Abstract
Objective
Trauma induces a complex immune response that requires a systems biology research approach. Here, we used a novel technology, mass cytometry by time-of-flight (CyTOF), to comprehensively characterize the multi-cellular response to trauma.
Design
Peripheral blood mononuclear cells (PBMCs) samples were stained with a 38-marker immunophenotyping CyTOF panel. Separately, matched PBMCs were stimulated in vitro with heat-killed S. pneumoniae or CD3/CD28 antibodies and stained with a 38-marker cytokine panel. Monocytes were studied for phagocytosis and oxidative burst.
Setting
Single-institution Level 1 Trauma Center.
Patients OR Subjects
Trauma patients with injury severity scores > 20 (n=10) at days 1, 3, and 5 after injury, and age- and gender-matched controls.
Interventions
None.
Measurements and Main Results
Trauma-induced expansion of Th17-type CD4+ T cells was seen with increased expression of IL-17 and IL-22 by day 5 after injury. NK cells showed reduced T-bet expression at day 1 with an associated decrease in TNFβ, IFNγ, and MCP-1. Monocytes showed robust expansion following trauma but displayed decreased stimulated pro-inflammatory cytokine production and significantly reduced HLA-DR expression. Further analysis of trauma-induced monocytes indicated that phagocytosis was no different from controls. However, monocyte oxidative burst after stimulation increased significantly after injury.
Conclusions
Using CyTOF, we were able to identify several major time-dependent phenotypic changes in blood immune cell subsets that occur following trauma, including induction of Th17-type CD4+ T cells, reduced T-bet expression by NK cells, and expansion of blood monocytes with less pro-inflammatory cytokine response to bacterial stimulation and less HLA-DR. We hypothesized that monocyte function might be suppressed after injury. However, monocyte phagocytosis was normal and oxidative burst was augmented, suggesting that their innate anti-microbial functions were preserved. Future studies will better characterize the cell subsets identified as being significantly altered by trauma using CyTOF, RNAseq technology, and functional studies.
Keywords: Trauma, immune phenotype, mass cytometry, CyTOF, immunology
Introduction
Clinical management of traumatic injury has significantly improved over the past several decades, reducing mortality (1,2). However, these incredible strides are somewhat mitigated when the trauma patient succumbs to post-injury sepsis, which increases mortality from 7.6% to 23.1% (3). Therefore, it is critically important to better understand the changes in the immune system that contribute to loss of immune function, in order to discover treatable cellular and molecular targets to reduce post-injury infection rates and reduce trauma-induced complications.
It has been known for some time that the immune system is altered by trauma (4–7), but the complexity of trauma has made it difficult to perform a comprehensive and in-depth analysis of the multiple immune cell types affected by injury in humans. In this study, we used mass cytometry by time-of-flight (CyTOF) as a systems biology tool to collect single cell phenotyping data on circulating PBMCs from severely injured trauma patients. The major advantage of CyTOF over conventional flow cytometry is that CyTOF uses antibodies that are conjugated to rare earth metal isotopes rather than fluorescent tags. This allows for improved discrimination between markers with reduced crosstalk and expands the number of markers that can be used simultaneously in a cell staining panel (8,9).
Methods
Patient Selection
Patients were enrolled from May to October 2015 from Brigham and Women’s Hospital who met the following inclusion criteria: over the age of 18 years, not pregnant, with Injury Severity Score greater than 20 (average ISS = 34), no medical history or medications predisposing immune dysregulation (for example, chemotherapy or steroid use) (10). ISS was estimated at admission and final calculation was performed after discharge. Blood was drawn at days 1, 3 and 5 after injury. Data collected included demographics, mechanism of injury and development of culture-proven infection. Blood samples were also collected from healthy, uninjured, age- and gender-matched volunteers. This study protocol was approved by the Brigham and Women’s Hospital Institutional Review Board (IRB).
Blood Sample Processing
Six ml of blood was collected in EDTA tubes. Peripheral blood mononuclear cells (PBMCs) were purified using a Ficoll density gradient (GE Healthcare Ficoll-Paque PLUS), then frozen using Cryostor CS10 (BioLife Solutions) by the manufacturer’s protocol.
CyTOF Staining Methods
PBMCs were thawed at 37° C for 3 minutes, then mixed into 37° C thawing medium consisting of RPMI 1640 supplemented with 5% heat-inactivated fetal calf serum, 1 mM glutamine, 10 mM HEPES, 2 mM nonessential amino acids, penicillin/streptomycin/amphotericin B, 2.5 × 10−5 M 2-mercaptoethanol, 20 units/ml heparin sodium salt, and 25 units/ml benzonase nuclease (Sigma-Aldrich). For the immunophenotyping stains, PBMCs were washed by centrifugation, plated, and immediately stained. For cytokine stains, PBMCs were plated into tissue culture plates (Corning) and stimulated with anti-CD3 (4 µg/ml)/anti-CD28 (2 µg/ml) antibodies (BioLegend), with 2 × 104 bacteria/ml heat-killed S. pneumoniae, or left unstimulated. Heat-killed bacteria (HKB) were prepared by exposing S. pneumonia to 95°C for 15 minutes. Cells were cultured with stimulants or no stimulation at 37°C for 30 minutes, after which Brefeldin A (Sigma) was added at a final concentration of 10 µg/ml to prevent cytokine release. After 4 hours incubation, cells were stained.
All CyTOF stains were performed at room temperature in 96-well round-bottom polypropylene plates (Corning). After washing cells by centrifugation at 200g for 5 minutes, 5 µM cisplatin viability staining reagent (Fluidigm) was added for 5 minutes. After centrifugation, TruStain FcX Fc receptor blocking reagent (BioLegend) was added for 10 minutes. CyTOF antibodies were labeled using purified antibodies from several different suppliers (BioLegend, eBioscience, R&D Systems). The CyTOF staining panels used in this study are shown in Supplemental Tables 1 and 2. The antibodies for cell-surface staining were incubated with cells for 30 minutes. After fixation and permeabilization of cells using the FoxP3 Staining Buffer Set (eBioscience), cells were barcoded using palladium barcoding reagents and combined into a single sample for pooled intracellular antibody staining for 30 minutes (11). After intracellular staining, the cells were fixed with 1.6% formaldehyde. To stain DNA, 18.75 µM iridium intercalator solution (Fluidigm) was added to the cells. Cells were subsequently washed and reconstituted in Milli-Q filtered distilled water with EQ four element calibration beads (Fluidigm), then analyzed on a Helios CyTOF Mass Cytometer (Fluidigm).
Bacteria Phagocytosis Assay
Five separate trauma patient samples and controls were selected to test monocyte phagocytosis. PBMCs were thawed and plated at 100,000 cells/well, then incubated at 37°C for 2 hours with or without the pHrodo Green S. aureus Bioparticles (ThermoFisher Scientific) prepared by the manufacturer’s protocol. After incubation, the cells were washed with PBS, stained with PE/Cy7-labeled CD14 antibody, and analyzed by flow cytometry. Percentage phagocytosis was calculated as .
Oxidative Burst Assay
Matched vials from the same 5 patients and controls were used to measure monocyte oxidative activity. PBMCs were plated and incubated at 37°C for 5 minutes with or without DHR123 (50 µM). Cells were stimulated with PMA (4 µM) for another 20 minutes, then centrifuged and fixed in 0.4% PFA for 10 minutes. The cells were stained with PE/Cy7-labeled CD14 antibody and analyzed by flow cytometry. The percentage of monocytes achieving oxidative burst was calculated as .
Statistics
CyTOF data was analyzed using Cytobank (Mountain View, CA). Gating was performed and statistics of mean expression intensity (MEI) and percentages of cell populations was exported. A sample gating strategy for CD4+ T cells, NK cells, and monocytes is provided in Supplemental Figure 1. Flow cytometry data was analyzed using FlowJo (Ashland, OR). All statistical analysis was performed in GraphPad Prism (La Jolla, CA). Paired one-way ANOVA was performed to identify statistically significant (p < 0.05) differences among multiple groups, with Tukey’s multiple comparison test where appropriate.
Results
Blood samples were collected from 10 patients within the inclusion criteria as well as 10 age- and gender-matched uninjured controls. The final demographics of the trauma patient and control groups are described in Table 1, with additional clinical data on the trauma patients in Supplemental Table 3. Of note, 4 of the patients developed subsequent infections after their injuries, ranging from post-injury day 2 to post-injury day 19. PBMCs from patients and controls were stained with CyTOF staining panels to measure immune cell subset phenotypes or to measure cytokine expression following stimulation with heat-killed bacteria (HKB) or a mixture of anti-CD3 and CD28 antibodies. Initial analysis of the trauma patient PBMC populations indicated that one patient had to be excluded from subsequent analysis due to a skewed monocytic condition.
Table 1.
Patient Demographics.
| Demographic Factor | Patient | Control |
|---|---|---|
| Mean Age (years ± SEM) | 49.4 ± 7.37 | 50 ± 6.47 |
| Male | 5 (50%) | 5 (50%) |
| Mean ISS (score ± SEM) | 38.3 ± 2.43 | n/a |
| Mean AIS (score ± SEM) | n/a | |
| Head and neck | 4.75 ± 0.25 | |
| Face | 2 ± 1 | |
| Chest | 3.6 ± 0.4 | |
| Abdomen | 3.75 ± 0.48 | |
| Extremity | 3.14 ± 0.26 | |
| External | 1.5 ± 0.5 | |
| Head Injury Present | 7 (70%) | n/a |
| Development of Culture-Proven Infection During Index Hospitalization | 4 (40%) | n/a |
| Types of Infection Developed | ||
| Urinary Tract Infection | 1 (10%) | n/a |
| Wound infection | 1 (10%) | n/a |
| Ventilator-Associated Pneumonia | 1 (10%) | n/a |
| Bacteremia | 1 (10%) | n/a |
SEM = standard error of the mean, ISS = Injury Severity Score, AIS = Abbreviated Injury Scale, n/a = not applicable.
The results from PBMC stains revealed multiple time-dependent changes in immune cell subset percentages occurring in trauma patients versus uninjured controls (Supplemental Figure 2). This report focuses primarily on the trauma-induced immune cell subset changes that were most significant.
Trauma induces an increase in Th17 CD4+ T cells
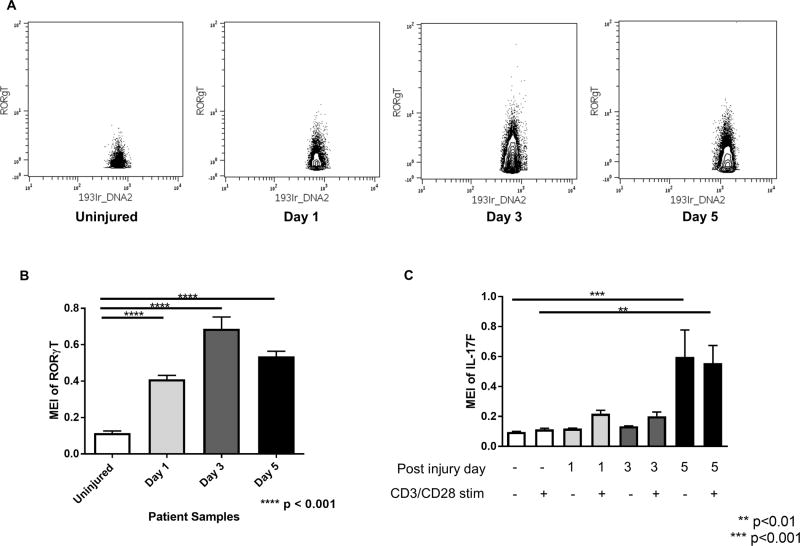
CD4+ T cells expressing RORγT, a transcription factor that identifies Th17-type CD4+ T cells, were found to expand in the blood of trauma patients at all time points after injury. The expansion of RORγT -expressing CD4+ T cells was observed visually in the CyTOF data by biaxial gating (Figure 1A) as well as statistically by plotting mean expression intensity (MEI) values for uninjured versus trauma patients (Figure 1B). We also measured the constitutive and anti-CD3/CD28 stimulated levels of cytokines associated with the Th17 phenotype, IL-17F and IL-22. We found that IL-17F and IL-22 expression in stimulated CD4+ T cells was increased, but did not demonstrate significance until day 5 after trauma at which time the production of these cytokines was constitutive (Figure 1C).
Figure 1. Trauma causes expansion of CD4+ Th17 cells all time points after injury, with constitutive activation at day 5.
A. Biaxial plots of representative samples from each experimental group showing increased RORγT expression in CD3+CD4+ gated T cells after injury, plotted as DNA+ vs RORγT+. B. RORγT expression in CD4+ T cells increases significantly at all time points after injury, with a peak at day 3. C. IL-17F expression in CD4+ T cells remains low after injury until day 5, at which time there is a high level of expression in both unstimulated samples as well as samples stimulated with anti CD3/CD28 antibodies. Statistics were performed using one-way ANOVA; results were considered significant if p < 0.05.
Changes in NK cell phenotype in response to trauma
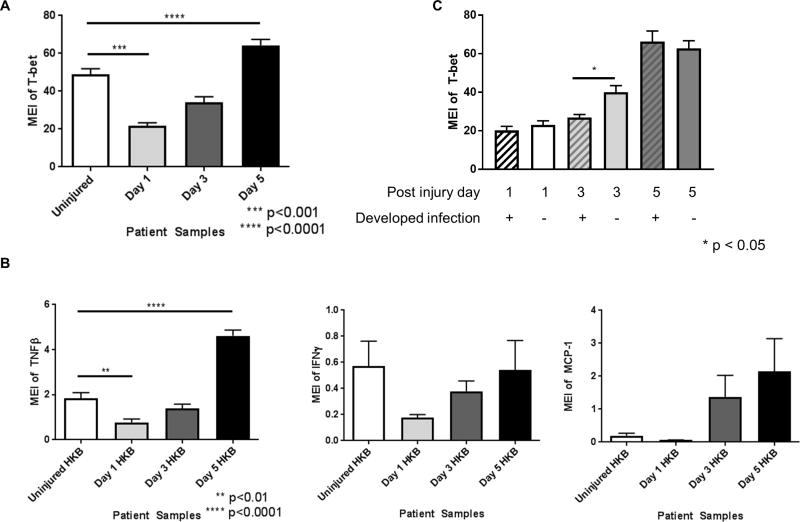
CyTOF staining data showed that T-bet transcription factor expression was significantly reduced in NK cells at day 1 after trauma. By day 3, we found that T-bet expression in NK cells returned to levels observed in uninjured controls, but by day 5 after trauma, T-bet expression was significantly higher than controls (Figure 2A). When PBMCs were stimulated with heat-killed S. pneumoniae and stained with the cytokine CyTOF panel, we observed that TNFβ, IFNγ, and MCP-1 cytokine expression in NK cells mirrored the expression pattern of T-bet (Figure 2B). Stratifying the CyTOF staining data based on the development of infection, we identified that patients with post-trauma infection (n=4) had a delayed increase in NK cell T-bet expression (Figure 2C).
Figure 2. Changes in T-bet expression in blood NK cells in trauma patients.
A. NK cells demonstrate decreased expression of the transcription factor T-bet on day 1 after injury, which returns to normal by day 3 and becomes significantly increased compared to baseline by day 5. B. Expression of TNFβ, IFNγ and MCP-1 showing similar expression patterns; IFNγ and MCP-1 show a similar trend as TNFβ although not statistically significant. C. Reduced T-bet expression in patients that subsequently develop an infection. Statistics were performed using one-way ANOVA; results were considered significant if p < 0.05.
Monocyte response to trauma
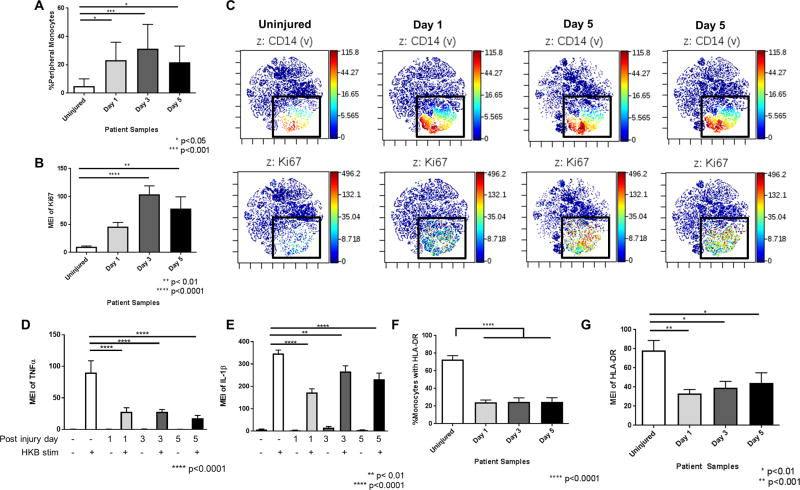
Monocyte percentages as a subset of all circulating PBMCs significantly increased after injury (Figure 3A). This increase in monocytes was likely due to proliferation, as evidenced by increased staining for the nuclear cell proliferation marker, Ki-67 (Figure 3B). This increase in expression of Ki-67 in monocytes was easily visualized in viSNE plots, which clusters groups of cells based on similar staining patterns (12). As shown in Figure 3C, Ki-67 staining colocalized with CD14+ monocytes, which are also shown to increase in density after trauma.
Figure 3. Circulating monocytes increase in percentage with increasing Ki-67 expression in trauma patients, but have reduced pro-inflammatory cytokine and HLA-DR expression.
A. Percentages of circulating monocytes in peripheral blood demonstrating that the percentage of circulating monocytes increases significantly at all time points after injury. B. Mean expression intensity (MEI) of Ki-67 expression in monocytes demonstrating a significant increase in Ki-67 expression at all time points after injury. C. viSNE plots illustrating the colocalization of CD14 and Ki67 staining of proliferating monocytes. Top panels show CD14 staining and bottom panels show Ki67 staining of same cell population. D. MEI of TNFα expression in monocytes in peripheral blood after stimulation with heat killed bacteria (HKB). Stimulated TNFα expression in monocytes decreased at all time points after injury. E. MEI of IL-1β in HKB-stimulated monocytes, showing a significant decrease in IL-1β expression at all time points after injury. F. Percentages of circulating HLA-DR+ monocytes from uninjured controls and trauma patients. The percentage of circulating monocytes that were HLA-DR+ was significantly reduced at all time points after injury. G. MEI of HLA-DR on monocytes is significantly decreased at all time points after injury. Statistics were performed using one-way ANOVA; results were considered significant if p < 0.05.
After stimulation with heat-killed S. pneumoniae, there was a marked difference in pro-inflammatory cytokine production between controls and trauma patients. Specifically, TNFα production, which was high in monocytes from controls, showed significantly decreased expression in monocytes from trauma patients at all time points (Figure 3D). Similarly, IL-1β was produced at high levels in monocytes after stimulation in controls, but was significantly lower in monocytes from trauma patients (Figure 3E). We also evaluated changes in HLA-DR. HLA-DR was robustly expressed on the cell surface of monocytes from controls. In contrast, the percentage of circulating HLA-DR+ monocytes decreased significantly after injury at all time points (Figure 3F). Moreover, among HLA-DR+ monocytes, the MEI for HLA-DR significantly decreased (Figure 3G).
Functional assessment of trauma-induced monocyte phagocytosis and oxidative burst responses
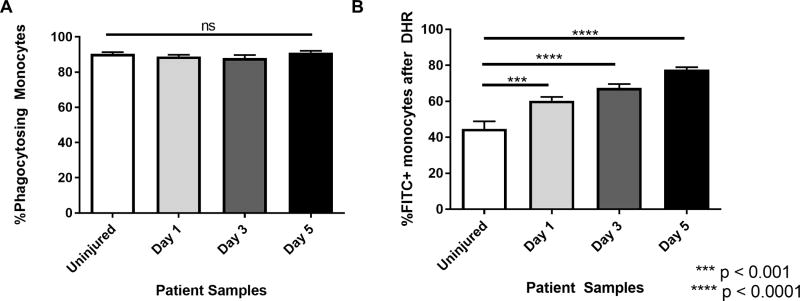
The findings above suggested that monocytes were significantly altered by trauma and may have suppressed anti-microbial function. Thus, we performed bacteria phagocytosis and oxidative burst assays on 5 separate patients to test for potential changes in anti-microbial immune reactivity by monocytes. Phagocytosis assays were performed using fluorescence-labeled S. aureus. Surprisingly, we found that there was no difference in phagocytic activity, as all monocytes showed nearly 90% uptake of fluorescence-labeled S. aureus (Figure 4A). Next, we tested monocytes for oxidative burst activity by measuring NADPH-oxidase dependent fluorescence of DHR123 rhodamine dye in cells. Rather than decreased oxidative burst activity by monocytes from trauma patients, we found increased oxidative burst over the entire time course as compared to controls (Figure 4B).
Figure 4. Phagocytosis and oxidative burst assays to assess anti-microbial function of monocytes from trauma patients after injury.
A. Cells were incubated with FITC-labeled S. aureus and opsonizing reagent to evaluate phagocytosis. No change is seen in monocyte phagocytosis after injury. B. Cells were incubated with DHR then stimulated with PMA and evaluated for oxidative burst. Monocyte oxidative burst in response to stimulation is augmented after injury. Statistics were performed using one-way ANOVA; results were considered significant if p < 0.05.
Discussion
Although trauma patients are now surviving traumatic injuries that would have been fatal decades ago, this leaves critically injured people vulnerable to post-injury infections (1,2), due in part to disruption of immune system homeostasis. Therefore, it is critical to map cellular changes and interactions that could predict which patients are more susceptible to post-trauma infections and to identify targets for immunotherapy. This challenging task is now more feasible with the development of systems biology tools like CyTOF, Luminex multiplex cytokine assays, and RNA sequencing. For example, Gaudillière et al used CyTOF to study blood samples in patients undergoing hip arthroplasty, identifying signaling changes in circulating CD14+ monocytes that correlated with functional impairment during recovery (13). Here, we used CyTOF to generate unbiased, blood immune cell phenotyping data to identify cells and markers that are significantly affected by traumatic injury (14–17).
In our trauma patient cohort, we found changes in cell populations and cytokine production that were specific to injury and dynamic over time. Further, these changes were common among the patients that were analyzed, despite their varied age, gender, and specific types of injuries. A prior report that measured gene expression profiles of blood cells from trauma and burn patients also found that there is high similarity between these different types of traumatic injuries (18).
In this study, the cell subsets showing the most significant changes were CD4+ T cells, NK cells, and monocytes. In CD4+ T cells, we identified an increase in Th17 cells, which are pro-inflammatory T cells that have detrimental activity in autoimmune diseases and beneficial activity for antimicrobial immune function by stimulating mobilization of neutrophils (19–21). Our data demonstrated increased CD4+ T cells expressing RORγT by day 1 after injury. By day 5, we found that the RORγT-expressing CD4+ T cells were producing detectable IL-17F or IL-22 even without stimulation. This suggests that trauma-induced Th17 cells take several days before being able to produce Th17-type cytokines. Furthermore, the fact that these cells are constitutively producing IL-17F and IL-22 demonstrates that injury alone is a sufficient stimulus to activate these cells. A recent report by Abboud et al used computational immunology methods on circulating cytokine results and patient outcome to identify a possible pathologic role for IL-17 or Th17 cells in trauma patients (22). Our findings by CyTOF concur that trauma induces an increase in circulating Th17 cells. The observed expansion of Th17 cells after injury compels further investigation to determine if immunotherapies to modulate Th17 cells or IL-17 protect trauma patients from opportunistic infections or organ injury.
NK cells are group 1 innate lymphoid cells (ILCs) that play critical roles in immune function by detecting tumor cells, virus-infected cells, and stressed cells (23). NK cells express the transcription factor T-bet and, when activated by IL-1β, IL-12, and/or IL-18, produce high-levels of the Th1-type cytokines, IFNγ and TNFβ. In this study, we discovered significant time-dependent changes in T-bet and inflammatory cytokine expression patterns. T-bet expression in NK cells is thought to regulate NK cell maturation and movement from the bone marrow and lymph nodes (24,25). The initial decrease seen in T-bet in NK cells suggests they may be recent emigrants from the bone marrow. Since T-bet and inflammatory cytokine expression in NK cells normalizes by day 3 and is induced beyond normal baseline levels by day 5, this observation supports that trauma induces NK cell maturation. Finally, despite the small sample size, we discovered a delayed rebound in T-bet expression in patients that developed infections, possibly indicating that NK cell maturation is involved in predisposition to infections after trauma. This finding requires further study with a larger cohort of patients to evaluate whether T-bet may be a prognostic marker or drug target.
Our data also demonstrated an increase in peripheral monocytes with evidence of proliferation. Though we anticipated that these monocytes would be pro-inflammatory, we found decreased TNFα and IL-1β expression in response to heat-killed bacteria. Furthermore, we found those monocytes from trauma patients expressed less cell-surface HLA-DR. This diminished HLA-DR implies a decreased capacity for monocytes to interact with CD4+ T cells. Several previous reports showed that a long-lived decrease in HLA-DR expression on monocytes after injury may predict poor clinical outcome and death for trauma patients (26–28). This suggests that early changes in monocytes may be a predictive measure for clinical trajectories in trauma patients.
Based on our phenotypic and cytokine production findings, we hypothesized monocytes might also have decreased innate anti-microbial function. While there is a paucity of data discussing monocyte oxidative burst after injury, it is known that monocyte oxidative burst is impaired in septic shock and that this could potentially be involved in the spread of infection during sepsis (29). Although a brief comparative delay in phagocytosis or oxidative burst during the short experimental incubations cannot be excluded, our findings support that trauma-induced monocytes retain their innate anti-microbial functions.
Our study has several limitations to note. While we were able to comprehensively phenotype multiple immune cell subsets after injury, these results do not identify the mechanisms responsible. Nevertheless, this information lays the groundwork for functional studies. Another limitation was that we were only able to evaluate blood. However, we will now pursue parallel blood and immune tissue sampling studies in a mouse trauma model. It should also be stated that while patients clinically developed an infection over a wide range of time, it is unclear at which time those patients were inoculated. Thus, there could be a component of underlying infection to the immune phenotypes identified. However, the commonality seen between these immune profiles despite the significant difference in time to infection leads us to believe that the changes identified are trauma-induced. Additionally, this report includes only 10 patients. Sample collection is ongoing and further studies including more patients would be able to validate and expand our findings. Finally, this cohort was severely injured and not all findings may be generalizable to less severely injured patients.
In conclusion, using CyTOF, we identified several novel time-dependent phenotypic changes in blood immune cell subsets following major trauma. Future directions will include in-depth study of specific immune cell subsets showing trauma-induced changes in a larger cohort of trauma patients. Relevant immune cell markers to include will be immunomodulatory receptors associated with strong T cells activation or exhaustion, NK cell maturation and functional markers, and markers to identify immature monocytes and M1 or M2 phenotypes. We will also profile more patients to allow us to stratify gender, types of injury, and clinical features. Future studies will use this data to create new insights and hypotheses about how to restore immune homeostasis in trauma patients and prevent the development of life-threatening infections.
Supplementary Material
Supplemental Table 1: CyTOF PBMC Cell-Surface Marker Phenotyping Panel
Supplemental Table 2: CyTOF PBMC Cytokine Production Panel
Supplemental Figure 1: Sample gating strategy of CyTOF data to identify monocytes, CD4+ T cells and NK cells.
A. Single cell events are gated by identifying the clustered population of appropriate DNA content with a short event length. B. Normalization beads are excluded. C. Cisplatin is used as a viability dye; cisplatin negative cells are identified as live cells. D. CD4+ T cells are identified as CD3+CD4+ cells. NK cells are identified as CD94+CD3− cells. Monocytes are identified as CD33+ cells.
Supplemental Figure 2: Changing cell subset percentages in the peripheral blood after injury. CyTOF staining data was analyzed to identify changes in the indicated cell subset percentages from uninjured controls and trauma patients, n=9 per group. The data is plotted as mean ± SEM of live cells and analyzed by one-way ANOVA; results were considered significant if p < 0.05.
Acknowledgments
Financial Support for the Study: NIH 5R01 AI092905-05
Drs. Keegan and Lederer received support for article research from the National Institutes of Health.
Footnotes
Institution where work was performed: Brigham and Women's Hospital, Boston, MA.
Copyright form disclosure: The remaining authors have disclosed that they do not have any potential conflicts of interest.
References
- 1.Dutton RP, Stansbury LG, Leone S, et al. Trauma mortality in mature trauma systems: are we doing better? An analysis of trauma mortality patterns, 1997–2008. J Trauma. 2010;69(3):620–626. doi: 10.1097/TA.0b013e3181bbfe2a. [DOI] [PubMed] [Google Scholar]
- 2.Gerber LM, Chiu YL, Carney N, et al. Marked reduction in mortality in patients with severe traumatic brain injury. J Neurosurg. 2016;119(6):1583–1590. doi: 10.3171/2013.8.JNS13276. [DOI] [PubMed] [Google Scholar]
- 3.Osborn TM, Tracy JK, Dunne J, et al. Epidemiology of sepsis in patient with traumatic injury. Crit Care Med. 2004;32(11):2234–2240. doi: 10.1097/01.ccm.0000145586.23276.0f. [DOI] [PubMed] [Google Scholar]
- 4.Osuka A, Ogura H, Ueyama M, et al. Immune response to traumatic injury: harmony and discordance of immune system homeostasis. Acute Med and Surg. 2014;1(2):63–69. doi: 10.1002/ams2.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stoecklein VM, Osuka A, Lederer JA. Trauma equals danger – damage control by the immune system. J Leukocyte Biology. 2012;92(3):539–551. doi: 10.1189/jlb.0212072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lord JM, Midwinter MJ, Chen Y-F, et al. The systemic immune response to trauma: an overview of pathophysiology and treatment. Lancet. 2014;384(9952):1455–1465. doi: 10.1016/S0140-6736(14)60687-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marik PE, Flemmer M. The immune response to surgery and trauma: implications for treatment. J Trauma and Acute Care Surg. 2012;73(4):801–808. doi: 10.1097/TA.0b013e318265cf87. [DOI] [PubMed] [Google Scholar]
- 8.Bandura DR, Baranov VI, Ornatsky OI, et al. Mass cytometry: technique for real time single cell multitarget immunoassay based on inductively coupled plasma time-of-flight mass spectrometry. Anal Chem. 2009;81(16):6813–6822. doi: 10.1021/ac901049w. [DOI] [PubMed] [Google Scholar]
- 9.Bendall SC, Nolan GP. From single cells to deep phenotypes in cancer. Nature Biotechnology. 2012;30(7):639–647. doi: 10.1038/nbt.2283. [DOI] [PubMed] [Google Scholar]
- 10.Baker SP, O’Neill B, Haddon W, Jr, et al. The Injury Severity Score: a method for describing patients with multiple injuries and evaluating emergency care. J Trauma. 1974;14(3):187–196. [PubMed] [Google Scholar]
- 11.Zunder ER, Finck R, Behbehani GK, et al. Palladium-based mass-tag cell barcoding with a doublet-filtering scheme and single cell deconvolution algorithm. Nat Protoc. 2015;10(2):316–333. doi: 10.1038/nprot.2015.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amir el-AD, Davis KL, Tadmor MD, et al. viSNE enables visualization of high dimensional single-cell data and reveals phenotypic heterogeneity of leukemia. Nat Biotechnol. 2013;31(6):545–552. doi: 10.1038/nbt.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaudillière B, Fragiadakis GK, Bruggner RV, et al. Clinical recovery from surgery correlates with single-cell immune signatures. Sci Transl Med. 2014;6(255):255ra131. doi: 10.1126/scitranslmed.3009701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kitano H. Systems biology: a brief overview. Science. 2002;295(5560):1662–1664. doi: 10.1126/science.1069492. [DOI] [PubMed] [Google Scholar]
- 15.Westerhoff HV, Palsson BO. The evolution of molecular biology into systems biology. Nature Biotechnology. 2004;22:1249–1252. doi: 10.1038/nbt1020. [DOI] [PubMed] [Google Scholar]
- 16.Aderem A. Systems biology: its practice and challenges. Cell. 2005;121(4):511–513. doi: 10.1016/j.cell.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 17.Hood L, Heath JR, Phelps ME, et al. Systems biology and new technologies enable predictive and preventative medicine. Science. 2004;306(5696):640–643. doi: 10.1126/science.1104635. [DOI] [PubMed] [Google Scholar]
- 18.Xiao W, Mindrinos MN, Seok J, et al. A genomic storm in critically injured humans. J Exp Medicine. 2011;208(13):2581–2590. doi: 10.1084/jem.20111354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stockinger B, Veldhoen M. Differentiation and function of Th17 T cells. Curr Opin Immunol. 2007;19(3):281–286. doi: 10.1016/j.coi.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 20.Rendon JL, Choudhry MA. Th17 cells: critical mediators of host responses to burn injury and sepsis. J Leukoc Biol. 2012;92(3):529–538. doi: 10.1189/jlb.0212083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dubin PJ, Kolls JK. Th17 cytokines and mucosal immunity. Immunol Rev. 2008;226:160–171. doi: 10.1111/j.1600-065X.2008.00703.x. [DOI] [PubMed] [Google Scholar]
- 22.Abboud A, Namas RA, Ramadan M, et al. Computational analysis supports an early, type 17 cell-associated divergence of blunt trauma survival and mortality. Crit Care Med. 2016;44(11):1074–1081. doi: 10.1097/CCM.0000000000001951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spits H, Di Santo JP. The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling. Nat Immunol. 2011;12(1):21–27. doi: 10.1038/ni.1962. [DOI] [PubMed] [Google Scholar]
- 24.Townsend MJ, Weinmann AS, Matsuda JL, et al. T-bet regulates the terminal maturation and homeostasis of NK and Valpha14i NKT cells. Immunity. 2004;20(4):477–494. doi: 10.1016/s1074-7613(04)00076-7. [DOI] [PubMed] [Google Scholar]
- 25.Jenne CN, Enders A, Rivera R, et al. T-bet-dependent S1P5 expression in NK cells promotes egress from lymph nodes and bone marrow. J Exp Med. 2009;206(11):2469–2481. doi: 10.1084/jem.20090525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hershman MJ, Cheadle WG, Wellhausen SR, et al. Monocyte HLA-DR antigen expression characterizes clinical outcome in the trauma patient. Br J Surg. 1990;77(2):204–207. doi: 10.1002/bjs.1800770225. [DOI] [PubMed] [Google Scholar]
- 27.Venet F, Tissot S, Debard AL, et al. Decreased monocyte human leukocyte antigen-DR expression after severe burn injury: correlation with severity and secondary septic shock. Crit Care Med. 2007;35(8):1910–1917. doi: 10.1097/01.CCM.0000275271.77350.B6. [DOI] [PubMed] [Google Scholar]
- 28.Gouel-Cheron A, Allaouchiche B, Guignant C, et al. Early interleukin-6 and slope of monocyte human leukocyte antigen-DR: a powerful association to predict the development of sepsis after major trauma. PLoS One. 2015;7(3):e33095. doi: 10.1371/journal.pone.0033095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams MA, White SA, Miller JJ, et al. Granulocyte-macrophage colony-stimulating factor induces activation and restores respiratory burst activity in monocytes from septic patients. J Infect Dis. 1998;177(1):107–115. doi: 10.1086/513802. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table 1: CyTOF PBMC Cell-Surface Marker Phenotyping Panel
Supplemental Table 2: CyTOF PBMC Cytokine Production Panel
Supplemental Figure 1: Sample gating strategy of CyTOF data to identify monocytes, CD4+ T cells and NK cells.
A. Single cell events are gated by identifying the clustered population of appropriate DNA content with a short event length. B. Normalization beads are excluded. C. Cisplatin is used as a viability dye; cisplatin negative cells are identified as live cells. D. CD4+ T cells are identified as CD3+CD4+ cells. NK cells are identified as CD94+CD3− cells. Monocytes are identified as CD33+ cells.
Supplemental Figure 2: Changing cell subset percentages in the peripheral blood after injury. CyTOF staining data was analyzed to identify changes in the indicated cell subset percentages from uninjured controls and trauma patients, n=9 per group. The data is plotted as mean ± SEM of live cells and analyzed by one-way ANOVA; results were considered significant if p < 0.05.