Abstract
L-Arginine (L-Arg), is a semi-essential amino acid involved in the formation of nitric oxide. The functional relevance of L-Arg in diabetes mellitus has been evaluated both in animal models and in human subjects. In the literature there are several lines of evidence indicating that L-Arg has beneficial effects in diabetes and numerous studies advocate its administration to attenuate glucose intolerance in diabetic patients. Here we present a comprehensive overview of the main studies exploring the effects of L-Arg in diabetes, including preclinical and clinical reports on this topic.
Keywords: L-Arginine, Diabetes mellitus, Endothelial dysfunction, GLP-1, Glucose metabolism, NO
Introduction
L-Arginine, hereinafter referred to as L-Arg, was first isolated from lupin seeds by E. Schulze and E. Steiger in 1886, who called it “áργυρος” (argiros), a Greek word meaning silver, due to the white-silverish appearance of its crystal. L-Arg is an essential or conditionally essential amino acid — because it can be synthesized by healthy individuals but not by premature newborns [1] — that has been shown to be safe for the human body [2].
L-Arg is a natural constituent of food proteins [3]. It is elemental to produce NO, which acts as a major vasodilator with favorable effects on the cardiovascular system [4]. Moreover, L-Arg is involved in the synthesis of creatine, L-Ornithine, L-Glutamate, collagen, polyamines, and agmatine [5]. L-Arg promotes the secretion of growth hormone from the pituitary gland [6] and is implicated in T cell proliferation and host immune responses [7–9]. The intake of L-Arg has been shown to improve oxidative metabolism, through an enhanced mitochondrial function, eventually improving physical performance [10].
Several studies advocate the implementation of L-Arg for the treatment of diabetes, both directly and indirectly. L-Arg is a powerful secretagogue of the endocrine system, as it induces the secretion of insulin [11] and glucagon [12], which are protagonists in glucose metabolism. Furthermore, investigations in rats have demonstrated that L-Arg can reduce plasma glucose levels, improving glucose tolerance [13]. L-Arg supplementation was also shown to reduce adiposity and improve insulin sensitivity in animal models of obesity as well as in patients with diabetes and obesity [14]. These findings are highly relevant considering that obesity is one of the main risk factors of diabetes. Consistently, L-Arg supplementation was also found to induce a decrease in white adipose tissue (WAT) [14] and a modulation of the BAT-WAT ratio (brown adipose tissue vs. white adipose tissue) in both clinical and preclinical investigations [15–17]. Notably, Hayde and collaborators observed that oral high-dose of L-Arg supplementation has an immunomodulatory effect that could cause an enhanced clearance of advanced-stage non-enzymatic glycosylation products, thereby ameliorating glucose tolerance in diabetic patients [18]. Interesting evaluations came from molecular dynamic simulations, which revealed that it is possible to have significant effects even with the association L-Arg/metformin. Indeed, when L-Arg is combined with metformin, it is displaced from the NOS activation site, reducing nitric oxide (NO) concentration. This side effect of the association could be useful in clinical conditions in which NO could give negative consequences such as shock and stroke [19].
The main studies evaluating L-Arg supplementation in clinical trials and in animal models are reported in Tables 1 and 2, respectively.
Table 1.
Summary of the main clinical trials investigating the effects of L-Arg supplementation in diabetes
| First Author, year [Ref.] | Study design | Participants | Dose and duration of L-Arg supplementation | Main results |
|---|---|---|---|---|
| Lubec B, 1997 [57] | Blind placebo-controlled trial | Patients with diabetes | 1 g twice/day for 3 months |
No significant differences In terms of blood glucose, fructosamine, and HbA1c |
| Wascher T C,1997 [45]. | Clinical trial | Patients with obesity and patients with T2DM | 0.052 g/kg/min for 180 min | L-Arg infusion improves insulin-sensitivity in patients |
| Marfella R, 2000 [43] | Clinical trial | Patients with T2DM | 1 g/min in infusion for 30 min | L-Arg infusion reverts the effects caused by acute hyperglycemia (increase of BP and alterations in baroreflex activity) |
| Piatti P.M, 2001 [46] | Double-blind trial | Patients with T2DM | 3 g daily for 3 months |
L-Arg administration significantly improves peripheral and hepatic insulin sensitivity in T2DM patients. |
| Lucotti P, 2006 [58] | Randomized controlled trial | Patients with T2DM | 8.3 g daily for 21 days | L-Arg therapy improves fasting and postprandial glycemic excursions and hyperinsulinemia. |
| Martina V,2008 [70] | Randomized, double-blind, placebo-controlled trial | Patients with T2DM | 1.2 g daily for 6 months | L-Arg improves endothelial function reducing oxidative stress and promotes NO anti-atherosclerotic effects. |
| Settergren M, 2008 [39] | Randomized controlled trial | Patients with T2DM and CAD | 0.2 g/min in infusion for 15 min | L-Arg and BH4 administration reduces I/R-induced endothelial dysfunction |
| Monti L.D, 2012–2018 [53, 54] | Randomized, double-blind, placebo-controlled trials | Patients with IGT and MS | 6.4 g daily for 18 months | L-Arg for 18 months significantly increases regression to NGT; 9 years from baseline the cumulative incidence of diabetes was less in the L-Arg group compared to placebo (40.6% vs. 57.4%). |
| Cherney D.Z.I, 2013 [62] | Clinical trial | Patients with T1DM | 100 mg/kg over 30 min and then 250 mg/kg over 30 min, in infusion | L-Arg inverts the hyperglycemia renal hemodynamic effects in women |
| Fayh A.P, 2013 [63] | Clinical trial | Men with T1DM | 7 g/day during 1 week |
L-Arg improves endothelial function |
| Costa G, 2022 [47] | Comparative study | Women with T2DM | 5 g/day for 14 days | L-Arg supplementation improves vascular and microvascular function. |
BP: Blood Pressure; CAD: Coronary Artery Disease; T1DM: Type 1 Diabetes Mellitus ; T2DM: Type 2 Diabetes Mellitus; IGT: Impaired Glucose Tolerance; I/R: ischemia-reperfusion; MS: Metabolic Syndrome; NGT: Normal Glucose Tolerance.
Table 2.
Summary of the main preclinical trials testing L-Arg effects in animal models of diabetes
| First Author, year [Ref.] | Model |
|---|---|
| Alba-Roth J, 1988 [6] | Rat anterior pituitary cells co-incubated with L-Arg and GHRH for 3 h |
| Mohan I.K, 2000 [55] | Insulin-dependent diabetes mellitus model of alloxan-induced diabetic rats |
| Lass A, 2002 [56] | Rat hearts with oxygen radical-induced myocardial injury. Oxygen radicals were obtained by electrolysis or to hypoxanthine and xanthine oxidase |
| El Missiry M.A, 2004 [49] | Insulin-dependent diabetes mellitus model of alloxan-induced diabetic rats |
| Vasilijevic A, 2007 [29]. | Insulin-dependent diabetes mellitus model of alloxan-induced diabetic rats |
| Clemmensen C, 2013 [24] | Diet-induced obese mice |
| Dubey H, 2022 [13] | Diet and streptozotocin induced diabetic rats |
GHRH: Growth Hormone Releasing Hormone
Role of L-Arg in glucose metabolism
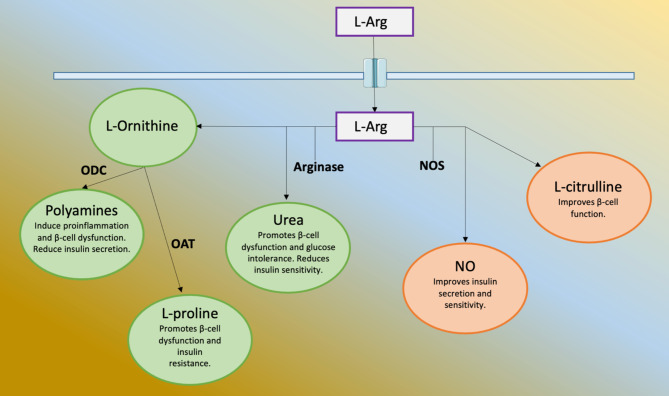
L-Arg plays essential roles in a number of metabolic pathways, including glucose metabolism [18, 20]. For instance, L-Arg can be metabolized by both arginase and NOS. If metabolized by arginase, L-Arg is cleaved to urea and L-Ornithine, causing a dysregulation of pancreatic β-cells determining an increase of insulin resistance and glucose intolerance alongside with a pro-inflammatory state. Urea is directly involved in such effects while L-Ornithine is transformed in polyamines by ornithine decarboxylase (ODC) and in L-proline by ornithine aminotransferase (OAT). When metabolized by NOS, L-Arg produces L-citrulline and NO, with the latter being crucial for endothelial function, for insulin secretion, and improvement of insulin sensitivity [21]. Thus, in physiologic conditions, the L-Arg NOS pathway is involved in a better response to glycemic levels increasing insulin secretion and sensitivity [22, 23], as shown in Fig. 1.
Fig. 1.
L-Arg once in the cell can be metabolized by Arginase. The products of this enzyme are: L-Ornithine, which is further cleaved to polyamines by ornithine decarboxylase (ODC), L-proline by ornithine aminotransferase (OAT), and urea. These compounds exert negative effects on glucose metabolism. When L-Arg is cleaved in nitric oxide (NO) and L-citrulline by the nitric oxide synthase (NOS), these compounds have positive effects on glucose metabolism, also exerting beneficial action on the cardiovascular system
L-Arg and diabetes: preclinical evidence
In a recent study performed in rodents by Clemmensen et al. [24], L-Arg was shown to stimulate the release of Glucagon-like peptide-1 (GLP-1), an intestinal hormone that plays an important role in the regulation of appetite and glucose metabolism [25, 26]. In this work, mice harboring genetically inactivated GLP-1 receptors were compared to mice with wild-type GLP-1 receptors, implementing the feeding of both groups with L-Arg. In the first group of mice, the intake of L-Arg did not bring great improvements in glucose metabolism, while in the second group there was a marked improvement in glucose metabolism and insulin secretion. A new indirect mechanism was therefore been suggested, in which the intake of L-Arg improves glucose metabolism, insulin resistance, and insulin sensitivity [24].
Claybaugh and collaborators demonstrated, in diet-induced diabetics rats, that L-Arg supplementation can preserve NO activity. This effect could contribute to delaying the onset of insulin resistance and renal dysfunction caused by hyperglycemic stress, suggesting a main role for NO in renal function and in the pathogenesis of diabetes [27].
Similarly, L-Arg administration in diabetic rats with Alzheimer’s disease demonstrated an improvement in terms of glucose tolerance and insulin levels. Intriguingly, L-Arg exhibited ameliorative effects on cognitive deficits, suggesting a potential therapeutic action to attenuate neurological deterioration mediated by diabetes [13].
L-Arg and diabetes: clinical trials
Drugs currently approved to treat type 2 diabetes mellitus (T2DM) mostly work by increasing insulin secretion or reducing glucose concentration but are unable to fully improve insulin sensitivity and protect beta-cells [28]. In fact, some of the main features of T2DM are the deterioration of beta-cells and the low insulin sensitivity. Conversely, L-Arg can cope with these problems since it has been shown to stimulate beta-cell neogenesis by increasing the area of beta-cells [29].
In diabetes, the condition of hyperglycemia reduces NO [10, 30], which, as mentioned above in this review, is important for the regulation of vasodilation, anticoagulation, the proliferation of smooth muscle, and the overall antioxidant capacity of endothelial cells [31–33]. L-Arg also serves as a basic substrate to produce NO in endothelial cells, thus regulating vascular tone and overall cardiovascular homeostasis [34–38]. Settergen and colleagues investigated the effect of L-Arg and tetrahydrobiopterin infusion on endothelial dysfunction induced by ischemia/reperfusion in patients with T2DM and coronary artery disease, observing that L-Arg supplementation significantly attenuated endothelial dysfunction in this type of patients [39]. Hence, an implementation of L-Arg could potentially reduce some of the main and most serious complications of diabetes, including heart failure; indeed, diabetic patients are more prone to develop cardiomyopathy than healthy subjects [40, 41].
Numerous clinical studies have confirmed the reduction of blood pressure and platelet aggregation in diabetic patients treated with intravenous L-Arg [23, 42, 43]. Additionally, the intravenous injection of L-Arg in obese T2DM patients has been shown to stimulate insulin reactivity, restoring insulin-dependent vasodilation [44, 45]. On the other hand, oral administration of L-Arg improves sensitivity to hepatic and peripheral insulin in a cGMP-dependent manner [46]. A recent comparative study has shown that oral supplementation with L-Arg (5 g/day for 14 days) improves vascular and microvascular health in elderly women with or without T2DM [47].
Other common occurrences in diabetes include oxidative stress and tissue damage [48]. In diabetic patients the redox balance is altered, leading to a high pro-oxidant enzymatic activity or a lower antioxidant enzymatic activity and is translated into augmented oxidative stress and dysfunction of endothelial cells. L-Arg has been linked to an attenuation of oxidative stress, preventing the reduction of regulation of cellular antioxidants, a finding demonstrated in a variety of species and cell lines [49–52]. Equally important, Monti and collaborators assessed the efficacy of long-term L-Arg therapy in preventing or delaying T2DM in patients with impaired glucose tolerance (IGT) and metabolic syndrome (MS); showing that the 18-month L-Arg supplementation induces a regression to normal glucose tolerance [53]. Having achieved these results, the same research grooup sought to determine whether the chronic L-Arg supplementation for 18 months maintained long-lasting effects on diabetes incidence, insulin secretion and sensitivity, oxidative stress, and endothelial function among subjects at high risk of developing T2DM. Thus, after the 18 months of L-Arg administration, people still free from diabetes were followed-up until the T2DM diagnosis. At the end of the study, the cumulative incidence of diabetes in the L-Arg group was of 40.6% and in the placebo-treated group was of 57.4%, strongly suggesting that the supplementation with L-Arg could retard the development of T2DM for a long period [54]. This effect could be linked to the L-Arg capacity of reducing oxidative stress. As discussed above, L-Arg acts as a substrate for NO, and there are in fact diverse theories regarding the L-Arg-NO system, emphasizing a protective role against oxidative stress [55, 56]. In a study authored by Lubec and co-workers, diabetic patients were treated with L-Arg for 3 months (two daily doses of 1 g), resulting in improved diabetes management, also observing a reduced lipid peroxidation [57]. Similarly, El-Missiry and collaborators reported a diminished oxidative stress following L-Arg supplementation, highlighted by lower levels of TBARS (thiobarbituric acid reactive substances), an indicator of lipid peroxidation and oxidative stress [49].
In 2006, Lucotti et al. evaluated the effects of a long-term oral L-Arg therapy. The Authors enrolled T2DM patients who followed a low-calorie diet with L-Arg (8.3 g/day) supplementation, in combination with physical training, for a period of 21 days. Such oral supplementation of L-Arg, in addition to improving endothelial function, oxidative stress, and adipokine release, ameliorated fasting glucose levels and normalized post-prandial glucose levels [58]. These results were somehow surprising, considering that previous studies had suggested that post-prandial hyperglycemia may be more significant than fasting glucose levels in terms of overall glycemic control [59–61].
In 2013, Cherney and collaborators demonstrated that L-Arg infusion reversed the exaggerated pressor response to clamped hyperglycemia in women with type 1 diabetes mellitus (T1DM), suggesting the importance of NO as a fundamental regulator of sex-dependent vascular responses to hyperglycemia [62]. In the same year, Farney and collaborators assessed in 10 men affected by non-complicated T1DM the efficacy of L-Arg administration as a critical tool for the treatment of diabetic complications, showing that L-Arg improved vascular function [63]. However, the Authors were unable to draw conclusions regarding the mechanisms by which L-Arg therapy is inducing improvements on cardiovascular function.
A recent meta-analysis of clinical trials confirmed that L-Arg is a safe compound able to reduce fasting blood glucose and serum insulin levels in patients with alterations of glucose metabolism [64].
Potential issues associated with oxidative stress
A problem resulting from the intake of L-Arg could be the risk of reaction with precursors of advanced glycosylated products [65] that are found to be abundant in diabetic patients. It has been shown in vivo that reacting with methylglyoxal, a molecule abundant in diabetic patients [66, 67], L-Arg might produce powerful superoxide radicals [68]. For this reason, it has been suggested to combine an antioxidant with the implementation of L-Arg [69, 70]. This aspect was confirmed in a study where 24 patients received oral treatment of L-Arg combined with N-acetylcysteine; such a treatment led to a reduction of blood pressure, total cholesterol, reactive C-proteins, and vascular adhesion molecules, all well-established risk factors for diabetes [70].
Conclusions
Overall, data currently available in the literature consider L-Arg supplementation safe and significant for the treatment of diabetes. Based on these considerations, L-Arg could represent an additional strategy for patients with diabetes, especially in the early stages of the disease, in order to prevent or at least slow down complications involving other organs. Among the various L-Arg formulations currently available, using the ones with a standardized formula in oral vials with no sugar should be preferred. Further randomized and long-term placebo-controlled clinical trials are warranted to definitively assess L-Arg beneficial effects on glucose metabolism and to define the cellular and molecular mechanisms underlying the metabolic benefits of L-Arg in diabetes.
Acknowledgements
We thank Dr. Xujun Wang for helpful discussion.
Author contributions
IF, RA, and GS contributed to manuscript writing. FV, SSJ, AC, PM, LS, UK, TT, and VT made substantial contributions to data research, intellectual direction, and revision of the drafting of the manuscript. All authors fully contributed to this research. All authors have read and approved the final manuscript and its submission.
Funding
The Santulli’s Lab is supported in part by the National Institutes of Health (NIH): National Heart, Lung, and Blood Institute (NHLBI: R01-HL164772, R01-HL159062, R01-HL146691, T32-HL144456), National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK: R01-DK123259, R01-DK033823), National Center for Advancing Translational Sciences (NCATS: UL1TR002556-06) to G.S., by the Diabetes Action Research and Education Foundation (to G.S.), and by the Monique Weill-Caulier and Irma T. Hirschl Trusts (to G.S.). F.V. is supported in part by a postdoctoral fellowship of the American Heart Association (AHA-22POST915561). S.S.J. is supported in part by a postdoctoral fellowship of the American Heart Association (AHA-21POST836407). U.K. is supported in part by a postdoctoral fellowship of the American Heart Association (AHA-23POST1026190).
Data availability
N/A.
Declarations
Ethics approval and consent to participate
N/A.
Consent for publication
All authors gave the consent for the publication of the article. All data and materials are available.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These Authors share the First Authorship.
Change history
5/18/2023
A Correction to this paper has been published: 10.1186/s12933-023-01852-1
References
- 1.Lopez MJ, Mohiuddin SS. Biochemistry, Essential Amino Acids. In: StatPearls Treasure Island (FL); 2022. [PubMed]
- 2.Wu G. Amino acids: metabolism, functions, and nutrition. Amino Acids. 2009;37(1):1–17. doi: 10.1007/s00726-009-0269-0. [DOI] [PubMed] [Google Scholar]
- 3.Rose WC. The role of the amino acids in human nutrition. Proc Am Philos Soc. 1947;91(1):112–6. [PubMed] [Google Scholar]
- 4.Wu G, Meininger CJ, McNeal CJ, Bazer FW, Rhoads JM. Role of L-Arginine in nitric oxide synthesis and health in humans. Adv Exp Med Biol. 2021;1332:167–87. doi: 10.1007/978-3-030-74180-8_10. [DOI] [PubMed] [Google Scholar]
- 5.Wu G, Morris SM Jr. Arginine metabolism: nitric oxide and beyond. Biochem J. 1998;336(Pt 1):1–17. [DOI] [PMC free article] [PubMed]
- 6.Alba-Roth J, Muller OA, Schopohl J, von Werder K. Arginine stimulates growth hormone secretion by suppressing endogenous somatostatin secretion. J Clin Endocrinol Metab. 1988;67(6):1186–9. doi: 10.1210/jcem-67-6-1186. [DOI] [PubMed] [Google Scholar]
- 7.Bromage DI, Yellon DM. The pleiotropic effects of metformin: time for prospective studies. Cardiovasc Diabetol. 2015;14:109. doi: 10.1186/s12933-015-0273-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gessner A, Gemeinhardt A, Bosch A, Kannenkeril D, Staerk C, Mayr A, Fromm MF, Schmieder RE, Maas R. Effects of treatment with SGLT-2 inhibitors on arginine-related cardiovascular and renal biomarkers. Cardiovasc Diabetol. 2022;21(1):4. doi: 10.1186/s12933-021-01436-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adebayo A, Varzideh F, Wilson S, Gambardella J, Eacobacci M, Jankauskas SS, Donkor K, Kansakar U, Trimarco V, Mone P et al. l-Arginine and COVID-19: An Update.Nutrients2021, 13(11). [DOI] [PMC free article] [PubMed]
- 10.Gambardella J, Fiordelisi A, Spigno L, Boldrini L, Lungonelli G, Di Vaia E, Santulli G, Sorriento D, Cerasuolo FA, Trimarco V et al. Effects of Chronic Supplementation of L-Arginine on Physical Fitness in Water Polo Players. Oxid Med Cell Longev 2021, 2021:6684568. [DOI] [PMC free article] [PubMed]
- 11.Sener A, Lebrun P, Blachier F, Malaisse WJ. Stimulus-secretion coupling of arginine-induced insulin release. Insulinotropic action of agmatine. Biochem Pharmacol. 1989;38(2):327–30. doi: 10.1016/0006-2952(89)90044-0. [DOI] [PubMed] [Google Scholar]
- 12.Aguilar-Parada E, Eisentraut AM, Unger RH. Pancreatic glucagon secretion in normal and diabetic subjects. Am J Med Sci. 1969;257(6):415–9. doi: 10.1097/00000441-196906000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Dubey H, Dubey A, Gulati K, Ray A. Protective effects of L-arginine on cognitive deficits and biochemical parameters in an experimental model of type-2 diabetes mellitus induced Alzheimer’s disease in rats.J Physiol Pharmacol2022, 73(1). [DOI] [PubMed]
- 14.McKnight JR, Satterfield MC, Jobgen WS, Smith SB, Spencer TE, Meininger CJ, McNeal CJ, Wu G. Beneficial effects of L-arginine on reducing obesity: potential mechanisms and important implications for human health. Amino Acids. 2010;39(2):349–57. doi: 10.1007/s00726-010-0598-z. [DOI] [PubMed] [Google Scholar]
- 15.Chen J, Wang J, Song P, Ma X. Determination of glycinin in soybean and soybean products using a sandwich enzyme-linked immunosorbent assay. Food Chem. 2014;162:27–33. doi: 10.1016/j.foodchem.2014.04.065. [DOI] [PubMed] [Google Scholar]
- 16.Ma X, Ding W, Wang J, Wu G, Zhang H, Yin J, Zhou L, Li D. LOC66273 isoform 2, a novel protein highly expressed in white adipose tissue, induces adipogenesis in 3T3-L1 cells. J Nutr. 2012;142(3):448–55. doi: 10.3945/jn.111.152108. [DOI] [PubMed] [Google Scholar]
- 17.Ma X, Zhang H, Yuan L, Jing H, Thacker P, Li D. CREBL2, interacting with CREB, induces adipogenesis in 3T3-L1 adipocytes. Biochem J. 2011;439(1):27–38. doi: 10.1042/BJ20101475. [DOI] [PubMed] [Google Scholar]
- 18.Hayde M, Vierhapper H, Lubec B, Popow C, Weninger M, Xi Z, Lubec G. Low-dose dietary L-arginine increases plasma interleukin 1 alpha but not interleukin 1 beta in patients with diabetes mellitus. Cytokine. 1994;6(1):79–82. doi: 10.1016/1043-4666(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 19.Irandoost M, Shabani M, Tavakoli-Yaraki M, Mirzaie S. The Effects of L-Arginine in patients with type 2 diabetes by experimental methods and Molecular Dynamics Simulations. Int J Pharm Phytopharmacological Res. 2018;8(5):94–107. [Google Scholar]
- 20.Morris SM Jr. Arginine metabolism revisited. J Nutr. 2016;146(12):2579S–86. [DOI] [PubMed]
- 21.Bahadoran Z, Mirmiran P, Ghasemi A. Role of nitric oxide in insulin secretion and glucose metabolism. Trends Endocrinol Metab. 2020;31(2):118–30. doi: 10.1016/j.tem.2019.10.001. [DOI] [PubMed] [Google Scholar]
- 22.Ren Y, Li Z, Li W, Fan X, Han F, Huang Y, Yu Y, Qian L, Xiong Y. Arginase: Biological and Therapeutic Implications in Diabetes Mellitus and Its Complications. Oxid Med Cell Longev 2022, 2022:2419412. [DOI] [PMC free article] [PubMed]
- 23.Hu S, Han M, Rezaei A, Li D, Wu G, Ma X. L-Arginine modulates glucose and lipid metabolism in obesity and diabetes. Curr Protein Pept Sci. 2017;18(6):599–608. doi: 10.2174/1389203717666160627074017. [DOI] [PubMed] [Google Scholar]
- 24.Clemmensen C, Smajilovic S, Smith EP, Woods SC, Brauner-Osborne H, Seeley RJ, D’Alessio DA, Ryan KK. Oral L-arginine stimulates GLP-1 secretion to improve glucose tolerance in male mice. Endocrinology. 2013;154(11):3978–83. doi: 10.1210/en.2013-1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kreymann B, Williams G, Ghatei MA, Bloom SR. Glucagon-like peptide-1 7–36: a physiological incretin in man. Lancet. 1987;2(8571):1300–4. doi: 10.1016/S0140-6736(87)91194-9. [DOI] [PubMed] [Google Scholar]
- 26.Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87(4):1409–39. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- 27.Claybaugh T, Decker S, McCall K, Slyvka Y, Steimle J, Wood A, Schaefer M, Thuma J, Inman S. L-Arginine supplementation in type II Diabetic rats preserves renal function and improves insulin sensitivity by altering the nitric oxide pathway. Int J Endocrinol. 2014;2014:171546. doi: 10.1155/2014/171546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.ElSayed NA, Aleppo G, Aroda VR, Bannuru RR, Brown FM, Bruemmer D, Collins BS, Hilliard ME, Isaacs D, Johnson EL, et al. Pharmacologic approaches to Glycemic Treatment: Standards of Care in Diabetes-2023. Diabetes Care. 2023;46(Suppl 1):140–S157. doi: 10.2337/dc23-S009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vasilijevic A, Buzadzic B, Korac A, Petrovic V, Jankovic A, Korac B. Beneficial effects of L-arginine nitric oxide-producing pathway in rats treated with alloxan. J Physiol. 2007;584(Pt 3):921–33. doi: 10.1113/jphysiol.2007.140277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Potenza MA, Gagliardi S, Nacci C, Carratu MR, Montagnani M. Endothelial dysfunction in diabetes: from mechanisms to therapeutic targets. Curr Med Chem. 2009;16(1):94–112. doi: 10.2174/092986709787002853. [DOI] [PubMed] [Google Scholar]
- 31.Hamed S, Brenner B, Aharon A, Daoud D, Roguin A. Nitric oxide and superoxide dismutase modulate endothelial progenitor cell function in type 2 diabetes mellitus. Cardiovasc Diabetol. 2009;8:56. doi: 10.1186/1475-2840-8-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Esper RJ, Nordaby RA, Vilarino JO, Paragano A, Cacharron JL, Machado RA. Endothelial dysfunction: a comprehensive appraisal. Cardiovasc Diabetol. 2006;5:4. doi: 10.1186/1475-2840-5-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.House LM 2nd, Morris RT, Barnes TM, Lantier L, Cyphert TJ, McGuinness OP, Otero YF. Tissue inflammation and nitric oxide-mediated alterations in cardiovascular function are major determinants of endotoxin-induced insulin resistance. Cardiovasc Diabetol. 2015;14:56. [DOI] [PMC free article] [PubMed]
- 34.Tripolt NJ, Aberer F, Riedl R, Url J, Dimsity G, Meinitzer A, Stojakovic T, Aziz F, Hodl R, Brachtl G, et al. Effects of linagliptin on endothelial function and postprandial lipids in coronary artery disease patients with early diabetes: a randomized, placebo-controlled, double-blind trial. Cardiovasc Diabetol. 2018;17(1):71. doi: 10.1186/s12933-018-0716-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luiking YC, Ten Have GA, Wolfe RR, Deutz NE. Arginine de novo and nitric oxide production in disease states. Am J Physiol Endocrinol Metab. 2012;303(10):E1177–1189. doi: 10.1152/ajpendo.00284.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tsikas D, Bollenbach A, Hanff E, Kayacelebi AA. Asymmetric dimethylarginine (ADMA), symmetric dimethylarginine (SDMA) and homoarginine (hArg): the ADMA, SDMA and hArg paradoxes. Cardiovasc Diabetol. 2018;17(1):1. doi: 10.1186/s12933-017-0656-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gambardella J, Khondkar W, Morelli MB, Wang X, Santulli G, Trimarco V. Arginine and Endothelial Function.Biomedicines2020, 8(8). [DOI] [PMC free article] [PubMed]
- 38.Yu Y, Rajapakse AG, Montani JP, Yang Z, Ming XF. p38 mitogen-activated protein kinase is involved in arginase-II-mediated eNOS-uncoupling in obesity. Cardiovasc Diabetol. 2014;13:113. doi: 10.1186/s12933-014-0113-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Settergren M, Bohm F, Malmstrom RE, Channon KM, Pernow J. L-arginine and tetrahydrobiopterin protects against ischemia/reperfusion-induced endothelial dysfunction in patients with type 2 diabetes mellitus and coronary artery disease. Atherosclerosis. 2009;204(1):73–8. doi: 10.1016/j.atherosclerosis.2008.08.034. [DOI] [PubMed] [Google Scholar]
- 40.Bell DS. Heart failure: the frequent, forgotten, and often fatal complication of diabetes. Diabetes Care. 2003;26(8):2433–41. doi: 10.2337/diacare.26.8.2433. [DOI] [PubMed] [Google Scholar]
- 41.Jankauskas SS, Kansakar U, Varzideh F, Wilson S, Mone P, Lombardi A, Gambardella J, Santulli G. Heart failure in diabetes. Metabolism. 2021;125:154910. doi: 10.1016/j.metabol.2021.154910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pieper GM. Review of alterations in endothelial nitric oxide production in diabetes: protective role of arginine on endothelial dysfunction. Hypertension. 1998;31(5):1047–60. doi: 10.1161/01.HYP.31.5.1047. [DOI] [PubMed] [Google Scholar]
- 43.Marfella R, Nappo F, De Angelis L, Paolisso G, Tagliamonte MR, Giugliano D. Hemodynamic effects of acute hyperglycemia in type 2 diabetic patients. Diabetes Care. 2000;23(5):658–63. doi: 10.2337/diacare.23.5.658. [DOI] [PubMed] [Google Scholar]
- 44.Kurz S, Harrison DG. Insulin and the arginine paradox. J Clin Invest. 1997;99(3):369–70. doi: 10.1172/JCI119166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wascher TC, Graier WF, Dittrich P, Hussain MA, Bahadori B, Wallner S, Toplak H. Effects of low-dose L-arginine on insulin-mediated vasodilatation and insulin sensitivity. Eur J Clin Invest. 1997;27(8):690–5. doi: 10.1046/j.1365-2362.1997.1730718.x. [DOI] [PubMed] [Google Scholar]
- 46.Piatti PM, Monti LD, Valsecchi G, Magni F, Setola E, Marchesi F, Galli-Kienle M, Pozza G, Alberti KG. Long-term oral L-arginine administration improves peripheral and hepatic insulin sensitivity in type 2 diabetic patients. Diabetes Care. 2001;24(5):875–80. doi: 10.2337/diacare.24.5.875. [DOI] [PubMed] [Google Scholar]
- 47.Costa G, Shushanof M, Bouskela E, Bottino D. Oral L-Arginine (5 g/day) for 14 days improves microcirculatory function in healthy Young Women and healthy and type 2 diabetes Mellitus Elderly Women. J Vasc Res. 2022;59(1):24–33. doi: 10.1159/000519428. [DOI] [PubMed] [Google Scholar]
- 48.Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991;40(4):405–12. doi: 10.2337/diab.40.4.405. [DOI] [PubMed] [Google Scholar]
- 49.El-Missiry MA, Othman AI, Amer MA. L-Arginine ameliorates oxidative stress in alloxan-induced experimental diabetes mellitus. J Appl Toxicol. 2004;24(2):93–7. doi: 10.1002/jat.952. [DOI] [PubMed] [Google Scholar]
- 50.Hsu CP, Hsu PF, Chung MY, Lin SJ, Lu TM. Asymmetric dimethylarginine and long-term adverse cardiovascular events in patients with type 2 diabetes: relation with the glycemic control. Cardiovasc Diabetol. 2014;13:156. doi: 10.1186/s12933-014-0156-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sibal L, Agarwal SC, Schwedhelm E, Luneburg N, Boger RH, Home PD. A study of endothelial function and circulating asymmetric dimethylarginine levels in people with type 1 diabetes without macrovascular disease or microalbuminuria. Cardiovasc Diabetol. 2009;8:27. doi: 10.1186/1475-2840-8-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Das UN, Repossi G, Dain A, Eynard AR. L-arginine, NO and asymmetrical dimethylarginine in hypertension and type 2 diabetes. Front Biosci (Landmark Ed) 2011;16(1):13–20. doi: 10.2741/3672. [DOI] [PubMed] [Google Scholar]
- 53.Monti LD, Setola E, Lucotti PC, Marrocco-Trischitta MM, Comola M, Galluccio E, Poggi A, Mammi S, Catapano AL, Comi G, et al. Effect of a long-term oral l-arginine supplementation on glucose metabolism: a randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2012;14(10):893–900. doi: 10.1111/j.1463-1326.2012.01615.x. [DOI] [PubMed] [Google Scholar]
- 54.Monti LD, Galluccio E, Villa V, Fontana B, Spadoni S, Piatti PM. Decreased diabetes risk over 9 year after 18-month oral L-arginine treatment in middle-aged subjects with impaired glucose tolerance and metabolic syndrome (extension evaluation of L-arginine study) Eur J Nutr. 2018;57(8):2805–17. doi: 10.1007/s00394-017-1548-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mohan IK, Das UN. Effect of L-arginine-nitric oxide system on the metabolism of essential fatty acids in chemical-induced diabetes mellitus. Prostaglandins Leukot Essent Fatty Acids. 2000;62(1):35–46. doi: 10.1054/plef.1999.0122. [DOI] [PubMed] [Google Scholar]
- 56.Lass A, Suessenbacher A, Wolkart G, Mayer B, Brunner F. Functional and analytical evidence for scavenging of oxygen radicals by L-arginine. Mol Pharmacol. 2002;61(5):1081–8. doi: 10.1124/mol.61.5.1081. [DOI] [PubMed] [Google Scholar]
- 57.Lubec B, Hayn M, Kitzmuller E, Vierhapper H, Lubec G. L-Arginine reduces lipid peroxidation in patients with diabetes mellitus. Free Radic Biol Med. 1997;22(1–2):355–7. doi: 10.1016/S0891-5849(96)00386-3. [DOI] [PubMed] [Google Scholar]
- 58.Lucotti P, Setola E, Monti LD, Galluccio E, Costa S, Sandoli EP, Fermo I, Rabaiotti G, Gatti R, Piatti P. Beneficial effects of a long-term oral L-arginine treatment added to a hypocaloric diet and exercise training program in obese, insulin-resistant type 2 diabetic patients. Am J Physiol Endocrinol Metab. 2006;291(5):E906–912. doi: 10.1152/ajpendo.00002.2006. [DOI] [PubMed] [Google Scholar]
- 59.Bastyr EJ 3rd, Stuart CA, Brodows RG, Schwartz S, Graf CJ, Zagar A, Robertson KE. Therapy focused on lowering postprandial glucose, not fasting glucose, may be superior for lowering HbA1c. IOEZ Study Group. Diabetes Care. 2000;23(9):1236–41. [DOI] [PubMed]
- 60.Ceriello A. Postprandial hyperglycemia and diabetes complications: is it time to treat? Diabetes 2005, 54(1):1–7. [DOI] [PubMed]
- 61.de Veciana M, Major CA, Morgan MA, Asrat T, Toohey JS, Lien JM, Evans AT. Postprandial versus preprandial blood glucose monitoring in women with gestational diabetes mellitus requiring insulin therapy. N Engl J Med. 1995;333(19):1237–41. doi: 10.1056/NEJM199511093331901. [DOI] [PubMed] [Google Scholar]
- 62.Cherney DZ, Scholey JW, Sochett EB. Sex differences in renal responses to hyperglycemia, L-arginine, and L-NMMA in humans with uncomplicated type 1 diabetes. Diabetes Care. 2013;36(5):1290–6. doi: 10.2337/dc12-1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fayh AP, Krause M, Rodrigues-Krause J, Ribeiro JL, Ribeiro JP, Friedman R, Moreira JC, Reischak-Oliveira A. Effects of L-arginine supplementation on blood flow, oxidative stress status and exercise responses in young adults with uncomplicated type I diabetes. Eur J Nutr. 2013;52(3):975–83. doi: 10.1007/s00394-012-0404-7. [DOI] [PubMed] [Google Scholar]
- 64.Yousefi Rad E, Nazarian B, Saboori S, Falahi E, Hekmatdoost A. Effects of l-arginine supplementation on glycemic profile: evidence from a systematic review and meta-analysis of clinical trials. J Integr Med. 2020;18(4):284–91. doi: 10.1016/j.joim.2020.05.001. [DOI] [PubMed] [Google Scholar]
- 65.Lai YL, Aoyama S, Nagai R, Miyoshi N, Ohshima H. Inhibition of L-arginine metabolizing enzymes by L-arginine-derived advanced glycation end products. J Clin Biochem Nutr. 2010;46(2):177–85. doi: 10.3164/jcbn.09-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fiory F, Lombardi A, Miele C, Giudicelli J, Beguinot F, Van Obberghen E. Methylglyoxal impairs insulin signalling and insulin action on glucose-induced insulin secretion in the pancreatic beta cell line INS-1E. Diabetologia. 2011;54(11):2941–52. doi: 10.1007/s00125-011-2280-8. [DOI] [PubMed] [Google Scholar]
- 67.Su Y, Qadri SM, Wu L, Liu L. Methylglyoxal modulates endothelial nitric oxide synthase-associated functions in EA.hy926 endothelial cells. Cardiovasc Diabetol. 2013;12:134. doi: 10.1186/1475-2840-12-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Polykretis P, Luchinat E, Boscaro F, Banci L. Methylglyoxal interaction with superoxide dismutase 1. Redox Biol. 2020;30:101421. doi: 10.1016/j.redox.2019.101421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Santulli G, Trimarco V, Trimarco B, Izzo R. Beneficial effects of vitamin C and L-Arginine in the treatment of post-acute sequelae of COVID-19. Pharmacol Res. 2022;185:106479. doi: 10.1016/j.phrs.2022.106479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Martina V, Masha A, Gigliardi VR, Brocato L, Manzato E, Berchio A, Massarenti P, Settanni F, Della Casa L, Bergamini S, et al. Long-term N-acetylcysteine and L-arginine administration reduces endothelial activation and systolic blood pressure in hypertensive patients with type 2 diabetes. Diabetes Care. 2008;31(5):940–4. doi: 10.2337/dc07-2251. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
N/A.