Abstract
Background
It is well-established that vitamins have many beneficial roles and protect humans against inflammatory diseases. Vitamin D, a lipid-soluble vitamin, plays a crucial role in viral infections. Therefore, this study aimed to investigate if serum 25(OH)D levels affect morbidity, mortality, and levels of inflammatory parameters in COVID-19 patients.
Methods
140 COVID-19 patients participated in this study (65 outpatients and 75 inpatients). Their blood samples were collected to determine TNFα, IL-6, D-dimer, zinc, Ca2+, and 25(OH)D levels. Patients with O2 saturation <93% were admitted and hospitalized in the infectious disease ward (inpatient group). Patients with O2 saturation >93% received routine treatment and were discharged (Outpatient group).
Results
The serum levels of 25(OH)D in the inpatient group were significantly lower than those in the outpatient group (p < 0.001). Serum TNF-α, IL-6, and D-dimer levels in the inpatient group were significantly higher than those in the outpatient group (p < 0.001). Serum TNF-α, IL-6, and D-dimer levels were inversely correlated with 25(OH)D levels. No significant differences were observed in the serum levels of zinc and Ca2+ between the studied groups (p = 0.96, p = 0.41 respectively). Ten out of 75 patients in the inpatient group were admitted to ICU (intubated). Nine out of them lost their lives (the mortality rate in ICU-admitted patients was 90%).
Conclusions
The lower mortality and severity of COVID-19 patients with higher 25(OH)D levels represented that this vitamin alleviates the severity of COVID-19.
Keywords: Sars-Cov-2, Vitamin D, TNF-α, IL-6, D-dimer, COVID-19
1. Introduction
The first detection of SARS-CoV-2 in Iran was reported in February 2020 [1]. Respiratory droplets and direct contact are the main routes for the transmission of Sars-CoV-2 [2]. The severity of the symptomatic disease can vary from mild respiratory and gastrointestinal symptoms to lung damage and multi-organ failure [3]. Factors such as smoking, congestive heart failure, old age, elevated creatinine, β-blocker use, presence of bilateral lung infiltrates, and severe vitamin D deficiency seem to be notable predictors of tragic outcomes [4].
The virus replication can cause overstimulation of the immune system, which finally leads to cytokine storm and progression to ARDS [5,6]. Many studies have shown that vitamins and minerals such as vitamin D and zinc may prevent the progression of ARDS, which might be related to antioxidant properties and the ability of these factors to strengthen the immune system [7]. Because of the pro-inflammatory state in ARDS, it is believed that increasing the antioxidant nutrients in the patient's body may have a beneficial effect [8]. For example, vitamin D upregulates the expression of some antioxidant genes, such as glutathione reductase, reducing free radicals resulting from inflammation which are believed to contribute to the progression of ARDS [9]. The beneficial effects of zinc include the regulation of the immune system, mucociliary clearance of the airways, reducing lung damage (therefore secondary infections), reducing inflammation, and even viral replication [10]. Vitamin D decreases Th17 lymphocytes and TNF-α and increases anti-inflammatory cytokines [11,12].
Studies on the efficacy of vitamin D in COVID-19 are controversial. Some studies claim vitamin D deficiency increases the risk of Sars-CoV-2 infection significantly [13,14], while others have shown the opposite [15]. Therefore, more studies are needed to clarify if vitamin D levels are associated with the severity of COVID-19 [[16], [17], [18], [19]].
In immune cells, such as T and B lymphocytes, calcium signaling pathways control: mitosis, apoptosis, differentiation, and transcriptional processes [[20], [21], [22]]. T cells' function and developing immunity against the infection are affected by the entry of calcium ions [23]. Studies suggested that COVID-19 patients with lower calcium levels in serum experience worse clinical conditions [24,25]. Therefore, calcium levels may act as a biomarker for the severity of COVID-19 [26].
One of the leading causes of deterioration in COVID-19 patients is cytokine storm, mainly due to the overproduction of pro-inflammatory cytokines such as IL-1β, IL-6, IL-15, and TNF-α [27]. Overproduction of these cytokines is associated with clinical symptoms of the disease. For example, the overproduction of TNF-α is associated with flu-like symptoms [28]. Studies have shown that in severe inflammatory disease induced by SARS-CoV-2, pro-inflammatory biomarkers levels such as D-dimer are elevated [19,29].
Antioxidants have the ability to decrease reactive oxygen species (markers of oxidative stress), mitigate the storm of cytokines, and activate several mechanisms to prevent viral inflammation [30]. The other beneficial effect of antioxidants is to alleviate the severity of diseases by reducing pro-inflammatory cytokines and preventing hyper-inflammation. Vitamins have been shown to inhibit or mitigate viral inflammation by impairing the replication of viruses, blocking spike proteins, and modulating the expression of the angiotensin-converting-enzyme-2 receptor [30]. Vitamin D plays crucial immunomodulatory roles by inducing the mRNA expression of antiviral peptides, which potentiate inborn immunity [31,32]. Many clinical reports have shown that acute respiratory tract diseases with low 25(OH)D levels in serum are associated [[33], [34], [35], [36]].
Therefore, this study aimed to investigate if serum 25(OH)D levels are related to levels of inflammatory parameters, morbidity, and mortality in patients with COVID-19.
2. Material and methods
2.1. Study design and participants
In this study, 140 COVID-19 patients participated (65 outpatients and 75 inpatients). Patients with COVID-19 symptoms referred to the infectious diseases clinic of Razi educational hospital in Ahvaz were screened for infection of Sars-Cov-2 in June and July 2021. Upon admission, their oro-nasopharyngeal swabs were collected and tested for Sars-Cov-2 using real-time PCR. In addition, their blood samples (in plain and EDTA tubes) were collected to determine the other studied variables. Patients with O2 saturation <93% were admitted and hospitalized in the infectious disease ward (considered inpatient group) and, according to national therapeutic protocol, received remdesivir (First day 200 mg/intravenously (IV), second to fifth days 100 mg/IV). Patients with O2 saturation >93% got conservative treatment and were discharged (considered outpatient group). The common morbidity in inpatients admitted to the ward was hypertension (7%) and diabetes mellitus (6%).
2.2. Ethical approval
Written informed consent forms were taken from patients, and all stages of the study were approved by the Iranian National Committee for Ethics in Biomedical Research (IR.AJUMS.REC.1399.948).
2.3. Laboratory data acquisition
In order to determine Sars-Cov-2 infection, total RNA was extracted from 200 μl oro-nasopharyngeal swab samples using Sinaclon RNA extraction Kit (Iran) as described in the manufacturer's instruction. The probe and primer mixture of the real-time PCR COVID-19 kit (Pishtaz Teb, Iran) was designed by the dual-target gene method and targeted the protected sequences of the RdRp and N region simultaneously. The kit also contained an internal control probe and primer for RNase P. FAM, HEX, and ROX channels selected for detecting the RdRp region, N region, and RNase P gene, respectively. Real-time PCR amplification was done under the following settings: an initial 20min reverse transcription step at 50 °C, a 3min cDNA initial denaturation step at 95 °C, followed by 45 amplification cycles, with each cycle including a 10 s denaturation step at 94 °C, and a 40s annealing, extension, and fluorescence measurement step at 55 °C.
In order to measure the levels of zinc, 25(OH)D, Ca2+, and D-dimer, blood samples were centrifuged, and the serum was isolated and kept at −80 °C until the start of subsequent procedures. Serum zinc and Ca2+ levels were measured using the biochemical analyzer (Mindray BS-600 and Biotecnica BT-3000, respectively). The limit of detection was 5 pg/ml. The serum 25-hydroxy vitamin D3 levels were measured by the ELISA method (Monobind, Iran).
In order to determine the TNF-α mRNA expression, Using a ficoll solution (DNA Bioteck, France), peripheral blood mononuclear cells (PBMCs) were separated from blood (containing EDTA). According to the manufacturer's instructions commercial RNA extraction kit (Annacell, Tehran, Iran) was used to extract the total RNAs from PBMCs. The purity and concentration of extracted RNAs were determined using nanodrop (Thermo Scientific, USA). Then, cDNA was synthesized using the Sinaclon cDNA Synthesis Kit (Sinaclon, Tehran, Iran). Semi-quantitative real-time PCR was performed using SYBR green PCR Master Mix (Ampliqon, Denmark) according to the protocol. The primer sequences of GAPDH (Glyceraldehyde-3-Phosphate Dehydrogenase), TNF-α, and the timing of real-time PCR reactions are listed in Tables S1 and S2. The relative TNF-α mRNA expression was analyzed using the 2-△△Ct method and normalized to internal control (GAPDH).
In order to determine the serum TNF-α and IL-6 levels, blood (containing EDTA) was centrifuged (5 min, 3000g), then serum was separated and kept at −80 °C. Frozen samples melted at laboratory temperature, and then ELISA kits (Karmania Pars Gene Company, Iran) were used to determine serum TNF-α and IL-6 levels according to the manufacturer's instructions.
2.4. Statistical analysis
IBM SPSS Statistics 26 was used for statistical analysis, including the Independent Samples T-Test (normal distribution), Mann–Whitney U Test (non-normal distribution), Correlation test (Spearman's rank correlation coefficient), and descriptive statistical tests. The p-value <0.05 was considered statistically significant. Data are expressed as mean ± SEM. The displayr tool was used to create the correlation matrix.
3. Results
3.1. Baseline characteristics
One hundred forty COVID-19 patients participated in this study (65 outpatients and 75 inpatients). Ten out of 75 patients in the inpatient group were admitted to ICU (intubated). Nine out of them lost their lives (the mortality rate in ICU-admitted patients was 90%).
3.2. Serum levels of 25(OH)D, IL-6, D-dimer, Ca2+, and zinc
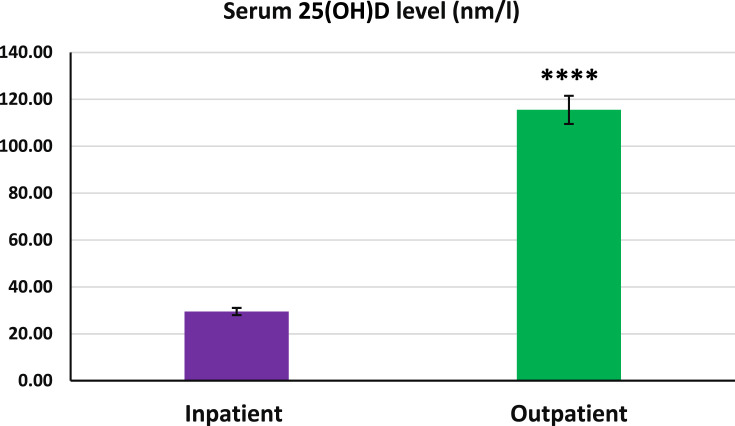
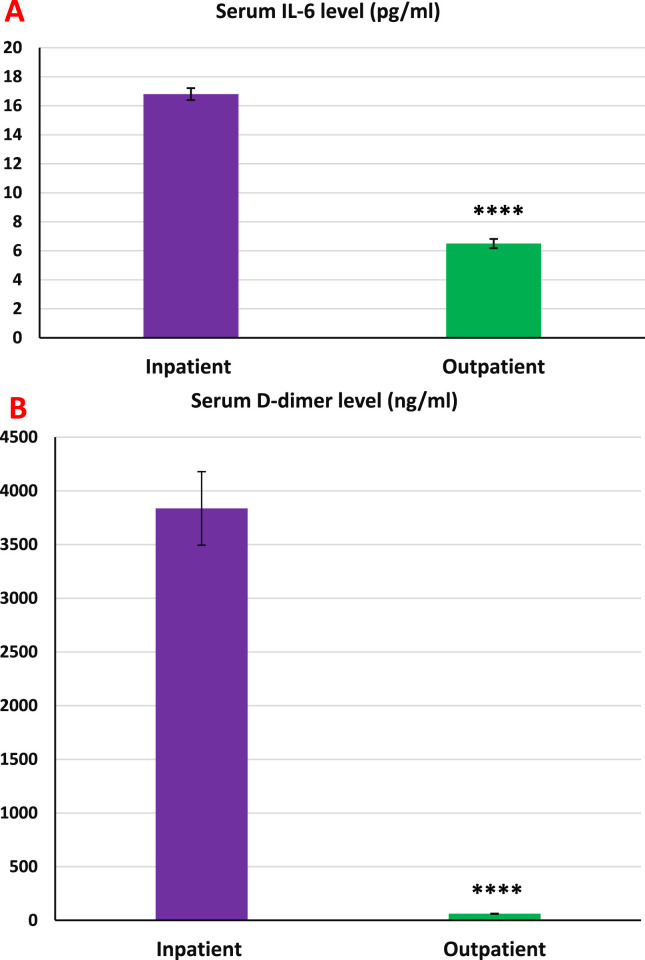
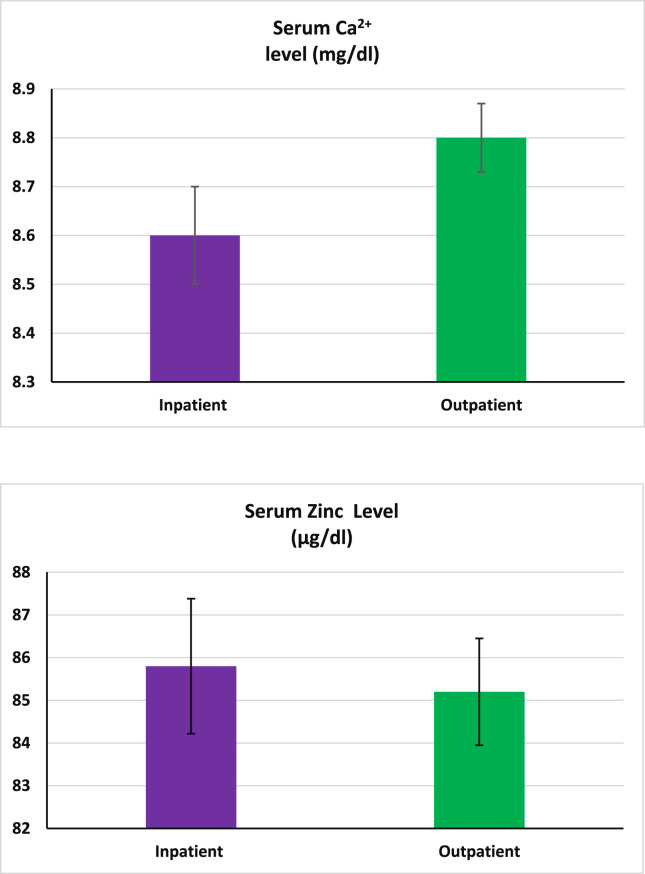
Figure 1 shows that the serum 25(OH)D levels in the inpatient group were significantly lower than those in the outpatient group (p < 0.001). Serum IL-6 and D-dimer levels in the inpatient group were significantly higher than the outpatient group (p < 0.001 in both cases, Fig. 2 , A and B). As shown in Fig. 3 (A and B), no significant differences in serum zinc and Ca2+ levels were observed between the studied groups.
Fig. 1.
Serum 25(OH)D levels in inpatient and outpatient groups. The 25(OH)D levels in the inpatient group were significantly lower than those in the outpatient group. S.E.M for inpatients and outpatients were 4.68 and 6.02, respectively. Data expressed as mean ± S.E.M. Independent sample statistical T-test was used to analyze the data. ∗∗∗∗p < 0.001.
Fig. 2.
Serum IL-6 and D-dimer levels in inpatient and outpatient groups. A: The levels of interleukine-6 in the inpatient group were significantly higher than in the outpatient group. S.E.M for inpatients and outpatients were 0.42 and 0.32, respectively. B: The D-dimer levels in the inpatient group were significantly higher than in the outpatient group. S.E.M for inpatients and outpatients were 342.38 and 3.39, respectively. Data expressed as mean ± S.E.M. Independent sample statistical T-test was used to analyze the data. ∗∗∗p < 0.001.
Fig. 3.
The serum Ca2+ and zinc levels in the inpatient and outpatient groups. The Ca2+ and zinc levels were not different between the studied groups. Ca2+ and zinc S.E.M for inpatients and outpatients were 0.11 and 0.08, and 1.52 and 1.26, respectively. Data expressed as mean ± S.E.M. Independent sample statistical T-test was used to analyze the data.
3.3. mRNA expression and serum levels of TNF-α
As illustrated in Figure S1 (A and B), both mRNA and serum levels of TNF-α in the inpatient group were significantly higher than those in the outpatient group (p < 0.001 in both cases).
3.4. Serum levels of pro-inflammatory biomarkers, zinc, and calcium in inpatient group (ICU-admitted and hospitalized non ICU patients)
As Table 2 shows, the serum levels of IL-6, D-dimer, and TNF-α in patients admitted to ICU were significantly higher than those in hospitalized non-ICU patients (p < 0.05, p < 0.05, and p < 0.002, respectively). The serum calcium levels in ICU-admitted patients were significantly lower than those in hospitalized non-ICU patients (p < 0.05). Zinc levels were not different between ICU-admitted and hospitalized non-ICU patients.
Table 2.
The serum levels of 25(OH)D, IL-6, D-dimer, TNF-α, calcium, and zinc in the inpatient group (ICU-admitted and hospitalized non ICU patients).
| hospitalized non ICU patients | ICU-admitted patients | p. Value | |
|---|---|---|---|
| Inpatients | 65 | 10 | |
| Male (n) | 35 | 7 | |
| Female (n) | 30 | 3 | |
| 25(OH)D (nm/l) | 46.72 ± 5.16 | 52.52 ± 10.75 | 0.63 |
| TNFα (pg/ml) | 44.24 ± 1.22 | 55.32 ± 3.46 | 0.001 |
| IL-6 (pg/ml) | 16.45 ± 0.43 | 19.49 ± 1.18 | 0.001 |
| D-Dimer (ng/ml) | 3485.93 ± 345 | 6126.3 ± 1023 | 0.001 |
| Zinc (mcg/dl) | 87.33 ± 1.6 | 84 ± 4.2 | 0.5 |
| Calcium (mg/dl) | 8.97 ± 0.1 | 8.24 ± 0.33 | 0.021 |
| Age (y) | 51.63 ± 14.51 | 59.5 ± 8.9 | 0.1 |
3.5. Correlation analysis
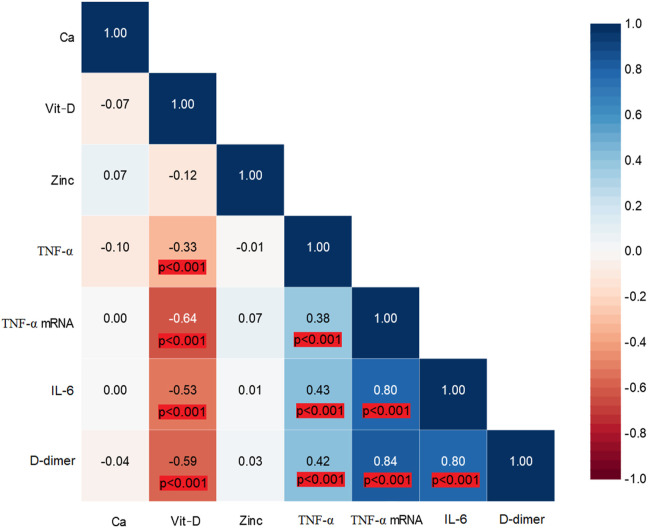
As illustrated in Fig. 4 , 25(OH)D levels were inversely correlated with TNFα, TNFα mRNA, IL-6, and D-dimer levels. There was a correlation between serum TNFα levels and IL-6, D-dimer, and TNFα mRNA levels. IL-6 levels were correlated with D-dimer levels, and TNFα mRNA levels were significantly correlated with IL-6 and D-dimer levels.
Fig. 4.
Correlation matrix for studied laboratory parameters (Blue color indicates positive correlation coefficient).
4. Discussion
This study illustrated that the morbidity and mortality rates in COVID-19 patients with higher serum 25(OH)D levels were significantly lower than those in patients with low levels of this vitamin. All patients with high 25(OH)D levels were discharged without needing hospitalization, which was considered the outpatient group. 10 out of 75 patients in the inpatient group were admitted to ICU and intubated (9 lost their lives). The common morbidities in inpatients admitted to ICU were hypertension (70%) and diabetes mellitus (30%). The serum levels of inflammatory cytokines, IL-6 and TNF-α, D-dimer, and mRNA expression of TNF-α in the inpatient group were significantly higher than those in the outpatient group. The serum Ca2+ and zinc levels were not different between the studied groups.
According to the used methodology, values of 25(OH)D less than 30 are sub-optimal, values between 30 and 50 are normal, and values between 70 and 150 ng/ml are high but not toxic [37]. The serum 25(OH)D levels in inpatient and outpatient groups were 29.5 ± 1.53 and 115.5 ± 6 ng/ml, respectively. Therefore, the current results indicated that the level of 25(OH)D in the inpatient group was already at lower (normal/borderline) or sub-optimal levels. This finding also represented that this level in the outpatient group was high. According to clinical studies, low 25(OH)D levels are associated with acute respiratory diseases such as influenza [33,34].
Platelets are crucial in the formation of thrombi, and platelet-activating factor (PAF) is their most potent trigger. Cells involved in host defense produce PAF, which bears similarities in biological actions with COVID-19 disease manifestations. Perivascular mast cell activation can be stimulated with PAF, leading to inflammation implicated in SARS. There are many mast cells in the lungs which are a rich source of inflammatory cytokines, such as IL-6 and IL-1β, and PAF which may contribute to COVID-19 disease [38]. In vitro data indicate that vitamin D reduces the secretion of the catabolic enzyme PAF-AH from placenta macrophages [39], which shows an interrelation between PAF and this vitamin [40]. The association between PAF and vitamin D is further substantiated by the known anti-thrombotic effects of this vitamin [41]. Hence, PAF inhibitors, such as vitamin D, may target inflammation and thrombosis and prevent the deleterious consequences of COVID-19 [40].
Additionally, a meta-analysis review reported that 25(OH)D levels <20 ng/ml increase the risk of pneumonia by more than 60% [42]. Recent hypotheses and studies suggest that vitamin D insufficiency and deficiency increase the severity and mortality of COVID-19 but not the chance of Sars-CoV-2 infection [[43], [44], [45]], while in some studies, it has been exhibited that 25(OH)D levels in covid-19 patients are lower than those in the control group [46]. Therefore, it can be concluded that 25(OH)D levels in the serum of the inpatient group were not enough to alleviate the severity of COVID-19.
An experimental animal study showed that co-administration of l-cysteine and vitamin D in mice with vitamin D deficiency increased circulating levels of 25(OH)D and glutathione but lowered oxidative stress and TNF-α levels [47]. According to studies, oral 5000 IU vitamin D supplementation for two weeks reduces the recovery time of cough and gustatory sensory loss in covid-19 patients with insufficient 25(OH)D levels and decreases serum IL-6 levels [48]. Present results indicatedd that serum TNF-α levels were inversely correlated with 25(OH)D levels (r = −0.33; p < 0.001). So it's possible that high serum 25(OH)D levels in the outpatient group protected them and mitigated the severity of COVID-19 illness by inhibiting cytokine release. In other words, cytokine storm as one of the leading causes of respiratory failure did not occur in patients with high enough serum levels of 25(OH)D.
As evidenced by in vitro studies, directly and indirectly, the protective roles of vitamin D on the homeostasis of the respiratory system were respectively exerted by inhibition of virus replication and induction of antiviral peptides production [49]. Moreover, it has been reported that heart failure and acute respiratory distress syndrome, which worsen the condition of the disease, were common in COVID-19 patients with vitamin D insufficiency [43]. Therefore, vitamin D deficiency by promoting the renin-angiotensin system (RAS) leads to chronic cardiovascular disease (CVD) and reduced lung function [50].
Serum levels of Ca2+ and zinc were not different. Shai et al. reported that hypocalcemia was common in COVID-19 patients, despite the significant difference in vitamin D levels between the studied groups (inpatient and outpatient groups) [51]. Similarly, Bara El Cardi et al. exhibited that keeping calcium levels high may prevent severe illness [52]. Therefore, hypocalcemia in patients with COVID-19 may worsen the severity of the disease. However, this study showed that calcium levels were not different between inpatient and outpatient groups, but this level in inpatients admitted to ICU was significantly lower than in non-ICU hospitalized inpatients (p < 0.05). Low serum calcium levels have been exhibited to worsen the severity of COVID-19 [24,25]. Therefore, poor prognosis, high mortality rate, and the need for ICU admission in inpatients with low calcium levels would be expected. These findings are in line with each other and emphasize that high calcium levels may mitigate the COVID-19 severity. However, the information mentioned in Table 1 demonstrated that lower calcium levels were not the main reason for mortality in ICU-admitted patients. In other words, comorbidities such as hypertension and diabetes mellitus in ICU-admitted patients increase the severity of illness and a higher rate of mortality as occurred in these patients. Therefore, further studies are needed to test the effectiveness of increasing serum calcium levels in the initial days of patients' hospitalization in preventing severe Covid-19.
Table 1.
Demographic data and Comorbidities of COVID-19 patients.
| Outpatients | Inpatients | ICU-admitted | |
|---|---|---|---|
| Number of patients | 65 | 75 | 10 |
| Demographics | |||
| Age (years) | 44.75 ± 3.9 | 52.69 ± 4.1 | 59.5 ± 2.81 |
| Male (n, %) | 36 (55%) | 42 (56%) | 7 (70%) |
| Comorbidities | |||
| Hypertension (n, %) | – | 5 (7%) | 7 (70%) |
| Diabetes mellitus (n, %) | – | 4 (6%) | 3 (30%) |
| Hyperlipidemia (n, %) | – | 2 (3%) | 1 (10%) |
| Chronic pulmonary disease (n, %) | – | – | 1 (10%) |
| Chronic ischemic heart disease (n, %) | – | – | 1 (10%) |
| Arthritis rheumatoid (n, %) | – | – | 1 (10%) |
| Hypothyroidism (n, %) | – | 1 (1.5%) | – |
| Mortality rate (n, %) | – | – | 9 (90%) |
As mentioned earlier, vitamin D has a wide range of inhibitory effects on viruses. Determination of serum levels of antiviral peptides was beyond the scope of this study, but as evidenced by Gombart et al., mRNA expression of antiviral peptides is increased by vitamin D [31,32]. Therefore, these results indicated that vitamin D protected patients against COVID-19 and possibly decreased the severity of the disease by increasing the production rate of antiviral peptides.
As demonstrated in the current findings, the serum levels of IL-6 and TNF-α in the inpatient group were significantly higher than those in the outpatient group. Additionally, their concentrations were inversely correlated with 25(OH)D serum levels. A report indicated that the overproduction of pro-inflammatory cytokines (IL-6 and TNF-α) is associated with disease symptoms [28]. These findings together suggested that vitamin D may decrease the rate of morbidity by exerting anti-inflammatory effects [11]. Current results illustrated that both mRNA expression and serum levels of TNF-α were lower in the outpatient group, which may imply genomic and non-genomic protective roles of vitamin D against COVID-19.
Studies have shown that in severe inflammatory disease induced by SARS-CoV-2, the levels of the pro-inflammatory biomarkers, such as D-dimer, are elevated [29,53]. The current results showed that levels of D-dimer and IL-6 in the inpatient group were significantly higher than in the outpatient group (p < 0.0001 and p < 0.001, respectively). Studies on inflammation biomarkers showed that elevated CRP, ESR, D-dimer, and IL-6 levels are involved in severe COVID-19 outcomes [54,55]. They indicate that patients receiving anti-TNF treatment were significantly less likely to develop severe illnesses requiring ICU admission [56]. Therefore, these reports suggest that vitamin D may play an anti-TNF role against COVID-19 by decreasing the serum levels of D-dimer, TNF-α, and IL-6 and mitigating the severity of COVID-19 complications.
The current results exhibited that 10 out of 75 patients in the inpatient group were admitted to ICU. Nine of them lost their lives. A study by Herold et al. showed that patients with IL-6 levels above 80 pg/ml suffered from respiratory failure and required intubation [57]. Consistent with Herold et al. report, the serum levels of D-dimer, TNF-α, and IL-6 in inpatients admitted to the ICU were significantly higher than those in hospitalized non-ICU patients (p < 0.05, p < 0.05, and p < 0.002, respectively; Table 2). Therefore, the level of IL-6 can be one of the determinant factors which cause respiratory distress and indicate the progression of the disease.
The present results displayed that serum IL-6 and D-dimer levels were inversely correlated with 25(OH)D levels (r = −0.53, p < 0.001; and r = −0.58, p < 0.001; respectively). Therefore, high serum levels of 25(OH)D in the outpatient group protected them against COVID-19 and reduced the disease severity by inhibiting cytokine release.
5. Conclusion
The present study showed that high serum 25(OH)D levels could be protective against COVID-19. The limitations of our study were the relatively small number of samples and the lack of examination of clinical symptoms to assess their association with each of the measured serum parameters. Our study suggested that vitamin D was related to reduction in the rate of mortality and morbidity by decreasing the levels of D-dimer, TNFα, and IL-6. These results strengthened the likelihood of requiring calcium supplementation early in the disease to prevent the patient's condition from worsening, as evidenced by lower calcium levels in ICU patients.
Author contributions
All authors agreed with the content and gave explicit consent to submit, and obtained consent from the responsible authorities at the institute/organization where the work has been carried out before the submission. The conception and design of the study were carried out by N.N, M.R, and R.N. Data acquisition was done by N.N and M.B. Measurement was done by M.B, N.N, and M.P. The data were analyzed by M.B and B.C and interpreted by N.N, R.N., and M.B. All authors drafted the work or revised it critically for important intellectual content; approved the version to be published; and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved based on ICMJE authorship criteria.
Financial disclosure
This study was issued from the M. Sc thesis of Masoud Beheshti and financially supported by the Infectious and Tropical Diseases Research Center (Grant no. OG-9964), Health Research Institute, Ahvaz Jundishapur University of Medical Sciences.
Ethical approvement
The study was approved by the Iranian National Committee for Ethics in Biomedical Research (IR.AJUMS.REC.1399.948).
Declaration of competing interest
All authors declare that they have no conflict of interest.
Acknowledgments
The authors would like to express their gratefulness to everyone who agreed to take part in the study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.clnesp.2023.04.012.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
Multimedia component 1
References
- 1.Yavarian J., Shafiei-Jandaghi N., Sadeghi K., Shatizadeh S., Salimi V., Nejati A., et al. First cases of SARS-CoV-2 in Iran, 2020: case series report. Iran J Public Health. 2020;49(8):1564–1568. doi: 10.18502/ijph.v49i8.3903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Greenhalgh T., Jimenez J., Prather K., Tufekci Z., Fisman D., Schooley R. Ten scientific reasons in support of airborne transmission of SARS-CoV-2. Lancet. 2021;397(10285):1603–1605. doi: 10.1016/S0140-6736(21)00869-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alguwaihes A.M., Al-Sofiani M., Megdad M., Albader S., Alsari M., Alelayan A., et al. Diabetes and covid-19 among hospitalized patients in Saudi Arabia: a single-centre retrospective study. Cardiovasc Diabetol. 2020;19:1–12. doi: 10.1186/s12933-020-01184-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46(5):846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jovic T.H., Ali S., Ibrahim N., Jessop Z., Tarassoli S., Dobbs T., et al. Could vitamins help in the fight against COVID-19? Nutrients. 2020;12(9) doi: 10.3390/nu12092550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soares A.D., Costa K., Wanner S., Santos R., Fernandes S., Martins F., et al. Dietary glutamine prevents the loss of intestinal barrier function and attenuates the increase in core body temperature induced by acute heat exposure. Br J Nutr. 2014;112(10):1601–1610. doi: 10.1017/S0007114514002608. [DOI] [PubMed] [Google Scholar]
- 9.Lei G.-S., Zhang C., Cheng B., Lee C. Mechanisms of action of vitamin D as supplemental therapy for Pneumocystis pneumonia. Antimicrob Agents Chemother. 2017;61(10) doi: 10.1128/AAC.01226-17. e01226–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wessels I., Rolles B., Rink L. The potential impact of zinc supplementation on COVID-19 pathogenesis. Front Immunol. 2020;11:1712. doi: 10.3389/fimmu.2020.01712. -1712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allen A.C., Kelly S., Basdeo S., Kinsella K., Mulready K., Mills K., et al. A pilot study of the immunological effects of high-dose vitamin D in healthy volunteers. Mult Scler. 2012;18(12):1797–1800. doi: 10.1177/1352458512442992. [DOI] [PubMed] [Google Scholar]
- 12.Ferreira A.O., Polonini H.C., Dijkers E.C.F. Postulated adjuvant therapeutic strategies for COVID-19. J Personalized Med. 2020;10(3) doi: 10.3390/jpm10030080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meltzer D.O., Best T., Zhang H., Vokes T., Arora V., Solway J. Association of vitamin D status and other clinical characteristics with COVID-19 test results. JAMA Netw Open. 2020;3(9) doi: 10.1001/jamanetworkopen.2020.19722. e2019722–e2019722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katz J., Yue S., Xue W. Increased risk for COVID-19 in patients with vitamin D deficiency. Nutrition. 2021;84:111106. doi: 10.1016/j.nut.2020.111106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hastie C.E., Mackay D., Ho F., Celis-Morales C., Katikireddi S., Niedzwiedz C., et al. Vitamin D concentrations and COVID-19 infection in UK Biobank. Diabetes Metabol Syndr: Clin Res Rev. 2020;14(4):561–565. doi: 10.1016/j.dsx.2020.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jain A., Chaurasia R., Sengar N., Singh M., Mahor S., Narain S. Analysis of vitamin D level among asymptomatic and critically ill COVID-19 patients and its correlation with inflammatory markers. Sci Rep. 2020;10(1):20191. doi: 10.1038/s41598-020-77093-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hernández J.L., Nan D., Fernandez-Ayala M., García-Unzueta M., Hernández-Hernández M., López-Hoyos M., et al. Vitamin D status in hospitalized patients with SARS-CoV-2 infection. J Clin Endocrinol Metab. 2021;106(3):e1343–e1353. doi: 10.1210/clinem/dgaa733. [DOI] [PubMed] [Google Scholar]
- 18.De Smet D., De Smet K., Herroelen P., Gryspeerdt S., Martens G. Serum 25(OH)D level on hospital admission associated with COVID-19 stage and mortality. Am J Clin Pathol. 2021;155(3):381–388. doi: 10.1093/ajcp/aqaa252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moutchia J., Pokharel P., Kerri A., McGaw K., Uchai S., Nji M., et al. Clinical laboratory parameters associated with severe or critical novel coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. PLoS One. 2020;15(10) doi: 10.1371/journal.pone.0239802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewis R.S. The molecular choreography of a store-operated calcium channel. Nature. 2007;446(7133):284–287. doi: 10.1038/nature05637. [DOI] [PubMed] [Google Scholar]
- 21.Hogan P.G., Rao A. Dissecting ICRAC, a store-operated calcium current. Trends Biochem Sci. 2007;32(5):235–245. doi: 10.1016/j.tibs.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 22.Hogan P.G., Chen L., Nardone J., Rao A. Transcriptional regulation by calcium, calcineurin, and NFAT. Gene Dev. 2003;17(18):2205–2232. doi: 10.1101/gad.1102703. [DOI] [PubMed] [Google Scholar]
- 23.Desvignes L., Weidinger C., Shaw P., Vaeth M., Ribierre T., Liu M., et al. STIM1 controls T cell-mediated immune regulation and inflammation in chronic infection. J Clin Invest. 2015;125(6):2347–2362. doi: 10.1172/JCI80273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mehta M., Ghani H., Chua F., Draper A., Calmonson S., Prabhakar M., et al. Retrospective case-control study to evaluate hypocalcaemia as a distinguishing feature of COVID-19 compared with other infective pneumonias and its association with disease severity. BMJ Open. 2021;11(12) doi: 10.1136/bmjopen-2021-053810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tanacan A., Erol S., Anuk A., Yetiskin F., Tokalioglu E., Sahin S., et al. The association of serum electrolytes with disease severity and obstetric complications in pregnant women with COVID-19: a prospective cohort study from a tertiary reference center. Geburtshilfe Frauenheilkd. 2022;82(3):326–332. doi: 10.1055/a-1577-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun J.-K., Zhang W., Zou L., Liu Y., Li J., Kan X., et al. Serum calcium as a biomarker of clinical severity and prognosis in patients with coronavirus disease 2019. Aging. 2020;12(12):11287–11295. doi: 10.18632/aging.103526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frisoni P., Neri M., D’Errico S., Alfieri L., Bonuccelli D., Cingolani M., et al. Cytokine storm and histopathological findings in 60 cases of COVID-19-related death: from viral load research to immunohistochemical quantification of major players IL-1β, IL-6, IL-15 and TNF-α. Forensic Sci Med Pathol. 2021:1–15. doi: 10.1007/s12024-021-00414-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun X., Wang T., Cai D., Hu Z., Liao H., Zhi L., et al. Cytokine storm intervention in the early stages of COVID-19 pneumonia. Cytokine Growth Factor Rev. 2020;53:38–42. doi: 10.1016/j.cytogfr.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gozalbo-Rovira R., Gimenez E., Latorre V., Frances-Gomez C., Albert E., Buesa J., et al. SARS-CoV-2 antibodies, serum inflammatory biomarkers and clinical severity of hospitalized COVID-19 patients. J Clin Virol. 2020;131:104611. doi: 10.1016/j.jcv.2020.104611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pisoschi A.M., Pop A., Iordache F., Stanca L., Geicu O., Bilteanu L., et al. Antioxidant, anti-inflammatory and immunomodulatory roles of vitamins in COVID-19 therapy. Eur J Med Chem. 2022:114175. doi: 10.1016/j.ejmech.2022.114175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gombart A.F., Borregaard N., Koeffler H.P. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1, 25-dihydroxyvitamin D3. Faseb J. 2005;19(9):1067–1077. doi: 10.1096/fj.04-3284com. [DOI] [PubMed] [Google Scholar]
- 32.Wang T.-T., Dabbas B., Laperriere D., Bitton A., Soualhine H., Tavera-Mendoza L., et al. Direct and indirect induction by 1, 25-dihydroxyvitamin D3 of the NOD2/CARD15-defensin β2 innate immune pathway defective in Crohn disease. J Biol Chem. 2010;285(4):2227–2231. doi: 10.1074/jbc.C109.071225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cannell J., Vieth R., Umhau JC., Holick MF., Grant WB., Madronich S., et al. Epidemic influenza and vitamin D. Epidemiol Infect. 2006;134(6):1129–1140. doi: 10.1017/S0950268806007175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cannell J.J., Vieth R., Willett W., Zasloff M., Hathcock J., White J., et al. Cod liver oil, vitamin A toxicity, frequent respiratory infections, and the vitamin D deficiency epidemic. Ann Otol Rhinol Laryngol. 2008;117(11):864–870. doi: 10.1177/000348940811701112. [DOI] [PubMed] [Google Scholar]
- 35.Ginde A.A., Mansbach J.M., Camargo C.A. Association between serum 25-hydroxyvitamin D level and upper respiratory tract infection in the third national Health and nutrition examination survey. Arch Intern Med. 2009;169(4):384–390. doi: 10.1001/archinternmed.2008.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ali N. Role of vitamin D in preventing of COVID-19 infection, progression and severity. J Infect Public Health. 2020;13(10):1373–1380. doi: 10.1016/j.jiph.2020.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Inc., M. Vit D Direct AccuBind ELISA Kits. [cited 2022; Available from: https://www.monobind.com/Products/immunoassays-bone-metabolism-vitamin-d. Vit D Direct AccuBind ELISA - IFU Rev 2, Page 2
- 38.Demopoulos C., Antonopoulou S., Theoharides T.C. COVID-19, microthromboses, inflammation, and platelet activating factor. Biofactors. 2020;46(6):927–933. doi: 10.1002/biof.1696. [DOI] [PubMed] [Google Scholar]
- 39.Narahara H., Miyakawa I., Johnston J. The inhibitory effect of 1, 25-dihydroxyvitamin D3 on the secretion of platelet-activating factor acetylhydrolase by human decidual macrophages. J Clin Endocrinol Metab. 1995;80(11):3121–3126. doi: 10.1210/jcem.80.11.7593412. [DOI] [PubMed] [Google Scholar]
- 40.Detopoulou P., Demopoulos C.A., Antonopoulou S. Micronutrients, phytochemicals and mediterranean diet: a potential protective role against COVID-19 through modulation of PAF actions and metabolism. Nutrients. 2021;13(2):462. doi: 10.3390/nu13020462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mohammad S., Mishra A., Ashraf M.Z. Emerging role of vitamin D and its associated molecules in pathways related to pathogenesis of thrombosis. Biomolecules. 2019;9(11):649. doi: 10.3390/biom9110649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou Y.-F., Luo B.-A., Qin L.-L. The association between vitamin D deficiency and community-acquired pneumonia: a meta-analysis of observational studies. Medicine. 2019;98(38) doi: 10.1097/MD.0000000000017252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grant W.B., Lahore H., McDonnell S., Baggerly C., French C., Aliano J., et al. Evidence that vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths. Nutrients. 2020;12(4):988. doi: 10.3390/nu12040988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pereira M., Dantas Damascena A., Mirella Galvão Azevedo L., de Almeida Oliveira T., da Mota Santana J., et al. Vitamin D deficiency aggravates COVID-19: systematic review and meta-analysis. Crit Rev Food Sci Nutr. 2022;62(5):1308–1316. doi: 10.1080/10408398.2020.1841090. [DOI] [PubMed] [Google Scholar]
- 45.Alguwaihes A.M., Sabico S., Hasanato R., Al-Sofiani M., Megdad M., Albader S., et al. Severe vitamin D deficiency is not related to SARS-CoV-2 infection but may increase mortality risk in hospitalized adults: a retrospective case–control study in an Arab Gulf country. Aging Clin Exp Res. 2021;33:1415–1422. doi: 10.1007/s40520-021-01831-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Amer O.E., Sabico S., Sheshah E., Alotaibi N., Aldisi D., Enani M., et al. Evaluation of 34 cytokines and vitamin D status reveal A sexually-dimorphic active immune response to SARS-CoV-2. Healthcare. 2022;10(12):2571. doi: 10.3390/healthcare10122571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jain S.K., Parsanathan R. Can vitamin D and L-cysteine co-supplementation reduce 25 (OH)-vitamin D deficiency and the mortality associated with COVID-19 in African Americans? J Am Coll Nutr. 2020;39(8):694–699. doi: 10.1080/07315724.2020.1789518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sabico S., Enani M., Sheshah E., Aljohani N., Aldisi D., Alotaibi N., et al. Effects of a 2-week 5000 IU versus 1000 IU vitamin D3 supplementation on recovery of symptoms in patients with mild to moderate covid-19: a randomized clinical trial. Nutrients. 2021;13(7):2170. doi: 10.3390/nu13072170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsujino I., Ushikoshi-Nakayama R., Yamazaki T., Matsumoto N., Saito I. Pulmonary activation of vitamin D3 and preventive effect against interstitial pneumonia. J Clin Biochem Nutr. 2019;65(3):245–251. doi: 10.3164/jcbn.19-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shi Y., Liu T., Yao L., Xing Y., Zhao X., Fu J., et al. Chronic vitamin D deficiency induces lung fibrosis through activation of the renin-angiotensin system. Sci Rep. 2017;7(1):1–10. doi: 10.1038/s41598-017-03474-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sun J.-K., Zhang W., Zou L., Liu Y., Li J., Kan X., et al. Serum calcium as a biomarker of clinical severity and prognosis in patients with coronavirus disease 2019. Aging (Albany NY) 2020;12(12):11287. doi: 10.18632/aging.103526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.El-Kurdi B., Khatua B., Rood C., Snozek C., Cartin-Ceba R., Singh V., et al. Mortality from coronavirus disease 2019 increases with unsaturated fat and may be reduced by early calcium and albumin supplementation. Gastroenterology. 2020;159(3):1015–1018. doi: 10.1053/j.gastro.2020.05.057. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Moutchia J., Pokharel P., Kerri A., McGaw K., Uchai S., Nji M., et al. Clinical laboratory parameters associated with severe or critical novel coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. PLoS One. 2020;15(10) doi: 10.1371/journal.pone.0239802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Henry B.M., De Oliveira M., Benoit S., Plebani M., Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin Chem Lab Med. 2020;58(7):1021–1028. doi: 10.1515/cclm-2020-0369. [DOI] [PubMed] [Google Scholar]
- 55.Petrilli C.M., Jones S., Yang J., Rajagopalan H., O’Donnell L., Chernyak Y., et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: prospective cohort study. BMJ. 2020;369:m1966. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kokkotis G., Kitsou K., Xynogalas I., Spoulou V., Magiorkinis G., Trontzas I., et al. Systematic review with meta-analysis: COVID-19 outcomes in patients receiving anti-TNF treatments. Aliment Pharmacol Ther. 2022;55(2):154–167. doi: 10.1111/apt.16717. [DOI] [PubMed] [Google Scholar]
- 57.Herold T., Jurinovic V., Arnreich C., Lipworth B., Hellmuth J., von Bergwelt-Baildon M., et al. Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J Allergy Clin Immunol. 2020;146(1):128–136. doi: 10.1016/j.jaci.2020.05.008. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Multimedia component 1