Abstract
Background
Reduced intake and absorption of antioxidants due to pain and malabsorption are probable causes of the lower levels of antioxidants observed in patients with chronic pancreatitis (CP). Improving the status of antioxidants might be effective in slowing the disease process and reducing pain in CP.
Objectives
To assess the benefits and harms of antioxidants for the treatment of pain in patients with CP.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE and the Conference Proceedings Citation Index from inception to October 2012. Two review authors performed the selection of trials independently.
Selection criteria
We included all randomised controlled trials (RCTs) evaluating antioxidants for treatment of pain in CP. All trials were included irrespective of blinding, numbers of participants randomly assigned and language of the article.
Data collection and analysis
Two review authors extracted data independently. The risk of bias of included trials was assessed. Study authors were asked for additional information in the case of missing data.
Main results
Twelve RCTs with a total of 585 participants were included. Six trials were double‐blinded, placebo‐controlled studies, and the other six trials were of less adequate methodology. Most trials were small and had high rates of dropout. Eleven of the 12 included trials described the effects of antioxidants on chronic abdominal pain in chronic pancreatitis. Pain as measured on a visual analogue scale (VAS, scale range 0 to 10) after one to six months was less in the antioxidant group than in the control group (mean difference (MD) ‐0.33, 95% confidence interval (CI) ‐0.64 to ‐0.02, P value 0.04, moderate‐quality evidence). The number of pain‐free participants was not statistically significantly different (risk ratio (RR) 1.73, 95% CI 0.95 to 3.15, P value 0.07, low‐quality evidence). More adverse events were observed in the antioxidant group, both in the parallel trials (RR 4.43, 95% CI 1.60 to 12.29, P value 0.0004, moderate‐quality evidence) and in the cross‐over trials (RR 5.80, 95% CI 1.56 to 21.53, P value 0.0009, moderate‐quality evidence). Adverse events occurred in 16% of participants and were mostly mild (e.g. headache, gastrointestinal complaints), but were sufficient to make participants stop antioxidant use. Other important outcomes such as use of analgesics, exacerbation of pancreatitis and quality of life were rarely reported. One trial from 1991 evaluated the effects of antioxidants on acute pain during exacerbation of chronic pancreatitis and found that a significantly higher proportion of participants in the antioxidant group experienced pain relief. This trial was conducted more than 25 years ago and has not been reproduced since that time. Therefore, additional trials are needed before reliable conclusions can be drawn.
Authors' conclusions
Current evidence shows that antioxidants can reduce pain slightly in patients with chronic pancreatitis. The clinical relevance of this small reduction is uncertain, and more evidence is needed. Adverse events in one of six patients may prevent the use of antioxidants. Effects of antioxidants on other outcome measures, such as use of analgesics, exacerbation of pancreatitis and quality of life remain uncertain because reliable data are not available.
Plain language summary
Antioxidants to reduce pain in chronic pancreatitis
Chronic pancreatitis is a persistent inflammation of the pancreas that in the long run can cause irreparable damage. The major causes of chronic pancreatitis are genetics, alcohol toxicity and other conditions that might damage or obstruct the pancreas. This inflammation can cause pain that often is severe and leaves patients socially isolated and unable to perform their jobs. Unfortunately, treatment options are scarce, and often strong morphine‐like pain medications are needed. Patients might benefit from alternative medication without the adverse effects associated with morphine‐like medication. This review summarises the evidence from randomised trials on the effects of antioxidants in chronic pancreatitis. Antioxidants are substances that prevent damage to cells caused by toxic byproducts of oxygen in the body. Levels of these byproducts are increased in chronic pancreatitis. Antioxidants constitute a large group that contains many natural and man‐made products. Examples include vitamin C, vitamin E, flavonoids (present in tea and cocoa) and many specialised medications. We found 12 randomised trials on this topic. The quality of these trials was mixed, and many had small sample sizes and high rates of dropout. Evidence shows that antioxidants may reduce pain in patients with chronic pancreatitis, but the reported reduction in pain was small. Whether this small decrease really had an impact on patients' complaints is not clear. Given the methodological problems of these trials, a strong conclusion could not be drawn. Use of antioxidants resulted in adverse effects in about 16% of study participants. Most adverse effects were mild, such as headache, nausea and constipation. However, participants who developed these adverse effects tended to stop using antioxidant medication. Other outcomes important for decision making such as use of analgesics, rate of exacerbation of pancreatitis and quality of life, were not very well reported. Therefore, we were unable to reach conclusions on these outcomes.
Summary of findings
Summary of findings for the main comparison. Antioxidant versus control intervention for pain in chronic pancreatitis.
| Antioxidant versus control intervention for pain in chronic pancreatitis | ||||||
| Patient or population: patients with pain in chronic pancreatitis Intervention: antioxidant versus control intervention | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Antioxidant versus control intervention | |||||
| Pain visual analogue score Scale from 0 to 10 | Mean pain visual analogue score in the intervention groups was 0.33 lower (0.64 to 0.02 lower) | 129 (4 studies) | ⊕⊕⊕⊝ moderatea | Clinical relevance is limited because of small absolute decrease (0.33 points on a scale of 10 points) | ||
| Pain‐free participants | 297 per 1000 | 514 per 1000 (282 to 935) | RR 1.73 (0.95 to 3.15) | 264 (3 studies) | ⊕⊕⊝⊝ lowb,c | |
| Adverse effects—parallel trials | 40 per 1000 | 177 per 1000 (64 to 492) | RR 4.43 (1.60 to 12.29) | 212 (3 studies) | ⊕⊕⊕⊝ moderated | Overall, adverse effects occurred in 16% of antioxidant group. Most adverse effects were mild in nature (headache, gastrointestinal symptoms) |
| Adverse effects—cross‐over trials (unpaired data) | 10 per 1000 | 60 per 1000 (16 to 224) | RR 5.80 (1.56 to 21.53) | 192 (5 studies) | ⊕⊕⊕⊝ moderated | Overall, adverse effects occurred in 16% of antioxidant group. Most adverse effects were mild in nature (headache, gastrointestinal symptoms) |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI. CI: Confidence interval; RR: Risk ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
a3 trials had high dropout rates. 1 trial also suffered from selective reporting of outcomes. bAll trials had high rates of dropout. 1 trial was not blinded, and another suffered from selective reporting. cHeterogeneity was high between trials (I2 = 71%). dMost trials had high rates of dropout. Some had additional methodological limitations (see Figure 1).
Background
Description of the condition
Chronic pancreatitis (CP) is an irreversible inflammatory process of the pancreas, characterised by damage to the pancreas parenchyme and loss of pancreatic function. The annual incidence and prevalence are estimated at around seven and 20 per 100,000, respectively (Dite 2001; Levy 2006; Spanier 2008). Development of CP is probably due to a complex interrelationship of etiological factors, of which the most important are alcohol toxicity, genetic predisposition, duct obstruction, trauma, pancreas divisum and autoimmune pancreatitis (Spanier 2008; Witt 2007).
Abdominal pain is the most prominent symptom in CP (van Esch 2006; Witt 2007). Pain in CP can be severe, debilitating and challenging to treat. Several options for treatment of pain are known, including lifestyle recommendations, use of analgesics and endoscopic or surgical intervention (Apte 1999; Gachago 2008). For many patients, however, these options may be inappropriate or may prove ineffective. Furthermore, long‐standing disease results in loss of pancreatic function. Exocrine insufficiency can lead to steatorrhoea, malnutrition, abdominal discomfort and weight loss. Endocrine insufficiency results in diabetes. CP thereby also leads to substantial impairment in quality of life for most patients (Pezzilli 2005; Wehler 2004).
Description of the intervention
Antioxidant supplements have been suggested as potentially useful treatment for pain in CP. Antioxidants are man‐made and natural substances that can inhibit the production of free radicals or can bind and inactivate them (Feng 2010). Examples of antioxidants include vitamin C, vitamin A, vitamin E, glutathione, flavonoids (in tea, cocoa and several fruits and vegetables), superoxide dismutase and various peroxidases. Free radicals are associated with many deleterious effects as a result of their chemical reactivity. Unbound, they can cause damage to all cellular macromolecules, including proteins, carbohydrates, lipids and nucleic acids (Ramos‐Márquez 2008). Epidemiological studies have reported that antioxidants may have both anti‐inflammatory and anticarcinogenic effects (Owen 2000; Sala 2002). Furthermore, some researchers suggest that intake of natural antioxidants reduces the risks of cancer, coronary heart disease, diabetes and Alzheimer's disease (Temple 2000; Willett 2002). In general, antioxidants are associated with few (direct) adverse effects, especially when doses are low (e.g. comparable normal diet intake). With high‐dose supplementation, headaches and gastrointestinal discomfort have been reported (Bhardwaj 2009; Bilton 1994a). However, over the long term, not all reports on the use of antioxidants are positive; for example, a recent Cochrane review comparing antioxidants versus placebo found that long‐term prophylactic use of some antioxidants like beta carotene, vitamin A and vitamin E may even increase mortality (Bjelakovic 2008). Other antioxidants were not associated with this effect (Bjelakovic 2008). Therefore, thorough evaluation is needed before antioxidants can be implemented as standard of care.
How the intervention might work
Studies have shown that patients with CP have a significantly lower level of circulating antioxidants and increased free radical activity compared with healthy controls (Bowrey 1999; Guyan 1990; Kalvaria 1986). Reduced intake of antioxidants and postprandial pain along with reduced resorption due to malabsorption caused by exocrine pancreatic insufficiency are probable causes of decreased antioxidant status in patients with CP (Bhardwaj 2004; Rose 1986). Improving the status of antioxidants might reduce antioxidant stress and provide a way to ameliorate the disease process while reducing pain in CP (Witt 2007).
Why it is important to do this review
No satisfactory treatment for pain in CP is available. Non‐opioid analgesics fail to relieve pain in many patients. Opioid analgesics are associated with many complications, like somnolence, obstipation and nausea, and present a serious risk of dependency. Antioxidants could be a promising alternative treatment that may relieve pain, improve health status and enhance quality of life in patients with CP. In contrast, potential harms of antioxidants should be thoroughly evaluated as well. This review aims to evaluate available evidence for both benefits and harms associated with the use of antioxidants in patients with CP.
Objectives
To assess the benefits and harms of antioxidants for the treatment of pain in patients with CP.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCTs) evaluating antioxidants for treatment of pain in CP. Trials were included irrespective of blinding, numbers of participants randomly assigned or language of publication. Quasi‐randomised trials were excluded.
Types of participants
We included all adult patients with established CP according to the criteria of at least one international guideline (Schneider 2007). Patients must have had some degree of pain, described as constant pain or as recurrent pain attacks.
Types of interventions
Trials with any of the following comparisons were included without restriction of dose, frequency, intensity, duration or route of administration.
Trials comparing any antioxidant regimen, single or compound, versus placebo.
Trials comparing different antioxidant regimens versus each other.
Trials comparing any antioxidant regimen versus any other control intervention.
The following definitions for the different treatment modalities were used.
Antioxidant: any medicinal product that inhibits the production of free radicals, or binds and inactivates them.
Single antioxidant: use of only one antioxidant product during the study period.
Combination antioxidants: use of more than one antioxidant product during the study period.
Other control intervention: any substance or intervention that may have a pharmacological effect and is used as a control.
Types of outcome measures
Primary outcomes
Pain: pain complaints after the intervention compared with before the intervention. Pain is a subjective outcome, and many different ways of measuring pain are used; therefore, no strict definition of pain can be provided. The pain outcome measures used in all trials are presented in a matrix table (Table 2).
1. Pain outcome measures.
| Study/Pain outcome measure | VAS pain score | Proportion of pain‐free participants | Numerical pain scale | Categorical pain scale | Descriptive pain score | Number of painful days | McGill Pain Questionnaire | SF‐36 pain component |
| Banks 1997 | X | ‐ | X | X | ‐ | ‐ | X | ‐ |
| Bhardwaj 2009 | ‐ | X | ‐ | ‐ | ‐ | X | ‐ | ‐ |
| Bilton 1994a | X | ‐ | ‐ | ‐ | X | ‐ | ‐ | ‐ |
| Bilton 1994b | X | ‐ | ‐ | ‐ | X | ‐ | ‐ | ‐ |
| Deprez 2003 | X | X | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Durgaprasad 2005 | X | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Jarosz 2010 | ‐ | X | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Kirk 2006 | X | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | X |
| Nandi 2002 | ‐ | ‐ | X | ‐ | ‐ | X | ‐ | ‐ |
| Salim 1991 | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ | ‐ |
| Siriwardena 2012 | X | X | ‐ | ‐ | X | ‐ | ‐ | ‐ |
| Uden 1990 | X | ‐ | ‐ | ‐ | X | ‐ | X | ‐ |
Secondary outcomes
Mortality.
Adverse effects, including nausea, constipation, allergic reaction or any other as reported.. Adverse effects were classified as minor (e.g. headache, gastrointestinal intolerance) and major complications (e.g. allergic reactions).
Pain medication: need for use of (additional) analgesic with no restriction on type of analgesic used.
Quality of life.
Number of admissions and duration of hospital stay during trial period.
Number of pancreatitis events.
Number of lost workdays.
Antioxidant status measures: dependent on the antioxidant marker reported by trial authors.
Search methods for identification of studies
Electronic searches
The following databases were searched.
Cochrane Central Register of Controlled Trials (CENTRAL) (Appendix 1).
MEDLINE via OVID (from 1950 to present) (Appendix 2).
EMBASE via OVID (from 1980 to present) (Appendix 3).
Conference Proceedings Citation Index‐Science (CPCI‐S) (from 1990 to present) (Appendix 4).
We developed these search strategies in cooperation with the Cochrane Upper Gastrointestinal and Pancreatic Diseases Group (see Acknowledgements).
Searching other resources
A cross‐reference search was performed of all included randomised trials and relevant reviews identified during the search process.
Data collection and analysis
This review was conducted according to the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008).
Selection of studies
Titles and abstracts were screened by two review authors independently. All potentially relevant hits were selected. In case of any uncertainty, hits were selected as well. Selection based on full text was performed by two review authors according to inclusion criteria. Disagreements were resolved by discussion. Excluded studies and reasons for exclusion are provided in the Characteristics of excluded studies table.
Data extraction and management
Two review authors independently extracted all relevant data. For each trial, participant characteristics, trial characteristics, data needed for methodological quality assessment of the trial and primary and secondary outcome measures were extracted according to availability. Data regarding participant characteristics included number of participants in each group, age and gender of participants, duration and etiology of disease, alcohol use, smoking and need for analgesic at baseline. Data regarding trial characteristics included study design, sample size calculation, inclusion and exclusion criteria of the trial, follow‐up period, loss to follow‐up and information regarding antioxidant supplements. The latter included the type of antioxidant supplement used, the duration of treatment and the timing of outcome assessment.
Assessment of risk of bias in included studies
Based on available empirical evidence and the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions, we assessed the methodological quality of RCTs by using the tool for assessing risk of bias (Higgins 2008; Kjaergard 2001; Moher 1998; Schulz 1995). The following definitions were used for items assessed by this tool.
Sequence allocation
Adequate: if the allocation sequence was generated by a computer or a random number table. Drawing lots, tossing a coin, shuffling cards and throwing dice were considered adequate if a person who was not otherwise involved in the recruitment of participants performed the procedure.
Unclear: if the trial was described as randomised, but the method used for generation of the allocation sequence was not described.
Inadequate: if a system involving dates, names or alternating allocation was used for allocation of participants.
Allocation concealment
Adequate; if allocation of participants involved a central independent unit, an on‐site locked computer or sealed envelopes.
Unclear: if the trial was described as randomised, but the method used to conceal the allocation was not described.
Inadequate: if the allocation sequence was known to the investigators who assigned participants.
Blinding
Adequate: if the trial was described (at least) as blind to participants or assessors and the method of blinding was described.
Unclear: if the trial was described as (double) blind, but the method of blinding was not described.
Inadequate: if the trial was not blinded.
Incomplete data outcome
Adequate: if the percentage of dropouts did not exceed 20%, and numbers of and reasons for dropouts and withdrawals in all intervention groups are described.
Unclear: if the report gives the impression that no dropouts or withdrawals occurred, but this is not specifically stated.
Inadequate: if the percentage of dropouts exceeds 20%, or the numbers of and reasons for dropouts and withdrawals are not described.
Selective outcome reporting
Adequate: if it was clear that published reports include all expected outcomes, including those that were prespecified.
Unclear: if insufficient information was provided to permit clear judgement of this aspect.
Inadequate: if not all relevant outcomes and prespecified outcomes were reported, or if they were incompletely reported.
Other sources of bias
Adequate: if the study appeared to be free of other sources of bias, with special attention to funding source and potential conflicts of interest.
Unclear: if a risk of potentially important bias exists, but sufficient information to assess this bias was lacking.
Inadequate: if one or more sources of potentially important bias could be identified in the study (e.g. extreme baseline imbalances, other imbalances in study design).
Cross‐over trials
For cross‐over trials, we have examined the following additional sources of bias according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008a).
Suitability of the cross‐over design.
Whether a carry‐over effect was present, and if first period data were presented.
These aspects are discussed and are noted under the heading 'Other sources of bias' when concerns are present in individual trials.
Measures of treatment effect
Statistical analyses of binary data were conducted using risk ratios (RRs). Trials with zero events in both arms were excluded from meta‐analyses. As a robustness assessment, meta‐analyses with zero event trials were performed using risk differences in a sensitivity analysis. For continuous outcomes, weighted mean differences (WMDs) were preferably used, but when different scales were used for the same outcome, we used the standardised mean difference (SMD) instead. When data were presented as medians with ranges, study authors were contacted and were asked to provide additional data. If data could not be retrieved, a sensitivity analysis imputing data for missing means and standard deviations (calculated from available medians and ranges) was performed as well (Hozo 2005).
Assessment of heterogeneity
Heterogeneity was calculated using the Higgins Chi2 test, and inconsistency in study effects was quantified by I2 (Higgins 2002). A Chi2 test with a P value < 0.10 was considered to indicate the presence of heterogeneity, and an I2 > 50% was considered to suggest marked inconsistency in effect between studies.
Assessment of reporting biases
Funnel plots were used to provide a visual assessment of whether treatment estimates were associated with study size. These depictions may reveal the presence of publication or other types of bias (Begg 1994; Egger 1997; Macaskill 2001).
Data synthesis
Parallel trials
The inverse variance and Mantel‐Haenzel methods were used for continuous and dichotomous outcomes, respectively.
Cross‐over trials
For continuous outcomes, the generic inverse variance method using mean differences and standard errors from paired analysis was used for meta‐analysis. If no paired data were available, we refrained from pooling data from cross‐over trials. In these cases, we performed a sensitivity analysis by combining parallel and cross‐over trials using unpaired data, as outlined below.
For dichotomous outcomes, the literature suggests that paired and unpaired analyses can be suitable for meta‐analysis (Curtin 2002; Elbourne 2002). Both types of analysis yield similar effect estimates, but the unpaired analysis yields a wider confidence interval (a more conservative estimate). If possible, we adjusted the variance using the Becker and Balagtas method (Elbourne 2002; Stedman 2011). Advantages of this approach are that values are easily calculated and this method allows for combinations of cross‐over and parallel trials while harnessing the power of cross‐over studies. The disadvantage is that this approach requires reporting of additional data, which might not be available. If such data were not available, an unpaired analysis was performed.
Combining parallel and cross‐over trials
When paired data from cross‐over trials were available, we combined these with data from parallel trials using the general inverse variance method. Paired data from cross‐over trials were entered into this model directly. For parallel trials, mean difference and standard error (calculated from the 95% confidence interval (CI)) were used for this purpose.
If no paired data were available, we performed a sensitivity analysis by combining unpaired data from cross‐over trials with data from parallel trials. For this approach, the usual methods of meta‐analysis were used.
For all meta‐analyses, the fixed‐effect model was used if no heterogeneity was present (Chi2 P value > 0.1 and I2 < 50%), or the random‐effects model was used. Statistical analysis was conducted using the statistical package RevMan v.5.2.5, as provided by The Cochrane Collaboration (RevMan 2014).
Results
Description of studies
Results of the search
We performed the search on 16 October 2012 and obtained a total of 489 citations. Upon selection, we found a total of 19 eligible citations describing 11 distinct RCTs (Figure 2). All studies excluded after the first selection are listed along with reasons for exclusion in the Characteristics of excluded studies table. Cross‐reference searching of all included randomised trials revealed one additional potentially eligible article (Nandi 2002). Cross‐reference searching of two relevant reviews (Bjelakovic 2008; Monfared 2009) yielded no further eligible articles. Therefore, a total of 20 citations describing 12 distinct trials were included. By means of personal communication, we identified one ongoing trial, EUROPAC‐2. Details of this trial are described in the Characteristics of ongoing studies table.
2.
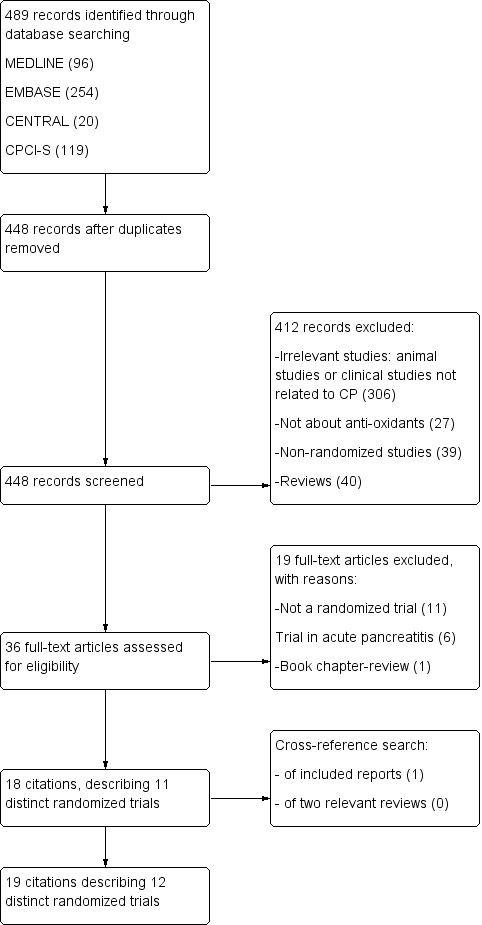
Study flow diagram.
Included studies
Eight of the 12 included trials were double‐blind, placebo‐controlled trials, and one trial was single‐blinded (Durgaprasad 2005). Six trials used a cross‐over design, and six a parallel‐group design. Two trials were published only in abstract form (Deprez 2003; Nandi 2002). Trial sizes varied from 14 to 147 participants. Three trials (Bilton 1994a; Bilton 1994b; Uden 1990) included only participants with recurrent pancreatitis of non‐gallstone origin (mostly alcohol). Durgaprasad 2005 excluded patients with alcoholic CP, and Kirk 2006 excluded patients with CP who had gallstones. The other trials included participants with established CP of all etiologies. Trials used a variety of antioxidants and reported on various outcomes. Most trials assessed pain using a visual analogue scale (VAS) (Hawker 2011); however different scales and methods of reporting were used (Table 2).
Eleven of the 12 included trials described the effects of antioxidants on chronic abdominal pain in CP. One trial (Salim 1991) evaluated the effects of antioxidants on acute pain during exacerbations of CP. As this is a different indication, results of this trial are described separately.
Ten trials compared antioxidant treatment versus placebo. Deprez 2003 compared antioxidants with dietary counselling versus dietary counselling alone but published no data that were suitable for meta‐analysis. Jarosz 2010 compared antioxidants versus no intervention (standard treatment). Given the availability of data, we performed only one of the three comparisons we had set out to perform (i.e. antioxidants vs placebo/no intervention).
Further characteristics of included trials are described in the Characteristics of included studies table. Baseline characteristics of included participants are described in Table 3.
2. Baseline characteristics of included trials.
| Study | Type of trial |
No. randomly assigned (IG vs PG) |
No. analysed (IG vs PG) | Age (years) (mean (SD)) | Gender (male, n (%)) | Disease | Disease duration (years) (mean (SD)) | Alcohol etiology (n (%)) | Alcohol intake (g/d) (mean (SD)) | Smokers (n (%)) | Initial pain levels |
| Banks 1997 | C | 16 | 13 | 42 (31‐51)1 | 8 (62) | All participants with CP | NA | NA | NA | NA | Continous pain, or > 2 pain episodes per week |
| Bhardwaj 2009 | P | 147 (76 vs 71) | 127 (71 vs 56) | 31.3 (11.4) vs 29.6 (9.3) |
24 (34) vs 17 (30) |
All participants with CP | 4.5 (4.2) vs 4.8 (5.4) | 15 (27) vs 25 (35) | 103 (82) vs 104 (71) | 22 (31) vs 14 (25) | Number of painful days: 9.1 ( SD 7.6) vs 7.2 ( SD 5.3) |
| Bilton 1994a | C | 30 | 20 | 45 (14) | 11 (55) | CP and ARP | 7.2 (4.1) | 2 (10) | NA | 8 (40) | NA |
| Bilton 1994b | C | 14 | 8 | NA | NA | NA | NA | NA | NA | NA | NA |
| Deprez 2003 | C | 30 | NA | NA | NA | All participants with CP | NA | NA | NA | NA | Overall mean VAS: 31.7% |
| Durgaprasad 2005 | P | 20 (10 vs 10) | 15 (8 vs 7) | 24 (13) vs 28 (17) | 7 (88) vs 7 (100) | Non‐alcoholic CP | 1 to 3 | 0 (0) | NA | NA | VAS: 5.5 ( SD 0.56) vs 5.9 ( SD 0.50) |
| Jarosz 2010 | P | 91 (46 vs 45) | 67 (32 vs 35) | 49 (27‐58) vs 46 (22‐60)2 | 26 (81) vs 27 (77) | Alcoholic CP | NA | 91 ( 100 ) | NA | NA | NA |
| Kirk 2006 | C | 36 | 19 | NA | 13 (68) | Non‐gallstone CP | NA | NA | NA | NA | NA |
| Nandi 2002 | P | 25 | NA | NA | NA | All participants with CP | NA | NA | NA | NA | NA |
| Salim 1991*** | P | 78 (25 vs 26 vs 27) | 66 (22 vs 21 vs 23) | 41 (32‐61) vs 42 (31‐62) vs 39 (31 vs. 65)3 | 21 (95) vs 21 (100) vs 22 (96) | Acute attack of alcoholic CP | 8.2 vs 7.7 vs 7.3 | 78 ( 100 ) | NA | NA | Mean number of attacks in previous 3 years: 6.7 vs 5.9 vs 6.1 |
| Siriwardena 2012 | P | 92 (NA) | 70 (33 vs 37) |
50 (13) vs 50 (9) | 23 (70) vs 27 (73) | All participants with CP | 4.2 (2.4) vs 4.9 (4.3) | IG: 24 (73) PG: 27 (73) |
IG: 222 (123) PG: 247 (202) |
IG: 28 (85) PG: 28 (76) |
IG: 3.6 PG: 3.9 |
| Uden 1990 | C | 23 | 20 | NA | NA | Non‐gallstone CP | NA | 7 (35) | NA | NA | NA |
All data presented as all participants (antioxidant group vs control group), unless otherwise specified.
Abbreviations: ARP: acute recurrent pancreatitis. CP: chronic pancreatitis. C: cross‐over. IG: intervention group. NA: not available. P: parallel. PG: placebo group. SD: standard deviation. VAS: visual analogue scale.
1Median (range). 2Mean (range).
3This is a 3‐arm trial. Data are presented in the following order: allopurinol vs dimethylsulfoxide vs control.
Excluded studies
Reports excluded after initial screening of titles and abstracts are listed along with reasons for exclusion in the Characteristics of excluded studies table.
Risk of bias in included studies
A risk of bias summary table of included trials is presented in Figure 1. The most common weakness of included trials was that outcome data were incomplete (high dropout rates, see below). Regarding other items, a division can be made between well‐conducted trials with relatively low risk of bias (Banks 1997; Bhardwaj 2009; Bilton 1994a; Bilton 1994b; Siriwardena 2012; Uden 1990) and poorly conducted trials with higher risk of bias.
1.
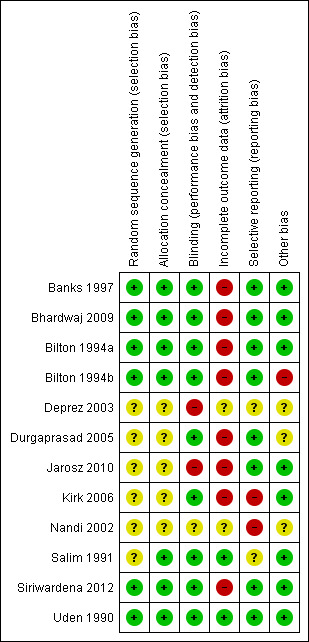
Summary of risk of bias: review authors' judgements about each risk of bias domain for included trials.
Dropout rates
The dropout rates of individual trials, the distribution of dropouts among trials arms and the reasons for dropout are stated in the 'Risk of bias table' sections of the Characteristics of included studies. In the studies Bilton 1994a and Bilton 1994b, most dropouts were in the antioxidant arms and most cases of dropout were due to adverse events. In all other trials, dropouts were similarly divided between trial arms.
Cross‐over trials
Appropriateness of the cross‐over design
CP is a chronic condition, making it a good candidate for cross‐over trials. The major outcomes of these studies (i.e. pain, quality of life, antioxidant levels, number of pancreatitis attacks) are reversible outcomes, which are suitable for this design. Antioxidant supplementation is a reversible treatment, and its effects are generally short‐lasting. However, two facts need to be noted: (1) Some antioxidants (e.g. vitamin E) are fat soluble, allowing for long‐term storage (in contrast to water‐soluble antioxidants, which are excreted immediately). This might result in some carry‐over effect if levels remain high in the second period; and (2) the mechanisms by which antioxidants might work in CP are not entirely elucidated. Although the major hypothessed action is reversible (i.e. countering the high free radical state in CP), it cannot be ruled out that some mechanisms might have longer‐lasting effects. Therefore, empirical data from these trials must be evaluated to rule out any carry‐over effect.
Carry‐over effect
Published reports of all cross‐over trials, except Deprez 2003 (published as abstract only), discussed the risk of carry‐over effect. Both Uden 1990 and Banks 1997 statistically investigated the presence of carry‐over effect and stated that they did not identify a significant carry‐over effect in clinical or biochemical outcomes. Uden 1990 used the fat‐soluble vitamin E, and its levels showed no signs of a carry‐over effect at the end of the second study period. Bilton 1994a and Bilton 1994b describe the analysis performed by Uden 1990 because these trials were performed by the same group. Kirk 2006 showed that biochemically the levels of fat‐soluble vitamin E tended to remain slightly elevated until the end of the study. These study authors identify this as a potential limitation of the study but conclude that it would have resulted in a bias towards the zero (no) effect, although this study showed a significant difference in clinical outcome. Based on these results, we can conclude that empirical evidence shows that the carry‐over effect does not play an important role in this comparison.
Publication bias
Publication bias was evaluated by means of funnels plots, but no clear evidence of such bias was observed (Figure 3).
3.
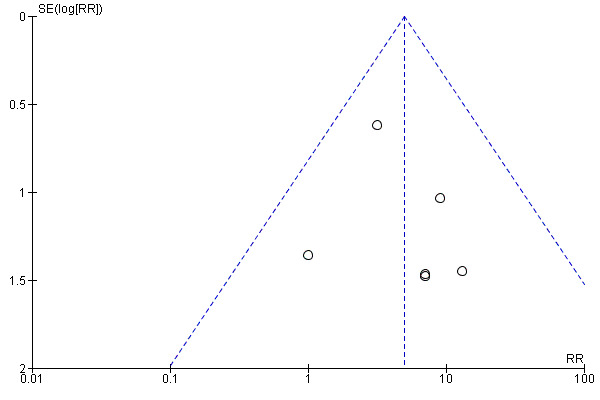
Evaluation of publication bias by funnel plot (based on the outcome 'adverse effects').
Effects of interventions
See: Table 1
Effects of antioxidants on chronic pain in chronic pancreatitis
Primary outcome—pain
An overview of the results of different pain outcome measures reported by the included trials is presented in Table 4.
3. Effects of antioxidants on chronic pain in chronic pancreatitis.
| Study | Outcome measure(s) | Results (antioxidants vs control) | |
| Banks 1997 |
|
|
|
| Bhardwaj 2009 |
|
|
|
| Bilton 1994a | VAS, descriptive pain score | No differences (no data shown) | |
| Bilton 1994b | VAS, descriptive pain score | No differences (no data shown) | |
| Deprez 2003 |
|
|
|
| Durgaprasad 2005 | VAS score (after intervention) (mean (SE)) | 5.81 (0.74) vs 6.57 (0.74), NS | |
| Jarosz 2010 |
|
|
|
| Kirk 2006 |
|
|
|
| Nandi 2002 |
|
|
|
| Siriwardena 2012 |
|
|
|
| Uden 1990 |
|
|
Abbreviations: NS: not significant. VAS: visual analogue scale.
Eight trials assessed pain using a VAS score (Table 2). Not all data were suitable for meta‐analysis. Bilton 1994a and Bilton 1994b reported that no significant difference was noted but did not provide any data. Kirk 2006 excluded the VAS score from analysis because of poor reporting by participants. Deprez 2003 reported only baseline VAS scores.
Pain VAS scores from two cross‐over trials were pooled (Analysis 1.1), showing a significant reduction in pain VAS scores in favour of the antioxidant group (MD ‐0.34 VAS points, 95% CI ‐0.67 to ‐0.01, P value 0.04) (Analysis 1.1). Two trials with a parallel‐group design were pooled, showing no difference in pain levels (MD ‐0.26, 95% CI ‐1.07 to 0.56, P value 0.5) (Analysis 1.2). When results of all trials were combined (118 participants), a significant reduction in VAS score was observed in the antioxidant groups (MD ‐0.33, 95% CI ‐0.64 to ‐0.02, P value 0.04) (Analysis 1.3).
1.1. Analysis.
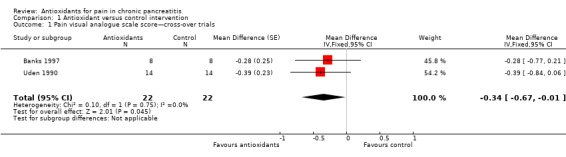
Comparison 1 Antioxidant versus control intervention, Outcome 1 Pain visual analogue scale score—cross‐over trials.
1.2. Analysis.
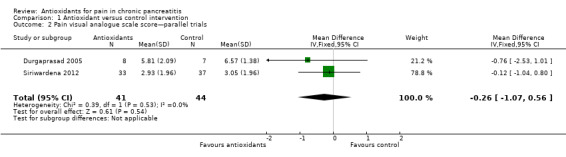
Comparison 1 Antioxidant versus control intervention, Outcome 2 Pain visual analogue scale score—parallel trials.
1.3. Analysis.
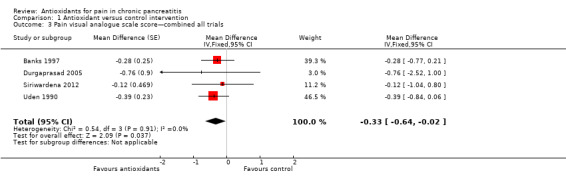
Comparison 1 Antioxidant versus control intervention, Outcome 3 Pain visual analogue scale score—combined all trials.
Three parallel trials reported the proportion of pain‐free participants as an outcome measure. Meta‐analysis showed a non–statistically significant difference between groups (RR 1.73, 95% CI 0.95 to 3.15, P value 0.07) (Analysis 1.4).
1.4. Analysis.
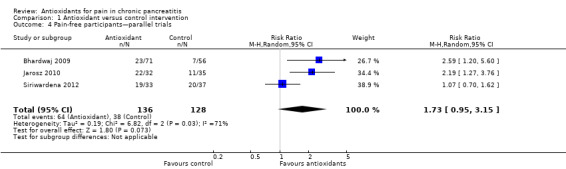
Comparison 1 Antioxidant versus control intervention, Outcome 4 Pain‐free participants—parallel trials.
Secondary outcomes
Adverse effects and mortality
Eight trials reported adverse effects. In total, 33 of 208 (16%) adverse events were reported in the antioxidant group compared with five of 196 (3%) in the placebo group. Separate analysis of cross‐over trials (RR 5.80, 95% CI 1.56 to 21.53, P value 0.009) and parallel trials (RR 4.43, 95% CI 1.60 to 12.29, P value 0.004) showed significantly higher adverse events in the antioxidant group (Analysis 1.5). Analysis of cross‐over trials was based on unpaired data because reported data did not allow for correction of variance. Sensitivity analyses combining cross‐over and parallel trials (Analysis 1.6) and data for zero event trials using risk differences produced similar results (Analysis 1.7). Most reported adverse events were minor complications and included headache, gastrointestinal intolerance, obstipation and nausea. Only two moderate to severe adverse effects were described. Banks 1997 reported that one participant developed swelling of joints, a rash and a puffy face. Siriwardena 2012 described one participant in the antioxidant group who developed convulsions as the result of hepatic encephalopathy, although the relation of this to antioxidant treatment was uncertain. No trials reported any mortality.
1.5. Analysis.
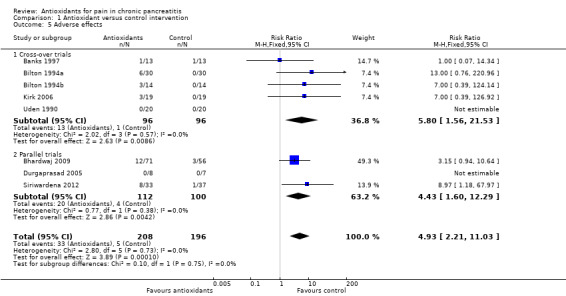
Comparison 1 Antioxidant versus control intervention, Outcome 5 Adverse effects.
1.6. Analysis.
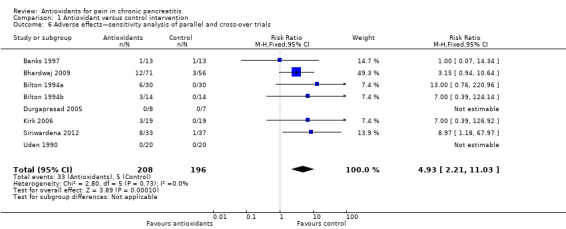
Comparison 1 Antioxidant versus control intervention, Outcome 6 Adverse effects—sensitivity analysis of parallel and cross‐over trials.
1.7. Analysis.
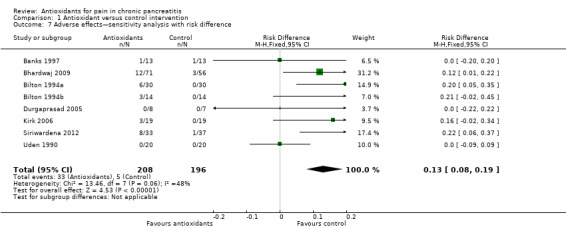
Comparison 1 Antioxidant versus control intervention, Outcome 7 Adverse effects—sensitivity analysis with risk difference.
Pain medication
Three trials including 210 participants reported on the need for pain medication during the study period. Data appeared unsuitable for meta‐analysis. Banks 1997 showed no difference in the need for morphine use between participants given antioxidants and those given placebo (increase of 5.5%, range ‐49% to +129%). Bhardwaj 2009 reported a positive effect of antioxidants compared with placebo when evaluating the numbers of oral analgesic tablets required per month (MD ‐6.15, 95% CI ‐2.65 to ‐9.65). Similar results were found for the numbers of analgesic injections required per month after adjustment for baseline differences (MD ‐0.44, 95% CI ‐0.07 to ‐0.81). Siriwardena 2012 described no difference in the need for opioid analgesic when antioxidants were used (MD ‐13.7 mg/d, 95% CI ‐38.0 to 10.6).
Quality of life
Three trials including 102 participants reported on quality of life. Data were unsuitable for meta‐analysis. Banks 1997 reported on activities of daily living and described no differences between antioxidants and placebo (MD ‐3.3, 95% CI ‐10.3 to 3.7, P value 0.32). Kirk 2006 assessed quality of life using the 36‐Item Short Form Health Survey (SF‐36) questionnaire. Results were presented for nine components separately. Six of the quality of life components (physical function, physical role, social function, pain, health perception and change in health) showed significant improvement in the antioxidant group compared with the placebo group. Siriwardena 2012 examined quality of life using four different quality of life questionnaires. None revealed a significant difference.
Admissions and duration of hospital stay
Two trials including 197 participants reported on this outcome. Bhardwaj 2009 reported on the need for hospitalisation. A small difference was observed in favour of antioxidant use after adjustment for baseline values (MD ‐0.034, 95% CI ‐0.069 to ‐0.002). Siriwardena 2012 showed no differences between study groups (MD ‐0.06, 95% CI ‐3.80 to 3.53).
Number of attacks of pancreatitis
Three cross‐over trials including 54 participants reported the frequency of severe attacks of pancreatic pain. Fifteen attacks occurred: five in the antioxidant period and 10 in the placebo period. This difference was not statistically significant (Analysis 1.8). This analysis was based on unpaired data, as reported data did not allow for correction of variance.
1.8. Analysis.
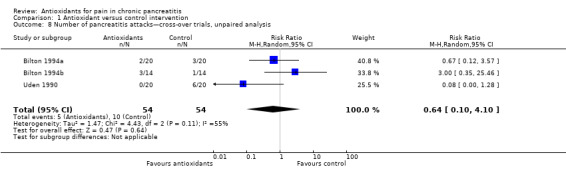
Comparison 1 Antioxidant versus control intervention, Outcome 8 Number of pancreatitis attacks—cross‐over trials, unpaired analysis.
Loss of workdays
Only Bhardwaj 2009 (127 participants) reported on the number of workdays lost. This trial reported a favourable larger decrease in workdays lost in the antioxidant group compared with the placebo group (11.4 ( SD 9.1) vs 7.6 ( SD 7.2), P value 0.014).
Antioxidant level measures
Most studies reported several measures of antioxidant status. Four of these measures were reported by three or more trials and were chosen for meta‐analysis (i.e. vitamin C and A, selenium and beta‐carotene). All cross‐trials reported unpaired data for this outcome and could be included only in sensitivity analyses. Main meta‐analyses based on parallel trials showed significantly higher levels of vitamins C and E in the antioxidant groups (Analysis 1.9; Analysis 1.11). Sensitivity analysis of these outcomes confirmed these findings (Analysis 1.10; Analysis 1.12). Finallly, sensitivity analysis of selenium and beta‐carotene suggested higher levels in the antioxidant groups (Analysis 1.13; Analysis 1.14).
1.9. Analysis.
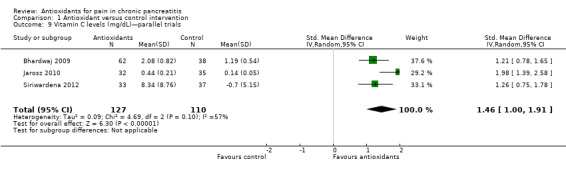
Comparison 1 Antioxidant versus control intervention, Outcome 9 Vitamin C levels (mg/dL)—parallel trials.
1.11. Analysis.
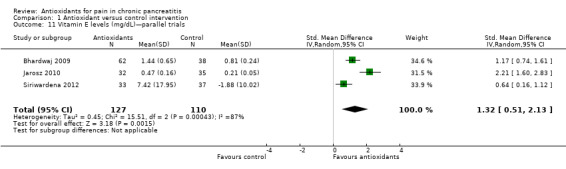
Comparison 1 Antioxidant versus control intervention, Outcome 11 Vitamin E levels (mg/dL)—parallel trials.
1.10. Analysis.
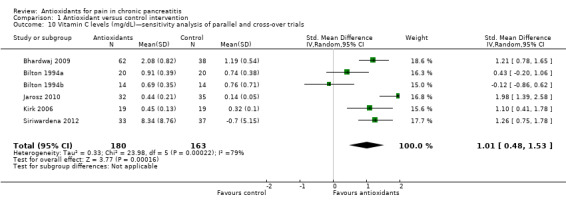
Comparison 1 Antioxidant versus control intervention, Outcome 10 Vitamin C levels (mg/dL)—sensitivity analysis of parallel and cross‐over trials.
1.12. Analysis.
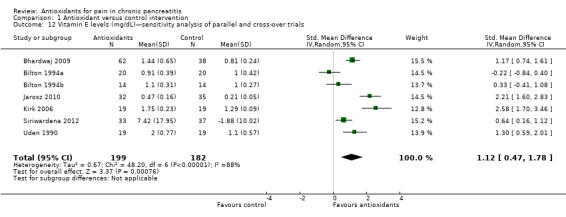
Comparison 1 Antioxidant versus control intervention, Outcome 12 Vitamin E levels (mg/dL)—sensitivity analysis of parallel and cross‐over trials.
1.13. Analysis.
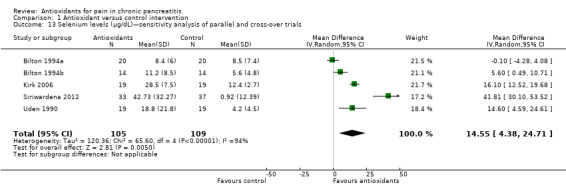
Comparison 1 Antioxidant versus control intervention, Outcome 13 Selenium levels (μg/dL)—sensitivity analysis of parallel and cross‐over trials.
1.14. Analysis.
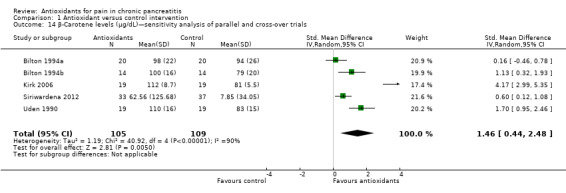
Comparison 1 Antioxidant versus control intervention, Outcome 14 β‐Carotene levels (μg/dL)—sensitivity analysis of parallel and cross‐over trials.
Effects of antioxidants on acute pain in chronic pancreatitis
Primary outcome—pain
Salim 1991 included patients with CP within two hours of onset of an acute pain episode. Participants were randomly assigned to three groups: two antioxidant groups (allopurinol and dimethylsulfoxide) and a placebo group. This trial assessed the proportions of pain‐free participants in the three study groups at different moments during admission. After 12 hours of admission, the proportions of pain‐free participants were significantly higher in the two antioxidant groups than in the placebo group (respectively, 13/22 (59%) and 12/21 (57%) vs 4/23 (17%); P value < 0.01). After 24 hours, all participants in the two antioxidant groups achieved pain relief versus 12 of 23 (52%) in the placebo group (P value < 0.01). Additionally, after two days, all participants in the placebo group experienced epigastric tenderness versus 12 of 22 (54%) in the allopurinol group and 11 of 21 (52%) in the dimethylsulfoxide group (P value < 0.01). After three days, only four of 22 (18%) and three of 21 (14%) participants, respectively, in the allopurinol and dimethylsulfoxide groups, experienced epigastric tenderness, and 17 of 23 (74%) in the placebo group had epigastric tenderness (P value < 0.01).
Secondary outcomes
This trial reported on only two of the secondary outcome measures (i.e. adverse effects and hospital stay) (Salim 1991). Five (23%) participants in the allopurinol group experienced adverse effects, including allergic reactions (rash) and headaches. A total of four (19%) participants in the dimethylsulfoxide group experienced adverse effects (intolerance to medication (1×) and headache (3×)). None of the participants in the placebo group reported any adverse effects.
This trial also reported the proportions of participants discharged from hospital after three days. All participants in the allopurinol (n = 22) and dimethylsulfoxide (n = 21) groups were discharged home after three days compared with five of 23 (22%) in the placebo group (P value < 0.01).
Discussion
Summary of main results
This systematic review shows several important findings regarding antioxidant treatment in chronic pancreatitis. First, it shows that antioxidant use may reduce pain in chronic pancreatitis. Second, it shows that antioxidant use is associated with adverse effects in 16% of patients. Although mostly mild in nature, these adverse effects sometimes result in discontinuation of antioxidant medication. Third, 12 randomised trials have been conducted, but these trials included small sample sizes, suffered high rates of dropout and were inadequate in reporting of outcomes critical for decision making.
Meta‐analysis of pain VAS scores showed a significant reduction favouring antioxidant treatment. This result was based on the findings of four trials, three of which had adequate methodology for most items included in the risk of bias tool (Figure 1). The contribution of the fourth trial was limited (weight in the analysis was 3%). No heterogeneity was observed between studies (I2 = 0%). All of these aspects increase the reliability of the findings. The marginal statistical significance (P value 0.04) on the other hand is probably an indication of the small numbers of included participants. The overall VAS score was only slightly reduced by antioxidants (0.33 of 10 points) (Analysis 1.3). Such a small difference is of unclear clinical relevance, and its clinical impact is uncertain.
A factor contributing to reported outcomes could be that most participants in the trials had only mild pain. (The pain VAS score under placebo treatment was around three points in most trials.) When the VAS pain score was higher, as in Durgaprasad 2005, the absolute reduction tended to be greater (e.g. a reduction of ‐0.76 from a placebo VAS of 6.57) (Analysis 1.2). The proportion of pain‐free participants offers a more clinically relevant outcome. Our meta‐analysis shows that the difference in this outcome was not statistically significant, although a trend favouring antioxidant treatment was observed (Analysis 1.4). It is clear that more evidence is needed to establish or reject potential differences.
Another important outcome for clinical practice is the adverse events observed in 16% of participants treated with antioxidants (Analysis 1.6). Although most adverse events were mild, trial authors reported that participants often decided to discontinue antioxidant treatment because of these events.
Other important secondary outcomes, such as use of pain medication, rate of exacerbation of pancreatitis and quality of life, were not well evaluated in the included trials, and data were insufficient to permit reliable conclusions. Future trials need to consider these outcomes and preferably present data in ways that facilitate meta‐analysis, by reporting complete outcome data and choosing outcome measures comparable with those of previous studies.
Overall completeness and applicability of evidence
Inclusion criteria varied between trials. Some trials included only non‐alcoholic participants with CP, and others recruited all patients with CP, including those with recurrent attacks of pancreatitis. This is representative of the heterogeneity of patients with CP and may justify an argument regarding the generalisability of the results of this review. A noteworthy aspect based on the hypothesised mechanism of antioxidant treatment is the duration of disease at the time of antioxidant therapy. Antioxidant therapy is hypothesised to reduce damage to the pancreas caused by oxidative stress. Maximal benefit is likely to be achieved when antioxidants are administered early in the disease process (before the damage has been done) and are continued for a substantial time. This aspect did not receive attention in the included trials. Only a few reported the duration of disease of included participants (Table 3), and none performed subgroup analysis based on this characteristic. The limited number of participants may have been a contributing factor in this regard.
Variation in reporting of outcome measures posed an important challenge for a summary of results (e.g. nearly all trials measured pain using a VAS score, but only four trials reported data that were suitable for meta‐analysis). Contacting study authors was not helpful, as most trials were conducted more than 15 years ago and original data were no longer available. In two studies, trial authors stated only the absence of a significant difference without presenting data (Bilton 1994a; Bilton 1994b). This way of reporting should be avoided because pooling of trial data could expose differences in treatments not observed in single trials. These trials also used different types of antioxidant regimens, with variations evident in types, numbers of preparations and doses of antioxidants used. Because of the small number of available trials, the influence of different regimens could not be evaluated in subgroups. Moreover, the lack of trials comparing different types of antioxidants makes direct comparison not feasible. Finally, only one trial studied the effects of antioxidants on acute pain in chronic pancreatitis. More evidence is needed before conclusions can be drawn.
Quality of the evidence
The 12 RCTs included a total of 585 participants. The most important limitation was the high rate of dropout due to adverse events or non‐compliance. Six trials were relatively well conducted in terms of adequate randomisation, concealment of allocation, blinding and placebo control (Banks 1997; Bhardwaj 2009; Bilton 1994a; Bilton 1994b; Siriwardena 2012; Uden 1990), but the remaining trials had serious methodological flaws (Figure 1). Another important limitation was the small sample size of most trials. Eight of the included trials recruited fewer than 40 participants. This is to some extent attenuated by a cross‐over design in some trials, in that this design allows more power than is attained by a parallel‐group design. Still, most trials were underpowered to detect any differences in clinically important outcomes.
Potential biases in the review process
Inconsistent reporting posed the most important challenge to this systematic review. Two randomised trials (Deprez 2003; Nandi 2002) were published only as abstracts and did not contribute data on any of the comparisons. This kind of publication bias has been widely acknowledged to be problematic, but solutions such as trial registration have already led to progress in resolution of this problem (McGee 2011). Second, we were unable to obtain suitable data for several outcomes. This was due mainly to incomplete reporting of trial data and to the fact that most trials were conducted some time ago. Third, the cross‐reference search identified one additional eligible report not identified by our electronic search. This report was published as an abstract in a supplement that was not indexed in any electronic database (Nandi 2002). This again shows that cross‐reference searching of included trials is an important step in the search process. Fourth, the use of unpaired data might lead to underestimation of the true level of statistical heterogeneity owing to the inflation of confidence intervals (as a result of the more conservative estimation). Although this can affect results in general, for our review the impact is probably limited. Heterogeneity estimates were consistent for all outcomes between estimates from parallel trial analysis and those from sensitivity analysis, including unpaired data. For the only outcome with exclusively unpaired data from cross‐over trials, heterogeneity was found to be significant, thus negating this potential bias. Finally, our search was conducted more than one year ago, meaning that some recent publications might have been missed. This lag is due to the fact that several steps in the process took more time than was anticipated. For practical reasons, we have planned an update of the review early next year, to keep results of this review recent and relevant.
Agreements and disagreements with other studies or reviews
A recent systematic review of antioxidant therapy in pancreatitis (Monfared 2009) was unable to provide clear conclusions about the benefit of antioxidant therapy and underlined the need for additional research. This review, however, included trials on both acute and chronic pancreatitis. These diseases were discussed simultaneously, and conclusions were not always clearly separated. Because of the distinct pathophysiological and clinical presentation of acute and chronic pancreatitis, combining trials on both diseases into a single analysis may be inappropriate. This review stratified the analysis per types of antioxidants used. Although this is a more precise approach, the lack of data for each type of antioxidant limits the possibility of useful conclusions. The fact that trials use various types of antioxidants indicates that clinicians are more interested in studying the hypothesis that reducing oxidative stress may improve health outcome than in evaluating which substance is more efficient. The review concluded that trials were heterogeneous and that drawing conclusions was impossible. The review authors stated that based on the results of the largest trial by Bhardwaj 2009, treatment with cocktails of oxidants could have a positive effect on pain reduction.
Another review (Braganza 2010) discussed the role of micronutrient therapy in CP and described the role of antioxidants as part of the review. This review concluded that antioxidants can control background pain and can curb acute attacks in chronic pancreatitis. A drawback of the Braganza 2010 review is the lack of assessment of risk of bias of the included trials. Moreover, since time of the Braganza review, two new trials have been published, which were not included in that review. Finally, both of the reviews discussed here (Braganza 2010; Monfared 2009) lacked quantitative assessment of various important outcomes, especially adverse events, although these data were available.
Authors' conclusions
Implications for practice.
Current evidence shows that antioxidants can reduce pain slightly in patients with CP, but the clinical relevance of the small observed difference is uncertain. With such small effects, routine use of antioxidants is questionable. In a minority of patients, the use of antioxidants can lead to mild adverse effects (headache and gastrointestinal intolerance), which can mandate cessation of treatment. Effects of antioxidants on other outcomes are still largely uncertain because of lack of data. Antioxidants also seem to benefit patients with CP during acute abdominal pain episodes (exacerbations), although evidence is insufficient for reliable conclusions.
Implications for research.
Topics that have not been sufficiently evaluated include:
providing additional data on the effects of antioxidants on pain, especially in terms of outcomes with clear clinical relevance, such as becoming pain free;
clarifying the effects of antioxidants on secondary outcomes such as quality of life and rate of pancreatitis flare‐ups; and
studying whether the timing of intervention (early intervention) can affect the outcome of antioxidant treatment.
Acknowledgements
We would like to thank Drs. Banks, Bhardwaj, Braganza, Bilton and Deprez for sharing their data with us. Finally, we would like to thank Racquel Simpson, Trials Search Co‐ordinator of the Cochrane Upper Gastrointestinal and Pancreatic Diseases Group, for help and assistance provided in the development of search strategies for this review.
Appendices
Appendix 1. CENTRAL search strategy
EBM reviews—Cochrane Central Register of Controlled Trials, 2010, 1st Quarter
exp Pancreatitis, Chronic/
exp Pancreatitis, Alcoholic/
(pancrea$ adj2 chronic$).mp.
(Alcohol$ adj2 pancrea$).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
(pancrea$ adj2 recurren$).mp.
or/1‐5
exp Free Radicals/ag, ai, ip [Agonists, Antagonists & Inhibitors, Isolation & Purification]
exp Antioxidants/
exp ascorbic acid/ or exp bilirubin/ or exp butylated hydroxyanisole/ or exp butylated hydroxytoluene/ or exp canthaxanthin/ or exp carotenoids/ or exp catalase/ or exp ergothioneine/ or exp grape seed extract/ or exp melatonin/ or exp nordihydroguaiaretic acid/ or exp probucol/ or exp propyl gallate/ or exp pyrogallol/ or exp quercetin/ or exp selenium/ or exp silymarin/ or exp thioctic acid/ or exp tocopherols/ or exp tocotrienols/ or exp uric acid/ or exp vitamin e/ or exp alpha‐tocopherol/ or exp beta‐tocopherol/ or exp gamma‐tocopherol/ or exp zeta carotene/ or exp beta‐carotene/ or exp curcumin/ or exp methionine/ or exp allopurinol/
exp Oxidants/
exp Oxidation‐Reduction/
*Reactive Oxygen Species/ai [Antagonists & Inhibitors]
exp Free Radical Scavengers/
exp Peroxides/ai [Antagonists & Inhibitors]
antioxidant$.mp.
or/7‐15
6 and 16
Appendix 2. MEDLINE search strategy
Ovid MEDLINE(R) 1950 to March Week 4 2010
exp Pancreatitis, Chronic/
exp Pancreatitis, Alcoholic/
(pancrea$ adj2 chronic$).mp.
(Alcohol$ adj2 pancrea$).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
(pancrea$ adj2 recurren$).mp.
or/1‐5
exp Free Radicals/ag, ai, ip [Agonists, Antagonists & Inhibitors, Isolation & Purification]
exp Antioxidants/
exp ascorbic acid/ or exp bilirubin/ or exp butylated hydroxyanisole/ or exp butylated hydroxytoluene/ or exp canthaxanthin/ or exp carotenoids/ or exp catalase/ or exp ergothioneine/ or exp grape seed extract/ or exp melatonin/ or exp nordihydroguaiaretic acid/ or exp probucol/ or exp propyl gallate/ or exp pyrogallol/ or exp quercetin/ or exp selenium/ or exp silymarin/ or exp thioctic acid/ or exp tocopherols/ or exp tocotrienols/ or exp uric acid/ or exp vitamin e/ or exp alpha‐tocopherol/ or exp beta‐tocopherol/ or exp gamma‐tocopherol/ or exp zeta carotene/ or exp beta‐carotene/ or exp curcumin/ or exp methionine/ or exp allopurinol/
exp Oxidants/
exp Oxidation‐Reduction/
*Reactive Oxygen Species/ai [Antagonists & Inhibitors]
exp Free Radical Scavengers/
exp Peroxides/ai [Antagonists & Inhibitors]
antioxidant$.mp.
or/7‐15
6 and 16
randomized controlled trial.pt.
controlled clinical trial.pt.
randomized.ab.
placebo.ab.
drug therapy.fs.
randomly.ab.
trial.ab.
groups.ab.
or/18‐25
exp animals/ not humans.sh.
26 not 27
17 and 28
Appendix 3. EMBASE search strategy
EMBASE 1980 to 2010 Week 12
exp alcoholic pancreatitis/
exp chronic pancreatitis/
(pancrea$ adj2 chronic$).mp.
(Alcohol$ adj2 pancrea$).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier]
(pancrea$ adj2 recurren$).mp.
or/1‐5
exp antioxidant/
exp ascorbic acid/ or exp bilirubin/ or exp butylated hydroxyanisole/ or exp butylated hydroxytoluene/ or exp canthaxanthin/ or exp carotenoids/ or exp catalase/ or exp ergothioneine/ or exp grape seed extract/ or exp melatonin/ or exp nordihydroguaiaretic acid/ or exp probucol/ or exp propyl gallate/ or exp pyrogallol/ or exp quercetin/ or exp selenium/ or exp silymarin/ or exp thioctic acid/ or exp tocopherols/ or exp tocotrienols/ or exp uric acid/ or exp vitamin e/ or exp alpha‐tocopherol/ or exp beta‐tocopherol/ or exp gamma‐tocopherol/ or exp zeta carotene/ or exp beta‐carotene/ or exp curcumin/ or exp methionine/ or exp allopurinol/
exp oxidizing agent/
exp oxidation reduction reaction/
exp antioxidant activity/
exp oxidation reduction state/
exp Free Radical Scavengers/
peroxide/cb, it, dt, pr, pk, pd [Drug Combination, Drug Interaction, Drug Therapy, Pharmaceutics, Pharmacokinetics, Pharmacology]
antioxidant$.mp.
or/7‐15
6 and 16
Clinical trial/
Randomized controlled trial/
Randomization/
Single‐Blind Method/
Double‐Blind Method/
Cross‐Over Studies/
Random Allocation/
Placebo/
Randomi?ed controlled trial$.tw.
Rct.tw.
Random allocation.tw.
Randomly allocated.tw.
Allocated randomly.tw.
(allocated adj2 random).tw.
Single blind$.tw.
Double blind$.tw.
((treble or triple) adj blind$).tw.
Placebo$.tw.
Prospective study/
or/18‐36
Case study/
Case report.tw.
Abstract report/ or letter/
or/38‐40
37 not 41
17 and 42
Appendix 4. CPCI‐S search strategy
Conference Proceedings Citation Index‐Science (CPCI‐S)—1990 to present
# 13. #12 AND #11
# 12. Topic=(pancreatitis)
# 11. #10 OR #8 OR #6 OR #3 OR #2 OR #1
# 10. #9 AND #4
# 9. Topic=(Isolation or Purification)
# 8. #7 AND #4
# 7. Topic=(Scavenger*)
# 6. #5 AND #4
# 5. Topic=(Agonist* or Antagonist* or Inhibitor*)
# 4. Topic=(Free Radical* or Peroxide*)
# 3. Topic=(Oxidation‐Reduction) OR Topic=(Oxidant*)
# 2. Topic=(ascorbic acid or bilirubin or butylated hydroxyanisole or butylated hydroxytoluene or canthaxanthin or carotenoids or catalase or ergothioneine or grape seed extract or melatonin or nordihydroguaiaretic acid or probucol or propyl gallate or pyrogallol or quercetin or selenium or silymarin or thioctic acid or tocopherols or tocotrienols or uric acid or vitamin e or ealpha‐tocopherol or beta‐tocopherol or gamma‐tocopherol or zeta carotene or beta‐carotene or curcumin or methionine or allopurinol)
# 1. Topic=(antioxidant*)
Appendix 5. Plain language definitions
This appendix contains definitions of specialised terms used in this review to make them more accessible for all users.
Ameliorating: to make or become better.
Anticarcinogenic: a substance that can inhibit or prevent the development of cancer.
Autoimmune pancreatitis: a rare form of pancreatitis thought to be caused by an immunological reaction of the body against its own organs (in this case, the pancreas).
Deleterious: causing harm or damage.
Endocrine pancreatic function: refers to the production of insulin by the pancreas to regulate blood sugar levels.
Epidemiology: science concerning the study of causes and patterns of disease.
Etiology: the cause of a disease.
Exocrine pancreatic function: refers to the production of digestive enzymes of the pancreas.
Lipids: fats.
Macromolecules: very large molecules, usually formed by combinations of many smaller subunits.
Nucleic acids: the building blocks of DNA.
Pancreatic divisum: a congenital anomaly in the anatomy of the ducts of the pancreas in which a single pancreatic duct is not formed, but rather remains as two distinct ducts.
Parenchyme: the body of an organ, used to mainly to distinguish the functional part of an organ from other structures, such as ducts and blood vessels within that organ.
Postprandial pain: pain after meals.
Somnolence: drowsiness.
Steatorhoea: the presence of excess fat in faeces.
Data and analyses
Comparison 1. Antioxidant versus control intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain visual analogue scale score—cross‐over trials | 2 | 44 | Mean Difference (Fixed, 95% CI) | ‐0.34 [‐0.67, ‐0.01] |
| 2 Pain visual analogue scale score—parallel trials | 2 | 85 | Mean Difference (IV, Fixed, 95% CI) | ‐0.26 [‐1.07, 0.56] |
| 3 Pain visual analogue scale score—combined all trials | 4 | Mean Difference (Fixed, 95% CI) | ‐0.33 [‐0.64, ‐0.02] | |
| 4 Pain‐free participants—parallel trials | 3 | 264 | Risk Ratio (M‐H, Random, 95% CI) | 1.73 [0.95, 3.15] |
| 5 Adverse effects | 8 | 404 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.93 [2.21, 11.03] |
| 5.1 Cross‐over trials | 5 | 192 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.8 [1.56, 21.53] |
| 5.2 Parallel trials | 3 | 212 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.43 [1.60, 12.29] |
| 6 Adverse effects—sensitivity analysis of parallel and cross‐over trials | 8 | 404 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.93 [2.21, 11.03] |
| 7 Adverse effects—sensitivity analysis with risk difference | 8 | 404 | Risk Difference (M‐H, Fixed, 95% CI) | 0.13 [0.08, 0.19] |
| 8 Number of pancreatitis attacks—cross‐over trials, unpaired analysis | 3 | 108 | Risk Ratio (M‐H, Random, 95% CI) | 0.64 [0.10, 4.10] |
| 9 Vitamin C levels (mg/dL)—parallel trials | 3 | 237 | Std. Mean Difference (IV, Random, 95% CI) | 1.46 [1.00, 1.91] |
| 10 Vitamin C levels (mg/dL)—sensitivity analysis of parallel and cross‐over trials | 6 | 343 | Std. Mean Difference (IV, Random, 95% CI) | 1.01 [0.48, 1.53] |
| 11 Vitamin E levels (mg/dL)—parallel trials | 3 | 237 | Std. Mean Difference (IV, Random, 95% CI) | 1.32 [0.51, 2.13] |
| 12 Vitamin E levels (mg/dL)—sensitivity analysis of parallel and cross‐over trials | 7 | 381 | Std. Mean Difference (IV, Random, 95% CI) | 1.12 [0.47, 1.78] |
| 13 Selenium levels (μg/dL)—sensitivity analysis of parallel and cross‐over trials | 5 | 214 | Mean Difference (IV, Random, 95% CI) | 14.55 [4.38, 24.71] |
| 14 β‐Carotene levels (μg/dL)—sensitivity analysis of parallel and cross‐over trials | 5 | 214 | Std. Mean Difference (IV, Random, 95% CI) | 1.46 [0.44, 2.48] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Banks 1997.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Study performed in Boston, United States of America | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence generated by hospital pharmacy |
| Allocation concealment (selection bias) | Low risk | Randomisation concealed by hospital pharmacy |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded. Placebo was identical to intervention |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 6 of 16 (38%) participants withdrew 3 did not come to the clinic before the start of study medication (all in allopurinol group). 2 participants (1 in each group) discontinued because of adverse experiences. 1 participant in the placebo first group withdrew from the study at the end of the washout period |
| Selective reporting (reporting bias) | Low risk | No protocol available. All outcomes mentioned in methods are shown in the results |
| Other bias | Low risk | No other biases identified |
Bhardwaj 2009.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Study performed in New Delhi, India | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random sequence was computer‐generated by independent statistician |
| Allocation concealment (selection bias) | Low risk | Concealed allocation. Separate individuals generated the allocation sequence, enrolled participants and assigned participants to groups |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded. Placebo was identical to intervention |
| Incomplete outcome data (attrition bias) All outcomes | High risk | In total, 40 (27%) participants (27 in the placebo group and 13 in the intervention group) were lost at some time during the study. Not all reasons for these losses are specified |
| Selective reporting (reporting bias) | Low risk | The study protocol is available. All outcomes in the protocol were reported. Additionally, the number of man‐days lost per month as the result of pain was reported in the article but was not specified in the protocol |
| Other bias | Low risk | No other biases identified |
Bilton 1994a.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Study performed in Manchester, England | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence |
| Allocation concealment (selection bias) | Low risk | Randomisation was concealed by envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded study, using placebo |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 10 of 30 (33%) participants withdrew (6 for gastrointestinal intolerance, 3 requiring urgent medical treatment, 1 who defaulted) |
| Selective reporting (reporting bias) | Low risk | No discrepancies between methods and results |
| Other bias | Low risk | No other biases identified |
Bilton 1994b.
| Methods |
|
|
| Participants |
|
|
| Interventions | Intervention: combination antioxidants (daily 800 mg S‐adenosylmethionine (SAMe) sulfate‐p‐toluenesulfonate and 600 μg selenium and 9000 IU β‐carotene) Control: placebo |
|
| Outcomes |
|
|
| Notes | Original goal was to include 30 participants. Study was terminated early because of adverse events Study performed in Manchester, England |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence |
| Allocation concealment (selection bias) | Low risk | Randomisation was concealed by envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded study, using placebo |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 6 (43%) of 14 patients withdrew (3 for gastrointestinal adverse effects, 2 with unrelated medical problems, 1 who defaulted) |
| Selective reporting (reporting bias) | Low risk | No discrepancies between methods and results |
| Other bias | High risk | Study was terminated early as the result of unexpected adverse events. No formal stopping rule was applied, and study authors did not state that analysis was corrected for early termination |
Deprez 2003.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not performed (open trial) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated |
| Selective reporting (reporting bias) | Unclear risk | Published only in abstract form. Pain data not well reported |
| Other bias | Unclear risk | Published only in abstract form |
Durgaprasad 2005.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Study performed in Manipal, India | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation is not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Single‐blind |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 5 (25%) participants did not return for evaluation and were not assessed |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in methods are shown in results. Data on use of analgesics not shown but use of analgesics is shortly described. No protocol available |
| Other bias | Unclear risk | Study authors say diabetic patients will be excluded, but in the characteristics of participants section, 6 are described as having diabetes mellitus |
Jarosz 2010.
| Methods |
|
|
| Participants | 91 participants (aged 18 to 60 years) with proven (by imaging) alcoholic CP (daily 20 mL for 7 years), with abdominal pain | |
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Study performed in Warsaw, Poland | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated. Stated only that a random code was used |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 24 (26%) of 91 participants were excluded: 10 in the standard treatment group, and 14 in the antioxidant group. Reasons for exclusion: continued alcohol consumption, loss to follow‐up and lack of compliance with study medication |
| Selective reporting (reporting bias) | Low risk | All outcomes in the methods section are reported |
| Other bias | Low risk | No other biases identified |
Kirk 2006.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Study performed in Belfast, Northern Ireland | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation was not specified |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind. Identical placebo |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 17 (47%) of 36 participants withdrew or were lost to follow‐up. 10 had first placebo and 7 first antioxidants. This was attributed to the length of the study period, poor participant motivation and, in some cases, ongoing problems with alcohol dependence |
| Selective reporting (reporting bias) | High risk | No protocol available. Pain diaries were excluded from analyses because of inconsistent completion |
| Other bias | Low risk | Fat‐soluble vitamins such as vitamin E tended to remain slightly elevated at the end of the study, but results of this study and of previous studies provide evidence against a significant bias due to carry‐over effect |
Nandi 2002.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not stated |
| Selective reporting (reporting bias) | High risk | Published only as an abstract |
| Other bias | Unclear risk | Published only as an abstract |
Salim 1991.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes | Participants were questioned 3 times each day and were physically examined twice daily.
|
|
| Notes | Study performed in Baghdad, Iraq | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of random sequence generation not stated |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blinded. Placebo was given in same amount (iv) and on same schedule |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Four of 27 participants in the placebo group, three of 25 in the allopurinol group and five of 26 in the dimethylsulfoxide group were not assessed. Reasons were given. Both per‐protocol and intention‐to‐treat analyses were performed |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. No clear specification of outcomes in the methods section |
| Other bias | Low risk | No other biases |
Siriwardena 2012.
| Methods |
|
|
| Participants | 70 patients with painful chronic pancreatitis (proven by imaging) with a baseline daily pain score of 5 or greater for at least 7 days during a prerandomisation run‐in period of 1 month | |
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Study performed in Manchester, United Kingdom | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random sequence |
| Allocation concealment (selection bias) | Low risk | Concealed by central allocation (by pharmacy) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind, identical placebo |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 22 (23%) of 92 participants withdrew or were lost to follow‐up. Withdrawals were similar, by treatment allocation and in age, sex and baseline pain scores |
| Selective reporting (reporting bias) | Low risk | According to the registration form, the study authors intended to also present 'Time in pain' and 'Economic evaluation' as part of their secondary outcomes. These outcomes are not reported in the published paper. However, these are secondary outcomes that are not likely to significantly affect the results of the trial |
| Other bias | Low risk | No other biases identified |
Uden 1990.
| Methods |
|
|
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Study performed in Manchester, England | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Low risk | Double‐blind, double‐dummy, coordinated by a senior pharmacist |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind. Identical placebos, except for subtle differences (i.e. the selenium‐placebo had a distinctive sweet taste, and the methionine‐placebo lacked the garlic‐like odour of the true substance) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 (14%) participants lost to follow‐up: 1 required surgery early in the trial, 1 got pregnant, 1 changed jobs and 1 was accidentally changed from placebo to antioxidant group 1 (4%) participant's data were not analysed because during the trial, after biochemical analysis, it turned out that the participant had high baseline levels of vitamin E (participant was taking vitamin E–containing supplement before the trial) |
| Selective reporting (reporting bias) | Low risk | All outcomes in the methods section are reported |
| Other bias | Low risk | No other biases identified |
Abbreviations: BMI: body mass index. CP: chronic pancreatitis. EORTC‐QLQC: European Organization for Research and Treatment of Cancer Quality of Life Questionnaire. EQ‐5D: EuroQOL 5‐Dimension Questionnaire. ERCP: endoscopic retrograde cholangiopancreatography. EuroQOL: European Quality of Life Group. QLQ‐PAN28: Quality of Life Questionnaire‐Pancreatic modification. SAMe: S‐adenosylmethionine. VAS: visual analogue scale.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bagul 2006 | Not a randomised study |
| Bhardwaj 2004 | Not a randomised study |
| Bhardwaj 2006 | Not a randomised study |
| Braganza 1991 | Book chapter. Review of topic |
| De las Heras 2000 | Not a randomised study |
| Klapdor 2012 | Not a randomised study. The intervention (vitamin D) is not a known antioxidant agent |
| Martinez‐Torres 2009 | Randomised controlled trial on acute pancreatitis |
| Matthew 1996 | Not a randomised study (cross‐sectional) |
| Milnerowicz 2005 | Not a randomised study |
| Mosler 2005 | Randomised controlled trial on acute pancreatitis |
| Nakamura 1996 | Not a randomised study. Study focused on effect of pancreatic insufficiency |
| Romagnuolo 2008 | Randomised controlled trial on acute pancreatitis |
| Shah 2010 | Not a randomised study |
| Shalimar 2011 | Not a randomised study |
| Sinwardena 2006 | Randomised controlled trial on acute pancreatitis |
| Uden 1988 | Not a randomised study (case‐control study) |
Characteristics of ongoing studies [ordered by study ID]
EUROPAC‐2.
| Trial name or title | Pain Treatment of Hereditary and Idiopathic Pancreatitis |
| Methods | 3‐armed, double‐blind, placebo‐controlled, randomised, parallel‐group study |
| Participants | Patients with hereditary pancreatitis or idiopathic chronic pancreatitis |
| Interventions | Group 1: daily doses of 300 µg organic selenium, 18 mg β‐carotene, 750 mg vitamin C, 240 mg vitamin E, 2700 mg methionine Group 2: magnesium‐L‐aspartate‐hydrochloride 365 mg/d Group 3: placebo |
| Outcomes | Primary outcome measures:
Secondary outcome measures:
|
| Starting date | June 2004 |
| Contact information | Markus M Lerch, Professor of Medicine; 03834‐86 ext 7230. lerch@uni‐greifswald.de Julia V Mayerle, MD; 03834‐86 ext 7244. mayerle@uni‐greifswald.de |
| Notes | Contact: Julia V Mayerle, MD |
Differences between protocol and review
A new secondary outcome (number of pancreatitis events) has been included in the review.
The protocol described under 'Searching for other resources' that review authors planned to “request additional information from all authors of included trials on any published, unpublished or ongoing trials, by letter or by e‐mail.” This is not included in the review.
The review authors have included assessment of suitability of cross‐over design in the assessment of risk of bias in the review methods.
The section on data synthesis has been updated with new methods for dealing with parallel/cross‐over/combining parallel and cross‐over trials.
Contributions of authors
Ahmed Ali U, Jens S, Busch ORC, Keus F, Gooszen HG and Boermeester MA participated in the design of this review and in drafting of the protocol.
Ahmed Ali U and Jens S performed the search, extracted the data, assessed the studies and drafted the first version of the review.
Ahmed Ali U, Busch ORC, Keus F, van Goor H and Boermeester MA participated in the statistical analysis and in interpretation of the results.
All review authors co‐authored the review and read and approved the final manuscript.
Sources of support
Internal sources
None, Other.
External sources
None, Other.
Declarations of interest
Authors have reported no conflicts of interest.
New
References
References to studies included in this review
Banks 1997 {published data only}
- Banks PA, Hughes M, Ferrante M, Noordhoek EC, Ramagopal V, Slivka A. Does allopurinol reduce pain of chronic pancreatitis?. International Journal of Pancreatology 1997;22(3):171‐6. [DOI] [PubMed] [Google Scholar]
Bhardwaj 2009 {published data only}
- Bhardwaj P, Garg PK, Maulik SK, Saraya A, Tandon RK, Acharya SK. A randomized controlled trial of antioxidant supplementation for pain relief in patients with chronic pancreatitis. Gastroenterology 2009;136(1):149‐59. [DOI] [PubMed] [Google Scholar]
- Bhardwaj P, Garg PK, Saraya A, Acharya S. Antioxidant supplementation for pain relief in chronic pancreatitis: a randomized placebo controlled double blind trial. Gastroenterology 2007;132:A51. [DOI] [PubMed] [Google Scholar]
- Bhardwaj PG. A randomized controlled trial of antioxidant supplementation for pain relief in patients with chronic pancreatitis. Gastroenterology 2009;136:abstract. [DOI] [PubMed] [Google Scholar]
Bilton 1994a {published data only}
- Bilton D, Schofield D, Mei G, Kay PM, Bottiglieri T, Braganza JM. Placebo‐controlled trials of antioxidant therapy including S‐adenosylmethionine in patients with recurrent non‐gallstone pancreatitis. Clinical Drug Investigation 1994;8:10‐20. [Google Scholar]
Bilton 1994b {published data only}
- Bilton D, Schofield D, Mei G, Kay PM, Bottiglieri T, Braganza JM. Placebo‐controlled trials of antioxidant therapy including S‐adenosylmethionine in patients with recurrent non‐gallstone pancreatitis. Clinical Drug Investigation 1994;8:10‐20. [Google Scholar]
Deprez 2003 {published data only}
- Deprez PH, Delazzer E, Galanti L, Lebrun J, Geubel A, Horsmans Y. Clinical and nutritional effects of anti‐oxidant supplementation: a prospective randomized study in patients with chronic pancreatitis. Gastroenterology 2003;124(4):A90. [Google Scholar]
Durgaprasad 2005 {published data only}
- Durgaprasad S, Pai CG, Vasanthkumar, Alvres JF, Namitha S. A pilot study of the antioxidant effect of curcumin in tropical pancreatitis. Indian Journal of Medical Research 2005;122(4):315‐8. [PubMed] [Google Scholar]
Jarosz 2010 {published data only}
- Jarosz M, Orzeszko M, Rychlik E, Kozuch M. Antioxidants in the treatment of chronic pancreatis [Antyoksydanty w leczeniu przewlekłego zapalenia trzustki]. Gastroenterologia Polska 2010;17:41‐6. [Google Scholar]
Kirk 2006 {published data only}
- Kirk GR, White JS, McKie L, Stevenson M, Young I, Clements WD, Rowlands BJ. Combined antioxidant therapy reduces pain and improves quality of life in chronic pancreatitis. Journal of Gastrointestinal Surgery 2006;10(4):499‐503. [DOI] [PubMed] [Google Scholar]
Nandi 2002 {published data only}
- Nandi B, Garg PK, Bhardwaj P, Prakash S, Tandon RK. Efficacy of antioxidants for pain relief in patients with chronic pancreatitis: a randomized controlled trial. Indian Journal of Gastroenterology 2002;21(Suppl 1):A43. [DOI] [PubMed] [Google Scholar]
Salim 1991 {published data only}
- Salim AS. Role of oxygen‐derived free radical scavengers in the treatment of recurrent pain produced by chronic pancreatitis. A new approach.. Archives of Surgery 1991;9:1109‐14. [DOI] [PubMed] [Google Scholar]
Siriwardena 2012 {published data only}
- Shah N, Mason JM, Makin AJ, Sheen AJ, Siriwardena AK. A randomised, double‐blind, placebo‐controlled trial of oral antioxidant therapy for chronic pancreatitis: the final results of the ANTICIPATE study. British Journal of Surgery 2012;99:2. [Google Scholar]
- Siriwardena A, Mason J, Sheen A, Makin A, Shah N. Antioxidant therapy for chronic pancreatitis: the final results of a randomised, double blind, placebo‐controlled trial (the ANTICIPATE STUDY). HPB 2012;14:663. [Google Scholar]
- Siriwardena AK, Mason JM, Shah NS, Sheen AJ. Antioxidant therapy for chronic pancreatitis: a randomized, controlled trial. Gastroenterology 2012;142:S113. [DOI] [PubMed] [Google Scholar]
- Siriwardena AK, Mason JM, Sheen AJ, Makin AJ, Shah NS. Antioxidant therapy does not reduce pain in patients with chronic pancreatitis: the ANTICIPATE study. Gastroenterology 2012;143:655–63. [DOI] [PubMed] [Google Scholar]
Uden 1990 {published data only}
- Uden S, Bilton D, Nathan L, Hunt LP, Main C, Braganza JM. Antioxidant therapy for recurrent pancreatitis: placebo‐controlled trial. Alimentary Pharmacology & Therapeutics 1990;4(4):357‐71. [DOI] [PubMed] [Google Scholar]
- Uden S, Main C. Placebo‐controlled double‐blind trial of antioxidant supplements in patients with recurrent pancreatitis. Clinical Science 1989;77(Suppl 21):26P‐27P. [Google Scholar]
- Uden S, Schofield D, Miller PF, Day JP, Bottiglier T, Braganza JM. Antioxidant therapy for recurrent pancreatitis: biochemical profiles in a placebo‐controlled trial. Alimentary Pharmacology & Therapeutics 1992;6(2):229‐40. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bagul 2006 {published data only}
- Bagul A, Siriwardena AK. Long‐term outcome of oral anti‐oxidant therapy in patients with painful chronic pancreatitis. Gastroenterology 2006;130(4):A517. [Google Scholar]
Bhardwaj 2004 {published data only}
- Bhardwaj P, Thareja S, Prakash S, Saraya A, Bhardwaj P, Thareja S, et al. Micronutrient antioxidant intake in patients with chronic pancreatitis. Tropical Gastroenterology 2004;25:69‐72. [PubMed] [Google Scholar]
Bhardwaj 2006 {published data only}
- Bhardwaj P, Garg PK, Saraya A. Free radical mediated oxidative stress and antioxidant status in patients with chronic pancreatitis. Free Radical Research 2006;40:S107. [Google Scholar]
Braganza 1991 {published data only}
- Braganza JM. Antioxidant therapy for pancreatitis—clinical experience. Pathogenesis of Pancreatitis. Manchester, UK: Manchester University Press, 1991:178‐97. [Google Scholar]
De las Heras 2000 {published data only}
- las Heras CG, Garcia de la Paz A, Fernandez MD, Fernandez‐Forcelledo JL. Use of antioxidants to treat pain in chronic pancreatitis. Revista Espanola de Enfermedades Digestivas 2000;92:375‐85. [PubMed] [Google Scholar]
Klapdor 2012 {published data only}
- Klapdor S, Richter E, Klapdor R. Vitamin D status and per‐oral vitamin D supplementation in patients suffering from chronic pancreatitis and pancreatic cancer disease. Anticancer Research 2012;32:1991‐8. [PubMed] [Google Scholar]
Martinez‐Torres 2009 {published data only}
- Martinez‐Torres HR‐L. Oral allopurinol to prevent hyperamylasemia and acute pancreatitis after endoscopic retrograde cholangiopancreatography. World Journal of Gastroenterology 2009;15:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Matthew 1996 {published data only}
- Mathew P, Wyllie R, Van LF, Steffen RM, Kay MH, Mathew P, et al. Antioxidants in hereditary pancreatitis. American Journal of Gastroenterology 1996;91:1558‐62. [PubMed] [Google Scholar]
Milnerowicz 2005 {published data only}
- Milnerowicz H, Jablonowska M, Milnerowicz S. The level of GSH and antioxidant enzyme activity: GPx and Cu/Zn SOD in patients with pancreatitis. FEBS Journal 2005;272:427. [Google Scholar]
Mosler 2005 {published data only}
- Mosler P, Sherman S, Marks J, Watkins JL, Geenen JE, Jamidar P, et al. Does prophylactic allopurinol administration reduce the risk and severity of post‐ERCP pancreatitis: randomized prospective multicenter study. Gastrointestinal Endoscopy 2005;61:AB100. [DOI] [PubMed] [Google Scholar]
Nakamura 1996 {published data only}
- Nakamura T, Takebe K, Imamura K, Tando Y, Yamada N, Arai Y, et al. Fat‐soluble vitamins in patients with chronic pancreatitis (pancreatic insufficiency). Acta Gastro‐enterologica Belgica 1996;59:10‐4. [PubMed] [Google Scholar]
Romagnuolo 2008 {published data only}
- Romagnuolo J, Hilsden R, Sandha GS, Cole M, Bass S, May G, et al. Allopurinol to prevent pancreatitis after endoscopic retrograde cholangiopancreatography: a randomized placebo‐controlled trial. Clinical Gastroenterology and Hepatology 2008;6:465‐71. [DOI] [PubMed] [Google Scholar]
- Romagnuolo J, Hilsden RJ, Sandha GS, Cole MJ, Bass S, May GR, et al. Allopurinol to prevent pancreatitis after endoscopic retrograde cholangiopancreatography (ERCP): a randomized placebo‐controlled trial. Gastrointestinal Endoscopy 2008;67:AB328. [DOI] [PubMed] [Google Scholar]
- Romagnuolo J, Sandha G, Kruger C, May G, Cole N, Bass S, et al. Allopurinol to prevent post‐ERCP pancreatitis: blind interim analysis of a randomized placebo‐controlled trial. Gastrointestinal Endoscopy 2005;61:AB195. [Google Scholar]
Shah 2010 {published data only}
- Shah NS, Makin AJ, Sheen AJ, Siriwardena AK. Quality of life assessment in patients with chronic pancreatitis receiving antioxidant therapy. World Journal of Gastroenterology 2010;16:4066‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shalimar 2011 {published data only}
- Shalimar S, Midha S, Bhardwaj P, Garg PK. Long‐term pain relief with optimized medical therapy including antioxidants in patients with chronic pancreatitis. Gastroenterology 2011;140:S547. [DOI] [PubMed] [Google Scholar]
Sinwardena 2006 {published data only}
- Sinwardena AK, Mason JM, Balachandra S, Bagul A, Galloway S, Formela L, et al. Randomized, double‐blind, placebo‐controlled, trial of high‐dose intravenous anti‐oxidant therapy in severe acute pancreatitis. Gastroenterology 2006;130:A83. [Google Scholar]
Uden 1988 {published data only}
- Uden S, Acheson DW, Reeves J, Worthington HV, Hunt LP, Brown S, et al. Antioxidants, enzyme induction, and chronic pancreatitis: a reappraisal following studies in patients on anticonvulsants. European Journal of Clinical Nutrition 1988;42:561‐9. [PubMed] [Google Scholar]
References to ongoing studies
EUROPAC‐2 {published data only}
- EUROPAC‐2.‐ Pain Treatment of Hereditary and Idiopathic Pancreatitis. Clinicaltrials.gov.
Additional references
Apte 1999
- Apte MV, Keogh GW, Wilson JS. Chronic pancreatitis: complications and management. Journal of Clinical Gastroenterology 1999;29(3):225‐40. [DOI] [PubMed] [Google Scholar]
Begg 1994
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50(4):1088‐101. [PUBMED: 7786990] [PubMed] [Google Scholar]
Bjelakovic 2008
- Bjelakovic G, Nikolova D, Simonetti RG, Gluud C. Antioxidant supplements for preventing gastrointestinal cancers. Cochrane Database of Systematic Reviews 2008, Issue 3. [DOI: 10.1002/14651858.CD004183.pub3] [DOI] [PubMed] [Google Scholar]
Bowrey 1999
- Bowrey DJ, Morris‐Stiff GJ, Puntis MC. Selenium deficiency and chronic pancreatitis: disease mechanism and potential for therapy. HPB Surgery 1999;11(4):207‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Braganza 2010
- Braganza JM, Dormandy TL. Micronutrient therapy for chronic pancreatitis: rationale and impact. Journal of the Pancreas 2010;11(2):99‐112. [PubMed] [Google Scholar]
Curtin 2002
- Curtin F, Elbourne D, Altman DG. Meta‐analysis combining parallel and cross‐over clinical trials. II: Binary outcomes. Statistics in Medicine 2002;21(15):2145‐59. [DOI] [PubMed] [Google Scholar]
Dite 2001
- Dite P, Stary K, Novotny I, Precechtelova M, Dolina J, Lata J, Zboril V. Incidence of chronic pancreatitis in the Czech Republic. The European Journal of Gastroenterology and Hepatology 2001;13:749‐50. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [DOI] [PubMed] [Google Scholar]
Feng 2010
- Feng Z, Liu Z, Li X, Jia H, Sun L, Tian C, et al. Alpha‐tocopherol is an effective phase II enzyme inducer: protective effects on acrolein‐induced oxidative stress and mitochondrial dysfunction in human retinal pigment epithelial cells. The Journal of Nutritional Biochemistry 2010;21(12):1222‐31. [DOI] [PubMed] [Google Scholar]
Gachago 2008
- Gachago C, Draganov PV. Pain management in chronic pancreatitis. World Journal of Gastroenterology 2008;14(20):3137‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyan 1990
- Guyan PM, Uden S, Braganza JM. Heightened free radical activity in pancreatitis. Free Radical Biology and Medicine 1990;8(4):347‐54. [DOI] [PubMed] [Google Scholar]
Hawker 2011
- Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short‐Form McGill Pain Questionnaire (SF‐MPQ), Chronic Pain Grade Scale (CPGS), Short Form‐36 Bodily Pain Scale (SF‐36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP). Arthritis Care and Research 2011;63(Suppl 11):S240‐52. [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21:1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Handbook for Systematic Reviews of Interventions. New York: John Wiley & Sons Ltd, 2008. [Google Scholar]
Higgins 2008a
- Higgins JPT, Green S. Section 16.4.3. Assessing risk of bias in cross‐over trials. Cochrane Handbook for Systematic Reviews of Interventions. New York: John Wiley & Sons Ltd, 2008. [Google Scholar]
Hozo 2005
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Medical Research Methodology 2005;5(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kalvaria 1986
- Kalvaria I, Labadarios D, Shephard GS, Visser L, Marks IN. Biochemical vitamin E deficiency in chronic pancreatitis. International Journal of Pancreatology 1986;1(2):119‐28. [DOI] [PubMed] [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodological quality and discrepancies between large and small randomised trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. [DOI] [PubMed] [Google Scholar]
Levy 2006
- Levy P, Barthet M, Mollard BR, Amouretti M, Marion‐Audibert AM, Dyard F. Estimation of the prevalence and incidence of chronic pancreatitis and its complications. Gastroenterology Clinical Biology 2006;30:838‐44. [DOI] [PubMed] [Google Scholar]
Macaskill 2001
- Macaskill P, Walter SD, Irwig L. A comparison of methods to detect publication bias in meta‐analysis. Statistics in Medicine 2001;20:641‐54. [DOI] [PubMed] [Google Scholar]
McGee 2011
- McGee RG, Su M, Kelly PJ, Higgins GY, Craig JC, Webster AC. Trial registration and declaration of registration by authors of randomized controlled trials. Transplantation 2011;92(10):1094‐100. [DOI] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. [DOI] [PubMed] [Google Scholar]
Monfared 2009
- Monfared SSMS, Vahidi H, Abdolghaffari AH, Nikfar S, Abdollahi M. Antioxidant therapy in the management of acute, chronic and post‐ERCP pancreatitis: a systematic review. World Journal of Gastroenterology 2009;15(36):4481‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Owen 2000
- Owen RW, Giacosa A, Hull WE, Haubner R, Spiegelhalder B, Bartsch H. The antioxidant/anticancer potential of phenolic compounds isolated from olive oil. European Journal of Cancer 2000;36(10):1235‐47. [DOI] [PubMed] [Google Scholar]
Pezzilli 2005
- Pezzilli R, Morselli Labate AM, Ceciliato R, Frulloni L, Cavestro GM, Comparato G, et al. Quality of life in patients with chronic pancreatitis. Digestive Liver Disease 2005;37:181‐9. [DOI] [PubMed] [Google Scholar]
Ramos‐Márquez 2008
- Ramos‐Márquez ME, Siller‐López F. Current antioxidant molecular therapies for oxidative stress–related ailments. Current Gene Therapy 2008;8(4):256‐63. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rose 1986
- Rose P, Fraine E, Hunt LP, Acheson DW, Braganza JM. Dietary antioxidants and chronic pancreatitis. Human Nutrition ‐ Clinical Nutrition 1986;40(2):151‐64. [PubMed] [Google Scholar]
Sala 2002
- Sala A, Recio MD, Giner RM, Manez S, Tournier H, Schinella G, et al. Anti‐inflammatory and antioxidant properties of Helichrysum italicum. The Journal of Pharmacy and Pharmacology 2002;54(3):365‐71. [DOI] [PubMed] [Google Scholar]
Schneider 2007
- Schneider A, Lohr JM, Singer MV. The M‐ANNHEIM classification of chronic pancreatitis: introduction of a unifying classification system based on a review of previous classifications of the disease. Journal of Gastroenterology 2007;42(2):101‐19. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayer R, Altman D. Empirical evidence of bias. JAMA 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]
Spanier 2008
- Spanier BW, Dijkgraaf MG, Bruno MJ. Epidemiology, aetiology and outcome of acute and chronic pancreatitis: an update. Best Practice and Research. Clinical Gastroenterology 2008;22:45‐63. [DOI] [PubMed] [Google Scholar]
Stedman 2011
- Stedman MR, Curtin F, Elbourne DR, Kesselheim AS, Brookhart MA. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2011;40(6):1732‐4. [DOI] [PubMed] [Google Scholar]
Temple 2000
- Temple NJ. Antioxidants and disease: more questions than answers. Nutrition Research 2000;20(3):449‐59. [Google Scholar]
van Esch 2006
- Esch AA, Wilder‐Smith OH, Jansen JB, Goor H, Drenth JP. Pharmacological management of pain in chronic pancreatitis. Digestive Liver Disease 2006;38(7):518‐26. [DOI] [PubMed] [Google Scholar]
Wehler 2004
- Wehler M, Nichterlein R, Fischer B, Farnbacher M, Reulbach U, Hahn EG, et al. Factors associated with health‐related quality of life in chronic pancreatitis. American Journal of Gastroenterology 2004;99:138‐46. [DOI] [PubMed] [Google Scholar]
Willett 2002
- Willett WC. Balancing life‐style and genomics research for disease prevention. Science 2002;296:695‐8. [DOI] [PubMed] [Google Scholar]
Witt 2007
- Witt H, Apte MV, Keim V, Wilson JS. Chronic pancreatitis: challenges and advances in the pathogenesis, genetics, diagnosis, and therapy. Gastroenterology 2007;132(4):1557‐73. [DOI] [PubMed] [Google Scholar]


