Abstract
Introduction
GERAS‐US prospectively characterized clinical and economic outcomes of early symptomatic Alzheimer's disease (AD). Societal cost changes were examined in amyloid‐positive patients with mild cognitive impairment due to AD (MCI) and mild dementia due to AD (MILD).
Methods
Cognition, function, and caregiver burden were assessed using Mini‐Mental State Examination (MMSE), Cognitive Function Index (CFI), and Zarit Burden Interview, respectively. Costs are presented as least square mean for the overall population and for MCI versus MILD using mixed model repeated measures.
Results
MMSE score and CFI worsened. Total societal costs (dollars/month) for MCI and MILD, respectively, were higher at baseline ($2430 and $4063) but steady from 6 ($1977 and $3032) to 36 months ($2007 and $3392). Direct non‐medical costs rose significantly for MILD. Caregiver burden was higher for MILD versus MCI at 12, 18, and 24 months.
Discussion
Function and cognition declined in MILD. Non‐medical costs reflect the increasing impact of AD even in its early stages.
HIGHLIGHTS
In the GERAS‐US study, total societal costs for patients with mild cognitive impairment due to Alzheimer's disease (MCI) and mild dementia due to Alzheimer's disease (MILD) were higher at baseline but steady from 6 to 36 months.
Mini‐Mental State Examination (MMSE) and Cognitive Function Index (CFI) worsened; the rate of decline was significant for patients with MILD but not for those with MCI.
There was a rise in direct non‐medical costs at 36 months for patients with MILD.
Caregiver burden was higher for MILD versus MCI at 12, 18, and 24 months.
Slowing the rate of disease progression in this early symptomatic population may allow patients to maintain their ability to carry out everyday activities longer.
Keywords: amyloid, dementia, mild cognitive impairment, societal burden
1. BACKGROUND
The costs and care‐related burden in the earlier symptomatic stages of Alzheimer's disease (AD) have not been well studied. There is a significant societal burden in the United States due to costs associated with the care of patients with AD, particularly as the disease progresses. These include direct health‐care costs and indirect costs associated with loss of earnings and reduction in the quality of life of caregivers of patients with dementia. 1 , 2
A previous 18‐month observational GERAS‐EU study (conducted in the UK, France, and Germany) found that the total societal costs increased as AD severity worsened. 3 Caregiver time contributed the most to societal costs. 4 However, this study does not reflect the health‐care patterns of patients with biomarker‐confirmed early symptomatic AD in the United States.
GERAS‐US (H8A‐US‐B004; ClinicalTrials.gov NCT02951598) is a prospective, 36‐month, US‐based, longitudinal cohort study of patients with AD characterizing the clinical and economic outcomes of early symptomatic AD 5 specifically in patients with amyloid‐positive mild cognitive impairment due to AD (MCI) or mild dementia due to AD (MILD). GERAS‐US examines societal costs in early symptomatic AD (MCI, MILD) in biomarker‐confirmed patients and bridges the gap in knowledge about earlier stages of AD. It also focuses on US societal costs, which have not been well characterized. This study was adapted from the previous GERAS‐EU study. 3 Previous cross‐sectional analyses of baseline data in this GERAS‐US study showed higher total societal costs/month among patients with MILD versus MCI. 5 Informal caregiver costs were responsible for the largest fraction of the overall total costs, especially for the MILD cohort. 5
A positive amyloid beta (Aβ) positron emission tomography (PET) scan is consistent with a diagnosis of AD, whereas symptomatic patients with a negative Aβ scan have cognitive impairment from other causes, likely related to vascular disease. 6 The previous GERAS‐EU study did not require amyloid testing, which is now considered confirmatory of an AD diagnosis. 7
Comprehensive longitudinal data are important to ascertain the long‐term societal burden of AD. Because treatments are currently being developed to slow disease progression in biomarker‐confirmed early symptomatic AD, it is important to characterize progression in the early stages of biomarker‐confirmed disease to evaluate the impact of such treatments on health outcomes. This study characterizes disease progression and its consequences over 3 years in a biomarker‐confirmed early symptomatic AD cohort. The objective of this article is to describe the changes in total societal costs over 3 years post baseline in amyloid‐positive patients with MCI and MILD in the United States.
2. METHODS
2.1. Study design
This study included patients with clinician‐diagnosed early symptomatic AD who were receiving routine care for memory problems and were invited by their health‐care providers to participate in the study during routine visits. Patients and study partners were enrolled in the study from October 30, 2016, through October 9, 2017. 6 The study, as detailed previously, 6 was conducted at 76 geographically diverse sites and included patients from 20 states. Patients with positive amyloid status continued assessments every 6 months (±6 weeks) for 36 months. The follow‐up period reported in this study extended for 36 months post baseline visit (in amyloid+ patients and their study partners).
2.2. Patients
All patients provided written informed consent prior to the start of the study. Patients (age 55–85 years) who met the criteria for early symptomatic AD were enrolled in the study. 6 Patients had to have a study partner who could communicate in English or Spanish and who was willing to participate for the duration of the study. Patients had to have a Mini‐Mental State Examination (MMSE) 8 score ≥20 (scores < 20 indicate more advanced disease). The Aβ status of patients was determined using PET scans or cerebrospinal fluid testing, and patients who were classified as having an amyloid‐positive status were included in the study. Overall, patients were classified as having MCI or MILD. Patients were excluded if they had evidence of amyloid negativity in the prior 2 years.
2.3. Cohort classification
Patient cohorts were classified using the MMSE 8 and the Functional Activities Questionnaire (FAQ). 9 , 10 Cut points consistent with a clinical diagnosis of MCI are an MMSE≥24 and an FAQ < 6 and those consistent with a clinical diagnosis of MILD are an MMSE ≥ 20 and an FAQ ≥ 6. 10 For 26 of the 28 patients with scores outside this range (i.e., MMSE ≥ 24 and FAQ ≥ 6), the diagnosis by the enrolling physician was used to classify patients, and two patients who had a diagnosis of “memory loss Not Elsewhere Classified” were excluded from the analyses. 5
2.4. Assessments
Data were collected at baseline and at 6, 12, 18, 24, 30, and 36 months. Patient demographic characteristics were collected. The clinical outcome measure of cognition was assessed using the MMSE. Everyday cognitive functioning was assessed using the Cognitive Function Instrument (CFI) study partner version, 11 in which the study partner responded to each question about the patient.
Health‐care resource use and caregiver time were assessed using the Resource Utilization in Dementia (RUD) questionnaire (version 4.0). 12 Trained site personnel administered the RUD to the study partner describing the patient and study partner information. The RUD includes questions on patient and study partner work status; use of hospital, emergency department, and outpatient services; and prescription drug use. Additional information includes caregiving time related to hours spent (averaged over the previous month) helping the patient with basic activities of daily life. Assistance with instrumental activities of daily living was also measured. In addition to measuring overall caregiver time, this instrument was used to calculate costs.
Caregiver burden was assessed at 12, 18, 24, and 36 months using the Zarit Burden Interview (ZBI), 13 which measures subjective caregiver burden in those caring for patients with AD. For this, the study partner completed a questionnaire that assessed caregiver burden. The score ranges from 0 to 88 (higher score indicates greater burden).
2.5. Cost estimation and statistics
Total societal costs included direct medical and non‐medical resources used for patients and direct medical resources and indirect non‐medical resources for the study partners. 5 Hours spent caring for patients or hours of work lost due to caring for the patient were classified as indirect non‐medical resources for the study partners, with the higher number being applied to the calculation of cost. The RUD was administered at baseline, 12, 18, 24, and 36 months. Costs (dollars/month) are estimated based on medical and non‐medical resources used in the 30 days prior to each assessment. Overestimation of caregiver time hours was avoided by allowing the response to caregiver time to truncate hours per day to 24 hours per day minus the reported hours slept. The cost unit for caregiver time was calculated using the opportunity cost approach (summing up the lost productive hours and multiplying them by the national average annual gross hourly wage for workers and by leisure time that was lost for non‐workers [35% of the hourly worker wage]) and the replacement costs approach (summing up the time related to lost productivity and multiplying it by the professional caregiver hourly wage market value for all caregivers regardless of the study partner working status), 5 and if the caregiver was paid for patient care, the hours were subtracted from the production loss time. If a cost component item was not recorded, it was considered 0 in the calculation of total costs.
RESEARCH IN CONTEXT
Systematic Review: The authors reviewed existing literature on health‐care payments for individuals with Alzheimer's disease (AD). Costs and caregiver burden in the earlier symptomatic stages of AD have not been well characterized. Previous analyses of baseline data in the GERAS‐US study in patients with amyloid‐positive mild cognitive impairment due to AD (MCI) or mild dementia due to AD (MILD) showed higher societal costs in MILD versus MCI. Here we examine disease progression and changes in societal costs over 36 months in the GERAS‐US study.
Interpretation: Over 36 months, function and cognition declined meaningfully in MILD but more modestly in MCI. There was an increase in costs and burden, especially with MILD. There was a rise in direct non‐medical costs at 36 months for patients with MILD. Caregiver burden was higher for MILD versus MCI.
Future Directions: Treatments aimed at slowing disease progression in early symptomatic AD may enable patients to stay in earlier stages of the disease longer, thereby delaying the burden associated with disease progression.
2.6. Statistical analysis
The cost analyses were based on total societal costs calculated using the opportunity cost approach. Categorial data are shown as the number of patients with percentages, and continuous variables are shown as mean ± standard deviation. Continuous characteristics were compared with t tests for MCI versus MILD and categorical variables with chi‐square test. Mean 30‐day total societal costs (direct and indirect costs using opportunity formula) were calculated. Mixed model repeated measures (MMRM) was used to estimate the cost by time and disease severity. The model was comprised of effects for time, disease severity, and their interaction. Using the central limit theorem, asymptotic normality was assumed for estimates of means at each time point and resulting P‐values and 95% confidence intervals. An unstructured covariance matrix was used and the denominator degrees of freedom for tests were calculated using the Kenward–Roger approximation. The model was fit at baseline, 6, 12, 18, 24, 30, and 36 months, and costs are presented as mean estimates (least squares [LS] mean) at each time point for the overall population and stratified by baseline severity (MCI vs. MILD). Formal tests for cost trends over time within disease severity are not presented but confidence intervals at each time point are provided. A Spearman correlation coefficient was calculated at various time points for the ZBI with both the RUD assessment and the change of RUD. P‐values < 0.05 were considered statistically significant. SAS Enterprise Guide 7.15 (SAS Institute Inc.) was used for all statistical analyses.
3. RESULTS
3.1. Demographic and socioeconomic characteristics
Patients in the MCI and MILD groups were ≈70 and 72 years old, respectively (Table 1). Approximately 53% of patients in both groups were female, and most patients were White (86% in the MCI group and 88% in the MILD group). Approximately 88% of the patients in the MCI group and 86% of patients in the MILD group had comorbid conditions. The most prevalent comorbid conditions were depression in ≈36% and 40% of patients in the MCI and MILD group, respectively, and sleep disorders in ≈22% and 27% of patients in the MCI and MILD group, respectively.
TABLE 1.
Baseline characteristics.
| Description | MCI N = 300 | MILD N = 317 | Total N = 617 |
|---|---|---|---|
| Age, mean (SD), years | 70.3 (7.4) | 71.7 (8.0) | 71.0 (7.8) |
| Sex, n (%) female | 158 (52.7) | 167 (52.7) | 325 (52.7) |
| Race, n (%) White | 259 (86.3) | 279 (88.0) | 538 (87.2) |
| Time since AD diagnosis, mean (SD), years | 1.0 (1.3) | 1.5 (2.3) | 1.3 (1.9) |
| Time since first symptoms, mean (SD), years | 2.7 (2.4) | 3.2 (3.1) | 3.0 (2.8) |
| Had any comorbid conditions, n (%) | 263 (87.7) | 272 (85.8) | 535 (86.7) |
| Depression, n (%) | 94 (35.7) | 110 (40.4) | 204 (38.1) |
| Sleep disorders, n (%) | 57 (21.7) | 72 (26.5) | 129 (24.1) |
| Number of comorbidities, mean (SD) | 2.3 (1.5) | 2.3 (1.7) | 2.3 (1.6) |
| CFI‐Study partner version (range 20–100) a | 47.0 (14.0) | 63.7 (13.4) | 55.6 (16.0) |
| MMSE (range 0–30) b , mean (SD) | 27.4 (1.8) | 24.4 (2.8) | 25.9 (2.8) |
Abbreviations: AD, Alzheimer's disease; CFI, Cognitive Function Index; MCI, mild cognitive impairment due to Alzheimer's disease; MILD, mild dementia due to Alzheimer's disease; MMSE, Mini‐Mental State Examination; n, number of patients in each category; N, total number of patients in the cohort; SD, standard deviation.
Higher scores equal poorer status.
Lower scores equal poorer status.
3.2. Clinical outcome measures
In both patients with MCI and those with MILD, the mean MMSE and mean CFI scores demonstrated worsening over 36 months (Figure 1). The CFI was higher in the MILD cohort than in the MCI cohort (63.7 vs. 47), indicating greater impairment. Though the MCI cohort showed only a modest decline of 1 point on the MMSE, the MILD cohort showed an approximately 4‐point decline on the MMSE over 3 years, which is considered clinically meaningful. 14 , 15
FIGURE 1.
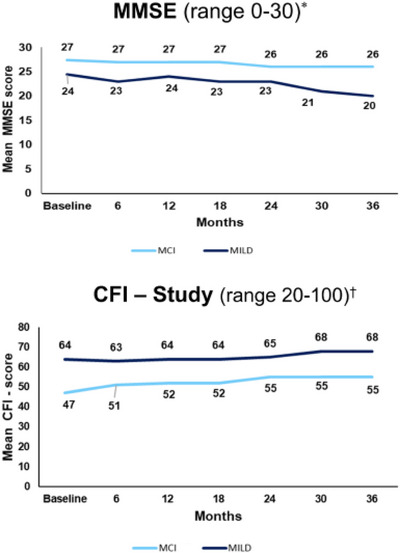
Clinical outcome measures (actual scores) at baseline to 36 months in patients with amyloid [+] MCI and MILD. *Lower scores equal poorer status. †Higher scores equal poorer status. CFI, Cognitive Function Index; MCI, mild cognitive impairment due to Alzheimer's disease; MILD, mild dementia due to Alzheimer's disease; MMSE, Mini‐Mental State Examination; +, positive.
3.3. Total societal costs
From baseline through 36 months, patients with MILD had greater total societal costs per month than those with MCI (Figure 2). The LS mean total societal costs per patient per month remained relatively steady from 6 months (MCI: $1977; MILD: $3032) to 36 months (MCI: $2007; MILD: $3392) after an initial decline from baseline (MCI: $2430; MILD: $4063).
FIGURE 2.
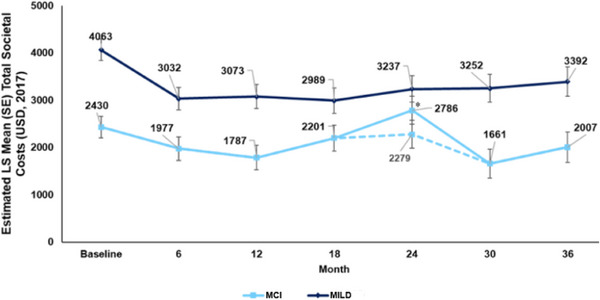
Total societal costs. Data were based on MMRM. The MMRM model was fitted at baseline, 6, 12, 18, 24, 30, and 36 months. Total societal costs were estimated on a 30‐day basis. Total societal costs (1‐month pre‐assessment) were calculated by summing the cost components at each time point. *A large increase in direct non‐medical costs for one MCI patient (structural changes to living accommodations costing 100,000 USD) drove total costs upward at 24 months, which then returned to the 6‐month levels by 36 months. The dashed line (—) represents the analysis with that patient removed. LS, least squares; MCI, mild cognitive impairment due to Alzheimer's disease; MILD, mild dementia due to Alzheimer's disease; MMRM, mixed‐model repeated measures; SE, standard error; USD, US dollar.
3.4. Patient and caregiver costs
Looking at the four components of total societal costs separately (Figure 3), the direct medical costs per person per month appeared to trend downward from the time the patient entered the study (MCI: $1080 [95% confidence interval (CI): 801, 1359]; MILD: $954 [95% CI: 683, 1225]) until the 36‐month time period (MCI: $701 [95% CI: 479, 924]; MILD: $730 [95% CI: 515, 945]; Table 2). Similarly, study partner direct medical costs also appeared to decrease over the course of 3 years from baseline (MCI: $410 [95% CI: −163, 983]; MILD: $861 [95% CI: 304, 1419]) to 36 months (MCI: $169 [95% CI: 11, 327]; MILD: $311 [95% CI: 157, 465]). Patient direct non‐medical costs decreased slightly for MCI and increased for MILD (MCI: $222 [95% CI: 2, 443]; MILD: $295 [95% CI: 81, 509]) over the follow‐up period of 3 years (MCI: $140 [95% CI: −167, 447]; MILD: $390 [95% CI: 93, 687]). There was a spike in direct non‐medical costs for MCI at Month 24. This was related to a large increase in direct non‐medical costs for one MCI patient. This cost involved structural changes to living accommodations costing $100,000 and this drove total costs upward at 24 months. When this patient was removed from the analysis, the cost returned to 6‐month levels by 36 months. For study partner indirect non‐medical costs, the initial decline in the MILD patients was seen in month 12 compared to baseline ($1953 [95% CI: 1741, 2164]) and then began to move upward until month 36 ($1964 [95% CI: 1748, 2180]). In the MCI patients, study partner indirect non‐medical costs changed little over the 3‐year period, but at 36 months these costs tended to be higher (MCI: $961 [95% CI: 736, 1185]) than they were at baseline (MCI: $718 [95% CI: 501, 935]).
FIGURE 3.
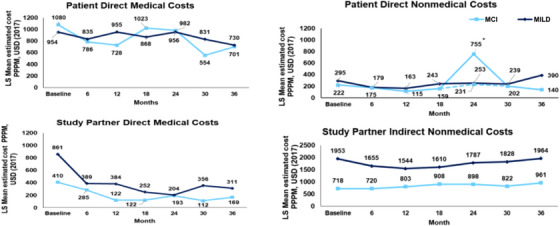
Patient and caregiver costsa over 3 yearsb in amyloid [+] MCI and MILDc. aAll cost estimates are derived from the Resource Utilization in Dementia scale. Opportunity cost sums lost productive hours and multiplies them by national average annual gross hourly wage for workers and by lost leisure time for non‐workers (35% of hourly wage for workers). MMRM was used to estimate the cost by time and disease severity and their interaction in the model. The MMRM model was fitted at baseline, 6, 12, 18, 24, 30, and 36 months. bCalculated per 30‐day basis. cThe classification of MCI and MILD is based on the baseline assessment. NOTE: The spike in direct non‐medical costs at 24 months was due to costly structural changes/adaptations required for the home of one patient at $100,000. The dashed line (—) represents the analysis with that patient removed. LS, least squares; MCI, mild cognitive impairment due to Alzheimer's disease; MILD, mild dementia due to Alzheimer's disease; MMRM, mixed‐model repeated measures; PPPM, per person per month; USD, US dollars; +, positive.
TABLE 2.
Direct and indirect opportunity costs a per 30‐day basis at baseline, 6 months, and 36 months: Amyloid [+] MCI versus MILD. b
| Baseline | 6 months | 36 months | ||||
|---|---|---|---|---|---|---|
| Description | MCI | MILD | MCI | MILD | MCI | MILD |
| Patients | N = 300 | N = 317 | N = 257 | N = 281 | N = 154 | N = 165 |
| Direct medical resources | ||||||
| LS mean estimated cost c (SE), USD (2017) | 1080 (142) | 954 (138) | 786 (83) | 835 (79) | 701 (113) | 730 (109) |
| 95% CI | [801, 1359] | [683, 1225] | [624, 948] | [679, 990] | [479, 924] | [515, 945] |
| Estimated LS mean change from 6 months (SE) | –87 (123) | –59 (118) | ||||
| 95% CI | [−329, 155] | [−291, 173] | ||||
| Median | 392 | 537 | 438 | 716 | 482 | 655 |
| Range | 0, 30,433 | 0, 16,693 | 0, 23,588 | 0, 11,302 | 0, 21,572 | 0, 5794 |
| Direct non‐medical resources | ||||||
| LS mean estimated cost (SE), USD (2017) | 222 (112) | 295 (109) | 175 (121) | 179 (116) | 140 (157) | 390 (151) |
| 95% CI | [2, 443] | [81, 509] | [−63, 413] | [−49, 406] | [−167, 447] | [93, 687] |
| Estimated LS mean change from 6 months (SE) | –34 (78) | 229 (74) | ||||
| 95% CI | [−187, 119] | [82, 375] | ||||
| Median | 0 | 0 | 0 | 0 | 0 | 0 |
| Range | 0, 6069 | 0, 10,390 | 0, 5013 | 0, 7000 | 0, 5000 | 0, 7500 |
| Study partners | ||||||
|---|---|---|---|---|---|---|
| Direct medical resources | N = 300 | N = 316 | N = 257 | N = 281 | N = 154 | N = 165 |
| LS mean estimated cost (SE), USD (2017) | 410 (292) | 861 (284) | 285 (61) | 389 (58) | 169 (73) | 311 (72) |
| 95% CI | [−163, 983] | [304, 1419] | [165, 404] | [274, 504] | [11, 327] | [157, 465] |
| Estimated LS mean change from 6 months (SE) | –118 (94) | –41 (91) | ||||
| 95% CI | [−307, 72] | [−222, 141] | ||||
| Median | 247 | 168 | 177 | 125 | 17 | 17 |
| Range | 0, 8452 | 0, 16,763 | 0, 4751 | 0, 15,105 | 0, 4094 | 0, 6181 |
| Indirect non‐medical resources d | N = 300 | N = 317 | N = 249 | N = 273 | N = 145 | N = 160 |
| LS mean estimated cost (SD), USD (2017) | 718 (111) | 1953 (108) | 720 (94) | 1655 (91) | 961 (114) | 1964 (110) |
| 95% CI | [501, 935] | [1741, 2164] | [535, 905] | [1477, 1834] | [736, 1185] | [1748, 2180] |
| Estimated LS mean change from 6 months (SE) | 177 (127) | 223 (120) | ||||
| 95% CI | [−72, 426] | [−12, 458] | ||||
| Median | 59 | 973 | 177 | 995 | 664 | 1659 |
| Range | 0, 7963 | 0, 13,272 | 0, 8627 | 0, 11,281 | 0, 7963 | 0, 7786 |
Abbreviations: +, positive; CI, confidence interval; LS, least squares; MCI, mild cognitive impairment due to Alzheimer's disease; MILD, mild dementia due to Alzheimer's disease; MMRM, mixed‐model repeated measures; SD, standard deviation; SE, standard error; USD, US dollars.
All cost estimates are derived from the Resource Utilization in Dementia scale. Opportunity cost sums lost productive hours and multiplies them by the national average annual gross hourly wage for workers and by lost leisure time for non‐workers (35% of hourly wage for workers). MMRM was used to estimate the cost by time and disease severity and their interaction in the model. The MMRM model was fitted at baseline, 6, 12, 18, 24, 30, and 36 months.
The classification of MCI and MILD is based on the baseline assessment.
LS mean cost estimates are presented as per person per month.
Costs based on informal caregiver time and work loss.
3.4.1. Caregiver burden using the Zarit Burden Inventory
Caregiver burden was higher for patients with MILD than for those with MCI over the 3‐year period. The score for caregivers of patients with MILD was significantly higher than that for caregivers of patients with MCI over the first 2 years (Figure 4A). The scores for caregiver burden decreased for caregivers of patients with MILD, while these increased for caregivers of patients with MCI over the 3 years. The extent of change was significantly different between patients with MCI and those with MILD at 36 months (LS mean change from baseline, estimate [standard error], MCI: 8.3 [1.33]; MILD: 3.3 [1.29]; P = 0.008). The Spearman correlation coefficient from the ZBI with caregiver time from the RUD showed that this burden positively correlates with the amount of time caregivers spent caring for patients. The correlations at the different time points between caregiver time and ZBI were positive (P < 0.05) for most time points for both MCI and MILD patients combined.
FIGURE 4.
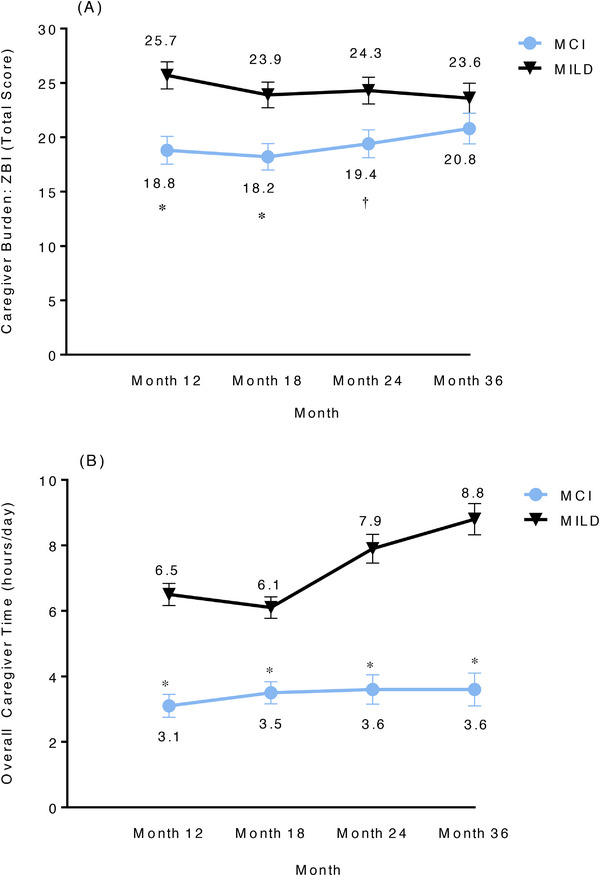
A, Zarit Burden Interview (total score)—actual value. B, Resource Utilization in Dementia questionnaire (overall caregiver time)—actual value. Data were based on MMRM. Actual value = disease severity (SD) + time (as categorical) + SD × time; the values represent the LS mean estimate at time points (SE). *P < .001, †P < .01. LS, least squares; MCI, mild cognitive impairment due to Alzheimer's disease; MILD, mild dementia due to Alzheimer's disease; MMRM, mixed‐model repeated measures; SD, standard deviation; SE, standard error; ZBI, Zarit Burden Interview.
3.4.2. Overall caregiver time
The overall caregiver time (hours/day) using the RUD showed that there was a significant difference in the LS mean estimate (standard error [SE]) of overall caregiver time between patients with MCI and those with MILD at 12 (3.1 [0.35] vs. 6.5 [0.34]), 18 (3.5 [0.34] vs. 6.1 [0.33]), 24 (3.6 [0.45] vs. 7.9 [0.44]), and 36 (3.6 [0.50] vs. 8.8 [0.48]) months (P < 0.001; Figure 4B). The caregiver time for patients with MILD was greater at 36 months (LS mean estimate [SE], 8.8 [0.48]) than at 6 months (6.6 [0.33]).
4. DISCUSSION
This study examined disease progression and changes in societal costs over 36 months in patients with biomarker‐confirmed early symptomatic AD and their caregivers who participated in the GERAS‐US study. Longitudinal studies have described socioeconomic and caregiver burden in cohorts of patients with AD who were diagnosed with the AD clinical syndrome but did not have biomarker confirmation of amyloid positivity. To our knowledge, this is the first study to characterize the socioeconomic and caregiver experience in a biomarker‐confirmed, early symptomatic AD cohort.
In this early symptomatic AD cohort, patients with MILD had a meaningful decline in cognition over 36 months while patients with MCI did not. The CFI, a measure of difficulty with complex activities of daily living, showed a similar pattern, where there was a more meaningful decline in cognition among patients with MILD but only a modest decline among patients with MCI.
Previous studies have shown an increase in costs in patients with AD‐related dementias and in those with MCI in the year leading up to diagnosis compared to matched controls. 16 , 17 This rise in costs related to increasing symptoms leads to several health‐care expenditures, including inpatient visits. 18 , 19 A study examining costs in Medicare patients with AD‐related dementia or MCI during a 2‐year follow‐up period found increased costs associated with caring for these patients. 18 Costs are highest in the year before and 6 months after diagnosis, then decline to some extent until these become more stable for a while and then begin to climb as the disease progresses. 20 , 21 The initial decline is likely due to more efficient clinical management and care planning after diagnosis. 22 In our study, the mean estimated total societal costs initially decreased compared to baseline. However, in the period from 6 to 36 months, the costs then remained relatively stable after 6 months although they increased slightly after 18 months in patients with MILD. Total societal costs were consistently higher for patients with MILD than for those with MCI, demonstrating the added economic burden with advancing disease even in earlier stages.
Direct medical costs for patients remained steady over the follow‐up period after an initial decline from baseline, possibly due to diagnostic certainty achieved at baseline. The definitive clinician diagnosis of MCI or MILD and a confirmed amyloid‐positive PET scan may have led to a more efficient care plan, hence the costs did not increase substantially over the 3‐year study period.
The rise in direct non‐medical costs at 36 months for patients with MILD and the trend toward rising indirect caregiver costs in those with MCI and MILD after an initial decline suggest additional economic impact beyond direct medical expenses that are associated with clinical progression in early symptomatic AD. Direct non‐medical costs and indirect caregiver costs include those for services such as nursing aide, home health care, food delivery, transportation, and caregiver time including unpaid volunteer time to care for the patient. Unexpected non‐medical expenses, such as home adaptations to accommodate patient needs, can accompany medical and other caregiver expenses as part of the comprehensive care strategy for the patient. Overall, the increase in cost and burden over 3 years among patients with MILD is greater than the increase seen in MCI over the same period. Although patients with MCI have less cognitive and functional impairment than those with MILD, 23 this study shows that indirect medical costs for the study partner in both the MCI and MILD cohorts trended upward over the 3‐year period. We examined the impact of the indirect cost component related to caregiver time and the burden associated with it. The increase in indirect non‐medical costs was important to examine because despite costs such as the patient direct medical and non‐medical costs as well as caregiver direct medical costs decreasing or remaining steady over 3 years, the increase in the caregiver indirect non‐medical costs (caregiver time) was associated with increasing burden as measured by ZBI, demonstrating the additional stress of steady disease progression in early symptomatic AD. Caregivers for patients with MILD had a greater burden than caregivers for patients with MCI. This finding reflects the impact of disease progression on caregivers beyond the hours spent caring for the patient. A previous study that described direct medical costs according to the severity of cognitive impairment found that direct medical costs increased over 2 years after the initial consultation in patients with AD dementia, and caregiver burden and direct medical costs increased from subjective cognitive complaint to AD dementia. 24 These data are important to better understand the economic burden of AD earlier in the disease course and the potential value of therapies that delay disease progression.
Potential limitations of the findings of this study need to be considered. These findings may not be generalizable to all community‐dwelling patients with AD. The GERAS‐US sample was largely White with only 13% of patients identifying as Black, Asian, or Other. 6 However, ≈21% of patients with MCI and > 40% of those with MILD identified as Hispanic or Latino. 6 Future studies should strive to include a racially and ethnically diverse population to fully characterize disease progression in early symptomatic AD. Attrition is also a potential limitation, as persons who dropped out of the study may have been sicker, leaving a relatively healthier cohort from which to estimate progression and associated costs over 3 years. The COVID‐19 pandemic may have also impacted the results of the study, because participants and caregivers may not have sought medical care otherwise needed during this time because of stay‐at‐home requirements and medical practice limitations. For participants and caregivers who could not come to sites for their regularly scheduled study visits, assessments were carried out by phone when feasible. Certain assessments such as the MMSE could not be completed by phone. The dropout rate during this time was higher than expected, and it is possible that sicker patients and their caregivers were those who were more likely to drop out.
Overall, function and cognition declined significantly over 36 months in patients with MILD. There was a rise in direct non‐medical costs at 36 months for patients with MILD. Direct medical costs remained steady over the follow‐up period after an initial decline from baseline, possibly because of diagnostic certainty achieved at baseline. The rise in direct non‐medical costs for patients with MILD and the trend toward rising indirect caregiver costs among patients with MCI and MILD over 36 months show the additional economic impact associated with clinical progression in early symptomatic AD. These findings demonstrate the broad impact of disease progression in early symptomatic AD especially on indirect costs related to caregiver time. Disease‐modifying treatments that can slow advancement of the disease for patients in the early symptomatic stages would delay the economic and caregiver burden associated with disease progression and enable patients and their caregivers to stay in a relatively early stage of disease for a longer duration.
CONFLICT OF INTEREST STATEMENT
Ronald L. Schwartz and Howard Fillit (unpaid) are investigators, consultants, and/or advisors of Eli Lilly and Company. Dorene M. Rentz is a consultant at Eli Lilly and Company, Biogen Idec, and Digital Cognition Technologies and was involved in a Scientific Advisory Board for Neurotrack. Julie M. Chandler and Anthony Zagar are employees and minor stockholders of Eli Lilly and Company. Yongin Kim is a former employee and minor stockholder of Eli Lilly and Company. Author disclosures are available in the supporting information.
CONSENT STATEMENT
All human subjects provided informed consent.
Supporting information
ACKNOWLEDGMENTS
The authors thank the study investigators, patients, site personnel, and study partners for participating in this study. This study was funded by Eli Lilly & Company, Indianapolis, IN, USA.
Chandler JM, Rentz DM, Zagar A, Kim Y, Schwartz RL, Fillit H. Disease progression and costs at the 3‐year follow‐up of the GERAS‐US study. Alzheimer's Dement. 2023;15:e12430. 10.1002/dad2.12430
The findings of this manuscript have been presented in part at the 2021 Alzheimer's Association International Conference.
Study Registry: H8A‐US‐B004; ClinicalTrials.gov: NCT02951598
REFERENCES
- 1. Argimon JM, Limon E, Vila J, Cabezas C. Health‐related quality of life in carers of patients with dementia. Fam Pract. 2004;21(4):454‐457. [DOI] [PubMed] [Google Scholar]
- 2. Schulz R, Beach SR. Caregiving as a risk factor for mortality: the Caregiver Health Effects Study. JAMA. 1999;282(23):2215‐2219. [DOI] [PubMed] [Google Scholar]
- 3. Wimo A, Reed CC, Dodel R, et al. The GERAS Study: a prospective observational study of costs and resource use in community dwellers with Alzheimer's disease in three European countries – study design and baseline findings. J Alzheimers Dis. 2013;36(2):385‐399. [DOI] [PubMed] [Google Scholar]
- 4. Reed C, Happich M, Argimon JM, et al. What drives country differences in cost of Alzheimer's disease? An explanation from resource use in the GERAS study. J Alzheimers Dis. 2017;57(3):797‐812. [DOI] [PubMed] [Google Scholar]
- 5. Robinson RL, Rentz DM, Andrews JS, et al. Costs of early stage Alzheimer's disease in the United States: cross‐sectional analysis of a prospective cohort study (GERAS‐US)1 . J Alzheimers Dis. 2020;75(2):437‐450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Robinson RL, Rentz DM, Bruemmer V, et al. Observation of patient and caregiver burden associated with early Alzheimer's disease in the United States: design and baseline findings of the GERAS‐US Cohort Study. J Alzheimers Dis. 2019;72(1):279‐292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Johnson KA, Minoshima S, Bohnen NI, et al. Appropriate use criteria for amyloid PET: a report of the Amyloid Imaging Taskforce, the Society of Nuclear Medicine and Molecular Imaging, and the Alzheimer's Association. J Nucl Med. 2013;9:476‐490. [DOI] [PubMed] [Google Scholar]
- 8. Folstein MF, Folstein SE, McHugh PR. Mini‐mental state: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189‐198. [DOI] [PubMed] [Google Scholar]
- 9. Pfeffer RI, Kurosaki TT, Harrah CH Jr, Chance JM, Filos S. Measurement of functional activities in older adults in the community. J Gerontol. 1982;37(3):323‐329. [DOI] [PubMed] [Google Scholar]
- 10. Teng E, Becker BW, Woo E, Knopman DS, Cummings JL, Lu PH. Utility of the functional activities questionnaire for distinguishing mild cognitive impairment from very mild Alzheimer's disease. Alzheimer Dis Assoc Disord. 2010;24(4):348‐353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Amariglio RE, Donohue MC, Marshall GA, et al. Tracking early decline in cognitive function in older individuals at risk for Alzheimer disease dementia: the Alzheimer's Disease Cooperative Study Cognitive Function Instrument. JAMA Neurol. 2015;72(4):446‐454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wimo A, Wetterholm AL, Mastey V, Winblad B. Evaluation of the resource utilization and caregiver time in anti‐dementia drug trials – a quantitative battery. In: Wimo A, Karlsson G, Jonsson B, Winblad B, eds. The Health Economics of Dementia. Wiley; 1998:465‐499. [Google Scholar]
- 13. Zarit SH, Reever KE, Bach‐Peterson J. Relatives of the impaired elderly: correlates of feelings of burden. Gerontologist. 1980;20(6):649‐655. [DOI] [PubMed] [Google Scholar]
- 14. Burback D, Molnar FJ, St John P, Man‐Son‐Hing M. Key methodological features of randomized controlled trials of Alzheimer's disease therapy. Minimal clinically important difference, sample size and trial duration. Dement Geriatr Cogn Disord. 1999;10(6):534‐540. [DOI] [PubMed] [Google Scholar]
- 15. Carcaillon L, Pérès K, Péré JJ, Helmer C, Orgogozo JM, Dartigues JF. Fast cognitive decline at the time of dementia diagnosis: a major prognostic factor for survival in the community. Dement Geriatr Cogn Disord. 2007;23(6):439‐445. [DOI] [PubMed] [Google Scholar]
- 16. Geldmacher DS, Kirson NY, Birnbaum HG, et al. Pre‐diagnosis excess acute care costs in Alzheimer's patients among a US Medicaid population. Appl Health Econ Health Policy. 2013;11(4):407‐413. [DOI] [PubMed] [Google Scholar]
- 17. Taipale H, Purhonen M, Tolppanen AM, Tanskanen A, Tiihonen J, Hartikainen S. Hospital care and drug costs from five years before until two years after the diagnosis of Alzheimer's disease in a Finnish nationwide cohort. Scand J Public Health. 2016;44(2):150‐158. [DOI] [PubMed] [Google Scholar]
- 18. Lin PJ, Zhong Y, Fillit HM, Chen E, Neumann PJ. Medicare expenditures of individuals with Alzheimer's Disease and related dementias or mild cognitive impairment before and after diagnosis. J Am Geriatr Soc. 2016;64(8):1549‐1957. [DOI] [PubMed] [Google Scholar]
- 19. Leibson CL, Long KH, Ransom JE, et al. Direct medical costs and source of cost differences across the spectrum of cognitive decline: a population‐based study. Alzheimers Dement. 2015;11(8):917‐932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gilden DM, Kubisiak JM, Sarsour K, Hunter CA. Diagnostic pathways to Alzheimer disease: costs incurred in a Medicare population. Alzheimer Dis Assoc Disord. 2015;29(4):330‐337. [DOI] [PubMed] [Google Scholar]
- 21. Albert SM, Glide S, Andrews H, Stern Y, Mayeux R. Primary care expenditures before the onset of Alzheimer's disease. Neurology. 2002;59(4):573‐578. [DOI] [PubMed] [Google Scholar]
- 22. Black CM, Lipton RB, Thiel E, Brouillette M, Khandker R. Relationship between treatment initiation and healthcare costs in Alzheimer's disease. J Alzheimers Dis. 2019;68(4):1575‐1585. [DOI] [PubMed] [Google Scholar]
- 23. Brown PJ, Devanand DP, Liu X, Caccappolo E, Alzheimer's Disease Neuroimaging Initiative . Functional impairment in elderly patients with mild cognitive impairment and mild Alzheimer disease. Arch Gen Psychiatry. 2011;68(6):617‐626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dauphinot V, Potashman M, Levitchi‐Benea M, Su R, Rubino I, Krolak‐Salmon P. Economic and caregiver impact of Alzheimer's disease across the disease spectrum: a cohort study. Alzheimers Res Ther. 2022;14(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


