Abstract
To determine the nature of neurologic dysfunction after deep hypothermic circulatory arrest during aortic arch surgery, we reconsidered the cases of 154 patients who had undergone aortic arch surgery (either of the ascending or transverse aorta, or both) between November 1993 and July 1999. Temporary postoperative neurologic dysfunction was seen in 9 patients (5.8%), and another 3 patients (1.9%) experienced stroke. Patients with temporary neurologic dysfunction had no new infarct and were discharged home with no residual symptoms. Computed tomographic scans revealed that 2 patients with stroke had multiple infarcts in the brainstem, and the 3rd had bilateral border-zone infarcts. The patients with brainstem infarcts died on postoperative days 7 and 15, and the patient with border-zone infarct was discharged home with no symptoms 3 months after surgery.
Univariate analysis revealed that patients with neurologic deficits had significantly higher rates of history of hypertension, concomitant coronary artery bypass grafting, cardiac ischemia times longer than 90 minutes, and chronic renal failure. A multivariate logistic regression analysis revealed that the significant preoperative variables associated with neurologic deficits were a history of hypertension and a cardiac ischemia time longer than 90 minutes.
Deep hypothermic circulatory arrest is a safe and useful technique for protection of the brain during surgery for complex aortic problems. In future, some patients at extreme risk for perioperative neurologic complications might be offered novel neuroprotective agents, in combination with deep hypothermia.
Key words: Aneurysm, dissecting/surgery; body temperature; brain/metabolism; brain ischemia; brain injuries/diagnosis/prevention & control
During aortic arch and cerebrovascular surgery, deep hypothermic circulatory arrest affords the brain some measure of protection by reducing cellular metabolic rate, excitatory transmitter release and generation of free radicals, vascular permeability, blood-brain barrier disruption, and postischemic cerebral edema. 1 Yet neurologic complications in this context are both well known and serious. 2–8 Although the mechanism of these neurologic deficits remains poorly defined, 9–11 both the timing and the types of neurologic complications may shed light on the underlying pathogenesis. In this retrospective study, we attempted to define the clinical variables associated with greater risk of neurologic complications, as well as to explore the clinical spectrum of patients who have undergone deep hypothermic circulatory arrest during aortic arch or cerebrovascular surgery.
Patients and Methods
The study was designed to evaluate neurologic complications in patients who had undergone aortic arch surgery (either of the ascending or transverse aorta, or both) between November 1993 and July 1999. Over the more than 5-year period, 154 patients (117 men and 37 women) underwent aortic arch surgery. One hundred two patients (66.2%) had aortic dissection and 52 (33.8%) had aortic aneurysms. Seventy patients (45.4%) had acute dissection and underwent operation on an emergency basis. Thirteen patients (8.4%) had undergone a previous cardiac operation. Patients with neurologic deficit (n=12) had a mean age of 53.3 ± 12.3 years (range, 36 to 71 years), and those without neurologic deficit (n=142) had a mean age of 53.2 ± 13.0 years (range, 16 to 78 years; P=0.97). Preoperatively, the associated concomitant diseases were hypertension in 58 patients (37.6%), coronary artery disease in 33 (21.4%), chronic obstructive pulmonary disease in 10 (6.5%), chronic renal failure in 9 (5.8%) (2 of whom were dialysis dependent), aortic coarctation in 1 (0.6%), stroke history in 6 (3.9%), Marfan syndrome in 4 (2.6%), and diabetes mellitus in 5 (3.2%).
Systematic neurologic assessments—including tests of motor, sensory, language, and right hemisphere functions—were performed in all patients just before the operation, and then postoperatively until discharge. Baseline characteristics, recorded in detail, included such risk factors as hypertension, diabetes mellitus, cigarette smoking, hypercholesterolemia, temporary ischemic attack, history of stroke or coronary artery disease, and peripheral vascular disease.
At our institution, surgeons have operated upon aortic lesions involving the ascending aorta, the aortic arch, or both, with the aid of retrograde cerebral perfusion as an adjunct to deep hypothermic circulatory arrest. After establishment of electrocerebral silence* during electroencephalography (EEG), patients were cooled for an additional 5 minutes and circulation was arrested. In this study, the alpha-stat pH management system was used, whereby the pH is kept at around 7.4 without compensating for the lower temperature, which means that the actual pH is alkaline.
In the event of stroke, the time of its occurrence was noted, and particular attention was paid to the duration of the surgical procedure. Neurologic complications were characterized either as temporary neurologic dysfunction (TND) or as permanent neurologic deficit (that is, completed stroke). Temporary neurologic dysfunction, defined as temporary disorientation, agitation, delirium, or character changes with no structural abnormality in the brain detectable by the usual imaging methods, was graded on the following clinical scale: simple confusion (grade 1), confusion and lethargy (grade 2), confusion and agitation (grade 3), overt psychosis (grade 4), and psychosis and parkinsonism (grade 5). Postoperative stroke was defined as any clinically evident focal or general neurologic deficit that was not present before surgery and that persisted for more than 48 hours. In our 3 patients with stroke, this diagnosis was confirmed by postoperative computed tomography (CT), which revealed new lesions. Any patient who was comatose or semicomatose, or who failed to recover neurologically within 48 postoperative hours, was considered to have suffered a stroke. Operative mortality was defined as death during hospitalization, or after discharge but within 30 days of the procedure, unless the cause was clearly unrelated to the operation.
Computed tomographic scans were performed in all patients with neurologic deficits, and readings were carried out by a neuroradiologist who was blinded to the findings of the neurologic evaluations. Infarcts imaged on CT were classified as territorial, border zone, or small deep. The clinical diagnosis of a syndrome and the imaging diagnosis of an infarct were made independently of one another. Outcome at discharge was classified as good if the patient went home or to inpatient rehabilitation, and poor if the patient went to a nursing home or had a severe cognitive and motor deficit. Unfortunately, we had incomplete long-term follow-up data on the patients in this study.
Evaluation of Data.
The Mann-Whitney U test was used to compare the mean values of cardiopulmonary bypass times, duration of cardiac ischemia, circulatory arrest times, and rectal temperatures of patients with and without neurologic dysfunction. Multiple logistic regression analysis was conducted, in which neurologic dysfunction was the dependent variable and the following risk factors were dichotomous variables: the preoperative variables were age greater than 65 years, female sex, history of hypertension, chronic renal failure, diabetes mellitus, peripheral vascular disease, temporary ischemic attack, chronic obstructive pulmonary disease, coronary artery disease, smoking, acute dissection, aortic aneurysm, end-organ ischemia, carotid bruit, and hemodynamic instability; and the operative variables were cardiac ischemia time, circulatory arrest time, cardiopulmonary bypass time, degree of hypothermia, reoperation, concomitant coronary artery bypass grafting, and type of aortic repair procedure. Odds ratios with 95% confidence intervals were calculated by univariate and multivariate analyses. Alpha-error levels of 0.05 for entry and 0.10 for removal were used in multiple logistic regression. The data were evaluated using SPSS for Windows, Release 7.5 (SPSS, Inc.; Chicago, Ill). All statistical significance was assumed at the P <0.05 level.
Results
The mean cardiopulmonary bypass time was longer in patients with postoperative neurologic deficit than in those without (186.2 ± 58.1 minutes vs 174.8 ± 50.2 minutes; P=0.48). The patients with neurologic deficits had a mean cardiac ischemia time similar to that of patients without neurologic deficit (92.1 ± 33.7 minutes vs 89.6 ± 27.3 minutes; P=0.72). The mean circulatory arrest time was significantly longer in patients with neurologic deficit than in those without (41.3 ± 11.7 minutes vs 31.4 ± 3.5 minutes; P=0.005). During the surgical procedure, there was no statistical difference between the mean rectal temperatures of patients in the 2 groups (16.5 ± 1.7 °C vs 16.8 ± 1.8 °C; P=0.58).
A total of 12 patients (7.8%) had postoperative neurologic deficits. Three patients (1.9%) experienced stroke during the early postoperative phase. Two of these had presented with ruptured acute type I dissection and had been taken to the operating room in shock, unconscious, and in unstable hemodynamic condition. These patients had decerebrate rigidity after the operation and died on postoperative days 7 and 15. Cerebral CT scans of these patients showed multiple infarcts involving the cerebellum, pons, and occipital lobe, which suggested embolic stroke. The 3rd patient had also presented with acute type I dissection. A postoperative CT scan showed bilateral border-zone infarcts between the middle cerebral artery and anterior cerebral artery territories in this patient. He experienced only mild tetraparesis during the early postoperative period and recovered completely—displaying no neurologic sequelae 3 months after surgery. Temporary neurologic dysfunctions were seen in the remaining 9 patients (5.8%). Four of the 9 had grade 1 TND, 4 had grade 2, and only 1 patient had grade 3. No patient had a temporary neurologic dysfunction greater than grade 3. In patients with TND, symptoms were resolved within 3 days. In 4 patients with TND, cerebral CT scans showed leukoaraiosis but no new infarction. All 9 patients with TND were normal at discharge.
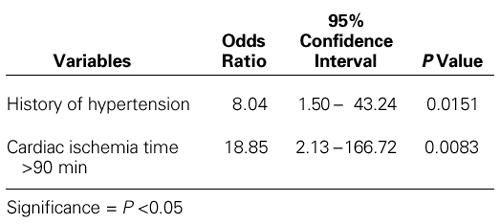
By univariate analysis, the preoperative variables that were significantly associated with increased risk for neurologic complications were a history of hypertension and a history of renal failure (Table I). Cardiac ischemia times longer than 90 minutes and concomitant coronary artery bypass grafting were operative risk factors for neurologic dysfunction on univariate analysis (Table II). There was a trend that suggested a higher risk of neurologic deficit among patients with a circulatory arrest time longer than 60 minutes. A multivariate analysis using stepwise logistic regression of preoperative and operative risk factors revealed that a history of hypertension (odds ratio, 8.04) and a cardiac ischemia time longer than 90 minutes (odds ratio, 18.84) were the only significant predictors of neurologic deficit (Table III).
TABLE I. Univariate Analysis of Preoperative Risk Factors for Neurologic Morbidity
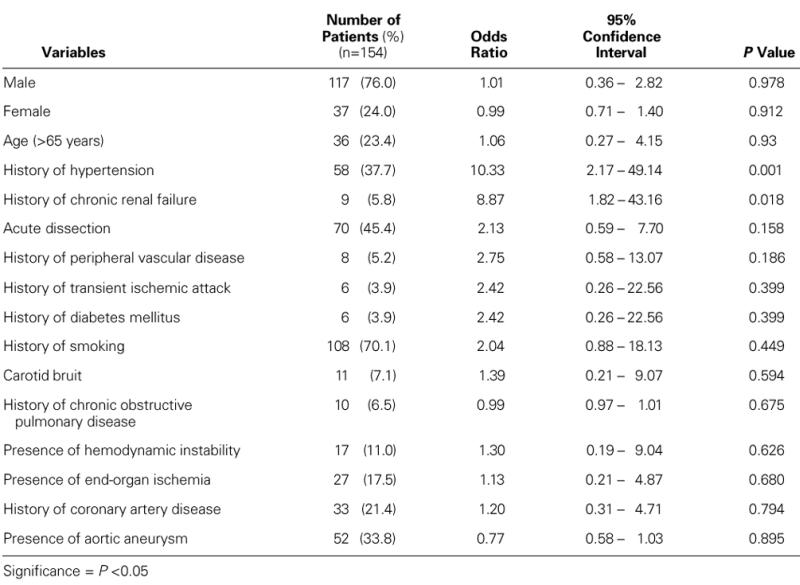
TABLE II. Univariate Analysis of Operative Risk Factors for Neurologic Morbidity
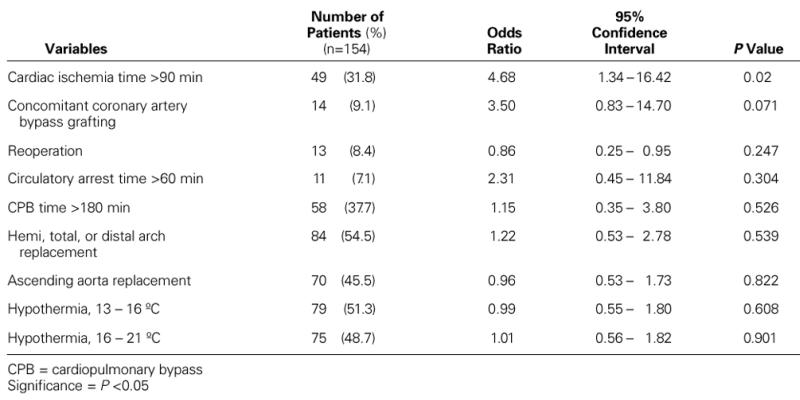
TABLE III. Multivariate Analysis of Risk Factors for Neurologic Morbidity
The in-hospital mortality rate was 18.2%, with 28 deaths overall. The reasons for death were sepsis and multiple organ failure in 10 patients, low cardiac output in 7, respiratory failure in 3, intestinal necrosis in 3, bleeding in 3, stroke in 1, and mediastinitis in 1 patient.
Discussion
Despite advances in surgical techniques and hypothermic circulatory arrest, stroke and other neurologic complications remain serious sequelae of aortic surgery. 12 The pathogenesis of these deficits varies. In hypothermic circulatory arrest, controlled hypothermia diminishes oxygen consumption and thus prolongs the viability of tissues under ischemic conditions. Previous empirical data 11–13 have indicated that core body temperatures of 18 °C to 20 °C are safe for periods as long as 45 to 60 minutes, yet there is no satisfactory means to accurately monitor cerebral function during surgery. An EEG measures only the brain's metabolic activity during surgery, with minimal activity indicated by a flat-line EEG. However, sufficient information on the safety of this procedure can be obtained by means of postoperative assessment.
In our study, we observed 2 types of neurologic deficit that occurred after operation upon the aortic arch. The 1st type—characterized by temporary signs and symptoms, including confusion and delirium—occurred in 9 of our patients (5.8%) and was not associated with any structural abnormality. Most of these patients had experienced longer cardiac ischemia times (>90 minutes) than had those without neurologic deficit. The 2nd type—characterized by stroke—occurred in 3 patients (1.9%), 2 of whom died. During the surgical procedure, there had been no differences between the TND and stroke groups in body temperature or in EEG activity.
Univariate analysis revealed that a history of hypertension and a history of renal failure were the only preoperative risk factors that were significantly associated with an increased risk for postoperative neurologic complications. Previous studies 14 have shown that hypertension is associated with intracranial atherosclerosis in large and small arteries; consequently, attenuated hemodynamic reserve capacity decreases regional blood oxygenation and can lead to temporary brain dysfunction. Chronic renal failure might cause temporary neurologic dysfunction, probably by metabolic mechanisms. Mendelowitsch and colleagues 7 measured metabolic indices of cerebral ischemia by inserting a microdialysis catheter into the cerebral cortex during hypothermia and found that there was an increase in glutamate and lactate concentrations, and in acidosis. Glutamate is an excitatory neurotransmitter that has been shown to cause irreversible neuronal injury during ischemia if excessive amounts are released into the extracellular space, or if its reuptake is inhibited. Glutamate promotes the entry of calcium and sodium into neuronal cells. 15,16
It has also been shown that neuronal nitric oxide synthase mediates neuronal necrosis after hypothermic circulatory arrest and plays an important role in neurotoxicity. 17 Inhibition of neuronal nitric oxide synthase substantially reduces neuronal cell death and results in clinically improved neurologic functions. 18
Although hypothermia protects the brain by decreasing the release of excitatory amino acids and lessening various detrimental enzymatic reactions, 1,19,20 the cerebral system is extremely sensitive to ischemia, and circulatory arrest appears to be safe for only about 40 minutes, if stroke is to be prevented. After 65 minutes of circulatory arrest, the mortality rate increases markedly. 3 Indeed, our data and those of others indicate that a safe circulatory arrest time cannot be determined with precision. The arrest times for patients in this study ranged from 15 to 60 minutes, but hypothermia was supplemented by retrograde cerebral perfusion at the approximate rate of 300 mL/min. On postoperative CT scan, patients with temporary neurologic dysfunction had no new lesion.
Early stroke after aortic surgery may develop either by embolism or by hemodynamic mechanisms. In our series, the frequency of stroke in aortic surgery performed under hypothermia was lower than that reported previously, among patients who underwent cardiac and aortic surgery. 21–24 In a study by Ergin and associates, 12 multivariate analysis suggested that the independent predictors of increased risk of stroke were advanced age (greater than 60 years), severe atheromatous disease of the aorta, and surgery involving the descending aorta. In their study, 39% of strokes were delayed, occurring between postoperative days 3 and 9. Engelman and co-authors 25 have stated that the mechanism of delayed stroke can be hypercoagulation, embolism from a calcific aorta or common carotid artery, or carotid stenosis. Brainstem infarct in our 2 patients was not delayed and was probably the result of undetected athero- or thromboembolism during surgery. Additionally, both of our patients presented with aortic arch dissection, which could have caused stroke either by obstruction or by embolism. In a recent study, 26 atheroma of the aorta was identified as a source of systemic emboli, and the frequency of stroke in cases of atheromatous aorta was 0.76%, depending on the severity of the sclerosis and on the surgical technique used.
In summation, our data show that deep hypothermia plus circulatory arrest is a relatively safe therapeutic approach to reduce ischemic brain injury. We conclude that stroke was the result of embolism and was not related to the use of deep hypothermia. Understanding the mechanism of perioperative stroke and temporary neurologic dysfunction after surgery might ultimately enable identification of patients at high risk for such complications and might also contribute to the development of novel neuroprotective methods, which could be used in the immediate preoperative period. To improve patients' quality of life, investigators must continue to find safer and more effective methods of cerebral protection for application to aortic arch surgery.
Footnotes
* Generally, electrocerebral silence was not achieved until a rectal temperature of 13 °C to 18 °C had been reached.
Address for reprints: Dr. Emre Kumral, Stroke Unit, Neurology Department, Ege University, Faculty of Medicine, Bornova, Izmir, 35100, Turkey
References
- 1.Svensson LG, Crawford ES. Cardiovascular and vascular disease of the aorta. Philadelphia: WB Saunders; 1997. p. 194–218.
- 2.Griepp EB, Griepp RB. Cerebral consequences of hypothermic circulatory arrest in adults. J Card Surg 1992;7:134–55. [DOI] [PubMed]
- 3.Svensson LG, Crawford ES, Hess KR, Coselli JS, Raskin S, Shenaq SA, Safi HJ. Deep hypothermia with circulatory arrest. Determinants of stroke and early mortality in 656 patients. J Thorac Cardiovasc Surg 1993;106:19–31. [PubMed]
- 4.Deeb GM, Jenkins E, Bolling SF, Brunsting LA, Williams DM, Quint LE, Deeb ND. Retrograde cerebral perfusion during hypothermic circulatory arrest reduces neurologic morbidity. J Thorac Cardiovasc Surg 1995;109:259–68. [DOI] [PubMed]
- 5.Ganzel BL, Edmonds HL Jr, Pank JR, Goldsmith LJ. Neurophysiologic monitoring to assure delivery of retrograde cerebral perfusion. J Thorac Cardiovasc Surg 1997;113:748–57. [DOI] [PubMed]
- 6.Griepp RB, Stinson EB, Hollingsworth JF, Buehler D. Prosthetic replacement of the aortic arch. J Thorac Cardiovasc Surg 1975;70:1051–63. [PubMed]
- 7.Mendelowitsch A, Mergner GW, Shuaib A, Sekhar LN. Cortical brain microdialysis and temperature monitoring during hypothermic circulatory arrest in humans. J Neurol Neurosurg Psychiatry 1998;64:611–8. [DOI] [PMC free article] [PubMed]
- 8.Solomon RA, Smith CR, Raps EC, Young WL, Stone JG, Fink ME. Deep hypothermic circulatory arrest for the management of complex anterior and posterior circulation aneurysms. Neurosurgery 1991;29:732–8. [DOI] [PubMed]
- 9.Barone FC, Feuerstein GZ, White RF. Brain cooling during transient focal ischemia provides complete neuroprotection. Neurosci Biobehav Rev 1997;21:31–44. [DOI] [PubMed]
- 10.Ausman JI, McCormick PW, Stewart M, Lewis G, Dujovny M, Balakrishnan G, et al. Cerebral oxygen metabolism during hypothermic circulatory arrest in humans. J Neurosurg 1993;79:810–5. [DOI] [PubMed]
- 11.Zickmann B, Wulf K, Dapper F, Hempelmann G. The place of neurophysiologic monitoring. In: Ennker J, Coselli JS, Treasure T, editors. Cerebral protection in surgery of the aortic arch. New York: Springer; 1997. p. 133–41.
- 12.Ergin MA, Galla JD, Lansman SL, Quintana C, Bodian C, Griepp RB. Hypothermic circulatory arrest in operations on the thoracic aorta. Determinants of operative mortality and neurologic outcome. J Thorac Cardiovasc Surg 1994;107:788–99. [PubMed]
- 13.Treasure T. Neurophysiological consequences of circulatory arrest with hypothermia. In: Ennker J, Coselli JS, Treasure T, editors. Cerebral protection in cerebrovascular and aortic surgery. New York: Springer; 1997. p. 143–55.
- 14.Kleinschmidt A, Steinmetz H, Sitzer M, Merboldt KD, Frahm J. Magnetic resonance imaging of regional cerebral blood oxygenation changes under acetazolamide in carotid occlusive disease. Stroke 1995;26:106–10. [DOI] [PubMed]
- 15.Lipton SA, Rosenberg PA. Excitatory amino acids as a final common pathway for neurologic disorders. N Engl J Med 1994;330:613–22. [DOI] [PubMed]
- 16.Choi DW, Rothman SM. The role of glutamate neurotoxicity in hypoxic-ischemic neuronal death. Annu Rev Neurosci 1990;13:171–82. [DOI] [PubMed]
- 17.Tseng EE, Brock MV, Lange MS, Troncoso JC, Lowenstein CJ, Blue ME, et al. Nitric oxide mediates neurologic injury after hypothermic circulatory arrest. Ann Thorac Surg 1999;67:65–71. [DOI] [PubMed]
- 18.Tseng EE, Brock MV, Kwon CC, Annanata M, Lange MS, Troncoso JC, et al. Increased intracerebral excitatory amino acids and nitric oxide after hypothermic circulatory arrest. Ann Thorac Surg 1999;67:371–6. [DOI] [PubMed]
- 19.Busto R, Globus MY, Dietrich WD, Martinez E, Valdes I, Ginsberg MD. Effect of mild hypothermia on ischemia-induced release of neurotransmitters and free fatty acids in rat brain. Stroke 1989;20:904–10. [DOI] [PubMed]
- 20.Illievich UM, Zornow MH, Choi KT, Scheller MS, Strnat MA. Effects of hypothermic metabolic suppression on hippocampal glutamate concentrations after transient global cerebral ischemia. Anesth Analg 1994;78:905–11. [DOI] [PubMed]
- 21.Ergin MA, Uysal S, Reich DL, Apaydn A, Lansman SL, McCullough JN, Griepp RB. Temporary neurological dysfunction after deep hypothermic circulatory arrest: a clinical marker of long-term functional deficit. Ann Thorac Surg 1999;67:1887–94. [DOI] [PubMed]
- 22.Libman RB, Wirkowski E, Neystat M, Barr W, Gelb S, Graver M. Stroke associated with cardiac surgery. Determinants, timing, and stroke subtypes. Arch Neurol 1997;54:83–7. [DOI] [PubMed]
- 23.Roach GW, Kanchuger M, Mangano CM, Newman M, Nussmeier N, Wolman R, et al. Adverse cerebral outcomes after coronary bypass surgery. Multicenter Study of Perioperative Ischemia Research Group and the Ischemia Research and Education Foundation Investigators. N Engl J Med 1996;335:1857–63. [DOI] [PubMed]
- 24.Moshkovitz Y, David TE, Caleb M, Feindel CM, de Sa MP. Circulatory arrest under moderate systemic hypothermia and cold retrograde cerebral perfusion. Ann Thorac Surg 1998;66:1179–84. [DOI] [PubMed]
- 25.Engelman RM, Pleet AB, Rousou JA, Flack JE 3rd, Deaton DW, Pekow PS, Gregory CA. Influence of cardiopulmonary bypass perfusion temperature on neurologic and he-matologic function after coronary artery bypass grafting. Ann Thorac Surg 1999;67:1547–56. [DOI] [PubMed]
- 26.Trehan N, Mishra M, Dhole S, Mishra A, Karlekar A, Kohli VM. Significantly reduced incidence of stroke during coronary artery bypass grafting using transesophageal echocardiography. Eur J Cardiothorac Surg 1997;11:234–42. [DOI] [PubMed]


