Summary
Serrated epithelial change (SEC) manifests in patients with long-standing inflammatory bowel disease (IBD) and is characterized by disorganized crypt architecture, irregular serrations, and goblet cell–rich epithelium. The serrated nature of SEC is reminiscent of serrated colorectal polyps, which frequently harbor KRAS /BRAF mutations. SEC is, however, not only histologically distinct from sporadic serrated polyps but also associated with colorectal neoplasia. Whether SEC is a precursor to IBD-associated neoplasia remains unclear. To further define the relationship of SEC with serrated colorectal polyps and IBD-associated neoplasia, we performed targeted next-generation sequencing on colorectal specimens to include the following: SEC without dysplasia/neoplasia (n = 10), SEC with separate foci of associated dysplasia/adenocarcinoma from the same patients (n = 17), and uninvolved mucosa (n = 10) from 14 patients. In addition, we molecularly profiled sessile serrated lesion (SSL)–like or serrated lesion, not otherwise specified (SL-NOS), specimens, from 11 patients who also had IBD. This control cohort included SSL-like/SL-NOS without dysplasia/neoplasia (n = 11), SSL-like/SL-NOS with associated low-grade dysplasia (n = 2), and uninvolved mucosa (n = 8). By next-generation sequencing, the most frequently mutated gene in SEC without neoplasia and associated dysplasia/adenocarcinoma from separate foci in the same patients was TP53. Recurrent TP53 mutations were present in 50% of SEC specimens without dysplasia/ neoplasia. In addition, alterations in TP53 were detected at a prevalence of 71% in low-grade dysplasia, 83% in high-grade dysplasia, and 100% in adenocarcinoma. Paired sequencing of SEC and associated neoplasia revealed identical TP53 missense mutations for 3 patients. In contrast, 91% of SSL-like/SL-NOS specimens without dysplasia/neoplasia harbored KRAS /BRAF mutations, which were conserved in associated low-grade dysplasia. No genomic alterations were found in uninvolved mucosa from either patients with SEC or patients with SSL-like/SL-NOS. Based on our findings, we conclude SEC is distinct from SSL-like serrated colorectal lesions in patients with IBD and an early precursor to IBD-associated neoplasia that warrants colonoscopic surveillance.
Keywords: Serrated dysplasia, Hyperplastic polyp, Sessile serrated adenoma/lesion, Crohn disease, Ulcerative colitis, Colon cancer
1. Introduction
Patients with inflammatory bowel disease (IBD) have an increased risk of developing colorectal adenocarcinoma [1,2]. Although the overall prevalence of colorectal adenocarcinoma in patients with IBD is only 3–5%, the cumulative risk of cancer increases with duration of disease [3]. Based on published studies, the incidence rates of IBD-associated colorectal adenocarcinoma range between 0.2% and 2.0% at 10 years, 1.4 and 8.0% at 20 years, and 3.1 and 18.0% at 30 years after diagnosis [4,5]. However, many of these studies were performed before the introduction of modern pharmacologic control of inflammation [6,7]. In addition, colonoscopic surveillance has resulted in increased detection of IBD-associated colorectal adenocarcinoma, but outcomes have improved with early diagnosis [8,9]. An estimated 15% of IBD-associated deaths are attributed to colorectal adenocarcinoma [3]. Consequently, current guidelines advocate surveillance colonoscopy to begin 8–10 years from disease onset and to continue surveillance within 1- to 5-year intervals thereafter [10,11]. The time interval of surveillance can be highly dependent on risk factors associated with the development of colorectal neoplasia in patients with IBD. These risk factors include extent of disease involvement, degree of histologic inflammation, the presence of a stricture, mucosal dysplasia, and a family history of colorectal adenocarcinoma in a first-degree relative.
Although dysplasia is a well-established risk factor for the development of colorectal adenocarcinoma in the setting of IBD, the diagnostic entity known as serrated epithelial change (SEC) has also been described as a potential risk factor for colorectal neoplasia in patients with IBD [12]. For decades, peculiar serrated proliferations adjoining IBD-associated colorectal adenocarcinoma have been described in the literature, thus raising the suspicion of SEC as a precursor lesion [13,14]. In fact, in a standardization study that attempted to codify the evaluation of dysplasia in patients with IBD, Riddell et al. [15] noted the presence of unusual serrated lesions and introduced the concept that such lesions may be regarded as indefinite for dysplasia. SEC is detected in both the flat and nodular colonic mucosa and, microscopically, characterized by disorganized crypt architecture, irregular serrations, and a goblet cell–rich epithelium. In a report by Parian et al. [12], the authors found that 8% of patients with SEC had synchronous colorectal dysplasia or adenocarcinoma. Furthermore, 21% of patients with SEC and no history of colorectal neoplasia developed dysplasia or adenocarcinoma on follow-up. As acknowledged by the authors, a limitation of their study was the absence of a control group for patients with IBD and without SEC to compare rates of colorectal neoplasia. Another point of contention is whether SEC is analogous to sporadic serrated colorectal polyps and sessile serrated lesions (SSLs) that arise in patients with IBD [16]. However, based on macroscopic and microscopic findings, we believe that SEC is histologically distinct from conventional serrated colorectal polyps. Ko et al. [17] reported their experience with serrated colorectal polyps in patients with IBD and detected frequent alterations in BRAF and KRAS, which have been previously described in conventional serrated colorectal polyps. The authors concluded that these polyps in the absence of dysplasia did not confer an increased long-term risk of neoplastic progression as compared with control patients with no dysplasia at baseline. It is, however, plausible that these authors were studying a different lesion than the one that has been termed SEC.
To further characterize SEC and its relationship with colorectal neoplasia, we performed targeted next-generation sequencing of SEC diagnosed using strict criteria and/or neoplastic specimens from the same patients for genes commonly altered in both sporadic and IBD-associated colorectal adenocarcinoma. In addition, we molecularly evaluated SSL-like and serrated lesion, not otherwise specified (SL-NOS), in patients with IBD that morphologically overlapped with SEC, but did not exhibit all of the key histologic features that define SEC [18]. The sequencing results between SEC and SSL-like/SL-NOS were compared to determine if SEC truly represents a distinct pathologic entity or should be classified within the spectrum of serrated colorectal polyps.
2. Materials and methods
2.1. Study population
Study approval was obtained from Institutional Review Boards at Cedars-Sinai Medical Center, Johns Hopkins University, and the University of Pittsburgh (STUDY19110319). The anatomic pathology archives from the Departments of Pathology at Cedars-Sinai Medical Center, the Johns Hopkins Hospital, and the University of Pittsburgh Medical Center were queried for the diagnosis of SEC in patients with a history of IBD. Both colonoscopic biopsy and surgical resection specimens were included. Corresponding hematoxylin and eosin–stained slides were evaluated (by A.D.S., K.M.W., and E.A.M.) for histologic findings associated with SEC as previously described [12]. Histologically, SEC is recognized as colonic mucosa that is characterized by a distorted architecture. The crypts in SEC lose their orientation to the lumen and are neithers perpendicular to the muscularis mucosae nor necessarily reach the muscularis mucosae. The serrations in SEC are also irregular, but present throughout the lesion. In addition, SEC is rich in goblet cells with enlarged goblet cells that extend to involve the bases of the crypts.
Fourteen patients with SEC were identified, and for a subset of these patients, available samples included an associated synchronous and/or metachronous low-grade dysplasia (n = 7), high-grade dysplasia (n = 6), and/or colorectal adenocarcinoma (n = 4). The corresponding formalin-fixed paraffin-embedded (FFPE) tissue blocks were retrieved for subsequent molecular analysis. In addition, 10 of the 14 patients had sufficient uninvolved colonic mucosa lacking SEC, dysplasia, or adenocarcinoma for further testing. Upon review for molecular analysis, the following number of specimens from 14 patients was sufficient for DNA microdissection: 10 uninvolved colonic mucosa specimens, 10 SEC specimens without dysplasia, 7 low-grade dysplasia specimens, 6 high-grade dysplasia specimens, and 4 colorectal adenocarcinoma specimens. The 4 colorectal adenocarcinoma cases were conventional in appearance without specific morphologic findings (eg, mucinous features, signet ring cells, and so on). As a control cohort, SSL-like/SL-NOS lesions from 11 patients with IBD were identified based on previously published criteria and chosen owing to their histologic overlap with SEC [18–22]. However, SSL-like/SL-NOS lesions lacked the full complement of all the aforementioned microscopic criteria and conventional dysplasia. Associated synchronous (n = 1) and metachronous (n = 1) low-grade dysplasia specimens were identified for 2 patients with SSL-like/SL-NOS. For 8 of 11 patients with SSL-like/SL-NOS lesions, uninvolved colonic mucosa was also available for further testing. The corresponding FFPE tissue blocks were obtained for molecular analysis and included the following specimens that were sufficient for DNA microdissection: 8 uninvolved colonic mucosa specimens, 11 SSL-like/SL-NOS specimens without dysplasia, and 2 low-grade dysplasia specimens. In addition, 35 surgical resection specimens of sporadic, primary colorectal adenocarcinomas were randomly selected from our surgical pathology archives to include juxtaposed uninvolved colonic mucosa. Both non-neoplastic and neoplastic tissues from these 35 surgical resections were also submitted for molecular analysis. For each patient, demographic data, clinical history, colonoscopic reports, and follow-up data were collected.
2.2. Targeted DNA next-generation sequencing
A total of 129 specimens were submitted for PancreaSeqV2 testing, an amplification-based targeted DNA next-generation sequencing panel (Supplementary Table 1) [23,24]. In brief, FFPE tissue was microdissected from 8.4-mm unstained histologic sections under stereomicroscopic visualization using an Olympus S=61 microscope (Olympus, Center Valley, PA). Genomic DNA was isolated using the DNeasy Blood and Tissue Kit on the automated QIAcube (Qiagen, Germantown, MD) instrument according to the manufacturer’s instructions. Extracted DNA was quantitated on the Glomax Discover Fluorometer using the QuantiFluor ONE dsDNA system (Promega, Madison, WI). PancreaSeqV2 includes customized AmpliSeq primers for targeted regions within the following genes associated with gastrointestinal tract and hepatopancreatobiliary neoplasms: AKT1, APC, BRAF, CTNNB1, GNAS, HRAS, IDH1, IDH2, KRAS, MEN1, MET, NF2, NRAS, PIK3CA, PTEN, RNF43, SMAD4, STK11, TERT, TP53, TSC2, and VHL. Amplicons were bar-coded, ligated with specific adapters, and purified. DNA library quantity and quality checks were performed using the 4200 TapeStation (Agilent Technologies, Santa Clara, CA). The Ion Chef was used to prepare and enrich templates and enable testing via Ion Sphere Particles on an Ion 540 semiconductor chip. Massive parallel sequencing was carried out on an Ion GeneStudio S5 System according to the manufacturer’s instructions (Thermo Fisher Scientific, Carlsbad, CA, USA), and data were analyzed using Torrent Suite Software version 5.8 for point mutations, small insertions/deletions, and copy number alterations. Variant Explorer (UPMC) was used for variant annotation and interpretation. Each variant was prioritized as per the 2017 Association for Molecular Pathology /American Society of Clinical Oncology/ College of American Pathologist joint consensus guidelines for interpretation of sequence variants in cancer using a tier-based system [25]. Tier I, tier II, and tier III variants were reported; however, only tier I and tier II variants were used for subsequent analysis. The limit of detection of the assay was at 2% mutant allele frequency (AF). The minimum depth of coverage for testing was 1000×. For each mutation identified, an AF was calculated based on the number of reads of the mutant allele versus the wild-type allele and reported as percentage [24]. Copy number variation analysis was performed as previously described [26]. The total depth of sequencing coverage of each sequenced region was normalized by the normal controls and calculated per sequenced case. A decrease in sequencing coverage below established cutoffs with simultaneous presence of sequence variant at high AF was considered a copy number loss. In contrast, an increase in sequencing coverage above established cutoffs was interpreted as a copy number gain. A gene amplification was defined by the presence of ≥6 copies of a variant as previously described and validated using fluorescence in situ hybridization analysis [26,27].
3. Results
3.1. Patient study cohort
The clinical and pathologic features of 14 patients with SEC are summarized in Table 1. At the time of initial diagnosis of SEC, patients ranged in age from 24 to 85 years (mean, 54.6 years; median, 55.5 years) and were predominantly men (10 of 14, 71%). All patients had a history of colitis that included either ulcerative colitis (n = 10, 71%) or Crohn disease (n = 4, 29%). Data regarding duration of colitis were available for 13 of 14 (93%) patients. Except for 1 patient known to have colitis for only one month, the remaining patients had a history of colitis that ranged between 7 and 38 years (mean, 18.5 years; median, 15 years) in duration. The presence or absence of coexisting primary sclerosing cholangitis (PSC) was documented for 13 patients, with 1 patient remarkable for a history of PSC. By colonoscopy, SEC was identified within the colon and located within the transverse colon (n = 4), descending colon (n = 3), sigmoid colon (n = 3), rectosigmoid colon (n = 1), and rectum (n = 3).
Table 1.
The clinicopathological features of 14 patients with serrated epithelial change (SEC).
| Case number | Gender | Age (years) at presentation | Type of colitis | Duration of colitis | Location of serrated epithelial change | Synchronous or metachronous neoplasia | Type(s) of associated neoplasiaa |
|---|---|---|---|---|---|---|---|
|
| |||||||
| 1 | M | 28 | Ulcerative colitis | 7 years | Transverse colon | Synchronous | LGD, HGD, AdenoCA, and metastatic AdenoCA |
| 2 | M | 42 | Ulcerative colitis | 14 years | Descending colon | No | N/A |
| 3 | F | 85 | Ulcerative colitis | 10 years | Descending colon | Synchronous | LGD and AdenoCA |
| 4 | F | 53 | Crohn disease | 30 years | Rectosigmoid colon | Metachronous | HGD |
| 5 | M | 49 | Crohn disease | 20 years | Sigmoid colon | No | N/A |
| 6 | F | 59 | Crohn disease | 38 years | Rectum | Synchronous | LGD, HGD, and AdenoCA |
| 7 | M | 58 | Ulcerative colitis | 17 years | Rectum | Synchronous | HGD and AdenoCA |
| 8 | M | 53 | Ulcerative colitis | 15 years | Descending colon | Synchronous | HGD |
| 9 | F | 68 | Ulcerative colitis | 15 years | Transverse colon | Synchronous | HGD |
| 10 | M | 24 | Crohn disease | 1 month | Transverse colon | Synchronous | LGD |
| 11 | M | 65 | Ulcerative colitis | 30 years | Transverse colon | Synchronous | LGD |
| 12 | M | 79 | Ulcerative colitis | Unknown | Sigmoid colon | Synchronous | LGD |
| 13 | M | 42 | Ulcerative colitis | 14 years | Distal sigmoid colon | Synchronous | LGD |
| 14 | M | 59 | Ulcerative colitis | 13 years | Rectum | Synchronous | HGD |
Abbreviations: AdenoCA, adenocarcinoma; F, female; HGD, high-grade dysplasia; LGD, low-grade dysplasia; M, male; N/A, not applicable.
Neoplasia found in association with SEC.
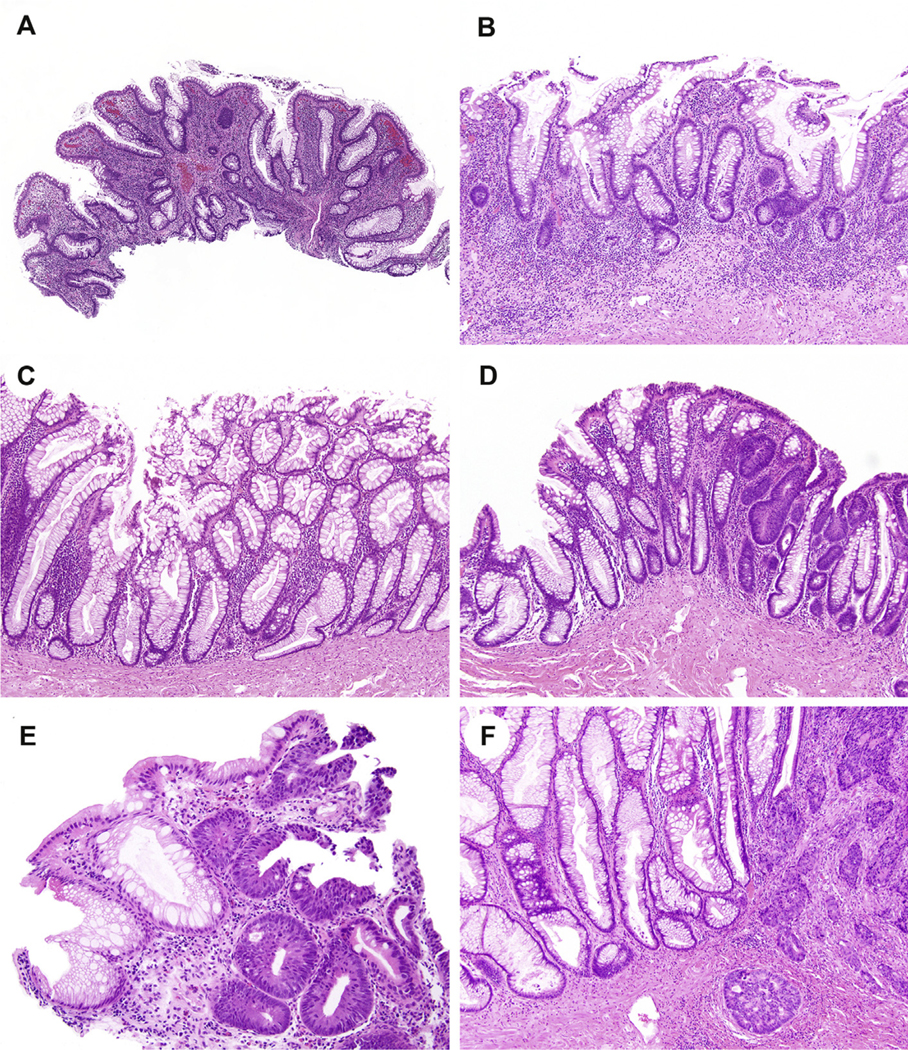
Consistent with findings reported by Parian et al. [12], SEC arose in both the flat and nodular colonic mucosa. Microscopically, the colonic mucosa was characterized by a strikingly distorted architecture with crypts showing loss of orientation, such that they were no longer perpendicular to the muscularis mucosae (Fig. 1A). For a subset of cases, the crypts did not touch the muscularis mucosae and were separated by chronic inflammation (Fig. 1B). Epithelial serrations were found at both the base and surface of the colonic mucosa. However, the serrations were irregular and not as prominent as those that characterize sessile serrated adenomas/SSLs and hyperplastic polyps. The colonic epithelium was also rich in goblet cells, which were enlarged and present throughout the mucosa (Fig. 1C). Cytoplasmic eosinophilia was seen, but nuclear atypia was absent. Other pathologic findings included neoplasia for 11 patients that consisted of low-grade dysplasia (n = 7, Fig. 1D), high-grade dysplasia (n = 6, Fig. 1E), and/or colorectal adenocarcinoma (n = 4, Fig. 1F). The dysplasia/neoplasia was detected close to the SEC in all 11 patients. Among the remaining three patients, one patient developed high-grade dysplasia upon follow-up colonoscopy.
Fig. 1.
Serrated epithelial change (SEC) is microscopically characterized by a strikingly distorted architecture with crypts exhibiting loss of orientation and loss of their perpendicular arrangement to the muscularis mucosae (A). For a subset of cases, the crypts of SEC do not touch the muscularis mucosae and are separated by a layer of chronic inflammation (B). In addition, the serrated epithelium is present throughout the lesion, but irregular, unlike sessile serrated adenomas/lesions and hyperplastic polyps, and is rich in goblet cells. In this particular example, a boot-shaped crypt akin to sessile serrated adenomas/lesions is present but differs by exhibiting subtle serrated and other cytologic differences (C). Additional pathologic findings seen in association with SEC include low-grade dysplasia (D), high-grade dysplasia (E), and invasive adenocarcinoma (F).
As a control cohort, SSL-like/SL-NOS lesions, which had some morphologic overlap, but lacked the full complement of diagnostic features of SEC, were collected from 11 additional patients with IBD (Table 2). At initial diagnosis, patients with SSL-like/SL-NOS were 41–69 years in age (mean, 54.6 years; median, 59.0 years), and the majority were men (n = 9, 82%). Similar to patients with SEC, all patients with an SSL-like/SL-NOS had a history of IBD that included either ulcerative colitis (n = 9, 82%) or Crohn disease (n = 2, 18%). The duration of IBD ranged from 10 to 57 years (mean, 28.2 years; median, 25.0 years). Two (18%) patients also had a history of PSC. Except for 3 cases with lesions within the cecum (n = 1) and ascending colon (n = 2), the SSL-like/SL-NOS lesions were detected within the left colon and found in the sigmoid colon (n = 2) and rectum (n = 6).
Table 2.
Clinicopathological features of 11 patients with a sessile serrated lesion (SSL)–like and serrated lesion, not otherwise specified (SL-NOS).
| Case number | Gender | Age (years) at presentation | Type of colitis | Duration of colitis | Location of the serrated epithelial lesion | Synchronous or metachronous neoplasia | Type(s) of associated neoplasiaa |
|---|---|---|---|---|---|---|---|
|
| |||||||
| 15 | M | 65 | Ulcerative colitis | 57 years | Rectum | Synchronous | LGD |
| 16 | M | 61 | Ulcerative colitis | 42 years | Sigmoid colon | No | N/A |
| 17 | M | 61 | Ulcerative colitis | 42 years | Cecum | No | N/A |
| 18 | M | 59 | Ulcerative colitis | 25 years | Rectum | No | N/A |
| 19 | F | 47 | Crohn disease | 23 years | Ascending colon | No | N/A |
| 20 | M | 46 | Ulcerative colitis | 14 years | Rectum | No | N/A |
| 21 | M | 69 | Ulcerative colitis | 29 years | Rectum | No | N/A |
| 22 | F | 46 | Ulcerative colitis | 33 years | Rectum | No | N/A |
| 23 | M | 64 | Ulcerative colitis | 16 years | Rectum | No | N/A |
| 24 | M | 42 | Crohn disease | 10 years | Ascending colon | No | N/A |
| 25 | M | 41 | Ulcerative colitis | 19 years | Sigmoid colon | Metachronous | LGD |
Abbreviations: F, female; LGD, low-grade dysplasia; M, male; N/A, not applicable.
Neoplasia found in association with SSL-like/SL-NOS.
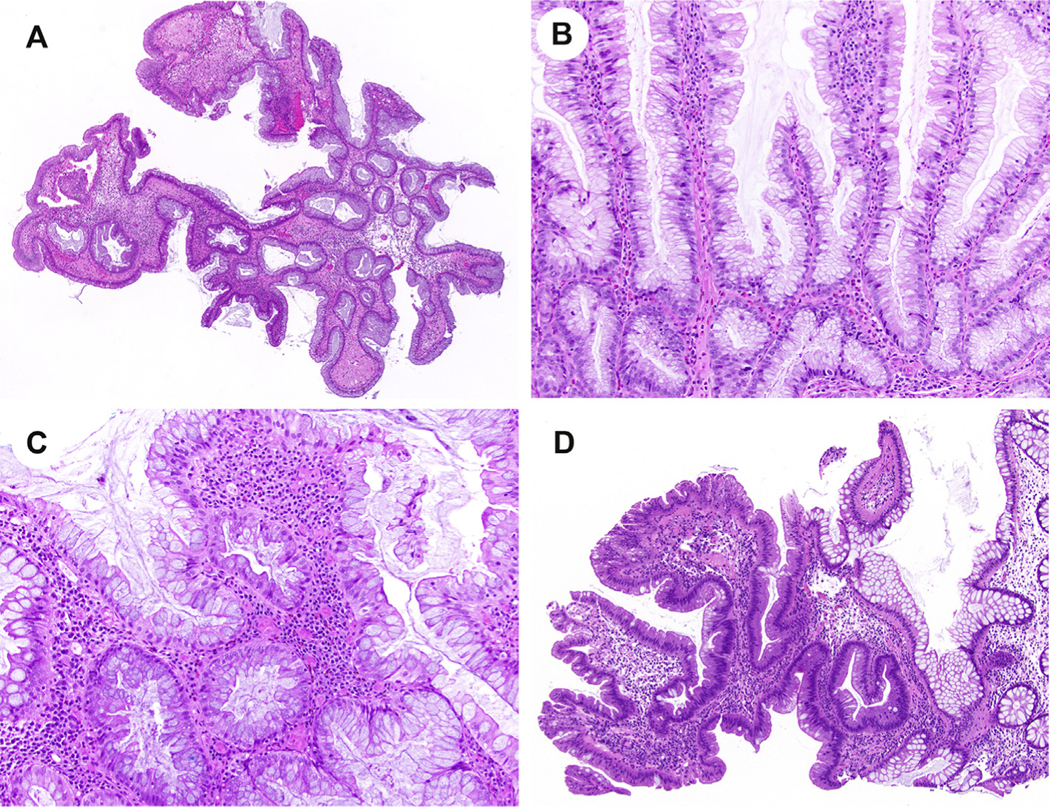
By colonoscopy, SSL-like/SL-NOS lesions were described as either flat or nodular. Histologically, these lesions were reminiscent of SEC, but displayed notable differences in that they either resembled conventional serrated colorectal polyps or assumed a bulbous appearance. There was no architectural distortion for 5 cases. Three cases had a villous or bulbous architecture (Fig. 2A). While serrations were present in all 11 SSL-like/SL-NOS lesions, there was focal dilation at the base in 3 cases. In addition, another 3 cases exhibited serrations that were more prominent at the mucosal surface (Fig. 2B). A goblet cell–rich epithelium was present in 7 cases, but for 3 cases, there were fewer goblet cells and a higher proportion of absorptive cells (Fig. 2C). Adjacent low-grade dysplasia was identified in a single case of SSL-like/SL-NOS (Fig. 2D). Among the remaining 10 patients, one patient developed low-grade dysplasia upon follow-up colonoscopy.
Fig. 2.
Sessile serrated lesion (SSL)–like and serrated lesion, not otherwise specified (SL-NOS), lesions can mimic an SEC owing to their serrated histologic findings. However, SSL-like/SL-NOS lesions show notable differences and do not exhibit all SEC-associated microscopic features. In contrast to SEC, SSL-like/SL-NOS can be villous or bullous in architecture (A) and exhibit serrations that are more prominent at either the mucosal surface (B) or base than throughout the lesion. In addition, a goblet cell–rich epithelium is typically absent in SSL-like/SL-NOS (C), but adjacent low-grade dysplasia can be seen in association (D). SEC, serrated epithelial change.
3.2. Molecular analysis of SEC and SSL-like/SL-NOS without neoplasia
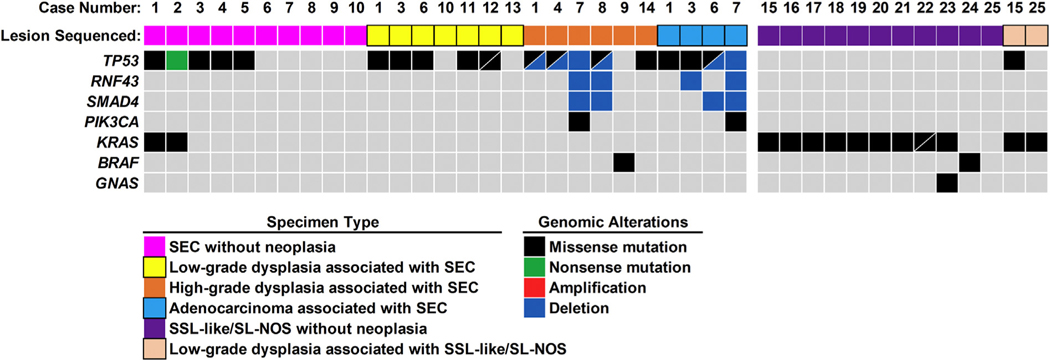
Among 14 patients with SEC, 10 specimens with SEC and no associated neoplasia were available for targeted next-generation sequencing (Fig. 3). Genomic alterations were identified in 5 of 10 (50%) cases, with TP53 alterations identified in all 5 cases. The TP53 alterations consisted of missense mutations (n = 4) and a nonsense mutation (n = 1). Mutant AFs for TP53 varied between 2% and 39%. In addition, case 1 and case 2 harbored co-occurring missense mutations in KRAS with mutant AFs of 3% and 10%, respectively, as compared with TP53 mutant AFs of 16% and 39%, respectively. Uninvolved colonic mucosa was available from the same patients for all 10 cases of SEC without neoplasia, and no genomic alterations were found by targeted next-generation sequencing.
Fig. 3.
Summary of detected genomic alterations in SEC, SSL-like/SL-NOS, and associated dysplasia. The most prevalent genomic alterations in SEC and associated dysplasia occurred in TP53, whereas SSL-like/SL-NOS and associated dysplasia predominantly harbored KRAS missense mutations. SEC, serrated epithelial change; SSL, sessile serrated lesion; SL-NOS, serrated lesion, not otherwise specified.
In contrast to SEC, genomic alterations were detected in nearly all (10 of 11, 91%) cases of SSL-like/SL-NOS without dysplasia/neoplasia. The most frequently mutated gene was KRAS (n = 9) and consisted of missense mutations with mutant AFs of 4–44%. Two different KRAS missense mutations with similar mutant AFs were identified for case 22. In addition, case 23 harbored a missense mutation in GNAS, with a mutant AF equivalent to KRAS. Although case 24 was KRAS wild-type, a class 3 BRAF missense mutation was identified. For 8 SSL-like/SL-NOS cases, the surrounding uninvolved colonic mucosa was available for molecular testing, and no genomic alterations were found.
As a separate control cohort, genomic profiling was performed for 35 resected primary, sporadic colorectal adenocarcinomas (Supplementary Fig. 1). Detectable genomic alterations involved the following genes from the most to least prevalent: TP53 (n = 24, 69%), KRAS (n = 22, 63%), SMAD4 (n = 14, 40%), BRAF (n = 6, 17%), PIK3CA (n = 6, 17%), PTEN (n = 5, 14%), NRAS (n = 2, 6%), RNF43 (n = 2, 6%), CTNNB1 (n = 2, 6%), and GNAS (n = 1, 3%). Considering genes classified into the mitogen-activated protein kinase pathway, 30 of 35 (86%) cases harbored missense alterations in KRAS, BRAF, or NRAS. Of note, juxtaposed, non-neoplastic colonic mucosa was also evaluated and was negative for genomic alterations.
3.3. Molecular analysis of colorectal neoplasia found in patients with SEC and SSL-like/SL-NOS
Targeted next-generation sequencing was also performed for 17 colonic neoplastic specimens from 12 patients with SEC. These specimens consisted of 7 cases of low-grade dysplasia, 6 cases of high-grade dysplasia, and 4 cases of colorectal adenocarcinoma. Similar to SEC specimens without neoplasia, TP53 alterations were the most frequent finding, with a prevalence of 71% in low-grade dysplasia (n = 5), 83% in high-grade dysplasia (n = 5), and 100% in colorectal adenocarcinoma (n = 4). Among the low-grade dysplastic cases, except for missense mutations in TP53, no additional genomic alterations were identified. Furthermore, two different TP53 missense mutations were found for a single case (case 12). The mutant AFs for TP53 ranged between 3% and 21%. In comparison, cases of high-grade dysplasia and colorectal adenocarcinoma were characterized by TP53 missense mutations (n = 7) with mutant AFs of 23–70% and TP53 deletions (n = 6). For 5 cases, both missense mutations and deletions in TP53 were identified. Other genomic alterations included RNF43 deletion (n = 4), SMAD4 deletion (n = 4), and PIK3CA missense mutation (n = 2). Case 9 was the only specimen with high-grade dysplasia that lacked TP53 mutations and harbored a BRAF V600E mutation.
A comparative analysis of matched SEC and neoplastic specimens from the same patient was able to be performed for 8 cases (Supplementary Fig. 2). For all 3 cases wherein the SEC without neoplasia had a TP53 alteration, TP53 missense mutations were identified within both SEC and colorectal neoplastic samples. These missense mutations in TP53 were conserved between matched specimens. Of note, for case 1, TP53 missense mutations were shared between SEC and high-grade dysplasia samples, but the missense mutations in low-grade dysplasia and adenocarcinoma were different. In addition, for case 6 and case 7, matched neoplastic specimens harbored identical TP53 alterations. Only two patients with SSL-like/SL-NOS lesions were found to have neoplasia, and both had low-grade dysplasia. A molecular analysis of case 15 and case 25 revealed KRAS missense mutations for both low-grade dysplastic samples. For case 15, a TP53 missense mutation was also detected with a mutant AF of 6% as compared with a mutant AF of 35% for KRAS. Furthermore, the KRAS missense mutations in the matched SSL-like/SL-NOS and low-grade dysplasia for case 15 were identical.
4. Discussion
Next-generation sequencing has been an invaluable tool in defining the genomic basis of not only invasive carcinomas but also precursor lesions. Identifying key genomic alterations in precursor lesions and determining their relationship with carcinoma allows for improvements in early detection strategies, development of surveillance protocols, and potentially guides subsequent management. Herein, alterations in TP53 were the most frequent genomic abnormality identified in patients with SEC. Furthermore, half of the tested SEC specimens without dysplasia or adenocarcinoma harbored TP53 mutations. Alterations in TP53 to include gene deletions were also detected in colorectal neoplasia, at a prevalence of 71% in low-grade dysplasia, 83% in high-grade dysplasia, and 100% in colorectal adenocarcinoma from patients with SEC. Furthermore, among 3 patients with SEC, paired analysis of SEC and colorectal dysplasia or adenocarcinoma revealed identical missense mutations in TP53. In comparison, no genomic alterations involving TP53 or other genes within the targeted next-generation sequencing panel were identified in the uninvolved colonic mucosa.
TP53 mutations are a common finding in colorectal adenocarcinoma and play a pivotal role in tumor initiation, promotion, and progression [28]. However, depending on etiology, mutations in TP53 can occur at different phases within the multistep progression model from normal colonic mucosa to carcinoma. For instance, in sporadic colorectal adenocarcinoma, TP53 mutations are a late genomic event and are unusual in adenomatous precursor neoplasms [29,30]. Conversely, TP53 mutations in IBD-associated colorectal adenocarcinoma are an early event and typically found in not only precancerous neoplasms but also the non-neoplastic colonic mucosa [31–33]. In fact, identical mutations in TP53 were previously reported to be detected within matched non-neoplastic and dysplastic colorectal specimens from the same IBD patient, supporting the concept of field cancerization in the development of IBD-associated colorectal adenocarcinoma [32]. Field cancerization refers to one or more colonic crypts or fields of the colon that become genomically unstable and predisposed to subsequent neoplastic transformation [34–36]. Based on our findings, we suspect that SEC is the morphologic manifestation of a colonic field in patients with IBD, but has not been previously recognized by prior publications [32]. In this study, half of the SEC specimens without neoplasia harbored TP53 mutations, and the prevalence of TP53 alterations increased with the grade of dysplasia. Moreover, the identification of TP53 missense mutations in SEC that are preserved in colorectal dysplasia and adenocarcinoma from the same patient indicates a clonal relationship between SEC and IBD-associated colorectal neoplasia.
In addition to TP53 mutations, the molecular pathogenesis of IBD-associated colorectal adenocarcinoma involves the acquisition of copy number alterations [31,37,38]. Copy number alterations begin to accrue during the transition from low-grade dysplasia to high-grade dysplasia [31]. Interestingly, the overall burden of chromosomal losses and gains between high-grade dysplasia and adenocarcinoma is equivalent and suggests stabilization of an altered genome upon malignant transformation. Analogous sequencing results were seen among advanced neoplasia specimens from patients with SEC. In contrast to SEC without neoplasia and specimens with low-grade dysplasia, deletions in TP53, RNF43, and SMAD4 were restricted to high-grade dysplasia and adenocarcinoma and had a similar prevalence for both neoplastic groups. Therefore, our findings would support that SEC is genetically related not only to IBD-associated colorectal neoplasia but also a precursor lesion that follows a similar molecular progression model as that reported for colorectal adenocarcinomas arising in patients with IBD.
Although the data presented here and prior clinical data support SEC as an IBD-associated precursor lesion, the differential diagnosis of serrated epithelium within SEC revolves around serrated colorectal polyps, especially hyperplastic polyps or sessile serrated adenomas/SSLs [39]. It is therefore understandable that many investigators have suggested that SEC should be categorized within the spectrum of sporadic serrated colorectal polyps but encountered within a background of IBD [16,18]. Serrated colorectal polyps that lack dysplasia are associated with a low risk of progression to carcinoma and, consequently, do not necessarily justify increased colonoscopic surveillance [17]. Hence, determining whether SEC as defined herein represents a discrete pathologic entity is clinically important and the impetus for this study. The macroscopic and microscopic features of SEC are distinct from those associated with serrated colorectal polyps. SEC is a subtle nodularity in the mucosa that is typically nonpolypoid, and although enhanced endoscopy techniques have improved the detection of SEC and other IBD-associated colorectal lesions, SEC may easily be overlooked by the unsuspecting gastroenterologist [12,39]. Histologically, SEC differs from serrated colorectal polyps on the basis of irregular serrations and different crypt architecture. Finally, the goblet cell–rich epithelium of SEC contrasts with most IBD-associated serrated colorectal polyps, which are often characterized by a microvesicular cytoplasm and indistinguishable from hyperplastic polyps [17,18].
Our genomic comparative analysis between serrated colorectal polyps and SEC further reinforces the dissimilarity between these two precursor lesions. Activating mutations in either BRAF or KRAS have been reported in 83% of serrated colorectal polyps without neoplasia [17]. In comparison, only 20% of SEC specimens without neoplasia in our series harbored a KRAS mutation. However, the AFs for mutant KRAS were appreciably lower than those for mutant TP53. Thus, it is plausible that the KRAS mutation within these two specimens is derived not from SEC, but another adjoining nonpolypoid to nodular precursor lesion, such as an SSL-like/SL-NOS. SSL-like/SL-NOS lesions within our cohort had overlapping morphologic features with SEC, but did not fulfill all three histologic requirements associated with SEC: disorganized crypt architecture, irregular serrations, and a goblet cell–rich epithelium. The premise that SEC specimens without neoplasia within this study are distinct from SSL-like/SL-NOS specimens is supported by the lack of KRAS mutations among SEC-associated neoplastic specimens. In addition, 91% of SSL-like/SL-NOS specimens without neoplasia harbored recurrent mutations in KRAS or BRAF. Considering the genomic similarities between SSL-like/SL-NOS and serrated colorectal polyps, these IBD-associated precursors are likely one and the same and differ from SEC defined by using strict morphologic criteria. Similarly, traditional serrated adenoma–like lesions have also been reported in patients with IBD and are presumably analogous to their sporadic counterparts [18,40].
Although our data highlight the distinctive nature of SEC as a precursor lesion to IBD-associated colorectal adenocarcinoma, appropriate clinical surveillance and management for patients with SEC remains unclear. Some investigators have chosen to manage SEC as equivalent to IBD-associated lesions that are classified as indefinite for dysplasia. In simplistic terms, the diagnosis of indefinite for dysplasia includes all mucosal changes for which it is not possible to determine whether the alterations are inflammatory/regenerative or constitute genuine neoplastic lesions. Interestingly, recent outcome studies on patients with long-standing IBD after surveillance for indefinite for dysplasia have shown a clinical course that is superimposable on that for patients with IBD and low-grade dysplasia [41,42]. Based on follow-up data reported by Parian et al. [12], albeit imperfect, SEC would appear to merit intensified colonoscopic surveillance. The 2015 SCENIC consensus statement (Surveillance for Colorectal Endoscopic Neoplasia Detection and Management in Inflammatory Bowel Disease Patients: International Consensus Recommendations) recommends endoscopic removal of all visible polypoid lesions rather than definitive treatment by colectomy, and this would seem reasonable for visible SEC [11]. However, considering the likelihood of progression of isolated SEC without dysplasia/neoplasia remains unclear, it is difficult to suggest surveillance intervals based on available data, but as noted previously, the identification of SEC would seem to merit a closer follow-up interval than nondysplastic colonic mucosa alone.
It is worth noting that there are a few limitations to our study. It is retrospective in design, and although it represents the largest series of SEC, associated dysplasia, and uninvolved colonic mucosa to be molecularly evaluated, the patient cohort size was relatively small. Furthermore, targeted next-generation sequencing was limited to known genes frequently associated with gastrointestinal tract and hepatopancreatobiliary malignancies, and a complete assessment of the entire genome of SEC and the corresponding neoplasia was not performed [24,43]. However, whole-genome sequencing and whole-exome sequencing studies can be challenging with mucosal biopsies owing to limited amounts of lesional DNA and contaminating normal DNA. The minimum depth of coverage for these sequencing methodologies can vary, with a median range of 30× to 150× [44]. Therefore, the detection of genomic mutations with a low mutant AF and copy number alterations by whole-genome sequencing and whole-exome sequencing is unlikely. In comparison, the minimum depth of coverage for targeted next-generation sequencing performed herein was at least 1000× for each genomic region. Another limitation of this study is the lack of an orthogonal method to confirm genomic alterations, such as those involving TP53 or APC/CTNNB1. Previous reports have found immunohistochemistry for p53 to be a reasonable surrogate for TP53 mutational analysis in colorectal neoplasia. But, equivocal staining patterns for p53 can occur and are not infrequent [45]. In addition, considering the low TP53 mutant AFs detected in SEC, these alterations are likely to be subclonal, and thus, p53 immunohistochemistry is expected to be inconclusive. Owing to scant nature of SEC and, in most cases, associated dysplasia, the corresponding tissue sections were exhausted in obtaining sufficient DNA for mutational analysis. Thus, we were unable to perform tandem p53 immunolabeling in the studied cases, but in our anecdotal experience, no aberrant expression for p53 has been detected. Interestingly, Galandiuk et al. [32] found the presence of TP53 alterations in patients with Crohn disease did not correlate with aberrant nuclear accumulation of p53. Regardless, the targeted next-generation sequencing panel used within the study has been previously published and both internally and externally validated for gastrointestinal and pancreatobiliary neoplasms [24]. Finally, while SEC is not only grossly, microscopically, and molecularly distinct from SSL-like/SL-NOS lesions found in patients with IBD, the relationship between SEC and other forms of nonconventional dysplasia, such as hypermucinous dysplasia, terminal epithelial differentiation/crypt cell dysplasia (TED/CCD), and serrated dysplasia, remains to be determined [18]. Nevertheless, p53 appears to play a major role in the development of hypermucinous dysplasia and TED/CCD, whereas both p53 and β-catenin have been implicated in serrated dysplasia [46].
5. Conclusion
In summary, the presence of recurrent TP53 alterations and the progressive accumulation of not only mutations but also gene deletions in TP53 strongly supports SEC as a precursor lesion to IBD-associated colorectal adenocarcinoma. Consequently, based on our findings and those of Parian et al. [12], the identification of SEC warrants intensified colonoscopic surveillance for patients with long-standing IBD. However, considering the serrated nature of these lesions, SEC can be easily mistaken for hyperplastic polyps and sessile serrated adenomas, but SEC is both histologically and molecularly distinct from sporadic serrated colorectal polyps. It is therefore important for the practicing pathologist to recognize the key microscopic findings of SEC and differentiate these lesions from conventional serrated polyps occurring in the background of IBD.
Supplementary Material
Acknowledgments
The authors would like to thank Mrs. Kate Smith for outstanding administrative assistance.
☆☆. Funding/Support:
This study was supported in part by the Pittsburgh Liver Research Center (NIH/NIDDK P30DK120531) at the University of Pittsburgh (to A.D.S.).
Footnotes
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.humpath.2021.03.002.
Competing interests: A.D.S. has received an honorarium from Foundation Medicine, Inc. The remaining authors have disclosed that they have no financial interests, arrangements, affiliations, or commercial interests with the manufacturers of any products discussed in this article or their competitors.
References
- [1].Farraye FA, Odze RD, Eaden J, Itzkowitz SH, McCabe RP, Dassopoulos T, et al. AGA medical position statement on the diagnosis and management of colorectal neoplasia in inflammatory bowel disease. Gastroenterology 2010;138(2):738–45. 10.1053/j.gastro.2009.12.037. [DOI] [PubMed] [Google Scholar]
- [2].Jess T, Gamborg M, Matzen P, Munkholm P, Sorensen TI. Increased risk of intestinal cancer in crohn’s disease: a meta-analysis of population-based cohort studies. Am J Gastroenterol 2005;100:2724–9. [DOI] [PubMed] [Google Scholar]
- [3].Mattar MC, Lough D, Pishvaian MJ, Charabaty A. Current management of inflammatory bowel disease and colorectal cancer. Gastrointest Canc Res 2011;4:53–61. [PMC free article] [PubMed] [Google Scholar]
- [4].Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut 2001;48:526–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Rutter MD, Saunders BP, Wilkinson KH, Rumbles S, Schofield G, Kamm MA, et al. Thirty-year analysis of a colonoscopic surveillance program for neoplasia in ulcerative colitis. Gastroenterology 2006;130:1030–8. [DOI] [PubMed] [Google Scholar]
- [6].Velayos FS, Terdiman JP, Walsh JM. Effect of 5-aminosalicylate use on colorectal cancer and dysplasia risk: a systematic review and metaanalysis of observational studies. Am J Gastroenterol 2005;100:1345–53. [DOI] [PubMed] [Google Scholar]
- [7].Dyson JK, Rutter MD. Colorectal cancer in inflammatory bowel disease: what is the real magnitude of the risk? World J Gastroenterol 2012;18:3839–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Mpofu C, Watson AJ, Rhodes JM. Strategies for detecting colon cancer and/or dysplasia in patients with inflammatory bowel disease. Cochrane Database Syst Rev 2004:CD000279. [DOI] [PubMed] [Google Scholar]
- [9].Choi CH, Rutter MD, Askari A, Lee GH, Warusavitarne J, Moorghen M, et al. Forty-Year analysis of colonoscopic surveillance program for Neoplasia in ulcerative colitis: an updated overview. Am J Gastroenterol 2015;110(7):1022–34. 10.1038/ajg.2015.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lamb CA, Kennedy NA, Raine T, Hendy PA, Smith PJ, Limdi JK, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019; 68(Suppl 3):s1–106. 10.1136/gutjnl-2019-318484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Laine L, Kaltenbach T, Barkun A, McQuaid KR, Subramanian V, Soetikno R. SCENIC international consensus statement on surveillance and management of dysplasia in inflammatory bowel disease. Gastroenterology 2015;148(3):639–51. 10.1053/j.gastro.2015.01.031. [DOI] [PubMed] [Google Scholar]
- [12].Parian AM, Limketkai BN, Chowdhury R, Brewer GG, Salem G, Falloon K, et al. Serrated epithelial change is associated with high rates of neoplasia in ulcerative colitis patients: a case-controlled study and systematic review with meta-analysis. Inflamm Bowel Dis 2020. 10.1093/ibd/izaa312. [DOI] [PubMed] [Google Scholar]
- [13].Kilgore SP, Sigel JE, Goldblum JR. Hyperplastic-like mucosal change in crohn’s disease: an unusual form of dysplasia? Mod Pathol 2000;13:797–801. [DOI] [PubMed] [Google Scholar]
- [14].Johnson DH, Khanna S, Smyrk TC, Loftus EV, Anderson KS, Mahoney DW, et al. Detection rate and outcome of colonic serrated epithelial changes in patients with ulcerative colitis or Crohn’s colitis. Aliment Pharmacol Ther 2014;39(12):1408–17. 10.1111/apt.12774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Riddell RH, Goldman H, Ransohoff DF, Appelman HD, Fenoglio CM, Haggitt RC, et al. Dysplasia in inflammatory bowel disease: standardized classification with provisional clinical applications. Hum Pathol 1983;14(11):931–68. 10.1016/s0046-8177(83)80175-0. [DOI] [PubMed] [Google Scholar]
- [16].Polydorides AD, Harpaz N. Serrated lesions in inflammatory bowel disease. Gastrointest Endosc 2017;85:461. [DOI] [PubMed] [Google Scholar]
- [17].Ko HM, Harpaz N, McBride RB, Cui M, Ye F, Zhang D, et al. Serrated colorectal polyps in inflammatory bowel disease. Mod Pathol 2015;28(12):1584–93. 10.1038/modpathol.2015.111. [DOI] [PubMed] [Google Scholar]
- [18].Choi WT, Yozu M, Miller GC, Shih AR, Kumarasinghe P, Misdraji J, et al. Nonconventional dysplasia in patients with inflammatory bowel disease and colorectal carcinoma: a multicenter clinicopathologic study. Mod Pathol 2020;33(5):933–43. 10.1038/s41379-019-0419-1. [DOI] [PubMed] [Google Scholar]
- [19].Batts KP. The pathology of serrated colorectal neoplasia: practical answers for common questions. Mod Pathol 2015;28:S80–7. [DOI] [PubMed] [Google Scholar]
- [20].Torlakovic EE, Gomez JD, Driman DK, Parfitt JR, Wang C, Benerjee T, et al. Sessile serrated adenoma (SSA) vs. traditional serrated adenoma (TSA). Am J Surg Pathol 2008;32(1):21–9. 10.1097/PAS.0b013e318157f002. [DOI] [PubMed] [Google Scholar]
- [21].Rex DK, Ahnen DJ, Baron JA, Batts KP, Burke CA, Burt RW, et al. Serrated lesions of the colorectum: review and recommendations from an expert panel. Am J Gastroenterol 2012;107(9):1315–29. 10.1038/ajg.2012.161.quiz1314,1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Snover DC. Update on the serrated pathway to colorectal carcinoma. Hum Pathol 2011;42:1–10. [DOI] [PubMed] [Google Scholar]
- [23].Singhi AD, Wood LD, Parks E, Torbenson MS, Felsenstein M, Hruban RH, et al. Recurrent rearrangements in PRKACA and PRKACB in intraductal oncocytic papillary neoplasms of the pancreas and bile duct. Gastroenterology 2020;158(3):573–82. 10.1053/j.gastro.2019.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Singhi AD, McGrath K, Brand RE, Khalid A, Zeh HJ, Chennat JS, et al. Preoperative next-generation sequencing of pancreatic cyst fluid is highly accurate in cyst classification and detection of advanced neoplasia. Gut 2018;67(12):2131–41. 10.1136/gutjnl-2016-313586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Li MM, Datto M, Duncavage EJ, Kulkarni S, Lindeman NI, Roy S, et al. Standards and Guidelines for the Interpretation and Reporting of Sequence Variants in Cancer: A Joint Consensus Recommendation of the Association for Molecular Pathology, American Society of Clinical Oncology, and College of American Pathologists. J Mol Diagn 2017; 19(1):4–23. 10.1016/j.jmoldx.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Grasso C, Butler T, Rhodes K, Quist M, Neff TL, Moore S, et al. Assessing copy number alterations in targeted, amplicon-based next-generation sequencing data. J Mol Diagn 2015;17(1):53–63. 10.1016/j.jmoldx.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Nikiforova MN, Wald AI, Melan MA, Roy S, Zhong S, Hamilton RL, et al. Targeted next-generation sequencing panel (GlioSeq) provides comprehensive genetic profiling of central nervous system tumors. Neuro Oncol 2016;18(3):379–87. 10.1093/neuonc/nov289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Cancer Genome Atlas N Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012;487:330–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hao XP, Frayling IM, Sgouros JG, Du MQ, Willcocks TC, Talbot IC, et al. The spectrum of p53 mutations in colorectal adenomas differs from that in colorectal carcinomas. Gut 2002;50(6):834–9. 10.1136/gut.50.6.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell 1990;61:759–67. [DOI] [PubMed] [Google Scholar]
- [31].Baker AM, Cross W, Curtius K, Al Bakir I, Choi CR, Davis HL, et al. Evolutionary history of human colitis-associated colorectal cancer. Gut 2019;68(6):985–95. 10.1136/gutjnl-2018-316191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Galandiuk S, Rodriguez-Justo M, Jeffery R, et al. Field cancerization in the intestinal epithelium of patients with crohn’s ileocolitis. Gastroenterology 2012;142:855–864 e858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Brentnall TA, Crispin DA, Rabinovitch PS, Haggitt RC, Rubin CE, Stevens AC, et al. Mutations in the p53 gene: an early marker of neoplastic progression in ulcerative colitis. Gastroenterology 1994; 107(2):369–78. 10.1016/0016-5085(94)90161-9. [DOI] [PubMed] [Google Scholar]
- [34].Al Bakir I, Curtius K, Graham TA. From colitis to cancer: an evolutionary trajectory that merges maths and biology. Front Immunol 2018;9:2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Leedham SJ, Graham TA, Oukrif D, McDonald SA, Rodriguez-Justo M, Harrison RF, et al. Clonality, founder mutations, and field cancerization in human ulcerative colitis-associated neoplasia. Gastroenterology 2009;136(2):542–50. 10.1053/j.gastro.2008.10.086. [DOI] [PubMed] [Google Scholar]
- [36].Choi CR, Bakir IA, Hart AL, Graham TA. Clonal evolution of colorectal cancer in ibd. Nature reviews. Gastroenterol Hepatol 2017; 14:218–29. [DOI] [PubMed] [Google Scholar]
- [37].Lai LA, Risques RA, Bronner MP, Rabinovitch PS, Crispin D, Chen R, et al. Pan-colonic field defects are detected by CGH in the colons of UC patients with dysplasia/cancer. Cancer Lett 2012; 320(2):180–8. 10.1016/j.canlet.2012.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Favazza LA, Parseghian CM, Kaya C, Nikiforova MN, Roy S, Wald AI, et al. KRAS amplification in metastatic colon cancer is associated with a history of inflammatory bowel disease and may confer resistance to anti-EGFR therapy. Mod Pathol 2020;33(9): 1832–43. 10.1038/s41379-020-0560-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Parian AM, Lazarev MG. Serrated colorectal lesions in patients with inflammatory bowel disease. Gastroenterol Hepatol 2018;14:19–25. [PMC free article] [PubMed] [Google Scholar]
- [40].Miller GC, Liu C, Bettington ML, Leggett B, Whitehall VLJ, Rosty C. Traditional serrated adenoma-like lesions in patients with inflammatory bowel disease. Hum Pathol 2020;97:19–28. [DOI] [PubMed] [Google Scholar]
- [41].Choi CH, Ignjatovic-Wilson A, Askari A, Lee GH, Warusavitarne J, Moorghen M, et al. Low-grade dysplasia in ulcerative colitis: risk factors for developing high-grade dysplasia or colorectal cancer. Am J Gastroenterol 2015;110(10):1461–71. 10.1038/ajg.2015.248.quiz1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Mahmoud R, Shah SC, Torres J, Castaneda D, Glass J, Elman J, et al. Association between indefinite dysplasia and advanced neoplasia in patients with inflammatory bowel diseases undergoing surveillance. Clin Gastroenterol Hepatol 2020;18(7):1518–27. 10.1016/j.cgh.2019.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Singhi AD, Nikiforova MN, Chennat J, Papachristou GI, Khalid A, Rabinovitz M, et al. Integrating next-generation sequencing to endoscopic retrograde cholangiopancreatography (ERCP)-obtained biliary specimens improves the detection and management of patients with malignant bile duct strictures. Gut 2020;69(1):52–61. 10.1136/gutjnl-2018-317817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Sims D, Sudbery I, Ilott NE, Heger A, Ponting CP. Sequencing depth and coverage: key considerations in genomic analyses. Nat Rev Genet 2014;15:121–32. [DOI] [PubMed] [Google Scholar]
- [45].Prall F, Huhns M. Quantitative evaluation of tp53 immunohistochemistry to predict gene mutations: lessons learnt from a series of colorectal carcinomas. Hum Pathol 2019;84:246–53. [DOI] [PubMed] [Google Scholar]
- [46].Patil DT, Goldblum JR, Odze RD. Immunohistochemical and molecular characterization of dysplasia subtypes in ulcerative colitis. Mod Pathol 2017;30:194A.27739436 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.