Abstract
The presence of renin production by the principal cells of the collecting duct has opened new perspectives for the regulation of intrarenal angiotensin II (Ang II). Angiotensinogen (AGT) and angiotensin converting enzyme (ACE) are present in the tubular fluid coming from the proximal tubule and collecting duct. All the components needed for Ang II formation are present along the nephron and much is known about the mechanisms regulating renin in juxtaglomerular cells (JG), however those in the collecting duct remain unclear. Ang II suppresses renin via protein kinase C (PKC) and calcium (Ca2+) in JG cells, but in the principal cells, Ang II increases renin synthesis and release through a pathophysiological mechanism that increases further intratubular Ang II de novo formation to enhance distal Na+ reabsorption. Transgenic mice overexpressing renin in the collecting duct demonstrate the role of collecting duct renin in the development of hypertension. The story became even more interesting after the discovery of a specific receptor for renin and prorenin: the prorenin receptor [(P)RR], which enhances renin activity and fully activates prorenin. The interactions between (P)RR and prorenin/renin may further increase intratubular Ang II levels. In addition to Ang II, other mechanisms have been described in the regulation of renin in the collecting duct, including vasopressin (AVP), bradykinin (BK), and prostaglandins. Current active investigations are aimed at elucidating the mechanisms regulating renin in the distal nephron segments and understand its role in the pathogenesis of hypertension.
Keywords: kidney, prorenin, prorenin receptor, hypertension, intracellular signaling
Introduction
In 1990 Hackenthal et al. was the first to report the observation of renin in the distal nephron segments [1]. However, it was not until 1999 that Rohrwasser et al. demonstrated specific renin immunostaining in the principal cells of the collecting duct, when technology allowed new views of the intrarenal renin angiotensin system (RAS) arose [2]. The kidney, is the only organ of synthetizing all of the components of the RAS. Evidence indicates that renin in the collecting duct is differentially regulated from that in the juxtaglomerular (JG) cells [3••]. While systemic angiotensin II (Ang II) inhibits JG renin synthesis and secretion [4, 5], it feeds forward renin in the principal cells [6, 7]. Renin and the prorenin receptor (P)RR have the potential to regulate blood pressure [5,14]. Prorenin secreted by the principal cells by binding (P)RR in neighboring intercalated cells [8] enhances local production of Ang II [9]. The (P)RR also activates intracellular signaling pathways linked to the enhancement of distal tubule Na+ reabsorption through the epithelial Na+ channel, ENaC) [10••]. Therefore, renin in the collecting ducts plays a critical role toward the augmentation of the intrarenal RAS. In this review, we discuss the most recent findings of the mechanisms regulating renin in the collecting duct and its functional role in the pathogenesis of hypertension.
Renin in the distal nephron segments
In 1999, Rohrwasser et al., showed that renin production and secretion by the connecting tubule occurs in a sodium-dependent fashion [11], which further supported the concept that the distal nephron segments harbor a paracrine mechanism contributing to the regulation of blood pressure [2]. We demonstrated that renin in the collecting ducts is exclusively expressed by the principal cells, co-localizing with aquaporin 2 (AQP2) in the mouse and rat kidneys [7, 3]. Renin expression has been also demonstrated in primary cultures from rat inner medullary collecting duct (IMCD) cells [12••] and mouse cortical M-1 cells [13] from the collecting ducts of mouse origin [14], where most of the renin detected corresponds to non-active prorenin [12••, 3••].
Mechanisms of renin regulation in the collecting ducts
The role of angiotensin II/AT1 receptors
In the JG cells, the activation of cAMP/PKA/CREB is the central pathway for regulation. In contrast to the inhibitory effect of Ang II/AT1 receptors on JG cells, the regulation of renin synthesis and secretion by the collecting duct cells seems to be dependent on AT1 receptor activation. To address this question, we used Ang II-dependent hypertensive models. Seminal studies demonstrated that most renin and prorenin proteins are augmented in the collecting ducts of chronic Ang II-infused hypertensive rats [7] [12••] and mice [15]. Similarly, Kang et al., demonstrated that M-1 cells treated with Ang II showed higher renin mRNA levels [13]. Furthermore, renin can be detected in the urine of Ang II hypertensive rodents regardless JG suppression [16]. Interestingly, the pharmacological blockade of AT1 receptor blunts the upregulation of renin in the collecting ducts in these models [17]. Angiotensin II-dependent upregulation of renin in the distal nephron segments occurs independent of blood pressure shown by 2K1C Goldblatt hypertensive rats in which there is stimulation of collecting duct renin in both clipped and non-clipped kidneys, despite differences in renal pressure [18]. One may ask what are the intracellular signaling mechanisms controlling renin in the distal nephron segments. We have found that the stimulatory effects of Ang II on prorenin and renin produced by the principal cells include the activation of AT1 receptors and downstream signals including PKC and cAMP/PKA/CREB [12••, 3••, 19••]. In cortical collecting duct M-1 cells, we have demonstrated that the AT1 receptor-dependent stimulation of renin involves cAMP production and activation of Ca2+-dependent PKC-α. Additional work is necessary to clarify whether and how these two pathways are synchronized.
The effect of Ang II on collecting duct renin is independent of epithelial sodium channels (ENaC) and mineralocorticoid receptors activation
To further determine the role of Na+ reabsorption on renin regulation in the collecting duct cells, we performed in vivo experiments to examine whether renin synthesis is directly mediated by AT1 receptors or modulated by ENaC and mineralocorticoid receptors. Treatment with ENaC inhibitor (amiloride; 5 mg/kg per day) in chronic Ang II-infused rats (80 ng/min, 2 weeks) demonstrated the independence of renin induction from the activity of ENaC in the rat renal inner medullary tissues, exempted of JG cells. Our in vitro studies using primary cultures of rat isolated IMCD cells demonstrated that Ang II-dependent stimulations of renin synthesis and secretion were also independent of mineralocorticoid receptor activation, but reliant on PKC [12••]. Those studies further revealed that activation of AT1 receptors increases intracellular Ca+2 levels in IMCD cells and suggested that Ca+2 is not a negative modulator of renin synthesis in the distal nephron segments.
Due to evidence demonstrating that dietary Na+ alters renin expression in the distal nephron [9], we also investigated the responses of renin in the collecting ducts from the renal inner medulla of rats subjected to low salt diet. We showed that low salt stimulates the expression of renin and (P)RR in the collecting ducts [20]. The concurrent induction of renin and (P)RR during chronic low salt diet might enhance the binding of renin to its receptor, which facilitates intratubular formation of Ang I and Ang II in the collecting duct, as a redundant mechanism to ensure distal sodium reabsorption.
The role of Vasopressin/V2 receptors
There is strong evidence showing that the cAMP/PKA and cAMP response element-binding protein (CREB) pathway is the central pathway for renin regulation in JG cells [21-24]. The formation of cAMP mediated by forskolin, an activator of adenylate cyclase (AC), increases the activity of renin promoter, thus stimulating renin gene transcription in JG cells. Because Ang II-dependent stimulation of renin in the collecting ducts involves augmentation of cAMP production, we further tested the hypothesis that activation of V2R increases renin synthesis in the collecting ducts via PKA/CREB, independently of Ang II/AT1 receptor. Using in vitro studies we showed that AVP/V2 receptor agonist DDAVP increased renin synthesis and secretion in M-1 cells [19••]. Furthermore, we demonstrated that mice subjected to water deprivation during 48 h exhibited augmented production of prorenin and renin in renal inner medullary tissues, circumvented of JG renin-producing cells. This effect was also observed in rats concomitantly treated with RAS blockers [19••]. Recently we found (unpublished data) that prostaglandin E2 (PGE2) through E prostanoid EP1 receptor coupled to the activation of PKC and cAMP pathways further increases renin synthesis in cultured collecting duct cells. Synergism between PKC and cAMP/PKA signals has been widely described in collecting duct cells. In these cells, Lee et al. demonstrated that Ang II plays a role in the regulation of AQP2 in the plasma membrane. This effect was mediated by the activation of the AT1 receptor [25]. Rozengurt et al. further showed that PKC activation enhanced cAMP accumulation induced by forskolin and that this effect was prevented by downregulation of PKC [5]. This modulatory effect of Ang II may be mediated by direct activation of adenylcyclase (AC) [4]. Thus, it seems clear that renin regulation depends on the activation of both, PKC and cAMP/PKA/CREB pathways.
The role of Bradykinin/B2 receptors
Most recently, we demonstrated that bradykinin B2R activation regulates renin in the collecting duct via two different mechanisms, the diacylglycerol-dependent PKC signaling and the nitric oxide (NO)-dependent pathways [26]. We explored whether the activation of B2R exerts a feed-forward effect on renin regulation in the collecting duct. We found that activation of B2R by bradykinin increased renin synthesis and release in M-1 cells via PKC and NO release, which supported our hypothesis. Because we also found B2R expression in the interstitial cells, we think it likely that BK further exerts a paracrine regulation of renin produced by neighboring principal cells. As discussed above, intercalated and interstitial cells in the renal inner medulla express cyclooxygenase-2 (COX-2), the enzyme responsible for PGE2 production [27]. It is also likely that activation of B2R in interstitial cells via the PGE2/cAMP/PKA pathway [28, 2] may act as a paracrine stimulator of renin synthesis by the neighboring principal cells. Efforts to understand whether NO directly contributes to renin synthesis or maturation are being explored in our laboratory.
Collecting duct renin and implications in the pathogenesis of hypertension
Renin might be filtered in the glomerulus; however, in Ang II-dependent hypertensive animals there is suppression of JG renin and plasma renin activity [16]. Given that AGT is secreted into the lumen of proximal tubules and then spilled over the distal nephron segments [29], it is likely that because of ACE along the nephron, renin is apically secreted by the principal cells [16] and acts on the substrate causing local Ang I and Ang II formation [30]. These actions seem to explain the de novo formation of Ang II in the kidneys of Ang II-dependent hypertensive animal models [31] and the hypertensinogenic effects of Ang II increasing distal Na+ reabsorption via ENaC [32, 33]. In these hypertensive models, Ang II further exerts a feed-forward stimulation of the intrarenal production of AGT [34], ACE [35], as well as renin in the collecting ducts [7, 18]. Ramkumar et al., showed that the site-specific overexpression of renin in the collecting ducts caused elevated systolic blood pressure [36••]. Moreover, the same group demonstrated that the specific deletion of renin from the distal nephron attenuates Ang II-induced hypertension through an effect associated with changes in ENaC expression [37••].
In line with the hypothesis that the lack of Ren-1c gene from the collecting duct attenuates hypertensive response during Ang II-induced hypertension, we used the conditional B2R knockout mice (UBBdkrb2−/−) which do not express B2R in the collecting ducts and showed a marked suppression of renin expression in the principal cells [26]. These knockout mice had decreased renin protein and mRNA expression and kidney Ang II levels [38]. Indeed, the conditional B2R knockout mice, which do not express renin in the collecting ducts, exhibited an attenuated blood pressure response during Ang II-dependent salt sensitive hypertension [39]. The functional roles of collecting duct renin are complex due to the local interaction with the (P)RR primarily expressed by the intercalated cells [8] and the potential for the paracrine regulation of blood pressure.
Physiological and pathophysiological consequences of the interactions between (P)RR and prorenin/renin in the kidney
The (P)RR binds renin and prorenin with similar affinity in the nanomolar range [40]. However, the biological characteristics of the (P)RR indicate that this receptor is the full-length form of the vacuolar H+-ATPase (v-ATPase) protein expressed by the ATP6AP2 gene (ATPase 6 accessory protein 2) [41]. Post-translational processing of the native (P)RR generates 3 different proteins: (i) the full-length integral transmembrane protein [(P)RR], which binds either renin or prorenin and mediates intracellular signaling; (ii) the soluble form [s(P)RR], which is found in plasma and urine and can activate prorenin or enhance the activity of renin in the extracellular environment; and (iii) the truncated form composed of the transmembrane and cytoplasmic domains and that is associated with the H+-ATPase pump [41].
It has been suggested that renin secreted by the principal cells binds to (P)RR at the neighboring intercalated cells, thus activating prorenin in a catalytic or non-catalytic form (Ang II-dependent mechanism) or triggering intracellular cascades (Ang II-independent mechanism) [42]. The pathophysiological consequences of intrarenal Ang II accumulation include the development of tissue injury mediated by AT1 receptor activation [43]. Evidence about Ang II-independent effects of (P)RR on kidney injury is scarce. We demonstrated that prorenin-(P)RR complex leads to COX-2 upregulation suggesting that its vasoconstrictor metabolites contributes to the late phase of Ang II-dependent hypertension [27]. As result, it is likely that prorenin-(P)RR interaction underlies pathological processes such as Ang II-dependent hypertension and diabetic nephropathy [44, 45]. Emerging in vitro and in vivo evidence supports a role of (P)RR in mediating ENaC regulation to stimulate Na+ reabsorption in the collecting ducts [10••]. The development of inducible distal nephron (P)RR knockout mice by using Cre-recombinase driven by the aquaporin-2 (AQP2) promoter has demonstrated a blunted response to AVP and reduced expression of AQP2 in the inner medullary collecting ducts leading to nephrogenic diabetes insipidus [46]. Furthermore, the activation of (P)RR also evokes inflammatory responses in diabetic kidneys through the augmentation of COX-2 via ERK1/2 pathway [47, 27] and kidney damage [48]. Indeed, we reported that (P)RR and COX-2 co-localize in intercalated cells, as well as in interstitial cells [27] and that the activation of (P)RR with recombinant prorenin stimulates the COX-2 synthesis and ERK1/2 phosphorylation [27]. Thus, it is likely that (P)RR plays a role in the control of prostaglandins production in the renal inner medulla to maintain Na+ and water balance. We demonstrated in male Sprague-Dawley rats that during the early phase of chronic Ang II infusion (between Days 0-5), when hypertension has not yet been developed, there was augmentation of full-length (P)RR expression on the plasma membrane and (P)RR-dependent stimulation of COX-2 expression in the renal inner medulla and excretion PGE2 in the urine. These changes were associated with increases in local renin and activity of ERK1/2. However, during late phase Ang II-dependent hypertension (after Day 7), (P)RR expression levels decreased significantly and only its soluble form, s(P)RR levels, increased [49••]. Together, these findings support the concept that (P)RR bound to cell membranes plays a crucial role in the activation of intracellular signals that stimulate COX-2 and PGE2, a mechanism that buffers the early local actions of Ang II. However, during late stages of chronic Ang II infusion, the s(P)RR assumes command, facilitating sustained intratubular Ang II formation and contributing to augmented distal Na+ reabsorption and to the development of hypertension.
Conclusions and Perspectives
The discovery of renin production and secretion by the principal cells of the collecting ducts provided better understanding of the intratubular RAS and its role in the development of hypertension. Many efforts have been done to comprehend the mechanisms regulating renin synthesis and release by the collecting ducts, but much more are needed to elucidate the specific functional contribution of renin in the distal nephron to the control of Na+ balance and blood pressure. Paracrine modulation of renin by local hormonal systems and its interaction with the (P)RR in the collecting ducts make the story complex. During Ang II-dependent hypertension, despite plasma renin activity suppression, there is upregulation of renin and (P)RR along with all the components necessary for de novo intratubular Ang II formation. Thus, targeting renin in the distal nephron segments or its interactions with the local (P)RR seems to be pharmacological tools needed to decrease Ang II content and its effects on the local stimulation of Na+ reabsorption and the development of hypertension.
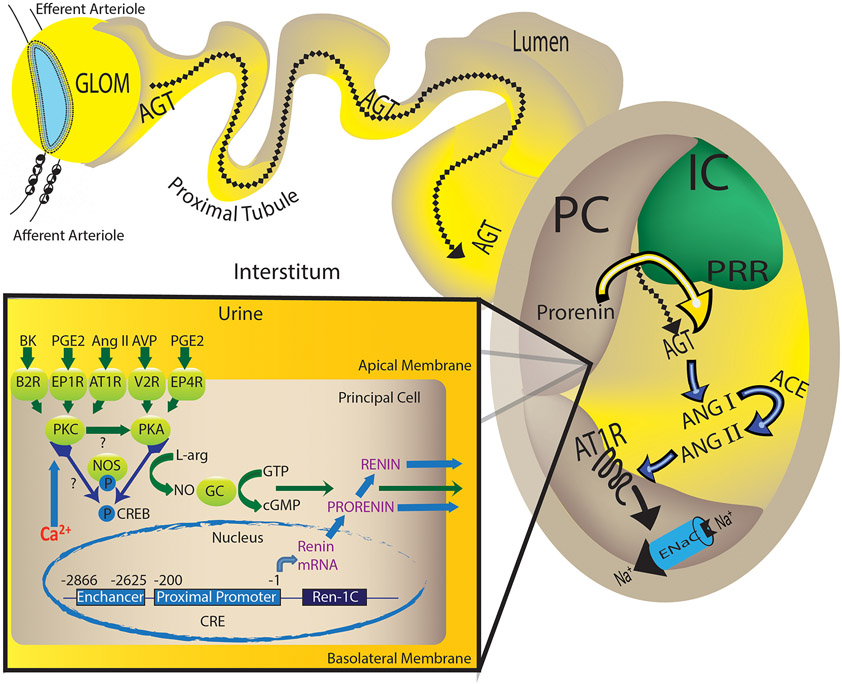
Proposed mechanisms involved in the regulation of prorenin/renin synthesis and secretion by the collecting duct.
In the collecting duct, the principal cell responds to the actions of angiotensin II (Ang II), vasopressin (AVP), bradykinin (BK) and prostaglandin (PGE2) via the AT1R, V2R, B2R, EP1 and EP4 receptors. Angiotensin (Ang) II-mediated augmentation of renin synthesis and secretion by the principal cells comprises the activation of AT1R and protein kinase alpha (PKCα), which is a calcium-dependent PKC. It is possible that PKCα potentiates G-couple receptor (GPCR)-mediated activation of adenylcyclase-6 or the inhibition of phosphodiesterase all of which causes the increased PKA activity, CREB phosphorylation turning on renin gene expression. Alternatively, independently of Ang II, the activation of AVP/V2R stimulates renin synthesis and secretion via the PKA/CREB pathway and BK/B2R via two different signals: the diacylglycerol-dependent PKC pathway as well as by releasing nitric oxide (NO) as a second messenger. Interestingly, during the early phase of Ang II-induced hypertension, PGE2 via EP1R and perhaps EP4R, might exert a buffer mechanism in the kidney, but also might be the initial stimuli for collecting duct renin-dependent intratubular Ang II formation. The specific localization of the prorenin receptor (PRR) at the neighboring intercalated cell (IC) in the kidney favors the hypertensinogenic effects of renin produced in the distal nephron segments. Secreted prorenin/renin into the lumen binds the PRR to enhance renin activity or activate prorenin, thus contributing to cleave AGT spilled from proximal tubules. Due to the presence of angiotensin converting enzyme (ACE) in the distal nephron segments, new Ang II is formed underwriting its actions on apical AT1R, which enhances ENaC activity, and sodium (Na+) reabsorption.
Acknowledgments
We thank Nancy B. Busija, M.A., CCC-SLP, Senior Editor, Department of Pharmacology, Tulane University School of Medicine for final edition of the article; and Lalo Gonzalez, MBA, Creative Designer from AVI (Art & Visual Ideas) for the excellence assistance in the figure design and preparation.
Source of Funding
The co-authors are supported by the National Institutes of Health (DK104375 grant) and LACaTS Center (1U54GM104940 grant) to M.C.P.; by FONDECYT-Chile (11121217 gran)t to A.A.G.; the National Council for Scientific and Technological Development (CNPq)-Brazil to L. S. L.; and the Science Without Borders grant from CNPq-Brazil, Especial Visiting Professor (420584/2013-7 grant) to L. S. L. and M. C. P.
Footnotes
Conflict of Interest: Drs. Gonzalez, Lara, and Prieto declare no conflicts of interest relevant to this manuscript.
Human and Animal Rights and Informed Consent: This review does not contain any new studies with human or animal subjects. Cited experiments previously performed by the authors have been approved by the Institutional Animal Care and Use Committee according to the National Institutes of Health (NIH).
References
- 1.Hackenthal E, Paul M, Ganten D, Taugner R. Morphology, Physiology, and Molecular-Biology of Renin Secretion. Physiol Rev. 1990;70(4):1067–1116. [DOI] [PubMed] [Google Scholar]
- 2.Rohrwasser A, Morgan T, Dillon HF, Zhao L, Callaway CW, Hillas E et al. Elements of a paracrine tubular renin-angiotensin system along the entire nephron. Hypertension. 1999;34(6):1265–1274. [DOI] [PubMed] [Google Scholar]
- ••3. Gonzalez AA, Liu L, Lara LS, Bourgeois CRT, Ibaceta-Gonzalez C, Salinas-Parra N et al. PKC-alpha-dependent augmentation of cAMP and CREB phosphorylation mediates the angiotensin II stimulation of renin in the collecting duct. Am J Physiol-Renal. 2015;309(10):E880–E888. This study demonstrated that Ang II feedforwards collecting duct renin via PKC-α, which allows the augmentation of cAMP production and the activation of PKA/CREB pathway via AC6.
- 4.Beazely MA, Watts VJ. G alpha(q)-coupled receptor signaling enhances adenylate cyclase type 6 activation. Biochem Pharmacol. 2005;70(1):113–120. [DOI] [PubMed] [Google Scholar]
- 5.Rozengurt E, Murray M, Zachary I, Collins M. Protein-Kinase-C Activation Enhances Camp Accumulation in Swiss 3t3 Cells - Inhibition by Pertussis Toxin. P Natl Acad Sci USA. 1987;84(8):2282–2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prieto-Carrasquero MC, Kobori H, Ozawa Y, Guttierrez A, Seth D, Navar LG. AT(1) receptor- mediated enhancement of collecting duct renin in angiotensin II-dependent hypertensive rats. Am J Physiol-Renal. 2005;289(3):F632–F637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prieto-Carrasquero MC, Harrison-Bernard LM, Kobori H, Ozawa Y, Hering-Smith KS, Hamm LL et al. Enhancement of collecting duct renin in angiotensin II-dependent hypertensive rats. Hypertension. 2004;44(2):223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Advani A, Kelly DJ, Cox AJ, White KE, Advani SL, Thai K et al. The (Pro) Renin Receptor Site-Specific and Functional Linkage to the Vacuolar H(+)-ATPase in the Kidney. Hypertension. 2009;54(2):261–269. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez AA, Lara LS, Luffman C, Seth DM, Prieto MC. Soluble Form of the (Pro) Renin Receptor Is Augmented in the Collecting Duct and Urine of Chronic Angiotensin II-Dependent Hypertensive Rats. Hypertension. 2011;57(4):859–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••10. Peng K, Lu X, Wang F, Nau A, Chen R, Zhou SF et al. Collecting duct (pro)renin receptor targets ENaC to mediate angiotensin II-induced hypertension. American journal of physiology Renal physiology. 2017;312(2):F245–F253. This study showed the functional role of (P)RR in the collecting ducts on Na+ transport contributing to Ang II-induced hypertension via activation of renal medullary ENaC.
- 11.Lantelme P, Rohrwasser A, Gociman B, Hillas E, Cheng T, Petty G et al. Effects of dietary sodium and genetic background on angiotensinogen and renin in mouse. Hypertension. 2002;39(5):1007–1014. [DOI] [PubMed] [Google Scholar]
- ••12. Gonzalez AA, Liu L, Lara LS, Seth DM, Navar LG, Prieto MC. Angiotensin II Stimulates Renin in Inner Medullary Collecting Duct Cells via Protein Kinase C and Independent of Epithelial Sodium Channel and Mineralocorticoid Receptor Activity. Hypertension. 2011;57(3):594–599. This study demonstrated for the first time the existence of a novel mechanism by which Ang II stimulates collecting duct renin, opposite to the well-known mechanism described for renin in the juxtaglomerular cells.
- 13.Kang JJ, Toma I, Sipos A, Meer EJ, Vargas SL, Peti-Peterdi J. The collecting duct is the major source of prorenin in diabetes. Hypertension. 2008;51(6):1597–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stoos BA, Narayfejestoth A, Carretero OA, Ito S, Fejestoth G. Characterization of a Mouse Cortical Collecting Duct Cell-Line. Kidney Int. 1991;39(6):1168–1175. [DOI] [PubMed] [Google Scholar]
- 15.Gonzalez-Villalobos RA, Satou R, Ohashi N, Semprun-Prieto LC, Katsurada A, Kim C et al. Intrarenal mouse renin-angiotensin system during ANG II-induced hypertension and ACE inhibition. Am J Physiol-Renal. 2010;298(1):F150–F157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu L, Gonzalez AA, McCormack M, Seth DM, Kobori H, Navar LG et al. Increased renin excretion is associated with augmented urinary angiotensin II levels in chronic angiotensin II-infused hypertensive rats. Am J Physiol-Renal. 2011;301(6):F1195–F201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kobori H, Prieto-Carrasquero MC, Ozawa Y, Navar LG. AT1 receptor mediated augmentation of intrarenal angiotensinogen in angiotensin II-dependent hypertension. Hypertension. 2004;43(5):1126–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prieto-Carrasquero MC, Botros FT, Pagan J, Kobori H, Seth DM, Casarini DE et al. Collecting duct renin is upregulated in both kidneys of 2-kidney, 1-clip Goldblatt hypertensive rats. Hypertension. 2008;51(6):1590–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••19. Gonzalez AA, Cifuentes-Araneda F, Ibaceta-Gonzalez C, Gonzalez-Vergara A, Zamora L, Henriquez R et al. Vasopressin/V2 receptor stimulates renin synthesis in the collecting duct. Am J Physiol-Renal. 2016;310(4):F284–F293. This work demonstrated that vasopressin/V2 receptor increases renin synthesis via protein kinase A/CREB, independently of Ang II type 1 (AT1) receptor activation in collecting duct cells. Furthermore, experiments using water deprivation and RAS blockade provided in vivo evidence that collecting duct renin is involved in water transport.
- 20.Gonzalez AA, Womack JP, Liu L, Seth DM, Prieto MC. Angiotensin II Increases the Expression of (Pro)Renin Receptor During Low-Salt Conditions. Am J Med Sci. 2014;348(5):416–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurtz A, Wagner C. Regulation of renin secretion by angiotensin II-AT1 receptors. J Am Soc Nephrol. 1999;10:S162–S168. [PubMed] [Google Scholar]
- 22.Kurtz A, Wagner C. Cellular control of renin secretion. J Exp Biol. 1999;202(3):219–225. [DOI] [PubMed] [Google Scholar]
- 23.Klar J, Sigl M, Obermayer B, Schweda F, Kramer BK, Kurtz A. Calcium inhibits renin gene expression by transcriptional and posttranscriptional mechanisms. Hypertension. 2005;46(6):1340–1346. [DOI] [PubMed] [Google Scholar]
- 24.Klar J, Sandner P, Muller MWH, Kurtz A. Cyclic AMP stimulates renin gene transcription in juxtaglomerular cells. Pflug Arch Eur J Phy. 2002;444(3):335–344. [DOI] [PubMed] [Google Scholar]
- 25.Lee YJ, Song IK, Jang KJ, Nielsen J, Frokiaer J, Nielsen S et al. Increased AQP2 targeting in primary cultured IMCD cells in response to angiotensin II through AT(1) receptor. Am J Physiol-Renal. 2007;292(1):F340–F350. [DOI] [PubMed] [Google Scholar]
- 26.Lara LS, Bourgeois CRT, El-Dahr SS, Prieto MC. Bradykinin/B-2 receptor activation regulates renin in M-1 cells via protein kinase C and nitric oxide. Physiol Rep. 2017;5(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gonzalez AA, Luffman C, Bourgeois CRT, Vio CP, Prieto MC. Angiotensin II-Independent Upregulation of Cyclooxygenase-2 by Activation of the (Pro) Renin Receptor in Rat Renal Inner Medullary Cells. Hypertension. 2013;61(2):443–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rohrwasser A, Ishigami T, Gociman B, Lantelme P, Morgan T, Cheng T et al. Renin and kallikrein in connecting tubule of mouse. Kidney Int. 2003;64(6):2155–21562. [DOI] [PubMed] [Google Scholar]
- 29.Kobori H, Harrison-Bernard LM, Navar LG. Urinary excretion of angiotensinogen reflects intrarenal angiotensinogen production. Kidney Int. 2002. Feb;61(2):579–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Casarini DE, Boim MA, Stella RCR, KriegerAzzolini MH, Krieger JE, Schor N. Angiotensin I-converting enzyme activity in tubular fluid along the rat nephron. Am J Physiol-Renal. 1997;272(3):F405–F409. [DOI] [PubMed] [Google Scholar]
- 31.Shao WJ, Seth DM, Navar LG. Augmentation of endogenous intrarenal angiotensin II levels in Val(5)-ANG II-infused rats. Am J Physiol-Renal. 2009;296(5):F1067–F1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mamenko M, Zaika O, Prieto MC, Jensen VB, Doris PA, Navar LG et al. Chronic Angiotensin II Infusion Drives Extensive Aldosterone-Independent Epithelial Na+ Channel Activation. Hypertension. 2013;62(6):1111–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mamenko M, Zaika O, Ilatovskaya DV, Staruschenko A, Pochynyuk O. Angiotensin II Increases Activity of the Epithelial Na+ Channel (ENaC) in Distal Nephron Additively to Aldosterone. J Biol Chem. 2012;287(1):660–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kobori H, Ozawa Y, Suzaki Y, Nishiyama A. Enhanced Intrarenal Angiotensinogen Contributes to Early Renal Injury in Spontaneously Hypertensive Rats. J Am Soc Nephrol. 2005. Jul; 16(7): 2073–2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gonzalez-Villalobos RA, Billett S, Kim C, Satou R, Fuchs S, Bernstein KE et al. Intrarenal Angiotensin-Converting Enzyme Induces Hypertension in Response to Angiotensin I Infusion. J Am Soc Nephrol. 2011;22(3):449–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••36. Ramkumar N, Ying J, Stuart D, Kohan DE. Overexpression of Renin in the Collecting Duct Causes Elevated Blood Pressure. Am J Hypertens. 2013;26(8):965–972. This study used mice with overexpression of renin in the collecting duct to demonstrate that collecting duct-derived renin modulates blood pressure independent of the systemic renin-angiotensin system.
- ••37. Ramkumar N, Stuart D, Rees S, Van Hoek A, Sigmund CD, Kohan DE. Collecting duct-specific knockout of renin attenuates angiotensin II-induced hypertension. Am J Physiol-Renal. 2014;307(8):F931–F938. By using a mouse model with specific collecting duct-specific knockout of renin, the authors demonstrated that collecting duct renin modulates blood pressure in Ang II-infused hypertension and that these effects are associated with changes in ENaC expression.
- 38.Imig JD, Zhao XY, Orengo SR, Dipp S, El-Dahr SS. The Bradykinin B2 receptor is required for full expression of renal COX-2 and renin. Peptides. 2003;24(8):1141–1147. [DOI] [PubMed] [Google Scholar]
- 39.Kopkan L, Huskova Z, Jichova S, Cervenkova L, Cervenka L, Saifudeen Z et al. Conditional knockout of collecting duct bradykinin B-2 receptors exacerbates angiotensin II-induced hypertension during high salt intake. Clin Exp Hypertens. 2016;38(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krop M, Lu XF, Danser AHJ, Meima ME. The (pro)renin receptor. A decade of research: what have we learned? Pflug Arch Eur J Phy. 2013;465(1):87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nguyen G. Renin and Prorenin Receptor in Hypertension: What's New? Curr Hypertens Rep. 2011;13(1):79–85. [DOI] [PubMed] [Google Scholar]
- 42.Gonzalez AA, Prieto MC. Renin and the (pro)renin receptor in the renal collecting duct: Role in the pathogenesis of hypertension. Clin Exp Pharmacol P. 2015;42(1):14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cuevas CA, Gonzalez AA, Inestrosa NC, Vio CP, Prieto MC. Angiotensin II increases fibronectin and collagen I through the beta-catenin-dependent signaling in mouse collecting duct cells. Am J Physiol-Renal. 2015;308(4):F358–F365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ichihara A, Hayashi M, Kaneshiro Y, Suzuki F, Nakagawa T, Tada Y et al. Inhibition of diabetic nephropathy by a decoy peptide corresponding to the "handle" region for nonproteolytic activation of prorenin. J Clin Invest. 2004;114(8):1128–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matavelli LC, Huang JQ, Siragy HM. (Pro)renin receptor contributes to diabetic nephropathy by enhancing renal inflammation. Clin Exp Pharmacol P. 2010;37(3):277–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang F, Lu XH, Peng KX, Fang H, Zhou L, Su JH et al. Antidiuretic Action of Collecting Duct (Pro)Renin Receptor Downstream of Vasopressin and PGE(2) Receptor EP4. J Am Soc Nephrol. 2016;27(10):3022–3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaneshiro Y, Ichihara A, Takemitsu T, Sakoda M, Suzuki F, Nakagawa T et al. Increased expression of cyclooxygenase-2 in the renal cortex of human prorenin receptor gene-transgenic rats. Kidney Int. 2006;70(4):641–646. [DOI] [PubMed] [Google Scholar]
- 48.Clavreul N, Sansilvestri-Morel P, Magard D, Verbeuren TJ, Rupin A. (Pro)renin promotes fibrosis gene expression in HEK cells through a Nox4-dependent mechanism. Am J Physiol-Renal. 2011;300(6):F1310–F318. [DOI] [PubMed] [Google Scholar]
- ••49. Gonzalez AA, Green T, Luffman C, Bourgeois CRT, Navar LG, Prieto MC. Renal medullary cyclooxygenase-2 and (pro)renin receptor expression during angiotensin II-dependent hypertension. Am J Physiol-Renal. 2014;307(8):F962–F70. Using in vivo and in vitro approaches, this work demonstrated the role of the full lengh and the soluble forms of the (P)RR to modulate COX-2 expression. At the early phase of the Ang II-dependent hypertension, the full lengh (P)RR activation increases COX-2/PGE2, while in the late phase, the secretion of the soluble form decreases COX-2 production associated with increased intratubular Ang II content.