Abstract
miRNA-93 is a member of the miR-106b-25 family and is encoded by a gene on chromosome 7q22.1. They play a role in the etiology of various diseases, including cancer, Parkinson’s disease, hepatic injury, osteoarthritis, acute myocardial infarction, atherosclerosis, rheumatoid arthritis, and chronic kidney disease. Different studies have found that this miRNA has opposing roles in the context of cancer. Recently, miRNA-93 has been downregulated in breast cancer, gastric cancer, colorectal cancer, pancreatic cancer, bladder cancer, cervical cancer, and renal cancer. However, miRNA-93 is up-regulated in a wide variety of malignancies, such as lung, colorectal, glioma, prostate, osteosarcoma, and hepatocellular carcinoma. The aim of the current review is to provide an overview of miRNA-93's function in cancer disorder progression and non-cancer disorders, with a focus on dysregulated signaling pathways. We also give an overview of this miRNA's function as a biomarker of prognosis in cancer and emphasize how it contributes to drug resistance based on in vivo, in vitro, and human studies.
Video Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s12964-023-01106-3.
Keywords: Cancer, miRNA-93, Biomarker, Drug resistance
Introduction
MicroRNAs (miRNAs) are small RNA molecules that regulate gene expression post-transcriptionally by acting on the stability and translation of transcribed mRNAs [1]. The RNA polymerase II enzyme generates polyadenylated and cap-coated pri-miRNAs [2]. This transcript is further processed by Drosha and Dicer ribonuclease to generate stem-loop precursor miRNA and mature miRNA [3]. Even this component is part of an RNA-induced silencing complex that can recognize certain mRNA targets, therefore preventing translation or making the mRNA unstable [4]. MiRNAs play regulatory roles in cancer, such as altering the signaling axis [5], modulation of gene expression [6], and biological processes like cell growth, division, apoptosis, and maintaining homeostasis [7], all of which suggest that they participate in the etiology of illness.
MiRNA-93 is encoded by a gene on chromosome 7q22.1 [8]. They are expressed in the nucleus and co-transcribed with the host minichromosome maintenance complex component 7 (MCM7) gene [9]. It is a paralog of the miRNA-17–92 cluster [10], a member of the pro-oncogenic miRNA-106b-25 cluster. This cluster has been shown to regulate the expression of various target genes involved in important cellular processes such as cell proliferation, apoptosis, and angiogenesis [10, 11].
Studies have shown that miRNA-93 is upregulated in several types of cancer, such as breast cancer (BC) [12], lung cancer [13], colorectal cancer [14], prostate cancer [15], and pancreatic cancer [16]. In these cancers, miRNA-93 acts as oncogenic miRNA and promotes tumor growth and metastasis through regulating the expression of targeted genes involved in cell proliferation, angiogenesis, and invasion [17, 18]. For example, Fang and his colleagues revealed that miRNA-93 might stimulate both tumor development and angiogenesis via inhibiting the expression of integrin-8 [19].
In contrast, miRNA-93 can inhibit tumor growth in a number of ways. For instance, in BC cells, overexpression of miR-93 reduced the protein level of Wiskott-Aldrich syndrome protein family member 3 (WASF3), a regulator of CSC characteristics and cytoskeleton remodeling, and WASF3 reversed the miRNA-93-mediated reduction of BC cell invasion [20]. These results contribute to the role of miRNA-93 as a metastasis inhibitor by reducing BC's invasiveness and stem cell characteristics.
In addition to cancer, miRNA-93 has also been implicated in non-malignant disorders, such as osteoarthritis [21], rheumatoid arthritis (RA) [22], atherosclerosis [23], hepatic injury [24], Parkinson's disease [25], acute myocardial infarction [26], and chronic kidney disease [27]. Matrix metalloproteinase 3 (MMP3), a proteolytic enzyme that breaks down collagen fiber that is primarily produced in inflamed joints and ultimately plays a substantial role in joint inflammation and bone erosion in RA, is a key participant in the etiology of the disease [28]. It was discovered that MMP3 is a potential miRNA-93 target. By specifically targeting the 3′UTR of MMP3, miRNA-93 reduced MMP3 expression [29]. Furthermore, the methylation of the promoter MMP3 gene is caused by the overexpression of the miRNA-93 gene [22].
Recently, miRNA-93 participates in drug resistance of tumor cells by targeting on genes or signaling pathways linked to resistance development, such as the PI3K/Akt signaling pathway and the expression of anti-apoptotic proteins in pancreatic cancer [30]. Moreover, miRNA-93, a potential PTEN/Akt signaling pathway inhibitor, controls the chemosensitivity of ovarian cancer cells to the chemotherapy agent cisplatin [31]. Further, miRNA-93 may play an important role in EMT and drug resistance of BC cells by targeting PTEN. For example, Chu et al. identified that miRNA-93 targets PTEN to help BC cells undergo EMT and acquire doxorubicin resistance [32].
The current study attempts to review miRNA-93's involvement in malignant and non-malignant diseases, focusing on its target mRNA and dysfunctional signaling cascades, as well as its function in drug resistance and cancer prognosis.
Search methodology
Based on the primary keywords (miRNA-93, malignant disorders, non-malignant disorders, drug resistance), we conducted searches on PubMed, a well-known database of biological literature. The real samples were included in accordance with the parameters of the review study on miRNA-93 related to the malignant and non-malignant disorders and drug resistance. Conversely, the study did not include the disqualifying data.
miRNA-93 expression in malignant conditions
Numerous miRNAs are affected by cancer, and depending on the situation, they might function as tumor suppressors or oncogenes. Recent studies in cancer cell lines, animal models of cancer, and cancer patient samples have highlighted the role of miRNA-93 in carcinogenesis. Utilizing these three datasets, we proceed to characterize miRNA-93's function in carcinogenesis in the following sections.
Cell line studies
Up-regulation of miRNA-93 in cancer cell lines
Different kinds of cell lines were used in vitro in order to find how miRNA-93 upregulation induces cancer progression (Table 2). For example, miRNA-93 significantly increased in TNBC by 6.92-fold and has been linked to TNM grade, Ki-67 staining, and lymph node metastases in TNBC patients when compared to non-TNBC tissues or normal tissues [33]. Shyamasundar et al. revealed that miRNA-93 was found to be increased by 60-fold and prevents the invasive potential of TNBC cells through the protein kinase WNK1 [34]. Likewise, Feng and his colleagues showed that in vascular smooth muscle cells (VSMCs), miRNA-93 is highly elevated in VSMCs in vivo and in vitro studies [35]. Their findings suggest that, via suppressing mitofusin 2 (Mfn2) expression, miRNA-93 promotes VSMC proliferation and migration.
Table 2.
Impact of miRNA-93 in carcinogenesis based on research in animal models
| Type of tumor | Types of miRNAs | Animal model | Methods of Manipulation and Cellular Grafting | Phenotypes linked to miRNA-93 dysregulation | Ref |
|---|---|---|---|---|---|
| NSCLC | miRNA-93 | Nude mice | Injecting miR-93- or control-transfected H1993 cells subcutaneously | ↑miRNA-93 (which targets DAB2): ↓tumor growth | [45] |
| Esophageal carcinoma | miRNA-93 | Nude mice | Injections of transfected SKGT4 cells (circ-21–93 or circ-scr) under the skin | ↑miRNA-93: ↓xenografted tumor growth | [92] |
| Hepatocellular carcinoma | miRNA-93-5p | BALB/c nude mice | Subcutaneous injection of lentiviral overexpression of miR-93-5p in SK-Hep-1 cells + lentiviral antagonist of miR-93-5p in MHCC-97H cells | ↑miRNA-93-5p (directly targets MAP3K2): ↑ cell proliferation | [59] |
| Mammary tumor | miRNA-93 | ACI rats | Injecting 3 mg of E2 pellets into MCF-10A and T47D cells infected with a lentiviral vector subcutaneously | ↑miRNA-93(targets NRF2): ↑Clonability, development of mamospheres, and migratory characteristics of MCF-10A cells, and ↓ apoptosis | [90] |
| Epithelial ovarian carcinoma | miRNA-93-5p | BALB/c nude mice | Injections of miR-93-5P or mock-transfected OVCAR3 cells under the skin | ↓miRNA-93-5p (directly targets RhoC): ↓ proliferation, ↑G1 or S arrest and apoptosis | [91] |
| Renal carcinoma | miRNA-93-3p | BALB/c nude mice | Transfected 786-O cells were injected subcutaneously with either a control lentivirus or an anti-miR-93-3p lentivirus | ↑miRNA-93-3p (directly targets PEDF): ↑cell tumorigenesis and metastasis | [93] |
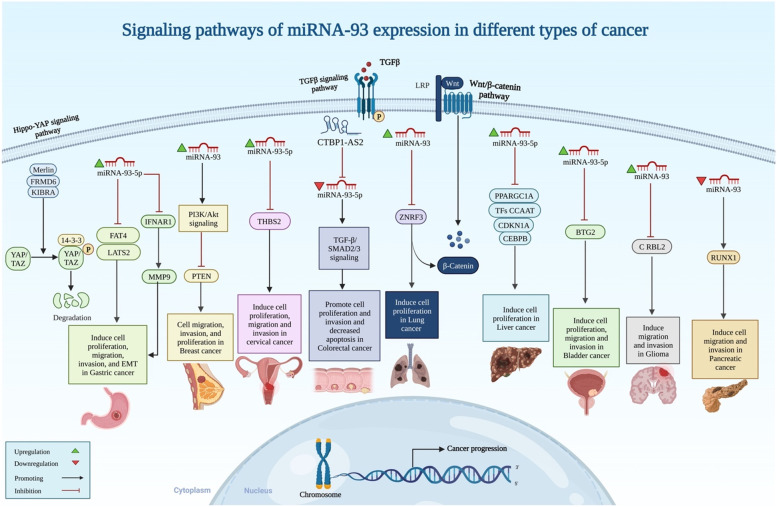
Furthermore, recent studies found that for the treatment of in-stent restenosis, miRNA-93 might be a potential target. For example, after controlling the standard risk factors (RFs), miRNA-93-5p was able to distinguish between individuals with stable coronary artery disease (CAD) and those without CAD [36]. Similarly, Feng et al. showed that Mfn2 is a direct target of miRNA-93, which encourages VSMC migration and proliferation. They showed that for the treatment of in-stent restenosis, miRNA-93 may be a novel target [37]. Additionally, miRNA-93 was found to be greatly enhanced in an in vitro investigation by Xio et al., promoting cell migration and proliferation, while miRNA-93 repression had the reverse effect [38]. They revealed that miRNA-93-3p increased significantly by 2.5-fold and inhibits ZFP36 Ring Finger Protein Like 1 (ZFP36L1), which then induces Zinc Finger Protein X-Linked (ZFX) expression and stimulates keratinocyte migration and proliferation during skin wound healing. Moreover, Li et al. proved that targeting the Phosphatase and tensin homolog (PTEN) gene with miRNA-93 controls the PTEN/PI3K/Akt pathway in BC cells, which increases tumor cell proliferation, invasion, and migration in vitro [12] (Fig. 1). Based on their data, PTEN is directly targeted by this miRNA and could act as a promising therapeutic target for BC.
Fig. 1.
The expression patterns and pivotal roles of miRNA-93 in tumor networks are graphically depicted, along with its signaling pathways in different types of cancer
According to these studies, miRNA-93 has been upregulated in cancer cell lines and promoted the progression of several kinds of cancer.
Down-regulation of miRNA-93 in cancer cell lines
MiRNA-93 is often upregulated in cancer cell lines; however, it can also be downregulated in other cancer cell lines. For instance, in colorectal cancer (CRC), miRNA-93 has anti-tumor properties such as reducing colon cancer cell motility, proliferation, and angiogenesis [39]. CTBP1-AS2 was shown to be overexpressed in colorectal cancer by Li et al., and it enhances the activation of the TGF-/SMAD2/3 signaling pathway by blocking miRNA-93-5p, which in turn allows rapid progression of CRC [40]. Furthermore, Qu et al. approved that miRNA-93 in lung cancer (LC) cells is overexpressed and directly attach to the 3′-UTR of the Neural precursor cell expressed developmentally downregulated gene 4-like (NEDD4L) messenger RNA (mRNA), which leads to the production of NEDD4L to be downregulated at the protein level and promoted TGF-β induced EMT [41]. Additionally, Xiang and his team demonstrated that in BC cells, megakaryoblastic leukemia 1 (MKL-1) gene and signal transducer and activator of transcription 3 (STAT3) expression are both suppressed by miRNA-93-5p through targeting their 3'UTR, which prevents BC cells from undergoing EMT [42]. They approved the fact that miRNA-93-5p regulates MKL-1 and STAT3, which influence the EMT process, and that it can control BC cell migration. These findings suggest that miRNA-93 is expressed differently in different cancer cell lines. The functions of miRNA-93 in a number of cancer cell lines are summarized in Table 1.
Table 1.
In various cancer cell lines, the functions of miRNA-93 and important targets with associated phenotypes are highlighted
| Tumor type | miRNAs | Levels incancer cell lines compared with normal cell lines | Interactions | Downstream target of miRNA | Effect of miRNA-93 up-regulation on its target | Cell line | Associated phenotypes with dysregulation of miRNA-93 | Ref |
|---|---|---|---|---|---|---|---|---|
| Lung Cancer | miRNA-93 | Up | FUS1 | FUS1 | Inhibition | NCI-H146, NCI-H157, NCI-H187, NCI-H209, NCI-H526, NCI-H889, NCIH1299, NCIH1648, NCI-H1672, NCI-H1770, NCI-H1819, NCI-H2052, NCI-H2107, NCI-H2171, NCI-H2195, NCI-H2122, NCI-H2887, HCC366, HCC970, HCC1195, HCC2450 | ↑ miRNA-93, ↓ FUS1: ↑ tumor progression | [43] |
| miRNA-93-5p | Up | PTEN/RB1 | PTEN/RB1 | Inhibition | A549, SK-MES-1 | ↑ miRNA-93-5p, ↓ PTEN/RB1: ↑ Cell proliferation, migration, and invasion | [44] | |
| miRNA-93 | Up | NEDD4L | NEDD4L | Inhibition | A549, H1650 | ↑ miRNA-93, ↓ NEDD4L: ↑ tumorigenesis and metastasis | [41] | |
| miRNA-93 | Up | DAB2 | DAB2 | Inhibition | H1993 | ↑ miRNA-93, ↓ DAB2: ↑ tumor growth | [45] | |
| miRNA-93 | Up | PI3K/Akt, LKB1, PTEN, p21, CDKN1A |
LKB1/PTEN /CDKN1A |
Inhibition | A549, NCI-H1975, NCI-H1299 | ↑ miRNA-93, ↓ LKB1/PTEN /CDKN1A/p21, ↑ PI3K/Akt: ↑ tumorigenesis and metastasis | [46] | |
| miRNA-93 | Up | ZNRF3, Wnt/β-catenin | ZNRF3 | Inhibition | A549, H460 | ↑ miRNA-93, ↓ZNRF3, ↓ Wnt/β-catenin: ↑ cell proliferation | [47] | |
| Breast Cancer | miRNA-93-5p | Up | MKL-1 /STAT3 | MKL-1 /STAT3 | Inhibition | MCF-7, MDA-MB-231, T47D | ↑ miRNA-93-5p, ↓ MKL-1 /STAT3: ↑ migration, ↓EMT | [42] |
| miRNA-93 | Up | PI3K/Akt, PTEN | PTEN | Inhibition | MDA-MB-231 | ↑ miRNA-93, ↓ PTEN, ↑ PI3K/Akt: ↑ cell migration, invasion, and proliferation | [12] | |
| miRNA-93 | Down | WNK1 | WNK1 | Inhibition | MDA-MB-231 | ↑miRNA-93, ↓ WNK1: ↓ invasive | [34] | |
| miRNA-93 | Downregulated (by lncRNA-H19) | STAT3, lncRNA-H19 | STAT3 | Inhibition | HEK293T, MCF‐7, MDA‐MB‐231 | ↑ lncRNA-H19, ↓ miRNA-93, ↑ STAT3: ↓proliferation | [48] | |
| miRNA-93 | Up | LATS2 | LATS2 | Inhibition | MT-1 | ↑ miRNA-93-5p, ↓ LATS2: ↑ angiogenesis and metastasis | [49] | |
| Colorectal Cancer | miRNA-93-5p | Up | MDR1, CDKN1A | CDKN1A | Inhibition | HCT‑8, MDR HCT‑8/vincristine (VCR) | ↑ miRNA-93-5p, ↓CDKN1A, ↑ MDR1: ↑ MDR | [14] |
| miRNA-93-5p | Up | FOXA1, TGFB3 | FOXA1 | Inhibition | HT-29, SW480, LoVo | ↑ miRNA-93-5p, ↓ FOXA1: ↑ tumor growth | [50] | |
| miRNA-93 | Down | Smad7, Wnt/β-catenin | Smad7 | Inhibition |
HCT116, HT29, SW480, SW620, LoVo, LS174T |
↓ miRNA-93, ↑ Wnt/β-catenin, ↓ Smad7: ↓ tumor growth | [51] | |
| miRNA-93-5p | Downregulated (by CTBP1-AS2) | TGF-β/SMAD2/3, CTBP1-AS2 | TGF-β | Inhibition | Caco-2, SW620, HT29, T84, HCT116, SW480 | ↑ CTBP1-AS2, ↓miRNA-93-5p, ↑ TGF-β/SMAD2/3 pathway: ↑ cell proliferation and invasion and decreased apoptosis | [40] | |
| miRNA-93 | Up | CCNB1, ERBB2, P21, VEGF | CCNB1, ERBB2, P21, VEGF | Inhibition | Caco2, LoVo, HCT116 (ATCC, Manassas, VA, USA) | ↑ miRNA-93-5p, ↓ CCNB1, ERBB2, P21, VEGF: ↓ tumorigenesis | [39] | |
| miRNA‐93‐5p | Down | MMP‐1, 2, MMP-9, IL‐2, IFN‐γ, TNF‐α, PD‐L1 | PD‐L1 | Inhibition | HCT116, SW480 | ↓ miRNA-93-5p, ↑ PD‐L1, ↓ MMP‐1, 2, MMP‐9: ↓ migration and invasion | [52] | |
| miRNA-93 | Downregulated by lncRNA CA3-AS1 | lncRNA CA3-AS1, PTEN | PTEN | Inhibition | HCT-116, SW480, SW620, SW1116, HT29 |
↑ CA3-AS1, ↓ miRNA-93, ↑ PTEN: ↑ apoptosis, ↓ proliferation, invasion |
[53] | |
| Liver Cancer | miRNA-93 | Up | TIMP2, TP53INP1, CDKN1A |
TIMP2/TP53INP1 /CDKN1A |
Inhibition | WRL68, HepG2, SMMC7721, SKHEP1, HUH7 | ↑ miRNA-93, ↓ TIMP2, TP53INP1, CDKN1A: ↑ proliferation and invasion | [54] |
| miRNA-93 | Up | PDCD4 | PDCD4 | Inhibition | HEK293T, SMMCC-7721, Huh-7 | ↑ miRNA-93, ↓ PDCD4: ↑migration and invasion | [55] | |
| miRNA-93 | Up | T-ICs, MTMR3 | MTMR3 | Inhibition | HCCLM3, EpCAM + , EpCAM − | ↑ miRNA-93, ↓ MTMR3, ↑T-ICs: ↑ resistance to sorafenib treatment | [56] | |
| miRNA-93-5p | Up | PPARGC1A, CDKN1A, CEBPB, TFs CCAAT | PPARGC1A, CDKN1A, CEBPB, TFs CCAAT | Inhibition | 293 T, L-02, a SMMC-7721, Huh-7, SK-Hep-1, HepG2, HCCLM3, and MHCC97H | ↑ miRNA-93-5p, ↓ PPARGC1A, CDKN1A, CEBPB, TFs CCAAT: ↑cell proliferation | [57] | |
| miRNA-93 | Up | PDCD4 | PDCD4 | Inhibition |
QGY-7703, SMMC-7721 |
↑ miRNA-93, ↓ PDCD4: ↑ cell proliferation | [58] | |
| miRNA-93-5p | Up | MAP3K2, MKK4, P38, JNK, c-Jun | - | - |
HepG2, BEL-7402, Hep3B, MHCC-97L, MHCC-97H, HCC-LM3 |
↑ miRNA-93-5p, ↑ MAP3K2, MKK4, P38, JNK, c-Jun: ↑ proliferation | [59] | |
| miRNA-93 | Up | PTEN, CDKN1A, c-Met/PI3K/Akt | PTEN, CDKN1A | Inhibition | HepG2, Hep3B, PLC/PRF/5, SNU398, SNU423, SNU449 | ↑ miRNA-93, ↓ PTEN, CDKN1A, ↑ c-Met/PI3K/Akt: ↑ cell proliferation, migration, and invasion | [60] | |
| Gastric Cancer | miRNA-93-5p | Up | IFNAR1, MMP9, STAT3 | IFNAR1 | Inhibition |
MGC803, MKN28, SGC-7901, HGC-27, BGC-823, MKN45, AGS, HEK293 |
↑ miRNA-93-5p, ↓IFNAR1, ↑ STAT3: ↑cell metastasis | [61] |
| miRNA-93 | Up | PDCD4 | PDCD4 | Inhibition | AGS | ↑ miRNA-93-5p, ↓ PDCD4: ↑ tumor growth | [62] | |
| miRNA-93-5p | Up | FAT4, LATS2 | FAT4, LATS2 | Inhibition | SGC-7901, HGC-27 | ↑ miRNA-93-5p, ↓FAT4, LATS2: ↑ tumor progression | [63] | |
| miRNA-93 | Up | TIMP2 | TIMP2 | Inhibition | SGC-7901, MKN-28, BGC-823, MGC-803, MKN-45, GES-1 | ↑ miRNA-93, ↓ TIMP2: ↑ proliferation and metastasis | [64] | |
| miRNA-93-5p | Up | AHNAK, DKK1 | AHNAK | Inhibition | SUN-216, BGC-823, MKN74, HGC-27, GES-1 | ↑ miRNA-93-5p, ↓ AHNAK: ↑cell migration, invasion and EMT | [65] | |
| miRNA-93-5p | Downregulated (by Matrine) | AHNAK | AHNAK | Inhibition | MKN‐28, SGC‐7901 | ↑ Matrine, ↓ miRNA-93-5p, ↑ AHNAK: ↑proliferation, migration and invasion | [66] | |
| Pancreatic Cancer | miRNA-93 | Up | CRMP-2, YES1, MAPRE1 | CRMP-2, YES1, MAPRE1 | Inhibition | HPDE, HEK-293 T, PANC-1, MIA PaCa-2 | ↑ miRNA-93, ↓ CRMP-2, YES1, MAPRE1: ↑tumor progression | [67] |
| miRNA-93 | Down | RUNX1, HMGA2 | RUNX1 | Inhibition | PANC-1, MIA PaCa-2 | ↓ miRNA-93, ↑RUNX1: ↑cell migration and invasion | [68] | |
| Bladder Cancer | miRNA-93-5p | Up | BTG2 | BTG2 | Inhibition | T24, UM-UC-3, SV-HUC-1 | ↑ miRNA-93-5p, ↓ BTG2: ↑ proliferation, migration and invasion | [69] |
| miRNA-93 | Up | PEDF | PEDF | Inhibition | TCCSUP, 5637, UM-UC-3, T24 | ↑ miRNA-93, ↓ PEDF: ↑ proliferation and invasion | [70] | |
| miRNA-93-5p | Up | KLF9 | KLF9 | Inhibition | SV-HUC-1, 5637, RT-112, RT4, BT-B | ↑ miRNA-93-5p, ↓ KLF9: ↑ proliferation and migration | [71] | |
| miRNA-93 | Down | LASS2 | LASS2 | Inhibition | RT4, T24 | ↓ miRNA-93, ↑ LASS2: ↑ chemo-sensitivity | [72] | |
| Cervical Cancer | miRNA-93-5p | Up | THBS2, MMPS | THBS2 | Inhibition | siHa, 293 T | ↑ miRNA-93-5p, ↓ THBS2: ↑ proliferation, invasion and migration | [73] |
| miRNA-93-5p | Up | BTG3, HR‑HPV | BTG3 | Inhibition | SiHa, CaSk, HeLa, C4-1, C33A | ↑ miRNA-93-5p, ↓BTG3: ↓ proliferation, invasion and migration | [74] | |
| miRNA-93 | Up | CDKN1A | CDKN1A | inhibition | Hela | ↑ miRNA-93, ↓ CDKN1A: ↑proliferation and invasion | [75] | |
| miRNA-93 | Downregulated (by MCM3AP-AS1) | - | - | - | C-33A (HPV-negative) and SiHa (HPV positive) | ↑ MCM3AP-AS1, ↓miRNA-93: ↓ cell proliferation | [76] | |
| Prostate Cancer | miRNA-93 | Up | TGFΒR2, ITGB8, LATS2 | - | - | RWPE-1, PC-3, LNCaP, DU145, 22RV1 | ↑ miRNA-93, ↑ TGFΒR2, ITGB8, LATS2: ↑proliferation and invasion | [15] |
| miRNA-93 | Up | DAB2, Akt/ERK1/2 | DAB2 | Inhibition | PC-3, DU145 | ↑ miRNA-93-5p, ↓ DAB2: ↑ progression and metastasis | [77] | |
| Glioma | miRNA-93 | Up | PTEN, PHLPP2, FOXO3, PI3K/Akt | PTEN, PHLPP2, FOXO3 | Inhibition | U251MG, A172, LN229, SF767, U118MG, U87MG, Hs683, LN18, SHG44 | ↑ miRNA-93, ↓ PTEN, PHLPP2, FOXO3, C PI3K/Akt signaling: ↑ Proliferation | [78] |
| miRNA-93 | Up | P21 | P21 | Inhibition | U87, U251, SF126, SF767, A172, SHG44 | ↑ miRNA-93, ↓ P21: ↑ proliferation, colony formation, drug resistance | [79] | |
| miRNA-93 | Up | RBL2 | RBL2 | Inhibition | NHAs, U251, U87 | ↑ miRNA-93, ↓ C RBL2: ↑ migration and invasion | [80] | |
| miRNA-93-5p | Down | MMP2 | MMP2 | Inhibition | U87-MG, LN-18 | ↓ miRNA-93, ↑MMP2: ↓ proliferation and metastasis | [81] | |
| miRNA-93 | Up | IL-8, VEGF | IL-8, VEGF | Inhibition | U251, T98G | ↑ miRNA-93, ↓ IL-8, VEGF: ↑angiogenesis | [82] | |
| Osteosarcoma | miRNA-93 | Up | PTEN, Akt | PTEN | Inhibition | HOS, SaOS, MG-63, NY, Hu09, | ↑ miRNA-93, ↓ PTEN: ↑proliferation | [83] |
| miRNA-93 | Up | P21 | P21 | Inhibition | Saos-2, U2OS, SW1353, MG63, hFOB1.19, HEK293 | ↑ miRNA-93, ↓ P21: ↑ proliferation | [84] | |
| miRNA-93 | Up | TIMP2, MMPs | TIMP2 | Inhibition | U-2OS, OS-732, HOS, Saos-2, hFOB | ↑ miRNA-93, ↓ TIMP2: ↑ cell viability, invasion, and EMT | [85] | |
| Renal cancer | miRNA-93 | Down | RBL2, TGF-beta | RBL2 | Inhibition | 786-O, 796-P | ↓ miRNA-93, ↑ RBL2: ↑ tumor progression | [86] |
| Esophageal carcinoma | miRNA-93-5p | Up | TGFβR2 | TGFβR2 | Inhibition | Het-1A (BNCC337688), and EC cell lines [TE-1 (BNCC100151), Eca-109 (BNCC337687) and EC9706 (BNCC339892)] | ↑ miRNA-93-5p, ↓ TGFβR2: ↑ proliferation, migration and invasion, ↓ apoptosis | [87] |
| miRNA-93-5p | Up | PTEN, p21, cyclin D1 | PTEN | Inhibition | EC9706 | ↑ miRNA-93-5p, ↓ PTEN, p21, ↑ cyclin D1: ↑ proliferation | [88] | |
| Papillary thyroid carcinoma | miRNA-93-3p | Downregulated (by ASMTL-AS1) | ASMTL-AS1, miR-93-3p, miR-660, FOXO1 | FOXO1 | Inhibition | Nthy-ori 3–1 | ↑ ASMTL-AS1, ↓ miRNA-93-3p, miR-660, ↑ FOXO1: ↑ tumor growth and glycolysis | [89] |
Animal studies
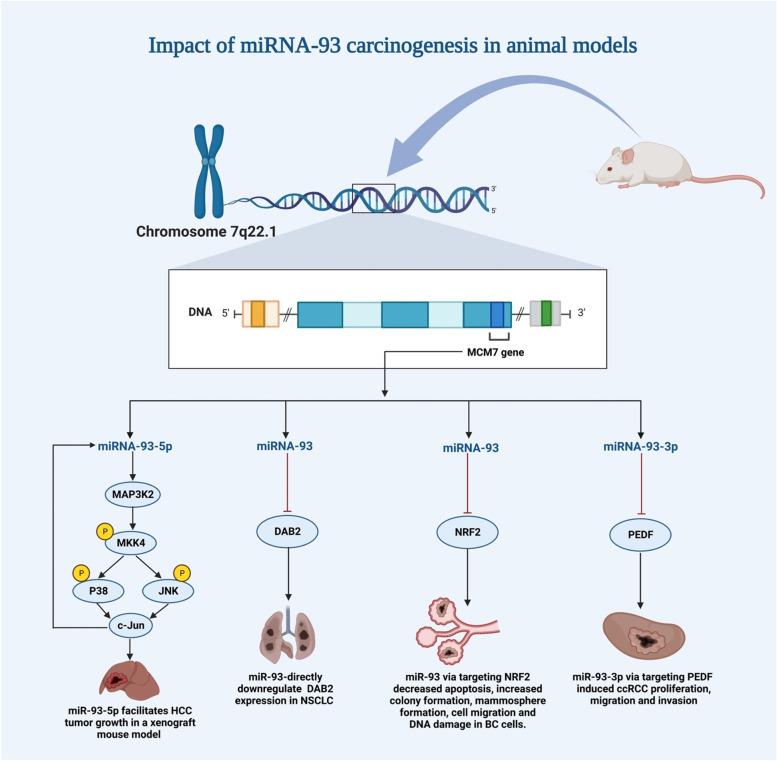
MiRNA93 enhances tumor development and tumor cell survival, according to extensive in vivo and in vitro experiments. For instance, Du et al. demonstrated that in mouse tumor xenografts of NSCLC, miRNA-93 overexpression enhances tumor growth and is mediated through down-regulating expression (Fig. 2). However, altering the levels of miRNA-93 and DAB2 has an impact on cell survival DAB2 protein [45]. They found that the miRNA-93/DAB2 pathway plays a key role in controlling how lung cancer gets worse. This is clear from the fact that high miRNA-93 expression levels are linked to both low DAB2 levels and poor patient survival. Similarly, CTBP1-AS2 was shown to promote proliferation and invasion of CRC cells in vitro and in vivo by sponging miRNA-93-5p and activating the TGF-/SMAD2/3 pathway [40]. Moreover, in another study, Singh and his team found that nuclear factor erythroid 2–related factor 2 (NRF2) and NRF2-regulated genes' protein expression was reduced by ectopic miRNA-93 expression in rat mammary tissue. As a result, miRNA-93 prevented the development of mamospheres, colony formation, apoptosis, cell migration, and DNA damage in breast epithelial cells, while silencing miRNA-93 in these cells promoted these cancer-causing activities [90]. They approved that miRNA-93 has a carcinogenic potential during E2-induced breast carcinogenesis. Additionally, Chen et al. showed in their study that ras homolog family member C (RhoC) is a target of miRNA-93-5P, which may prevent the growth and advancement of EOC tumors. Although, they suggested that miRNA-93-5P has the capacity to inhibit the growth of ovarian cells [91]. This result shows that RhoC is being downregulated in tumor xenografts in vivo by miRNA-93-5P in order to prevent EOC aggression, which may offer a deeper understanding of the molecular pathways behind cancer aggression. However, according to these studies, miRNA-93 might be an important factor in the advancement of cancers in vivo. Based on research in animal models, Table 2 shows how miRNA-93 affects carcinogenesis, along with the genes it affects and how it affects the progression of cancer.
Fig. 2.
MiRNA-93's role in animal cancer models. By interacting with specific targets, miR-93-5p makes tumors grow rapidly in a xenograft mouse model of different types of cancer, such as, miR-93 directly inhibits the expression of DAB2 in NSCLC. Targeting PEDF made RCC cells multiply and targeting NRF2 inhibits cells from dying and made more colonies form in BC
Studies in clinical samples
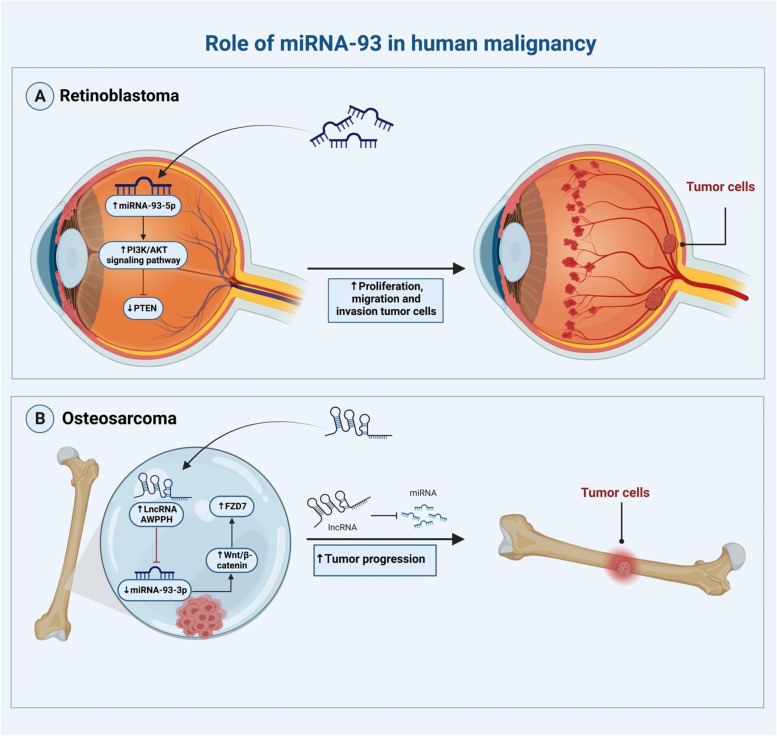
Expression of miRNA-93 has been differentially expressed in different types of malignant tissues. Experimental studies in these tissues showed that changes in miRNA-93 expression are associated either positively or negatively with its target genes (Table 3). For instance, Xu et al., by using the qRT-PCR method for EC tissues and cells, found that the vitality and migration rate of EC cells were markedly boosted by miRNA-93-5p overexpression, which was responded to Interferon Alpha And Beta Receptor Subunit 1 (IFNAR1) up-regulation [94]. Likewise, miRNA-93-5p could accelerate the course of retinoblastoma by controlling apoptosis, cell proliferation, migration, and invasion in a way that is dependent on the PTEN/PI3K/AKT signaling cascade [95] (Fig. 3a). Similarly, Li and his team demonstrated that lncRNA AWPPH accelerates the development of osteosarcoma via modifying the miR-93-3p/FZD7 axis, which activates the Wnt/b-catenin pathway [96] (Fig. 3b). Further, Xiao et al. showed that miRNA-93 targets cyclin G2 (CCNG2), which it in turn uses to increase proliferation, apoptosis inhibition, and increase migration and invasion of LSCC cells [97]. Moreover, according to Chen et al., miRNA-93-5p is likely increased by CCND2 overexpression in ovarian cancer malignancy, which favors the growth and survival of ovarian cancer tumors [98]. Additionally, in SCCHN samples, miRNA-93-5p acts as an oncogene to inhibit repulsive guidance molecule BMP co-receptor b (RGMB), which in turn regulates invasion and migration [99]. These findings suggest that miRNA-93-5p may serve as a helpful biomarker for assessing the prognosis of cancer patients as well as a possible therapeutic target.
Table 3.
miRNA-93 dysregulation in clinical samples and correlation between clinicopathologic traits and its expression in different types of cancer
| Tumor/ disorder type | Samples | miRNA-93 expression (Tumor vs. Normal) | Cox regression and Kaplan–Meier analysis | Association of miRNA-93 expression with clinicopathologic characteristics | Ref |
|---|---|---|---|---|---|
| Breast carcinoma | 20 BC patient tissue |
miRNA-93 (Up) |
- | Lymph node metastasis | [49] |
| Colon cancer | 138 pairs of CC sample |
miRNA-93 (Down) |
Downregulation is associated with poor survival in patients | Positive nodal metastasis (P = 0.006), positive distant metastases (P = 0.01), and advanced tumor stage (P = 0.02) | [100] |
| Uterine cancer | 176 UC sample + 100 healthy controls |
miRNA-93 (Up) |
The prognosis of UC patients is correlated with upregulation | Lymph node metastases and pathological stage | [8] |
| Laryngeal squamous cell carcinoma | 59 pairs of LSCC samples |
miRNA‐93‐5p (Up) |
- | Histological grade, lymph node metastasis | [97] |
| Colorectal cancer |
-First cohort: (35 non-early relapse CRC patients + 42 early relapse CRC patients) -Second cohort 45 CRC patients |
miRNA-93 (Up) |
An early UICC stage of CRC is linked to upregulation | G2 phase cell cycle arrest | [39] |
| Endometrial carcinoma | 50 paired of EC tissues |
miRNA‐93‐5p (Up) |
Upregulation is associated with patients' low survival rates from EC | The EC's FIGO stage and lymph node metastases | [94] |
| Lacrimal gland adenoid cystic carcinoma | 5 ACC patient tissues + 3 healthy controls |
miRNA‐93‐5p (Up |
- | Tumor migration, invasion, and proliferation | [101] |
| Ovarian cancer | - |
miRNA-93-5p (Down) |
Downregulation is linked to a poor prediction of patient survival time | Weakly increase OC cell apoptosis and inhibit cell migration | [98] |
| Esophageal cancer | 30 ESCA samples + 30 healthy controls |
miRNA-93 (Up) |
- | - | [102] |
| Squamous cell carcinoma of the head and neck | 522 SCCHN samples + 44 healthy controls |
miRNA‐93‐5p (Up) |
Upregulation in SCCHN is associated with a poor prognosis | Metastasis of lymph nodes | [99] |
| Renal cell carcinoma | 138 paired ccRCC sample |
miRNA-93-3p (Up) |
Upregulation is correlated with poor prognosis | - | [93] |
| Endometrial cancer | 100 EC patient |
miRNA-93 (Up) |
Upregulation is associated with Poor overall median survival | Lymph node involvement | [103] |
| Retinoblastoma | 23 human RB + 12 normal retinae |
miRNA-93-5p (Up) |
- | - | [95] |
| Osteosarcoma | - |
miRNA-93-3p (Downregulated (by LncRNA AWPPH)) |
Downregulation is associated with OS poor prognosis | TNM stage, metastasis | [96] |
Fig. 3.
The function of miRNA-93 in human Retinoblastoma and Osteosarcoma. In a clinical sample of retinoblastoma and osteosarcoma, miRNA-93-5p interacts with particular targets to cause tumors to grow quickly. A Through the PI3K/AKT signaling pathway, miRNA-93 directly reduces the expression of PTEN, a tumor suppressor gene, which promotes the growth of tumors in RB. B The expression of FZD7, which promotes tumor growth, is stimulated by the Wnt/B-catenin pathway and the rising amount of lncRNA AWPPH, which sponges miRNA-93
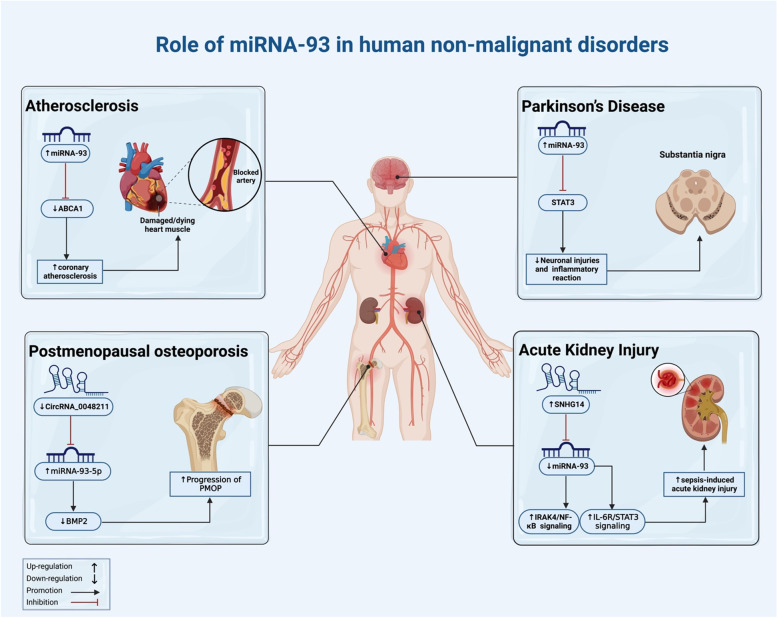
Numerous studies have used cell lines, animal models, and human clinical data to study the role of miRNA-93 in human disease (Fig. 4). Based on the data from three main sources, we outlined miRNA-93's function in human disorders in the sections below.
Fig. 4.
The diagram depicts the primary roles that miRNA-93 plays in the pathophysiology of non-cancerous illnesses. The expression level of miRNA-93, which modulates a large number of signaling pathways, has a role in the progression of a wide variety of diseases
miRNA-93 in human disorder cell lines
miRNA-93 is showing to be one of the most crucial regulators of gene expression, and studies have demonstrated that its dysregulation has a role in a variety of diseases (Table 4). For instance, Shi et al. found that SNHG14 is increased in LPS-induced HK-2 cells and that sepsis accelerates the cellular injury of AKI caused by IL-1β, LPS, and IL-6 [104]. As a result, the IRAK4/NF-B, IL-6R/STAT3, and miRNA-93 signaling pathways may be activated by SNHG14 via miRNA-93 as a potential method [104]. Similarly, Liu et al.’s study revealed that exosomal miRNA-93-5p inhibits toll like receptor 4 (TLR4)-mediated inflammation and Atg7-mediated autophagy to prevent myocardial injury, according to in vitro and in vivo research [105]. Further, in individuals with coronary atherosclerosis, up-regulated serum miR-93 is positively associated with raising serum cholesterol levels through targeting ATP binding cassette subfamily A member 1 (ABCA1) [23]. These data suggest that miRNA-93 dysregulation is one of the most significant variables influencing the development of human diseases.
Table 4.
Based on the results of cell line investigations, it can be seen how miRNA-93 contributes to the pathogenesis of diseases
| Disease type | miRNA type | Interactions | Cell lines | Associated phenotype with dysregulation of miRNA-93 | Ref |
|---|---|---|---|---|---|
| Acute myocardial infraction | miRNA-93-5p | TLR4/NF-κB-Mediated Inflammatory Response | H9c2 | ↑miRNA-93-5p, ↓Atg7-mediated autophagy and TLR4-mediated inflammatory: ↓myocardial injury | [105] |
| Acute kidney injury | miRNA-93 | IRAK4/NF-κB and IL-6R/STAT3 | HK-2 | ↑SNHG14, ↓ miRNA-93, ↑IRAK4/NF-κB and IL-6R/STAT3 signaling: ↑Sepsis-Induced Acute Kidney Injury | [104] |
| Atherosclerosis | miRNA-93 | ABCA1 | THP1 | ↑miRNA-93, ↓ ABCA1: ↑ disease progression |
Animal studies
To assess the effect of miRNA-93 dysregulation on the development of disease, numerous animal experiments have been carried out. For instance, according to Wang et al., mice's substantia nigra lost less tyrosine hydroxylase in 13 (PD) because miRNA-93 expression was higher. This decreased the production of STAT3, the activation of microglia, and the inflammatory response caused by MPTP [25]. Consequently, miRNA-93 promotes Parkinson's disease through regulating STAT3 expression. Accordingly, Xiong et al. showed that rat hepatic I-R injury is associated with both STAT3 up-regulation and miRNA-93 down-regulation. By overexpressing miRNA-93, which also reduced inflammation and enhanced liver function, the expression of STAT3 in rat liver I-R damage was significantly reduced [106]. Moreover, based on the Wu et al. study, miRNA-93 plays a significant role in regulating the cytotoxic effects of CAB, which can increase prolactinoma drug resistance by specifically targeting autophagy-related genes and decreasing autophagy related 7 (ATG7). They proved that pituitary cancers' drug resistance to CAB can be decreased by upregulating ATG7 or silencing miRNA-93 expression in vivo xenograft models in nude mice [107]. Additionally, Yang and his team demonstrated that miRNA-93-5p expression was increased in diabetic nephropathy following the downregulation of the lncRNA XIST, which prevented the production of CDKN1A and renal interstitial fibrosis [108]. Based on these data, lncRNA XIST has been proposed as a novel prognostic biomarker and potential therapeutic target for people with DN. Table 5 provides additional details on the function of miRNA-93 in disease models in animals.
Table 5.
Studies performed on animals to investigate the function of miRNA-93 in non-cancerous disease
| Disease type | micRNA type | Animal model | Result | Ref |
|---|---|---|---|---|
| Parkinson’s Disease | miRNA‑93 |
A mouse model of PD induced by 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP) |
By controlling STAT3 expression in the MPTP-induced PD mouse model, miRNA-93 lessens neuronal damage and decreases inflammatory response | [25] |
| Hepatic injury | miRNA-93 | Rat I-R hepatic injury model | MiRNA-93 inhibits STAT3, to reduce hepatic damage during ischemia–reperfusion | [106] |
| Prolactinoma | miRNA-93 | Rat, Female athymic nude mice | MicroRNA-93 targets ATG7 in prolactinoma to mediate cabergoline resistance | [107] |
| Diabetic nephropathy | miRNA-93-5p | A total of 48 C57BL/6 mice (age: 6–8 weeks, weight: 20–24 g) were used | The prevention of renal interstitial fibrosis in DN was enhanced by silenced XIST causing miRNA-93-5p-dependent CDKN1A suppression, suggesting a potential future method for DN progression prevention | [108] |
Studies in clinical samples
According to evidence from recent studies, the expression of miRNA-93 varies among various human datasets. Research on these samples shows that changes in miRNA-93 expression are associated either positively or negatively with its target genes (Table 6). For instance, hsa-miRNA-93-5p expression and MMP-3 promoter methylation were found to be potential biomarkers for the etiology of RA and the development of the disease, as reported by Celik et al. [22]. Likewise, Qiao et al. found that miRNA-93-5p is negatively targeted by circRNA 0,048,211 in order to upregulate BMP2, which increases the progression of postmenopausal osteoporosis [109]. Similarly, in peripheral arterial disease, Shu et al. demonstrated that miRNA-93 promotes endothelial cell proliferation, migration, and tube formation, which is linked to decreased CDKN1A expression and is a factor in angiogenesis [110]. Moreover, Qiao et al. investigated that circRNA_0048211 was overexpressed, which increased RUNX family transcription factor 2 (RUNX2), osteopontin (OPN), and osteocalcin (OCN) and increased ALP activity. MiRNA-93-5p has a direct target in BMP2 and could be sponged by CircRNA 0,048,211. As a result, circRNA 0,048,211 prevents postmenopausal osteoporosis [109]. Furthermore, Chen et al. revealed that, through direct targeting of the GLUT4 3' UTR in adipocytes, miRNA-93 overexpression caused glucose transporter type 4 (GLUT4) gene expression to be downregulated, whereas miRNA-93 activity inhibition caused GLUT4 expression to be upregulated. These findings show that miRNA-93 expression is elevated in all PCOS patients as well as in non-PCOS women who have IR, potentially explaining the IR of the disease [111]. Although, they suggest a unique method for controlling insulin-stimulated glucose uptake via miRNA-93.
Table 6.
Studies of clinical samples investigate the effect of miRNA-93 in diseases that are not malignant
| Disease type | miRNA type | Number of clinical samples | Targets/pathways | Expression | Function | Ref |
|---|---|---|---|---|---|---|
| Polycystic ovary syndrome | miRNA-93 | 25- PCOS and biochemical hyperandrogenemia | - | Upregulated | MiRNA-93 levels were higher in the blood of PCOS patients who had higher insulin and testosterone levels | [112] |
| miRNA-93 | 41 subjects (20 control and 21 PCOS) | GLUT4 | Upregulated | In PCOS-associated adipose tissue (AT), overexpression of miRNA-93 decreases GLUT4 expression | [111] | |
| Postmenopausal osteoporosis | miRNA-93-5p | Patients with PMOP (n = 30) and controls (n = 30) |
CircRNA_0048211/ miRNA-93- 5p/BMP2 |
Downregulated (by circRNA_0048211) | MiRNA-93-5p is negatively targeted by CircRNA 004,211, which increases BMP2 and slows the course of PMOP | [109] |
| Peripheral arterial disease | miRNA-93 | 146 sample with PAD (79 male and 67 female) | CDKN1A | Upregulated | MiRNA-93 increases angiogenesis by increasing EA. hy926 endothelial cell proliferation, migration, and tube formation, which decreases CDKN1A expression | [110] |
| Mild Head Trauma | miRNA 93 | 59 sample and 91 controls | - | Upregulated | Validity of Serum miRNA 93 Can Reduce the Need for CT scans in Patients with Mild Head Injury | [113] |
| Multiple blunt trauma | miRNA-93 | A total of 60 healthy controls and 50 consecutive persons with MBT who are matched for age and sex | - | Upregulated | In individuals with multiple traumas, miRNA-93 may be a helpful biomarker for assessing the severity of the injuries | [114] |
| Periodontitis | miRNA-93 | 3 sample | HIF-1α, NFAT5 | Upregulated | MiRNA-93 expression increased in periodontitis patients, whereas NFAT5 mRNA expression decreased. Additionally, hypoxic environments cause GMSCs to increase HIF-1 expression | [115] |
| Chronic kidney disease | miRNA-93-5p | 67 CKD patients with KT, 73 patients with CKD stages 3 to 5, and 36 healthy controls | - | Downregulated | Levels of miRNA-93-5p are linked to CKD stage, inflammation, and bone metrics | [116] |
| Rheumatoid arthritis | miRNA-93-5p | 49 RA sample and 38 controls | MMP-3, IL-16 | Upregulated | MMP-3 promoter methylation and miRNA-93-5p expression levels may serve as helpful biomarkers for the pathophysiology of RA | [22] |
miRNA-93 related signaling pathways in human disorders
miRNA-93 can target different signaling pathways and induce the progression of the vast majority of malignancies [117]. Wu et al. showed that miRNA-93-5p produces drug resistance in pancreatic cancer (PCa) cells and promotes cancer growth using the PTEN-mediated PI3K/Akt signaling pathway [30]. Furthermore, the AKT/mTOR/VEGF pathway might be regulated by miR-93 in AML. For example, it was reported that mTOR is involved in AML's tumor-associated angiogenesis, vascular endothelial growth factor (VEGF) production, and leukemic cell proliferation [118]. The molecular analysis showed that miR-93 was found to suppress AKT's phosphorylation [119]. Additionally, miR-93 induced PI3K/AKT signaling, which facilitated the proliferation, invasion, and metastasis of cancer cells [120, 121]. The miR-93/PTEN/AKT signaling pathway has been linked to drug resistance in cancer cells when miR-93 is overexpressed [122]. AML cells also have an increased PI3K-Akt-mTOR signaling pathway, which eventually contributes to the metabolic remodeling of AML [123].
Additionally, in cancer cells, miRNA-93 enhances TGF-β and induces epithelial-to-mesenchymal transformation through specific gene targeting. QU et al. showed that miRNA-93 overexpression in LC cells facilitated TGF-induced EMT by suppressing NEDD4L [124]. Reducing NEDD4L improves TGF- signal transduction and promotes TGF-β induced EMT by protecting activated SMAD2/SMAD3 from degradation [124]. Likewise, by stimulating the Hippo pathway, miRNA-93-5p improves the proliferative, migratory, and invasive properties of GC cells. The Hippo pathway could be inhibited by miRNA-93-5p overexpression, whereas miRNA-93-5p knockdown may potentially promote Hippo signaling [125]. The protein levels of Hippo pathway regulators, protocadherin fat 4 (AKA, cadherin family member 14 (CDHF14)), and Large Tumor Suppressor Kinase 2 (LATS2) have also been shown to be repressed by the upregulation of miRNA-93-5p, which may be used as a diagnostic and therapeutic target for GC [125].
On the other hand, miRNA-93 inhibits the progression of a number of malignancies by impeding some signaling pathways, such as the Wnt/β-catenin pathway. For instance, using an experiment that measures the expression of β-catenin, axin, c-Myc, and cyclin-D1, Tang and his colleagues found evidence that miRNA-93 may downregulate the Wnt/β-catenin pathway in CRC cells [126].
Moreover, in vitro miRNA-93 overexpression greatly reduced the ability of BC cells to invade and proliferate in 3D organoids, and it reduced their capacity to spread to the liver in vivo [127].
Furthermore, Shang et al. found that through the TLR4/NF-β signaling pathway, miRNA-93 controls neurological function, cerebral edema, and neuronal death in rats with intracerebral hemorrhage [128].
In breast tumors, miRNA-93 acts as a metastasis inhibitor by repressing invasion and stem cell characteristics. Shibuya et al. found that the protein level of WASF3 in BC cells was decreased by miR-93 overexpression, and WASF3 restored the miR-93-mediated inhibition of BC development [129].
Taken together, these studies suggest that miRNA-93 is responsible for regulating signaling pathways in the tumor growth of human malignancies, and it can be used as a possible therapeutic target.
MiRNA-93 and drug resistance
Drug resistance remains a significant health concern that restricts the effectiveness of cancer chemotherapy [130] and is a significant issue in the care of cancer patients. miRNAs play a significant role in tumor growth and therapeutic resistance [131]. Further, cancer chemotherapeutic resistance is a major challenge to the fight cancer disease. According to the statistics, over 90% of cancer patient mortality is associated with drug resistance [132]. Cancer drug resistance can be developed by numerous factors, including reduced anticancer drug absorption, changed drug targets, altered cell cycle checkpoints, and enhanced DNA damage repair. Many researches have revealed that miRNAs target drug-resistance genes or influence cell growth, and apoptosis genes to make cancer cell therapy resistant.
One of these, miRNA-93, is essential for the development of drug resistance in a variety of malignancies by interacting either with coding genes [133] or non-coding genes [40], as well as by altering the targeted pathways [134]. For instance, Wu et al. revealed that miRNA-93-5p induces resistance to gemcitabine via targeting the PTEN-mediated PI3K/Akt signaling pathway in pancreatic cancer (PCa) cells [30]. Likewise, miRNA-93 promotes cabergoline resistance in prolactinoma through targeting ATG7 [107]. Further, lncRNA CTBP1-AS2 induces the activation of the TGF-β/SMAD2/3 pathway via inhibiting miR-93-5p, thereby accelerating the development of CRC [40]. Similarly, upregulated miRNA-93 induces cisplatin-resistant ovarian cancer cells through directly targeting PTEN, which in turn co-regulates the PTEN/Akt signaling pathway [131]. Additionally, tumor initiating cells (T-ICs) play an important role in tumor development, metastasis, recurrence, and drug resistance in liver cancer [135]. Li et al. demonstrated that miRNA-93 was significantly upregulated and regulated liver T-ICs by binding to the 3′-UTR of myotubularin-related protein 3 (MTMR3) in cisplatin- or sorafenib-resistant liver cancer tissues [56]. Furthermore, it has been demonstrated that miRNA-93 plays a role in the development of MDR in prolactinoma [136] and ovarian cancer cells [131]. Interestingly, Hu et al. showed that elevated miRNA-93 expression is correlated with BC resistance, and they proved that miRNA-93 expression is controlled by DNA demethylation [137].
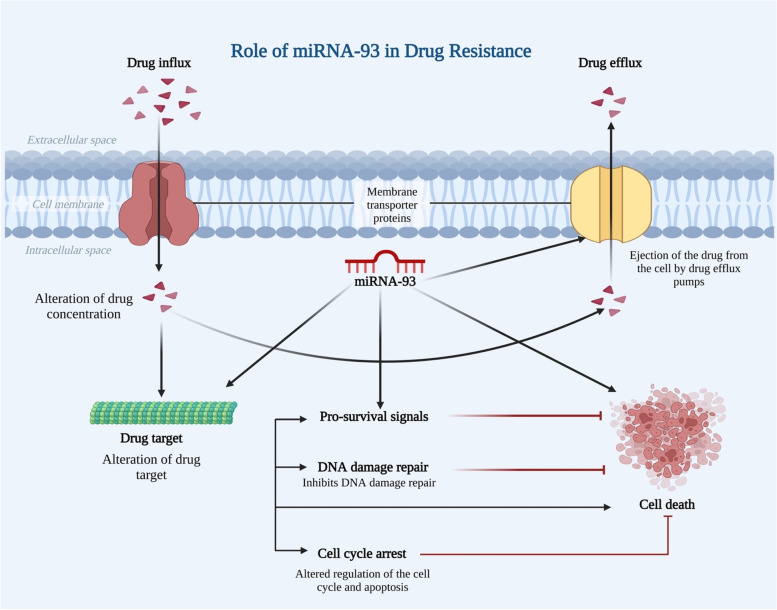
According to the above data, miRNA-93 plays a significant role in driving therapeutic resistance in various cancer types; nevertheless, additional research will be needed to understand the mechanisms that miRNA-93 in cancer patients triggers to resist chemotherapy (Fig. 5).
Fig. 5.
Illustration shows the role of miRNA-93 in drug resistance through different mechanisms such as alteration of drug concentration, drug target, cell cycle and apoptosis, and inhibition of DNA damage repair mechanisms
Discussion
Recent studies indicate that miRNAs have significant roles in human disorders as well as the initiation, development, and metastasis of cancer, which are expressed abnormally [138, 139]. Multiple cancers have abnormal miRNA-93 levels, and their expression levels are associated with a poor prognosis [60]. Furthermore, miRNA-93 is significantly dysregulated in chemo-resistant cell lines, animal models, and clinical tumor samples.
Several studies revealed that upregulated levels of lncRNAs and circRNAs can target miRNA-93 or specific parts of this miRNA. For example miRNA-93 has been found to be sponged by some lncRNAs and circRNAs, namely lncRNA PTENP1 [140], lncRNA H19 [48], lncRNA-XIST [141], LINC01116 [142], lncRNA MEG3 [143], LINC00472 [144], lncRNA SNHG14 [145], lncRNA AWPPH [96], lncRNA CA3-AS1 [146], lncRNA ASMTL-AS1 [89], lncRNA ZNF667‐AS1 [147], and lncRNA SNHG16 [148], circRNF13 [149], cESRP1 [150], circRNA VPRBP [151]. These findings highlighted the complexity of the network that miRNA-93 uses to carry out its actions. Along with chromosomal polymorphisms at the miRNA-93 gene locus, it is thought that abnormal up-regulation of the circRNAs or lncRNAs could cause miRNA-93 to be turned down. As a result, the up-regulation of lncRNAs and circRNAs that sponge miRNA-93 is a well-known mechanism for its downregulation in many cancers. Although loss in the genomic region that codes for miRNA-93 is a putative explanation, the mechanism behind miRNA-93's down-regulation in malignant tissues is not fully understood.
Furthermore, the link between miRNA-93 expression levels and patient outcomes shows how this miRNA could be used as a biomarker to predict how well a cancer patient will do. However, miRNA-93 performs diverse roles in different cancers; therefore, the patterns and orientations of these relationships depend on the roles that miRNA-93 plays in each cancer type.
Additionally, miRNA-93 plays a crucial role in the pathophysiology of illnesses that are not cancerous, such as atherosclerosis, hepatic injury, diabetic nephropathy, rheumatoid arthritis, prolactinoma, osteoarthritis, Parkinson’s disease, rheumatoid arthritis, and acute myocardial infarction. However, the diagnostic use of this miRNA is complicated by the dysregulation of miRNA-93 in cancerous and non- cancerous diseases originating from a particular tissue.
Meanwhile, the best explanation for how this miRNA contributes to the pathophysiology of both malignant and non-malignant illnesses is provided by its substantial role in the regulation of signaling pathways that control cell proliferation and death.
Conclusions
miRNA-93 is an example of a miRNA having tissue-specific effects on cancer development. miRNA-93 may be useful in the clinical diagnosis and prognosis of cancer, which play a role in the progression of malignancy and chemotherapy resistance. Its role in this process depends on the type of tissue, because it can help cancer cells grow in some tissues and stop them in others. However, studies on miRNA-93 have been conducted in cell lines, animal models, and clinical samples in malignant and non-malignant conditions. In cell line studies of malignant condition, miRNA-93 has been shown to regulate various cellular processes such as cell proliferation, migration, and apoptosis. Although, animal studies have also explored the role of miRNA-93 dysregulation in different cancers by using different animal models. In clinical samples, miRNA-93 has been found to be dysregulated in various cancers and may serve as a potential biomarker for diagnosis and prognosis. In cancer cell lines and animal models of the disease, targeting miRNA-93 has been shown to be a practical and effective way to stop cancer cells from spreading and reduce the size of tumors. In non-malignant conditions, miRNA-93 has also been shown to play a role in various diseases based on cell line, animal, and clinical studies. Further, studies on miRNA-93 suggested that it plays a role in various cellular processes and may be a promising target for the development of novel therapies in both malignant and non-malignant conditions. The viability of these strategies in a clinical environment has not yet been assessed. To expand new insights into this area, additional study is required.
Acknowledgements
The authors would like to thank the clinical Research Development Unit (CRDU) of Loghman Hakim Hospital, Shahid Beheshti University of Medical Sciences, Tehran, Iran for their support, cooperation and assistance throughout the period of study.
Authors’ contributions
BMH and MFR wrote the draft and revised it. MT and AK designed and supervised the study. SRA, ZHJ and GSHF collected the data and designed the figures and tables. All the authors read the submitted version and approved it.
Funding
Not applicable.
Availability of data and materials
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participant
Not applicable.
Consent of publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Arda Kiani, Email: ardakiani@sbmu.ac.ir.
Mohammad Taheri, Email: mohammad.taheri@uni-jena.de.
References
- 1.Hussen BM, Hidayat HJ, Salihi A, Sabir DK, Taheri M, Ghafouri-Fard S. MicroRNA: a signature for cancer progression. Biomed Pharmacother. 2021;138:111528. doi: 10.1016/j.biopha.2021.111528. [DOI] [PubMed] [Google Scholar]
- 2.Dozier C, Plaza S. Functions of animal microRNA-encoded peptides: the race is on! EMBO reports. 2022;23(5):e54789. doi: 10.15252/embr.202254789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Rooij LA, Mastebroek DJ, Ten Voorde N, van der Wall E, van Diest PJ, Moelans CB. The microRNA Lifecycle in Health and Cancer. Cancers. 2022;14(23):5748. doi: 10.3390/cancers14235748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vilimova M, Pfeffer S. Post‐transcriptional regulation of polycistronic microRNAs. Wiley Interdisciplinary Reviews: RNA. 2023;14(2):e1749. [DOI] [PubMed]
- 5.Inui M, Martello G, Piccolo S. MicroRNA control of signal transduction. Nat Rev Mol Cell Biol. 2010;11(4):252–263. doi: 10.1038/nrm2868. [DOI] [PubMed] [Google Scholar]
- 6.Shi X, Yang H, Birchler JA. MicroRNAs play regulatory roles in genomic balance. BioEssays. 2023;45(2):2200187. [DOI] [PubMed]
- 7.Olcum M, Tufekci KU, Genc S. MicroRNAs in genetic etiology of human diseases. miRNomics: MicroRNA Biology and Computational Analysis. 2022. pp. 255-68. [DOI] [PubMed]
- 8.Fang S, Gao M, Xiong S, Chen Q, Zhang H. Expression of serum Hsa-miR-93 in uterine cancer and its clinical significance. Oncol Lett. 2018;15(6):9896–9900. doi: 10.3892/ol.2018.8553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sperling R. Small non-coding RNA within the endogenous spliceosome and alternative splicing regulation. Biochim Biophys Acta Gene Regul Mech. 2019;1862(11-12):194406. doi: 10.1016/j.bbagrm.2019.07.007. [DOI] [PubMed] [Google Scholar]
- 10.Petrocca F, Vecchione A, Croce CM. Emerging role of miR-106b-25/miR-17-92 clusters in the control of transforming growth factor β signaling. Can Res. 2008;68(20):8191–8194. doi: 10.1158/0008-5472.CAN-08-1768. [DOI] [PubMed] [Google Scholar]
- 11.Zhang R, Li F, Wang W, Wang X, Li S, Liu J. The effect of antisense inhibitor of miRNA 106b∼ 25 on the proliferation, invasion, migration, and apoptosis of gastric cancer cell. Tumor Biology. 2016;37:10507–10515. doi: 10.1007/s13277-016-4937-x. [DOI] [PubMed] [Google Scholar]
- 12.Li N, Miao Y, Shan Y, Liu B, Li Y, Zhao L, et al. MiR-106b and miR-93 regulate cell progression by suppression of PTEN via PI3K/Akt pathway in breast cancer. Cell Death Dis. 2017;8(5):e2796-e. doi: 10.1038/cddis.2017.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qu M-H, Han C, Srivastava AK, Cui T, Zou N, Gao Z-Q, et al. miR-93 promotes TGF-β-induced epithelial-to-mesenchymal transition through downregulation of NEDD4L in lung cancer cells. Tumor Biology. 2016;37:5645–5651. doi: 10.1007/s13277-015-4328-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang SJ, Cao YF, Yang ZQ, Jiang ZY, Cai B, Guo J, et al. MicroRNA-93-5p increases multidrug resistance in human colorectal carcinoma cells by downregulating cyclin dependent kinase inhibitor 1A gene expression. Oncol Lett. 2017;13(2):722–730. doi: 10.3892/ol.2016.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu J-J, Zhang X, Wu X-H. miR-93 promotes the growth and invasion of prostate cancer by upregulating its target genes TGFBR2, ITGB8, and LATS2. Mol Ther Oncolytics. 2018;11:14–19. doi: 10.1016/j.omto.2018.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vila-Navarro E, Fernandez-Castañer E, Rovira-Rigau M, Raimondi G, Vila-Casadesus M, Lozano JJ, et al. MiR-93 is related to poor prognosis in pancreatic cancer and promotes tumor progression by targeting microtubule dynamics. Oncogenesis. 2020;9(5):43. doi: 10.1038/s41389-020-0227-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang L, Huang J, Li L, Chen Y, Chen X, Zhao X, et al. MicroRNA-93 promotes ovarian granulosa cells proliferation through targeting CDKN1A in polycystic ovarian syndrome. J Clin Endocrinol Metab. 2015;100(5):E729–E738. doi: 10.1210/jc.2014-3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liang L, Zhao L, Zan Y, Zhu Q, Ren J, Zhao X. MiR-93-5p enhances growth and angiogenesis capacity of HUVECs by down-regulating EPLIN. Oncotarget. 2017;8(63):107033. doi: 10.18632/oncotarget.22300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fang L, Deng Z, Shatseva T, Yang J, Peng C, Du W, et al. MicroRNA miR-93 promotes tumor growth and angiogenesis by targeting integrin-β8. Oncogene. 2011;30(7):806–821. doi: 10.1038/onc.2010.465. [DOI] [PubMed] [Google Scholar]
- 20.Shibuya N, Kakeji Y, Shimono Y. MicroRNA-93 targets WASF3 and functions as a metastasis suppressor in breast cancer. Cancer Sci. 2020;111(6):2093–2103. doi: 10.1111/cas.14423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Meng Y, Qiu S, Sun L, Zuo J. Knockdown of exosome-mediated lnc-PVT1 alleviates lipopolysaccharide-induced osteoarthritis progression by mediating the HMGB1/TLR4/NF-κB pathway via miR-93-5p. Mol Med Rep. 2020;22(6):5313–5325. doi: 10.3892/mmr.2020.11594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Celik ZB, Tural S, Cengiz AK, Kara N, Alayli G. Upregulation of microRNA-93-5p/microRNA-4668-5p, and promoter methylation of matrix metalloproteinase-3 and interleukin-16 genes in Turkish patients with rheumatoid arthritis. The Egyptian Rheumatologist. 2021;43(1):35–39. doi: 10.1016/j.ejr.2020.06.004. [DOI] [Google Scholar]
- 23.He Y, Lin L, Cao J, Mao X, Qu Y, Xi B. Up-regulated miR-93 contributes to coronary atherosclerosis pathogenesis through targeting ABCA1. Int J Clin Exp Med. 2015;8(1):674. [PMC free article] [PubMed] [Google Scholar]
- 24.Li N, Muthusamy S, Liang R, Sarojini H, Wang E. Increased expression of miR-34a and miR-93 in rat liver during aging, and their impact on the expression of Mgst1 and Sirt1. Mech Ageing Dev. 2011;132(3):75–85. doi: 10.1016/j.mad.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 25.Wang X, Liu Z, Wang F. MicroRNA-93 Blocks Signal Transducers and Activator of Transcription 3 to Reduce Neuronal Damage in Parkinson’s Disease. Neurochem Res. 2021;46(7):1859–1868. doi: 10.1007/s11064-021-03333-x. [DOI] [PubMed] [Google Scholar]
- 26.Lv J, Zhu Y, Yao S. LncRNAMORT is upregulated in myocardial infarction and promotes the apoptosis of cardiomyocyte by downregulating miR-93. BMC Cardiovasc Disord. 2020;20(1):1–7. doi: 10.1186/s12872-020-01522-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Peng J, Qin C, Tian S-Y, Peng J-Q. MiR-93 inhibits the vascular calcification of chronic renal failure by suppression of Wnt/β-catenin pathway. Int Urol Nephrol. 2022;54(1):225–235. doi: 10.1007/s11255-021-02907-6. [DOI] [PubMed] [Google Scholar]
- 28.Fadda S, Abolkheir E, Afifi R, Gamal M. Serum matrix metalloproteinase-3 in rheumatoid arthritis patients: Correlation with disease activity and joint destruction. Egypt Rheumatol. 2016;38(3):153–159. doi: 10.1016/j.ejr.2016.01.001. [DOI] [Google Scholar]
- 29.Jing W, Jiang W. MicroRNA-93 regulates collagen loss by targeting MMP3 in human nucleus pulposus cells. Cell Prolif. 2015;48(3):284–292. doi: 10.1111/cpr.12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu Y, Xu W, Yang Y, Zhang Z. miRNA-93-5p promotes gemcitabine resistance in pancreatic cancer cells by targeting the PTEN-mediated PI3K/Akt signaling pathway. Ann Clin Lab Sci. 2021;51(3):310–320. [PubMed] [Google Scholar]
- 31.Fu X, Tian J, Zhang L, Chen Y, Hao Q. Involvement of microRNA-93, a new regulator of PTEN/Akt signaling pathway, in regulation of chemotherapeutic drug cisplatin chemosensitivity in ovarian cancer cells. FEBS Lett. 2012;586(9):1279–1286. doi: 10.1016/j.febslet.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 32.Chu S, Liu G, Xia P, Chen G, Shi F, Yi T, et al. miR-93 and PTEN: Key regulators of doxorubicin-resistance and EMT in breast cancer. Oncol Rep. 2017;38(4):2401–2407. doi: 10.3892/or.2017.5859. [DOI] [PubMed] [Google Scholar]
- 33.Hu J, Xu J, Wu Y, Chen Q, Zheng W, Lu X, et al. Identification of microRNA-93 as a functional dysregulated miRNA in triple-negative breast cancer. Tumor Biology. 2015;36(1):251–258. doi: 10.1007/s13277-014-2611-8. [DOI] [PubMed] [Google Scholar]
- 34.Shyamasundar S, Lim JP, Bay BH. miR-93 inhibits the invasive potential of triple-negative breast cancer cells in vitro via protein kinase WNK1. Int J Oncol. 2016;49(6):2629–2636. doi: 10.3892/ijo.2016.3761. [DOI] [PubMed] [Google Scholar]
- 35.Feng S, Gao L, Zhang D, Tian X, Kong L, Shi H, et al. MiR-93 regulates vascular smooth muscle cell proliferation, and neointimal formation through targeting Mfn2. Int J Biol Sci. 2019;15(12):2615. doi: 10.7150/ijbs.36995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O'Sullivan JF, Neylon A, Fahy EF, Yang P, McGorrian C, Blake GJ. MiR-93-5p is a novel predictor of coronary in-stent restenosis. Heart Asia. 2019;11(1):e011134. doi: 10.1136/heartasia-2018-011134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feng S, Gao L, Zhang D, Tian X, Kong L, Shi H, et al. MiR-93 regulates vascular smooth muscle cell proliferation, and neointimal formation through targeting Mfn2. Int J Biol Sci. 2019;15(12):2615–2626. doi: 10.7150/ijbs.36995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feng X, Zhou S, Cai W, Guo J. The miR-93-3p/ZFP36L1/ZFX axis regulates keratinocyte proliferation and migration during skin wound healing. Molecular Therapy-Nucleic Acids. 2021;23:450–463. doi: 10.1016/j.omtn.2020.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yang I-P, Tsai H-L, Hou M-F, Chen K-C, Tsai P-C, Huang S-W, et al. MicroRNA-93 inhibits tumor growth and early relapse of human colorectal cancer by affecting genes involved in the cell cycle. Carcinogenesis. 2012;33(8):1522–1530. doi: 10.1093/carcin/bgs166. [DOI] [PubMed] [Google Scholar]
- 40.Li Q, Yue W, Li M, Jiang Z, Hou Z, Liu W, et al. Downregulating long Non-coding RNAs CTBP1-AS2 inhibits colorectal cancer development by modulating the miR-93-5p/TGF-β/SMAD2/3 pathway. Front Oncol. 2021;11:626620. doi: 10.3389/fonc.2021.626620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qu M-H, Han C, Srivastava AK, Cui T, Zou N, Gao Z-Q, et al. miR-93 promotes TGF-β-induced epithelial-to-mesenchymal transition through downregulation of NEDD4L in lung cancer cells. Tumor Biology. 2016;37(4):5645–5651. doi: 10.1007/s13277-015-4328-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xiang Y, Liao X-H, Yu C-X, Yao A, Qin H, Li J-P, et al. MiR-93-5p inhibits the EMT of breast cancer cells via targeting MKL-1 and STAT3. Exp Cell Res. 2017;357(1):135–144. doi: 10.1016/j.yexcr.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 43.Du L, Schageman JJ, Subauste MC, Saber B, Hammond SM, Prudkin L, et al. miR-93, miR-98, and miR-197 regulate expression of tumor suppressor gene FUS1. Mol Cancer Res. 2009;7(8):1234–1243. doi: 10.1158/1541-7786.MCR-08-0507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang W, Bai J, Liu D, Wang S, Zhao N, Che R, et al. MiR-93-5p up-regulation is involved in non-small cell lung cancer cells proliferation and migration and poor prognosis. Gene. 2018;647:13–20. doi: 10.1016/j.gene.2018.01.024. [DOI] [PubMed] [Google Scholar]
- 45.Du L, Zhao Z, Ma X, Hsiao T-H, Chen Y, Young E, et al. miR-93-directed downregulation of DAB2 defines a novel oncogenic pathway in lung cancer. Oncogene. 2014;33(34):4307–4315. doi: 10.1038/onc.2013.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li C, Lyu J, Meng QH. MiR-93 promotes tumorigenesis and metastasis of non-small cell lung cancer cells by activating the PI3K/Akt pathway via inhibition of LKB1/PTEN/CDKN1A. J Cancer. 2017;8(5):870. doi: 10.7150/jca.17958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shi J, Jiang X, Yu Z, He G, Ning H, Wu Z, et al. ZNRF3 contributes to the growth of lung carcinoma via inhibiting Wnt/β-catenin pathway and is regulated by miR-93. Tumor Biology. 2016;37(3):3051–3057. doi: 10.1007/s13277-015-3949-2. [DOI] [PubMed] [Google Scholar]
- 48.Li JP, Xiang Y, Fan LJ, Yao A, Li H, Liao XH. Long noncoding RNA H19 competitively binds miR-93-5p to regulate STAT3 expression in breast cancer. J Cell Biochem. 2019;120(3):3137–3148. doi: 10.1002/jcb.27578. [DOI] [PubMed] [Google Scholar]
- 49.Fang L, Du WW, Yang W, Rutnam ZJ, Peng C, Li H, et al. MiR-93 enhances angiogenesis and metastasis by targeting LATS2. Cell Cycle. 2012;11(23):4352–4365. doi: 10.4161/cc.22670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen X, Liu J, Zhang Q, Liu B, Cheng Y, Zhang Y, et al. Exosome-mediated transfer of miR-93-5p from cancer-associated fibroblasts confer radioresistance in colorectal cancer cells by downregulating FOXA1 and upregulating TGFB3. J Exp Clin Cancer Res. 2020;39(1):1–15. doi: 10.1186/s13046-019-1507-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tang Q, Zou Z, Zou C, Zhang Q, Huang R, Guan X, et al. MicroRNA-93 suppress colorectal cancer development via Wnt/β-catenin pathway downregulating. Tumor Biology. 2015;36(3):1701–1710. doi: 10.1007/s13277-014-2771-6. [DOI] [PubMed] [Google Scholar]
- 52.Chen YL, Wang GX, Lin BA, Huang JS. MicroRNA-93-5p expression in tumor tissue and its tumor suppressor function via targeting programmed death ligand-1 in colorectal cancer. Cell Biol Int. 2020;44(5):1224–1236. doi: 10.1002/cbin.11323. [DOI] [PubMed] [Google Scholar]
- 53.Wei H, Yang Z, Lin B. Overexpression of long non coding RNA CA3-AS1 suppresses proliferation, invasion and promotes apoptosis via miRNA-93/PTEN axis in colorectal cancer. Gene. 2019;687:9–15. doi: 10.1016/j.gene.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 54.Xue X, Wang X, Zhao Y, Hu R, Qin L. Exosomal miR-93 promotes proliferation and invasion in hepatocellular carcinoma by directly inhibiting TIMP2/TP53INP1/CDKN1A. Biochem Biophys Res Commun. 2018;502(4):515–521. doi: 10.1016/j.bbrc.2018.05.208. [DOI] [PubMed] [Google Scholar]
- 55.Ji C, Liu H, Yin Q, Li H, Gao H. miR-93 enhances hepatocellular carcinoma invasion and metastasis by EMT via targeting PDCD4. Biotech Lett. 2017;39(11):1621–1629. doi: 10.1007/s10529-017-2403-5. [DOI] [PubMed] [Google Scholar]
- 56.Li J, Zhong X, Wang X, Xu F, Yang J, Lu J, et al. miR-93 regulates liver tumor initiating cells expansion and predicts chemotherapeutic response of patients. Arch Biochem Biophys. 2021;703:108871. doi: 10.1016/j.abb.2021.108871. [DOI] [PubMed] [Google Scholar]
- 57.Wang X, Liao Z, Bai Z, He Y, Duan J, Wei L. MiR-93-5p promotes cell proliferation through down-regulating PPARGC1A in hepatocellular carcinoma cells by bioinformatics analysis and experimental verification. Genes. 2018;9(1):51. doi: 10.3390/genes9010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Huang H, Wang X, Wang C, Zhuo L, Luo S, Han S. The miR-93 promotes proliferation by directly targeting PDCD4 in hepatocellular carcinoma. Neoplasma. 2017;64(5):770–777. doi: 10.4149/neo_2017_516. [DOI] [PubMed] [Google Scholar]
- 59.Shi X, Liu T-T, Yu X-N, Balakrishnan A, Zhu H-R, Guo H-Y, et al. microRNA-93-5p promotes hepatocellular carcinoma progression via a microRNA-93-5p/MAP3K2/c-Jun positive feedback circuit. Oncogene. 2020;39(35):5768–5781. doi: 10.1038/s41388-020-01401-0. [DOI] [PubMed] [Google Scholar]
- 60.Ohta K, Hoshino H, Wang J, Ono S, Iida Y, Hata K, et al. MicroRNA-93 activates c-Met/PI3K/Akt pathway activity in hepatocellular carcinoma by directly inhibiting PTEN and CDKN1A. Oncotarget. 2015;6(5):3211. doi: 10.18632/oncotarget.3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ma D-H, Li B-S, Liu J-J, Xiao Y-F, Yong X, Wang S-M, et al. miR-93-5p/IFNAR1 axis promotes gastric cancer metastasis through activating the STAT3 signaling pathway. Cancer Lett. 2017;408:23–32. doi: 10.1016/j.canlet.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 62.Liang H, Wang F, Chu D, Zhang W, Liao Z, Fu Z, et al. miR-93 functions as an oncomiR for the downregulation of PDCD4 in gastric carcinoma. Sci Rep. 2016;6(1):1–11. doi: 10.1038/srep23772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li L, Zhao J, Huang S, Wang Y, Zhu L, Cao Y, et al. MiR-93-5p promotes gastric cancer-cell progression via inactivation of the Hippo signaling pathway. Gene. 2018;641:240–247. doi: 10.1016/j.gene.2017.09.071. [DOI] [PubMed] [Google Scholar]
- 64.Guan H, Li W, Li Y, Wang J, Li Y, Tang Y, et al. MicroRNA-93 promotes proliferation and metastasis of gastric cancer via targeting TIMP2. PLoS One. 2017;12(12):e0189490. doi: 10.1371/journal.pone.0189490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shen E, Wang X, Liu X, Lv M, Zhang L, Zhu G, et al. MicroRNA-93-5p promotes epithelial-mesenchymal transition in gastric cancer by repressing tumor suppressor AHNAK expression. Cancer Cell Int. 2020;20(1):1–13. doi: 10.1186/s12935-019-1092-7. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 66.Liu ZM, Yang XL, Jiang F, Pan YC, Zhang L. Matrine involves in the progression of gastric cancer through inhibiting miR-93-5p and upregulating the expression of target gene AHNAK. J Cell Biochem. 2020;121(3):2467–2477. doi: 10.1002/jcb.29469. [DOI] [PubMed] [Google Scholar]
- 67.Vila-Navarro E, Fernandez-Castañer E, Rovira-Rigau M, Raimondi G, Vila-Casadesus M, Lozano JJ, et al. MiR-93 is related to poor prognosis in pancreatic cancer and promotes tumor progression by targeting microtubule dynamics. Oncogenesis. 2020;9(5):1–14. doi: 10.1038/s41389-020-0227-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cheng Y, Yang H, Sun Y, Zhang H, Yu S, Lu Z, et al. RUNX1 promote invasiveness in pancreatic ductal adenocarcinoma through regulating miR-93. Oncotarget. 2017;8(59):99567. doi: 10.18632/oncotarget.20433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lin H, Shi X, Li H, Hui J, Liu R, Chen Z, et al. Urinary Exosomal miRNAs as biomarkers of bladder Cancer and experimental verification of mechanism of miR-93-5p in bladder Cancer. BMC Cancer. 2021;21(1):1–17. doi: 10.1186/s12885-021-08926-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jiang H, Bu Q, Zeng M, Xia D, Wu A. MicroRNA-93 promotes bladder cancer proliferation and invasion by targeting PEDF. In Urologic oncology: seminars and original investigations. 2019;37(2):150-7. [DOI] [PubMed]
- 71.Li T, Xu Q, Wei Y, Lin R, Hong Z, Zeng R, Hu W, Wu X. Overexpression of miRNA-93-5p promotes proliferation and migration of bladder urothelial carcinoma via inhibition of KLF9. Comput Math Methods Med. 2022. p. 2022. [DOI] [PMC free article] [PubMed] [Retracted]
- 72.Liu J, Wang Y, Li Z, Pan Y, Liu Q, Yang M, Wang J. Repression of the miR-93-enhanced sensitivity of bladder carcinoma to chemotherapy involves the regulation of LASS2. Onco Targets Ther. 2016. pp. 1813-22. [DOI] [PMC free article] [PubMed]
- 73.Sun X, Han X, Zhao X, Cheng X, Zhang Y. MiR-93-5p promotes cervical cancer progression by targeting THBS2/MMPS signal pathway. Eur Rev Med Pharmacol Sci. 2019;23(12):5113–5121. doi: 10.26355/eurrev_201906_18175. [DOI] [PubMed] [Google Scholar]
- 74.Li J, Chu Z-P, Han H, Zhang Y, Tian F, Zhang J-Q, et al. Suppression of miR-93-5p inhibits high-risk HPV-positive cervical cancer progression via targeting of BTG3. Hum Cell. 2019;32(2):160–171. doi: 10.1007/s13577-018-00225-1. [DOI] [PubMed] [Google Scholar]
- 75.Zhang X, Li F, Zhu L. Clinical significance and functions of microRNA-93/CDKN1A axis in human cervical cancer. Life Sci. 2018;209:242–248. doi: 10.1016/j.lfs.2018.08.021. [DOI] [PubMed] [Google Scholar]
- 76.Lan L, Liang Z, Zhao Y, Mo Y. LncRNA MCM3AP-AS1 inhibits cell proliferation in cervical squamous cell carcinoma by down-regulating miRNA-93. Biosci Rep. 2020;40(2):BSR20193794. doi: 10.1042/BSR20193794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yang K, Li Y-W, Gao Z-Y, Xiao W, Li T-Q, Song W, et al. MiR-93 functions as a tumor promoter in prostate cancer by targeting disabled homolog 2 (DAB2) and an antitumor polysaccharide from green tea (Camellia sinensis) on their expression. Int J Biol Macromol. 2019;125:557–565. doi: 10.1016/j.ijbiomac.2018.12.088. [DOI] [PubMed] [Google Scholar]
- 78.Jiang L, Wang C, Lei F, Zhang L, Zhang X, Liu A, et al. miR-93 promotes cell proliferation in gliomas through activation of PI3K/Akt signaling pathway. Oncotarget. 2015;6(10):8286. doi: 10.18632/oncotarget.3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen R, Liu H, Cheng Q, Jiang B, Peng R, Zou Q, et al. MicroRNA-93 promotes the malignant phenotypes of human glioma cells and induces their chemoresistance to temozolomide. Biology open. 2016;5(6):669–677. doi: 10.1242/bio.015552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu D, Wei Y, Guo Y, Wang J, Wang G. MiRNA-93 functions as an oncogene in glioma by directly targeting RBL2. Eur Rev Med Pharmacol Sci. 2018;22(8):2343–2350. doi: 10.26355/eurrev_201804_14825. [DOI] [PubMed] [Google Scholar]
- 81.Wu H, Liu L, Zhu J. MiR-93-5p inhibited proliferation and metastasis of glioma cells by targeting MMP2. Eur Rev Med Pharmacol Sci. 2019;23(21):9517–9524. doi: 10.26355/eurrev_201911_19446. [DOI] [PubMed] [Google Scholar]
- 82.Fabbri E, Brognara E, Montagner G, Ghimenton C, Eccher A, Cantù C, et al. Regulation of IL-8 gene expression in gliomas by microRNA miR-93. BMC Cancer. 2015;15(1):1–15. doi: 10.1186/s12885-015-1659-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kawano M, Tanaka K, Itonaga I, Ikeda S, Iwasaki T, Tsumura H. microRNA-93 promotes cell proliferation via targeting of PTEN in Osteosarcoma cells. J Exp Clin Cancer Res. 2015;34(1):1–11. doi: 10.1186/s13046-015-0192-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.He Y, Yu B. MicroRNA-93 promotes cell proliferation by directly targeting P21 in osteosarcoma cells. Exp Ther Med. 2017;13(5):2003–2011. doi: 10.3892/etm.2017.4204. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 85.Zhang H, Zhang J, Meng F, Zhu H, Yan H, Guo Y, et al. MicroRNA-93 promotes the tumorigenesis of osteosarcoma by targeting TIMP2. Biosci Rep. 2019;39(8):BSR20191237. doi: 10.1042/BSR20191237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shi J, Zhuang Y, Liu X, Zhang Y, Zhang Y. TGF-beta induced RBL2 expression in renal cancer cells by down-regulating miR-93. Clin Transl Oncol. 2014;16(11):986–992. doi: 10.1007/s12094-014-1185-7. [DOI] [PubMed] [Google Scholar]
- 87.Cai Y, Ruan W, Ding J, Wei N, Wang J, Zhang H, et al. miR-93-5p regulates the occurrence and development of esophageal carcinoma epithelial cells by targeting TGFβR2. Int J Mol Med. 2021;47(3):1. doi: 10.3892/ijmm.2020.4836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Liu MX, Juan L, Ming X, Gao ZK, Wang XH, Zhang Y, et al. miR-93-5p transferred by exosomes promotes the proliferation of esophageal cancer cells via intercellular communication by targeting PTEN. Biomed Environ Sci. 2018;31(3):171–185. doi: 10.3967/bes2018.023. [DOI] [PubMed] [Google Scholar]
- 89.Feng Z, Chen R, Huang N, Luo C. Long non-coding RNA ASMTL-AS1 inhibits tumor growth and glycolysis by regulating the miR-93-3p/miR-660/FOXO1 axis in papillary thyroid carcinoma. Life Sci. 2020;244:117298. doi: 10.1016/j.lfs.2020.117298. [DOI] [PubMed] [Google Scholar]
- 90.Singh B, Ronghe AM, Chatterjee A, Bhat NK, Bhat HK. MicroRNA-93 regulates NRF2 expression and is associated with breast carcinogenesis. Carcinogenesis. 2013;34(5):1165–1172. doi: 10.1093/carcin/bgt026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen X, Chen S, Xiu Y-L, Sun K-X, Zong Z-H, Zhao Y. RhoC is a major target of microRNA-93-5P in epithelial ovarian carcinoma tumorigenesis and progression. Mol Cancer. 2015;14(1):1–11. doi: 10.1186/s12943-015-0304-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang Z, Ma K, Cheng Y, Abraham JM, Liu X, Ke X, et al. Synthetic circular multi-miR sponge simultaneously inhibits miR-21 and miR-93 in esophageal carcinoma. Lab Invest. 2019;99(10):1442–1453. doi: 10.1038/s41374-019-0273-2. [DOI] [PubMed] [Google Scholar]
- 93.Wang L, Yang G, Zhu X, Wang Z, Wang H, Bai Y, et al. miR-93-3p inhibition suppresses clear cell renal cell carcinoma proliferation, metastasis and invasion. Oncotarget. 2017;8(47):82824. doi: 10.18632/oncotarget.20458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xu J. MicroRNA-93-5p/IFNAR1 axis accelerates metastasis of endometrial carcinoma by activating the STAT3 pathway. Eur Rev Med Pharmacol Sci. 2019;23(13):5657–5666. doi: 10.26355/eurrev_201907_18302. [DOI] [PubMed] [Google Scholar]
- 95.Cao Y, Xia F, Wang P, Gao M. MicroRNA-93-5p promotes the progression of human retinoblastoma by regulating the PTEN/PI3K/AKT signaling pathway. Mol Med Rep. 2018;18(6):5807–5814. doi: 10.3892/mmr.2018.9573. [DOI] [PubMed] [Google Scholar]
- 96.Li C, Wang F, Wei B, Wang L, Kong D. LncRNA AWPPH promotes osteosarcoma progression via activation of Wnt/β-catenin pathway through modulating miR-93-3p/FZD7 axis. Biochem Biophys Res Commun. 2019;514(3):1017–1022. doi: 10.1016/j.bbrc.2019.04.203. [DOI] [PubMed] [Google Scholar]
- 97.Xiao X, Zhou L, Cao P, Gong H, Zhang Y. MicroRNA-93 regulates cyclin G2 expression and plays an oncogenic role in laryngeal squamous cell carcinoma. Int J Oncol. 2015;46(1):161–174. doi: 10.3892/ijo.2014.2704. [DOI] [PubMed] [Google Scholar]
- 98.Chen G, Yan Y, Qiu X, Ye C, Jiang X, Song S, et al. miR-93-5p suppresses ovarian cancer malignancy and negatively regulate CCND2 by binding to its 3′ UTR region. Discover Oncology. 2022;13(1):1–11. doi: 10.1007/s12672-022-00478-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zhang S, He Y, Liu C, Li G, Lu S, Jing Q, et al. miR-93-5p enhances migration and invasion by targeting RGMB in squamous cell carcinoma of the head and neck. J Cancer. 2020;11(13):3871. doi: 10.7150/jca.43854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Xiao Z-G, Deng Z-S, Zhang Y-D, Zhang Y, Huang Z-C. Clinical significance of microRNA-93 downregulation in human colon cancer. Eur J Gastroenterol Hepatol. 2013;25(3):296–301. doi: 10.1097/MEG.0b013e32835c077a. [DOI] [PubMed] [Google Scholar]
- 101.Hao J, Jin X, Shi Y, Zhang H. miR-93-5p enhance lacrimal gland adenoid cystic carcinoma cell tumorigenesis by targeting BRMS1L. Cancer Cell Int. 2018;18(1):1–12. doi: 10.1186/s12935-018-0552-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ansari MH, Irani S, Edalat H, Amin R, Mohammadi RA. Deregulation of miR-93 and miR-143 in human esophageal cancer. Tumor Biology. 2016;37(3):3097–3103. doi: 10.1007/s13277-015-3987-9. [DOI] [PubMed] [Google Scholar]
- 103.Zheng W, Yang J, Wang Y, Liu X. Exosomal miRNA-93 and miRNA-205 expression in endometrial cancer. Journal of King Saud University-Science. 2020;32(1):1111–1115. doi: 10.1016/j.jksus.2019.10.006. [DOI] [Google Scholar]
- 104.Shi C, Zhao Y, Li Q, Li J. lncRNA SNHG14 plays a role in sepsis-induced acute kidney injury by regulating miR-93. Mediators Inflamm. 2021;2021:5318369. doi: 10.1155/2021/5318369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liu J, Jiang M, Deng S, Lu J, Huang H, Zhang Y, et al. miR-93-5p-containing exosomes treatment attenuates acute myocardial infarction-induced myocardial damage. Mol Ther Nucleic Acids. 2018;11:103–115. doi: 10.1016/j.omtn.2018.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Xiong L, Yu K, Zhen S. MiR-93 blocks STAT3 to alleviate hepatic injury after ischemia-reperfusion. Eur Rev Med Pharmacol Sci. 2018;22(16):5295–5304. doi: 10.26355/eurrev_201808_15729. [DOI] [PubMed] [Google Scholar]
- 107.Wu Z, Cai L, Lu J, Wang C, Guan J, Chen X, et al. MicroRNA-93 mediates cabergoline resistance by targeting ATG7 in prolactinoma. J Endocrinol. 2019;240(1):1–13. doi: 10.1530/JOE-18-0203. [DOI] [PubMed] [Google Scholar]
- 108.Yang J, Shen Y, Yang X, Long Y, Chen S, Lin X, et al. Silencing of long noncoding RNA XIST protects against renal interstitial fibrosis in diabetic nephropathy via microRNA-93-5p-mediated inhibition of CDKN1A. Am J Physiol Renal Physiol. 2019;317(5):F1350–F1358. doi: 10.1152/ajprenal.00254.2019. [DOI] [PubMed] [Google Scholar]
- 109.Qiao L, Li C, Liu D. CircRNA_0048211 protects postmenopausal osteoporosis through targeting miRNA-93-5p to regulate BMP2. Eur Rev Med Pharmacol Sci. 2020;24(7):3459–3466. doi: 10.26355/eurrev_202004_20804. [DOI] [PubMed] [Google Scholar]
- 110.Shu X, Mao Y, Li Z, Wang W, Chang Y, Liu S, et al. MicroRNA-93 regulates angiogenesis in peripheral arterial disease by targeting CDKN1A. Mol Med Rep. 2019;19(6):5195–5202. doi: 10.3892/mmr.2019.10196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chen Y-H, Heneidi S, Lee J-M, Layman LC, Stepp DW, Gamboa GM, et al. miRNA-93 inhibits GLUT4 and is overexpressed in adipose tissue of polycystic ovary syndrome patients and women with insulin resistance. Diabetes. 2013;62(7):2278–2286. doi: 10.2337/db12-0963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sathyapalan T, David R, Gooderham NJ, Atkin SL. Increased expression of circulating miRNA-93 in women with polycystic ovary syndrome may represent a novel, non-invasive biomarker for diagnosis. Sci Rep. 2015;5(1):1–8. doi: 10.1038/srep16890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tas D, Kaplan O, Sogut O. Validity of Serum miRNA 93 and miRNA 191 to Reduce Unnecessary Computed Tomography in Patients With Mild Head Trauma. J Clin Med Res. 2020;12(9):579. doi: 10.14740/jocmr4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Söğüt Ö, Metiner M, Kaplan O, Çalık M, Çakmak S, Ümit TB, et al. The utility of serum miRNA-93 and miRNA-191 levels for determining injury severity in adults with multiple blunt trauma. Turkish J Trauma Emerg Surg. 2021;27(6):631–638. doi: 10.14744/tjtes.2020.45470. [DOI] [PubMed] [Google Scholar]
- 115.Chaparro A, Lozano M, Gaedechens D, López C, Albers D, Hernández M, et al. Exploring the expression of pro-inflammatory and hypoxia-related MicroRNA-20a, MicroRNA-30e, and MicroRNA-93 in periodontitis and gingival mesenchymal stem cells under hypoxia. Int J Mol Sci. 2022;23(18):10310. doi: 10.3390/ijms231810310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ulbing M, Kirsch A, Leber B, Lemesch S, Münzker J, Schweighofer N, et al. MicroRNAs 223–3p and 93–5p in patients with chronic kidney disease before and after renal transplantation. Bone. 2017;95:115–123. doi: 10.1016/j.bone.2016.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Niveditha D, Jasoria M, Narayan J, Majumder S, Mukherjee S, Chowdhury R, et al. Common and unique microRNAs in multiple carcinomas regulate similar network of pathways to mediate cancer progression. Sci Rep. 2020;10(1):1–11. doi: 10.1038/s41598-020-59142-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Böhm A, Aichberger KJ, Mayerhofer M, Herrmann H, Florian S, Krauth MT, et al. Targeting of mTOR is associated with decreased growth and decreased VEGF expression in acute myeloid leukaemia cells. Eur J Clin Invest. 2009;39(5):395–405. doi: 10.1111/j.1365-2362.2009.02101.x. [DOI] [PubMed] [Google Scholar]
- 119.Bao C, Chen J, Chen D, Lu Y, Lou W, Ding B, et al. MiR-93 suppresses tumorigenesis and enhances chemosensitivity of breast cancer via dual targeting E2F1 and CCND1. Cell Death Dis. 2020;11(8):618. doi: 10.1038/s41419-020-02855-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Li N, Miao Y, Shan Y, Liu B, Li Y, Zhao L, et al. MiR-106b and miR-93 regulate cell progression by suppression of PTEN via PI3K/Akt pathway in breast cancer. Cell Death Dis. 2017;8(5):e2796. doi: 10.1038/cddis.2017.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Jiang L, Wang C, Lei F, Zhang L, Zhang X, Liu A, et al. miR-93 promotes cell proliferation in gliomas through activation of PI3K/Akt signaling pathway. Oncotarget. 2015;6(10):8286–8299. doi: 10.18632/oncotarget.3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chen Q, Qin R, Fang Y, Li H. Berberine Sensitizes Human Ovarian Cancer Cells to Cisplatin Through miR-93/PTEN/Akt Signaling Pathway. Cell Physiol Biochem. 2015;36(3):956–965. doi: 10.1159/000430270. [DOI] [PubMed] [Google Scholar]
- 123.Nepstad I, Hatfield KJ, Grønningsæter IS, Reikvam H. The PI3K-Akt-mTOR Signaling Pathway in Human Acute Myeloid Leukemia (AML) Cells. Int J Mol Sci. 2020;21(8):2907. doi: 10.3390/ijms21082907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Qu MH, Han C, Srivastava AK, Cui T, Zou N, Gao ZQ, et al. miR-93 promotes TGF-β-induced epithelial-to-mesenchymal transition through downregulation of NEDD4L in lung cancer cells. Tumour Biol. 2016;37(4):5645–5651. doi: 10.1007/s13277-015-4328-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Astamal RV, Maghoul A, Taefehshokr S, Bagheri T, Mikaeili E, Derakhshani A, et al. Regulatory role of microRNAs in cancer through Hippo signaling pathway. Pathol Res Pract. 2020;216(12):153241. doi: 10.1016/j.prp.2020.153241. [DOI] [PubMed] [Google Scholar]
- 126.Tang Q, Zou Z, Zou C, Zhang Q, Huang R, Guan X, et al. MicroRNA-93 suppress colorectal cancer development via Wnt/β-catenin pathway downregulating. Tumour Biol. 2015;36(3):1701–1710. doi: 10.1007/s13277-014-2771-6. [DOI] [PubMed] [Google Scholar]
- 127.Loveless R, Teng Y. Targeting WASF3 signaling in metastatic cancer. Int J Mol Sci. 2021;22(2):836. doi: 10.3390/ijms22020836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Shang Y, Dai S, Chen X, Wen W, Liu X. RETRACTED ARTICLE: MicroRNA-93 regulates the neurological function, cerebral edema and neuronal apoptosis of rats with intracerebral hemorrhage through TLR4/NF-κB signaling pathway. Cell Cycle. 2019;18(22):3160–3176. doi: 10.1080/15384101.2019.1670509. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 129.Shibuya N, Kakeji Y, Shimono Y. MicroRNA-93 targets WASF3 and functions as a metastasis suppressor in breast cancer. Cancer Sci. 2020;111(6):2093–2103. doi: 10.1111/cas.14423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Longley D, Johnston P. Molecular mechanisms of drug resistance. J Pathol. 2005;205(2):275–292. doi: 10.1002/path.1706. [DOI] [PubMed] [Google Scholar]
- 131.Fu X, Tian J, Zhang L, Chen Y, Hao Q. Involvement of microRNA-93, a new regulator of PTEN/Akt signaling pathway, in regulation of chemotherapeutic drug cisplatin chemosensitivity in ovarian cancer cells. FEBS Lett. 2012;586(9):1279–1286. doi: 10.1016/j.febslet.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 132.Emran TB, Shahriar A, Mahmud AR, Rahman T, Abir MH, Siddiquee MFR, et al. Multidrug resistance in cancer: understanding molecular mechanisms immunoprevention and therapeutic approaches. Front Oncol. 2022;12:891652. doi: 10.3389/fonc.2022.891652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Hu B, Mao Z, Du Q, Jiang X, Wang Z, Xiao Z, et al. miR-93-5p targets Smad7 to regulate the transforming growth factor-β1/Smad3 pathway and mediate fibrosis in drug-resistant prolactinoma. Brain Res Bull. 2019;149:21–31. doi: 10.1016/j.brainresbull.2019.03.013. [DOI] [PubMed] [Google Scholar]
- 134.Chen Q, Qin R, Fang Y, Li H. Berberine sensitizes human ovarian cancer cells to cisplatin through miR-93/PTEN/Akt signaling pathway. Cell Physiol Biochem. 2015;36(3):956–965. doi: 10.1159/000430270. [DOI] [PubMed] [Google Scholar]
- 135.Tovar V, Cornella H, Moeini A, Vidal S, Hoshida Y, Sia D, et al. Tumour initiating cells and IGF/FGF signalling contribute to sorafenib resistance in hepatocellular carcinoma. Gut. 2017;66(3):530–540. doi: 10.1136/gutjnl-2015-309501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wu ZB, Li WQ, Lin SJ, De Wang C, Cai L, Lu JL, et al. MicroRNA expression profile of bromocriptine-resistant prolactinomas. Mol Cell Endocrinol. 2014;395(1–2):10–18. doi: 10.1016/j.mce.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 137.Hu J, Yi T, Chu S, Zeng H, Xia P, Liu G, et al. DNA methylation of miR-93: an important event in acquiring drug resistance in breast cancer cells. Int J Clin Exp Med. 2019;12(5):4697–4706. [Google Scholar]
- 138.Yoshino H, Seki N, Itesako T, Chiyomaru T, Nakagawa M, Enokida H. Aberrant expression of microRNAs in bladder cancer. Nat Rev Urol. 2013;10(7):396–404. doi: 10.1038/nrurol.2013.113. [DOI] [PubMed] [Google Scholar]
- 139.John F, Neylon A, McGorrian C, Blake GJ. miRNA-93-5p and other miRNAs as predictors of coronary artery disease and STEMI. Int J Cardiol. 2016;224:310–316. doi: 10.1016/j.ijcard.2016.09.016. [DOI] [PubMed] [Google Scholar]
- 140.Zhang R, Guo Y, Ma Z, Ma G, Xue Q, Li F, et al. Long non-coding RNA PTENP1 functions as a ceRNA to modulate PTEN level by decoying miR-106b and miR-93 in gastric cancer. Oncotarget. 2017;8(16):26079. doi: 10.18632/oncotarget.15317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yang LG, Cao MZ, Zhang J, Li XY, Sun Ql LncRNA XIST modulates HIF‐1A/AXL signaling pathway by inhibiting miR‐93‐5p in colorectal cancer. Mol Genetics Genomic Med. 2020;8(4):e1112. doi: 10.1002/mgg3.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Liu W, Liang F, Yang G, Xian L. LncRNA LINC01116 sponges miR-93-5p to promote cell invasion and migration in small cell lung cancer. BMC Pulm Med. 2021;21(1):1–6. doi: 10.1186/s12890-020-01369-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zhang L, Liang X, Li Y. Long non-coding RNA MEG3 inhibits cell growth of gliomas by targeting miR-93 and inactivating PI3K/AKT pathway Retraction in/103892/or. 20207828. Oncol Rep. 2017;38(4):2408–16. doi: 10.3892/or.2017.5871. [DOI] [PubMed] [Google Scholar]
- 144.Chen C, Zheng Q, Kang W, Yu C. Long non-coding RNA LINC00472 suppresses hepatocellular carcinoma cell proliferation, migration and invasion through miR-93-5p/PDCD4 pathway. Clin Res Hepatol Gastroenterol. 2019;43(4):436–445. doi: 10.1016/j.clinre.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 145.Zhang K, Cai Y, Zhou Q, Sun H, Wei J. Long non-coding RNA SNHG14 impedes viability, migration and invasion of endometrial carcinoma cells through modulating miR-93-5p/ZBTB7A axis. Cancer Manag Res. 2020;12:9515. doi: 10.2147/CMAR.S257419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Zhang XY, Zhuang HW, Wang J, Shen Y, Bu YZ, Guan BG, Xu F, Dou J. Long noncoding RNA CA3-AS1 suppresses gastric cancer migration and invasion by sponging miR-93-5p and targeting BTG3. Gene Therapy. 2022;29(9):566-74. [DOI] [PubMed]
- 147.Li YJ, Yang Z, Wang YY, Wang Y. Long noncoding RNA ZNF667-AS1 reduces tumor invasion and metastasis in cervical cancer by counteracting microRNA-93-3p-dependent PEG3 downregulation. Mol Oncol. 2019;13(11):2375–2392. doi: 10.1002/1878-0261.12565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Xu F, Zha G, Wu Y, Cai W, Ao J. Overexpressing lncRNA SNHG16 inhibited HCC proliferation and chemoresistance by functionally sponging hsa-miR-93. Onco Targets Ther. 2018;11:8855. doi: 10.2147/OTT.S182005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wang L, Liu S, Mao Y, Xu J, Yang S, Shen H, et al. CircRNF13 regulates the invasion and metastasis in lung adenocarcinoma by targeting miR-93-5p. Gene. 2018;671:170–177. doi: 10.1016/j.gene.2018.04.069. [DOI] [PubMed] [Google Scholar]
- 150.Huang W, Yang Y, Wu J, Niu Y, Yao Y, Zhang J, et al. Circular RNA cESRP1 sensitises small cell lung cancer cells to chemotherapy by sponging miR-93-5p to inhibit TGF-β signalling. Cell Death Differ. 2020;27(5):1709–1727. doi: 10.1038/s41418-019-0455-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Shen L, Dang J, Liu S, Xian B, Deng Y, Qu D. CircRNA VPRBP inhibits tumorigenicity of cervical cancer via miR-93-5p/FRMD6 axis. Reprod Sci. 2022;29(8):2251-64. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.