Summary
Background
The impact of environmental hygiene on the occurrence of hospital-acquired infections (HAIs) remains a subject of debate. We determined the effect of three different surface-cleaning strategies on the incidence of HAIs.
Methods
Between June 2017 and August 2018 we conducted a pragmatic, cluster-randomized controlled crossover trial at 18 non-ICU wards in the university hospital of Berlin, Germany. Surfaces in patient rooms on the study wards were routinely cleaned using one of three agents: Soap-based (reference), disinfectant and probiotic. Each strategy was used on each ward for four consecutive months (4m-4m-4m). There was a one-month wash-in period at the beginning of the study and after each change in strategy. The order of strategies used was randomized for each ward. Primary outcome was the incidence of HAIs. The trial was registered with the German Clinical Trials Register, DRKS00012675.
Findings
13,896 admitted patients met the inclusion criteria, including 4708 in the soap-based (reference) arm, 4535 in the disinfectant arm and 4653 in the probiotic arm. In the reference group, the incidence density of HAIs was 2.31 per 1000 exposure days. The incidence density was similar in the disinfectant arm 2.21 cases per 1000 exposure days (IRR 0.95; 95% CI 0.69–1.31; p = 0.953) and the probiotic arm 2.21 cases per 1000 exposure days (IRR 0.96; 95% CI 0.69–1.32; p = 0.955).
Interpretation
In non-ICU wards, routine surface disinfection proved not superior to soap-based or probiotic cleaning in terms of HAI prevention. Thus, probiotic cleaning could be an interesting alternative, especially in terms of environmental protection.
Funding
Federal Ministry of Education and Research of Germany (03Z0818C). Bill and Melinda Gates Foundation (INV-004308).
Keywords: Hospital-aquired infection, Multidrug-resistant pathogen, Environmental cleaning, Probiotic, Randomized-controlled trial, Environmental hygiene, MRSA, VRE, Multidrug-resistant Gram-negative
Research in context.
Evidence before this study
Our study was designed in 2016 in response to an international debate about the impact of different environmental cleaning agents on the prevention of hospital-acquired infections. We searched PubMed on December 31, 2016, for original research articles published up to that date that associated daily environmental cleaning or disinfection with the incidence of hospital-acquired infections in general. No language restrictions were applied. We used the search terms ("environmental cleaning" OR "environmental decontamination" OR "environmental disinfection" OR "surface disinfection" OR "surface cleaning" OR "surface decontamination" OR "environmental hygiene" OR "hospital surface" OR "probiotic-based sanitation") AND ("hospital-acquired infections" OR "nosocomial infections" OR "healthcare-associated infections"). Our search yielded 78 articles, none of which met our inclusion criteria. We repeated this search on November 23, 2022, and found 106 additional articles, 3 of which met our inclusion criteria. Of these 3 studies, one performed a before-after approach and the other 2 were modeling studies to evaluate the cost-effectiveness of environmental cleaning.
Added value of this study
To our knowledge, our randomized controlled trial (RCT) of three different environmental cleaning agents is the first RCT to evaluate the added value of daily environmental cleaning for preventing HAIs in general. An important strength of our study is its pragmatic design, which compares different cleaning agents in a randomized controlled trial and reflects current German practice and possible alternatives for hospital infection control.
Implications of all the available evidence
Our study of environmental cleaning in hospitalized patients showed that disinfection or probiotic cleaning was not superior to soap-based agents for the prevention of hospital-acquired infections. The non-superiority of either environmental cleaning strategy could change the current preference for environmental cleaning, thus expanding the options for alternatives with potential human and environmental safety benefits.
Introduction
Environmental cleaning is considered an important pillar of hospital infection prevention and control.1, 2, 3, 4 Hospital surfaces are contaminated by patients and hospital staff and represent the microbiome of their users.5,6 This includes potentially harmful pathogens and multidrug-resistant organisms (MDRO). Therefore, routine daily cleaning of frequently touched surfaces in patient rooms and in the hospital in general is a standard procedure performed to prevent the transmission of pathogens to vulnerable patients, eventually causing infection.7 A plethora of agents and technologies are in use internationally and new technologies evolve frequently in this field.2 Although there are many studies available, scientific evidence has not resulted in universally accepted guidelines or practical recommendations.8, 9, 10 Moreover, there has been suspicion that an increased use of environmental sanitization could have a profound influence on the environmental microbiome, driving the rise of further resistant organisms.11
There are currently three major strategies utilized for the manual maintenance cleaning of surfaces: Soap-based,12,13 disinfectant14,15 and probiotic-based cleaning.11,16,17 Manually used soap-based formulations provide visible cleanliness and reduce the bioburden on surfaces.12,13,18,19 Disinfectant substances reduce pathogen quantity more effectively through chemical disintegration, but they might be toxic to humans and show e.g. potential cytotoxicity, mutagenicity and carcinogenicity.20 The vapours can irritate the mucous membranes of the respiratory tract, and repeated contact has been linked to dermatitis.21 In addition, unlike detergents, they must be disposed of in designated landfills and must not be poured down drains or onto the ground to prevent their release into the environment.22 Moreover, they are suspected of producing highly resistant pathogens.2,11,23 Van Dijk et al. analysed outbreak reports and looked for disinfectant susceptibility tests of the respective outbreak pathogen.23 They found 13 papers that contained this information, 12 showed highly resistant strains to the disinfectant. The effect of these two strategies (soap-based and disinfectant) is time-limited, as surfaces quickly become re-contaminated.13 The idea of probiotic cleaning is based on the principle of biological competition.18,24 The products usually contain Bacillus spp. spores that germinate after dilution in water and application to surfaces and inhibit the multiplication and survival of other potentially harmful pathogens.18,24,25 Cleaning agents based on Bacillus spp. have been shown to reduce the overall pathogenic bioburden as well as burden of multidrug-resistant pathogens.17,19 Probiotic agents, therefore, might have the potential for reducing the discharge of toxic effluents in hospitals and reducing the transmission of pathogens via surfaces sustainably.17
Two recent systematic reviews on environmental hygiene in hospitals highlighted the weaknesses of the currently available literature.9,10 Peters et al. argued that there are major problems with the heterogeneity of the interventions, the study settings and the quality of the studies. Compared to other fields, there are very few high-quality studies. In particular, the use of RCTs in this field is exceptionally rare. Most studies showed no effect on HAIs or patient colonisation.9 The authors of the other analysis also concluded that the results and methods of studying environmental hygiene measures are still inconsistent. Composite outcomes or specific marker microorganisms are often used. They emphasised that none of the included RCTs examined comprehensive approaches to cleaning surfaces in hospitals in terms of risk for HAIs.10 The most commonly assessed endpoint was a reduction in the bioburden of pathogens.9,10,18 However, since the primary goal is to prevent hospital-acquired infections (HAIs), there is an urgent necessity for high quality studies that assess the reduction in HAIs associated with different cleaning strategies.9,10 Both author teams s suggested, therefore, that environmental hygiene in healthcare deserves further and better-designed field research and recommended developing a set of standardised primary and secondary outcomes to enable comparative studies.9,10
To our knowledge, no randomized controlled trial (RCT) that compared soap-based, disinfection and probiotic cleaning has yet been conducted.10 Therefore, we designed the KARMIN RCT to assess these effects on the incidence of HAIs and on the acquisition of multidrug-resistant organisms (MDRO) in a real life setting.
Methods
Study design and participants
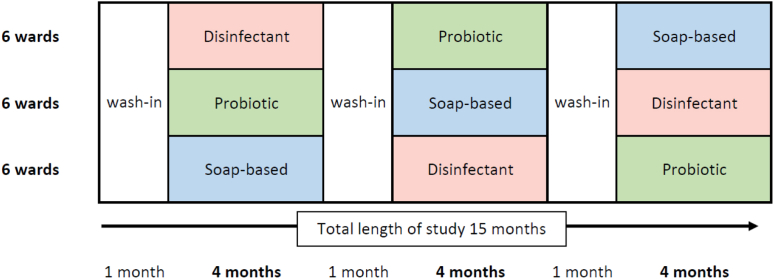
We performed a pragmatic cluster-randomized, crossover trial on eighteen non-ICU wards at Charité Universitätsmedizin Berlin between June 2017 and August 2018. Charité Universitätsmedizin Berlin is a tertiary care university hospital with about 3100 beds and about 21 intensive care units distributed over four locations within the city of Berlin, Germany. Each year about 125,000 in-hospital patients and about 680,000 outpatients are cared for including all existing medical disciplines. The study was performed in one of the main bedding houses including 10 surgical and 8 medicine wards (see Supplementary Material for more details). We tested three different strategies for daily routine environmental room cleaning. The three strategies were used on each entire ward as the standard cleaning agent for all cleaning of surfaces. During the reference period, surfaces were cleaned with a soap-based agent, in the other two groups either a disinfectant agent and or a probiotic agent was used as an alternative. In a previous study, it was observed that the composition of the hospital microbiome stabilises in the four weeks following a significant change in environmental conditions.6,17 Therefore, each study period started with a 1-month wash-in period, followed by a 4-month period of data collection (Fig. 1, Study setting). Each strategy was applied consecutively on every ward for 5-months.
Fig. 1.
Study setting.
We received a waiver of informed consent for this study from our institutional review board (internal process number EA1/387/16). The trial was registered with the German Clinical Trials Register, DRKS00012675.
Randomization and masking
Each strategy was used in each ward for three consecutive 5-month study periods. The sequence of cleaning strategies was randomly selected for each ward. The hospital staff was completely blinded as to the cleaning agent used. This was also true for the staff performing the surveillance of HAIs and MDROs. Cleaning staff and post hoc analysis staff were also blinded to the type of cleaning strategy in use and analysed. We selected different types of wards (10 surgical, 8 internal medicine) in order to yield a representative mix of medical disciplines (Table S1).
Cleaning procedures
For environmental cleaning, the following agents were used: In the soap-based arm, the agent used contained non-ionic surfactants, anionic surfactants, and fragrances in a total concentration of 1% (Brial Top®, Ecolab Inc.). For the disinfectant arm: 2-phenoxyethanol (10%), 3-aminopropyldodecylamine (8%), benzalkonium chloride (7.5%) at a total concentration of 1.5%, with a contact time of 15 min (Incidin Pro®, Ecolab Inc.). In the probiotic arm, the agent contained a combination of bacteria: overall, 5 × 107 CFUs/ml of Bacillus subtilis (ATCC6051), Bacillus megaterium (ATCC14581), Bacillus licheniformis (ATCC12713), Bacillus pumilus (ATCC14884), and Bacillus amyloliquefaciens (DSL13563-0) with a total concentration of 1% (SYNBIO®, HeiQ Chrisal NV). The material of the cloths used for all surface cleaning procedures was made up of 80% viscose and 20% polyester. The concentration of the agents used was based on national recommendations or, if not available, on manufacturers' specifications, the latter especially for non-enveloped viruses.26
Cleaning procedures were divided into maintenance cleaning and terminal cleaning:
-
(a)
Maintenance cleaning was performed once a day in all patient rooms. This type of cleaning was broken down into four types of surfaces. To avoid cross-contamination from the wipes used, each of these surfaces was treated with a cleaning agent from separate, color-coded buckets (Figure S1). In patient rooms, these were frequently-touched surfaces, such as door handles and handrails (blue), in wet room surfaces, such as washbasins and shower cubicles (yellow), and toilet surfaces (pink). A fourth surface was the floor in patient rooms and wet rooms (grey).
-
(b)
Terminal cleaning was defined as cleaning in rooms with potential infection risks. Staff trained exclusively in this standard operating procedure carried out the cleaning of these rooms. The following rooms were subjected to targeted cleaning based on potential risks of infection after stays by patients who displayed the following infections or colonization by the following: multidrug-resistant pathogens (methicillin-resistant Staphylococcus aureus (MRSA), multidrug-resistant Gram-negative Enterobacterales (MDR-GN), vancomycin-resistant Enterococcus spp. (VRE)), infections with non-enveloped viruses (norovirus, rotavirus, adenovirus), measles, or infections with overt pulmonary tuberculosis. Terminal cleaning used the disinfectant agent described above exclusively.
Outcomes
The primary endpoint of the study was acquisition of hospital-acquired infections (HAIs). The secondary endpoint was acquisition of multidrug-resistant organisms (MDROs). The assessment of hospital-acquired infections (HAI) was based on the surveillance definitions of the Centers for Disease Control and Prevention (CDC).27 The general classification of an infection as hospital-acquired was based on the time interval between the patient's admission to a study ward and the onset of the first symptoms of infection. Only infections that provided signs or symptoms after the second day of admission to a study ward were classified as HAIs. Only patients that stayed ≥3 days on the study wards were included in the analysis.
Incidence was calculated as incidence per 100 patients and incidence density per 1000 exposure days. Hand hygiene compliance was assessed at the ward level based on direct observations prior to the study period and was based on the recommendations of the WHO 5 moments for hand hygiene. We obtained demographic data and comorbid conditions for all exposed patients through administrative databases to calculate the Charlson comorbidity index.9,28
Our study staff also assessed the biological burden and compliance with the cleaning strategy on a weekly basis. The former was examined by reviewing the cleaning agent used on the ward. Also on a weekly basis, we performed direct microbiological examination by Rodac plate sampling. Here, we examined a predefined floor segment in patient rooms in each study ward. The specimens were examined quantitatively for growth of Enterococcus spp. and Escherichia coli.
Statistical analysis
We did power calculations based on HAI rates published in the 2016 European Point Prevalence Survey of healthcare-associated infections (PPS2016).29 They detected a prevalence of hospital-acquired infections in 3.6% of patients in German hospitals. The power calculation was performed with a two-sided significance level of 0.025. In order to detect a reduction of 30%4 with a power of 80%, the sample size of each arm would be 4939 patients. Based on our hospital admission data, we estimated an average length of stay at 7 days. The average ward size in the study site was 35 beds. Thus, within a 4-month intervention period 32,760 patient-days of care would occur for 5259 patients. As we performed a crossover study, each cluster serves as their own control. We therefore set the intracluster correlation coefficient (ICC) at 0.0001.
To compare the occurrence of HAI, we calculated incidence rate ratios with 95% confidence intervals. In the analyses, the soap-based strategy was used as reference. All analyses were performed with SPSS (Chicago, Illinois, USA, version 25) and SAS (Cary, North Carolina, USA, version 9.4).
Role of funding source
The study was funded primarily by the German Federal Ministry of Education and Research (Bundesministerium für Bidung und Forschung) within the framework of InfectControl 2020 (Project KARMIN – 03Z0818C) and was partly funded by the Bill and Melinda Gates foundation (Investment ID INV-004308). The funder played no role in the development or implementation of the study protocol and had no influence or access to either the data or the analysis and interpretation at any time. All authors had full access to all data in the study and had final responsibility for the decision to submit for publication.
The funding sources had no role in the writing of the manuscript or the decision to submit it for publication. No author has received a fee for writing this article from a pharmaceutical company or other organization. No authors of the study were precluded from accessing study data, and they accept full responsibility for its submission for publication.
Results
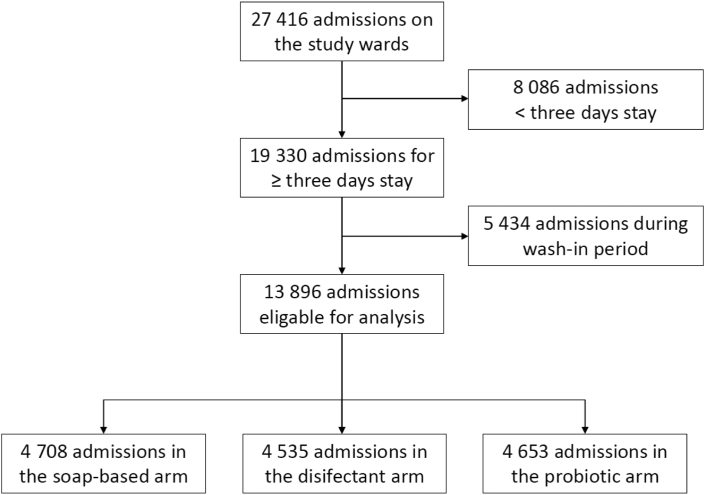
The study took place from June 2017 until August 2018 on 18 non-ICU wards (Table S1). During the study period, 20,130 patients accounted for 27,416 admissions (on average 1.36 admissions per patient). 8086 patient admissions (19.8%) had to be excluded because of a stay shorter than three days and 5434 admissions (29.5%) were excluded because they took place during a wash-in period (Fig. 2). A total of 13,896 admissions (11,428 patients) met all inclusion criteria, accounting for a total of 98,933 exposure days. The average number of patient admissions per arm was 4632 and the average number of exposure days per arm was 32,977. There was no relevant difference between study arms. The compliance with study protocols was 96.6% overall. The hand hygiene compliance across all wards was 63.4% (Table S1). We performed two analysis: (1) the incidence of HAI among admissions of patients to a study ward and (2) the incidence of HAIs with MDROs (MRSA, MDR-GN, VRE) (Table 1).
Fig. 2.
Recruitment flowchart.
Table 1.
Baseline characteristics.
| Total n = 13,896 | Soap-based (reference) n = 4708 | Disinfection n = 4535 | Probiotic n = 4653 | |
|---|---|---|---|---|
| Basic demographics | ||||
| Total length of stay, median (IQR) | 6 (4–10) | 6 (4–10) | 7 (4–11) | 6 (4–10) |
| Exposure days, median (IQR) | 5 (4–8) | 5 (4–8) | 5 (4–8) | 5 (4–8) |
| Age, median (IQR) | 61 (47–74) | 62 (49–74) | 61 (46–73) | 60 (47–74) |
| Male gender | 53% (n = 7368) | 53% (n = 2504) | 53% (n = 2418) | 53% (n = 2446) |
| Comorbidities | ||||
| Heart disease | 9% (n = 1177) | 9% (n = 417) | 8% (n = 379) | 8% (n = 381) |
| Diabetes | 16% (n = 2267) | 16% (n = 760) | 16% (n = 740) | 17% (n = 767) |
| Petptic ulcer | 1% (n = 108) | 1% (n = 38) | 1% (n = 37) | 1% (n = 33) |
| Liver disease | 5% (n = 742) | 5% (n = 250) | 5% (n = 240) | 5% (n = 252) |
| Neurological disease | 4% (n = 520) | 4% (n = 169) | 4% (n = 196) | 3% (n = 155) |
| Cancer | 19% (n = 2633) | 20% (n = 928) | 19% (n = 852) | 18% (n = 853) |
| Rheuma | 8% (n = 1154) | 9% (n = 420) | 8% (n = 378) | 8% (n = 356) |
| AIDS/HIV | <1% (n = 37) | <1% (n = 17) | <1% (n = 11) | <1% (n = 9) |
| Lung disease | 9% (n = 1198) | 9% (n = 402) | 9% (n = 402) | 9% (n = 394) |
| Renal disease | 20% (n = 2773) | 18% (n = 865) | 20% (n = 912) | 21% (n = 996) |
| Leukemia | <1% (n = 35) | <1% (n = 10) | <1% (n = 10) | <1% (n = 15) |
| Charlson Comorbidity Index, median (IQR) | 3 (1–5) | 3 (1–5) | 3 (1–5) | 3 (1–5) |
| All cause mortality | <1% (n = 116) | <1% (n = 29) | <1% (n = 45) | <1% (n = 42) |
Data are presented as % (n) unless stated otherwise.
IQR, interquartile range.
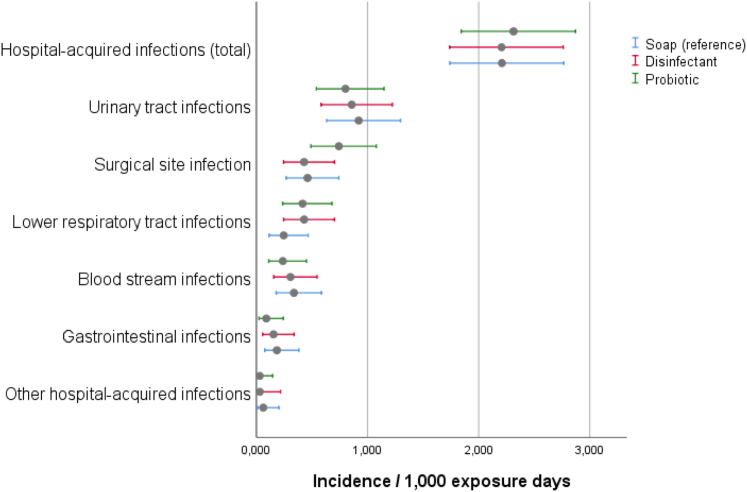
We detected 222 HAIs in 219 patients. The overall incidence was 1.59 per 100 patients (corresponding to a prevalence of 2.39%, see Supplementary Material S1) and an incidence density of 2.243 HAIs per 1000 exposure days. Patients with HAI had longer overall hospital stays than patients without HAI (17 vs. 5 days, p < 0.001), were older (68 years vs. 61 years, p < 0.001), had a higher Charlson comorbidity index (5 vs. 3, p < 0.001) and were more likely to die during their hospital stay (8% vs. 1%, p < 0.001) (Table S4). There was no statistically significant difference between the HAI incidences densities of the three arms (Table 2, Fig. 3).
Table 2.
Incidence of hospital-acquired infection and multidrug-resistant organisms.
| Soap-based (reference) | Disinfectant | Probiotic | |
|---|---|---|---|
| Protocol compliance | 95.6% | 96.9% | 96.9% |
| Exposed patients | 4708 | 4535 | 4653 |
| Exposure days | 33,704 | 32,633 | 32,596 |
| Hospital-acquired infections | |||
| Incident cases (incidence per 100 patients) | 78 (1.65) | 72 (1.59) | 72 (1.55) |
| Incidence density (per 1000 exposure days) (95% CI) | 2.314 (1.842, 2.873) | 2.206 (1.739, 2.762) | 2.209 (1.741, 2.766) |
| Risk reduction ID (95% CI) | Reference | −0.108 (−0.831, 0.616) | −0.105 (−0.829, 0.619) |
| IRR (95% CI); p value | 1 (ref) | 0.953 (0.692, 1.313); 0.8337 | 0.955 (0.692, 1.315); 0.839 |
| Overall MDRO infection | |||
| Incident cases (incidence density per 100 patients) | 18 (0.382) | 16 (0.353) | 15 (0.322) |
| Rate (per 1000 exposure days) (95% CI) | 0.534 (0.3164, 0.8441) | 0.490 (0.2902, 0.7792) | 0.460 (0.2574, 0.7590) |
| Risk reduction (95% CI) | Reference | −0.044 (−0.388, 0.301) | −0.074 (−0.413, 0.2654) |
| IRR (95% CI); p value | 1 (ref) | 0.919 (0.468, 1.800); 0.8073 | 0.862 (0.434, 1.710); 0.6757 |
| Methicillin-resistant Staphylococcus aureus | |||
| Incident cases (%) | 6 (0.127) | 1 (0.022) | 2 (0.043) |
| Rate (per 1000 exposure days) (95% CI) | 0.178 (0.072, 0.370) | 0.031 (0.002, 0.151) | 0.061 (0.010, 0.203) |
| Risk reduction ID (95% CI) | Reference | −0.1474 (−0.302, 0.072) | −0.117 (−0.283, 0.049) |
| IRR (95% CI); p value | 1 (ref) | 0.172 (0.021, 1.43); 0.136 | 0.345 (0.069, 1.707); 0.312 |
| Vancomycin-resistant enterococci | |||
| Incident cases (%) | 6 (0.127) | 6 (0.018) | 3 (0.065) |
| Rate (per 1000 exposure days) (95% CI) | 0.178 (0.072, 0.370) | 0.184 (0.075, 0.382) | 0.092 (0.023, 0.25) |
| Risk reduction ID (95% CI) | Reference | 0.058 (−0.199, 0.211) | −0.0856 (−0.262, 0.091) |
| IRR (95% CI); p value | 1 (ref) | 1.033 (0.333, 3.202); 1.000 | 0.517 (0.129, 2.067); 0.541 |
| Multidrug-resistant Gram-negative bacteria | |||
| Incident cases (%) | 6 (0.127) | 9 (0.198) | 10 (0.215) |
| Rate (per 1000 exposure days) (95% CI) | 0.178 (0.0722, 0.370) | 0.276 (0.135, 0.506) | 0.307 (0.156, 0.547) |
| Risk reduction ID (95% CI) | Reference | 0.098 (−0.132, 0.327) | 0.1289 (−0.109, 0.366) |
| IRR (95% CI); p value | 1 (ref) | 1.549 (0.552, 4.352); 0.564 | 1.723 (0.626, 4.741); 0.415 |
ID, incidence density; IRR, incidence rate ratio; CI, confidence interval; MDRO, multidrug-resistant organisms.
Fig. 3.
Hospital-acquired infections given as incidence per 1000 exposure days and 95% confidence intervals (error bars).
The most commonly detected HAI was urinary tract infection (38.3%, n = 85), followed by surgical site infection (24.3%, n = 54), lower respiratory tract infection (16.2%, n = 36), blood stream infection (13.1%, n = 29), and gastrointestinal infection (6.3%, n = 14). The prevalence of all other infections was 1.8%, n = 4 (Supplementary Table S2). Using routine clinical microbiological methods, we found 238 pathogens in 88.7% (197 of 222) of hospital-acquired infections. They accounted for an average of 1.2 pathogens per infection. 11.3% (n = 25) of infections were detected based on clinical definitions. The six most commonly detected pathogens were E. coli (29.4%, n = 70), S. aureus (13.4%, n = 32), Enterococcus feacium (10.1%, n = 24), Klebsiella pneumoniae (8.4%, n = 20), Staphylococcus epidermidis (5.9%, n = 14), and Clostridoides difficile (5.9%, n = 14) (Table S3). During the entire study, no clinical specimen grew bacillus species. Furthermore, no relevant outbreak was detected on the study wards during the trial. The most commonly detected MDRO overall was MDR-GN (n = 25), followed by VRE (n = 15) and MRSA (n = 9). There was no relevant statistical difference between both cleaning strategy and the reference (Table 2, Fig. 3).
The results of the biological burden examination showed low overall contamination detecting 8% (48 of 585) of samples Enterococci spp. and in <1% (2 of 585) E. coli with no significant differences between the groups (Supplementary Table S5).
Discussion
Improving environmental hygiene in hospitals contributes to patient safety. Many studies have shown that interventions in the hospital environment could improve hygiene conditions. However, two recent systematic reviews showed that the body of literature concerning this topic has yielded varying results, from studies of heterogeneous quality, of which, notably, only a few assessed the effect on HAIs.9,10 The present prospective, cluster-randomized study is, to our knowledge, the first to examine the effects of three environmental cleaning strategies on the risk of HAIs.
Compared to earlier studies, we observed a lower overall incidence of hospital-acquired infections.29, 30, 31, 32 However, our institution participated several times in ECDCs point prevalence study.33, 34, 35 The results on non-ICU wards were similar to the present study, showing an average HAI prevalence and comparable antimicrobial use in European tertiary care hospitals. Moreover, previous studies included intensive care units, while our study took place in non-ICUs only. Thus, our baseline results appear plausible. Under these conditions, cleaning with either surface disinfection or probiotic cleaning showed no statistical significant difference. When compared with other studies, we found the same distribution of types of HAIs and HAI-associated pathogens.29, 30, 31, 32 Though not statistically significant, disinfection and probiotic cleaning where associated with a trend towards a lower risk of surgical site infection and probiotic cleaning alone towards a decreased risk of lower respiratory tract infection. The overall incidence of HAIs with multidrug-resistant organisms was similar to earlier reports from Germany.36 Our results showed a decreasing trend in the incidence of MRSA infections which was associated with environmental disinfection and probiotic cleaning, as well as of VRE infections during probiotic cleaning. However, those trends were not statistically significant.
The overall evidence regarding the effect of environmental disinfection on the prevention of HAIs is limited.9,10 In most cases, studies did not assess the effect on HAI incidence but on surrogate parameters, such as the MDRO colonization rate or biological burden. Moreover, most studies examined bundles of interventions, making it difficult to estimate the effect of individual components.9,10 In their systematic review from relevant literature until June 2021, Thomas et al. found 14 relevant RCTs of which only four showed significantly changed primary outcomes. However, as the authors of this review pointed out as problematic methodological limitation in their overall conclusion, two studies assessed composite outcomes or specific marker microorganisms instead of assessing the overall risk for hospital-acquired infections.14,15,37 One study assessed the use of copper surfaces as primary intervention and one assessed enhanced education and precautions but no change cleaning agents.38,39
In Germany, routine disinfection of frequently touched surfaces in intensive care units is recommended by the Commission for Hospital Hygiene and Infection Prevention (KRINKO).40 However, daily disinfection can lead to a relevant exposure of hospital staff and patients to potentially toxic substances, to wastewater pollution, and to the biological selection of resistant bacteria.2,41 A recent study indicated that resistance to disinfectants might play a relevant role in hospital outbreaks, indicating possible disinfection failure through selection of disinfectant-resistant pathogens.23 The cleaning procedures investigated in this study appeared to be comparable to environmental disinfection. However, both procedures (soap-based and probiotics) are less toxic than disinfection, and the selection of disinfection-resistant pathogens is biologically unlikely. Another experimental study showed that both soap-based and probiotic cleaning were more effective than disinfectants in promoting competitor exclusion.18 An Italian study demonstrated using a before-after approach that probiotic cleaning could be associated with the reduction of HAIs by up to 50%.42 However, they started with a much higher baseline of HAIs and conducted a before-and-after study (instead of a cross-over trial) in which the probiotic phase followed the baseline period in each center. Dancer et al. pointed out that a protective effect of more than 30% seems biologically implausible, and the maximum potential effect is likely to be much lower overall.4,43
In our study, probiotic cleaning yielded similar results when compared to the other two cleaning strategies, especially disinfection. Furthermore, we did not turn up a single Bacillus spp. infection. Thus, probiotic cleaning appears to be a safe alternative to conventional cleaning agents. Recently, two experimental studies showed that the effect of probiotic cleaning on the hospital microbiome—Bacillus spp. reproduction and displacement of clinically relevant pathogens—is time-dependent.17,24 It is likely that the protective effect for HAIs also is time-dependent. Therefore, it is possible that our study was not long enough to capture the maximum effect of this strategy, particularly since we had to disinfect the rooms occasionally as part of the terminal cleaning after patients with certain infections or MDRO colonisation vacated their room. As a recent RCT showed, terminal room disinfection on ICUs has a significant protective effect against the transmission of MDROs. Thus, it is unclear whether terminal room disinfection can be omitted during a probiotic cleaning strategy.14 Disinfection in certain circumstances was also allowed in a previously published work.44 This study showed different outcomes, but also focused on surface pathogens rather than HAIs and had a different study design. Nevertheless, future studies should document the timing of probiotic use after disinfection, which is important to assess the impact on Bacillus spore viability and germination.
We did not find any overall significant effect on the incidence of MDRO infections. However, we found trends suggesting that both—disinfection and probiotic cleaning—lower the risk of HAIs resulting from MRSA. An earlier experimental study also found a significant reduction in MRSA resistance genes (mecA) during probiotic cleaning compared to soap-based and disinfection.17 Yet another experimental study showed a decrease in gene expression in A. baumanii and K. pneumoniae associated with probiotic cleaner.24 However, this effect is not yet fully understood and its epidemiological relevance needs to be assessed in further studies.
Our study has limitations. The incidence of HAIs in our study was lower than expected, and we included slightly fewer patients than planned in the sample estimate. However, we did not detect a relevant trend between the groups, so it is questionable whether a higher baseline incidence of HAIs or a larger study population would show relevant differences between the groups. We collected the data retrospectively, but the study staff were blinded in regards to the cleaning protocol they analyzed. We did not assess the hand hygiene compliance specific to each study arm, only the overall hand hygiene compliance per ward. However, as the entire ward staff was fully blinded concerning the cleaning protocol, a relevant influence on study outcome is unlikely. We did not perform post-discharge surveillance. That could have led to an underestimation of late onset HAIs. The environmental examination showed low overall contamination. Therefore, in environments with higher levels of contamination or significantly other settings notably low- or middle income countries and countries with higher HAI rates, the results could be different. Our study was designed to assess the impact of different cleaning agents on the risk for hospital-acquired infections as this is the most relevant clinical outcome. This is in clear contrast to the earlier studies that assessed the impact on the surface microbiome in Italy.44 Moreover, the colonization pressure for MDROs is much higher in Italy compared to Germany. Hence, we don't think the results are comparable. Since the infection incidence on non-ICU wards is significantly lower than ICUs, this should be investigated in future studies. German regulations required us to perform terminal disinfection after discharging patients with certain infections and MDROs. This could have led to a breach of the probiotic biofilm during the probiotic cleaning strategy and might as a result have altered the results.
In conclusion, our study showed that on non-ICU wards, routine surface disinfection is not superior to soap-based or probiotic cleaning in preventing HAIs and MDRO infections. Thus, probiotic cleaning agents may serve as an interesting alternative, especially as they provide other advantages such as non-toxicity, environmental sustainability, and potential long-term protection. Regardless our results, surface disinfection should continue to be used in outbreak situations. Considering that the baseline rates for HAIs and MDROs were low in our setting, results might be different under other conditions with higher baseline rates.
Contributors
Literature search: RL/PG, figures: RL/FS, study design: RL/PG, data collection: RL/BK/AB/EL, data access and data verification: RL/BK/AB/EL/PG, data analysis: RL/BK/AB/PG, data interpretation: RL/PG, writing: RL/PG, conceptualisation: RL/GZ/PG, data curation: RL/BK/AB, formal analysis: RL/FS/PG, funding acquisition: RL/PG, investigation: RL/BK/AB/EL/GZ/PG, methodology: RL/FS/PG, project administration: RL/PG, resources: RL/PG, software: RL/PG, supervision: FS/PG, validation: RL/BK/AB/EL/GZ/PG, visualisation: RL/FS, writing: original draft: RL/PG, writing: review & editing: RL/BK/AB/EL/GZ/PG.
Data sharing statement
Individual participant data that underlie the results reported in this article will be made available, after de-identification. This includes also the study protocol. The data will be available beginning 9 months and ending 36 months following article publication. Data will be shared with researchers who provide a methodologically sound proposal for any types of analyses to achieve aims in the approved proposal. Proposals should be directed to rasmus.leistner@charite.de. To gain access, data requestors will need to sign a data access agreement.
Declaration of interests
The authors declare that they have no conflict of interest.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2023.101958.
Appendix A. Supplementary data
References
- 1.Dancer S.J. The role of environmental cleaning in the control of hospital-acquired infection. J Hosp Infect. 2009;73(4):378–385. doi: 10.1016/j.jhin.2009.03.030. [DOI] [PubMed] [Google Scholar]
- 2.Dancer S.J. Controlling hospital-acquired infection: focus on the role of the environment and new technologies for decontamination. Clin Microbiol Rev. 2014;27(4):665–690. doi: 10.1128/CMR.00020-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weber D.J., Anderson D., Rutala W.A. The role of the surface environment in healthcare-associated infections. Curr Opin Infect Dis. 2013;26(4):338–344. doi: 10.1097/QCO.0b013e3283630f04. [DOI] [PubMed] [Google Scholar]
- 4.Dancer S.J. Oxford University Press US; 2020. How much impact do antimicrobial surfaces really have on healthcare-acquired infection? pp. 1814–1816. [DOI] [PubMed] [Google Scholar]
- 5.Lax S., Sangwan N., Smith D., et al. Bacterial colonization and succession in a newly opened hospital. Sci Transl Med. 2017;9(391) doi: 10.1126/scitranslmed.aah6500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klassert T.E., Leistner R., Zubiria-Barrera C., et al. Bacterial colonization dynamics and antibiotic resistance gene dissemination in the hospital environment after first patient occupancy: a longitudinal metagenetic study. Microbiome. 2021;9(1):1–17. doi: 10.1186/s40168-021-01109-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lax S., Gilbert J.A. Hospital-associated microbiota and implications for nosocomial infections. Trends Mol Med. 2015;21(7):427–432. doi: 10.1016/j.molmed.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 8.Assadian O., Harbarth S., Vos M., Knobloch J.K., Asensio A., Widmer A.F. Practical recommendations for routine cleaning and disinfection procedures in healthcare institutions: a narrative review. J Hosp Infect. 2021;113:104–114. doi: 10.1016/j.jhin.2021.03.010. [DOI] [PubMed] [Google Scholar]
- 9.Peters A., Schmid M.N., Parneix P., et al. Impact of environmental hygiene interventions on healthcare-associated infections and patient colonization: a systematic review. Antimicrob Resist Infect Control. 2022;11(1):38. doi: 10.1186/s13756-022-01075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thomas R.E., Thomas B.C., Conly J., Lorenzetti D. Cleaning and disinfecting surfaces in hospitals and long-term care facilities for reducing hospital- and facility-acquired bacterial and viral infections: a systematic review. J Hosp Infect. 2022;122:9–26. doi: 10.1016/j.jhin.2021.12.017. [DOI] [PubMed] [Google Scholar]
- 11.D'Accolti M., Soffritti I., Bini F., Mazziga E., Mazzacane S., Caselli E. Pathogen control in the built environment: a probiotic-based system as a remedy for the spread of antibiotic resistance. Microorganisms. 2022;10(2):225. doi: 10.3390/microorganisms10020225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bogusz A., Stewart M., Hunter J., et al. How quickly do hospital surfaces become contaminated after detergent cleaning? Healthc Infect. 2013;18(1):3–9. [Google Scholar]
- 13.Stewart M., Bogusz A., Hunter J., et al. Evaluating use of neutral electrolyzed water for cleaning near-patient surfaces. Infect Control Hosp Epidemiol. 2014;35(12):1505–1510. doi: 10.1086/678595. [DOI] [PubMed] [Google Scholar]
- 14.Anderson D.J., Chen L.F., Weber D.J., et al. Enhanced terminal room disinfection and acquisition and infection caused by multidrug-resistant organisms and Clostridium difficile (the Benefits of Enhanced Terminal Room Disinfection study): a cluster-randomised, multicentre, crossover study. Lancet. 2017;389(10071):805–814. doi: 10.1016/S0140-6736(16)31588-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson D.J., Moehring R.W., Weber D.J., et al. Effectiveness of targeted enhanced terminal room disinfection on hospital-wide acquisition and infection with multidrug-resistant organisms and Clostridium difficile: a secondary analysis of a multicentre cluster randomised controlled trial with crossover design (BETR Disinfection) Lancet Infect Dis. 2018;18(8):845–853. doi: 10.1016/S1473-3099(18)30278-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fauci Vl, Costa G., Anastasi F., Facciolà A., Grillo O., Squeri R. An innovative approach to hospital sanitization using probiotics: in vitro and field trials. J Microb Biochem Technol. 2015;7(3):160–164. [Google Scholar]
- 17.Klassert T.E., Zubiria-Barrera C., Neubert R., et al. Comparative analysis of surface sanitization protocols on the bacterial community structures in the hospital environment. Clin Microbiol Infect. 2022;28(8):1105–1112. doi: 10.1016/j.cmi.2022.02.032. [DOI] [PubMed] [Google Scholar]
- 18.Stone W., Tolmay J., Tucker K., Wolfaardt G.M. Disinfectant, soap or probiotic cleaning? Surface microbiome diversity and biofilm competitive exclusion. Microorganisms. 2020;8(11):1726. doi: 10.3390/microorganisms8111726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vandini A., Temmerman R., Frabetti A., et al. Hard surface biocontrol in hospitals using microbial-based cleaning products. PLoS One. 2014;9(9) doi: 10.1371/journal.pone.0108598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Srivastav A.L., Patel N., Chaudhary V.K. Disinfection by-products in drinking water: occurrence, toxicity and abatement. Environ Pollut. 2020;267 doi: 10.1016/j.envpol.2020.115474. [DOI] [PubMed] [Google Scholar]
- 21.Doll M., Stevens M., Bearman G. Environmental cleaning and disinfection of patient areas. Int J Infect Dis. 2018;67:52–57. doi: 10.1016/j.ijid.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 22.Rutala W., Weber D. Surface disinfection: should we do it? J Hosp Infect. 2001;48:S64–S68. doi: 10.1016/s0195-6701(01)90017-9. [DOI] [PubMed] [Google Scholar]
- 23.van Dijk H.F., Verbrugh H.A. Resisting disinfectants. Community Med. 2022;2(1):1–5. doi: 10.1038/s43856-021-00070-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu J., Shuai W., Sumner J.T., Moghadam A.A., Hartmann E.M. Clinically relevant pathogens on surfaces display differences in survival and transcriptomic response in relation to probiotic and traditional cleaning strategies. NPJ Biofilms Microbiomes. 2022;8(1):1–12. doi: 10.1038/s41522-022-00335-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dancer S.J. Dos and don'ts for hospital cleaning. Curr Opin Infect Dis. 2016;29(4):415–423. doi: 10.1097/QCO.0000000000000289. [DOI] [PubMed] [Google Scholar]
- 26.des Robert Koch-Institutes B. Liste der vom Robert Koch-Institut geprüften und anerkannten Desinfektionsmittel und-verfahren. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2017;11:1274. doi: 10.1007/s00103-017-2633-7. [DOI] [PubMed] [Google Scholar]
- 27.Horan T.C., Gaynes R.P., Martone W.J., Jarvis W.R., Emori T.G. CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Am J Infect Control. 1992;20(5):271–274. doi: 10.1016/s0196-6553(05)80201-9. [DOI] [PubMed] [Google Scholar]
- 28.Thygesen S.K., Christiansen C.F., Christensen S., Lash T.L., Sørensen H.T. The predictive value of ICD-10 diagnostic coding used to assess Charlson comorbidity index conditions in the population-based Danish National Registry of Patients. BMC Med Res Methodol. 2011;11(1):1–6. doi: 10.1186/1471-2288-11-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Behnke M., Aghdassi S.J., Hansen S., Pen A. The prevalence of nosocomial infection and antibiotic use in German hospitals. Deutsches Ärzteblatt Int. 2017;114(50):851. doi: 10.3238/arztebl.2017.0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vandael E., Latour K., Goossens H., et al. Point prevalence survey of antimicrobial use and healthcare-associated infections in Belgian acute care hospitals: results of the Global-PPS and ECDC-PPS 2017. Antimicrob Resist Infect Control. 2020;9(1):1–13. doi: 10.1186/s13756-019-0663-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zingg W., Metsini A., Balmelli C., et al. National point prevalence survey on healthcare-associated infections in acute care hospitals, Switzerland, 2017. Euro Surveill. 2019;24(32) doi: 10.2807/1560-7917.ES.2019.24.32.1800603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Suetens C., Latour K., Kärki T., et al. Prevalence of healthcare-associated infections, estimated incidence and composite antimicrobial resistance index in acute care hospitals and long-term care facilities: results from two European point prevalence surveys, 2016 to 2017. Euro Surveill. 2018;23(46) doi: 10.2807/1560-7917.ES.2018.23.46.1800516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Behnke M., Aghdassi S.J., Hansen S., Pen A., Gastmeier P., Piening B. The prevalence of nosocomial infection and antibiotic use in German hospitals. Deutsches Ärzteblatt Int. 2017;114(50):851. doi: 10.3238/arztebl.2017.0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aghdassi S.J.S., Gastmeier P., Piening B.C., et al. Antimicrobial usage in German acute care hospitals: results of the third national point prevalence survey and comparison with previous national point prevalence surveys. J Antimicrob Chemother. 2018;73(4):1077–1083. doi: 10.1093/jac/dkx494. [DOI] [PubMed] [Google Scholar]
- 35.Behnke M., Hansen S., Leistner R., et al. Nosocomial infection and antibiotic use: a second national prevalence study in Germany. Deutsches Ärzteblatt Int. 2013;110(38):627. doi: 10.3238/arztebl.2013.0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gastmeier P., Geffers C., Herrmann M., et al. Nosocomial infections and infections with multidrug-resistant pathogens-frequency and mortality. Dtsch Med Wochenschr. 2016;141(6):421–426. doi: 10.1055/s-0041-106299. [DOI] [PubMed] [Google Scholar]
- 37.Mitchell B.G., Hall L., White N., et al. An environmental cleaning bundle and health-care-associated infections in hospitals (REACH): a multicentre, randomised trial. Lancet Infect Dis. 2019;19(4):410–418. doi: 10.1016/S1473-3099(18)30714-X. [DOI] [PubMed] [Google Scholar]
- 38.Mody L., Krein S.L., Saint S., et al. A targeted infection prevention intervention in nursing home residents with indwelling devices: a randomized clinical trial. JAMA Intern Med. 2015;175(5):714–723. doi: 10.1001/jamainternmed.2015.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Salgado C.D., Sepkowitz K.A., John J.F., et al. Copper surfaces reduce the rate of healthcare-acquired infections in the intensive care unit. Infect Control Hosp Epidemiol. 2013;34(5):479–486. doi: 10.1086/670207. [DOI] [PubMed] [Google Scholar]
- 40.von Flächen D. 2004. Anforderungen an die Hygiene bei der Reinigung. [Google Scholar]
- 41.Tong C., Hu H., Chen G., Li Z., Li A., Zhang J. Disinfectant resistance in bacteria: mechanisms, spread, and resolution strategies. Environ Res. 2021;195 doi: 10.1016/j.envres.2021.110897. [DOI] [PubMed] [Google Scholar]
- 42.Caselli E., Brusaferro S., Coccagna M., et al. Reducing healthcare-associated infections incidence by a probiotic-based sanitation system: a multicentre, prospective, intervention study. PLoS One. 2018;13(7) doi: 10.1371/journal.pone.0199616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dancer S.J., Adams C.E., Smith J., Pichon B., Kearns A., Morrison D. Tracking Staphylococcus aureus in the intensive care unit using whole-genome sequencing. J Hosp Infect. 2019;103(1):13–20. doi: 10.1016/j.jhin.2019.04.016. [DOI] [PubMed] [Google Scholar]
- 44.Soffritti I., D'Accolti M., Cason C., et al. Introduction of probiotic-based sanitation in the emergency ward of a children's hospital during the COVID-19 pandemic. Infect Drug Resist. 2022:1399–1410. doi: 10.2147/IDR.S356740. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.