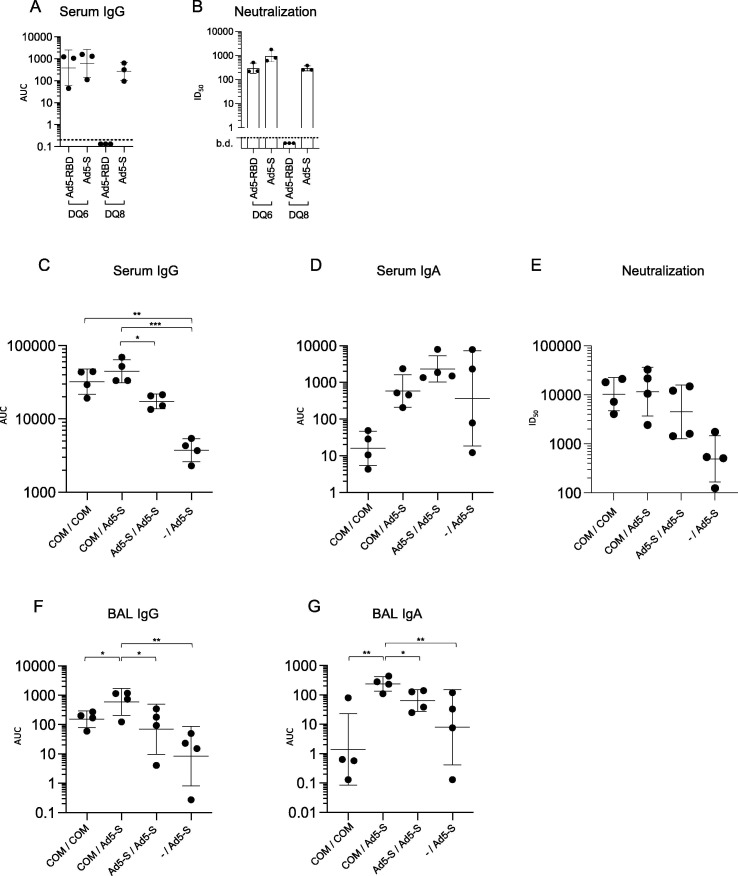
Fig. 8.
Humoral responses to single treatment with Ad5-RBD or Ad5-S administered intranasally in HLA-DQ6 vs. HLA-DQ8 mice, and responses to Ad5-S administered intranasally after priming with mRNA vaccine Comirnaty (Pfizer-BioNTech, “COM”) intramuscularly. Groups of adult female HLA-DQ6 or -DQ8 mice (n = 3) were inoculated with 107 viral particles (vp)/mouse of either Ad5-RBD or Ad5-S (a, b), as indicated. Anti-spike IgG serum antibodies (a; AUC) and SARS-CoV-2 pseudovirus neutralization (b; pooled sera, dots representing the values of triplicates; calculated serum dilution producing 50% inhibition (ID50)) were measured after three weeks. Groups of adult male and female HLA-DQ8 mice (n = 4) were inoculated either with Comirnaty (2 µg/dose, i.m.), Ad5-S (109 vp/dose, i.n.) or were not pre-treated (c-g). Three weeks later, they received either Comirnaty i.m. or Ad5-S i.n. at the same doses, as indicated. The Ad5-S vector used in this experiment encoded for the beta variant strain of SARS-CoV-2, containing three major receptor-binding domain mutations. Anti-spike IgG and IgA serum or bronchoalveolar lavage (BAL) antibodies (c, d, f, g; AUC) and pseudovirus neutralization (e; individual sera; ID50) were measured after six weeks. For statistical comparisons, one-way ANOVA and Tukey’s test for multiple comparisons (a, c, d, f, g) or Kruskal-Wallis test and Dunn’s test for multiple comparisons (b, e) were used. Adjusted p-values are displayed as * <0.05, ** <0.01 and *** <0.001. Lines and bars represent geometric means and standard deviations. Dotted line represents the limit of detection of the assay.