Abstract
Background
Non-syndromic orofacial clefts (NSOFC) are among the most common congenital malformations. Several studies have investigated the association between stress and NSOFC; however, they have reported different and heterogeneous results. Therefore, this systematic review was conducted to investigate the association between maternal periconceptional stress and non-syndromic orofacial clefts in infants.
The research question was “Is maternal periconceptional stress an etiological factor for non-syndromic orofacial clefts in infants”?
Methods
Search strategy, inclusion/exclusion criteria, and data extraction from studies reporting periconceptional maternal exposure to stress and NSOFC were implemented without language restrictions. The risks of bias in the identified studies was assessed, and this information was used in the sensitivity analyses to explain heterogeneity. A meta-analysis of the extracted data was performed.
Results
Twelve eligible studies were included. Forest plot for meta-analysis of the association between maternal periconceptional exposure to stress and NSOFC among studies with adjustment for potential confounders showed a statistically significant association with an increased risk of NSOFC (odds ratio [OR]:1.17; P = 0.03), which was apparent for both cleft lip with and without palate (OR:2.07; P = 0.007) and cleft palate (OR:1.72; P = 0.003). There was a substantial heterogeneity between studies, which improved when analyzing only studies that were adjusted for potential confounders.
Conclusion
Based on the currently available evidence, maternal exposure to periconceptional stress could be considered a risk factor for NSOFCs. Therefore, we strongly recommend research investigating the effect of stress caused by the coronavirus disease-2019 pandemic on the incidence of clefts.
Keywords: Orofacial clefts, Maternal stress, Environmental etiology
1. Introduction
Orofacial clefts are among the most common congenital malformations (World Health Organization, 2003). The global prevalence of orofacial clefts is 0.45/1,000 births (Salari et al., 2022). Clefts result from the failure of tissue fusion during development (Ray and Niswander, 2012). Orofacial clefts affect speech, hearing, nutritional intake, and psychological and social development (Zeraatkar et al., 2019). As clefts impact an individual’s life from a variety of aspects, they affect the quality of life of those suffering from the disease (Zeraatkar et al., 2019).
The etiology of non-syndromic orofacial clefts (NSOFC) may fall into three main categories: environmental, genetic-environmental interactions, and genetics alone (Martinelli et al., 2020). Different environmental factors have been investigated to determine the etiology of NSOFC; however, the results are still inconclusive and require further investigation (Cheshmi et al., 2020, Hao et al., 2015, Regina Altoé et al., 2020, Shu et al., 2010, Xu et al., 2018).
The effects of cortisol administration in animal models were investigated years ago, and cortisol has been shown to cause orofacial clefts in animal models (Spain et al., 1975). To investigate these results in human models, researchers have studied the association between stress, which increases blood cortisol levels, and orofacial clefts (Carmichael et al., 2014, Carmichael and Shaw, 2000, Carmichael et al., 2007, Cheshmi et al., 2020, Chincharadze et al., 2017, Fraser and Warburton, 1964, Hao et al., 2015, Ingstrup et al., 2013, Regina Altoé et al., 2020, Sabbagh et al., 2016, Shu et al., 2010, Xu et al., 2018). In a systematic review investigating the environmental etiology of NSOFC, Moline et al. found that stress was associated with NSOFC (P < 0.001, 95% confidence interval [CI] 1.21,1.65, odds ratio [OR] 1.4) (Molina-Solana et al., 2013). Moreover, a recent systematic review by Tran et al. found an association between personal stressful life events and NSOFC (P = 0.001, 95% CI: 1.16, 2.30, OR: 1.63) (Tran et al., 2022). However, these systematic reviews have reported limited evidence and weak associations, indicating the need for further investigation. Therefore, this systematic review was conducted to investigate the association between maternal periconceptional stress and NSOFC in infants.
The research question was “Is maternal periconceptional stress an etiological factor for non-syndromic orofacial clefts in infants?
PECO (P: population, E: exposure, C: comparison, O: outcome)
Population: Mothers
Exposure: Periconceptual Stress
Comparison: Mothers of unaffected children
Outcome: NSOFC
2. Methods
2.1. Search strategy and study selection
This study aimed to determine the role of maternal periconceptional stress in the etiology of NSOFC. The study was registered with the Prospero ID CRD42022344044. The database search ended on June 3, 2022. The following databases were searched to retrieve the articles for this study: PubMed, Scopus, EBSCOhost, ProQuest, Cochrane Library, and PsycNET APA. Search terms that were used to conduct the research were: (“Mother” OR “Maternal” OR “Parental” OR “Pregnancy” OR “Gestating” OR “Gestational”) AND (“Stress” OR “life event” OR “traumatic life event” OR “life change” OR “stressful event” OR “life experience” OR “cortisol”) AND (“Cleft lip” OR “Cleft palate” OR “Cleft lip and palate” OR “Orofacial cleft”). To maximize the comprehensiveness of the search, the list of sources used in all related articles found in the above search was manually reviewed. Additionally, no restrictions were placed on the year, publication status, or language of the retrieved articles. After retrieval of all included articles, duplicates were removed. Screening was performed by carefully examining the titles and abstracts of the final article list, and unrelated articles were removed based on the inclusion and exclusion criteria. The second stage was the evaluation of articles at a full-text level, which was conducted by the three authors independently. Any article in dispute was discussed in a meeting, and a consensus was reached.
2.2. Inclusion and exclusion criteria
Inclusion criteria were studies with control or comparison groups, including case-control and cohort studies that evaluated maternal periconceptional stress. Exclusion criteria was studies with no comparison groups, genetics studies, studies comprised syndromic orofacial cleft cases and studies with missing information.
2.3. Quality assessment
Quality assessment of the included studies was performed in two ways: using the New Castle Ottawa Scale (NOS) and Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) checklist (for methodological validity and results).
2.3.1. The new Castle Ottawa
The NOS for case-control studies has three main domains that are most critical in this type of study: selection, comparability, and exposure. The three domains form a checklist with a maximum score of 10 points. We used cut-off points to categorize studies as follows: 0–6 points were considered low quality, while studies with scores of 7 and above were considered high-quality studies (Islam et al., 2016).
2.3.2. Strengthening the reporting of Observational studies in Epidemiology
The STROBE checklist consists of six main domains: the title, abstract, introduction, methods, results, and discussion. We used the methods and results sections to comprehensively evaluate the methodological validity. The scale consisted of 24 points. These 24 items represent different methodological aspects of the study, such as the sampling method, sample size determination, definition of variables and procedures, data collection tools, statistical analysis methods, and findings. The cut-off points to sort studies as poor, good, or excellent were as follows: 0–8 poor, 9–16 good, and 16 –26 excellent quality.
2.3.3. Grading of Recommendations, Assessment, development and evaluations (GRADE) for evidence certainty
GRADE was used to assess the certainty of evidence for the research question. It includes the following five domains: risk of bias, inconsistency, indirectness, imprecision, and publication bias. Each domain was evaluated and considered high, moderate, low, or very low.
2.4. Synthesis of the results
Data, frequencies, percentages, and ORs were extracted from the included studies. In studies in which the OR was not calculated, it was calculated using the reported numbers in the study. In studies in which cleft lip with/without palate (CL/P) and cleft palate (CP) were calculated separately, we added both the groups and created a new group of NSOFC. For studies that presented results of several units (cleft lip and cleft lip and palate as different outcomes), changes in the stressful life index were converted into a one-unit change in the stressful life index.
2.5. Statistical analysis
A meta-analysis was performed using the Review Manager statistical software (RevMan 5.4.1). Statistical heterogeneity was assessed using Cochran chi2 test (Q-test) and I2 statistics. I2 statistic was conducted, categorized as follows: 30 to 60%: represent moderate heterogeneity; 50 to 90%: substantial heterogeneity; and 75 to 100%, considerable heterogeneity (Higgins et al., 2022). A random-effects model was used to overcome the heterogeneity of the studies. A funnel plot was used to assess the small-study effects. The Egger’s test was used to evaluate the publication bias. The significance level was set at 0.05.
2.6. Sensitivity analysis:
A sensitivity analysis was performed to evaluate the stability of the results using subgrouping analyses. We conducted subgrouping according to the type of NSOFC (CL/P or CP), study risk of bias, and reported an adjusted OR.
3. Results
3.1. Review of the studies
The studies were reviewed based on the four-step process of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2009 flowchart, including the identification of articles, screening, review of admission criteria, and articles submitted to the meta-analysis (Fig. 1). We were able to retrieve all the studies sought for retrieval, except for one study published in 1964 that was not found through a digital or manual search (Jaworska et al., 1964). Twelve studies were eligible for inclusion in this systematic review (Table 1) (Chincharadze et al., 2017, Fraser and Warburton, 1964, Hao et al., 2015, Ingstrup et al., 2013, Regina Altoé et al., 2020, Sabbagh et al., 2016, Shu et al., 2010, Xu et al., 2018) (Carmichael et al., 2014, Carmichael and Shaw, 2000). Carmichael and Shaw studies have been conducted in various parts of the world, including the United States (n = 3), Canada (n = 1) (Fraser and Warburton, 1964), Brazil (n = 1) (Regina Altoé et al., 2020), China (n = 3) (Hao et al., 2015, Shu et al., 2010, Xu et al., 2018), Saudi Arabia (n = 1) (Sabbagh et al., 2016), Iran (n = 1) (Cheshmi et al., 2020), and Georgia (n = 1). A 10-year gap in the studies was identified between 1989 and 1999. In most studies, ascertainment of exposure and stress was conducted via a questionnaire on mothers experiencing stress or stressful life events during the periconceptional period (Carmichael and Shaw, 2000, Chincharadze et al., 2017, Fraser and Warburton, 1964, Hao et al., 2015, Regina Altoé et al., 2020, Sabbagh et al., 2016). Some studies used stressful life events, and the number of questions ranged from 3 to 11 (Carmichael et al., 2014, Carmichael and Shaw, 2000, Carmichael et al., 2007). Three studies asked direct questions with answers as yes/no about experiencing stress (Chincharadze et al., 2017, Regina Altoé et al., 2020, Sabbagh et al., 2016). These two inferred stresses were based on a history of abnormal mood (Shu et al., 2010, Xu et al., 2018). One study assessed the death of a close relative as an indicator of stress (Ingstrup et al., 2013). One inferred stressor was a history of abortion or miscarriage (Cheshmi et al., 2020). Most studies defined the periconception period as 1, 2, or 3 months before conception until the end of the first trimester (Carmichael and Shaw, 2000, Carmichael et al., 2007, Hao et al., 2015, Sabbagh et al., 2016, Xu et al., 2018). One study defined it as 6 months prior to conception till 2 years after (Carmichael et al., 2014), and three studies considered the first trimester of pregnancy (Chincharadze et al., 2017, Fraser and Warburton, 1964, Regina Altoé et al., 2020). One study defined the period as 1 year before conception to the end of the first trimester. In studies that reported response rates, there was a high and similar response rate in both cases and controls (Carmichael et al., 2014). Most studies reported results for CL/P and CP subtypes (n = 5) (Carmichael et al., 2014, Carmichael and Shaw, 2000, Carmichael et al., 2007, Fraser and Warburton, 1964). One study reported a separate CL subtype (Sabbagh et al., 2016). Six studies collectively reported NSOFC (Cheshmi et al., 2020, Ingstrup et al., 2013, Regina Altoé et al., 2020, Shu et al., 2010, Xu et al., 2018). One study reported only the OR of NSOFC (Chincharadze et al., 2017). Eight studies found a statistically significant difference between mothers of NSOFC cases and controls (Carmichael et al., 2014, Carmichael and Shaw, 2000, Carmichael et al., 2007, Fraser and Warburton, 1964, Hao et al., 2015, Sabbagh et al., 2016). However, more recent studies conducted in 2020 found no statistically significant difference between mothers of NSOFC cases and controls (Cheshmi et al., 2020, Regina Altoé et al., 2020). Some studies adjusted for confounding factors, such as race, smoking, vitamin use, obesity, folate intake, corticosteroids, gestational age, negative reproductive history, stress, and family history of NSOFC.
Fig. 1.
PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) flow chart of included studies.
Table 1.
Characteristics of included studies.
| Reference | Country | Time | Design | Sample size | Exposure Ascertainment (stress) | Timing of exposure | Sample sub categories/Exposure (%) |
AOR/OR 95% CI P-value |
Adjusting Factors |
|---|---|---|---|---|---|---|---|---|---|
| Fraser at al. 1964 | Canada | Not mentioned | case-control | Cases 246 Controls 460 |
Reported experiences of mothers were read out loud and classified as positive or negative by the researcher | First trimester | NSOFC 43/246(17.49)a | OR 4.156a95 % CI 2.42 to 7.1299a P < 0.0001a |
Not adjusted |
| Controls 22/460(4.78)a | |||||||||
| CL/P 34/187 (18.2) | OR 3.978a 95% CI 2.175a to 7.264 P < 0.0001a |
||||||||
| Controls CL/P 18/340(5.3) | |||||||||
| CP 9/59 (15.3) | OR 5.9a 95% CI 1.536 to 17.745a P = 0.0081a |
||||||||
| Controls CP 4/120 (3.3) | |||||||||
| Carmichael et al. 2000 | United States, California | 1987–1989 | case-control | CL/P 338 CP 140 Controls 710 |
Questionnaire on 3 Stressful life events (Yes/No) Death of a close relative, Divorce, Occupational loss |
1 month pre + the 1st trimester. |
NSOFC 166/478(34.73)a | OR 1.456a95% CI 1.133 to 1.872a P = 0.0034a |
Race-ethnicity and nativity mother’s education ,smoking, binge drinking, vitamin use, obesity, folate intake, corticosteroids, medications, valium, presence of abnormal TGF alpha allele |
| CL/P 120/338(35.5) | OR 1.5 95% CI 1.1–2 | ||||||||
| CP 46/140 (32.8) | OR 1.3 95% CI 0.9–2 | ||||||||
| Controls 190/710 (26.76) | |||||||||
|
Carmichael et al. 2007 |
United States | July 1999 - June 2004 | case-control | Cases 1189 Controls 695 |
Questionnaire on 18 life events (Yes/ No) |
2 months pre + 2 months after conception | CL/P 364/1189(30.6) | AOR 1.3495% CI (1.06–1.71) |
Maternal Race-ethnicity, education, age obesity, smoking, alcohol intake, neighborhood crime, folic acid intake, food insecurity |
| CP 122 /1189 (9.4) |
AOR 1.4595% CI (1.03–2.06) |
||||||||
| Controls 556/695 (80) | |||||||||
| Shu et al. 2010 | China | September 2009 to March 2010 | case-control | Cases 105 Controls 110 |
History of mood disorders in early pregnancy | Not mentioned | NSOFC 17/105 (16) Controls 8/110 (7.2) |
OR 2.46395% CI (1.014–5.982) P-value 0.041 |
Results were NS after adjustment |
| Ingstrup et al. 2013. | Denmark | 1978–2008 | cohort | Cases 3043 Control 1,768,620 |
Maternal bereavement due to death of close relatives | 1 year before con– ception to the end of the first trimester | NSOFC 78/3043 Controls 35040/1768620 |
AOR 1.2895% CI (1.01–1.61) |
|
| Carmichael et al. 2014 | United States | January 2006 – December 2009 | case-control | CP 413 CL/P 797 Controls 2974 |
Questionnaire on 7 life events (Yes/ No) |
6 months pre + 2 years post |
NSOFC 724/1210(59.8)a | OR 1.0063a95% CI (0.139 to 0.215)a P < 0.0001a |
Maternal race-ethnicity, age, education, body mass index, smoking, drinking, and intake of vitamin supplements. |
| CL/P 488/797 (61.23)a | OR 1.067a95% CI (0.9089 to 1.25)a P = 0.4290a |
||||||||
| CP 236/413 (57.143)a | OR 0.901a95% CI (0.731–1.109)a P = 0.3246a |
||||||||
| Controls 1775/2974 (59.68)a | |||||||||
| Hao et al. 2015 | China | 2009–2014 | case-control | 499 cases 480 controls |
Maternal negative life events (relationship difficulties, legal/financial problems, violence/crime, illness/injury, or a relative’s death) |
1 months pre + 3 months post | NSOFC 248/499(49.69)a |
OR 14.467a 95% CI (9.696 to 21.59)a P < 0.0001a | Maternal gestational age, Maternal and paternal education, Income, Gender, Gravidity, negative reproductive history |
| CL/P 179/499 (49.4) | AOR 2.56 95% CI (1.92–3.42) P < 0.001 | ||||||||
| CP 69/499 (50.4) | AOR 2.66 95% CI (1.81–3.90) P < 0.001 | ||||||||
| Controls 32/480 (27.5) | |||||||||
|
Sabbagh et al. 2016 |
Saudi Arabia | 2010–2012 | case-control | 217 Cases 244 Controls |
Questionnaire maternal reporting experiencing stress (Yes/No) | 3 months pre + 3 months post | NSOFC 95/204 (46.6) |
AOR 2 95% CI (1.27–3.14) P = 0.003 | Family history of NSOFC, twins versus singleton birth frequency,antibiotics in the pre-gestation and 1st trimester periods, antipyretic medication in the pre-gestation period, anti-emetic medication in the 1st trimester, maternal illness in the pre-gestation and 1st trimester periods, flu/ common cold during pre-gestation and 1st trimester periods, fever in the pre- gestation period, folic acid in the 1st trimester periods, calcium supplementation in the 1st trimester period, mothers complaining of stress, mothers with family problems, abdominal pain in the 1st trimester period, paternal waterpipe smoking, and type of maternal drinking water source. |
| CL 32/77 (41.6) | OR 1.7495% CI (1.01–3) P = 0.04 |
||||||||
| CLP 38/74 (51.4) |
AOR 395% CI (1.49–6.03) P = 0.002 |
Family history of NSOFC, twins versus singleton birth frequency, folic acid in the 1st trimester period, antibiotic in the pre-gestation period, maternal illness in the pre-gestation period, flu/ common cold in the pre-gestation period, maternal fever in the pre- gestation period, mothers complaining of stress, mothers with family problems, paternal waterpipe smoking, maternal exposure to chemicals in the pre-gestation and 1st trimester periods, maternal solvent exposure during the pre-gestation period, and type of maternal drinking water source. | |||||||
| CP 25/53 (47.3) |
AOR 2.195% CI (1.08–4.06) P = 0.028 |
calcium supplementation in the 1st trimester period, maternal illness in pre-gestation period, flu/ common cold in the pre-gestation and 1st trimester period, abdominal pain in the 1st trimester period, mothers complaining of stress, and mothers with family problems | |||||||
| Controls71/244 (29.1) |
|||||||||
| Chinchaga et al. 2017 | Georgia | 2015–2016 | case-control | Cases 41 Controls 61 |
History of stressful situation | 1st trimester | Not mentioned | AOR = 7.7695% CI (1.93 – 31.33) |
Infectious disease Non-communicable disease, use of medicine severe economic, condition, stress |
| Xu et al. 2018 | China | September 2013 and December 2016 | case-control | Cases 236 Controls 208 |
Abnormal mood | 3 months pre + 3 months post | NSOFC 36/236 Controls 19//208 (short tongue frenum |
OR 1.80095% CI (0.998 – 3.248) P = 0.049 |
Results were NS after adjustment |
| Altoe et al. 2020 | Brazil | March 2012 – September 2014 | case-control | Cases 150 Control 300 |
Questionnaire maternal reporting experiencing stress (Yes/No) | 1st trimester | Cases 67/300 (35.64) Controls 121/150 (64.36%) |
OR 1.1995 % CI (0.8–1.7) P = 0.38 |
Results were NS after adjustment |
| Cheshmi et al. 2020 | Iran | 2014–2020 | case-control | NSOFC 323 Control 400 |
History of abortion or miscarriage | Not specified | NSOFC 42/323(13) Controls 28/400 (7) |
AOR 1.33995% CI (0.761–2.355) P = 0.311026 |
Adjusted |
AOR Adjusted odds ratuo.
Calculated by the author.
3.2. Quality assessment and risk of bias
Most studies had low (high risk of bias) or moderate (moderate risk of bias) quality on both scales (Carmichael et al., 2014, Carmichael and Shaw, 2000, Carmichael et al., 2007, Cheshmi et al., 2020, Chincharadze et al., 2017, Fraser and Warburton, 1964, Hao et al., 2015, Ingstrup et al., 2013, Regina Altoé et al., 2020, Sabbagh et al., 2016, Shu et al., 2010, Xu et al., 2018) (Supplementary Tables 3-4). The low scores in most studies can be attributed to the inability to objectively assess stress (Fraser and Warburton, 1964, Hao et al., 2015, Sabbagh et al., 2016, Shu et al., 2010, Xu et al., 2018), (Carmichael et al., 2007, Chincharadze et al., 2017), (Carmichael et al., 2014, Carmichael and Shaw, 2000, Cheshmi et al., 2020, Ingstrup et al., 2013). Additionally, not all studies matched their cases and controls, even though it is known in the literature that the sex distribution of NSOFC differs, therefore comparability is affected (Meng et al., 2006). However, no study was ranked as high-quality (low risk of bias) (Supplementary Tables 3 and 4).
3.3. Meta-analysis
A meta-analysis was conducted to assess NSOFC, CL/P, and CP. A summary of the results of the meta-analysis are presented in Supplementary Table 1.
3.3.1. Non-Syndromic orofacial clefts
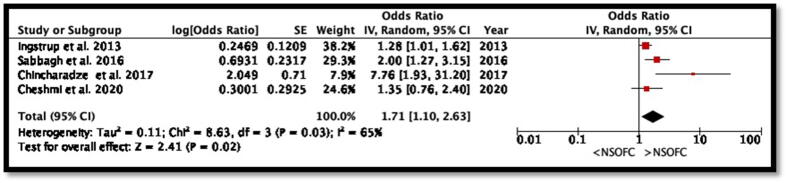
Six studies reported the ORs for NSOFC. However, five other studies calculated ORs and prevalence using the available data presented in this study. Although the association was not statistically significant; the over-all stress seems to increases the tendency of NSOFC (p = 0.09, OR: 1.77 and 95% CI: 0.92 to 3.43) (Fig. 2). However, the study heterogeneity was high (I2 98%, p < 0.00001). Four studies reported the ORs for NSOFC after adjusting for potential confounders. The OR for the meta-analysis decreased to 1.71 and was statistically significant (p = 0.03, OR: 1.71, 95% CI: 1.1 to 2.63) (Fig. 3). Additionally, although the heterogeneity from the studies that adjusted for covariates was still high, it decreased to I2 65% (p = 0.03).
Fig. 2.
Forest plot for meta-analysis of the association between maternal prenatal exposure to stress and NSOFC.
Fig. 3.
Forest plot for meta-analysis of the association between maternal prenatal exposure to stress and NSOFC among studies that adjusted for potential confounders.
3.3.2. Cleft lip with or without cleft palate
Six studies examined CL/P as a separate group, all reported ORs, and three of these were adjusted ORs. Data from all of the six studies showed no significant relationship between stress and CL/P (P = 0.48, OR: 1.54, 95% CI: 0.46 to 5.1) with high heterogeneity (I2 99%, p < 0.00001) (Supplementary Fig. 2). However, when meta-analysis was conducted on studies that were adjusted for potential confounders, the association between stress and CL/P became statistically significant (p = 0.007), and the OR increased to 2.07 (Fig. 4). The heterogeneity of the studies decreased; however, it was still significant (I2 86%, p < 0.00001).
Fig. 4.
Forest plot for meta-analysis of the association between maternal prenatal exposure to stress and CL/P in studies that adjusted for potential confounders.
3.3.3. Cleft lip
One study that reported the OR of CL alone was by Sabbagh et al. (2016) who concluded that there was a significant association between stress and CL with p = 0.04, OR: 1.74, 95% CI: 1.01 to 3.
3.3.4. Cleft palate
Five studies examined CP. Three studies reported adjusted ORs, while the others reported crude ORs. The overall effect of all the studies combined did not support that stress leads to increased incidence of CP (p = 0.55, OR: 0.54, and 95% CI: 0.07 to 4.13) (Supplementary Fig. 3). However, a meta-analysis of the three studies that reported the adjusted OR showed statistically significant association between maternal stress and CP, with adjusted OR of 1.98 (p = 0.001, and 95% CI: 1.31 to 3.01). The heterogeneity between studies was not statistically significant (I2 = 62%; p = 0.07) (Fig. 5).
Fig. 5.
Forest plot for meta-analysis of the association between maternal prenatal exposure to stress and CP among studies that adjusted for potential confounders.
3.4. Heterogeneity and small study effect
The heterogeneity between the studies investigating the association with NSOFC was high. This phenomenon can be attributed to the small study effect shown in the funnel plot (Supplementary Fig. 4). To overcome this limitation, a meta-analysis of the NSOFC phenotypes CL/P and CP was performed.
Even after subgrouping, the heterogeneity remained, but it improved. The inclusion of studies that adjusted for covariates decreased the heterogeneity from 98% to 65%. Moreover, after excluding low-quality studies from the adjusted OR analysis, it decreased even more to 10%. The Egger's test for regression intercept yielded a P-value of 0.5675, indicating no evidence of publication bias.
3.5. Certainty of evidence
A GRADE profile table was constructed to evaluate the magnitude of the effect, quality and confidence of evidence, and certainty of the outcome. The total number of infants from the 12 studies included 7,843 NSOFC infants and 1,774,711 controls. The ORs ranged from 0.173 to 14.47. Downgrading for the risk of bias was found to be related to the study design, reporting bias, and maternal factors. However, no downgrading was observed for inconsistencies. We downgraded the imprecision to one level because not all CIs were narrow. The quality of evidence was considered moderate (Supplementary Table 2).
4. Discussion
This systematic review and meta-analysis found a two-fold increase in the risk of NSOFC associated with maternal periconceptional stress. There are two theories that explain how maternal stress leads to birth defects. The first categorizes cortisol as a teratogen (Yaribeygi et al., 2017). The mechanism by which corticosteroids cause teratogenic effects has not yet been established (Xiao et al., 2017). However, two theories have been suggested to explain the mechanism of teratogenic effects on craniofacial development (Salomon and Pratt, 1979). The second is explained through the direct effect of stress on the body systems; stress activates the fight and flight response that require muscular blood profusion, as the blood in the mothers body redirects to vital organs to serve the fight and flight response, the placenta will be hypo-profused leading to in fetal damage (Wallace et al., 2011). Most of the included studies solely investigated the effect of stress on NSOFC; however, some studies examined the etiology of NSOFC by examining multiple factors. The lack of focus on stress in these studies resulted in a lower reliability of the stress ascertainment. Stress cannot be measured objectively; therefore, efforts should be made to minimize bias. When assessing stress, most studies asked mothers a question with an answer as yes/no about stress. This not only carries recall and reporter biases, but also maternal memory bias. Maternal memory bias is where mothers with affected children would look harder for reasons to explain the resulting malformation in their children. When examining stress, it is better to use validated scales or quantification of multiple questions to eliminate as many of the biases included as possible. In addition, blinding of the reporter would decrease the assumed reporter bias.
There was a considerable heterogeneity between studies, which means that there was variability in the data between the studies. Subgrouping of the data was attempted to overcome the high heterogeneity; however, high results remained. Therefore, to overcome the effect of heterogeneity on our results, a random-effects model was used.
Although the association was not statistically significant; the over-all stress seems to increases the tendency of NSOFC (p = 0.11, OR: 1.81 and 95% CI: 0.88 to 3.73) (Fig. 2). As Chinchaga et al. (2017) only reported adjusted OR, their study was included in a meta-analysis that assessed the association between NSOFC and stress using adjusted OR. The OR decreased to 1.71 when examining the studies that were adjusted for covariates. This could be attributed to the effect of Chinchaga et al., who reported a high adjusted OR without reporting its incidence. His study was considered of a poor quality according to the STROBE checklist; therefore, the latter OR should be interpreted with caution. After removing their study from the analysis, the OR was not significantly affected (OR:1.72); however, the heterogeneity between the studies decreased significantly. Therefore, the most accurate association was the following (OR: 1.72, 95% CI: 1.21–3.53) that adjusted for confounders and excluded lowest quality studies according to the NOS.
We assumed that most studies did not address or subgroup CL as a separate entity from CL/P because CL is believed to be a mild form of CL/P (Fogh-Andersen, 1971, Harville et al., 2005).
Examining the effect of stress on CL/P through a meta-analysis of the studies that reported CL/P subgroup showed that there is an association between CL/P and stress (p = 0.01. OR: 2.59, 95% CI 1.25–5.38). When investigating the studies that were adjusted for covariates, the association remains significant (p = 0.007, OR 2.02, 95% CI 1.22–3.53). However, the heterogeneity among the studies remained high because of the different approaches used in the analysis. Lastly, the analysis of the effect of stress on CP showed that stress was not associated with the development of CP (P = 0.11, OR 1.48, 95% CI 0.92–2.4). However, after that sub-grouping of the studies that adjusted for covariates was done and analyzed the association became significant (P = 0.0001, OR 1.98, 95% CI 95% CI 1.3–3.01).
We compared the methodology of our systematic review and meta-analysis with that of the recently published meta-analysis by Tran et al. (2022) who also investigated the effect of stress on orofacial clefts. We noted that, although they had no limitation on the type of studies included, they only reported the results of 4 case-control studies, compared to the 12 studies identified in our work. Additionally, our study had no language restrictions, which was not the case in a previous review that excluded studies in other languages. Language restrictions may have resulted in some studies being overlooked. Tran et al. found an association between stressful life events and orofacial clefts, which is consistent with our findings. Tran et al.’s association between stress and NSOFC is slightly weaker than ours, and this could be attributed to the larger number of pooled samples in our study. Understanding the environmental etiology of NSOFC is crucial for controlling the prevalence and incidence of the disease. A knowledge of the environmental etiology can facilitate the implementation of primary prevention programs.
5. Conclusion
Based on currently available evidence, maternal exposure to periconceptional stress could be considered a risk factor for NSOFC.
6. Future recommendations
Although some biases are inevitable, studies assessing the effect of stress on the development of NSOFC should assess stress with the least possible number of biases. This can be achieved by blinding the interviewer to eliminate the reporter bias. Additionally, stress can be better measured using either validated scales or quantification of multiple questions to increase the comprehensiveness of the measure. In addition, better-quality studies are needed to fully determine the role of stress in the etiology of NSOFC. Finally, as none of these studies were conducted during the coronavirus disease-2019 (COVID-19) pandemic, we strongly recommend further research investigating the effect of stress caused by the COVID-19 pandemic and the incidence of facial cleft.
7. Ethics approval and consent to participate
Not applicable.
8. Availability of data and materials
Data generated or analyzed during this study are available upon request from the corresponding author.
Funding
This research did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
10. Submission statement
We believe that this submission is an original work and is not under consideration for publication in any other journal.
CRediT authorship contribution statement
Mona Talal AlSharif: Conceptualization, Methodology, Resources, Data curation, Writing – original draft, Writing – review & editing. Rana Abdullah Alamoudi: Conceptualization, Methodology, Resources, Data curation, Writing – original draft, Writing – review & editing. Heba Jafar Sabbagh: Conceptualization, Methodology, Project administration, Resources, Data curation, Formal analysis, Writing – original draft, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University. Production and hosting by Elsevier.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.sdentj.2023.02.004.
Contributor Information
Mona Talal AlSharif, Email: mthalsharif@kau.edu.sa.
Rana Abdullah Alamoudi, Email: rasalamoudi@kau.edu.sa.
Heba Jafar Sabbagh, Email: hsabbagh@kau.edu.sa.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- Carmichael S.L., Ma C., Tinker S., Rasmussen S.A., Shaw G.M. Maternal stressors and social support as risks for delivering babies with structural birth defects. Paediatr. Perinat. Epidemiol. 2014;28(4):338–344. doi: 10.1111/ppe.12123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael S.L., Shaw G.M. Maternal life event stress and congenital anomalies. Epidemiology. 2000;11(1):30–35. doi: 10.1097/00001648-200001000-00008. [DOI] [PubMed] [Google Scholar]
- Carmichael S.L., Shaw G.M., Yang W., Abrams B., Lammer E.J. Maternal stressful life events and risks of birth defects. Epidemiology. 2007;18(3):356–361. doi: 10.1097/01.ede.0000259986.85239.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheshmi B., Jafari Z., Naseri M.A., Davari H.A. Assessment of the correlation between various risk factors and orofacial cleft disorder spectrum: a retrospective case-control study. Maxillofac. Plast. Reconstr. Surg. 2020;42(1) doi: 10.1186/s40902-020-00270-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chincharadze S., Vadachkoria Z., McHedlishvili I. Risk factors of cleft lip and palate in Georgia. Georgian Med. News. 2017;264:31–35. [PubMed] [Google Scholar]
- Fogh-Andersen P. Epidemiology and etiology of clefts. Birth Defects Orig. Artic. Ser. 1971;7(7):50–53. [PubMed] [Google Scholar]
- Fraser F.C., Warburton D. No association of emotional stress or vitamin supplement during pregnancy to cleft lip or palate in man. Plast. Reconstr. Surg. 1964;33:395–399. doi: 10.1097/00006534-196404000-00009. [DOI] [PubMed] [Google Scholar]
- Hao Y., Tian S., Jiao X., Mi N., Zhang B., Song T., An L., Zheng X., Zhuang D. Association of parental environmental exposures and supplementation intake with risk of nonsyndromic orofacial clefts: a case-control study in Heilongjiang Province, China. Nutrients. 2015;7(9):7172–7184. doi: 10.3390/nu7095328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harville E.W., Wilcox A.J., Lie R.T., Vindenes H., Abyholm F. Cleft lip and palate versus cleft lip only: are they distinct defects? Am. J. Epidemiol. 2005;162(5):448–453. doi: 10.1093/aje/kwi214. [DOI] [PubMed] [Google Scholar]
- Higgins, J.P.T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M.J., Welch, V.A., 2022. Cochrane handbook for systematic reviews of interventions version 6.3.
- Ingstrup K.G., Liang H., Olsen J., Nohr E.A., Bech B.H., Wu C.S., Christensen K., Li J. Maternal bereavement in the antenatal period and oral cleft in the offspring. Hum. Reprod. 2013;28(4):1092–1099. doi: 10.1093/humrep/des434. [DOI] [PubMed] [Google Scholar]
- Islam M.M., Iqbal U., Walther B., Atique S., Dubey N.K., Nguyen P.A., Poly T.N., Masud J.H.B., Li Y.C., Shabbir S.A. Benzodiazepine Use and Risk of Dementia in the Elderly Population: A Systematic Review and Meta-Analysis. Neuroepidemiology. 2016;47(3–4):181–191. doi: 10.1159/000454881. [DOI] [PubMed] [Google Scholar]
- Jaworska M., Reszke S., Kawiak H. Attempted etiologic analysis of cleft palate in 438 cases. Pol. Tyg. Lek. 1964;19:1517–1519. [PubMed] [Google Scholar]
- Martinelli M., Palmieri A., Carinci F., Scapoli L. Non-syndromic cleft palate: an overview on human genetic and environmental risk factors. Front. Cell Dev. Biol. 2020;8 doi: 10.3389/fcell.2020.592271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng T., Shi B., Zheng Q., Wang Y., Li S. Clinical and epidemiologic studies of nonsyndromic cleft lip and palate in China: analysis of 4268 cases. Ann. Plast. Surg. 2006;57(3) doi: 10.1097/01.sap.0000221623.15710.b9. [DOI] [PubMed] [Google Scholar]
- Molina-Solana R., Yáñez-Vico R.M., Iglesias-Linares A., Mendoza-Mendoza A., Solano-Reina E. Current concepts on the effect of environmental factors on cleft lip and palate. Int. J. Oral Maxillofac. Surg. 2013;42(2):177–184. doi: 10.1016/j.ijom.2012.10.008. [DOI] [PubMed] [Google Scholar]
- Ray H.J., Niswander L. Mechanisms of tissue fusion during development. Development. 2012;139(10):1701–1711. doi: 10.1242/dev.068338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regina Altoé S., Borges Á.H., Neves A., Aranha A.M.F., Borba A.M., Espinosa M.M., Volpato L.E.R. Influence of parental exposure to risk factors in the occurrence of oral clefts. J. Dent. (Shiraz) 2020;21(2):119–126. doi: 10.30476/DENTJODS.2019.77620.0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabbagh H.J., Alamoudi N.M., Abdulhameed F.D., Innes N.P., Al-Aama J.Y., Hummaida T., Almalik M., El Derwi D.A., Mossey P.A. Environmental risk factors in the etiology of nonsyndromic orofacial clefts in the western region of Saudi Arabia. Cleft Palate Craniofac. J. 2016;53(4):435–443. doi: 10.1597/14-136. [DOI] [PubMed] [Google Scholar]
- Salari N., Darvishi N., Heydari M., Bokaee S., Darvishi F., Mohammadi M. Global prevalence of cleft palate, cleft lip and cleft palate and lip: a comprehensive systematic review and meta-analysis. J. Stomatol. Oral Maxillofac. Surg. 2022;123(2):110–120. doi: 10.1016/j.jormas.2021.05.008. [DOI] [PubMed] [Google Scholar]
- Salomon D.S., Pratt R.M. Involvement of glucocorticoids in the development of the secondary palate. Differentiation. 1979;13(3):141–154. doi: 10.1111/j.1432-0436.1979.tb01577.x. [DOI] [PubMed] [Google Scholar]
- Shu S., Tang S., Wu S., Chen W. Study on risk factors of nonsyndromic cleft lip and palate in Chinese Guangdong population. Chin. J. Reparative Reconstr. Surg. 2010;24(8):962–966. [PubMed] [Google Scholar]
- Spain K.M., Kisieleski W., Wood N.K. Cleft palate induction: quantitative studies of 3H corticoids in A/Jax mouse tissues after maternal injections of 3H cortisol. J. Dent. Res. 1975;54(5):1069–1077. doi: 10.1177/00220345750540051101. [DOI] [PubMed] [Google Scholar]
- Tran C., Crawford A.A., Hamilton A., French C.E., Wren Y., Sandy J., Sharp G. Maternal stressful life events during the periconceptional period and orofacial clefts: a systematic review and meta-analysis. Cleft Palate Craniofac. J. 2022 doi: 10.1177/10556656211045553. 10556656211045553. [DOI] [PubMed] [Google Scholar]
- Wallace G.H., Arellano J.M., Gruner T.M. Non-syndromic cleft lip and palate: could stress be a causal factor? Women Birth. 2011;24(1):40–46. doi: 10.1016/j.wombi.2010.08.001. [DOI] [PubMed] [Google Scholar]
- World Health Organization, 2003. Global registry and database on craniofacial anomalies. Report of a WHO registry meeting on craniofacial anomalies. Available: https://cir.nii.ac.jp/crid/1571135650521182592. Accessed: 12 October 2022.
- Xiao W.L., Liu X.Y., Liu Y.S., Zhang D.Z., Xue L.F. The relationship between maternal corticosteroid use and orofacial clefts-a meta-analysis. Reprod. Toxicol. 2017;69:99–105. doi: 10.1016/j.reprotox.2017.02.006. [DOI] [PubMed] [Google Scholar]
- Xu D.P., Qu W.D., Sun C., Cao R.Y., Liu D.W., Du P.G. A Study on Environmental Factors for Nonsyndromic Cleft Lip and/or Palate. J. Craniofac. Surg. 2018;29(2):364–367. doi: 10.1097/SCS.0000000000004214. [DOI] [PubMed] [Google Scholar]
- Yaribeygi H., Panahi Y., Sahraei H., Johnston T.P., Sahebkar A. The impact of stress on body function: a review. Excli. J. 2017;16:1057–1072. doi: 10.17179/excli2017-480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeraatkar M., Ajami S., Nadjmi N., Faghihi S.A., Golkari A. A qualitative study of children's quality of life in the context of living with cleft lip and palate. Pediatric Health Med. Ther. 2019;10:13–20. doi: 10.2147/PHMT.S173070. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data generated or analyzed during this study are available upon request from the corresponding author.