Abstract
Introduction
Plasma concentrations of gut microbial metabolites are associated with cardiomyocyte viability and platelet reactivity. We hypothesized that increased concentrations of gut metabolites may predict major adverse cardiac and cerebrovascular events (MACCE) after acute myocardial infarction (AMI).
Aim
The primary objective of this study was to evaluate the association between elevated plasma concentrations of gut metabolites and MACCE after AMI.
Material and methods
We compared plasma concentrations of gut metabolites (trimethylamine-N-oxide (TMAO) and indoxyl sulphate (IS)) and platelet reactivity in 57 patients with AMI and 27 healthy controls. We assessed the predictive value of gut metabolites for MACCE (stroke, recurrent AMI, death) over a median of 3.5-years.
Results
The concentrations of TMAO and IS did not differ between AMI patients and controls. The concentrations of TMAO and IS were higher in patients who developed MACCE than in those who did not (p ≤ 0.015 for all). The concentration of TMAO was the only independent predictor of MACCE in a multivariate analysis (OR = 35.041, 95% CI: 1.269–967.307, p = 0.036). Patients with the concentration of TMAO and indoxyl sulphate above the cut-off value predictive of MACCE had higher platelet activity (p ≤ 0.149 for all).
Conclusions
Increased plasma concentration of TMAO is an independent predictor of MACCE and may contribute to post-AMI cardiac dysfunction.
Keywords: acute myocardial infarction, major adverse cardiovascular events, gut microbiome, gut metabolites, TMAO, prognosis
Summary
Elevated plasma concentration of TMAO occured to be independent and firm predictor of major cardiac and cerebrovascular events after acute myocardial infarction in the course of the 3.5 years follow-up. We assume that the main cause might be the correlation between TMAO concentration and increased platelet reactivity.
Introduction
Cardiovascular disease (CVD), including acute myocardial infarction (AMI), is the leading cause of death worldwide [1]. In 2009, the global healthcare costs for CVD were estimated at €106 billion [2]. Despite the progress in the pharmacological and interventional treatment of AMI, recurrent ischaemic events such as cardiovascular death, recurrent AMI, or stroke occur in ~10% of patients within 1 year of the initial AMI [3]. At present, there is no tool to predict recurrent major adverse cardiac and cerebrovascular events (MACCE) after AMI. Accumulating data shows that gut microbiome plays an important role in the pathogenesis of CVD [4–6]. Among gut microbial metabolites, trimethylamine-N-oxide (TMAO) and indoxyl sulphate (IS) are the focus of extensive research in CVD [7, 8]. TMAO originates from the liver, which oxidizes trimethylamine (TMA), a TMAO precursor produced by conversion of carnitine, betaine, and choline by intestinal symbiotic bacteria [9]. IS, in turn, is a metabolite of dietary tryptophan that acts as a cardiotoxin and uremic toxin [10]. For example, TMAO was shown to have a dose-dependent association with platelet reactivity and cumulative incidence of thrombotic events in a cohort of over 4000 patients presenting for elective cardiac evaluations [11]. TMAO was shown to enhance platelet responsiveness to multiple agonists (adenosine diphosphate, thrombin, collagen) by enhancing the release of calcium from intracellular stores [11]. Elevated serum TMAO levels were also predictive of thrombus formation in atrial fibrillation patients [12]. Similarly, IS was shown to promote arterial thrombosis in a rat model [13] and induce platelet hyperactivity, thus contributing to chronic kidney disease (CKD)-associated thrombosis in mice [14]. Furthermore, IS exacerbated fibrosis and proliferation of cardiomyocytes [15].
Aim
We hypothesized that plasma concentrations of gut microbial metabolites differ between patients with AMI and healthy volunteers, and plasma concentrations of metabolites may be used as biomarkers to predict MACCE after AMI. The goal of this study was to (I) compare plasma concentrations of TMAO and IS in patients with AMI and healthy volunteers, as well as in patients with AMI who did and who did not experience MACCE during the median follow-up of 3.5 years, (II) to evaluate the predictive value of TMAO and IS for MACCE, and (III) to assess the correlation between TMAO and IS and platelet reactivity.
Material and methods
Study design
This was a prospective, observational study including patients participating in the AFFECT EV Metabolite Substudy. AFFECT EV was an investigator-initiated, prospective study conducted at the 1st Chair and Department of Cardiology, Medical University of Warsaw, Poland [16]. The study protocol, designed in compliance with the Declaration of Helsinki, was approved by the Ethics Committee of the Medical University of Warsaw (approval number KB/112/2016), registered in the Clinical Trials database (NCT02931045), and published previously [17]. All participants provided written informed consent.
Study participants
Study inclusion and exclusion criteria are listed in Table I. Patients were eligible for enrolment if they were (i) admitted to the hospital due to the first ST-segment elevation of AMI (STEMI) or non-STEMI (NSTEMI) with an onset of symptoms during the previous 24 h, and (ii) underwent PCI with stent implantation. STEMI was defined as persistent ST-segment elevation of at least 0.1 mV in at least 2 contiguous electrocardiography leads, or a new left bundle-branch block [18]. NSTEMI was diagnosed in patients with typical anginal chest pain accompanied by ST-segment changes (ST depression, T-wave changes, transient ST elevation) on electrocardiogram and an elevation of cardiac troponin concentration in the peripheral blood [19]. When the study was initiated, patients with STEMI were pre-treated with clopidogrel before hospital admission. Because antiplatelet therapy with P2Y12 inhibitors affects platelet reactivity, only patients who received clopidogrel prior to PCI were enrolled in the study to obtain a homogenous study group. Because gut metabolites are excreted by the urinary tract, patients with CKD with estimated glomerular filtration rate (eGFR) < 45 ml/min/1.73 m2, calculated using the Modification of Diet in Renal Disease (MDRD) formula, were excluded [20]. Because the intestinal metabolism is affected by the state of gastrointestinal tract and its microbiota, patients with acute or chronic gastrointestinal diseases, autoimmune disease, treated with antibiotics within the last 2 months or taking dietary supplements within the last 7 days, were excluded from the study [21].
Table I.
Eligibility criteria for the study
| Inclusion criteria | Age ≥ 18 years Informed consent First acute myocardial infarction treated with percutaneous coronary intervention with stent implantation |
| Exclusion criteria | Chronic kidney disease (estimated glomerular filtration rate < 45 ml/min) Chronic inflammatory disease Chronic intestinal disease Acute gastrointestinal disease within the last month Antibiotic administration in the last 2 months Dietary supplements in the last 7 days Autoimmune disease Active neoplasm Pregnancy or breast-feeding |
Healthy volunteers were recruited among the hospital stuff and included people aged 18–99 years, without any medical history of chronic diseases, chronic pharmacotherapy, acute gastrointestinal disease within the last month, antibiotic therapy within the last 2 months, and dietary supplements within the last 7 days.
Trial schedule and blinding
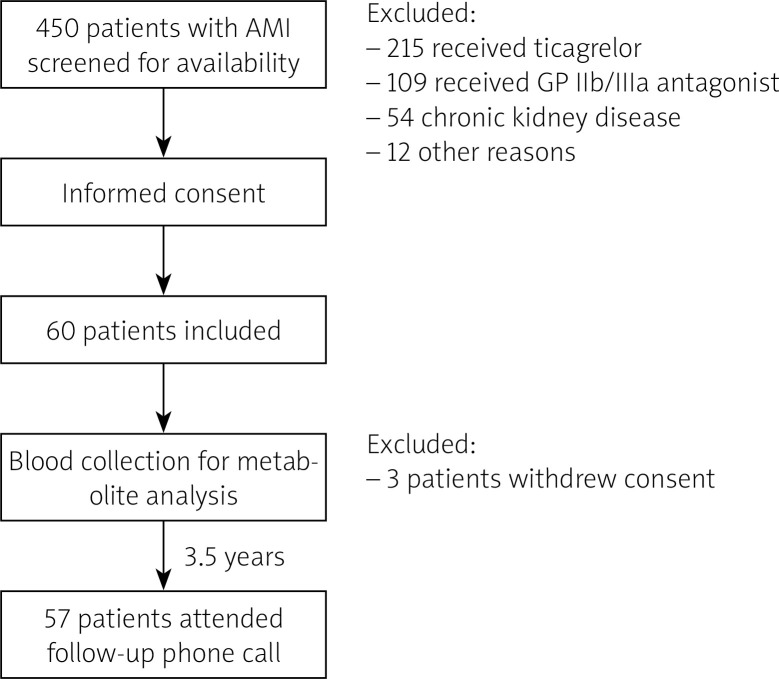
The trial schedule is presented in Figure 1. Blood was collected from patients by an independent operator (CE), who was otherwise not involved in sample analysis. Aggregometry was conducted by an independent operator (AG). During the trial, participants were identified by an individual number, and samples were coded with a sample number. Bacterial metabolite concentration analysis was performed by an independent operator, blinded to clinical data (MU). Statistical analysis was performed by independent operators (PS, KJ) [22].
Figure 1.
Inclusion and exclusion chart
AMI – acute myocardial infarction, GP – glycoprotein.
Treatment
All patients received standard treatment after AMI according to the guidelines, including double antiplatelet therapy, β-blocker, angiotensin-converting enzyme inhibitor or angiotensin receptor blocker, aldosterone receptor antagonist, and protein pump inhibitor [18, 19].
Clinical data collection
Data collected at baseline include demographics (age, gender), body mass index, initial diagnosis, and cardiovascular risk factors, including arterial hypertension, diabetes, hyperlipidaemia, and smoking. In addition, routine laboratory parameters were recorded. At discharge, pharmacotherapy was recorded. Data regarding MACCE (recurrent AMI, stroke, cardiovascular death, all-cause death) were collected during a follow-up phone-call at a median of 3.5 years [22].
Blood collection and handling
Peripheral venous blood samples were collected from fasting patients at a single time-point (within 24 h after AMI). Briefly, blood was collected into ethylenediaminetetraacetic acid (EDTA) tubes for metabolites analysis and into hirudin tubes for platelet reactivity analysis. The first 2 ml were disposed of to avoid pre-activation of platelets. Within a maximum of 15 min from blood collection, EDTA samples were centrifuged for 15 min at 2500 g. Plasma was frozen at –80°C until analysed [22].
Evaluation of gut bacterial metabolite concentration
The concentrations of TMAO and IS were measured using a Waters Acquity Ultra Performance Liquid Chromatograph coupled with a Waters TQ-S Triple-Quadrupole Mass Spectrometer. The mass spectrometer operated in the multiple-reaction monitoring (MRM)-positive electrospray ionization (ESI) mode, as we have previously described [23].
Platelet reactivity
Platelet reactivity was assessed by multiple electrode aggregometry using the adenosine diphosphate test (ADP, 6.5 μmol/l), and the thrombin receptor-activating peptide-6 (SFLLRN) test (TRAP, 32 μmol/l) was used as a positive control. Unstimulated whole blood was used as a negative control.
Endpoints
The primary endpoint of the study was the difference in plasma TMAO and IS concentrations between patients with AMI and healthy volunteers. The secondary endpoint was the prognostic value of TMAO and IS for the occurrence of MACCE during the median 3.5-year follow-up time. The exploratory endpoint was the correlation between TMAO and IS and platelet reactivity [22].
Statistical analysis
Statistical analysis was done using IBM SPSS Statistics, version 24.0 (IBM). Qualitative variables were presented as numbers and percentages and compared with the use of Fischer’s exact test. A Shapiro-Wilk test was used to assess the normal distribution of continuous variables. Continuous variables were presented as median with interquartile range or as mean and SD and compared using the Mann-Whitney U test or an unpaired t-test. The diagnostic ability of gut microbial metabolites to discriminate between patients with and without MACCE and the cut-offs were calculated using a receiver operating characteristic (ROC) curve. A logistic regression model incorporating the gut microbial metabolites with significant sensitivity and specificity (area under the ROC curve – AUC) and clinical characteristics was used to determine the best model for MACCE prediction. Mortality and other adverse events were reported depictively. A p-value below 0.05 was considered significant [22].
Results
Between January 2017 and July 2018, 60 patients were enrolled. Due to withdrawal of permission to participate in the study by 3 patients, 57 patients attended the final analysis and completed follow-up. Control group consisted of 27 age- and gender-matched healthy persons. MACCE was developed by 5 (8.8%) patients during the follow-up: 4 deaths (2 patients from unknown cause, 2 from cardiovascular cause) and a relapse of AMI.
Metabolite concentrations in patients with AMI and in healthy controls
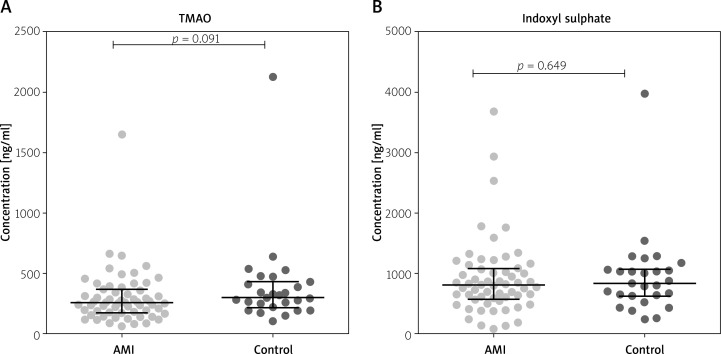
The concentrations of IS and TMAO concentrations were similar in patients with AMI and in the healthy controls (Figure 2).
Figure 2.
Gut microbial metabolites in plasma of patients with acute myocardial infarction and healthy controls. A – Trimethylamine-N-oxide (TMAO). B – Indoxyl sulphate
Metabolites as predictors of MACCE after AMI
Table II shows the characteristics of patients who developed major adverse cardiovascular events and persons who did not. Patients with MACCE were older (p = 0.031), had increased creatinine levels (p = 0.001), and elevated levels of peak troponin I (p = 0.048) at baseline, in comparison to patients who did not experience MACCE. Nevertheless, the remaining cardiovascular risk factors and laboratory data were similar within the groups. Pharmacotherapy did not differ much between the patients. All of them received dual antiplatelet therapy – atorvastatin (except one person). The majority received a β-blocker, ACEI, and proton pump inhibitor (PPI).
Table II.
Comparison of baseline characteristics between patients who experienced MACE and those who did not during the median follow-up of 3.5 years
| Variable | MACE (n = 5) | No MACE (n =52) | P-value | ||
|---|---|---|---|---|---|
| Characteristic: | |||||
| Age [years] mean (SD) | 75.0 | 9.9 | 63.4 | 9.5 | 0.031 |
| Male gender, n (%) | 5 | 100 | 37 | 71 | 0.162 |
| BMI, mean (SD) | 22.6 | 3.3 | 29.7 | 4.3 | 0.311 |
| STEMI at admission, n (%) | 2 | 40 | 42 | 81 | 0.072 |
| CV risk factors, n (%): | |||||
| Arterial hypertension | 4 | 80 | 32 | 62 | 0.642 |
| Diabetes mellitus | 1 | 20 | 15 | 29 | 1.000 |
| Dyslipidaemia | 2 | 60 | 35 | 67 | 0.332 |
| Smoking | 1 | 20 | 24 | 46 | 0.372 |
| Clinical data: | |||||
| CrCl [ml/min] median (IQR) | 57 | 43.50–73 | 90 | 68.50–113 | 0.007 |
| Hb [g/dl] mean (SD) | 12.9 | 1.3 | 13.9 | 1.3 | 0.335 |
| LDL-C, median (IQR) | 136 | 52–188 | 123 | 91–151 | 0.922 |
| NT-proBNP, median (IQR) | 3307 | 1034–3579 | 764 | 305–1893 | 0.109 |
| Plt count [× 103/µl ] mean (SD) | 212 | 28 | 226 | 69 | 0.129 |
| TnI max [ng/ml] median (IQR) | 52.5 | 23.2–104.8 | 12.1 | 3.1–36.4 | 0.048 |
| LVEF, % – mean (SD) | 40.0 | 7.1 | 49.8 | 8.9 | 0.446 |
| Pharmacotherapy at discharge, n (%): | |||||
| Aspirin | 5 | 100 | 52 | 100 | 1.000 |
| P2Y12 inhibitor | 5 | 100 | 52 | 100 | 1.000 |
| Statin | 5 | 100 | 51 | 98 | 0.754 |
| β-blocker | 5 | 100 | 47 | 90 | 0.468 |
| ACE-inhibitor or ARB | 5 | 100 | 50 | 96 | 0.655 |
| Diuretics | 2 | 40 | 13 | 25 | 0.467 |
| Aldosterone antagonists | 3 | 60 | 10 | 19 | 0.072 |
| Protein pump inhibitor | 5 | 100 | 49 | 94 | 0.581 |
| Cardiac rehabilitation, n (%) | 4 | 80 | 40 | 77 | 0.437 |
ACE – angiotensin-converting enzyme, ARB – angiotensin-receptor blockers, BMI – body mass index, weight in kilograms divided by square of the height in metres, CrCl – creatinine clearance, calculated according to the Cockcroft-Gault equation, CRP – C-reactive protein, Hb – haemoglobin, IQR – interquartile range, LDL-C – low-density lipoprotein-cholesterol, LVEF – left ventricle ejection fraction, NSTEMI – non-ST-segment elevation myocardial infarction, NT-proBNP – N-terminal pro-B-type natriuretic peptide, Plt – platelets, SD – standard deviation, STEMI – ST-segment elevation myocardial infarction.
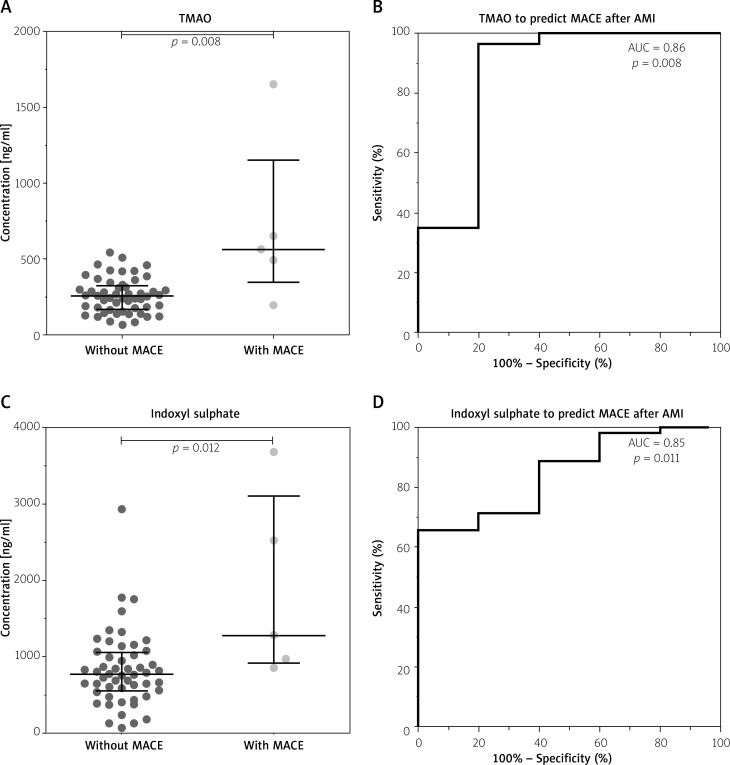
The concentrations of intestinal microbial metabolites in the plasma of patients with and without MACCE after AMI during the 3.5-year follow-up are shown in Figure 3. TMAO (Figure 3 A) and IS (Figure 3 C) levels were elevated in patients who experienced MACCE, in contrast to those who did not develop any major adverse cardiovascular events (p ≤ 0.012 for all), and distinguished between the groups (area under ROC curve (AUC) ≥ 0.85, p ≤ 0.011 for all) in univariate analysis (Figures 3 B, D).
Figure 3.
Gut microbial metabolites in plasma of patients with acute myocardial infarction predict major adverse cardiovascular events (MACE) during the median follow-up of 3.5 years. A, B – Trimethylamine-N-oxide (TMAO). C, D – Indoxyl sulphate
Table III indicates the statistical estimates of MACCE hazard via TMAO and IS, which also include the cut-off values, which were set based on the ROC curves. We include TMAO and IS in a logistic regression model alongside with patients’ gender and other clinical data (age, creatinine, troponin), to examine if these metabolites are independent MACCE predictors. In Supplementary Materials we have included a table with multivariable analysis, where MACE was used as a dependent variable, and predictors that were significant in univariable analysis were used as independent variables. The analysis indicates that the plasma concentration of TMAO at baseline was the only independent predictor of MACCE during the observation period (OR = 35.041, 95% CI: 1.269–967.307, p = 0.036), whereas the baseline concentrations of IS lost statistical significance to predict MACCE in multivariate analysis (OR = 9.260, 95% CI: 0.287–298.409, p = 0.209). This model, however, should be interpreted cautiously due to the small sample size (Table IV).
Table III.
Statistical estimates for prediction of major adverse cardiovascular events by gut microbial metabolites
| Metabolite | AUC (95% CI) | P-value | Cut-off [ng/ml] | Sensitivity | Specificity | PPV | NPV |
|---|---|---|---|---|---|---|---|
| TMAO | 0.86 (0.62–1.00) | 0.008 | 478 | 80% | 96% | 67% | 98% |
| Indoxyl sulphate | 0.85 (0.71–0.99) | 0.011 | 962 | 80% | 71% | 21% | 97% |
AUC – area under the curve, CI – confidence interval, PPV – positive predictive value, NPV – negative predictive value.
Correlation between platelet reactivity and SDMA
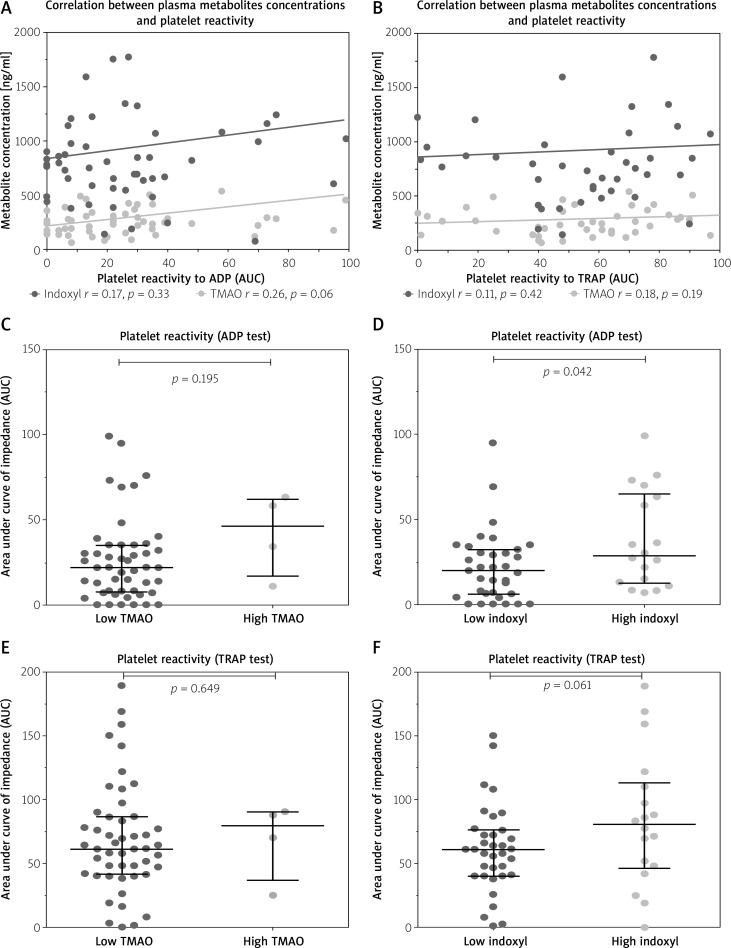
Figure 4 shows the correlation between plasma metabolites concentrations and platelets reactivity in response to ADP and TRAP in patients after AMI. Although there was no significant correlation between plasma TMAO and IS concentrations and platelet reactivity (Figures 4 A, B), patients with concentrations of TMAO and IS above the cut-off value predictive of MACCE (“high TMAO”, “high IS”) had higher platelet reactivity compared to patients with metabolite concentration below the cut-off value (Figures 4 C–F). However, the statistical significance was reached only for IS in the ADP test (p = 0.042; Figure 4 D).
Figure 4.
Correlations between platelet reactivity in response to ADP and TRAP and plasma gut microbial metabolite concentrations (A, B) and comparison of platelet reactivity in patients with the concentrations of TMAO and IS above the cut-off value predictive of MACE (“high TMAO”, “high IS”), compared to those with metabolite concentration below the cut-off value (C–F)
Discussion
The main finding of our study is that the plasma concentration of TMAO was a firm and independent predictor of major adverse cardiovascular events after AMI in the course of the 3.5-year observation period, with 80% sensitivity and 96% specificity. Moreover, other studies indicate that elevated plasma levels of TMAO were associated with a higher risk of MACCE (death, myocardial infarction, or stroke) independently of conventional risk factors, for example chronic kidney disease, obesity, or diabetes mellitus, in stable patients with coronary artery disease managed with optimal medical treatment [24, 25] or undergoing elective coronary angiography [26], in patients presenting to the emergency department with chest pain [27], in patients after AMI [28], with chronic heart failure [29], and those after out-of-hospital cardiac arrest [30]. The association of plasma TMAO and adverse outcomes in cardiovascular patients was also confirmed in a recent meta-analysis [31]. Nonetheless, there are also studies that have shown no association between the plasma concentration of TMAO and adverse outcomes in cardiovascular patients, indicating that TMAO results tend to be confounded by impaired kidney function and poor metabolic control [32, 33]. In addition, it has been suggested that the TMAO precursor trimethylamine, but not TMAO itself, is involved in cardiovascular pathology by exerting negative effects on cardiomyocytes, probably due to disturbing their protein structure [7]. Hence, our study adds to the discussion in the literature on this topic.
In our study, the plasma concentration of IS predicted MACCE after AMI in univariate analysis, but it was not an independent predictor in multivariate analysis. In contrast to our results, other authors found that IS predicted adverse cardiovascular outcomes in patients after AMI and those with CKD undergoing dialysis [34, 35]. Again, further studies are required to determine the real prognostic value of IS for cardiovascular outcomes and potentially implement both TMAO and IS in daily clinical routine.
The association between plasma TMAO and IS concentrations and adverse cardiovascular events is multifactorial. First, chronic dietary L-carnitine supplementation in mice enhanced synthesis of TMA and TMAO by gut microbiota and increased atherosclerosis, thus contributing to the well-established link between high levels of red meat consumption and CVD risk [25]. There is evidence that an increased concentration of TMAO activates platelets and triggers clot formation, resulting in a higher risk of atherothrombotic events and cardiovascular death [11, 36]. In our study, patients with concentrations of TMAO above the cut-off value predictive of MACCE had higher platelet reactivity, compared to patients with metabolite concentrations below the cut-off value, although the result was not statistically significant. Nevertheless, based on previous evidence from the literature [35], an association between TMAO concentration and platelet reactivity might be one of the mechanisms underlying adverse outcomes.
The uremic toxin IS, in turn, was shown to activate inflammation and coagulation signalling pathways in the rat aorta in the short-term and induce calcification in the aorta and peripheral arteries in the long-term [37]. Furthermore, IS induced the production of reactive oxygen species and the expression of osteoblast-specific proteins in human aortic smooth muscle cells [38] and stimulated the proliferation of rat vascular smooth muscle cells [39]. These observations, derived from cell cultures and animal models, might at least partly explain the association between extensive calcification and faster progression of atherosclerosis in CKD patients. In our cohort of post-AMI patients without CKD (eGFR < 45 ml/min/1.73 m2) we could not confirm the independent predictive value of IS, suggesting that IS might be specifically used to predict adverse prognostic effects in CKD patients [40] but not in I CVD patients. Nevertheless, the main limitations of our study are the small sample size and wide confidence interval of TMAO predictive value in multivariate analysis. Therefore, we can only hypothesize rather than ultimately prove that an elevated concentration of TMAO in the plasma of AMI patients is related to MACCE. Accordingly, the data should not only be considered with caution but also confirmed in a larger study group.
The main limitation of our study is the small sample size and MACCE, which affects the statistical results and should be furtherly validated. We also did not assess the occurrence of MACE in the control group. Also, the impact of diet and PPI on gut microbiome and metabolites was not considered.
Conclusions
In our study elevated plasma concentration of TMAO was shown to be an independent and firm predictor of major adverse cardiovascular events after AMI in the course of the 3.5-year follow-up. We assume that the main cause might be the correlation between TMAO concentration and increased platelet reactivity. These results, however, may be affected by the small sample size and MACCE and are only hypothesis-generating.
Supplementary Material
Acknowledgments
The study was funded by the National Science Centre, Poland, grant no. 2020/37/B/NZ5/00366.
Conflict of interest
The authors declare no conflict of interest.
References
- 1.Sanchis-Gomar F, Perez-Quilis C, Leischik R, Lucia A. Epidemiology of coronary heart disease and acute coronary syndrome. Ann Transl Med 2016; 4: 256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nicholson G, Gandra SR, Halbert RJ, et al. Patient-level costs of major cardiovascular conditions: a review of the international literature. Clinicoecon Outcomes Res 2016; 8: 495-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rossello X, Bueno H, Pocock SJ, et al. Predictors of all-cause mortality and ischemic events within and beyond 1 year after an acute coronary syndrome: Results from the EPICOR registry. Clin Cardiol 2019; 42: 111-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Z, Klipfell E, Bennett BJ, et al. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 2011; 472: 57-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trøseid M, Andersen GØ, Broch K, et al. The gut microbiome in coronary artery disease and heart failure: current knowledge and future directions. EBioMedicine 2020; 52: 102649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hung SC, Kuo KL, Wu CC, et al. Indoxyl sulfate: a novel cardiovascular risk factor in chronic kidney disease. J Am Heart Assoc 2017; 6: e005022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ufnal M, Jazwiec R, Dadlez M, et al. Trimethylamine-N-oxide: a carnitine-derived metabolite that prolongs the hypertensive effect of angiotensin II in rats. Can J Cardiol 2014; 30: 1700-5. [DOI] [PubMed] [Google Scholar]
- 8.Ufnal M, Zadlo A, Ostaszewski R. TMAO: a small molecule of great expectations. Nutrition 2015; 31: 1317-23. [DOI] [PubMed] [Google Scholar]
- 9.Sonnenburg JL, Bäckhed F. Diet-microbiota interactions as moderators of human metabolism. Nature 2016; 535: 56-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Konopelski P, Ufnal M. Indoles-gut bacteria metabolites of tryptophan with pharmacotherapeutic potential. Curr Drug Metab 2018; 19: 883-90. [DOI] [PubMed] [Google Scholar]
- 11.Risk T, Zhu W, Gregory JC, et al. Gut microbial metabolite TMAO enhances platelet article gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell 2016; 165: 111-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gong D, Zhang L, Zhang Y, et al. Gut microbial metabolite trimethylamine N-oxide is related to thrombus formation in atrial fibrillation patients. Am J Med Sci 2019; 358: 422-8. [DOI] [PubMed] [Google Scholar]
- 13.Karbowska M, Kaminski TW, Znorko B, et al. Indoxyl sulfate promotes arterial thrombosis in rat model via increased levels of complex TF/VII, PAI-1, platelet activation as well as decreased contents of SIRT1 and SIRT3. Front Physiol 2018; 9: 1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang K, Du C, Wang X, et al. Indoxyl sulfate induces platelet hyperactivity and contributes to chronic kidney disease-associated thrombosis in mice. Blood J Am Soc Hematol 2017; 129: 2667-79. [DOI] [PubMed] [Google Scholar]
- 15.Lekawanvijit S, Kompa AR, Manabe M, et al. Chronic kidney disease-induced cardiac fibrosis is ameliorated by reducing circulating levels of a non-dialysable uremic toxin, indoxyl sulphate. PLoS One 2012; 7: e41281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gasecka A, Nieuwland R, Budnik M, et al. Ticagrelor attenuates the increase of extracellular vesicle concentrations in plasma after acute myocardial infarction compared to clopidogrel. J Thromb Haemost 2020; 18: 609-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gasecka A, Nieuwland R, Budnik M, et al. Randomized controlled trial protocol to investigate the antiplatelet therapy effect on extracellular vesicles (AFFECT EV) in acute myocardial infarction. Platelets 2020; 31: 26-32. [DOI] [PubMed] [Google Scholar]
- 18.Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J 2018; 39: 119-77. [DOI] [PubMed] [Google Scholar]
- 19.Collet JP, Thiele H, Barbato E, et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevationThe Task Force for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J 2021; 42: 1289-367. [DOI] [PubMed] [Google Scholar]
- 20.Cosola C, Rocchetti MT, Sabatino A, et al. Microbiota issue in CKD: how promising are gut-targeted approaches? J Nephrol 2019; 32: 27-37. [DOI] [PubMed] [Google Scholar]
- 21.Visconti A, Le Roy CI, Rosa F, et al. Interplay between the human gut microbiome and host metabolism. Nat Commun 2019; 10: 4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gąsecka A, Szwed P, Jasińska K, et al. Symmetric dimethylarginine is altered in patients after myocardial infarction and predicts adverse outcomes. J Inflamm Res 2021; 14: 3797-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jaworska K, Hering D, Mosieniak G, et al. TMA, a forgotten uremic toxin, but not TMAO, is involved in cardiovascular pathology. Toxins 2019; 11: 490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Senthong V, Wang Z, Li XS, et al. Intestinal microbiota-generated metabolite trimethylamine-N-oxide and 5-year mortality risk in stable coronary artery disease: the contributory role of intestinal microbiota in a COURAGE-like patient cohort. J Am Heart Assoc 2016; 5: e002816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koeth RA, Wang Z, Levison BS, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med 2013; 19: 576-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tang WHW, Wang Z, Levison BS, et al. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med 2013; 368: 1575-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li XS, Obeid S, Klingenberg R, et al. Gut microbiota-dependent trimethylamine N-oxide in acute coronary syndromes: a prognostic marker for incident cardiovascular events beyond traditional risk factors. Eur Heart J 2017; 38: 814-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Suzuki T, Heaney LM, Jones DJL, et al. Trimethylamine N-oxide and risk stratification after acute myocardial infarction. Clin Chem 2017; 63: 420-8. [DOI] [PubMed] [Google Scholar]
- 29.Zhou X, Jin M, Liu L, et al. Trimethylamine N-oxide and cardiovascular outcomes in patients with chronic heart failure after myocardial infarction. ESC Heart Fail 2020; 7: 189-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hochstrasser SR, Metzger K, Vincent AM, et al. Trimethylamine-N-oxide (TMAO) predicts short-and long-term mortality and poor neurological outcome in out-of-hospital cardiac arrest patients. Clin Chem Lab Med 2020; 59: 393-402. [DOI] [PubMed] [Google Scholar]
- 31.Heianza Y, Ma W, Manson JE, et al. Gut microbiota metabolites and risk of major adverse cardiovascular disease events and death: a systematic review and meta-analysis of prospective studies. J Am Heart Assoc 2017; 6: e004947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mueller DM, Allenspach M, Othman A, et al. Plasma levels of trimethylamine-N-oxide are confounded by impaired kidney function and poor metabolic control. Atherosclerosis 2015; 243: 638-44. [DOI] [PubMed] [Google Scholar]
- 33.Kaysen GA, Johansen KL, Chertow GM, et al. Associations of trimethylamine N-oxide with nutritional and inflammatory biomarkers and cardiovascular outcomes in patients new to dialysis. J Ren Nutr 2015; 25: 351-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watanabe I, Tatebe J, Fujii T, et al. Prognostic utility of indoxyl sulfate for patients with acute coronary syndrome. J Atheroscler Thromb 2019; 26: 64-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fan PC, Chang JCH, Lin CN, et al. Serum indoxyl sulfate predicts adverse cardiovascular events in patients with chronic kidney disease. J Formos Med Assoc 2019; 118: 1099-106. [DOI] [PubMed] [Google Scholar]
- 36.Berger M, Kleber ME, Delgado GE, et al. Trimethylamine N-oxide and adenosine diphosphate-induced platelet reactivity are independent risk factors for cardiovascular and all-cause mortality. Circ Res 2020; 126: 660-2. [DOI] [PubMed] [Google Scholar]
- 37.Opdebeeck B, Maudsley S, Azmi A, et al. Indoxyl sulfate and p-cresyl sulfate promote vascular calcification and associate with glucose intolerance. J Am Soc Nephrol 2019; 30: 751-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muteliefu G, Enomoto A, Jiang P, et al. Indoxyl sulphate induces oxidative stress and the expression of osteoblast-specific proteins in vascular smooth muscle cells, Nephrol Dial Transplant 2009; 24: 2051-8. [DOI] [PubMed] [Google Scholar]
- 39.Yamamoto H, Tsuruoka S, Ioka T, et al. Indoxyl sulfate stimulates proliferation of rat vascular smooth muscle cells. Kidney Int 2006; 69: 1780-5. [DOI] [PubMed] [Google Scholar]
- 40.Lin CJ, Wu CJ, Pan CF, et al. Serum protein-bound uraemic toxins and clinical outcomes in haemodialysis patients. Nephrol Dial Transplant 2010; 25: 3693-700. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.