Abstract
Sonic Hedgehog (SHH) signaling has a critical role in mediating developmental neurogenesis and has been implicated in adult subventricular (SVZ) neurogenesis. However, the precise role of Smoothened (SMO) receptor mediated SHH signaling in adult neurogenesis during aging especially in hippocampal subgranular zone (SGZ) neurogenesis remain undefined. Additionally, our previous study showed that stimulation of SHH signaling post-stroke leads to increased neurogenesis and improved behavioral functions after stroke. However, it is not clear whether SHH signaling in neural stem cells (NSCs) are required for stroke-induced neurogenesis and functional recovery post stroke. In this study, using conditional knockout (cKO) of SHH signaling receptor Smo gene in NSCs, we show a decreased neurogenesis at both SVZ and SGZ in young-adult mice and an accelerated depletion of neurogenic cells in the process of aging suggesting that SHH signaling is critical in maintaining neurogenesis during aging. Behavior studies revealed that compromised neurogenesis in Smo cKO mice leads to increased anxiety/depression-like behaviors without affecting general locomotor function or spatial and fear-related learning. Importantly we also show that NSCs cKO of SHH signaling abolishes stroke-induces neurogenesis in Smo cKO mice. Compared to control mice, Smo cKO mice also show delayed motor function recovery and increased anxiety level after stroke. Our data highlight the essential role of Smo function in regulating adult neurogenesis and emotional behaviors during both aging and CNS injury such as stroke.
Keywords: Stroke, Neurogenesis, Sonic hedgehog, Smoothened, Behavior
Introduction
Across the lifespan, neurogenesis continuously to produce new functional neurons to support central nervous system development and maintain normal neurological functions [1]. Meanwhile, neural stem cells (NSCs) in adult brain potentially contribute to neurorepair following brain insults such as ischemic stroke, traumatic brain injury, etc. [2, 3]. It is well established that neurogenic niches such as subventricular (SVZ) and hippocampal dentate gyrus (DG) subgranular zone (SGZ) are major locations where adult neurogenesis takes place [4, 5]. Under physiological conditions, newly born neurons from these niches incorporate into the olfactory bulb (derived from SVZ) or DG granular cell layer (derived from SGZ) to become part of the neuronal circuitry [5]. It has been speculated that some of the key molecules might have played an important role in adult neurogenesis under both physiological and pathological conditions [6, 7, 8]. It is well known now that SHH signaling controls the patterning of cells in the neural tube development and limb development [9, 10, 11]. Mutations of human SHH gene can cause severe central nervous system (CNS) abnormalities such as Holoprosencephaly [12]. Shortly after the initial report of its functions in cell fate decision in spinal cord morphogenesis [13], the regulatory role of SHH signaling in mouse adult brain neurogenesis was first discovered [14, 15, 16]. Using transgenic mouse models, studies focusing on SHH signaling gain/loss of functions have demonstrated its indispensable requirement in modulating adult neurogenesis in the SVZ [4, 17]. However, it is not clear whether loss of function in SHH signaling pathway affects adult neurogenesis in hippocampal DG in vivo, especially during the process of aging or CNS injury. Moreover, the functional relevance of SHH signaling in NSCs pertinent to neurological function is unclear. Additionally, our recent study demonstrates that administration of Smoothened agonist (SAG) promotes neurogenesis in stroke mice and improve post-stroke behavioral recovery [18], suggesting that SHH signaling is also involved in CNS-injury induced neurogenesis and neurorepair. However, whether endogenous SHH signaling in NSCs is essential for stroke-induced neurogenesis and motor/cognitive functional recovery remains unknown.
In this study, utilizing NSCs specific SHH signaling receptor Smoothened (Smo) conditional knockout mouse model, we examined the population of proliferating neurogenic cells and immature neurons at SVZ and SGZ regions in adult and middle-aged mice following disruption of SHH signaling in NSCs. We focus on two main scenarios. One is natural aging where neurogenesis is known to decline. The other is stroke where it is known to have stimulated neurogenesis but the exact functional contribution of neurogenesis to motor or non-motor behavioral functional outcome is not known. We demonstrated necessary requirement of Smo in maintaining SVZ neurogenesis during young adulthood to middle-aged stage. For the first time, we discovered that compared to SVZ region, there is a higher requirement of SHH signaling in maintaining adult neurogenesis at hippocampal SGZ region. Meanwhile, our results from behavior analysis on young-adult and middle-aged mice showed no correlation between adult neurogenesis and spatial and fear related memory tasks. Instead, anxiety/depression-like behaviors are correlated with decreased adult neurogenesis.
Our study also addresses the role of SHH signaling in adult neurogenesis and its functional relevance under ischemic stroke conditions. Our results demonstrates that post-stroke neurogenesis in response to ischemia are compromised following disruption of SHH singling in NSCs at both SVZ and SGZ regions. In addition, post-stroke Smo cKO mice showed delayed motor functional recovery supporting a critical role of neurogenesis to motor function recovery after stroke. Moreover, we report increased anxiety-like behavior in stroke mice while disruption of SHH signaling further aggravates anxiety-like behavior in conditional knockout mice subjected to stroke. Together, these data support that SHH signaling acts as a critical modulator in adult neurogenesis both during aging and stroke and further demonstrates the important role of SHH signaling functions in regulating animal behaviors under both physiological and post-stroke conditions.
Materials and Methods
Animals
All animal protocols were approved by the Institutional Animal Care and Use Committee at the University of Cincinnati. Smo cKO mouse line was generated as described in Supplementary Information.
Adeno Associated Virus transfection assay
AAV-CMV-Cre (105537-AAV1) or AAV-CMV-RFP (105548-AAV1) were purchased from Addgene (Watertown, MA). Transfection experiment details are described in Supplementary Information.
Neural stem cell culture and exogenous Sonic Hedgehog protein responding assay
Primary neurogenic cells were isolated from 6 weeks old Smo−/− or Smo+/+ or Smofl/fl mice and cultured into neurosphere according to our previous studies [19]. Details could be found in Supplementary Information. We used neurospheres at passage P3-P8 in this study. We used AAV-CMV-Cre to infect SGZ NSCs which derived from Smofl/fl mice to generate Smo cKO NSCs as described in Supplementary Information. Mouse Sonic Hedgehog protein (464-SH) were purchased from Novus Biologicals (Centennial CO) and reconstitute with 0.1% bovine serum albumin in PBS solution. Neurospheres were dissociated into single cells and placed in differentiation media with vehicle or 10nM SHH or 20nM of SHH. Neurospheres dimension was quantified as described in Supplementary Information.
Murine Model of Transient Cerebral Ischemia
Transient middle cerebral artery occlusion (tMCAo) was performed by occlusion of the middle cerebral artery for 45 min with a silicone tip-coated filament (Cat.602212PK10Re and 602312PK10Re, Doccol Corporation). Surgery details can be found in Supplementary Information. Laser Doppler flowmetry monitoring and T2-weighted MRI imaging were performed during and post-surgery to ensure consistency of the surgery. See Supplementary Information for details.
Immunohistochemistry
Immunostaining for guinea pig anti-DCX (1:500, Millipore), rabbit anti-DCX (1:1000, Cell Signaling Technology), and rat anti-Ki67 (1:200, Invitrogen) was carried out as described in Supplementary Information. All immunohistochemical measurements were done by blinded observers.
Behavior studies
All behavioral tests were performed during the light phase of animals. To reduce stress, mice were acclimated in the behavioral test room 1h before test start. All apparatuses were cleaned with either water or cleaning solutions in between animals to avoid odor influences. Multiple cohorts were used in behavior studies ranging from lowest to highest anxiety stimulation. Details for all behavioral tests are listed in Supplementary Information.
Data acquisition and statistical analyses
All studies were analyzed using SigmaPlot. Results are expressed by mean±SEM of the indicated number of experiments. Statistical analysis was performed using the students’ t-test, 1way or 2way analysis of variance (ANOVA), as appropriate, with Tukey’s post-hoc tests for repeated behavioral measurements. A p value equal to or less than 0.05 was considered significant. Details for all statistical analyses results are listed in Supplementary Information.
Results
Sonic Hedgehog signaling modulates neurogenic niche cell proliferation directly in vitro.
To study SHH signaling function in SVZ neurogenesis, we established primary NSCs culture from Smo cKO (mouse-Glial Fibrillary Acidic Protein promoter driven Cre crossed with Smofl/fl mice) or control mice as previously described [20]. Fig S1 B showed successful Smo exon1 deletion in NSCs derived from Smo cKO mice. To test if SHH ligand directly regulates growth of primary NSCs, we cultured Smo deficient or control neurospheres in the presence of different concentrations of SHH and showed increased neurosphere diameter with 10nM or 20nM SHH treatment compared to vehicle group Which effect was abolished in Smo cKO neurospheres (Fig S1, E).
In Smo cKO mouse line, mGFAP promoter-driven Cre expression starts around the neonatal stage [21]. To rule out any potential compensatory effects on Smo gene deletion in NSCs, we also isolated primary SGZ NSCs from Smofl/fl mice. AAV-Cre infected Smofl/fl neurospheres expressed lower levels of Smo and Smo Exon1 mRNA than AAV-RFP infected Smofl/fl neurospheres (Fig S1, C-D). Treatment of control neurospheres (AAV-RFP infected) with 10nM or 20nM SHH leads to increased neurosphere diameter compared to vehicle group. In contrast, treatment of AAV-Cre infected Smo-floxed neurospheres with either 10nM or 20nM SHH did not lead to increase of neurosphere diameter (Fig S1, F). Importantly, the effects of SHH treatment on the NSCs is only observed in decreased epidermal growth factor (EGF) and basic fibroblast growth factor (bFGF) culturing concentration (2 ng/ml EGF and 2ng/ml bFGF, 1/10 concentration of regular (20 ng/ml EGF and 20ng/ml bFGF) NSC culture media). This suggests that growth factors such as bFGF and EGF in the culture media could compensate SHHsignaling function in neurosphere culture in vitro.
Disruption of Sonic Hedgehog signaling results in accelerated aging phenotypes in both SVZ and SGZ adult neurogenesis.
Through genetic knockout studies, it has been shown previously that SVZ adult neurogenesis relies on SHH signaling function [21]. However, the role of SHH signaling in SGZ neurogenesis, and its function in neurogenesis during aging is unknown. Recent studies have shown that although both niches continue to generate adult born neurons, many key features including molecular transcriptome can be substantially different for NSCs from these two distinct niches [22, 23]. To address these questions, brain sections from 3month (m), 7-9m, and 12m old Smo cKO and control mice were stained with Ki67 and Doublecortin (DCX) antibodies to label proliferating cells and immature neurons, respectively.
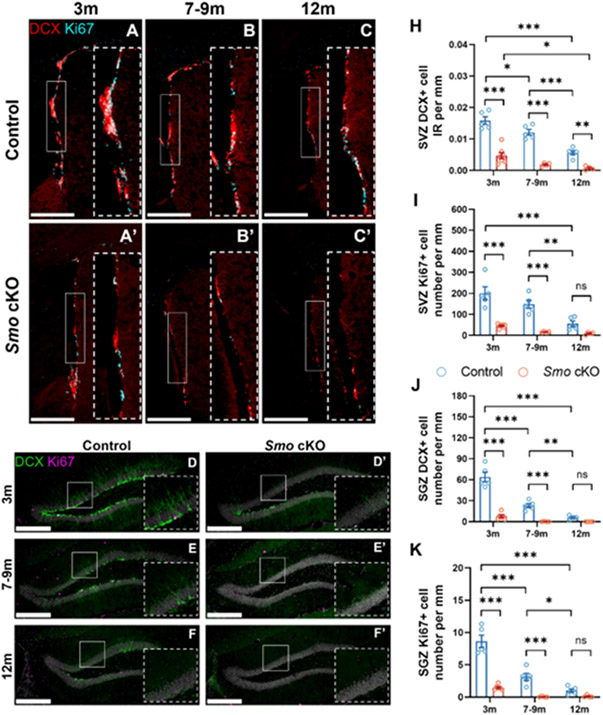
We observed a significant reduction of Ki67+ cell numbers and DCX+ cells immunoreactivity (IR) at SVZ region in 3m Smo cKO mice compared to control littermates (Fig 1). Additionally, we observed decreased number of Ki67+ or DCX+ cells during aging in control mice (Fig 1, A-C), consistent with previous studies[24]. Importantly, compared to aged-matched control mice, Smo cKO mice showed a more severe decline of Ki67+ and DCX+ cell population at SVZ (Fig 1, A’-C’). These data demonstrate the indispensable role of SHH signaling in modulating adult neurogenesis at SVZ across the young-adult (3m) to middle-aged (12m) stage. Previous studies have suggested that dorsal and ventral NSCs exhibit heterogeneity in transcriptome and cell fate [25, 26], therefore we also quantified DCX+ or Ki67+ cells in the dorsal or ventral part of SVZ. Interestingly, when calculated as percentage of cells remaining (compared to dorsal or ventral cells in control mice), compared to ventral SVZ, dorsal SVZ showed a more substantial depletion in DCX+ cells, with only 25% remaining in dorsal SVZ vs 45% remaining ventral SVZ in cKO compared to control (p=0.021, Students’ t-test, Fig S2). Similarly, Ki67+ cells also demonstrated a more severe reduction in dorsal SVZ compared to ventral SVZ, with 15% of Ki67+ cells remaining in dorsal SVZ vs 32% remaining ventral SVZ in cKO compared to control (p<0.001, Students’ t-test, Fig S2). This result suggests that dorsal SVZ NSCs might have a higher reliance on SHH signaling compared to ventral SVZ NSCs.
Fig 1. Disruption of Sonic Hedgehog signaling results in pre-mature aging phenotype in adult neurogenesis.
Immunostaining of DCX-labeled immature neurons (Red) and Ki67-labeled proliferating cells (Cyan) from age matched control (A-C, D-F) or Smo cKO(A’-C’, D’-F’) mice at different age. Significant reduction of DCX+ cells (H, J) and Ki67+ cells (I, K) were observed at SVZ (H, I) and SGZ (J, K) in Smo cKO mice compared to control mice. (Control: 3m, n=5; 7-9m, n=5; 12m, n=5. Smo cKO: 3m, n=7; 7-9m, n=4; 12m, n=4, 2way ANOVA with Tukey’s post-hoc test). Scale bar =500 μm. All data shown are Mean±SEM. * p<0.05, ** p<0.01, *** p<0.001. For detail in statistical analysis, see Table S1.
In the hippocampal SGZ region, we observed a faster decline of Ki67+ and DCX+ cells in control mice during aging indicated by about 64% and 64% decrease in SGZ at 7-9m than 3m, in contrast to 23% and 26% decrease in SVZ at this age. Moreover, we observed an even more severe reduction of Ki67+ and DCX+ cells as early as 3m in Smo cKO mice compared to control littermates (Fig 1, D-F’). This suggests a pre-mature aging phenotype of SGZ neurogenesis in Smo cKO mice. Quantification results also showed similar trends of Ki67+ or DCX+ cell population decline at suprapyramidal or infrapyramidal blades between Smo cKO and control littermates (Fig S3) which suggesting a similar requirement of Smo function across the whole DG.
Deficits in adult neurogenesis do not impair general locomotor function, spatial or fear-related memory learning ability under physiological condition
To investigate the functions of SHH signaling mediated adult neurogenesis in animal behaviors, we characterized young adult and middle-aged Smo cKO and control mice in three categories: general locomotion function, spatial memory learning ability, and anxiety/depression-related behaviors.
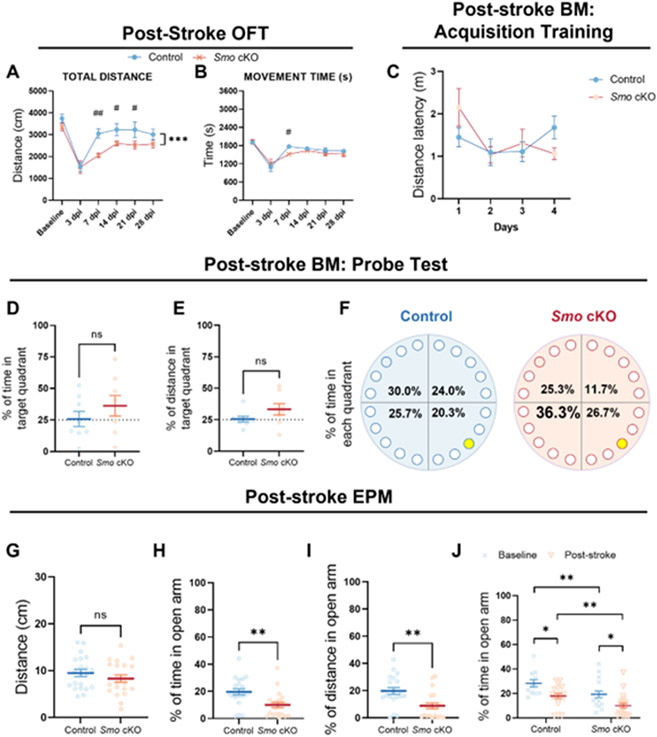
For general locomotion function, we performed open field test (OFT, 23 hours duration) on 3m and 12m old Smo cKO and control mice. No significant difference in total distance, resting time, movement time, or vertical movement time was observed between Smo cKO and control mice (Fig 2, A-B). This suggests that decreased adult neurogenesis in the Smo cKO mice does not affect the locomotion function in either young adult or middle-aged mice.
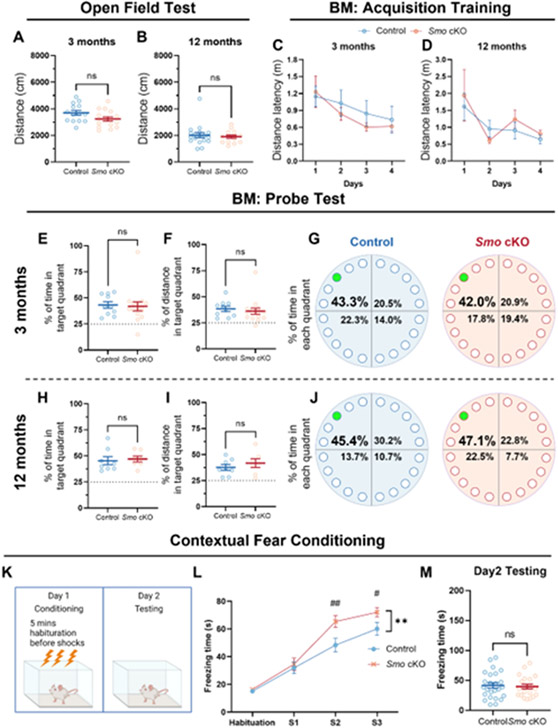
Fig 2. Deficits in adult neurogenesis in Smo cKO mice do not impact general locomotor function, spatial memory learning in Barnes Maze test or fear related memory.
Decreased adult neurogenesis in the Smo cKO mice does not affect the general locomotor function in either young adult (A, 3m Control, n=14. 3m Smo cKO n=16, Students’ t-test,) or middle-aged (B, 12m Control, n=18. 12m Smo cKO, n=14, Students’ t-test,) groups. No significant impairment in spatial memory consolidation was observed in Smo cKO mice during 4-day BM training sessions in either young adult (C, 3m Control, n=11. 3m Smo cKO, n=15, 2way ANOVA with Tukey’s post-hoc test) or middle-aged (D, 12m Control, n=8. 12m Smo cKO, n=7, 2way ANOVA with Tukey’s post-hoc test) groups. No significant impairment in spatial memory retrieval was observed in Smo cKO mice during probe trial in either young adult (E-G, 3m Control, n=10. 3m Smo cKO, n=15, Students’ t-test) or middle-aged (H-J, 12m Control, n=8. 12m Smo cKO, n=7, Students’ t-test) groups. Exposure to contextual fear conditioning (K) revealed significantly higher post shock freezing (L) in Smo cKO mice (L). However, exposure to context on Day 2 revealed comparable freezing between genotypes (M). (K-M, Control, n=25. Smo cKO, n=21, 2way ANOVA with Tukey’s post-hoc test or Students’ t-test). All data shown are Mean±SEM. For statistical detail, see Table S1.
To evaluate spatial learning ability, we performed two different paradigms of the Barnes maze (BM) test (the standard 5-day BM and a more challenging 2-day BM test) on 3m or 12m old Smo cKO and control mice. For the standard BM tests on 3m old animals, results showed a significant difference in distance latency among days (F(3,92)=3.201, P=0.027) but not between genotypes during the 4-days acquisition training phase, suggesting both cKO and control mice learned the position of escape hole during the training phase (Fig 2, C). Probe trail showed comparable percentage of time spent or percentage of distance traveled in the target quadrant between Smo cKO and control mice (Fig 2, E-F). Meanwhile, both groups spent more than 40% of total time in the target quadrant, suggesting both groups successfully retrieved the spatial memory of the escape hole position (Fig 2, G). Similar to 3m old animals, 12m old Smo cKO and control mice showed no difference in spatial memory learning and retrieval ability during the training phase and probe trail (Fig 2, D, H-J). Consistently, challenging BM test results showed no significant difference between Smo cKO and control mice at age of 3m or 12m in the latency of time or distance traveled to the escape hole (Fig S4).
Using a contextual fear conditioning (CFC) paradigm (Fig 2, K) we assessed fear acquisition and conditioned fear memory in 3m control and Smo cKO mice. While no significant group differences were noted during pre-shock habituation on Day 1, Smo cKO mice elicited significantly higher post-shock freezing (Fig 2, L). 2way ANOVA revealed a significant effect of genotype (F(1,172)=8.943, P=0.003) and time (F(3,172)=68.96, P<0.001). Despite increased post-shock freezing on Day 1, context conditioned freezing 24h later showed no significant differences between control and Smo cKO mice (Fig 2, M), suggesting that conditioned fear memory was not impacted by Smo deficiency. Taken together, these results demonstrated that deficiency of adult neurogenesis in the Smo cKO mice does not affect spatial memory learning or the formation of contextual fear related memory.
Deficits in adult neurogenesis elevate anxiety-related behavior in young adult mice
Recently, a regulatory role of hippocampal neurogenesis in determining young adult animal anxiety-related behaviors has been suggested [27]. Here, we evaluated Smo function in regulating anxiety-related behavior in Smo cKO mice using elevated plus maze (EPM). During the test, Smo cKO and control mice traveled comparable total distance in the apparatus (Fig 3, A, D). Interestingly, 3m old Smo cKO mice showed a significantly lower percentage of time spent (P=0.0079), or distance traveled in the open arm (P=0.0164) than control mice(Fig 3, B-C), indicating elevated anxiety in Smo cKO mice. For the 12m mice, a time point when the adult neurogenesis levels are significantly declined in both groups, we did not observe any change in anxiety in Smo cKO mice compared to control mice (Fig 3, E-F).
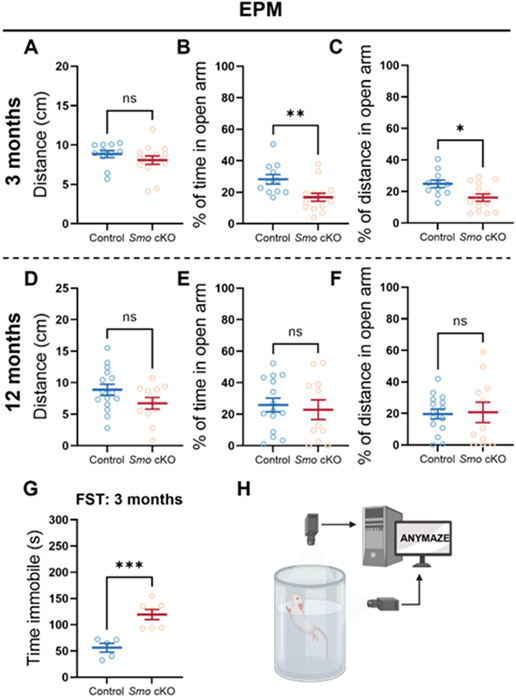
Fig 3. Deficits in adult neurogenesis in Smo cKO mice increase anxiety levels in young adult mice.
Comparable total distance traveled in the EPM during the EPM test (5min) was observed between Smo cKO and control mice (A, 3m Control, n=11. 3m Smo cKO, n=14; D, 12m Control, n=15. 12m Smo cKO, n=11, Students’ t-test). EPM test results on 3m old young adult mice showed a significantly lower percentage of time spent, or distance traveled in the open arm in Smo cKO group compared to control group, indicating elevated anxiety level (B, C). EPM test results on 12m old middle-aged mice showed comparable percentage of time spent, or distance traveled in the open arm between Smo cKO group and control group, indicating similar anxiety level (E, F). Smo cKO mice exhibit increased immobile time compared to control group mice in Forced Swim Test, indicating increased depression-like behavior in young-adult cKO mice. (G, H, Control, n=5. Smo cKO, n=7, Students’ t-test). All data shown are Mean±SEM. * p<0.05, ** p<0.01, *** p<0.001. For statistical detail, see Table S1.
Next, to evaluate anxiety level in a less stressful environment, we performed zone-based open field test [28]. During 20min test, both groups at 3m traveled similar total distance (Fig S5, B). Interestingly, 3m old Smo cKO mice spent significantly less time in the center region (P=0.0177) than control mice (Fig S5, C). Further analysis showed animals from both groups spent similar average time in the center area in the initial 10 minutes, then control mice start to explore more in the center area from 11 minutes to 20 minutes while Smo cKO mice spent significantly less time in the center zone (Fig S5, D). Again, these results indicated elevated animal anxiety level or less exploratory motivation in Smo cKO mice. For 12m mice, both groups traveled similar total distance during the test. However, we did not observe any difference in time spent in center zone between Smo cKO and control mice. (Fig S5, E-G).
Next, we performed the forced swim tests (FST) on 3m mice. We only performed this test on 3m old mice since middle-aged mice have a higher risk in this test. Results showed that Smo cKO mice exhibit increased immobile time (P=0.0008) than control mice (Fig 3, G-H). This result indicated that the deficits of adult neurogenesis in Smo cKO affected depression-like behavior in young-adult mice as well.
Disruption of Sonic Hedgehog signaling in GFAP+ NSCs compromise stroke-induced neurogenesis increase.
Our previous study suggests that stimulation of SHH signaling leads to increased neurogenesis and functional recovery after stroke. However, it is not clear whether SHH signaling in NSCs are required for stroke stimulated enhanced neurogenesis. To examine the role of Smo function in stroke-induced neurogenesis, we used a transient middle cerebral artery occlusion (tMCAo) stroke model in 3m Smo cKO and control group mice to mimic human stroke ischemia/reperfusion injury. To validate successful ischemic injury and to evaluate infarct size during the post-stroke acute stage, T2-weighted MRI were performed at 24hours post-surgery. Percentage of edema volume was calculated, and results showed no significant difference in average of edema volume caused by stroke between Smo cKO and control group mice (Fig 4, A-B).
Fig 4. Disruption of Sonic Hedgehog signaling impedes post-stroke neurogenesis.
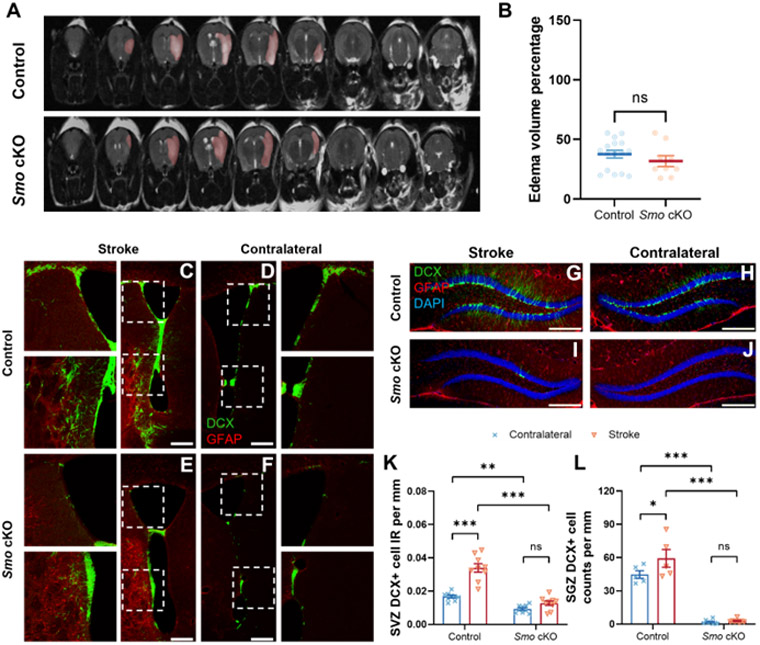
A T2-weighted MRI scan visualized infarction area following tMCAo stroke (highlighted by pink color). B Calculated infarction index showed statistical comparable infarct volume between Smo cKO and control mice (Control, n=16. Smo cKO, n=9, Students’ t-test). At one month after stroke, Immunostaining of DCX-labeled immature neurons (Green) and GFAP-labeled reactive astrocytes (Red) at SVZ region from control (C, D) or Smo cKO mice (E, F) at ipsilateral lesion side (C, E) and contralateral non-lesion side (D, F). SGZ region DCX-labeled immature neurons (Green) and GFAP-labeled reactive astrocytes (Red) from control (G, H) or Smo cKO mice (I, J) at ipsilateral lesion side (G, I) and contralateral non-lesion side (H, J). Quantification results showed significant increase of DCX+ cell IR at lesion side SVZ (K, Control: n=9. Smo cKO: n=9, 2way ANOVA with Tukey’s post-hoc test) or cell counts at lesion side SGZ (L, Control: n=5. Smo cKO: n=8, 2way ANOVA with Tukey’s post-hoc test) were observed in control stroke mice but not in Smo cKO mice. Scale bar= 250 μm. All data shown are Mean±SEM. * p<0.05, ** p<0.01, *** p<0.001. For statistical detail, see Table S1.
To evaluate the impact of SHH signaling deficiency on post-stroke neurogenesis, we analyzed neurogenesis in control or Smo cKO mice at 30 days after stroke onset, a time point we previously evaluated the stimulatory effect of SHH agonist following stroke [18]. Smo cKO mice showed significant decreased neurogenesis (DCX+ cells) at the contralateral side SVZ compared to control mice (Fig 4, D, F, K), confirming that disruption of SHH signaling leads to decreased adult neurogenesis on the non-lesion SVZ. Meanwhile, a significant increase of DCX+ cells IR at the stroke side SVZ was observed in control stroke mice, however, in Smo cKO stroke mice, SVZ DCX+ cell IR at the stroke side was at comparable low level with contralateral side, which suggests hypoxia failed to stimulate post-stroke SVZ neurogenesis in the absence of SHH signaling in NSCs (Fig 4, C, E, K).
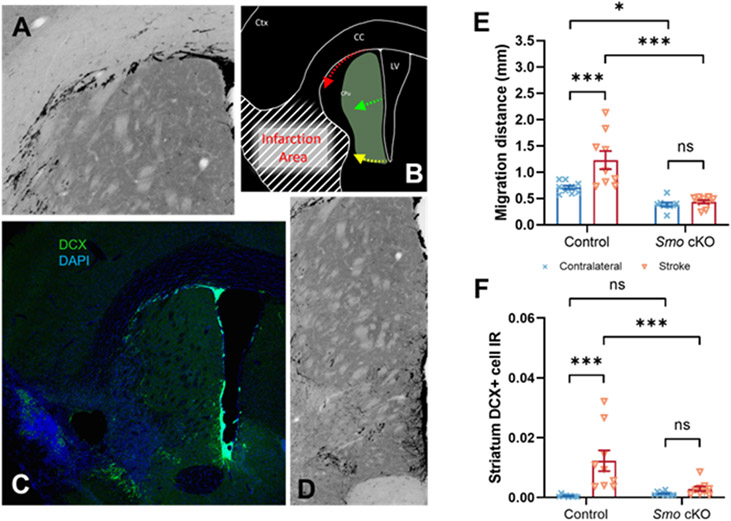
Previous studies focusing on mapping cell migration demonstrated a distinct migration pathway in the SVZ region. Neurogenic cells derived from SVZ dorsolateral corner migrate along the white matter tract (Pathway 1), while neurogenic cells derived from striatal side SVZ region migrate via perpendicular or parallel direction to the surface of ventricle wall into the striatum (Pathway 2) [29] (Fig 5, A-D). Our data showed in 30 days post injury (dpi) control mice, we observed a significant increase in migration distance via Pathway 1 which compared to the contralateral side. However, in 30dpi Smo cKO mice, this trend was not observed. DCX+ cells at either ipsilateral or contralateral side SVZ dorsolateral corner migrated similar short distance via Pathway 1 (Fig 5, E). In addition, data showed an increase of migrating striatum DCX+ cells IR via Pathway 2 at the stroke side compared to the contralateral side in 30dpi control mice. However, in 30dpi Smo cKO mice, striatum DCX+ cell IR showed a similar level at either ipsilateral or contralateral side which indicated SVZ neurogenic cell migration ability was impeded following disruption of SHH signaling (Fig 5, F).
Fig 5. Disruption of Sonic Hedgehog signaling affects post-stroke immature neuron migration.
Newly born neuroblasts derived from SVZ dorsolateral corner migrate along the white matter tract (A, B, C, Pathway 1), while newly born neuroblasts derived from striatal side SVZ region migrate via perpendicular or parallel direction to the surface of ventricle wall into the striatum (B, C, D, Pathway 2). Quantification data showed significantly decreased migration distance via Pathway 1 (E) or Pathway 2 (F) at stroke side in Smo cKO mice (Control, n=9. Smo cKO, n=9, 2way ANOVA with Tukey’s post-hoc test). All data shown are Mean±SEM. * or # p<0.05, ** or ## p<0.01, *** or ### p<0.001. For statistical detail, see Table S1.
For post-stroke hippocampal SGZ neurogenesis, we observed a significant reduction of DCX+ cell counts in 30dpi Smo cKO mice contralateral side compared to control stroke mice (Fig 4, G-J), consistent with the results observed in non-stroke mice. In response to hypoxic stimulation, SGZ region DCX+ cell counts increased significantly at the stroke side than contralateral side in control stroke mice at 30dpi. However, in stroke Smo cKO mice, few SGZ DCX+ cells were observed at stroke side suggesting that hypoxia-induced factors were not able to compensate disruption of SHH signaling to enhance post-stroke SGZ neurogenesis (Fig 4, L).
Disruption of SHH signaling delays the recovery of locomotor function in post-stroke mice.
tMCAo induced ischemic damage in sensorimotor cortex and striatum contributes to impaired animal locomotor function [30]. Meanwhile, the functional contribution of newly generated neuroblasts and neurons to the motor functional recovery is still not clear. To determine the impact of SHH signaling deficiency on post-stroke behavioral outcome, we first focused on investigating animal locomotion activity. 1 hour open-field tests were performed on the day before tMCAo surgery, and at 3, 7, 14, 21, and 28dpi. Total traveled distance and total movement time were evaluated using automated computer software. In the post-stroke acute stage, results showed comparable locomotor activity at 3dpi between Smo cKO and control mice. At 7dpi, 14dpi, and 21dpi, Smo cKO mice showed a significantly shorter total travel distance count (at 7dpi, 14dpi and 21dpi) and shorter movement time (at 7dpi) compared to the control stroke mice. Smo cKO mice recovered back to a statistically comparable level with control stroke mice at 28dpi in total distance traveled and total movement time (Fig 6, A-B). These results demonstrated that SHH signaling disruption delayed post-stroke locomotor function recovery in mice but does not affect the final plateaued level of recovery at later stage (28dpi).
Fig 6. Disruption of SHH signaling delays locomotor function recovery in post-stroke mice.
A-B Smo cKO stroke mice showed a significantly shorter total travel distance count (A, at 7dps, 14dps and 21dps) and shorter movement time (B, at 7dps) compared to the control stroke mice (Control, n=13. Smo cKO, n=12, 2way ANOVA with Tukey’s post-hoc test). BM training results demonstrated deficits in spatial learning ability after stroke in both Smo cKO and control group mice (C, 2way ANOVA with Tukey’s post-hoc test), and BM probe test results showed a comparable level between both groups on the percentage of time spent or distance traveled in the target quadrant of the apparatus that containing the escape hole (D-F, Control, n=8. Smo cKO, n=8, Students’ t-test). Comparable total distance traveled in the EPM during the test (5min) was observed between two groups (G), while Smo cKO stroke mice showed a significantly lower percentage of time spent (H), or distance traveled (I) in the open arm than control stroke mice (Control, n=21. Smo cKO, n=21, Students’ t-test). Group study results showed aggravated anxiety in Smo cKO mice after stroke (J, Baseline control: n=11, Smo cKO: n=17. Post-stroke control: n=18, Smo cKO: n=21, 2way ANOVA with Tukey’s post-hoc test). All data shown are Mean±SEM. * or # p<0.05, ** or ## p<0.01, *** p<0.001. For statistical detail, see Table S1.
Post-stoke spatial memory deficits are comparable in Smoothened conditional knockout and control mice.
Among stroke patients, cognitive impairment and memory loss are very common symptoms, and about 30% of stroke patient experience cognitive failure after stroke onset [31]. Our results in naïve control and Smo cKO mice show comparable spatial learning ability in Barnes Maze tests (Fig 2, C-J, Fig S4). To determine the post-stroke spatial learning ability, we performed standard Barnes maze tests on Smo cKO and control group mice at 30dpi. During the 4-day acquisition training phase, results showed no significant difference in distance latency between training days (F(3,56)=2.544, P=0.065) or genotypes (F(1,56)=0.081, P=0.778) or interactions (F(3,56)=1.879, P=0.144) which demonstrates deficits in spatial learning ability after stroke in both Smo cKO and control group mice (Fig 6, C). Probe test results showed both groups spent similar time or traveled similar distance in target quadrant (Fig 6, D-E). In addition, both groups showed similar percentage of time spent or distance traveled across the whole apparatus (Fig 6, F). Taken together, these data demonstrated that stroke could induce memory learning ability impairment in control mice, and neurogenic deficits in the Smo cKO did not affect the extent of cognitive deficits at 30 days after stroke.
Stroke increases animal anxiety levels while disruption of SHH signaling further aggravates anxiety in stroke mice.
Lastly, to evaluate the anxiety-related behavior in post-stroke mice following with the disruption of SHH signaling, we performed the elevated plus maze (EPM) test on Smo cKO and control mice at 14dpi. Interestingly, 14dpi Smo cKO group showed a significantly lower percentage of time spent (P=0.0024), or distance traveled (P=0.0014) in the open arm than 14dpi control mice, respectively (Fig 6, H-I (F(1,63)=18.347, P<0.001). Compared to naïve mice, stroke mice also showed significant decrease of the percentage of time spent in the open arm (F(1,63)=10.006, P=0.002). Post-hoc analysis results showed significantly increased anxiety levels in post-stroke Smo cKO mice compared to the other three groups (Fig 6, J). Taken together, these data demonstrated that stroke leads to elevated anxiety level in control mice, while decreased neurogenesis in Smo cKO mice could further aggravate anxiety in post-stroke phase.
Discussion
Role of SHH signaling in adult neurogenesis under physiological conditions.
Previous studies using either genetic or pharmacological method have demonstrated the important role of SHH signaling in adult SVZ neurogenesis [4, 15, 32], however, whether SHH signaling is critical for SGZ adult neurogenesis is unknown. Our in vitro neurosphere assay demonstrated that in the presence of EGF and bFGF, SHH is not required for the self-renewal and proliferation of SVZ or SGZ NSCs. Instead, SHH signaling plays a modulatory role in promoting proliferation of in vitro NSCs in low EGF and bFGF concentrations (1/10 of standard neurosphere culturing conditions). This response to exogenously added SHH is abolished in NSCs derived from Smo cKO mice which further validates functional loss of SHH signaling pathway in these cells. Consistent with a previous study [33] which showed Gli1+ cells at SVZ region decreasing after 2-4month from the initial Smo deletion in vivo in GFAP+ cells [34], our results also confirmed that SVZ adult neurogenesis is decreased in the Smo cKO mice (by DCX and Ki67 immunohistochemistry). Additionally, our results following the longitudinal dynamic of adult neurogenesis in 3 to 12m mice further illustrate that adult neurogenesis decline naturally with aging in control mice and the loss of SHH signaling in GFAP+ cells further accelerate this decline, leading to a premature loss of adult neurogenesis in Smo cKO mice at as early as 7m at the SVZ and SGZ. More importantly, our study, for the first time, demonstrates SGZ adult neurogenesis highly relies on normal Smo function in GFAP+ cells which is demonstrated by the almost complete depletion of SGZ DCX+ or Ki67+ cells in 7m Smo cKO mice. All these suggest that potential relevance of SHH signaling functions in regulating neurogenesis during aging. Natural reduction of neurogenesis during the aging process could potentially be the result of decreased secretion of SHH protein during aging or decreased receptor expression or downstream signaling response to SHH ligand. These hypotheses warrant further investigation in future studies.
Heterogeneity in the adult neurogenic niche has recently started to gain attention in the field and is an understudied area [35]. Previous studies have reported regional specific expression of Gli1/3 in adult mice SVZ region, which could contribute to possible functional difference of SHH signaling between the dorsal and the ventral sub-regions of SVZ[13, 32, 34]. In this study, we also discovered a heterogeneity in the dependence on Smo in dorsal vs ventral SVZ neurogenesis. Compared to the ventral SVZ region, we observed a more severe reduction of neurogenesis at the dorsal region in Smo cKO mice (Fig S2). While dorsal SVZ neurogenesis are nearly abolished in the 3m Smo cKO mice, the ventral SVZ neurogenesis are partially spared even in 7m old cKO mice. One potential explanation could be the expression pattern of the transcriptional repressor effector Gli3. Since Gli3 level is higher in the dorsal SVZ [34], constituent SHH binding and disinhibition of Smo from Patched might be required for the dorsal SVZ neurogenesis. Other possible mechanisms also include redundant non-SHH signaling mechanisms that could compensate for its function specifically at the ventral SVZ region.
Role of Sonic Hedgehog signaling pathway in post-stroke neurogenesis.
Given the limited time window to prevent neuronal loss after stroke, promoting post-stroke neurogenesis is becoming an area of interest in therapeutic development for post-stroke treatment strategy. Other group studies have demonstrated that promoting endogenous neurogenesis is a potential therapeutic strategy in the treatment of stroke disease [2, 3, 36, 37]. We have also reported that post-stroke treatment of SHH agonist (SAG) leads to increased neurogenesis and enhanced functional recovery after stroke [18]. However, whether loss of Smo in NSCs would lead to compromised neurogenesis in stroke mice or deficits in functional recovery after stroke is not clear. Our data demonstrated that SHH signaling in GFAP+ cells are required for adult neurogenesis under physiological conditions and in aged mice, however, extrinsic factors such as those upregulated during exercise or stroke could potentially compensate for loss of endogenous signaling pathways critical for neurogenesis, an example being the Notch signaling pathway [8, 38, 39, 40, 41, 42]. Our data showed injury-stimulated SVZ and SGZ neurogenesis increased at stroke side in control mice but not in Smo cKO mice. Moreover, our data showed significantly less migration of immature neurons at the peri-lesion region in stroke Smo cKO mice compared to control stroke mice. These results suggest that SHH signaling is indispensable to the post-stroke neurogenesis.
Role of Sonic Hedgehog signaling in regulating animal behaviors under physiological and post-stroke conditions.
The relevance of adult born neurons in various behavioral functions has been a focus of the adult neurogenesis field [43]. The Smo cKO mice show decreased neurogenesis at both SVZ and SGZ at different ages, which allows us to examine the functional outcome of compromised adult neurogenesis in vivo. In this study, we investigated the impact of SHH signaling modulated neurogenesis on general locomotor-related, memory-related, and anxiety-related behavioral outcomes under physiological and stroke conditions.
Locomotor function
Under physiological conditions, results either from the open-field locomotion test or from the locomotor parameter of other tests (such as total distance traveled in EPM test) demonstrate that decreased neurogenesis following with disruption of SHH signaling did not impact overall locomotor function on young adult (3m old) mice or middle-aged (12m old) mice. This is not surprising since physiologically, SVZ derived neuroblasts mainly migrate into olfactory bulb but not the adjacent striatum. During acute and subacute stage of stroke, ischemic induced locomotion deficits are common in stroke patients. In our study, we observed a significant decline of locomotor ability (such as total distance traveled and total movement time) which begins from 3dpi following with a gradual spontaneous recovery in control mice, consistent with previous reports [18]. Importantly previous studies have shown that ablation of DCX+ cells lead to more severe deficits in motor function after stroke, consistent with our data showing delayed locomotion functional recovery in Smo cKO after stroke.
Spatial memory learning/retrieval
Over the past decade, functions of adult neurogenesis in cognitive behaviors are still under debate [43, 44]. Here, our results support the notion that deficits in adult neurogenesis do not impair spatial memory learning and retrieval ability. Consistent with the study results from Hen’s group [45], our standard BM tests showed no differences in spatial memory learning or retrieval between 3m or 12 m old Smo cKO and aged matched control mice. Results from our challenging BM or CFC tests also showed no spatial or contextual fear memory impairment in Smo cKO mice. Based on our IHC analysis, a severe reduction of DCX+ at hippocampal SGZ region is already substantial at 3m following disruption of SHH signaling (reduction of 88% in DCX+ and 83% in Ki67+ cells at SGZ, Fig 1), therefore this lack of impairment in learning ability in Smo cKO is not likely due to mild or moderate loss of neurogenesis.
Previous study reported significant impairment of spatial learning ability on tMCAo induced stroke mice by performing Morris water maze test [46]. Here in this study, we chose BM in stroke mice to avoid potential confounding effects of motor deficits on the cognitive function as Morris water maze is more demanding on the motor function than the BM. We observed an impairment of spatial memory learning/retrieval function in the control stroke mice at 30dpi in the BM. However, we didn’t observe any difference between Smo cKO mice and peer control mice, supporting that compromised adult neurogenesis does not lead to further decline in general cognitive function. Taken together, we conclude that an adult neurogenesis decline under either physiological or pathological conditions does not impact the spatial memory learning/retrieval function. Interestingly, our previous study showed that treatment with SHH signaling agonist SAG led to increased adult neurogenesis and a significant improved spatial learning ability in stroke mice [18]. This suggests that although compromised adult neurogenesis does not exacerbate cognitive deficits after stroke, boosting endogenous adult neurogenesis after stroke might lead to improved outcome.
Anxiety/depression-like behavior
Post-stroke anxiety disorders are commonly reported in stroke patients, and 20% or 24% of stroke patients were reported with anxiety during the acute or in the long-term stage, respectively [47]. Meanwhile, recent studies have suggested the novel role of hippocampal neurogenesis in anxiety-related behavior [48, 49]. Our data from EPM and the zone based open field tests clearly demonstrated increased anxiety-like behavior following disruption of SHH signaling in young adult (3m) mice but not in middle-aged (12m) mice. Meanwhile, FST results also showed increased depression-like behavior in 3m old Smo cKO mice compared to control mice. In addition, although contextual fear memory at 24h post shock (day 2) was not impacted by Smo deficiency, we did observe higher post-shock freezing in Smo cKO mice on day 1. While the exact explanation for this observation is not evident, it may be an outcome of increased sensitivity to aversive exposures and is consistent with the higher anxiety-like phenotype observed in Smo cKO mice in other tests. However, the lack of difference between Smo cKO and control mice in freezing time on day 2 indicated that deficits of adult hippocampal neurogenesis minimally affect fear memory retrieval, which is consistent with previous studies [44, 50]. All together, these results suggest a critical role of Smo in regulating animal anxiety level through modulating SGZ neurogenesis. Previous studies on elderly depressed patients showed a decreased progenitor cell population at hippocampus [51] which supports a potential association of adult neurogenesis with depression. In addition, the relationship of post-stroke neurogenesis with anxiety-related behavior is not known. In this study, our data from the EPM test on post-stroke mice indicated that stroke results in elevated anxiety level while disruption of SHH signaling further aggravated the anxiety level in young Smo cKO stroke mice. Our results point to the role of adult neurogenesis in post-stroke anxiety while provides a potential pharmacological target to alleviate anxiety/depression in stroke patients.
One of the caveats in our study is that we utilized a non-inducible Cre line driven by mGFAP promoter (B6.Cg-Tg(Gfap-cre)73.12Mvs/J) to genetically knockout the loxP flanked Smo exon1 in perinatal NSCs. This Cre line is chosen based on previous reports showing the advantages of mGFAP-Cre line in studying phenotype on a populational level due to its high recombination efficiency in NSCs [34]. Previous study using Nestin-CreER mice to delete Smo gene in NSCs has demonstrated low recombination efficiency in adult Nestin-creER mice [52]. To achieve moderate reduction (about 80%) in SVZ NSCs, 16 tamoxifen injection had to be administrated over the course of 5 weeks, a timeline that is not feasible for stroke study. However, it is important to note that this mGFAP-Cre line will also mediate gene deletion in early postnatal precursors which leads to the disruption of SHH signaling in both astrocytes and NSCs in this study [21]. Taken all together, to investigate the effects of Smo loss of function in adult neurogenesis in the process of aging and to investigate the contribution of adult neurogenesis to behavioral functions under physiological and ischemic conditions, we believe this mGFAPcre driver line serves this purpose better given its high efficiency in deleting Smo gene in NSCs compared to the lower efficient in other Nestin-creER lines [52].
Supplementary Material
Acknowledgements
We would like to thank Dr. Masato Nakafuku from Cincinnati Children’s Hospital Medical Center (CCHMC) for valuable discussions during the study, Drs. Diana M. Lindquist and Elizabeth Fugate from University of Cincinnati (UC)/CCHMC NMR/MRI Animal Imaging Lab for all MRI scans, Dr. Chet Closson from UC live microscopy core for assistance in confocal microscope images. This project was supported by NIH grants to Dr. Yu Luo (R01NS091213 and R01NS107365). Dr. Sah would like to acknowledge support from her VA Merit award (2I01BX001075).
Footnotes
Declaration of interests
The authors declare no competing interests.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
REFERENCE:
- 1.Ming GL and Song H. Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron. 2011;70:687–702. 10.1016/j.neuron.2011.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dillen Y, Kemps H, Gervois P, Wolfs E and Bronckaers A. Adult Neurogenesis in the Subventricular Zone and Its Regulation After Ischemic Stroke: Implications for Therapeutic Approaches. Transl Stroke Res. 2020;11:60–79. 10.1007/s12975-019-00717-8 [DOI] [PubMed] [Google Scholar]
- 3.Rahman AA, Amruta N, Pinteaux E and Bix GJ. Neurogenesis After Stroke: A Therapeutic Perspective. Transl Stroke Res. 2021;12:1–14. 10.1007/s12975-020-00841-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahn S and Joyner AL. In vivo analysis of quiescent adult neural stem cells responding to Sonic hedgehog. Nature. 2005;437:894–7. 10.1038/nature03994 [DOI] [PubMed] [Google Scholar]
- 5.Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–8. 10.1126/science.287.5457.1433 [DOI] [PubMed] [Google Scholar]
- 6.Zhang W, Cheng J, Vagnerova K, Ivashkova Y, Young J, Cornea A, et al. Brambrink AM. Effects of androgens on early post-ischemic neurogenesis in mice. Transl Stroke Res. 2014;5:301–11. 10.1007/s12975-013-0298-6 [DOI] [PubMed] [Google Scholar]
- 7.Jiang Y, Han J, Li Y, Wu Y, Liu N, Shi SX, et al. Wang X. Delayed rFGF21 Administration Improves Cerebrovascular Remodeling and White Matter Repair After Focal Stroke in Diabetic Mice. Transl Stroke Res. 2021. 10.1007/s12975-021-00941-1 [DOI] [PubMed] [Google Scholar]
- 8.Li Z, Wang J, Zhao C, Ren K, Xia Z, Yu H and Jiang K. Acute Blockage of Notch Signaling by DAPT Induces Neuroprotection and Neurogenesis in the Neonatal Rat Brain After Stroke. Transl Stroke Res. 2016;7:132–40. 10.1007/s12975-015-0441-7 [DOI] [PubMed] [Google Scholar]
- 9.Briscoe J and Ericson J. The specification of neuronal identity by graded Sonic Hedgehog signalling. Semin Cell Dev Biol. 1999;10:353–62. 10.1006/scdb.1999.0295 [DOI] [PubMed] [Google Scholar]
- 10.Johnson RL, Laufer E, Riddle RD and Tabin C. Ectopic expression of Sonic hedgehog alters dorsal-ventral patterning of somites. Cell. 1994;79:1165–73. 10.1016/0092-8674(94)90008-6 [DOI] [PubMed] [Google Scholar]
- 11.Ericson J, Muhr J, Placzek M, Lints T, Jessell TM and Edlund T. Sonic hedgehog induces the differentiation of ventral forebrain neurons: a common signal for ventral patterning within the neural tube. Cell. 1995;81:747–56. 10.1016/0092-8674(95)90536-7 [DOI] [PubMed] [Google Scholar]
- 12.Roessler E, Belloni E, Gaudenz K, Jay P, Berta P, Scherer SW, et al. Muenke M. Mutations in the human Sonic Hedgehog gene cause holoprosencephaly. Nat Genet. 1996;14:357–60. 10.1038/ng1196-357 [DOI] [PubMed] [Google Scholar]
- 13.Ribes V and Briscoe J. Establishing and interpreting graded Sonic Hedgehog signaling during vertebrate neural tube patterning: the role of negative feedback. Cold Spring Harb Perspect Biol. 2009;1:a002014. 10.1101/cshperspect.a002014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Charytoniuk D, Traiffort E, Hantraye P, Hermel JM, Galdes A and Ruat M. Intrastriatal sonic hedgehog injection increases Patched transcript levels in the adult rat subventricular zone. Eur J Neurosci. 2002;16:2351–7. 10.1046/j.1460-9568.2002.02412.x [DOI] [PubMed] [Google Scholar]
- 15.Lai K, Kaspar BK, Gage FH and Schaffer DV. Sonic hedgehog regulates adult neural progenitor proliferation in vitro and in vivo. Nat Neurosci. 2003;6:21–7. 10.1038/nn983 [DOI] [PubMed] [Google Scholar]
- 16.Palma V, Lim DA, Dahmane N, Sanchez P, Brionne TC, Herzberg CD, et al. Ruiz i Altaba A. Sonic hedgehog controls stem cell behavior in the postnatal and adult brain. Development. 2005;132:335–44. 10.1242/dev.01567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Antonelli F, Casciati A, Tanori M, Tanno B, Linares-Vidal MV, Serra N, et al. Pazzaglia S. Alterations in Morphology and Adult Neurogenesis in the Dentate Gyrus of Patched1 Heterozygous Mice. Front Mol Neurosci. 2018;11:168. 10.3389/fnmol.2018.00168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jin Y, Barnett A, Zhang Y, Yu X and Luo Y. Poststroke Sonic Hedgehog Agonist Treatment Improves Functional Recovery by Enhancing Neurogenesis and Angiogenesis. Stroke. 2017;48:1636–45. 10.1161/STROKEAHA.117.016650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Luo F, Zhang Z, Barnett A, Bellinger TJ, Turcato F, Schmidt K and Luo Y. Cuprizone-induced demyelination under physiological and post-stroke condition leads to decreased neurogenesis response in adult mouse brain. Exp Neurol. 2020;326:113168. 10.1016/j.expneurol.2019.113168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guo W, Patzlaff NE, Jobe EM and Zhao X. Isolation of multipotent neural stem or progenitor cells from both the dentate gyrus and subventricular zone of a single adult mouse. Nat Protoc. 2012;7:2005–12. 10.1038/nprot.2012.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcia AD, Petrova R, Eng L and Joyner AL. Sonic hedgehog regulates discrete populations of astrocytes in the adult mouse forebrain. J Neurosci. 2010;30:13597–608. 10.1523/JNEUROSCI.0830-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mizrak D, Levitin HM, Delgado AC, Crotet V, Yuan J, Chaker Z, et al. Doetsch F. Single-Cell Analysis of Regional Differences in Adult V-SVZ Neural Stem Cell Lineages. Cell Rep. 2019;26:394–406 e5. 10.1016/j.celrep.2018.12.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O'Keeffe S, et al. Wu JQ. An RNA-sequencing transcriptome and splicing database of glia, neurons, and vascular cells of the cerebral cortex. J Neurosci. 2014;34:11929–47. 10.1523/JNEUROSCI.1860-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuhn HG, Dickinson-Anson H and Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chaker Z, Codega P and Doetsch F. A mosaic world: puzzles revealed by adult neural stem cell heterogeneity. Wiley Interdiscip Rev Dev Biol. 2016;5:640–58. 10.1002/wdev.248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cebrian-Silla A, Nascimento MA, Redmond SA, Mansky B, Wu D, Obernier K, et al. Alvarez-Buylla A. Single-cell analysis of the ventricular-subventricular zone reveals signatures of dorsal and ventral adult neurogenesis. Elife. 2021;10. 10.7554/eLife.67436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Revest JM, Dupret D, Koehl M, Funk-Reiter C, Grosjean N, Piazza PV and Abrous DN. Adult hippocampal neurogenesis is involved in anxiety-related behaviors. Mol Psychiatry. 2009;14:959–67. 10.1038/mp.2009.15 [DOI] [PubMed] [Google Scholar]
- 28.Walsh RM, Shen EY, Bagot RC, Anselmo A, Jiang Y, Javidfar B, et al. Hochedlinger K. Phf8 loss confers resistance to depression-like and anxiety-like behaviors in mice. Nat Commun. 2017;8:15142. 10.1038/ncomms15142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kakita A and Goldman JE. Patterns and dynamics of SVZ cell migration in the postnatal forebrain: monitoring living progenitors in slice preparations. Neuron. 1999;23:461–72. 10.1016/s0896-6273(00)80800-4 [DOI] [PubMed] [Google Scholar]
- 30.Soleman S, Yip P, Leasure JL and Moon L. Sustained sensorimotor impairments after endothelin-1 induced focal cerebral ischemia (stroke) in aged rats. Exp Neurol. 2010;222:13–24. 10.1016/j.expneurol.2009.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Al-Qazzaz NK, Ali SH, Ahmad SA, Islam S and Mohamad K. Cognitive impairment and memory dysfunction after a stroke diagnosis: a post-stroke memory assessment. Neuropsychiatr Dis Treat. 2014;10:1677–91. 10.2147/NDT.S67184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ihrie RA, Shah JK, Harwell CC, Levine JH, Guinto CD, Lezameta M, et al. Alvarez-Buylla A. Persistent sonic hedgehog signaling in adult brain determines neural stem cell positional identity. Neuron. 2011;71:250–62. 10.1016/j.neuron.2011.05.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dorr A, Sled JG and Kabani N. Three-dimensional cerebral vasculature of the CBA mouse brain: a magnetic resonance imaging and micro computed tomography study. Neuroimage. 2007;35:1409–23. 10.1016/j.neuroimage.2006.12.040 [DOI] [PubMed] [Google Scholar]
- 34.Petrova R, Garcia AD and Joyner AL. Titration of GLI3 repressor activity by sonic hedgehog signaling is critical for maintaining multiple adult neural stem cell and astrocyte functions. J Neurosci. 2013;33:17490–505. 10.1523/JNEUROSCI.2042-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Obernier K and Alvarez-Buylla A. Neural stem cells: origin, heterogeneity and regulation in the adult mammalian brain. Development. 2019;146. 10.1242/dev.156059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Parent JM, Vexler ZS, Gong C, Derugin N and Ferriero DM. Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann Neurol. 2002;52:802–13. 10.1002/ana.10393 [DOI] [PubMed] [Google Scholar]
- 37.Egawa N, Lok J, Washida K and Arai K. Mechanisms of Axonal Damage and Repair after Central Nervous System Injury. Transl Stroke Res. 2017;8:14–21. 10.1007/s12975-016-0495-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Imayoshi I, Sakamoto M, Yamaguchi M, Mori K and Kageyama R. Essential roles of Notch signaling in maintenance of neural stem cells in developing and adult brains. J Neurosci. 2010;30:3489–98. 10.1523/JNEUROSCI.4987-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xiao MJ, Han Z, Shao B and Jin K. Notch signaling and neurogenesis in normal and stroke brain. Int J Physiol Pathophysiol Pharmacol. 2009;1:192–202 [PMC free article] [PubMed] [Google Scholar]
- 40.Ables JL, Decarolis NA, Johnson MA, Rivera PD, Gao Z, Cooper DC, et al. Eisch AJ. Notch1 is required for maintenance of the reservoir of adult hippocampal stem cells. J Neurosci. 2010;30:10484–92. 10.1523/JNEUROSCI.4721-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stasiulewicz M, Gray SD, Mastromina I, Silva JC, Bjorklund M, Seymour PA, et al. Dale JK. A conserved role for Notch signaling in priming the cellular response to Shh through ciliary localisation of the key Shh transducer Smo. Development. 2015;142:2291–303. 10.1242/dev.125237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ringuette R, Atkins M, Lagali PS, Bassett EA, Campbell C, Mazerolle C, et al. Wallace VA. A Notch-Gli2 axis sustains Hedgehog responsiveness of neural progenitors and Muller glia. Dev Biol. 2016;411:85–100. 10.1016/j.ydbio.2016.01.006 [DOI] [PubMed] [Google Scholar]
- 43.Goncalves JT, Schafer ST and Gage FH. Adult Neurogenesis in the Hippocampus: From Stem Cells to Behavior. Cell. 2016;167:897–914. 10.1016/j.cell.2016.10.021 [DOI] [PubMed] [Google Scholar]
- 44.Zhang CL, Zou Y, He W, Gage FH and Evans RM. A role for adult TLX-positive neural stem cells in learning and behaviour. Nature. 2008;451:1004–7. 10.1038/nature06562 [DOI] [PubMed] [Google Scholar]
- 45.Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, Monckton JE, et al. Drew MR. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci U S A. 2006;103:17501–6. 10.1073/pnas.0607207103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gibson CL and Murphy SP. Progesterone enhances functional recovery after middle cerebral artery occlusion in male mice. J Cereb Blood Flow Metab. 2004;24:805–13. 10.1097/01.WCB.0000125365.83980.00 [DOI] [PubMed] [Google Scholar]
- 47.Campbell Burton CA, Murray J, Holmes J, Astin F, Greenwood D and Knapp P. Frequency of anxiety after stroke: a systematic review and meta-analysis of observational studies. Int J Stroke. 2013;8:545–59. 10.1111/j.1747-4949.2012.00906.x [DOI] [PubMed] [Google Scholar]
- 48.Sakalem ME, Seidenbecher T, Zhang M, Saffari R, Kravchenko M, Wordemann S, et al. Ambree O. Environmental enrichment and physical exercise revert behavioral and electrophysiological impairments caused by reduced adult neurogenesis. Hippocampus. 2017;27:36–51. 10.1002/hipo.22669 [DOI] [PubMed] [Google Scholar]
- 49.Schloesser RJ, Lehmann M, Martinowich K, Manji HK and Herkenham M. Environmental enrichment requires adult neurogenesis to facilitate the recovery from psychosocial stress. Mol Psychiatry. 2010;15:1152–63. 10.1038/mp.2010.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ko HG, Jang DJ, Son J, Kwak C, Choi JH, Ji YH, et al. Kaang BK. Effect of ablated hippocampal neurogenesis on the formation and extinction of contextual fear memory. Mol Brain. 2009;2:1. 10.1186/1756-6606-2-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lucassen PJ, Stumpel MW, Wang Q and Aronica E. Decreased numbers of progenitor cells but no response to antidepressant drugs in the hippocampus of elderly depressed patients. Neuropharmacology. 2010;58:940–9. 10.1016/j.neuropharm.2010.01.012 [DOI] [PubMed] [Google Scholar]
- 52.Balordi F and Fishell G. Mosaic removal of hedgehog signaling in the adult SVZ reveals that the residual wild-type stem cells have a limited capacity for self-renewal. J Neurosci. 2007;27:14248–59. 10.1523/JNEUROSCI.4531-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.