Abstract
Background and objectives
In spite of bone’s healing capacity, critical-size bone defect regeneration and peri-implant osseointegration are challenging. Tissue engineering provides better outcomes, but requires expensive adjuncts like stem cells, growth factors and bone morphogenic proteins. Vitamin D (Vit.D) regulates calcium and phosphorus metabolism, and helps maintain bone health. Vit.D supplements in deficient patients, accentuates bone healing and regeneration. Therefore the aim of this systematic review was to evaluate the role of adjunctive Vit.D on bone defect regeneration.
Methods
Comprehensive database search of indexed literature, published between January 1990 and June 2022, was carried out. English language articles fulfilling inclusion criteria (clinical/in vivo studies evaluating bone regeneration including osseointegration and in vitro studies assessing osteogenic differentiation, with adjunct Vit.D) were identified and screened.
Results
Database search identified 384 titles. After sequential title, abstract and full-text screening, 23 studies (in vitro – 9/in vivo – 14) were selected for review. Vit.D as an adjunct with stem cells and osteoblasts resulted in enhanced osteogenic differentiation and upregulation of genes coding for bone matrix proteins and alkaline phosphatase. When used in vivo, Vit.D resulted in early and increased new bone formation and mineralization within osseous defects, and better bone implant contact and osseointegration, around implants. Adjunct Vit.D in animals with induced systemic illnesses resulted in bone defect regeneration and osseointegration comparable to healthy animals. While systemic and local administration of Vit.D resulted in enhanced bone defect healing, outcomes were superior with systemic route.
Conclusions
Based on this review, adjunct Vit.D enhances bone defect regeneration and osseointegration. In vitro application of Vit.D to stem cells and osteoblasts enhances osteogenic differentiation. Vit.D is a potentially non-invasive and inexpensive adjunct for clinical bone regeneration and osseointegration. Long term clinical trials are recommended to establish protocols relating to type, dosage, frequency, duration and route of administration.
Keywords: Vitamin D, Cholecalciferol, Calcitriol, Ergocalciferol, Bone defect regeneration, Osseointegration
1. Introduction
One of the major challenges routinely faced in the fields of orthopedic, maxillofacial and dental surgery is the need for replacing bone lost due to trauma and pathology. In dentistry and oral surgery, dentoalveolar bone loss may further occur as a result of teeth loss, periodontal disease, systemic illnesses and ageing process (Muresan, Hedesiu et al. 2022). Over the last few decades bone defect reconstruction strategies have evolved from simple metallic implants, to vascularized and non-vascularized bone grafts, and more recently towards tissue engineering techniques (Alkindi, Ramalingam et al. 2021). Maxillofacial segmental and non-segmental defects were traditionally reconstructed using reconstruction plates and meshes, which only aimed at providing anatomic continuity of the maxilla and mandible (Hayden, Mullin et al. 2012). Although implantable meshes enabled placement of particulate graft material within the defect site, the use of autologous block bone grafts, either vascularized or non-vascularized, has facilitated functional rehabilitation of the maxillofacial skeleton through dental implants (van Gemert, Holtslag et al. 2015). In this regard, autologous vascularized bone flaps have become the reconstruction modality of choice for large critical sized segmental defects of the maxilla and mandible (Hayden et al., 2012, van Gemert et al.Holtslag, 2015).
Guided bone regeneration (GBR) was first reported as a periodontal treatment procedure to help heal dentoalveolar bone defects surrounding the tooth supporting periodontal apparatus (Retzepi and Donos 2010). GBR involves the use of particulate bone graft placed within a defect and covered by a barrier membrane to promote unhindered healing, and was borne out of the clinical successes encountered with guided tissue regeneration (GTR). In contemporary clinical practice, GBR employs a wide variety of particulate grafts including autografts, allografts, xenografts and alloplastic materials as a scaffold and resorbable collagen membranes as a barrier (Retzepi and Donos, 2010, Ramalingam et al., 2019). Nevertheless, GBR was and is still being used widely to treat small defects with at least one intact margin. Recently, translational studies have claimed the efficacy of GBR for repairing segmental bone defects stabilized by an implant (Binsalah et al., 2019, Badwelan et al., 2020, Alkindi et al., 2021). Interestingly, while GBR by itself is a promising tissue engineering technique, the challenge lies in identifying the best bone substitute which could help in complete bone defect healing within an optimal span of time (Wang, Feng et al. 2022).
Although autograft bone is considered as the gold standard for comparison, harvesting it is often associated with donor site morbidity and paucity of volume obtained (Wang, Feng et al. 2022). While allografts and xenografts are readily available with differing physical characteristics to fulfil bone grafting requirements, they are associated with the risk of disease transmission (Ramalingam, Al-Rasheed et al. 2016). Over the last few decades, natural and synthetic alloplastic bone substitutes have been preferred as a bone graft material of choice, as they avoid the pitfalls faced with autografts, allografts and xenografts (Badwelan and Alkindi (2020)). Nevertheless, alloplastic bone materials are predominantly osteoconductive and are amongst the poorest in terms of quality and quantity of regenerated bone. Thereby necessitating the use of bone regenerative adjuncts such as growth factors, stem cells and pharmacological substances with pleiotropic bone stimulation effect (Wang, Feng et al. 2022). One such adjunct factor which has been the subject of several researches involving bone regeneration and bone osseointegration is vitamin D (Vit. D) (Muresan et al., 2022, Werny et al., 2022).
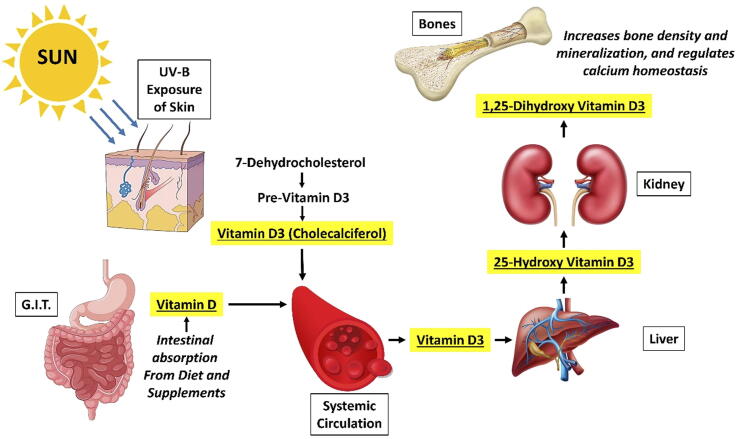
Vit. D is a fat soluble vitamin and steroid hormone which plays a major role in calcium metabolism within the body. It is most commonly obtained through diet, supplementation and exposure to ultraviolet-B/C (UV-B/UV-C) rays of sunlight (Muresan, Hedesiu et al. 2022). The two naturally available inactive forms of Vit. D, namely ergosterol (in plants) and 7-dehydrocholesterol (in animals), are activated through UV irradiation into the pro-vitamins, ergocalciferol (Vit. D2) and cholecalciferol (Vit. D3), respectively. Following absorption by the human body, the pro-vitamin is converted to its active forms through hydroxylation in the liver (25-hydroxyergocalciferol and 25-hydroxycholecalciferol) and kidney (1,25-dihydroxyergocalciferol and 1,25-dihydroxycholecalciferol) (Muresan et al., 2022, Werny et al., 2022). While both 1,25-dihydroxy forms of Vit. D2 and Vit. D3 are capable of activating the Vit. D receptor, Vit. D3 is more potent and is popularly known as calcitriol (1,25-dihydroxycholecalciferol). Biosynthesis of Vit.D, its activation and mechanism of action are graphically represented in Fig. 1.
Fig. 1.
Graphical representation of vitamin D biosynthesis and absorption, activation and mechanism of action.G.I.T. – Gastrointestinal tract; UV-B - Ultraviolet-B rays.
Vit. D plays an important role in the metabolism of calcium and phosphorus, including their intestinal absorption, renal excretion and reabsorption, and utilizing them for bone turnover and mineralization through activation of osteoclasts and osteoblasts (Werny, Sagheb et al. 2022). In addition to bone and mineral metabolism, Vit. D also influences the immune system, thereby playing a significant role in maintaining normal bone health and osseous defect healing. Studies have shown that supplementation with Vit. D and calcium, based on percentage daily requirements, reduces bone resorption and risk of fracture, through enhancement of bone mineral density (Muresan, Hedesiu et al. 2022). On the contrary, Vit. D deficiency is known to cause a wide variety of diseases affecting the bone, including osteoporosis, periodontitis and impaired fracture healing. Based on a clinical, randomized controlled trial, Schulze-Späte, Dietrich et al. (2016) reported increased osteoclastic activity in grafted sites of patients receiving Vit. D supplementation, indicating effective bone turnover leading to accentuated healing.
In light of the above evidences, it is intriguing to know the actual effect of Vit. D, administered as an adjunct either locally or systemically, on bone tissue engineering and regeneration. Therefore the aim of the present study was to systematically review the literature to identify evidences pertaining to the effect of adjunctive Vit. D on bone defect regeneration.
2. Materials and methods
Since the present study was a systematic review, only institutional research approval was obtained along with a waiver for ethical committee clearance. Based on PRISMA 2020 (Preferred Reporting Items for Systematic Reviews and Meta-analysis) guidelines (Page, McKenzie et al. 2021), the current systematic review was conducted to address the question, “What is the role of Vit. D as an adjunct in bone defect regeneration?”.
2.1. Study selection criteria and search strategy
A comprehensive literature search of published articles in English language, between January 1990 and June 2022, was conducted on popular scientific databases, including PubMed (Medline), Scopus (Elsevier), ScienceDirect (Elsevier), Cochrane database of clinical trials, Web of science (Clarivate Analytics) and Scholar (Google). The initial search was based on a combination of MeSH (Medical Subject Headings) terms including “vitamin D”, “cholecalciferol”, “ergocalciferol”, “calcitriol”, “eldecalcitol”, “Vit D3″, “bone regeneration”, “bone grafting”, “bone healing”, and “implant osseointegration” along with Boolean operators “AND”, “OR” and “NOT”. Original research articles including clinical prospective and retrospective studies, randomized controlled trials (RCTs), in vivo studies and in vitro researches fulfilling the following (PICO – Problem Intervention Comparison & Outcome) criteria were selected for the systematic review.
Problem – Bone defect regeneration including bone osseointegration around implants (clinical and in vivo), and osteogenic differentiation of cells (in vitro).
Intervention – Use of Vit. D as an adjunct, either in its active form or precursor or analogue.
Comparison – When available, clinical, in vivo or in vitro evidence of bone regeneration, implant osseointegration or osteogenic differentiation in the absence of adjunct Vit. D.
Outcome – Clinical, radiographic, histological and/or molecular biological evidence of new bone formation, new bone mineralization, implant osseointegration, osteogenic differentiation and/or expression of osteogenic mediators.
Studies which reported using Vit. D or its analogues as an adjunct for reasons other than enhancement of bone healing and regeneration mechanisms, and studies which reported the effects of Vit. D deficiency on bone metabolism were excluded from the systematic review.
2.2. Review process and data extraction
Based on the literature search, all retrieved articles were exported to a reference management software (EndNote X7, Clarivate Analytics, Philadelphia, USA). The entire review process was qualitatively evaluated by two independent observers with a kappa coefficient of 0.72 (k = 0.72). The reviewers initially screened the titles of articles to exclude duplicates and include relevant studies. This was followed by abstract screening to consider the articles’ suitability based on inclusion and exclusion criteria. Abstracts were selected when at least one of the reviewers found the study suitable for the review process. Full texts of the selected abstracts were retrieved through open access sources, institutional repositories, scientific peer-to-peer sharing databases (Researchgate, Academia…etc.), author correspondence and paid access, when other aforementioned options were ruled-out. Both the reviewers read the full text manuscripts thoroughly, with special focus on the methods and results. Additionally, the reference lists were checked to identify potentially relevant articles which may have been missed during the database search. The full texts were finally included in the review only when both reviewers agreed to the fulfillment of inclusion and exclusion criteria, and any disagreement was resolved through discussion. Cumulative data from the selected full texts were extracted onto a spreadsheet (Microsoft Excel 2016), including information about author name and year of reporting, study design, objectives, demographics, methods, type of Vit. D adjunct used and its route of administration, and outcomes (Table 1).
Table 1.
Characteristics of all the included in vitro studies in the systematic review.
| Author (year) | Study design | Study objective | Target cell/tissue/organ | Vit. D form (adjuncts if any) | Outcome |
|---|---|---|---|---|---|
| Breitbart, Grande et al. (1998) | In vitro | Study the tissue engineering capability of cultured periosteal cells for bone repair. | Cultured periosteal cells from rat calvarial bone | 1,25 Dihydroxy Vitamin D3 (Calcitriol) | Periosteal cells when cultured, exhibited osteoblastic differentiation and addition of Vit. D to the cultured induced OCN expression. |
| Saad, Casotti et al. (2000) | In vitro | Effect of Vit. D on bio functionality of cultured osteoblasts. | Osteoblasts from rat tibia, cultured on DegraPol-foam (highly porous polyesterurethane foam) | 1,25 Dihydroxy Vitamin D3 (Calcitriol) | Cultured osteoblasts in the presence of Vit. D exhibited greater spreading and reduced spindle-like morphology. Vit. D enhanced upregulation of ALP and OCN, and downregulation of collagen type 1. |
| Bosetti, Boccafoschi et al. (2007) | In vitro | Effect of Vit. D and growth factors (TGF and FGF) on osteoblast differentiation. | Cultured human primary osteoblasts | 1,25 Dihydroxy Vitamin D3 (Calcitriol) | Vit. D in combination with growth factors enhanced osteodifferentiation, ALP activity and calcium content. |
| Mansell, Nowghani et al. (2011) | In vitro | Effect of Vit. D and LPA on osteoblast differentiation. | Human bone marrow MSCs | 1,25 Dihydroxy Vitamin D3 (Calcitriol) | Vit. D and LPA exhibit a cooperative role in osteoblast differentiation of bone marrow MSCs, which has a potential role in bone repair and regeneration. |
| Nebel, Svensson et al. (2015) | In vitro | Effect of Vit. D on osteogenic differentiation and anti-inflammatory activity. | Human PDL stem cells | 1,25 Dihydroxy Vitamin D3 (Calcitriol) | Stimulation of the PDL cells in culture with Vit. D resulted in enhanced expression of OPN, OCN and ALP, indicative of osteogenic differentiation. Also, Vit. D downregulated proinflammatory cytokine IL-6, in response to LPS evoked inflammatory response in PDL cells. |
| Bosetti, Sabbatini et al. (2016) | In vitro | Compare the effect of retinoic acid and Vit. D on osteoblast activity and differentiation based on PPARγ2 activity. | Cultured human primary osteoblasts | 1,25 Dihydroxy Vitamin D3 (Calcitriol) | Considering the fact that PPARγ2 expression is an inhibitory signal in osteoblast differentiation and development, Vit. D decreased expression of PPARγ2. On the contrary, retinoic acid increased PPARγ2 expression. |
| Nah, Lee et al. (2019) | In vitro | Effect of Vit. D conjugated AuNPs on osteogenic differentiation. | Human adipose derived stem cells | Vitamin D3 conjugated with AuNPs (using thiol-polyethylene glycol) | Vit. D conjugated AuNPs promotes osteogenic differentiation of adipose derived stem cells and has potential role in bone tissue engineering and regeneration. |
| Abdelgawad, Abdelaziz et al. (2020) | In vitro | Influence of Vit. D and laser photobiomodulation on human PDL stem cells. | Human PDL stem cells | Vitamin D3 | Vit. D enhanced osteoblastic differentiation of human PDL stem cells. |
| Petrescu, Jurj et al. (2020) | In vitro | Effect of Vit. D on osteogenic differentiation. | Human dental MSCs | Vitamin D3 + Cannabidiol | Vit. D and cannabidiol enhance osteogenic differentiation of dental derived MSCs which is evidence through expression of OPN, OCN and ON. However, the response to osteoinductive stimulus varied between MSCs derived from dental pulp, dental follicle and apical papilla. |
Vit. D - Vitamin D; PDL - Periodontal ligament; TGF - Transforming growth factor; FGF - Fibroblast growth factor; PPARγ2 - Peroxisome proliferator activated receptor gamma-2; OCN - Osteocalcin; OPN - Osteopontin; PLGA - poly(D,L-lactide-co-glycolide); BIC - Bone-to-implant contact; DM - Diabetes mellitus; GBR - Guided bone regeneration; RANKL - Receptor activator of nuclear factor kappa-Β ligand; OPG - Osteoprotegerin; CKD - Chronic kidney disease; LPA - Lysophosphatidic acid; MSCs - Mesenchymal stem cells; AuNPs - Gold nanoparticles; ALP - Alkaline phosphatase; ON - Osteonectin; RT-PCR - Reverse transcription polymerase chain reaction; LPS - Lipopolysaccharide.
3. Results
3.1. Database search and study selection
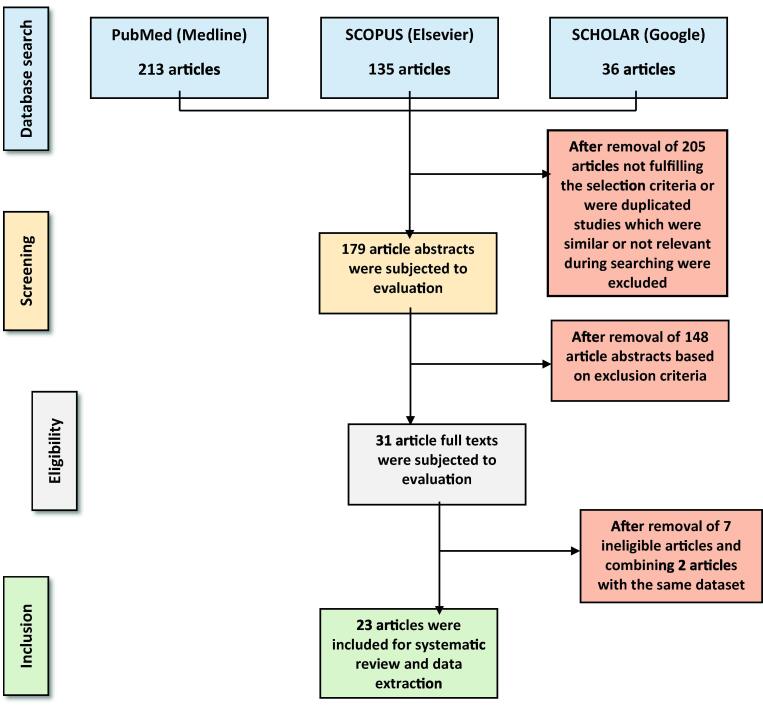
A comprehensive literature search based on specific MeSH terms returned a total of 384 articles from the different databases. After scrutinizing the article titles, 179 abstracts were identified for abstract evaluation, and the remaining were excluded as they were either duplicated or were not fulfilling the selection criteria. A thorough review of the abstracts based on inclusion and exclusion criteria resulted in the selection of 31 articles for full text evaluation. Full texts of the 31 articles were obtained and reviewed by both the reviewers until an agreement was reached regarding the selection of the article for systematic review. Finally, 24 articles agreed upon by the reviewers were considered for data extraction and systematic review. Although, two of the studies included in the full text review and published by the same authors (Salomó-Coll et al., 2016, Salomó-Coll et al., 2016) were not duplicated, they still used the same study sample and reported different outcomes. Therefore, the two studies were considered as one for the sake of systematic review and data synthesis. The entire study selection process has been illustrated in Fig. 2. While the selected studies along with the extracted data are as shown in Table 1, Table 2, studies excluded after full text review and the reasons for their exclusion are shown in Table 3.
Fig. 2.
Flow chart illustrating the selection process of articles for the systematic review.
Table 2.
Characteristics of all the included in vivo studies in the systematic review.
| Author (year) | Study design | Study objective | Target cell/tissue/organ | Vit. D form (adjuncts if any) | Route of administration of Vit. D | Outcome |
|---|---|---|---|---|---|---|
| Yoon, Park et al. (2007) | In vivo | Effect of Vit. D loaded PLGA scaffolds on critical size bone defect regeneration. | Critical size (1.5 cm), segmental, diaphyseal defect in a rabbit femur model + MSCs in PLGA scaffold | 1,25 Dihydroxy Vitamin D3 (Calcitriol) | Locally, through Vit. D loaded PLGA scaffold | Based on radiographic evidence, after 9 weeks of healing bony union was seen in defects grafted with Vit. D loaded PLGA scaffold, and restoration of normal femur anatomy after 20 weeks. RT-PCR showed overexpression of ALP, ON and m-RNA for type 1 collagen by the MSCs seeded in PLGA. |
| Uysal, Amasyali et al. (2009) | In vivo | Effect of Vit. D analog on bone regeneration in orthodontically expanded sutures. | Orthodontically expanded mid palatal suture in a rat model | ED-71 (a hydroxypropoxy derivative of 1,25 Dihydroxy Vitamin D3) | Locally administered as an injection in the mid palatal suture (0.8 µg/kg) | Vit. D analog (ED-71) administration resulted in early bone regeneration within the expanded mid palatal sutures. |
| Cho, Heo et al. (2011) | In vivo | Effect of coating anodized titanium implant surfaces with submicroscopic PLGA/Vit. D mixture (electrospray technique) on bone tissue response. | Bone implant interface in a rabbit tibia model | 1,25 Dihydroxy Vitamin D3 (Calcitriol) + PLGA | Local (in the form electro sprayed submicron coating) | After 4 weeks and 12 weeks of healing, Vit. D/PLGA coated implants had an overall increased BIC% than uncoated implants. This difference was statistically significant (p < 0.05) for the first four consecutive implant threads. |
| Dvorak, Fügl et al. (2012) | In vivo | Effect of dietary Vit. D supplementation on implant osseointegration. | Bone implant interface in the tibia of a rat model, with ovariectomy induced osteoporosis | 1,25 Dihydroxy Vitamin D3 (Calcitriol) | Systemic (2400 IU/kg; orally; once weekly) | Vit. D depleted diet in ovariectomized rats resulted in decreased cortical BIC%, which returned to the level of control animals following dietary Vit. D repletion. |
| Hong, Chou et al. (2012) | In vivo | Effect of Vit. D and calcium supplementation on alveolar bone regeneration. | Standardized defects created in healed mandibular extraction sockets in a beagle dog model | Cholecalciferol | Systemic (1.56 µg; orally; once weekly) | After 4 weeks of healing, supplementation with Vit. D and calcium resulted in more new bone formation, greater bone density and lesser vertical ridge reduction, in both grafted and non-grafted defects. Although grafting significantly reduced vertical ridge resorption, it led to lower bone density and poorer primary implant stability. |
| Zhou, Li et al. (2012) | In vivo | Effect of Vit. D on implant osseointegration in osteoporosis. | Bone implant interface in the tibia of an ovariectomized rat model | 1,25 Dihydroxy Vitamin D3 (Calcitriol) | Systemic (0.1 µg/kg; intragastrically; once daily) | After 8 weeks of healing, implant osseointegration was significantly superior in Vit. D treated osteoporotic rats, based on increased bone area and density, higher BIC% and greater resistance to push-out. |
| Wu, Yu et al. (2013) | In vivo | Effect of Vit. D and insulin on implant osseointegration in diabetic rats. | Bone implant interface in the femur of a rat model with streptozotocin induced type 1 DM | 1,25 Dihydroxy Vitamin D3 (Calcitriol) | Systemic (12 µg/kg; intragastrically; once weekly) | After 12 weeks of healing, implants in the “Vit. D + insulin” treatment group showed enhanced peri-implant new bone formation, better BIC% and greater resistance to push-out, comparable to that of control animals. |
| Liu, Zhang et al. (2014) | In vivo | Effect of Vit. D supplementation on improving implant stability in CKD. | Bone implant interface in the femur of a mouse model, with nephrectomy induced CKD | 1,25 Dihydroxy Vitamin D3 (Calcitriol) | Systemic (100 ng/kg; intraperitoneally; thrice weekly) | After 2 weeks of implant placement, there was significantly greater bone volume and BIC% in CKD mice supplemented with Vit. D. Moreover, systemic Vit. D supplementation showed evidence of reversal of hyperparathyroidism caused due to CKD. |
| Fügl, Gruber et al. (2015) | In vivo | Effect of local applied Vit. D on alveolar bone regeneration. | Maxillary and mandibular bone defects in a Vit. D deficient rat model | 1,25 Dihydroxy Vitamin D3 (Calcitriol) | Local (soaked in collagen) | Single local application of Vit. D in maxillary and mandibular alveolar bone defects does not enhance bone regeneration. |
| Hong, Yen et al. (2015) | In vivo | Effect of systemic and local Vit. D on alveolar bone defect regeneration. | Standardized defects created in healed mandibular extraction sockets in a beagle dog model | Cholecalciferol (systemic) and 1,25 Dihydroxy Vitamin D3 (Calcitriol - local) | Systemic (1.56 µg; orally; once weekly) and Calcitriol 80 IU (locally through injection; once weekly + mixing with alloplast) | After 4 weeks of healing, both systemic supplementation and local application of Vit. D resulted in accelerated bone regeneration. However, systemic Vit. D administration produced a greater stimulating effect in terms of new bone formation and bone density, leading to better primary implant stability. |
| Salomó-Coll, Maté-Sánchez de Val et al. (2016a, 2016b) | In vivo | Effect of Vit. D surface coating on immediate implant osseointegration. | Mandibular extraction socket in a dog model | 1,25 Dihydroxy Vitamin D3 (Calcitriol) | Surface coating by soaking in a 10% Vit. D solution | After 12 weeks of healing, Vit. D coated implants had reduced crestal bone loss and a 10% increase in BIC. |
| Han, Du et al. (2017) | In vivo | Effect of systemic Vit. D analog on GBR. | Femoral bone defect in a rat model | Eldecalcitol | Systemic (50 ng/kg; intragastrically; once in 2 days) | Systemic eldecalcitol administration enhanced GBR with increased bone volume and mineralization. Additionally, eldecalcitol administration suppressed osteoclastic activity through down regulation of RANKL/OPG and upregulation of OCN. |
| Cignachi, Ribeiro et al. (2020) | In vivo | Effect of Vit. D and insulin on bone regeneration in type 1 DM. | Femoral bone defect in a mouse model with streptozotocin induced type 1 DM | 1,25 Dihydroxy Vitamin D3 (Calcitriol) and insulin | Systemic (4 μg/kg; orally; once daily) | Either Vit. D or insulin improved bone regeneration in mice with type 1 DM. Combination of insulin and Vit. D had no adjunctive effect. |
| He, Zhang et al. (2020) | In vivo | Effect of Vit. D loaded titanium nanotubes on early osseointegration. | Bone implant interface in a rabbit femur model | 1,25 Dihydroxy Vitamin D3 (Calcitriol) | Local delivery through incorporation in porous titanium nanotubes | Histological analysis of the implanted porous titanium nanotube scaffolds revealed effective osseointegration with bone ingrowth and early mineralization. |
Vit. D - Vitamin D; PDL - Periodontal ligament; TGF - Transforming growth factor; FGF - Fibroblast growth factor; PPARγ2 - Peroxisome proliferator activated receptor gamma-2; OCN - Osteocalcin; OPN - Osteopontin; PLGA - poly(D,L-lactide-co-glycolide); BIC - Bone-to-implant contact; DM - Diabetes mellitus; GBR - Guided bone regeneration; RANKL - Receptor activator of nuclear factor kappa-Β ligand; OPG - Osteoprotegerin; CKD - Chronic kidney disease; LPA - Lysophosphatidic acid; MSCs - Mesenchymal stem cells; AuNPs - Gold nanoparticles; ALP - Alkaline phosphatase; ON - Osteonectin; RT-PCR - Reverse transcription polymerase chain reaction; LPS - Lipopolysaccharide.
Table 3.
Details of studies excluded after full text review and the reason for exclusion.
| Author(year) | Reason for exclusion from review |
|---|---|
| Cooper (2000) | Reported the effect of Vit. D as a systemic factor along with other systemic effectors on alveolar bone healing and repair. |
| Gogolewski and Gorna (2006) | Reported the effect of Vit. D as a confounding variable in polymer based, biodegradable bone graft substitutes used for regeneration of iliac crest defects in a sheep model. |
| Kelly, Lin et al. (2009) | Reported the effect of systemic Vit. D deficiency on implant osseointegration in a rat model. |
| Bashutski and Kinney (2012) | A single clinical case reporting primarily the effect of teriparatide along with Vit. D and calcium supplementation on bone regeneration within an intrabony periodontal defect. |
| Mostafa, Fitzsimmons et al. (2012) | Reported the simultaneous influence of Vit. D, dexamethasone, FGF and BMP on the osteogenic differentiation of MSCs. |
| Pimentel, Casarin et al. (2016) | Reported the effect of micronutrient supplementation including Vit. D, calcium, magnesium and zinc on peri-implant bone healing in a rat model. |
| Xiong, Zhang et al. (2017) | Reported the combined effect of Vit. D administration and local FoxO1 inhibition on implant osseointegration in a mouse model, with induced diabetes mellitus. |
Vit. D - Vitamin D; FGF - Fibroblast growth factor; BMP - Bone morphogenetic protein; MSCs - Mesenchymal stem cells; FoxO1 - Forkhead transcription factor 1.
3.2. General characteristics of included studies
Out of the 23 studies included for systematic review, nine studies were in vitro experimental studies conducted using either cultured osteoblasts, periosteal cells or mesenchymal stem cells (MSCs). The remaining 14 studies were based on in vivo evaluation of bone defect regeneration in either rat, rabbit, mouse or dog animal models. While the biochemically active, “1,25-dihydroxycholecalciferol” (1,25-Dihydroxy Vitamin D3 / Calcitriol) was the commonly used adjunctive form of Vit. D, studies also reported using vitamin D3 (cholecalciferol) and eldecalcitol. The detailed characteristics of all the included studies are elucidated in Table 1, Table 2.
3.3. Use of vitamin D as an in vitro adjunct
Among the reviewed in vitro studies, Vit. D was mainly used as an adjunct to enhance osteogenic differentiation of cultured cells. Mesenchymal stem cells derived from human bone marrow, adipose and dental tissues (pulp, periodontal ligament, dental follicle and apical papilla) where predominantly used as the target cell. Additionally, human primary osteoblasts and osteoblasts derived from rat calvarium and tibia were reportedly used as target cells. All studies excepting two, used only Vit. D as the adjunct. In those two studies reported by Nah et al., 2019, Petrescu et al., 2020, in addition to Vit. D, gold nanoparticles and cannabidiol were also used as adjuncts, respectively. In terms of evaluating osteogenic expression and osteoblastic differentiation of the target cells upon exposure to Vit. D, both morphological and biochemical parameters were studied. While this was evidenced morphologically by greater cell spreading and reduction in spindle-shaped cells, expression of several osteogenic markers, enzymes and proteins served as biochemical evidence. The reviewed studies reported enhanced expression of osteocalcin (OCN), alkaline phosphatase (ALP), osteoprotegerin (OPG), Osteopontin (OPN) and osteonectin (ON). Moreover, downregulation of collagen type-1 and pro-inflammatory interleukin-6 (IL 6) were also reported in the review. (Table 1).
3.4. Use of vitamin D as an in vivo adjunct
Among the 14 reviewed in vivo studies, eight studies evaluated the role of Vit. D on bone defect regeneration and five studies evaluated implant osseointegration through bone formation in the bone implant interface. Enhancement of bone formation in the mid palatal suture with Vit. D analogue after orthodontic expansion, in a rat model, was evaluated in one study (Uysal, Amasyali et al. 2009). Majority of the studies (6 out of 14) used a rat animal model with bone defects or mini implants situated in the maxilla, mandible, tibia or femur bones. While rabbits and beagle dogs were used as the animal model in three studies, respectively, a mouse model was used only in two studies. The studies using a dog model were mainly conducted to evaluate bone regeneration in mandibular extraction sockets. On the other hand, the rabbit, rat and mouse models were used for evaluation of both bone defect regeneration and implant osseointegration. The active form of Vit. D (calcitriol) was used as the adjunct in all in vivo studies, except for three studies which either used a hydroxypropoxy derivative of calcitriol (ED-71) (Uysal, Amasyali et al. 2009), cholecalciferol (Hong, Chou et al. 2012), or eldecalcitol (Han, Du et al. 2017).
Vitamin D or its analogues where administered as adjuncts through local and systemic routes of administration. Studies wherein the effect of locally administered Vit. D was evaluated, it was done with the help of a scaffold such as PLGA [poly(D,L-lactide-co-glycolide)], collagen or porous titanium nanotubes. Additionally, implants electro sprayed with a submicron coating of Vit. D + PLGA were tested for enhanced osseointegration, in a rabbit tibia model, by Cho, Heo et al. (2011). Systemic administration of Vit. D as an adjunct was predominantly through the enteral route (orally or intragastrically), and none of the studies reported parenteral injection of Vit. D. Nevertheless, there was heterogeneity between the studies in terms of the dose and frequency of systemically administered Vit. D. Only one study reported using combined systemic (oral) and local administration (mixing with alloplast) of Vit. D, for GBR of mandibular extraction sockets in a dog model (Hong, Yen et al. 2015). They inferred better outcomes of GBR with systemic administration than with local application, in spite of both strategies generally enhancing bone regeneration with Vit. D as an adjunct. While majority of the in vivo studies reported in this review were conducted on healthy animals, few studies also reported the role of Vit. D as an adjunct for bone regeneration in animals with systemic illnesses. Osteoporosis induced through ovariectomy in a rat model was reported by two studies (Dvorak Fügl et al., 2012, Zhou et al., 2012), streptozotocin induced diabetes mellitus (DM) in rat and mouse models were reported by two studies (Wu et al., 2013, Cignachi et al., 2020), and one study reported nephrectomy induced chronic kidney disease (CKD) in a mouse model (Liu, Zhang et al. 2014). In all the above studies, mimicking an in vivo scenario of compromised health, Vit. D was administered systemically as an adjunct and was shown to enhance bone regeneration and implant osseointegration.
Among the 14 reviewed in vivo studies reporting the role of Vit. D as an adjunct, half of them (7 studies) evaluated bone defect regeneration and the remaining assessed osseointegration and bone formation around implants. Within studies evaluating role of Vit. D on bone defect regeneration, there was considerable heterogeneity with respect to the defect site, graft or scaffold used, method and timing of assessment, and route of Vit. D administration. Both radiographic and histologic assessments of osseous defect healing were reportedly used to detect newly formed bone and its mineral density, and this was in addition to studying expression of osteogenic markers. Majority of the defects were grafted with bone substitutes or were treated using scaffolds such as PLGA or collagen, and the defects were evaluated after approximately 4 – 9 weeks of healing. Similarly, heterogeneity was also observed among reviewed studies evaluating osseointegration in terms of site of implant placement, implant surface coating, local or systemic Vit. D administration, time allowed for osseointegration and testing methods. In almost all of these cases, bone formation around the implant was measured using histological bone implant contact (BIC %), after around 4 – 12 weeks of implant placement. Additionally, few studies also employed an implant push out test to physically evaluate osseointegration. (Table 2).
4. Discussion
The physiology of bone bestows it with an inherent healing capacity, which forms the basis of treatment and recovery following reduction and fixation of fractures. However, bone defects of a critical nature, either segmental or non-segmental, would not heal spontaneously without grafting with autografts, allografts, xenografts or alloplasts (Wang, Feng et al. 2022). While the concept of GBR, first described by Dahlin in 1988, was originally applied to treat non-segmental bone defects, the advent of tissue engineering strategies which could be used along with GBR have enabled treatment of segmental bone defects too (Ramalingam et al., 2019, Alkindi et al., 2021, Wang et al., 2022). Studies about tissue engineering as an adjunct to GBR have predominantly employed growth factors, stem cells and cytokines incorporated within the graft materials, scaffolds and barrier membranes (Wang, Feng et al. 2022). Interestingly, the role of Vit. D as an adjunct to bone regeneration has not been evaluated extensively, in spite of knowledge about the biochemical and physiological functions of Vit. D in bone metabolism (Markopoulos, Lepetsos et al. 2021).
The present review was therefore undertaken to identify evidence from the literature which could prove a potentially beneficial role for Vit. D, when used as an adjunct for bone regeneration. Since the number of studies evaluating the role of Vit. D on bone regeneration alone was limited, the review was expanded to include studies assessing bone regeneration around implants and those studying Vit. D as an adjunct to tissue engineering through target cells (Table 1, Table 2, Table 3). Unfortunately, no clinically relevant studies evaluating the adjunct role of Vit. D on bone regeneration, either within osseous defects or around implants, were identified during the selection process. Incidentally, in a similar review published recently, Muresan, Hedesiu et al. (2022) identified no more than three clinical studies and that too reporting only a causal relationship between Vit. D deficiency, bone defect healing and osseointegration.
Among all the studies reviewed currently, majority of them were conducted on animal models (14 studies, Table 2) and the remainder were in vitro researches (Table 1). Nevertheless all of the studies specifically evaluated the role of Vit. D or its analogues as an adjunct. The primary focus of all the reviewed in vitro studies was to evaluate osteogenic differentiation in target cells and evaluating their ability to express osteogenic markers such as OCN, ALP, OPN, and ON. In addition to promoting gene expression of the aforementioned osteogenic proteins, Vit. D has also been reported to inhibit osteoclast formation, decrease breakdown of extracellular matrix and accentuate differentiation of MSCs into the osteogenic lineage (Markopoulos, Lepetsos et al. 2021). Similar outcomes were also observed when periosteal cells and periodontal ligament (PDL) cells were cultured with Vit. D as an adjunct, as reported respectively by Breitbart et al., 1998, Nebel et al., 2015.
Based on the present review, in vitro culture of Vit. D and MSCs from varied sources, including bone marrow, adipose tissue, dental pulp, developing tooth germ, and PDL, resulted invariably in osteogenic differentiation of the stem cells. Additionally, osteoblasts when cultured with Vit. D resulted in morphological characteristics favoring bone formation (increased cell spreading and decreased spindle shape), and biochemical responses inhibiting pro-inflammatory cytokines and anti-osteogenic receptors (Saad et al., 2000, Bosetti et al., 2007, Bosetti et al., 2016). It is important to note that gene expression of bone matrix proteins (ON, OCN, OPG, and OPN) in a site of osseous healing, along with expression of the enzyme ALP, is indicative of matrix mineralization (Berezin, 2017, Abdelgawad et al., 2020). This was reflected in the outcomes of the reviewed in vitro studies, wherein Vit. D as an adjunct induced expression of bone matrix proteins and ALP in the target cells, as and when they differentiated into an osteogenic lineage.
Out of the 14 reviewed in vivo studies, Vit. D was used as an adjunct for enhancing either bone defect regeneration or implant osseointegration, in equal proportions, and there was a discernible benefit evidenced in all the studies. The use of scaffolds such as osteoconductive bone substitutes, collagen, PLGA, etc. form an integral part of GBR and critical size osseous defect healing protocols (Wang, Feng et al. 2022). Not only do these scaffolds act in space maintenance, but also help in bone formation through migration of osteoblasts, and mineralization of the newly formed bone matrix, like in the calcium phosphate containing bioceramics (Al-Qutub and Al-Omar (2016)). Furthermore, the presence of a scaffold within a bone regeneration site, with or without a barrier membrane, aids in preventing fibrous soft tissue migration which may compromise defect healing (Ramalingam, Al-Rasheed et al. 2016). Among the reviewed studies which evaluated bone regeneration, all of them except one study by Uysal, Amasyali et al. (2009), used a resorbable scaffold which also doubled up as a carrier for Vit. D, whenever it was locally applied (Yoon et al., 2007, Fügl et al., 2015).
Based on the review, irrespective of whether Vit. D was administered locally or systemically, there was greater new bone formation in the defects as early as 4 weeks and until 9 weeks. Similarly, mineralization of the newly formed bone was also observed as a result of adjunctive Vit. D usage. This was further reinforced by the findings of Yoon, Park et al. (2007), who reported near complete restoration of femur anatomy after 20 weeks of treating bone defects with Vit. D loaded PLGA scaffold. They further reported, through RT-PCR, an upregulation of genes for bone matrix proteins, ALP and collagen type 1 (Yoon, Park et al. 2007). Similar increased gene expression for OPG, OPN and RANKL (Receptor activator of nuclear factor kappa-Β ligand) was reported also with eldecalcitol, a Vit. D analogue (Han, Du et al. 2017). Eldecalcitol or ED-71, with the chemical name “1α, 25-dihydroxy-2β-[3-hydroxypropyloxy] vitamin D3”, is known to dually affect bone and calcium metabolism, and is used to treat osteoporosis and as a prophylactic supplement in elderly patients. Interestingly, eldecalcitol (ED-71) was reported to help in early bone formation of orthodontically expanded mid-palatal sutures in a rat model and even in the absence of a scaffold (Uysal, Amasyali et al. 2009). The only study in the present review reporting no potential benefit of Vit. D during bone regeneration with Vit. D was by Fügl, Gruber et al. (2015). However, their study was based on local application of Vit. D soaked collagen scaffold in jaw bone defects of a rat model, with induced Vit. D deficiency. The authors claimed that a single local application of Vit. D within a bone defect site provides is of no use when there is underlying systemic deficiency (Fügl, Gruber et al. 2015). The same was concurred by Hong, Yen et al. (2015), who reported a greater stimulating effect on bone regeneration through systemic administration of Vit. D, than by local application.
The key criterion for success of dental implants is the functional and mechanical integration of the implant surface with the surrounding bone, rightly termed as osseointegration (Werny, Sagheb et al. 2022). Although several local and systemic factors affect osseointegration, the most important are those that are related bone turnover and healing in the implantation site, which in turn is similar to conventional osseous healing albeit surrounding the implant surface. In the present review, seven studies evaluated the role of adjunct Vit. D on osseointegration through animal models, and all of them were in agreement with the beneficial effect of Vit. D, irrespective of the route of administration. Majority of the studies used systemic Vit. D as an adjunct, and noted increased BIC % and resistance to push out as early as 2 – 4 weeks after placement and until 12 weeks. Only three studies employed a local route of administration for Vit. D, and they were through implant surface coating (Cho, Heo et al. 2011), soaking the implant in 10% Vit. D solution (Salomó-Coll, Maté-Sánchez de Val et al. 2016), and porous titanium nanotubes (He, Zhang et al. 2020).
Similar to systemic administration, locally applied adjunct Vit. D also resulted in enhanced BIC % and in addition demonstrated reduced crestal bone loss (Cho et al., 2011, Salomó-Coll et al., 2016). Comparing local and systemically administered Vit. D for grafted mandibular extraction sockets in a dog model, Hong, Yen et al. (2015) reported better primary implant stability based on ISQ (Implant stability quotient) values. While their study did not assess osseointegration through BIC % or push out test, they noted that the overall outcomes were favorable with systemic Vit. D administration. Systemic illnesses such as DM, CKD and osteoporosis hinder osseointegration and contribute to implant failure (Werny, Sagheb et al. 2022). Based on the reviewed in vivo studies conducted on animal models with induced systemic illness, Vit. D significantly enhanced implant osseointegration. Systemically administered calcitriol in osteoporotic rats (induced by ovariectomy) led to implant osseointegration, which was quantitatively comparable to that of healthy animals (Dvorak Fügl et al., 2012, Zhou et al., 2012). Similarly, Vit. D and insulin administered systemically to rats with streptozotocin induced DM significantly enhanced implant osseointegration parameters to the level of healthy animals (Wu, Yu et al. 2013). Moreover, intraperitoneal Vit. D administration in mice with nephrectomy induced CKD not only increased BIC % around implants, but also helped reverse hyperparathyroidism associated with end stage renal failure (Liu, Zhang et al. 2014).
In an effort to mitigate issues faced with different bone grafting techniques, bone tissue engineering using bone substitutes in combination with MSCs and growth factors has gained prominence (Alkindi et al., 2021, Wang et al., 2022). As has been observed by several studies in the present review, Vit. D by itself is capable of fulfilling the role of an adjunct in bone tissue engineering, thereby eliminating the need for MSCs or growth factors (Markopoulos et al., 2021, Muresan et al., 2022, Werny et al., 2022). However, it must be noted through the in vitro findings of this review that Vit. D could accentuate the cellular response of stem cells and push them to preferentially differentiate into osteogenic cells. This is in fact proven to a great extent through the results of the study by Yoon, Park et al. (2007), wherein critical size rabbit femoral defects could be healed to normalcy using a combination of Vit. D, MSC and PLGA scaffold. Surprisingly, despite PLGA scaffold being only osteoconductive and not contributing in any part to matrix mineralization, the authors reported radiographic evidence of complete bone defect healing (Yoon, Park et al. 2007). All of the above make it interesting to surmise that a combination of Vit. D, with MSCs and bone substitute at a GBR site could possibly be the bone tissue engineering option to vouch for in the future.
Another area of tissue engineering that has gained significance in the last decade is the incorporation of nanotechnology (Wang, Feng et al. 2022). In the present review, two studies reported using nanotechnology along with Vit. D as an adjunct. Nah, Lee et al. (2019) reported the use of Vit. D conjugated with gold nanoparticles resulting in in vitro osteogenic differentiation of adipose derived stem cells. Using Vit. D incorporated porous titanium nanotube scaffolds in a rabbit femur defect model, He, Zhang et al. (2020) reported early bone ingrowth and mineralization. Although these findings indicate the potential promise which nanotechnology holds for optimizing delivery and use of Vit. D as an adjunct, they need to be established through further long term in vitro and in vivo studies. Evidences from the reviewed studies and also from the literature clearly point to the beneficial role of using Vit. D as an adjunct in bone matrix formation, early mineralization and osseointegration (Muresan, Hedesiu et al. 2022). However, in order to realize the real benefit of using Vit. D as an adjunct to bone regeneration, it is imperative that more clinical studies are conducted through extrapolation of outcomes already published based on in vitro and in vivo studies.
Although the present review was comprehensively conducted to address the focused question about the role of adjunct Vit. D on bone regeneration, it was not without its share of limitations. One major limitation was the heterogeneity of reviewed studies, which prevailed in terms of study sample, target cell used in vitro, animal model used in vivo, type of Vit. D or its analogue used, route of Vit. D administration, nature of bone substitute or scaffold used, and outcomes evaluated. Owing to the heterogeneity of collated data, no statistically relevant meta-analysis could be conducted despite the availability of quantitative results in few studies. Nevertheless, the review was able to provide evidence from literature regarding the relevance of Vit. D as an adjunct for bone regeneration and implant osseointegration. Furthermore, this was in line with what was inferred from the outcomes of a recently published, similar systematic review (Muresan, Hedesiu et al. 2022). Yet another limitation of this review was the inability to qualitatively assess the included studies using a risk of bias assessment tool. This could be attributed to the predominantly in vitro or in vivo nature of the included studies, without control groups and no clear prospective or retrospective sampling.
5. Conclusion
Based on the evidences obtained through the present review, it can be concluded that Vit. D when used as an adjunct enhances bone regeneration within osseous defects and improves osseointegration around implants. Moreover, in vitro application of Vit. D to target cell culture comprising of osteoblasts or stem cells results in expression of osteogenic factors, and upregulation of genes coding for bone matrix proteins and alkaline phosphatase. Although no formidable clinical evidence was identified through the review, considering the non-invasive and relatively inexpensive nature of Vit. D, its use as an adjunct in clinical bone regeneration and osseointegration should be highly encouraged. In this regard, long term multicentric clinical studies and RCTs are highly recommended to establish clinical protocols relating to type, dosage, frequency, duration and route of Vit. D administration.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University. Production and hosting by Elsevier.
Contributor Information
Ramalingam Sundar, Email: drsundar_mds@yahoo.com.
A. Bhagavandas Rai, Email: drbrai@yahoo.com.
J. Naveen Kumar, Email: naveenjayakumar@gmail.com.
Darshan Devang Divakar, Email: darshandevang@gmail.com.
References
- Abdelgawad L.M., Abdelaziz A.M., et al. Influence of photobiomodulation and vitamin D on osteoblastic differentiation of human periodontal ligament stem cells and bone-like tissue formation through enzymatic activity and gene expression. Biomol. Concepts. 2020;11(1):172–181. doi: 10.1515/bmc-2020-0016. [DOI] [PubMed] [Google Scholar]
- Alkindi, M., S. Ramalingam, et al. (2021). “Guided bone regeneration with osteoconductive grafts and PDGF: A tissue engineering option for segmental bone defect reconstruction.” J Appl Biomater Funct Mater19: 2280800020987405. [DOI] [PubMed]
- Al-Qutub M.N., Al-Omar N.A., et al. Guided bone regeneration using biphasic calcium phosphate with adjunct recombinant human bone morphogenetic protein-2 with and without collagen membrane in standardized calvarial defects in rats: a histologic and biomechanical analysis. Int. J. Periodontics Restorative Dent. 2016;36(Suppl):s11–s20. doi: 10.11607/prd.2376. [DOI] [PubMed] [Google Scholar]
- Badwelan M., Alkindi M., et al. The efficacy of recombinant platelet-derived growth factor on beta-tricalcium phosphate to regenerate femoral critical sized segmental defects: longitudinal in vivo micro-CT study in a rat model. J. Invest. Surg. 2020;33(5):476–488. doi: 10.1080/08941939.2018.1519048. [DOI] [PubMed] [Google Scholar]
- Bashutski J.D., Kinney J.S., et al. Systemic teriparatide administration promotes osseous regeneration of an intrabony defect: a case report. Clin. Adv. Periodontics. 2012;2(2):66–71. doi: 10.1902/cap.2012.110043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berezin A.E. In: Biomarkers in Bone Disease. Patel V.B., Preedy V.R., editors. Dordrecht, Springer; Netherlands: 2017. Bone-Related Proteins as Markers in Vascular Remodeling; pp. 1023–1043. [Google Scholar]
- Binsalah M.A., Ramalingam S., et al. Guided bone regeneration of femoral segmental defects using equine bone graft: an in-vivo micro-computed tomographic study in rats. J. Invest. Surg. 2019;32(5):456–466. doi: 10.1080/08941939.2018.1441343. [DOI] [PubMed] [Google Scholar]
- Bosetti M., Boccafoschi F., et al. Effect of different growth factors on human osteoblasts activities: a possible application in bone regeneration for tissue engineering. Biomol. Eng. 2007;24(6):613–618. doi: 10.1016/j.bioeng.2007.08.019. [DOI] [PubMed] [Google Scholar]
- Bosetti M., Sabbatini M., et al. Effect of retinoic acid and vitamin D3 on osteoblast differentiation and activity in aging. J. Bone Miner. Metab. 2016;34(1):65–78. doi: 10.1007/s00774-014-0642-2. [DOI] [PubMed] [Google Scholar]
- Breitbart, A. S., D. A. Grande, et al. (1998). “Tissue engineered bone repair of calvarial defects using cultured periosteal cells.” Plast Reconstr Surg101(3): 567-574; discussion 575-566. [DOI] [PubMed]
- Cho Y.J., Heo S.J., et al. Promotion of osseointegration of anodized titanium implants with a 1α,25-dihydroxyvitamin D3 submicron particle coating. Int. J. Oral Maxillofac. Implants. 2011;26(6):1225–1232. [PubMed] [Google Scholar]
- Cignachi N.P., Ribeiro A., et al. Bone regeneration in a mouse model of type 1 diabetes: influence of sex, vitamin D3, and insulin. Life Sci. 2020;263 doi: 10.1016/j.lfs.2020.118593. [DOI] [PubMed] [Google Scholar]
- Cooper L.F. Systemic effectors of alveolar bone mass and implications in dental therapy. Periodontol. 2000;2000(23):103–109. doi: 10.1034/j.1600-0757.2000.2230110.x. [DOI] [PubMed] [Google Scholar]
- Dvorak G., Fügl A., et al. Impact of dietary vitamin D on osseointegration in the ovariectomized rat. Clin. Oral Implants Res. 2012;23(11):1308–1313. doi: 10.1111/j.1600-0501.2011.02346.x. [DOI] [PubMed] [Google Scholar]
- Fügl A., Gruber R., et al. Alveolar bone regeneration in response to local application of calcitriol in vitamin D deficient rats. J. Clin. Periodontol. 2015;42(1):96–103. doi: 10.1111/jcpe.12342. [DOI] [PubMed] [Google Scholar]
- Gogolewski S., Gorna K., et al. Regeneration of bicortical defects in the iliac crest of estrogen-deficient sheep, using new biodegradable polyurethane bone graft substitutes. J. Biomed. Mater. Res. A. 2006;77(4):802–810. doi: 10.1002/jbm.a.30669. [DOI] [PubMed] [Google Scholar]
- Han X., Du J., et al. Histochemical examination of systemic administration of eldecalcitol combined with guided bone regeneration for bone defect restoration in rats. J. Mol. Histol. 2017;48(1):41–51. doi: 10.1007/s10735-016-9705-0. [DOI] [PubMed] [Google Scholar]
- Hayden R.E., Mullin D.P., et al. Reconstruction of the segmental mandibular defect: current state of the art. Curr. Opin. Otolaryngol. Head Neck Surg. 2012;20(4):231–236. doi: 10.1097/MOO.0b013e328355d0f3. [DOI] [PubMed] [Google Scholar]
- He P., Zhang H., et al. 1α,25-Dihydroxyvitamin D3-loaded hierarchical titanium scaffold enhanced early osseointegration. Mater. Sci. Eng. C Mater. Biol. Appl. 2020;109 doi: 10.1016/j.msec.2019.110551. [DOI] [PubMed] [Google Scholar]
- Hong H.H., Chou T.A., et al. The potential effects of cholecalciferol on bone regeneration in dogs. Clin. Oral Implants Res. 2012;23(10):1187–1192. doi: 10.1111/j.1600-0501.2011.02284.x. [DOI] [PubMed] [Google Scholar]
- Hong H.H., Yen T.H., et al. Association of vitamin D3 with alveolar bone regeneration in dogs. J. Cell Mol. Med. 2015;19(6):1208–1217. doi: 10.1111/jcmm.12460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly J., Lin A., et al. Vitamin D and bone physiology: demonstration of vitamin D deficiency in an implant osseointegration rat model. J. Prosthodont. 2009;18(6):473–478. doi: 10.1111/j.1532-849X.2009.00446.x. [DOI] [PubMed] [Google Scholar]
- Liu W., Zhang S., et al. Vitamin D supplementation enhances the fixation of titanium implants in chronic kidney disease mice. PLoS One. 2014;9(4):e95689. doi: 10.1371/journal.pone.0095689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansell J.P., Nowghani M., et al. Lysophosphatidic acid and calcitriol co-operate to promote human osteoblastogenesis: requirement of albumin-bound LPA. Prostaglandins Other Lipid Mediat. 2011;95(1–4):45–52. doi: 10.1016/j.prostaglandins.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Markopoulos G., Lepetsos P., et al. Possible roles of Vitamin D in bone grafting. Cureus. 2021;13(4):e14688. doi: 10.7759/cureus.14688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostafa N.Z., Fitzsimmons R., et al. Osteogenic differentiation of human mesenchymal stem cells cultured with dexamethasone, vitamin D3, basic fibroblast growth factor, and bone morphogenetic protein-2. Connect. Tissue Res. 2012;53(2):117–131. doi: 10.3109/03008207.2011.611601. [DOI] [PubMed] [Google Scholar]
- Muresan G.C., Hedesiu M., et al. Effect of Vitamin D on bone regeneration: a review. Medicina (Kaunas) 2022;58(10) doi: 10.3390/medicina58101337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nah H., Lee D., et al. Vitamin D-conjugated gold nanoparticles as functional carriers to enhancing osteogenic differentiation. Sci. Technol. Adv. Mater. 2019;20(1):826–836. doi: 10.1080/14686996.2019.1644193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebel D., Svensson D., et al. 1α,25-dihydroxyvitamin D3 promotes osteogenic activity and downregulates proinflammatory cytokine expression in human periodontal ligament cells. J. Periodontal. Res. 2015;50(5):666–673. doi: 10.1111/jre.12249. [DOI] [PubMed] [Google Scholar]
- Page M.J., McKenzie J.E., et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372 doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrescu N.B., Jurj A., et al. Cannabidiol and Vitamin D3 Impact on osteogenic differentiation of human dental mesenchymal stem cells. Medicina (Kaunas) 2020;56(11) doi: 10.3390/medicina56110607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimentel S.P., Casarin R.C., et al. Impact of micronutrients supplementation on bone repair around implants: microCT and counter-torque analysis in rats. J. Appl. Oral Sci. 2016;24(1):45–51. doi: 10.1590/1678-775720150293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramalingam S., Al-Rasheed A., et al. Guided bone regeneration in standardized calvarial defects using beta-tricalcium phosphate and collagen membrane: a real-time in vivo micro-computed tomographic experiment in rats. Odontology. 2016;104(2):199–210. doi: 10.1007/s10266-015-0211-8. [DOI] [PubMed] [Google Scholar]
- Ramalingam S., Alrayyes Y.F., et al. Lateral periodontal cyst treated with enucleation and guided bone regeneration: a report of a case and a review of pertinent literature. Case Rep. Dent. 2019;2019:4591019. doi: 10.1155/2019/4591019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retzepi M., Donos N. Guided Bone Regeneration: biological principle and therapeutic applications. Clin. Oral Implants Res. 2010;21(6):567–576. doi: 10.1111/j.1600-0501.2010.01922.x. [DOI] [PubMed] [Google Scholar]
- Saad B., Casotti M., et al. In vitro evaluation of the biofunctionality of osteoblasts cultured on DegraPol-foam. J. Biomater. Sci. Polym. Ed. 2000;11(8):787–800. doi: 10.1163/156856200744011. [DOI] [PubMed] [Google Scholar]
- Salomó-Coll, O., J. E. Maté-Sánchez de Val, et al. (2016). “Osseoinductive elements for promoting osseointegration around immediate implants: a pilot study in the foxhound dog.” Clin Oral Implants Res27(12): e167-e175. [DOI] [PubMed]
- Salomó-Coll O., Maté-Sánchez de Val J.E., et al. Topical applications of vitamin D on implant surface for bone-to-implant contact enhance: a pilot study in dogs part II. Clin. Oral Implants Res. 2016;27(7):896–903. doi: 10.1111/clr.12707. [DOI] [PubMed] [Google Scholar]
- Schulze-Späte U., Dietrich T., et al. Systemic vitamin D supplementation and local bone formation after maxillary sinus augmentation - a randomized, double-blind, placebo-controlled clinical investigation. Clin. Oral Implants Res. 2016;27(6):701–706. doi: 10.1111/clr.12641. [DOI] [PubMed] [Google Scholar]
- Uysal T., Amasyali M., et al. Effect of ED-71, a new active Vitamin D analog, on bone formation in an orthopedically expanded suture in rats. a histomorphometric study. Eur. J. Dent. 2009;3(3):165–172. [PMC free article] [PubMed] [Google Scholar]
- van Gemert J., Holtslag I., et al. Health-related quality of life after segmental resection of the lateral mandible: free fibula flap versus plate reconstruction. J. Craniomaxillofac. Surg. 2015;43(5):658–662. doi: 10.1016/j.jcms.2015.03.018. [DOI] [PubMed] [Google Scholar]
- Wang B., Feng C., et al. Recent advances in biofunctional guided bone regeneration materials for repairing defective alveolar and maxillofacial bone: a review. Jpn. Dent. Sci. Rev. 2022;58:233–248. doi: 10.1016/j.jdsr.2022.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werny J.G., Sagheb K., et al. Does vitamin D have an effect on osseointegration of dental implants? a systematic review. Int. J. Implant Dent. 2022;8(1):16. doi: 10.1186/s40729-022-00414-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y.Y., Yu T., et al. Vitamin D3 and insulin combined treatment promotes titanium implant osseointegration in diabetes mellitus rats. Bone. 2013;52(1):1–8. doi: 10.1016/j.bone.2012.09.005. [DOI] [PubMed] [Google Scholar]
- Xiong Y., Zhang Y., et al. 1α,25-Dihydroxyvitamin D(3) increases implant osseointegration in diabetic mice partly through FoxO1 inactivation in osteoblasts. Biochem. Biophys. Res. Commun. 2017;494(3–4):626–633. doi: 10.1016/j.bbrc.2017.10.024. [DOI] [PubMed] [Google Scholar]
- Yoon S.J., Park K.S., et al. Repair of diaphyseal bone defects with calcitriol-loaded PLGA scaffolds and marrow stromal cells. Tissue Eng. 2007;13(5):1125–1133. doi: 10.1089/ten.2006.0287. [DOI] [PubMed] [Google Scholar]
- Zhou, C., Y. Li, et al. (2012). “1,25Dihydroxy vitamin D(3) improves titanium implant osseointegration in osteoporotic rats.” Oral Surg Oral Med Oral Pathol Oral Radiol114(5 Suppl): S174-178. [DOI] [PubMed]