Abstract
Asthma affects over 300 million people worldwide and its prevalence is increasing. COPD is the third leading cause of death globally. Asthma and COPD are complex inflammatory diseases of the airways in which impaired host defences lead to increased susceptibility to pathogens, pollutants and allergens. There is a constant interplay between host and the environment. Environmental exposures can alter the lung microbiome and influence the development of sensitisation by disrupting normal immunoregulation. The underlying airway inflammation in severe asthma is heterogeneous, with upregulation of type 2 cytokines in most cases but increased neutrophilic inflammation and activated T-helper 17 mediated immunity in others. COPD may also comprise several different phentoypes that are driven by different molecular mechanisms or endotypes. This disease heterogeneity is affected by comorbidities, treatments and environmental exposures. Recent intervention trials have shed light on the pathways beyond type 2 inflammation that can lead to beneficial outcomes versus potentially deleterious effects. We have made a great deal of progress over the last 10 years in terms of immunology and the pathophysiology of asthma and this has led to the development of novel treatments and major improvements in severe asthma outcomes. In COPD, however, no targeted treatments have demonstrated great improvements. This article reviews the mechanism of action and efficacy of the available biologics in asthma and COPD.
Short abstract
Asthma and COPD are complex inflammatory diseases of the airways. Targeted treatments have drastically improved severe asthma outcomes, but in COPD no targeted treatments have at present demonstrated great improvement. https://bit.ly/40ebm6H
Introduction
Over the last two decades, the understanding of the complex pathophysiology of asthma has led to the development of targeted treatments for uncontrolled severe asthma. It is increasingly recognised that asthma is a heterogeneous disease, with numerous clinical and inflammatory phenotypes showing different responses to treatment [1–8]. The most important clinical phenotypes are based on the age of onset. Early/childhood onset of asthma is mostly associated with a sensitisation to common aeroallergens [9]. In this phenotype, a T-helper (Th) 2-driven T-cell receptor-specific allergic response leads to eosinophilic inflammation. A subgroup of this early allergic asthma may become severe, exhibit frequent exacerbations and develop fixed airflow obstruction [10]. In contrast to this phenotype, late/adult-onset asthma is mostly nonallergic. In this phenotype, it is important to exclude occupational asthma or aspirin-sensitive asthma as avoidance of exposure may lead to an improvement in asthma status [11].
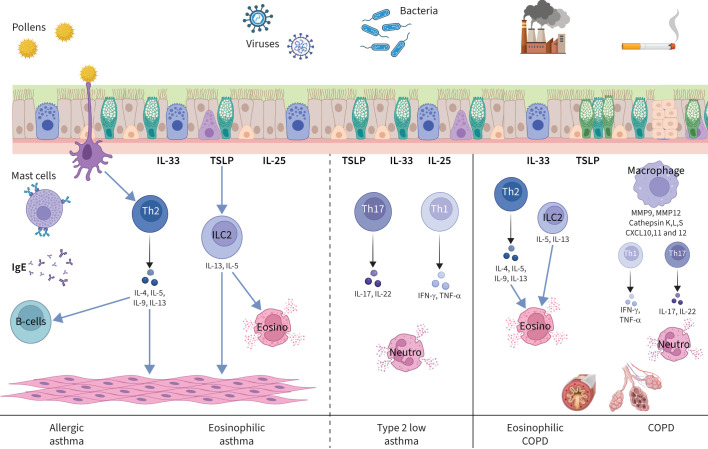
Besides the clinical phenotypes of asthma, there are also asthma inflammatory phenotypes. Type 2 asthma is characterised by eosinophilic airway inflammation that can be either allergen driven or secondary to the activation of innate lymphoid cell type 2 (ILC2) [12]. ILC2 cells do not express an antigen-specific T-cell receptor but respond to epithelial cytokines such as thymic stromal lymphopoietin (TSLP) and interleukin (IL) 33 (figure 1). When activated, ILC2 cells may produce both IL-5, leading to hypereosinophilia, and IL-13, leading to the production of exhaled nitric oxide and smooth muscle cell contraction with subsequent bronchial hyperresponsiveness. ILC2 cells are, however, more resistant to inhaled corticosteroids (ICS) [13]. Type 2 low asthma can be either neutrophilic or paucigranulocytic [14, 15]. Patients with the latter may exhibit persistent symptoms of asthma despite the low levels of granulocytic inflammation or type 2 biomarkers [16]. The inflammatory phenotypes are not fixed over time [17] and have to be re-assessed in cases of loss of control. The only feature that is common to all asthma inflammatory phenotypes is airway hyperresponsiveness, measured by direct or indirect bronchial challenges, which has been related to the number of mast cells in airway smooth muscle [18].
FIGURE 1.
Airway inflammation in severe asthma and COPD. CXCL: C-X-C motif ligand; Eosino: eosinophil; IFN-γ: interferon-γ; IL: interleukin; ILC2: innate lymphoid cell type 2; MMP: matrix metalloproteinase; Neutro: neutrophil; TNF-α: tumour necrosis factor-α; TSLP: thymic stromal lymphopoietin.
Several treatments targeting the cytokines involved in the pathophysiology of asthma, such as IL-5, IL-4, IL-13, IgE and TSLP, have proven efficacy in the management of severe asthma. COPD is also a heterogeneous disease with distinct inflammatory phenotypes, including eosinophilia, which may drive acute exacerbations in a subgroup of patients. Therefore, monoclonal antibodies developed to improve asthma status have been tested in COPD to see if they can similarly provide a therapeutic benefit for people with COPD of an eosinophilic phenotype.
This article reviews the mechanism of action and efficacy of the available biologics in asthma and COPD and is based on discussions that took place during the European Respiratory Society (ERS) Lung Science Conference 2022 in Estoril, Portugal. This review focuses on targeted therapies that have been developed based on the emerging classification of patients by phenotypes and endotypes in asthma and COPD. We performed a search using pre-defined specific keywords and summarised main studies in the field.
Severe asthma
Diagnosis and pheno-endotyping
Before thinking about a biologic treatment for a patient suffering from asthma, we must confirm the diagnosis of severe asthma, improve the patient's adherence to ICS, check and correct the inhaler technique, identify and eliminate exposure to risk factors and triggers such as allergens, irritants and smoking, and assess and treat co-existing conditions such as obstructive sleep apnoea, dysfunctional breathing, chronic rhinosinusitis and nasal polyps [19]. After this adequate management, in some patients, asthma may remain poorly controlled despite high-dose ICS and other controllers [20]. These patients could be candidates for targeted treatments. Clinicians need to establish clinical and inflammatory phenotypes through measurements of sputum inflammatory cells, blood eosinophil count and exhaled nitric oxide and look at coexisting conditions such as severe atopic dermatitis, chronic rhinosinusitis with nasal polyps, eosinophilic pneumonia and eosinophilic granulomatosis with polyangiitis (EGPA) to make the best treatment choice [21].
Induced sputum is the gold standard to phenotype the airway inflammatory pattern in severe asthma and is recommended in current guidelines on the management of this disease [19, 22]. Eosinophilic asthma is defined by sputum eosinophil counts greater than 1–3%, while neutrophilic asthma is defined by sputum neutrophil counts greater than 60–76%. When both cell types are increased, asthma is defined as mixed granulocytic; while if both cell types are below the normal range, asthma is considered as paucigranulocytic [15, 16]. As induced sputum analysis is technically demanding, time-consuming and not widely available, several surrogate markers have been discovered to establish the inflammatory phenotype. An increase in sputum eosinophils can be suspected in cases of increased blood eosinophils or exhaled nitric oxide fraction (FENO) but there is no correlation between sputum and blood neutrophils [15, 23]. The only surrogate biomarkers for sputum neutrophils were identified in volatile organic compounds [24].
Type 2 high asthma is the most frequently encountered form of severe asthma. In a recent paper, Hinks et al. [25] proposed to consider severe asthma as “type 2 high” in cases of blood eosinophils ≥150 μL−1, FENO ≥25 ppb or sputum eosinophils ≥2%. In the absence of these type 2 biomarkers, other measurements should be performed to determine type 2 low asthma, such as the measurement of sputum neutrophils (≥61%), sputum myeloperoxidase or volatile organic compounds, in order to discriminate between neutrophilic and paucigranulocytic asthma. The absence of type 2 biomarkers is suggestive of paucigranulocytic or neutrophilic severe asthma, but a diagnosis of type 2 low asthma should be suspected after at least three measurements of biomarkers below the cut-offs. In cases of neutrophilic asthma, smoking and occupational exposure should be assessed, imaging should be performed to exclude bronchiectasis and sputum cultures, and microbiology should be done to exclude aggravator or situations mimicking neutrophilic asthma [25].
Frøssing et al. [26] reported the absence of elevated type 2 biomarkers in 30% of patients with severe asthma, while 70% had type 2 severe asthma defined by FENO ≥25 ppb, total serum IgE ≥150 KU·L−1 or blood eosinophil counts ≥300 mm−3. Only 15% of patients with severe asthma had an increase in all type 2 biomarkers. Exhaled nitric oxide and blood eosinophil counts are the two most important type 2 biomarkers for the management of severe asthma; the first reflecting the production of airway IL-13 and the second associated with elevated systemic IL-5. These biomarkers can be used to phenotype severe asthma and to predict the response to targeted treatments.
Targeted treatments for severe asthma
Six targeted treatments have been approved by the United States Food and Drug Administration for the management of severe asthma [21]. The first targeted treatment available in clinical practice for the management of severe asthma is the anti-IgE omalizumab. This treatment has been found to reduce exacerbations and improve asthma quality of life in patients sensitised to perennial aeroallergens, who are exacerbation prone and who most often have early-onset asthma [27–40] (table 1). Some data in the literature suggest that omalizumab could be discontinued after 5 years in 60% of patients without relapse for at least 4 years [41]. This could be due to a disease modification effect, as Chanez et al. [42] found that omalizumab was able to reduce FcεRI expression in basophils (82.6%) and to a lesser extent in plasmacytoid dendritic cells. Future long-term clinical studies must be conducted to confirm these issues. Regarding safety, hypersensitivity reactions at the site of injection have been reported and anaphylaxis occurs in 0.1–0.2% of patients, most frequently with one of the first three doses.
TABLE 1.
Targeted treatments for severe asthma: effect on exacerbations, oral corticosteroid (OCS) dose reduction, OCS withdrawal, symptoms, quality of life (QoL), lung function and prediction of response
| Biologic agent | Exacerbations | OCS dose reduction | OCS withdrawal | Symptoms | QoL | FEV1 | Prediction of response |
| Omalizumab | |||||||
| Humbert et al. [ 37 ] (INNOVATE)# | 26% reduction | NA | NA | p=0.039 | AQLQ +0.45 | p=0.043 | NA |
| Busse et al. [ 36 ]# | 27% reduction | NA | NA | ns | NA | ns | BEC >300 mm−3 |
| Sorkness et al. [ 38 ]# | 50% reduction | NA | NA | p<0.01 | NA | NA | High FENO, blood eosinophils and BMI >25 kg·m−2 |
| Hanania et al. [ 39 ]# | 53% reduction | NA | NA | p<0.05 | p<0.05 | NA | FENO >20 ppb, BEC >260 mm−3, high periostin |
| Hanania et al. [ 27 ]# | 25% reduction | NA | NA | p<0.05 | AQLQ +0.29 | NA | NA |
| Vignola et al. [ 40 ]# | 30% reduction | NA | NA | p=0.023 | p<0.001 | NA | NA |
| Adachi et al. [ 29 ]¶ | 44% reduction | 80% reduction | NA | NA | NA | NA | NA |
| Casale et al. [ 34 ]¶ | 39% reduction | NA | NA | ACT +4.4 | NA | ns | NA |
| Cazzola et al. [ 30 ]¶ | 78% reduction | 71% reduction | NA | NA | NA | NA | NA |
| Yorgancıoğlu et al. [ 31 ]¶ | 71% reduction at 5 years | NA | NA | p<0.001 | NA | NA | NA |
| Humbert et al. [ 28 ]¶ | 78.5% had a ≥40% reduction | NA | NA | NA | NA | NA | Not BEC |
| Frix et al. [ 33 ]¶ | 83% reduction at 5 years | p<0.05 | NA | ACQ −0.8 after 5 years | AQLQ +1.5 after 5 years | FEV1 +6% at 1 year | NA |
| Brusselle et al. [32]¶ | 65% reduction at week 52 | ns | NA | NA | 84% had AQLQ increase >0.5 | NA | NA |
| Mepolizumab | |||||||
| Pavord et al. [ 44 ] (DREAM)# | 48% reduction (75 mg i.v.) | NA | NA | ACQ −0.16 (ns) | AQLQ +0.08 (ns) | FEV1 +61 mL | Baseline BEC and history of exacerbations |
| Ortega et al. [ 45 ] (MENSA)# | 53% reduction (100 mg s.c.) | NA | NA | ACQ −0.44 | SGRQ −7 | FEV1 +98 mL(p=0.03) | NA |
| Chupp et al. [ 46 ] (MUSCA)# | 58% reduction | NA | NA | NA | SGRQ −7.7 | NA | NA |
| Bel et al. [ 49 ] (SIRIUS)# | 32% reduction | 50% reduction | OR 1.67 (p=0.41) | ACQ −0.52 | SGRQ −5.8 at week 24 (p=0.02) | pre-BD FEV1 +114 mL (ns) | NA |
| Harrison et al. [ 48 ] (REALITI-A)¶ | 69% reduction at 5 years | 52% reduction at week 49 | 34% reduction | NA | NA | NA | NA |
| Schleich et al. [ 47 ]¶ | 85% reduction | 75% at 30 months | NA | ACQ −1.13 | AQLQ +1.24 | FEV1 +190 mL | NA |
| Reslizumab | |||||||
| Bjermer et al. [ 53 ]# | NA | NA | NA | ACQ −0.36 (p=0.03) | AQLQ +0.36 (p=0.02) | FEV1 +160 mL | NA |
| Castro et al. [ 51 ]# | 67% reduction (ns) | NA | NA | ACQ −0.38 (ns) | NA | FEV1 +240 mL (p=0.002) | Nasal polyposis – improvement in ACQ |
| Corren et al. [ 52 ]# | NA | NA | NA | ACQ-7 −0.195 (p<0.05) | NA | FEV1 +68 mL | NA |
| Benralizumab | |||||||
| Bleecker et al. [ 54 ] (SIROCCO)# | 51% reduction | NA | NA | ACQ −0.25 | NA | FEV1 +159 mL | NA |
| FitzGerald et al. [ 57 ] (CALIMA)# | 28% reduction | NA | NA | ACQ-6 –0.25 | AQLQ +0.24 | pre-BD FEV1 +116 mL | BEC >300 mm−3 |
| Nair et al. [ 56 ] (ZONDA)# | 70% reduction | 75% reduction | 52% reduction | ns at week 28 | ns at week 28 | ns at week 28 | NA |
| Harrison et al. [ 55 ] (ANDHI)# | 49% reduction | NA | NA | ACQ-6 –0.36 | SGRQ −8.11 | FEV1 +160 mL | NA |
| Kavanagh et al. [ 58 ]¶ | 72.8% reduction | Median reduction of 100% | 51.4% | ACQ-6 −0.75 | AQLQ +0.89 | post-BD FEV1 +140 mL | Higher BEC, adult-onset, nasal polyposis |
| Dupilumab | |||||||
| Castro et al. [ 60 ]# | 48% reduction | NA | NA | ACQ-5 −0.22 (week 52) | AQLQ +0.26 (week 52) | FEV1 +140 mL | BEC >300, FENO >25 ppb: better improvement in lung function and exacerbations |
| Rabe et al. [ 62 ]# | 59% reduction | 28% reduction | 52% versus 29% in placebo group | ACQ-5 −0.47 | NA | FEV1 +220 mL | BEC >300, higher FENO |
| Corren et al. [ 61 ]# | 45% reduction | NA | NA | ACQ-5 −0.26 (allergic asthma) and ns in nonallergic asthma | NA | FEV1 +130 mL | Elevated type 2 biomarkers |
| Sher et al. [ 63 ]# | 66% reduction | 41–89% | 0.41 | ACQ-5 −1.06–−1.25 | NA | FEV1 +250–360 mL | |
| Dupin et al. [66]¶ | 75% reduction | Reduction from 20 mg to 5 mg prednisolone | NA | ACT +7 | NA | FEV1 +10% | NA |
| Tezepelumab | |||||||
| Corren et al. [ 69 ]# | 66–78% reduction | NA | NA | NA | NA | FEV1 +150 mL | Irrespective of allergic status |
| Menzies-Gow et al. [ 68 ]# | 66% reduction | NA | NA | ACQ-6 −0.33 | AQLQ +0.34 | FEV1 +130 mL | High blood eosinophils, high FENO |
| Corren et al. [ 71 ]# | NA | NA | NA | Significant improvement in ACQ-6 (OR 1.94) | Significant improvement in AQLQ (OR 1.96) | NA | NA |
| Corren et al. [ 70 ]# | 62–71% reduction | NA | NA | ACQ-6 −0.29 | AQLQ +0.20 ns | FEV1 +120–150 mL | Irrespective of blood eosinophils |
ACQ: Asthma Control Questionnaire; ACT: Asthma Control Test; AQLQ: Asthma Quality Of Life Questionnaire; BD: bronchodilator; BEC: blood eosinophil count; BMI: body mass index; FENO: exhaled nitric oxide fraction; FEV1: forced expiratory volume in 1 s; NA: not available; ns: nonsignificant; SGRQ: St George's Respiratory Questionnaire. #: Randomised controlled trial. ¶: Real-word evidence study.
Anti-IL-5 mepolizumab has been shown to improve severe eosinophilic asthma irrespective of the allergic status [43]. The DREAM study concluded that elevated baseline blood eosinophils and history of exacerbations in the previous year were predictors of a good response to mepolizumab in terms of reduction in exacerbations [44]. In the MENSA trial, mepolizumab reduced asthma exacerbations by 53% as compared to placebo and improved asthma quality of life in adolescents and adults treated with high-dose ICS+long-acting β-agonists (LABAs) exhibiting at least two exacerbations within the last year and having blood eosinophil counts ≥300 μL−1 in the past year or >150 μL−1 at screening [45]. In the MUSCA study, mepolizumab was associated with significant improvements in health-related quality of life and had a safety profile similar to that of placebo [46]. Real-life data confirmed the drastic improvement of severe hypereosinophilic asthma treated with mepolizumab [47, 48] (table 1). Mepolizumab also allowed a 50% reduction in the dose of oral corticosteroids (OCS) with one third of patients able to withdraw OCS [48, 49].
Anti-IL-5 reslizumab given intravenously was also found to significantly reduce exacerbations in adolescents and adults with uncontrolled asthma despite medium to high dose ICS+LABAs and who had at least one exacerbation in the past year and blood eosinophil counts >400 μL−1 at screening [50]. Moreover, reslizumab led to a better reduction in exacerbations in patients with late-onset asthma [50]. The presence of nasal polyposis was associated with a better response in terms of asthma control [51]. Some studies found a significant improvement in asthma control [52, 53], quality of life [53] and lung function [51, 53] (table 1).
The anti-IL-5 receptor benralizumab targets the IL-5 receptor on the surface of eosinophils and basophils. Benralizumab depletes eosinophils and basophils by antibody-dependent cell-mediated cytotoxicity. In the SIROCCO study [54], benralizumab was administered to adolescents and adults with persistently uncontrolled asthma and who had at least two exacerbations within the last 12 months despite high-dose ICS+LABA. This targeted treatment induced a reduction in exacerbations by 51% only in patients with blood eosinophil counts ≥300 μL−1. The best predictors of response to benralizumab were history of previous exacerbations and blood eosinophil counts. Harrison et al. [55] found that age of onset was also important to take into account, as patients with onset of asthma earlier than 18 years of age had no significant improvement in exacerbations, quality of life, asthma control and lung function with benralizumab. According to the ZONDA trial, benralizumab allowed a 75% reduction in OCS dose with 52% of patients able to stop OCS [56]. The ANDHI and CALIMA trials reported significant improvements in asthma control, quality of life and lung function [55, 57]. Results were confirmed by real-life studies, where it was found that benralizumab significantly decreased exacerbation rate and OCS use and improved asthma symptoms, quality of life and lung function [58] (table 1). Anti-IL-5 and anti-IL-5 receptor treatment has to be continued for life as there is a rapid recurrence of systemic eosinophilia after discontinuation of these targeted treatments.
Anti-IL-4R dupilumab targets the IL-4 receptor shared by IL-4 and IL-13. In the Liberty Asthma Quest study [59], dupilumab led to a rapid improvement in lung function in patients with uncontrolled asthma despite medium-to-high dose ICS plus up to two additional controllers and at least one severe exacerbation in the previous year. Moreover, there was an overall 50% reduction in asthma exacerbations as compared to placebo with a better decrease in patients exhibiting blood eosinophil counts greater than 300 μL−1 and FENO ≥50 ppb [60]. In this phase 3 study, dupilumab reduced exacerbations by 54–90%, with greater improvements in patients with more exacerbations prior to study initiation [61]. In another study, dupilumab was able to significantly reduce the dose of OCS, with 52% of patients able to withdraw OCS as compared to 29% in the placebo group [62]. Sher et al. [63] confirmed an important reduction in exacerbations and OCS use, together with a significant improvement in lung function (table 1). In TRAVERSE, an open-label extension study performed across 27 countries, 2282 adults and adolescents were enrolled and data showed that the safety and efficacy of dupilumab are sustained when treatment is extended up to 148 weeks [64]. Bacharier et al. [65], however, reported a risk of increase in blood eosinophil counts and some parasitic infections, requiring the exclusion of parasitic infections before the start of anti-IL-4R treatment. In the study by Dupin et al. [66], 25% of patients had an hypereosinophilia greater than 1500 mm−3 at least once during follow-up, persisting after 6 months in 14% of patients.
Anti-alarmins such as anti-TSLP block upstream cytokines and can therefore be offered to a broader population of severe asthma patients. TSLP is produced by epithelial cells in response to exposure to pollutants, allergens or viral infection [67]. TSLP is able to induce the production of IL-4, IL-13 and IL-5, leading to an increase in IgE, eosinophils and FENO with a subsequent increase in bronchial hyperresponsiveness, mucus hypersecretion and airway remodelling [67]. Targeting TSLP [68] allows an overall reduction of 66% in exacerbations, with a significant effect in type 2 low severe asthma. A better reduction in exacerbations was observed in patients with higher blood eosinophil counts or higher FENO levels. In patients that had both low FENO and low blood eosinophils, tezepilumab was still able to reduce exacerbations by 25%. This is the first targeted treatment that shows a significant reduction in exacerbations in patients with severe asthma and blood eosinophils below 150 μL−1 and FENO <25 ppb. Corren et al. [69] confirmed the important reduction in exacerbations and a significant improvement in lung function. Two other studies also found an impact of tezepelumab on asthma symptom control and quality of life [70, 71]. The improvement in asthma outcomes is probably driven, at least in part, by reductions in airway submucosal eosinophils, IL-5 and IL-13 and a reduction in hyperresponsiveness to mannitol, pointing to a possible direct effect of tezepelumab on mast cells [72, 73]. Of note, tezepelumab did not affect innate immunity because it did not decrease neutrophil and T-cell numbers.
According to the results of randomised controlled trials, the choice of monoclonal antibody treatment in uncontrolled severe asthma should be directed by patient characteristics. Anti-IgE is indicated in patients with severe asthma who are sensitised to perennial allergens, most often with childhood onset of asthma or in patients with coexisting conditions such as allergic rhinitis, chronic rhinosinusitis with nasal polyposis or chronic urticaria. Total serum IgE has to be assessed for dosing according to local criteria and the response to omalizumab has been found to be slightly better in patients with increased blood eosinophils or FENO [21]. Treatments targeting IL-5 and its receptor have dramatically improved asthma outcomes in severe hypereosinophilic asthma irrespective of the presence of allergy. These treatments have been found to be more effective in adult-onset asthma and in patients combining chronic rhinosinusitis with nasal polyps, eosinophilic pneumonia or EGPA [47, 74, 75]. According to current data, anti-IL-4R treatments are good options to improve asthma outcomes in patients with severe type 2 childhood or adult-onset asthma, irrespective of allergy, and show a better response in cases of increased blood eosinophil counts and FENO. Co-existing conditions in favour of this treatment involve atopic dermatitis and chronic rhinosinusitis with nasal polyposis [21]. As mentioned previously, the treatment should be selected according to patient characteristics. When focusing on biomarkers, patients with severe asthma and levels of eosinophils >1500 μL−1 should be treated with anti-IL-5(R) after ruling out parasitic infections, haematologic diseases and other hypereosinophilic conditions [21]. If both type 2 biomarkers – blood eosinophils and FENO – are low, allergic status has to be checked. In cases of sensitisation to perennial allergens, anti-IgE and anti-TSLP are good options; while, in the absence of sensitisation, anti-TSLP is the only option for the patient. If blood eosinophils <150 μL−1 are associated with FENO ≥25 ppb, according to allergic status, the pulmonologist should propose anti-IgE, anti-IL-4R or anti-TSLP.
Finally, for patients with blood eosinophil counts between 150 and 1500 μL−1, clinical characteristics, biomarkers and coexisting conditions need to be integrated in order to choose between anti-IgE, anti-IL-4R, anti-IL5, anti-IL-5(R) or anti-TSLP [21].
Safety
Drug-related adverse events have been assessed in randomised controlled trials.
The incidence of treatment-related side-effects was similar in patients receiving omalizumab versus placebo [39]. Mepolizumab was not associated with an increased risk of adverse events as compared to placebo [45, 46], even in a population of patients with chronic OCS use [49]. There might be rare cases of hypersensitivity reactions. Reslizumab was well tolerated, with only rare cases of anaphylactic reactions after injections [50]. The most common adverse events observed with benralizumab were nasopharyngitis, but its occurrence was not significantly different from the placebo group [57]. This observation was confirmed in a study assessing the corticosteroid-sparing effect of benralizumab, showing that the frequencies of adverse events were similar between benralizumab and placebo [56].
The most common adverse events with dupilumab compared with placebo were upper respiratory tract infections and injection-site reactions [76]. Hypereosinophilia has been observed in 4–25% of patients treated with dupilumab [60] and is transient in most cases, although there have been reports of persistent cases of symptomatic hypereosinophilia consistent with EGPA, eosinophilic pneumonia, eosinophilic vasculitis and sudden worsening of asthma symptoms. One hypothesis for this hypereosinophilia is that blockade of the IL-4/IL-13 pathway reduces eosinophil migration, thereby inducing an increase in blood eosinophils by inhibiting eotaxin-3, vascular cell adhesion protein 1 and thymus and activation-regulated chemokine without simultaneously inhibiting eosinophilopoiesis in bone marrow. Therefore, when choosing the optimal biologic, it seems necessary to consider the presence of hypereosinophilia (>1500 μL−1), in which case an anti-IL-5/IL-5R agent is preferable. When dupilumab is started in severe asthma patients, blood eosinophilia should be monitored on a regular basis.
Finally, the frequencies and types of adverse events with tezepelumab did not differ meaningfully from the placebo group. Longer-term follow-up of patients treated with biologics is of upmost importance to confirm the absence of later events and the inclusion of patients suffering from severe asthma in registries will offer clinicians data on long-term safety.
Concept of remission
Recently, the concept of remission has been developed and different definitions have been used [77]. It seems clear that the definition of remission must include the absence of exacerbations, withdrawal of systemic corticosteroid use and asthma symptom control. The inclusion of the fourth criteria – improvement in lung function – remains under debate as some patients with long disease duration and fixed airflow obstruction are sometimes unable to improve or normalise their lung function while being asymptomatic and exacerbation-free. The patient's view also needs to be taken into account when developing a consensus on the definition of remission.
Menzies-Gow et al. [78] evaluated the proportion of patients reaching remission with benralizumab. After 1 year of treatment, 23.9% (140/586) of SIROCCO/CALIMA patients had no exacerbations, no OCS use, a pre-bronchodilator (BD) forced expiratory volume in 1 s (FEV1) increase of at least 100 mL and an Asthma Control Questionnaire (ACQ)-6 score lower than 1.5. A very recent observational study evaluated predictors of remission with mepoilzumab [79] defined as patients who combined 1 year after therapy: no chronic treatment with oral corticosteroids, no exacerbation, asthma control, FEV1 >80% or an improvement of FEV1 >10% and a blood eosinophil count <300 mm−3. Patients reaching remission were characterised at baseline by increased levels of sputum eosinophils, sputum eotaxin-1, sputum TSLP, sputum IL-5, sputum eosinophil peroxydase and sputum IgE while blood eosinophils were not able to discriminate between patients in remission after 1 year of treatment. Other analyses have been performed with other biologics and preliminary results were presented during the ERS International Congress 2022 in Barcelona, Spain. It is of upmost importance to define remission to allow comparison between different studies.
Future therapeutic options
New drugs have been recently studied such as anti-ST2, astegolimab [80]. This treatment, which targets the IL-33 pathway, led to a slightly better reduction in exacerbation in patients with low blood eosinophil counts; suggesting that the IL-33 pathway could be more important in type 2 low severe asthma.
Itepekimab is a human IgG4P monoclonal antibody against IL-33. In a phase 2 trial [81], 296 moderate-to-severe asthmatics were randomly assigned 300 mg subcutaneous itepekimab, itepekimab plus dupilumab (both at 300 mg), dupilumab (300 mg) or placebo every 2 weeks for 12 weeks. Eligibility criteria were a pre-BD FEV1 value between 50 and 85% predicted and at least one severe asthma exacerbation. After randomisation, LABA was discontinued at week 4 and ICS were tapered over weeks 6–9. The primary end-point was an event indicating a loss of asthma control defined as a reduction of at least 30% in the morning peak expiratory flow (PEF) or at least six additional uses of rescue medication on two consecutive days, an increase by four of the most recent doses of ICS or an asthma exacerbation. Patients with a loss of asthma control returned to maintenance LABA+ICS and the trial drug was discontinued. By 12 weeks, loss of asthma control occurred in 22% of the itepekimab group (OR versus placebo 0.42), 27% of the combination group (OR 0.52) and 19% of the dupilumab group (OR 0.33), as compared with 41% in the placebo group. The combination of itepekimab and dupilumab was not superior to either treatment alone. The incidence of adverse events was similar in all four trial groups.
Anti-IL-23 risankizumab [82] has been studied in moderate to severe asthma. IL-23 promotes Th17-cell proliferation, neutrophil recruitment and Th2 cytokine production. This phase 2a, multicentre, randomised, double-blind, placebo-controlled, 24-week trial assessed the efficacy and safety of risankizumab, an anti-IL-23p19 monoclonal antibody in adults with severe asthma. Eligible patients had at least two exacerbations or one severe exacerbation resulting in hospitalisation or emergency visit. FEV1 was between 40 and 85% and the smoking history was less than 10 pack-years. A total of 105 patients received 90 mg risankizumab subcutaneously once every 4 weeks while 109 were assigned to the placebo group. The primary end-point was the time to first asthma worsening (decrease in PEF, increase in rescue medication use, exacerbation or increase in ACQ-5 >0.75). The time to first asthma worsening was shorter with risankizumab than with placebo (40 days versus 86 days, p=0.03) and the annualised rate of asthma worsening was higher. Risankizumab did not affect sputum cell counts, but downregulated genes involved in the activation of natural killer cells and cytotoxic T-cells and the activation of Th1 and Th17 transcription factors. These findings support the view that risankizumab exerted a biologic effect on airway immunity, which may have contributed to the poor clinical outcome.
Treatments targeting neutrophilic asthma such as anti-IL-17 and anti-CXCR2 have not shown any improvement in asthma outcomes in this asthma phenotype [83, 84]. This could be due to the fact that neutrophilic inflammation reflects microbiome dysbiosis. The microbiome is defined as the combined genetic material from microorganisms in a particular environment. As such, it represents the communities of microorganisms including bacteria, viruses, fungi, protists and archea that inhabit a specific niche. Most studies into the respiratory microbiome focus on bacteria, but it is important to remember that other microorganisms may play a role in modifying health and disease [85]. The microbiome in airway diseases is altered mostly by a loss of bacterial diversity and expansion of specific pathogenic taxa. There might be airway dysbiosis in neutrophilic asthma with upregulation of sputum IL-1β and Proteobacteria [86], in particular Haemophilus. Perturbation in the normal healthy microbiome in chronic airway diseases is caused by different factors, such as airway structural damage and changes in the metabolic activity and immune system within the lungs. Moreover, recurrent courses of systemic corticosteroids and antibiotics may also contribute to changes in the microbiome. Several studies have demonstrated a link between the microbiome and inflammatory phenotype. Dysbiosis could in part explain the effect of azithromycin in reducing exacerbations, as shown in the AMAZES trial [7], irrespective of the phenotype. Patients who were bacteria positive showed the best improvement with azithromycin 500 mg three times per week over 48 weeks.
The only feature that is common to all asthma inflammatory phenotypes is the airway hyperresponsiveness measured by direct or indirect bronchial challenges. Bronchial thermoplasty has been shown to reduce airway smooth muscle mass and can lead to improvement of asthma control and reduction in asthma exacerbations [87]. It also led to repair of epithelial cells [88, 89]. The number of mast cells in airway smooth muscle has been found to be related to asthma severity and bronchial hyperresponsiveness to methacholine [18]. Imatinib, a KIT inhibitor targeting mast cell survival [90], was able to reduce airway hyperresponsiveness. Tezepilumab was also found to reduce airway hyperresponsiveness [72] and this could be due to a direct effect of TSLP on smooth muscle cells. Future strategies must be developed to reduce intrinsic airway smooth muscle hypercontractility. Previous studies supported a critical role for NADPH oxidase-4 overexpression in the promotion of oxidative stress and consequent airway smooth muscle hypercontractility in asthma [91]. The study by Prihandoko et al. [92] also provides evidence that pharmacological targeting of lung free fatty acid receptor 4 has in vivo efficacy and might have therapeutic value in the treatment of bronchoconstriction.
COPD
Diagnosis and pheno-endotyping
COPD is a complex heterogeneous syndrome, having both pulmonary and extrapulmonary features [93]. The diagnosis of COPD is based on the presence of chronic airflow limitation, as assessed by post-BD FEV1 and FEV1/forced vital capacity measurements. COPD may comprise several different phenotypes that are driven by different molecular mechanisms or endotypes [94]. Genetic, clinical and inflammatory phenotypes of COPD such as alpha-1 antitrypsin deficiency or telomerase polymorphisms, smoking versus nonsmoking, small airways disease and emphysema, as well as frequent exacerbators or inflammatory phenotypes such as eosinophilic or neutrophilic, have been recognised and this could lead to the prescription of targeted treatments [95]. Most patients have increased neutrophils and macrophages in sputum, reflecting the increased secretion of neutrophil and monocyte chemotactic mediators in the lungs [96]. However, some patients also have increased eosinophils in sputum and this may be reflected by increased blood eosinophils [97]. Identification of elevated blood or sputum eosinophils [98] has been associated with an increased risk of exacerbations [99] and has been associated with a better response to ICS in reducing and treating exacerbations [100, 101]. Other studies have demonstrated that ICS do not have a major role in reducing exacerbations in patients with eosinophil counts <150 cells·μL−1 and relatively increased efficacy in patients with higher eosinophil counts [102, 103].
Patients with COPD should be assessed and treated according to their individual treatable characteristics.
Targeted treatment for COPD
Several innate and adaptive immune responses could be targeted with biologicals in COPD. However, recent studies failed to demonstrate a significant reduction in exacerbations in COPD using targeted treatments [104–106]. The reasons for this poor response to biologics in COPD as compared to what can be achieved in severe asthma remains unclear.
The treatable traits concept has been proposed to manage chronic airway diseases such as asthma and COPD with the same drugs according to inflammatory characteristics [107]. One important treatable trait in chronic airway diseases is the eosinophilic airway inflammation that has been shown to predict the response to ICS [108] and systemic corticosteroids [109], while blood eosinophilia was associated with a better response to anti-IgE [110], anti-IL-5(R) [44, 54] and anti-IL-4R [60] in asthma. However, several studies suggest that the results observed in asthma cannot be generalised in COPD patients.
Anti-IL5 mepolizumab has been found to reduce exacerbations by 50% in hypereosinophilic severe asthma [45], while the reduction in exacerbation was only 15% in hypereosinophilic COPD patients [104]. As in studies with asthma, efficacy again tracked with higher eosinophil counts at entry. More importantly, mepolizumab significantly improved quality of life in severe eosinophilic asthma but not in COPD in these studies.
The same observation was made with anti-IL-5R benralizumab [106] in eosinophilic COPD. This targeted treatment was able to reduce exacerbations by 50% in severe eosinophilic asthma with blood eosinophils >300 μL−1 [54], while this reduction was not significant in hypereosinophilic COPD patients exhibiting blood eosinophils higher than 220 μL−1 [106]. In the study by Brightling et al. [111], no significant reduction in exacerbations was shown with benralizumab as compared to placebo.
Different hypotheses could explain the difference in response to biologicals in asthma and COPD despite the presence of elevated blood eosinophils as a treatable trait in both conditions. The first being the absence of randomisation according to smoking status and the absence of phenotyping of COPD exacerbations. COPD exacerbations may indeed be treated with either OCS or antibiotics or both. It is of upmost importance to evaluate the effect of biologics on the reduction of COPD exacerbations according to the exacerbations’ profiles. Moreover, COPD is a disease affecting the airways and the parenchyma. Patients suffering from COPD should undergo a chest computed tomography scan to exclude a dominant emphysema component, in which case parenchymal destruction should not be improved by biologics.
A phase 2a trial with itepekimab, a monoclonal antibody targeting IL-33, was reported in patients with moderate–severe COPD on standard therapy. No reduction in exacerbation rate was observed in active smokers, while a 42% reduction occurred in former smokers [112]. This study may suggest that active smoking, with a resultant predominance of neutrophilic inflammation, may cloud the underlying contribution of coexisting Th2 responses to the pathogenesis of COPD.
The BOREAS study (a pivotal study to assess the efficacy, safety and tolerability of dupilumab in patients with moderate-to-severe COPD with type 2 inflammation) is evaluating the role of dupilumab in patients with eosinophilic COPD (NCT03930732).
Promising future phenotypic characteristics
Studies looking at the microbiome in COPD have found a link between inflammatory phenotypes and the microbiome [86]. In healthy individuals, the lung microbiome is shaped very early in life, starting with inheriting the mother's microbiome during delivery and then evolving during childhood according to different factors such as breastfeeding and early-life exposures like infections and the presence of pets. The microbiome is quite stable during adulthood and then changes occur during aging due to immunosenescence. In patients with chronic airway diseases, the microbiome may evolve not only according to increased microbial immigration upon inhalation of bacteria and microaspiration but also decreased microbial elimination in cases of impaired innate and adaptative host defences [113]. Regional growth conditions such as the presence of inflammatory cells, oxygen tension, temperature or local pH may also impact the lung microbiome [114]. Several studies have reported the composition of the airway microbiome in patients suffering from COPD and found a loss in microbiome diversity and an increase in Proteobacteria, particularly the genus Haemophilus. These bacteria were associated with increased severity reflected by increased exacerbations characterised by a greater fall in lung function, especially when associated with eosinophilic inflammation [115]. Dysbiosis was also associated with increased mortality [116] compared to patients with a more balanced profile. Haemophilus has been found to be associated with increased neutrophilic inflammation [115] while healthy regulatory bacteria such as Campylobacter, Rothia and Fusobacterium were predominant in eosinophilic phenotypes. Neutrophilic COPD, reflected by the amount of sputum neutrophil extracellular traps, was associated with lower microbiome diversity and a significant increase in Pseudomonas and Haemophilus. Antibiotics have been found to modulate the microbiome and reduce the neutrophil degranulation pathway, thereby improving microbiome dysbiosis and patient status [117]. ICS were found to increase Proteobacteria in patients with sputum eosinophil counts below 2% while antibiotics decrease these bacteria [118]. In another study, ICS were found to alter the microbiome by modulating host immunity [119]. Macrolide antibiotics have powerful effects on host immunity and can reduce exacerbations [120]. Macrolides may decrease Haemophilus influenzae but this will lead to an increase in Pseudomonas aeruginosa [121, 122].
All these studies looking at the microbiome in COPD highlight that it is of upmost importance to phenotype exacerbations to avoid giving antibiotics when this is not necessary. There are still many unanswered questions around the effect of antibiotics on the microbiome and more longitudinal studies are needed.
Conclusion
Asthma and COPD are complex inflammatory diseases of the airways in which impaired host defences lead to increased susceptibility to pathogens, pollutants and allergens. Environmental exposures can alter the microbiome and influence the development of sensitisation by disrupting normal immunoregulation.
A great deal of progress has been made in gaining insights into the immunology and pathophysiology of asthma and this has led to the development of novel treatments and major improvement in severe asthma outcomes. In COPD, however, no targeted treatments have at present demonstrated great improvement.
Acknowledgements
This paper summarises data presented during the Lung Science Conference held in Estoril in 2022 based on the presentations of Guy Brusselle, Chris Brightling and James Chalmers.
Provenance: Commissioned article, peer reviewed.
Conflict of interest: F. Schleich has received grants or contracts from GSK, AstraZeneca and Chiesi; payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from GSK, AstraZeneca, Chiesi and TEVA; and has participated on a Data Safety Monitoring Board or Advisory Board for GSK and AstraZeneca.
Conflict of interest: N. Bougard has nothing to disclose.
Conflict of interest: C. Moermans has nothing to disclose.
Conflict of interest: M. Sabbe has nothing to disclose.
Conflict of interest: R. Louis has received grants or contracts from GSK, AstraZeneca and Chiesi; payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from GSK, AstraZeneca, Chiesi and TEVA; and has participated on a Data Safety Monitoring Board or Advisory Board for GSK and AstraZeneca.
References
- 1.Schleich FN, Chevremont A, Paulus V, et al. . Importance of concomitant local and systemic eosinophilia in uncontrolled asthma. Eur Respir J 2014; 44: 97–108. doi: 10.1183/09031936.00201813 [DOI] [PubMed] [Google Scholar]
- 2.Green RH, Brightling CE, McKenna S, et al. . Asthma exacerbations and sputum eosinophil counts: a randomised controlled trial. Lancet 2002; 360: 1715–1721. doi: 10.1016/S0140-6736(02)11679-5 [DOI] [PubMed] [Google Scholar]
- 3.Berry M, Morgan A, Shaw DE, et al. . Pathological features and inhaled corticosteroid response of eosinophilic and non-eosinophilic asthma. Thorax 2007; 62: 1043–1049. doi: 10.1136/thx.2006.073429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pavord ID, Brightling CE, Woltmann G, et al. . Non-eosinophilic corticosteroid unresponsive asthma. Lancet 1999; 353: 2213–2214. doi: 10.1016/S0140-6736(99)01813-9 [DOI] [PubMed] [Google Scholar]
- 5.Haldar P, Pavord ID. Noneosinophilic asthma: a distinct clinical and pathologic phenotype 1. J Allergy Clin Immunol 2007; 119: 1043–1052. doi: 10.1016/j.jaci.2007.02.042 [DOI] [PubMed] [Google Scholar]
- 6.Brusselle GG, Vanderstichele C, Jordens P, et al. . Azithromycin for prevention of exacerbations in severe asthma (AZISAST): a multicentre randomised double-blind placebo-controlled trial. Thorax 2013; 68: 322–329. doi: 10.1136/thoraxjnl-2012-202698 [DOI] [PubMed] [Google Scholar]
- 7.Gibson PG, Yang IA, Upham JW, et al. . Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double-blind, placebo-controlled trial. Lancet 2017; 390: 659–668. doi: 10.1016/S0140-6736(17)31281-3 [DOI] [PubMed] [Google Scholar]
- 8.Wenzel SE. Asthma: defining of the persistent adult phenotypes. Lancet 2006; 368: 804–813. doi: 10.1016/S0140-6736(06)69290-8 [DOI] [PubMed] [Google Scholar]
- 9.Kaur R, Chupp G. Phenotypes and endotypes of adult asthma: moving toward precision medicine. J Allergy Clin Immunol 2019; 144: 1–12. doi: 10.1016/j.jaci.2019.05.031 [DOI] [PubMed] [Google Scholar]
- 10.Hanania NA, Fortis S, Haselkorn T, et al. . Omalizumab in asthma with fixed airway obstruction: post hoc analysis of EXTRA. J Allergy Clin Immunol Pract 2022; 10: 222–228. doi: 10.1016/j.jaip.2021.08.006 [DOI] [PubMed] [Google Scholar]
- 11.Stevens WW, Jerschow E, Baptist AP, et al. . The role of aspirin desensitization followed by oral aspirin therapy in managing patients with aspirin-exacerbated respiratory disease: a work group report from the Rhinitis, Rhinosinusitis and Ocular Allergy Committee of the American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol 2021; 147: 827–844. doi: 10.1016/j.jaci.2020.10.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jonckheere A-C, Bullens DMA, Seys SF. Innate lymphoid cells in asthma: pathophysiological insights from murine models to human asthma phenotypes. Curr Opin Allergy Clin Immunol 2019; 19: 53–60. doi: 10.1097/ACI.0000000000000497 [DOI] [PubMed] [Google Scholar]
- 13.van der Ploeg EK, Golebski K, van Nimwegen M, et al. . Steroid-resistant human inflammatory ILC2s are marked by CD45RO and elevated in type 2 respiratory diseases. Sci Immunol 2021; 6: eabd3489. doi: 10.1126/sciimmunol.abd3489 [DOI] [PubMed] [Google Scholar]
- 14.Schleich F. Diagnosis and clinical interest of asthma inflammatory phenotypes. https://orbi.uliege.be/bitstream/2268/177575/1/2014_SCHLEICH_THESE.pdf
- 15.Schleich FN, Manise M, Sele J, et al. . Distribution of sputum cellular phenotype in a large asthma cohort: predicting factors for eosinophilic vs neutrophilic inflammation. BMC Pulm Med 2013; 13: 11. doi: 10.1186/1471-2466-13-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Demarche S, Schleich F, Henket M, et al. . Detailed analysis of sputum and systemic inflammation in asthma phenotypes: are paucigranulocytic asthmatics really non-inflammatory? BMC Pulm Med 2016; 16: 46. doi: 10.1186/s12890-016-0208-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Green RH, Pavord I. Stability of inflammatory phenotypes in asthma. Thorax 2012; 67: 665–667. doi: 10.1136/thoraxjnl-2012-201657 [DOI] [PubMed] [Google Scholar]
- 18.Brightling CE, Bradding P, Symon FA, et al. . Mast-cell infiltration of airway smooth muscle in asthma. N Engl J Med 2002; 346: 1699–1705. doi: 10.1056/NEJMoa012705 [DOI] [PubMed] [Google Scholar]
- 19.Global Initiative for Asthma . Global strategy for asthma management and prevention. https://ginasthma.org/gina-reports/ Date last updated: 2022.
- 20.Schleich F, Brusselle G, Louis R, et al. . Heterogeneity of phenotypes in severe asthmatics. The Belgian Severe Asthma Registry (BSAR). Respir Med 2014; 108: 1723–1732. doi: 10.1016/j.rmed.2014.10.007 [DOI] [PubMed] [Google Scholar]
- 21.Brusselle GG, Koppelman GH. Biologic therapies for severe asthma. N Engl J Med 2022; 386: 157–171. doi: 10.1056/NEJMra2032506 [DOI] [PubMed] [Google Scholar]
- 22.Chung KF, Wenzel SE, Brozek JL, et al. . International ERS/ATS guidelines on definition, evaluation and treatment of severe asthma. Eur Respir J 2014; 43: 343–373. doi: 10.1183/09031936.00202013 [DOI] [PubMed] [Google Scholar]
- 23.Demarche SF, Schleich FN, Paulus VA, et al. . Is it possible to claim or refute sputum eosinophils ≥3% in asthmatics with sufficient accuracy using biomarkers? Respir Res 2017; 18: 133. doi: 10.1186/s12931-017-0615-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schleich FN, Zanella D, Stefanuto P-H, et al. . Exhaled volatile organic compounds are able to discriminate between neutrophilic and eosinophilic asthma. Am J Respir Crit Care Med 2019; 200: 444–453. doi: 10.1164/rccm.201811-2210OC [DOI] [PubMed] [Google Scholar]
- 25.Hinks TSC, Levine SJ, Brusselle GG. Treatment options in type-2 low asthma. Eur Respir J 2021; 57: 2000528. doi: 10.1183/13993003.00528-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frøssing L, Silberbrandt A, Von Bülow A, et al. . The prevalence of subtypes of type 2 inflammation in an unselected population of patients with severe asthma. J Allergy Clin Immunol Pract 2021; 9: 1267–1275. doi: 10.1016/j.jaip.2020.09.051 [DOI] [PubMed] [Google Scholar]
- 27.Hanania NA, Alpan O, Hamilos DL, et al. . Omalizumab in severe allergic asthma inadequately controlled with standard therapy: a randomized trial. Ann Intern Med 2011; 154: 573–582. doi: 10.7326/0003-4819-154-9-201105030-00002 [DOI] [PubMed] [Google Scholar]
- 28.Humbert M, Taillé C, Mala L, et al. . Omalizumab effectiveness in patients with severe allergic asthma according to blood eosinophil count: the STELLAIR study. Eur Respir J 2018; 51: 1702523. doi: 10.1183/13993003.02523-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adachi M, Kozawa M, Yoshisue H, et al. . Real-world safety and efficacy of omalizumab in patients with severe allergic asthma: a long-term post-marketing study in Japan. Respir Med 2018; 141: 56–63. doi: 10.1016/j.rmed.2018.06.021 [DOI] [PubMed] [Google Scholar]
- 30.Cazzola M, Camiciottoli G, Bonavia M, et al. . Italian real-life experience of omalizumab. Respir Med 2010; 104: 1410–1416. doi: 10.1016/j.rmed.2010.04.013 [DOI] [PubMed] [Google Scholar]
- 31.Yorgancıoğlu A, Öner Erkekol F, Mungan D, et al. . Long-term omalizumab treatment: a multicenter, real-life, 5-year trial. Int Arch Allergy Immunol 2018; 176: 225–233. doi: 10.1159/000488349 [DOI] [PubMed] [Google Scholar]
- 32.Brusselle G, Michils A, Louis R, et al. . “Real-life” effectiveness of omalizumab in patients with severe persistent allergic asthma: the PERSIST study. Respir Med 2009; 103: 1633–1642. doi: 10.1016/j.rmed.2009.06.014 [DOI] [PubMed] [Google Scholar]
- 33.Frix AN, Schleich F, Paulus V, et al. . Effectiveness of omalizumab on patient reported outcomes, lung function, and inflammatory markers in severe allergic asthma. Biochem Pharmacol 2020; 179: 113944. doi: 10.1016/j.bcp.2020.113944 [DOI] [PubMed] [Google Scholar]
- 34.Casale TB, Luskin AT, Busse W, et al. . Omalizumab effectiveness by biomarker status in patients with asthma: evidence from PROSPERO, a prospective real-world study. J Allergy Clin Immunol Pract 2019; 7: 156–164. doi: 10.1016/j.jaip.2018.04.043 [DOI] [PubMed] [Google Scholar]
- 35.Busse WW, Massanari M, Kianifard F, et al. . Effect of omalizumab on the need for rescue systemic corticosteroid treatment in patients with moderate-to-severe persistent IgE-mediated allergic asthma: a pooled analysis. Curr Med Res Opin 2007; 23: 2379–2386. doi: 10.1185/030079907X226258 [DOI] [PubMed] [Google Scholar]
- 36.Busse W, Spector S, Rosén K, et al. . High eosinophil count: a potential biomarker for assessing successful omalizumab treatment effects. J Allergy Clin Immunol 2013; 132: 485–486.e11. doi: 10.1016/j.jaci.2013.02.032 [DOI] [PubMed] [Google Scholar]
- 37.Humbert M, Beasley R, Ayres J, et al. . Benefits of omalizumab as add-on therapy in patients with severe persistent asthma who are inadequately controlled despite best available therapy (GINA 2002 step 4 treatment): INNOVATE. Allergy 2005; 60: 309–316. doi: 10.1111/j.1398-9995.2004.00772.x [DOI] [PubMed] [Google Scholar]
- 38.Sorkness CA, Wildfire JJ, Calatroni A, et al. . Reassessment of omalizumab-dosing strategies and pharmacodynamics in inner-city children and adolescents. J Allergy Clin Immunol Pract 2013; 1: 163–171. doi: 10.1016/j.jaip.2013.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hanania NA, Wenzel S, Rosen K, et al. . Exploring the effects of omalizumab in allergic asthma: an analysis of biomarkers in the EXTRA study. Am J Respir Crit Care Med 2013; 187: 804–811. doi: 10.1164/rccm.201208-1414OC [DOI] [PubMed] [Google Scholar]
- 40.Vignola AM, Humbert M, Bousquet J, et al. . Efficacy and tolerability of anti-immunoglobulin E therapy with omalizumab in patients with concomitant allergic asthma and persistent allergic rhinitis: SOLAR. Allergy 2004; 59: 709–717. doi: 10.1111/j.1398-9995.2004.00550.x [DOI] [PubMed] [Google Scholar]
- 41.Vennera MDC, Sabadell C, Picado C, et al. . Duration of the efficacy of omalizumab after treatment discontinuation in “real life” severe asthma. Thorax 2018; 73: 782–784. doi: 10.1136/thoraxjnl-2017-210017 [DOI] [PubMed] [Google Scholar]
- 42.Chanez P, Contin-Bordes C, Garcia G, et al. . Omalizumab-induced decrease of FcεRI expression in patients with severe allergic asthma. Respir Med 2010; 104: 1608–1617. doi: 10.1016/j.rmed.2010.07.011 [DOI] [PubMed] [Google Scholar]
- 43.Sposato B, Scalese M, Camiciottoli G, et al. . Mepolizumab effectiveness and allergic status in real life. Int Arch Allergy Immunol 2021; 182: 311–318. doi: 10.1159/000511147 [DOI] [PubMed] [Google Scholar]
- 44.Pavord ID, Korn S, Howarth P, et al. . Mepolizumab for severe eosinophilic asthma (DREAM): a multicentre, double-blind, placebo-controlled trial. Lancet 2012; 380: 651–659. doi: 10.1016/S0140-6736(12)60988-X [DOI] [PubMed] [Google Scholar]
- 45.Ortega HG, Liu MC, Pavord ID, et al. . Mepolizumab treatment in patients with severe eosinophilic asthma. N Engl J Med 2014; 371: 1198–1207. doi: 10.1056/NEJMoa1403290 [DOI] [PubMed] [Google Scholar]
- 46.Chupp GL, Bradford ES, Albers FC, et al. . Efficacy of mepolizumab add-on therapy on health-related quality of life and markers of asthma control in severe eosinophilic asthma (MUSCA): a randomised, double-blind, placebo-controlled, parallel-group, multicentre, phase 3b trial. Lancet Respir Med 2017; 5: 390–400. doi: 10.1016/S2213-2600(17)30125-X [DOI] [PubMed] [Google Scholar]
- 47.Schleich F, Graff S, Nekoee H, et al. . Real-word experience with mepolizumab: does it deliver what it has promised? Clin Exp Allergy 2020; 50: 687–695. doi: 10.1111/cea.13601 [DOI] [PubMed] [Google Scholar]
- 48.Harrison T, Canonica GW, Chupp G, et al. . Real-world mepolizumab in the prospective severe asthma REALITI-A study: initial analysis. Eur Respir J 2020; 56: 2000151. doi: 10.1183/13993003.00151-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bel EH, Wenzel SE, Thompson PJ, et al. . Oral glucocorticoid-sparing effect of mepolizumab in eosinophilic asthma. N Engl J Med 2014; 371: 1189–1197. doi: 10.1056/NEJMoa1403291 [DOI] [PubMed] [Google Scholar]
- 50.Castro M, Zangrilli J, Wechsler ME, et al. . Reslizumab for inadequately controlled asthma with elevated blood eosinophil counts: results from two multicentre, parallel, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet Respir Med 2015; 3: 355–366. doi: 10.1016/S2213-2600(15)00042-9 [DOI] [PubMed] [Google Scholar]
- 51.Castro M, Mathur S, Hargreave F, et al. . Reslizumab for poorly controlled, eosinophilic asthma: a randomized, placebo-controlled study. Am J Respir Crit Care Med 2011; 184: 1125–1132. doi: 10.1164/rccm.201103-0396OC [DOI] [PubMed] [Google Scholar]
- 52.Corren J, Weinstein S, Janka L, et al. . Phase 3 Study of reslizumab in patients with poorly controlled asthma: effects across a broad range of eosinophil counts. Chest 2016; 150: 799–810. doi: 10.1016/j.chest.2016.03.018 [DOI] [PubMed] [Google Scholar]
- 53.Bjermer L, Lemiere C, Maspero J, et al. . Reslizumab for inadequately controlled asthma with elevated blood eosinophil levels: a randomized phase 3 study. Chest 2016; 150: 789–798. doi: 10.1016/j.chest.2016.03.032 [DOI] [PubMed] [Google Scholar]
- 54.Bleecker ER, FitzGerald JM, Chanez P, et al. . Efficacy and safety of benralizumab for patients with severe asthma uncontrolled with high-dosage inhaled corticosteroids and long-acting β2-agonists (SIROCCO): a randomised, multicentre, placebo-controlled phase 3 trial. Lancet 2016; 388: 2115–2127. doi: 10.1016/S0140-6736(16)31324-1 [DOI] [PubMed] [Google Scholar]
- 55.Harrison TW, Chanez P, Menzella F, et al. . Onset of effect and impact on health-related quality of life, exacerbation rate, lung function, and nasal polyposis symptoms for patients with severe eosinophilic asthma treated with benralizumab (ANDHI): a randomised, controlled, phase 3b trial. Lancet Respir Med 2021; 9: 260–274. doi: 10.1016/S2213-2600(20)30414-8 [DOI] [PubMed] [Google Scholar]
- 56.Nair P, Wenzel S, Rabe KF, et al. . Oral glucocorticoid-sparing effect of benralizumab in severe asthma. N Engl J Med 2017; 376: 2448–2458. doi: 10.1056/NEJMoa1703501 [DOI] [PubMed] [Google Scholar]
- 57.FitzGerald JM, Bleecker ER, Nair P, et al. . Benralizumab, an anti-interleukin-5 receptor α monoclonal antibody, as add-on treatment for patients with severe, uncontrolled, eosinophilic asthma (CALIMA): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2016; 388: 2128–2141. doi: 10.1016/S0140-6736(16)31322-8 [DOI] [PubMed] [Google Scholar]
- 58.Kavanagh JE, Hearn AP, Dhariwal J, et al. . Real-world effectiveness of benralizumab in severe eosinophilic asthma. Chest 2021; 159: 496–506. doi: 10.1016/j.chest.2020.08.2083 [DOI] [PubMed] [Google Scholar]
- 59.Corren J, Maspero JF, Valero Santiago AL, et al. . Dupilumab improves asthma-related patient reported outcomes in asthma patients with chronic rhinosinusitis or nasal polyposis (CRS/NP) in Liberty Asthma Quest. Eur Respir J 2018; 52: Suppl. 62, PA1124. doi: 10.1183/13993003.congress-2018.PA1124 [DOI] [Google Scholar]
- 60.Castro M, Corren J, Pavord ID, et al. . Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med 2018; 378: 2486–2496. doi: 10.1056/NEJMoa1804092 [DOI] [PubMed] [Google Scholar]
- 61.Corren J, Katelaris CH, Castro M, et al. . Effect of exacerbation history on clinical response to dupilumab in moderate-to-severe uncontrolled asthma. Eur Respir J 2021; 58: 204498. doi: 10.1183/13993003.04498-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rabe KF, Nair P, Brusselle G, et al. . Efficacy and safety of dupilumab in glucocorticoid-dependent severe asthma. N Engl J Med 2018; 378: 2475–2485. doi: 10.1056/NEJMoa1804093 [DOI] [PubMed] [Google Scholar]
- 63.Sher LD, Wechsler ME, Rabe KF, et al. . Dupilumab reduces oral corticosteroid use in patients with corticosteroid-dependent severe asthma: an analysis of the phase 3, open-label extension TRAVERSE trial. Chest 2022; 162: 46–55. doi: 10.1016/j.chest.2022.01.071 [DOI] [PubMed] [Google Scholar]
- 64.Wechsler ME, Ford LB, Maspero JF, et al. . Long-term safety and efficacy of dupilumab in patients with moderate-to-severe asthma (TRAVERSE): an open-label extension study. Lancet Respir Med 2022; 10: 11–25. doi: 10.1016/S2213-2600(21)00322-2 [DOI] [PubMed] [Google Scholar]
- 65.Bacharier LB, Maspero JF, Katelaris CH, et al. . Dupilumab in children with uncontrolled moderate-to-severe asthma. N Engl J Med 2021; 385: 2230–2240. doi: 10.1056/NEJMoa2106567 [DOI] [PubMed] [Google Scholar]
- 66.Dupin C, Belhadi D, Guilleminault L, et al. . Effectiveness and safety of dupilumab for the treatment of severe asthma in a real-life French multi-centre adult cohort. Clin Exp Allergy 2020; 50: 789–798. doi: 10.1111/cea.13614 [DOI] [PubMed] [Google Scholar]
- 67.Porsbjerg CM, Sverrild A, Lloyd CM, et al. . Anti-alarmins in asthma: targeting the airway epithelium with next-generation biologics. Eur Respir J 2020; 56: 2000260. doi: 10.1183/13993003.00260-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Menzies-Gow A, Corren J, Bourdin A, et al. . Tezepelumab in adults and adolescents with severe, uncontrolled asthma. N Engl J Med 2021; 384: 1800–1809. doi: 10.1056/NEJMoa2034975 [DOI] [PubMed] [Google Scholar]
- 69.Corren J, Ambrose CS, Sałapa K, et al. . Efficacy of tezepelumab in patients with severe, uncontrolled asthma and perennial allergy. J Allergy Clin Immunol Pract 2021; 9: 4334–4342.e6. doi: 10.1016/j.jaip.2021.07.045 [DOI] [PubMed] [Google Scholar]
- 70.Corren J, Parnes JR, Wang L, et al. . Tezepelumab in adults with uncontrolled asthma. N Engl J Med 2017; 377: 936–946. doi: 10.1056/NEJMoa1704064 [DOI] [PubMed] [Google Scholar]
- 71.Corren J, Garcia Gil E, Griffiths JM, et al. . Tezepelumab improves patient-reported outcomes in patients with severe, uncontrolled asthma in PATHWAY. Ann Allergy Asthma Immunol 2021; 126: 187–193. doi: 10.1016/j.anai.2020.10.008 [DOI] [PubMed] [Google Scholar]
- 72.Diver S, Khalfaoui L, Emson C, et al. . Effect of tezepelumab on airway inflammatory cells, remodelling, and hyperresponsiveness in patients with moderate-to-severe uncontrolled asthma (CASCADE): a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Respir Med 2021; 9: 1299–1312. doi: 10.1016/S2213-2600(21)00226-5 [DOI] [PubMed] [Google Scholar]
- 73.Puzzovio PG, Eliashar R, Levi-Schaffer F. Tezepelumab administration in moderate-to-severe uncontrolled asthma: is it all about eosinophils? J Allergy Clin Immunol 2022; 149: 1582–1584. doi: 10.1016/j.jaci.2022.01.019 [DOI] [PubMed] [Google Scholar]
- 74.Brenard E, Pilette C, Dahlqvist C, et al. . Real-life study of mepolizumab in idiopathic chronic eosinophilic pneumonia. Lung 2020; 198: 355–360. doi: 10.1007/s00408-020-00336-3 [DOI] [PubMed] [Google Scholar]
- 75.Schleich F, Vaia E-S, Pilette C, et al. . Mepolizumab for allergic bronchopulmonary aspergillosis: report of 20 cases from the Belgian Severe Asthma Registry and review of the literature. J Allergy Clin Immunol Pract 2020; 8: 2412–2413.e2. doi: 10.1016/j.jaip.2020.03.023 [DOI] [PubMed] [Google Scholar]
- 76.Wenzel S, Castro M, Corren J, et al. . Dupilumab efficacy and safety in adults with uncontrolled persistent asthma despite use of medium-to-high-dose inhaled corticosteroids plus a long-acting β2 agonist: a randomised double-blind placebo-controlled pivotal phase 2b dose-ranging trial. Lancet 2016; 388: 31–44. doi: 10.1016/S0140-6736(16)30307-5 [DOI] [PubMed] [Google Scholar]
- 77.Menzies-Gow A, Bafadhel M, Busse WW, et al. . An expert consensus framework for asthma remission as a treatment goal. J Allergy Clin Immunol 2020; 145: 757–765. doi: 10.1016/j.jaci.2019.12.006 [DOI] [PubMed] [Google Scholar]
- 78.Menzies-Gow A, Hoyte FL, Price DB, et al. . Clinical remission in severe asthma: a pooled post hoc analysis of the patient journey with benralizumab. Adv Ther 2022; 39: 2065–2084. doi: 10.1007/s12325-022-02098-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Moermans C, Brion C, Bock G, et al. . Sputum type 2 markers could predict remission in severe asthma treated with anti-interleukin-5. Chest 2023; in press [ 10.1016/j.chest.2023.01.037]. [DOI] [PubMed] [Google Scholar]
- 80.Kelsen SG, Agache IO, Soong W, et al. . Astegolimab (anti-ST2) efficacy and safety in adults with severe asthma: a randomized clinical trial. J Allergy Clin Immunol 2021; 148: 790–798. doi: 10.1016/j.jaci.2021.03.044 [DOI] [PubMed] [Google Scholar]
- 81.Wechsler ME, Ruddy MK, Pavord ID, et al. . Efficacy and safety of itepekimab in patients with moderate-to-severe asthma. N Engl J Med 2021; 385: 1656–1668. doi: 10.1056/NEJMoa2024257 [DOI] [PubMed] [Google Scholar]
- 82.Brightling CE, Nair P, Cousins DJ, et al. . Risankizumab in severe asthma – a phase 2a, placebo-controlled trial. N Engl J Med 2021; 385: 1669–1679. doi: 10.1056/NEJMoa2030880 [DOI] [PubMed] [Google Scholar]
- 83.Busse WW, Holgate S, Kerwin E, et al. . Randomized, double-blind, placebo-controlled study of brodalumab, a human anti-IL-17 receptor monoclonal antibody, in moderate to severe asthma. Am J Respir Crit Care Med 2013; 188: 1294–1302. doi: 10.1164/rccm.201212-2318OC [DOI] [PubMed] [Google Scholar]
- 84.O'Byrne PM, Metev H, Puu M, et al. . Efficacy and safety of a CXCR2 antagonist, AZD5069, in patients with uncontrolled persistent asthma: a randomised, double-blind, placebo-controlled trial. Lancet Respir Med 2016; 4: 797–806. doi: 10.1016/S2213-2600(16)30227-2 [DOI] [PubMed] [Google Scholar]
- 85.Gosens R, Hiemstra PS, Adcock IM, et al. . Host–microbe cross-talk in the lung microenvironment: implications for understanding and treating chronic lung disease. Eur Respir J 2020; 56: 1902320. doi: 10.1183/13993003.02320-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ghebre MA, Pang PH, Diver S, et al. . Biological exacerbation clusters demonstrate asthma and chronic obstructive pulmonary disease overlap with distinct mediator and microbiome profiles. J Allergy Clin Immunol 2018; 141: 2027–2036.e12. doi: 10.1016/j.jaci.2018.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Castro M, Rubin AS, Laviolette M, et al. . Effectiveness and safety of bronchial thermoplasty in the treatment of severe asthma: a multicenter, randomized, double-blind, sham-controlled clinical trial. Am J Respir Crit Care Med 2010; 181: 116–124. doi: 10.1164/rccm.200903-0354OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sun Q, Fang L, Roth M, et al. . Bronchial thermoplasty decreases airway remodelling by blocking epithelium-derived heat shock protein-60 secretion and protein arginine methyltransferase-1 in fibroblasts. Eur Respir J 2019; 54: 1900300. doi: 10.1183/13993003.00300-2019 [DOI] [PubMed] [Google Scholar]
- 89.Chernyavsky IL, Russell RJ, Saunders RM, et al. . In vitro, in silico and in vivo study challenges the impact of bronchial thermoplasty on acute airway smooth muscle mass loss. Eur Respir J 2018; 51: 1701680. doi: 10.1183/13993003.01680-2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cahill KN, Katz HR, Cui J, et al. . KIT inhibition by imatinib in patients with severe refractory asthma. N Engl J Med 2017; 376: 1911–1920. doi: 10.1056/NEJMoa1613125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sutcliffe A, Hollins F, Gomez E, et al. . Increased nicotinamide adenine dinucleotide phosphate oxidase 4 expression mediates intrinsic airway smooth muscle hypercontractility in asthma. Am J Respir Crit Care Med 2012; 185: 267–274. doi: 10.1164/rccm.201107-1281OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Prihandoko R, Kaur D, Wiegman CH, et al. . Pathophysiological regulation of lung function by the free fatty acid receptor FFA4. Sci Transl Med 2020; 12: eaaw9009. doi: 10.1126/scitranslmed.aaw9009 [DOI] [PubMed] [Google Scholar]
- 93.Nussbaumer-Ochsner Y, Rabe KF. Systemic manifestations of COPD. Chest 2011; 139: 165–173. doi: 10.1378/chest.10-1252 [DOI] [PubMed] [Google Scholar]
- 94.Barnes PJ. Inflammatory endotypes in COPD. Allergy 2019; 74: 1249–1256. doi: 10.1111/all.13760 [DOI] [PubMed] [Google Scholar]
- 95.Vanfleteren LEGW, Spruit MA, Wouters EFM, et al. . Management of chronic obstructive pulmonary disease beyond the lungs. Lancet Respir Med 2016; 4: 911–924. doi: 10.1016/S2213-2600(16)00097-7 [DOI] [PubMed] [Google Scholar]
- 96.Barnes PJ. Inflammatory mechanisms in patients with chronic obstructive pulmonary disease. J Allergy Clin Immunol 2016; 138: 16–27. doi: 10.1016/j.jaci.2016.05.011 [DOI] [PubMed] [Google Scholar]
- 97.Schleich F, Corhay JL, Louis R. Blood eosinophil count to predict bronchial eosinophilic inflammation in COPD. Eur Respir J 2016; 47: 1562–1564. doi: 10.1183/13993003.01659-2015 [DOI] [PubMed] [Google Scholar]
- 98.McDonald VM, Gibson PG. Treatable Traits in Asthma and COPD. Arch Bronconeumol 2022; 58: 583–585. doi: 10.1016/j.arbres.2021.07.003 [DOI] [PubMed] [Google Scholar]
- 99.Hastie AT, Martinez FJ, Curtis JL, et al. . Association of sputum and blood eosinophil concentrations with clinical measures of COPD severity: an analysis of the SPIROMICS cohort. Lancet Respir Med 2017; 5: 956–967. doi: 10.1016/S2213-2600(17)30432-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pascoe S, Locantore N, Dransfield MT, et al. . Blood eosinophil counts, exacerbations, and response to the addition of inhaled fluticasone furoate to vilanterol in patients with chronic obstructive pulmonary disease: a secondary analysis of data from two parallel randomised controlled trials. Lancet Respir Med 2015; 3: 435–442. doi: 10.1016/S2213-2600(15)00106-X [DOI] [PubMed] [Google Scholar]
- 101.Pavord ID, Lettis S, Locantore N, et al. . Blood eosinophils and inhaled corticosteroid/long-acting β-2 agonist efficacy in COPD. Thorax 2016; 71: 118–125. doi: 10.1136/thoraxjnl-2015-207021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu T, Xiang Z-J, Hou X-M, et al. . Blood eosinophil count-guided corticosteroid therapy and as a prognostic biomarker of exacerbations of chronic obstructive pulmonary disease: a systematic review and meta-analysis. Ther Adv Chronic Dis 2021; 12: 20406223211028770. doi: 10.1177/20406223211028768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Harries TH, Rowland V, Corrigan CJ, et al. . Blood eosinophil count, a marker of inhaled corticosteroid effectiveness in preventing COPD exacerbations in post-hoc RCT and observational studies: systematic review and meta-analysis. Respir Res 2020; 21: 3. doi: 10.1186/s12931-019-1268-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pavord ID, Chanez P, Criner GJ, et al. . Mepolizumab for eosinophilic chronic obstructive pulmonary disease. N Engl J Med 2017; 377: 1613–1629. doi: 10.1056/NEJMoa1708208 [DOI] [PubMed] [Google Scholar]
- 105.Pavord ID, Chapman KR, Bafadhel M, et al. . Mepolizumab for eosinophil-associated COPD: analysis of METREX and METREO. Int J Chron Obstruct Pulmon Dis 2021; 16: 1755–1770. doi: 10.2147/COPD.S294333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Criner GJ, Celli BR, Brightling CE, et al. . Benralizumab for the prevention of COPD exacerbations. N Engl J Med 2019; 381: 1023–1034. doi: 10.1056/NEJMoa1905248 [DOI] [PubMed] [Google Scholar]
- 107.Agusti A, Bel E, Thomas M, et al. . Treatable traits: toward precision medicine of chronic airway diseases. Eur Respir J 2016; 47: 410–419. doi: 10.1183/13993003.01359-2015 [DOI] [PubMed] [Google Scholar]
- 108.Green RH, Brightling CE, Woltmann G, et al. . Analysis of induced sputum in adults with asthma: identification of subgroup with isolated sputum neutrophilia and poor response to inhaled corticosteroids. Thorax 2002; 57: 875–879. doi: 10.1136/thorax.57.10.875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kupczyk M, Haque S, Middelveld RJM, et al. . Phenotypic predictors of response to oral glucocorticosteroids in severe asthma. Respir Med 2013; 107: 1521–1530. doi: 10.1016/j.rmed.2013.07.014 [DOI] [PubMed] [Google Scholar]
- 110.van Rensen ELJ, Evertse CE, van Schadewijk WAAM, et al. . Eosinophils in bronchial mucosa of asthmatics after allergen challenge: effect of anti-IgE treatment. Allergy 2009; 64: 72–80. doi: 10.1111/j.1398-9995.2008.01881.x [DOI] [PubMed] [Google Scholar]
- 111.Brightling CE, Bleecker ER, Panettieri RA Jr, et al. . Benralizumab for chronic obstructive pulmonary disease and sputum eosinophilia: a randomised, double-blind, placebo-controlled, phase 2a study. Lancet Respir Med 2014; 2: 891–901. doi: 10.1016/S2213-2600(14)70187-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rabe KF, Celli BR, Wechsler ME, et al. . Safety and efficacy of itepekimab in patients with moderate-to-severe COPD: a genetic association study and randomised, double-blind, phase 2a trial. Lancet Respir Med 2021; 9: 1288–1298. doi: 10.1016/S2213-2600(21)00167-3 [DOI] [PubMed] [Google Scholar]
- 113.Budden KF, Shukla SD, Rehman SF, et al. . Functional effects of the microbiota in chronic respiratory disease. Lancet Respir Med 2019; 7: 907–920. doi: 10.1016/S2213-2600(18)30510-1 [DOI] [PubMed] [Google Scholar]
- 114.Dickson RP, Martinez FJ, Huffnagle GB. The role of the microbiome in exacerbations of chronic lung diseases. Lancet 2014; 384: 691–702. doi: 10.1016/S0140-6736(14)61136-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang Z, Singh R, Miller BE, et al. . Sputum microbiome temporal variability and dysbiosis in chronic obstructive pulmonary disease exacerbations: an analysis of the COPDMAP study. Thorax 2018; 73: 331–338. doi: 10.1136/thoraxjnl-2017-210741 [DOI] [PubMed] [Google Scholar]
- 116.Dicker AJ, Huang JTJ, Lonergan M, et al. . The sputum microbiome, airway inflammation, and mortality in chronic obstructive pulmonary disease. J Allergy Clin Immunol 2021; 147: 158–167. doi: 10.1016/j.jaci.2020.02.040 [DOI] [PubMed] [Google Scholar]
- 117.Keir HR, Shoemark A, Dicker AJ, et al. . Neutrophil extracellular traps, disease severity, and antibiotic response in bronchiectasis: an international, observational, multicohort study. Lancet Respir Med 2021; 9: 873–884. doi: 10.1016/S2213-2600(20)30504-X [DOI] [PubMed] [Google Scholar]
- 118.Ubags NDJ, Marsland BJ. Mechanistic insight into the function of the microbiome in lung diseases. Eur Respir J 2017; 50: 1602467. doi: 10.1183/13993003.02467-2016 [DOI] [PubMed] [Google Scholar]
- 119.Contoli M, Pauletti A, Rossi MR, et al. . Long-term effects of inhaled corticosteroids on sputum bacterial and viral loads in COPD. Eur Respir J 2017; 50: 1700451. doi: 10.1183/13993003.00451-2017 [DOI] [PubMed] [Google Scholar]
- 120.Albert RK, Connett J, Bailey WC, et al. . Azithromycin for prevention of exacerbations of COPD. N Engl J Med 2011; 365: 689–698. doi: 10.1056/NEJMoa1104623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Rogers GB, Bruce KD, Martin ML, et al. . The effect of long-term macrolide treatment on respiratory microbiota composition in non-cystic fibrosis bronchiectasis: an analysis from the randomised, double-blind, placebo-controlled BLESS trial. Lancet Respir Med 2014; 2: 988–996. doi: 10.1016/S2213-2600(14)70213-9 [DOI] [PubMed] [Google Scholar]
- 122.Taylor SL, Leong LEX, Mobegi FM, et al. . Long-term azithromycin reduces Haemophilus influenzae and increases antibiotic resistance in severe asthma. Am J Respir Crit Care Med 2019; 200: 309–917. doi: 10.1164/rccm.201809-1739OC [DOI] [PubMed] [Google Scholar]