Summary
Background
Autism has long been viewed as a paediatric condition, meaning that many autistic adults missed out on a diagnosis as children when autism was little known. We estimated numbers of diagnosed and undiagnosed autistic people in England, and examined how diagnostic rates differed by socio-demographic factors.
Methods
This population-based cohort study of prospectively collected primary care data from IQVIA Medical Research Data (IMRD) compared the prevalence of diagnosed autism to community prevalence to estimate underdiagnosis. 602,433 individuals registered at an English primary care practice in 2018 and 5,586,100 individuals registered between 2000 and 2018 were included.
Findings
Rates of diagnosed autism in children/young people were much higher than in adults/older adults. As of 2018, 2.94% of 10- to 14-year-olds had a diagnosis (1 in 34), vs. 0.02% aged 70+ (1 in 6000). Exploratory projections based on these data suggest that, as of 2018, 463,500 people (0.82% of the English population) may have been diagnosed autistic, and between 435,700 and 1,197,300 may be autistic and undiagnosed (59–72% of autistic people, 0.77%–2.12% of the English population). Age-related inequalities were also evident in new diagnoses (incidence): c.1 in 250 5- to 9-year-olds had a newly-recorded autism diagnosis in 2018, vs. c.1 in 4000 20- to 49-year-olds, and c.1 in 18,000 people aged 50+.
Interpretation
Substantial age-related differences in the proportions of people diagnosed suggest an urgent need to improve access to adult autism diagnostic services.
Funding
Dunhill Medical Trust, Economic and Social Research Council, Medical Research Council, National Institute for Health Research, the Wellcome Trust, and the Royal College of Psychiatrists.
Keywords: Autism spectrum condition, Primary care, Underdiagnosis, Under-diagnosis, Incidence, Prevalence
Research in context.
Evidence before this study
We searched PubMed from database inception to October 17th, 2022 using the search terms: (1) ‘autism’, and (2) ‘incidence’ or ‘prevalence’ or ‘underdiagnosis’ or ‘under-diagnosis’, without language restrictions. This identified articles describing the global incidence and prevalence of autism. Some studies identified rates of diagnosed autism, and others used active sampling approaches (community case-finding) to identify the numbers of autistic individuals both diagnosed and undiagnosed in a population. The vast majority of studies focused on rates of autism in children. There was a high degree of variability in estimated autism prevalence by year, by region, and by method of case ascertainment. Most studies investigating time-trends reported increasing rates of diagnosed autism over the past 20 years. No studies estimated the extent to which autism was underdiagnosed at a national level or inequalities in diagnosis by comparing diagnosed autism prevalence for a nationally-representative population of children and adults with estimates of true autism prevalence.
Added value of this study
This study is the first to estimate underdiagnosis of autism using data from English primary care for more than 5 million individuals, a critical question given the key role of diagnosis in providing health and social services to autistic people. We are the first to provide upper and lower bound projected estimates of autism underdiagnosis in England, and to establish how these vary according to key demographic and clinical indices. Applying estimates of true prevalence derived from community case-finding studies and the highest rate of diagnosed autism in any age-band in this dataset, we found evidence suggesting high levels of underdiagnosis, particularly in older age groups. We estimate that between 150,000 and 500,000 people aged 20–49 years, and between 250,000 and 600,000 people aged 50+ in England may be autistic but undiagnosed.
Implications of all the available evidence
Community case-finding studies indicate that the true prevalence of autism has been stable over the last 70–80 years. Therefore, these findings highlight continuing inequalities in access to autism diagnostic assessments for adults in England, and suggest that policy initiatives designed to address underdiagnosis in adults have not yet been effective.
Introduction
Autism spectrum disorder is a lifelong developmental condition diagnosed on the basis of (i) difficulties with social communication and social interaction, and (ii) restricted and repetitive patterns of behaviours, interests, and activities.1 Rates of autism diagnoses have risen rapidly since the 1990s.2 Community case-finding surveys, where a population-representative sample undergoes in-depth screening to identify those meeting diagnostic criteria, have found similar numbers of people of different ages meeting criteria for autism.3, 4, 5 This suggests that broadening diagnostic criteria, growing awareness, and improved access to diagnostic services explain the increase in diagnosed autism prevalence.2 Swedish data indicate that the average level of autism characteristics in people with a diagnosis decreased by 30% from 1992 to 2002, and by 50% from 2004 to 2014 for those diagnosed at age 7 or older.6 This suggests that the threshold at which a person is considered to be autistic has become more inclusive, leading to increasing numbers of people meeting diagnostic criteria.
English primary care data for 2020–2021 indicate that 2.9–3% of children aged 10–17 years have an autism diagnosis without co-occurring intellectual disability (ID) in a dataset covering 56% of the population.7 This exceeds the diagnosed prevalence of 1.76% for those aged 5–19 years based on UK pupil census data for 2017.8 Potential explanations for this rate difference are the inclusion of younger as-yet-undiagnosed children in the census data, plus exclusion of children not in school, and incomplete recording of autism for those not receiving extra support at school.8 Other factors are the recency of the English primary care data given that rates of new diagnoses are increasing, and the fact that it covers only 56% of general practices.2,7
Autism has long been viewed as a paediatric condition. Many autistic adults missed out on a diagnosis as children when autism was little known, and have reached adulthood without a diagnosis, despite significant challenges in their daily lives.9 English primary care data for 2020–2021 indicate that 0.1% or fewer of adults in the 50+ age-bands had a diagnosis,7 despite the evidence from community case-finding studies that true autism prevalence is similar across different ages.3 Undiagnosed autistic adults likely overrepresent those with fewer support needs.10 Evidence has also highlighted under- and mis-diagnosis in females,11 and regional variation in diagnostic rates, potentially reflecting local variation in services.12 Higher rates of diagnosed autism have been reported in socioeconomically disadvantaged children.8,12
Many autistic people need adjustments to facilitate healthcare access, inclusion in the workplace, and access to local authority support.13, 14, 15 Not having a diagnosis is a barrier to advocacy, and limits access to specialist services.13,14 Efforts have been made to address poor diagnostic coverage in adults16; yet timely access to adult diagnostic services is still overlooked (e.g., in the 2019 NHS Long-term plan17).
We aimed to quantify whether rates of diagnosed autism differ by sociodemographic factors and co-occurring intellectual disability (ID) in a large dataset of primary care records from practices in England. In an exploratory analysis, we used projections of true autism prevalence derived from community studies and rates of diagnosed autism in children in this dataset to estimate potential numbers of undiagnosed autistic people in England. We also report historical trends in autism diagnosis, and how these varied by socio-demographic factors and ID, between 2000 and 2018.
Methods
Study design
A population-based retrospective cohort study.
Data sources
We investigated rates of autism diagnoses using electronic primary care data for practices in England from IQVIA Medical Research Data (IMRD). IMRD contains anonymised data drawn from routinely collected primary care records exported from computer systems that use Cegedim Healthcare Systems (formerly VISION GP practice systems). The IMRD database includes >18 million individuals from 794 UK primary care practices (c. 10% of all practices), and is broadly representative of the population.18 IMRD incorporates data from THIN, a Cegedim Database. Reference made to THIN is intended to be descriptive of the data asset licensed by IQVIA.
In the United Kingdom, almost all of the population are registered with an NHS primary care practice and access is free of charge.19 Primary care practitioners (GPs) act as gatekeepers for non-emergency specialist care. GP practice staff input communications received from secondary care and make referrals. Practices keep records of patient consultations coded using a hierarchical system. Diagnoses made in secondary care (including autism), are communicated to the patient's general practitioner and the outcome coded in the patient's record. Electronic transfer of records, effective for the majority of practices by 2009,20 means that when someone changes practice, their medical record follows them to their new practice.
IMRD holds ethical approval to collect and supply data for research purposes from the NHS London—South East Research Ethics Committee (reference 18/LO/0441). Use of the IMRD for this study was obtained and approved by IQVIA World Publications Scientific Review Committee in June 2021 (reference 21SRC014).
Participants
We included individuals registered at general practices in England born in 1901 or later, for whom record quality was deemed acceptable by IQVIA, for whom data on socioeconomic deprivation were available. Other inclusion and exclusion are described in eTable S3, and a flow diagram detailing numbers meeting inclusion criteria is provided eFig. S1.
To calculate the percentage of individuals with an autism diagnosis as of 2018, we included individuals with data for the full calendar year 2018, meaning that they were registered at a general practice that met data quality standards for the entirety of 2018. To analyse historical trends, we included individuals with at least one calendar year's data between 2000 and 2018, who did not have an autism diagnosis prior to 1st January 2000; meaning that each person was enrolled at a contributing practice for a full calendar year after the practice met acceptable computing standards within the time-period under study.
Variables
The outcome variable was a code indicating an autism spectrum condition (e.g., autism, Asperger's, pervasive developmental disorder). Identification of codes and the code-list are described in eFig. S3 and eTable S1.
The indicator variables were calendar year, sex, age, ID diagnosis, and socioeconomic deprivation. Identification of ID codes and the code-list are described in eFig. S4 and eTable S2. Socioeconomic deprivation was quantified via Townsend neighbourhood deprivation score quintiles, which index deprivation within a locality (see Supplementary Methods).
Statistical methods
Percentage of individuals with an autism diagnosis as of 2018
Crude estimates of the percentage of individuals diagnosed autistic as of 2018 were calculated by dividing the number of individuals diagnosed on or before 31st December 2018 present in the database for the calendar year 2018 by the total number of individuals in the database for the calendar year 2018, and multiplying the result by 100. Estimates were calculated separately for each age-band, sex, Townsend quintile and for people with/without ID. Poisson regression was used to obtain confidence intervals.
Multilevel random intercept negative binomial regression models with log person-time as an offset were used to estimate fixed effects of predictors (age-band, sex, Townsend quintile and presence/absence of ID), adjusting for each of the other predictors. GP practice was included as a nested random effect. Wald tests were used to examine linear relationships between rates of diagnosed autism and Townsend quintile and age-band. Nested fixed-effect negative binomial regression models were used to examine multiplicative interactions between age-band and sex, age-band and Townsend quintile, sex and Townsend quintile, sex and ID, and ID and age-band. A random intercept model was used to account for clustering by practice. Likelihood ratio tests were used to examine interactions.
There was a significant sex by age-band interaction in rates of diagnosed autism (LR χ2 (9) = 20.76, p = 0.014), so we present results for males and females separately.
Exploratory analysis estimating numbers of diagnosed and undiagnosed autistic people
To estimate the number of diagnosed autistic people in England, we first applied the percentage of males and females in each age-band with an existing autism diagnosis as of 31st December 2018 to the total numbers of people in England based on mid-2020 population estimates.21 We made these projections separately for people with and without ID, estimating true ID prevalence at 1%.22
To estimate underdiagnosis, we first estimated the total true number of autistic people (both diagnosed and undiagnosed). We adopted two estimates of true autism prevalence for autistic people without ID. Lower bound estimates derive from Brugha et al. community case-finding work,3,4 which suggested that around 1.82% of males and 0.20% of females are autistic.a Upper bound estimates for autistic people without ID were the highest diagnosed autism prevalence across any age-group in our dataset: 4.14% for males and 1.22% for females (see eTable S9).
For autistic people with ID, we used a single true autism prevalence estimate: the highest diagnosed prevalence across any age-band in our dataset: 41.07% for males and 23.67% for females (see eTable S9). Estimates from Brugha et al.,4 pertain to moderate to profound ID, so were not applicable here, where ID also included mild ID.
We then compared the proportion of people in each age-band/sex/ID group who had a diagnosis to the hypothesised upper- and lower-bound true numbers of autistic people. We summed the totals of potentially undiagnosed autistic people across these groupings to generate an overall total.
Historical trends in diagnosis
The rate of new autism diagnoses was estimated per 100,000 person-years as the total number of people with a newly-recorded autism diagnosis between 2000 and 2018, divided by the total number of person years of follow up. Crude rates were estimated at level of predictors (e.g., at the year-level) by calculating the number of new diagnoses recorded in that year by the number of person-years-at-risk (i.e., person-time with no recorded autism diagnosis contributed to the dataset for that year). Confidence intervals were calculated assuming a Poisson distribution. Tables and figures present crude rates: no standardised rates are presented.
For the analysis of historical trends, person-time was calculated from the start of the first calendar year after all of the following criteria were met (a) the person had been registered at the practice for at least 1 monthb; (b) the person was at least 1 year old; (c) the date was 1st January 2000 or later; (d) the practice contributed data to IMRD and met quality assurance measures for electronic recording. Persons were censored from the cohort from the earliest date of the following: (a) the date of the first record of an autism diagnosis; (b) the 31st December prior to the individual's date of death, date of leaving the practice, or date of last data collection from the practice; (c) the 31st December 2018 (eTable S3).
Multilevel random intercept negative binomial regression models with log person-time as an offset were used to estimate fixed effects of predictors (calendar year, age-band, sex, Townsend quintile and presence/absence of ID), adjusting for other predictors, on the rate of new diagnoses between 2000 and 2018, with nested fixed effects for region and practice. A random intercept model was used to account for clustering by practice. A Wald test was used to examine the linear relationship between rates of new diagnoses and year. Nested fixed-effect negative binomial regression models were used to examine multiplicative interactions between age-band and sex, and year and age-band, sex, Townsend quintile, and ID.
Analysis of the rate of new diagnoses annually revealed a significant sex by age-band interaction (LR χ2 (9) = 224.74, p < 0.001). We therefore present results for males and females separately.
All analyses were performed using Stata 16.
Patient and public involvement
Autistic adults were involved in the design and conduct of this research. Four autistic adults provided consultancy via an Experts by Experience Steering Group, giving feedback on project outcomes. General practitioners with expertise in autism also provided input.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
Calculating the percentage of individuals diagnosed autistic as of 2018 and estimating underdiagnosis
Characteristics of the cohort
602,433 individuals from 75 GP practices were included. 298,761 (49.59%) were male, and 303,672 (50.41%) female. Median (IQR) age at cohort entry was 42.51 (22.51–59.50) years (eTable S4).
4704 individuals (0.78% of the cohort) were diagnosed autistic as of 31st December 2018 (eTable S5). Median (IQR) age at diagnosis was 8.34 years in males and 10.58 years in females. 3635 (77.27%) were male, and 1069 (22.73%) female. 711 (15.11%) also had a record of ID. 274 (5.82%) had a recorded diagnosis before 2000; 1266 (26.91%) from 2000 to 2009, and 3164 (67.26%) from 2010 to 2018 (see eTable S5). ID status of individuals diagnosed autistic is presented in eTable S6, split by age and sex.
Main results
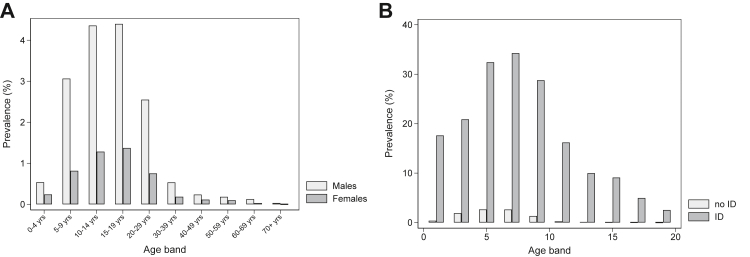
As of 31st December 2018, 1.22% of males (1 in 80) and 0.35% of females (1 in 280) had been diagnosed autistic (Table 1). Lower rates of diagnosed autism were found in older age-groups (Wald test χ2 (9) = 3475.69, p < 0.00005). For both males and females, the highest diagnosed prevalence was in 15- to 19-year-olds (males: 4.40% or 1 in 23; females: 1.37% or 1 in 75; Table 1, Fig. 1A). The lowest diagnosed prevalence was found in those aged 70+: 0.03% or 1 in 3000 males and <0.01% or 1 in 44,000 females were diagnosed (Table 1): a c. 150-fold difference vs. the 15–19 age-group (adjusted rate ratio for 15- to 19-year-olds: 6.38 (95% CI: 5.03–8.10); adjusted rate ratio for people aged 70+: 0.04 (95% CI: 0.02–0.08); eTable S7).
Table 1.
Percentage of individuals with an existing autism diagnosis as of 31st December 2018 and adjusted rate ratios for sex, age, calendar year, social deprivation, and ID.
| Covariate | Males |
Females |
||||
|---|---|---|---|---|---|---|
| Crude percentage diagnosed autistic (95% CI) | Approximate proportion already diagnosed autistic by end of 2018 | Adjusted rate ratioa | Crude percentage diagnosed autistic (95% CI) | Approximate proportion already diagnosed autistic by end of 2018 | Adjusted rate ratioa | |
| Sex | ||||||
| Male | 1.22 (1.18–1.26) | 1 in 80 | – | |||
| Female | 0.35 (0.33–0.38) | 1 in 280 | – | |||
| Age band | ||||||
| 0–4 years | 0.54 (0.40–0.71) | 1 in 190 | 1 | 0.24 (0.15–0.37) | 1 in 420 | 1 |
| 5–9 years | 3.07 (2.83–3.32) | 1 in 33 | 5.36 (4.03–7.13) | 0.82 (0.70–0.96) | 1 in 120 | 3.24 (2.07–5.07) |
| 10–14 years | 4.36 (4.08–4.66) | 1 in 23 | 7.42 (5.60–9.85) | 1.29 (1.13–1.45) | 1 in 80 | 4.84 (3.12–7.49) |
| 15–19 years | 4.40 (4.09–4.72) | 1 in 23 | 7.05 (5.31–9.36) | 1.37 (1.20–1.56) | 1 in 75 | 4.93 (3.18–7.65) |
| 20–29 years | 2.55 (2.38–2.73) | 1 in 39 | 3.91 (2.95–5.19) | 0.75 (0.66–0.86) | 1 in 130 | 2.45 (1.58–3.81) |
| 30–39 years | 0.54 (0.46–0.62) | 1 in 190 | 0.90 (0.66–1.23) | 0.19 (0.15–0.23) | 1 in 550 | 0.66 (0.41–1.06) |
| 40–49 years | 0.24 (0.20–0.29) | 1 in 420 | 0.43 (0.30–0.59) | 0.12 (0.09–0.15) | 1 in 850 | 0.42 (0.26–0.70) |
| 50–59 years | 0.18 (0.15–0.23) | 1 in 550 | 0.32 (0.23–0.46) | 0.10 (0.07–0.13) | 1 in 1000 | 0.37 (0.22–0.61) |
| 60–69 years | 0.13 (0.09–0.17) | 1 in 800 | 0.23 (0.15–0.34) | 0.03 (0.01–0.05) | 1 in 3400 | 0.11 (0.05–0.24) |
| 70+ years | 0.03 (0.02–0.06) | 1 in 3000 | 0.06 (0.03–0.12) | <0.01 (0.00–0.01) | <1 in 9500 | 0.01 (0.00–0.07) |
| Townsend deprivation quintile | ||||||
| 1 (least) | 0.95 (0.88–1.02) | 1 in 110 | 1 | 0.27 (0.23–0.30) | 1 in 380 | 1 |
| 2 | 1.12 (1.04–1.21) | 1 in 90 | 1.14 (1.02–1.27) | 0.36 (0.32–0.41) | 1 in 280 | 1.33 (1.10–1.61) |
| 3 | 1.33 (1.24–1.42) | 1 in 75 | 1.27 (1.14–1.41) | 0.38 (0.34–0.43) | 1 in 260 | 1.30 (1.08–1.56) |
| 4 | 1.41 (1.31–1.51) | 1 in 70 | 1.25 (1.12–1.40) | 0.39 (0.34–0.45) | 1 in 260 | 1.21 (1.00–1.48) |
| 5 (most) | 1.60 (1.47–1.75) | 1 in 60 | 1.43 (1.26–1.62) | 0.44 (0.37–0.52) | 1 in 220 | 1.38 (1.10–1.73) |
| ID diagnosis | ||||||
| No ID diagnosis | 1.05 (1.02–1.09) | 1 in 95 | 1 | 0.29 (0.27–0.31) | 1 in 340 | 1 |
| ID diagnosis | 23.14 (21.20–25.21) | 1 in 4 | 16.89 (15.30–18.64) | 11.63 (10.02–13.42) | 1 in 9 | 35.32 (29.95–41.64) |
95% CIs provided in parentheses.
Abbreviation: ID: Intellectual Disability.
Adjusted for other variables considered: sex, age band, year, Townsend deprivation quintile, co-occurring ID; Adjusted rate ratios compared with the reference group for each categorical variable. Estimated with clustering at the practice level. Adjusted rate ratio for the main effect of sex: 0.33 (0.31–0.35) (male sex is reference category).
Fig. 1.
Percentage of individuals diagnosed autistic as of 2018: (A) stratified by sex and age-band; (B) stratified by co-occurring intellectual disability (ID) and age band.
Higher levels of socioeconomic deprivation were associated with higher rates of autism diagnosis (Wald test χ2 (4) = 43.96, p < 0.00005). In the least deprived quintile, 0.60% of people were diagnosed, vs. 1.02% in the most deprived quintile (eTable S7). This pattern was evident across age-groups, though for people aged 40+ years, the least deprived quintile was about half as likely to be diagnosed as the most (eTable S8).
Just over 18.36% of people with ID had an autism diagnosis: 23.14% or 1 in 4 males and 11.63% or 1 in 9 females (Table 1, eTable S7). Males with ID aged 15–19 years and females with ID aged 10–14 years had the highest rate of diagnosed autism: 41.07% (c. 2 in 5) males and 23.67% (c. 1 in 4) females (Fig. 1B, eTable S9). The male to female ratio for autistic people varied across age-bands and in relation to ID (eTable S9; LR test for sex x ID interaction: χ2 (1) = 50.03, p < 0.001).c
The proportion of people diagnosed autistic who had ID differed across age-groups (LR test for age-band x ID interaction: LR χ2 (9) = 309.27, p < 0.001). For those aged 19 years or younger, 3.67–11.42% of autistic males and 8.84–10.18% of autistic females had diagnosed ID, while for those aged 50–59 years, the figure was 41.86% for males, and 41.30% for females (eTable S6). Lower rates were also seen in males aged 70+ and females aged 60+.
Estimating underdiagnosis in autistic people
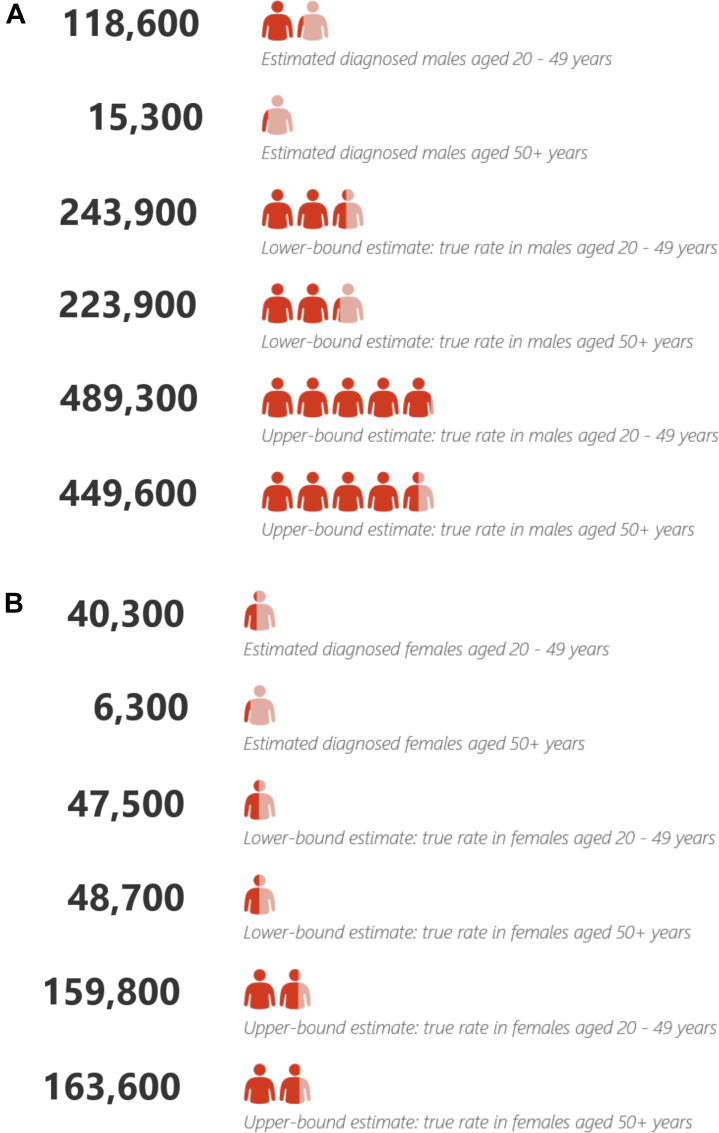
Out of a population of 56.5 million; we estimate that 463,500 (0.82% of the population) have been diagnosed autistic, and between 435,700 and 1,197,300 (58.63–72.11% of autistic people; 0.77%–2.12% of the English population) may be autistic but undiagnosed as of 2018 (see eTables S12–S14).d Fig. 2 provides estimates of numbers of diagnosed autistic people and lower- and upper-bound figures for true prevalence based on our projections. We estimate that between c. 152,900 and 489,900 people aged 20–49 years (52.47–75.47% of autistic people) and between 251,100 and 591,600 people aged 50+ (92.11–96.48% of autistic people) may be autistic but undiagnosed.
Fig. 2.
Estimated numbers of individuals diagnosed autistic, plus upper- and lower-bound estimates of true autism prevalence in England for (A) males and (B) females.
Estimating historical trends in autism diagnosis from 2000 to 2018
Characteristics of the cohort
5,586,100 individuals from 432 GP practices were included. 2,750,668 (49.24%) were male, and 2,835,432 (50.76%) female. Median (IQR) age at cohort entry was 33.5 years (17.50–51.50). Median (IQR) duration in the cohort was 6 years (3.00–12.00), reflecting the median length of time that a person was registered at a practice contributing data to IMRD that met quality thresholds prior to their death, their transfer out of the practice, or the practice no longer contributing data to IMRD (eTable S4).
We identified 12,098 individuals (0.22% of the cohort) with a first record of an autism diagnosis between 2000 and 2018. Median age at first recorded diagnosis was 8.64 years (5.27–13.18) for males and 10.82 years (6.31–16.28) for females. 9555 (78.98%) were male and 2543 (21.02%) were female. 1497 (12.37%) also had a diagnosis of ID (eTable S5).
Main results
The average rate of new diagnoses from 2000 to 2018 was 29.24 per 100,000 individuals (95% CI: 28.72–29.77). Across the time-period, the rate of new diagnoses in children aged 0–4 years was 1 in 750; approximately 40 times higher than for adults aged 50–59 years (c. 1 in 1 in 32,000; adjusted rate ratio: 0.02 (0.02–0.03); and approximately 116 times higher than for adults aged 60–69 (c. 1 in 90,000; adjusted rate ratio: 0.01 (0.01–0.01); eTable S15).
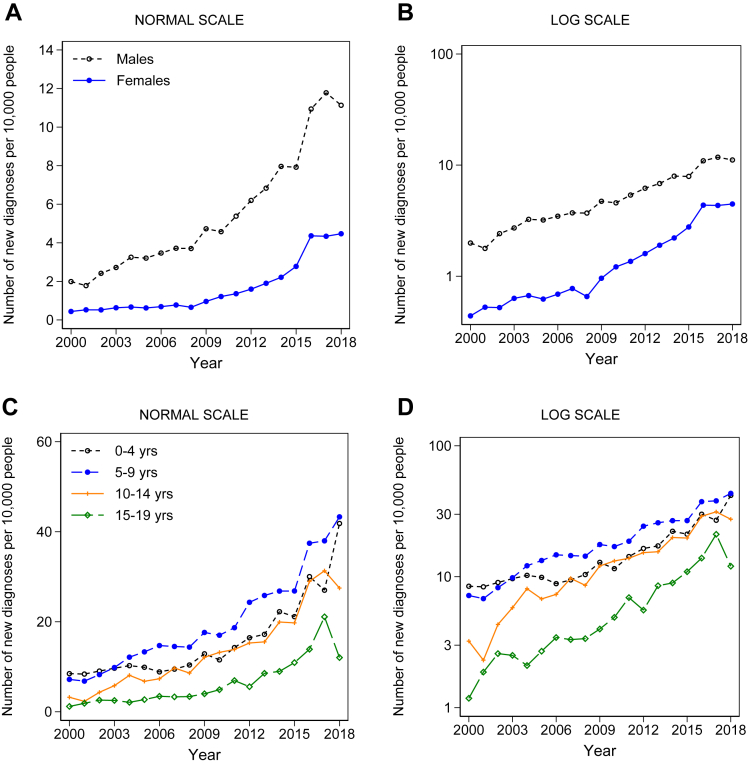
Annual rates of new diagnoses increased six-fold between 2000 and 2018, from c. 1 in 8500 individuals newly diagnosed in 2000, to 1 in 1300 in 2018 (adjusted rate ratio for 2018: 6.05 (95% CI: 5.09–7.19); Wald test χ2 (18) = 2244.81, p < 0.00005) (eTable S15). For females, the rate-increase from 2000 to 2018 was 8.6-fold (adjusted rate ratio for 2018: 8.60 (95% CI: 5.89–12.55)), and for males, 5.5-fold (adjusted rate ratio for 2018: 5.49 (95% CI: 4.51–6.68)) (eTable S16, Fig. 3A and B). The annual rate increase varied by age-band (LR test for age-band x year interaction: χ2 (9) = 100.71, p < 0.001; Fig. 3C–F, eTable S17).
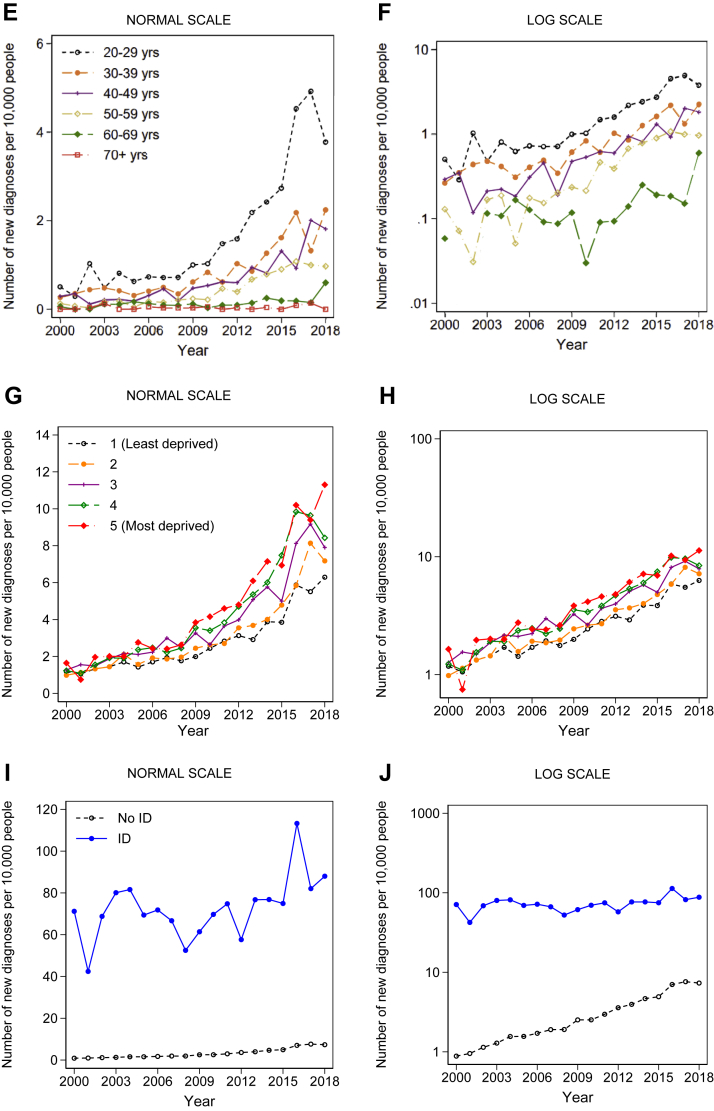
Fig. 3.
Historical trends in annual rates of diagnoses from 2000 to 2018 stratified by: (A) sex, (B) sex on a log scale; (C) age-band: people aged under 20 (D) age-band: people aged under 20 on a log scale; (E) age-band: people aged 20+ (F) age-band: people aged 20+ on a log scale; (G) socioeconomic deprivation; (H) socioeconomic deprivation on a log scale; (I) intellectual disability (ID); (J) intellectual disability (ID) on a log scale. Note: F: there were no new autism diagnoses in the 60- to 69-year age-group in 2002. eFigs. S5–S8 present these results for males and females separately. eFigs. S9–S12 present these results for individuals with and without ID separately.
In 2018, around 1 in 250 5- to 9-year-olds were newly diagnosed autistic, compared to 1 in 4000 people aged 20–49 years, and 1 in 18,000 people aged 50+ (eTable S17). Looking at males and females separately, the group with the highest rate of new diagnoses was boys aged 5–9 years, of whom 1 in 150 were newly diagnosed in 2018 (Fig. 3C and D, eTable S18). Of the 84,000 people aged 70+ in the database, there were no new autism diagnoses recorded in 2018 (Fig. 3E and F, eTable S18).
There was a significant interaction between social deprivation and year in terms of rates of new diagnoses (LR test: χ2 (4) = 19.42, p < 0.001; Fig. 3G and H). By 2018, the crude rate of new diagnoses was nearly twice as high in people in the lowest vs. highest SES quintile (1 in 900 for the lowest vs. 1 in 1600 for the highest) (eTable S19). There was a significant interaction between ID and year (LR test: χ2 (1) = 132.86, p < 0.001). For people without ID, the crude rate of new diagnoses was 8-fold higher by 2018 compared to 2000, whilst for people with ID, the crude rate was only 1.2 times higher (Fig. 3I and J, eTable S20; LR test for ID x year interaction: χ2 (1) = 132.86, p < 0.001).e
Discussion
The present study is the first to our knowledge to examine age-related inequalities in autism diagnoses affecting adults in England. Adults aged 20–49 years and those aged 50+ were less likely to have been diagnosed compared to people aged <20 years. Our exploratory projections suggest that 152,900 to 489,900 people aged 20–49 years, and 251,100–591,600 people aged 50+ in England may be autistic but undiagnosed. The midpoint of these estimates is approximately 750,000 undiagnosed autistic people aged 20 and above. These projections suggest that the total autistic population in England could be over 1.2 million; nearly twice the widely-cited figure of 700,000 given for the entirety of the UK.23
By our projected estimates, only 3.5–7.9% of autistic people aged 50+ have a diagnosis. Even assuming a very conservative estimate of true autism prevalence of c. 1%, this means that more than nine out of 10 autistic over-50s are undiagnosed. This highlights the imperative to improve access to adult autism diagnostic assessments so that lack of a diagnosis does not impede access to services.
These findings indicate that there remains a substantial diagnostic gap in adults compared to children and young people in England. This may partly reflect lack of awareness and understanding of autism in adults on the part of healthcare professionals, and poor communication between autistic adults and healthcare professionals.24 Older adults might also be less likely to self-identify as autistic, meaning that they do not come to the attention of services; and providers may be hesitant to raise the issue of autism given uncertainty around waiting times for a diagnosis and the availability of support or specialist services post-diagnosis.
Being undiagnosed prevents autistic people from accessing vital statutory support (e.g., see Box 1). Underdiagnosis also impedes efforts to plan resources to meet the needs of this population, who are often poorly served by routine services.14,15,25 Autistic adults are more likely to report unmet physical and mental health needs, are less likely to access preventive interventions, and are more likely to require emergency care.25 Unmet support needs and poor mental health have been linked to suicidality.26 Better access to diagnostic assessments is an important step towards reducing health inequalities that shorten autistic peoples’ lives.15
Box 1. Personal account describing challenges accessing adult autism diagnostic services (Public Health England, 2017).
“My son is a 50 year old who has been in full-time employment all his adult life and is able to live on his own with some support from his family. Four years ago, I discovered that he had been financially exploited by several people. As a result, he was heavily in debt with payday loan companies. The police were unable to help, so I went with my son to his GP. She referred him to the Adult in Need Services [local authority Adult Social Services]. He was interviewed over the telephone and told that there were people a lot worse off than him. On the advice of the GP, my son was seen by a psychologist. The recommendations in his report were that my son should be assessed for ASD and treated for moderate depression. The GP referred him for this assessment, which was refused because he was not a danger to himself or anyone else and there were, according to the psychiatrist, no co-morbidities. My son is now on medication and has had CBT for his depression. I then went to my local councillor and my son was referred to Adult Social Services. An assessment for ASD was necessary, so the GP applied again for this. This second referral was refused because the CCG [NHS Clinical Commissioning Group] were unable to fund for ASD assessment. In the meantime, the result of a genetic test I had requested revealed that my son has a chromosomal disorder, which is probably the source of his autism. As a result, he can now have support and protection from the Adults in Need Service. This whole process has taken over 3 years at considerable financial cost to me and caused a great deal of anxiety.” (p. 16, Public Health England, 2017).
Our pooled estimate of diagnosed autism prevalence for people of all ages in 2018 (1.22% of males and 0.35% of females) is lower than recent estimates based on school-aged children.8 This difference reflects very low rates of diagnoses in older age-groups in our sample, also evident in NHS digital data for 56% of English general practices.7
Rates of autism diagnoses increased six-fold from 2000 to 2018, similar to previous findings.2 In people without ID, rates rose eight-fold, but the rate in people with ID showed only a 1.2-fold increase. Our projections suggest that 55% of autistic people with ID in England may be undiagnosed (nearly 100,000 people). Underdiagnosis in people with ID may result in unmet needs (e.g., unrecognized sensory sensitivities), which could contribute to behaviour that challenges, motivating use of anti-psychotics with adverse health sequelae.27 Therefore, autistic adults with ID must not be overlooked in terms of access to diagnostic assessments.
People in the most socio-economically deprived localities were about twice as likely to be newly diagnosed autistic in 2018 compared to those in the least (Fig. 3G and H, eTable S18). This could reflect underdiagnosis in the least deprived localities, and/or over-diagnosis in the most. Reverse causality may also play a role: being autistic may increase the likelihood of experiencing deprivation. On average, autistic adults in England have fewer educational qualifications and higher rates of rented social housing compared to national norms.3 Socio-economically disadvantaged young people may be more likely to be referred for an assessment; and long waiting times may lead socio-economically advantaged families seek private assessments, and not necessarily inform their GP about the outcome.
Study strengths and limitations
Strengths of the present study include the comprehensive primary care database on which analyses were conducted. Limitations include the fact that diagnoses made in secondary care are not directly linked to primary care. Lack of direct linkage means some records may be missed, leading to underestimation of diagnostic rates. It may also take time for the outcome of diagnostic assessments to be reported back to the GP practice, meaning that dates of first recorded diagnoses may lag behind the actual date of diagnosis.
Given that we did not have complete data on all English GP practices, nor information on which NHS trust each practice belonged to, mapping local variation in diagnostic rates at a granular level was not possible. This is an important limitation, given the evidence for considerable local variation in rates of autism diagnoses in children.8 If we had had a complete dataset, we would have been able to nest analyses by GP practice and by trust, and therefore account for the impact of local variation in access to diagnostic services nationally.
The practices that we had data for may not be representative of English general practices; though the diagnosed prevalence rates we report are similar to those from NHS digital for 56–57% of English practices.7 Future studies exploring trust-level variation using a more complete dataset could identify local inequalities in the provision of adult autism diagnostic services. We also did not provide age-standardised estimates of autism prevalence.
By restricting our analyses to time-frames where practices met quality control thresholds for electronic record keeping, we may estimate a higher level of recorded autism diagnoses compared to general practices as a whole across the time-period studied. High rates of missing data on ethnicity in primary care datasets, and the fact that these data are not missing at random, meant that we could not examine whether rates of autism diagnosis differed by ethnicity without potentially introducing bias.
In making projections regarding the number of undiagnosed autistic people, we used data from Brugha et al.3,4 as a lower-bound estimate of true autism prevalence, the basis of the widely-stated figure that around 1% of people in England are autistic. This is likely an underestimate given the broadening of diagnostic criteria since the research was undertaken, and the limitations of the measures used to detect autism in females suggested by the reported male to female ratio of 8.6:1 for autistic people with no or mild ID.
We used data from the present sample for young people to derive upper-bound estimates of true autism prevalence. Children and young people are the section of the population in whom autism is most likely to have been identified and assessed against current criteria. The upper bound rates we used were similar to the highest rates of diagnosed autism in England from NHS digital for 2019–2020 in any age-band (which was in the 10–17 years age-group): 1.28% of females without ID, and 4.05% of males without ID of this age had a diagnosis.7
Our approach to estimating the number of undiagnosed autistic people in England has several limitations. Our upper bound estimate of true autism prevalence gives a male to female ratio of 3.4:1 for those without ID. Although this approaches the c. 3:1 ratio reported in a meta-analysis of community case-finding studies,28 a study suggested that girls with equivalent autism characteristics to boys require additional behavioural challenges to receive a diagnosis.29 There is also regional variation in the male to female ratio across local authority districts, indicating that in certain areas, females may be particularly likely to miss out on a diagnosis.8 Therefore, there could be even more undiagnosed autistic females than we have estimated.
In estimating numbers of undiagnosed autistic people, we did not adjust for premature mortality in autistic people. A 2016 Swedish study with 27,122 autistic people, representing c. 0.3% of the total population, reported a 2.2-fold increased odds of dying during the observation period in autistic people without ID, and a 5.8-fold increased odds in autistic people with ID.30 A 2019 Australian study including 35,929 autistic people aged 5–64 years reported a 2.1-fold increased risk of death vs. a population comparison group, with excess mortality mostly found in autistic people with an intellectual disability.31 A 2021 study using Scottish data reported no significantly increased mortality among nearly 10,000 autistic people aged 5–24, representing 1.2% of the sampled population32; whilst a 2022 South Korean study reported that 35,529 autistic children, representing 0.7% of the sampled population, were at a 2.3-fold increased risk of dying over an 8-year follow-up.33
We did not adjust for mortality in our sample because age-specific mortality ratios stratified by ID status are not available, and there is variation across published studies in terms of age-ranges, data sources, and diagnosed autism prevalence. We speculate that diagnosed autistic adults, and especially older adults, are likely to have more co-occurring health problems, bringing them to the attention of clinical services who identified their autism. More co-occurring health problems could lead to more premature mortality than that seen in the autistic population as a whole (both diagnosed and undiagnosed autistic people).
Our estimation of rates of underdiagnosis assumed that age is not related to whether a person meets diagnostic criteria. A study that followed individuals until age of 25 showed that 1 in 5 of those with a higher IQ “lost” their diagnosis by age 25 based on DSM-5 criteria.34 Therefore, some people who meet criteria as children might no longer experience challenges as adults. However, a modest degree of attrition in terms of the proportions meeting diagnostic criteria in adulthood would not explain the difference in rates of diagnoses by age that we observed.
Given these uncertainties, our estimates of autism underdiagnosis should be viewed as preliminary. Further community case-finding studies are needed that provide statistics on true prevalence using current diagnostic criteria stratified by socio-demographic variables and region. This could facilitate a more precise estimate of the level of autism underdiagnosis in England.
Clinical implications
The present findings illustrating differences in rates of autism diagnosis by age are in line with data from NHS digital,7 suggest a pressing need for better identification of autism in adults and better access to adult autism diagnostic services. An autism diagnosis on a medical record offers the opportunity to target measures to accommodate access challenges and other barriers to healthcare and services, and to facilitate advocacy and access to statutory support. Underdiagnosis means that the needs of most autistic adults in England are going unrecognized. A similar pattern of ADHD underdiagnosis in adults has also been reported,35,36 with a report suggesting waiting times of up to five years for an adult ADHD assessment in the UK.37
Autism characteristics are on a continuum in the population with no natural cut-point.38 The point at which diagnostic criteria are considered to be met is a matter of clinical judgement, which is impacted by clinicians’ own interpretation of criteria; and pragmatically, the perceived benefits of giving vs. not giving a diagnosis. How the criteria are interpreted and applied is likely to vary between individual clinicians. Prevalence is also impacted by societal awareness, which relates to whether individuals come forward for an assessment. Efforts to promote understanding and reduce stigma mean that more people are finding that identifying as autistic helps them to understand and not judge themselves,39,40 providing a stronger rationale for making a diagnosis where there is uncertainty.
Closing the diagnostic gap affecting adults is not without challenges. An autism diagnosis based on ICD-11 criteria requires that autism characteristics were evident during the developmental period, typically in early childhood.41 It is therefore challenging for clinicians to accurately diagnose adults when they find it hard to attain accurate information about this period. Often, an informant who can provide a developmental history is not available. In one high-quality epidemiological study, 44% of adults with suspected autism did not have a parent or sibling who was available to give a historical autism report.4 There are also limitations of existing diagnostic tools to aid the accuracy of diagnostic practice applied to adults, particularly for adults who have co-occurring mental health diagnoses.42
Whilst addressing underdiagnosis of autism is key to reducing health inequalities, it is not sufficient without other policy initiatives, including better post-diagnostic support, upskilling of providers, and a national public information campaign to “shift the attitude and behaviour of millions of people” towards better understanding of autism and inclusion of autistic people.43 p.7 These initiatives are of paramount importance to ensure that having a diagnosis is of meaningful benefit for autistic people.
Contributors
W.M., J.S., & I.P. conceived of the study. E.O., J.S., & I.P. accessed and verified the data. E.O. and I.P. undertook the analysis. E.O., I.P., J.E.J.B., R.C., C.C., A.C., F.H., J.M., M.R., R.S., C.Z., W.M., and J.S. interpreted the findings. E.O. wrote the first draft of the manuscript; all other authors revised the manuscript for critically important content and approved the final version of the manuscript. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted. All authors accept responsibility to submit for publication.
Data sharing statement
Individual participant data cannot be shared.
Declaration of interests
E.O. received a post-doctoral fellowship from the Dunhill Medical Trust which funded completion of the work (grant awarded to J.S., W.M., I.P., R.C., C.C., F.H., A.C., J.M., M.R., and C.Z.). J.S. was supported by the ESRC and NIHR. M.R. was supported by the Medical Research Council and J.E.J.B. was supported by the Wellcome Trust and the Royal College of Psychiatrists. R.S. declares no support from any organisation for the submitted work. J.M. was supported by the NIHR Applied Research Collaboration (ARC) South London. WM was supported by the MRC and NIHR. The views expressed are those of the authors and not necessarily those of the NHS, NIHR, the Wellcome Trust, the Dunhill Medical Trust, the ESRC, or the MRC. All authors declare that they have no financial relationships with any organisations that might have an interest in the submitted work in the previous three years, and no other relationships or activities that could appear to have influenced the submitted work.
Acknowledgements
We are grateful to the members of the Experts by Experience Steering Group, and other autistic people with whom we have discussed our work, for sharing their experiences with us and offering helpful feedback. We also thank Doug McKechnie and Carole Buckley for their assistance in developing code-lists.
Footnotes
More recently, Brugha et al. suggest that autism is likely underdiagnosed in women using standardised tools, and thus estimates of autism prevalence are likely to underestimate true prevalence.4
eFig. S2 illustrates that rates of new records of autism were elevated during the first month of registration at contributing practices, potentially reflecting recording of existing rather than new diagnoses.
Main effects and interactions are presented in eTables S10 and S11.
Estimates of numbers of undiagnosed autistic people stratified by ID and for the pooled sample are presented in eTables S12–S14.
Main effects and interactions are presented in eTables S20 and S21.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.lanepe.2023.100626.
Appendix A. Supplementary data
References
- 1.American Psychiatric Association . 5th ed. American Psychiatric Association; Washington, D.C.: 2013. Diagnostic and statistical manual of mental disorders: DSM-5. [Google Scholar]
- 2.Russell G., Stapley S., Newlove-Delgado T., et al. Time trends in autism diagnosis over 20 years: a UK population-based cohort study. J Child Psychol Psychiatry. 2022;63:674–682. doi: 10.1111/jcpp.13505. [DOI] [PubMed] [Google Scholar]
- 3.Brugha T.S., McManus S., Bankart J., et al. Epidemiology of autism spectrum disorders in adults in the community in England. Arch Gen Psychiatry. 2011;68:459–465. doi: 10.1001/archgenpsychiatry.2011.38. [DOI] [PubMed] [Google Scholar]
- 4.Brugha T.S., Spiers N., Bankart J., et al. Epidemiology of autism in adults across age groups and ability levels. Br J Psychiatry. 2016;209:498–503. doi: 10.1192/bjp.bp.115.174649. [DOI] [PubMed] [Google Scholar]
- 5.Lundstrom S., Reichenberg A., Anckarsater H., Lichtenstein P., Gillberg C. Autism phenotype versus registered diagnosis in Swedish children: prevalence trends over 10 years in general population samples. BMJ. 2015;350:h1961. doi: 10.1136/bmj.h1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arvidsson O., Gillberg C., Lichtenstein P., Lundström S. Secular changes in the symptom level of clinically diagnosed autism. J Child Psychol Psychiatry. 2018;59:744–751. doi: 10.1111/jcpp.12864. [DOI] [PubMed] [Google Scholar]
- 7.NHS Digital. 2021. https://digital.nhs.uk/data-and-information/publications/statistical/health-and-care-of-people-with-learning-disabilities/experimental-statistics-2019-to-2020 [Google Scholar]
- 8.Roman-Urrestarazu A., Van Kessel R., Allison C., Matthews F.E., Brayne C., Baron-Cohen S. Association of race/ethnicity and social disadvantage with autism prevalence in 7 million school children in England. JAMA Pediatr. 2021;175 doi: 10.1001/jamapediatrics.2021.0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lai M.C., Baron-Cohen S. Identifying the lost generation of adults with autism spectrum conditions. Lancet Psychiatry. 2015;2:1013–1027. doi: 10.1016/S2215-0366(15)00277-1. [DOI] [PubMed] [Google Scholar]
- 10.National Audit Office Supporting people with autism through adulthood. London. 2009. https://www.nao.org.uk/reports/supporting-people-with-autism-through-adulthood/
- 11.Lockwood Estrin G., Milner V., Spain D., Happé F., Colvert E. Barriers to autism spectrum disorder diagnosis for young women and girls: a systematic review. Rev J Autism Dev Disord. 2021;8:454–470. doi: 10.1007/s40489-020-00225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roman-Urrestarazu A., Yang J.C., van Kessel R., et al. Autism incidence and spatial analysis in more than 7 million pupils in english schools: a retrospective, longitudinal, school registry study. Lancet Child Adolesc Health. 2022;6:857–868. doi: 10.1016/S2352-4642(22)00247-4. [DOI] [PubMed] [Google Scholar]
- 13.Department for Education and Department of Health and Social Care . 2021. Summary of findings from the government's review of the National Autism Strategy ‘Think Autism’: call for evidence.https://www.gov.uk/government/consultations/review-of-the-national-autism-strategy-think-autism-call-for-evidence/outcome/summary-of-findings-from-the-governments-review-of-the-national-autism-strategy-think-autism-call-for-evidence [Google Scholar]
- 14.Public Health England . 2017. Autism self-assessment exercise 2016 personal stories.https://www.gov.uk/government/publications/autism-self-assessment-framework-exercise [Google Scholar]
- 15.The Westminster Commission on Autism . 2016. A spectrum of obstacles: an inquiry into access to healthcare for autistic people.https://westminsterautismcommission.files.wordpress.com/2016/03/ar1011_ncg-autism-report-july-2016.pdf [Google Scholar]
- 16.Autism Act 2009. United Kingdom. 2009. https://www.legislation.gov.uk/ukpga/2009/15/contents [Google Scholar]
- 17.The NHS long term plan. 2019. https://www.longtermplan.nhs.uk/wp-content/uploads/2019/08/nhs-long-term-plan-version-1.2.pdf [Google Scholar]
- 18.Blak B.T., Thompson M., Dattani H., Bourke A. Generalisability of The Health Improvement Network (THIN) database: demographics, chronic disease prevalence and mortality rates. Inform Prim Care. 2011;19:251–255. doi: 10.14236/jhi.v19i4.820. [DOI] [PubMed] [Google Scholar]
- 19.NHS Digital Patients registered at a GP practice, July 2022. https://digital.nhs.uk/data-and-information/publications/statistical/patients-registered-at-a-gp-practice/july-2022
- 20.NHS Connecting for Health . 2009. 5000 practices now live with GP2GP.https://webarchive.nationalarchives.gov.uk/ukgwa/20100408232237/http://www.connectingforhealth.nhs.uk/newsroom/news-stories/5000live/ [Google Scholar]
- 21.Office for National Statistics . 2021. Mid-year population estimates, UK, June 2020. London, United Kingdom.https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/datasets/populationestimatesforukenglandandwalesscotlandandnorthernireland [Google Scholar]
- 22.Maulik P.K., Mascarenhas M.N., Mathers C.D., Dua T., Saxena S. Prevalence of intellectual disability: a meta-analysis of population-based studies. Res Dev Disabil. 2011;32:419–436. doi: 10.1016/j.ridd.2010.12.018. [DOI] [PubMed] [Google Scholar]
- 23.HM Government . 2021. The national strategy for autistic children, young people and adults.https://www.gov.uk/government/publications/national-strategy-for-autistic-children-young-people-and-adults-2021-to-2026 [Google Scholar]
- 24.Au-Yeung S.K., Bradley L., Robertson A.E., Shaw R., Baron-Cohen S., Cassidy S. Experience of mental health diagnosis and perceived misdiagnosis in autistic, possibly autistic and non-autistic adults. Autism. 2019;23:1508–1518. doi: 10.1177/1362361318818167. [DOI] [PubMed] [Google Scholar]
- 25.Nicolaidis C., Raymaker D., McDonald K., et al. Comparison of healthcare experiences in autistic and non-autistic adults: a cross-sectional online survey facilitated by an academic-community partnership. J Gen Intern Med. 2013;28:761–769. doi: 10.1007/s11606-012-2262-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cassidy S., Bradley L., Shaw R., Baron-Cohen S. Risk markers for suicidality in autistic adults. Mol Autism. 2018;9:42. doi: 10.1186/s13229-018-0226-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.NHS England . 2019. Psychotropic drugs and people with learning disabilities or autism: introduction.https://www.gov.uk/government/publications/psychotropic-drugs-and-people-with-learning-disabilities-or-autism/psychotropic-drugs-and-people-with-learning-disabilities-or-autism-introduction [Google Scholar]
- 28.Loomes R., Hull L., Mandy W.P.L. What is the male-to-female ratio in autism spectrum disorder? A systematic review and meta-analysis. J Am Acad Child Adolesc Psychiatry. 2017;56:466–474. doi: 10.1016/j.jaac.2017.03.013. [DOI] [PubMed] [Google Scholar]
- 29.Duvekot J., van der Ende J., Verhulst F.C., et al. Factors influencing the probability of a diagnosis of autism spectrum disorder in girls versus boys. Autism. 2017;21:646–658. doi: 10.1177/1362361316672178. [DOI] [PubMed] [Google Scholar]
- 30.Hirvikoski T., Mittendorfer-Rutz E., Boman M., Larsson H., Lichtenstein P., Bölte S. Premature mortality in autism spectrum disorder. Br J Psychiatry. 2016;208:232–238. doi: 10.1192/bjp.bp.114.160192. [DOI] [PubMed] [Google Scholar]
- 31.Hwang Y.I.J., Srasuebkul P., Foley K., Arnold S., Trollor J.N. Mortality and cause of death of Australians on the autism spectrum. Autism Res. 2019;12:806–815. doi: 10.1002/aur.2086. [DOI] [PubMed] [Google Scholar]
- 32.Smith G.S., Fleming M., Kinnear D., et al. Mortality in 787,666 school pupils with and without autism: a cohort study. Autism. 2021;25:300–304. doi: 10.1177/1362361320944037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoo S.M., Kim K.-N., Kang S., Kim H.J., Yun J., Lee J.Y. Prevalence and premature mortality statistics of autism spectrum disorder among children in Korea: a nationwide population-based birth cohort study. J Korean Med Sci. 2022;37 doi: 10.3346/jkms.2022.37.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elias R., Lord C. Diagnostic stability in individuals with autism spectrum disorder: insights from a longitudinal follow-up study. J Child Psychol Psychiatry. 2022;63:973–983. doi: 10.1111/jcpp.13551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raman S.R., Man K.K.C., Bahmanyar S., et al. Trends in attention-deficit hyperactivity disorder medication use: a retrospective observational study using population-based databases. Lancet Psychiatry. 2018;5:824–835. doi: 10.1016/S2215-0366(18)30293-1. [DOI] [PubMed] [Google Scholar]
- 36.Asherson P., Leaver L., Adamou M., et al. Mainstreaming adult ADHD into primary care in the UK: guidance, practice, and best practice recommendations. BMC Psychiatry. 2022;22:640. doi: 10.1186/s12888-022-04290-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.BBC . 2020. ADHD assessment system ‘broken’ with five-year waiting times.https://www.bbc.com/news/uk-england-53526174 [Google Scholar]
- 38.Ruzich E., Allison C., Smith P., et al. Measuring autistic traits in the general population: a systematic review of the Autism-Spectrum Quotient (AQ) in a nonclinical population sample of 6,900 typical adult males and females. Mol Autism. 2015;6:2. doi: 10.1186/2040-2392-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hickey A., Crabtree J., Stott J. ‘Suddenly the first fifty years of my life made sense’: experiences of older people with autism. Autism. 2018;22:357–367. doi: 10.1177/1362361316680914. [DOI] [PubMed] [Google Scholar]
- 40.Punshon C., Skirrow P., Murphy G. The not guilty verdict: psychological reactions to a diagnosis of Asperger syndrome in adulthood. Autism. 2009;13(3):265–283. doi: 10.1177/1362361309103795. [DOI] [PubMed] [Google Scholar]
- 41.World Health Organization International classification of diseases, eleventh revision (ICD-11) https://icd.who.int/browse11 Licensed under Creative%0ACommons Attribution-NoDerivatives 3.0 IGO licence (CC BY-ND 3.0 IGO).
- 42.Wigham S., Rodgers J., Berney T., Le Couteur A., Ingham B., Parr J.R. Psychometric properties of questionnaires and diagnostic measures for autism spectrum disorders in adults: a systematic review. Autism. 2019;23:287–305. doi: 10.1177/1362361317748245. [DOI] [PubMed] [Google Scholar]
- 43.All Party Parliamentary Group on Autism . 2019. The autism act, 10 years on. London, United Kingdom.https://pearsfoundation.org.uk/wp-content/uploads/2019/09/APPGA-Autism-Act-Inquiry-Report.pdf [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.