Abstract
Although obesity is a preventable disease, maintaining a normal body weight can be very challenging and difficult, which has led to a significant increase in the demand for surgical subcutaneous fat removal (SSFR) to improve physical appearance. The need for SSFR is further exacerbated because of the global rise in the number of bariatric surgeries, which is currently the single most durable intervention for mitigating obesity. Fat tissue is now recognized as a vital endocrine organ that produces several bioactive proteins. Thus, SSFR-mediated weight (fat) loss can potentially have significant metabolic effects; however, currently, there is no consensus on this issue. This review focuses on the metabolic sequelae after SSFR interventions for dealing with cosmetic body appearance. Data were extracted from existing systematic reviews and the diversity of possible metabolic changes after SSFR are reported along with gaps in the knowledge and future directions for research and practice. We conclude that there is a potential for metabolic sequelae after SSFR interventions and their clinical implications for the safety of the procedures as well as for our understanding of subcutaneous adipose tissue biology and insulin resistance are discussed.
Keywords: Surgical subcutaneous fat removal (SSFR), body contouring surgery, metabolism, insulin resistance, inflammation, adipokines
Introduction
Obesity has reached pandemic levels and currently affects all age groups and socioeconomic classes worldwide. Obesity prevalence has almost tripled in the last 50 years according to the World Health Organization and this, in turn, has led to more fatality than malnutrition and being underweight combined [1]. The rising obesity rate has led to a substantial rise in metabolic diseases, such as diabetes mellitus type 2 (T2D), hypertension, cardiovascular disease, non-alcoholic hepato-steatosis, and dyslipidemia [2].
Lipids comprise a wide range of molecules, such as phospholipids, fatty acids, and triglycerides [3]. These molecules represent a highly efficient energy resource. Recent studies have advanced our view of adipose tissue from being simply an energy store, into an active endocrine organ, which secretes several metabolically active adipokines, such as leptin, adiponectin, and resistin. The latter plays an essential role in glucose hemostasis and energy metabolism in our body [2]. These molecules have been ascribed to have a critical role in energy homeostasis through communication with organs that maintain system-wide metabolic homeostasis such as the liver. Of the adipocyte-derived factors, adiponectin and leptin are among the essential adipokines. Indeed, adiponectin analogs are now considered one of the promising new therapeutic targets for obesity-linked hyperglycemia, that mitigates obesity and improves insulin sensitivity [4].
Insulin resistance, as a consequence of such dysregulation associated with obesity, is what links the latter to T2D. Insulin resistance leads to dysregulation of glucose homeostasis via a combination of impaired glucose clearance and elevated glucose production in the liver. Adipose tissue is a major contributor to insulin sensitivity/resistance status. Too little fat mass, as seen in patients with lipodystrophy, results in a severe form of insulin resistance, and too much adipose mass can also result in a similar condition [5]. The primary reason for the latter form of insulin resistance may be hypoxia in adipose tissue that leads to inflammatory lipo-toxicity [6].
Currently, it is unknown if the removal of excess subcutaneous fat tissue through surgical subcutaneous fat removal (SSFR; also known as body contouring surgeries such as liposuction or abdominoplasty) ameliorates the mass of hypoxic fat thus reducing its consequences. Such surgeries have become very common because, although obesity can be prevented, maintaining a normal body weight can be very challenging and difficult and the increase in demand for SSFR has been driven by patients seeking an improved physical appearance [7]. However, the precise effect of sudden removal of a patient’s body fat on metabolism is still not fully understood.
Surgical subcutaneous fat removal
The current drift toward cosmetic plastic surgeries, especially the body contouring surgeries which aim to produce a more attractive body shape by removing the excess of skin and fat tissue from multiple body areas, is due to several reasons such as the increase in the safety of these procedures, the increase in the availability of these operations, and largely due to the recent increase in the number of bariatric surgeries. Bariatric surgery is performed for morbidly obese patients to facilitate loss of a significant amount of their body fat mass. Because of the rapid and massive weight loss following bariatric surgery such as sleeve gastrectomy, many patients tend to require body contouring plastic surgery to remove redundant skin and excess body fat [8]. The body-contouring surgery is also done for purely cosmetic purposes in patients not undergoing bariatric surgery.
A typical example of these body contouring surgeries is the abdominoplasty (as known as Tummy Tuck) surgery which suddenly removes around 2–3 kg of abdominal subcutaneous fat (ASF) tissue, and usually is followed by tightening of the abdominal wall muscles, to correct divarication of recti muscles [9]. The other commonly undertaken surgery is suction-assisted lipectomy and, together with abdominoplasty, these represent the commonest plastic surgery procedures that target subcutaneous fat from unwanted areas such as the abdominal wall and flanks. The accelerating demand for these surgical procedures has gradually moved the practice from removing a small amount of intractable fat tissue to the removal of a large volume (more than five liters) of subcutaneous fat tissue, which eventually can result in a significant metabolic effect [10]. However, whether the metabolic effects of these two surgeries are the same or different is not known. In fact, previous reviews and meta-analyses (MAs) have combined these two procedures together which might not be accurate. For example, the repair of the abdominal wall in abdominoplasty might result in an increased intra-abdominal pressure with reduced space for the future expansion of intra-abdominal fat tissue, which might result in different metabolic effects than liposuction [10, 11].
Finally, a distinction needs to be made between SSFR and other modalities of fat loss (such as diet, exercise, or bariatric surgeries) in that non-SSFR modalities result in a gradual decrease in both the subcutaneous and intra-abdominal fat tissue. This gradual reduction occurs through a decrease in the size of the adipocytes while with SSFR there is actual loss of subcutaneous adipocyte numbers, but without impact on intra-abdominal adipocytes.
Fat removal sites in SSFR
SSFR classically is from abdominal and thigh areas, although other sites may less commonly be targets for surgery. Abdominal (or upper-fat) distribution is correlated more strongly with obesity-associated metabolic risks and consequences than the gluteo-femoral (or lower-fat) distribution in the gluteal and thigh regions [12]. Fat in the abdomen may be subcutaneous (ASF) or as abdominal visceral fat (AVF) tissue and it should be noted that only ASF is the target for abdominal SSFR [9]. AVF is intraperitoneal fat that represents both the mesenteric as well as the omental fat cells [13]. AVF is typically formed of large adipocytes and contains necrotic and inflammatory tissues. There is also retroperitoneal fat in humans of unclear significance.
Central obesity in the abdominal area represents one of the essential components of metabolic syndrome, along with insulin resistance, elevated serum triglyceride, blood pressure, and low high-density lipoproteins. The distribution of fat deposits in the abdomen (ASF vs AVF) has thus been thought to determine metabolic outcomes and that AVF tissue is more “pathogenic” [14] and is what has been linked to metabolic syndrome and T2D [15]. Other studies have also proposed that both ASF and AVF play a role in metabolic risk [10] but largely the metabolic risk of obesity has been linked mainly to AVF because it is directly involved in the delivery of free fatty acids as well as inflammatory proteins such as interleukin-6 (IL-6), to the liver via the portal circulation [16]. It is nevertheless probable that ASF may also play a role given that more than 80% of the free fatty acids and other inflammatory proteins reach the liver via the systemic circulation [17]. This is supported by studies that report the intrahepatic triglyceride rather than AVF is a better marker for obesity-associated metabolic risk [18]. Therefore, it has recently been suggested that the metabolic risk in obesity is a shared effect of molecules secreted by both these compartments. Thus, there is an expectation that SSFR may alter glucose homeostasis and insulin resistance as a direct consequence of surgical ASF removal.
Potential for metabolic sequelae after SSFR
Research has found that even a small weight loss of ten percent can result in a significant improvement of obesity-linked metabolic abnormalities, such as insulin resistance, high blood pressure, and abnormal inflammatory marker levels [19, 20]. Additionally, increased knowledge of the metabolic consequences of excess body fat and observations after bariatric surgeries [21] have suggested that there could possibly be a similar effect after SSFR. This has been examined in several studies, which measure hormonal changes before and after SSFR at different time points. These studies have been small and heterogeneous and have reported inconsistent effects on metabolic parameters, such as insulin resistance, adipokine levels, and inflammation [22–34] To improve power and resolve the inconsistency, these studies have been combined in several syntheses, both systematic reviews (SRs) and MAs. The aim of this umbrella review therefore is to now examine these syntheses and summarize their findings as well as define current knowledge gaps in the metabolic impact of SSFR, particularly, changes in insulin resistance, inflammatory markers, and adipokines levels.
Materials and methods
Study inclusion and exclusion criteria
A search was conducted for evidence syntheses that synthesized data on the metabolic changes after SSFR. PubMed, Embase, and Scopus databases were searched without any date, language, or publication restriction but exclusion of non-English and animal studies, as well as non-surgical body fat removal and bariatric surgeries.
Search strategy
Search was conducted on 8 November 2021 by two independent authors using the polyglot Search Translator [35]. The search strings used are given in the supplementary material (Figure S1) for the syntheses that report changes in insulin sensitivity, inflammatory markers, and adipokines levels after SSFR. Data were extracted regarding synthesis type (SR or MA), title and author, year of publication, type of SSFR, a summary of included studies, follow-up duration after SSFR, and possible evidence gaps. Main findings were summarized regarding metabolic changes in terms of potential inflammatory and anti-inflammatory adipokines and other metabolic markers.
Quality assessment
A MeaSurement Tool to Assess systematic Reviews-2 (AMSTAR-2) was used to assess the quality of the included reviews and each included synthesis was examined against 16 quality safeguards to assess their methodological quality [36].
Data synthesis
A structured summary of findings was done for the eligible and included SRs and MAs. Metabolic change findings were assessed in three categories: insulin resistance, inflammatory markers, and adipokines. For each of the categories, a separate table of findings was formulated.
Results
Study selection
A search in the three databases: PubMed, Embase, and Scopus on (08/11/2021) resulted in 444 studies. A total of 186 duplicate studies were excluded. The remaining 258 articles were screened by title and manuscript for eligibility of which six met inclusion criteria. One synthesis was in French and was excluded from this umbrella review [37], while another was excluded as it reported changes in weight and fat mass only [38]. There were thus three MAs and one SR included, and Figure 1 depicts the PRISMA flow diagram for the selection of studies.
Figure 1.
PRISMA flow diagram of the study selection process.
The first synthesis was conducted in 2013 [39], and since then, another three syntheses have been published [10, 11, 40]. None of the four included syntheses (15, 14, 12, and 11 studies included) examined the time trend after SSFR, and thus they looked at metabolic changes through quantitative analyses (if any) did not consider the heterogeneity in follow-up duration across studies. This umbrella review summarizes the changes reported in three categories: insulin resistance, inflammatory markers, and adipokines levels. Quality assessment of the included syntheses demonstrated that most of them included PICO components in the review, explanation of inclusion criteria, justification for the excluded studies, use of a satisfactory quality assessment tool in studies included in the review, and adequate description of the included studies. See supplementary material Figure S2.
Impact of SSFR on insulin resistance
Several studies have measured changes in insulin resistance status after SSFR using different tests, such as measuring fasting glucose, fasting insulin, and the Homeostatic Model Assessment for Insulin Resistance (HOMA-IR) [31, 34, 41], insulin tolerance test (ITT) [42], oral glucose tolerance test [30, 43], and the gold standard glucose clamp test [29, 44]. Apart from the glucose clamp test, most of these tests are not accurate in assessing the change in insulin sensitivity, and the studies that used the glucose clamp test had a small sample size and a lot of variability among participants in terms of diabetic status, and degree of obesity. The challenge behind using accurate tests such as the hyper insulinemic glucose clamp and the intravenous glucose tolerance test is the fact that they are very demanding [45].
Across three MAs and one SR examining the effect of SSFR on insulin sensitivity, most of the evidence suggests a possible improvement in obesity-associated insulin resistance, however, there was a lack of clarity regarding the extent of the effect and clinical significance. This was because there were major problems in the design and analysis of the MAs and therefore results couldn’t be thus interpreted. In terms of the SRs, there was no clarity on the extent of the changes across the studies since there was a focus on statistical significance only. In summary, syntheses were inconsistent, there was a trend toward improvement in insulin sensitivity, and the clinical extent or duration of any improvement remains unclear. The impact of SSFR on insulin resistance thus remains unknown given the data reported in (Table 1) and we recommend that a dose response MA be conducted to answer this question.
Table 1.
Syntheses that report changes in insulin sensitivity after SSFR
| Synthesis author and year | Synthesis type | Type of SSFR | Included studies | Follow up | Main finding | Remaining evidence gaps | |
|---|---|---|---|---|---|---|---|
| 1 | Sailon et al. 2017 [10] | SR | Liposuction | Ten prospective studies (346 participants), which examined large volume liposuction (>3.5 liters) | 3 weeks–6 months | Author reported conflicting results but stated that surgical fat removal by large volume liposuction can improve insulin sensitivity. No clear extent of change was reported. | This SR focused examining the statistical significance of these changes post SSFR, without reporting the extent of change, or its clinical importance. The review had substantial heterogeneity in terms of participants baseline characteristics, included studies sample size, and different assessment tools for insulin resistance. |
| 2 | Seretis et al. 2015 [11] | MA | Liposuction + Abdominoplasty | Four studies (140 participants) | 2 months–2 years | Fasting glucose levels changes after SSFR were not statistically significant (1.42, 95% CI: −1.57, 4.40). Changes in insulin sensitivity were also assessed either by insulin tolerance test or HOMA index, however the result reported a lack of significant change after SSFR (0.14, 95% CI -0.69–0.96). | This MA included studies that were so contrived in terms of control group that no conclusion was possible. The small number of studies limited its validity and prevented subgroup analysis according to certain confounders such as age or BMI. |
| 3 | Boriani et al. 2014 [40] | MA | Liposuction | Five prospective studies (190 participants) | 3 months–1 year | Fasting insulin levels were significantly higher before SSFR by a weighted mean difference of 3.49 mIU/ml (95% CI 1.12, 5.87). | There was a degree of heterogeneity among studies (p ═ 0.02, I2 ═ 67%). Fasting insulin levels were used as a surrogate for insulin resistance, which is an indirect measure. |
| 4 | Danilla et al., 2013 [39] | MA | Liposuction | Five quasi experiment studies (111 participants) | 3 weeks–1 year | Analysis reported that SSFR result in decreased fasting insulin levels, and the amount of reduction was associated with the amount of aspirated fat, independent with the baseline BMI. No significant change was reported in HOMA-IR levels after SSFR. | Although this MA studied the effect of time on the SSFR induces changes in insulin resistance, the sample size of the included studies was small. |
SSFR: Surgical subcutaneous fat removal; SR: Systemic review; MA: Meta-analysis; CI: Confidence interval; BMI: Body mass index; HOMA-IR: Homeostatic model assessment for insulin resistance.
Impact of SSFR on inflammation
Obesity is associated with chronic low-grade inflammation. This is a result of the increased influx of immune cells to the fat tissue, as well as the increased secretion of inflammatory cytokines such as tumor necrosis factor-alpha (TNF-α) [2]. Adipocytes have an equal proinflammatory effect on the macrophages [46]. This inflammatory status is thought to be the mechanism behind most of obesity-linked metabolic disorders [2].
One SR and one MA examined the effect of SSFR on multiple inflammatory markers such as TNF-α, C-reactive protein (CRP), and IL-6, and the findings are detailed in Table 2. In summary, the syntheses combined heterogeneous studies with different follow-up times. Conclusions varied between no change after SSFR or lower levels of IL-6 and TNF-α after surgery. However, the extent and time-trend were not reported, thus a dose response MA remains a needed future task.
Table 2.
Syntheses that report changes in inflammatory markers after SSFR
| Synthesis author and year | Synthesis type | Type of SSFR | Included studies | Follow up | Main finding | Remaining evidence gaps | |
|---|---|---|---|---|---|---|---|
| 1 | Sailon et al. 2017 [10] | SR | Liposuction | Four prospective studies (210 participants). The review examined the effect of large volume liposuction (more than 3.5 liters) on IL-6 and TNF-α | 10 weeks–6 months | Two studies reported a statistically significant decrease in plasma IL-6 and TNF-α levels. | Neither a clear extent of change nor the clinical significance was reported. |
| 2 | Danilla et al. 2013 [39] | MA | Liposuction | Eight prospective studies (239 participants) examined the changes in CRP (4 studies), IL-6 (3 studies), and TNF-α (3 studies) | 1–6 months | No association between the amount of aspirated fat and serum levels of CRP, IL-6, and TNF-α. | No clear report on the results, rather than just a general conclusion of no association. |
SR: Systemic review; MA: Meta-analysis; CI: Confidence interval; CRP: C-reactive protein; IL-6: Interleukin 6; TNF-α: Tumor necrosis factor-alpha; SSFR: Surgical subcutaneous fat removal.
Impact of SSFR on adipokine levels
Changes in the adipokines have been examined by only one SR and one MA, and both reported a reduction of leptin levels after SSFR. However, there was heterogeneity in the reported changes in other adipokines, such as adiponectin and resistin (Table 3).
Table 3.
Syntheses that report changes in adipokines after SSFR
| Synthesis author and year | Synthesis Type | Type of SSFR | Included studies | Follow up | Main finding | Remaining evidence gaps | |
|---|---|---|---|---|---|---|---|
| 1 | Sailon et al. 2017 [10] | SR | Liposuction | Five prospective studies (225 participants) examined the effect of large volume liposuction (>3.5 liters) on adipokines levels (namely leptin and adiponectin) | 10 weeks–6 months | Leptin was examined by 4 studies, which all reported a statistically significant reduction. Adiponectin was assessed in all studies, two of which reported a significant increase. | Neither a clear extent of change, nor the clinical significance was reported. Other adipokines were not assessed. |
| 2 | Danilla et al. 2013 [39] | MA | Liposuction | Six quasi experiment studies (191 participants) examined the effect of SSFR on leptin levels | 6 weeks–6 months | The MA showed a statistically significant reduction in leptin levels (Coefficient: 0.18). This reduction was proportional to the amount of aspirated fat, and patient BMI. | The study didn’t report the changes in other adipokines, nor the clinical significance of the reported changes. |
SR: Systemic review; MA: Meta-analysis; SSFR: Surgical subcutaneous fat removal; BMI: Body mass index.
Summary of findings
This umbrella review summarizes four attempts at evidence synthesis on the metabolic changes after surgical fat removal, with a total of 29 unique studies included and 759 total participants. There was a possible improvement in obesity-associated insulin resistance, however, there was a lack of clarity regarding the extent of the effect and clinical significance. Nevertheless, it seems likely that ASF removal is associated with improved insulin sensitivity. In terms of inflammation, one of the two syntheses reported that ASF removal results in a lower degree of IL-6 and TNF-α, and thus potentially a more favorable metabolic risk profile. These syntheses also reported a reduction of leptin levels after ASF removal through surgery. There was heterogeneity in the reported changes in other adipokines, such as adiponectin and resistin. Clearly, the data from the previous studies are not conclusive, nevertheless, it seems likely that SSFR is associated with improved insulin sensitivity and lower levels of inflammatory cytokines.
Implications for future research
The role of ASF vs AVF in human metabolism
The central obesity in the abdominal area represents one of the essential components of metabolic syndrome, along with insulin resistance, elevated serum triglyceride, blood pressure, and low high-density lipoproteins, and it is distributed between the ASF and AVF compartments [11] Although some studies have linked the metabolic risk of obesity mainly to the AVF tissue [16, 47], others have proposed that both AVF and ASF play a role in metabolic risk [10]. Generally, subcutaneous fat mass is more than twice the visceral fat mass, especially among females [48]. As a result, 85% of bloodstream free fatty acids are coming from the subcutaneous fat stores, which is a major contributor to systemic insulin resistance by inhibiting glucose uptake by skeletal muscles [49]. There is evidence from some studies among healthy men [50] and those with T2DM [51] that ASF may be more strongly correlated with insulin resistance than AVF. There has also been a report from a study of a healthy cohort of mixed genders that ASF correlates with insulin resistance independently of AVF, but not the other way around [52]. To sum this up, there is some evidence from the umbrella review as well as other studies suggesting that ASF may make an important contribution to obesity-related metabolic change, and this thus can be a mechanism through which SSFR can create a more favorable metabolic profile.
When studies have looked directly at the added impact of AVF on metabolism, by examining the effect of adding omentectomy to bariatric procedures, results were inconsistent. Some studies reported that it could result in better glucose homeostasis and lower inflammatory markers [53, 54]. Conversely, others reported a lack of clinical improvement in the metabolic profile [55–57]. Many open questions remain therefore about the role of AVF vs ASF and part of the problem lies in their study design, for example, the lack of clarity regarding patient selection, determining the type of surgery, the parameters that needed to be measured, and accounting for patient factors [58]. In addition, there were also technical limitations of older studies regarding advanced imaging technologies to measure visceral adipose tissue accurately. At a more fundamental level, improved knowledge of all aspects of adipose biology, including adipose tissue cellular heterogeneity [59, 60] as well as divergent responses to metabolic and endocrine stimuli that will be required to make significant advances and resolve the problem highlighted above [61]. In addition, a recent genome-wide association study also shows the contribution of genetics to visceral adiposity and its relation to ethnicities and gender in the context of metabolic disease. In particular, the study suggests that increased AVF is more harmful compared with ASF, but it is not clear why this should be the case [62].
Adipokines
To determine why SSFR impacts adipokines levels, one needs to understand the roles of adipocyte-derived factors, as well as their effects on intermediary metabolism. Adipocyte-derived factors need to be understood in terms of source, relation to obesity, and main function. Tables 4 and 5 summarize the inflammatory and anti-inflammatory adipokines, the most well-known candidates are leptin and IL-6.
Table 4.
Description of the potential inflammatory adipokines
| Hormone | Source | Observed changes in obesity | Main function | |
|---|---|---|---|---|
| 1 | Leptin [89] | Mainly from adipocytes | Well-known marker of obesity | Satiety hormone that regulates body weight by suppressing the feeling of hunger, inhibits fat storage, and promotes fatty acid oxidization; also promotes inflammation |
| 2 | Resistin [90] | Adipocytes, monocytes, and macrophages | Increased in obesity, insulin resistance, and diabetic patients | Proinflammatory adipokine; thought to play a role in insulin resistance |
| 3 | Fatty acid binding protein-4 (FABP-4) [91] | Adipocytes and macrophages | Increased in obesity, insulin resistance, and diabetic patients | Plays a role insulin resistance and inflammation |
| 4 | Retinol binding protein (RBP-4) [92] | Adipocytes (especially visceral fat), macrophages, and liver | Increased in obesity, insulin resistance, and diabetic patients. Associated with hypertension, and dyslipidemia | Acts as a transporter for retinol and plays a role in insulin resistance development |
| 5 | Acylation stimulating protein (ASP) [93] | Adipocyte | Increased in obesity and dyslipidemia patients | Autocrine function that leads to increasing triglyceride synthesis |
| 6 | Lipocalin-2 (LCN2) [94] | Adipose tissue, liver, kidney, lung, macrophages, and neutrophils | Increased in obesity (especially in severely obese females) | Plays a role in inflammation and insulin resistance |
| 7 | Chemerin [95] | Adipose tissue, liver, as well as innate immune cells | Elevated with obesity and diabetic patients | Plays a role in insulin resistance, adipocyte metabolism, and diabetic induced cardiovascular disease |
| 8 | Visfatin [96] | Adipose tissue and neutrophils | Increased in obesity, and diabetic patients | Acts as a proinflammatory mediator |
| 9 | Vaspin [97] | Adipose tissue, liver, pancreas, stomach, muscles and skin | Increased in obesity, insulin resistance and diabetic patients | Acts as a member of the serine protease inhibitor family |
| 10 | Apelin [98] | Adipose tissue, hypothalamus, heart, and skeletal muscles | Increased in obesity, insulin resistance and diabetic patients | Plays a role in regulating glucose metabolism, by inducing glucose uptake |
| 11 | Gremlin-1 [99] | Preadipocytes | Increased in obesity | Acts as an inhibitor of bone morphogenetic protein (BMP), which is one of the transforming growth factor-beta family |
Table 5.
Description of the potential anti-inflammatory adipokines
| Hormone | Source | Observed changes in obesity | Main function | |
|---|---|---|---|---|
| 1 | Adiponectin [100] | Adipose tissue and skeletal muscles | Lower levels in diabetic patients | Anti-obesity, anti-atherogenic, anti-inflammatory, and anti-diabetic effects |
| 2 | Omentin-1 [101] | Visceral adipose tissue | Lower levels in obese and diabetic patients | Anti-inflammatory, anti-obesity, anti-diabetic properties, and insulin sensitizing effects |
| 3 | Secreted frizzled related protein 5 (SSFRP5) [102, 103] | Adipose tissue | Lower levels in obese and diabetic patients | Anti-inflammatory and insulin sensitizing effects |
| 4 | Cardiotrophin-1 (CT-1) [103] | Adipose tissue, liver, kidney, muscle, heart, and lung, brain and testis | Controverial results regarding the changes in serum levels of obese patients | One of the IL-6 cytokine family, plays a role in glucose and lipid metabolism, has an insulin sensitizing potential effect |
IL-6: Interleukin 6.
Leptin, ASF and insulin sensitivity
Leptin is a 167-residue peptide hormone encoded by the Ob gene, and it is secreted mainly by the adipocytes but also from the gastric epithelium and other tissues [63]. Since its identification in 1994 by positional cloning [64], leptin has gained much recognition as a crucial peripheral and central signaling molecule associated with energy balance. This, in turn, has contributed to changing the perception of the adipose tissue from being a form of passive energy depot (primarily in the form of energy-rich triglycerides (9 kilocalories per gram) to that of an active endocrine organ that actively modulates food intake and systemic energy metabolism.
Leptin levels are positively associated with BMI, HOMA-IR, and serum triglycerides and negatively with serum HDL in mostly normal weight health individuals suggesting that leptin increases with BMI as well as in those with insulin resistance [65]. The latter study suggests that leptin was coming mainly from ASF given correlation with hip and waist circumference but not with waist–hip ratio [65]. Under normal physiological conditions, bloodstream levels of leptin are proportional to fat mass for a given individual [66] suggesting that the increase in leptin is driven by fat mass and that both leptin and insulin resistance are consequences of an increase in fat mass. Nevertheless, basal plasma leptin concentrations are significantly lower in insulin-sensitive than in insulin-resistant men (1.90 ± 0.4 vs. 4.35 ± 1.21 ng/ml, P < 0.05) of identical body fat composition [67] suggesting either that excess leptin may also lead to increases in insulin resistance independent of adiposity or that leptin production increases in insulin resistant men in response to unknown feedback mechanisms in an effort to ameliorate the insulin resistance. The latter seems more plausible given that a direct action of leptin on its hypothalamic neuronal target is required to maintain normal glucose homeostasis data and insulin sensitivity [68, 69] and therefore the rising leptin level and insulin resistance in obesity lends plausibility to the conclusion that another fat derived molecule required for the leptin effect on glucose homeostasis may be downregulated in obesity for this paradoxical observation to hold. It remains to be determined if this molecule does indeed exist and what it could be.
Interleukin-6 (IL-6), ASF and inflammation
IL-6 is a 212-residue protein cytokine encoded by the IL-6 gene [70]. Since its identification in 1986 by molecular cloning of B-cell stimulatory factor-2 [71], IL-6 has been recognized as a cytokine with various biological activities implicated with a detrimental role in a wide range of inflammation-associated disease states, including susceptibility to diabetes mellitus [72]. IL-6 is synthesized by various cell types of which white adipocytes are responsible for one-third of basal serum levels in humans [73].
The IL-6 level is probably the single most important factor associated with the hepatic acute-phase response and this is a response to tissue damage or infection that initiates host defense mechanisms and whose goal is to eliminate the threat and facilitate tissue repair [74]. Obesity however is associated with chronic low-grade inflammation possibly from hypoxia in adipocytes, resulting in the release of IL-6 and activation of other factors that positively feedback and amplify IL-6 release [75]. This leads to the metabolic syndrome and similar to leptin, in vitro studies have shown that ASF produces more IL-6 than VSF [76] making the link between ASF and metabolic syndrome stronger than that for VSF [77].
Leptin, IL-6 and the SSFR- bariatric surgery interaction
It is important to note that some SSFR patients tend to have had bariatric surgery, which is associated with enhanced postprandial gut hormone release, particularly GLP-1, a hormone interlinked with factors released from adipose tissue, e.g., leptin and IL-6 highlighted above. However, what remains unclear is whether or to what extent this crosstalk gets perturbed in patients undergoing SSFR and/or bariatric surgery. Furthermore, what are the long-term metabolic sequelae? Thus, a robust examination of the changes of IL-6 after the sudden removal of fat surgically by body contouring procedures might widen our understanding of the mechanisms behind these metabolic changes.
Other considerations and future tasks
Apart from the potentially favorable effects of SSFR on metabolism and adipokines discussed above, many studies also support the effectiveness of bariatric surgery for treating obesity and weight-related disease [21, 78]. However, the question about the combined impacts of these surgical interventions has been relatively under-studied, and the results remain inconclusive. Future studies that can link the metabolic improvement after bariatric surgery and bariatric medications such as Semaglutide to the preferential loss of AVF or ASF will be of great benefit. Additionally, a dose response MAs is needed to examine the time trend of the metabolic changes after SSFR, which can answer important questions regarding the durability and extent of changes induced by these procedures over time.
When a negative energy balance is induced by interventions such as SSFR, resulting in a moderate initial reduction of 5% to 10% from baseline body weight, the physiological adaptations certainly favor weight regain; thus, most people recover weight post-SSFR or at the end of lifestyle interventions. With the common SSFR procedures, this loss is of abdominal fat that constitutes <15% of total adipose tissue [79], with the main component of the latter being ASF.
Given that fat distribution is one parameter that modifies the impact of obesity on health, knowledge about whether fat tissue removed through SSFR is replaced by new fat tissue and if this occurs in the same or at different anatomical sites is important since the latter may have worse effects. Previous studies reported that the fat could return to sites other than that from which fat has been removed, such as the breast, hip, and thigh regions [80, 81], but this is not always the case [82]. There is also the possibility that new fat may accumulate at sites where fat does not commonly accumulate (ectopic fat) and such ectopic adipose tissues may deposit in several organs/tissues (intramuscular/cardiac/hepatic) in the body with adverse consequences [83, 84]. However, recent studies of the heart [85, 86] have suggested that ectopic fat is protective against the risk of developing cardiovascular complications by increasing glycolysis, as a physiological healing response. In the context of SSFR, it is unclear to what extent the redistributed fat contributes to the ectopic fat accumulation in tissues, such as intramuscular, intrahepatic, and myocardial fat and if it has a protective or detrimental effect. Furthermore, it is unclear if and how or which specific factors drive the fat redistribution to ectopic regions in preference to the rest of the body spatiotemporally. Identifying such factors can be helpful surrogate biomarkers for predicting potential risk factors in epidemiological studies. However, it should be noted that rodent models of fat biology do not adequately represent what happens in humans, and higher mammals such as baboons may be a better model that closely resembles human adipocyte function [87].
Thus far, results from studies designed to identify the factors that address the regulation of energetics and body fat redistribution/ regeneration post-SSFR in rats, mice, or hamsters have limited contribution in closing the knowledge gap because of insufficient mechanistic data, inadequate sample size, or lack of proper statistical tests reported [88]. Therefore, future studies in appropriate animal models or human clinical trials should account for the biological consequences of ectopic fat redistribution following weight gain post-SSFR. However, there is a need to ascertain the beneficial or detrimental nature of fat redistribution at specific anatomical sites, in relation to its quantity, rate, and time of accumulation following weight gain post-SSFR.
Conclusion
We conclude that there is a gap in terms of the probability of weight gain or accumulation of fat post-SSFR, but there is data that in the short term there might be a metabolic benefit of excess ASF removal. Longer-term data are needed to determine if this benefit is sustained in the longer term. Patients going for SSFR represent a unique population with a sudden removal of their ASF. However, the metabolic changes after these procedures are still unclear, and existing studies suggest a trend toward benefit rather than harm. There is thus no immediate harm from these procedures but there is a need for properly designed dose-response MAs as well as well-conducted prospective clinical studies to unravel these putative changes. In turn, this will help us not only to confirm the safety of these procedures but also to define if these procedures can be used for metabolic benefit and to broaden our knowledge about the mechanisms underpinning excess ASF and associated metabolic consequences.
Supplemental Data
Figure S1.

Search strings.
Figure S2.
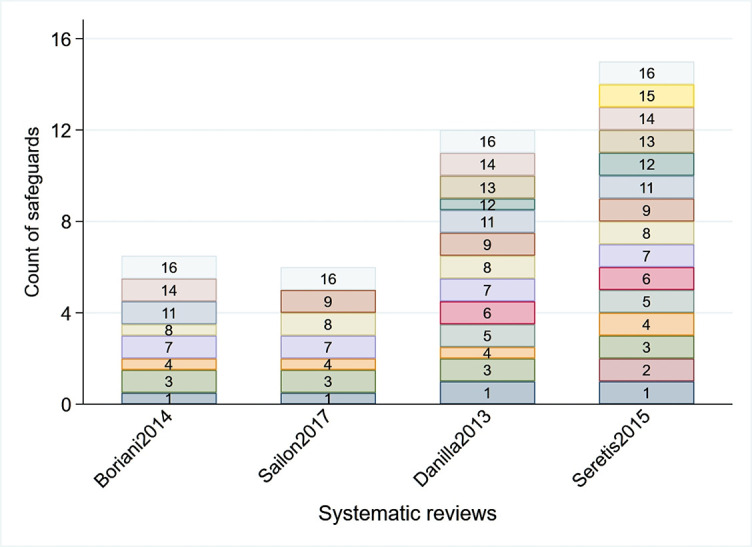
Quality assessment of the four syntheses. The thick boxes indicate presence of the quality safeguard and the thin boxes indicate they are partially present. Missing numbers indicate the safeguard was missing.
Acknowledgments
The authors thank the Department of Plastic Surgery at Hamad General Hospital and the Department of Population Medicine at the College of Medicine at Qatar University for their contribution to this paper.
Footnotes
Conflict of interest: The authors declare no conflicts of interest.
Funding: This project was supported by the Medical Research Office at Hamad Medical Corporation (#01-20-466) and the Qatar National Research Fund (Projects #NPRP13S-0209-200315 and #NPRP14S-0406-210153). The responsibility for the paper lies with the authors and there was no influence of the funder.
References
- 1. Obesity and overweight [Internet]. [cited 2021 Mar 14]. Available from: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight.
- 2.Cao H. Adipocytokines in obesity and metabolic disease. J Endocrinol. 2014 Feb 1;220(2):T47–59. doi: 10.1530/JOE-13-0339. https://doi.org/10.1530/JOE-13-0339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bergé J-P, Barnathan G. Fatty acids from lipids of marine organisms: molecular biodiversity, roles as biomarkers, biologically active compounds, and economical aspects. Adv Biochem Eng Biotechnol. 2005 Aug 24;96:49–125. doi: 10.1007/b135782. https://doi.org/10.1007/b135782. [DOI] [PubMed] [Google Scholar]
- 4.Achari AE, Jain SK. Adiponectin, a therapeutic target for obesity, diabetes, and endothelial dysfunction. Int J Mol Sci. 2017 Jun 21;18(6):1321. doi: 10.3390/ijms18061321. https://doi.org/10.3390/ijms18061321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frayn KN. Adipose tissue and the insulin resistance syndrome. Proc Nutr Soc. 2001 Aug;60(3):375–80. doi: 10.1079/pns200195. https://doi.org/10.1079/pns200195. [DOI] [PubMed] [Google Scholar]
- 6.Kusminski CM, Holland WL, Sun K, Park J, Spurgin SB, Lin Y, et al. MitoNEET-driven alterations in adipocyte mitochondrial activity reveal a crucial adaptive process that preserves insulin sensitivity in obesity. Nat Med. 2012 Oct;18(10):1539–49. doi: 10.1038/nm.2899. https://doi.org/10.1038/nm.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9•1 million participants. Lancet. 2011 Feb 12;377(9765):557–67. doi: 10.1016/S0140-6736(10)62037-5. https://doi.org/10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kitzinger HB, Abayev S, Pittermann A, Karle B, Bohdjalian A, Langer FB, et al. After massive weight loss: patients’ expectations of body contouring surgery. Obes Surg. 2012 Apr;22(4):544–8. doi: 10.1007/s11695-011-0551-6. https://doi.org/10.1007/s11695-011-0551-6. [DOI] [PubMed] [Google Scholar]
- 9.Almutairi K, Gusenoff JA, Rubin JP. Body contouring. Plast Reconstr Surg. 2016 Mar;137(3):586e–602e. doi: 10.1097/PRS.0000000000002140. https://doi.org/10.1097/PRS.0000000000002140. [DOI] [PubMed] [Google Scholar]
- 10.Sailon AM, Wasserburg JR, Kling RR, Pasick CM, Taub PJ. Influence of large-volume liposuction on metabolic and cardiovascular health: a systematic review. Ann Plast Surg. 2017 Dec;79(6):623–30. doi: 10.1097/SAP.0000000000001195. https://doi.org/10.1097/SAP.0000000000001195. [DOI] [PubMed] [Google Scholar]
- 11.Seretis K, Goulis DG, Koliakos G, Demiri E. The effects of abdominal lipectomy in metabolic syndrome components and insulin sensitivity in females: a systematic review and meta-analysis. Metabolism. 2015 Dec 1;64(12):1640–9. doi: 10.1016/j.metabol.2015.09.015. https://doi.org/10.1016/j.metabol.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 12.Pinkhasov BB, Selyatitskaya VG, Karapetyan AR, Astrakhantseva EL. Metabolic syndrome in men and women with upper or lower types of body fat distribution. Health N Hav. 2012 Dec 31;4(12):1381–9. https://doi.org/10.4236/health.2012.412A200. [Google Scholar]
- 13.Després J-P, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006 Dec 14;444(7121):881–7. doi: 10.1038/nature05488. https://doi.org/10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 14.Fu J, Hofker M, Wijmenga C. Apple or pear: size and shape matter. Cell Metab. 2015 Apr 7;21(4):507–8. doi: 10.1016/j.cmet.2015.03.016. https://doi.org/10.1016/j.cmet.2015.03.016. [DOI] [PubMed] [Google Scholar]
- 15.Kwon H, Kim D, Kim JS. Body fat distribution and the risk of incident metabolic syndrome: a longitudinal cohort study. Sci Rep. 2017 Dec 1;7(1):1–8. doi: 10.1038/s41598-017-09723-y. https://doi.org/10.1038/s41598-017-09723-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klein S. Is visceral fat responsible for the metabolic abnormalities associated with obesity? Diabetes Care. 2010 Jul;33(7):1693–4. doi: 10.2337/dc10-0744. https://doi.org/10.2337/dc10-0744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klein S. The case of visceral fat: argument for the defense. J Clin Invest. 2004 Jun 1;113(11):1530–2. doi: 10.1172/JCI22028. https://doi.org/10.1172/JCI22028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fabbrini E, Magkos F, Mohammed BS, Pietka T, Abumrad NA, Patterson BW, et al. Intrahepatic fat, not visceral fat, is linked with metabolic complications of obesity. Proc Natl Acad Sci USA. 2009 Sep 8;106(36):15430–5. doi: 10.1073/pnas.0904944106. https://doi.org/10.1073/pnas.0904944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ziccardi P, Nappo F, Giugliano G, Esposito K, Marfella R, Cioffi M, et al. Reduction of inflammatory cytokine concentrations and improvement of endothelial functions in obese women after weight loss over one year. Circulation. 2002 Feb 19;105(7):804–9. doi: 10.1161/hc0702.104279. https://doi.org/10.1161/hc0702.104279. [DOI] [PubMed] [Google Scholar]
- 20.Stevens VJ, Obarzanek E, Cook NR, Lee IM, Appel LJ, Smith West D, et al. Long-term weight loss and changes in blood pressure: results of the trials of hypertension prevention, phase II. Ann Intern Med. 2001 Jan 2;134(1):1–11. doi: 10.7326/0003-4819-134-1-200101020-00007. https://doi.org/10.7326/0003-4819-134-1-200101020-00007. [DOI] [PubMed] [Google Scholar]
- 21.Madsbad S, Dirksen C, Holst JJ. Mechanisms of changes in glucose metabolism and bodyweight after bariatric surgery. Lancet Diabetes Endocrinol. 2014 Feb 1;2(2):152–64. doi: 10.1016/S2213-8587(13)70218-3. https://doi.org/10.1016/S2213-8587(13)70218-3. [DOI] [PubMed] [Google Scholar]
- 22.Cazes L, Deitel M, Levine RH. Effect of abdominal lipectomy on lipid profile, glucose handling and blood pressure in patients with truncal obesity. Obes Surg. 1996;6(2):159–66. doi: 10.1381/096089296765557123. https://doi.org/10.1381/096089296765557123. [DOI] [PubMed] [Google Scholar]
- 23.Rizzo MR, Paolisso G, Grella R, Barbieri M, Grella E, Ragno E, et al. Is dermolipectomy effective in improving insulin action and lowering inflammatory markers in obese women? Clin Endocrinol (Oxf) 2005 Sep;63(3):253–8. doi: 10.1111/j.1365-2265.2005.02337.x. https://doi.org/10.1111/j.1365-2265.2005.02337.x. [DOI] [PubMed] [Google Scholar]
- 24.Swanson E. Prospective clinical study reveals significant reduction in triglyceride level and white blood cell count after liposuction and abdominoplasty and no change in cholesterol levels. Plast Reconstr Surg. 2011 Sep;128(3):182e–197e. doi: 10.1097/PRS.0b013e31822213c6. https://doi.org/10.1097/PRS.0b013e31822213c6. [DOI] [PubMed] [Google Scholar]
- 25.Gonzalez-Ortiz M, Robles-Cervantes JA, Cardenas-Camarena L, Bustos-Saldana R, Martinez-Abundis E. The effects of surgically removing subcutaneous fat on the metabolic profile and insulin sensitivity in obese women after large-volume liposuction treatment. Horm Metab Res. 2002 Aug;34(08):446–9. doi: 10.1055/s-2002-33603. https://doi.org/10.1055/s-2002-33603. [DOI] [PubMed] [Google Scholar]
- 26.Ybarra J, Blanco-Vaca F, Fernández S, Castellví A, Bonet R, Palomer X, et al. The effects of liposuction removal of subcutaneous abdominal fat on lipid metabolism are independent of insulin sensitivity in normal-overweight individuals. Obes Surg. 2008 Apr;18(4):408–14. doi: 10.1007/s11695-007-9261-5. https://doi.org/10.1007/s11695-007-9261-5. [DOI] [PubMed] [Google Scholar]
- 27.Busetto L, Bassetto F, Zocchi M, Zuliani F, Nolli ML, Pigozzo S, et al. The effects of the surgical removal of subcutaneous adipose tissue on energy expenditure and adipocytokine concentrations in obese women. Nutr Metab Cardiovasc Dis. 2008 Feb 1;18(2):112–20. doi: 10.1016/j.numecd.2006.09.009. https://doi.org/10.1016/j.numecd.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 28.Davis DA, Pellowski DM, Donahoo WT. Acute and 1-month effect of small-volume suction lipectomy on insulin sensitivity and cardiovascular risk. Int J Obes (Lond) 2006 Aug;30(8):1217–22. doi: 10.1038/sj.ijo.0803120. https://doi.org/10.1038/sj.ijo.0803120. [DOI] [PubMed] [Google Scholar]
- 29.D’Andrea F, Grella R, Rizzo MR, Grella E, Grella R, Nicoletti G, et al. Changing the metabolic profile by large-volume liposuction: a clinical study conducted with 123 obese women. Aesthetic Plast Surg. 2005 Nov-Dec;29(6):472–8. doi: 10.1007/s00266-005-0089-x. https://doi.org/10.1007/s00266-005-0089-x. [DOI] [PubMed] [Google Scholar]
- 30.Samdal F, Birkeland KI, Ose L, Amland PF. Effect of large-volume liposuction on sex hormones and glucose-and lipid metabolism in females. Aesthetic Plast Surg. 1995 Mar;19(2):131–5. doi: 10.1007/BF00450248. https://doi.org/10.1007/BF00450248. [DOI] [PubMed] [Google Scholar]
- 31.Giugliano G, Nicoletti G, Grella E, Giugliano F, Esposito K, Scuderi N, et al. Effect of liposuction on insulin resistance and vascular inflammatory markers in obese women. Br J Plast Surg. 2004;57(3):190–4. doi: 10.1016/j.bjps.2003.12.010. https://doi.org/10.1016/j.bjps.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 32.Giese SY, Neborsky R, Bulan EJ, Spear SL, Yanovski JA. Improvements in cardiovascular risk profile after large-volume lipoplasty: a 1-year follow-up study. Aesthet Surg J. 2001;21(6):527–31. doi: 10.1067/maj.2001.120434. https://doi.org/10.1067/maj.2001.120434. [DOI] [PubMed] [Google Scholar]
- 33.Giese SY, Bulan EJ, Commons GW, Spear SL, Yanovski JA. Improvements in cardiovascular risk profile with large-volume liposuction: a pilot study. Plast Reconstr Surg. 2001;108(2):510–9. doi: 10.1097/00006534-200108000-00035. https://doi.org/10.1097/00006534-200108000-00036. [DOI] [PubMed] [Google Scholar]
- 34.Esposito K, Giugliano G, Giugliano D, Arner P, Busetto L, Bassetto F, et al. Metabolic effects of liposuction–yes or no? N Engl J Med. 2004 Sep 23;351(13):1354–7. doi: 10.1056/NEJM200409233511320. https://doi.org/10.1056/NEJM200409233511320. [DOI] [PubMed] [Google Scholar]
- 35.Clark JM, Sanders S, Carter M, Honeyman D, Cleo G, Auld Y, et al. Improving the translation of search strategies using the polyglot search translator: a randomized controlled trial. J Med Libr Assoc. 2020 Apr 1;108(2):195–207. doi: 10.5195/jmla.2020.834. https://doi.org/10.5195/jmla.2020.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shea BJ, Grimshaw JM, Wells GA, Boers M, Andersson N, Hamel C, et al. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. 2007 Dec;7(1):1–7. doi: 10.1186/1471-2288-7-10. https://doi.org/10.1186/1471-2288-7-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crahay FX, Nizet JL. Metabolic and cardiovascular consequences of suction-assisted lipectomy: systematic review. Ann Chir Plast Esthet. 2016 Aug 1;61(4):270–86. doi: 10.1016/j.anplas.2016.03.005. https://doi.org/10.1016/j.anplas.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 38.Seretis K, Goulis DG, Koliakos G, Demiri E. Short- and long-term effects of abdominal lipectomy on weight and fat mass in females: a systematic review. Obes Surg. 2015 Oct;25(10):1950–8. doi: 10.1007/s11695-015-1797-1. https://doi.org/10.1007/s11695-015-1797-1. [DOI] [PubMed] [Google Scholar]
- 39.Danilla S, Longton C, Valenzuela K, Cavada G, Norambuena H, Tabilo C, et al. Suction-assisted lipectomy fails to improve cardiovascular metabolic markers of disease: a meta-analysis. J Plast Reconstr Aesthet Surg. 2013 Nov;66(11):1557–63. doi: 10.1016/j.bjps.2013.07.001. https://doi.org/10.1016/j.bjps.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 40.Boriani F, Villani R, Morselli PG. Metabolic effects of large-volume liposuction for obese healthy women: a meta-analysis of fasting insulin levels. Aesthetic Plast Surg. 2014 Sep 25;38(5):1050–6. doi: 10.1007/s00266-014-0386-3. https://doi.org/10.1007/s00266-014-0386-3. [DOI] [PubMed] [Google Scholar]
- 41.Robles-Cervantes JA, Yánez-Diaz S, Cárdenas-Camarena L. Modification of insulin, glucose and cholesterol levels in nonobese women undergoing liposuction: is liposuction metabolically safe? Ann Plast Surg. 2004 Jan;52(1):64–7. doi: 10.1097/01.sap.0000096448.59407.43. https://doi.org/10.1097/01.sap.0000096448.59407.43. [DOI] [PubMed] [Google Scholar]
- 42.Martínez-Abundis E, Molina-Villa CA, González-Ortiz M, Robles-Cervantes JA, Saucedo-Ortiz JA. Effect of surgically removing subcutaneous fat by abdominoplasty on leptin concentrations and insulin sensitivity. Ann Plast Surg. 2007 Apr;58(4):416–9. doi: 10.1097/01.sap.0000240033.00047.cc. https://doi.org/10.1097/01.sap.0000240033.00047.cc. [DOI] [PubMed] [Google Scholar]
- 43.Mohammed BS, Cohen S, Reeds D, Young VL, Klein S. Long-term effects of large-volume liposuction on metabolic risk factors for coronary heart disease. Obesity (Silver Spring) 2008 Dec;16(12):2648–51. doi: 10.1038/oby.2008.418. https://doi.org/10.1038/oby.2008.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klein S, Fontana L, Young VL, Coggan AR, Kilo C, Patterson BW, et al. Absence of an effect of liposuction on insulin action and risk factors for coronary heart disease. N Engl J Med. 2004 Jun 17;350(25):2549–57. doi: 10.1056/NEJMoa033179. https://doi.org/10.1056/NEJMoa033179. [DOI] [PubMed] [Google Scholar]
- 45.Pratt-Phillips SE, Geor RJ, McCutcheon LJ. Comparison among the euglycemic-hyperinsulinemic clamp, insulin-modified frequently sampled intravenous glucose tolerance test, and oral glucose tolerance test for assessment of insulin sensitivity in healthy standardbreds. Am J Vet Res. 2015;76(1):84–91. doi: 10.2460/ajvr.76.1.84. https://doi.org/10.2460/ajvr.76.1.84. [DOI] [PubMed] [Google Scholar]
- 46.Rehman K, Akash MSH. Mechanisms of inflammatory responses and development of insulin resistance: how are they interlinked? J Biomed Sci. 2016 Dec;23(1):1–8. doi: 10.1186/s12929-016-0303-y. https://doi.org/10.1186/s12929-016-0303-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fontana L, Eagon JC, Trujillo ME, Scherer PE, Klein S. Visceral fat adipokine secretion is associated with systemic inflammation in obese humans. Diabetes. 2007 Apr;56(4):1010–3. doi: 10.2337/db06-1656. https://doi.org/10.2337/db06-1656. [DOI] [PubMed] [Google Scholar]
- 48.Ross R, Shaw KD, Martel Y, de Guise J, Avruch L. Adipose tissue distribution measured by magnetic resonance imaging in obese women. Am J Clin Nutr. 1993;57(4):470–5. doi: 10.1093/ajcn/57.4.470. https://doi.org/10.1093/ajcn/57.4.470. [DOI] [PubMed] [Google Scholar]
- 49.Patel P, Abate N. Body fat distribution and insulin resistance. Nutrients. 2013 Jun 5;5(6):2019–27. doi: 10.3390/nu5062019. https://doi.org/10.3390/nu5062019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abate N, Garg A, Peshock RM, Stray-Gundersen J, Grundy SM. Relationships of generalized and regional adiposity to insulin sensitivity in men. J Clin Invest. 1995;96(1):88–98. doi: 10.1172/JCI118083. https://doi.org/10.1172/JCI118083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abate N, Garg A, Peshock RM, Stray-Gundersen J, Adams-Huet B, Grundy SM. Relationship of generalized and regional adiposity to insulin sensitivity in men with NIDDM. Diabetes. 1996;45(12):1684–93. doi: 10.2337/diab.45.12.1684. https://doi.org/10.2337/diab.45.12.1684. [DOI] [PubMed] [Google Scholar]
- 52.Goodpaster BH, Thaete FL, Simoneau JA, Kelley DE. Subcutaneous abdominal fat and thigh muscle composition predict insulin sensitivity independently of visceral fat. Diabetes. 1997;46(10):1579–85. doi: 10.2337/diacare.46.10.1579. https://doi.org/10.2337/diacare.46.10.1579. [DOI] [PubMed] [Google Scholar]
- 53.Wolfe BM, Kvach E, Eckel RH. Treatment of obesity: weight loss and bariatric surgery. Circ Res. 2016 May 27;118(11):1844–55. doi: 10.1161/CIRCRESAHA.116.307591. https://doi.org/10.1161/CIRCRESAHA.116.307591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lima MM, Pareja JC, Alegre SM, Geloneze SR, Kahn SE, Astiarraga BD, et al. Visceral fat resection in humans: effect on insulin sensitivity, beta-cell function, adipokines, and inflammatory markers. Obesity (Silver Spring) 2013 Mar;21(3):E182–9. doi: 10.1002/oby.20030. https://doi.org/10.1002/oby.20030. [DOI] [PubMed] [Google Scholar]
- 55.Fabbrini E, Tamboli RA, Magkos F, Marks–Shulman PA, Eckhauser AW, Richards WO, et al. Surgical removal of omental fat does not improve insulin sensitivity and cardiovascular risk factors in obese adults. Gastroenterology. 2010;139(2):448–55. doi: 10.1053/j.gastro.2010.04.056. https://doi.org/10.1053/j.gastro.2010.04.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dunn JP, Abumrad NN, Breitman I, Marks-Shulman PA, Flynn CR, Jabbour K, et al. Hepatic and peripheral insulin sensitivity and diabetes remission at 1 month after Roux-en-Y gastric bypass surgery in patients randomized to omentectomy. Diabetes Care. 2012 Jan;35(1):137–42. doi: 10.2337/dc11-1383. https://doi.org/10.2337/dc11-1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Herrera MF, Pantoja JP, Velázquez-Fernández D, Cabiedes J, Aguilar-Salinas C, García-García E, et al. Potential additional effect of omentectomy on metabolic syndrome, acute-phase reactants, and inflammatory mediators in grade III obese patients undergoing laparoscopic Roux-en-Y gastric bypass: a randomized trial. Diabetes Care. 2010 Jul;33(7):1413–8. doi: 10.2337/dc09-1833. https://doi.org/10.2337/dc09-1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shi J, Bao G, Hong J, Wang S, Chen Y, Zhao S, et al. Deciphering CT texture features of human visceral fat to evaluate metabolic disorders and surgery-induced weight loss effects. EBioMedicine. 2021 Jul 1;69:103471. doi: 10.1016/j.ebiom.2021.103471. https://doi.org/10.1016/j.ebiom.2021.103471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lenz M, Arts ICW, Peeters RLM, de Kok TM, Ertaylan G. Adipose tissue in health and disease through the lens of its building blocks. Sci Rep. 2020 Dec;10(1):10433. doi: 10.1038/s41598-020-67177-1. https://doi.org/10.1038/s41598-020-67177-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ferrero R, Rainer P, Deplancke B. Toward a consensus view of mammalian adipocyte stem and progenitor cell heterogeneity. Trends Cell Biol. 2020 Dec 1;30(12):937–50. doi: 10.1016/j.tcb.2020.09.007. https://doi.org/10.1016/j.tcb.2020.09.007. [DOI] [PubMed] [Google Scholar]
- 61.Merlotti C, Ceriani V, Morabito A, Pontiroli AE. Subcutaneous fat loss is greater than visceral fat loss with diet and exercise, weight-loss promoting drugs and bariatric surgery: a critical review and meta-analysis. Int J Obes (Lond) 2017 May 1;41(5):672–82. doi: 10.1038/ijo.2017.31. https://doi.org/10.1038/ijo.2017.31. [DOI] [PubMed] [Google Scholar]
- 62.Karlsson T, Rask-Andersen M, Pan G, Höglund J, Wadelius C, Ek WE, et al. Contribution of genetics to visceral adiposity and its relation to cardiovascular and metabolic disease. Nat Med. 2019 Sep 1;25(9):1390–5. doi: 10.1038/s41591-019-0563-7. https://doi.org/10.1038/s41591-019-0563-7. [DOI] [PubMed] [Google Scholar]
- 63.Seth M, Biswas R, Ganguly S, Chakrabarti N, Chaudhuri AG. Leptin and obesity. Physiol Int. 2020 Dec 1;107(4):455–68. doi: 10.1556/2060.2020.00038. https://doi.org/10.1556/2060.2020.00038. [DOI] [PubMed] [Google Scholar]
- 64.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994 Dec 1;372(6505):425–32. doi: 10.1038/372425a0. https://doi.org/10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 65.Yadav A, Jyoti P, Jain SK, Bhattacharjee J. Correlation of adiponectin and leptin with insulin resistance: a pilot study in healthy North Indian population. Indian Clin Biochem. 2011 Feb 18;26(2):193–6. doi: 10.1007/s12291-011-0119-1. https://doi.org/10.1007/s12291-011-0119-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Considine RV, Sinha MK, Heiman ML, Kriauciunas A, Stephens TW, Nyce MR, et al. Serum immunoreactive-leptin concentrations in normal-weight and obese humans. N Engl J Med. 1996 Feb 20;334(5):292–5. doi: 10.1056/NEJM199602013340503. https://doi.org/10.1056/NEJM199602013340503. [DOI] [PubMed] [Google Scholar]
- 67.Segal KR, Landt M, Klein S. Relationship between insulin sensitivity and plasma leptin concentration in lean and obese men. Diabetes. 1996 Jul 1;45(7):988–91. doi: 10.2337/diab.45.7.988. https://doi.org/10.2337/diab.45.7.988. [DOI] [PubMed] [Google Scholar]
- 68.Berglund ED, Vianna CR, Donato J, Kim MH, Chuang JC, Lee CE, et al. Direct leptin action on POMC neurons regulates glucose homeostasis and hepatic insulin sensitivity in mice. J Clin Invest. 2012 Mar 1;122(3):1000–9. doi: 10.1172/JCI59816. https://doi.org/10.1172/JCI59816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schwartz MW, Baskin DG, Bukowski TR, Kuijper JL, Foster D, Lasser G, et al. Specificity of leptin action on elevated blood glucose levels and hypothalamic neuropeptide Y gene expression in ob/ob mice. Diabetes. 1996;45(4):531–5. doi: 10.2337/diab.45.4.531. https://doi.org/10.2337/diab.45.4.531. [DOI] [PubMed] [Google Scholar]
- 70.Zilberstein A, Ruggieri R, Korn JH, Revel M. Structure and expression of cDNA and genes for human interferon-beta-2, a distinct species inducible by growth-stimulatory cytokines. EMBO J. 1986 Oct;5(10):2529–37. doi: 10.1002/j.1460-2075.1986.tb04531.x. https://doi.org/10.1002/j.1460-2075.1986.tb04531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hirano T, Yasukawa K, Harada H, Taga T, Watanabe Y, Matsuda T, et al. Complementary DNA for a novel human interleukin (BSF-2) that induces B lymphocytes to produce immunoglobulin. Nature. 1986 Nov;324(6092):73–6. doi: 10.1038/324073a0. https://doi.org/10.1038/324073a0. [DOI] [PubMed] [Google Scholar]
- 72.Kang S, Tanaka T, Narazaki M, Kishimoto T. Targeting interleukin-6 signaling in clinic. Immunity. 2019 Apr 16;50(4):1007–23. doi: 10.1016/j.immuni.2019.03.026. https://doi.org/10.1016/j.immuni.2019.03.026. [DOI] [PubMed] [Google Scholar]
- 73.Mohamed-Ali V, Goodrick S, Rawesh A, Katz DR, Miles JM, Yudkin JS, et al. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-α, in vivo. J Clin Endocrinol Metab. 1997;82(12):4196–200. doi: 10.1210/jcem.82.12.4450. https://doi.org/10.1210/jcem.82.12.4450. [DOI] [PubMed] [Google Scholar]
- 74.Heinrich PC, Castell JV, Andus T. Interleukin-6 and the acute phase response. Biochem J. 1990;265(3):621–36. doi: 10.1042/bj2650621. https://doi.org/10.1042/bj2650621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hirano T. IL-6 in inflammation, autoimmunity and cancer. Int Immunol. 2021 Mar 1;33(3):127–48. doi: 10.1093/intimm/dxaa078. https://doi.org/10.1093/intimm/dxaa078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jonas MI, Kurylowicz A, Bartoszewicz Z, Lisik W, Jonas M, Wierzbicki Z, et al. Interleukins 6 and 15 levels are higher in subcutaneous adipose tissue, but obesity is associated with their increased content in visceral fat depots. Int J Mol Sci. 2015 Oct 1;16(10):25817–30. doi: 10.3390/ijms161025817. https://doi.org/10.3390/ijms161025817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wisse BE. The inflammatory syndrome: the role of adipose tissue cytokines in metabolic disorders linked to obesity. J Am Soc Nephrol. 2004 Nov;15(11):2792–800. doi: 10.1097/01.ASN.0000141966.69934.21. https://doi.org/10.1097/01.ASN.0000141966.69934.21. [DOI] [PubMed] [Google Scholar]
- 78.Laferrère B. Diabetes remission after bariatric surgery: is it just the incretins? Int J Obes (Lond) 2011 Sep;35:S22–5. doi: 10.1038/ijo.2011.143. https://doi.org/10.1038/ijo.2011.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Klein S, Allison DB, Heymsfield SB, Kelley DE, Leibel RL, Nonas C, et al. Waist circumference and cardiometabolic risk: a consensus statement from shaping America’s health: association for weight management and obesity prevention; NAASO, the obesity society; the American society for nutrition; and the American diabetes association. Am J Clin Nutr. 2007 May 1;85(5):1197–202. doi: 10.1093/ajcn/85.5.1197. https://doi.org/10.1093/ajcn/85.5.1197. [DOI] [PubMed] [Google Scholar]
- 80.Hernandez TL, Kittelson JM, Law CK, Ketch LL, Stob NR, Lindstrom RC, et al. Fat redistribution following suction lipectomy: defense of body fat and patterns of restoration. Obesity (Silver Spring) 2011 Jul;19(7):1388–95. doi: 10.1038/oby.2011.64. https://doi.org/10.1038/oby.2011.64. [DOI] [PubMed] [Google Scholar]
- 81.Van der Lei B, Halbesma GJ, van Nieuwenhoven CA, van Wingerden JJ. Spontaneous breast enlargement following liposuction of the abdominal wall: does a link exist? Plast Reconstr Surg. 2007 Apr;119(5):1584–9. doi: 10.1097/01.prs.0000256069.54596.0b. https://doi.org/10.1097/01.prs.0000256069.54596.0b. [DOI] [PubMed] [Google Scholar]
- 82.Gurunluoglu R. Discussion: photographic measurements in 301 cases of liposuction and abdominoplasty reveal fat reduction without redistribution. Plast Reconstr Surg. 2012 Aug;130(2):323e–4e. doi: 10.1097/PRS.0b013e31825904d8. https://doi.org/10.1097/PRS.0b013e31825904d8. [DOI] [PubMed] [Google Scholar]
- 83.Okamura T, Hashimoto Y, Hamaguchi M, Obora A, Kojima T, Fukui M. Ectopic fat obesity presents the greatest risk for incident type 2 diabetes: a population-based longitudinal study. Int J Obes (Lond) 2019 Jan 1;43(1):139–48. doi: 10.1038/s41366-018-0076-3. https://doi.org/10.1038/s41366-018-0076-3. [DOI] [PubMed] [Google Scholar]
- 84.Bosy-Westphal A, Braun W, Albrecht V, Müller MJ. Determinants of ectopic liver fat in metabolic disease. Eur J Clin Nutr. 2019 Feb 1;73(2):209–14. doi: 10.1038/s41430-018-0323-7. https://doi.org/10.1038/s41430-018-0323-7. [DOI] [PubMed] [Google Scholar]
- 85.De Munck TJI, Soeters PB, Koek GH. The role of ectopic adipose tissue: benefit or deleterious overflow? Eur J Clin Nutr. 2021 Jan 1;75(1):38–48. doi: 10.1038/s41430-020-00713-4. https://doi.org/10.1038/s41430-020-00713-4. [DOI] [PubMed] [Google Scholar]
- 86.Flaherty SE, Grijalva A, Xu X, Ables E, Nomani A, Ferrante AW. A lipase-independent pathway of lipid release and immune modulation by adipocytes. Science. 2019;363(6430):989–93. doi: 10.1126/science.aaw2586. https://doi.org/10.1126/science.aaw2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Andrew MS, Huffman DM, Rodriguez-Ayala E, Williams NN, Peterson RM, Bastarrachea RA. Mesenteric visceral lipectomy using tissue liquefaction technology reverses insulin resistance and causes weight loss in baboons. Surg Obes Relat Dis. 2018 Jun 1;14(6):833–41. doi: 10.1016/j.soard.2018.03.004. https://doi.org/10.1016/j.soard.2018.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Murillo AL, Kaiser KA, Smith Jr. DL, Peterson CM, Affuso O, Tiwari HK, et al. A systematic scoping review of surgically manipulated adipose tissue and the regulation of energetics and body fat in animals. Obesity (Silver Spring) 2019;27(9):1404–17. doi: 10.1002/oby.22511. https://doi.org/10.1002/oby.22511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang Y, Scarpace PJ. The role of leptin in leptin resistance and obesity. Physiol Behav. 2006 Jun 30;88(3):249–56. doi: 10.1016/j.physbeh.2006.05.038. https://doi.org/10.1016/j.physbeh.2006.05.038. [DOI] [PubMed] [Google Scholar]
- 90.Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, et al. The hormone resistin links obesity to diabetes. Nature. 2001 Jan 18;409(6818):307–12. doi: 10.1038/35053000. https://doi.org/10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- 91.Maeda K, Uysal KT, Makowski L, Gorgun CZ, Atsumi G, Parker RA, et al. Role of the fatty acid binding protein mal1 in obesity and insulin resistance. Diabetes. 2003 Feb 1;52(2):300–7. doi: 10.2337/diabetes.52.2.300. https://doi.org/10.2337/diabetes.52.2.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM, et al. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005 Jul 21;436(7049):356–62. doi: 10.1038/nature03711. https://doi.org/10.1038/nature03711. [DOI] [PubMed] [Google Scholar]
- 93.Cianflone K, Xia Z, Chen LY. Critical review of acylation-stimulating protein physiology in humans and rodents. Biochim Biophys Acta Biomembr. 2003 Jan 31;1609(2):127–43. doi: 10.1016/s0005-2736(02)00686-7. https://doi.org/10.1016/s0005-2736(02)00686-7. [DOI] [PubMed] [Google Scholar]
- 94.Yan QW, Yang Q, Mody N, Graham TE, Hsu CH, Xu Z, et al. The adipokine lipocalin 2 is regulated by obesity and promotes insulin resistance. Diabetes. 2007 Oct 1;56(10):2533–40. doi: 10.2337/db07-0007. https://doi.org/10.2337/db07-0007. [DOI] [PubMed] [Google Scholar]
- 95.Ernst MC, Sinal CJ. Chemerin: at the crossroads of inflammation and obesity. Trends Endocrinol Metab. 2010 Nov 1;21(11):660–7. doi: 10.1016/j.tem.2010.08.001. https://doi.org/10.1016/j.tem.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 96.Chang YH, Chang DM, Lin KC, Shin SJ, Lee YJ. Visfatin in overweight/obesity, type 2 diabetes mellitus, insulin resistance, metabolic syndrome and cardiovascular diseases: a meta-analysis and systemic review. Diabetes Metab Res Rev. 2011 Sep 1;27(6):515–27. doi: 10.1002/dmrr.1201. https://doi.org/10.1002/dmrr.1201. [DOI] [PubMed] [Google Scholar]
- 97.Feng R, Li Y, Wang C, Luo C, Liu L, Chuo F, et al. Higher vaspin levels in subjects with obesity and type 2 diabetes mellitus: a meta-analysis. Diabetes Res Clin Pract. 2014 Oct 1;106(1):88–94. doi: 10.1016/j.diabres.2014.07.026. https://doi.org/10.1016/j.diabres.2014.07.026. [DOI] [PubMed] [Google Scholar]
- 98.Castan-Laurell I, Dray C, Attané C, Duparc T, Knauf C, Valet P. Apelin, diabetes, and obesity. Endocrine. 2011;40:1–9. doi: 10.1007/s12020-011-9507-9. https://doi.org/10.1007/s12020-011-9507-9. [DOI] [PubMed] [Google Scholar]
- 99.Hedjazifar S, Khatib Shahidi R, Hammarstedt A, Bonnet L, Church C, Boucher J, et al. The novel adipokine Gremlin 1 antagonizes insulin action and is increased in type 2 diabetes and NAFLD/NASH. Diabetes. 2020 Mar 1;69(3):331–41. doi: 10.2337/db19-0701. https://doi.org/10.2337/db19-0701. [DOI] [PubMed] [Google Scholar]
- 100.Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005 May 1;26(3):439–51. doi: 10.1210/er.2005-0005. https://doi.org/10.1210/er.2005-0005. [DOI] [PubMed] [Google Scholar]
- 101.Pan X, Kaminga AC, Wen SW, Acheampong K, Liu A. Omentin-1 in diabetes mellitus: a systematic review and meta-analysis. PLoS One. 2019 Dec 10;14(12):e0226292. doi: 10.1371/journal.pone.0226292. https://doi.org/10.1371/journal.pone.0226292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ouchi N, Higuchi A, Ohashi K, Oshima Y, Gokce N, Shibata R, et al. Sfrp5 is an anti-inflammatory adipokine that modulates metabolic dysfunction in obesity. Science. 2010 Jul 23;329(5990):454–7. doi: 10.1126/science.1188280. https://doi.org/10.1126/science.1188280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Moreno-Aliaga MJ, Pérez-Echarri N, Marcos-Gómez B, Larequi E, Gil-Bea FJ, Viollet B, et al. Cardiotrophin-1 is a key regulator of glucose and lipid metabolism. Cell Metab. 2011 Aug 3;14(2):242–53. doi: 10.1016/j.cmet.2011.05.013. https://doi.org/10.1016/j.cmet.2011.05.013. [DOI] [PubMed] [Google Scholar]


