10.1080/10298420290023954. [DOI] [PubMed] [Google Scholar]
- 93.Doyle MA, Mazei-Robison MS. Opioid-induced molecular and cellular plasticity of ventral tegmental area dopamine neurons. Cold Spring Harb Perspect Med. 2021;11:a039362. doi: 10.1101/cshperspect.a039362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Oliva I, Saberi SA, Rangel-Barajas C, Iyer V, Bunner KD, Lai YY, et al. Inhibition of PSD95-nNOS protein-protein interactions decreases morphine reward and relapse vulnerability in rats. Addict Biol. 2022;27:e13220. doi: 10.1111/adb.13220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Johnson LR, McGuire J, Lazarus R, Palmer AA. Pavlovian fear memory circuits and phenotype models of PTSD. Neuropharmacology. 2012;62:638–646. doi: 10.1016/j.neuropharm.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 96.Li LP, Dustrude ET, Haulcomb MM, Abreu AR, Fitz SD, Johnson PL, et al. PSD95 and nNOS interaction as a novel molecular target to modulate conditioned fear: Relevance to PTSD. Transl Psychiatry. 2018;8:155. doi: 10.1038/s41398-018-0208-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Smith AE, Xu Z, Lai YY, Kulkarni PM, Thakur GA, Hohmann AG, et al. Source memory in rats is impaired by an NMDA receptor antagonist but not by PSD95-nNOS protein-protein interaction inhibitors. Behav Brain Res. 2016;305:23–29. doi: 10.1016/j.bbr.2016.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Song S, Lee J, Park S, Choi S. Fear renewal requires nitric oxide signaling in the lateral amygdala. Biochem Biophys Res Commun. 2020;523:86–90. doi: 10.1016/j.bbrc.2019.12.038. [DOI] [PubMed] [Google Scholar]
- 99.Deyama S, Sugano Y, Mori S, Amano T, Yoshioka M, Kaneda K, et al. Activation of the NMDA receptor-neuronal nitric oxide synthase pathway within the ventral bed nucleus of the stria terminalis mediates the negative affective component of pain. Neuropharmacology. 2017;118:59–68. doi: 10.1016/j.neuropharm.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 100.Chen ZJ, Su CW, Xiong S, Li T, Liang HY, Lin YH, et al. Enhanced AMPAR-dependent synaptic transmission by S-nitrosylation in the vmPFC contributes to chronic inflammatory pain-induced persistent anxiety in mice. Acta Pharmacol Sin. 2022 doi: 10.1038/s41401-022-01024-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Penninx BW, Pine DS, Holmes EA, Reif A. Anxiety disorders. Lancet. 2021;397:914–927. doi: 10.1016/S0140-6736(21)00359-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kalin NH. Novel insights into pathological anxiety and anxiety-related disorders. Am J Psychiatry. 2020;177:187–189. doi: 10.1176/appi.ajp.2020.20010057. [DOI] [PubMed] [Google Scholar]
- 103.Rupprecht R, Rammes G, Eser D, Baghai TC, Schüle C, Nothdurfter C, et al. Translocator protein (18 kD) as target for anxiolytics without benzodiazepine-like side effects. Science. 2009;325:490–493. doi: 10.1126/science.1175055. [DOI] [PubMed] [Google Scholar]
- 104.Wang J, Jin L, Zhu Y, Zhou X, Yu R, Gao S. Research progress in NOS1AP in neurological and psychiatric diseases. Brain Res Bull. 2016;125:99–105. doi: 10.1016/j.brainresbull.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 105.Zhu LJ, Ni HY, Chen R, Chang L, Shi HJ, Qiu D, et al. Hippocampal nuclear factor kappa B accounts for stress-induced anxiety behaviors via enhancing neuronal nitric oxide synthase (nNOS)-carboxy-terminal PDZ ligand of nNOS-Dexras1 coupling. J Neurochem. 2018;146:598–612. doi: 10.1111/jnc.14478. [DOI] [PubMed] [Google Scholar]
- 106.Zhu LJ, Shi HJ, Chang L, Zhang CC, Si M, Li N, et al. nNOS-CAPON blockers produce anxiolytic effects by promoting synaptogenesis in chronic stress-induced animal models of anxiety. Br J Pharmacol. 2020;177:3674–3690. doi: 10.1111/bph.15084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shi HJ, Wu DL, Chen R, Li N, Zhu LJ. Requirement of hippocampal DG nNOS-CAPON dissociation for the anxiolytic and antidepressant effects of fluoxetine. Theranostics. 2022;12:3656–3675. doi: 10.7150/thno.70370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Monfils MH, Cowansage KK, Klann E, LeDoux JE. Extinction-reconsolidation boundaries: Key to persistent attenuation of fear memories. Science. 2009;324:951–955. doi: 10.1126/science.1167975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mamiya N, Fukushima H, Suzuki A, Matsuyama Z, Homma S, Frankland PW, et al. Brain region-specific gene expression activation required for reconsolidation and extinction of contextual fear memory. J Neurosci. 2009;29:402–413. doi: 10.1523/JNEUROSCI.4639-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Burgos-Robles A, Vidal-Gonzalez I, Santini E, Quirk GJ. Consolidation of fear extinction requires NMDA receptor-dependent bursting in the ventromedial prefrontal cortex. Neuron. 2007;53:871–880. doi: 10.1016/j.neuron.2007.02.021. [DOI] [PubMed] [Google Scholar]
- 111.Cestari V, Rossi-Arnaud C, Saraulli D, Costanzi M. The MAP(K) of fear: From memory consolidation to memory extinction. Brain Res Bull. 2014;105:8–16. doi: 10.1016/j.brainresbull.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 112.Kou X, Xian J, Huang Z, Tao Y, Lin Y, Qin C, et al. Disrupting the interaction of nNOS with CAPON prevents the reinstatement of morphine conditioned place preference. Cereb Cortex. 2022;32:569–582. doi: 10.1093/cercor/bhab234. [DOI] [PubMed] [Google Scholar]
- 113.Shen Y, Lv F, Min S, Hao X, Yu J. Ketamine alleviating depressive-like behaviors is associated with regulation of nNOS-CAPON-Dexras1 complex in chronic unpredictable mild stress rats. Transl Neurosci. 2022;13:309–319. doi: 10.1515/tnsci-2022-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gao S, Zhang T, Jin L, Liang D, Fan G, Song Y, et al. CAPON is a critical protein in synaptic molecular networks in the prefrontal cortex of mood disorder patients and contributes to depression-like behavior in a mouse model. Cereb Cortex. 2019;29:3752–3765. doi: 10.1093/cercor/bhy254. [DOI] [PubMed] [Google Scholar]
- 115.Freudenberg F, Candemir E, Chen X, Li LL, Esen-Sehir D, Schenk N, et al. Hippocampal overexpression of NOS1AP promotes endophenotypes related to mental disorders. EBioMedicine. 2021;71:103565. doi: 10.1016/j.ebiom.2021.103565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Candemir E, Kollert L, Weißflog L, Geis M, Müller A, Post AM, et al. Interaction of NOS1AP with the NOS-I PDZ domain: Implications for schizophrenia-related alterations in dendritic morphology. Eur Neuropsychopharmacol. 2016;26:741–755. doi: 10.1016/j.euroneuro.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 117.Leng F, Edison P. Neuroinflammation and microglial activation in Alzheimer disease: Where do we go from here? Nat Rev Neurol. 2021;17:157–172. doi: 10.1038/s41582-020-00435-y. [DOI] [PubMed] [Google Scholar]
- 118.Shao B, Jiang J, Wu Q, Xu Y, Lv Q, Li X, et al. The nuclear localization of CAPON in hippocampus and cerebral cortex neurons after lipopolysaccharide stimulation. Neuroimmunomodulation. 2011;18:89–97. doi: 10.1159/000320419. [DOI] [PubMed] [Google Scholar]
- 119.Li LL, Ginet V, Liu X, Vergun O, Tuittila M, Mathieu M, et al. The nNOS-p38MAPK pathway is mediated by NOS1AP during neuronal death. J Neurosci. 2013;33:8185–8201. doi: 10.1523/JNEUROSCI.4578-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Qin Y, Feng L, Fan X, Zheng L, Zhang Y, Chang L, et al. Neuroprotective effect of N-cyclohexylethyl-[A/G]-[D/E]-X-V peptides on ischemic stroke by blocking nNOS-CAPON interaction. ACS Chem Neurosci. 2021;12:244–255. doi: 10.1021/acschemneuro.0c00739. [DOI] [PubMed] [Google Scholar]
- 121.Li LL, Melero-Fernandez de Mera RM, Chen J, Ba W, Kasri NN, Zhang M, et al. Unexpected heterodivalent recruitment of NOS1AP to nNOS reveals multiple sites for pharmacological intervention in neuronal disease models. J Neurosci. 2015;35:7349–7364. doi: 10.1523/JNEUROSCI.0037-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ni HY, Song YX, Lin YH, Cao B, Wang DL, Zhang Y, et al. Dissociating nNOS (neuronal NO synthase)-CAPON (carboxy-terminal postsynaptic density-95/discs large/zona occludens-1 ligand of nNOS) interaction promotes functional recovery after stroke via enhanced structural neuroplasticity. Stroke. 2019;50:728–737. doi: 10.1161/STROKEAHA.118.022647. [DOI] [PubMed] [Google Scholar]
- 123.Lee WH, Li LL, Chawla A, Hudmon A, Lai YY, Courtney MJ, et al. Disruption of nNOS-NOS1AP protein-protein interactions suppresses neuropathic pain in mice. Pain. 2018;159:849–863. doi: 10.1097/j.pain.0000000000001152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lee WH, Carey LM, Li LL, Xu Z, Lai YY, Courtney MJ, et al. ZLc002, a putative small-molecule inhibitor of nNOS interaction with NOS1AP, suppresses inflammatory nociception and chemotherapy-induced neuropathic pain and synergizes with paclitaxel to reduce tumor cell viability. Mol Pain. 2018;14:1744806918801224. doi: 10.1177/1744806918801224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cui Z, Lv Q, Yan M, Cheng C, Guo Z, Yang J, et al. Elevated expression of CAPON and neuronal nitric oxide synthase in the sciatic nerve of rats following constriction injury. Vet J. 2011;187:374–380. doi: 10.1016/j.tvjl.2010.01.014. [DOI] [PubMed] [Google Scholar]
- 126.Shen A, Chen M, Niu S, Sun L, Gao S, Shi S, et al. Changes in mRNA for CAPON and Dexras1 in adult rat following sciatic nerve transection. J Chem Neuroanat. 2008;35:85–93. doi: 10.1016/j.jchemneu.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 127.Murphy DL, Lesch KP. Targeting the murine serotonin transporter: Insights into human neurobiology. Nat Rev Neurosci. 2008;9:85–96. doi: 10.1038/nrn2284. [DOI] [PubMed] [Google Scholar]
- 128.Chancellor D. The depression market. Nat Rev Drug Discov. 2011;10:809–810. doi: 10.1038/nrd3585. [DOI] [PubMed] [Google Scholar]
- 129.Chaki S, Watanabe M. Antidepressants in the post-ketamine Era: Pharmacological approaches targeting the glutamatergic system. Neuropharmacology. 2023;223:109348. doi: 10.1016/j.neuropharm.2022.109348. [DOI] [PubMed] [Google Scholar]
- 130.Corriger A, Pickering G. Ketamine and depression: A narrative review. Drug Des Devel Ther. 2019;13:3051–3067. doi: 10.2147/DDDT.S221437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Coplan JD, Gopinath S, Abdallah CG, Berry BR. A neurobiological hypothesis of treatment-resistant depression - mechanisms for selective serotonin reuptake inhibitor non-efficacy. Front Behav Neurosci. 2014;8:189. doi: 10.3389/fnbeh.2014.00189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Okaty BW, Commons KG, Dymecki SM. Embracing diversity in the 5-HT neuronal system. Nat Rev Neurosci. 2019;20:397–424. doi: 10.1038/s41583-019-0151-3. [DOI] [PubMed] [Google Scholar]
- 133.Albert PR, Le François B, Millar AM. Transcriptional dysregulation of 5-HT1A autoreceptors in mental illness. Mol Brain. 2011;4:21. doi: 10.1186/1756-6606-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bradley SA, Steinert JR. Nitric oxide-mediated posttranslational modifications: Impacts at the synapse. Oxid Med Cell Longev. 2016;2016:5681036. doi: 10.1155/2016/5681036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Chenouard N, Xuan F, Tsien RW. Synaptic vesicle traffic is supported by transient actin filaments and regulated by PKA and NO. Nat Commun. 2020;11:5318. doi: 10.1038/s41467-020-19120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Steinert JR, Robinson SW, Tong H, Haustein MD, Kopp-Scheinpflug C, Forsythe ID. Nitric oxide is an activity-dependent regulator of target neuron intrinsic excitability. Neuron. 2011;71:291–305. doi: 10.1016/j.neuron.2011.05.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang HG, Lu FM, Jin I, Udo H, Kandel ER, de Vente J, et al. Presynaptic and postsynaptic roles of NO, cGK, and RhoA in long-lasting potentiation and aggregation of synaptic proteins. Neuron. 2005;45:389–403. doi: 10.1016/j.neuron.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 138.Biojone C, Casarotto P, Cannarozzo C, Fred SM, Herrera-Rodríguez R, Lesnikova A, et al. nNOS-induced tyrosine nitration of TRKB impairs BDNF signaling and restrains neuronal plasticity. Prog Neurobiol. 2023;222:102413. doi: 10.1016/j.pneurobio.2023.102413. [DOI] [PubMed] [Google Scholar]
- 139.Steinert JR, Chernova T, Forsythe ID. Nitric oxide signaling in brain function, dysfunction, and dementia. Neuroscientist. 2010;16:435–452. doi: 10.1177/1073858410366481. [DOI] [PubMed] [Google Scholar]
- 140.Chakroborty S, Kim J, Schneider C, West AR, Stutzmann GE. Nitric oxide signaling is recruited as a compensatory mechanism for sustaining synaptic plasticity in Alzheimer’s disease mice. J Neurosci. 2015;35:6893–6902. doi: 10.1523/JNEUROSCI.4002-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Richter-Levin G, Xu L. How could stress lead to major depressive disorder? IBRO Rep. 2018;4:38–43. doi: 10.1016/j.ibror.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]