Abstract
We studied the structural and functional heart adaptations of 52 male triathletes compared with those of 22 active, nonathletic men, by 2-dimensional Doppler echocardiography. Left ventricular diastolic function was evaluated by recording transmitral flow velocities. To exclude the influences of preload, left atrial pressure, and aortic pressure, left ventricular diastolic function was also evaluated by pulsed Doppler tissue imaging.
Significant differences in cardiac structure and function were observed between the 2 groups. In the triathletes, the left ventricular diastolic function was completely normal, despite signs of mixed eccentric and concentric left ventricular hypertrophy, and this function was better than that in the control group. We measured 2 aspects of the late passive diastolic filling period in the triathletes: ASEAC value (the amplitude of excursion of the interventricular septal endocardium at the end of left ventricular di–astole just after atrial contraction); and the time between onset of the P wave on the electrocardiographic tracing and onset of systolic septal movement on M-mode echo-cardiography. Pulsed Doppler tissue imaging confirmed these results. The E/A ratios (peak early left ventricular diastolic motion velocity divided by the peak atrial systolic motion velocity), measured by pulsed Doppler tissue imaging, yielded even more evidence for supernormal left ventricular diastolic function in the triathletes. Left ventricular relaxation and filling properties were measured along the longitudinal and transverse axes by pulsed Doppler tissue imaging, which was useful for evaluating left ventricular diastolic function. We determined that triathletes may develop supernormal left ventricular diastolic function with increased diastolic reserves.
Key words: Diastole; echocardiography, Doppler; exercise/physiology; heart septum; heart ventricle/physiology; myocardial contraction; physical endurance; sports; systole; ventricular function, left
The triathlon, which consists of 3.9 km of swimming, 180 km of bicycling, and 42 km of running, is an example of an extreme endurance sport. To finish a triathlon successfully, the triathlete must engage in extensive aerobic training to ensure that only minimal changes occur in the homeostasis of his cardiovascular, hemodynamic, and metabolic functions over the duration of the competition (between 9:45 and 10:45 hours). To increase the performance capacity, a variety of physiological adaptations—both central and peripheral—are required. The heart is the central concern and is also the most important limiting factor.
It is generally accepted that increased physical activity and training cause structural and functional heart adaptations with important hemodynamic implications. In this study, we investigated the structural and functional cardiac changes that occur in triathletes. The left ventricular (LV) diastolic function was investigated by recording transmitral flow velocities. To exclude influences of preload, left atrial pressure, aortic pressure, and viscoelastic myocardial properties, the LV diastolic function was also evaluated by means of pulsed Doppler tissue imaging.
Methods
Fifty-two male triathletes were examined and compared with 22 men in the control group. All 52 triathletes were regular competitors in the Belgian superprestige category.
The 22 men in the control group were young, healthy, and active, but did not participate in athletic competition or recreational sports. Eleven of these men were police officers and 11 were senior nurses.
All 52 triathletes were tested at the same stage of their training program and at the same time of day, between 4 and 8 PM; the 22 members of the control group underwent the same testing protocol. The protocol consisted of a traditional, detailed clinical examination with special attention to cardiac components; determination of general physical and anthropometric characteristics; determination of vital capacity; and a 2-dimensional echocardiographic Doppler examination. In addition, an electrocardiogram at rest was administered, followed by a programmed incremental exercise test on the bicycle under continuous electrocardiographic monitoring with a lactic acid determination every 3 minutes and with measurement of the lactate threshold and the ventilatory threshold.
A Vingmed System FiVe® apparatus (GE Medical Systems; New Orleans, La) was used for the 2–dimensional Doppler echocardiographic examination and for the pulsed Doppler tissue imaging. All echocardiographic examinations were performed at the end of expiration with the subject lying on his left side. All Doppler echocardiographic measurements were performed for 5 consecutive heartbeats. The average value of those 5 measurements was used for statistical analysis. To determine the end diastole, we used the 1st deflection of the QRS complex on the accompanying electrocardiographic trace as the point of reference. The systolic diameters were measured between the apex of the systolic movement of the posterior wall and the nadir of the interventricular septal (IVS) movement. The same points of reference were used for both the left and right ventricular measurements. 1
The 2-dimensional echocardiographic image was used to determine the LV mass in grams by means of the formula:
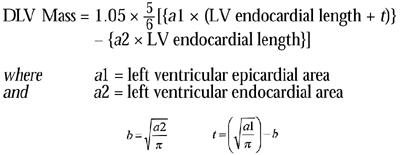 |
To study cardiac function, we measured the LV systolic and diastolic function. The systolic wall tension in the left ventricle was also calculated, because it provided a quantitative index for myocardial afterload. We were particularly interested in the LV diastolic function. 2,3
We measured the velocity of the IVS during early diastole, as well as the velocity of the LV posterior wall during early diastole.
To evaluate the late diastolic passive filling period, we explored 2 parameters: the 1st was ASEAC, which is the amplitude (in mm) of the excursion of the endocardium of the IVS, at the end of LV diastole, that occurs just after atrial contraction. The ASEAC is measured on the M-mode of the echo-cardiographic image of the left ventricle. From the parasternal long axis of the 2-dimensional echocardiogram, we switched to the M-mode image of the left ventricle through a cross-section just distal to the mitral valve leaflets. Referring to the P wave on the accompanying electrocardiogram, we measured the excursion of the IVS at the end of diastole for 5 consecutive heartbeats. The average of those 5 values was used for statistical analysis (Fig. 1). The ASEAC value was corrected for the R-R distance but did not seem to be rhythm dependent.
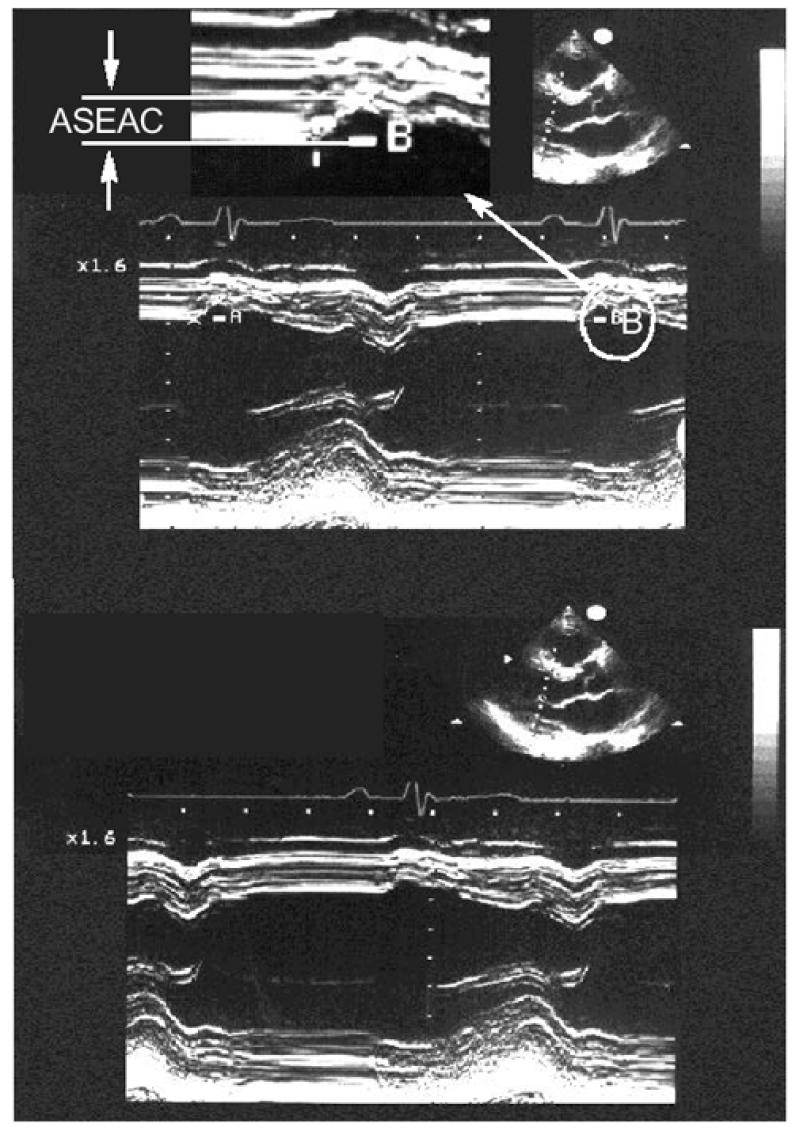
Fig. 1 Two-dimensional echocardiography in the parasternal long-axis (transverse) view, and M-mode echocardiography showing a cross-section just distal to the leaflets of the mitral valve, illustrate ASEAC (the amplitude of the excursion of the endocardium of the interventricular septum at the end of the diastole of the left ventricle, occurring just after atrial contraction). The top and bottom figures are similar examples of ASEAC, and A and B are 2 of the 5 consecutive measurements.
The 2nd parameter for evaluating the late diastolic filling period was the time between the onset of the P wave on the accompanying electrocardiographic trace and the onset of the systolic IVS movement on the M-mode of the echocardiographic image. The time between the onset of the P wave and the onset of the systolic contraction of the LV posterior wall was also determined. Both results were corrected for the R-R interval.
The Doppler inflow signals in the left ventricle during diastole at the mitral valve were studied to evaluate the relaxation and compliance of the left ventricle. The sample volume was set at the tip of the mitral valve leaflets on the apical long-axis view of the left ventricle. A clear, diastolic, biphasic waveform was obtained. From the transmitral flow velocity pattern recorded, peak early diastolic (E) and atrial systolic (A) velocities were measured. 4
By 2-dimensional Doppler echocardiography, we determined the isovolumetric relaxation period. At the mitral valve, the deceleration time and the duration of the diastolic filling period were measured.
The wall motion velocity patterns along the longitudinal axes were recorded by the pulsed Doppler tissue imaging method. 5–9
In the parasternal long-axis (transverse-axis) echo-cardiogram and the apical long-axis (longitudinal-axis) echocardiogram of the left ventricle, sample volumes were set at the endocardial portions of the basal, middle, and apical sites of the LV posterior wall and the IVS (Fig. 2).
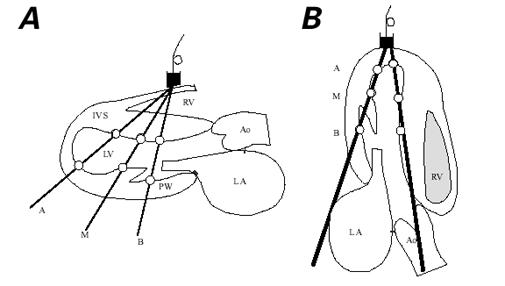
Fig. 2 Sample sites for recording motion velocity patterns of the IVS and of the LV posterior wall by pulsed Doppler tissue imag–ing in A) the parasternal long-axis (transverse) view of the left ventricle and B) the apical long-axis (longitudinal) view of the left ventricle. Sample volumes (white circles) were set at the endocardial portions of the apical (A), middle (M), and basal (B) sites of the LV posterior wall and at the endocardial portions of the apical (A), middle (M), and basal or ASEAC (B) sites of the IVS.
Ao = ascending aorta; IVS = interventricular septum; LA = left atrium; LV = left ventricle; PW = posterior wall; RV = right ventricle; T = transducer
In the parasternal long-axis echocardiogram of the left ventricle, the sample volume at the endocardial portion of the basal site of the IVS corresponded with the site where the ASEAC was measured (Fig. 3). From the obtained patterns, the peak early diastolic (E) and peak atrial systolic (A) velocities of the LV posterior wall were measured in the same axis (Fig. 4). The same parameters for the LV posterior wall (Fig. 5) and for the IVS (Fig. 6) were measured in the apical long-axis view. Figures 3 through 6 all depict the basal sites of the IVS and the LV posterior wall. Using both echocardiographic views of the left ventricle, we calculated the pulsed Doppler tissue imaging E/A ratios at the different sites of the IVS and the LV posterior wall.
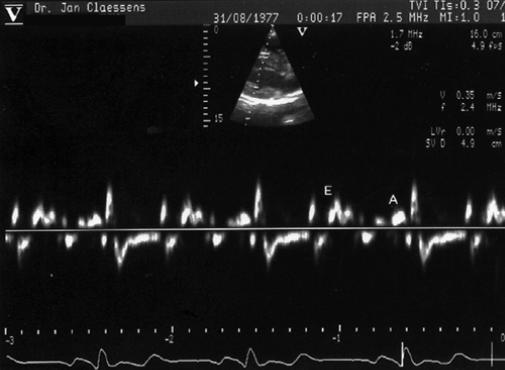
Fig. 3 Pulsed Doppler tissue imaging showing the peak early diastolic velocity of the interventricular septum (E) and peak atrial systolic velocity of the interventricular septum (A), with sample volume at the endocardial portion of the basal or ASEAC site of the interventricular septum in the parasternal long-axis echocardiogram.
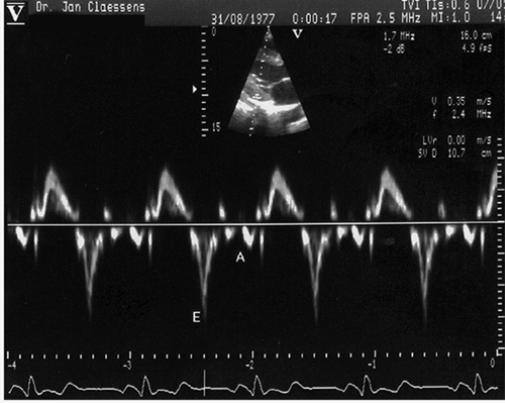
Fig. 4 Pulsed Doppler tissue imaging showing the peak early diastolic velocity (E) and peak atrial systolic velocity (A) of the LV posterior wall, with sample volume at the endocardial portion of the basal site of the LV posterior wall in the parasternal long-axis echocardiogram.
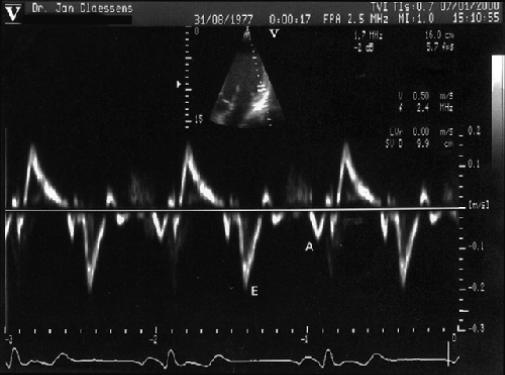
Fig. 5 Pulsed Doppler tissue imaging showing the peak early diastolic velocity (E) and peak atrial systolic velocity (A) of the LV posterior wall, with sample volume at the endocardial portion of the basal site of the LV posterior wall in the apical long-axis echocardiogram.
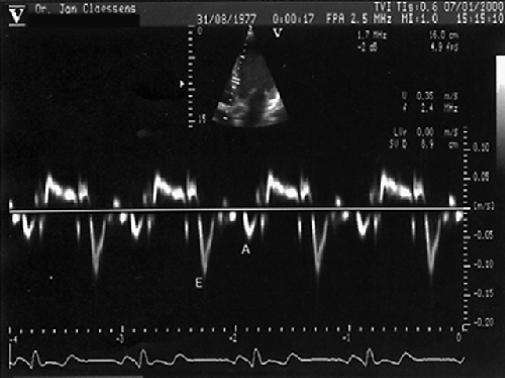
Fig. 6 Pulsed Doppler tissue imaging showing the peak early diastolic velocity (E) and peak atrial systolic velocity (A) of the interventricular septum, with sample volume at the endocardial portion of the basal site of the interventricular septum in the apical long-axis echocardiogram.
All data were subjected to statistical analysis of variance (ANOVA). Values are expressed as mean ± SD. A P value of <0.05 was considered statistically significant.
We classified the 52 triathletes into 4 groups of 13 athletes as a function of their results in the Super-prestige competitions. The 13 best triathletes were placed in group 1 and the 13 with the poorest results in group 4. In groups 2 and 3, we placed the athletes with average and moderate-to-poor results, respectively. To determine whether there were statistically significant differences among the subgroups of triathletes, we compared the 4 groups of triathletes by means of the Scheffé test.
Results
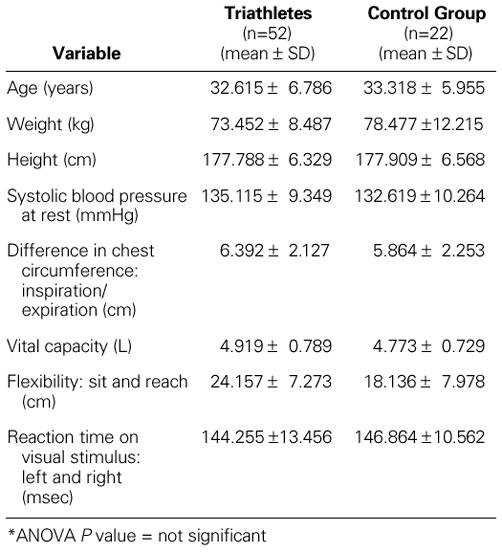
There were no significant differences in the general physical characteristics and the anthropometric data between the triathletes and the control group (Table I).
TABLE I. General Physical Characteristics of Triathletes and the Control Group*
There were significant differences in cardiac structure between the triathletes and the control group. The maximal end-diastolic internal diameter of the left ventricle was significantly higher in the triathletes than in the control group. The same differences were found with regard to the maximal end-diastolic diameter of the right ventricle, and in the thickness of the IVS and the LV posterior wall. Statistically significant differences between the 2 groups were observed with regard to diastolic LV mass in grams (Table II).
TABLE II. Cardiac Structure of the Triathletes and the Control Group
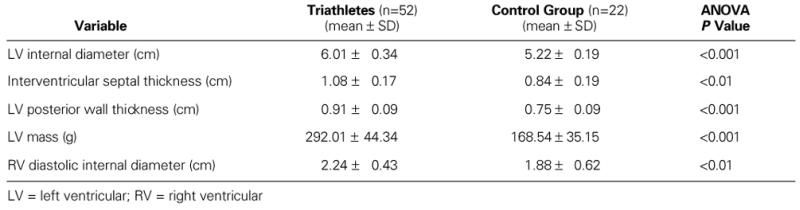
The LV systolic function was completely normal in both groups. There were no significant differences between the triathletes and the control group regarding the values of fractional shortening, cardiac output, ejection fraction, stroke volume, or systolic LV wall stress (Table III).
TABLE III. Cardiac Function: Systolic Left Ventricular Function in the Triathletes and the Control Group*
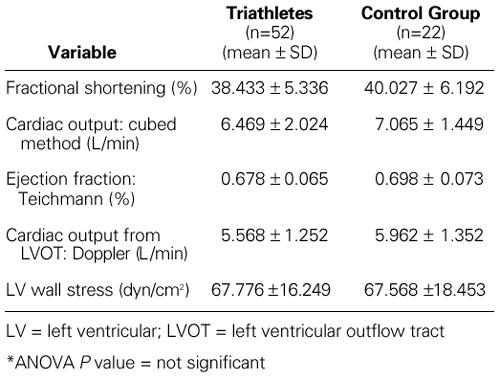
In contrast with the LV systolic function, there were distinct significant differences in LV diastolic function between the groups. The E-point velocity was distinctly higher (P <0.05) and the A-point velocity distinctly lower (P <0.05) in the triathletes, compared with the control group.
The E/A ratio was clearly higher in the triathletes. The isovolumetric relaxation period was distinctly shorter in the triathletes than in the control group. The corrected time between the onset of the P wave and the onset of the systolic IVS contraction was also significantly shorter in the triathletes than in the control group. The same applies to the corrected time between the onset of the P wave and the onset of the systolic contraction of the LV posterior wall, which was statistically much shorter in the triathletes than in the control group. The ASEAC value was obviously much higher and significantly very different in the triathletes, compared with the control group. Those findings indicate distinct differences between the 2 groups in the late diastolic passive filling period (Table IV). The passive elasticity and expansibility of the myocardium was much higher in the triathletes than in the control group.
TABLE IV. Cardiac Function: Left Ventricular Diastolic Function in the Triathletes and the Control Group
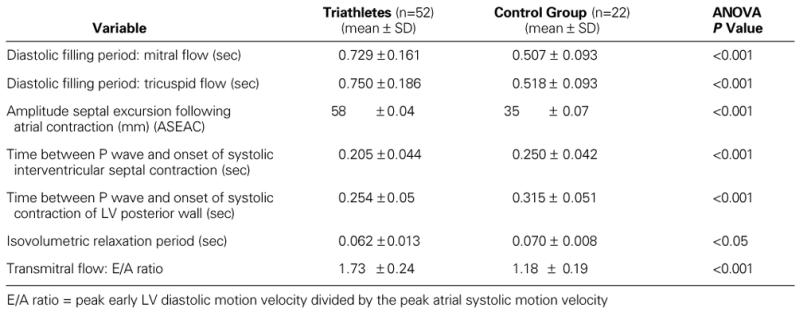
There was only a small difference in the velocity and amplitude of the IVS motion and in the velocity and amplitude of the LV posterior wall motion during the early active diastolic relaxation period. In this respect, the values in the triathletes and the control group were normal and statistically identical (Table V).
TABLE V. Cardiac Function: Left Ventricular Diastolic Function in the Triathletes and the Control Group*
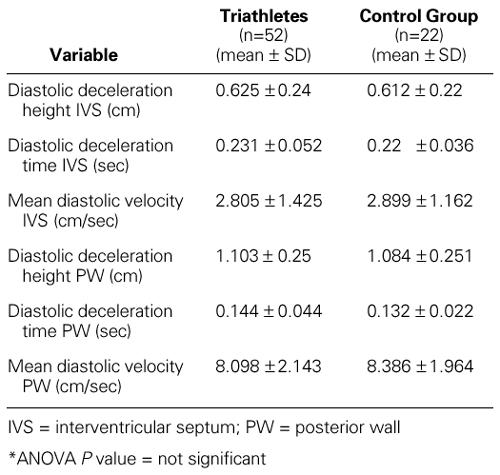
Pulsed Doppler tissue imaging demonstrated that in all subjects the peak early diastolic velocities of the IVS and the LV posterior wall decreased gradually between the basal and apical sites. Moreover, the peak early diastolic velocity at each site of the IVS was significantly lower than that at the corresponding sites of the posterior wall.
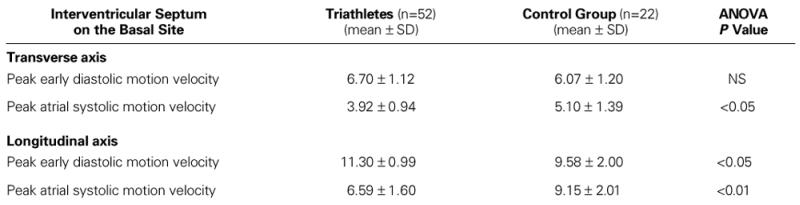
In both echocardiographic views (transverse and longitudinal), at the basal site along the IVS and the LV posterior wall, the peak early diastolic wall motion velocities were higher in the triathletes than in the control group, and the peak atrial systolic wall motion velocities were lower (Table VI).
TABLE VI. Left Ventricular Diastolic Function: Pulsed Doppler Tissue Findings in the Triathletes and the Control Group
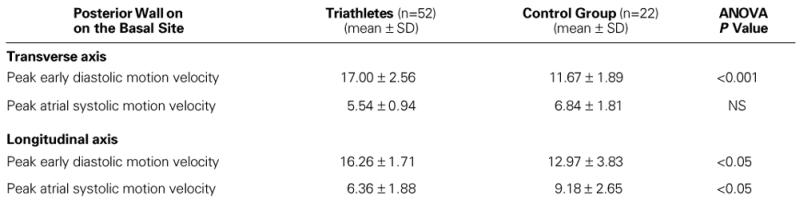
Along the transverse axis, the peak early diastolic motion velocity of the IVS with sample volume at the endocardial portion of the basal (ASEAC) site was higher in the triathletes than in the control group. The peak atrial systolic motion velocity of the IVS with sample volume at the same site was lower in the triathletes than in the control group (Table VII).
TABLE VII. Left Ventricular Diastolic Function: Pulsed Doppler Tissue Findings in the Triathletes and the Control Group
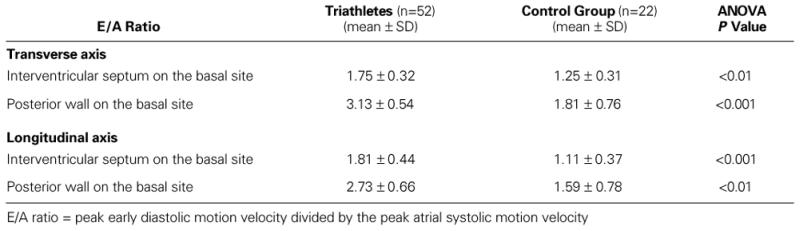
On both axes and at each site, the pulsed Doppler tissue imaging E/A ratios were significantly higher in the triathletes than in the control group (Table VIII).
TABLE VIII. Left Ventricular Diastolic Function: Pulsed Doppler Tissue Findings (E/A Ratios) in the Triathletes and the Control Group
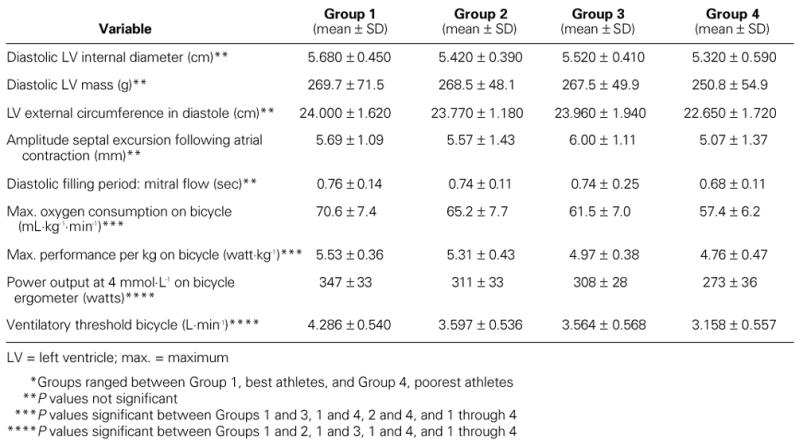
Analysis of the 4 subgroups of triathletes showed no significant differences in structural heart changes. In addition, there were no significant differences among the 4 subgroups in systolic or diastolic cardiac function. When the performance characteristics of the 4 subgroups of triathletes were compared to evaluate the athletic capacities of a triathlete and to distinguish the better triathletes from the others, the maximal exercise test on the bicycle permitted a distinction among the 4 subgroups, to a certain extent. The 4 subgroups of triathletes clearly distinguished themselves from one another in the maximal oxygen consumption on the bicycle, in the power output at 4 mmol/L on bicycle ergometer, and in the ventilatory threshold on the bicycle (Table IX).
TABLE IX. Cardiac Structure, Cardiac Function, and Performance Characteristics of 52 Triathletes Divided into 4 Distinct Groups* of 13, as a Function of Their Competition Results
Discussion
Important structural heart adaptations are found in triathletes. 10–15 These adaptations show eccentric, as well as concentric, LV hypertrophy with a prepon–derance of eccentric hypertrophy. However, unlike pathologic LV hypertrophy, such as that occurring in aortic valve disease and arterial hypertension (in which LV diastolic dysfunction is always observed), the LV diastolic function in the triathletes in our study was completely normal, yet clearly different from that in the control group. 16–18
Myocardial alterations can manifest themselves as abnormalities in myocardial relaxation connected with a retarded force decay or as an alteration in the distensibility of the myocardium with secondary effects on the late passive diastolic filling period. 3
During the early active diastolic relaxation period, the hypertrophic left ventricle of the triathletes in our study was normal. No differences were noted between the triathletes and the control group. The most important differences between the groups were seen during the late diastolic passive filling period: there was evidence of increased flexibility and elasticity of the LV muscle in the triathletes. In this respect, we noted in particular a significantly higher ASEAC value in the triathletes, compared with the control group. Although there was probably a certain degree of volume overloading in the triathletes, this finding could also be a sign of increased elasticity and increased myocardial distensibility at the end of diastole. Such structural myocardial alterations can be genetically determined, and not connected with athletics or training.
Before correction for the R-R interval, the time between the onset of the P wave and the onset of systolic IVS movement did not differ significantly between the triathletes and the control group, nor did the time between the onset of the P wave and the onset of LV posterior wall motion at the onset of systole. After correcting both of these values for the R-R interval, however, we saw a very significant difference between the control group and the athletes. The corrected time from the P wave to the onset of the IVS movement and to the onset of LV posterior wall movement was shorter in the triathletes than in the control group, which could be an indication of a much faster passive filling period in the triathletes. In our opinion, this suggests that there is an increased diastolic reserve in triathletes. Most likely, this reserve is very useful to the triathletes in their athletic performance, because with a faster heart rate and shorter diastolic time, the left ventricle can fill more efficiently.
To evaluate the diastolic function of the left ventricle accurately, it is necessary to assess LV relaxation and compliance. 19 Conventional clinical evaluation of LV relaxation involves determining the time constant of pressure decay during isovolumic diastole as calculated from the LV pressure curve. This requires an invasive procedure. However, with pulsed Doppler echocardiography, LV diastolic function can now be evaluated noninvasively by the recording of transmitral flow velocities. Left ventricular early diastolic filling is affected by many factors, such as LV relaxation, the pressure difference between the left atrium and left ventricle, and viscoelastic myocardial properties. Left ventricular late diastolic filling is controlled by passive compliance or myocardial stiffness. The peak E velocity of the transmitral flow is affected by loading conditions, and the flow velocity pattern changes to pseudonormalization in patients with a marked decrease in LV diastolic compliance. Therefore, there are limitations in the evaluation of LV relaxation abnormalities with the use of these methods.
During the past several years, techniques have been developed to evaluate LV diastolic function without the influence of preload by using diastolic measurements obtained from the motion velocities of the LV posterior wall and the IVS, recorded by pulsed Doppler tissue imaging along the transverse and longitudinal axes. This method provides important information that cannot be obtained from transmitral flow velocities. 20–24
In our study, the peak atrial systolic motion velocity of the IVS with the sample volume at the endocardial position of the basal (ASEAC) site along the transverse axis, and also along the longitudinal axis, was significantly higher in the control group, compared with the triathletes. Although the peak early diastolic motion velocity of the IVS with sample volume at the basal (ASEAC) site of the endocardium in each of the echocardiographic axes was higher in the triathletes compared with the control group, we could not determine any significant differences. At the level of the IVS, the most important differences between both groups were noted in the peak atrial systolic motion velocities.
Regarding the peak early diastolic motion velocities, however, we noted the most statistically significant differences at the level of the posterior wall. As shown in Table VI, these values at the basal site along the transverse and longitudinal axes were significantly higher in the triathlete group, compared with the control group.
When the pulsed Doppler tissue imaging E/A ratios were calculated, there were statistically significant differences between the 2 groups as well. In all axes and at each site, the pulsed Doppler tissue-imaging E/A ratios were significantly higher in the triathletes than in the control group. This clearly indicated that the triathletes had supernormal LV diastolic function in comparison with the nonathletic men. Both the early active diastolic relaxation period and the late passive diastolic filling period showed supernormal characteristics in the triathletes, notwithstanding undeniable signs of eccentric and concentric LV hypertrophy.
These findings were partially based on the results of 2-dimensional echocardiographic Doppler examination. Pulsed Doppler tissue imaging, which excludes the influence of preload, left atrial pressure, and aortic pressure, confirmed our results. Two–dimensional Doppler echocardiography revealed specific characteristics of the late LV diastolic passive filling period in the triathletes, and pulsed Doppler tissue imaging demonstrated specific properties of the early active diastolic relaxation period.
We found no significant differences in the structural and functional heart adaptations of the 4 subgroups of triathletes. Not all of the triathletes showed an enlarged internal diameter of the left ventricle or a manifest wall hypertrophy. It is apparently not necessary to have an “athletic heart” to become a triathlete or to win a triathlon. In our study, there were several excellent athletes with normal LV diameters who had a nonhypertrophic myocardial wall and normal LV mass. It was not the triathletes with the best competition results who had the most characteristics of eccentric and concentric LV hypertrophy. Moreover, it was not the triathletes with the greatest amount of training who had the most extensive heart adaptations. This variable physiologic response of the heart to training in the different subgroups of triathletes indicates that other factors (very likely genetic) affect the structural heart adaptations in triathletes.
Conclusions
Male triathletes undergo undeniable structural heart changes, which apparently are caused by pressure and volume overload and result in signs of combined eccentric and concentric hypertrophy. In spite of these changes, the LV systolic and diastolic function remain normal, and sometimes supernormal in the case of diastolic function. Indeed, LV diastolic function distinguishes the triathlete from the healthy, active control subject. Two-dimensional echocardiographic Doppler examination enabled us to show that, in particular, the late diastolic passive filling period in the triathlete has specific characteristics.
Pulsed Doppler tissue imaging was found to be useful for evaluating LV function without the influence of preload or other loading conditions. With this method, we were able to measure LV relaxation and filling properties in competitive male triathletes and to compare these results with those of the male control group. Pulsed Doppler tissue imaging demonstrated specific characteristics of both the late passive diastolic filling period in triathletes and the early active diastolic relaxation period. By means of such imaging, we were able to determine specific characteristics of the left ventricle during the entire diastolic phase. Our findings also led us to speculate that genetic factors may play a role in the structural and functional heart adaptations in male triathletes. Further study of the genetics of the hearts of triathletes would be of interest.
Footnotes
Address for reprints: Dr. Philip Claessens, Rubenslei 35, B-2018 Antwerp, Belgium
References
- 1.Sahn DJ, DeMaria A, Kisslo J, Weyman A. Recommendations regarding quantitation in M-mode echocardiography: results of a survey of echocardiographic measurements. Circulation 1978;58:1072–83. [DOI] [PubMed]
- 2.Oh JK, Appleton CP, Hatle LK, Nishimura RA, Seward JB, Tajik AJ. The noninvasive assessment of LV diastolic function with two-dimensional and Doppler echocardiography. J Am Soc Echocardiogr 1997;10:246–70. [DOI] [PubMed]
- 3.Brutsaert DL, Housmans PR, Goethals MA. Dual control of relaxation. Its role in the ventricular function in the mammalian heart. Circ Res 1980;47:637–52. [DOI] [PubMed]
- 4.Fagard R, Van den Broeke C, Bielen E, Vanhees L, Amery A. Assessment of stiffness of the hypertrophied left ventricle of bicyclists using left ventricular inflow Doppler velocimetry. J Am Coll Cardiol 1987;9:1250–4. [DOI] [PubMed]
- 5.Galiuto L, Ignone G, DeMaria AN. Contraction and relaxation velocities of the normal left ventricle using pulsed-wave tissue Doppler echocardiography. Am J Cardiol 1998; 81:609–14. [DOI] [PubMed]
- 6.Garcia MJ, Thomas JD, Klein AL. New Doppler echocardiographic applications for the study of diastolic function. J Am Coll Cardiol 1998;32:865–75. [DOI] [PubMed]
- 7.Oki T. State of the art: “diastology” research 1998. J Med Invest 1998;45(1–4):9–25. [PubMed]
- 8.Oki T, Tabata T, Yamada H, Wakatsuki T, Shinohara H, Nishikado A, et al. Clinical application of pulsed Doppler tissue imaging for assessing abnormal left ventricular relaxation. Am J Cardiol 1997;79:921–8. [DOI] [PubMed]
- 9.Vitarelli A, Gheorghiade M. Diastolic heart failure: standard Doppler approach and beyond. Am J Cardiol 1998; 81(12A):115G–121G. [DOI] [PubMed]
- 10.Douglas PS, O'Toole ML, Hiller WD, Reichek N. Left ventricular structure and function by echocardiography in ultra-endurance athletes. Am J Cardiol 1986;58:805–9. [DOI] [PubMed]
- 11.Fagard RH. Impact of different sports and training on cardiac structure and function. Cardiol Clin 1992;10:241–56. [PubMed]
- 12.Liu Y, Steinacker JM, Dehnert C, Menold E, Baur S, Lormes W, Lehmann M. Effect of “living high-training low” on the cardiac functions at sea level. Int J Sports Med 1998;19:380–4. [DOI] [PubMed]
- 13.Shapiro LM. Morphologic consequences of systematic training. Cardiol Clin 1992;10:219–26. [PubMed]
- 14.Shapiro LM. Physiological left ventricular hypertrophy. Br Heart J 1984;52:130–5. [DOI] [PMC free article] [PubMed]
- 15.Pelliccia A. Outer limits of physiologic hypertrophy and relevance to the diagnosis of primary cardiac disease. Cardiol Clin 1992;10:267–79. [PubMed]
- 16.Colan SD. Mechanics of left ventricular systolic and diastolic function in physiologic hypertrophy of the athlete heart. Cardiol Clin 1992;10:227–40. [PubMed]
- 17.Cuspidi C, Lonati L, Sampieri L, Leonetti G, Zanchetti A. Similarities and differences in structural and functional changes of left ventricle and carotid arteries in young borderline hypertensives and in athletes. J Hypertens 1996;14: 759–64. [DOI] [PubMed]
- 18.Mockel M, Stork T. Diastolic function in various forms of left ventricular hypertrophy: contribution of active Doppler stress echo. Int J Sports Med 1996;17(Suppl 3):S184–90. [DOI] [PubMed]
- 19.Oki T, Tabata T, Mishiro Y, Yamada H, Abe M, Onose Y, et al. Pulsed tissue Doppler imaging of left ventricular systolic and diastolic wall motion velocities to evaluate differences between long and short axes in healthy subjects. J Am Soc Echocardiogr 1999;12:308–13. [DOI] [PubMed]
- 20.Gorcsan J, Strum DP, Mandarino WA, Gulati VK, Pinsky MR. Quantitative assessment of alterations in regional left ventricular contractility with color-coded tissue Doppler echocardiography. Comparison with sonomicrometry and pressure-volume relations. Circulation 1997;95:2423–33. [DOI] [PubMed]
- 21.Miyatake K, Yamagishi M, Tanaka N, Uematsu M, Yamazaki N, Mine Y, et al. New method for evaluating left ventricular wall motion by color-coded tissue Doppler imaging: in vitro and in vivo studies. J Am Coll Cardiol 1995;25:717–24. [DOI] [PubMed]
- 22.Nagueh SF, Lakkis NM, Middleton KJ, Spencer WH, Zoghbi WA, Quinones MA. Doppler estimation of left ventricular filling pressures in patients with hypertrophic cardiomyopathy. Circulation 1999;99:254–61. [DOI] [PubMed]
- 23.Oki T, Tabata T, Yamada H, Wakatsuki T, Mishiro Y, Abe M, et al. Left ventricular diastolic properties of hypertensive patients measured by pulsed tissue Doppler imaging. J Am Soc Echocardiogr 1998;11:1106–12. [DOI] [PubMed]
- 24.Pai RG, Gill KS. Amplitudes, durations, and timings of apically directed left ventricular myocardial velocities: II. Systolic and diastolic asynchrony in patients with left ventricular hypertrophy. J Am Soc Echocardiogr 1998;11:112–8. [DOI] [PubMed]


