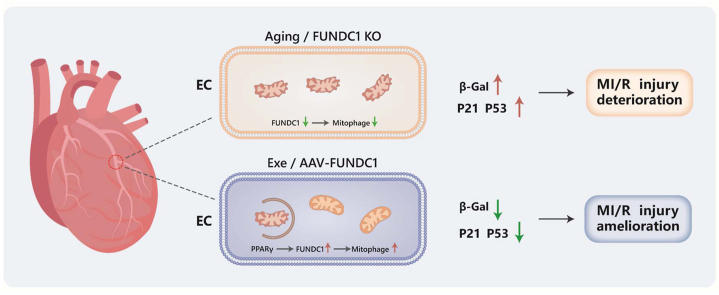
Abstract
Vascular aging contributes to adverse changes in organ function and is a significant indicator of major cardiac events. Endothelial cells (ECs) participate in aging-provoked coronary vascular pathology. Regular exercise is associated with preservation of arterial function with aging in humans. However, the molecular basis is not well understood. The present study was aimed to determine the effects of exercise on coronary endothelial senescence and whether mitochondrial clearance regulator FUN14 domain containing 1 (FUNDC1)-related mitophagy and mitochondrial homeostasis were involved. In mouse coronary arteries, FUNDC1 levels showed gradually decrease with age. Both FUNDC1 and mitophagy levels in cardiac microvascular endothelial cells (CMECs) were significantly reduced in aged mice and were rescued by exercise training. Exercise also alleviated CMECs senescence as evidenced by senescence associated β-galactosidase activity and aging markers, prevented endothelial abnormal cell migration, proliferation, and eNOS activation in CMECs from aged mice, and improved endothelium-dependent vasodilation of coronary artery, reduced myocardial neutrophil infiltration and inflammatory cytokines evoked by MI/R, restored angiogenesis and consequently alleviated MI/R injury in aging. Importantly, FUNDC1 deletion abolished the protective roles of exercise and FUNDC1 overexpression in ECs with adeno-associated virus (AAV) reversed endothelial senescence and prevented MI/R injury. Mechanistically, PPARγ played an important role in regulating FUNDC1 expressions in endothelium under exercise-induced laminar shear stress. In conclusion, exercise prevents endothelial senescence in coronary arteries via increasing FUNDC1 in a PPARγ-dependent manner, and subsequently protects aged mice against MI/R injury. These findings highlight FUNDC1-mediated mitophagy as potential therapeutic target that prevents endothelial senescence and myocardial vulnerability.
Keywords: Exercise, Coronary arteries, FUNDC1, Mitophagy, Endothelial senescence
Graphical abstract
Highlights
-
•
Loss of FUNDC1 contributes to coronary endothelial senescence and deterioration of MI/R injury.
-
•
Exercise improves aging-related coronary arterial dysfunction via upregulation of FUNDC1.
-
•
Exercise-induced laminar shear stress regulates FUNDC1 expression in a PPARγ-dependent manner.
1. Introduction
Cardiovascular disease is responsible for the highest mortality rates in older individuals, accounting for approximately 40% of all deaths [1]. Aging causes dysfunction in conducting arteries, arterioles and microcirculation, including coronary arteries [2]. Recent research has provided evidence for the Vascular Theory of Aging, which states that vascular aging is a driver of organismal aging at large [3,4], leading to a progressive decline in organ function and generating increased adverse changes and susceptibility to cardiovascular disease in the elderly. Endothelial cells (ECs) are the most abundant cell type in the heart [5] and perform many diverse but specialized functions. Considering that the age-related decline in endothelial function is associated with microvasculature pathologic remodeling and contributes to enhanced cardiovascular vulnerability to stress in elderly [6,7], prevention strategies to optimize cardiovascular health with age are urgently needed [8]. Lifestyle modifications, especially regular exercise, are associated with a reduced risk of cardiovascular damage [9] and preserve large elastic arterial function concomitant with aging [10,11]. However, the underlying pathophysiological mechanisms of coronary vascular aging and effects of physical exercise on coronary arteries during aging remains to be elucidate.
Mitochondria are sensors and integrators of environmental cues in ECs [12]. Senescence heralds an indisputable decline in mitochondrial function [13] and mediated several interrelated cellular processes such as calcium handling, apoptosis, and redox signaling. Mitochondrial reactive oxygen species (ROS) accumulation primarily caused EC damage [14]. Additionally, decreased mitochondrial membrane potential is associated with increased mitochondrial membrane hyperpermeability, resulting in the apoptosis activation and ultimately ECs dysfunction. Thus, failures in mitochondrial signaling in the endothelium may be an initial alteration observed at an early stage and constitute causative events in the development of cardiovascular disease [15,16]. In mammalian cells, mitophagy is a vital mitochondrial quality control mechanism; it centrally regulates cardiovascular homeostasis by governing and fine-controlling cellular metabolism, redox equilibrium, and ATP generation. Mitophagy controls various factors that may drive pathologies such as aging-related disorders [17]. On the other hand, aging is also a key determinant that alters mitophagic homeostasis in cardiovascular systems [18]. A recent study has demonstrated that acute exercise provokes the removal of damaged mitochondria via mitophagy, regulating through the AMPK-ULK1 signaling axis in skeletal muscle [19]. The above evidence strongly supports that targeting mitophagy may be a promising approach for cardiac and coronary vascular protection against myocardial ischemia/reperfusion (MI/R) injury in aging.
FUN14 domain containing 1 (FUNDC1) is an integral mitochondrial outer-membrane protein that plays a key role as a receptor for hypoxia-induced mitophagy. A number of studies support the view that FUNDC1 levels are closely related to the occurrence, progression, and prognosis of various diseases, including heart diseases, metabolic disorders and cancer [[20], [21], [22]], suggesting that FUNDC1 may be a promising biomarker and potential therapeutic target. It has been proven that FUNDC1-mediated mitophagy is linked with inflammatory responses [23], metabolic state [24], function of mitochondria-associated endoplasmic reticulum membranes [22]. It was reported that casein kinase 2α (CK2α) suppresses FUNDC1-mediated mitophagy and aggravates MI/R injury [25,26]. However, the role of FUNDC1 in endothelium, especially whether disturbance of FUNDC1-mediated mitophagy is responsible for endothelial cellular senescence remains to be elucidate. Therefore, the present study was aimed to investigate the roles of exercise in coronary endothelial senescence and explore the potential underlying effects of FUNDC1-related mitophagy on EC processes.
2. Materials and methods
2.1. Study animals
This study was adhered to the National Institutes of Health Guidelines for the Use of Laboratory Animals and approved by the Fourth Military Medical University Committee on Animal Care. Male C57BL6J mice 8 weeks old (young animal cohort) and 18 months old (aged animal cohort) were supplied by the Experimental Animal Center of the University. EC specific FUNDC1 knockout mice (Fundc1fl/Y/Tek-Cre) were established using CRISPR/Cas9 system driven by TEK promoter (Gembio Co. Ltd., Chengdu, China). The sgRNA target sequences included: sgRNA1 (5′-CTCTAAGCAAGTAATATCCC-3′) and sgRNA2 (5′-TATAGTCCTATTTCCGCAAC-3′). Fundc1fl/Y and Fundc1fl/Y/Tek-Cre with 6-month-old age were used in this study. Animals had ad libitum access to food and water and were housed under a 12:12 h light-dark cycle at 22–24 °C.
Mice were anesthetized by isoflurane inhalation (5% for induction and 1–2% for maintenance) for surgeries or functional detections. The adequacy of anesthesia was verified by the lack of a toe-pinch withdrawal response. To obtain tissues, CO2 inhalation were used for mice euthanasia. All experimental procedures were performed in accordance with ethical guidelines from the European Parliament on the protection of animals used for scientific purposes.
2.2. Exercise training
Mice in exercise groups underwent endurance swim training 5 days/week for 4 weeks followed previously described protocol [27,28], with minor modifications. On the first day, swimming training lasted 15 min. Then, exercise time progressively increased to 90 min/day in the first week and were maintained at this level for the rest of the days. Constant monitoring ensured the safety of the mice and prevented them from floating or holding their breath under water. Caged sedentary mice served as controls.
2.3. Functional assessment of coronary arteries
Left anterior descending (LAD) coronary artery was removed and sectioned into ring segments as described [29]. To assess arterial function, arteriolar segments (approximately 1 mm) were mounted on wires connected to isometric force transducers in a temperature-regulated myograph multichamber (620 M, Danish Myo Technology). Arteriolar segments were continuously perfused with aerated (95% O2/5% CO2) physiological saline solution (118.99 mM NaCl, 4.69 mM KCl, 2.50 mM CaCl2·2H2O, 1.18 mM KH2PO4, 1.17 mM MgSO4·7H2O, 25.0 mM NaHCO3, 0.03 mM EDTA, and 5.50 mM glucose, pH 7.4). After equilibrium for 1 h, ET-1 of 10−7 M were used for preconstriction. We examined the responses of small coronary arteries to sodium nitroprusside (SNP) and acetylcholine (ACh). A dose-response curve was obtained by cumulative addition of ACh (10−10 M to 10−5 M) and SNP (10−10 to 10−6 M). Relaxation at each concentration was measured and expressed as the percentage of force generated in response to ET-1. The endothelium-dependent vasodilation was defined as the percentage of ACh induced relaxation when endothelium was intact.
2.4. The MI/R model
As described previously, MI was induced around the LAD coronary artery [30]. Briefly, mice were anesthetized and a left thoracotomy was performed in the fourth intercostal space. The LAD artery was ligated with a 7-0 silk suture slipknot at 1–2 mm distal to its emergence from under the left atrium. MI was confirmed by the presence of myocardial blanching in the ischemic area and ST segment elevation on the electrocardiogram. The thoracotomy was closed with 5–0 silk sutures. After 30 min MI, the slipknot was released and the heart was reperfused for 180 min (for biochemical and apoptosis studies) or 24 h [for cardiac function studies and protein analysis]. Cardiac caspase-3 activity and creatine kinase-MB (CK-MB) levels were measured using caspase-3 colorimetric assay kit (Sigma, USA) and CK-MB kit (Nanjing Jiancheng Bioengineering Institute, China) respectively, according to manufacturer's instructions. Cardiac function of isoflurane anesthetized mice was evaluated using transthoracic echocardiography at the end of the 24 h reperfusion session.
2.5. Echocardiography
Transthoracic echocardiography was performed in anesthetized mice using a Vevo 3100 high resolution ultrasound device (Visualsonics, Toronto, Canada). Echocardiographic images were recorded along parasternal short axis to measure LV end-diastolic dimension (LVEDD) and LV end-systolic dimension (LVESD). The ejection fraction was calculated with the formula: [(LVEDV−LVESV)/LVEDV] × 100, where EDV is end-diastolic volume and ESV is end-systolic volume.
2.6. Angiogenesis assay
Angiogenesis was detected by immunofluorescence co-labelling Ki67 with CD31 7 days after MI/R with the primary antibodies anti-CD31 (ab281583, 1:100) and anti-Ki67 (ab279653, 1:100, Abcam, UK). Then, the sections were incubated with the Alexa-Fluor-coupled secondary antibodies for 1 h at room temperature, and nuclei were stained with DAPI.
2.7. Mouse cardiac microvascular endothelial cells (CMECs) isolation
We isolated CMECs from hearts as previously reported [31]. Briefly, to prevent blood coagulation, mice were intraperitoneally injected with 0.1 ml heparin. The heart was then excised, dissected, minced, digested using 1 mg/ml collagenase II and 0.6 U/ml dispase II, and filtered through a sterile 40 μm nylon mesh. After this, cells were incubated with magnetic beads which are conjugated to an anti-CD31 antibody. The beads with endothelial cells were washed and were ready for assays. CMECs were verified by the fluorescent dye Dil-Ac-LDL and cultured in EC medium.
2.8. Senescence-associated β-galactosidase (SA-β-gal) staining
SA-β-gal staining was performed using a commercial kit (Abcam, UK) per instructions. Firstly, cells were fixed for 10 min in fixative at room temperature. Then cells were washed in phosphate buffered saline (PBS), and incubated at 37 °C overnight in staining solution containing X-gal. The reaction was stopped by rinsing several times in PBS for 5 min, after which cells were microscopically assessed for blue color development.
2.9. In vitro 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2H-tetrazolium bromide (MTT), migration, and tube formation assays
MTT assays were performed on primary CMECs (5 × 103) which were seeded into 96-wells plates and incubated for 24 h. Then, 10 μl MTT (5 mg/ml) per well was added to wells (after changing the medium) and followed by a 4 h incubation. Supernatants were then removed and replaced by 200 μl DMSO. The absorbance was read at 492 nm using a spectrophotometer (Thermo Fisher Scientific, Inc.). To evaluate CMEC migration, 1 × 104 primary CMECs (in approximately 150 μl medium) were seeded into the upper chambers of transwell plates (8 μm pore; Corning, NY, USA) and incubated at 37 °C for 12 h. Migrated CMECs in lower chambers were stained with 1% crystal violet for 5 min and cell numbers counted in five fields for each chamber. For the in vitro tube formation assay, a 96-well plate was pre-coated with Matrigel (Corning, NY, USA). Then, primary CMECs (0.7 × 104 cells) were seeded into wells and images captured after 6 h.
2.10. In vitro measurement of mitophagy with mt-Keima
Primary CMECs were seeded into glass-bottomed dishes and infected with a lentivirus overexpressing the pH-dependent fluorescent mt-Keima (MBL Medical & Biological Laboratories Co., JP) following manufacturer's instructions. This was followed by carbonyl cyanide p-(trifluoromethoxy)phenyl-hydrazone (FCCP, 10 μM) treatment for 6 h prior to imaging. Fluorescent images were captured using a Leica TCS SP8 confocal spectral microscope. Ratio (534/458 nm) of mt-Keima emission light were calculated to reflect mitophagy [32].
2.11. Mitochondrial membrane permeability and mitochondrial ROS measurements
A JC-1 assay kit (Beyotime Institute of Biotechnology, Jiangsu, China) and MitoSOX indicator (Yeasen Biotechnology, Shanghai, China) to evaluate mitochondrial membrane potential and ROS generation, respectively, in primary CMECs. After washing three times in PBS, cells were photographed using an inverted fluorescence microscope (DMI6000B, Leica, Germany).
2.12. Adeno-associated virus (AAV) infection
GeneChem Biotechnology (China) generated serotype 9 AAV vectors (AAV9) with TIE promoters encoding FUNDC1 to overexpress FUNDC1. Aged sedentary animals had either AAV9 encoding FUNDC1 or negative control (NC) at 5 × 1011 viral genomes/mouse and animals were subjected to MI/R surgery 2 weeks after the AAV injection.
2.13. Extraction of mRNA and real-time quantitative polymerase chain reaction
Total RNA was extracted using Trizol reagent according to manufacturer's instructions. RNA concentration and purity were measured using absorbance at 260 and 280 nm on a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, USA). Total RNA was then reverse transcribed by a PrimeScript RT regent Kit (TaKaRa). We next measured levels of individual genes by qPCR in a Bio-Rad CFX-96 System (Bio-Rad, USA) using a SYBR Premix (TaKaRa, Japan). Reactions were performed over three technical replications. The levels of mouse Fundc1 was determined using primers (forward 5′-TATCATGGCATCCCGGAACC-3'; and reverse 5′-AGTCACGCCACCCATTACAA-3′). Relative gene expression was calculated using the relative standard curve method (2−ΔΔCt) and β-actin used as a housekeeping control for internal normalization.
2.14. Mitochondrial fraction isolation
Mitochondrial fractionation was performed using the Mitochondrial Fractionation kit (Thermo Fisher Scientific) according to the manufacturer's instructions. Briefly, cells were collected and lysed in mitochondria isolation reagent supplemented with protease inhibitors on ice. Unlysed cells and nuclei were pelleted by spinning for 10 min at 700×g. The supernatants were centrifuged at 12,000×g for 15 min at 4 °C to obtain mitochondrial pellets. The remaining supernatant was taken as the cytosol fraction. The mitochondrial and cytosolic fraction was collected for immunoblot analysis.
2.15. Western blotting
Proteins were subjected to sodium dodecyl sulfate-polyacrylamide electrophoresis, transferred to polyvinylidene difluoride membranes, blocked in 5% bovine serum albumen, and incubated overnight with primary antibodies: anti-FUNDC1 (ab224722, 1:1000, from Abcam, UK), anti-p-eNOS (#9571, 1:1000), anti-LC3 (#12741, 1:1000), and anti-p62 (#8025, 1:1000, from Cell Signaling Technology, MA, USA), and anti-eNOS (27120-1-AP, 1:1000), anti-ET-1 (12191-1-AP, 1:1000), anti-VEGF (19003-1-AP, 1:1000), anti-p16 (10883-1-AP, 1:2000), anti-p21 (10355-1-AP, 1:2000), anti-p53 (10442-1-AP, 1:5000), VDAC (10866-1-AP, 1:1000, from Proteintech, China) and β-actin (AB0035, 1:5000, from Abways, China). Blots were then washed, incubated with secondary antibodies, and proteins visualized using enhanced chemiluminescent-plus reagent. VDAC and β-actin proteins were used as internal loading controls for the mitochondrial fraction and cytosolic lysates, respectively.
2.16. Chromatin immunoprecipitation (ChIP) assay
For this assay, we used 1% formaldehyde to cross link CMECs for 15 min at room temperature. Chromatin was sheared by sonication using the Bioruptor Plus (30” on and 30” off for 10 cycles) and immunoprecipitated using an anti-PPARγ (ab226183, Abcam, UK) or an isotype control IgG. Eluted DNA was used for quantitative PCR.
2.17. PPARγ activity assay
PPARγ DNA-binding activity were measured from the CMECs or nuclear extracts of vascular tissue using the PPARγ Transcription Factor Assay Kit (ab133101, Abcam, UK) according to the manufacturer's protocol.
2.18. Laminar shear stress
Laminar flow studies were conducted using a parallel-plate flow system at 37 °C [33]. Briefly, a constant shear stress was applied by perfusing CMECs with a continuous culture medium flow circulating at 20 dyn/cm2. CMECs at static condition were used as controls.
2.19. Statistical analyses
Data were represented as the mean ± standard error of the mean (SEM). Gaussian distribution and the homogeneity of variance have been tested. Comparisons between two data groups were analyzed with Student's unpaired t-test was used to indicate significance for normally distributed data or Mann-Whitney U test for non-normally distributed data. Multiple groups were compared using One-way ANOVA analysis of variance followed by Tukey's post hoc test for normally distributed data or Mann-Whitney test for non-normally distributed data. Concentration-response curves were evaluated by Two-way ANOVA and subsequent Bonferroni post-test. Significance was accepted at P < 0.05.
3. Results
3.1. Low FUNDC1 caused coronary arterial dysfunction and deterioration of MI/R injury
We first examined age-related coronary arterial functions. Coronary arteries were isolated from young and aged mice. The functional state of the coronary arteries was investigated by assaying endothelium-dependent vascular responses. We observed that ACh-induced, but not SNP-induced coronary arterial dilation was much lower in 18-month-old aged mice when compared with that in 8-week-old young mice, suggesting impaired endothelium-dependent vasodilation in aging coronary arteries (Figs. S1A and B). Coronary microcirculation is associated with myocardial perfusion and energy metabolism, myocardial glucose uptake was determined by 18F-FDG microPET/CT which showed impaired in aged mice when compared with that in young mice (Fig. S1C).
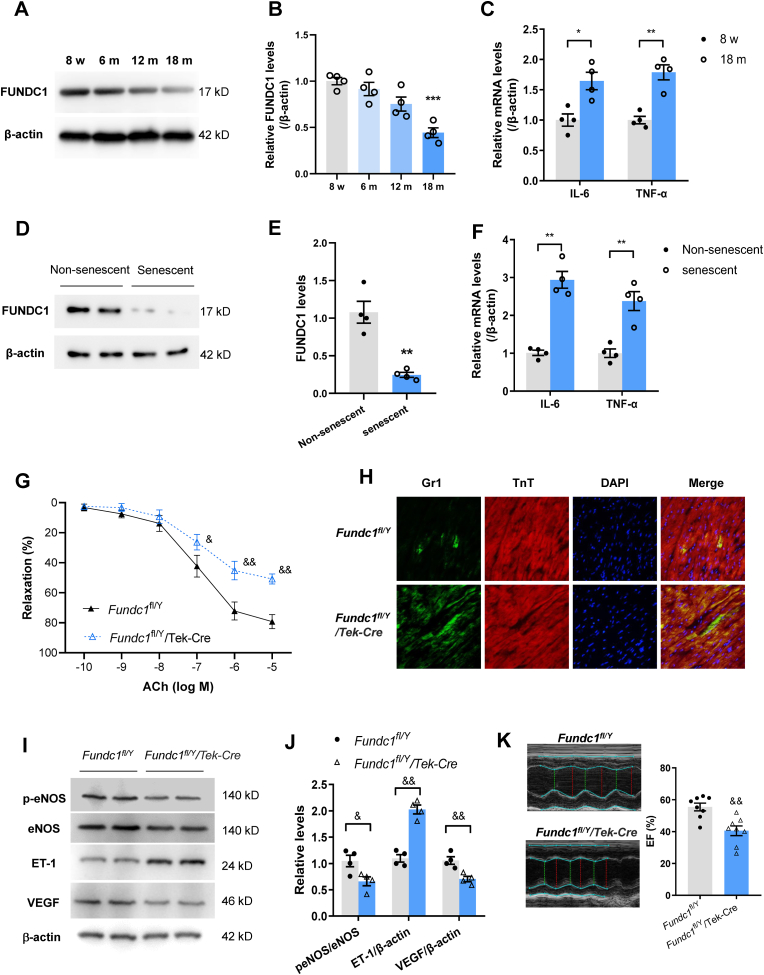
We next assessed the change in FUNDC1 expression under natural aging and senescence conditions. Compared with 8-week-old young mice, FUNDC1 protein levels in coronary arterial tissues were gradually reduced with age. Eighteen-month-old aged mice showed markedly decrease FUNDC1 in coronary arteries (Fig. 1A and B). The mRNA levels of interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α), two important inflammatory factors that promote senescence, were significantly enhanced in the coronary arteries of aged mice (Fig. 1C). Consistently, FUNDC1 expression in replicative-senescent human umbilical vein endothelial cells (HUVECs, passage number 40) was much lower than that in non-senescent HUVECs (passage number 5) (Fig. 1D and E). In the cultured senescent cells, the IL-6 and TNF-α levels were higher (Fig. 1F).
Fig. 1.
FUNDC1 levels are reduced under aging. (A) Representative Western Blotting of FUNDC1 in coronary arteries tissues isolated from mice with different ages from 8 weeks to 18 months. (B) Quantification of FUNDC1 protein levels in coronary arteries isolated from mice with different ages. (C) Inflammatory cytokines IL-6 and TNF-α were determined in mouse coronary arteries with different ages. (D) and (E) Representative and quantification of Western Blotting analysis of FUNDC1 protein levels in non-senescent and replicative-senescent of HUVECs. (F) IL-6 and TNF-α mRNA levels in non-senescent and replicative-senescent HUVECs. (G) ACh induced coronary arteries dilation in floxed (Fundc1fl/Y) and endothelial-specific FUNDC1 knockout mice (Fundc1fl/Y/Tek-Cre). (H) Immunofluorescence staining of Gr1+ neutrophils and TnT+ cardiomyocytes in floxed and endothelial-specific FUNDC1 knockout mice after subjected to myocardial ischemia/reperfusion (MI/R). (I) and (J) Western blotting analysis of eNOS expression and phosphorylation at Ser1177, ET-1 and VEGF expressions in the infarct border zone of myocardial tissue. (K) Echocardiographic assessment of LV ejection fraction (EF) in floxed and endothelial-specific FUNDC1 knockout mice after MI/R. *P < 0.05, **P < 0.01, ***P < 0.001 versus 8 w-old mice or non-senescent cells. &P < 0.05, &&P < 0.01 versus Fundc1fl/Y mice.
Since coronary vascular dysfunction is associated with exacerbation of MI/R injury, we observed the myocardial inflammatory responses by assessing accumulation of Gr-1+ neutrophils. After undergoing MI/R, accumulated Gr-1+ neutrophils were markedly increased in aged mice compared with those in young mice (Fig. S1D), indicating that aggravative microvascular hyperpermeability in aging condition. Moreover, reduced endothelial nitric oxide synthase (eNOS) phosphorylation and upregulated endothelial vasoconstrictor endothelin-1 (ET-1) (Fig. S1E) levels in the infarct border zone, confirmed the observation of endothelium dysfunction in aged mice. Furthermore, angiogenesis in the infarct border zone was markedly reduced as shown by CD31 and Ki67 positive cells by immunofluorescence staining and VEGF levels by WB analysis in aged hearts (Figs. S1E and F). In accordance with these data, aged MI/R hearts exhibited deteriorated contractile functions when compared with young mice as indicated by decreased LV ejection fraction (EF) (Fig. S1G). Aging also exacerbated cardiac damage after MI/R as shown by more CK-MB release and elevated caspase-3 activity (Figs. S1H and I). These data suggested aging caused coronary arterial dysfunction and deterioration of MI/R injury.
To further elucidate the direct role of FUNDC1 in coronary vascular function, we generated EC-specific FUNDC1 knockout (Fundc1fl/Y/Tek-Cre) mice and FUNDC1 levels were significantly reduced in both endothelium of aorta and isolated CMECs (Figs. S2A and B). As FUNDC1 is mitophagy-related protein, we measured mitophagy using mt-Keima fluorescence. CMECs from FUNDC1 knockout mice exhibited marked reduction in overall red mt-Keima fluorescence when treated with FCCP (a mitophagy inducer), indicating impaired mitophagy (Fig. S2C). FUNDC1 deficiency in ECs impaired vasodilation induced by ACh but not SNP, suggesting FUNDC1 was responsible for maintaining normal endothelium-dependent vasodilated function in coronary arteries (Fig. 1G). After MI/R, accumulated Gr-1+ neutrophils were markedly augmented in FUNDC1 knockout mice compared with those in floxed (Fundc1fl/Y) mice (Fig. 1H), indicating more neutrophils migration and endothelial permeability followed by MI/R injury. Reduced eNOS phosphorylation and VEGF expression, and upregulated ET-1 also suggested the endothelium dysfunction in FUNDC1 knockout mice (Fig. 1I and J). Importantly, the EC-specific FUNDC1 deletion aggravated cardiac injury following MI/R as evidenced by reduced cardiac contractile function (Fig. 1K), enhanced CK-MB releases and caspase-3 activity (Figs. S2D and E) when compared with those in the floxed mice.
3.2. Exercise prevented FUNDC1 downregulation and coronary arterial dysfunction with age and reduced the deterioration of MI/R injury in the aging heart
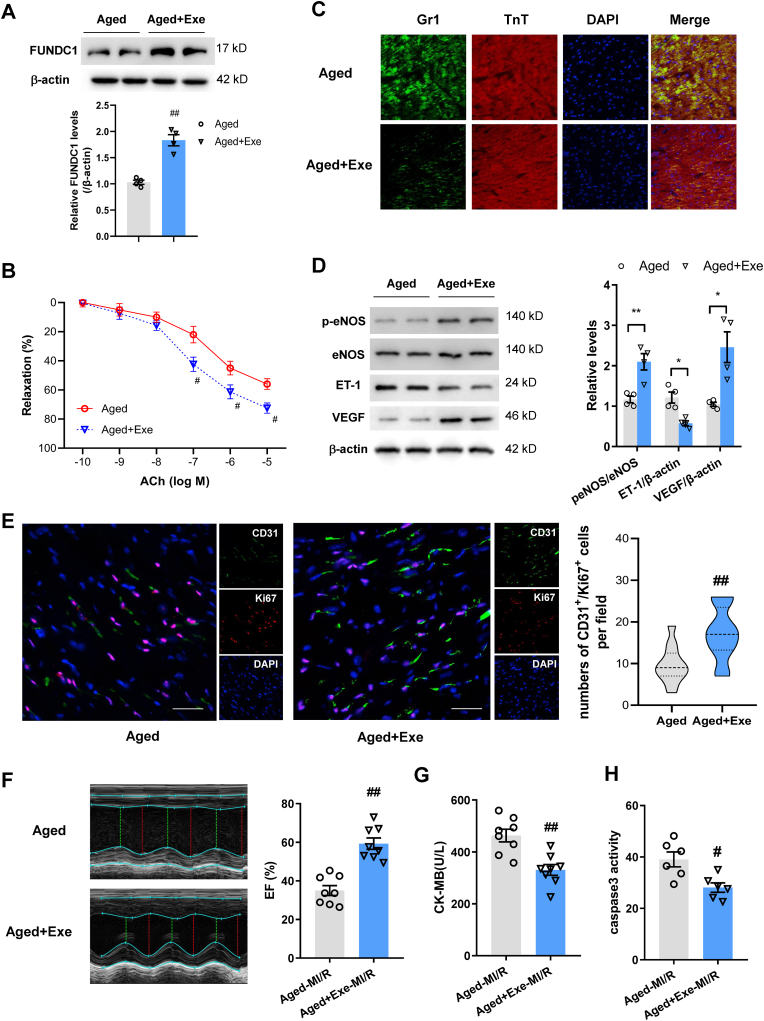
To elucidate the effects of exercise on age-related FUNDC1 downregulation and coronary arterial function, mice underwent regular exercise for 4 weeks. We then isolated coronary arteries from aged mice undergone regular exercise or their sedentary control. We noted that FUNDC1 protein were significantly upregulated in coronary arterial tissues and ACh-induced coronary arterial dilation was significantly enhanced in aged mice with exercise training when compared with those in sedentary mice (Fig. 2A and B), indicating improved endothelial-dependent vasodilation induced by exercise. Endothelium-independent vasodilation in coronary arteries did not show changes between groups (Fig. S3A). Next, we examined myocardial glucose uptake using microPET/CT. Myocardial glucose uptake was significantly enhanced in aged exercise mice compared with that in sedentary ones (Fig. S3B). After undergoing MI/R, Gr-1+ neutrophils migration was reduced in aged mice with exercise compared with those without exercise (Fig. 2C). In line with the observations, inflammation markers such as cytokines (IL-6, IL-8 and TNF-α) and adhesion molecule (VCAM-1, E-selectin and ICAM-1) were showed significant reduction in the infarct border zone of left ventricular tissue in aged MI/R mice with exercise training (Fig. S3C), indicating that exercise protected MI/R evoked microvascular hyperpermeability in aging. In addition, Western blot showed restored eNOS phosphorylation and decreased ET-1 levels in aged exercised mice (Fig. 2D). Angiogenesis in the infarct border zone was markedly restored as showed by CD31 and Ki67 positive cells by immunofluorescence staining and VEGF levels by WB analysis in aged exercised hearts (Fig. 2D and E). When measuring cardiac function and injury, MI/R hearts displayed alleviated contractile dysfunction in aged exercise mice compared with the sedentary control as evidenced by enhanced LV EF (Fig. 2F). Exercise also mitigated cardiac injury after MI/R as indicated by reduced CK-MB levels and less caspase 3 activity (Fig. 2G and H).
Fig. 2.
Exercise restored FUNDC1 expression and coronary artery dilation, contributing to prevention of MI/R injury. (A) Western blotting analysis of FUNDC1 expression in coronary arteries. (B) ACh-mediated coronary arteries dilation in aged mice with or without exercise training. (C) Immunofluorescence staining of Gr1+ neutrophils and TnT+ cardiomyocytes in aged mice with or without exercise training after MI/R. (D) Western blotting analysis of eNOS expression and phosphorylation at Ser1177, ET-1 and VEGF expression in the infarct border zone. (E) Representative angiogenesis images and quantification of CD31 and Ki67 positive cells in risk areas in aged mice with or without exercise after 7 days of MI/R. Scale bar, 25 μm. (F) Echocardiography of LV EF in aged mice with or without exercise after MI/R. (G) Myocardial injury was determined using serum CK-MB level after MI/R. (H) Myocardial apoptosis was assessed using the caspase-3 activity assay. Data were shown as mean ± SEM or median (25th, 75th). #P < 0.05, ##P < 0.01 versus aged mice.
3.3. Exercise alleviated endothelial senescence and dysfunction
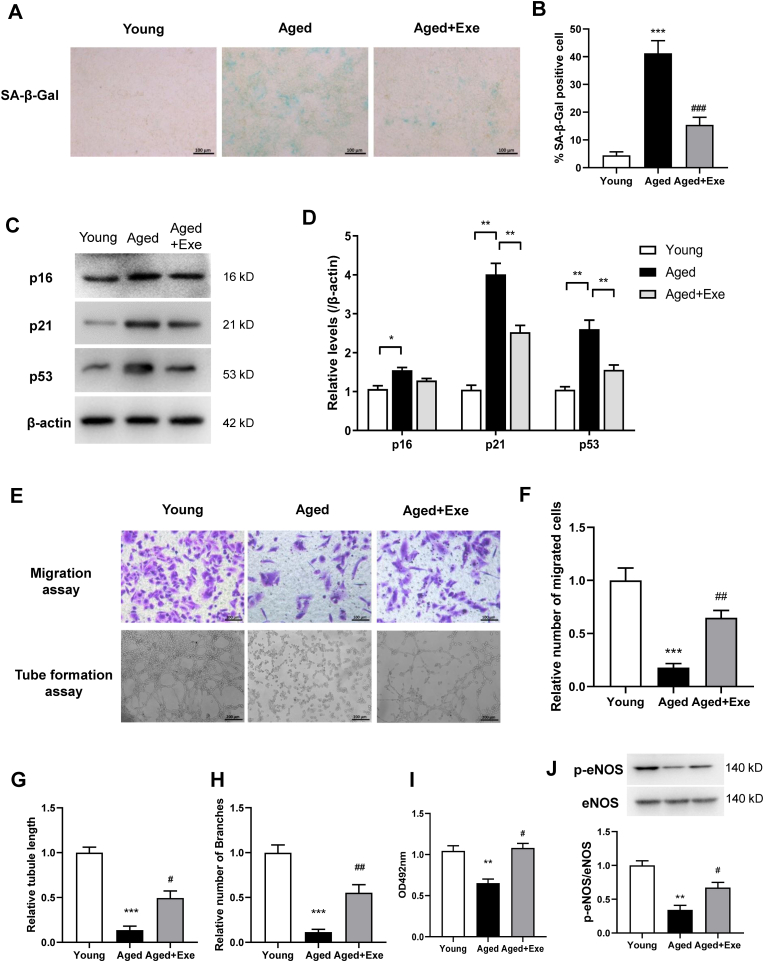
To examine the role of exercise on endothelial senescence and function, SA-β-gal staining was performed on primary CMECs from exercised or sedentary aged mice. SA-β-gal activity (at pH 6.0) permits senescent cell identification as cells lose proliferation abilities. As expected, primary CMECs from aged mice exhibited an obvious rise in SA-β-gal activity, with exercise dramatically reducing these levels (Fig. 3A and B). This observation was supported by the protein expressions of senescence markers p21 and p53 which were significantly increased in aged CMECs, and were restored in cells from exercised aged animals (Fig. 3C and D). p16 was also increased in sedentary aged mice but was slightly reduced in exercised aged mice. Thus, we observed considerable senescence in aged CMECs, and importantly, exercise significantly reversed CMECs senescence. Furthermore, angiogenic function was impaired in CMECs from aged hearts when assessed by tube formation assay, migration and proliferation assay, but were restored by exercise (Fig. 3E–I). eNOS phosphorylation in CMECs was markedly reduced in aged mice and restored by exercise (Fig. 3J).
Fig. 3.
Exercise alleviated CMECs senescence and dysfunction. (A) and (B) Representative images and quantification of cellular senescence associated with β-galactosidase (SA-β-Gal) staining in isolated CMECs. (C) and (D) Western blotting and quantification showing levels of the senescence markers p16, p21, and p53 in CMECs. (E) to (H) Representative images and quantification of migration and tube formation assays on CMECs from different mouse groups. (I) Cell proliferation assay and (J) eNOS phosphorylation in CMECs from different mouse groups. Data represented the mean ± SEM. n = 4 independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001 versus young mice. #P < 0.05, ##P < 0.01, ###P < 0.001 versus aged mice.
3.4. Exercise reduced mitochondrial injury and restored mitophagy in senescent CMECs
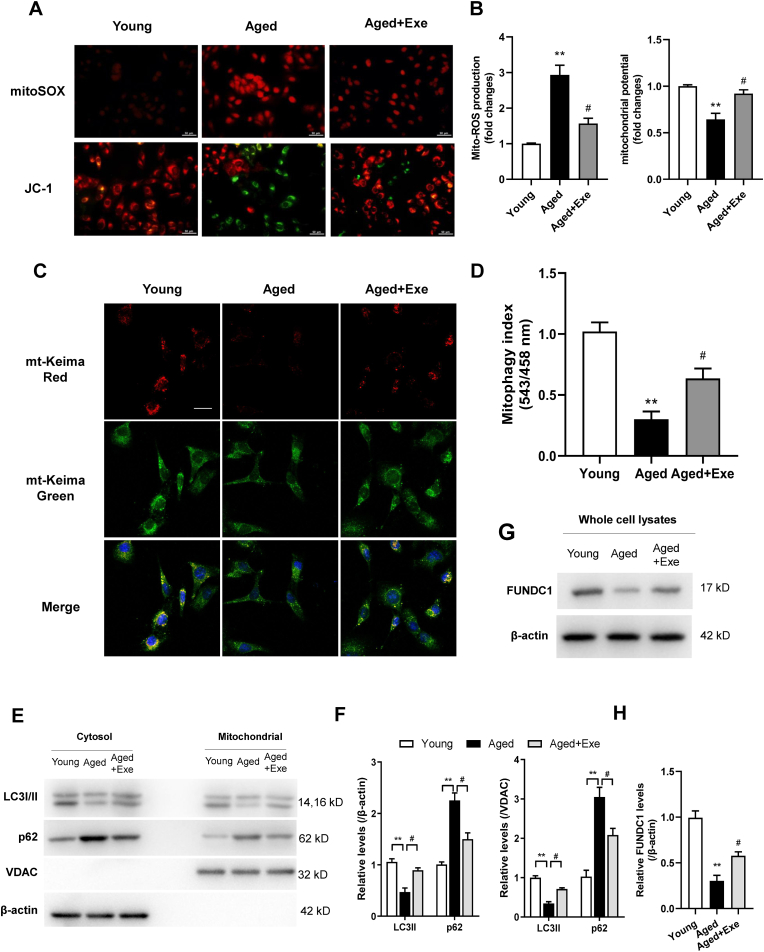
As endothelial mitochondria are required for EC functional integrity, we investigated whether mitochondrial damage was involved in age-related endothelial dysfunction. Mitochondrial ROS levels and membrane potential in mitochondria were examined in primary CMECs. Accumulated mitochondrial ROS and aberrant mitochondrial membrane potential were observed in aged CMECs, which were mitigated in CMECs from aged mice with exercise training when compared with sedentary animals (Fig. 4A and B).
Fig. 4.
Exercise reduced mitochondrial injury and restores mitophagy in senescent CMECs. (A) Mitochondrial O2•− was detected by MitoSOX staining (Top). Fluorescence images of JC-1 monomers (Green) showing hyperpolarized mitochondria and JC-1 aggregates (red) showing normal mitochondria in CMECs from different groups (Bottom). Scale bar, 50 μm. (B) Quantification of relative levels of mitochondrial ROS and potential (Red/Green fluorescence ratio). (C) and (D) CMECs transfected with mt-Keima followed by FCCP (10 μM) treatment showing the mitochondrial network in green and mitophagic vesicles in red. Scale bar, 25 μm. (E) and (F) Western blotting showing levels of LC3 and p62 in mitochondrial fraction and cytosolic fraction of CMECs. (G) and (H) Western blotting showing the mitophagy receptor FUNDC1 level in whole cell lysates of CMECs. n = 4 independent cell isolations per group. **P < 0.01 versus young mice. #P < 0.05, ##P < 0.01 versus aged mice.
As mitophagy selectively targets and removes damaged or dysfunctional mitochondria thereby ensuring normal homeostatic functions. FUNDC1 has essential roles in mitochondrial quality control system to maintain cellular functions during stress. We monitored mitophagy using mt-Keima fluorescence, and assaying LC3 and p62 levels by Western blot. As expected, CMECs from aged mice exhibited an impaired mitophagy when treated with FCCP (a mitophagy inducer). Senescence caused a marked reduction in overall red mt-Keima fluorescence (Fig. 4C and D), reduced LC3-II levels and accumulation of p62 in the mitochondrial fraction, as well as in cytosolic fraction of CMECs, and these conditions were reversed by exercise (Fig. 4E and F). Importantly, the impaired mitophagy in senescent CMECs were reserved by exercise (Fig. 4C–F). Levels of mitophagy receptor FUNDC1 in CMECs were also verified lower in aged mice and its expression were restored in exercised aged mice (Fig. 4G and H).
3.5. Endothelial-specific FUNDC1 deletion blocked the role of exercise in coronary vascular protection
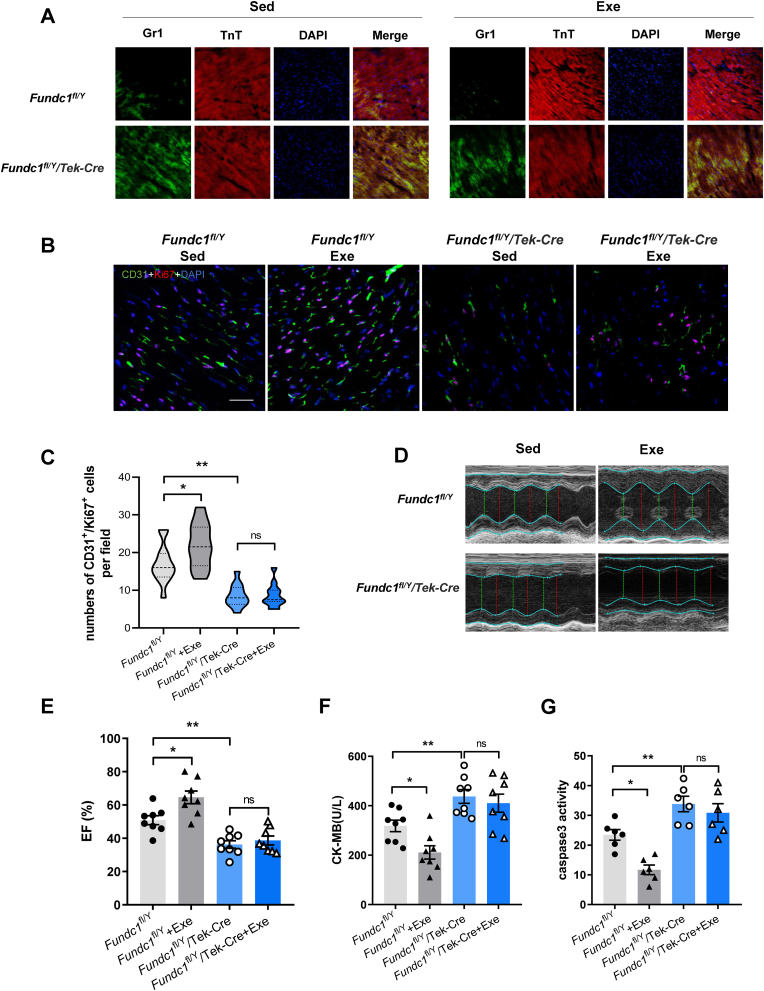
To verified the role of FUNDC1 in exercise-induced coronary arterial protection, Fundc1fl/Y and Fundc1fl/Y/Tek-Cre mice were performed regular exercise training for 4 weeks. In the Fundc1fl/Y mice, exercise protected against MI/R-evoked endothelial hyperpermeability as shown by reduced Gr-1+ neutrophils migration. Angiogenesis in the infarct border zone was markedly restored in exercised Fundc1fl/Y mice as evidenced by CD31 and Ki67 positive cells by immunofluorescence staining. However, even trained with regular exercise, Gr-1+ neutrophils migration following MI/R were still significant increase in FUNDC1 deleted mice compared with that in floxed mice (Fig. 5A), indicating impairment of endothelial integrity. Co-staining with CD31 and Ki67 showed comparable level of angiogenesis in the infarct border zone of MI/R in exercise and sedentary Fundc1fl/Y/Tek-Cre mice (Fig. 5B and C). Consistently, FUNDC1 knockout abrogated the exercise's effect of the improvement of cardiac contractile function as evidenced by comparable EF (Fig. 5D and E). Furthermore, the effects of exercise on alleviated MI/R injury were abolished by FUNDC1 knockout as indicated by comparable CK-MB releases and caspase-3 activity (Fig. 5F and G). These data suggested that FUNDC1 deletion blocked the improvement of coronary arteries function and reduction of MI/R injury induced by exercise.
Fig. 5.
Endothelial-specific FUNDC1 deletion blocked the role of exercise in coronary arteries protection. (A) Immunofluorescence staining of Gr1+ neutrophils and TnT+ cardiomyocytes in sedentary or exercised Fundc1fl/Y and Fundc1fl/Y/Tek-Cre mice subjected to MI/R. (B) and (C) Representative angiogenesis images and quantification of CD31 and Ki67 positive cells in risk areas Fundc1fl/Y and Fundc1fl/Y/Tek-Cre mice with or without exercise after 7 days of MI/R. Scale bar, 25 μm. (D) and (E) Echocardiography of LV EF in Fundc1fl/Y and Fundc1fl/Y/Tek-Cre mice with or without exercise training after MI/R. (F) Myocardial injury was determined using serum CK-MB level after MI/R. (G) Myocardial apoptosis was assessed using the caspase-3 activity assay. Data were shown as mean ± SEM or median (25th, 75th). *P < 0.05, **P < 0.01.
3.6. FUNDC1 overexpression reversed endothelial senescence and dysfunction
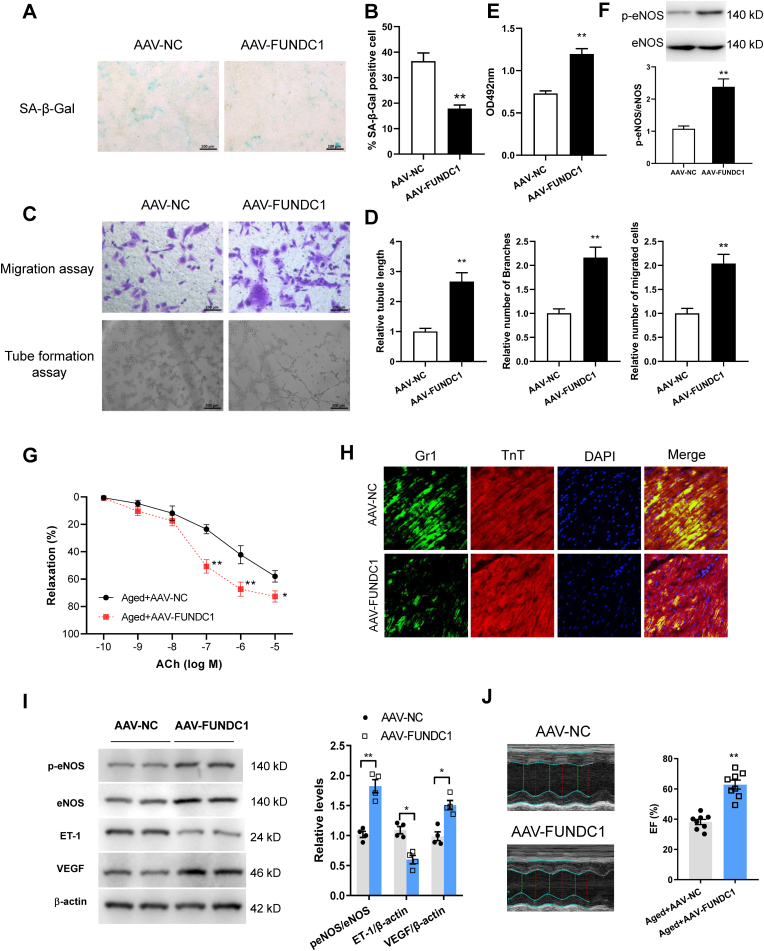
To evaluate the therapeutic potential of FUNDC1 for EC senescence, we next engineered a AAV9-FUNDC1 under the control of the TIE promoter. Aged mice were injected with this AAV intravenously to overexpress FUNDC1 in endothelium. Using immunofluorescence and qPCR assays respectively, FUNDC1 levels in aorta endothelium and primary CMECs were found increased in AAV9-FUNDC1 injected mice (Figs. S4A and B). Importantly, FUNDC1 overexpression markedly decreased SA-β-gal activity in CMECs (Fig. 6A and B), suggesting overexpressed mitophagy receptor FUNDC1 reversed endothelial senescence. Accordingly, FUNDC1 overexpression improved EC function as indicated by tube formation, migration and proliferation (Fig. 6C–E). eNOS phosphorylation in CMECs was markedly upregulated in aged mice after FUNDC1 overexpression (Fig. 6F).
Fig. 6.
Overexpressing FUNDC1 by AAV reversed endothelial senescence and dysfunction. (A) and (B) Images and quantification of SA-β-Gal staining in primary CMECs after EC-specific FUNDC1 overexpression. (C) and (D) Migration and tube formation assays in CMECs upon FUNDC1 overexpression. (E) Cell proliferation assay in CMECs from FUNDC1 overexpressed mice. (F) eNOS phosphorylation at Ser1177 in CMECs. (G) ACh-induced coronary arteriole dilation in aged mice injected with AAV-NC or AAV-FUNDC1. (H) Immunofluorescence staining of Gr1+ neutrophils and TnT+ cardiomyocytes in aged animals injected with AAV-NC or AAV-FUNDC1 after MI/R. (I) Western blotting analysis of eNOS expression and phosphorylation at Ser1177, ET-1 and VEGF expression in the infarct border area. (J) Echocardiography of LV EF after MI/R in aged mice injected with AAV-NC or AAV-FUNDC1. Data represented the mean ± SEM. *P < 0.05, **P < 0.01 versus aged mice with AAV-NC treatment.
Furthermore, consistent with the protective effects of exercise, FUNDC1 overexpression enhanced ACh-stimulated coronary arteriole dilation (Fig. 6G), integrity of endothelial barrier (Fig. 6H), restored eNOS phosphorylation and VEGF expression and decreased ET-1 levels in aged mice (Fig. 6I). Importantly, AAV-FUNDC1 treatment significantly improved cardiac contractile function following MI/R (Fig. 6J).
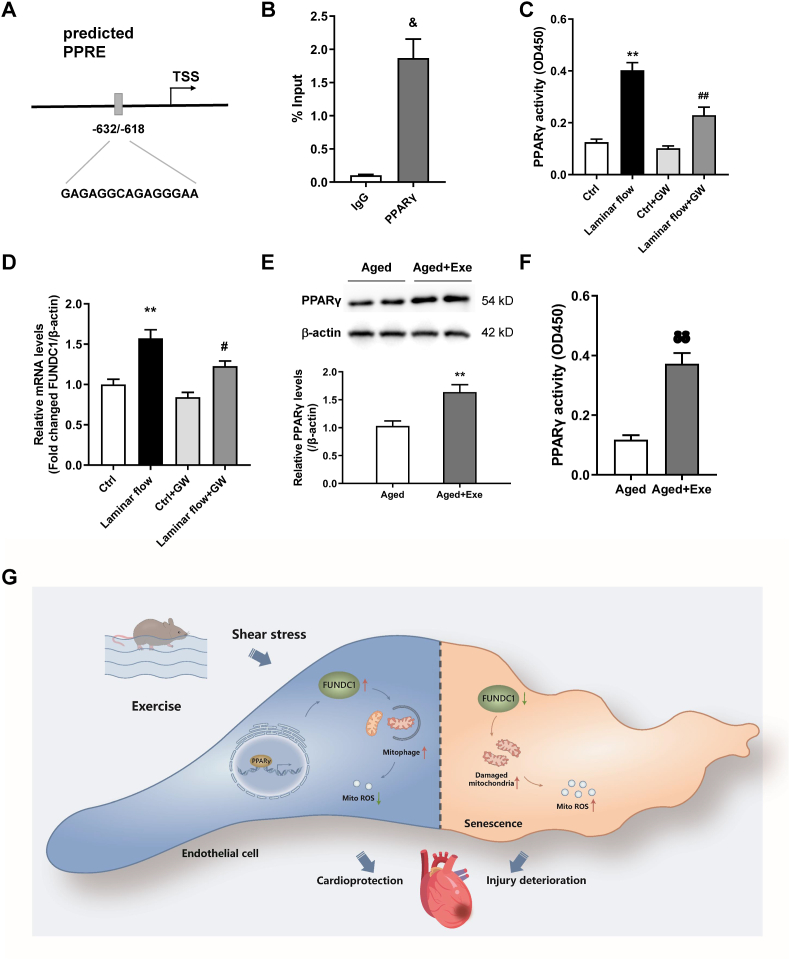
3.7. PPARγ was responsible for the laminar flow regulation of FUNDC1 expression in CMECs
Laminar flow-induced increases in laminar shear stress (LSS) are vital mechanisms underpinning exercise-induced endothelial adaptation during exercise. Thus, we used laminar flow studies to assess the regulatory mechanisms of FUNDC1 gene expression during exercise. We also potentially identified the transcription factor initiating transcription of FUNDC1. Bioinformatics predictions suggested that FUNDC1 contained a PPARγ response element (PPRE) (Fig. 7A). Previous studies also showed that laminar flow activated PPARγ in ECs. Therefore, we assessed whether laminar flow-enhanced FUNDC1 gene expression in a PPARγ-dependent manner in CMECs. Our ChIP assays showed that PPARγ interacted with the FUNDC1 promoter region in CMECs (Fig. 7B). To reinforce this finding, cultured CMECs were pretreated with a PPARγ-specific antagonist, GW9662 (10 μM for 30 min) and exposed to laminar flow at 20 dyne/cm2 for 6 h. Laminar flow-induced LSS increased PPARγ DNA-binding activity CMECs and flow-induced PPARγ activation was depressed under GW9662 treatment (Fig. 7C). In addition, GW9662 significantly attenuated FUNDC1 expression, but not under static conditions, suggesting PPARγ contributed to shear-induced FUNDC1 gene expression (Fig. 7D). In addition, PPARγ expression and activity were enhanced in vascular tissue of aged mice with exercise training compared with those in sedentary animals (Fig. 7E and F).
Fig. 7.
PPARγ was responsible for laminar flow-enhanced FUNDC1 gene expression in CMECs. (A) Schematic diagram showing the predicted PPRE in the transcription initiation region of FUNDC1. (B) ChIP assays in CMECs using an antibody against PPARγ or IgG as control. (C) PPARγ activity assay in CMECs exposed to laminar flow, treated with or without the GW9662. (D) FUNDC1 expression levels in CMECs exposed to laminar flow, with or without the PPARγ-specific antagonist GW9662 treatment. (E) and (F) PPARγ expression and activity in coronary arteries from aged mice with or without exercise training. (G) Model of FUNDC1 function in protective role of exercise on endothelial senescence. Data represented the mean ± SEM. n = 4 independent experiments. &P < 0.05 versus IgG; **P < 0.01 versus control; P < 0.05, ##P < 0.01 versus laminar flow; δP < 0.05 versus aged mice.
4. Discussion
In the present study, we demonstrated a pivotal role of FUNDC1 in endothelial homeostasis. Firstly, loss of FUNDC1 directly contributed to coronary endothelial senescence and dysfunction, and subsequent deterioration of MI/R injury. Secondly, exercise protected against age-related coronary arterial dysfunction and the exacerbated MI/R injury; upregulation of FUNDC1 was responsible for coronary arterial protective roles of exercise. Thirdly, exercise-induced laminar flow regulated Fundc1 gene expression in a PPARγ-dependent manner in CMECs. To the best of our knowledge, these findings are the first demonstration that exercise protected against coronary endothelial senescence and dysfunction via FUNDC1-dependent mitophagy, and identified a key function for PPARγ in mediating FUNDC1 expression induced by shear stress.
The rapidly changing composition of the human population necessitates a deeper understanding of cardiovascular senescence. Aging is a central risk factor for vascular disorders, with vascular aging per se possibly contributing to organ dysfunction, disease development and worse prognosis [34,35]. Adverse changes mediated by arterial aging include large elastic artery stiffness and microvascular susceptibility to injury. Therefore, it is critical to consider the effects of aging on the postischemic heart, particularly on the blood vessels of the heart. Among these, endothelial disorders are the central mechanisms by which aging promotes vascular pathology [36]. Exercise is widely considered a cardiovascular protective factor and data from rodent models have suggested that exercise effectively modulates cardiac aging processes. Current guidelines recommend at least 150 min of moderate exercise or 75 min of vigorous exercise per week [37]. However, impact of exercise training on vascular aging phenotypes and its significance remains ill-defined. In this study, the mitophagy-receptor FUNDC1 expressions in coronary arteries were gradually reduced with age. FUNDC1 levels, as well as mitophagy levels, were significantly lowered in CMECs of aged mice and preserved in aged mice with exercise training.
Endothelial mitochondria act as signal transduction mediators for proliferation, mobilization, paracrine signaling and cell death. Mitochondrial functionality depends on dynamic networks and continuous surveillance. Several mitophagy mediators such as PINK1/Parkin, FUNDC1 and NIX/BNIP3 selectively target and remove dysfunctional mitochondria [38,39]. Emerging evidence has shown controlled mitophagy in cardiomyocytes during healthy aging. Thus, systems that downregulate mitophagy could induce cardiac dysfunction [40,41]. Although the protective effects of mitophagy on cardiovascular disorders have been carefully investigated, the roles of mitophagy in ECs senescence are not fully recognized. Different from the cardiomyocytes neighbors, ECs primarily rely on glycolysis rather than mitochondria oxidative phosphorylation for ATP production, thus the potential role of ECs mitochondria has been neglected, to some degree [42]. Intriguingly and importantly, EC-specific FUNDC1 deletion using CRISPR/Cas9 system exhibited impaired mitophagy, synchronized with significant ECs senescence and reduced functional integrity. Recent work have increasingly recognized the critical role of endothelial mitochondria to serve as biosynthetic organelles for ECs proliferation, differentiation and pathological sensor [12,43,44], supporting our findings that restored mitophagy in ECs protects endothelial and myocardial function under stress. In addition, liver specific knockout of FUNDC1 facilitates the cytosolic release of mtDNA, resulting in elevated release of proinflammatory cytokine IL-1β in hepatocytes [23], suggesting that mitochondria quality control participates in cellular damage. FUNDC1 also interacts with the chaperone HSC70, promoting the mitochondrial translocation and degradation of unfolded cytosolic proteins. Excessive accumulation of unfolded proteins impairs mitochondrial integrity, leading to cellular senescence [45]. Together with our findings, disturbance of FUNDC1-mediated mitophagy with age is responsible for endothelial function disorders and impairment of coronary vascular function. These data further verified that FUNDC1 is responsible for microvascular protection of exercise.
Mitochondrial homeostasis, including free radical generation in modulating aging and senescence, has received considerable attention in recent studies [46]. Mitochondrial quality control mechanisms degrade dysfunctional mitochondria via mitophagy. Mitophagy may function more broadly to limit the deleterious effects of ROS on cellular function [47]. In the present study, mitochondrial ROS were accumulated and mitochondrial inner membrane potential (ΔΨm), which directly relate to the opening of mPTP and mitochondrial damage [48], was dissipated in CMECs of aged mice. Exercise training also decreased mitochondrial damage in aged mice. Recently, it was reported that FUNDC1 silencing reduced mitochondria-associated endoplasmic reticulum membrane formation and inhibited angiogenesis by decreasing VEGFR2 expression [49]. ROS-mediated oxidative stress promotes endothelial senescence [50], leading to impaired angiogenesis. Inhibition of mitochondrial ROS regulated by mitochondrial Ca2+ attenuated skeletal muscle disorders associated with aging [51]. Protection against oxidative stress mediates longevity and human healthful aging [52]. These observations are consistent with our data showing accumulated mitochondrial ROS and defective endothelial function upon FUNDC1 downregulation during aging. Thus, impairment of FUNDC1-dependent mitochondrial quality control in the endothelium triggers cellular senescence and dysfunction. Our results provide evidence, for the first time, that endothelial FUNDC1 profoundly and adversely affects myocardial outcomes.
Furthermore, we identified that coronary vessels in aged mice underwent a series of functional changes reflecting impaired endothelium-dependent vasodilation, endothelial integrity and angiogenesis after stress. In the present study, we also demonstrated that exercise restored endothelial-dependent coronary vasodilation. As a hallmark of age-related vascular endothelial dysfunction is impaired endothelial-dependent dilation and/or a reduction in the critical endothelium-derived vasodilator, nitric oxide (NO), which is predictive of future cardiovascular events [60], our data suggest the important endothelial protective role of exercise against aging. Exercise also rejuvenated ECs in the coronary circulation and affected EC functional integrity in aged mice. eNOS activity which is related to activating telomerase and delaying EC senescence [61], was markedly decreased in aging and reserved by exercise in our observation. Insufficient moderate-to-vigorous intensity physical activity has been associated with increased risk of cardiovascular diseases [62]. While some studies find that exercise's effects on some aspect wanes with advancing age [63]. Although the effects of exercise training on elders are still controversial that cardiovascular risk profile in older adults undergoing supervised exercise for 5 years was not shown significant changed in a very recent randomized clinical trial [64], out data intriguingly suggest that regular exercise, as a therapeutic intervention, can potentially mitigate coronary endothelial senescence, which is consistent with previous observations on vasculature [[65], [66], [67], [68], [69]].
Endothelial dysfunction is increasingly recognized as being involved in early and late pathogenic events during myocardial ischemia and the severity of endothelial dysfunction is correlated with the risk of primary or recurrent cardiovascular events [70]. Additionally, slow- or no-reflow after percutaneous coronary intervention would discount the benefits of coronary reperfusion after infarction in patients. The present study provides valuable insights into a functional association between exercise-mediated alleviations of coronary vascular disorder and cardiac damage in aged mice. In this study, impaired endothelium-dependent vasodilation was observed in aging coronary arteries, which may lead to reduced perfusion, especially coronary blood flow reserves under stress conditions [71]. Regular exercise training improved endothelium-dependent vasodilation, resulting in restoration blood glucose perfusion and uptake and alleviation of MI/R injury. Moreover, exercise training preserved endothelial integrity, suppressed inflammatory responses and adhesive protein expressions, which may play an important role in reducing injury and improving microvascular perfusion during MI/R. The eNOS activation in ECs plays an obligatory role in regulating local vascular tone and cardiac blood supply, which was improved in mice undergoing exercise. Angiogenesis is also vital for cardiomyocytes perfusion and metabolism after MI/R [72]. We observed that endothelial senescence was correlated with reduced microvessel density in the infarct border zone after MI/R, which was preserved in mice undergoing exercise, indicating that elevated microvascular density was induced by exercise. Importantly, FUNDC1 deficiency impaired endothelium-dependent vasodilation and increased endothelial permeability in coronary arteries, and further aggravated cardiac dysfunction and injury after MI/R. Meanwhile, endothelium specific deletion of FUNDC1 abolished protective roles of exercise in coronary arteries and MI/R hearts. FUNDC1 overexpression reversed age-related coronary dysfunction and prevented aged hearts from aggravated I/R injury, which were consistent with the protective effects of exercise. Taken together, FUNDC-mediated mitophagy and ECs function participated in the beneficial effects of exercise on MI/R hearts during aging.
Exercise-induced LSS is a primary driving force underpinning EC adaptation. Substantial evidence has shown that local hemodynamic forces are key determinants of EC phenotypes [53,54]. Exercise-induced LSS reportedly increased mitochondrial biogenesis in ECs by upregulating PGC-1α and TFAM [55]. Different from the disturbed blood flow in atherosclerosis, which contributes to mitochondrial impairment and endothelial dysfunction [56,57], the unidirectional laminar flow induced by exercise upregulates regulators of mitochondrial remodeling [58]. However, the regulation of FUNDC1 expression and activity in ECs, particularly in response to exercise-induced LSS, were largely unknown. In our study, bioinformatics predictions indicated that FUNDC1 contained a PPRE, suggesting FUNDC1 expression may be mediated by PPARγ. As a previous laminar flow study showed that PPARγ was activated in ECs in a ligand-dependent manner [59], we assessed whether laminar flow mediated FUNDC1 expression, with or without PPARγ inhibition. The selective PPARγ antagonist GW9662 treatment significantly reduced laminar flow-induced PPARγ activation and FUNDC1 expression. Consistently, PPARγ expression and activation were significantly increased in coronary vascular tissue of aged mice after exercise. Our data provided the first evidence that enhanced laminar flow improved FUNDC1 gene expressions, with PPARγ potentially responsible for LSS-induced FUNDC1 upregulation in ECs.
In conclusion, upregulated FUNDC1 and restored mitophagy contribute to decreased endothelial senescence and MI/R injury induced by exercise training in aging. PPARγ plays an important role in regulating FUNDC1 expression during exercise. Suppressing FUNDC1 per se leads to endothelial senescence and abolished the protective effects of exercise against endothelium and MI/R injury. In light of these findings, FUNDC1 may be a promising target for maintaining endothelial mitochondrial homeostasis, preventing endothelial senescence and reducing MI/R injury via vascular endothelium “cross-talk” with heart tissue mechanisms (Fig. 7G).
Funding
This work was supported by National Natural Science Foundation of China (Nos. 32071107, 81870280, 81800088), China Postdoctoral Science Foundation (No. 2020M673670), Shaanxi Nova Program (No. 2021KJXX-21) and Young Talent Fund in Fourth Military Medical University.
Declaration of competing interest
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.redox.2023.102693.
Contributor Information
Haifeng Zhang, Email: hfzhang@fmmu.edu.cn.
Wenjuan Xing, Email: xwjfmmu@126.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
Data availability
Data will be made available on request.
References
- 1.Go A.S., Mozaffarian D., Roger V.L., Benjamin E.J., Berry J.D., Borden W.B., Bravata D.M., Dai S., Ford E.S., Fox C.S., Franco S., Fullerton H.J., Gillespie C., Hailpern S.M., Heit J.A., Howard V.J., Huffman M.D., Kissela B.M., Kittner S.J., Lackland D.T., Lichtman J.H., Lisabeth L.D., Magid D., Marcus G.M., Marelli A., Matchar D.B., McGuire D.K., Mohler E.R., Moy C.S., Mussolino M.E., Nichol G., Paynter N.P., Schreiner P.J., Sorlie P.D., Stein J., Turan T.N., Virani S.S., Wong N.D., Woo D., Turner M.B., American Heart Association Statistics C., Stroke Statistics S. Heart disease and stroke statistics--2013 update: a report from the American Heart Association. Circulation. 2013;127(1):e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.LeBlanc A.J., Kelm N.Q., George M. Current themes in myocardial and coronary vascular aging. Curr Opin Physiol. 2018;1:27–33. doi: 10.1016/j.cophys.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grunewald M., Kumar S., Sharife H., Volinsky E., Gileles-Hillel A., Licht T., Permyakova A., Hinden L., Azar S., Friedmann Y., Kupetz P., Tzuberi R., Anisimov A., Alitalo K., Horwitz M., Leebhoff S., Khoma O.Z., Hlushchuk R., Djonov V., Abramovitch R., Tam J., Keshet E. Counteracting age-related VEGF signaling insufficiency promotes healthy aging and extends life span. Science. 2021;373(6554) doi: 10.1126/science.abc8479. [DOI] [PubMed] [Google Scholar]
- 4.Le Couteur D.G., Lakatta E.G. A vascular theory of aging. J Gerontol A Biol Sci Med Sci. 2010;65(10):1025–1027. doi: 10.1093/gerona/glq135. [DOI] [PubMed] [Google Scholar]
- 5.Calmac L., Bataila V., Ricci B., Vasiljevic Z., Kedev S., Gustiene O., Trininic D., Knezevic B., Milicic D., Dilic M., Manfrini O., Cenko E., Badimon L., Bugiardini R., Scafa-Udriste A., Tautu O., Dorobantu M. Factors associated with use of percutaneous coronary intervention among elderly patients presenting with ST segment elevation acute myocardial infarction (STEMI): results from the ISACS-TC registry. Int. J. Cardiol. 2016;217(Suppl):S21–S26. doi: 10.1016/j.ijcard.2016.06.227. [DOI] [PubMed] [Google Scholar]
- 6.Ungvari Z., Tarantini S., Kiss T., Wren J.D., Giles C.B., Griffin C.T., Murfee W.L., Pacher P., Csiszar A. Endothelial dysfunction and angiogenesis impairment in the ageing vasculature. Nat. Rev. Cardiol. 2018;15(9):555–565. doi: 10.1038/s41569-018-0030-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.LeBlanc A.J., Shipley R.D., Kang L.S., Muller-Delp J.M. Age impairs Flk-1 signaling and NO-mediated vasodilation in coronary arterioles. Am. J. Physiol. Heart Circ. Physiol. 2008;295(6):H2280–H2288. doi: 10.1152/ajpheart.00541.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Augustin H.G., Kipnis J. Vascular rejuvenation is geroprotective. Science. 2021;373(6554):490–491. doi: 10.1126/science.abj8674. [DOI] [PubMed] [Google Scholar]
- 9.Roh J., Rhee J., Chaudhari V., Rosenzweig A. The role of exercise in cardiac aging: from physiology to molecular mechanisms. Circ. Res. 2016;118(2):279–295. doi: 10.1161/CIRCRESAHA.115.305250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kitzman D.W., Brubaker P.H., Herrington D.M., Morgan T.M., Stewart K.P., Hundley W.G., Abdelhamed A., Haykowsky M.J. Effect of endurance exercise training on endothelial function and arterial stiffness in older patients with heart failure and preserved ejection fraction: a randomized, controlled, single-blind trial. J. Am. Coll. Cardiol. 2013;62(7):584–592. doi: 10.1016/j.jacc.2013.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seals D.R., Nagy E.E., Moreau K.L. Aerobic exercise training and vascular function with ageing in healthy men and women. J. Physiol. 2019;597(19):4901–4914. doi: 10.1113/JP277764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caja S., Enriquez J.A. Mitochondria in endothelial cells: sensors and integrators of environmental cues. Redox Biol. 2017;12:821–827. doi: 10.1016/j.redox.2017.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lopez-Otin C., Blasco M.A., Partridge L., Serrano M., Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu H., Toan S., Mui D., Zhou H. Mitochondrial quality surveillance as a therapeutic target in myocardial infarction. Acta Physiol. 2021;231(3) doi: 10.1111/apha.13590. [DOI] [PubMed] [Google Scholar]
- 15.Bravo-San Pedro J.M., Kroemer G., Galluzzi L. Autophagy and mitophagy in cardiovascular disease. Circ. Res. 2017;120(11):1812–1824. doi: 10.1161/CIRCRESAHA.117.311082. [DOI] [PubMed] [Google Scholar]
- 16.Ren J., Wu N.N., Wang S., Sowers J.R., Zhang Y. Obesity cardiomyopathy: evidence, mechanisms, and therapeutic implications. Physiol. Rev. 2021;101(4):1745–1807. doi: 10.1152/physrev.00030.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen G., Kroemer G., Kepp O. Mitophagy: an emerging role in aging and age-associated diseases. Front. Cell Dev. Biol. 2020;8:200. doi: 10.3389/fcell.2020.00200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gustafsson A.B., Dorn G.W. 2nd, evolving and expanding the roles of mitophagy as a homeostatic and pathogenic process. Physiol. Rev. 2019;99(1):853–892. doi: 10.1152/physrev.00005.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laker R.C., Drake J.C., Wilson R.J., Lira V.A., Lewellen B.M., Ryall K.A., Fisher C.C., Zhang M., Saucerman J.J., Goodyear L.J., Kundu M., Yan Z. Ampk phosphorylation of Ulk1 is required for targeting of mitochondria to lysosomes in exercise-induced mitophagy. Nat. Commun. 2017;8(1):548. doi: 10.1038/s41467-017-00520-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Munoz J.P., Zorzano A. FUNDC1: a novel protein in cardiac health. Circulation. 2017;136(23):2267–2270. doi: 10.1161/CIRCULATIONAHA.117.031417. [DOI] [PubMed] [Google Scholar]
- 21.Poole L.P., Macleod K.F. Mitophagy in tumorigenesis and metastasis. Cell. Mol. Life Sci. 2021;78(8):3817–3851. doi: 10.1007/s00018-021-03774-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu S., Lu Q., Ding Y., Wu Y., Qiu Y., Wang P., Mao X., Huang K., Xie Z., Zou M.H. Hyperglycemia-driven inhibition of AMP-activated protein kinase alpha2 induces diabetic cardiomyopathy by promoting mitochondria-associated endoplasmic reticulum membranes in vivo. Circulation. 2019;139(16):1913–1936. doi: 10.1161/CIRCULATIONAHA.118.033552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li W., Li Y., Siraj S., Jin H., Fan Y., Yang X., Huang X., Wang X., Wang J., Liu L., Du L., Chen Q. FUN14 domain-containing 1-mediated mitophagy suppresses hepatocarcinogenesis by inhibition of inflammasome activation in mice. Hepatology. 2019;69(2):604–621. doi: 10.1002/hep.30191. [DOI] [PubMed] [Google Scholar]
- 24.Wu H., Wang Y., Li W., Chen H., Du L., Liu D., Wang X., Xu T., Liu L., Chen Q. Deficiency of mitophagy receptor FUNDC1 impairs mitochondrial quality and aggravates dietary-induced obesity and metabolic syndrome. Autophagy. 2019;15(11):1882–1898. doi: 10.1080/15548627.2019.1596482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou H., Zhu P., Wang J., Zhu H., Ren J., Chen Y. Pathogenesis of cardiac ischemia reperfusion injury is associated with CK2alpha-disturbed mitochondrial homeostasis via suppression of FUNDC1-related mitophagy. Cell Death Differ. 2018;25(6):1080–1093. doi: 10.1038/s41418-018-0086-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou H., Zhu P., Guo J., Hu N., Wang S., Li D., Hu S., Ren J., Cao F., Chen Y. Ripk3 induces mitochondrial apoptosis via inhibition of FUNDC1 mitophagy in cardiac IR injury. Redox Biol. 2017;13:498–507. doi: 10.1016/j.redox.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin H., Zhu Y., Zheng C., Hu D., Ma S., Chen L., Wang Q., Chen Z., Xie J., Yan Y., Huang X., Liao W., Kitakaze M., Bin J., Liao Y. Antihypertrophic memory after regression of exercise-induced physiological myocardial hypertrophy is mediated by the long noncoding RNA Mhrt779. Circulation. 2021;143(23):2277–2292. doi: 10.1161/CIRCULATIONAHA.120.047000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DeBosch B., Treskov I., Lupu T.S., Weinheimer C., Kovacs A., Courtois M., Muslin A.J. Akt1 is required for physiological cardiac growth. Circulation. 2006;113(17):2097–2104. doi: 10.1161/CIRCULATIONAHA.105.595231. [DOI] [PubMed] [Google Scholar]
- 29.Choi S.K., Galan M., Kassan M., Partyka M., Trebak M., Matrougui K. Poly(ADP-ribose) polymerase 1 inhibition improves coronary arteriole function in type 2 diabetes mellitus. Hypertension. 2012;59(5):1060–1068. doi: 10.1161/HYPERTENSIONAHA.111.190140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xing W., Tan Y., Li K., Tian P., Tian F., Zhang H. Upregulated hepatokine fetuin B aggravates myocardial ischemia/reperfusion injury through inhibiting insulin signaling in diabetic mice. J. Mol. Cell. Cardiol. 2021;151:163–172. doi: 10.1016/j.yjmcc.2020.03.002. [DOI] [PubMed] [Google Scholar]
- 31.Makino A., Dai A., Han Y., Youssef K.D., Wang W., Donthamsetty R., Scott B.T., Wang H., Dillmann W.H. O-GlcNAcase overexpression reverses coronary endothelial cell dysfunction in type 1 diabetic mice. Am. J. Physiol. Cell Physiol. 2015;309(9):C593–C599. doi: 10.1152/ajpcell.00069.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oblong J.E., Bowman A., Rovito H.A., Jarrold B.B., Sherrill J.D., Black M.R., Nelson G., Kimball A.B., Birch-Machin M.A. Metabolic dysfunction in human skin: restoration of mitochondrial integrity and metabolic output by nicotinamide (niacinamide) in primary dermal fibroblasts from older aged donors. Aging Cell. 2020;19(10) doi: 10.1111/acel.13248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu L.H., Chang H.C., Ting P.C., Wang D.L. Laminar shear stress promotes mitochondrial homeostasis in endothelial cells. J. Cell. Physiol. 2018;233(6):5058–5069. doi: 10.1002/jcp.26375. [DOI] [PubMed] [Google Scholar]
- 34.North B.J., Sinclair D.A. The intersection between aging and cardiovascular disease. Circ. Res. 2012;110(8):1097–1108. doi: 10.1161/CIRCRESAHA.111.246876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thijssen D.H., Carter S.E., Green D.J. Arterial structure and function in vascular ageing: are you as old as your arteries? J. Physiol. 2016;594(8):2275–2284. doi: 10.1113/JP270597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ungvari Z., Tarantini S., Donato A.J., Galvan V., Csiszar A. Mechanisms of vascular aging. Circ. Res. 2018;123(7):849–867. doi: 10.1161/CIRCRESAHA.118.311378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Parry-Williams G., Gati S., Sharma S. The heart of the ageing endurance athlete: the role of chronic coronary stress. Eur. Heart J. 2021;42(28):2737–2744. doi: 10.1093/eurheartj/ehab095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fu T., Xu Z., Liu L., Guo Q., Wu H., Liang X., Zhou D., Xiao L., Liu L., Liu Y., Zhu M.S., Chen Q., Gan Z. Mitophagy directs muscle-adipose crosstalk to alleviate dietary obesity. Cell Rep. 2018;23(5):1357–1372. doi: 10.1016/j.celrep.2018.03.127. [DOI] [PubMed] [Google Scholar]
- 39.Pickrell A.M., Youle R.J. The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson's disease. Neuron. 2015;85(2):257–273. doi: 10.1016/j.neuron.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shirakabe A., Ikeda Y., Sciarretta S., Zablocki D.K., Sadoshima J. Aging and autophagy in the heart. Circ. Res. 2016;118(10):1563–1576. doi: 10.1161/CIRCRESAHA.116.307474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zha Z., Wang J., Wang X., Lu M., Guo Y. Involvement of PINK1/Parkin-mediated mitophagy in AGE-induced cardiomyocyte aging. Int. J. Cardiol. 2017;227:201–208. doi: 10.1016/j.ijcard.2016.11.161. [DOI] [PubMed] [Google Scholar]
- 42.Davidson S.M., Duchen M.R. Endothelial mitochondria: contributing to vascular function and disease. Circ. Res. 2007;100(8):1128–1141. doi: 10.1161/01.RES.0000261970.18328.1d. [DOI] [PubMed] [Google Scholar]
- 43.Diebold L.P., Gil H.J., Gao P., Martinez C.A., Weinberg S.E., Chandel N.S. Mitochondrial complex III is necessary for endothelial cell proliferation during angiogenesis. Nat Metab. 2019;1(1):158–171. doi: 10.1038/s42255-018-0011-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Farber G., Parks M.M., Lustgarten Guahmich N., Zhang Y., Monette S., Blanchard S.C., Di Lorenzo A., Blobel C.P. ADAM10 controls the differentiation of the coronary arterial endothelium. Angiogenesis. 2019;22(2):237–250. doi: 10.1007/s10456-018-9653-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li Y., Xue Y., Xu X., Wang G., Liu Y., Wu H., Li W., Wang Y., Chen Z., Zhang W., Zhu Y., Ji W., Xu T., Liu L., Chen Q. A mitochondrial FUNDC1/HSC70 interaction organizes the proteostatic stress response at the risk of cell morbidity. EMBO J. 2019;38(3) doi: 10.15252/embj.201798786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Park S.Y., Kwon O.S., Andtbacka R.H.I., Hyngstrom J.R., Reese V., Murphy M.P., Richardson R.S. Age-related endothelial dysfunction in human skeletal muscle feed arteries: the role of free radicals derived from mitochondria in the vasculature. Acta Physiol. 2018;222(1) doi: 10.1111/apha.12893. [DOI] [PubMed] [Google Scholar]
- 47.Su L., Zhang J., Gomez H., Kellum J.A., Peng Z. Mitochondria ROS and mitophagy in acute kidney injury. Autophagy. 2023;19(2):401–414. doi: 10.1080/15548627.2022.2084862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rottenberg H., Hoek J.B. The path from mitochondrial ROS to aging runs through the mitochondrial permeability transition pore. Aging Cell. 2017;16(5):943–955. doi: 10.1111/acel.12650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang C., Dai X., Wu S., Xu W., Song P., Huang K. FUNDC1-dependent mitochondria-associated endoplasmic reticulum membranes are involved in angiogenesis and neoangiogenesis. Nat. Commun. 2021;12(1):2616. doi: 10.1038/s41467-021-22771-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jia G., Aroor A.R., Jia C., Sowers J.R. Endothelial cell senescence in aging-related vascular dysfunction. Biochim. Biophys. Acta, Mol. Basis Dis. 2019;1865(7):1802–1809. doi: 10.1016/j.bbadis.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 51.Yang Y.F., Yang W., Liao Z.Y., Wu Y.X., Fan Z., Guo A., Yu J., Chen Q.N., Wu J.H., Zhou J., Xiao Q. MICU3 regulates mitochondrial Ca(2+)-dependent antioxidant response in skeletal muscle aging. Cell Death Dis. 2021;12(12):1115. doi: 10.1038/s41419-021-04400-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vatner S.F., Zhang J., Oydanich M., Berkman T., Naftalovich R., Vatner D.E. Healthful aging mediated by inhibition of oxidative stress. Ageing Res. Rev. 2020;64 doi: 10.1016/j.arr.2020.101194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tinken T.M., Thijssen D.H., Hopkins N., Dawson E.A., Cable N.T., Green D.J. Shear stress mediates endothelial adaptations to exercise training in humans. Hypertension. 2010;55(2):312–318. doi: 10.1161/HYPERTENSIONAHA.109.146282. [DOI] [PubMed] [Google Scholar]
- 54.Hou Z., Qin X., Hu Y., Zhang X., Li G., Wu J., Li J., Sha J., Chen J., Xia J., Wang L., Gao F. Longterm exercise-derived exosomal miR-342-5p: a novel exerkine for cardioprotection. Circ. Res. 2019;124(9):1386–1400. doi: 10.1161/CIRCRESAHA.118.314635. [DOI] [PubMed] [Google Scholar]
- 55.Kim J.S., Kim B., Lee H., Thakkar S., Babbitt D.M., Eguchi S., Brown M.D., Park J.Y. Shear stress-induced mitochondrial biogenesis decreases the release of microparticles from endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2015;309(3):H425–H433. doi: 10.1152/ajpheart.00438.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Souilhol C., Serbanovic-Canic J., Fragiadaki M., Chico T.J., Ridger V., Roddie H., Evans P.C. Endothelial responses to shear stress in atherosclerosis: a novel role for developmental genes. Nat. Rev. Cardiol. 2020;17(1):52–63. doi: 10.1038/s41569-019-0239-5. [DOI] [PubMed] [Google Scholar]
- 57.Liu W., Song H., Xu J., Guo Y., Zhang C., Yao Y., Zhang H., Liu Z., Li Y.C. Low shear stress inhibits endothelial mitophagy via caveolin-1/miR-7-5p/SQSTM1 signaling pathway. Atherosclerosis. 2022;356:9–17. doi: 10.1016/j.atherosclerosis.2022.07.014. [DOI] [PubMed] [Google Scholar]
- 58.Shin J., Hong S.G., Choi S.Y., Rath M.E., Saredy J., Jovin D.G., Sayoc J., Park H.S., Eguchi S., Rizzo V., Scalia R., Wang H., Houser S.R., Park J.Y. Flow-induced endothelial mitochondrial remodeling mitigates mitochondrial reactive oxygen species production and promotes mitochondrial DNA integrity in a p53-dependent manner. Redox Biol. 2022;50 doi: 10.1016/j.redox.2022.102252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu Y., Zhu Y., Rannou F., Lee T.S., Formentin K., Zeng L., Yuan X., Wang N., Chien S., Forman B.M., Shyy J.Y. Laminar flow activates peroxisome proliferator-activated receptor-gamma in vascular endothelial cells. Circulation. 2004;110(9):1128–1133. doi: 10.1161/01.CIR.0000139850.08365.EC. [DOI] [PubMed] [Google Scholar]
- 60.Donato A.J., Machin D.R., Lesniewski L.A. Mechanisms of dysfunction in the aging vasculature and role in age-related disease. Circ. Res. 2018;123(7):825–848. doi: 10.1161/CIRCRESAHA.118.312563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Herrera M.D., Mingorance C., Rodriguez-Rodriguez R., Alvarez de Sotomayor M. Endothelial dysfunction and aging: an update. Ageing Res. Rev. 2010;9(2):142–152. doi: 10.1016/j.arr.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 62.Piercy K.L., Troiano R.P., Ballard R.M., Carlson S.A., Fulton J.E., Galuska D.A., George S.M., Olson R.D. The physical activity guidelines for Americans. JAMA. 2018;320(19):2020–2028. doi: 10.1001/jama.2018.14854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Das A., Huang G.X., Bonkowski M.S., Longchamp A., Li C., Schultz M.B., Kim L.J., Osborne B., Joshi S., Lu Y., Trevino-Villarreal J.H., Kang M.J., Hung T.T., Lee B., Williams E.O., Igarashi M., Mitchell J.R., Wu L.E., Turner N., Arany Z., Guarente L., Sinclair D.A. Impairment of an endothelial NAD(+)-H2S signaling network is a reversible cause of vascular aging. Cell. 2018;173(1):74–89 e20. doi: 10.1016/j.cell.2018.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Letnes J.M., Berglund I., Johnson K.E., Dalen H., Nes B.M., Lydersen S., Viken H., Hassel E., Steinshamn S., Vesterbekkmo E.K., Stoylen A., Reitlo L.S., Zisko N., Baekkerud F.H., Tari A.R., Ingebrigtsen J.E., Sandbakk S.B., Carlsen T., Anderssen S.A., Singh M.A.F., Coombes J.S., Helbostad J.L., Rognmo O., Wisloff U., Stensvold D. Eur Heart J; 2021. Effect of 5 Years of Exercise Training on the Cardiovascular Risk Profile of Older Adults: the Generation 100 Randomized Trial. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Woodman C.R., Seawright J.W., Luttrell M.J., Shin S.Y., Trache A. Importance of mechanical signals in promoting exercise-induced improvements in vasomotor function of aged skeletal muscle resistance arteries. Am. J. Physiol. Heart Circ. Physiol. 2018;315(3):H602–H609. doi: 10.1152/ajpheart.00732.2017. [DOI] [PubMed] [Google Scholar]
- 66.Rossman M.J., Kaplon R.E., Hill S.D., McNamara M.N., Santos-Parker J.R., Pierce G.L., Seals D.R., Donato A.J. Endothelial cell senescence with aging in healthy humans: prevention by habitual exercise and relation to vascular endothelial function. Am. J. Physiol. Heart Circ. Physiol. 2017;313(5):H890–H895. doi: 10.1152/ajpheart.00416.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Koller A., Laughlin M.H., Cenko E., de Wit C., Toth K., Bugiardini R., Trifunovits D., Vavlukis M., Manfrini O., Lelbach A., Dornyei G., Padro T., Badimon L., Tousoulis D., Gielen S., Duncker D.J. Functional and structural adaptations of the coronary macro- and micro-vasculature to regular aerobic exercise by activation of physiological, cellular and molecular mechanisms: esc Working Group on Coronary Pathophysiology & Microcirculation Position Paper. Cardiovasc. Res. 2022;118(2):357–371. doi: 10.1093/cvr/cvab246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fujie S., Hasegawa N., Horii N., Uchida M., Sanada K., Hamaoka T., Padilla J., Martinez-Lemus L.A., Maeda S., Iemitsu M. Aerobic exercise restores aging-associated reductions in arterial adropin levels and improves adropin-induced nitric oxide-dependent vasorelaxation. J. Am. Heart Assoc. 2021;10(10) doi: 10.1161/JAHA.120.020641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xing W., Li Y., Zhang H., Mi C., Hou Z., Quon M.J., Gao F. Improvement of vascular insulin sensitivity by downregulation of GRK2 mediates exercise-induced alleviation of hypertension in spontaneously hypertensive rats. Am. J. Physiol. Heart Circ. Physiol. 2013;305(8):H1111–H1119. doi: 10.1152/ajpheart.00290.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Heitzer T., Schlinzig T., Krohn K., Meinertz T., Munzel T. Endothelial dysfunction, oxidative stress, and risk of cardiovascular events in patients with coronary artery disease. Circulation. 2001;104(22):2673–2678. doi: 10.1161/hc4601.099485. [DOI] [PubMed] [Google Scholar]
- 71.Dal Lin C., Tona F., Osto E. Coronary microvascular function and beyond: the crosstalk between hormones, cytokines, and neurotransmitters. Internet J. Endocrinol. 2015;2015 doi: 10.1155/2015/312848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chang X., Lochner A., Wang H.H., Wang S., Zhu H., Ren J., Zhou H. Coronary microvascular injury in myocardial infarction: perception and knowledge for mitochondrial quality control. Theranostics. 2021;11(14):6766–6785. doi: 10.7150/thno.60143. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.