Abstract
Dietary guanidinoacetic acid (GAA) has been shown to affect creatine (Cr) metabolic pathways resulting in increased cellular Cr and hitherto broiler performances. Yet, the impact of dietary GAA on improving markers of oxidative status remains equivocal. A model of chronic cyclic heat stress, known to inflict oxidative stress, was employed to test the hypothesis that GAA could modify bird's oxidative status. A total of 720-day-old male Ross 308 broilers were allocated to 3 treatments: 0, 0.6 or 1.2 g/kg GAA was added to corn-SBM diets and fed for 39 d, with 12 replicates (20 birds each) per treatment. The chronic cyclic heat stress model (34°C with 50–60% RH for 7 h daily) was applied in the finisher phase (d 25–39). Samples from 1 bird per pen were taken on d 26 (acute heat stress) and d 39 (chronic heat stress). GAA and Cr in plasma were linearly increased by feeding GAA on either sampling day, illustrating efficient absorption and methylation, respectively. Energy metabolism in breast and heart muscle was greatly supported as visible by increased Cr and phosphocreatine: ATP, thus providing higher capacity for rapid ATP generation in cells. Glycogen stores in breast muscle were linearly elevated by incremental GAA, on d 26 only. More Cr seems to be directed to heart muscle as opposed to skeletal muscle during chronic heat stress as tissue Cr was higher in heart but lower in breast muscle on d 39 as opposed to d 26. The lipid peroxidation marker malondialdehyde, and the antioxidant enzymes superoxide dismutase and glutathione peroxidase showed no alterations by dietary GAA in plasma. Opposite to that, superoxide dismutase activity in breast muscle was linearly lowered when feeding GAA (trend on d 26, effect on d 39). Significant correlations between the assessed parameters and GAA inclusion were identified on d 26 and d 39 using principal component analysis. To conclude, beneficial performance in heat-stressed broilers by GAA is associated with enhanced muscle energy metabolism which indirectly may also support tolerance against oxidative stress.
Key words: broiler, creatine, guanidinoacetic acid, heat stress, oxidative stress
INTRODUCTION
Dietary guanidinoacetic acid (GAA) has been shown to affect creatine (Cr) metabolic pathways resulting in increased cellular Cr levels and hitherto performance in various animal species (e.g., in broilers: Lemme et al., 2007; Michiels et al., 2012; DeGroot et al., 2019; Majdeddin et al., 2020). Elevated Cr in the muscle cell provokes increased synthesis of phosphocreatine which is then diffusing to sites of ATP usage (Brosnan and Brosnan, 2010; Guimarães-Ferreira, 2014), for immediate rephosphorylation of ADP back to ATP (Supplementary Figure 1). Thus, in most studies showing improvements of bird performance by supplementing GAA to the diet, these effects were ascribed in part to increased physiological levels of Cr which benefits cellular energy status and, as a consequence, protein synthesis directly and indirectly, for example, through Arg sparing (Khajali et al., 2020; Dao and Swick, 2021; Portocarero and Braun, 2021). However, the performance supporting effects of elevated physiological Cr levels may also be attributed to other well described functions, such as the attenuation of acute stress responses by quenching superoxide anions and other aqueous reactive species (Lawler et al., 2002; Deminice and Jordao, 2012). It is well known that excessive production of reactive species can cause cellular damage resulting from lipid peroxidation, protein oxidation and DNA modification. Sestili et al. (2006) showed in cultured mammalian cells exposed to various oxidizing agents that Cr, at concentrations comparable to those attainable in plasma upon oral supplementation in human, exerted direct antioxidant activity. Additional investigations showed protective effects of Cr exposure on oxidatively injured mitochondrial DNA (Guidi et al., 2008) and against RNA-damaging agents (Fimognari et al., 2009). Similar reports are available in vivo; for example, in rats, it was reported that short-term Cr supplementation (5 g/d for 6 d) decreases reactive oxygen species content in skeletal muscle, possibly due to the direct action of Cr on scavenging superoxide radicals (Guimarães-Ferreira, 2014). Cr may also exert antioxidant outcomes through its primary action on cellular energy status. The putative benefits of Cr in a number of muscular, neurological, and cardiovascular diseases have been generally attributed and not surprisingly to the Cr-induced buffering of cellular ATP levels, whose fall would lead to the accumulation of intracellular Ca2+, and stimulation of formation of reactive species leading to tissue oxidative damage (Persky and Brazeau, 2001). Contrary, Zugno et al. (2006, 2008) showed that GAA administration to the rat brain led to a decrease of the nonenzymatic antioxidant capacity likely due to oxidation of sulfhydryl groups, leading to lower glutathione (GSH) levels, a major endogenous intracellular antioxidant. Furthermore, Aksentijevic et al. (2014) questioned any physiologically relevant antioxidant activity of Cr in oxidatively challenged mice heart and guanidine compounds, like GAA, were even discussed to induce the formation of free radicals (Hiramatsu, 2003). It is thus equivocal what the effect would be of dietary GAA on the organism when imposed to an oxidative stress challenge. Regarding poultry, limited evidence shows positives responses of the antioxidant system to GAA in normal bird rearing (Wang et al., 2016; Zhao et al., 2021) and challenge models (Nasirolesami et al., 2018, cold stress; Amiri et al., 2019, heat stress; Khalil et al., 2021, T3-hormone challenge). Therefore, in the current study, we applied a heat challenge to birds in order to induce oxidative stress and to investigate the interaction with GAA feeding and Cr loading. Indeed, the involvement of heat stress as an inducer of oxidative stress has been widely acknowledged (Akbarian et al., 2016). In our previous report we presented the beneficial effects of GAA on performance and mortality during heat stress (Majdeddin et al., 2020). In the current report, we investigated whether the identified performance benefits are due to a protective effect of GAA related to mitochondrial function and consequently the antioxidant status in tissues.
Altogether, the purpose of this study was to evaluate the effect of GAA supplementation to broilers subjected to acute and chronic heat stress during the finisher period, on different vital organs’ oxidative status, such as plasma, breast muscle, heart, liver, and kidney, and as well to deepen the insights in GAA and Cr metabolism in key organs. Broilers were fed increasing levels of GAA and organs were sampled on the 2nd and 14th day of heat stress (i.e., d 26 and 39 of age to reflect acute and chronic stress, respectively). Data were subjected to analysis of variance, and additionally data were used in a principal component analysis (PCA) to explore the correlation structure across variables.
MATERIALS AND METHODS
Animals, Housing, and Diets
Samples were taken from the same sampled broilers reported by Majdeddin et al. (2020). Hence, experimental procedures including heat stress protocol and treatments were accordingly. In brief, 720 male Ross 308 broilers were allocated to 3 treatments: 0, 0.6 or 1.2 g/kg GAA added to corn-SBM diets and fed for 39 d, with 12 replicates (20 birds each). A chronic cyclic heat stress model (T increase to 34°C with 50–60% RH for 7 h daily) was applied in the finisher phase (d 25–39). Heat stress was confirmed by high rectal temperatures and highly elevated panting frequency (Majdeddin et al., 2020).
Sampling During Acute and Chronic Heat Stress
On d 26 (acute heat stress) and d 39 (chronic heat stress), 1 animal per pen with weight close to average weight of the pen was selected, leading to 12 replicates per sampling day. It is important to notify that sampling started >4 h after inducing heat stress on that day, which means that broilers experienced heat stress at the moment of sampling. The latter was verified by measuring rectal T and was reported in Majdeddin et al. (2020). After euthanasia as described in Majdeddin et al. (2020), blood was taken by puncture from heart and plasma was harvested from K2EDTA tubes after centrifugation (3,000 × g, 15 min) and stored at −80°C pending analysis. Subsequently, the animal was bled and immediately, within seconds, the skin was removed from the breast, and a 2 × 2 × 0.5 cm3 (length × width × depth) flat piece of the right breast (pectoralis major), middle of right breast in length and 2 cm away from median plane of the body, was transferred to liquid N2. Snap frozen tissues were broken, collected in precooled cryovials and submerged in liquid N2 and stored at −80°C pending analysis. Next, the abdomen was opened, and the heart, liver, and kidney were collected. Ventricular myocardium was dissected from the heart and adhering fat and large vessels were removed. The middle part of the right lob of the liver was sampled. Heart and liver tissue samples were submerged in liquid N2 and stored at −80°C pending analysis. In case of kidney, the complete organ was sampled, wrapped in aluminum foil to preserve integrity, and snap frozen in liquid N2 and stored at −80°C pending analysis. All samples were rinsed with saline prior to processing and freezing.
Protein and Amino Acids in Plasma
GAA, homocysteine, and amino acids in plasma were determined by means of LC-MS/MS (Majdeddin et al., 2020). Sample preparation included protein precipitation and addition of stable isotopic labeled internal standards prior to analysis. Cr was quantified photometrically according to the Barrit reaction. Creatinine was analyzed photometrically using the Jaffé reaction.
Energy Metabolites in Breast Muscle, Heart, Liver, and Kidney
ATP, phosphocreatine, free Cr, and glycogen were determined as described by DeGroot et al. (2018, 2019) in freeze dried breast muscle biopsies (Swiss BioQuant AG, Reinach, Switzerland). The analytical method was based on enzymatic determinations, which ultimately resulted in either reduction of NADP to NADPH (for ATP and phosphocreatine) or oxidation of NADH to NAD (for free Cr). Final data included the concentration of each energy and Cr-related metabolite (ATP, phosphocreatine, free Cr, and glycogen) and are reported on wet tissue basis. In our previous report (Majdeddin et al., 2020), these metabolites were expressed as molar concentrations to dry tissue weight of breast muscle. In the current paper, the concentrations were converted to weight concentrations on wet tissue basis to make it comparable to other tissues (heart, liver, and kidney). The conversion of concentrations on dry matter basis to wet tissue basis was based on a dry matter content of 22.7% in breast muscle tissue for all samples. This number was taken from an assessment of dry matter in breast muscle tissue in few of these chickens. In addition, total Cr (combined phosphocreatine and free Cr) and the phosphocreatine:ATP ratio were calculated. As muscle ATP is an indicator for the muscle tissue content in the sample, the phosphocreatine:ATP ratio is more appropriate than phosphocreatine values since it cancels out variance due to differences in biopsy samples in their contents of blood and connective tissue (Harris et al., 1992). Similarly, the analysis of Cr in heart was performed in freeze dried samples, and a dry matter content of 23.0% was taken for conversion (DeGroot et al., 2018, 2019; Swiss BioQuant AG, Reinach, Switzerland). The analysis of GAA, Cr, and creatinine in liver and kidney was performed in original substance by separation using reverse phase chromatography followed by detection with triple stage quadrupole MS/MS in the selected monitoring mode (Khalil et al., 2021; Swiss BioQuant AG, Reinach, Switzerland). Creatine kinase, lactate, cholesterol, triglycerides, and glucose in plasma were assayed using an AU 5800 Beckman Coulter.
Oxidative Status Parameters
Malonaldehyde (MDA), glutathione peroxidase (GPx) activity, and SOD activity were determined in plasma, breast muscle, heart, liver, and kidney samples. After thawing of organ samples, samples were kept on ice during the procedure. A 5 g subsample of breast muscle and a 1 g subsample of heart, liver, and kidney were homogenized with Turax T2S in 10 mL of 0.05 mol/L ice-cold phosphate buffer (pH = 7.0) during 45 s and centrifuged at 4°C for 15 min at 10,000 × g. The supernatant fraction was filtered through glass wool before determining MDA and enzyme activities. The thiobarbituric acid reactive substances (TBARS) method was used to quantify MDA as a marker for lipid peroxidation (Grotto et al., 2007). MDA was allowed to react with 2-thiobarbituric acid in an acid environment. Absorbance of the colored complex was measured at 532 nm after 1-butanol extraction. A standard curve with 1,1,3,3-tetramethoxypropane was used. The activity of GPx was determined by measuring the oxidation of NADPH according to Hernandez et al. (2004). One unit of GPx activity was defined as the amount of plasma or extract needed to oxidize 1 μmol of NADPH per min at 25°C. The SOD activity assay for breast muscle, heart, liver and kidney was performed as described by Marklund and Marklund (1974) by measuring the inhibition of pyrogallol autoxidation. One unit of enzyme activity was defined as the amount of extract needed to inhibit the rate of oxidation by the control (no SOD) by 50%. SOD in plasma was determined using the commercial SOD assay kit according to the manufacturer's instructions (19160 SOD determination kit, Sigma-Aldrich, St. Louis, MO).
Calculations and Statistical Analysis
Statistical analytical techniques appropriate to a completely randomized design were used (SAS Enterprise Guide 7, SAS Institute, Cary, NC). As 1 individual animal per pen was taken, the bird was considered the experimental unit. Boxplots were constructed to detect outliers and hence removed from the dataset. Further, data were checked for normality by Kolmogorov-Smirnov and Shapiro-Wilk tests and for homogeneity of variances by Levene's test. Analysis was run for each sampling day separately. All parameters were analyzed by 1-way ANOVA or the nonparametric Kruskall-Wallis test if normality was violated, with GAA supplementation being the fixed effect. Linear and quadratic contrasts were included as well. Post hoc Tukey test was used to compare means. Level of P < 0.05 was considered significant, 0.05 < P < 0.10 was considered a trend.
Initially it was aimed to deduce correlations between all physiological variables by employing a PCA. However, as minimal treatment effects on oxidative status parameters were found and because of the absence of correlations between variables representing oxidative status on the one side and Cr and energy metabolism on the other side during a preliminary PCA, it was decided to restrict the PCA to variables of Cr and energy metabolism in all tissues. Hence, 21 variables were included, and the PCA was carried out for each sampling day separately. The methodology as outlined by Montagne et al. (2007) and Michiels et al. (2013) was applied. Briefly, the data of continuous variables were standardized, that is, the data were diminished by the mean and divided by the standard deviation of that variable. A first PCA, including a scree plot was done to determine the number of principal components to be retained. Five principal components at both d 26 and d 39 appeared before a clear break where the eigenvalues leveled off. The eigenvalues of these 5 components were greater than 1.6. Then, variables that did not load on any principal component retained (correlation coefficient between variable and principal components ≤0.4) were excluded. This was the case for ATP in breast muscle on d 26 and for creatine in heart on d 39 only, resulting in 20 variables on d 26 and d 39 finally included in the analysis. Only loadings ≥|0.4| are shown in the results, and finally a 1-way ANOVA was done on scores for all principal components for the broilers. Here, all calculations were carried out using the IBM SPSS Statistics Version 24.0 program for Windows (SPSS Inc., Chicago, IL).
RESULTS
Creatine Metabolism, Energy Metabolites, and Antioxidant Enzyme Activities in Plasma
GAA and total Cr in plasma were highly and linearly increased by feeding GAA to broilers on both sampling days (all P < 0.001, Table 1). Creatinine was below the detection limit in all samples and timepoints. Interestingly, while both GAA and Cr for control birds did not differ between sampling days, the elevation of both metabolites by feeding GAA was smaller on d 39 vs. d 26. Homocysteine was not affected, thus ruling out hyperhomocysteinemia by feeding GAA at any timepoint. Next, linear increases in plasma Arg with supplemental GAA were seen on d 26 (P = 0.009), which was different from control in 1.2 g/kg GAA-fed broilers (P < 0.05), while only a trend for linear increase could be perceived for d 39 (P = 0.070). A linear reduction in plasma glucose by feeding GAA was noticed on d 26 (P = 0.041), but not on d 39. Also, a linear decrease in plasma cholesterol was found by feeding GAA (P = 0.048 on d 26, P = 0.055 on d 39). Neither creatine kinase activity, lactate, cholesterol, or triglycerides were different across treatments on any day. Also, no alterations were detected for either the lipid peroxidation marker MDA, or the antioxidant enzymes SOD and GPx by dietary GAA.
Table 1.
Effect of guanidinoacetic acid (GAA) supplementation on plasma creatine metabolism, energy metabolites, malondialdehyde (MDA) concentration, and antioxidant enzyme activities (superoxide dismutase, SOD, and glutathione peroxidase, GPx) of male broilers subjected to heat stress in the finisher phase at d 26 and 391 (n = 12).
| Item | Dietary treatment2 |
SEM |
P value |
||||
|---|---|---|---|---|---|---|---|
| Supplemental GAA, g/kg | 0.0 | 0.6 | 1.2 | Model | Linear | Quadratic | |
| Guanidinoacetic acid, μmol/L | |||||||
| d 26 | 0.5c | 6.3b | 14.3a | 1.15 | <0.001 | <0.001 | 0.458 |
| d 39 | 0.5c | 3.8b | 7.4a | 0.50 | <0.001 | <0.001 | 0.620 |
| Total creatine, μmol/L | |||||||
| d 26 | 78b | 127ab | 170a | 11.3 | 0.002 | <0.001 | 0.906 |
| d 39 | 72b | 84b | 121a | 5.0 | <0.001 | <0.001 | 0.122 |
| Homocysteine, μmol/L | |||||||
| d 26 | 57 | 57 | 61 | 1.7 | 0.558 | 0.324 | 0.688 |
| d 39 | 48 | 45 | 50 | 1.5 | 0.427 | 0.661 | 0.222 |
| Arginine, μmol/L | |||||||
| d 26 | 263b | 311ab | 344a | 12.8 | 0.029 | 0.009 | 0.752 |
| d 39 | 226 | 271 | 302 | 16.9 | 0.185 | 0.070 | 0.847 |
| Creatine kinase, U/mL | |||||||
| d 26 | 17 | 16 | 13 | 1.5 | 0.543 | 0.277 | 0.878 |
| d 39 | 20 | 17 | 25 | 1.8 | 0.229 | 0.268 | 0.187 |
| Lactate, mmol/L | |||||||
| d 26 | 3.1 | 3.4 | 3.3 | 0.33 | 0.943 | 0.817 | 0.803 |
| d 39 | 2.2 | 2.0 | 2.0 | 0.12 | 0.720 | 0.477 | 0.704 |
| Cholesterol, mmol/L | |||||||
| d 26 | 3.9 | 3.8 | 3.6 | 0.07 | 0.111 | 0.048 | 0.498 |
| d 39 | 3.9 | 3.5 | 3.4 | 0.10 | 0.116 | 0.055 | 0.421 |
| Triglycerides, mmol/L | |||||||
| d 26 | 1.6 | 1.3 | 1.7 | 0.08 | 0.108 | 0.745 | 0.038 |
| d 39 | 1.0 | 1.2 | 1.0 | 0.09 | 0.708 | 0.923 | 0.413 |
| Glucose, mmol/L | |||||||
| d 26 | 18 | 18 | 16 | 0.4 | 0.060 | 0.041 | 0.219 |
| d 39 | 17 | 17 | 16 | 0.2 | 0.133 | 0.360 | 0.073 |
| MDA, μmol/L | |||||||
| d 26 | 14 | 14 | 13 | 0.2 | 0.261 | 0.225 | 0.267 |
| d 39 | 14 | 14 | 13 | 0.2 | 0.876 | 0.615 | 0.927 |
| SOD, U/mL | |||||||
| d 26 | 42 | 41 | 49 | 4.4 | 0.739 | 0.510 | 0.689 |
| d 39 | 49 | 72 | 67 | 5.1 | 0.472 | 0.378 | 0.353 |
| GPx, U/mL | |||||||
| d 26 | 1.4 | 1.6 | 1.4 | 0.05 | 0.318 | 0.970 | 0.133 |
| d 39 | 1.3 | 1.2 | 1.2 | 0.03 | 0.256 | 0.187 | 0.318 |
Broilers were fed a corn-soybean starter diet from d 0 to 10, a grower diet from d 10 to 25, and a finisher diet from d 25 to 39.
Values with different superscripts (a–c) within a row are significantly different at P < 0.05.
Creatine Metabolism, Energy Metabolites, and Antioxidant Enzyme Activities in Breast Muscle, Heart, Liver, and Kidney
Breast muscle samples revealed at both sampling days increases of phosphocreatine (P = 0.029, linear effect at d 26; P = 0.005, linear effect at d 39), free Cr (P = 0.056, linear trend at d 26, P < 0.001 at d 39), total Cr (P < 0.001, both days), and, phosphocreatine:ATP (P = 0.033, linear effect at d 26; P = 0.002, linear effect at d 39) with increasing dietary GAA (Table 2). For instance, phosphocreatine:ATP in 1.2 g/kg GAA-fed broilers was 27 and 23% higher than control on d 26 and d 39, respectively, underlining a higher energy load by dietary GAA inclusion. On d 26, muscle energy reserve as glycogen also linearly increased by GAA (P = 0.006), but no increase was detected on d 39. Interestingly, muscle total Cr levels appeared lower on d 39 as compared to d 26. Strikingly, the opposite was observed for phosphocreatine and hence phosphocreatine:ATP, leading to a higher proportion of PCr in total creatine (PCr/TCr) on d 39. Regarding markers of oxidative status in breast muscle only SOD activity was affected by feeding GAA, that is, it was linearly reduced on d 26 (trend, P = 0.057) and on d 39 (P = 0.015).
Table 2.
Effect of guanidinoacetic acid (GAA) supplementation on breast muscle creatine metabolism, energy metabolites, malondialdehyde (MDA) concentration, and antioxidant enzyme activities (superoxide dismutase, SOD, and glutathione peroxidase, GPx) of male broilers subjected to heat stress in the finisher phase at d 26 and 391 (n = 12).
| Item Supplemental GAA, g/kg | Dietary treatment2 |
SEM |
P value |
||||
|---|---|---|---|---|---|---|---|
| 0.0 | 0.6 | 1.2 | Model | Linear | Quadratic | ||
| ATP, μmol/g | |||||||
| d 26 | 5.9 | 6.1 | 5.9 | 0.14 | 0.703 | 0.898 | 0.410 |
| d 39 | 6.6 | 6.6 | 6.4 | 0.11 | 0.472 | 0.387 | 0.388 |
| Phosphocreatine, mg/kg | |||||||
| d 26 | 2351 | 2639 | 3119 | 143.9 | 0.085 | 0.029 | 0.784 |
| d 39 | 4078b | 4558ab | 4750a | 105.6 | 0.016 | 0.005 | 0.532 |
| Free creatine, mg/kg | |||||||
| d 26 | 3308 | 3815 | 3845 | 116.2 | 0.097 | 0.056 | 0.310 |
| d 39 | 1341b | 1699a | 1818a | 56.6 | 0.001 | <0.001 | 0.245 |
| Total creatine, mg/kg | |||||||
| d 26 | 4769b | 5454a | 5782a | 116.2 | <0.001 | <0.001 | 0.357 |
| d 39 | 3875b | 4500a | 4769a | 101.3 | <0.001 | <0.001 | 0.274 |
| Phosphocreatine/ATP, μmol/μmol | |||||||
| d 26 | 1.9 | 2.1 | 2.6 | 0.13 | 0.080 | 0.033 | 0.488 |
| d 39 | 3.0b | 3.3ab | 3.7a | 0.09 | 0.008 | 0.002 | 0.785 |
| Glycogen, μmol/g | |||||||
| d 26 | 239b | 291ab | 306a | 10.2 | 0.016 | 0.006 | 0.373 |
| d 39 | 277 | 305 | 305 | 7.1 | 0.172 | 0.102 | 0.353 |
| MDA, nmol/g | |||||||
| d 26 | 7.1 | 5.9 | 7.0 | 0.40 | 0.433 | 0.920 | 0.202 |
| d 39 | 7.0 | 6.9 | 7.8 | 0.36 | 0.561 | 0.384 | 0.517 |
| SOD, U/g | |||||||
| d 26 | 29 | 28 | 27 | 0.5 | 0.156 | 0.057 | 0.803 |
| d 39 | 30 | 28 | 26 | 0.6 | 0.049 | 0.015 | 0.781 |
| GPx, U/g | |||||||
| d 26 | 0.36 | 0.33 | 0.35 | 0.01 | 0.608 | 0.688 | 0.365 |
| d 39 | 0.52 | 0.51 | 0.50 | 0.01 | 0.837 | 0.562 | 0.906 |
Broilers were fed a corn-soybean starter diet from d 0 to 10, a grower diet from d 10 to 25, and a finisher diet from d 25 to 39.
Mean values with different superscripts (a, b) per sampling day are significantly different at P < 0.05.
A linear increase in Cr in myocardium on both sampling days upon incremental GAA in the diet was noticed (P = 0.003 and P = 0.029, on d 26 and d 39, respectively; Table 3). Contrary to breast muscle, heart Cr showed higher levels on d 39 as compared to d 26. Heart tissue did not reveal any treatment effect on antioxidant enzyme activities.
Table 3.
Effect of guanidinoacetic acid (GAA) supplementation on heart malondialdehyde (MDA) concentration and antioxidant enzyme activities (superoxide dismutase, SOD, and glutathione peroxidase, GPx) of male broilers subjected to heat stress in the finisher phase at d 26 and 391 (n = 12).
| Item | Dietary treatment 2 |
SEM |
P value |
||||
|---|---|---|---|---|---|---|---|
| Supplemental GAA, g/kg | 0.0 | 0.6 | 1.2 | Model | Linear | Quadratic | |
| Total creatine, mg/kg | |||||||
| d 26 | 676b | 795ab | 953a | 39.3 | 0.011 | 0.003 | 0.801 |
| d 39 | 960 | 1120 | 1209 | 46.2 | 0.084 | 0.029 | 0.706 |
| MDA, nmol/g | |||||||
| d 26 | 85 | 84 | 103 | 5.6 | 0.317 | 0.209 | 0.397 |
| d 39 | 73 | 72 | 71 | 3.2 | 0.972 | 0.813 | 0.992 |
| SOD, U/g | |||||||
| d 26 | 52 | 52 | 49 | 3.0 | 0.872 | 0.658 | 0.787 |
| d 39 | 56 | 52 | 59 | 1.8 | 0.285 | 0.540 | 0.145 |
| GPx, U/g | |||||||
| d 26 | 1.2 | 1.2 | 1.2 | 0.03 | 0.692 | 0.651 | 0.470 |
| d 39 | 1.3 | 1.2 | 1.3 | 0.04 | 0.687 | 0.640 | 0.469 |
Broilers were fed a corn-soybean starter diet from d 0 to 10, a grower diet from d 10 to 25, and a finisher diet from d 25 to 39.
Mean values with different superscripts (a, b) per sampling day are significantly different at P < 0.05.
In liver and kidney, levels of Cr and metabolites were substantially lower as compared to other tissues, while intratreatment variation was very large, as seen by high SEM values in these cases, in particular for kidney. This might have been caused by heterogeneity in the organ and possibly variances from further subsampling for analysis. In liver, GAA was not affected by graded GAA in the diet suggesting rapid turnover of GAA to Cr (Table 4). Cr was almost 2-fold higher in GAA-fed broilers vs. control on d 26 (P < 0.05), however no difference was observed on d 39. Interestingly, contrary to breast muscle and heart, d 39 and d 26 values ranked in a more similar range for Cr, while GAA levels ranked lower on d 39 as compared to d 26. Data show a linear increase in liver creatinine on both sampling days (P = 0.004 and P = 0.003, on d 26 and d 39, respectively), and levels were overall low. MDA and antioxidant enzyme activities were not affected by treatment. No significant effects on Cr metabolism and antioxidant enzyme activities in kidney were found, apart from a quadratic (P = 0.042) and linear (P = 0.042) effect on total Cr on d 26 and 39, respectively, and a linear elevation in creatinine on d 26 (P = 0.038, Table 5). Yet, similar to liver, much lower overall levels for GAA seemed to be present in kidney on d 39 as compared to d 26.
Table 4.
Effect of guanidinoacetic acid (GAA) supplementation on liver creatine metabolism, malondialdehyde (MDA) concentration, and antioxidant enzyme activities (superoxide dismutase, SOD, and glutathione peroxidase, GPx) of male broilers subjected to heat stress in the finisher phase at d 26 and 391 (n = 12).
| Item | Dietary treatment2 |
SEM |
P value |
||||
|---|---|---|---|---|---|---|---|
| Supplemental GAA, g/kg | 0.0 | 0.6 | 1.2 | Model | Linear | Quadratic | |
| Total creatine, mg/kg | |||||||
| d 26 | 2.3b | 4.1a | 4.3a | 0.26 | 0.001 | 0.001 | 0.095 |
| d 39 | 3.2 | 3.6 | 3.9 | 0.23 | 0.497 | 0.243 | 0.951 |
| Creatinine, mg/kg | |||||||
| d 26 | 0.21b | 0.25ab | 0.27a | 0.09 | 0.010 | 0.004 | 0.383 |
| d 39 | 0.21b | 0.24ab | 0.30a | 0.01 | 0.009 | 0.003 | 0.417 |
| GAA, mg/kg | |||||||
| d 26 | 6.4 | 7.4 | 5.7 | 0.40 | 0.190 | 0.457 | 0.104 |
| d 39 | 3.7 | 3.6 | 2.5 | 0.28 | 0.147 | 0.093 | 0.353 |
| MDA, nmol/g | |||||||
| d 26 | 96 | 87 | 94 | 2.7 | 0.396 | 0.707 | 0.194 |
| d 39 | 77 | 77 | 82 | 1.8 | 0.418 | 0.247 | 0.532 |
| SOD, U/g | |||||||
| d 26 | 172 | 164 | 159 | 5.3 | 0.591 | 0.312 | 0.909 |
| d 39 | 283 | 260 | 257 | 11.7 | 0.631 | 0.386 | 0.691 |
| GPx, U/g | |||||||
| d 26 | 3.9 | 4.2 | 3.8 | 0.09 | 0.237 | 0.807 | 0.095 |
| d 39 | 3.7 | 3.6 | 3.6 | 0.08 | 0.727 | 0.435 | 0.889 |
Broilers were fed a corn-soybean starter diet from d 0 to 10, a grower diet from d 10 to 25, and a finisher diet from d 25 to 39.
Mean values with different superscripts (a, b) per sampling day are significantly different at P < 0.05.
Table 5.
Effect of guanidinoacetic acid (GAA) supplementation on kidney creatine metabolism, malondialdehyde (MDA) concentration, and antioxidant enzyme activities (superoxide dismutase, SOD, and glutathione peroxidase, GPx) of male broilers subjected to heat stress in the finisher phase at d 26 and 391 (n = 12).
| Item | Dietary treatment |
SEM |
P value |
||||
|---|---|---|---|---|---|---|---|
| Supplemental GAA, g/kg | 0.0 | 0.6 | 1.2 | Model | Linear | Quadratic | |
| Total creatine, mg/kg | |||||||
| d 26 | 4.7 | 11.9 | 6.4 | 1.5 | 0.120 | 0.678 | 0.042 |
| d 39 | 8.7 | 9.1 | 10.8 | 4.2 | 0.102 | 0.042 | 0.476 |
| Creatinine, mg/kg | |||||||
| d 26 | 1.2 | 1.2 | 1.6 | 0.07 | 0.073 | 0.038 | 0.320 |
| d 39 | 1.1 | 1.2 | 1.1 | 0.06 | 0.843 | 0.976 | 0.565 |
| GAA, mg/kg | |||||||
| d 26 | 33.7 | 65.4 | 62.0 | 12.9 | 0.583 | 0.394 | 0.527 |
| d 39 | 15.1 | 14.3 | 15.5 | 1.75 | 0.957 | 0.913 | 0.789 |
| MDA, nmol/g | |||||||
| d 26 | 33 | 34 | 34 | 1.1 | 0.871 | 0.807 | 0.644 |
| d 39 | 31 | 32 | 32 | 0.27 | 0.344 | 0.366 | 0.251 |
| SOD, U/g | |||||||
| d 26 | 209 | 203 | 204 | 5.2 | 0.898 | 0.727 | 0.764 |
| d 39 | 218 | 210 | 223 | 3.9 | 0.446 | 0.613 | 0.246 |
| GPx, U/g | |||||||
| d 26 | 3.8 | 4.0 | 4.0 | 0.06 | 0.249 | 0.159 | 0.370 |
| d 39 | 4.7 | 4.7 | 4.6 | 0.08 | 0.983 | 0.869 | 0.939 |
Broilers were fed a corn-soybean starter diet from d 0 to 10, a grower diet from d 10 to 25, and a finisher diet from d 25 to 39.
Principal Component Analysis of Variables Describing Creatine Metabolism and Energy Metabolites
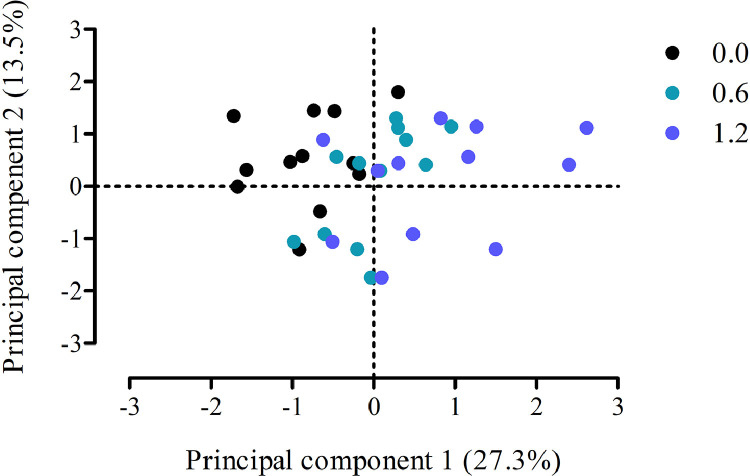
A PCA was run for each sampling day separately, including 20 variables for both d 26 and d 39, representing Cr and energy metabolism in all tissues. For d 26, the 5 principal components with highest variance explained were retained, for which 3 (principal components 1, P < 0.001; 2, P = 0.011; and 3, P = 0.029) were affected by treatment (Table 6). Representation of the most affected principal components, that is, principal component 1 and 2, allowed to discriminate treatments visually (Figure 1). Principal component 1, containing 27.3% variance explained, showed high positive loadings for GAA, Cr, Arg, and lactate in plasma, phosphocreatine, and glycogen in breast muscle, Cr in heart, Cr and creatinine in liver, and creatinine in kidney. Not surprisingly, this was associated with higher dietary GAA inclusion, as means for treatments 0.0, 0.6, and 1.2 g/kg GAA were −0.81, 0.02, and 0.80, respectively (P < 0.001). Interestingly, the negative loading of −0.43 for GAA in liver suggests that feeding GAA reduces this metabolite in liver. Principal component 2 shows negative loadings for free Cr and total Cr in breast (−0.67 and −0.75 loadings, respectively) and positive loadings for lactate, cholesterol and glucose in plasma and Cr in kidney. Here, negative loadings are correlated with higher dietary GAA as 1.2 g/kg GAA-fed broilers had a mean of −0.63 for the scores of principal component 2, vs. 0.53 for control birds. Principal component 3 suggests that control and 0.6 g/kg GAA-fed broilers can be discriminated by plasma triglycerides and glucose, phosphocreatine and free Cr in breast, and liver creatinine, whereas 1.2 g/kg GAA-fed broilers are intermediate. On d 39, only principal component 1 showed differences between treatments (Table 7). Means and loadings for the variables of this principal component seem to be very similar to principal component 1 on d 26, and underlines consistent differences in Cr metabolic pathways as affected by feeding GAA to birds. However, worth mentioning is that on d 26, Cr in liver was included in this principal component and not on d 39; and further on d 26 creatinine in kidney was seen while on d 39 it was Cr in kidney.
Table 6.
Description of the major principal components obtained by principal component analysis (PCA) of 20 variables characterizing creatine metabolism and energy metabolites in male broilers subjected to heat stress in the finisher phase at d 261.
| Principal component | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Eigenvalues | 5.2 | 2.6 | 1.8 | 1.8 | 1.6 |
| % variance explained | 27.3 | 13.5 | 9.0 | 8.8 | 8.1 |
| % cumulative | 27.3 | 40.8 | 49.8 | 58.6 | 66.7 |
| Means for treatments2, supplemental GAA, g/kg | |||||
| 0.0 | −0.81c | 0.53a | −0.47b | −0.02 | −0.17 |
| 0.6 | 0.02b | 0.10ab | 0.58a | -0.14 | 0.24 |
| 1.2 | 0.80a | −0.63b | −0.10ab | 0.15 | −0.08 |
| SEM | 0.17 | 0.17 | 0.17 | 0.17 | 0.17 |
| P value | <0.001 | 0.011 | 0.029 | 0.784 | 0.584 |
| Guanidinoacetic acid, plasma | 0.80 | ||||
| Creatine, plasma | 0.78 | ||||
| Homocysteine, plasma | 0.67 | ||||
| Arginine, plasma | 0.79 | ||||
| Creatine kinase, plasma | −0.67 | ||||
| Lactate, plasma | 0.41 | 0.46 | -0.44 | ||
| Cholesterol, plasma | 0.56 | 0.46 | |||
| Triglycerides, plasma | −0.53 | 0.46 | |||
| Glucose, plasma | 0.75 | 0.40 | |||
| Phosphocreatine, breast muscle | 0.65 | −0.44 | |||
| Free creatine, breast muscle | −0.67 | 0.57 | |||
| Total creatine, breast muscle | 0.40 | −0.75 | |||
| Glycogen, breast muscle | 0.69 | ||||
| Creatine, heart | 0.58 | ||||
| Creatine, liver | 0.70 | ||||
| Creatinine, liver | 0.64 | 0.53 | |||
| GAA, liver | −0.43 | ||||
| Creatine, kidney | 0.41 | ||||
| Creatinine, kidney | 0.77 | ||||
| GAA, kidney | 0.59 |
Broilers were fed a corn-soybean starter diet from d 0 to 10, a grower diet from d 10 to 25, and a finisher diet from d 25 to 39.
Values with different superscripts (a–c) within a column are significantly different at P < 0.05.
Figure 1.
Representation of broilers supplemented with guanidinoacetic acid (GAA) at 0.0, 0.6, and 1.2 g/kg and sampled at d 26 according to their principal component scores for principal component 1 and 2 from the principal component analysis. Broilers can be discriminated visually according to treatment; principal component 1 (27.3%), P < 0.001, and principal component 2 (13.6%), P = 0.004.
Table 7.
Description of the major principal components obtained by principal component analysis (PCA) of 20 variables characterizing creatine metabolism and energy metabolites in male broilers subjected to heat stress in the finisher phase at d 391.
| Principal component | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| Eigenvalues | 5.4 | 2.0 | 1.9 | 1.7 | 1.7 |
| % variance explained | 27.2 | 10.2 | 9.4 | 8.7 | 8.3 |
| % cumulative | 27.2 | 37.4 | 46.9 | 55.6 | 63.8 |
| Means for treatments2, supplemental GAA, g/kg | |||||
| 0.0 | −0.85c | 0.14 | −0.16 | 0.12 | −0.14 |
| 0.6 | 0.01b | −0.11 | −0.08 | 0.10 | −0.34 |
| 1.2 | 0.84a | −0.04 | 0.23 | −0.21 | 0.48 |
| SEM | 0.17 | 0.17 | 0.17 | 0.17 | 0.17 |
| P value | <0.001 | 0.826 | 0.619 | 0.679 | 0.107 |
| Guanidinoacetic acid, plasma | 0.74 | ||||
| Creatine, plasma | 0.89 | ||||
| Homocysteine, plasma | 0.77 | ||||
| Arginine, plasma | 0.74 | ||||
| Creatine kinase, plasma | 0.64 | ||||
| Lactate, plasma | 0.50 | 0.46 | |||
| Cholesterol, plasma | −0.58 | ||||
| Triglycerides, plasma | 0.76 | −0.40 | |||
| Glucose, plasma | 0.70 | ||||
| ATP, breast muscle | −0.72 | ||||
| Phosphocreatine, breast muscle | 0.74 | ||||
| Free creatine, breast muscle | 0.75 | ||||
| Total creatine, breast muscle | 0.87 | ||||
| Glycogen, breast muscle | 0.52 | −0.40 | |||
| Creatine, liver | 0.40 | ||||
| Creatinine, liver | 0.74 | ||||
| GAA, liver | −0.43 | −0.46 | |||
| Creatine, kidney | 0.61 | ||||
| Creatinine, kidney | 0.42 | ||||
| GAA, kidney | 0.58 | ||||
Broilers were fed a corn-soybean starter diet from d 0 to 10, a grower diet from d 10 to 25, and a finisher diet from d 25 to 39.
Values with different superscripts (a–c) within a column are significantly different at P < 0.05.
DISCUSSION
Dietary Guanidinoacetic Acid Has Limited Effect on Oxidative Status, But Demonstrates Benefits for Energy Metabolism
The present study aimed to evaluate the effects of dietary GAA on markers of energy metabolism and oxidative status in broiler chickens. Hence, a model of chronic cyclic heat stress was adapted and a cohort of different endpoints in various organs was assessed. However, the study failed to demonstrate convincing effects from GAA feeding on oxidative status variables apart from reductions in SOD activity in breast muscle on d 26 (linear trend) and d 39 (linear effect). These effects were also notably small, but as breast muscle is a major source of reactive species, the observed reduced SOD activity may still be relevant. Heat stress reduces the efficiency of electron transfer along the electron transport chain which in turn leads to aberrant leakage of electrons and eventually results in higher superoxide anion formation (Mujahid et al., 2009). Various isoforms of SOD, that is, CuZnSOD in the intermembrane space (also present in cytosol) and MnSOD in the matrix of mitochondria, have a counteractive effect and prevent oxidative damage as they reduce superoxide anion to hydrogen peroxide (H2O2) to prevent chain reactions in forming other radicals such as ONOO−. H2O2 in turn can act as signaling molecule or is further metabolized to harmless water by peroxidase activity (Akbarian et al., 2016). A lower SOD activity may point at a lower need for this catalysis in line with postulations that Cr has a direct action on scavenging superoxide anion (Guimarães-Ferreira, 2014), or at a lower production of superoxide anion through the Cr-induced buffering of cellular ATP levels (Persky and Brazeau, 2001). Mitochondria are equipped with uncoupling proteins (UCP) that assist in the control of superoxide anion production (Pamplona and Costantini, 2011). Mild uncoupling by UCP, that is, transferring protons back into the matrix, slightly stimulates electron transport and reduces superoxide anion production, but redox energy is dissipated as heat instead of being used for ATP synthesis. It was shown that chronic heat stress enhanced avian UCP transcript levels in breast muscle mitochondria by no less than 71% (Dridi et al., 2008) and 100% (Toyomizu et al., 2011). Therefore, higher Cr load and phosphocreatine:ATP may act as buffer for this loss of ATP generation and support efficiency of electron transfer. Indeed, in poultry, Wang et al. (2015) who studied the effect of creatine monohydrate on muscle lipid peroxidation and antioxidant capacity in transported broilers in summer reported that transport stress accelerated muscle lipid peroxidation shown by increased TBARS levels. The SOD and GPx activities increased and the upregulation of avian UCP, avian peroxisome proliferator-activated receptor γ coactivator-1α, and HSP70 were insufficient to reduce muscle TBARS and prevent muscle from transport-induced oxidative stress. Despite creatine monohydrate elevated muscle Cr load, it did not demonstrate potential for scavenging free radicals and activation of antioxidant enzymes such as SOD and GPx. These authors supposed that the protective effect of creatine monohydrate for maintaining meat quality during transport stress is possibly correlated to loading more phosphocreatine to generate ATP and consequently reduce muscle glycolysis instead of any direct antioxidant activity. However, Zhao et al. (2021), studying GAA as a source of Cr in broilers, found that dietary 0.6 g/kg GAA altered various markers of oxidative status in breast muscle of broilers, that is, reactive oxygen species and MDA were decreased, and total antioxidant capacity was increased, yet no effect on enzyme activities of SOD and GPx was found. Wang et al. (2016) found further ambiguous evidence for antioxidant outcomes by feeding GAA to Cherry valley ducks. Ducks fed 0.5 g/kg GAA exhibited reduced MDA, however, contrary to the expectation, blood GPx activity and GSH as well as activity of catalase, SOD, and GPx and GSH in the liver increased. They concluded that GAA could improve the body's antioxidative capacity to some extent due to the ability of GAA to increase the level of Cr in the body. Such evidence for liver may look surprising as Cr is mainly present in skeletal muscle and thus antioxidant effects are expected in that tissue. In our study we see that Cr levels in liver are 1,000-fold lower than in breast muscle, yet it was linearly increased on d 26, but not on d 39, by feeding GAA, and that MDA levels and SOD and GPx activities are around 10-fold higher in liver as compared to breast muscle. It appears that liver must cope with a much larger oxidative stress burden, either from local or systemic origin, and that dietary GAA may support mitigation. Interestingly, in challenge models, Nasiroleslami et al. (2018) demonstrated increased liver GPx and decreased serum MDA in cold-stressed broilers by supplementing GAA to the broilers; while in T3 administered broilers (a model to increase ascites syndrome, basal metabolic rate, and to induce mitochondrial-dependent reactive species generation), GAA was able to mitigate MDA increase and SOD decrease in liver (Khalil et al., 2021). Finally, in a cyclic heat stress model, Amiri et al. (2019) found trends for increased GPx and SOD activity in plasma with graded GAA, which could not be repeated in our study. Overall, literature and to a limited extent our study show that GAA can improve oxidative status of the bird. It is plausible to state that these antioxidant effects are indirect and based on improved muscle Cr loading. Thus, the performance supporting effects of dietary GAA may be in part attributed to improvement of oxidative status. However, Ostojic (2015) postulated a number of physiological roles of supplemental GAA next to muscle Cr loading, such as insulin stimulator and sensitizer and γ-aminobutyric acid antagonist and neuromodulation, and acknowledged that GAA may act as antioxidant and pro-oxidant. It is plausible that all these intertwined physiological roles determine the outcome, not in the least leading to inconsistencies in simple measurements of oxidative status. Moreover, it may largely depend on dietary concentration, tissue studied, and physiological state of the body, but literature is fragmented in order to draw firm conclusions hitherto. Since it can donate an electron from its conjugate base, GAA can generate superoxide, and be a potent pro-oxidant. For example, pro-oxidant effects were found after intrastriatal accumulation of GAA (100 µmol/L) (Zugno et al., 2008). In contrast, antioxidant effects evoked by the related metabolites Cr and Arg were demonstrated upon GAA supplementation that resulted in low serum levels of GAA (5 µmol/L). Interestingly, GAA in plasma of the broilers in our study varied between 0.5 and 14.3 µmol/L, depending on dietary GAA and age. Thus, the outcome of oxidative status may on the balance of pro- and antioxidant effects. Ostojic (2015) concluded that an explicit exposure-response relationship concerning pro-oxidant and antioxidant status of supplementary GAA remains unknown.
The Cr loading potential in breast muscle by feeding GAA to broilers, and concomitant higher phosphocreatine:ATP ratio are well established in literature. Here, we were able to correlate this to other energy metabolites in plasma, heart, and liver. First, breast muscle energy reserves as glycogen were higher in GAA-fed birds on d 26, while a linear trend was seen on d 39. Also, DeGroot et al. (2018) reported elevations of muscle glycogen from GAA feeding to broilers under thermoneutral conditions. Apart from improving energy status as such, higher glycogen stores may prevent substantial pH drop in muscle postmortem, which is particularly relevant for muscle from heat-stressed birds as it was found that glycogen reserves are consumed faster postmortem than in muscle from nonstressed birds (Song and King, 2015). On this note, for both d 26 and d 39, glycogen in breast muscle showed positive loadings for principal component 1, albeit higher on d 26 vs. d 39, thus indicating that GAA feeding fosters glycogen stores associated with Cr loading and ATP buffering. On d 26, GAA did not affect plasma lactate or triglycerides, and minimal linear decreases were found for plasma cholesterol and glucose. However, principal component 2 highlights an interesting observation. This principal component groups negatively free Cr and total Cr in breast and positively lactate, cholesterol, and glucose in plasma. Here, positive loadings are associated with lower dietary GAA. This suggests that dietary GAA dose-dependently reduces blood lactate, cholesterol, and glucose for this sampling day (d 26), concomitant to an elevation of free Cr and total Cr in breast muscle. This supports the assumption that dietary GAA shifts energy generation toward the use of readily available phosphocreatine instead of anaerobic metabolism of glycogen (lower lactate) and oxidative phosphorylation (lower glucose). Also, lower lactate could simply indicate higher tolerance to cellular stress. Peculiar is principal component 3 on d 26, with a negative loading for plasma triglycerides, which actually corroborates with the quadratic effect for this variable. Further, reports in humans agree with our findings for reduced plasma cholesterol by GAA, which occurred on both sampling days. In fact, Kreider et al. (1998), studying Cr supplementation in human, demonstrated that high density lipoprotein concentrations were increased (+13%), while there was some evidence that very low density lipoprotein levels (−13%) and the ratio of total cholesterol to high density lipoprotein levels (−7%) were decreased in the Cr group; corroborating with earlier results by Earnest et al. (1996). In this latter study, Cr supplementation to humans decreased total cholesterol, triglycerides, and very low density lipoprotein in moderately hyperlipidemic, physically active male and female subjects. In Majdeddin et al. (2020) we yet alluded to the role of AMP-activated protein kinase (AMPK) herein. AMPK is the main sensor of cellular energy status and is activated in response to energy stress to restore energy balance by inhibiting ATP-consuming processes and promoting ATP-generating pathways; driven by fluctuations in the AMP:ATP ratio (Corton et al., 1994, 1995). It was postulated that GAA feeding might enhance cellular energy in liver and thus lower stimulation of the AMPK pathway (Majdeddin et al., 2020). Recently Duan et al. (2022) proved that 0.9 g/kg GAA could reduce mRNA levels of AMPKα2 and its upstream kinase LKB1 in breast muscle, thus inhibiting the activation of the AMPK pathway. Improved cellular energy in liver would, in turn, alter energy metabolism and potentially increase liver cholesterol 7-alpha-hydroxylase activity (Hu et al., 2019). This enzyme converts cholesterol to 7-alpha-hydroxycholesterol, the first and rate limiting step in bile acid synthesis. Higher activity of this enzyme might thus cause lower circulating cholesterol, congruent to our observations. On the other hand, AMPK phosphorylates and inactivates 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMG-CoA reductase), the rate-controlling enzyme of the mevalonate pathway that produces cholesterol, among others. Cholesterol lowering effects in broilers via modulating lipid metabolism were also reported from increased dietary Arg (Fouad et al., 2013). Arg inclusion reduced HMG-CoA reductase mRNA expression. Liver is the most important vital organ for fatty acid synthesis in avian species, whereby almost 85% of the fat accumulated by growing birds is produced in the liver (Molette et al., 2012). As GAA is able to spare Arg in broilers (Dilger et al., 2013; DeGroot et al., 2018), it can be speculated whether this effect of GAA on lipid metabolism is mediated by its Arg sparing properties. The effects of feeding GAA to subjects on liver energy status and AMPK and lipid metabolism therefore remains enigmatic even more so as we did not measure phosphocreatine, ATP, or AMPK in hepatic tissue. In our study, liver total Cr increased linearly with increasing GAA dose on d 26, but only numerically on d 39. Similar reports are documented in EFSA (2009) in broilers under thermoneutral conditions. EFSA (2009) shows studies in which graded GAA has no (up to 0.6 g/kg GAA) or elevating effects on liver Cr (up to 1.5 g/kg GAA) in poultry.
Effects of Dietary Guanidinoacetic Acid on Creatine Metabolism in Key Organs
Increases in GAA and total Cr in plasma by feeding GAA to broilers suggests efficient absorption in the gastrointestinal tract and methylation of GAA, respectively. Interestingly, for both plasma GAA and total Cr elevations were markedly higher on d 26 as compared to d 39. It is fair to speculate that differences between these days are primarily related to the sustained heat stress broilers faced. For example, it could be hypothesized that intestinal absorption of GAA might be hampered by chronic heat stress, since GAA-digestibility information is available only in thermoneutral conditions (Tossenberger et al., 2016). To the best of our knowledge, a full description of transport mechanisms for GAA in the intestine is not available. However, it is well described that GAA is taken up by a variety of cell types through several transporters such as SCL6A8, and protein carriers for taurine (SLC6A6) and γ-aminobutyric acid (GAT2) (Ostojic, 2017). SLC6A6 was also shown to be highly expressed in the human intestine; but likely GAA might have affinity for other intestinal amino acid transporters as well. Habashy et al. (2017) demonstrated that heat stress downregulated the expression of several ileal amino acid transporters. It is thus plausible that lower increases in plasma GAA on d 39 are caused by reduced absorption. Next, lower augment of plasma Cr may indicate limited S-adenosyl-L-methionine:N-guanidinoacetate methyltransferase (GAMT) activity. GAMT is the catalyzer for the methylation of GAA to produce Cr in liver (Wyss and Kaddurah-Daouk, 2000). Notably, Chamruspollert et al. (2004) reported that higher temperatures slowed Arg metabolism negatively affecting Cr synthesis pathways. However, lower plasma Cr is more likely a simple consequence of lower GAA availability due to lower GAA absorption, since plasma Cr was at similar levels on d 26 and d 39 in control birds. Plasma homocysteine was not affected at either sampling day. It means that methylation of GAA did not cause systemic accumulation of this metabolite.
Following increased plasma Cr levels in GAA-fed birds, muscle Cr load increased, possibly facilitated by SLC6A8, which is highly expressed in brain, liver, intestine and skeletal muscle (Ostojic, 2017). For all treatments, notably including control birds, breast total Cr levels appeared to be substantially lower on d 39 vs. d 26. This might be a consequence of lower plasma Cr on d 39, but it cannot be completely ruled out that there is also an influence of age, since it is reported in humans, that muscle Cr may decline during aging, specifically during the third life span (>60 yr) (Kerksick et al., 2016). However, no such age dependent decline in muscle Cr is reported in chickens with references measuring up to 57 d (Fisher et al., 1956; Ngo et al., 1977; Majdeddin et al., 2018, 2019). Strikingly, there seems to be a shift between d 26 and d 39 in the proportion of PCr in total Cr, where PCr:TCr was found much higher on d 39 compared to d 26 despite lower overall breast muscle Cr levels on d 39. It appears as with prolonged heat stress followed by elevated body temperature the CK-reaction promotes the formation of PCr. In contrast, and remarkable, total Cr levels in heart were higher on d 39 as compared to d 26. On both days, dietary GAA linearly increased total Cr concentrations. It appears that the body is prioritizing Cr synthesis to supply heart, rather than breast muscle, and that GAA is fostering Cr deposition in heart. Nain et al. (2008) stated that the regeneration of ATP from the Cr and phosphocreatine system is of paramount importance in the cardiac energy management of fast-growing broilers. Stressed cardiac muscle by time may lose its ability to sustain the work overload and may develop right ventricular heart failure or cause even sudden death syndrome. Nonetheless, Khalil et al. (2021) clearly demonstrated that 0.6 g/kg improved mitochondrial respiration and complex chain activities. It is fair to state that lowered mortality by GAA found from this study and reported in Majdeddin et al. (2020) can be partly ascribed to improved cardiac energy metabolism, yet no effects on oxidative status in this organ were found.
CONCLUSIONS
In this work we found that feeding GAA to heat-stressed finisher broilers had limited effects on the oxidative status as solely evidenced by linearly reduced breast muscle SOD activity. Even if other oxidative status markers were not affected, this finding may support indirect effects of dietary GAA on the oxidative status due to Cr loading protecting muscle function, in particular after chronic heat stress. As these effects were linear it can be hypothesized that higher GAA dosages may be required for more notorious effects on oxidative status. Dietary GAA greatly supported energy metabolism in muscle: Cr, phosphocreatine:ATP, and glycogen were improved. Chronic heat stress may reduce GAA uptake from the gut, leading to overall lower physiological Cr levels and a shift to a greater proportion of readily available PCr to muscle Cr. Furthermore, circulating Cr appears to be directed more to heart muscle as opposed to skeletal muscle in chronically heat-stressed broilers.
ACKNOWLEDGMENTS
The study was funded by AlzChem Trostberg GmbH (Trostberg, Germany) and Evonik Nutrition & Care GmbH (Hanau-Wolfgang, Germany). The Medizinisches Labor Bremen GmbH (Bremen, Germany) is acknowledged for analysis of creatine, creatinine, GAA, arginine, and homocysteine in plasma and Swiss BioqQuant AG (Reinach, Switzerland) ATP, PCr, free Cr, and TCr in breast muscle and TCr, Crn, and GAA in liver and kidney. M. Majdeddin was supported by the Ferdowsi University of Mashhad, Iran (Grant number 3/28524). The funders had no role in data collection, sample analysis, and statistical evaluation of data.
DISCLOSURES
Ulrike Braun is employee of AlzChem Trostberg GmbH and Andreas Lemme is employee of Evonik Operations GmbH. Other authors have no interest to declare. Yet, all authors read and approved the final manuscript.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2023.102653.
Appendix. Supplementary materials
REFERENCES
- Akbarian A., Michiels J., Degroote J., Majdeddin M., Golian A., De Smet S. Association between heat stress and oxidative stress in poultry; mitochondrial dysfunction and dietary interventions with phytochemicals. J. Anim. Sci. Biotechnol. 2016;7:37. doi: 10.1186/s40104-016-0097-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksentijevic D., Zervou S., Faller K.M.E., McAndrew D.J., Schneider J.E., Neubauer S., Lygate C.A. Myocardial creatine levels do not influence response to acute oxidative stress in isolated perfused heart. PLoS One. 2014;9 doi: 10.1371/journal.pone.0109021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amiri M., Ghasemi H.A., Hajkhodadadi I., Khaltabadi Farahani A.H. Efficacy of guanidinoacetic acid at different dietary crude protein levels on growth performance, stress indicators, antioxidant status, and intestinal morphology in broiler chickens subjected to cyclic heat stress. Anim. Feed Sci. Technol. 2019;254 [Google Scholar]
- Brosnan J.T., Brosnan M.E. Creatine metabolism and the urea cycle. Mol. Genet. Metab. 2010;100:S49–S52. doi: 10.1016/j.ymgme.2010.02.020. [DOI] [PubMed] [Google Scholar]
- Chamruspollert M., Pesti G.M., Bakalli R.I. Chick responses to dietary arginine and methionine levels at different environmental temperatures. Br. Poult. Sci. 2004;45:93–100. doi: 10.1080/00071660410001668914. [DOI] [PubMed] [Google Scholar]
- Corton J.M., Gillespie J.G., Hardie D.G. Role of the AMP-activated protein-kinase in the cellular stress-response. Curr. Biol. 1994;4:315–324. doi: 10.1016/s0960-9822(00)00070-1. [DOI] [PubMed] [Google Scholar]
- Corton J.M., Gillespie J.G., Hawley S.A., Hardie D.G. 5-Aminoimidazole-4-carboxamide ribonucleoside - a specific method for activating amp-activated protein-kinase in intact-cells. Eur. J. Biochem. 1995;229:558–565. doi: 10.1111/j.1432-1033.1995.tb20498.x. [DOI] [PubMed] [Google Scholar]
- Dao H.T., Swick R.A. New insights into arginine and arginine-sparing effects of guanidinoacetic acid and citrulline in broiler diets. World's Poult. Sci. J. 2021;77:753–773. [Google Scholar]
- DeGroot A.A., Braun U., Dilger R.N. Efficacy of guanidinoacetic acid on growth and muscle energy metabolism in broiler chicks receiving arginine-deficient diets. Poult. Sci. 2018;97:890–900. doi: 10.3382/ps/pex378. [DOI] [PubMed] [Google Scholar]
- DeGroot A.A., Braun U., Dilger R.N. Guanidinoacetic acid is efficacious in improving growth performance and muscle energy homeostasis in broiler chicks fed arginine-deficient or arginine-adequate diets. Poult. Sci. 2019;98:2896–2905. doi: 10.3382/ps/pez036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deminice R., Jordao A.A. Creatine supplementation reduces oxidative stress biomarkers after acute exercise in rats. Amino Acids. 2012;43:709–715. doi: 10.1007/s00726-011-1121-x. [DOI] [PubMed] [Google Scholar]
- Dilger R.N., Bryant-Angeoni K., Payne R.L., Lemme A., Parsons C.M. Dietary guanidino acetic acid is an efficacious replacement for arginine for young chicks. Poult. Sci. 2013;92:171–177. doi: 10.3382/ps.2012-02425. [DOI] [PubMed] [Google Scholar]
- Dridi S., Temim S., Derouet M., Tesseraud S., Taouis M. Acute cold-and chronic heat-exposure upregulate hepatic leptin and muscle uncoupling protein (UCP) gene expression in broiler chickens. J. Exp. Zool. A Ecol. Integr. Biol. 2008;309:381–388. doi: 10.1002/jez.461. [DOI] [PubMed] [Google Scholar]
- Duan B.B., Xu J.W., Xing T., Li J.L., Zhang L., Gao F. Creatine nitrate supplementation strengthens energy status and delays glycolysis of broiler muscle via inhibition of LKB1/AMPK pathway. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2021.101653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnest C.P., Almada A.L., Mitchell T.L. High-performance capillary electrophoresis - pure creatine monohydrate reduces blood lipids in men and women. Clin. Sci. 1996;91:113–118. doi: 10.1042/cs0910113. [DOI] [PubMed] [Google Scholar]
- EFSA Safety and efficacy of guanidinoacetic acid as feed additive for chickens for fattening. EFSA J. 2009;7:988. [Google Scholar]
- Fimognari C., Sestili P., Lenzi M., Cantelli-Forti G., Hrelia P. Protective effect of creatine against RNA damage. Mutat. Res. 2009;670:59–67. doi: 10.1016/j.mrfmmm.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Fisher H., Salander R.C., Taylor M.W. The influence of creatine biosynthesis on the arginine requirement of the chick. J. Nutr. 1956;59:491–499. doi: 10.1093/jn/59.4.491. [DOI] [PubMed] [Google Scholar]
- Fouad A.M., El-Senousey H.K., Yang X.J. Dietary L-arginine supplementation reduces abdominal fat content by modulating lipid metabolism in broiler chickens. Animal. 2013;7:1239–1245. doi: 10.1017/S1751731113000347. [DOI] [PubMed] [Google Scholar]
- Grotto D., Santa Maria L.D., Boeira S., Valentini J., Charão M.F., Moro A.M., Nascimento P.C., Pomblum V.J., Garcia S.C. Rapid quantification of malondialdehyde in plasma by high performance liquid chromatography-visible detection. J. Pharm. Biomed. Anal. 2007;43:619–624. doi: 10.1016/j.jpba.2006.07.030. [DOI] [PubMed] [Google Scholar]
- Guidi C., Potenza L., Sestill P., Martinelli C., Guescini M., Stocchi L., Zeppa S., Polidori E., Annibalini G., Stocchi V. Differential effect of creatine on oxidatively-injured mitochondrial and nuclear DNA. Biochim. Biophys. Acta Gen. Subj. 2008;1780:16–26. doi: 10.1016/j.bbagen.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Guimarães-Ferreira L. Role of the phosphocreatine system on energetic homeostasis in skeletal and cardiac muscles. Einstein. 2014;12:126–131. doi: 10.1590/S1679-45082014RB2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habashy W.S., Milfort M.C., Fuller A.L., Attia Y.A., Rekaya R., Aggrey S.E. Effect of heat stress on protein utilization and nutrient transporters in meat-type chickens. Int. J. Biometeorol. 2017;61:2111–2118. doi: 10.1007/s00484-017-1414-1. [DOI] [PubMed] [Google Scholar]
- Harris R.C., Söderlund K., Hultman E. Elevation of creatine in resting and exercised muscle of normal subjects by creatine supplementation. Clin. Sci. 1992;83:367–374. doi: 10.1042/cs0830367. [DOI] [PubMed] [Google Scholar]
- Hernandez P., Zomeno L., Arino B., Blasco A. Antioxidant, lipolytic and proteolytic enzyme activities in pork meat from different genotypes. Meat Sci. 2004;66:525–529. doi: 10.1016/S0309-1740(03)00155-4. [DOI] [PubMed] [Google Scholar]
- Hiramatsu M. A role for guanidino compounds in the brain. Mol. Cell Biochem. 2003;244:57–62. [PubMed] [Google Scholar]
- Hu X., Wang Y.F., Sheikhahmadi A., Li X.L., Buyse J., Lin H., Song Z.G. Effects of glucocorticoids on lipid metabolism and AMPK in broiler chickens' liver. Comp. Biochem. Physiol. B. 2019;232:23–30. doi: 10.1016/j.cbpb.2019.02.001. [DOI] [PubMed] [Google Scholar]
- Kerksick C.M., Roberts M.D., Dalbo V.J., Sunderland K.L. Intramuscular phosphagen status and the relationship to muscle performance across the age spectrum. Eur. J. Appl. Physiol. 2016;116:115–127. doi: 10.1007/s00421-015-3246-1. [DOI] [PubMed] [Google Scholar]
- Khajali F., Lemme A., Rademacher-Heilshorn M. Guanidinoacetic acid as a feed supplement for poultry. World's Poult. Sci. J. 2020;76:270–291. [Google Scholar]
- Khalil S., Al-Sagan A.A., Abdellatif H.A., Prince A., El-Banna R. Effects of guanidinoacetic acid supplementation on zootechnical performance and some biometric indices in broilers challenged with T-3-hormone. Ital. J. Anim. Sci. 2021;20:611–622. [Google Scholar]
- Kreider R.B., Ferreira M., Wilson M., Grindstaff P., Plisk S., Reinardy J., Cantler E., Almada A.L. Effects of creatine supplementation on body composition, strength, and sprint performance. Med. Sci. Sport. Exer. 1998;30:73–82. doi: 10.1097/00005768-199801000-00011. [DOI] [PubMed] [Google Scholar]
- Lawler J.M., Barnes W.S., Wu G.Y., Song W., Demaree S. Direct antioxidant properties of creatine. Biochem. Biophys. Res. Co. 2002;290:47–52. doi: 10.1006/bbrc.2001.6164. [DOI] [PubMed] [Google Scholar]
- Lemme A., Ringel J., Sterk A.R., Young J.F. Supplemental guanidino acetic acid affects energy metabolism of broilers. Page 208 in 16th Eur. Poult. Nutr. Symposium; Strasbourg, France; 2007. [Google Scholar]
- Majdeddin M., Braun U., Lemme A., Golian A., Kermanshahi H., De Smet S., Michiels J. Guanidinoacetic acid supplementation improves feed conversion in broilers subjected to heat stress associated with muscle creatine loading and arginine sparing. Poult. Sci. 2020;99:4442–4453. doi: 10.1016/j.psj.2020.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majdeddin M., Golian A., Kermanshahi H., De Smet S., Michiels J. Guanidinoacetic acid supplementation in broiler chickens fed on corn-soybean diets affects performance in the finisher period and energy metabolites in breast muscle independent of diet nutrient density. Br. Poult. Sci. 2018;59:443–451. doi: 10.1080/00071668.2018.1476678. [DOI] [PubMed] [Google Scholar]
- Majdeddin M., Golian A., Kermanshahi H., Michiels J., De Smet S. Effects of methionine and guanidinoacetic acid supplementation on performance and energy metabolites in breast muscle of male broiler chickens fed corn-soybean diets. Br. Poult. Sci. 2019;60:554–563. doi: 10.1080/00071668.2019.1631447. [DOI] [PubMed] [Google Scholar]
- Marklund S., Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974;47:469–474. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- Michiels J., De Vos M., Missotten J., Ovyn A., De Smet S., Van Ginneken C. Maturation of digestive function is retarded and plasma antioxidant capacity lowered in fully weaned low birth weight piglets. Br. J. Nutr. 2013;109:65–75. doi: 10.1017/S0007114512000670. [DOI] [PubMed] [Google Scholar]
- Michiels J., Maertens L., Buyse J., Lemme A., Rademacher M., Dierick N.A., De Smet S. Supplementation of guanidinoacetic acid to broiler diets: effects on performance, carcass characteristics, meat quality, and energy metabolism. Poult. Sci. 2012;91:402–412. doi: 10.3382/ps.2011-01585. [DOI] [PubMed] [Google Scholar]
- Molette C., Theron L., Marty-Gasset N., Fernandez X., Remignon H. Current advances in proteomic analysis of (fatty) liver. J. Proteomics. 2012;75:4290–4295. doi: 10.1016/j.jprot.2012.04.041. [DOI] [PubMed] [Google Scholar]
- Montagne L., Boudry G., Favier C., Huerou-Luron I.Le, Lalles J.P., Seve B. Main intestinal markers associated with the changes in gut architecture and function in piglets after weaning. Br. J. Nutr. 2007;97:45–57. doi: 10.1017/S000711450720580X. [DOI] [PubMed] [Google Scholar]
- Mujahid A., Akiba Y., Toyomizu M. Progressive changes in the physiological responses of heat-stressed broiler chickens. J. Poult. Sci. 2009;46:163–167. [Google Scholar]
- Nain S., Ling B., Alcorn J., Wojnarowicz C.M., Laarveld B., Olkowski A.A. Biochemical factors limiting myocardial energy in a chicken genotype selected for rapid growth. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2008;149:36–43. doi: 10.1016/j.cbpa.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Nasiroleslami M., Torki M., Saki A.A., Abdolmohammadi A.R. Effects of dietary guanidinoacetic acid and betaine supplementation on performance, blood biochemical parameters and antioxidant status of broilers subjected to cold stress. J. Appl. Anim. Res. 2018;46:1016–1022. [Google Scholar]
- Ngo A., Coon C.N., Beecher G.R. Dietary glycine requirements for growth and cellular development in chicks. J. Nutr. 1977;107:1800–1808. doi: 10.1093/jn/107.10.1800. [DOI] [PubMed] [Google Scholar]
- Ostojic S.M. Advanced physiological roles of guanidinoacetic acid. Eur. J. Nutr. 2015;54:1211–1215. doi: 10.1007/s00394-015-1050-7. [DOI] [PubMed] [Google Scholar]
- Ostojic S.M. Co-administration of creatine and guanidinoacetic acid for augmented tissue bioenergetics: a novel approach? Biomed. Pharmacother. 2017;91:238–240. doi: 10.1016/j.biopha.2017.04.075. [DOI] [PubMed] [Google Scholar]
- Pamplona R., Costantini D. Molecular and structural antioxidant defenses against oxidative stress in animals. Am. J. Physiol. Reg. I. 2011;301:843–863. doi: 10.1152/ajpregu.00034.2011. [DOI] [PubMed] [Google Scholar]
- Persky A.M., Brazeau G.A. Clinical pharmacology of the dietary supplement creatine monohydrate. Pharmacol. Rev. 2001;53:161–176. [PubMed] [Google Scholar]
- Portocarero N., Braun U. The physiological role of guanidinoacetic acid and its relationship with arginine in broiler chickens. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sestili P., Martinelli C., Bravi G., Piccoli G., Curci R., Battistelli M., Falcieri E., Agostini D., Gioacchini A.M., Stocchi V. Creatine supplementation affords cytoprotection in oxidatively injured cultured mammalian cells via direct antioxidant activity. Free Radic. Biol. Med. 2006;40:837–849. doi: 10.1016/j.freeradbiomed.2005.10.035. [DOI] [PubMed] [Google Scholar]
- Song D.J., King A.J. Effects of heat stress on broiler meat quality. Worlds Poult. Sci. J. 2015;71:701–709. [Google Scholar]
- Tossenberger J., Rademacher M., Nemeth K., Halas V., Lemme A. Digestibility and metabolism of dietary guanidino acetic acid fed to broilers. Poult. Sci. 2016;95:2058–2067. doi: 10.3382/ps/pew083. [DOI] [PubMed] [Google Scholar]
- Toyomizu M., Kikusato M., Kawabata Y., Azad M.A.K., Inui E., Amo T. Meat-type chickens have a higher efficiency of mitochondrial oxidative phosphorylation than laying-type chickens. Comp. Biochem. Phys. A. 2011;159:75–81. doi: 10.1016/j.cbpa.2011.01.020. [DOI] [PubMed] [Google Scholar]
- Wang X.F., Zhu X.D., Li Y.J., Liu Y., Li J.L., Gao F., Zhou G.H., Zhang L. Effect of dietary creatine monohydrate supplementation on muscle lipid peroxidation and antioxidant capacity of transported broilers in summer. Poult. Sci. 2015;94:2797–2804. doi: 10.3382/ps/pev255. [DOI] [PubMed] [Google Scholar]
- Wang Y., Liu Q., Jiang F., Yuan Q., Yan R., Zhuang S. Effects of guanidinoacetic acid on performance and antioxidant capacity in Cherry Valley ducks. J. Nanjing Agric. Univ. 2016;39:269–274. [Google Scholar]
- Wyss M., Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiol. Rev. 2000;80:1107–1213. doi: 10.1152/physrev.2000.80.3.1107. [DOI] [PubMed] [Google Scholar]
- Zhao W., Li J.L., Xing T., Zhang L., Gao F. Effects of guanidinoacetic acid and complex antioxidant supplementation on growth performance, meat quality, and antioxidant function of broiler chickens. J. Sci. Food Agric. 2021;101:3961–3968. doi: 10.1002/jsfa.11036. [DOI] [PubMed] [Google Scholar]
- Zugno A.I., Scherer E.B., Schuck P.F., Oliveira D.L., Wofchuk S., Wannmacher C.M., Wajner M., Wyse A.T. Intrastriatal administration of guanidinoacetate inhibits Na+, K+-ATPase and creatine kinase activities in rat striatum. Metab. Brain Dis. 2006;21:39–48. doi: 10.1007/s11011-006-9003-8. [DOI] [PubMed] [Google Scholar]
- Zugno A.I., Stefanello F.M., Scherer E.B., Mattos C., Pederzolli C.D., Andrade V.M., Wannmacher C.M., Wajner M., Dutra-Filho C.S., Wyse A.T. Guanidinoacetate decreases antioxidant defenses and total protein sulfhydryl content in striatum of rats. Neurochem. Res. 2008;33:1804–1810. doi: 10.1007/s11064-008-9636-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.