Abstract
Objective
To investigate the intake of specific types of beverages in relation to mortality and cardiovascular disease (CVD) outcomes among adults with type 2 diabetes.
Design
Prospective cohort study.
Setting
Health professionals in the United States.
Participants
15 486 men and women with a diagnosis of type 2 diabetes at baseline and during follow-up (Nurses’ Health Study: 1980-2018; and Health Professionals Follow-Up Study: 1986-2018). Beverage consumption was assessed using a validated food frequency questionnaire and updated every two to four years.
Main outcome measures
The main outcome was all cause mortality. Secondary outcomes were CVD incidence and mortality.
Results
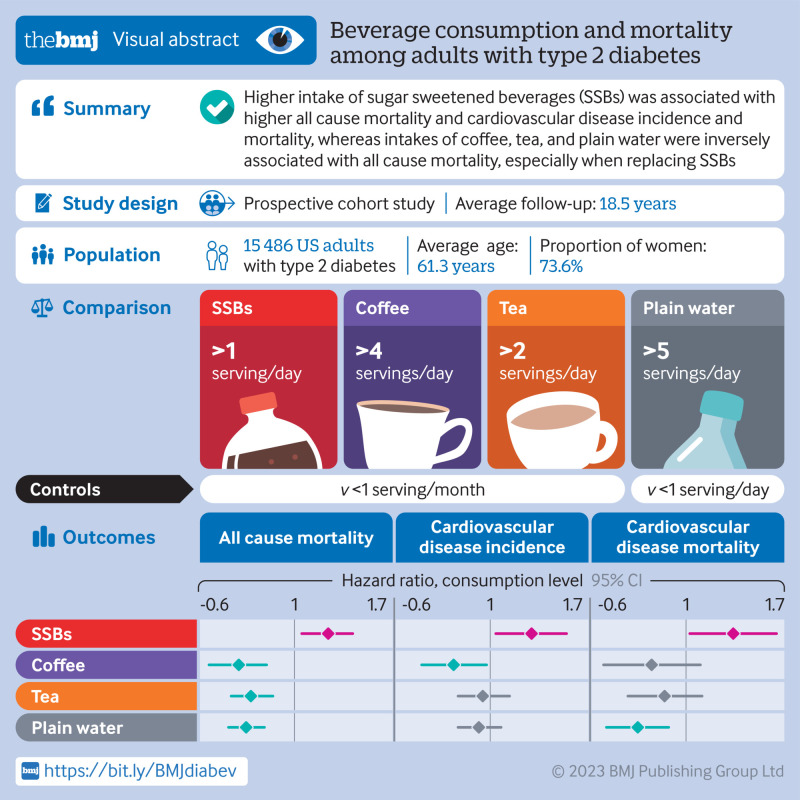
During an average of 18.5 years of follow-up, 3447 (22.3%) participants with incident CVD and 7638 (49.3%) deaths were documented. After multivariable adjustment, when comparing the categories of lowest intake of beverages with the highest intake, the pooled hazard ratios for all cause mortality were 1.20 (95% confidence interval 1.04 to 1.37) for sugar sweetened beverages (SSBs), 0.96 (0.86 to 1.07) for artificially sweetened beverages (ASBs), 0.98 (0.90 to 1.06) for fruit juice, 0.74 (0.63 to 0.86) for coffee, 0.79 (0.71 to 0.89) for tea, 0.77 (0.70 to 0.85) for plain water, 0.88 (0.80 to 0.96) for low fat milk, and 1.20 (0.99 to 1.44) for full fat milk. Similar associations were observed between the individual beverages and CVD incidence and mortality. In particular, SSB intake was associated with a higher risk of incident CVD (hazard ratio 1.25, 95% confidence interval 1.03 to 1.51) and CVD mortality (1.29, 1.02 to 1.63), whereas significant inverse associations were observed between intake of coffee and low fat milk and CVD incidence. Additionally, compared with those who did not change their consumption of coffee in the period after a diabetes diagnosis, a lower all cause mortality was observed in those who increased their consumption of coffee. A similar pattern of association with all cause mortality was also observed for tea, and low fat milk. Replacing SSBs with ABSs was significantly associated with lower all cause mortality and CVD mortality, and replacing SSBs, ASBs, fruit juice, or full fat milk with coffee, tea, or plain water was consistently associated with lower all cause mortality.
Conclusions
Individual beverages showed divergent associations with all cause mortality and CVD outcomes among adults with type 2 diabetes. Higher intake of SSBs was associated with higher all cause mortality and CVD incidence and mortality, whereas intakes of coffee, tea, plain water, and low fat milk were inversely associated with all cause mortality. These findings emphasize the potential role of healthy choices of beverages in managing the risk of CVD and premature death overall in adults with type 2 diabetes.
Introduction
In 2021 about 537 million adults worldwide had diabetes and this number is projected to rise to 783 million by 2045.1 The risk of cardiovascular disease (CVD), other morbidities, and premature death is particularly increased in adults with type 2 diabetes.2 Dietary interventions play a fundamental role in the glycemic management of adults with type 2 diabetes, although the prevailing dietary recommendations and nutritional guidelines for the general population may not necessarily be directly relevant to adults with diabetes because of their altered metabolism of carbohydrates and other macronutrients.3 4 It is therefore important to evaluate various dietary intakes, such as beverages, in relation to disease outcomes and mortality among adults with diabetes.
That different types of beverages may have distinct health effects depending on the contents of sugar and other constituents has been well documented.5 6 Several meta-analyses of prospective cohort studies have shown that high intake of beverages with a low energy density, such as plain water, low fat milk, and coffee was clearly associated with a lower incidence of obesity, type 2 diabetes, CVD, and all cause and cause specific mortality, primarily in the general population.7 8 9 In contrast with these findings, intake of sugar sweetened beverages (SSBs) was associated with a higher risk of type 2 diabetes and CVD.10 11 As a potential alternative to SSBs, artificially sweetened beverages (ASBs) contain little to no sugar or calories. The American Heart Association and American Diabetes Association have recommended that non-nutritive sweeteners could replace added sugar within a balanced diet to maintain a healthy weight and minimize cardiometabolic risk.12 To date, the association of individual beverage consumption with risk of CVD and mortality among adults with type 2 diabetes remains largely unexplored. Nonetheless, several clinical trials have shown some favorable effects of intakes of ASBs, coffee, or tea on body weight, lipid profile, and cardiometabolic risk factors among adults with type 2 diabetes.13 14 15
To fill this knowledge gap, we prospectively investigated individual beverage consumption after a diagnosis of type 2 diabetes mellitus, as well as changes in individual beverage consumption before and after the diagnosis, in relation to subsequent risk of CVD and mortality among adults with type 2 diabetes participating in the Nurses’ Health Study and Health Professionals Follow-Up Study in the United States. We also estimated associations between substituting one beverage for another and CVD risk and mortality.
Methods
Study population
The Nurses’ Health Study, a prospective cohort study initiated in 1976, enrolled 121 700 female registered nurses aged 30 to 55 years.16 The Health Professionals Follow-Up Study cohort was established in 1986 and enrolled 51 529 male health professionals aged 40 to 75 years.17 In both studies, detailed information on dietary and lifestyle factors, medical history, and disease status was collected at baseline and updated every two to four years through validated questionnaires.18 The cumulative response rate exceeded 90% for both cohorts. In the current analysis, we included participants with prevalent type 2 diabetes at baseline (1980 for the Nurses’ Health Study, and 1986 for the Health Professionals Follow-Up Study, when dietary information was first collected using a validated food frequency questionnaire), as well as participants with a diagnosis of incident type 2 diabetes during follow-up to 2018. We excluded participants if they had type 1 diabetes, CVD, or cancer at baseline; reported CVD or cancer before the diagnosis of type 2 diabetes during follow-up; left more than nine blank responses on the food frequency questionnaire or reported implausible daily energy intakes (<2510 or >14 644 kJ/day for women, and <3347 or >17 573 kJ/day for men); or had incomplete information on beverage consumption or dietary data at diabetes diagnosis. After exclusions, a total of 11 399 participants of the Nurses’ Health Study and 4087 participants of the Health Professionals Follow-Up Study with type 2 diabetes were included in the current analysis. For the analysis of changes in beverage consumption from before to after the diabetes diagnosis, we further excluded participants with type 2 diabetes at baseline or those with missing data on beverage consumption assessed before the diabetes diagnosis (n=2715), which left 9252 women and 3519 men for the change analysis.
Assessment of beverage intake
Intake of beverages was assessed using the validated food frequency questionnaires administered every two to four years. Participants were asked how often, on average (never to >6 times per day), they had consumed SSBs, ASBs, fruit juice, coffee, tea, low fat milk (skimmed, 1% or 2% fat), full fat milk, or plain water of a prespecified portion size (cup, glass, can, or bottle). SSBs included caffeinated colas, caffeine-free colas, other carbonated SSBs, and non-carbonated SSBs (fruit punches, lemonades, or other fruit drinks). ASBs included low calorie cola with caffeine, low calorie caffeine-free cola, and other low calorie beverages. Fruit juices included orange, apple, grapefruit, or other fruit juices. Coffee included caffeinated and decaffeinated varieties. A validation study conducted among a subsample of the participants in the Health Professionals Follow-Up Study showed reasonable validity for the assessment of beverage intake.19 The correlation coefficients between the food frequency questionnaire and multiple diet records were 0.84 for colas, 0.73 for low calorie colas, 0.75-0.89 for fruit juices, 0.93 for coffee, 0.77 for tea, 0.88 for low fat milk, 0.67 for full fat milk, and 0.52 for plain water.19 Similar correlation coefficients were found in a validation study conducted among a subsample of participants in the Nurses’ Health Study.20 Our primary dietary factors of interest were specific types of beverage consumption assessed after the diabetes diagnosis, and changes in beverage consumption before and after the diagnosis. We assessed the pre-diabetes beverage intake from the most proximal questionnaires before diabetes was ascertained.
Ascertainment of type 2 diabetes
Participants who reported a physician diagnosis of diabetes mellitus in the biennial questionnaires were sent a validated supplementary questionnaire about diagnostic tests, symptoms, and hypoglycemic treatment. The National Diabetes Data Group and American Diabetes Association criteria were applied to ascertain a diagnosis of type 2 diabetes (see supplementary appendix).21 22 We excluded from the current analysis those participants who reported a diagnosis of type 1 diabetes on the supplementary questionnaire. Studies among 62 participants in the Nurses’ Health Study and 59 participants in the Health Professionals Follow-Up Study showed high validity in the supplementary questionnaire, with 98% and 97% of questionnaire confirmed type 2 diabetes diagnoses validated by medical record review in these women and men, respectively.23 24
Ascertainment of outcomes
The primary endpoint was all cause mortality. We also examined the secondary outcomes of CVD incidence and mortality. Deaths were identified from reports by the next of kin or postal authorities or from searches of the National Death Index (see supplementary appendix).25 ICD-9 (international classification of diseases, ninth revision) codes were used to classify deaths from CVD (codes 390-459), cancer (codes 140-208.32), or other causes. Incident CVD was defined as fatal and non-fatal coronary heart disease, including coronary artery bypass graft surgery and non-fatal myocardial infarction, and as fatal and non-fatal stroke (see supplementary appendix).
Assessment of covariates
In both cohorts, information on lifestyle factors and medical history was collected at baseline and in biennial questionnaires. The supplementary appendix provides details of the assessments of covariates. To assess overall diet quality, we calculated the Alternate Healthy Eating Index (AHEI) score based on intakes of 11 foods and nutrients predictive of chronic disease risk, including vegetables, fruits, whole grains, SSBs and fruit juice, nuts and legumes, red and processed meat, trans fatty acids, long chain omega 3 fatty acids, other polyunsaturated fats, sodium, and alcohol.26 In the current study, we modified the AHEI score by excluding the consumption of SSBs and fruit juices.
Statistical analysis
The Kolmogorov-Smirnov normality test was used to assess distributions of continuous variables for normality, and natural logarithm transformations of skewed variables were applied before analyses. In descriptive analyses, continuous variables were expressed as means (standard deviations) for normally distributed variables or medians (interquartile ranges) for skewed variables, and categorical variables were represented by frequency and percentage. General linear models were used to calculate mean characteristics of the study participants at the time of diabetes diagnosis, and a test for linear trend using the Wald test was performed by assigning the median value to each category of beverage consumption and modeling this variable as a continuous variable.
For each participant, we calculated person years of follow-up from the date of diabetes diagnosis to the date of occurrence of study outcomes, last return of a valid follow-up questionnaire, or end of follow-up (30 June 2018 for the Nurses’ Health Study, and 30 January 2018 for the Health Professionals Follow-Up Study), whichever came first. Because changes in diet after a diagnosis of cancer could distort the associations of interest, for the CVD incidence analyses we stopped updating dietary variables after participants reported a diagnosis of cancer. For mortality analyses, dietary intake was not updated after a diagnosis of cancer or CVD. Time varying Cox proportional hazards models, conditioned on age and follow-up cycle, were applied to estimate hazard ratios and 95% confidence intervals for the associations of each beverage intake with all cause mortality, CVD incidence, and CVD mortality. Changes in beverage intake from before to after the diabetes diagnosis were defined as the absolute difference in beverage consumption (time varying post-diabetes beverage intake minus pre-diabetes beverage intake). The time varying covariates assessed during follow-up were considered in the multivariable models. Missing data for beverage consumptions and covariates during follow-up were replaced by the most recent valid assessments. In the multivariable model, we adjusted for age (years), duration of diabetes (years), sex (men or women), white ethnicity (yes or no), physical activity (<3.0, 3.0-8.9, 9.0-17.9, 18.0-26.9, ≥27.0 metabolic equivalents of task-hours/week), smoking status (never, former, current 1-14 cigarettes/day, current ≥15 cigarettes/day), alcohol consumption (0, 0.1-4.9, 5.0-14.9, ≥15.0 g/day), menopausal status and post-menopausal hormone use (pre-menopause, post-menopause (never, former, or current hormone use), or missing; Nurses’ Health Study only), family history of type 2 diabetes (yes or no) or myocardial infarction (yes or no), intake of total energy, and modified AHEI score (all in fourths). To further reduce the impact of confounding by existing comorbidities, disease management, and weight change, we further included history of hypertension (yes or no) or hypercholesterolemia (yes or no), use of antihypertensive (yes or no) or lipid lowering drug (yes or no), aspirin use (yes or no), diabetes drug use (oral drug only, insulin use, or others), and change in body mass index (BMI) before to after the diabetes diagnosis in the fully adjusted model.27 We mutually adjusted for different types of beverage intakes in the analysis of specific types of beverages. To obtain overall estimates for men and women and to increase statistical power, we pooled the hazard ratios from each model from the two cohorts with the use of an inverse variance weighted meta-analysis by the random effects model, which accounted for between study heterogeneity.28 CVD incidence and mortality were also examined according to the per serving intake of beverages. In the analysis of changes in beverage consumption from before to after the diabetes diagnosis, we further adjusted for beverage intake before the diagnosis in the multivariable model.
In the current study, we tested the proportional hazards assumption by using a likelihood ratio test comparing models with and without multiplicative interaction terms between beverage consumptions and calendar year, and we did not find evidence of violation of the assumption. Tests for trend were performed by assigning a median value to each beverage consumption category as a continuous variable. To examine the dose-response relationships between beverage intake and the outcomes, we used restricted cubic spline regression with three knots. Tests for non-linearity were based on the likelihood ratio test comparing two models: one with only the linear term and the other with the linear and the cubic spline terms.
We estimated the association of substituting a serving of one beverage for another by including both as continuous variables in the same multivariable model. Differences in their β coefficients were used to calculate the hazard ratios for the substitution effects, and their variances and covariance matrix were used to derive the 95% confidence intervals for the point estimate.
Several sensitivity analyses were conducted to test the robustness of our findings. First, we restricted our analyses to adults with incident type 2 diabetes by excluding those with prevalent diabetes at baseline. Second, we excluded deaths that occurred within four years after the diabetes diagnosis to examine whether the results were impacted by reverse causation bias. Third, a four year and eight year lag were placed between the assessment of beverage intake and outcome incidence, respectively. In these analyses, beverage intake was used to predict disease occurring four years or eight years later. Fourth, given that weight change can be an intermediate outcome, in our final model we adjusted for BMI before the diabetes diagnosis, instead of change in BMI before to after the diagnosis to examine the robustness of our observed associations. Fifth, we examined potential confounding from measures of socioeconomic status by adding partner’s education and self-rated socioeconomic status to the final model. Sixth, we used beverage intake assessed before the diabetes diagnosis instead of the cumulative average after diagnosis to evaluate whether changes in consumption pattern immediately after the diagnosis might impact the associations of interest. Seventh, as it is likely that participants might quit drinking unhealthy drinks immediately after the diabetes diagnosis, we skipped the first food frequency questionnaire after the diagnosis and used the rest to calculate cumulative averages and re-examine the associations. Eighth, we also conducted a sensitivity analysis excluding current and former smokers to further reduce confounding by smoking status. Ninth, we performed an analysis restricted to adults with asymptomatic type 2 diabetes to assess the impact of diabetes screening on associations of interest. Tenth, we controlled for the number of diabetes related symptoms as a measure of disease severity. Lastly, to reduce potential confounding by glucose control, we further adjusted for the self-reported levels of glycated hemoglobin HbA1c in a subset of the participants (n=5192).
All statistical analyses were performed with SAS software, version 9.4 (SAS Institute, Cary, NC). Two sided P<0.05 was considered statistically significant.
Patient and public involvement
Participants were not involved in the design, development of outcome measures, or other aspects of the conduct of the study. Participants are given updates on main findings of the cohort studies through newsletters and the study websites (https://www.nurseshealthstudy.org and https://www.hsph.harvard.edu/hpfs/).
Results
Table 1 shows the baseline characteristics of the study participants at diagnosis of type 2 diabetes and according to consumption of different beverages. Participants with higher intakes of SSBs were younger and more likely to have a higher consumption of total energy than participants with lower intakes. Those who consumed more ASBs also had a higher BMI at diabetes diagnosis (P for trend <0.001). Greater coffee consumption was positively associated with smoking. Participants who consumed a higher volume of low fat milk and plain water were more likely to use aspirin, antihypertensives, and lipid lowering drugs. Individual beverages were weakly correlated with each other; the highest Spearman correlation coefficients were 0.25 between low fat milk and plain water and 0.19 between low fat milk and fruit juice (see supplementary figure 1).
Table 1.
Baseline characteristics of adults with type 2 diabetes according to consumption of different beverages.* Values are numbers (percentages) unless stated otherwise
| Characteristics | SSBs | ASBs | Fruit juice | Coffee | Plain water | Low fat milk | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| <1 serving/month | >1 serving/day | <1 serving/month | >2 servings/day | <1 serving/month | >1 serving/day | <1 serving/month | >4 servings/day | <1 serving/day | >5 servings/day | <1 serving/day | >1 serving/day | ||||||
| No of participants | 8142 | 1152 | 6918 | 1527 | 4545 | 2103 | 6618 | 1086 | 4891 | 2182 | 5225 | 2131 | |||||
| Mean (SD) age† (years) | 61.6 (9.8) | 58.2 (10.2) | 62.5 (9.9) | 56.7 (9.4) | 61.9 (9.3) | 62.9 (9.4) | 61.6 (9.8) | 57.1 (9.9) | 60.7 (10.1) | 61.7 (9.0) | 60.7 (10.1) | 61.7 (9.0) | |||||
| Men | 2005 (24.6) | 298 (25.9) | 1985 (28.7) | 326 (21.4) | 1202 (26.5) | 734 (34.9) | 1964 (26.7) | 255 (23.5) | 1511 (309) | 385 (17.6) | 1544 (29.6) | 522 (24.5) | |||||
| White ethnicity | 7752 (95.2) | 1077 (93.5) | 6492 (93.8) | 1480 (96.9) | 4279 (94.2) | 2011 (95.6) | 6222 (94.0) | 1068 (98.3) | 4567 (93.4) | 2077 (95.2) | 4842 (92.7) | 2072 (97.2) | |||||
| Mean (SD) BMI | 30.2 (6.0) | 30.8 (6.0) | 29.7 (5.9) | 32.2 (6.1) | 30.6 (6.0) | 29.9 (5.6) | 30.4 (6.1) | 29.4 (5.7) | 30.2 (5.8) | 31.1 (6.1) | 30.2 (5.8) | 31.1 (6.1) | |||||
| Mean (SD) MET-hours/week | 17.4 (26.1) | 16.3 (30.2) | 17.2 (27.6) | 16 (25.7) | 16.8 (25.2) | 19.4 (29.8) | 17.5 (28.2) | 18.5 (29.8) | 17.4 (29.1) | 18.0 (24.6) | 17.4 (29.1) | 18.0 (24.6) | |||||
| Current smoker | 1084 (13.3) | 180 (15.6) | 983 (14.2) | 234 (15.3) | 603 (13.3) | 200 (9.5) | 674 (10.2) | 383 (35.3) | 728 (14.9) | 215 (9.9) | 867 (16.6) | 238 (11.2) | |||||
| Former smoker | 3536 (43.4) | 425 (36.9) | 2734 (39.5) | 659 (43.2) | 1923 (42.3) | 865 (41.1) | 2537 (38.3) | 376 (34.6) | 1914 (39.1) | 944 (43.3) | 1992 (38.1) | 832 (39.0) | |||||
| Postmenopausal‡ | 5094 (83.0) | 626 (73.7) | 4176 (84.7) | 857 (71.4) | 2882 (86.2) | 1156 (84.4) | 4022 (82.9) | 582 (70.0) | 2687 (79.5) | 1533 (85.3) | 2898 (78.7) | 1315 (81.7) | |||||
| Ever menopausal hormone use‡ | 2803 (45.7) | 355 (41.6) | 2213 (44.9) | 490 (40.8) | 1519 (45.4) | 657 (48.0) | 2235 (46.0) | 276 (33.2) | 1287 (38.1) | 938 (52.2) | 1325 (36.0) | 797 (49.5) | |||||
| Hypertension | 5123 (62.9) | 683 (59.2) | 4263 (61.6) | 945 (61.9) | 2857 (62.9) | 1389 (66.1) | 4226 (63.9) | 494 (45.5) | 2919 (59.7) | 1469 (67.3) | 3077 (58.9) | 1365 (64.1) | |||||
| Hypercholesterolemia | 4167 (51.2) | 547 (47.5) | 3525 (51.0) | 715 (46.8) | 2452 (54.0) | 1088 (51.7) | 3452 (52.2) | 381 (35.1) | 2378 (48.6) | 1182 (54.2) | 2349 (45.0) | 1072 (50.3) | |||||
| Family history of MI | 1791 (22.0) | 630 (21.2) | 1455 (21.0) | 346 (22.7) | 1003 (22.1) | 499 (23.7) | 1422 (21.5) | 233 (21.5) | 1081 (22.1) | 450 (20.6) | 1131 (21.7) | 444 (20.8) | |||||
| Use of antihypertensive | 2730 (33.5) | 395 (34.3) | 2270 (32.8) | 500 (32.7) | 1442 (31.7) | 897 (42.7) | 2238 (33.8) | 220 (20.3) | 1286 (26.3) | 950 (43.5) | 1296 (24.8) | 891 (41.8) | |||||
| Use of lipid lowering drug | 1473 (18.1) | 195 (16.9) | 1158 (16.7) | 223 (14.6) | 801 (17.6) | 401 (19.1) | 1111 (16.8) | 124 (11.4) | 670 (13.7) | 454 (20.8) | 611 (11.7) | 395 (18.5) | |||||
| Aspirin use | 4064 (49.9) | 618 (53.7) | 3364 (48.6) | 779 (51.0) | 2079 (45.7) | 1190 (56.6) | 3152 (47.6) | 576 (53.0) | 2311 (47.3) | 1195 (54.8) | 2431 (46.5) | 1183 (55.5) | |||||
| Mean (SD) total energy intake (kcal/day) | 1685.6 (557.9) | 2246.7 (640.9) | 1815.7 (607.9) | 1853.1 (603.7) | 1711.3 (591.4) | 2049.2 (596.8) | 1764.8 (590.8) | 1901.9 (627.9) | 1760.9 (603.3) | 1844.3 (610.9) | 1760.9 (603.3) | 1844.3 (610.9) | |||||
| Alcohol consumption (g/day) | 0.9 (0-5.8) | 0.8 (0-3.4) | 0.9 (0-5.8) | 0.8 (0-3.4) | 0.9 (0-4.9) | 1.2 (0-6.0) | 0.0 (0-3.1) | 1.0 (0-5.6) | 1.0 (0-5.6) | 0.0 (0-3.5) | 1.0 (0-6.0) | 0.0 (0-3.5) | |||||
| Mean (SD) modified AHEI score | 49.9 (10.8) | 44.2 (9.7) | 48.0 (10.7) | 47.3 (10.3) | 48.8 (11.0) | 49.5 (10.3) | 48.7 (10.6) | 46.8 (10.7) | 47.7 (11.0) | 47.9 (10.0) | 47.7 (11.0) | 47.9 (10.0) | |||||
AHEI=Alternative Healthy Eating Index; ASBs=artificially sweetened beverages; BMI=body mass index; MET=metabolic equivalent of task; MI=myocardial infarction; SSBs=sugar sweetened beverages.
Values standardized to age distribution of the study population.
Values were not age adjusted.
Women only.
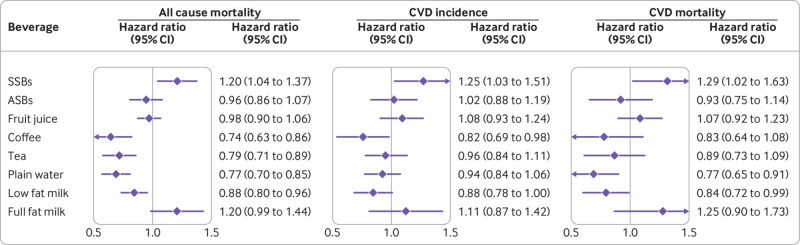
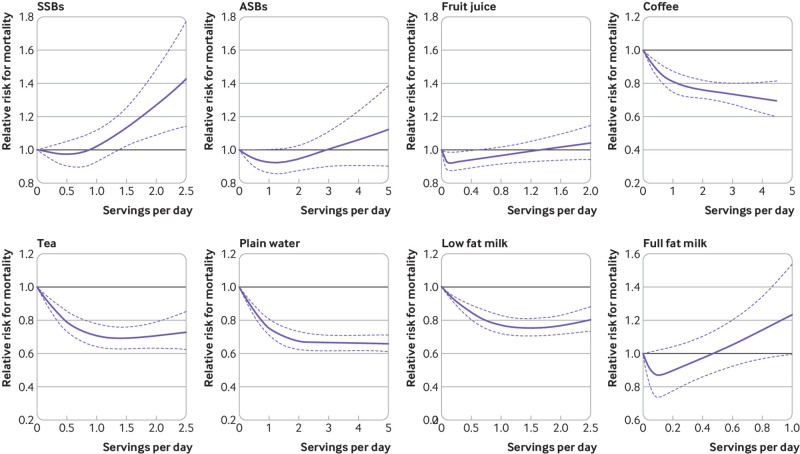
We documented 7638 (49.3%) deaths during 285 967 person years of follow-up. The pooled hazard ratio for all cause mortality in participants with the lowest intake of SSB compared with highest intake of SSB was 1.20 (95% confidence interval 1.04 to 1.37) (fig 1 and table 2). In contrast, high intakes of certain beverages were associated with lower mortality: 0.74 (0.63 to 0.86) for coffee, 0.79 (0.71 to 0.89) for tea, 0.77 (0.70 to 0.85) for plain water, and 0.88 (0.80 to 0.96) for low fat milk. We did not observe clear association patterns for other beverages, such as ASBs, fruit juice, or full fat milk. The associations of intake of specific types of beverages with CVD incidence and mortality were also evaluated separately, and the pattern of associations was largely similar to those for all cause mortality (fig 1 and supplementary tables 1 and 2). Results from the multivariable adjusted restricted cubic spline regression showed that the risk of all cause mortality decreased non-linearly with increasing intake of coffee, tea, plain water, and low fat milk (all P for non-linearity <0.001), whereas the association between SSBs and all cause mortality was more linear (fig 2). Each one serving/day increment in SSBs was associated with 8% (95% confidence interval 2% to 14%) higher all cause mortality.
Fig 1.
Hazard ratios (95% CIs) of all cause mortality, CVD incidence, and CVD mortality according to consumption of specific types of beverages among adults with type 2 diabetes. Hazard ratios for CVD incidence comparing extreme categories of consumption of specific types of beverages (SSB: <1 serving/month v >1 serving/day; ASB: <1 serving/month v >2 servings/day; fruit juice: <1 serving/month v >1 serving/day; coffee: <1 serving/month v >4 servings/day; tea: <1 serving/month v >2 servings/day; plain water: <1 serving/day v >5 servings/day; low fat milk: <1 serving/month v >2 servings/day; full fat milk: <1 serving/month v >1 serving/day) among individuals with type 2 diabetes were adjusted for age (continuous), duration of diabetes mellitus (years), sex (men or women), white ethnicity (yes or no), physical activity (<3.0, 3.0-8.9, 9.0-17.9, 18.0-26.9, ≥27.0 metabolic equivalents of task-hours/week), smoking status (never, former, current 1-14 cigarettes/day, current ≥15 cigarettes/day), alcohol consumption (0, 0.1-4.9, 5.0-14.9, ≥15.0 g/day), menopausal status and post-menopausal hormone use (pre-menopause, post-menopause (never, former, or current hormone use), or missing; Nurses’ Health Study only), family history of type 2 diabetes (yes or no) or myocardial infarction (yes or no), intake of total energy (continuous), the modified Alternative Healthy Eating Index score (fourths), history of hypertension (yes or no) or hypercholesterolemia (yes or no), use of antihypertensive (yes or no) or lipid lowering drug (yes or no), aspirin use (yes or no), diabetes drug use (oral drug only, insulin use, or others), and change in body mass index before to after diabetes diagnosis. Individual beverage consumption was mutually adjusted. ASB=artificially sweetened beverage; CI=confidence interval; CVD=cardiovascular disease; SSB=sugar-sweetened beverage
Table 2.
Hazard ratios for all cause mortality according to consumption of specific types of beverages among adults with type 2 diabetes
| Hazard ratio (95% CI) by consumption level | Every 1 serving/day | P value for trend | |||||
|---|---|---|---|---|---|---|---|
| SSBs | <1 serving/month | <1 serving/week | 1-3 servings/week | 4-7 servings/week | >1 serving/day | ||
| No of participants/person years | 5669/200 809 | 942/40 299 | 485/21 511 | 304/13 109 | 238/10 239 | ||
| Age adjusted model | 1 | 0.84 (0.79 to 0.90) | 0.85 (0.77 to 0.93) | 0.93 (0.83 to 1.05) | 1.11 (0.97 to 1.26) | 1.03 (0.98 to 1.09) | 0.65 |
| Multivariable model* | 1 | 0.96 (0.89 to 1.03) | 0.99 (0.90 to 1.09) | 1.01 (0.90 to 1.14) | 1.19 (1.04 to 1.36) | 1.07 (1.02 to 1.13) | 0.02 |
| Fully adjusted model† | 1 | 0.97 (0.91 to 1.05) | 1.01 (0.92 to 1.11) | 1.02 (0.91 to 1.15) | 1.20 (1.04 to 1.37) | 1.08 (1.02 to 1.14) | 0.01 |
| ASBs | <1 serving/month | <3 servings/week | 3-6 servings/week | 1-2 servings/day | >2 servings/day | ||
| No of participants/person years | 3868/117 911 | 1216/49 777 | 1269/54 548 | 698/34 472 | 587/29 260 | ||
| Age adjusted model | 1 | 0.74 (0.70 to 0.79) | 0.83 (0.77 to 0.88) | 0.89 (0.82 to 0.96) | 1.16 (1.06 to 1.26) | 1.05 (1.02 to 1.08) | 0.002 |
| Multivariable model* | 1 | 0.78 (0.72 to 0.83) | 0.82 (0.76 to 0.89) | 0.80 (0.73 to 0.89) | 0.91 (0.81 to 1.01) | 0.98 (0.94 to 1.01) | 0.04 |
| Fully adjusted model† | 1 | 0.83 (0.77 to 0.89) | 0.88 (0.81 to 0.94) | 0.85 (0.77 to 0.94) | 0.96 (0.86 to 1.07) | 0.98 (0.94 to 1.01) | 0.34 |
| Fruit juice | <1 serving/month | <1 serving/week | 1-3 servings/week | 4-7 servings/week | >1 serving/day | ||
| No of participants/person years | 3284/103 402 | 944/42 993 | 928/40 754 | 1321/54 625 | 1161/44 193 | ||
| Age adjusted model | 1 | 0.80 (0.75 to 0.86) | 0.86 (0.80 to 0.93) | 0.83 (0.78 to 0.89) | 0.93 (0.86 to 1.01) | 0.99 (0.96 to 1.03) | <0.001 |
| Multivariable model* | 1 | 0.84 (0.78 to 0.90) | 0.90 (0.83 to 0.97) | 0.86 (0.80 to 0.92) | 0.96 (0.88 to 1.04) | 1.00 (0.96 to 1.04) | 0.03 |
| Fully adjusted model† | 1 | 0.87 (0.81 to 0.93) | 0.92 (0.86 to 1.00) | 0.89 (0.83 to 0.95) | 0.98 (0.90 to 1.06) | 1.01 (0.97 to 1.05) | 0.12 |
| Coffee | <1 serving/month | <1 serving/day | 1-2 servings/day | 3-4 servings/day | >4 servings/day | ||
| No of participants/person years | 4897/138 144 | 833/39 261 | 872/41 830 | 852/53 379 | 184/13 354 | ||
| Age adjusted model | 1 | 0.61 (0.57 to 0.66) | 0.57 (0.53 to 0.62) | 0.51 (0.48 to 0.55) | 0.60 (0.52 to 0.70) | 0.81 (0.79 to 0.83) | <0.001 |
| Multivariable model* | 1 | 0.81 (0.75 to 0.87) | 0.79 (0.73 to 0.85) | 0.69 (0.64 to 0.74) | 0.71 (0.61 to 0.82) | 0.90 (0.87 to 0.92) | <0.001 |
| Fully adjusted model† | 1 | 0.84 (0.78 to 0.91) | 0.83 (0.77 to 0.90) | 0.72 (0.67 to 0.78) | 0.74 (0.63 to 0.86) | 0.91 (0.89 to 0.93) | <0.001 |
| Tea | <1 serving/month | <3 servings/week | 3-6 servings/week | 1-2 servings/day | >2 servings/day | ||
| No of participants/person years | 5473/158 479 | 871/51 426 | 615/33 659 | 365/21 457 | 314/20 947 | ||
| Age adjusted model | 1 | 0.84 (0.78 to 0.91) | 0.83 (0.77 to 0.90) | 0.72 (0.67 to 0.78) | 0.74 (0.63 to 0.86) | 0.90 (0.88 to 0.93) | <0.001 |
| Multivariable model* | 1 | 0.57 (0.53 to 0.61) | 0.58 (0.53 to 0.63) | 0.61 (0.55 to 0.68) | 0.60 (0.53 to 0.67) | 0.78 (0.74 to 0.82) | <0.001 |
| Fully adjusted model† | 1 | 0.79 (0.74 to 0.86) | 0.84 (0.77 to 0.92) | 0.83 (0.74 to 0.92) | 0.79 (0.71 to 0.89) | 0.94 (0.90 to 0.97) | <0.001 |
| Plain water | <1 serving/day | 1 serving/day | 2-3 servings/day | 3-5 servings/day | >5 serving/day | ||
| No of participants/person years | 4246/103 523 | 486/24 751 | 1374/67 553 | 960/54 188 | 572/35 953 | ||
| Age adjusted model | 1 | 0.58 (0.53 to 0.64) | 0.51 (0.48 to 0.54) | 0.45 (0.42 to 0.48) | 0.48 (0.44 to 0.52) | 0.84 (0.83 to 0.85) | <0.001 |
| Multivariable model* | 1 | 0.79 (0.72 to 0.87) | 0.75 (0.70 to 0.80) | 0.69 (0.64 to 0.75) | 0.74 (0.68 to 0.82) | 0.93 (0.92 to 0.95) | <0.001 |
| Fully adjusted model† | 1 | 0.84 (0.76 to 0.92) | 0.79 (0.74 to 0.85) | 0.73 (0.68 to 0.79) | 0.77 (0.70 to 0.85) | 0.94 (0.93 to 0.96) | <0.001 |
| Low fat milk | <1 serving/month | <3 servings/week | 3-6 servings/week | 1-2 servings/day | >2 servings/day | ||
| No of participants/person years | 4138/103 028 | 616/32 359 | 1177/61 638 | 1050/53 761 | 657/35 183 | ||
| Age adjusted model | 1 | 0.53 (0.48 to 0.57) | 0.50 (0.47 to 0.53) | 0.51 (0.48 to 0.55) | 0.52 (0.47 to 0.56) | 0.79 (0.76 to 0.81) | <0.001 |
| Multivariable model* | 1 | 0.81 (0.74 to 0.89) | 0.80 (0.74 to 0.86) | 0.83 (0.77 to 0.89) | 0.83 (0.75 to 0.91) | 0.96 (0.93 to 0.99) | <0.001 |
| Fully adjusted model† | 1 | 0.86 (0.79 to 0.94) | 0.85 (0.79 to 0.92) | 0.87 (0.81 to 0.94) | 0.88 (0.80 to 0.96) | 0.97 (0.94 to 1.00) | 0.02 |
| Full fat milk | <1 serving/month | <1 serving/week | 1-3 servings/week | 4-7 servings/week | >1 serving/day | ||
| No of participants/person years | 7178/26 0187 | 111/8917 | 84/4682 | 147/6827 | 118/5353 | ||
| Age adjusted model | 1 | 0.70 (0.58 to 0.84) | 0.88 (0.71 to 1.10) | 1.03 (0.87 to 1.21) | 1.17 (0.97 to 1.40) | 1.10 (1.00 to 1.20) | 0.19 |
| Multivariable model* | 1 | 0.81 (0.67 to 0.98) | 0.98 (0.79 to 1.22) | 1.19 (1.01 to 1.41) | 1.20 (1.00 to 1.45) | 1.11 (1.02 to 1.21) | 0.01 |
| Fully adjusted model† | 1 | 0.81 (0.67 to 0.98) | 1.01 (0.81 to 1.26) | 1.18 (1.00 to 1.40) | 1.20 (0.99 to 1.44) | 1.12 (1.02 to 1.22) | 0.02 |
ASBs=artificially sweetened beverages; CI=confidence interval; SSBs=sugar sweetened beverages.
Adjusted for age (continuous), duration of diabetes mellitus (years), sex (men or women), white ethnicity (yes or no), physical activity (<3.0, 3.0-8.9, 9.0-17.9, 18.0-26.9, ≥27.0 metabolic equivalents of task-hours/week), smoking status (never, former, current 1-14 cigarettes/day, current ≥15 cigarettes/day), alcohol consumption (0, 0.1-4.9, 5.0-14.9, ≥15.0 g/day), menopausal status and post-menopausal hormone use (pre-menopause, post-menopause (never, former, or current hormone use), or missing; Nurses’ Health Study only), family history of type 2 diabetes (yes or no) or myocardial infarction (yes or no), intake of total energy (continuous), and the modified Alternative Healthy Eating Index score (fourths). Individual beverage consumption was mutually adjusted.
Further adjusted for history of hypertension (yes or no) or hypercholesterolemia (yes, no), use of antihypertensive (yes or no) or lipid lowering drug (yes or no), aspirin use (yes or no), diabetes drug use (oral drug only, insulin use, or others), and change in body mass index before to after diabetes diagnosis.
Fig 2.
Restricted cubic spline analysis of association between individual beverage consumption and all cause mortality among adults with type 2 diabetes. Adjusted for age (continuous), duration of diabetes (years), sex (men or women), white ethnicity (yes or no), physical activity (<3.0, 3.0-8.9, 9.0-17.9, 18.0-26.9, ≥27.0 metabolic equivalents of task-hours/week), smoking status (never, former, current 1-14 cigarettes/day, current ≥15 cigarettes/day), alcohol consumption (0, 0.1-4.9, 5.0-14.9, ≥15.0 g/day), menopausal status and post-menopausal hormone use (pre-menopause, post-menopause (never, former, or current hormone use), or missing; Nurses’ Health Study only), family history of type 2 diabetes (yes or no) or myocardial infarction (yes or no), intake of total energy (continuous), the modified Alternative Healthy Eating Index score (fourths), history of hypertension (yes or no) or hypercholesterolemia (yes or no), use of antihypertensive (yes or no) or lipid lowering drug (yes or no), aspirin use (yes or no), diabetes drug use (oral drug only, insulin use, or others), and change in body mass index before to after diabetes diagnosis. Individual beverage consumption was mutually adjusted. ASB=artificially sweetened beverage; CI=confidence interval; CVD=cardiovascular disease; HR=hazard ratio; SSB=sugar sweetened beverage
A total of 3447 (22.3%) adults with incident CVD were documented during 248 447 person years of follow-up. In the fully adjusted model, higher intake of SSBs was significantly associated with a higher risk of CVD; multivariable hazard ratio of CVD comparing the highest intake with lowest intake was 1.25 (95% confidence interval 1.03 to 1.51) (fig 1 and table 3). Conversely, increased consumption of coffee and low fat milk was inversely associated with CVD incidence: hazard ratio 0.82 (0.69 to 0.98) for coffee and 0.88 (0.78 to 1.00) for low fat milk.
Table 3.
Hazard ratios for incident cardiovascular disease according to consumption of specific types of beverages among adults with type 2 diabetes
| Hazard ratio (95% CI) by consumption level | Every 1 serving/day | P value for trend | ||||||
|---|---|---|---|---|---|---|---|---|
| SSBs | <1 serving/month | <1 serving/week | 1-3 servings/week | 4-7 servings/week | >1 serving/day | |||
| No of participants/person years | 2430/174 971 | 498/34 626 | 265/18 619 | 132/11 357 | 122/8874 | |||
| Age adjusted model | 1 | 0.98 (0.89 to 1.08) | 1.01 (0.89 to 1.14) | 0.82 (0.69 to 0.98) | 1.08 (0.90 to 1.30) | 1.01 (0.94 to 1.09) | 0.88 | |
| Multivariable model* | 1 | 1.06 (0.96 to 1.17) | 1.13 (0.99 to 1.29) | 0.91 (0.76 to 1.09) | 1.24 (1.03 to 1.51) | 1.08 (1.00 to 1.16) | 0.10 | |
| Fully adjusted model† | 1 | 1.06 (0.96 to 1.17) | 1.13 (0.99 to 1.28) | 0.90 (0.75 to 1.08) | 1.25 (1.03 to 1.51) | 1.08 (1.00 to 1.16) | 0.10 | |
| ASBs | <1 serving/month | <3 servings/week | 3-6 servings/week | 1-2 servings/day | >2 servings/day | |||
| No of participants/person years | 1372/103 280 | 621/43 031 | 657/47 303 | 421/29 713 | 376/25 121 | |||
| Age adjusted model | 1 | 1.05 (0.95 to 1.15) | 1.04 (0.94 to 1.14) | 1.13 (1.01 to 1.27) | 1.31 (1.16 to 1.47) | 1.08 (1.04 to 1.11) | <0.001 | |
| Multivariable model* | 1 | 1.04 (0.93 to 1.17) | 0.97 (0.87 to 1.09) | 1.06 (0.93 to 1.22) | 1.04 (0.90 to 1.21) | 1.01 (0.97 to 1.06) | 0.58 | |
| Fully adjusted model† | 1 | 1.03 (0.92 to 1.16) | 0.97 (0.86 to 1.09) | 1.05 (0.91 to 1.20) | 1.02 (0.88 to 1.19) | 1.01 (0.97 to 1.05) | 0.78 | |
| Fruit juice | <1 serving/month | <1 serving/week | 1-3 servings/week | 4-7 servings/week | >1 serving/day | |||
| No of participants/person years | 1178/90 915 | 510/37 294 | 473/35 285 | 708/46 918 | 578/38 035 | |||
| Age adjusted model | 1 | 1.00 (0.90 to 1.10) | 1.02 (0.92 to 1.14) | 1.01 (0.92 to 1.11) | 0.98 (0.87 to 1.10) | 1.01 (0.96 to 1.07) | 0.97 | |
| Multivariable model* | 1 | 1.06 (0.94 to 1.19) | 1.07 (0.94 to 1.22) | 1.03 (0.92 to 1.16) | 1.09 (0.94 to 1.25) | 1.02 (0.96 to 1.09) | 0.38 | |
| Fully adjusted model† | 1 | 1.05 (0.93 to 1.18) | 1.06 (0.93 to 1.20) | 1.02 (0.91 to 1.15) | 1.08 (0.93 to 1.24) | 1.02 (0.95 to 1.08) | 0.48 | |
| Coffee | <1 serving/month | <1 serving/day | 1-2 servings/day | 3-4 servings/day | >4 servings/day | |||
| No of participants/person years | 1749/117 872 | 438/33 919 | 507/36 224 | 605/48 083 | 148/12 350 | |||
| Age adjusted model | 1 | 0.87 (0.78 to 0.97) | 0.91 (0.82 to 1.00) | 0.87 (0.79 to 0.96) | 0.90 (0.76 to 1.06) | 0.97 (0.94 to 1.00) | 0.01 | |
| Multivariable model* | 1 | 0.91 (0.82 to 1.02) | 0.97 (0.87 to 1.07) | 0.89 (0.81 to 0.98) | 0.80 (0.67 to 0.96) | 0.96 (0.93 to 0.99) | 0.004 | |
| Fully adjusted model† | 1 | 0.90 (0.81 to 1.00) | 0.95 (0.86 to 1.05) | 0.89 (0.81 to 0.98) | 0.82 (0.69 to 0.98) | 0.96 (0.94 to 0.99) | 0.006 | |
| Tea | <1 serving/month | <3 servings/week | 3-6 servings/week | 1-2 servings/day | >2 servings/day | |||
| No of participants/person years | 1943/135 675 | 585/45 187 | 405/29 547 | 278/19 127 | 236/18 911 | |||
| Age adjusted model | 1 | 0.91 (0.83 to 1.00) | 0.98 (0.88 to 1.09) | 1.01 (0.89 to 1.15) | 0.93 (0.81 to 1.07) | 1.01 (0.97 to 1.05) | 0.58 | |
| Multivariable model* | 1 | 0.99 (0.90 to 1.09) | 1.08 (0.96 to 1.20) | 1.09 (0.96 to 1.24) | 0.96 (0.84 to 1.11) | 1.01 (0.97 to 1.05) | 0.99 | |
| Fully adjusted model† | 1 | 0.98 (0.89 to 1.08) | 1.06 (0.95 to 1.18) | 1.07 (0.94 to 1.22) | 0.96 (0.84 to 1.11) | 1.01 (0.97 to 1.05) | 0.95 | |
| Plain water | <1 serving/day | 1 serving/day | 2-3 servings/day | 4-5 servings/day | >5 servings/day | |||
| No of participants/person years | 1298/88 004 | 262/21 915 | 761/59 357 | 668/47 427 | 458/31 745 | |||
| Age adjusted model | 1 | 0.87 (0.76 to 1.00) | 0.86 (0.79 to 0.95) | 0.87 (0.79 to 0.96) | 0.87 (0.78 to 0.97) | 0.97 (0.96 to 0.99) | 0.002 | |
| Multivariable model* | 1 | 0.90 (0.78 to 1.03) | 0.93 (0.84 to 1.03) | 0.98 (0.89 to 1.09) | 0.98 (0.87 to 1.10) | 0.99 (0.98 to 1.01) | 0.94 | |
| Fully adjusted model† | 1 | 0.89 (0.77 to 1.02) | 0.91 (0.82 to 1.00) | 0.95 (0.86 to 1.05) | 0.94 (0.84 to 1.06) | 0.94 (0.90 to 0.98) | 0.45 | |
| Low fat milk | <1 serving/month | <3 servings/week | 3-6 servings/week | 1-2 servings/day | >2 servings/day | |||
| No of participants/person years | 1275/88 826 | 349/28 287 | 722/53 292 | 666/46 836 | 435/31 205 | |||
| Age adjusted model | 1 | 0.86 (0.76 to 0.97) | 0.88 (0.80 to 0.96) | 0.87 (0.79 to 0.96) | 0.82 (0.73 to 0.92) | 0.95 (0.92 to 0.99) | 0.003 | |
| Multivariable model* | 1 | 0.89 (0.79 to 1.01) | 0.96 (0.86 to 1.06) | 0.95 (0.85 to 1.05) | 0.89 (0.79 to 1.01) | 0.98 (0.94 to 1.02) | 0.18 | |
| Fully adjusted model† | 1 | 0.87 (0.76 to 0.99) | 0.93 (0.84 to 1.03) | 0.93 (0.83 to 1.03) | 0.88 (0.78 to 1.00) | 0.98 (0.94 to 1.02) | 0.16 | |
| Full fat milk | <1 serving/month | <1 serving/week | 1-3 servings/week | 4-7 servings/week | >1 serving/day | |||
| No of participants/person years | 3138/224 780 | 99/8231 | 51/4232 | 89/6291 | 70/4915 | |||
| Age adjusted model | 1 | 0.94 (0.77 to 1.15) | 0.97 (0.73 to 1.28) | 1.06 (0.86 to 1.32) | 1.13 (0.88 to 1.43) | 1.14 (1.02 to 1.28) | 0.30 | |
| Multivariable model* | 1 | 0.94 (0.77 to 1.15) | 0.96 (0.72 to 1.27) | 1.05 (0.85 to 1.31) | 1.09 (0.85 to 1.39) | 1.13 (1.00 to 1.27) | 0.45 | |
| Fully adjusted model† | 1 | 0.93 (0.76 to 1.14) | 0.95 (0.72 to 1.25) | 1.05 (0.85 to 1.31) | 1.11 (0.87 to 1.42) | 1.14 (1.01 to 1.28) | 0.37 | |
ASBs=artificially sweetened beverages; CI=confidence interval; SSBs=sugar sweetened beverages.
Analyses were adjusted for age (continuous), duration of diabetes mellitus (years), sex (men or women), white ethnicity (yes or no), physical activity (<3.0, 3.0-8.9, 9.0-17.9, 18.0-26.9, ≥27.0 metabolic equivalents of task-hours/week), smoking status (never, former, current 1-14 cigarettes/day, current ≥15 cigarettes/day), alcohol consumption (0, 0.1-4.9, 5.0-14.9, ≥15.0 g/day), menopausal status and post-menopausal hormone use (pre-menopause, post-menopause (never, former, or current hormone use), or missing; Nurses’ Health Study only), family history of type 2 diabetes (yes or no) or myocardial infarction (yes or no), intake of total energy (continuous), and the modified Alternative Healthy Eating Index score (fourths). Individual beverage consumption was mutually adjusted.
Fully adjusted model, further adjusted for history of hypertension (yes or no) or hypercholesterolemia (yes or no), use of antihypertensive (yes or no) or lipid lowering drug (yes or no), aspirin use (yes or no), diabetes drug use (oral drug only, insulin use, or others), and change in body mass index before to after diabetes diagnosis.
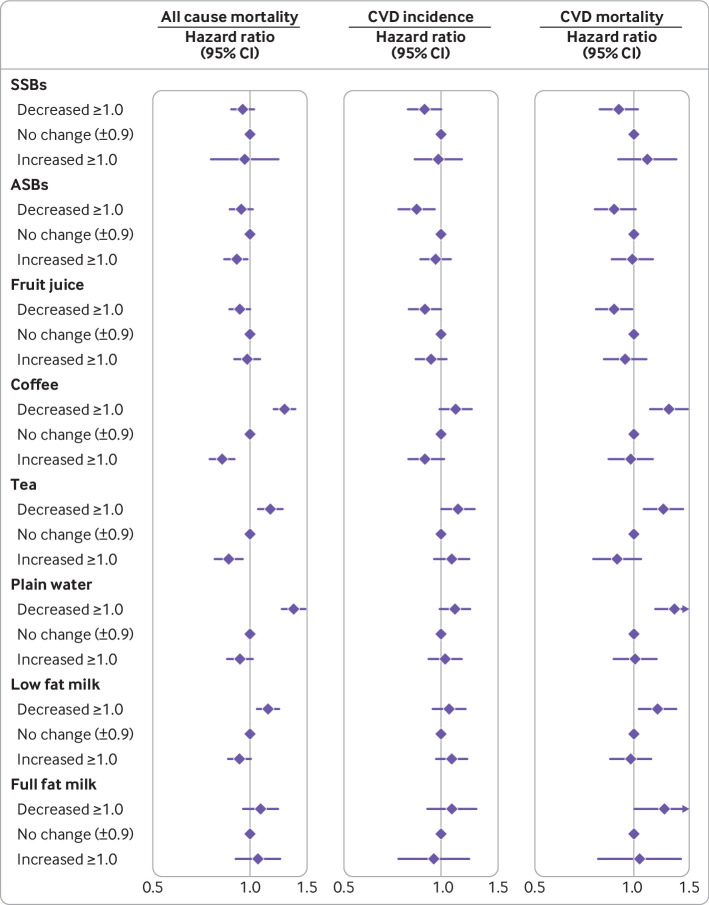
Increment in coffee consumption from before to after the diabetes diagnosis was also significantly associated with a lower risk of all cause mortality (fig 3 and supplementary table 3). Compared with participants whose coffee intake did not change, those who increased coffee consumption after a diabetes diagnosis had an associated 18% lower risk of all cause mortality. A similar pattern of associations in relation to all cause mortality was observed for tea and low fat milk. Associations between changes in intakes of these beverage and CVD incidence were similar but largely null owing to lower power.
Fig 3.
Hazard ratios (95% CIs) of all cause mortality and CVD incidence according to changes in consumption of specific types of beverages from before to after a diagnosis of type 2 diabetes. Hazard ratios were adjusted for age (continuous), duration of diabetes (years), sex (men or women), white (yes or no), physical activity (<3.0, 3.0-8.9, 9.0-17.9, 18.0-26.9, ≥27.0 metabolic equivalents of task-hours/week), smoking status (never, former, current 1-14 cigarettes/day, current ≥15 cigarettes/day), alcohol consumption (0, 0.1-4.9, 5.0-14.9, ≥15.0 g/day), menopausal status and post-menopausal hormone use (pre-menopause, post-menopause (never, former, or current hormone use), or missing; Nurses’ Health Study only), family history of type 2 diabetes (yes or no) or myocardial infarction (yes or no), intake of total energy (continuous), the modified Alternative Healthy Eating Index score (fourths), history of hypertension (yes or no) or hypercholesterolemia (yes or no), use of antihypertensive (yes or no) or lipid lowering drug (yes or no), aspirin use (yes or no), diabetes drug use (oral drug only, insulin use, or others), and change in body mass index before to after diabetes diagnosis. Individual beverage consumption was mutually adjusted. ASBs=artificially sweetened beverages; CI=confidence interval; CVD=cardiovascular disease; HR=hazard ratio; SSBs=sugar sweetened beverages
In estimating the associations of substituting beverages for each other, when replacing one serving/day of SSBs, one serving/day of coffee was associated with an 18% (95% confidence interval 12% to 23%) lower risk of all cause mortality, tea with a 16% (9% to 22%) lower risk, plain water with a 16% (10% to 21%) lower risk, and low fat milk with a 12% (5% to 17%) lower risk (table 4). The corresponding substitution estimates for CVD mortality were 20%, 24%, 20%, and 19%. Replacing one serving/day of SSBs with ASBs was also significantly associated with 8% (95% 1% to 14%) and 15% (4% to 24%) lower all cause mortality and CVD mortality, respectively. Replacing one serving/day of ASBs with coffee, tea, or plain water was also associated with lower all cause mortality.
Table 4.
Associations for replacing one serving/week of specific types of beverages on all cause mortality and cardiovascular disease incidence and mortality among adults with type 2 diabetes*
| Beverage and substitutions | All cause mortality | CVD incidence | CVD mortality | |||||
|---|---|---|---|---|---|---|---|---|
| Relative risk (95% CI) | P value | Relative risk (95% CI) | P value | Relative risk (95% CI) | P value | |||
| SSBs | ||||||||
| ASB | 0.92 (0.86 to 0.99) | 0.02 | 0.94 (0.86 to 1.04) | 0.23 | 0.85 (0.76 to 0.96) | 0.007 | ||
| Fruit juice | 0.97 (0.89 to 1.04) | 0.22 | 0.96 (0.85 to 1.07) | 0.46 | 0.92 (0.81 to 1.05) | 0.21 | ||
| Coffee | 0.82 (0.77 to 0.88) | <0.001 | 0.91 (0.83 to 1.00) | 0.05 | 0.80 (0.72 to 0.89) | <0.001 | ||
| Tea | 0.84 (0.78 to 0.91) | <0.001 | 0.93 (0.84 to 1.03) | 0.18 | 0.76 (0.66 to 0.86) | <0.001 | ||
| Plain water | 0.84 (0.79 to 0.90) | <0.001 | 0.94 (0.86 to 1.03) | 0.16 | 0.80 (0.72 to 0.89) | <0.001 | ||
| Low fat milk | 0.88 (0.83 to 0.95) | 0.001 | 0.94 (0.85 to 1.04) | 0.23 | 0.81 (0.72 to 0.91) | <0.001 | ||
| Full fat milk | 1.00 (0.89 to 1.13) | 0.97 | 0.99 (0.82 to 1.20) | 0.95 | 0.86 (0.69 to 1.07) | 0.17 | ||
| ASBs | ||||||||
| Fruit juice | 1.05 (0.99 to 1.11) | 0.11 | 1.01 (0.94 to 1.10) | 0.73 | 1.08 (0.98 to 1.18) | 0.13 | ||
| Coffee | 0.89 (0.85 to 0.93) | <0.001 | 0.96 (0.91 to 1.02) | 0.19 | 0.94 (0.87 to 1.01) | 0.08 | ||
| Tea | 0.91 (0.86 to 0.96) | <0.001 | 0.99 (0.93 to 1.06) | 0.73 | 0.88 (0.80 to 0.98) | 0.02 | ||
| Plain water | 0.91 (0.88 to 0.95) | <0.001 | 0.99 (0.95 to 1.04) | 0.74 | 0.93 (0.88 to 0.99) | 0.03 | ||
| Low fat milk | 0.96 (0.91 to 1.00) | 0.07 | 0.99 (0.93 to 1.06) | 0.87 | 0.95 (0.87 to 1.03) | 0.22 | ||
| Full fat milk | 1.08 (0.97 to 1.20) | 0.16 | 1.05 (0.89 to 1.24) | 0.56 | 1.00 (0.82 to 1.23) | 0.97 | ||
| Fruit juice | ||||||||
| ASB | 0.96 (0.90 to 1.01) | 0.11 | 0.99 (0.91 to 1.07) | 0.73 | 0.93 (0.84 to 1.02) | 0.13 | ||
| Coffee | 0.85 (0.81 to 0.90) | <0.001 | 0.95 (0.88 to 1.03) | 0.20 | 0.87 (0.79 to 0.95) | 0.002 | ||
| Tea | 0.87 (0.82 to 0.93) | <0.001 | 0.97 (0.89 to 1.06) | 0.55 | 0.82 (0.73 to 0.92) | <0.001 | ||
| Plain water | 0.87 (0.83 to 0.92) | <0.001 | 0.98 (0.91 to 1.05) | 0.55 | 0.87 (0.80 to 0.94) | <0.001 | ||
| Low fat milk | 0.91 (0.86 to 0.97) | 0.003 | 0.98 (0.90 to 1.07) | 0.67 | 0.93 (0.76 to 1.15) | 0.51 | ||
| Full fat milk | 1.03 (0.92 to 1.15) | 0.59 | 1.04 (0.87 to 1.24) | 0.70 | 0.93 (0.76 to 1.14) | 0.49 | ||
| Full fat milk | ||||||||
| Coffee | 0.82 (0.74 to 0.92) | <0.001 | 0.92 (0.77 to 1.09) | 0.31 | 0.88 (0.71 to 1.09) | 0.24 | ||
| Tea | 0.84 (0.76 to 0.94) | 0.003 | 0.94 (0.79 to 1.12) | 0.48 | 0.93 (0.76 to 1.13) | 0.48 | ||
| Plain water | 0.85 (0.76 to 0.94) | 0.002 | 0.94 (0.80 to 1.11) | 0.49 | 0.90 (0.72 to 1.13) | 0.38 | ||
| Low fat milk | 0.89 (0.80 to 0.99) | 0.02 | 0.95 (0.80 to 1.12) | 0.51 | 0.94 (0.77 to 1.15) | 0.57 | ||
ASBs=artificially sweetened beverages; CI=confidence interval; CVD=cardiovascular disease; SSBs=sugar sweetened beverages.
Hazard ratios were adjusted for age (continuous), duration of diabetes mellitus (years), sex (men or women), white ethnicity (yes or no), physical activity (<3.0, 3.0-8.9, 9.0-17.9, 18.0-26.9, ≥27.0 metabolic equivalents of task-hours/week), smoking status (never, former, current 1-14 cigarettes/day, current ≥15 cigarettes/day), alcohol consumption (0, 0.1-4.9, 5.0-14.9, ≥15.0 g/day), menopausal status and post-menopausal hormone use (pre-menopause, post-menopause (never, former, or current hormone use), or missing; Nurses’ Health Study only), family history of type 2 diabetes (yes or no) or myocardial infarction (yes or no), intake of total energy (continuous), the modified Alternative Healthy Eating Index score (fourths), history of hypertension (yes or no) or hypercholesterolemia (yes or no), use of antihypertensive (yes or no) or lipid lowering drugs (yes or no), aspirin use (yes or no), diabetes drug use (oral drug only, insulin use, or others), and change in body mass index before to after diabetes diagnosis. Individual beverage consumption was mutually adjusted.
In sensitivity analyses, the associations between beverage consumptions and CVD risk and mortality remained robust when we excluded participants with prevalent diabetes at baseline, when we excluded deaths that occurred within four years after a diabetes diagnosis, and when we further adjusted for intakes of major dietary variables or beverage consumptions with a four year lag or eight year lag (see supplementary table 4). In sensitivity analyses adjusting for BMI before diabetes diagnosis instead of the change in BMI before to after diabetes diagnosis, the associations were largely similar to the results from primary analyses (see supplementary table 4). Estimates of associations were similar when further adjusting for socioeconomic status (see supplementary table 4). The J-shaped dose-response relationships for SSBs, ASBs, fruit juice, and full fat milk were also largely robust when we used beverage intake before the diabetes diagnosis instead of the cumulative average (see supplementary figure 2), or when we left out the first food frequency questionnaire data after the diabetes diagnosis (see supplementary figure 3). The results remained virtually unchanged when we restricted our analyses to never smokers (see supplementary table 4). Restricting to participants with asymptomatic type 2 diabetes at the time of diagnosis did not materially alter the associations (see supplementary table 4). In addition, further adjustment for the number of diabetes related symptoms or for HbA1c levels did not appreciably change the results (see supplementary table 4).
Discussion
In these two prospective cohorts of men and women with type 2 diabetes in the United States, we found that higher SSB intake was associated with higher all cause mortality and CVD incidence, whereas intakes of coffee, low fat milk, and plain water were inversely associated with CVD incidence and mortality. In addition, greater increase in coffee consumption from before to after a diabetes diagnosis was significantly associated with lower mortality. Replacing SSBs with ASBs was significantly associated with lower all cause and CVD mortality. In addition, replacing SSBs or ASBs with coffee, tea, low fat milk, or plain water was associated with lower all cause mortality and CVD mortality.
Comparison with other studies and possible explanations
Abundant evidence links the intake of various beverages with cardiometabolic conditions and mortality in the general population. For example, meta-analyses of prospective cohort studies indicated that a higher consumption of SSBs was associated with weight gain, type 2 diabetes, and all cause mortality and CVD mortality.10 11 29 Several prospective cohort studies have suggested that ASB as a replacement for SSB consumption may increase the risk of obesity, metabolic diseases, and mortality in the general population.30 31 However, the results from meta-analysis of randomized controlled trials showed that ASB consumption did not appear to influence blood glucose levels.32 In contrast, consumption of coffee, tea, and plain water was strongly associated with a lower risk of CVD, type 2 diabetes, cancer, and mortality.9 33 34 35 In addition, evidence is accumulating that consumption of full fat milk is associated with a higher risk of all cause, CVD, and cancer mortality, whereas consumption of low fat milk was inversely associated with type 2 diabetes and risk of stroke.7 8,36 In the European Prospective Investigation into Cancer and Nutrition-InterAct study, replacing SSBs for coffee or tea by 250 g/day was associated with a 21% or 22% lower incidence of type 2 diabetes across eight European populations.37
In contrast with the evidence from general populations, evidence from prospective epidemiological studies specifically for adults with type 2 diabetes is largely lacking. In the current analyses, we found a pattern of associations between individual beverages and mortality that largely mirrors that observed in general populations. In particular, we found that higher SSB intake was associated with higher CVD incidence and mortality among individuals with type 2 diabetes, whereas intakes of coffee, low fat milk, or plain water were inversely associated with CVD incidence and mortality. Our observed associations were also consistent with findings in some controlled trials that examined beverages in relation to cardiometabolic risk factors among adults with diabetes or those at higher risk of developing diabetes. In overweight participants who received four cups of coffee daily, 24 week intervention led to a significant reduction in fat mass.38 An eight week supplementation of coffee extract significantly reduced fasting insulin level and the homeostasis model assessment of insulin resistance in adults with metabolic syndrome.39 Daily supplementary intake of tea extract powder lowered the HbA1c level and decreased serum C reactive protein levels in adults with diabetes.14 40 A 12 week randomized trial suggested that supplementary intake of green tea extract may improve arterial stiffness in patients with type 2 diabetes.15 Overall, the consistency of evidence regarding individual beverage intake between populations with and without diabetes suggests that the altered metabolism among adults with diabetes does not play a critical role in modifying the associations of beverages and health outcomes.
Several biological mechanisms may explain the putative distinct associations of specific types of beverages with all cause mortality and CVD incidence among adults with diabetes. The positive association between SSB intake and adverse health outcomes may relate to the high fructose content in liquid form.41 42 43 44 The added high fructose corn syrup and sucrose in SSBs may lead to weight gain, insulin resistance, and inflammation.42 A postprandial spike in blood glucose and insulin concentrations after SSB consumption may potentially lead to hyperinsulinemia, lipogenesis, and insulin resistance over time, which is particularly harmful for adults with diabetes.43 Also, calories in liquid from SSB consumption may decrease satiety and lead to incomplete compensatory reduction in energy intake, which consequently does not suppress consumption of solid foods.44 In addition to relatively higher contents of simple sugars in fruits juices, in comparison with whole fruits, fruit juice, on average, may have lower contents of some beneficial constituents, such as fiber, polyphenols, and other phytochemicals, which are partially lost during the juicing process.45 In contrast, coffee contains various beneficial bioactive constituents, such as chlorogenic acids, melanoidins, and trigonelline, which may reduce oxidative stress and inflammation.46 47 48 Regular consumption of coffee may significantly reduce systematic inflammation.47 48 In particular, chlorogenic acid has also been shown to delay intestinal glucose uptake and inhibit hepatic gluconeogenesis.49 50 51 Tea is also a good source of polyphenols, especially catechins, which bear antioxidant and anti-inflammatory properties.52 Catechins could also modulate several key genes in triacylglycerol biosynthesis,53 protect pancreatic β cells,54 and improve the cellular redox state.55 The divergent associations between intake of full fat and low fat milk are in line with the evidence base that collectively suggests detrimental effects of saturated fat intake on blood lipids, systemic inflammation, and insulin sensitivity.56
In restricted cubic spline analysis, we observed a slight J-shaped relationship between consumption of some specific beverages (SSBs, ASBs, fruit juice, and full fat milk) and overall mortality. It is possible that underreporting or recent quitting of these beverages may potentially account for these observations, although the sensitivity analyses in which we modeled consumption levels before diabetes diagnosis or left out the first food frequency questionnaire assessment since diagnosis yielded similar findings. It would seem that more studies are warranted to replicate and further explore these important associations.
Our results suggested that replacing SSBs with ASBs was associated with a significantly lower risk of CVD incidence and mortality in adults with diabetes, after controlling for several covariates, including weight change from before to after the diabetes diagnosis, which is a strong predictor of adverse outcomes in our previous study.27 The observations are in accordance with the recommendations of the American Heart Association and American Diabetes Association, which suggest replacement of SSBs with non-caloric sweetened beverages for weight and glycemic control.13 Compared with SSBs that are high in sugars, ASBs contain sweeteners that provide limited or no energy content. The evidence is largely inconsistent about the effects of non-nutritive sweeteners and ASBs on glucose metabolism, gut microbiota, and cardiometabolic risk.57 58 59 In contrast with findings of a positive association of ASB consumption with weight gain, metabolic diseases, and mortality in prospective cohort studies, evidence from randomized controlled trials did not find a substantial impact of ASB consumption compared with SSB consumption on increasing blood glucose levels and inducing changes to gut microbiota.31 60 61 In addition, evidence from trials in humans had suggested that using non-caloric sweetened beverages as an intended substitute for beverages that contain sugar could result in a modest improvement in body weight and cardiometabolic risk factors, especially among people with obesity.62 63 64 The reason for inconsistent findings between cohort studies and trials is unclear, although the differences in study duration between trials and observational studies and confounding by weight loss attempts in observational studies may partially explain the discrepancies.
Strengths and limitations of this study
The strengths of the current study include the large sample size, long duration of follow-up from two prospective cohort studies, high response rates during follow-up, and detailed and repeated assessments of dietary and lifestyle variables before and after a diabetes diagnosis.
Several potential limitations need to be considered as well. First, individual beverage consumption may be correlated with other dietary and lifestyle risk factors for CVD incidence and mortality among adults with diabetes. Given the observational nature of the study design, the possibility of residual confounding due to measurement errors of covariates (including severity of diabetes, glucose control, and dietary and lifestyle factors) and unmeasured confounding (including genetic susceptibility and psychosocial stress) cannot be completely ruled out, especially in this patient population—even though we controlled for a wide range of potential confounders. Therefore, our results should be interpreted with caution and warrant intervention studies to help establish causal relationships. Second, some measurement errors and misclassification are inevitable in estimates of food and nutrient intakes. The validation studies showed that our food frequency questionnaires have reasonable reproducibility and validity compared with diet record assessments.19 20 65 In particular, food frequency questionnaire assessments of beverage intake are, in general, strongly correlated with diet record assessments, probably because the consumption pattern is habitual. Use of repeated measurements of dietary intake to calculate cumulative averages is likely to help minimize random measurement errors and reflect long term dietary intakes. Third, self-reported diabetes diagnoses were ascertained using a validated supplementary questionnaire, although the possibility of some underdiagnosis of type 2 diabetes may exist. Previous studies have, however, clearly shown the validity of the supplementary questionnaire.23 24 Moreover, the incidence rate of diabetes in our study populations was also comparable with that in other cohort studies.66 67 68 Because of the study participants’ professions and ready access to healthcare, underreporting of diabetes was expected to be less than that in the general population. A validation study assessing the prevalence of undiagnosed diabetes among a random sample of participants without type 2 diabetes in the Nurses’ Health Study showed a low false negative rate (0.5%) in this cohort.69 In addition, results did not significantly change when the analysis was restricted to adults with asymptomatic type 2 diabetes. Fourth, diabetes in participants in our study was diagnosed during an extended period since the 1980s. The risk profile of adults with diabetes might significantly change over time owing to better control of hypertension, blood lipids, and other risk factors in recent years; although similar results were found in analyses stratified by follow-up time. Fifth, our study did not have direct measurements of glycemic control and severity of diabetes, although the results did not materially change after further adjustment for duration of diabetes, use of diabetes drugs, diabetes symptoms, or HbA1c levels. Lastly, the participants in our cohorts were predominantly white, US health professionals, which could potentially limit the generalizability of the findings to the general population. However, the range and frequency distribution of dietary macronutrient composition and diet quality for our cohort were similar to national estimates among US adults.70 71 Although it is unlikely that the biologic mechanisms underlying these associations would differ substantially among different populations, further studies are warranted to replicate our findings in other populations.
Conclusions
Findings from two large prospective cohort studies suggested that among adults with type 2 diabetes, coffee, plain water, and low fat milk are associated with lower risk of CVD or premature death, whereas the opposite association was found for excess intake of SSBs. Overall, these results provide additional evidence that emphasizes the importance of beverage choices in maintaining overall health among adults with diabetes.
What is already known on this topic
Depending on the content of sugar and other constituents, different types of beverages may have distinct health effects
The prevailing dietary recommendations are largely based on findings in the general US population
Evidence is limited among adults with type 2 diabetes, who have altered metabolism of energy and macronutrients
What this study adds
Among adults with type 2 diabetes, higher intake of sugar sweetened beverages (SSBs) was associated with higher all cause mortality and incidence of cardiovascular disease, whereas intakes of coffee, tea, plain water, or low fat milk were inversely associated with all cause mortality
Greater increase in coffee and tea consumption from before to after a diabetes diagnosis was significantly associated with lower all cause mortality
Replacing SSBs with coffee, tea, or plain water was statistically significantly associated with lower all cause mortality among adults with diabetes
Web extra.
Extra material supplied by authors
Supplementary information: Additional methods, figures 1-3, and tables 1-4
Contributors: QS and LM participated in project conception and development of research methods. QS, FBH, EBR, and JEM obtained funding and provided oversight. LM, YH, DA, GL, VM, and QS analyzed data and performed analysis. LM and QS drafted the manuscript. All authors provided final approval of the version to be published. QS is the guarantor. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: This study was sponsored by the National Institutes of Health (UM1 CA186107, U01 CA167552, R01 HL034594, R01 HL035464, DK126698, DK120870, and DK119268). The funders had no role in considering the study design or in the collection, analysis, interpretation of data, writing of the report, or decision to submit the article for publication.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/disclosure-of-interest/ and declare: support from the National Institutes of Health (NIH) for the submitted work; JEM has been supported by grants from NIH. FBH has been supported by grants HL60712, HL118264, and DK112940 from NIH and received research support from the California Walnut Commission, honorariums for lectures from Metagenics and Standard Process, and honorariums from Diet Quality Photo Navigation, outside the submitted work; VM has received research support from Canada Research Chairs Program, Connaught New Researcher Award, University of Toronto, and The Joannah and Brian Lawson Centre for Child Nutrition, University of Toronto, received consulting fees from the World Health Organization Nutrition Guidance Expert Advisory Group, and has held a leadership role in Canadian Institutes of Health Research Advisory Board, Institute of Nutrition, Metabolism and Diabetes. No other relationships or activities that could appear to have influenced the submitted work.
The lead author (QS) affirms that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Dissemination to participants and related patient and public communities: Findings from the current work will be disseminated through open access publication at The BMJ, our newsletters to study participants, the Health Professionals Follow-Up Study Twitter handle, and potential press releases.
Provenance and peer review: Not commissioned; externally peer reviewed.
Ethics statements
Ethical approval
The study protocol (P10628-104) was approved by the institutional review board of the Brigham and Women’s Hospital and Committee on the Use of Human Subjects review board of Harvard T.H. Chan School of Public Health, Boston, MA, UAS. The completion of the self-administered questionnaire was considered to imply informed consent.
Data availability statement
Data of the Nurses’ Health Study and Health Professionals Follow-Up Study can be shared through mechanisms detailed at https://www.nurseshealthstudy.org or https://www.hsph.harvard.edu/hpfs/.
References
- 1.International Diabetes Federation. IDF Diabetes Atlas 9th Edition. https://www.diabetesatlas.org/en/. Accessed Feb 10 2022.
- 2. Rawshani A, Rawshani A, Franzén S, et al. Mortality and Cardiovascular Disease in Type 1 and Type 2 Diabetes. N Engl J Med 2017;376:1407-18. 10.1056/NEJMoa1608664. [DOI] [PubMed] [Google Scholar]
- 3. Ley SH, Hamdy O, Mohan V, Hu FB. Prevention and management of type 2 diabetes: dietary components and nutritional strategies. Lancet 2014;383:1999-2007. 10.1016/S0140-6736(14)60613-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lichtenstein AH, Appel LJ, Vadiveloo M, et al. 2021 Dietary Guidance to Improve Cardiovascular Health: A Scientific Statement From the American Heart Association. Circulation 2021;144:e472-87. 10.1161/CIR.0000000000001031. [DOI] [PubMed] [Google Scholar]
- 5. Pallazola VA, Davis DM, Whelton SP, et al. A Clinician’s Guide to Healthy Eating for Cardiovascular Disease Prevention. Mayo Clin Proc Innov Qual Outcomes 2019;3:251-67. 10.1016/j.mayocpiqo.2019.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. de Oliveira Otto MC, Anderson CAM, Dearborn JL, et al. American Heart Association Behavioral Change for Improving Health Factors Committee of the Council on Lifestyle and Cardiometabolic Health and Council on Epidemiology and Prevention; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; and Stroke Council . Dietary Diversity: Implications for Obesity Prevention in Adult Populations: A Science Advisory From the American Heart Association. Circulation 2018;138:e160-8. 10.1161/CIR.0000000000000595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Naghshi S, Sadeghi O, Larijani B, Esmaillzadeh A. High vs. low-fat dairy and milk differently affects the risk of all-cause, CVD, and cancer death: A systematic review and dose-response meta-analysis of prospective cohort studies. Crit Rev Food Sci Nutr 2022;62:3598-612. 10.1080/10408398.2020.1867500. [DOI] [PubMed] [Google Scholar]
- 8. Gijsbers L, Ding EL, Malik VS, de Goede J, Geleijnse JM, Soedamah-Muthu SS. Consumption of dairy foods and diabetes incidence: a dose-response meta-analysis of observational studies. Am J Clin Nutr 2016;103:1111-24. 10.3945/ajcn.115.123216. [DOI] [PubMed] [Google Scholar]
- 9. Di Maso M, Boffetta P, Negri E, La Vecchia C, Bravi F. Caffeinated Coffee Consumption and Health Outcomes in the US Population: A Dose-Response Meta-Analysis and Estimation of Disease Cases and Deaths Avoided. Adv Nutr 2021;12:1160-76. 10.1093/advances/nmaa177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yin J, Zhu Y, Malik V, et al. Intake of Sugar-Sweetened and Low-Calorie Sweetened Beverages and Risk of Cardiovascular Disease: A Meta-Analysis and Systematic Review. Adv Nutr 2021;12:89-101. 10.1093/advances/nmaa084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pan B, Ge L, Lai H, et al. Association of soft drink and 100% fruit juice consumption with all-cause mortality, cardiovascular diseases mortality, and cancer mortality: A systematic review and dose-response meta-analysis of prospective cohort studies. Crit Rev Food Sci Nutr 2022;62:8908-19. 10.1080/10408398.2021.1937040. [DOI] [PubMed] [Google Scholar]
- 12. Gardner C, Wylie-Rosett J, Gidding SS, et al. American Heart Association Nutrition Committee of the Council on Nutrition, Physical Activity and Metabolism, Council on Arteriosclerosis, Thrombosis and Vascular Biology, Council on Cardiovascular Disease in the Young, and the American D . Nonnutritive sweeteners: current use and health perspectives: a scientific statement from the American Heart Association and the American Diabetes Association. Circulation 2012;126:509-19. 10.1161/CIR.0b013e31825c42ee. [DOI] [PubMed] [Google Scholar]
- 13. McGlynn ND, Khan TA, Wang L, et al. Association of Low- and No-Calorie Sweetened Beverages as a Replacement for Sugar-Sweetened Beverages With Body Weight and Cardiometabolic Risk: A Systematic Review and Meta-analysis. JAMA Netw Open 2022;5:e222092. 10.1001/jamanetworkopen.2022.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Neyestani TR, Shariatzade N, Kalayi A, et al. Regular daily intake of black tea improves oxidative stress biomarkers and decreases serum C-reactive protein levels in type 2 diabetic patients. Ann Nutr Metab 2010;57:40-9. 10.1159/000312666. [DOI] [PubMed] [Google Scholar]
- 15. Sirichaiwetchakoon K, Churproong S, Kupittayanant S, Eumkeb G. The Effect of Pluchea indica (L.) Less. Tea on Blood Glucose and Lipid Profile in People with Prediabetes: A Randomized Clinical Trial. J Altern Complement Med 2021;27:669-77. 10.1089/acm.2020.0246. [DOI] [PubMed] [Google Scholar]
- 16. Belanger CF, Hennekens CH, Rosner B, Speizer FE. The nurses’ health study. Am J Nurs 1978;78:1039-40. [PubMed] [Google Scholar]
- 17. Grobbee DE, Rimm EB, Giovannucci E, Colditz G, Stampfer M, Willett W. Coffee, caffeine, and cardiovascular disease in men. N Engl J Med 1990;323:1026-32. 10.1056/NEJM199010113231504. [DOI] [PubMed] [Google Scholar]
- 18. Hu FB, Manson JE, Stampfer MJ, et al. Diet, lifestyle, and the risk of type 2 diabetes mellitus in women. N Engl J Med 2001;345:790-7. 10.1056/NEJMoa010492. [DOI] [PubMed] [Google Scholar]
- 19. Feskanich D, Rimm EB, Giovannucci EL, et al. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc 1993;93:790-6. 10.1016/0002-8223(93)91754-E. [DOI] [PubMed] [Google Scholar]
- 20. Salvini S, Hunter DJ, Sampson L, et al. Food-based validation of a dietary questionnaire: the effects of week-to-week variation in food consumption. Int J Epidemiol 1989;18:858-67. 10.1093/ije/18.4.858. [DOI] [PubMed] [Google Scholar]
- 21. National Diabetes Data Group . Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes 1979;28:1039-57. 10.2337/diab.28.12.1039. [DOI] [PubMed] [Google Scholar]
- 22. Expert Committee on the Diagnosis and Classification of Diabetes Mellitus . Report of the expert committee on the diagnosis and classification of diabetes mellitus. Diabetes Care 2003;26(Suppl 1):S5-20. 10.2337/diacare.26.2007.S5. [DOI] [PubMed] [Google Scholar]
- 23. Manson JE, Rimm EB, Stampfer MJ, et al. Physical activity and incidence of non-insulin-dependent diabetes mellitus in women. Lancet 1991;338:774-8. 10.1016/0140-6736(91)90664-B. [DOI] [PubMed] [Google Scholar]
- 24. Hu FB, Leitzmann MF, Stampfer MJ, Colditz GA, Willett WC, Rimm EB. Physical activity and television watching in relation to risk for type 2 diabetes mellitus in men. Arch Intern Med 2001;161:1542-8. 10.1001/archinte.161.12.1542. [DOI] [PubMed] [Google Scholar]
- 25. Bao Y, Han J, Hu FB, et al. Association of nut consumption with total and cause-specific mortality. N Engl J Med 2013;369:2001-11. 10.1056/NEJMoa1307352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wang T, Heianza Y, Sun D, et al. Improving adherence to healthy dietary patterns, genetic risk, and long term weight gain: gene-diet interaction analysis in two prospective cohort studies. BMJ 2018;360:j5644. 10.1136/bmj.j5644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hu J, Hu Y, Hertzmark E, et al. Weight Change, Lifestyle, and Mortality in Patients With Type 2 Diabetes. J Clin Endocrinol Metab 2022;107:627-37. 10.1210/clinem/dgab800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 29. Kazemi A, Soltani S, Mokhtari Z, et al. The relationship between major food sources of fructose and cardiovascular disease, cancer, and all-cause mortality: a systematic review and dose-response meta-analysis of cohort studies. Crit Rev Food Sci Nutr 2021:1-14. 10.1080/10408398.2021.2000361. [DOI] [PubMed] [Google Scholar]
- 30. Azad MB, Sharma AK, de Souza RJ, et al. Canadian Healthy Infant Longitudinal Development Study Investigators . Association Between Artificially Sweetened Beverage Consumption During Pregnancy and Infant Body Mass Index. JAMA Pediatr 2016;170:662-70. 10.1001/jamapediatrics.2016.0301. [DOI] [PubMed] [Google Scholar]
- 31. Malik VS, Li Y, Pan A, et al. Long-Term Consumption of Sugar-Sweetened and Artificially Sweetened Beverages and Risk of Mortality in US Adults. Circulation 2019;139:2113-25. 10.1161/CIRCULATIONAHA.118.037401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nichol AD, Holle MJ, An R. Glycemic impact of non-nutritive sweeteners: a systematic review and meta-analysis of randomized controlled trials. Eur J Clin Nutr 2018;72:796-804. 10.1038/s41430-018-0170-6. [DOI] [PubMed] [Google Scholar]
- 33. Kim Y, Je Y, Giovannucci E. Coffee consumption and all-cause and cause-specific mortality: a meta-analysis by potential modifiers. Eur J Epidemiol 2019;34:731-52. 10.1007/s10654-019-00524-3. [DOI] [PubMed] [Google Scholar]
- 34. Zhang C, Qin YY, Wei X, Yu FF, Zhou YH, He J. Tea consumption and risk of cardiovascular outcomes and total mortality: a systematic review and meta-analysis of prospective observational studies. Eur J Epidemiol 2015;30:103-13. 10.1007/s10654-014-9960-x. [DOI] [PubMed] [Google Scholar]
- 35. Majdi M, Hosseini F, Naghshi S, Djafarian K, Shab-Bidar S. Total and drinking water intake and risk of all-cause and cardiovascular mortality: A systematic review and dose-response meta-analysis of prospective cohort studies. Int J Clin Pract 2021;75:e14878. 10.1111/ijcp.14878. [DOI] [PubMed] [Google Scholar]
- 36. Chen Z, Ahmed M, Ha V, et al. Dairy Product Consumption and Cardiovascular Health: a Systematic Review and Meta-Analysis of Prospective Cohort Studies. Adv Nutr 2021:13:439-54. 10.1093/advances/nmab118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Imamura F, Schulze MB, Sharp SJ, et al. Estimated Substitution of Tea or Coffee for Sugar-Sweetened Beverages Was Associated with Lower Type 2 Diabetes Incidence in Case-Cohort Analysis across 8 European Countries in the EPIC-InterAct Study. J Nutr 2019;149:1985-93. 10.1093/jn/nxz156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Alperet DJ, Rebello SA, Khoo EY, et al. The effect of coffee consumption on insulin sensitivity and other biological risk factors for type 2 diabetes: a randomized placebo-controlled trial. Am J Clin Nutr 2020;111:448-58. 10.1093/ajcn/nqz306. [DOI] [PubMed] [Google Scholar]
- 39. Roshan H, Nikpayam O, Sedaghat M, Sohrab G. Effects of green coffee extract supplementation on anthropometric indices, glycaemic control, blood pressure, lipid profile, insulin resistance and appetite in patients with the metabolic syndrome: a randomised clinical trial. Br J Nutr 2018;119:250-8. 10.1017/S0007114517003439. [DOI] [PubMed] [Google Scholar]
- 40. Fukino Y, Ikeda A, Maruyama K, Aoki N, Okubo T, Iso H. Randomized controlled trial for an effect of green tea-extract powder supplementation on glucose abnormalities. Eur J Clin Nutr 2008;62:953-60. 10.1038/sj.ejcn.1602806. [DOI] [PubMed] [Google Scholar]
- 41. Malik VS, Hu FB. The role of sugar-sweetened beverages in the global epidemics of obesity and chronic diseases. Nat Rev Endocrinol 2022;18:205-18. 10.1038/s41574-021-00627-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Cho YE, Kim DK, Seo W, Gao B, Yoo SH, Song BJ. Fructose Promotes Leaky Gut, Endotoxemia, and Liver Fibrosis Through Ethanol-Inducible Cytochrome P450-2E1-Mediated Oxidative and Nitrative Stress. Hepatology 2021;73:2180-95. 10.1002/hep.30652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Malik VS, Hu FB. Fructose and Cardiometabolic Health: What the Evidence From Sugar-Sweetened Beverages Tells Us. J Am Coll Cardiol 2015;66:1615-24. 10.1016/j.jacc.2015.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kuzma JN, Cromer G, Hagman DK, et al. No difference in ad libitum energy intake in healthy men and women consuming beverages sweetened with fructose, glucose, or high-fructose corn syrup: a randomized trial. Am J Clin Nutr 2015;102:1373-80. 10.3945/ajcn.115.116368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pepin A, Stanhope KL, Imbeault P. Are Fruit Juices Healthier Than Sugar-Sweetened Beverages? A Review. Nutrients 2019;11:1006. 10.3390/nu11051006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bakuradze T, Lang R, Hofmann T, et al. Consumption of a dark roast coffee decreases the level of spontaneous DNA strand breaks: a randomized controlled trial. Eur J Nutr 2015;54:149-56. 10.1007/s00394-014-0696-x. [DOI] [PubMed] [Google Scholar]
- 47. Martínez-López S, Sarriá B, Mateos R, Bravo-Clemente L. Moderate consumption of a soluble green/roasted coffee rich in caffeoylquinic acids reduces cardiovascular risk markers: results from a randomized, cross-over, controlled trial in healthy and hypercholesterolemic subjects. Eur J Nutr 2019;58:865-78. 10.1007/s00394-018-1726-x. [DOI] [PubMed] [Google Scholar]
- 48. Hang D, Kværner AS, Ma W, et al. Coffee consumption and plasma biomarkers of metabolic and inflammatory pathways in US health professionals. Am J Clin Nutr 2019;109:635-47. 10.1093/ajcn/nqy295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kajikawa M, Maruhashi T, Hidaka T, et al. Coffee with a high content of chlorogenic acids and low content of hydroxyhydroquinone improves postprandial endothelial dysfunction in patients with borderline and stage 1 hypertension. Eur J Nutr 2019;58:989-96. 10.1007/s00394-018-1611-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Johnston KL, Clifford MN, Morgan LM. Coffee acutely modifies gastrointestinal hormone secretion and glucose tolerance in humans: glycemic effects of chlorogenic acid and caffeine. Am J Clin Nutr 2003;78:728-33. 10.1093/ajcn/78.4.728. [DOI] [PubMed] [Google Scholar]
- 51. Pallauf K, Günther I, Kühn G, Chin D, de Pascual-Teresa S, Rimbach G. The Potential of Resveratrol to Act as a Caloric Restriction Mimetic Appears to Be Limited: Insights from Studies in Mice. Adv Nutr 2021;12:995-1005. 10.1093/advances/nmaa148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bag S, Mondal A, Majumder A, Banik A. Tea and its phytochemicals: Hidden health benefits & modulation of signaling cascade by phytochemicals. Food Chem 2022;371:131098. 10.1016/j.foodchem.2021.131098. [DOI] [PubMed] [Google Scholar]
- 53. Felisbino K, Granzotti JG, Bello-Santos L, Guiloski IC. Nutrigenomics in Regulating the Expression of Genes Related to Type 2 Diabetes Mellitus. Front Physiol 2021;12:699220. 10.3389/fphys.2021.699220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Han MK. Epigallocatechin gallate, a constituent of green tea, suppresses cytokine-induced pancreatic beta-cell damage. Exp Mol Med 2003;35:136-9. 10.1038/emm.2003.19. [DOI] [PubMed] [Google Scholar]
- 55. Rowley TJ, 4th, Bitner BF, Ray JD, et al. Monomeric cocoa catechins enhance β-cell function by increasing mitochondrial respiration. J Nutr Biochem 2017;49:30-41. 10.1016/j.jnutbio.2017.07.015. [DOI] [PubMed] [Google Scholar]
- 56. Unger AL, Torres-Gonzalez M, Kraft J. Dairy Fat Consumption and the Risk of Metabolic Syndrome: An Examination of the Saturated Fatty Acids in Dairy. Nutrients 2019;11:2200. 10.3390/nu11092200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Seferidi P, Millett C, Laverty AA. Sweetened beverage intake in association to energy and sugar consumption and cardiometabolic markers in children. Pediatr Obes 2018;13:195-203. 10.1111/ijpo.12194. [DOI] [PubMed] [Google Scholar]
- 58. Yarmolinsky J, Duncan BB, Chambless LE, et al. Artificially Sweetened Beverage Consumption Is Positively Associated with Newly Diagnosed Diabetes in Normal-Weight but Not in Overweight or Obese Brazilian Adults. J Nutr 2016;146:290-7. 10.3945/jn.115.220194. [DOI] [PubMed] [Google Scholar]
- 59. Suez J, Korem T, Zeevi D, et al. Artificial sweeteners induce glucose intolerance by altering the gut microbiota. Nature 2014;514:181-6. 10.1038/nature13793. [DOI] [PubMed] [Google Scholar]
- 60. Serrano J, Smith KR, Crouch AL, et al. High-dose saccharin supplementation does not induce gut microbiota changes or glucose intolerance in healthy humans and mice. Microbiome 2021;9:11. 10.1186/s40168-020-00976-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ahmad SY, Friel J, Mackay D. The Effects of Non-Nutritive Artificial Sweeteners, Aspartame and Sucralose, on the Gut Microbiome in Healthy Adults: Secondary Outcomes of a Randomized Double-Blinded Crossover Clinical Trial. Nutrients 2020;12:3408. 10.3390/nu12113408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Miller PE, Perez V. Low-calorie sweeteners and body weight and composition: a meta-analysis of randomized controlled trials and prospective cohort studies. Am J Clin Nutr 2014;100:765-77. 10.3945/ajcn.113.082826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. de Ruyter JC, Olthof MR, Seidell JC, Katan MB. A trial of sugar-free or sugar-sweetened beverages and body weight in children. N Engl J Med 2012;367:1397-406. 10.1056/NEJMoa1203034. [DOI] [PubMed] [Google Scholar]
- 64. Ebbeling CB, Feldman HA, Steltz SK, Quinn NL, Robinson LM, Ludwig DS. Effects of Sugar-Sweetened, Artificially Sweetened, and Unsweetened Beverages on Cardiometabolic Risk Factors, Body Composition, and Sweet Taste Preference: A Randomized Controlled Trial. J Am Heart Assoc 2020;9:e015668. 10.1161/JAHA.119.015668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yuan C, Spiegelman D, Rimm EB, et al. Validity of a Dietary Questionnaire Assessed by Comparison With Multiple Weighed Dietary Records or 24-Hour Recalls. Am J Epidemiol 2017;185:570-84. 10.1093/aje/kww104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Bhole V, Choi JW, Kim SW, de Vera M, Choi H. Serum uric acid levels and the risk of type 2 diabetes: a prospective study. Am J Med 2010;123:957-61. 10.1016/j.amjmed.2010.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Zheng JS, Sharp SJ, Imamura F, et al. Association of plasma biomarkers of fruit and vegetable intake with incident type 2 diabetes: EPIC-InterAct case-cohort study in eight European countries. BMJ 2020;370:m2194. 10.1136/bmj.m2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Hirahatake KM, Jacobs DR, Shikany JM, et al. Cumulative intake of artificially sweetened and sugar-sweetened beverages and risk of incident type 2 diabetes in young adults: the Coronary Artery Risk Development In Young Adults (CARDIA) Study. Am J Clin Nutr 2019;110:733-41. 10.1093/ajcn/nqz154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Field AE, Coakley EH, Must A, et al. Impact of overweight on the risk of developing common chronic diseases during a 10-year period. Arch Intern Med 2001;161:1581-6. 10.1001/archinte.161.13.1581. [DOI] [PubMed] [Google Scholar]
- 70. Shan Z, Rehm CD, Rogers G, et al. Trends in Dietary Carbohydrate, Protein, and Fat Intake and Diet Quality Among US Adults, 1999-2016. JAMA 2019;322:1178-87. 10.1001/jama.2019.13771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Yin J, Huang Y, Liu G, Wang L, Shan Z, Liu L. Trends in dietary macronutrient composition and diet quality among US adults with diagnosed and undiagnosed elevated glycemic status: a serial cross-sectional study. Am J Clin Nutr 2022;115:1602-11. 10.1093/ajcn/nqac061. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information: Additional methods, figures 1-3, and tables 1-4
Data Availability Statement
Data of the Nurses’ Health Study and Health Professionals Follow-Up Study can be shared through mechanisms detailed at https://www.nurseshealthstudy.org or https://www.hsph.harvard.edu/hpfs/.