Version Changes
Revised. Amendments from Version 1
In this version of the article, the authors made some edits as follows: 1. In the Abstract part of the paper, we made modifications depending on the suggestions of the reviewers. 2. The introduction has been revised, and more references have been included in response to the reviewers' comments. 3. In accordance to the suggestions made by the reviewers, the section relating to the ongoing 2022 mpox outbreak has been amended, and more references have been provided. Also, in response to the suggestions made by the reviewers, we added a figure that is relevant to the most recent mpox that has been confirmed worldwide. 4. The transmission and replication section has been revised, and more references have been included in response to the reviewers' comments. 5. The mpox in immunosuppressed patients’ section has been revised, and more references have been included in response to the reviewers' comments. 6. The management and prevention section changed to (prevention strategies and counter-measures for immunocompromised individuals). In addition, we revised, and more references have been included in response to the reviewers' comments. 7. The conclusion and future prospects section has been revised, in response to the reviewers' comments. 8. The name of (Eman Abdelaziz Ahmed Rashad Dabou) changed to (Eman Abdelaziz Dabou)
Abstract
The World Health Organization (WHO) proclaimed a public health emergency in July 2022 due to the emergence of Mpox (formerly monkeypox) while the globe was still dealing with the COVID-19 epidemic. The characteristics of mpox in immunocompetent individuals are well-characterized, despite difficulties in diagnostics, immunization, and access to treatment that persist in low-income countries. Patients with weakened immune systems are more likely to spread an illness and die from it than healthy people because they cannot mount a protective immune response against it, such as a neutralizing IgG and poxvirus-specific Th1 response. A health warning on severe mpox in people who are immunocompromised due to Human Immunodeficiency virus (HIV) and other illnesses was released by the U.S. Centers for Disease Control and Prevention (CDC) on September 29, 2022. The advice does not specifically include primary immunodeficiency, but it does define other immunocompromising disorders as “having autoimmune disease with immunodeficiency as a clinical component”. Both those with healthy immune systems and those with weakened immune systems, such as those who are immunosuppressed, older people, children, etc., have encountered serious health issues, but the latter group is more likely to do so. According to the advisory, “of the people with severe mpox manifestations for whom CDC has been consulted, the majority have had HIV with CD4 counts 200 cells/ml, indicating substantial immunosuppression”. However, new cases are still expected to be discovered, especially in low-income countries with limited access to diagnosis, treatment, and prevention, and where a large percentage of the mpox-infected population also has advanced HIV infection. Thus, further research is always needed to determine the best way to treat mpox in immunocompromised people. In this context, we discussed /reviewed the mpox clinical presentation, available treatment options and current preventive guidelines in immunocompromised patients.
Keywords: monkeypox, immunocompromised patients, autoimmune disease, human immunodeficiency virus, management, prevention, control
Introduction
The monkeypox virus (MPXV) is an Orthopoxvirus that can spread to humans and result in mpox (formerly monkeypox) disease quite similar to the smallpox. 1 Large respiratory droplets, inadvertent or intentional contact with bodily fluids or lesion material or contact with biota—including bedding or towels—are some of the main ways that viruses are spread. Symptoms like malaise, headache, lymphadenopathy, myalgia, and cutaneous symptoms have been documented within one to three days of the onset of fever. Macules may initially be present when a lesion first develops, followed by papules, pustules, and vesicles, which then dry up and peel off. Immunocompromised individuals should be examined for other diseases, such as primary or secondary infection with the varicella zoster virus or other pathogens such as Cryptococcus neoformans, Histoplasma capsulatum, or Bartonella henselae, when skin lesions arise along with viral prodromal symptoms. 2 Despite ongoing challenges with diagnoses, immunization, and availability to treatment in low-income countries, the characteristics of mpox in immunocompetent persons are well-characterized. Individuals with compromised immune systems are at a higher risk of spreading an infection and succumbing to it than the general population because they lack the ability to produce a protective immunological response, such as a neutralizing IgG and poxvirus-specific Th1 response. Yet, more instances will likely be found, particularly in low-income countries with sluggish healthcare systems and a high proportion of mpox-infected people who are also HIV-positive. 3 , 4 Research on the most effective treatment for mpox in immunocompromised patients is, thus, an ongoing necessity. In-depth discussion of mpox disease in immunocompromised people and recommended preventative measures is provided in the current article.
The epidemiological history of the mpox revealed that the first recognition of this disease in human beings was in 1970 in the Democratic Republic of the Congo among children. 5 The mpox disease was endemic to central and western Africa region where 12 countries were identified, including Benin, Cameroon, the Central African Republic, the Democratic Republic of the Congo, Gabon, the Ivory Coast, Liberia, Nigeria, Sierra Leone, and South Sudan. 6 – 7 Since then, human cases of mpox have occurred rarely in that area. 5 , 10 – 16 The first outbreak of the disease reported outside Africa was in the USA in 2003. 17 Investigation revealed that the USA outbreak was linked to an infected Prairie dog with MPXV. These pets were housed near rodents shipped from Ghana in 2003. 18 After the 2003 USA outbreak, several countries reported mpox cases with a history of travel from Nigeria in 2018, 19 including Israel, 20 the UK, 21 and Singapore. 22 Now, some 34 years later, we find ourselves in an eerily similar predicament. 23 The World Health Organization (WHO) closely tracked human mpox cases after the 1980 eradication and subsequent end of routine smallpox immunization out of worry that decreased immunity rates to smallpox would increase population vulnerability to the MPXV. In the Democratic Republic of the Congo, 760 cases were confirmed in laboratories between 2005 and 2007, and there were three spatial clusters from 2000 to 2015 that are indicative of outbreaks of probable mpox cases. From 1981-1986 to 2006-2007, the number of instances grew by 20 (from 0.72 per 10000 to 14.2 per 10000). There has been an uptick in reported cases of mpox in Nigeria since 2017, following an almost 40-year absence of such reports. 24 , 25 WHO reported in 2022 that mpox was endemic in several African countries, including Benin, Cameroon, the Central African Republic, the Democratic Republic of the Congo, Gabon, Ghana (identified in animals only), Côte d’Ivoire, Liberia, Nigeria, Sierra Leone, and South Sudan. There were 1284 probable cases of mpox and 58 deaths reported between January and May of 2022, with the majority of cases occurring in the Democratic Republic of the Congo. The discrepancy between confirmed and suspected cases is large and concerning, suggesting insufficient laboratory testing capability. In addition to rising rates in endemic regions, mpox has been detected in non-endemic regions (e.g., the United Kingdom, the United States, Israel, and Singapore) through either imported animals or tourists. In the United States, prairie dogs were linked to 71 human infections in 2003 (35 of which were confirmed by laboratories). Seven cases of mpox were identified in the United Kingdom between 2018 and 2021, four of which were linked to international travel to endemic regions. 26 – 28
The documented incubation period of mpox is 5-21 days and clinical symptoms range from widespread rashes with secondary fungal or bacterial skin infections or tissue death (necrosis) to intestine obstruction, and difficulties with the heart, lungs, urinary system, and nervous systems. 25 , 27
Since several Orthopoxvirus species share genetic and antigenic characteristics, getting infected with one of them may provide significant protection from getting infected with the others. The vaccinia virus vaccine offers defense against illnesses brought on by the variola major, mpox, or cowpox viruses. 29 , 30 Vaccine-induced cross-protection appears to be mediated by a variety of immunologic pathways, with neutralizing antibodies among the key players. 26 , 31 , 32 Monkeys can be immunized with the human smallpox vaccine to prevent mpox, which is consistent with the ability of the smallpox vaccine to give cross-protection for humans against mpox. Since the end of smallpox vaccinations in 1978, cross-protective immunity to different orthopoxviruses has decreased, especially in younger people without vaccinia-induced immunity, and the number of unvaccinated, susceptible people has increased globally. In fact, during the past few years, these changes have been accompanied by a rise in the number and geographic dispersion of human mpox cases. 33 , 34 A health warning on severe mpox in people who are immunocompromised due to HIV and other illnesses was released by the U.S. Centers for Disease Control and Prevention (CDC) on 29 th September 2022. 35
Notwithstanding difficulties in diagnostics, immunization, and availability to therapy in low-income countries, the characteristics of mpox in immunocompetent persons are well-defined. 28 Sexual contact was suspected as the mode of transmission in 95% of the 528 instances of mpox reported, with 98% of patients being gay or bisexual men. There were no fatalities, although 13% of patients needed hospitalization due to discomfort; a few patients needed hospitalization due to visual lesions, decreased oral intake, myocarditis, or acute kidney disease. The majority of patients exhibited a rash upon presentation, with the majority having less than 10 lesions and 11% having more extensive lesions. Patients had a 41% HIV infection rate, but after receiving antiretroviral medication, their viral loads dropped to undetectable levels. Most importantly, no coexisting immunocompromising diseases were observed, indicating that this is an immunologically healthy group. Hence, it is not surprising that only about 5% of people needed mpox-specific antiviral medication, as this demonstrates that mpox is self-limiting in immunocompetent people. 36 Although “having autoimmune disease with immunodeficiency as a clinical component” is included in the advisory's definition of other immunocompromising disorders, primary immunodeficiency is not specifically included in it. There have been reports of widespread rashes, subsequent bacterial or fungal skin infections, tissue death (necrosis), bowel obstruction, heart, lung, urinary, and neurological problems as severe symptoms of mpox. 26 Serious problems have happened in both immunocompromised and immunocompetent individuals, although immunocompromised individuals are more prone to experience them. The majority of those with severe mpox presentations for whom the CDC has been contacted have Human Immunodeficiency Virus (HIV) and CD4 counts below 200 cells/ml, indicating significant immunosuppression, according to the advice. 37
The ongoing 2022 mpox outbreak
In the 2022 outbreak, the mpox disease was reported by numerous non-endemic countries. The first case of mpox was reported by the United Kingdom Health Security Agency (UKHSA) in the United Kingdom. 38 Afterwards, cases of the illness were reported in Spain, Portugal, Italy, the United Kingdom, and the United States. 39 – 48 Up to January 06, 2023, the Centers for Disease Control and Prevention (CDC) confirmed 84,075 mpox cases in 110 countries worldwide with 75 deaths, 49 and clinical research has shown that the disease has distinct epidemiological and clinical features. 36 , 39 , 40 , 50 Statistics show that one-third of the reported cases were highly concentrated in the USA (the highest), Spain, Germany, and the UK. 51 The mortality rate of this disease is less severe as investigation revealed that the MPXV genome involved in the current outbreak belongs to West African (clade II) strain, which is less virulent than the Central African clade also known as the Congo Basin (clade I) strain. 52 The case fatality rate of the clade I and clade II strains of MPXV are documented to be 10.6% and 3.6% worldwide. 53 , 54
The demographic characteristics of reported cases distinguished from the previous outbreak were 99% male with a median age of 37 years, and 44% were HIV-positive. 55 Furthermore, 99% of reported cases were gay or bisexual, 55 indicating sexual transmission as a new mode of transmission. 33 Interestingly, Pluart et al. declared that mpox occurred among healthcare workers. 56 However, the protection of healthcare workers shoud be a top prority during this public health emergency. 57 , 58 Scientists have espoused that the affected people represent the population who did not receive smallpox vaccination due to disease eradication, indicating its possible contribution to their susceptibility to mpox disease. 59 , 60
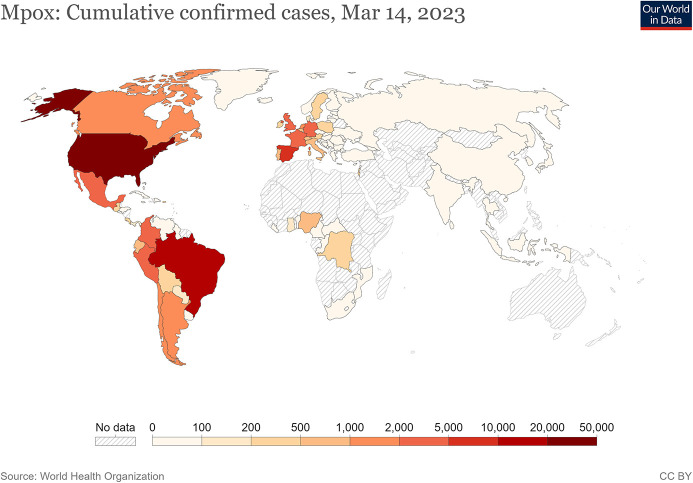
The WHO received its first reports of the 2022 global outbreak of mpox in May, and the number of confirmed cases has steadily increased ( Figure 1). Since then, many of the earliest cases were found in people who had attended a Pride celebration for LGBT+ people from all over the world. This event served as a transmission hub that spread the disease throughout several different European countries. By the end of May 2022, however, locally acquired illnesses and community transmission had become the norm throughout all impacted nations. An epidemic of mpox was labeled a Public Health Emergency of International Concern by the WHO on July 23, 2022. 3 , 26 , 27 , 62 , 63 Even though evidence is just starting to emerge, it is possible that mpox can present with more severe symptoms in immunocompromised adults than in the general population. 64 , 65 The CDC found that among 57 patients hospitalized with severe mpox infection between August and October 2022, 90% had underlying immunocompromising diseases, including 31 people with HIV who had a CD4 cell count of fewer than 50 cells/mm. Among the most striking aspects of this report is the notably high fatality rate of 21%, with at least 5 deaths directly attributable to mpox. 66
Figure 1. Geographical distribution of confirmed cases of mpox globally. 61 .
Genital lesions have been reported as one of the distinguishing features of the current outbreak. 67 The majority of confirmed cases have reported to clinics with at least one symptom, including systemic rashes, fever, or genital rashes, which is not consistent with the typical presentation of mpox disease, which usually includecentrifugal rashes preceded by fever and swollen lymph nodes. 45 , 53 In addition, the number of rashes is less in most cases, with the first rash occurring in the genital and perineal area followed by monographic and centrifugal distribution all over the body. 30 , 68 The other sites of lesions were as follows: 55% on the truck, arms, and legs, 25% on the face, 10% on the palms and soles. 36 Most rashes (58%) were identified as vesiculopustular. 36
In the current outbreak, there is a disappearance of an epidemiological link to the endemic region. 33 Yet, data showed that 91% of confirmed cases reported an immediate sexual exposure before symptoms started, particularly with men having sex with men, 30 while 98% of the patients identified as gay or bisexual. 36 Therefore, WHO considered sexual exposure in the 21 days before symptoms onset as a risk factor, 51 , 53 especially among men who have sex with other men. Evidence showed that all confirmed cases of mpox among men who have sex with other men have a history of sexually transmitted disease (STD), hepatitis C, syphilis, and HIV. 41 Epidemiologists reported that the reproductive ratio value of MPXV is between 1.10 and 2.40, suggesting the ability of an infected individual to infect one to two other persons. 68 Such value highlights the need to initiate preventive measures to contain the spread of the disease. 57 , 69 , 70
Transmission and replication
The MPXV is considered one of the largest DNA viruses 72 and its size ranges from 130 to 360 kbp. 73 It therefore stimulates the host immune system rapidly. The MPXV has two distinctive sets of modulatory proteins responsible for invading the immune system, including intracellular modulatory proteins and extracellular modulatory proteins. Another unique feature of MPXV is its exclusive replication in the cytoplasm. 73 The replication process of MPXV starts with viral attachment initiated by glycosaminoglycans including chondroitin, heparin sulfates, and laminin, 74 followed by viral fusion to the host cell involving 11 to 12 non-glycosylated, transmembrane proteins. 75 Once the viral entry is complete, viral transcription takes place by virus-encoded multi-subunit DNA-dependent RNA polymerase. 76 It is proceeded by the translation of proteins on the host ribosome, which occurs at three levels: early, intermediate and late translation. 76 Studies reveal that DNA synthesis and replication occur at cytoplasmic structures called factories, 20 proving its effectiveness in viral RNA synthesis. 72 Each factory derives from the cell's rough endoplasmic reticulum (RER). 77 Lastly, some of the mature virions transported via microtubules and coated by endoplasmic reticulum or Golgi-derived membranes will exit the cell by fusing with the cytoplasmic membrane and become extracellular enveloped virus (EEV). 78
The orthopoxviral physical stability contributes to their varied modes of transmission. 52 The mpox information chain includes animal-to-human transmission and human-to-human transmission. Since the virus is present in the infected host lesions, crust, and secretions, direct contact with these secretions can promote viral transmission, whether the host is animal or human. Animal to human transmission occurs when there is direct contact with the bodily fluids of affected animals, such as blood, saliva, and cutaneous and mucosal lesions of these animals. Furthermore, respiratory droplets and eating raw meat or poorly cooked meat products of affected animals can transmit the MPXV. Another transmission mode includes the bites and scratches caused by infected animals. 70 Monkeys, rats, and squirrels are the primary host of MPXV in Africa. 58
The source of MPXV has not been determined, however rats are a leading possibility. Consumption of raw or undercooked meat or other animal products from infected animals is a possible risk factor. Also, people who reside in or near forested areas may experience low-level or indirect exposure to sick animals. Direct or indirect contact with infected body fluids or lesion materials can result in transmission of mpox, despite the disease’s low transmissibility. Fomites, respiratory secretions, and skin-to-skin contact are all examples of direct exposure in the context of MPX. Patients can be exposed to you through indirect contact if you are in their room or within 6 feet of them during procedures that may produce aerosols from oral secretions, skin lesions, or the resuspension of dry exudates. Intimate contact during and after childbirth is another potential route of transmission, as is the placenta (resulting in congenital mpox). 3 , 27 The virus replicates at the site of infection, where it was initially detected by mononuclear phagocytic cells after infection. Afterwards, it leaves the skin and circulates through the body before returning to the dermal layer. Once the virus has completed its initial round of replication, it spreads to nearby lymph nodes, resulting in viremia 10–14 days later (the possible incubation period). Nonspecific prodromal symptoms such as fever, chills, myalgia, headache, fatigue, and lymphadenopathy typically occur before the hallmark clinical manifestation of a vesiculo-pustular rash. It is important to note that patients are contagious from the onset of prodromal symptoms until the scabs that formed over the lesions peel off. The oropharynx is typically the first area affected, followed by the skin. 26 , 79
Similarly, transmission from human to human occurs when there is direct contact with an infected person's rashes, sores, scabs, respiratory droplets, or oral fluids. 51 , 53 Furthermore, sharing the same household of infected individuals increases the risk of contamination. 70 The common mechanism used by MPXV in the 2022 outbreak is attacking the host defense by encoded proteins produced during transcriptions named MHC class II antigen presentation inhibitor, an IFN-alpha/beta receptor glycoprotein and IL-1/TLR signaling inhibitor. 80 Moreover, vertical transmission, such as from mother to fetus, was also identified. 81 Another unique feature that contributed to the current outbreak is the excessive genome mutations that result from the action of apolipoprotein B mRNA-editing catalytic polypeptide-like 3(APOBEC3) enzymes. 80 The action of these enzymes results in hypermutated, viable variants of the virus as revealed by phylogenomic analysis. 80
Immunosuppression
The immune system plays a vital role in defending the body against microorganisms and other foreign bodies. 82 Failure of the immune system to protect the body indicates the body's immunosuppressed status. Immunosuppression is defined as “a state of temporary or permanent dysfunction of the immune response resulting from insults to the immune system and leading to increased susceptibility to disease”, originally proposed by Dohms and Saif in 1984. 83 , 84 Immune dysfunction is classified as primary and secondary immune dysfunction. The immunosuppression status occurs due to disease condition or medication-induced immunosuppressed state. Consequently, hematologic malignancies, solid-organ transplant, chimeric antigen receptor (CAR)-T-cell therapy, or hematopoietic stem cell transplant are all common causes of immunosuppression, as are moderate to severe levels of immunodeficiency disease, such as DiGeorge syndrome, Wiskott-Aldrich syndrome, HIV cases with CD4 cell counts less than 200/mm 3, and the history of an AIDS-defining illness without immune recrudescence. The degree of immunosuppression can vary among patients. 85 Furthermore, medication that induces immunosuppression status includes high-dose corticosteroids (i.e., ≥20 mg of prednisone for two or more weeks), alkylating agents, antimetabolites, transplant-related immunosuppressive drugs, tumor necrosis factor (TNF) blockers, and other biologic agents that are immunosuppressive or immunomodulatory. Evidence has shown a steady rise in the number of acquired immunity deficiency (secondary immunodeficiency) conditions in response to an increasing number of individuals with transplantation of solid organ or hematopoietic stem cells 86 as such medical treatment requires an intensive immunosuppressive regimen which can cause the patient to develop severe adenovirus infections. 86 A US cross-sectional study revealed that 2.8% of patients experienced drug-induced immunosuppression between 2018 and 2019, particularly those who were prescribed oral corticosteroids for 30 days or longer (40.9%). 87 Oral corticosteroids, methotrexate, and other disease-modifying anti-rheumatic therapies, transplant antirejection drugs, tumor necrosis factor inhibitors, antineoplastic treatments, and other biological product medications all contribute to immunosuppression. 87 Immunosuppressive medication has a significant role in patient survival such as keeping a person from rejecting an organ transplant and treating the overactivity of the immune system in cases like autoimmune disease and allergies. On the other hand, immunosuppressant medication can negatively impact a patients' health as it increases their likelihood of having infections. 88
The most commonly reported infection among immunocompromised patients is a protracted infection characterized by the intra-host viral revolution and the generation of multiply mutated viruses. 88 The common form of protracted infection is protracted bacterial bronchitis which occurs among children. 89 In addition, other types of infections associated with immunosuppression are viral, bacterial, and fungal infections of the blood, lungs, and central nervous system. 90 Furthermore, the immunocompromised status might increase patients' susceptibility to prolonged infection, resulting in a prolonged length of stay in hospital and more complications. 91 Studies on immunosuppressed patients' response to infection vary in terms of mortality rate, complication, and length of stay. For example, authors of a study conducted in Spain among immunocompromised patients admitted with Influenza A (H1N1) virus in 2009 reported that immunocompromised patients had higher mortality than non-immunosuppressed individuals. 92 In addition, the complicated cases experienced a bacterial coinfection, specifically gram-negative bacilli and Staphylococcus aureus infections. 92 Inversely, a retrospective cohort study in the U.S. in 2020 was conducted on the impact of COVID-19 on drug-induced immunosuppressed patients. It revealed that the chronic use of immunosuppressive drugs was neither associated with worse nor better clinical outcomes among hospitalized cases with COVID-19 in terms of the risk of using mechanical ventilation, in-hospital mortality, or length of stay. 93 Similarly, another study revealed that immunosuppressed patients infected with COVID-19 are not at increased risk of severe pulmonary disease compared to other populations, highlighting the necessity of continuing patients' treatment, such as chemotherapy and radiotherapy. 94 The controversial condition of immune suppression was pregnancy. There was a debate regarding pregnancy being considered an immunosuppression state. 95 However, the authors highlight that the susceptibility of pregnant women to infection depends on the placental immune response to certain pathogens. 95 , 96
Monkeypox in immunosuppressed patients
According to studies, there is a greater chance of severe manifestation of MPXV infection in immunosuppressed patients. 50 , 97 – 106 Immunosuppressed patients include patients who have undertaken cancer treatment, organ transplant, HIV infection, primary immune deficiency disorders, some severe autoimmune disorders, and medications to treat autoimmune diseases and other illnesses that can weaken the immune system. The disease appears to have a lethal prognosis, especially in children who have not been vaccinated against smallpox. 97 Individuals with compromised immune systems include those with HIV, hematological malignancies, usage of immunosuppressive medicines such corticosteroids, organ transplant patients, autoimmune illness, or innate immunodeficiencies.
A CDC investigation of 57 people hospitalized with severe mpox showed a substantially more dismal natural history among immunocompromised persons. Around 90% of people had CD4 counts 200 cells/mm 3, and roughly 70% had CD4 levels 50 cells/mm 3. Less than 10% of people were getting antiretroviral therapy. Just about 5% of patients had undergone a solid organ transplant, and another 3.5% had been diagnosed with a hematologic malignancy. Severe mucosal lesions were present in the majority of patients, and all of them had severe skin lesions. In addition, mpox often spread to other organs, affecting the lungs (21%), eyes (7%) and brain (7%). Patients were treated with tecovirimat (93%), vaccinia immune globulin intravenous (51%), or cidofovir (23%), all of which are antivirals that target mpox. Certain mpox lesions may persist even when treated with antivirals, as evidenced by two patients’ individual stories. The most alarming statistic is that 21% of patients who were treated in the intensive care unit ultimately died, with 5 out of 12 deaths being directly attributed to mpox. 36 , 107 , 108 These results paint a bleak picture of the prevalence and lethality of mpox in immunocompromised patients, who, in contrast to immunocompetent persons, are unable to mount a neutralizing IgG and poxvirus-specific Th1 response after infection. 3 Two case reports, one from a patient receiving cytotoxic chemotherapy for Hodgkin’s lymphoma and the other from a patient who had undergone a kidney transplant in the context of well-controlled HIV, are published in the current edition of Transplant Infectious Diseases. Both patients were guys who engage in anal intercourse with other men and who did not use any form of barrier protection in the weeks leading up to the development of lesions. Large, disfiguring, and necrotic face lesions progressed in the lymphoma patient despite the early use of tecovirimat to combat the disease. When his lesions progressed, he was also given tecovirimat, cidofovir, and vaccinia immune globulin intravenous for 28 days (instead of the usual 14). The kidney transplant recipient contracted mpox despite having received a smallpox immunization as a child, indicating that the vaccine’s protective effects had worn off. In response to his over 50 lesions, he was started on tecovirimat 12 days after the onset of symptoms and treated for a total of 14 days. There was at least one lesion that appeared while he was on tecovirimat and tested positive for orthopoxvirus even after treatment ended; resistance testing is currently underway. The pictures of the recovered patients show that the cancer patient’s disease progressed significantly more severely than the kidney transplant patient’s, despite the fact that both patients survived. This finding likely reflects the kidney transplant recipient’s reduced net state of immunosuppression, given he had received his transplant 5 years prior to his illness, had not experienced any rejection episodes recently, and was only taking low doses of tacrolimus and azathioprine. Both these instances demonstrate the diversity of this infection’s clinical presentation in immunocompromised patients and the difficulties in controlling them in the absence of more information. 4 Although over 80000 instances of mpox have been diagnosed globally, including over 29000 in the United States, there is a lack of data from randomized controlled trials (RCTs) of antiviral medication. 107
The immunocompromised patient is more liable to get the mpox infection with severe manifestations of mpox, including extensive skin rashes with secondary bacterial or fungal infections or necrosis, bowel blockage, and heart, lung, urinary, and neurological complications. 42 , 60 , 100 Guarner et al. (2022) 33 reported that an immunosuppressed patient had several scattered maculopapular rashes and pustules on the trunk, upper and lower extremities, groin, and peri-anal area and palpable cervical lymph nodes. Moreover, immunocompromised patients are more likely to develop complications, including respiratory deterioration, acute kidney injury, and multiple organ dysfunctions. 97 Simon-Gozalbo et al. (2022) 109 reported a case of a 30-year-old male patient diagnosed with HIV infection. The manifestations were maculopapular rash affecting mainly the trunk, buttocks, upper and lower extremities, and multiple demarcated purpuric macules with central umbilicated pustules and crusts, along with palpable cervical lymph nodes.
Prevention strategies and counter-measures for immunocompromised individuals
Data on mpox in HIV patients are sparse; however, early identification, treatment, and prevention may lessen the severity of potential complications and slow the disease’s transmission. 97 No licensed treatment or proper evidence-based guideline is available for treating human mpox. However, the viruses belonging to the Orthopoxvirus genus are genetically similar. Hence, antivirals used to treat smallpox might be effective against mpox. Antiviral drugs such as tecovirimat, cidofovir, and brincidofovir can be considered mainly for those with severe symptoms or who may be at risk of poor outcomes, such as those with immune suppression. 110
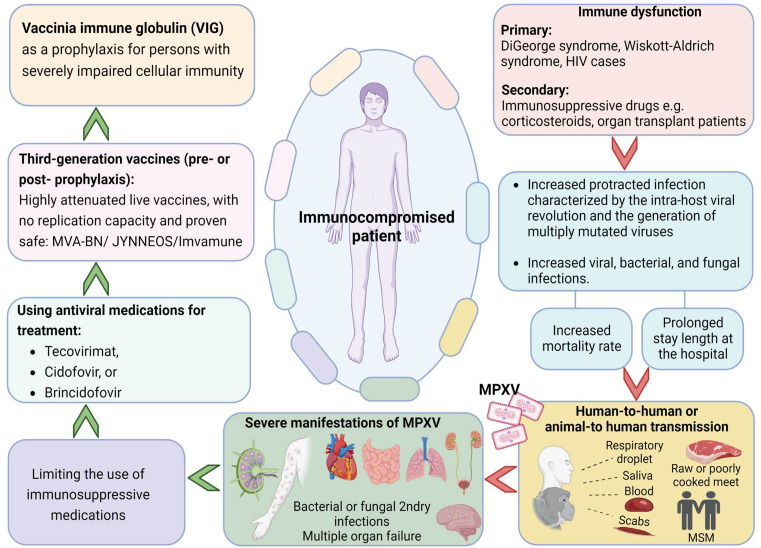
Two anti-viral medications, tecovirimat and brincidofovir, have been given the green light by the Food and Drug Administration (FDA) to treat smallpox. The CDC offers tecovirimat for use in the treatment of mumps as a first-line agent. Co-administration of tecovirimat and antiretroviral therapy for HIV infection is safe because there are no known medication interactions that would prevent it. The JYNNEOS vaccination can be used for prophylaxis both before and after exposure if necessary. Although the European Medicines Agency (EMA) has given its approval for the use of tecovirimat in mpox, there is a need for innovative therapies with different mechanisms due to the danger of resistance. In terms of effectiveness and safety, tecovirimat is the drug of choice for treating mpox. Consequently, the accessibility and diversity of effective anti- Orthopoxvirus medicines will be improved through the target-based design of novel antivirals. Patients who did not show a response to tecovirimat run the risk of developing resistance. An immunocompromised HIV/AIDS patient was reported to have contracted mpox by Viguier et al. (2022). 102 He had a serious, prolonged infection that was treated with tecovirimat for 14 days after his clinical status deteriorated. Almost immediately, he began to feel better, and both the skin lesions and MPXV burdens dropped with no negative consequences. In this instance, tecovirimat shows promise as an effective treatment. The success of tecovirimat in this patient suggests that it may be used to treat other immunocompromised people who have contracted MPXV. Hernandez et al. (2022) 100 reported a case study about an immunocompromised patient and treated him with tecovirimat. Tecovirimat could effectively and safely treat severe mpox infections among immunocompromised patients. Last but not least, there is an immediate need to focus on minimizing psychological distress, particularly among immunocompromised individuals and healthcare workers. 111 – 113 An overview of mpox in immunosuppressed patients, preventive measures and clinical management is presented in Figure 2.
Figure 2.
An overview on monkeypox in immunosuppressed patients, prevention and clinical management (Designed with Biorender premium software; https://app.biorender.com/).
Patients with immune impairment run the risk of contracting serious illness. As a result, efforts should be undertaken to strengthen their immune system in addition to using tecovirimat (e.g., limiting the use of immunosuppressive medications, initiating antiretroviral therapy for those with HIV). Following a tecovirimat 14-day therapy, certain individuals with significant immunocompromise may continue to develop lesions. 113 If viral resistance is suspected in this situation, physicians may think about sending a second swab for sequencing. As long as there is no sign of viral resistance, it is reasonable to continue tecovirimat (with emphasis on the need for a fatty meal for optimal absorption) until there is clinical improvement, but no longer than 90 days. 115 In contrast, if there is sign of viral resistance, a second anti-viral medication, such as cidofovir (or brincidofovir, if it becomes available), can be added. An infectious disease expert or public health official should be consulted for the management of such patients (e.g., the CDC mpox consultation team in the United States. 32 , 35 , 70 , 110
Prevention is essential for the immunocompromised patient to protect them from infection. There are two types of vaccines: second and third generation. Second-generation vaccines called replication-competent vaccinia virus vaccines due to their replication capacity in mammalian cells (examples include ACAM2000 and APSV approved for >18 years old) are associated with complications among immunocompromised patients. 116 This type of vaccine is not recommended for any group of immunosuppressed patients, especially those with inflammatory/autoimmune diseases of the central nervous system where there can be an outbreak of the disease. Third-generation vaccines, for example, MVA-BN/JYNNEOS/Imvamune, are highly attenuated live vaccines. In contrast to replication-competent smallpox vaccines, these replication-deficient vaccinia vaccines pose a lower risk of side effects, making them suitable for use in healthy people as well as those with immune system deficits, HIV infection, atopic dermatitis, or allergic rhinitis. However, those with compromised immune systems may not respond as well to the vaccine. 117 , 118 The vaccination of those with immunosuppression, who are at high risk for developing mpox sequelae, should proceed with a 0.5 ml SC/IM dose. People living with HIV whose CD4 count is greater than 200 cells/mm 3 can get MVA-BN through intradermal administration. 119
JYNNEOS (also known as Imvamune and Imvanex) is a vaccine against a modified Vaccinia Ankara virus that does not replicate. In the US, it was approved for use in preventing both mpox and smallpox. 110 Because JYNNEOS does not cause the development of live viruses in vaccinated patients, it is safer for use in immunocompromised individuals than ACAM2000. Importantly, however, immunocompromised persons may have a lower immunological response to the JYNNEOS vaccination. Thus, protection can be weaker than in immunocompetent people. Both vaccines can legally be administered to those aged 18 and up. However, information on JYNNEOS's ability to protect humans from MPXV infection is scant. 32 , 53 , 121 There is a lack of information regarding the efficacy of vaccinia immune globulin (VIG) in the treatment of mpox sequelae. In severe cases of human mpox, VIG may be considered, albeit its efficacy is uncertain. In the case of an exposed person with significantly compromised cellular immunity, for whom smallpox vaccination is not an option, VIG may be considered as a prophylactic measure. 1
Evidence from other immunosuppressive diseases suggests that people with HIV who are getting antiretroviral medication and have healthy CD4 cell counts are not at elevated risk for most infections, including opportunistic infections like mpox. The approved first-line treatment for mpox is tecovirimat, and clinicians should consider taking this drug when determining a patient’s risk of serious illness due to both HIV and mpox. Tecovirimat and antiretroviral therapy can be used together, if necessary, because of the low risk of medication interactions. Pre- and post-exposure prophylaxis with a mpox vaccination are options that clinicians should consider. The JYNNEOS vaccine, which is licensed for the protection of smallpox and mpox, is recommended for patients with HIV since it uses a live, nonreplicating modified vaccinia Ankara virus. 25 , 122
To sum up, the 85% efficacy offered by cross-immunity means that the current smallpox vaccinations are also licensed for mpox. However, before recommending or not vaccinating immunosuppressed patients, it is essential to be well informed of the type of vaccine that local authorities are obtaining. If it is a second-generation live attenuated vaccine, these patients should not receive it. If it is a third generation live attenuated vaccine with no replication capacity and proven safe in immunosuppressed patients. In that case, vaccination should be advised, always considering that the underlying disease should not be active at the moment of the inoculation. 2 , 123
However, new cases are still expected to be discovered, especially in low-income countries with limited access to diagnosis, treatment, and prevention, and where a large percentage of the mpox-infected population also has advanced HIV infection. Thus, further research is always needed to determine the best way to treat mpox in immunocompromised people. First, it is crucial that all occurrences of mpox infection in immunocompromised individuals continue to be recorded so that we may learn more about mpox’s ability to spread and kill in this patient population. To further define the host immune response to mpox in patients with and without underlying immunodeficiency, the duration of viral infectivity, and the prevalence of treatment-emergent resistance, all providers caring for immunocompromised patients with mpox infection in settings where resources are available should attempt to enroll these patients into biorepositories. 124 Third, there is a need for prospective research to determine appropriate therapeutic approaches of immunocompromised patients with mpox, including treatment duration and the impact of combination antivirals. While a randomized controlled trial (RCT) of tecovirimat for mpox infection is currently being conducted with funding from the National Institutes of Health (NIH) (NCT05534984), the declining number of mpox cases worldwide makes it unlikely that RCTs focusing solely on immunocompromised patients will ever be conducted. Similar to the feasibility of conducting trials of cidofovir, brincidofovir, vaccinia immune globulin intravenous, or combinations thereof, their implementation is highly improbable. Thus, any application of tecovirimat should be documented as part of observational research to fill this data vacuum, whether through currently established procedures or once the NIH-sponsored trial (hopefully) proves clinical efficacy. Fourth, further information is needed on the safety and immunogenicity of the smallpox and monkeypox, non-replicating vaccine (JYNNEOS), notwithstanding the fact that clinical efficacy trials of this vaccine cannot be undertaken in immunocompromised persons. In the same way as COVID-19 vaccination data were generated by academic medical centers, so too should JYNNEOS data about the safety and immunogenicity of the vaccine in immunocompromised individuals. 4 , 108 , 122
Conclusion and future prospects
Immunotherapeutics and preventative strategies are critical public health interventions that complement rigorous contact tracing in stopping the spread of mpox. In a similar manner, serology-based investigations are effective surveillance tools for tracing contacts and determining exposure histories. However, given that vaccination provides a baseline of protection against poxviruses, these serological diagnostic approaches must be MPXV-specific. Expanding the surveillance network and identifying gaps are also crucial for an efficient ring-fencing system. Importantly, there is a fundamental need in public health to alert individuals who may be exposed about the advantages and hazards of vaccination.
Numerous important scientific queries are yet unanswered. The mechanisms of immune defense against the MPXV will need to be better understood, which will necessitate more research on the human systemic and mucosal immune responses during MPXV infection. It is significant to note that it is not yet known whether previous exposure to variola, mpox, or smallpox vaccine results in any type of mucosal immunity. Understanding the mucosal immune responses will be critical due to the respiratory aerosol transmission of MPXV and other poxviruses. To further understand the respiratory difficulties produced by MPXV, it would be especially necessary to understand the functions of tissue-resident memory T cells and IgA in infection. Because MPXV DNA has been detected in semen, it is also necessary to define the immunity of the preputial mucosa. A key objective for assessing more recent vaccines, particularly those intended for the at-risk populations of immune compromised populations such as older people, pregnant women and children, is defining the immunological correlates of protection. What other factors, such as those related to behavior, geography, nutrition, health, immunology, or genetics, besides not having received a vaccine, could be at play? Recent research suggests that the severity of a viral respiratory infection in young children is tied to the efficiency of their innate immune responses. Children who are infected typically have weaker T cell and B cell responses than adults, similar to reports for SARS-CoV-2 infections. Children with MPXV tend to manifest a more severe disease and have lower vaccine effectiveness, which may be explained by the characterization of adaptive immune responses in these children. Understanding the risks of vaccination in immunocompromised populations, particularly children and expectant women, is also crucial.
It is feasible for MPXV to co-infect with any of the several infectious diseases that are endemic at global level. A co-infection with malaria and an alphavirus, for instance, can drastically alter host immunity and influence the course of an illness. Co-infection with other illnesses that disproportionately affect the group of men who have sex with men makes it especially important to understand mpox disease and immunization, especially given the continuous transmission among these communities in non-endemic countries. This is especially true for HIV-1, which can significantly inhibit adaptive immune reactions. Additionally, it is important to identify the risk factors for severe MPXV. The most vulnerable groups are known to be unvaccinated people, pregnant women, and young children. Other immunocompromised populations, such as older people, those using long-term drugs, and those with underlying metabolic illnesses that may exhibit the disease in a different way, also need to be characterized though.
Patients, including immunocompromised humans such as geriatric patients or babies born to mothers infected with MPXV should be closely monitored for the development of chronic health problems. The Zika virus epidemic provided evidence that children who were exposed to the virus in utero may be at risk of developmental issues later in life. 125 Evidence suggests that just 25% of pregnant women infected with MPXV would be successful in giving birth 126 ; there should be a higher priority placed on this area of research. Furthermore, infants and young children appear to be more vulnerable to severe mpox. 127 , 128 Data on fetal development after congenital MPXV infection are currently missing. The ability to determine whether MPXV infection can have long-term repercussions, as was seen after SARS-CoV-2 infection during the current pandemic, would also be made possible by longitudinal surveillance of patients with the MPXV.
Because mpox has not previously been documented in many countries, there is a lack of clinical experience with the infection, inadequate diagnostic resources, an unclear illness course, and an incomplete understanding of both therapeutic and preventative approaches. Physicians should keep a high index of suspicion for this disease, record cases according to protocol, and isolate them to reduce public worry and misinformation. Even though the disease in the nonendemic nations has garnered international attention, it is in Africa that the vast majority of new cases and fatalities have been reported. To avoid making the same mistake in the future, we must not overlook neglected tropical diseases. Nowadays, when the world is so interconnected, the phrase “no one is safe unless everyone is safe” rings especially true. There is a need for more study into the length of mpox treatment and the efficacy of antivirals.
The scientific community needs to rapidly gather data, especially in extremely vulnerable communities that are unlikely to be included in clinical trials, as this second worldwide pandemic in as many years demonstrates the need for more strong infrastructure for diagnosis, treatment, and prevention. Many more pandemics are likely to strike within our lives. Well coordinated multicenter networks, with early backing from the Centers for Disease Control and Prevention, the Food and Drug Administration, and the National Institutes of Health, are necessary for generating evidence while simultaneously delivering clinical care. Local IRBs at individual hospitals should be consulted on the feasibility of rapid observational data gathering, and hospitals as a whole should work together to develop uniform guidelines for the prospective collecting of data in advance of any potential pandemic. These methods could speed up our understanding of how to deal with new infectious risks in immunocompromised individuals, which would be especially useful given the time lags experienced in responding to COVID-19 and mpox.
Acknowledgements
We thank Ramiar Kamal Kheder and Safin Hassan Hussein for their comments on the manuscript.
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 2; peer review: 2 approved
Data availability
No data are associated with this article.
References
- 1. Di Giulio DB, Eckburg PB: Human monkeypox: an emerging zoonosis. Lancet Infect. Dis. 2004;4(1):15–25. 10.1016/S1473-3099(03)00856-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chakraborty S, Chandran D, Mohapatra RK, et al. : Clinical management, antiviral drugs and immunotherapeutics for treating monkeypox. An update on current knowledge and futuristic prospects. Int. J. Surg. 2022;105:106847. 10.1016/j.ijsu.2022.106847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Saeed S, Shabbir H, Basit J, et al. : Monkeypox: Potentially another pandemic or a mere hoax? Ann. Med. Surg (Lond). 2022;81:104364. 10.1016/j.amsu.2022.104364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sigler R, Haidar G: Mpox in immunocompromised patients: with more data, more questions. Transpl. Infect. Dis. 2023 Feb;25(1):e14023. Epub 2023 Jan 30. 10.1111/tid.14023 [DOI] [PubMed] [Google Scholar]
- 5. Ladnyj ID, Ziegler P, Kima E: A human infection caused by monkeypox virus in Basankusu Territory, Democratic Republic of the Congo. Bull. World Health Organ. 1972;46(5):593–597. [PMC free article] [PubMed] [Google Scholar]
- 6. Ježek Z, Szczeniowski M, Paluku KM, et al. : Human monkeypox: clinical features of 282 patients. J. Infect. Dis. 1987;156(2):293–298. 10.1093/infdis/156.2.293 [DOI] [PubMed] [Google Scholar]
- 7. Likos AM, Sammons SA, Olson VA, et al. : A tale of two clades: monkeypox viruses. J. Gen. Virol. 2005;86(10):2661–2672. 10.1099/vir.0.81215-0 [DOI] [PubMed] [Google Scholar]
- 8. Chen N, Li G, Liszewski MK, et al. : Virulence differences between monkeypox virus isolates from West Africa and the Congo basin. Virology 2005;340(1):46–63. 10.1016/j.virol.2005.05.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sbrana E, Xiao S-Y, Newman PC, et al. : Comparative pathology of North American and central African strains of monkeypox virus in a ground squirrel model of the disease. Am. J. Trop. Med. Hyg. 2007;76(1):155–164. 10.4269/ajtmh.2007.76.155 [DOI] [PubMed] [Google Scholar]
- 10. Jezek Z, Marennikova SS, Mutumbo M, et al. : Human monkeypox: a study of 2,510 contacts of 214 patients. J. Infect. Dis. 1986;154(4):551–555. 10.1093/infdis/154.4.551 [DOI] [PubMed] [Google Scholar]
- 11. Jezek Z, Nakano JH, Arita I, et al. : Serological survey for human monkeypox infections in a selected population in Zaire. J. Trop. Med. Hyg. 1987;90(1):31–38. [PubMed] [Google Scholar]
- 12. Jezek Z, Grab B, Paluku KM, et al. : Human monkeypox: disease pattern, incidence and attack rates in a rural area of northern Zaire. Trop. Geogr. Med. 1988;40(2):73–83. [PubMed] [Google Scholar]
- 13. Mukinda VBK, Mwema G, Kilundu M, et al. : Re-emergence of human monkeypox in Zaire in 1996. Lancet 1997;349(9063):1449–1450. 10.1016/S0140-6736(05)63725-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Heymann DL, Szczeniowski M, Esteves K: Re-emergence of monkeypox in Africa: a review of the past six years. Br. Med. Bull. 1998;54(3):693–702. 10.1093/oxfordjournals.bmb.a011720 [DOI] [PubMed] [Google Scholar]
- 15. Meyer H, Perrichot M, Stemmler M, et al. : Outbreaks of disease suspected of being due to human monkeypox virus infection in the Democratic Republic of Congo in 2001. J. Clin. Microbiol. 2002;40(8):2919–2921. 10.1128/JCM.40.8.2919-2921.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hutin YJ, Williams RJ, Malfait P, et al. : Outbreak of human monkeypox, Democratic Republic of Congo, 1996 to 1997. Emerg. Infect. Dis. 2001;7(3):434–438. 10.3201/eid0703.017311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Reed KD, Melski JW, Graham MB, et al. : The detection of monkeypox in humans in the Western Hemisphere. N. Engl. J. Med. 2004;350(4):342–350. 10.1056/NEJMoa032299 [DOI] [PubMed] [Google Scholar]
- 18. Centers for Disease Control and Prevention (CDC): Multistate outbreak of monkeypox--Illinois, Indiana, and Wisconsin, 2003. MMWR Morb. Mortal. Wkly Rep. 2003;52(23):537–540. [PubMed] [Google Scholar]
- 19. Yinka-Ogunleye A, Aruna O, Dalhat M, et al. : Outbreak of human monkeypox in Nigeria in 2017–18: a clinical and epidemiological report. Lancet Infect. Dis. 2019;19(8):872–879. 10.1016/S1473-3099(19)30294-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Erez N, Achdout H, Milrot E, et al. : Diagnosis of imported monkeypox, Israel, 2018. Emerg. Infect. Dis. 2019;25(5):980–983. 10.3201/eid2505.190076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vaughan A, Aarons E, Astbury J, et al. : Two cases of monkeypox imported to the United Kingdom, September 2018. Eurosurveillance. 2018;23(38):1800509. 10.2807/1560-7917.ES.2018.23.38.1800509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ng OT, Lee V, Marimuthu K, et al. : A case of imported Monkeypox in Singapore. Lancet Infect. Dis. 2019;19(11):1166. 10.1016/S1473-3099(19)30537-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sherwat A, Brooks JT, Birnkrant D, et al. : Tecovirimat and the treatment of monkeypox—past, present, and future considerations. N. Engl. J. Med. 2022;387(7):579–581. 10.1056/NEJMp2210125 [DOI] [PubMed] [Google Scholar]
- 24. Beer EM, Rao VB: A systematic review of the epidemiology of human monkeypox outbreaks and implications for outbreak strategy. PLoS Negl. Trop. Dis. 2019;13(10):e0007791. 10.1371/journal.pntd.0007791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chandran D, Nandanagopal VG, Gopan M, et al. : Major Advances in Monkeypox Vaccine Research and Development – An Update. J. Pure Appl. Microbiol. 2022;16(suppl 1):3083–3095. 10.22207/JPAM.16.SPL1.0 [DOI] [Google Scholar]
- 26. Bunge EM, Hoet B, Chen L, et al. : The changing epidemiology of human monkeypox—A potential threat? A systematic review. PLoS Negl. Trop. Dis. 2022;16(2):e0010141. 10.1371/journal.pntd.0010141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chandran D, Hridya P, Prasanth D, et al. : Changing Patterns in the Spread of Human Monkeypox: A Dangerous New Development in Disease Epidemiology. J. Pure Appl. Microbiol. 2022;16(suppl 1):3106–3118. 10.22207/JPAM.16.SPL1.11 [DOI] [Google Scholar]
- 28. Sharma V, Aggarwal D, Sharma AK, et al. : An overview on Monkeypox, Current Paradigms and Advances in its Vaccination, Treatment and Clinical Management: Trends, Scope, Promise and Challenges. J. Pure Appl. Microbiol. 2022;16(suppl 1):3000–3012. 10.22207/JPAM.16.SPL1.21 [DOI] [Google Scholar]
- 29. Brown K, Leggat PA: Human monkeypox: current state of knowledge and implications for the future. Trop. Med. Infect. Dis. 2016;1(1):8. 10.3390/tropicalmed1010008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bragazzi NL, Kong JD, Mahroum N, et al. : Epidemiological trends and clinical features of the ongoing monkeypox epidemic: A preliminary pooled data analysis and literature review. J. Med. Virol. 2022;95. 10.1002/jmv.27931 [DOI] [PubMed] [Google Scholar]
- 31. Nguyen P-Y, Ajisegiri WS, Costantino V, et al. : Reemergence of human monkeypox and declining population Immunity in the context of urbanization, Nigeria, 2017–2020. Emerg. Infect. Dis. 2021;27(4):1007–1014. 10.3201/eid2704.203569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sah R, Humayun M, Baig E, et al. : FDA’s authorized “JYNNEOS” vaccine for counteracting monkeypox global public health emergency; an update–Correspondence. Int. J. Surg. 2022;107:106971. 10.1016/j.ijsu.2022.106971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Guarner J, Del Rio C, Malani PN: Monkeypox in 2022—What Clinicians Need to Know. JAMA 2022;328:139–140. 10.1001/jama.2022.10802 [DOI] [PubMed] [Google Scholar]
- 34. Harapan H, Ophinni Y, Megawati D, et al. : Monkeypox: A Comprehensive Review. Viruses 2022;14(10):2155. 10.3390/v14102155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chandran D, Dhama K, Chakraborty S, et al. : Monkeypox: An update on current knowledge and research advances. J. Exp. Biol. Agric. Sci. 2022;10:679–688. 10.18006/2022.10(4).679.688 [DOI] [Google Scholar]
- 36. Thornhill JP, Barkati S, Walmsley S, et al. : Monkeypox virus infection in humans across 16 countries—April–June 2022. N. Engl. J. Med. 2022;387(8):679–691. 10.1056/NEJMoa2207323 [DOI] [PubMed] [Google Scholar]
- 37. Lum F-M, Torres-Ruesta A, Tay MZ, et al. : Monkeypox: disease epidemiology, host immunity and clinical interventions. Nat. Rev. Immunol. 2022;22(10):597–613. 10.1038/s41577-022-00775-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ward T, Christie R, Paton RS, et al. : Transmission dynamics of monkeypox in the United Kingdom: contact tracing study. BMJ. 2022;379:e073153. 10.1136/bmj-2022-073153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Català A, Clavo-Escribano P, Riera-Monroig J, et al. : Monkeypox outbreak in Spain: clinical and epidemiological findings in a prospective cross-sectional study of 185 cases. Br. J. Dermatol. 2022;187(5):765–772. 10.1111/bjd.21790 [DOI] [PubMed] [Google Scholar]
- 40. Tarín-Vicente EJ, Alemany A, Agud-Dios M, et al. : Clinical presentation and virological assessment of confirmed human monkeypox virus cases in Spain: a prospective observational cohort study. Lancet 2022;400(10353):661–669. 10.1016/S0140-6736(22)01436-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Antinori A, Mazzotta V, Vita S, et al. : Epidemiological, clinical and virological characteristics of four cases of monkeypox support transmission through sexual contact, Italy, May 2022. Eurosurveillance. 2022;27(22):2200421. 10.2807/1560-7917.ES.2022.27.22.2200421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Duque MP, Ribeiro S, Martins JV, et al. : Ongoing monkeypox virus outbreak, Portugal, 29 April to 23 May 2022. Eurosurveillance. 2022;27(22):2200424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Caria J, Pinto R, Leal E, et al. : Clinical and Epidemiological Features of Hospitalized and Ambulatory Patients with Human Monkeypox Infection: A Retrospective Observational Study in Portugal. Infect Dis Rep. 2022;14(6):810–823. 10.3390/idr14060083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Adler H, Gould S, Hine P, et al. : Clinical features and management of human monkeypox: a retrospective observational study in the UK. Lancet Infect. Dis. 2022;22(8):P1153–P1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Girometti N, Byrne R, Bracchi M, et al. : Demographic and clinical characteristics of confirmed human monkeypox virus cases in individuals attending a sexual health centre in London, UK: an observational analysis. Lancet Infect. Dis. 2022;22(9):1321–1328. 10.1016/S1473-3099(22)00411-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Philpott D: Epidemiologic and clinical characteristics of monkeypox cases—United States, May 17–July 22, 2022. MMWR Morb. Mortal. Wkly Rep. 2022;71:1018–1022. 10.15585/mmwr.mm7132e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Miller MJ, Cash-Goldwasser S, Marx GE, et al. : Severe Monkeypox in Hospitalized Patients-United States, August 10-October 10, 2022. MMWR Morb. Mortal. Wkly Rep. 2022;71(44):1412–1417. 10.15585/mmwr.mm7144e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kraemer MUG, Tegally H, Pigott DM, et al. : Tracking the 2022 monkeypox outbreak with epidemiological data in real-time. Lancet Infect. Dis. 2022;22:941–942. 10.1016/S1473-3099(22)00359-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Centers for Disease Control and Prevention (CDC): 2022 Monkeypox Outbreak Global Map. 2022 [cited 2022 Oct 5]. Reference Source
- 50. Patel A, Bilinska J, Tam JCH, et al. : Clinical features and novel presentations of human monkeypox in a central London centre during the 2022 outbreak: descriptive case series. BMJ 2022;378. 10.1136/bmj-2022-072410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sah R, Abdelaal A, Reda A, et al. : Monkeypox and its Possible Sexual Transmission: Where are we now with its evidence? Pathogens. 2022;11(8):924. 10.3390/pathogens11080924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Schnierle BS: Monkeypox Goes North: Ongoing Worldwide Monkeypox Infections in Humans. Viruses 2022;14(9):1874. 10.3390/v14091874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ahmed SK, Rashad EAA, Mohamed MG, et al. : The global human monkeypox outbreak in 2022: An overview. Int. J. Surg. 2022;104:106794. 10.1016/j.ijsu.2022.106794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ejaz H, Junaid K, Younas S, et al. : Emergence and dissemination of monkeypox, an intimidating global public health problem. J. Infect. Public Health 2022;15:1156–1165. 10.1016/j.jiph.2022.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Angelo KM, Smith T, Camprubí-Ferrer D, et al. : Epidemiological and clinical characteristics of patients with monkeypox in the GeoSentinel Network: a cross-sectional study. Lancet Infect. Dis. 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Le Pluart D, Ruyer-Thompson M, Ferré VM, et al. : A healthcare-associated infection with Monkeypox virus of a healthcare worker during the 2022 outbreak. Open Forum Infectious Diseases Oxford University Press US;2022; p.ofac520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ibrahim PK, Abdulrahman DS, Ali HM, et al. : The 2022 monkeypox outbreak—Special attention to nurses’ protection should be a top priority. Ann Med Surg. 2022;82:104615. 10.1016/j.amsu.2022.104615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Goyal L, Ajmera K, Pandit R, et al. : Prevention and treatment of monkeypox: a step-by-step guide for healthcare professionals and general population. Cureus. 2022;14(8). 10.7759/cureus.28230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Okyay RA, Bayrak E, Kaya E, et al. : Another Epidemic in the Shadow of Covid 19 Pandemic: A Review of Monkeypox. Proteins 2022;7:10. 10.14744/ejmo.2022.2022 [DOI] [Google Scholar]
- 60. Petersen E, Kantele A, Koopmans M, et al. : Human monkeypox: epidemiologic and clinical characteristics, diagnosis, and prevention. Infect. Dis. Clin. 2019;33(4):1027–1043. 10.1016/j.idc.2019.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Mathieu E, Spooner F, Dattani S, et al. : Mpox (monkeypox). 2022. OurWorldInData.org https://ourworldindata.org/monkeypox [Google Scholar]
- 62. Sharma V, Panwar A, Garg VK, et al. : Tecovirimat as a Potential Bioavailable Iinhibitor against MPXVgp158 Established through Molecular Dynamic Simulations and Docking Studies. J. Pure Appl. Microbiol. 2022;16(suppl 1):3168–3178. 10.22207/JPAM.16.SPL1.13 [DOI] [Google Scholar]
- 63. Ahmed SK, Abdulqadir SO, Omar RM, et al. : Knowledge, Attitude and Worry in the Kurdistan Region of Iraq during the Mpox (Monkeypox) Outbreak in 2022: An Online Cross-Sectional Study. Vaccines. 2023;11:610. 10.3390/vaccines11030610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ahmed SK, El-Kader RGA, Lorenzo JM, et al. : Hospital-based salient prevention and control measures to counteract the 2022 monkeypox outbreak. Health Sci. Rep. 2023;6: e1057. 10.1002/hsr2.1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ahmed SK, El-Kader, Rabab GA, et al. : Monkeypox clinical symptoms, pathology, and advances in management and treatment options: an update. Int. J. Surg. 2023. 10.1097/JS9.0000000000000091 [DOI] [PMC free article] [PubMed]
- 66. Jang W, Kandimalla L, Rajan S, et al. : Monkeypox in an immunocompromised patient with underlying human immunodeficiency virus and syphilis infections in Southern Florida of the United States: a case report. AIDS Res. Ther. 2023 Feb 18;20(1):12. 10.1186/s12981-023-00504-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Otu A, Ebenso B, Walley J, et al. : Global human monkeypox outbreak: atypical presentation demanding urgent public health action. Lancet Microbe. 2022;3:e554–e555. 10.1016/S2666-5247(22)00153-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Kmiec D, Kirchhoff F: Monkeypox: a new threat? Int. J. Mol. Sci. 2022;23(14):7866. 10.3390/ijms23147866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Grant R, Nguyen L-BL, Breban R: Modelling human-to-human transmission of monkeypox. Bull. World Health Organ. 2020;98(9):638–640. 10.2471/BLT.19.242347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kaler J, Hussain A, Flores G, et al. : Monkeypox: A Comprehensive Review of Transmission, Pathogenesis, and Manifestation. Cureus. 2022;14(7). 10.7759/cureus.26531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ahmed SK, Omar RM, Hussein SH, et al. : Middle East countries preparedness for Monkeypox outbreak: A call to action. Int. J. Surg. 2022;106:106948. 10.1016/j.ijsu.2022.106948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Katsafanas GC, Moss B: Colocalization of transcription and translation within cytoplasmic poxvirus factories coordinates viral expression and subjugates host functions. Cell Host Microbe 2007;2(4):221–228. 10.1016/j.chom.2007.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Stanford MM, McFadden G, Karupiah G, et al. : Immunopathogenesis of poxvirus infections: forecasting the impending storm. Immunol. Cell Biol. 2007;85(2):93–102. 10.1038/sj.icb.7100033 [DOI] [PubMed] [Google Scholar]
- 74. Hsiao J-C, Chung C-S, Chang W: Vaccinia virus envelope D8L protein binds to cell surface chondroitin sulfate and mediates the adsorption of intracellular mature virions to cells. J. Virol. 1999;73(10):8750–8761. 10.1128/JVI.73.10.8750-8761.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Moss B: Poxvirus cell entry: how many proteins does it take? Viruses 2012;4(5):688–707. 10.3390/v4050688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Challberg MD, Englund PT: Purification and properties of the deoxyribonucleic acid polymerase induced by vaccinia virus. J. Biol. Chem. 1979;254(16):7812–7819. 10.1016/S0021-9258(18)36019-8 [DOI] [PubMed] [Google Scholar]
- 77. Kieser Q, Noyce RS, Shenouda M, et al. : Cytoplasmic factories, virus assembly, and DNA replication kinetics collectively constrain the formation of poxvirus recombinants. PLoS One. 2020;15(1):e0228028. 10.1371/journal.pone.0228028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Smith GL, Vanderplasschen A, Law M: The formation and function of extracellular enveloped vaccinia virus. J. Gen. Virol. 2002;83(12):2915–2931. 10.1099/0022-1317-83-12-2915 [DOI] [PubMed] [Google Scholar]
- 79. Dammann F, Raja M, Camargo JF: Progression of human monkeypox infection despite tecovirimat in an immunocompromised adult. Transpl. Infect. Dis. 2023 Feb;25(1): e14022. Epub 2023 Jan 30. 10.1111/tid.14022 [DOI] [PubMed] [Google Scholar]
- 80. Isidro J, Borges V, Pinto M, et al. : Phylogenomic characterization and signs of microevolution in the 2022 multi-country outbreak of monkeypox virus. Nat. Med. 2022;28(8):1569–1572. 10.1038/s41591-022-01907-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Kumar N, Acharya A, Gendelman HE, et al. : The 2022 outbreak and the pathobiology of the monkeypox virus. J. Autoimmun. 2022;131:102855. 10.1016/j.jaut.2022.102855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Marshall JS, Warrington R, Watson W, et al. : An introduction to immunology and immunopathology. Allergy, Asthma Clin. Immunol. 2018;14(2):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Dohms JE, Saif YM: Guest Editorial: Criteria for Evaluating Immunosuppression. Avian Dis. 1984;28(2):305–310. 10.2307/1590336 [DOI] [PubMed] [Google Scholar]
- 84. Kaspers B, Schat KA, Göbel T, et al. : Avian immunology. Academic Press;2021. [Google Scholar]
- 85. Lu X, Wang X, Gao Y, et al. : Efficacy and safety of corticosteroids for septic shock in immunocompromised patients: A cohort study from MIMIC. Am. J. Emerg. Med. 2021;42:121–126. 10.1016/j.ajem.2020.02.002 [DOI] [PubMed] [Google Scholar]
- 86. Echavarría M: Adenoviruses in immunocompromised hosts. Clin. Microbiol. Rev. 2008;21(4):704–715. 10.1128/CMR.00052-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Wallace BI, Kenney B, Malani PN, et al. : Prevalence of immunosuppressive drug use among commercially insured US adults, 2018-2019. JAMA Netw. Open 2021;4(5):e214920–e214920. 10.1001/jamanetworkopen.2021.4920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Haidar G, Mellors JW: Improving the outcomes of immunocompromised patients with coronavirus disease 2019. Clin. Infect. Dis. 2021;73(6):e1397–e1401. 10.1093/cid/ciab397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Chang AB, Upham JW, Masters IB, et al. : Protracted bacterial bronchitis: the last decade and the road ahead. Pediatr. Pulmonol. 2016;51(3):225–242. 10.1002/ppul.23351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Hoffman JJ, Sullivan NJ: Davis Advantage for Medical-Surgical Nursing: Making Connections to Practice FA Davis;2020. [Google Scholar]
- 91. Choi B, Choudhary MC, Regan J, et al. : Persistence and evolution of SARS-CoV-2 in an immunocompromised host. N. Engl. J. Med. 2020;383(23):2291–2293. 10.1056/NEJMc2031364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Cordero E, Aydillo T, Farinas MC, et al. : Immunosuppressed patients with pandemic influenza A 2009 (H1N1) virus infection. Eur. J. Clin. Microbiol. Infect. Dis. 2012;31(4):547–556. 10.1007/s10096-011-1346-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Andersen KM, Mehta HB, Palamuttam N, et al. : Association between chronic use of immunosuppresive drugs and clinical outcomes from coronavirus disease 2019 (COVID-19) hospitalization: a retrospective cohort study in a large US health system. Clin. Infect. Dis. 2021;73(11):e4124–e4130. 10.1093/cid/ciaa1488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. D’Antiga L: Coronaviruses and immunosuppressed patients: the facts during the third epidemic. Liver Transpl. 2020;26(6):832–834. 10.1002/lt.25756 [DOI] [PubMed] [Google Scholar]
- 95. Armenti VT, Moritz MJ, Cardonick EH, et al. : Immunosuppression in pregnancy. Drugs 2002;62(16):2361–2375. 10.2165/00003495-200262160-00004 [DOI] [PubMed] [Google Scholar]
- 96. Mor G, Cardenas I: The immune system in pregnancy: a unique complexity. Am. J. Reprod. Immunol. 2010;63(6):425–433. 10.1111/j.1600-0897.2010.00836.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Menezes YR, Miranda AB: Severe disseminated clinical presentation of monkeypox virus infection in an immunosuppressed patient: first death report in Brazil. Rev. Soc. Bras. Med. Trop. 2022;55:e0392. 10.1590/0037-8682-0392-2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. O’Shea J: Interim guidance for prevention and treatment of monkeypox in persons with HIV infection—United States, August 2022. MMWR Morb. Mortal. Wkly Rep. 2022;71:1023–1028. 10.15585/mmwr.mm7132e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Hoffmann C, Jessen H, Wyen C, et al. : Clinical characteristics of monkeypox virus infections among men with and without HIV: a large outbreak cohort in Germany. HIV Med. 2022. 10.1111/hiv.13378 [DOI] [PubMed] [Google Scholar]
- 100. Hernandez LE, Jadoo A, Kirsner RS: Human monkeypox virus infection in an immunocompromised man: trial with tecovirimat. Lancet 2022;400(10355):e8. 10.1016/S0140-6736(22)01528-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Manoharan A, Braz BX, McBride A, et al. : Severe monkeypox with superimposed bacterial infection in an immunocompetent patient: A case report. IDCases. 2022;30:e01626. 10.1016/j.idcr.2022.e01626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Viguier C, Kermel T, Boumaza X, et al. : A severe monkeypox infection in a patient with an advanced HIV infection treated with Tecovirimat: clinical and virological outcome. Int. J. Infect. Dis. 2022;125:135–137. 10.1016/j.ijid.2022.10.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Martínez CAP, Flores GAS, Santamaría FP, et al. : Monkeypox and its broad clinical spectrum in immunocompromised patients: Two case reports. IDCases. 2022;31:e01651. 10.1016/j.idcr.2022.e01651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Sousa D, Patrocínio J, Frade J, et al. : Human monkeypox coinfection with acute HIV: an exuberant presentation. Int. J. STD AIDS 2022;33(10):936–938. 10.1177/09564624221114998 [DOI] [PubMed] [Google Scholar]
- 105. Wong L, Gonzales-Zamora JA, Beauchamps L, et al. : Clinical presentation of Monkeypox among people living with HIV in South Florida: a case series. Le Infez Med. 2022;30(4):610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Raccagni AR, Candela C, Mileto D, et al. : Breakthrough Monkeypox Infection among Individuals Previously Immunized with Smallpox or Monkeypox Vaccination. J. Infect. 2022;86:154–225. 10.1016/j.jinf.2022.12.001 Reference Source [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Kumbhar N, Agarwala P: The lurking threat of monkeypox in current times. Indian J. Med. Microbiol. 2022 Oct-Dec;40(4):475–479. 10.1016/j.ijmmb.2022.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Mitjà O, Ogoina D, Titanji BK, et al. : Monkeypox. Lancet. 2023 Jan 7;401(10370):60–74. Erratum in: Lancet.2022 Dec 3; 400(10367): 1926. 10.1016/S0140-6736(22)02075-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Simon-Gozalbo A, Gamo-Guerrero M, Alonso-Garcia S, et al. : Haemorrhagic monkeypox infection in an immunosuppressed patient with human immunodeficiency virus: beyond the pustules. Clin. Microbiol. Infect. 2022;29:122–124. 10.1016/j.cmi.2022.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Farahat RA, Sah R, El-Sakka AA, et al. : Human monkeypox disease (MPX). Infez. Med. 2022;30(3):372–391. 10.53854/liim-3003-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Ahmed SK, Abdulqadir SO, Hussein SH, et al. : The impact of monkeypox outbreak on mental health and counteracting strategies: A call to action. Int. J. Surg. 2022;106:106943. 10.1016/j.ijsu.2022.106943 Reference Source [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Ahmed SK, M-Amin HI, Abdulqadir SO, et al. : Timely mental health care for the 2022 novel monkeypox outbreak is urgently needed. Ann. Med. Surg. 2022;82:104579. 10.1016/j.amsu.2022.104579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Ahmed SK, Saied AA, Ravi RK, et al. : The 2022 monkeypox outbreak and associated psychiatric morbidities. Int. J. Surg. 2022;106:106913. 10.1016/j.ijsu.2022.106913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Peter OJ, Kumar S, Kumari N, et al. : Transmission dynamics of Monkeypox virus: a mathematical modelling approach. Model Earth Syst Environ. 2022;8(3):3423–3434. 10.1007/s40808-021-01313-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Ranganath N, Tosh PK, O’Horo J, et al. : Monkeypox 2022: Gearing Up for Another Potential Public Health Crisis. Mayo Clin. Proc. 2022;97(9):1694–1699. 10.1016/j.mayocp.2022.07.011 [DOI] [PubMed] [Google Scholar]
- 116. Kwon SL, Ban S, Shin J, et al. : Monkeypox vaccination in the Republic of Korea: Identifying the high-risk target Group. J. Korean Med. Sci. 2022;37(29). 10.3346/jkms.2022.37.e239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Islam MR, Hossain MJ, Roy A, et al. : Repositioning potentials of smallpox vaccines and antiviral agents in monkeypox outbreak: a rapid review on comparative benefits and risks. Heal. Sci. Reports. 2022;5(5):e798. 10.1002/hsr2.798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Galleguillos L: Monkeypox and Immunosuppressed Neurological Patients: What can we Advise Vaccination in Multiple Sclerosis and Neuromyelitis Optica Patients? J. ISSN. 2022;3:1177–1179. 10.37871/jbres1573 [DOI] [Google Scholar]
- 119. UK Health Security Agency: Recommendations for the use of preand post-exposure vaccination during a monkeypox incident. 2022 [cited 2022 Dec 12]. Reference Source
- 120. Ahmed SK: Prevention, Vaccination, Management and Infection Control of Monkeypox Outbreak: an Update Global Recommendation for the Next Year 2023. J. Pure Appl. Microbiol. 2022;16(1):3189–3191. 10.22207/JPAM.16.SPL1.15 [DOI] [Google Scholar]
- 121. Titanji BK, Tegomoh B, Nematollahi S, et al. : Monkeypox: A Contemporary Review for Healthcare Professionals. Open Forum Infectious Diseases Oxford University Press;2022; p.ofac310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Singhal T, Kabra SK, Lodha R: Monkeypox: A Review. Indian J. Pediatr. 2022 Oct;89(10):955–960. 10.1007/s12098-022-04348-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Chakraborty S, Mohapatra RK, Chandran D, et al. : Monkeypox vaccines and vaccination strategies: Current knowledge and advances. An update–Correspondence. Int. J. Surg. 2022;105:106869. 10.1016/j.ijsu.2022.106869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Agrati C, Cossarizza A, Mazzotta V, et al. : Immunological signature in human cases of monkeypox infection in 2022 outbreak: an observational study. Lancet Infect. Dis. 2023 Mar;23(3):320–330. 10.1016/S1473-3099(22)00662-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Aguilar Ticona JP, Nery N, Jr, Ladines-Lim JB, et al. : Developmental outcomes in children exposed to Zika virus in utero from a Brazilian urban slum cohort study. PLoS Negl. Trop. Dis. 2021;15(2):e0009162. 10.1371/journal.pntd.0009162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Mbala PK, Huggins JW, Riu-Rovira T, et al. : Maternal and fetal outcomes among pregnant women with human monkeypox infection in the Democratic Republic of Congo. J. Infect. Dis. 2017;216(7):824–828. 10.1093/infdis/jix260 [DOI] [PubMed] [Google Scholar]
- 127. Lopes Moreira ME, Nielsen-Saines K, Brasil P, et al. : Neurodevelopment in infants exposed to Zika virus in utero. N. Engl. J. Med. 2018 Dec 13;379(24):2377–2379. 10.1056/NEJMc1800098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Peçanha PM, Gomes Junior SC, Pone SM, et al. : Neurodevelopment of children exposed intra-uterus by Zika virus: A case series. PLoS One. 2020;15(2):229434. 10.1371/journal.pone.0229434 [DOI] [PMC free article] [PubMed] [Google Scholar]