Abstract
Background
Viscosupplementation for knee osteoarthritis (OA) may raise concerns regarding conflicts of interest (COI). Evidence of inconclusive study results and publication bias in previous studies has led to concern that financial COI have influenced viscosupplementation outcomes. It is critical to ensure that clinical practice is guided by informed decision making and evidence-based medicine.
Methods
A systematic review was conducted following PRISMA guidelines. PubMed, MEDLINE, and Web of Science databases were searched for articles pertaining to hyaluronic acid (or similarly derived) injections to native knees with primary OA only. Bibliometric data, financial COI, and study outcomes were assessed.
Results
67 studies met inclusion criteria for analysis, 53 of which (79.1%) presented Level I evidence, and 21 of which (31.3%) reported at least one author with COI. All studies reporting COI also disclosed industry funding. There were no relationships between reported COI and study outcomes (Χ2 = 0.31, P = 0.577), levels of evidence (Χ2 = 3.48, P = 0.176), or relative citation ratio (RCR) (S = 743, P = 0.591). Studies reporting COIs/industry funding tended to be published in journals with significantly higher impact factors (IF) (reporting COI: IF = 3.5 ± 2.0; no COI: IF = 1.8 ± 1.1; S = 950, P < 0.001). Study outcomes were not related to the probability of being published in an open access journal (Χ2 = 0.01, P = 0.960), nor to level of evidence (Χ2 = 2.67, P = 0.263), RCR (S = 618, P = 0.835), or IF (S = 563, P = 0.655).
Conclusions
Investigator COIs (and commercial funding of studies) have not significantly influenced the frequency of favorable outcomes or study level of evidence regarding contemporary viscosupplementation for the treatment of knee OA. Studies reporting COIs/industry funding tended to be published in journals with significantly higher impact factors. Results overwhelmingly supported using viscosupplementation to treat knee OA.
Level of evidence
Level V Systematic Review.
Keywords: Conflict of interest, Funding, Viscosupplementation, Hyaluronic acid, Knee osteoarthritis
Highlights
-
•
Viscosupplementation may represent a promising treatment modality for knee osteoarthritis.
-
•
Wide variation in reported efficacy necessitates further analysis for sources of bias.
-
•
Author conflicts and industry funding had no significant effect on study results or conclusions.
-
•
Conflicted papers had higher impact factors, yet similar evidence level and relative citation ratio to non-conflicted papers.
-
•
Most papers reported favorable outcomes regardless of presence of industry funding.
Abbreviations
- OA
Osteoarthritis
- COI
Conflict of interest
- RCR
Relative citation ratio
- IF
Impact factor
- HA
Hyaluronic acid
- PPSA
Physicians Payments Sunshine Act
- CMS
Centers for Medicare and Medicaid Services
- TKA
Total knee arthroplasty
1. Introduction
Knee osteoarthritis (OA) is a degenerative joint disease associated with progressive destruction of articular cartilage, inflammation, and diminished remodeling of adjacent bone.1, 2, 3 Given the increasing prevalence of knee OA, novel therapies aimed at improving biomechanical function and patient symptomatology have been devised, including viscosupplementation.4 Recognized for its rheological properties, hyaluronic acid (HA) acts to absorb shock and lubricate during joint movement.1,4,5 In osteoarthritis, HA is clears at higher rates than usual, which reduces synovial fluid viscoelasticity and leads to cartilage loss.5,6 Intraarticular administration of HA aims to maintain joint lubrication, protect against cartilage erosion, lessen inflammation of synovium, and increase the synovial fluid elasticity.1,5
The American Academy of Orthopaedic Surgeons (AAOS) 3rd edition evidence-based clinical practice guideline, “Management of Osteoarthritis of the Knee (Non-Arthroplasty),” reports a recommendation strength of moderate against the routine use of HA in the treatment of symptomatic OA of the knee.7 This is a downgrade in strength from the 2nd edition released in 2013, which made a strong recommendation against the use of HA for patients with symptomatic OA of the knee.8 The updated recommendation recognizes the potential for HA to benefit patients but is limited by inconsistency in the available evidence, highlighting the need for physicians to critically evaluate the published literature when trying to determine the true clinical efficacy of this treatment modality.7
An important consideration when critically reviewing published studies is the potential for bias, including author conflicts of interest (COI). COI refers to scenarios where a physician's secondary interest may compromise the integrity of the physician-patient relationship.9,10 Although potential COI are sometimes unavoidable, there is a societal expectation that providers are free from of conflicted interests throughout medical decision making.10 Thus, transparency to the public is expected to maintain an impartial arrangement, and concerns may arise when industry-sponsored medical devices dictate clinical practice.9,10 Both industry participation in scientific research and payments to surgery practices have been implicated with publication of positive outcomes and underreporting of negative findings.9,10 Accordingly, the Physicians Payments Sunshine Act (PPSA) requires medical industries to disclose any transfers of value to physicians or hospitals to the Centers for Medicare and Medicaid Services (CMS).10 In addition, the CMS's Open Payments program encourages transparency by maintaining a public database of payments that reporting entities make to covered recipients.
Given the literature supporting the advantages of delaying total knee arthroplasty (TKA), conservative treatment modalities for OA, including viscosupplementation, have correspondingly been on the rise.3,11,12 Evidence of inconclusive study results and publication bias in these studies has led to concern that financial COI have influenced viscosupplementation outcomes.11 Previous investigations assessing the role of author COIs/industry funding as sources of bias in studies on viscosupplementation for knee OA have come to differing conclusions, with one study concluding that industry funding did not consistently affect the estimates of viscosupplementation efficacy, and another reporting an observed significant association between author COIs and qualitative study conclusions.13,14 These investigations, however, were limited by incomplete COI/funding information and included studies only as recent as the year 2012, and thus the conclusions may not translate to the more recent body of published literature. The purpose of the present study was systematically review and assess bibliometric data, financial COIs, and overall outcomes of contemporary viscosupplementation studies. We hypothesize that manuscripts favorably detailing viscosupplementation with HA are more likely to have COI.
2. Materials and methods
2.1. Literature search
All publications on the subject of viscosupplementation for knee OA published between January 2011 and April 2021 were identified by searching the PubMed, MEDLINE, and Web of Science databases. PRISMA guidelines were adhered to throughout literature identification and screening. Articles prior to 2011 were excluded to facilitate obtaining the most comprehensive information concerning potential conflicts of interest from the Physician Payments Sunshine Act. Primary keywords of interest related to any hyaluronic acid (or similarly derived) injections to native knees with primary osteoarthritis only. Other biologic injections such as platelet-rich plasma were not included in this study.
The search strategy utilized for identifying potential manuscripts was “Knee AND (Arthrit* OR Arthro* OR Osteo* OR Chondr* OR Intraarticular OR Intra-articular OR Inject* OR Joint OR Pain) AND (Viscosupplement* OR Regen* Hyaluron* OR Hyalgan OR Adant OR Arthrum OR Artz OR Artzal OR Arthrease OR Supartz OR Orthovisc OR Euflexxa OR Nuflexxa OR Durolane OR Hyruan OR Suvenyl OR Ostenil OR Replasyn OR Suplasyn OR Synject OR IA-BioHA OR Synvisc OR Gel-Syn OR Hylan*) NOT (ankle OR shoulder OR hip OR foot OR hand OR elbow).” This search was run March 2021.
2.2. Quality of studies
Articles were screened in 3 rounds using the web-based version of Rayyan Intelligent Systematic Review (Rayyan Systems Inc., Qatar Computing Research Institute, Doha, Qatar). Articles were first screened by title, by abstract, and then by full-text, respectively. A total of 3 independent reviewers completed the screening process, and a 2 out of 3 majority was required for study inclusion during each stage. Articles were included only if they were written in English and were available in their full-text form. All included manuscripts reported resulted that included functional outcomes utilizing a validated scoring tool. It was also required that the analysis included a comparison between a control and viscosupplementation. Also excluded were biomechanical and cadaveric studies. Any clinical studies containing treatment arms with fewer than 20 patients each, as well as commentaries, editorials, case reports, systematic reviews, and meta-analyses were excluded. Articles were excluded if disclosures or conflicts of interest were not reported within the manuscript. This review process was based upon and adapted from similar studies that have been successfully published regarding conflicts of interest.9,15,16
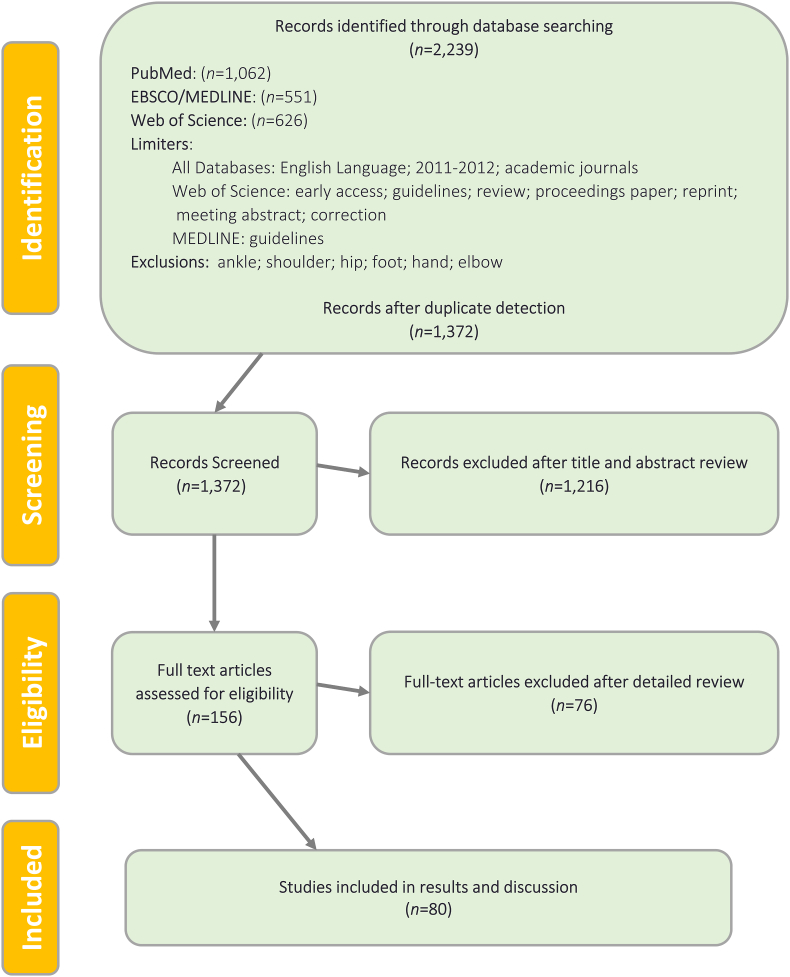
A PRISMA flow diagram depicting the manuscript screening process is presented in [Fig. 1].
Fig. 1.
PRISMA flow diagram for viscosupplementation in primary knee OA.
2.3. Data extraction
Data were collected through manual review of the full-text manuscripts that were selected for inclusion after the screening process. The year of publication, authors, open access status, industry funding presence and the specific industry/manufacturer name (if applicable), the years from which the study's data were collected until publication (for conflict of interest determination), impact factor (IF; InCites Journal Citations Report), and relative citation ratio (RCR; NIH iCite) were recorded.17 Level of evidence was defined based upon the criteria previously established by the Journal of Bone and Joint Surgery.
Conflicts of interest were identified in 2 ways for each study. First, each manuscript was manually reviewed for any disclosed conflicts of interest and/or industry funding. Additionally, the online Centers for Medicare and Medicaid Services (CMS) Open Payments reporting database was used to search all U.S.-based authors (non-international). Specifically, authors were searched for monetary payment disclosures, ownership of stocks/bonds, and/or funding provided for research. Conflicts of interest were deemed as relevant to a study when an industry/manufacturer that produced the viscosupplement referenced in the study provided any form of support to the study. Finally, any monetary payments/funding received during the time in which a study was conducted were recorded.
Manuscripts were categorized as having favorable, equivocal, or unfavorable outcomes. Favorable studies demonstrated statistical superiority (p < 0.05) of viscosupplementation versus a control. Equivocal studies had either no statistical significance (p 0.05), equivalent outcomes, or inconclusive results (i.e. neither the control nor the viscosupplementation was found to demonstrate superiority). Unfavorable studies demonstrated statistical inferiority (p < 0.05) and/or a lack of benefit for viscosupplementation when compared to a control. This protocol for analysis was based upon and adapted from similar research.9,15,16
2.4. Statistical analysis
Statistical analysis was performed in SAS 9.4 (SAS Institute, Cary, NC), and significance was set to α = 0.05. Between-groups differences in frequencies of categorical (study outcome) and ordinal (level of evidence) variables were analyzed with Pearson chi square tests. Between-groups differences in continuous variables (RCR, IF) were analyzed utilizing nonparametric Wilcoxon rank sum tests.
3. Results
After excluding studies lacking a COI statement or for which the level of evidence was <3, 67 studies were retained for analysis. A full list of the included studies, along with payment information is provided in [APPENDIX 1]. A large majority of studies presented Level I evidence (53, 79.1%). Over half were published in open access journals (42, 62.7%), with mean IF of 2.34 ± 1.65 and mean RCR of 3.77 ± 3.94. The large majority of studies reported favorable outcomes (48, 71.6%), with substantially fewer reporting equivocal (15, 22.4%) or negative (4, 6.0%) outcomes. Within this sample, 21 studies (31.3%) reported that at least one author had a COI, and all of those studies also disclosed industry funding. None of the remaining studies without a disclosed COI had any industry funding.
There were no relationships between reported COI and the outcomes of studies (Χ2 = 0.31, P = 0.577), levels of evidence (Χ2 = 3.48, P = 0.176), or RCR (S = 743, P = 0.591). However, studies reporting COIs/industry funding tended to be published in significantly higher impact journals (reporting COI: IF = 3.5 ± 2.0; no COI: IF = 1.8 ± 1.1; S = 950, P < 0.001). Study outcomes were not related to the probability of being published in an open access journal (Χ2 = 0.01, P = 0.960), nor to level of evidence (Χ2 = 2.67, P = 0.263), RCR (S = 618, P = 0.835), or IF (S = 563, P = 0.655). Level of evidence and the probability of being published in an open access journal were also unrelated (Χ2 = 0.51, P = 0.776).
4. Discussion
The current study reviewed the contemporary literature regarding viscosupplementation for the treatment of knee OA and sought to determine whether author COIs and/or industry funding affect study conclusions, level of evidence, and scientific impact (as measured by RCR and journal IF). Due to an observed lack of consistency in the available evidence regarding the efficacy of viscosupplementation for knee OA, several authors have previously investigated the published literature for factors to explain the discrepant study results, including potential sources of bias.4,11,18,19 One such source which has remained incompletely studied is potential investigator bias related to COIs and/or industry funding.
We hypothesized that “conflicted” studies (i.e., those in which authors disclosed COI and/or industry funding) would be more likely to report favorable conclusions than “nonconflicted” studies. This is due to the fact that author financial COIs and industry funding are more frequently associated with statistically significant results and favorable study outcomes.20,21 We found, on the other hand, that neither author COIs nor industry funding significantly affected study outcomes and conclusions. Additionally, there was no relationship between author COIs/industry funding and the level of evidence or RCR of the included studies.
It was, however, found with statistical significance that “conflicted” studies tended to be published in journals with significantly higher IFs than “nonconflicted” studies (IF = 3.5 vs. IF = 1.8). The same finding was also observed in the earlier Printz et al. study.13 The exact reason(s) for these findings remains unclear. Such a finding could indicate that these conflicted studies have the potential for being read by a wider audience than studies published in lower impact journals. In turn, the conflicted articles could become more impactful on general practice through increased reader attention and future citations in other impactful publications. Despite the significance of IF, neither level of evidence nor RCR between conflicted and nonconflicted studies demonstrated statistical significance, suggesting that the scientific quality/impact of contemporary viscosupplementation studies is not substantially influenced by COI or funding status.
Intraarticular HA remains controversial in the treatment of knee OA, owing in part to conflicting reports in the literature regarding its efficacy; even systematic reviews have produced discordant results, and this has been attributed to a high degree of various forms of bias and/or methodological flaws among published studies.4,7,8,11,18,19,22,23 Investigator COIs/industry funding can be one potential source of bias and carries the risk of influencing study design along with conduction, analysis, and reporting of outcomes. However, little is known regarding the specific impact of COIs on the contemporary viscosupplementation literature.
Earlier studies in this area have come to different conclusions.13,14 Wang et al. performed a meta-analysis of randomized controlled trials on the therapeutic effects of HA on OA of the knee, which included 20 trials published up to the year 2001.14 The authors concluded that industry funding did not consistently affect the estimates of HA efficacy based on 3 outcome end points (pain with activities, pain without activities, and function). More recently, Printz et al. performed an updated systematic review of prospective, randomized, placebo-controlled studies on the effects of HA injections for knee OA to evaluate whether industry sponsorship or author COIs were associated with study conclusions.13 Their investigation included 48 studies published from 1987 up to March/April 2012. The authors were unable to demonstrate any significant differences among qualitative study conclusions (favorable, neutral, unfavorable) between industry- and non-industry-sponsored studies, which was attributed to the small number of studies (only 3/48) which were non-industry-sponsored. However, they observed a significant difference in qualitative conclusions when studies were compared according to author affiliations. The studies in which any author had pharmaceutical company affiliations were significantly more likely to report favorable/neutral conclusions and significantly less likely to report unfavorable conclusions. The authors concluded that qualitative conclusions were associated with a financial COI with the sponsoring pharmaceutical company among viscosupplementation for knee OA studies.
The conflicting conclusions from these two previous investigations may be due to differences among the included studies, as the latter investigation considered studies published up to the year 2012, whereas the former only included studies up to the year 2001. Furthermore, the findings of those studies do not reflect the most recent decade of published literature and may have been limited by incomplete COI/funding information. For example, Printz et al. reported that study sponsorship information was unidentifiable in 31% of studies, which limited the strength of the findings and conclusions of that investigation.
For these reasons, in the current study we sought to review the contemporary literature and to include only those studies published during or after 2011, which coincides with the implementation of Physician Payments Sunshine Act. This ensured that the most accurate and detailed information pertaining to COIs/funding could be accessed. Having complete and accurate COI/funding information represents a strength of the current investigation, but it must be noted that the results of this study apply specifically to the contemporary viscosupplementation literature (i.e., those studies which have been published after the year 2010). The results may not translate to the older literature and it's unclear whether similar findings would have been observed when considering the entire body of viscosupplementation literature, had complete COI/funding been available for all studies.
Overall, only a small percentage of the studies included in this investigation, roughly one-third (31%), were “conflicted.” It should also be noted that all COIs were found to be related to industry funding of the studies. This percentage is lower than that reported in the previously mentioned systematic reviews, which both observed that the majority of studies were industry funded. Wang et al. reported that 60% of studies were industry funded, while Printz et al. reported that figure to be 63%.13,14 This discrepancy may reflect a decreasing industry influence on the viscosupplementation literature in the most recent decade.
On the other hand, a large majority of studies, nearly three-fourths (72%), reported favorable outcomes, regardless of COI/funding status. Furthermore, no statistical association was found between the presence of COI or industry funding and how often studies described favorable outcomes, with 76% of “conflicted” studies and 70% of “nonconflicted” studies reporting favorable outcomes. These findings contrast with those of Printz et al., who observed a much lower percentage of all studies reporting favorable qualitative outcomes (only 39.5% favorable).13 In addition, they found that none (0/17) of the “industry-authored” studies (i.e., those in which any of the authors had pharmaceutical company affiliation), reported unfavorable conclusions, while 35% (11/31) of studies with “academic authorship,” i.e., those in which all authors had academic affiliations, reported unfavorable conclusions.
It was postulated that this difference may have been the result of publication bias, with industry-sponsored trials with unfavorable results less likely to be presented and/or published as a result of influence from sponsoring companies/authors. It is unclear why such a large difference in the percentage of studies reporting favorable outcomes has been observed between our investigation and that of Printz et al. (72% versus 39.5%, respectively). This may be due to differing methodological quality, patient populations, and outcome instruments among the published studies. It could also reflect continued publication bias in the contemporary viscosupplementation literature, with studies demonstrating favorable outcomes being more frequently published than those reporting negative outcomes, regardless of author COIs and/or funding sources.
There are several limitations to this study. As mentioned previously, the findings reflect only the most recent literature, as we relied in part on Open Payments Data to determine COI/funding status. In addition, the accuracy of our analysis depends on the correctness of the reported COI/funding data, and it is possible that inaccurate reporting exists. The authors also acknowledge that there may also be underlying relationships between COI/funding across time with the evolution of the molecules/drugs formulations of various viscosupplements and their respective frequencies of administration. Finally, our assessments regarding the qualitative conclusions of the included studies were based upon the reported outcome measures and statistical analyses from each individual study. We did not attempt to interpret each study's results in the context of clinical significance, and it's possible that some studies reported “favorable” conclusions despite having clinically insignificant results. If the frequency of such reporting was influenced by COI/funding status, this could potentially have had an impact on the results.
Despite these limitations, this study presents a comprehensive review of the contemporary literature regarding viscosupplementation for the treatment of knee OA, offering additional insights into the impact of an author's COIs and industry funding on a study's outcomes and scientific quality. Industry and commercial support is a valuable and often necessary aspect of modern research, and a better understanding of its influence on a particular field can help clinicians and researchers effectively interpret the body of published literature.
5. Conclusions
The results of this review suggest that investigator COIs, and more specifically commercial funding of studies, do not significantly influence the frequency of favorable outcomes or study level of evidence within the contemporary body of published literature on viscosupplementation in treating knee OA. The percentage of recent viscosupplementation studies that were sponsored by industry was lower than previously reported, but the frequency of favorable outcomes was higher than previously reported, regardless of COI/funding status. It is, however, worth noting that studies reporting COIs/industry funding tended to be published in journals with significantly higher impact factors. The authors recommend that physicians carefully assess the published literature for potential COIs and consider the effects of hidden biases when interpreting study results.
Funding/sponsorship
None.
Informed consent
Not applicable.
Institutional ethical committee approval
Institutional Review Board approval was not required as this manuscript did not involve the use of human subjects.
Authors contribution
GGV: Conceptualization, Methodology, Data curation, Writing – Original Draft, Writing – Review & Editing. DAB: Conceptualization, Methodology, Data curation, Writing – Review & Editing. JGL: Conceptualization, Methodology, Writing – Original Draft, Writing – Review & Editing. TCF: Data curation, Writing – Original Draft, Writing – Review & Editing. AWF: Conceptualization, Methodology, Formal analysis, Writing – Original Draft, Writing – Review & Editing. ABK: Conceptualization, Data curation, Supervision, Validation, Writing – Review & Editing.
Declaration of competing interest
The authors have no relevant financial or non-financial interests to declare.
Acknowledgements
None.
Appendix 1. Included primary knee osteoarthritis viscosupplementation studies
| Author (et al.) | Article Title | Journal | Year | Conclusiona | Level of Evidence | Disclosed Conflict | Mean Author Payment ($USD) | Journal Impact Factor | Relative Citation Ratio | Open Access |
|---|---|---|---|---|---|---|---|---|---|---|
| Navarro-Sarabia | A 40-Month Multicentre, Randomized Placebo-Controlled Study to Assess the Efficacy and Carry-Over Effect of Repeated Intra-Articular Injections of Hyaluronic Acid in Knee Osteoarthritis: The AMELIA Project | Ann Rheum Dis | 2011 | Favorable | 1 | Y | 0.00 | 8.727 | 4.66 | Y |
| Huang | Intra-Articular Injections of Sodium Hyaluronate (Hyalgan®) in Osteoarthritis of the Knee. A Randomized, Controlled, Double-Blind, Multicenter Trial in the Asian Population | BMC Musculoskelet Disord | 2011 | Favorable | 1 | N | 0.00 | 1.577 | 3.17 | Y |
| Altman | Safety and Efficacy of Retreatment with a Bioengineered Hyaluronate for Painful Osteoarthritis of the Knee: Results of the Open-Label Extension Study of the FLEXX Trial | Osteoarthritis Cartilage | 2011 | Favorable | 1 | Y | 0.00 | 3.904 | 1.54 | Y |
| DeCaria | The Effect of Intra-Articular Hyaluronic Acid Treatment on Gait Velocity in Older Knee Osteoarthritis Patients: A Randomized, Controlled Study | Arch Gerontol Geriatr | 2012 | Against | 1 | N | 0.00 | 1.704 | 1.87 | Y |
| Strand | A Multicenter, Randomized Controlled Trial Comparing a Single Intra-Articular Injection of Gel-200, A New Cross-Linked Formulation of Hyaluronic Acid, to Phosphate Buffered Saline for Treatment of Osteoarthritis of the Knee | Osteoarthritis Cartilage | 2012 | Favorable | 1 | Y | 0.00 | 4.262 | 3.76 | Y |
| Spakova | Treatment of Knee Joint Osteoarthritis with Autologous Platelet-Rich Plasma in Comparison with Hyaluronic Acid | Am J Phys Med Rehabil | 2012 | Favorable | 1 | N | 0.00 | 2.358 | 9.68 | N |
| Cerza | Comparison Between Hyaluronic Acid and Platelet-Rich Plasma, Intra-Articular Infiltration in the Treatment of Gonarthrosis | Am J Sports Med | 2012 | Favorable | 1 | N | 0.00 | 4.439 | 11.07 | N |
| Sánchez | A Randomized Clinical Trial Evaluating Plasma Rich in Growth Factors (PRGF-Endoret) Versus Hyaluronic Acid in the Short-Term Treatment of Symptomatic Knee Osteoarthritis | Arthroscopy | 2012 | Favorable | 1 | N | 0.00 | 0.000 | 10.66 | N |
| Strand | Effectiveness and Safety of a Multicenter Extension and Retreatment Trial of Gel-200 in Patients with Knee Osteoarthritis | Cartilage | 2012 | Favorable | 1 | Y | 0.00 | 0.000 | 0.40 | Y |
| Filardo | Platelet-Rich Plasma vs Hyaluronic Acid to Treat Knee Degenerative Pathology: Study Design and Preliminary Results of a Randomized Controlled Trial | BMC Musculoskelet Disord | 2012 | Favorable | 1 | N | 0.00 | 1.875 | 9.81 | Y |
| Vaquerizo | Comparison of Intra-Articular Injections of Plasma Rich in Growth Factors (PRGF-Endoret) Versus Durolane Hyaluronic Acid in the Treatment of Patients with Symptomatic Osteoarthritis: A Randomized Controlled Trial | Arthroscopy | 2013 | Favorable | 1 | Y | 0.00 | 1.144 | 7.36 | N |
| Vincent | “Functional Pain,” Functional Outcomes, and Quality of Life After Hyaluronic Acid Intra-Articular Injection for Knee Osteoarthritis | PM R | 2013 | Favorable | 3 | N | 0.00 | 1.662 | 0.59 | N |
| Oka | The Mid-Term Efficacy of Intra-Articular Hyaluronic Acid Injections on Joint Structure: A Nested Case Control Study | Mod Rheumatol | 2013 | Favorable | 3 | N | 0.00 | 2.206 | 0.19 | N |
| Chen | Comparison of Intra-Articular Hyaluronic Acid Injections with Transcutaneous Electric Nerve Stimulation for the Management of Knee Osteoarthritis: A Randomized Controlled Trial | Arch Phys Med Rehabil | 2013 | Favorable | 1 | N | 0.00 | 2.441 | 0.92 | Y |
| Say | Platelet-Rich Plasma Injection is More Effective than Hyaluronic Acid in the Treatment of Knee Osteoarthritis | Acta Chir Orthop Traumatol Cech | 2013 | Favorable | 2 | N | 0.00 | 0.415 | 2.69 | N |
| Housman | Intra-Articular Hylastan Versus Steroid for Knee Osteoarthritis | Knee Surg Sports Traumatol Arthrosc | 2014 | Against | 1 | Y | 0.00 | 3.053 | 1.84 | N |
| Khalaj | Effect of Intra-Articular Hyaluronic Injection on Postural Stability and Risk of Fall in Patients with Bilateral Knee Osteoarthritis | Scientific World Journal | 2014 | Favorable | 1 | N | 0.00 | 1.524 | 0.35 | Y |
| Ishijima | Intra-Articular Hyaluronic Acid Injection Versus Oral Non-Steroidal Anti-Inflammatory Drug for the Treatment of Knee Osteoarthritis: A Multi-Center, Randomized, Open-Label, Non-Inferiority Trial | Arthritis Res Ther | 2014 | Equivocal | 1 | N | 0.00 | 3.753 | 2.65 | Y |
| Arden | A Randomized Saline-Controlled Trial of NASHA Hyaluronic Acid for Knee Osteoarthritis | Curr Med Res Opin | 2014 | Equivocal | 1 | Y | 0.00 | 2.653 | 2.12 | N |
| Giarratana | A Randomized Double-Blind Clinical Trial on the Treatment of Knee Osteoarthritis: The Efficacy of Polynucleotides Compared to Standard Hyaluronin Viscosupplementation | Knee | 2014 | Favorable | 1 | N | 0.00 | 0.000 | 0.89 | N |
| Leighton | NASHA Hyaluronic Acid vs. Methylprednisolone for Knee Osteoarthritis: A Prospective, Multi-Centre, Randomized, Non-Inferiority Trial | Osteoarthritis Cartilage | 2014 | Favorable | 1 | Y | 0.00 | 4.165 | 3.92 | Y |
| Guler | Comparison of Short-Term Results of Intraarticular Platelet-Rich Plasma (PRP) and Hyaluronic Acid Treatments in Early-Stage Gonarthrosis Patients | Eur J Orthop Surg Traumatol | 2015 | Favorable | 3 | N | 0.00 | 0.000 | 1.41 | N |
| Abate | Efficacy and Safety Profile of a Compound Composed of Platelet-Rich Plasma and Hyaluronic Acid in the Treatment for Knee Osteoarthritis (Preliminary Results) | Eur J Orthop Surg Traumatol | 2015 | Equivocal | 2 | N | 0.00 | 0.000 | 1.47 | N |
| Kilincoglu | Short Term Results Comparison of Intraarticular Platelet-Rich Plasma (PRP) and Hyaluronic Acid (HA) Applications in Early Stage of Knee Osteoarthritis | Int J Clin Exp Med | 2015 | Favorable | 3 | N | 0.00 | 0.000 | 1.06 | Y |
| Davalillo | Clinical Efficacy of Intra-Articular Injections in Knee osteoarthritis: A Prospective Randomized Study Comparing Hyaluronic acid and Betamethasone | Open Access Rheumatol | 2015 | Equivocal | 1 | N | 0.00 | 0.000 | 1.05 | Y |
| Raeissadat | Knee Osteoarthritis Injection Choices: Platelet- Rich Plasma (PRP) Versus Hyaluronic Acid (A one-year randomized clinical trial) | Clin Med Insights Arthritis Musculoskelet Disord | 2015 | Favorable | 1 | N | 0.00 | 0.000 | 11.22 | Y |
| Filardo | Platelet-Rich Plasma Intra-articular Knee Injections Show No Superiority Versus Viscosupplementation: A Randomized Controlled Trial | Am J Sports Med | 2015 | Equivocal | 1 | Y | 0.00 | 4.517 | 12.94 | Y |
| van der Weegen | No Difference Between Intra-Articular Injection of Hyaluronic Acid and Placebo for Mild to Moderate Knee Osteoarthritis: A Randomized, Controlled, Double-Blind Trial | J Arthroplasty | 2015 | Against | 1 | N | 0.00 | 2.515 | 2.51 | N |
| Petrella | Pain Relief and Improved Physical Function in Knee Osteoarthritis Patients Receiving Ongoing Hylan G-F 20, a High-Molecular-Weight Hyaluronan, Versus Other Treatment Options: Data from a Large Real-World Longitudinal Cohort in Canada | Drug Des Devel Ther | 2015 | Favorable | 3 | Y | 0.00 | 0.000 | 0.80 | Y |
| Rosen | Cost-Effectiveness of Different Forms of Intra-Articular Injections for the Treatment of Osteoarthritis of the Knee | Adv Ther | 2016 | Favorable | 3 | Y | 209,585.31 | 3.847 | 1.77 | Y |
| Montañez-Heredia | Intra-Articular Injections of Platelet-Rich Plasma Versus Hyaluronic Acid in the Treatment of Osteoarthritic Knee Pain: A Randomized Clinical Trial in the Context of the Spanish National Health Care System | Int J Mol Sci | 2016 | Favorable | 1 | N | 0.00 | 3.226 | 5.31 | Y |
| Saccomanno | Efficacy of Intra-Articular Hyaluronic Acid Injections and Exercise-Based Rehabilitation Programme, Administered as Isolated or Integrated Therapeutic Regimens for the Treatment of Knee Osteoarthritis | Knee Surg Sports Traumatol Arthrosc | 2016 | Favorable | 1 | N | 0.00 | 3.227 | 0.81 | N |
| Giombini | Comparison Between Intrarticular Injection of Hyaluronic Acid, Oxygen Ozone, and the Combination of Both in the Treatment of Knee Osteoarthrosis | J Biol Regul Homeost Agents | 2016 | Favorable | 1 | N | 0.00 | 1.469 | 1.90 | N |
| Bisicchia | HYADD 4 Versus Methylprednisolone Acetate in Symptomatic Knee Osteoarthritis: A Single-Centre Single Blind Prospective Randomized Controlled Clinical Study with 1-Year Follow-Up | Clin Exp Rheumatol | 2016 | Favorable | 1 | N | 0.00 | 2.634 | 1.45 | N |
| Askari | Hyaluronic Acid Compared with Corticosteroid Injections for the Treatment of Osteoarthritis of the Knee: A Randomized Control Trail | Springerplus | 2016 | Against | 1 | N | 0.00 | 1.130 | 1.81 | Y |
| Tammachote | Intra-Articular, Single-Shot Hylan G-F 20 Hyaluronic Acid Injection Compared with Corticosteroid in Knee Osteoarthritis: A Double-Blind, Randomized Controlled Trial | J Bone Joint Surg Am | 2016 | Favorable | 1 | N | 0.00 | 4.840 | 3.63 | N |
| Martin | A Double Blind Randomized Active-Controlled Clinical Trial on the Intra-Articular Use of Md-Knee Versus Sodium Hyaluronate in Patients with Knee Osteoarthritis (“Joint") | BMC Musculoskelet Disord | 2016 | Equivocal | 1 | N | 0.00 | 1.739 | 1.33 | Y |
| Lana | Randomized Controlled Trial Comparing Hyaluronic Acid, Platelet-Rich Plasma and the Combination of Both in the Treatment of Mild and Moderate Osteoarthritis of the Knee | J Stem Cells Regen Med | 2016 | Favorable | 1 | N | 0.00 | 0.000 | 5.04 | Y |
| Cole | Hyaluronic Acid Versus Platelet-Rich Plasma: A Prospective, Double-Blind Randomized Controlled Trial Comparing Clinical Outcomes and Effects on Intra-articular Biology for the Treatment of Knee Osteoarthritis | Am J Sports Med | 2017 | Equivocal | 1 | Y | 1,452,516.02 | 6.057 | 13.88 | N |
| Duymus | Choice of Intra-Articular Injection in Treatment of Knee Osteoarthritis: Platelet-Rich Plasma, Hyaluronic Acid or Ozone Options | Knee Surg Sports Traumatol Arthrosc | 2017 | Equivocal | 1 | N | 0.00 | 3.210 | 11.52 | N |
| Lee | Safety and Efficacy of Bi-Annual Intra-Articular LBSA0103 Injections in Patients with Knee Osteoarthritis | Rheumatol Int | 2017 | Favorable | 1 | N | 0.00 | 1.952 | 0.52 | N |
| Campos | Viscosupplementation in Patients with Severe Osteoarthritis of the Knee: Six Month Follow-Up of a Randomized, Double-Blind Clinical Trial | Int Orthop | 2017 | Equivocal | 1 | N | 0.00 | 2.377 | 1.47 | N |
| Vaishya | Intra-Articular Hyaluronic Acid is Superior to Steroids in Knee Osteoarthritis: A Comparative, Randomized Study | J Clin Orthop Trauma | 2017 | Equivocal | 1 | N | 0.00 | 0.000 | 1.16 | Y |
| Raeissadat | Efficacy of Intra-articular Injection of a Newly Developed Plasma Rich in Growth Factor (PRGF) Versus Hyaluronic Acid on Pain and Function of Patients with Knee Osteoarthritis: A Single-Blinded Randomized Clinical Trial | Clin Med Insights Arthritis Musculoskelet Disord | 2017 | Equivocal | 1 | N | 0.00 | 0.760 | 2.97 | Y |
| Kizhedath | Comparative Study of Intra-Articular Hyaluronic Acid and Intra-Articular Triamcinolone Hexacetonide in Primary Osteoarthritis of Knee | J Evol Med Dent Sci | 2017 | Equivocal | 2 | N | 0.00 | 0.010 | 0.00 | N |
| Görmeli | Multiple PRP Injections are More Effective than Single injections and Hyaluronic Acid in Knees with Early Osteoarthritis: A Randomized, Double-Blind, Placebo-Controlled Trial | Knee Surg Sports Traumatol Arthrosc | 2017 | Favorable | 1 | N | 0.00 | 3.210 | 16.36 | N |
| Buendía‐López | Clinical and Radiographic Comparison of a Single LP-PRP Injection, a Single Hyaluronic Acid Injection and Daily NSAID Administration with a 52-week Follow-up: A Randomized Controlled Trial | J Orthop Traumatol | 2018 | Favorable | 2 | N | 0.00 | 1.826 | 5.73 | Y |
| Hangody | Intraarticular Injection of a Cross-Linked Sodium Hyaluronate Combined with Triamcinolone Hexacetonide (Cingal) to Provide Symptomatic Relief of Osteoarthritis of the Knee: A Randomized, Double-Blind, Placebo-Controlled Multicenter Clinical Trial | Cartilage | 2018 | Favorable | 1 | Y | 0.00 | 2.961 | 3.78 | Y |
| Liu | Longterm Effectiveness of Intraarticular Injections on Patient-Reported Symptoms in Knee Osteoarthritis | J Rheumatol | 2018 | Against | 2 | N | 0.00 | 3.634 | 1.45 | Y |
| Bao | Effect of Therapeutic Exercise on Knee Osteoarthritis After Intra-Articular Injection of Botulinum Toxin Type A, Hyaluronate or Saline: A Randomized Controlled Trial | J Rehabil Med | 2018 | Favorable | 1 | N | 0.00 | 1.907 | 1.48 | Y |
| Lisi | Treatment of Knee Osteoarthritis: Platelet-Derived Growth Factors vs. Hyaluronic Acid. A Randomized Controlled Trial | Clin Rehabil | 2018 | Favorable | 1 | N | 0.00 | 2.738 | 3.52 | N |
| Yu | Clinical Therapy of Hyaluronic Acid Combined with Platelet-Rich Plasma for the Treatment of Knee Osteoarthritis | Exp Ther Med | 2018 | Equivocal | 1 | N | 0.00 | 1.448 | 4.28 | Y |
| Raeissadat | Intra-Articular Ozone or Hyaluronic Acid Injection: Which One is Superior in Patients with Knee Osteoarthritis? A 6-month Randomized Clinical Trial | J Pain Res | 2018 | Equivocal | 1 | N | 0.00 | 2.236 | 4.13 | Y |
| Conrozier | Getting Better or Getting Well? The Patient Acceptable Symptom State (PASS) Better Predicts Patient's Satisfaction than the Decrease of Pain, in Knee Osteoarthritis Subjects Treated with Viscosupplementation | Cartilage | 2018 | Favorable | 3 | Y | 0.00 | 2.961 | 1.66 | Y |
| Guo | Origin and Efficacy of Hyaluronan Injections in Knee Osteoarthritis: Randomized, Double-Blind Trial | Med Sci Monit | 2018 | Favorable | 1 | N | 0.00 | 1.980 | 1.37 | Y |
| Huang | Intra-Articular Injections of Platelet-Rich Plasma, Hyaluronic Acid or Corticosteroids for Knee Osteoarthritis: A Prospective Randomized Controlled Study | Orthopade | 2019 | Favorable | 1 | N | 0.00 | 0.823 | 9.54 | N |
| Maia | Viscosupplementation Improves Pain, Function and Muscle Strength, but Not Proprioception, in Patients with Knee Osteoarthritis: A Prospective Randomized Trial | Clinics (Sao Paulo) | 2019 | Favorable | 1 | N | 0.00 | 0.000 | 0.92 | Y |
| Lin | Intra-articular Injection of Platelet-Rich Plasma Is Superior to Hyaluronic Acid or Saline Solution in the Treatment of Mild to Moderate Knee Osteoarthritis: A Randomized, Double-Blind, Triple-Parallel, Placebo-Controlled Clinical Trial | Arthroscopy | 2019 | Favorable | 1 | N | 0.00 | 1.313 | 10.16 | N |
| Hermans | The Effectiveness of High Molecular Weight Hyaluronic Acid for Knee Osteoarthritis in Patients in the Working Age: A Randomized Controlled Trial | BMC Musculoskelet Disord | 2019 | Favorable | 1 | N | 0.00 | 1.879 | 2.00 | Y |
| Takamura | A Single Intra-Articular Injection of Gel-200 for Treatment of Symptomatic Osteoarthritis of the Knee Is More Effective than Phosphate Buffered Saline at 6 Months: A Subgroup Analysis of a Multicenter, Randomized Controlled Trial | Cartilage | 2019 | Favorable | 1 | Y | 0.00 | 2.961 | 1.82 | Y |
| Petterson | Single Intra-Articular Injection of Lightly Cross-Linked Hyaluronic Acid Reduces Knee Pain in Symptomatic Knee Osteoarthritis: A Multicenter, Double-Blind, Randomized, Placebo-Controlled Trial | Knee Surg Sports Traumatol Arthrosc | 2019 | Favorable | 1 | Y | 7905.76 | 3.166 | 3.50 | N |
| Parisi | Ultrasound-Guided Intra-Articular Injection: Efficacy of Hyaluronic Acid Compared to Glucocorticoid in the Treatment of Knee Osteoarthritis | Minerva Med | 2019 | Equivocal | 3 | N | 0.00 | 3.031 | 1.50 | N |
| Farr | A Randomized Controlled Single-Blind Study Demonstrating Superiority of Amniotic Suspension Allograft Injection Over Hyaluronic Acid and Saline Control for Modification of Knee Osteoarthritis Symptoms | J Knee Surg | 2019 | Favorable | 1 | Y | 34,712.30 | 1.986 | 2.26 | Y |
| Hosseini | Periarticular Hypertonic Dextrose vs Intraarticular Hyaluronic Acid Injections: A Comparison of Two Minimally Invasive Techniques in the Treatment of Symptomatic Knee Osteoarthritis | Open Access Rheumatol | 2019 | Against | 1 | N | 0.00 | 0.430 | 0.25 | Y |
| Tavassoli | Single- and Double-Dose of Platelet-Rich Plasma Versus Hyaluronic Acid for Treatment of Knee Osteoarthritis: A Randomized Controlled Trial | World J Orthop | 2019 | Favorable | 1 | N | 0.00 | 0.610 | 3.68 | Y |
| Pereira | Gait Analysis Following Single-Shot Hyaluronic Acid Supplementation: A Pilot Randomized Double-Blinded Controlled Trial | Pilot Feasibility Stud | 2019 | Favorable | 1 | N | 0.00 | 0.000 | 0.45 | Y |
| Di Martino | Platelet-Rich Plasma Versus Hyaluronic Acid Injections for the Treatment of Knee Osteoarthritis: Results at 5 Years of a Double-Blind, Randomized Controlled Trial | Am J Sports Med | 2019 | Equivocal | 1 | Y | 0.00 | 5.810 | 12.33 | Y |
| Annaniemi | Platelet-Rich Plasma Versus Hyaluronic Acid Injections for Knee Osteoarthritis: A Propensity-Score Analysis | Scand J Surg | 2019 | Favorable | 3 | N | 0.00 | 1.950 | 0.33 | Y |
| de Sire | Long-Term Effects of Intra-Articular Oxygen-Ozone Therapy Versus Hyaluronic Acid in Older People Affected by Knee Osteoarthritis: A Randomized Single-Blind Extension Study | J Back Musculoskelet Rehabil | 2020 | Favorable | 1 | N | 0.00 | 1.398 | 7.12 | N |
| Rezasoltani | Physical Therapy, Intra-Articular Dextrose Prolotherapy, Botulinum Neurotoxin, and Hyaluronic Acid for Knee Osteoarthritis: Randomized Clinical Trial | Int J Rehabil Res | 2020 | Favorable | 1 | N | 0.00 | 1.479 | 1.56 | N |
| Jokar | Intra-Articular Hyaluronic Acid Injection vs. Atorvastatin; Which Treatment is More Effective in Controlling Symptoms of Knee Osteoarthritis? A Clinical Trial | Acta Reumatol Port | 2020 | Equivocal | 1 | N | 0.00 | 1.290 | 0.00 | Y |
| Kandel | A Novel Approach for Knee Osteoarthritis Using High Molecular Weight Hyaluronic Acid Conjugated to Plasma Fibrinogen - Interim Findings of a Double-Blind Clinical Study | Heliyon | 2020 | Favorable | 1 | Y | 0.00 | 0.280 | 0.61 | Y |
| Kesiktas | Comparison of the Short-Term Results of Single-Dose Intra-Articular Peptide with Hyaluronic Acid and Platelet-Rich Plasma Injections in Knee Osteoarthritis: A Randomized Study | Clin Rheumatol | 2020 | Favorable | 1 | N | 0.00 | 2.980 | 2.30 | Y |
| Kim | Comparative Matched-Pair Cohort Analysis of the Short-Term Clinical Outcomes of Mesenchymal Stem Cells Versus Hyaluronic Acid Treatments Through Intra-Articular Injections for Knee Osteoarthritis | J Exp Orthop | 2020 | Favorable | 3 | N | 0.00 | 0.000 | 0.44 | Y |
| Mackowiak | A Comparison of 4-Year Total Medical Care Costs, Adverse Outcomes, and Opioid/Prescription Analgesic Use for 3 Knee Osteoarthritis Pain Treatments: Intra-Articular Hyaluronic Acid, Intra-Articular Corticosteroids, and Knee Arthroplasty | Semin Arthritis Rheum | 2020 | Favorable | 3 | Y | 194,758.64 | 5.532 | 0.97 | Y |
| Raeissadat | Platelet-Rich Plasma-Derived Growth Factor vs Hyaluronic Acid Injection in the Individuals with Knee Osteoarthritis: A One Year Randomized Clinical Trial | J Pain Res | 2020 | Favorable | 1 | N | 0.00 | 3.133 | 1.48 | Y |
| Etter | High-Concentration Nonavian High-Molecular Weight Hyaluronan Injections and Time-to-Total Knee Replacement Surgery | J Comp Eff Res | 2020 | Favorable | 3 | Y | 0.00 | 1.744 | 0.38 | Y |
| Yaradilmis | Comparison of Two Platelet Rich Plasma Formulations with Viscosupplementation in Treatment of Moderate Grade Gonarthrosis: A Prospective Randomized Controlled Study | J Orthop | 2020 | Favorable | 1 | N | 0.00 | 0.470 | 2.30 | Y |
| Khurana | Efficacy of Autologous Conditioned Serum (ACS), Platelet-Rich Plasma (PRP), Hyaluronic Acid (HA) and Steroid for Early Osteoarthritis Knee: A Comparative Analysis | Indian J Orthop | 2020 | Favorable | 2 | N | 0.00 | 1.251 | 0.34 | Y |
| Raeissadat | The Comparison Effects of Intra-Articular Injection of Platelet Rich Plasma (PRP), Plasma Rich in Growth Factor (PRGF), Hyaluronic Acid (HA), and Ozone in Knee Osteoarthritis; A One Year Randomized Clinical Trial | BMC Musculoskelet Disord | 2021 | Favorable | 1 | N | 0.00 | 0.000 | 0.00 | Y |
aOutcome conclusion reported as viscosupplementation vs. control.
References
- 1.He W.-W., Kuang M.-J., Zhao J., et al. Efficacy and safety of intraarticular hyaluronic acid and corticosteroid for knee osteoarthritis: a meta-analysis. Int J Surg. 2017;39:95–103. doi: 10.1016/j.ijsu.2017.01.087. [DOI] [PubMed] [Google Scholar]
- 2.Altman R., Hackel J., Niazi F., Shaw P., Nicholls M. Efficacy and safety of repeated courses of hyaluronic acid injections for knee osteoarthritis: a systematic review. Semin Arthritis Rheum. 2018;48(2):168–175. doi: 10.1016/j.semarthrit.2018.01.009. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y., Chen X., Tong Y., Luo J., Bi Q. Development and prospect of intra-articular injection in the treatment of osteoarthritis: a review. J Pain Res. 2020;13:1941–1955. doi: 10.2147/JPR.S260878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rutjes A.W.S., Jiini P., Da Costa B.R., Trelle S., Niiesch E., Reichenbach S. Viscosupplementation for osteoarthritis of the knee A systematic review and meta-analysis. Ann Intern Med. 2012;157:180–191. doi: 10.7326/0003-4819-157-3-201208070-00473. www.annals.org [DOI] [PubMed] [Google Scholar]
- 5.Hunter D.J. Viscosupplementation for osteoarthritis of the knee. N Engl J Med. 2015;372(11):1040–1047. doi: 10.1056/NEJMct1215534. [DOI] [PubMed] [Google Scholar]
- 6.Reid M.C. Viscosupplementation for osteoarthritis: a primer for primary care physicians. Adv Ther. 2013;30(11):967–986. doi: 10.1007/s12325-013-0068-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brophy R.H., Fillingham Y.A. AAOS clinical practice guideline summary: management of osteoarthritis of the knee (nonarthroplasty), third edition. J Am Acad Orthop Surg. 2022;30(9):e721–e729. doi: 10.5435/JAAOS-D-21-01233. [DOI] [PubMed] [Google Scholar]
- 8.Jevsevar D.S. Treatment of osteoarthritis of the knee: evidence-based guideline, 2nd edition. J Am Acad Orthop Surg. 2013;21(9):571–576. doi: 10.5435/JAAOS-21-09-571. [DOI] [PubMed] [Google Scholar]
- 9.Narain A.S., Hijji F.Y., Yom K.H., Kudaravalli K.T., Singh K. Cervical disc arthroplasty: do conflicts of interest influence the outcome of clinical studies? Spine J. 2017;17(7):1026–1032. doi: 10.1016/j.spinee.2017.03.018. [DOI] [PubMed] [Google Scholar]
- 10.Walcott B.P., Sheth S.A., Nahed B.V., Coumans J.-V. Conflict of interest in spine research reporting. PLoS One. 2012;7(8) doi: 10.1371/journal.pone.0044327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jevsevar D., Donnelly P., Brown G.A., Cummins D.S. Viscosupplementation for Osteoarthritis of the Knee: a systematic review of the evidence. J Bone Jt Surg - Am. 2014;97(24):2047–2060. doi: 10.2106/JBJS.N.00743. [DOI] [PubMed] [Google Scholar]
- 12.Bannuru R.R., Natov N.S., Dasi U.R., Schmid C.H., McAlindon T.E. Therapeutic trajectory following intra-articular hyaluronic acid injection in knee osteoarthritis - meta-analysis. Osteoarthritis Cartilage. 2011;19(6):611–619. doi: 10.1016/j.joca.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 13.Printz J.O., Lee J.J., Knesek M., Urquhart A.G. Conflict of interest in the assessment of hyaluronic acid injections for osteoarthritis of the knee: an updated systematic review. J Arthroplasty. 2013;28(8 SUPPL):30–33.e1. doi: 10.1016/j.arth.2013.05.034. [DOI] [PubMed] [Google Scholar]
- 14.Wang C.-T., Lin J., Chang C.-J., Lin Y.-T., Hou S.-M. Therapeutic effects of hyaluronic acid on osteoarthritis of the knee. J Bone Jt Surg. 2004;86(3):538–545. doi: 10.2106/00004623-200403000-00012. [DOI] [PubMed] [Google Scholar]
- 15.DeFrance M.J., Yayac M.F., Courtney P.M., Squire M.W. The impact of author financial conflicts on robotic-assisted joint arthroplasty research. J Arthroplasty. 2020 doi: 10.1016/j.arth.2020.10.033. [DOI] [PubMed] [Google Scholar]
- 16.Cavinatto L., Bronson M.J., Chen D.D., Moucha C.S. Robotic-assisted versus standard unicompartmental knee arthroplasty—evaluation of manuscript conflict of interests, funding, scientific quality and bibliometrics. Int Orthop. 2019;43(8):1865–1871. doi: 10.1007/s00264-018-4175-5. [DOI] [PubMed] [Google Scholar]
- 17.Hutchins B.I., Yuan X., Anderson J.M., Santangelo G.M. Relative citation ratio (RCR): a new metric that uses citation rates to measure influence at the article level. PLoS Biol. 2016;14(9):1–25. doi: 10.1371/journal.pbio.1002541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colen S., Van Den Bekerom M.P.J., Mulier M., Haverkamp D. Hyaluronic acid in the treatment of knee osteoarthritis A systematic review and meta-analysis with emphasis on the efficacy of different products. BioDrugs. 2012;26(4):257–268. doi: 10.2165/11632580-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 19.Johansen M., Bahrt H., Altman R.D., et al. Exploring reasons for the observed inconsistent trial reports on intra-articular injections with hyaluronic acid in the treatment of osteoarthritis: meta-regression analyses of randomized trials. Semin Arthritis Rheum. 2016;46(1):34–48. doi: 10.1016/j.semarthrit.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 20.Ahn R., Woodbridge A., Abraham A., et al. Financial ties of principal investigators and randomized controlled trial outcomes: cross sectional study. BMJ. 2017;356 doi: 10.1136/bmj.i6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lundh A., Lexchin J., Mintzes B., Schroll J.B., Bero L. Industry sponsorship and research outcome: systematic review with meta-analysis. Intensive Care Med. 2018;44(10):1603–1612. doi: 10.1007/s00134-018-5293-7. [DOI] [PubMed] [Google Scholar]
- 22.Bellamy N., Campbell J., Welch V., Gee T.L., Bourne R., Wells G.A. Viscosupplementation for the treatment of osteoarthritis of the knee. Cochrane Database Syst Rev. 2006;2014(11) doi: 10.1002/14651858.CD005321.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller L.E., Block J.E. US-approved intra-articular hyaluronic acid injections are safe and effective in patients with knee osteoarthritis: systematic review and meta-analysis of randomized, saline-controlled trials. Clin Med Insights Arthritis Musculoskelet Disord. 2013;6:57–63. doi: 10.4137/CMAMD.S12743. [DOI] [PMC free article] [PubMed] [Google Scholar]