Abstract
Background
A substantial proportion of tuberculosis patients remain with pulmonary symptoms and reduced physical capacity despite successful treatment. We performed a systematic review to analyse the burden of post-tuberculosis lung impairment measured by lung function testing.
Methods
We searched the PubMed database for articles published between database inception and November 2020 and performed meta-analyses to estimate the prevalence, type and severity of lung impairment among drug-susceptible and multidrug-resistant tuberculosis survivors. Methodological quality of included studies was assessed using the Newcastle–Ottawa scale.
Results
54 articles were included in this review. For subjects with former drug-susceptible tuberculosis, the combined estimated mean was 76.6% (95% CI 71.6–81.6) of predicted for forced expiratory volume in 1 s (FEV1) and 81.8% (95% CI 77.4–86.2) for forced vital capacity (FVC). In former patients with multidrug-resistant tuberculosis, it was 65.9% (95% CI 57.1–74.7) for FEV1 and 76.0% (95% CI 66.3–85.8) for FVC, respectively. The analysis of impairment types in former patients with drug-susceptible and multidrug-resistant tuberculosis showed that 22.0% versus 19.0% had obstructive, 23.0% versus 22.0% restrictive and 15.0% versus 43.0% had mixed impairment type, respectively. In the majority of studies, at least 10–15% of tuberculosis survivors had severe lung impairment.
Conclusions
This systematic review showed long-term abnormal spirometry results in a significant proportion of tuberculosis survivors.
Short abstract
These findings highlight a significant burden of functional lung impairment following tuberculosis. There is a need to implement effective strategies to diagnose post-tuberculosis lung impairment and to improve lung health in survivors of tuberculosis. https://bit.ly/3woP9oQ
Introduction
Tuberculosis (TB) remains one of the greatest public health challenges worldwide, with an estimated 9.9 million affected patients and associated 1.3 million deaths in 2020 [1]. TB survivors suffer from relevant residual morbidity [2, 3] and have a higher mortality risk compared to the standard population [4, 5]. In addition to evidence of an association between COPD and prior pulmonary TB from epidemiological studies [6, 7], it has been estimated that up to half of all former TB patients are left with post-TB lung disease (PTLD) at the end of TB treatment [8, 9].
While there is consensus among experts that spirometry can be a useful test to measure lung impairment after TB [3] and can also inform treatment decisions and treatment success evaluation in pulmonary rehabilitation in patients with PTLD [10], no evidence-based international guidelines are available to provide recommendations for the evaluation and treatment of PTLD [11]. Spirometry is one of the basic diagnostic methods in pulmonary medicine and key for the diagnosis of common chronic lung diseases such as asthma and COPD. Several international guidelines on spirometry and reference values for spirometry values are available, guiding the investigation and analysis of results [12, 13]. Despite the unclear role of spirometry and its parameters in the diagnosis and monitoring of PTLD, it has already been used in many studies of PTLD [8, 9, 14], including host-directed therapy trials that are aiming for an improved outcome in pulmonary function in TB patients [15].
This systematic review aims to comprehensively describe the scientific evidence on the prevalence of pulmonary function impairment measured by spirometry in former patients with drug-susceptible and multidrug-resistant TB.
Methods
The protocol for this review was registered with PROSPERO (CRD42018095000). The review methods and reporting of results follow PRISMA guidelines [16].
Search strategy and selection criteria
Full reports of studies investigating pulmonary function in former TB patients at any time after active pulmonary TB were searched via PubMed (US National Library of Medicine National Institutes of Health Search database) from inception until November 2020, with keywords such as “pulmonary tuberculosis”, “lung impairment”, “lung function”, “spirometry” (a list including all search terms can be found in Supplement 1). Search terms including 6-min walk test and St. George's Respiratory Questionnaire were also used as these assessments are often performed together with spirometry.
In addition to the systematic search, eligible articles on the topic known to the authors and articles identified by hand search, but not identified by the systematic search in PubMed, were also included. All articles were reviewed for relevance by title and abstract. Duplicates were removed. Inclusion criteria were original studies that reported spirometry measurements, full-text access and written in English. We excluded those studies that did not report spirometry outcomes in a format that could be further used for meta-analysis, i.e. figures only, editorials, commentaries, conference papers, abstracts only or brief communications, and studies targeting specific study populations such as patients with lobectomy or with previously known specific comorbidities, e.g. advanced COPD or exclusively elderly people.
Data extraction
Relevant data, extracted from eligible articles by one reviewer (O.I., V.H. or A.R.) and cross checked by another reviewer (O.I., V.H. or A.R.), included author name, year of publication, study region, sample size, type of TB (drug-susceptible disease versus multidrug-resistant disease) and time point of lung function testing in relation to the last TB episode. The following spirometry data were also retrieved: absolute values and percentage (%) of predicted for expiratory volume in 1 s (FEV1), forced vital capacity (FVC) and FEV1/FVC ratio including means, medians and sds, interquartile range (IQR) as well as impairment type (i.e. normal, reduced FEV1, reduced FVC (restriction), reduced FEV1/FVC ratio (obstruction) or both reduced FVC plus reduced FEV1/FVC ratio present (mixed)) and severity of lung impairment (i.e. mild, moderate, moderately severe, severe or very severe). Discrepancies between the reviewers were resolved by discussion and re-examination of the corresponding publications.
Quality assessment
Methodological quality of the included studies was assessed by one reviewer (O.I., V.H. or A.R.) using the Newcastle–Ottawa scale (NOS). A “star system” was used to place publications in three categories – low, moderate and good [17]. The NOS is a valid tool to assess the internal and external quality of a study according to the Cochrane Collaboration [18].
Data analysis
Accounting for possible heterogeneity between the studies, we fitted random effects models to derive pooled estimators of the means of spirometry using the restricted maximum-likelihood estimator for estimation of the between study variance τ2 [19, 20]. If the standard deviations of the means were not reported in the manuscript, they were estimated from confidence intervals, IQRs, or by combining standard deviations of groups (as recommended in the Cochrane handbook [18]).
For meta-analysis of proportions of patients with obstructive, restricted and mixed impairment patterns on spirometry, logit-transformed and generalised linear mixed models (more specific, random intercept logistic regression models [21]) were fitted [22]. Confidence intervals for individual studies were calculated as Wilson score intervals [23]. The maximum-likelihood method was used for estimation of the between study variance τ2.
To assess the extent of between-study heterogeneity, I2 was calculated [24]. Sources of heterogeneity were investigated using meta-regression analysis. The following pre-specified variables were analysed: year of publication, country, world region, patient selection, patient age, proportion of males, proportion of smokers and study quality. Pooled estimators of means and proportions were calculated for relevant subgroups. Publication bias was assessed by plotting study results against precision of the study [25] and the according regression tests [26].
All calculations were performed using the package “meta” in the statistical software package R (version 4.0.0, R Foundation for Statistical Computing, Vienna, Austria). p-values below 0.05 were considered significant and all confidence limits were calculated on the 95% level.
Role of the funding source
The funders of the study played no role in study design, data collection, data analysis, data interpretation or writing of the report.
Results
Study selection and general characteristics
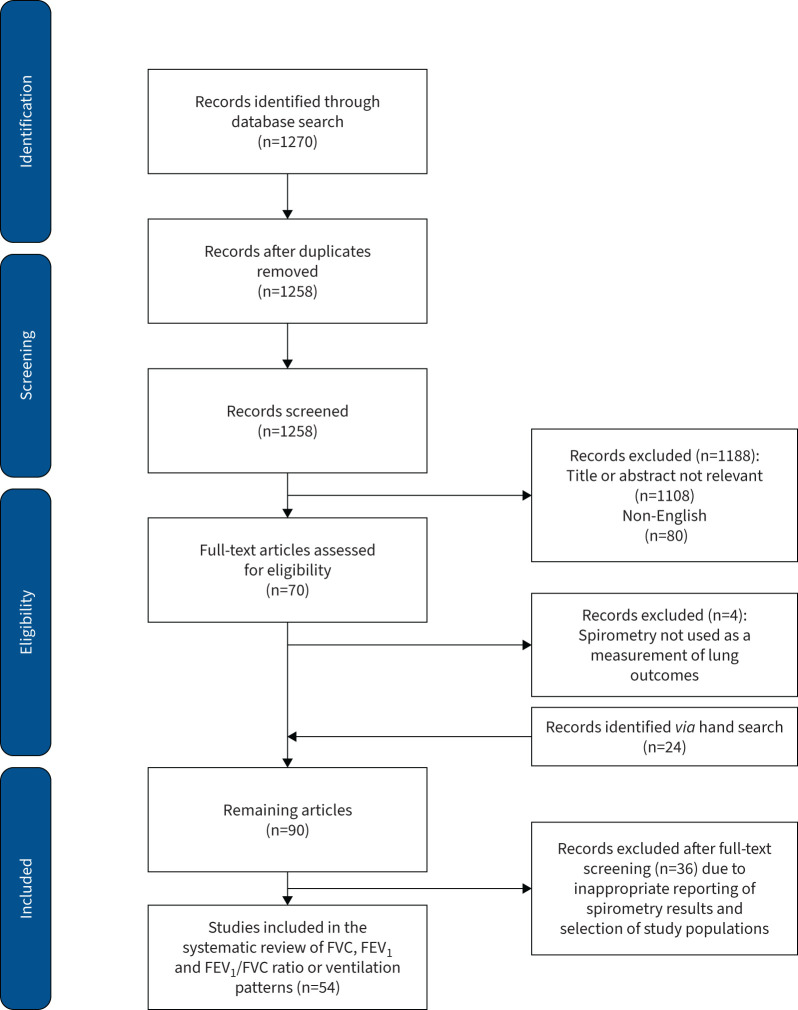
The database search yielded 1270 records. After title and content screening and eligibility assessment, a total of 54 [6, 8, 9, 27–77] studies were included in this review (figure 1).
FIGURE 1.
Flow chart diagram of studies for the systematic review (PRISMA). FVC: forced vital capacity; FEV1: forced expiratory volume in 1 s.
The earliest study included was from 1971 and the latest from 2020. Included publications represented all World Health Organization regions [78] except for the Eastern Mediterranean region, with the smallest number of studies from Europe (n=3) and the largest from Africa (n=18).
In the majority of studies (patient series, clinical studies and cohort studies, n=46, 8725 people), participants were selected based on a clinical TB diagnosis and/or microbiologically based TB diagnosis or TB treatment in the past in order to assess the pulmonary TB outcome in these subjects. In this review, we named these studies “patient series”. The remaining studies (n=8, 5896 people) were population-based surveys, in which a TB history (either based on self-reporting or on chest X-ray analysis) was assessed as one of several risk factors for lung function impairment.
Almost all studies reported the distribution of sex (male/female) and age among participants. Less than half of the studies (n=18) clearly stated whether there was anti-TB drug resistance diagnosed in the included participants. Studies that did not specify the TB resistance status of participants were usually older and from settings known for having a low multidrug-resistant TB prevalence. Thus, studies that did not explicitly state the resistance profile of participants were merged into one group with the studies that explicitly included drug-susceptible TB patients only and analysed separately from studies that stated that they included exclusively multidrug-resistant TB patients. The total sample size out of 54 studies was 14 621 included people. Detailed study characteristics of all 54 studies are presented in Supplement 2.
Quality assessment
The quality assessment using the NOS showed that more than half of the studies (n=31) were of a good quality, 22 had moderate quality and one had low quality (Supplement 2).
Spirometry methodology and time point
Around 87.0% of the studies clearly stated the guidelines according to which spirometry measurement and quality control were performed, of which the vast majority followed American Thoracic Society/European Respiratory Society standards. Only 36/54 (66.7%) studies stated the reference standard used to analyse spirometry results. The time point for spirometry assessment in relation to end of TB treatment varied significantly between studies, with the median time of 6 months (IQR 0–26.5).
Differences in reporting of spirometry results
Supplement 2 shows the different spirometric parameters and spirometric impairment types reported by each study. The quality, scope and comprehensiveness of spirometry data analysis and reporting differed greatly between the articles. 21 out of 54 (38.9%) studies reported absolute values for FVC and/or FEV1 in litres, 41/54 (76.0%) studies reported values for FEV1 and FVC as a percentage of predicted and in 11/54 (20.4%) studies none of these measurements for FVC, FEV1 or FEV1/FVC ratio were included in the article or only presented graphically without measures of precision (i.e. sd and IQR). A total of 47/54 (87.0%) articles reported on the type of ventilation impairment seen in spirometry (obstruction, restriction or mixed pattern), with some of them (n=21; 44.4%) also analysing the severity grade of lung impairment. Based on the type of data reported by each study, they were included in one or both of the following meta-analyses.
Meta-analysis of spirometric parameters: FVC and FEV1
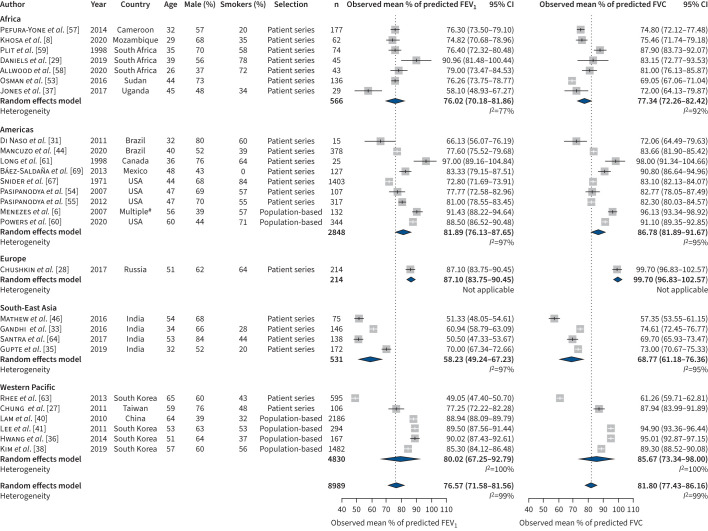
Those 27/54 (50.0%) studies that reported values for FVC and/or FEV1 as a percentage of predicted, as well as an indicator of variance, were included in this meta-analysis. For studies reporting on drug-susceptible TB, the combined estimated mean of percentage of predicted was 76.6% (95% CI 71.6–81.6) for FEV1 and 81.8% (95% CI 77.4–86.2) for FVC (figure 2). Heterogeneity in this analysis was substantial for both estimates (I2=99%). Main sources of heterogeneity were world region and study type (tests for subgroup differences p<0.0001). Patient series tend to give significant lower estimates and more heterogeneous results than population-based studies, with a combined estimate of 72.9 (95% CI 67.4–78.4, I2=99%) for FEV1 and 79.0 (95% CI 74.3–83.6, I2=98%) for FVC compared to population-based studies with estimates of 88.7 (95% CI 87.0–90.4, I2=85%) for FEV1 and 93.2 (95% CI 90.5–95.8, I2=94%) for FVC (Supplement 3, figures A and B). Meta-regression and subgroup analysis for age, percentage of males, percentage of smokers and the NOS did not yield significant results.
FIGURE 2.
Forced vital capacity (FVC) and forced expiratory volume in 1 s (FEV1) for drug-susceptible tuberculosis studies. #: Countries: Brazil, Uruguay, Mexico, Chile and Venezuela.
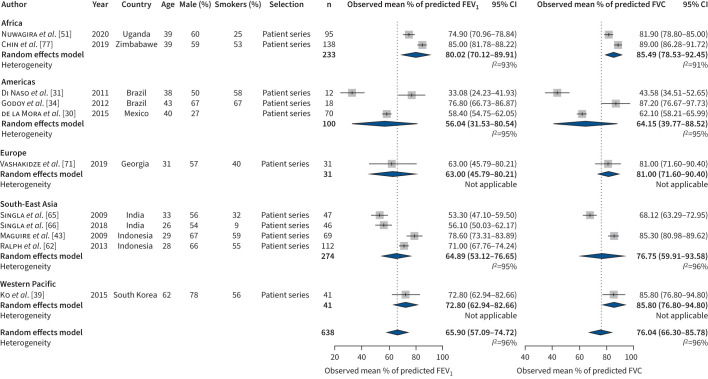
For former patients with multidrug-resistant TB, the combined estimated mean of percentage of predicted was 65.9% (95% CI 57.1–74.7, I2=96%) for FEV1 and 76.0% (95% CI 66.3–85.8, I2=96%) for FVC (figure 3). Similar to studies reporting on drug-susceptible TB, the estimated percentage of predicted for FEV1 was lower compared to that for FVC. Visual inspection and tests for funnel plot asymmetry did not indicate publication bias.
FIGURE 3.
Forced vital capacity (FVC) and forced expiratory volume in 1 s (FEV1) for multidrug-resistant tuberculosis studies.
Meta-analysis of abnormal ventilation patterns on spirometry
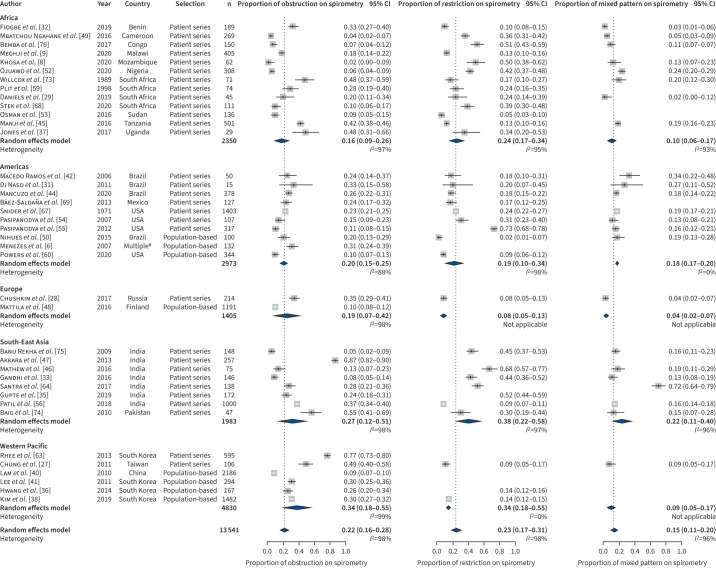
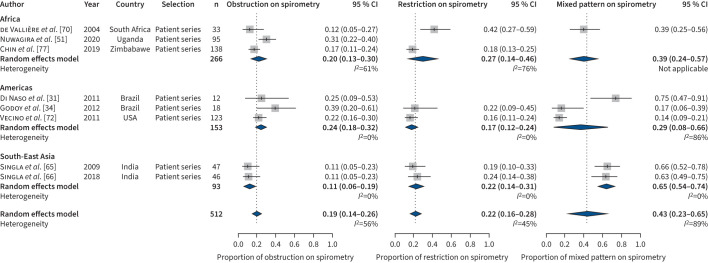
A total of 47/54 (87.1%) studies reported the impairment type identified by spirometry. Among them, all studies assessed pulmonary obstruction, many also assessed restriction (n=37) and some less mixed patterns (n=31). The combined estimated proportions of people with obstruction and restriction were almost equal in studies reporting on drug-susceptible TB (figure 4) and multidrug-resistant TB (figure 5), with 0.2 (95% CI 0.2–0.3, I2=98%) and 0.2 (95% CI 0.2–0.3, I2=98%), respectively, in studies on drug-susceptible TB (figure 4) versus 0.2 (95% CI 0.1–0.3, I2=56%) and 0.2 (95% CI 0.2–0.3, I2=45%), respectively, in studies on multidrug-resistant TB (figure 5). However, a mixed impairment pattern was more prevalent among studies on multidrug-resistant TB with 0.4 (95% CI 0.2–0.7, I2=89%) compared to studies on drug-susceptible TB with 0.2 (95% CI 0.1–0.2, I2=96%). Notably, while the combined estimates for obstruction (Supplement 3, figure C) and mixed impairment (Supplement 3, figure D) were almost similar in patients’ series versus population-based surveys in survivors of drug-susceptible TB, the proportion of survivors of drug-susceptible TB with restriction was significantly higher in patient series compared to population surveys (Supplement 3, figure E). In this analysis too, the amount of heterogeneity was high. Again, world region and study type (only for pulmonary restriction) were significant sources of heterogeneity, while age, percentage of males, percentage of smokers and NOS score were not. In addition, in this analysis visual inspection and tests for funnel plot asymmetry did not indicate publication bias.
FIGURE 4.
Type of ventilation disorder for drug-susceptible tuberculosis studies. #: Countries: Brazil, Uruguay, Mexico, Chile and Venezuela.
FIGURE 5.
Type of ventilation disorder for multidrug-resistant tuberculosis studies.
Severity of lung impairment
A total of 21/54 (38.9%) studies provided a grading of severity of lung impairment. The majority of studies graded the severity of lung impairment for obstruction only (11 studies, table 1). Nine of them followed the Global Initiative for Chronic Obstructive Lung Disease classification for obstruction severity, table 1. The proportion of patients with moderate or severe lung impairment was extremely heterogenous across the 21 studies, which can partially be explained by the different denominators (e.g. all participants versus participants with lung impairment) used by the respective authors. The data indicate that, in the majority of studies, about 10–15% of participants had severe lung impairment, which is less than 50% of predicted.
TABLE 1.
Severity of lung impairment
| Author | Year | Type of TB | Mild (%) | Moderate (%) | Severe (%) | Any impairment (%) | Severity classification used by authors | ||
| Moderate | Moderately severe | Severe | Very severe | ||||||
| Akkara et al. [47]# | 2013 | TB | 21.5 | 37.2 | 41.3 | 86.8 | ATS | ||
| Báez-Saldaña et al. [69]# | 2013 | TB | 13 | 3 | 5 | 1.5 | 1.5 | 41 | ATS/ERS |
| Baig et al. [74]# | 2010 | TB | 5.9 | 23 | 69.2 | 100 | GOLD | ||
| Bemba et al. [76] (obstruction) | 2017 | TB | 4.9 | 0 | 4.9 | 68.7 | ATS/ERS | ||
| Bemba et al. [76] (restriction) | 2017 | TB | 34.9 | 25.2 | 14.6 | 68.7 | ATS/ERS | ||
| Bemba et al. [76] (mixed) | 2017 | TB | 0 | 6.8 | 8.7 | 68.7 | ATS/ERS | ||
| Chin et al. [77]# | 2019 | TB (MDR) | – | 64 | 14 | 35 | GLI 2012 | ||
| Chushkin et al. [28]# | 2017 | TB | 2.8 | 15.9 | 9.3 | 47.6 | ATS/ERS | ||
| Daniels et al. [29]# | 2019 | TB | 20 | 10 | 50 | 10 | 48 | GOLD | |
| Di Naso et al. [31] (DS TB) | 2011 | TB (DS) | 53.3 | 13.3 | 13.3 | 80 | NA | ||
| Di Naso et al. [31] (MDR TB) | 2011 | TB (MDR) | 8.3 | 16.7 | 75 | 100 | NA | ||
| Fiogbe et al. [32] (obstruction) | 2019 | TB (DS) | 23 | 10 | 0 | 45 | ATS/ERS | ||
| Fiogbe et al. [32] (restriction) | 2019 | TB (DS) | 3.2 | 6 | 1 | 45 | ATS/ERS | ||
| Fiogbe et al. [32] (mixed) | 2019 | TB (DS) | 0 | 0 | 2.1 | 45 | GOLD | ||
| Hwang et al. [36]# | 2014 | TB | 45 | 43.7 | 11.3 | NA | GOLD | ||
| Khosa et al. [8] (obstruction) | 2020 | TB (DS) | 1.6 | – | – | 64.5 | Criée et al. [91] | ||
| Khosa et al. [8] (restriction) | 2020 | TB (DS) | 27.4 | 22.6 | – | 64.5 | Criée et al. [91] | ||
| Khosa et al. [8] (mixed) | 2020 | TB (DS) | – | 1.6 | 11.3 | 64.5 | Criée et al. [91] | ||
| Mancuzo et al. [44] (obstruction) | 2020 | TB | 21.4 | 4.5 | 0.5 | 63 | Pereira [92] | ||
| Mancuzo et al. [44] (restriction) | 2020 | TB | 16.7 | 0.3 | 1.3 | 63 | Pereira [92] | ||
| Mancuzo et al. [44] (mixed) | 2020 | TB | 3.2 | 7.7 | 7.4 | 63 | Pereira [92] | ||
| Manji et al. [45] (obstruction) | 2016 | TB | 56 | 23 | 21 | 74 | ATS/ERS/NLHEP | ||
| Manji et al. [45] (restriction) | 2016 | TB | 43 | 30 | 27 | 74 | ATS/ERS/NLHEP | ||
| Mattila et al. [48]# | 2017 | TB | 10 | 57 | 38 | NA | GOLD | ||
| Nuwagira et al. [51]# | 2020 | TB (MDR) | 0 | 55 | 45 | NA | GOLD | ||
| Ojuawo et al. [52] (obstruction) | 2020 | TB | 1.9 | 3.9 | 0 | 0 | 0 | 72 | ATS/ERS |
| Ojuawo et al. [52] (restriction) | 2020 | TB | 5.2 | 22.1 | 0 | 8.4 | 6.5 | 72 | ATS/ERS |
| Ojuawo et al. [52] (mixed) | 2020 | TB | 0 | 0 | 4.5 | 13.6 | 5.8 | 72 | ATS/ERS |
| Osman et al. [53]# | 2016 | TB | 8.3 | 58.3 | 16.7 | 16.7 | NA | GOLD | |
| Ralph et al. [62] | 2013 | TB (DS and MDR) | – | 27 | NA | Pellegrino et al. [93] | |||
| Macedo Ramos et al. [42] | 2006 | TB | 29.4 | 22.6 | 23.6 | 76 | Brazilian Society of Pulmonology and Phthisiology 2000 | ||
| Santra et al. [64]# | 2017 | TB | 12 | 52 | 44 | 30 | 100 | GOLD | |
| Vecino et al. [72] | 2011 | TB (DS and MDR) | – | 11 | 9 | 52 | ATS and Cocchiarella et al. [94] | ||
The denominator used to calculate the proportion of subjects with a specific severity grade of impairment types varied among the studies, e.g. denominator=total study population with both normal and impaired lung function combined versus denominator=only participants with lung impairment. #: Only the severity of pulmonary obstruction was presented in the respective manuscript. ATS: American Thoracic Society; DS: drug susceptible; ERS: European Respiratory Society; GLI: Global Lung Function Initiative; GOLD: Global Initiative for Chronic Obstructive Lung Disease; MDR: multidrug resistant; NA: not applicable; NLHEP: National Lung Health Education Program; TB: tuberculosis.
Discussion
This systematic review and meta-analyses included the spirometry results from 54 studies with highly heterogenous study populations, study designs and geographical origins published from 1971 to 2020, leading to a substantial heterogeneity in the data. In summary, our analyses showed a globally significant burden of abnormal lung function in spirometry of TB survivors. Although FEV1 and FVC were on average reduced by 24.0% and 20.0%, respectively, which is just below the limit of normality, a relevant proportion of previous TB patients were affected by obstructive (low FEV1/FVC ratio), restrictive (low FVC) or combined obstructive/restrictive pulmonary disease, with about 10–15% having severe impairment. FEV1 and FVC were on average significantly lower in patient series compared to population-based studies. This could be due to two main reasons. First, previous data showed that lung function continues to recover significantly for up to 12–18 months after the end of TB treatment [9, 79]. Therefore, in patient series that included former TB patients at around 6 months after TB treatment, lung function was measured at a time when improvement is possible in many patients. Second, there is a possibility of selection bias due to lower secondary mortality [5] and morbidity [9] in patients with less pulmonary sequelae, leading to increased recruitment of former TB patients with less severe PTLD compared to those with more severe PTLD in population-based surveys.
All types of lung impairment, obstruction, restriction and mixed, were found in former TB patients, indicating that several additional co-factors (genetic background of host and pathogen, comorbidities, risk-behaviour, environment, etc.) may be contributing to and modulating the development of PTLD. About 22% of former TB patients had pulmonary obstruction. This is a higher proportion than in general populations included in the Burden of Obstructive Lung Disease (BOLD) study [7]. TB was shown to be associated with COPD diagnosis in several studies [6, 7, 80, 81]. A major driver of both COPD and pulmonary TB is past or ongoing exposure to cigarette smoke [7]. Smoking was also shown to be associated with delayed time to culture conversion [82] and less favourable outcomes of TB treatments [7]. There was a high prevalence of smoking as a co-risk factor in the included studies. As nonsmoking populations are usually used as a reference, the individual and interactive effect of TB and smoking on the development of lung impairment in former TB patients could not be investigated in most studies. Unfortunately, the fact that smoking habits were inconsistently defined, queried and reported in the reviewed studies also prevented the analysis of the effect of smoking in this meta-analysis.
23% of people with a history of drug-susceptible TB had pulmonary restriction, meaning a low value for FVC. Worldwide, TB ranks among the leading causes of restrictive lung diseases [83]. In addition, in the BOLD study, an association between TB and restrictive spirometry pattern was shown [7]. The proportions of patients with restriction were highly variable and reached up to 68% in some studies. The timing of spirometry assessment could be one reason. While restriction seemed to be the prevailing spirometric abnormality early, during and after TB treatment, there is an indication for a decrease in restriction and increase in the proportion and severity of obstruction towards later time points when TB treatment had been completed [9, 58, 84, 85]. This observation could be explained by a relative improvement in vital capacity due to a decrease in parenchymal inflammation during and after treatment, but would need further investigations in future studies.
While in studies reporting on drug-susceptible TB only 15% of participants had mixed impairment, in patients with multidrug-resistant TB, mixed impairment was the prevailing abnormality in up to 70% of participants. Further, patients with multidrug-resistant TB suffered from substantially greater FEV1 and FVC impairment in spirometry than patients with drug-susceptible TB. This is plausible given that, in many settings, appropriate diagnosis of multidrug-resistant TB and initiation of antimycobacterial treatment are often delayed and treatment efficacy less than optimal, both of which can lead to a higher degree of chronicity of TB with greater lung damage in affected patients. Thus, there is an indication in the data for an association between severity of lung damage, duration of illness and mixed impairment type, which needs confirmation by greater studies.
Apart from differences in study design, there could be other sources for the heterogeneity observed in our data. For instance, the use of inadequate reference standards or diagnostic thresholds, especially for study populations that are not represented by common standards like the Global Lung Function Initiative, could lead to over- or under-reporting of lung impairment [13, 86, 87]. In addition, there was an indication for a systematic under-reporting of restriction or mixed impairment types within the reviewed studies. We observed a much greater focus on COPD and airways disease in studies on post-TB lung diseases. This could possibly be explained by the tremendous disease burden caused by chronic airways diseases such as COPD and asthma globally and the importance of spirometry for the diagnosis of COPD.
Furthermore, differences in the quality of spirometry data, which are not captured by the NOS, can also contribute to heterogeneity in the data. Some studies contributing to heterogeneity can be identified as outliers: e.g. Rhee et al. [63] included only patients with more than one lung lobe destroyed by TB, Santra et al. [64] selected patients based on obstruction or mixed pattern spirometry results and Mathew et al. [46] included 29% patients who defaulted from TB treatment. Some of the observed heterogeneity is an inherent effect of the many studies with large sample sizes, resulting in the very exact estimates with narrow confidence intervals that were included into the meta-analyses. Those very small confidence intervals lead to a large I2 that is induced by differences between studies, which may not be clinically relevant. Therefore, the summary estimates should not be interpreted as exact numbers but as indicators for the relevance of the overall problem of TB sequelae resulting in spirometric impairment. Results of this systematic review document a clearly heterogeneous study situation.
Although this study presents robust evidence for impaired lung function by spirometry in people with a history of TB, the clinical relevance of this finding is not clear. Only few studies systematically assessed different health outcomes such as physiological capacity tests (e.g. 6-min walk test), health-related quality of life (HRQoL) or frequency of exacerbations and hospitalisation together with spirometry results in a relevant number of post-TB patients. Publications on systematically performed prospective long-term evaluations of lung function and the prognosis of TB are not available to date. However, there is convincing evidence for the association of impaired FEV1 with a greater risk of death in normal populations and in COPD patients [88, 89]. FEV is also a predictor for hospitalisation risk and associated with HRQoL in COPD patients [89, 90]. There is supporting evidence for a similar relationship between morbidity and mortality and spirometry parameters in former TB patients. A population-based study from Brazil showed that respiratory diseases were among the main causes of death among previous TB patients [5]. Also, Meghji et al. [9] showed in a Malawian cohort that post-TB lung impairment in spirometry was associated with adverse clinical outcomes during the first 12 months after end of treatment.
This systematic review has some limitations leading to the fact that not all available research data from other databases (i.e. Embase or Cochrane) could be included in the review and meta-analysis and the effect of co-morbidities and cigarette smoking could not be addressed. Additionally, the search terms used were broad but probably not exhaustive as no consistent terminology has been used in this field for decades. Further, a significant number of studies were excluded because of language barriers. Also, studies that presented outcome measures that were either not comparable to other studies (e.g. absolute values for FEV1 and FVC in litres or graphical data) or that did not include estimates for precision could not be included in this meta-analysis. Strengths of this work include the diversity of study settings and the use of objective outcome measures produced by spirometry.
To conclude, this is the first systematic review and meta-analysis of spirometry outcomes in TB survivors. The findings highlight a significant burden of lung impairment after TB, especially in survivors of multidrug-resistant TB. Our results underpin the need to improve TB control to prevent chronic lung impairment in affected populations. Effective strategies to follow up TB survivors with sequelae are needed to improve health and wellbeing for these individuals. For future clinical and programmatic interventions, to be effective to reduce the burden of post-TB sequelae, a better understanding of the pathomechanisms and risk factors leading to different pulmonary impairment types and long-term adverse clinical outcomes will be important.
Points for future research
• Explore the relevance of these findings for clinical practice.
• Understand the pathomechanisms and risk factors leading to different pulmonary impairment types and long-term adverse clinical outcomes after TB.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplement 1: Search terms ERR-0221-2022.SUPPLEMENT1 (104.1KB, pdf)
Supplement 2: Characteristics of the selected studies ERR-0221-2022.SUPPLEMENT2 (244.7KB, pdf)
Supplement 3: Supplementary figures ERR-0221-2022.SUPPLEMENT3 (2.2MB, pdf)
Acknowledgements
The authors would like to acknowledge Julia Witzleb (Klinikum Bremen-Mitte, Bremen, Germany), who performed the initial literature search and data extraction for her master thesis. We would also like to thank the TB Sequel consortium members for their support and encouragement during the conduct of this study. We want to specifically mention Celso Khosa (Instituto Nacional de Saúde, Maputo, Mozambique) for his participation in brainstorming and discussions about the review.
Provenance: Submitted article, peer reviewed.
Data sharing: The data are available on request from the corresponding author.
Author contributions: A. Rachow, O. Ivanova and V.S. Hoffmann conceived and designed the review. O. Ivanova, A. Rachow and V.S. Hoffmann performed literature search and quality appraisal of the included studies. O. Ivanova, A. Rachow and V.S. Hoffmann extracted the data. V.S. Hoffmann performed a meta-analysis and created figures. A. Rachow, O. Ivanova, V.S. Hoffmann and C. Lange drafted the manuscript. C. Lange and M. Hoelscher advised on study design and conduct. M. Hoelscher supported funding acquisition. All authors critically reviewed and approved the manuscript.
Conflict of interest: The authors declare no conflict of interest.
Support statement: The study was funded by the Friedrich-Baur-Stiftung (Medical Faculty of Ludwig Maximilian University of Munich (LMU), Germany) and the German Ministry for Education and Research (BMBF), 01KA1613. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.World Health Organization . Global Tuberculosis Report 2021. www.who.int/publications/digital/global-tuberculosis-report-2021 Date last updated: 14 October 2021.
- 2.Quaife M, Houben RMGJ, Allwood B, et al. . Post-tuberculosis mortality and morbidity: valuing the hidden epidemic. Lancet Respir Med 2020; 8: 332–333. doi: 10.1016/S2213-2600(20)30039-4 [DOI] [PubMed] [Google Scholar]
- 3.Allwood BW, Van Der Zalm MM, Amaral AFS, et al. . Post-tuberculosis lung health: perspectives from the First International Symposium. Int J Tuberc Lung Dis 2020; 24: 820–828. doi: 10.5588/ijtld.20.0067 [DOI] [PubMed] [Google Scholar]
- 4.Romanowski K, Baumann B, Basham CA, et al. . Long-term all-cause mortality in people treated for tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis 2019; 19: 1129–1137. doi: 10.1016/S1473-3099(19)30309-3 [DOI] [PubMed] [Google Scholar]
- 5.Ranzani OT, Rodrigues LC, Bombarda S, et al. . Long-term survival and cause-specific mortality of patients newly diagnosed with tuberculosis in São Paulo state, Brazil, 2010–15: a population-based, longitudinal study. Lancet Infect Dis 2020; 20: 123–132. doi: 10.1016/S1473-3099(19)30518-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Menezes AMB, Hallal PC, Perez-Padilla R, et al. . Tuberculosis and airflow obstruction: evidence from the PLATINO study in Latin America. Eur Respir J 2007; 30: 1180–1185. doi: 10.1183/09031936.00083507 [DOI] [PubMed] [Google Scholar]
- 7.Amaral AFS, Coton S, Kato B, et al. . Tuberculosis associates with both airflow obstruction and low lung function: BOLD results. Eur Respir J 2015; 46: 1104–1112. doi: 10.1183/13993003.02325-2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khosa C, Bhatt N, Massango I, et al. . Development of chronic lung impairment in Mozambican TB patients and associated risks. BMC Pulm Med 2020; 20: 127. doi: 10.1186/s12890-020-1167-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meghji J, Lesosky M, Joekes E, et al. . Patient outcomes associated with post-tuberculosis lung damage in Malawi: a prospective cohort study. Thorax 2020; 75: 269–278. doi: 10.1136/thoraxjnl-2019-213808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Migliori GB, Marx FM, Ambrosino N, et al. . Clinical standards for the assessment, management and rehabilitation of post-TB lung disease. Int J Tuberc Lung Dis 2021; 25: 797–813. doi: 10.5588/ijtld.21.0425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wanner A, Edwards M, Harries AD, et al. . International research and guidelines on post-tuberculosis chronic lung disorders: a systematic scoping review. BMJ Glob Heal 2018; 3: e000745. doi: 10.1136/bmjgh-2018-000745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Graham BL, Steenbruggen I, Barjaktarevic IZ, et al. . Standardization of spirometry 2019 update. An official American Thoracic Society and European Respiratory Society technical statement. Am J Respir Crit Care Med 2019; 200: E70–E88. doi: 10.1164/rccm.201908-1590ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Quanjer PH, Stanojevic S, Cole TJ, et al. . Multi-ethnic reference values for spirometry for the 3–95-yr age range: the global lung function 2012 equations. Eur Respir J 2012; 40: 1324–1343. doi: 10.1183/09031936.00080312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rachow A, Ivanova O, Wallis R, et al. . TB sequel: incidence, pathogenesis and risk factors of long-term medical and social sequelae of pulmonary TB a study protocol. BMC Pulm Med 2019; 19: 4. doi: 10.1186/s12890-018-0777-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wallis RS, Ginindza S, Beattie T, et al. . Adjunctive host-directed therapies for pulmonary tuberculosis: a prospective, open-label, phase 2, randomised controlled trial. Lancet Respir Med 2021; 9: 897–908. doi: 10.1016/S2213-2600(20)30448-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, et al. . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg 2010; 8: 336–341. doi: 10.1016/j.ijsu.2010.02.007 [DOI] [PubMed] [Google Scholar]
- 17.Wells G, Shea B, O'Connell D, et al. . The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. www.ohri.ca/programs/clinical_epidemiology/oxford.asp Date last accessed: 21 December 2017.
- 18.Reeves BC, Deeks JJ, Higgins JPT, et al. . 13.5.2.3 Tools for assessing methodological quality or risk of bias in nonrandomized studies. In: Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0. Cochrane, 2011. https://handbook-5-1.cochrane.org/chapter_13/13_5_2_3_tools_for_assessing_methodological_quality_or_risk_of.htm Date last accessed: 24 August 2021. Date last updated: March 2011. [Google Scholar]
- 19.Viechtbauer W. Bias and efficiency of meta-analytic variance estimators in the random-effects model. J Educ Behav Stat 2005; 30: 261–293. doi: 10.3102/10769986030003261 [DOI] [Google Scholar]
- 20.Veroniki AA, Jackson D, Viechtbauer W, et al. . Methods to estimate the between-study variance and its uncertainty in meta-analysis. Res Synth Methods 2016; 7: 55–79. doi: 10.1002/jrsm.1164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stijnen T, Hamza TH, Özdemir P. Random effects meta-analysis of event outcome in the framework of the generalized linear mixed model with applications in sparse data. Stat Med 2010; 29: 3046–3067. doi: 10.1002/sim.4040 [DOI] [PubMed] [Google Scholar]
- 22.Schwarzer G, Chemaitelly H, Abu-Raddad LJ, et al. . Seriously misleading results using inverse of Freeman–Tukey double arcsine transformation in meta-analysis of single proportions. Res Synth Methods 2019; 10: 476–483. doi: 10.1002/jrsm.1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Newcombe RG. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat Med 1998; 17: 857–872. doi: [DOI] [PubMed] [Google Scholar]
- 24.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002; 21: 1539–1558. doi: 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 25.Egger M, Smith GD, Schneider M, et al. . Bias in meta-analysis detected by a simple, graphical test. Br Med J 1997; 315: 629–634. doi: 10.1136/bmj.315.7109.629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994; 50: 1088–1101. doi: 10.2307/2533446 [DOI] [PubMed] [Google Scholar]
- 27.Chung KP, Chen JY, Lee CH, et al. . Trends and predictors of changes in pulmonary function after treatment for pulmonary tuberculosis. Clinics 2011; 66: 549–556. doi: 10.1590/S1807-59322011000400005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chushkin MI, Ots ON. Impaired pulmonary function after treatment for tuberculosis: the end of the disease? J Bras Pneumol 2017; 43: 38–43. doi: 10.1590/s1806-37562016000000053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Daniels KJ, Irusen E, Pharaoh H, et al. . Post-tuberculosis health-related quality of life, lung function and exercise capacity in a cured pulmonary tuberculosis population in the Breede Valley District, South Africa. South African J Physiother 2019; 75: 1319. doi: 10.4102/sajp.v75i1.1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de la Mora IL, Martínez-Oceguera D, Laniado-Laborín R. Chronic airway obstruction after successful treatment of tuberculosis and its impact on quality of life. Int J Tuberc Lung Dis 2015; 19: 808–810. doi: 10.5588/ijtld.14.0983 [DOI] [PubMed] [Google Scholar]
- 31.Di Naso FC, Pereira JS, Schuh SJ, et al. . Functional evaluation in patients with pulmonary tuberculosis sequelae. Rev Port Pneumol 2011; 17: 216–221. doi: 10.1016/j.rppneu.2011.06.010 [DOI] [PubMed] [Google Scholar]
- 32.Fiogbe AA, Agodokpessi G, Tessier JF, et al. . Prevalence of lung function impairment in cured pulmonary tuberculosis patients in Cotonou, Benin. Int J Tuberc Lung Dis 2019; 23: 195–202. doi: 10.5588/ijtld.18.0234 [DOI] [PubMed] [Google Scholar]
- 33.Gandhi K, Gupta S, Singla R. Risk factors associated with development of pulmonary impairment after tuberculosis. Indian J Tuberc 2016; 63: 34–38. doi: 10.1016/j.ijtb.2016.01.006 [DOI] [PubMed] [Google Scholar]
- 34.Godoy MDP, Mello FCQ, Lopes AJ, et al. . The functional assessment of patients with pulmonary multidrug-resistant tuberculosis. Respir Care 2012; 57: 1949–1954. doi: 10.4187/respcare.01532 [DOI] [PubMed] [Google Scholar]
- 35.Gupte AN, Paradkar M, Selvaraju S, et al. . Assessment of lung function in successfully treated tuberculosis reveals high burden of ventilatory defects and COPD. PLoS One 2019; 14: e0217289. doi: 10.1371/journal.pone.0217289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hwang YI, Kim JH, Lee CY, et al. . The association between airflow obstruction and radiologic change by tuberculosis. J Thorac Dis 2014; 6: 471–476. doi: 10.3978/j.issn.2072-1439.2014.04.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jones R, Kirenga BJ, Katagira W, et al. . A pre–post intervention study of pulmonary rehabilitation for adults with post-tuberculosis lung disease in Uganda. Int J COPD 2017; 12: 3533–3539. doi: 10.2147/COPD.S146659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim MS, Choi CJ, Kwon KM, et al. . Association of lung function with serum 25-hydroxyvitamin D level according to the presence of past pulmonary tuberculosis in Korean adults. Korean J Fam Med 2019; 40: 93–99. doi: 10.4082/kjfm.17.0083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ko Y, Lee YM, Lee HY, et al. . Changes in lung function according to disease extent before and after pulmonary tuberculosis. Int J Tuberc Lung Dis 2015; 19: 589–595. doi: 10.5588/ijtld.14.0454 [DOI] [PubMed] [Google Scholar]
- 40.Lam KBH, Jiang CQ, Jordan RE, et al. . Prior TB, smoking, and airflow obstruction: a cross-sectional analysis of the Guangzhou Biobank Cohort Study. Chest 2010; 137: 593–600. doi: 10.1378/chest.09-1435 [DOI] [PubMed] [Google Scholar]
- 41.Lee SW, Kim YS, Kim DS, et al. . The risk of obstructive lung disease by previous pulmonary tuberculosis in a country with intermediate burden of tuberculosis. J Korean Med Sci 2011; 26: 268–273. doi: 10.3346/jkms.2011.26.2.268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Macedo Ramos LM, Sulmonett N, Ferreira CS, et al. . Functional profile of patients with tuberculosis sequelae in a university hospital. J Bras Pneumol 2006; 32: 43–47. doi: 10.1590/S1806-37132006000100010 [DOI] [PubMed] [Google Scholar]
- 43.Maguire GP, Anstey NM, Ardian M, et al. . Pulmonary tuberculosis, impaired lung function, disability and quality of life in a high-burden setting. Int J Tuberc Lung Dis 2009; 13: 1500–1506. [PubMed] [Google Scholar]
- 44.Mancuzo EV, Netto EM, Sulmonett N, et al. . Spirometry results after treatment for pulmonary tuberculosis: comparison between patients with and without previous lung disease: a multicenter study. J Bras Pneumol 2020; 46: e20180198. doi: 10.36416/1806-3756/e20180198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Manji M, Shayo G, Mamuya S, et al. . Lung functions among patients with pulmonary tuberculosis in Dar es Salaam – a cross-sectional study. BMC Pulm Med 2016; 16: 56. doi: 10.1186/s12890-016-0213-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mathew D, Kirthana G, Krishnapriya R, et al. . To assess the pulmonary impairment in treated pulmonary tuberculosis patients using spirometry. IAIM 2016; 3: 94–99. [Google Scholar]
- 47.Akkara AS, Shah AD, Adalja M, et al. . Pulmonary tuberculosis: the day after. Int J Tuberc Lung Dis 2013; 17: 810–813. doi: 10.5588/ijtld.12.0317 [DOI] [PubMed] [Google Scholar]
- 48.Mattila T, Heliövaara M, Rissanen H, et al. . Tuberculosis, airway obstruction and mortality in a Finnish population. COPD 2017; 14: 143–149. doi: 10.1080/15412555.2016.1250253 [DOI] [PubMed] [Google Scholar]
- 49.Mbatchou Ngahane BH, Nouyep J, Nganda Motto M, et al. . Post-tuberculous lung function impairment in a tuberculosis reference clinic in Cameroon. Respir Med 2016; 114: 67–71. doi: 10.1016/j.rmed.2016.03.007 [DOI] [PubMed] [Google Scholar]
- 50.Nihues SSE, Mancuzo EV, Sulmonetti N, et al. . Chronic symptoms and pulmonary dysfunction in post-tuberculosis Brazilian patients. Braz J Infect Dis 2015; 19: 492–497. doi: 10.1016/j.bjid.2015.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nuwagira E, Stadelman A, Baluku JB, et al. . Obstructive lung disease and quality of life after cure of multi-drug-resistant tuberculosis in Uganda: a cross-sectional study. Trop Med Health 2020; 48: 34. doi: 10.1186/s41182-020-00221-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ojuawo OB, Fawibe AE, Desalu OO, et al. . Spirometric abnormalities following treatment for pulmonary tuberculosis in Ilorin, Nigeria. Niger Postgrad Med J 2020; 27: 163–170. doi: 10.4103/npmj.npmj_18_20 [DOI] [PubMed] [Google Scholar]
- 53.Osman RK, Mortimer K, Bjune G, et al. . Chronic respiratory disease in adults treated for tuberculosis in Khartoum. Sudan Public Heal Action 2016; 6: 199–204. doi: 10.5588/pha.16.0030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pasipanodya JG, Miller TL, Vecino M, et al. . Pulmonary impairment after tuberculosis. Chest 2007; 131: 1817–1824. doi: 10.1378/chest.06-2949 [DOI] [PubMed] [Google Scholar]
- 55.Pasipanodya JG, Vecino E, Miller TL, et al. . Non-hispanic whites have higher risk for pulmonary impairment from pulmonary tuberculosis. BMC Public Health 2012; 12: 119. doi: 10.1186/1471-2458-12-119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Patil S, Patil R, Jadhav A. Pulmonary functions’ assessment in post-tuberculosis cases by spirometry: obstructive pattern is predominant and needs cautious evaluation in all treated cases irrespective of symptoms. Int J Mycobacteriol 2018; 7: 128–133. doi: 10.4103/ijmy.ijmy_56_18 [DOI] [PubMed] [Google Scholar]
- 57.Pefura-Yone EW, Kengne AP, Tagne-Kamdem PE, et al. . Clinical significance of low forced expiratory flow between 25% and 75% of vital capacity following treated pulmonary tuberculosis: a cross-sectional study. BMJ Open 2014; 4: e005361. doi: 10.1136/bmjopen-2014-005361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Allwood BW, Maasdorp E, Kim GJ, et al. . Transition from restrictive to obstructive lung function impairment during treatment and follow-up of active tuberculosis. Int J COPD 2020; 15: 1039–1047. doi: 10.2147/COPD.S219731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Plit ML, Anderson R, Van Rensburg CEJ, et al. . Influence of antimicrobial chemotherapy on spirometric parameters and pro-inflammatory indices in severe pulmonary tuberculosis. Eur Respir J 1998; 12: 351–356. doi: 10.1183/09031936.98.12020351 [DOI] [PubMed] [Google Scholar]
- 60.Powers M, Sanchez TR, Welty TK, et al. . Lung function and respiratory symptoms after tuberculosis in an American Indian population. The strong heart study. Ann Am Thorac Soc 2020; 17: 38–48. doi: 10.1513/AnnalsATS.201904-281OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Long R, Maycher B, Dhar A, et al. . Pulmonary tuberculosis treated with directly observed therapy: serial changes in lung structure and function. Chest 1998; 113: 933–943. doi: 10.1378/chest.113.4.933 [DOI] [PubMed] [Google Scholar]
- 62.Ralph AP, Kenangalem E, Waramori G, et al. . High morbidity during treatment and residual pulmonary disability in pulmonary tuberculosis: under-recognised phenomena. PLoS One 2013; 8: e80302. doi: 10.1371/journal.pone.0080302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rhee CK, Yoo KH, Lee JH, et al. . Clinical characteristics of patients with tuberculosis-destroyed lung. Int J Tuberc Lung Dis 2013; 17: 67–75. doi: 10.5588/ijtld.12.0351 [DOI] [PubMed] [Google Scholar]
- 64.Santra A, Dutta P, Manjhi R, et al. . Clinico-radiologic and spirometric profile of an Indian population with post-tuberculous obstructive airway disease. J Clin Diagnostic Res 2017; 11: OC35–OC38. doi: 10.7860/JCDR/2017/24555.9529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Singla N, Singla R, Fernandes S, et al. . Post treatment sequelae of multi-drug resistant tuberculosis patients. Indian J Tuberc 2009; 56: 206–212. [PubMed] [Google Scholar]
- 66.Singla R, Mallick M, Mrigpuri P, et al. . Sequelae of pulmonary multidrug-resistant tuberculosis at the completion of treatment. Lung India 2018; 35: 4–8. doi: 10.4103/lungindia.lungindia_269_16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Snider GL, Doctor L, Demas TA, et al. . Obstructive airway disease in patients with treated pulmonary tuberculosis. Am Rev Respir Dis 1971; 103: 625–640. doi: 10.1164/arrd.1971.103.5.625 [DOI] [PubMed] [Google Scholar]
- 68.Stek C, Allwood B, Du Bruyn E, et al. . The effect of HIV-associated tuberculosis, tuberculosis-IRIS and prednisone on lung function. Eur Respir J 2020; 55: 1901692. doi: 10.1183/13993003.01692-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Báez-Saldaña R, López-Arteaga Y, Bizarrón-Muro A, et al. . A novel scoring system to measure radiographic abnormalities and related spirometric values in cured pulmonary tuberculosis. PLoS One 2013; 8: e78926. doi: 10.1371/journal.pone.0078926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.de Vallière S, Barker RD. Residual lung damage after completion of treatment for multidrug-resistant tuberculosis. Int J Tuberc Lung Dis 2004; 8: 767–771. [PubMed] [Google Scholar]
- 71.Vashakidze SA, Kempker JA, Jakobia NA, et al. . Pulmonary function and respiratory health after successful treatment of drug-resistant tuberculosis. Int J Infect Dis 2019; 82: 66–72. doi: 10.1016/j.ijid.2019.02.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vecino M, Pasipanodya JG, Slocum P, et al. . Evidence for chronic lung impairment in patients treated for pulmonary tuberculosis. J Infect Public Health 2011; 4: 244–252. doi: 10.1016/j.jiph.2011.08.005 [DOI] [PubMed] [Google Scholar]
- 73.Willcox PA, Ferguson AD. Chronic obstructive airways disease following treated pulmonary tuberculosis. Respir Med 1989; 83: 195–198. doi: 10.1016/S0954-6111(89)80031-9 [DOI] [PubMed] [Google Scholar]
- 74.Baig IM, Saeed W, Khalil KF. Post-tuberculous chronic obstructive pulmonary disease. J Coll Physicians Surg Pak 2010; 20: 542–544. [PubMed] [Google Scholar]
- 75.Banu Rekha VV, Ramachandran R, Kuppu Rao KV, et al. . Assessment of long term status of sputum positive pulmonary TB patients successfully treated with short course chemotherapy. Indian J Tuberc 2009; 56: 132–140. [PubMed] [Google Scholar]
- 76.Bemba ELP, Moyikoua R, Ouedraogo AR, et al. . Spirometric and radiographic profile of patients with pulmonary tuberculosis treated and cured at the Department of Pulmonology of Brazzaville University Hospital. Rev Pneumol Clin 2017; 73: 217–224. doi: 10.1016/j.pneumo.2017.08.009 [DOI] [PubMed] [Google Scholar]
- 77.Chin AT, Rylance J, Makumbirofa S, et al. . Chronic lung disease in adult recurrent tuberculosis survivors in Zimbabwe: A cohort study. Int J Tuberc Lung Dis 2019; 23: 203–211. doi: 10.5588/ijtld.18.0313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.World Health Organization . The Global Health Observatory. www.who.int/data/gho/data/countries Date last accessed: 5 July 2021.
- 79.Hnizdo E, Singh T, Churchyard G. Chronic pulmonary function impairment caused by initial and recurrent pulmonary tuberculosis following treatment. Thorax 2000; 55: 32–38. doi: 10.1136/thorax.55.1.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Allwood BW, Myer L, Bateman ED. A systematic review of the association between pulmonary tuberculosis and the development of chronic airflow obstruction in adults. Respiration 2013; 86: 76–85. doi: 10.1159/000350917 [DOI] [PubMed] [Google Scholar]
- 81.Byrne AL, Marais BJ, Mitnick CD, et al. . Tuberculosis and chronic respiratory disease: a systematic review. Int J Infect Dis 2015; 32: 138–146. doi: 10.1016/j.ijid.2014.12.016 [DOI] [PubMed] [Google Scholar]
- 82.Reimann M, Schaub D, Kalsdorf B, et al. . Cigarette smoking and culture conversion in patients with susceptible and M/XDR-TB. Int J Tuberc Lung Dis 2019; 23: 93–98. doi: 10.5588/ijtld.18.0354 [DOI] [PubMed] [Google Scholar]
- 83.Grippi MA, Elias JA, Fishman JA, et al. , eds. Fishman's pulmonary diseases and disorders. 5th Edn. New York, McGraw-Hill & Co., 2015. [Google Scholar]
- 84.Auld SC, Kornfeld H, Maenetje P, et al. . Pulmonary restriction predicts long-term pulmonary impairment in people with HIV and tuberculosis. BMC Pulm Med 2021; 21: 19. doi: 10.1186/s12890-020-01377-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Radovic M, Ristic L, Ciric Z, et al. . Changes in respiratory function impairment following the treatment of severe pulmonary tuberculosis – limitations for the underlying COPD detection. Int J COPD 2016; 11: 1307–1316. doi: 10.2147/COPD.S106875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cerveri I, Corsico AG, Accordini S, et al. . Underestimation of airflow obstruction among young adults using FEV1/FVC <70% as a fixed cut-off: a longitudinal evaluation of clinical and functional outcomes. Thorax 2008; 63: 1040–1045. doi: 10.1136/thx.2008.095554 [DOI] [PubMed] [Google Scholar]
- 87.Ivanova O, Khosa C, Bakuli A, et al. . Lung function testing and prediction equations in adult population from Maputo, Mozambique. Int J Environ Res Public Health 2020; 17: 4535. doi: 10.3390/ijerph17124535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Celli B, Locantore N, Yates JC, et al. . Markers of disease activity in COPD: an 8-year mortality study in the ECLIPSE cohort. Eur Respir J 2021; 57: 2001339. doi: 10.1183/13993003.01339-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Duong ML, Islam S, Rangarajan S, et al. . Mortality and cardiovascular and respiratory morbidity in individuals with impaired FEV1 (PURE): an international, community-based cohort study. Lancet Glob Heal 2019; 7: e613–e623. doi: 10.1016/S2214-109X(19)30070-1 [DOI] [PubMed] [Google Scholar]
- 90.Stöber A, Lutter JI, Schwarzkopf L, et al. . Impact of lung function and exacerbations on health-related quality of life in COPD patients within one year: real-world analysis based on claims data. Int J Chron Obstruct Pulmon Dis 2021; 16: 2637–2651. doi: 10.2147/COPD.S313711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Criée C-P, Baur X, Berdel D, et al. Leitlinie zur Spirometrie. Leitlinie der Deutschen Atemwegsliga, der Deutschen Gesellschaft für Pneumologie und Beatmungsmedizin und der Deutschen Gesellschaft für Arbeitsmedizin und Umweltmedizin zur Spirometrie. [Standardization of spirometry: 2015 update. Published by German Atemwegsliga, German Respiratory Society and German Society of Occupational and Environmental Medicine]. Pneumologie 2015; 69: 147–164. doi: 10.1055/s-0034-1391345 [DOI] [PubMed] [Google Scholar]
- 92.Pereira CA. Espirometria. J Bras Pneumol 2002; 28: Suppl., S1–S82. [Google Scholar]
- 93.Pellegrino R, Viegi G, Brusasco V, et al. Interpretative strategies for lung function tests. Eur Respir J 2005; 26: 948–968. doi: 10.1183/09031936.05.00035205 [DOI] [PubMed] [Google Scholar]
- 94.Cocchiarella L, Andersson GBJ, eds. Guides to the Evaluation of Permanent Impairment. 5th edn. Chicago, AMA Press, 2000. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplement 1: Search terms ERR-0221-2022.SUPPLEMENT1 (104.1KB, pdf)
Supplement 2: Characteristics of the selected studies ERR-0221-2022.SUPPLEMENT2 (244.7KB, pdf)
Supplement 3: Supplementary figures ERR-0221-2022.SUPPLEMENT3 (2.2MB, pdf)