Abstract
Despite significant advances in pharmacological, electrophysiological and valve therapies for heart failure with reduced ejection fraction (HFrEF), the associated morbidity, mortality and healthcare costs remain high. With a constantly growing heart failure population, the existing treatment gap between current and advanced heart failure therapies (e.g., left ventricular [LV] assist devices, heart transplantation) reflects a large unmet need, calling for novel therapeutic approaches. Left ventricular remodelling and dilatation, with or without scar formation, is the hallmark of cardiomyopathy and is associated with poor prognosis. In the era of exciting advances in structural heart interventions, the advent of minimally invasive, device-based therapies directly targeting the LV geometry and promoting physical reverse remodelling has created a new frontier in the battle against heart failure. Interventional heart failure therapy is a rapidly emerging field, encompassing structural heart and minimally invasive hybrid procedures, with two left ventriculoplasty devices currently under investigation in pivotal clinical trials in the US. This review addresses the rationale for left ventriculoplasty, presents the prior surgical and percutaneous attempts in the field, provides an overview of the novel transcatheter left ventriculoplasty devices and their respective trials, and highlights potential challenges associated with establishing such device-based therapies in our armamentarium against heart failure.
Introduction: the unmet heart failure needs
According to recent data from the American Heart Association, heart failure (HF) is an epidemic of our era, with an estimated 6.2 million affected people in the US and a projected 46% increase in its prevalence by 20301. Despite advances in pharmacologic and device therapies for heart failure with reduced ejection fraction (HFrEF), the associated morbidity, mortality, and healthcare expenditures remain high2. Furthermore, currently available advanced therapies are either too invasive (left ventricular assist devices; LVAD) or limited by donor availability (heart transplantation), patient age, and comorbidities.
Adverse left ventricular (LV) remodelling and dilatation is the hallmark of cardiomyopathy, both ischaemic and non-ischaemic. Preventing and/or reversing the naturally progressive remodelling process remains the main target of contemporary pharmacologic and device therapies, as improvements in left ventricular ejection fraction (LVEF) and volumes are associated with improved survival in HFrEF3. While these therapies focus mainly on biologic mechanisms (neurohormonal pathways, electrical conduction and loading conditions) associated with pathologic remodelling, there is a paucity of interventional therapies that can lead to immediate and direct physical reverse remodelling of the LV.
With the exponential growth of transcatheter interventions for structural heart disease, novel applications of minimally invasive, device-based therapies that directly modify the adversely remodelled LV have been developed. Percutaneous left ventriculoplasty encompasses transcatheter procedures that accomplish direct, physical reverse remodelling of the LV by excluding non-viable scar/aneurysmal tissue, reducing the LV circumference through endocardial cinching or scaffolding of the LV myocardium (Central illustration).
Central illustration. Novel transcatheter ventriculoplasty procedures and their effects on reverse left ventricular remodelling.
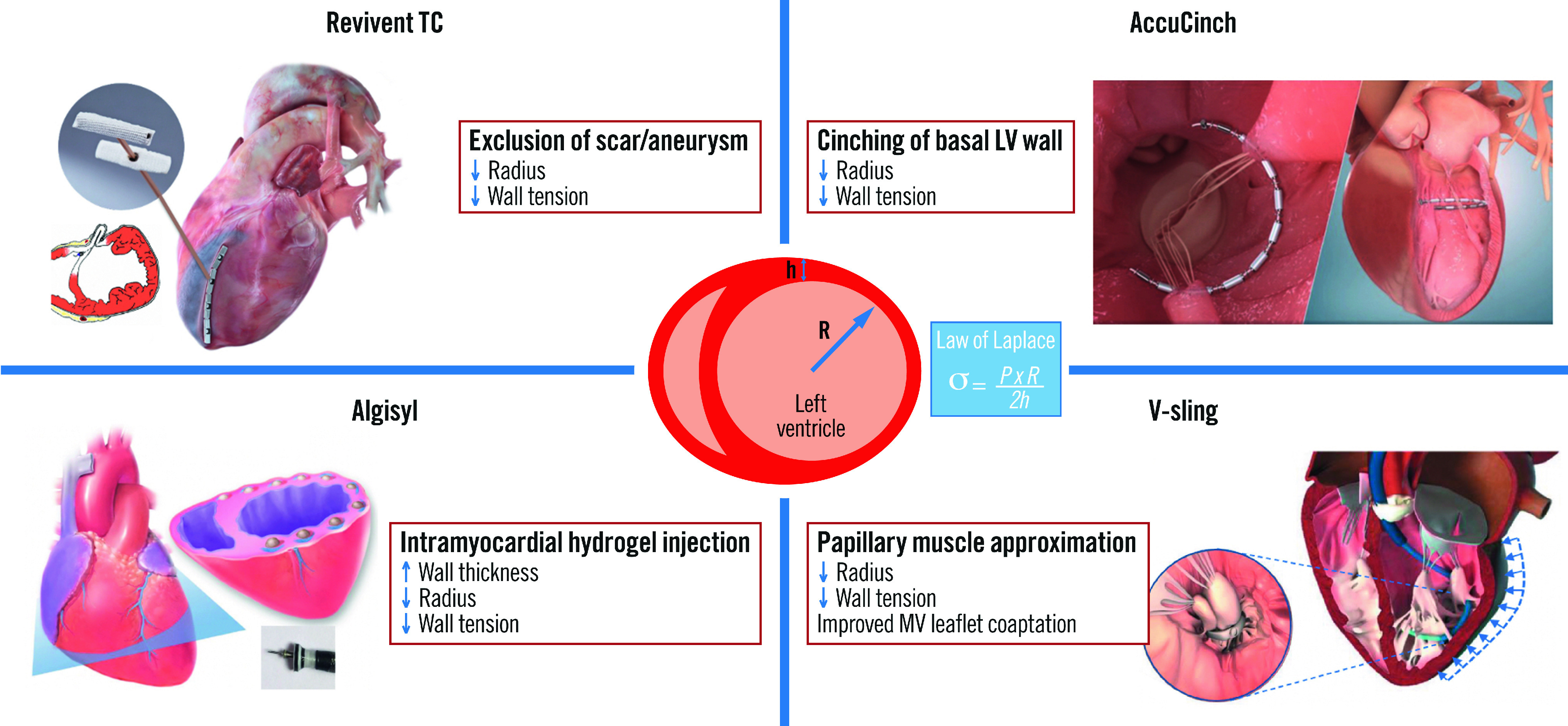
h: wall thickness; LV: left ventricular; MV: mitral valve; P: pressure; R: radius; σ: wall tension
This review focuses on the rationale for the development of two transcatheter left ventriculoplasty devices that are currently being assessed in pivotal randomised trials and provides a brief overview of some emerging novel LV reshaping therapies for HFrEF.
Current HF therapies and rationale for ventriculoplasty
The microscopic changes at the myocyte and extracellular matrix level during LV remodelling ultimately lead to macroscopic changes in LV function, geometry, and volumes. The LVEF, LV end-systolic volume (LVESV) and end-diastolic volume (LVEDV) are all used as markers of remodelling and provide important information on prognosis and response to therapy in HFrEF3,4,5.
Therapies to reverse LV remodelling can be classified into two major categories based on their principal mechanism of action: biologic and physical. Biologic therapies target the neurohormonal pathways associated with HF, optimise LV preload and/or afterload, or modify the electrical substrate with the potential to indirectly achieve reverse remodelling and improve prognosis (Table 1)6,7,8,9,10,11,12. On the contrary, physical reverse remodelling refers to therapies that exert a direct and immediate mechanistic effect on LV size and geometry, leading to secondary biologic changes in the long term.
Table 1. Effect of current pharmacologic and device therapies on mortality and LV remodelling of HFrEF patients compared to placebo.
| Treatment | Average delta EF (%) | Average delta EDV (mL) | Average delta ESV (mL) | Mortality (%) |
|---|---|---|---|---|
| Carvedilol | 6.9 | −26.7 | −33.9 | −38.0 |
| Metoprolol succinate | 4.5 | −27.6 | −30.8 | −33.0 |
| Bisoprolol | 12.0 | −52.5 | −63.0 | −36.0 |
| Enalapril | 3.7 | −11.1 | −19.6 | −18.0 |
| Candesartan | 4.0 | −8.2 | −11.3 | −18.0** |
| Sacubitril-valsartan | 9.4 | −12.25* | 15.29* | −16.0*** |
| Spironolactone | 3.0 | −26.9 | −27.7 | −38.0 |
| Empagliflozin | 0.3 | −8.2* | −6.0* | −25.0** |
| Hydralazine-ISDN | 2.9 | −5.6 | −8.8 | −43.0^ |
| CRT | 11.0 | −52.2 (−26.2*) | −57.3 (−28.7*) | −17.0-21.0+ |
| *Indexed volume (mL/m2). **Cardiovascular death or heart failure hospitalisation. ***Compared to enalapril. ^In African Americans. +Decrease in mortality per 10% decrease in LVEDV/LVESV. CRT: cardiac resynchronisation therapy; EDV: end-diastolic volume; EF: ejection fraction; ESV: end-systolic volume; HFrEF: heart failure with reduced ejection fraction; ISDN: isosorbide dinitrate; LV: left ventricular | ||||
Left ventriculoplasty encompasses surgical and minimally invasive procedures that directly modulate the LV geometry and promote an immediate and direct physical effect, followed by ongoing reverse remodelling as a result of reduced chamber size and wall tension. According to Laplace’s law, LV wall stress is directly proportional to the internal radius and pressure and inversely proportional to wall thickness (Central illustration). Interventions that optimise this relationship and restore the elliptical shape of the ventricle should theoretically improve chamber compliance, reduce wall stress, and ultimately enhance contractile performance.
Furthermore, the observation that myocardial infarction can indirectly affect the function of remote, non-infarcted areas of the myocardium13,14,15 supports further the hypothesis that excluding the non-functional scar tissue post-myocardial infarction (MI) may improve global LV function through secondarily affected, remote areas of the myocardium. Castelvecchio and colleagues found improved LV fibre shortening and function in the remote mid- and basal LV regions with 3-dimensional speckle-tracking echocardiography after surgical LV apical reconstruction, suggesting the important role of remote myocardium in LV function recovery16. Similarly, in non-ischaemic dilated cardiomyopathy, a direct reduction of LV volumes after ventriculoplasty may initiate a more global reverse remodelling process with sustained results.
Such interventions have been attempted both surgically and percutaneously, by either resecting, compressing, or isolating non-functional myocardium. Understanding the historical development and efficacy of, as well as the lessons learned from, surgical ventricular reconstruction (SVR) is important to gain insight into the contemporary era of transcatheter-based LV remodelling techniques.
Surgical ventricular reconstruction and previous percutaneous attempts
The first SVR procedure was described by Cooley in 1958, when he performed a left ventricular aneurysmectomy using a dacron patch17. This technique was further developed by Dor, who used a circular endoventricular patch and published the first SVR results in 198918. As a result of the increasing interest in SVR, the Surgical Treatment for Ischemic Heart Failure II (STICH II) randomised trial was conducted, comparing SVR plus coronary artery bypass grafting (CABG) to CABG alone in patients with ischaemic cardiomyopathy, obstructive CAD, and a large anterior wall scar19. The study did not show reduction in all-cause mortality or hospitalisation for cardiac causes; however, one of the main criticisms was that the 19% LVESV index (LVESVi) reduction in the SVR group was insufficient to show an improvement in clinical outcomes (average decrease of 16 mL/m2, from 83 to 67 mL/m2) compared to previous observational studies, possibly attenuating the potential benefits of SVR. Further analysis of LV volume subgroups showed that SVR resulted in improved long-term survival when the postoperative LVESVi was 70 mL/m2 or less20. In addition, data from the RESTORE registry suggested that a more drastic LVESVi reduction, to the lowest achievable LVESVi (less than 45-60 mL/m2), was associated with improved outcomes21,22.
Based on these data, the most recent European Society of Cardiology/European Association of Cardio-Thoracic Surgery (ESC/EACTS) guidelines include SVR as a reasonable surgical option combined with CABG in selected HF patients with a scar in the left anterior descending (LAD) territory, especially if a postoperative LVESVi <70 mL/m2 can be achieved (class of recommendation IIb; level of evidence B)23.
Given the enduring belief in the potential benefits of SVR, more devices were developed focusing on SVR optimisation and transition to less invasive approaches, as summarised in Supplementary Figure 121,22,24,25,26,27,28,29.
The Parachute device (CardioKinetix), consisting of a self-expanding nitinol frame with an expanded polytetrafluoroethylene (ePTFE) membrane placed in the LV apex via the femoral artery, was the first percutaneous device tried in patients with ischaemic cardiomyopathy and an anterior-apical scar. The device was intended to partition the non-contractile anterior-apical scar, create a new apex, and restore the elliptical shape of the LV. Despite promising early data from 19 patients (Supplementary Figure 2)30, a phase III randomised controlled trial was terminated in 2017 after enrolling 338 patients without published results31.
On the whole, the above studies demonstrated improvements in quality-of-life metrics and functional status but did not consistently show a substantial survival benefit. Their data were mostly limited to observational cohorts or patients undergoing surgery for a separate indication, such as CABG or mitral valve repair/replacement. Thus, the question remains whether patients with dilated cardiomyopathy who receive optimal guideline-directed medical therapy (GDMT) but continue to demonstrate symptomatic HF could benefit from an isolated structural intervention targeting the LV. Because of the complexity and highly invasive nature of SVR in this high-risk patient population, percutaneous ventriculoplasty procedures may be more feasible.
Transcatheter left ventriculoplasty devices currently in pivotal trials
Revivent myocardial anchoring system
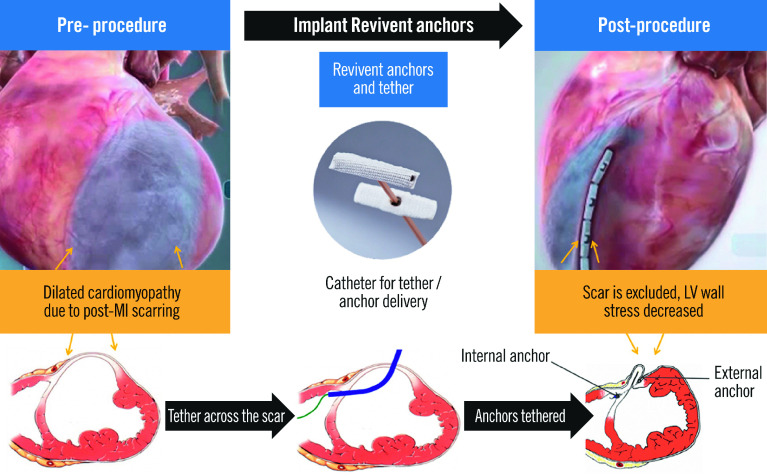
The Revivent TC system (BioVentrix) offers a minimally invasive strategy for LV reconstruction in HF patients with an LV antero-apical scar and/or aneurysm. Instead of surgically resecting the LV scar/aneurysm, this procedure excludes the non-functional tissue through the application of pairs of titanium anchors on the beating heart. The reduced LV radius lowers myocardial wall stress (according to Laplace’s law), leading to more efficient contractile function. Preprocedural planning with multimodality imaging (transthoracic echocardiography [TTE] to screen and computerised tomography [CT] or magnetic resonance imaging [MRI] to assess LV volumes and scar location) is key.
While the first-generation Revivent TC delivery system required a median sternotomy, the second-generation system allows a hybrid approach via a mini-thoracotomy and right internal jugular (IJ) access, which is performed off-pump. The mini-thoracotomy provides direct access to the anterolateral LV epicardial surface, where a needle enters the LV through the scar and traverses the interventricular septum into the right ventricular (RV) apex. A guidewire is then delivered to the RV and retrieved by a prepositioned snare through the 14 Fr right IJ venous sheath, creating a continuous track rail over which the internal anchor can be advanced and applied against the RV side of the septum. A tether along the same rail through the LV wall is then affixed with a locking anchor on the LV epicardial surface and is used to draw the two anchors towards each other until sufficient contact between the two opposing walls is achieved, resulting in the exclusion of a portion of the LV chamber. This action is repeated along the long axis of the LV, until a portion of the anterolateral wall is in contact with the corresponding portion of the septum, resulting in the exclusion of the non-functioning scar tissue from the rest of the myocardium (Figure 1). Transoesophageal echocardiography (with deep transgastric views) and fluoroscopy are used to guide crucial steps of the procedure, such as ventricular septal puncture and wire snaring in the RV.
Figure 1. The Revivent TC system.
Implantation of the Revivent TC anchor pairs across the right side of the septum and anterolateral left ventricular wall, resulting in the exclusion of the aneurysmal and/or akinetic scar tissue. Reproduced with permission from BioVentrix. LV: left ventricular; MI: myocardial infarction
A prospective, multicentre, single-arm study was conducted in Europe between 2010 and 2016 to evaluate the safety and efficacy of the Revivent TC system in 86 patients with dilated ischaemic cardiomyopathy (LVEF 25-45%, LVESVi 60-120 mL/m2, New York Heart Association [NYHA] Class II-IV) and an akinetic or dyskinetic scar in the anteroseptal, anterolateral and/or apical regions. Fifty-one patients received the first-generation system through median sternotomy and 35 received the hybrid procedure, with 4.5% 30-day in-hospital mortality and a 90.6% survival rate at 12 months. Haemodynamic and clinical data showed significant improvements in LVEF, LVESVi, LVEDVi, secondary mitral regurgitation (MR), NYHA class, 6-minute walk test (6MWT) distance and Minnesota Living with Heart Failure (MLHF) score32. The adverse event rates were relatively low: ventricular arrhythmias (14%), bleeding (8.1%), increase in tricuspid regurgitation (5.8%), pleural effusion (5.8%), stroke (4.7%), atrial fibrillation (3.5%) and ventricular septal defect (2.3%). Based on the above, CE marking was awarded, and the Revivent TC system has been available in Europe since 2016.
To further assess the clinical benefit of the Revivent TC system, the Randomized Evaluation and Verification of Ventricular Enhancement (REVIVE-HF) randomised controlled trial is currently being conducted in Europe, where 120 patients with ischaemic cardiomyopathy will receive the investigational device and 60 will remain on GDMT. Preliminary data have demonstrated significant improvement in LVESVi, LVEDVi, LVEF and cardiac output assessed with cardiac magnetic resonance (CMR) at 12 months ([Hennig F, et al. Impact of Less Invasive Ventricular Enhancement TM (LIVE TM) Compared to Optimal Medical Therapy on Cardiac Output in Patients with HFREF - Preliminary Results of a 12-month Multi-center CMR Trial. J Am Coll Cardiol. 2019;73:810] and [Stoiber L, et al. Abstract 20772: In a multi-center trial Less Invasive Ventricular Enhancement (LIVETM) Technique with the Revivent TM system (Bioventrix) significantly improves LV function in HFrEF patients. A 12 months-CMR-follow-up study. Circulation. 2017;136:A20772]). Furthermore, longitudinal data from Revivent TC recipients in Europe demonstrated sustained LVESVi reduction at 5 years (from 73.2±27 ml at baseline to 56.1±16 ml, p=0.047), along with improvement in NYHA class and 6MWT distance33. Various refinements of the procedure over time, as well as imaging advances resulting in better identification of suitable pathology and intraprocedural guidance, have been associated with more effective LV volume reduction and better safety outcomes.
The American Less Invasive Ventricular Enhancement (ALIVE) study is a prospective, multicentre, non-randomised, dual-arm pivotal trial of the Revivent TC system being conducted at US and European sites. A total of 126 patients will be allocated in a 2:1 fashion to the study device and GDMT groups, respectively. The key qualifying criteria are LVEF <45%, LVESVi >50 mL/m2, NYHA III-IVa symptoms despite GDMT, and presence of a contiguous, akinetic scar involving the septum, anterior, apical or anterolateral LV walls. The control group will consist of patients who meet the inclusion criteria, except for the LV aneurysm/scar location, or patients with previous sternotomy or left thoracotomy, and patients who elect to be enrolled in the control group. Safety data from patients treated with the Revivent TC system will be compared with surgical outcomes from the Society of Thoracic Surgeons database on LV aneurysm repair. The primary endpoint is freedom from device-related major adverse events including all-cause death, myocardial infarction, stroke, prolonged mechanical ventilation, renal failure, non-elective cardiac surgery, and worsening HF requiring mechanical support (e.g., intra-aortic balloon pump, ventricular assist device, or extracorporeal membrane oxygenation). Secondary endpoints include improvement in quality of life and clinical parameters (NYHA class, 6MWT distance and MLHF score) and reduction in HF-related hospitalisation rates. Supplementary Table 1 summarises the key aspects of ALIVE.
AccuCinch Ventricular Restoration System
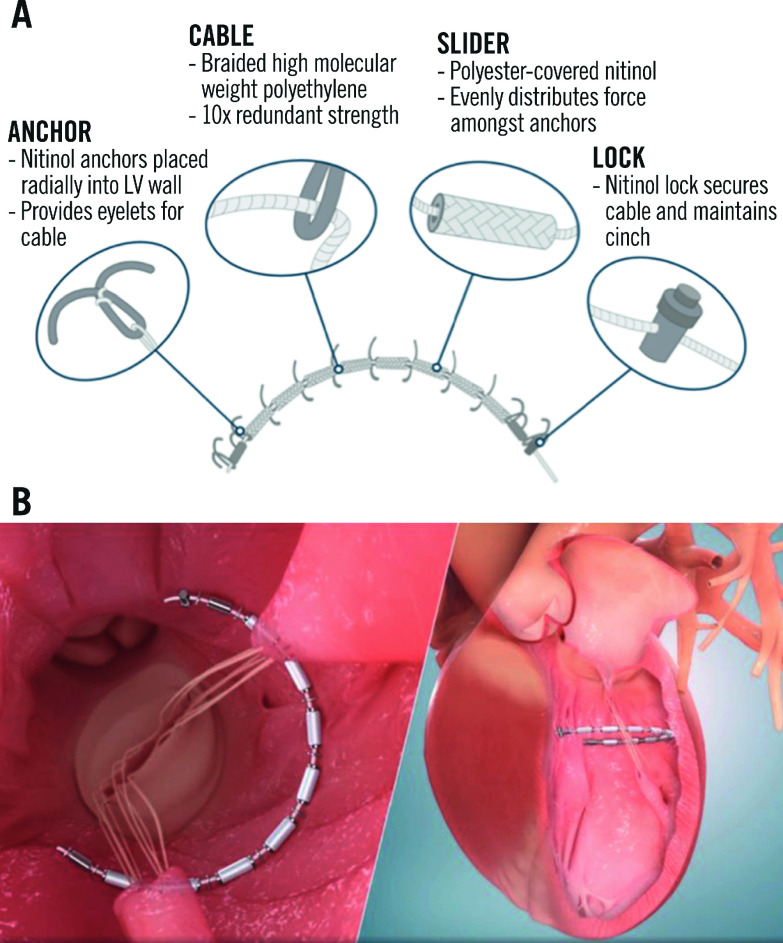
The AccuCinch Ventricular Restoration System (Ancora Heart) consists of anchors, radiopaque sliders, locks, and a cable that connects the anchors in the endocardial surface of the basal LV wall. It is delivered through the femoral artery and aortic valve retrogradely into the LV under fluoroscopic and echocardiographic guidance. A tracking catheter with a semi-circular shape is positioned at the basal aspect of the LV, serving as a guide for device deployment. The anchors and sliders are placed in series approximately 10-20 mm below the mitral annulus in the basal to mid-LV wall, starting under the medial aspect of the posterior mitral leaflet (P3). Special caution is applied to avoid entanglement in the subvalvular mitral apparatus. Tension is finally applied on the cable, which is cinched, leading to a reduction of the basal LV wall radius and volume (Figure 2). This results not only in a reduction of wall stress, but also in the approximation of the papillary muscles and reduction of mitral leaflet tethering, leading to improved LV systolic performance and secondary MR reduction.
Figure 2. The AccuCinch ventricular restoration system.
A) AccuCinch implant configuration. B) The AccuCinch device is implanted 10-20 mm below the mitral valve annulus. Tension is applied on the cable connecting the anchors, leading to a reduction of basal left ventricular diameter. Reproduced with permission from Ancora Heart. LV: left ventricular
Initial experience with the AccuCinch was described in a multicentre, single-arm, prospective early feasibility study in 19 patients with HF and functional MR (CorCinch-FMR). Preliminary results showed a 40% reduction in LV systolic and diastolic volumes, reduction in mitral regurgitation, and improvements in LVEF, NYHA class and quality of life (Kansas City Cardiomyopathy Questionnaire [KCCQ]) at 6 months, without any major safety concerns (Reisman M, et al. TCT-88 6-Month Outcomes of an Early Feasibility Study of the AccuCinch Left Ventricular Repair System in Patients With Heart Failure and Functional Mitral Regurgitation. JACC. 2019;74:B88).
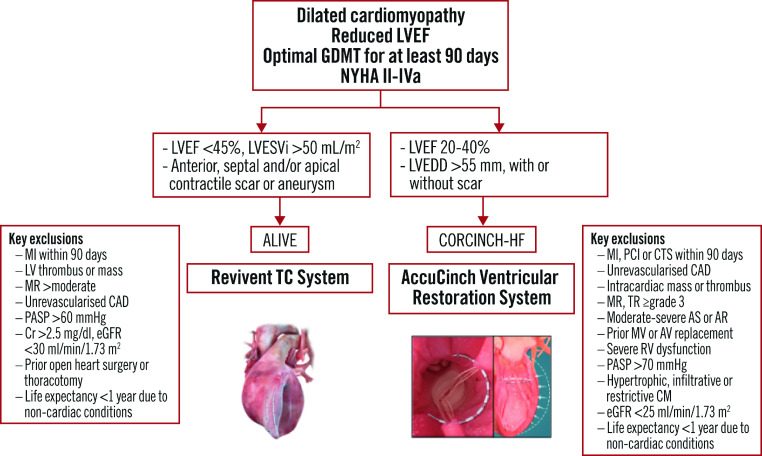
The Clinical Evaluation of the AccuCinch Ventricular Restoration System in Patients Who Present with Symptomatic Heart Failure with Reduced Ejection Fraction (HFrEF) (CORCINCH-HF) study is a prospective, randomised, controlled, open-label, multicentre trial intending to evaluate the safety and efficacy of the investigational device in patients with dilated cardiomyopathy. The trial intends to randomise 400 patients to the investigational device and GDMT groups. Key inclusion criteria are LVEF 20-40%, left ventricular end-diastolic diameter (LVEDD) >55 mm and persistent NYHA II-IVa symptoms, despite optimal GDMT and cardiac resynchronisation therapy (if indicated) for at least 90 days. Similar to ALIVE, patients with more-than-moderate functional or degenerative MR are excluded. The primary endpoints are freedom from device-related major adverse events, changes in the KCCQ score, the 6MWT distance, and the composite of all-cause death, LVAD implant or heart transplant, and HF hospitalisations. Figure 3 outlines the patient selection process for the Revivent TC and AccuCinch ventriculoplasty devices.
Figure 3. Algorithm for the appropriate selection of patients for the Revivent TC and AccuCinch ventricular repair systems.
AR: aortic regurgitation; AS: aortic stenosis; AV: aortic valve; CAD: coronary artery disease; CM: cardiomyopathy; Cr: creatinine; CTS: cardiothoracic surgery; eGFR: estimated glomerular filtration rate; GDMT: guideline-directed medical therapy; LV: left ventricular; LVEDD: left ventricular end-diastolic diameter; LVEF: left ventricular ejection fraction; LVEFi: LVEF index; LVESVi: left ventricular end-systolic volume index; MI: myocardial infarction; MR: mitral regurgitation; MV; mitral valve; NYHA: New York Heart Association; PASP: pulmonary arterial systolic pressure; PCI: percutaneous coronary intervention; RV: right ventricular; TR: tricuspid regurgitation
Emerging dedicated LV reshaping technologies
Algisyl-LVR
Left ventricular augmentation with intramyocardial injection of Algisyl (Algisyl-LVR; LoneStar Heart), a calcium alginate biopolymer, increases the LV wall thickness, resulting in reduction of the LV cavity diameter, volume, and ultimately wall stress according to Laplace’s law (Central illustration). The biopolymer serves as a prosthetic scaffold that reduces the size and sphericity of the LV, leading to a more efficient contractile function. The AUGMENT-HF safety and efficacy trial randomised 78 patients with advanced dilated cardiomyopathy (LVEF ≤35%, peak VO2 of 9.0-14.5 mL/min/kg, and LVEDD index [LVEDDi] 30-40 mm/m2) to Algisyl and GDMT versus GDMT alone. Algisyl was administered by a series of circumferential injections in the mid-LV wall from the anterior to posterior intraventricular groove via a limited left anterior thoracotomy. Treatment with Algisyl was associated with significant improvements in peak VO2, 6MWT distance, and NYHA Functional Class at 12 months, compared with the controls34. The development of a completely percutaneous, catheter-based delivery system for intramyocardial administration of Algisyl with fluoroscopic and echocardiographic guidance is currently underway. This novel technology may expand the interventional treatment options for dilated cardiomyopathy and allow for hybrid left ventriculoplasty procedures with other devices.
V-sling
V-sling (Cardiac Success) is a left ventricular transcatheter repair system that targets the subvalvular mitral apparatus and aims at papillary muscle approximation in patients with dilated cardiomyopathy and secondary MR. The sling procedure was first performed surgically in combination with mitral ring annuloplasty, with the placement of a 4 mm polytetrafluoroethylene (Gore-Tex) tube around the papillary muscles, which is tightened together, thereby reducing mitral leaflet tethering and the LV diameter (Central illustration). A single-centre randomised trial of 96 patients with severe ischaemic MR and reduced LVEF undergoing CABG showed that surgical papillary muscle approximation in addition to mitral annuloplasty was associated with significant reverse LV remodelling (LVEDD, LVEF), lower major adverse cardiovascular events and lower recurrence of moderate-severe MR at 5 years, without a difference in overall mortality compared to mitral annuloplasty alone35. A fully dedicated transcatheter device, delivered via a steerable sheath that is inserted into the LV via retrograde (transfemoral) aortic access was anticipated to undergo a first-in-human early feasibility evaluation later in 2022 in Europe and Israel. The study will enrol patients with an LVEF 20-40%, secondary MR 1-2+, LVEDD ≥55 mm and end-systolic interpapillary distance ≥20 mm. This minimally invasive transcatheter approach may directly address the underlying mechanism of secondary MR, with the potential to restore the elliptical LV shape and promote reverse LV remodelling, without precluding future valvular or other transcatheter ventriculoplasty procedures.
Other devices, such as the Heart Damper (Eucardia), are currently in preclinical stages. The Heart Damper is a memory-shaped, dynamic structure implanted in the LV apex. Besides reducing the effective LV volume, its property of changing shape during systole (pointing upward) and diastole (flat) is intended to transmit the energy generated by LV contraction to increase stroke volume36.
Current challenges and future directions
With preliminary data outlining the safety and feasibility of both the Revivent TC and AccuCinch percutaneous ventriculoplasty devices, the ALIVE and CORCINCH-HF pivotal trials are positioned not only to demonstrate safety in a larger number of HFrEF patients, but also efficacy, beyond the established pharmacologic and device HF therapies. Safety will be key, followed by reduction in HF hospitalisations and quality of life/functional improvement, which are increasingly being shown to correlate with significant clinical endpoint reductions across a range of device therapies37,38,39.
Preprocedural imaging is key for appropriate patient selection, planning, and device success. The effects of transcatheter ventriculoplasty on global LV function and the response of the remote, non-infarct-related myocardium to the geometric changes of the ventricle may require a more comprehensive assessment of regional LV function with advanced echocardiographic (3D speckle tracking) and/or CT/CMR techniques.
Despite the potential advantages of transcatheter ventriculoplasty, related to its less invasive nature compared to surgery, there are certain limitations inherent to the current procedures. For example, the Revivent TC device is intended to treat left anterior descending territory scars, which need to be transmural. Post-sternotomy patients, who represent a significant portion of patients with ischaemic cardiomyopathy, are currently excluded from ALIVE. The presence of intracavitary RV pacemaker/defibrillator leads in close proximity to the interventricular septum can pose a technical challenge for internal anchor deployment. A small or trabeculated RV apex poses considerable challenges for undertaking the traditional LV-to-RV first anchor placement, which is felt to be the substrate for achieving the greatest LV volume reduction. In such circumstances, 3 direct LV-LV stitches are applied by the cardiac surgeon, obviating the need to create an LV-RV anchor from the jugular vein. For the AccuCinch device, the need for large-bore iliofemoral arterial access (20 Fr) may be a limitation for patients with peripheral vascular disease. A minimum thickness (5-6 mm) of the basal LV wall is required to safely deploy and accommodate the anchors of the cinching device, which may result in the exclusion of a significant number of patients.
The presence of more-than-moderate secondary MR currently serves as an exclusion criterion in both the ALIVE and CORCINCH-HF trials; however preliminary data suggest MR reduction in certain patients who received the respective investigational device. Given that secondary MR is considered a left ventricular disease (present in about 20% of the HFrEF population), patients may benefit from left ventriculoplasty upfront, possibly obviating the need for mitral valve intervention. On the other hand, reducing LV volume with left ventriculoplasty may uncover patients with “disproportionate” MR who may gain additional benefit from subsequent percutaneous MV repair or replacement. Including patients with more-than-moderate secondary MR in future trials may be helpful to adequately formulate the treatment algorithm associated with best outcomes: upfront transcatheter ventriculoplasty versus early percutaneous mitral valve intervention.
Another important question is whether certain patients with significantly dilated LV and an anterior and/or apical scar might benefit from combined left ventriculoplasty procedures. Further innovation in transcatheter left ventriculoplasty devices to address all categories of dilated cardiomyopathy, irrespective of the presence, size, and location of the scar or prior open heart surgery, will be important to expand the applicability of such procedures to a broader HFrEF population. Lessons learned from earlier translational models of surgical ventricular restoration shed important light onto the potential adverse effects of indiscriminately targeting extensive areas of compliant/potentially contractile myocardium for LV volume reduction40. Any future hybrid transcatheter LV-reshaping technique designed to treat a broader HfrEF population will need advanced preprocedural imaging assessment to identify the specific myocardial regions most likely to serve as a volume reduction target whilst also promoting reverse remote LV remodelling.
Conclusions
Despite early challenges with surgical LV reconstruction, there is growing interest in percutaneous physical reverse remodelling for dilated cardiomyopathy, with numerous transcatheter devices under development and investigation. While transcatheter left ventriculoplasty appears a promising strategy based on preliminary anatomical, pathophysiological, and clinical data, the efficacy of these procedures has yet to be proven in the HfrEF population. To that end, two devices are currently under investigation in pivotal clinical trials in the US. With the emergence of interventional heart failure therapy - a new field spanning interventional cardiology, heart failure, imaging, and cardiac surgery - innovative interventional and structural therapies aimed at physical LV remodelling may expand our armamentarium against the heterogeneous heart failure syndrome.
Supplementary data
The CORCINCH-HF and ALIVE clinical trials.
Surgical ventricular restoration procedures and relevant studies.
The Parachute left ventricular partitioning device.
Acknowledgments
Conflict of interest statement
J.D. Estep serves as the national coprincipal investigator of the ALIVE trial and as a consultant to BioVentrix. R. Puri serves as a consultant to BioVentrix. H. Sievert serves as a consultant to Cardiac Success (developer of V-sling device). The other authors have no conflicts of interest to declare.
Abbreviations
- CMR
cardiac magnetic resonance
- GDMT
guideline-directed medical therapy
- HFrEF
heart failure with reduced ejection fraction
- LV
left ventricle/ventricular
- LVEF
left ventricular ejection fraction
- MI
myocardial infarction
- MR
mitral regurgitation
- NYHA
New York Heart Association
- SVR
surgical ventricular reconstruction
- LVEDV
left ventricular end-diastolic volume
- LVESV
left ventricular end-systolic volume
Contributor Information
Nikolaos Spilias, Heart, Vascular & Thoracic Institute, Cleveland Clinic, Cleveland, OH, USA.
Travis M. Howard, Heart, Vascular & Thoracic Institute, Cleveland Clinic, Cleveland, OH, USA.
Chris M. Anthony, Heart, Vascular & Thoracic Institute, Cleveland Clinic, Cleveland, OH, USA.
Balint Laczay, Heart, Vascular & Thoracic Institute, Cleveland Clinic, Cleveland, OH, USA.
Edward G. Soltesz, Heart, Vascular & Thoracic Institute, Cleveland Clinic, Cleveland, OH, USA; CardioVascular Center Frankfurt, Frankfurt, Germany.
Randall C. Starling, Heart, Vascular & Thoracic Institute, Cleveland Clinic, Cleveland, OH, USA.
Horst Sievert, CardioVascular Center Frankfurt, Frankfurt, Germany.
Jerry D. Estep, Heart, Vascular & Thoracic Institute, Cleveland Clinic, Cleveland, OH, USA.
Samir R. Kapadia, Heart, Vascular & Thoracic Institute, Cleveland Clinic, Cleveland, OH, USA.
Rishi Puri, Heart, Vascular & Thoracic Institute, Cleveland Clinic, Cleveland, OH, USA; CardioVascular Center Frankfurt, Frankfurt, Germany.
References
- Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Jordan LC, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, O'Flaherty M, Pandey A, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Spartano NL, Stokes A, Tirschwell DL, Tsao CW, Turakhia MP, VanWagner LB, Wilkins JT, Wong SS, Virani SS American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation. 2019;139:e56–528. doi: 10.1161/CIR.0000000000000659. [DOI] [PubMed] [Google Scholar]
- Shah KS, Xu H, Matsouaka RA, Bhatt DL, Heidenreich PA, Hernandez AF, Devore AD, Yancy CW, Fonarow GC. Heart Failure With Preserved, Borderline, and Reduced Ejection Fraction: 5-Year Outcomes. J Am Coll Cardiol. 2017;70:2476–86. doi: 10.1016/j.jacc.2017.08.074. [DOI] [PubMed] [Google Scholar]
- Konstam MA, Kramer DG, Patel AR, Maron MS, Udelson JE. Left ventricular remodeling in heart failure: current concepts in clinical significance and assessment. JACC Cardiovasc Imaging. 2011;4:98–108. doi: 10.1016/j.jcmg.2010.10.008. [DOI] [PubMed] [Google Scholar]
- McManus DD, Shah SJ, Fabi MR, Rosen A, Whooley MA, Schiller NB. Prognostic value of left ventricular end-systolic volume index as a predictor of heart failure hospitalization in stable coronary artery disease: data from the Heart and Soul Study. J Am Soc Echocardiogr. 2009;22:190–7. doi: 10.1016/j.echo.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White HD, Norris RM, Brown MA, Brandt PW, Whitlock RM, Wild CJ. Left ventricular end-systolic volume as the major determinant of survival after recovery from myocardial infarction. Circulation. 1987;76:44–51. doi: 10.1161/01.cir.76.1.44. [DOI] [PubMed] [Google Scholar]
- Kramer DG, Trikalinos TA, Kent DM, Antonopoulos GV, Konstam MA, Udelson JE. Quantitative evaluation of drug or device effects on ventricular remodeling as predictors of therapeutic effects on mortality in patients with heart failure and reduced ejection fraction: a meta-analytic approach. J Am Coll Cardiol. 2010;56:392–406. doi: 10.1016/j.jacc.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MMY, Brooksbank KJM, Wetherall K, Mangion K, Roditi G, Campbell RT, Berry C, Chong V, Coyle L, Docherty KF, Dreisbach JG, Labinjoh C, Lang NN, Lennie V, McConnachie A, Murphy CL, Petrie CJ, Petrie JR, Speirits IA, Sourbron S, Welsh P, Woodward R, Radjenovic A, Mark PB, McMurray JJV, Jhund PS, Petrie MC, Sattar N. Effect of Empagliflozin on Left Ventricular Volumes in Patients With Type 2 Diabetes, or Prediabetes, and Heart Failure With Reduced Ejection Fraction (SUGAR-DM-HF). Circulation. 2021;143:516–25. doi: 10.1161/CIRCULATIONAHA.120.052186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhaert D, Grimm RA, Puntawangkoon C, Wolski K, De S, Wilkoff BL, Starling RC, Tang WH, Thomas JD, Popović ZB. Long-term reverse remodeling with cardiac resynchronization therapy: results of extended echocardiographic follow-up. J Am Coll Cardiol. 2010;55:1788–95. doi: 10.1016/j.jacc.2010.01.022. [DOI] [PubMed] [Google Scholar]
- McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- Januzzi JL, Prescott MF, Butler J, Felker GM, Maisel AS, McCague K, Camacho A, Piña IL, Rocha RA, Shah AM, Williamson KM, Solomon SD PROVE-HF Investigators. Association of Change in N-Terminal Pro-B-Type Natriuretic Peptide Following Initiation of Sacubitril-Valsartan Treatment With Cardiac Structure and Function in Patients With Heart Failure With Reduced Ejection Fraction. JAMA. 2019;322:1085–95. doi: 10.1001/jama.2019.12821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, Jamal W, Kimura K, Schnee J, Zeller C, Cotton D, Bocchi E, Böhm M, Choi DJ, Chopra V, Chuquiure E, Giannetti N, Janssens S, Zhang J, Gonzalez Juanatey, Kaul S, Brunner-La Rocca, Merkely B, Nicholls SJ, Perrone S, Pina I, Ponikowski P, Sattar N, Senni M, Seronde MF, Spinar J, Squire I, Taddei S, Wanner C, Zannad F EMPEROR-Reduced Trial Investigators. Cardiovascular and Renal Outcomes with Empagliflozin in Heart Failure. N Engl J Med. 2020;383:1413–24. doi: 10.1056/NEJMoa2022190. [DOI] [PubMed] [Google Scholar]
- Solomon SD, Foster E, Bourgoun M, Shah A, Viloria E, Brown MW, Hall WJ, Pfeffer MA, Moss AJ MADIT-CRT Investigators. Effect of cardiac resynchronization therapy on reverse remodeling and relation to outcome: multicenter automatic defibrillator implantation trial: cardiac resynchronization therapy. Circulation. 2010;122:985–92. doi: 10.1161/CIRCULATIONAHA.110.955039. [DOI] [PubMed] [Google Scholar]
- Bogaert J, Bosmans H, Maes A, Suetens P, Marchal G, Rademakers FE. Remote myocardial dysfunction after acute anterior myocardial infarction: impact of left ventricular shape on regional function: a magnetic resonance myocardial tagging study. J Am Coll Cardiol. 2000;35:1525–34. doi: 10.1016/s0735-1097(00)00601-x. [DOI] [PubMed] [Google Scholar]
- Neizel M, Lossnitzer D, Korosoglou G, Schäufele T, Peykarjou H, Steen H, Ocklenburg C, Giannitsis E, Katus HA, Osman NF. Strain-encoded MRI for evaluation of left ventricular function and transmurality in acute myocardial infarction. Circ Cardiovasc Imaging. 2009;2:116–22. doi: 10.1161/CIRCIMAGING.108.789032. [DOI] [PubMed] [Google Scholar]
- Götte MJ, van Rossum, Twisk JWR, Kuijer JPA, Marcus JT, Visser CA. Quantification of regional contractile function after infarction: strain analysis superior to wall thickening analysis in discriminating infarct from remote myocardium. J Am Coll Cardiol. 2001;37:808–17. doi: 10.1016/s0735-1097(00)01186-4. [DOI] [PubMed] [Google Scholar]
- Castelvecchio S, Frigelli M, Sturla F, Milani V, Pappalardo OA, Citarella M, Menicanti L, Votta E. Elucidating the mechanisms underlying left ventricular function recovery in patients with ischemic heart failure undergoing surgical remodeling: A 3-dimensional ultrasound analysis. J Thorac Cardiovasc Surg. 2021;S0022-5223:00381. doi: 10.1016/j.jtcvs.2021.02.067. [DOI] [PubMed] [Google Scholar]
- Cooley DA, Collins HA, Morris GC, Chapman DW. Ventricular aneurysm after myocardial infarction; surgical excision with use of temporary cardiopulmonary bypass. J Am Med Assoc. 1958;167:557–60. doi: 10.1001/jama.1958.02990220027008. [DOI] [PubMed] [Google Scholar]
- Dor V, Saab M, Coste P, Kornaszewska M, Montiglio F. Left ventricular aneurysm: a new surgical approach. Thorac Cardiovasc Surg. 1989;37:11–9. doi: 10.1055/s-2007-1013899. [DOI] [PubMed] [Google Scholar]
- Jones RH, Velazquez EJ, Michler RE, Sopko G, Oh JK, O'Connor CM, Hill JA, Menicanti L, Sadowski Z, Desvigne-Nickens P, Rouleau JL, Lee KL STICH Hypothesis 2 Investigators. Coronary bypass surgery with or without surgical ventricular reconstruction. N Engl J Med. 2009;360:1705–17. doi: 10.1056/NEJMoa0900559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michler RE, Rouleau JL, Al-Khalidi HR, Bonow RO, Pellikka PA, Pohost GM, Holly TA, Oh JK, Dagenais F, Milano C, Wrobel K, Pirk J, Ali IS, Jones RH, Velazquez EJ, Lee KL, Di Donato STICH Trial Investigators. Insights from the STICH trial: change in left ventricular size after coronary artery bypass grafting with and without surgical ventricular reconstruction. J Thorac Cardiovasc Surg. 2013;146:1139–45. doi: 10.1016/j.jtcvs.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athanasuleas CL, Buckberg GD, Stanley AW, Siler W, Dor V, Di Donato, Menicanti L, Almeida de, Beyersdorf F, Kron IL, Suma H, Kouchoukos NT, Moore W, McCarthy PM, Oz MC, Fontan F, Scott ML, Accola KA RESTORE group. Surgical ventricular restoration in the treatment of congestive heart failure due to post-infarction ventricular dilation. J Am Coll Cardiol. 2004;44:1439–45. doi: 10.1016/j.jacc.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Di Donato, Toso A, Maioli M, Sabatier M, Stanley AW, Dor V RESTORE Group. Intermediate survival and predictors of death after surgical ventricular restoration. Semin Thorac Cardiovasc Surg. 2001;13:468–75. doi: 10.1053/stcs.2001.29972. [DOI] [PubMed] [Google Scholar]
- Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet JP, Falk V, Head SJ, Jüni P, Kastrati A, Koller A, Kristensen SD, Niebauer J, Richter DJ, Seferović PM, Sibbing D, Stefanini GG, Windecker S, Yadav R, Zembala MO. 2018 ESC/EACTS Guidelines on myocardial revascularization. EuroIntervention. 2019;14:1435–534. doi: 10.4244/EIJY19M01_01. [DOI] [PubMed] [Google Scholar]
- Castelvecchio S, Pappalardo OA, Menicanti L. Myocardial reconstruction in ischaemic cardiomyopathy. Eur J Cardiothorac Surg. 2019;55:i49–56. doi: 10.1093/ejcts/ezy367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann DL, Acker MA, Jessup M, Sabbah HN, Starling RC, Kubo SH Acorn Trial Principal Investigators and Study Coordinators. Clinical evaluation of the CorCap Cardiac Support Device in patients with dilated cardiomyopathy. Ann Thorac Surg. 2007;84:1226–35. doi: 10.1016/j.athoracsur.2007.03.095. [DOI] [PubMed] [Google Scholar]
- Starling RC, Jessup M, Oh JK, Sabbah HN, Acker MA, Mann DL, Kubo SH. Sustained benefits of the CorCap Cardiac Support Device on left ventricular remodeling: three year follow-up results from the Acorn clinical trial. Ann Thorac Surg. 2007;84:1236–42. doi: 10.1016/j.athoracsur.2007.03.096. [DOI] [PubMed] [Google Scholar]
- Costanzo MR, Ivanhoe RJ, Kao A, Anand IS, Bank A, Boehmer J, Demarco T, Hergert CM, Holcomb RG, Maybaum S, Sun B, Vassiliades TA, Rayburn BK, Abraham WT. Prospective evaluation of elastic restraint to lessen the effects of heart failure (PEERLESS-HF) trial. J Card Fail. 2012;18:446–58. doi: 10.1016/j.cardfail.2012.04.004. [DOI] [PubMed] [Google Scholar]
- Fukamachi K, McCarthy PM. Initial safety and feasibility clinical trial of the myosplint device. J Card Surg. 2005;20:S43–7. doi: 10.1111/j.1540-8191.2005.00157.x. [DOI] [PubMed] [Google Scholar]
- Grossi EA, Patel N, Woo YJ, Goldberg JD, Schwartz CF, Subramanian V, Feldman T, Bourge R, Baumgartner N, Genco C, Goldman S, Zenati M, Wolfe JA, Mishra YK, Trehan N, Mittal S, Shang S, Mortier TJ, Schweich CJ RESTOR-MV Study Group. Outcomes of the RESTOR-MV Trial (Randomized Evaluation of a Surgical Treatment for Off-Pump Repair of the Mitral Valve). J Am Coll Cardiol. 2010;56:1984–93. doi: 10.1016/j.jacc.2010.06.051. [DOI] [PubMed] [Google Scholar]
- Costa MA, Mazzaferri EL, Sievert H, Abraham WT. Percutaneous ventricular restoration using the parachute device in patients with ischemic heart failure: three-year outcomes of the PARACHUTE first-in-human study. Circ Heart Fail. 2014;7:752–8. doi: 10.1161/CIRCHEARTFAILURE.114.001127. [DOI] [PubMed] [Google Scholar]
- Costa MA, Pencina M, Nikolic S, Engels T, Templin B, Abraham WT. The PARACHUTE IV trial design and rationale: percutaneous ventricular restoration using the parachute device in patients with ischemic heart failure and dilated left ventricles. Am Heart J. 2013;165:531–6. doi: 10.1016/j.ahj.2012.12.022. [DOI] [PubMed] [Google Scholar]
- Klein P, Anker SD, Wechsler A, Skalsky I, Neuzil P, Annest LS, Bifi M, McDonagh T, Frerker C, Schmidt T, Sievert H, Demaria AN, Kelle S. Less invasive ventricular reconstruction for ischaemic heart failure. Eur J Heart Fail. 2019;21:1638–50. doi: 10.1002/ejhf.1669. [DOI] [PubMed] [Google Scholar]
- Naar J, Skalský I, Krűger A, Málek F, Van Bladel, Annest LS, Moučka P, Mráz T, Reddy VY, Neužil P. Long-Term Results of Hybrid Left Ventricular Reconstruction in the Treatment of Ischemic Cardiomyopathy. J Cardiovasc Transl Res. 2021;14:1043–50. doi: 10.1007/s12265-021-10133-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann DL, Lee RJ, Coats AJ, Neagoe G, Dragomir D, Pusineri E, Piredda M, Bettari L, Kirwan BA, Dowling R, Volterrani M, Solomon SD, Sabbah HN, Hinson A, Anker SD. One-year follow-up results from AUGMENT-HF: a multicentre randomized controlled clinical trial of the efficacy of left ventricular augmentation with Algisyl in the treatment of heart failure. Eur J Heart Fail. 2016;18:314–25. doi: 10.1002/ejhf.449. [DOI] [PubMed] [Google Scholar]
- Nappi F, Lusini M, Spadaccio C, Nenna A, Covino E, Acar C, Chello M. Papillary Muscle Approximation Versus Restrictive Annuloplasty Alone for Severe Ischemic Mitral Regurgitation. J Am Coll Cardiol. 2016;67:34–2346. doi: 10.1016/j.jacc.2016.03.478. [DOI] [PubMed] [Google Scholar]
- Brener M, Uriel N, Burkhoff D. Left Ventricular Volume Reduction and Reshaping as a Treatment Option for Heart Failure. Structural Heart. 2020;4:264–83. [Google Scholar]
- Arnold SV, Stone GW, Jain SS, Mack MJ, Saxon JT, Zhang Z, Lindenfeld J, Abraham WT, Cohen DJ COAPT Investigators. Prognostic Importance of Health Status Versus Functional Status in Heart Failure and Secondary Mitral Regurgitation. JACC Heart Fail. 2021;9:684–92. doi: 10.1016/j.jchf.2021.04.012. [DOI] [PubMed] [Google Scholar]
- Arnold SV, Stone GW, Mack MJ, Chhatriwalla AK, Austin BA, Zhang Z, Ben-Yehuda O, Kar S, Lim DS, Lindenfeld J, Abraham WT, Cohen DJ COAPT Investigators. Health Status Changes and Outcomes in Patients With Heart Failure and Mitral Regurgitation: COAPT Trial. J Am Coll Cardiol. 2020;75:2099–106. doi: 10.1016/j.jacc.2020.03.002. [DOI] [PubMed] [Google Scholar]
- Baron SJ, Arnold SV, Wang K, Magnuson EA, Chinnakondepali K, Makkar R, Herrmann HC, Kodali S, Thourani VH, Kapadia S, Svensson L, Brown DL, Mack MJ, Smith CR, Leon MB, Cohen DJ PARTNER 2 Investigators. Health Status Benefits of Transcatheter vs Surgical Aortic Valve Replacement in Patients With Severe Aortic Stenosis at Intermediate Surgical Risk: Results From the PARTNER 2 Randomized Clinical Trial. JAMA Cardiol. 2017;2:837–45. doi: 10.1001/jamacardio.2017.2039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Artrip JH, Oz MC, Burkhoff D. Left ventricular volume reduction surgery for heart failure: a physiologic perspective. J Thorac Cardiovasc Surg. 2001;122:775–82. doi: 10.1067/mtc.2001.116208. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The CORCINCH-HF and ALIVE clinical trials.
Surgical ventricular restoration procedures and relevant studies.
The Parachute left ventricular partitioning device.