Abstract
Objectives: The aim was to examine the association of post-injury heart rate variability (HRV), coping with injury (fighting-acceptance), and depression symptoms in individuals with spinal cord injury (SCI).
Study Design: Cross-sectional study.
Setting: Tertiary care spinal cord injury hospital.
Participants: Ninety-one individuals with SCI.
Methods: All participants were assessed for HRV using polar heart rate monitor RS 800 CX and completed the Patient Health Questionnaire and Spinal Cord Lesion Coping Strategy questionnaire. Participants were grouped based on level of injury (tetraplegic, high paraplegia, and low paraplegia) and injury duration (early vs. late). Odds ratio calculated the risk of depression using HRV and coping as factors for early and late duration groups. Spearman rho estimated the correlation between three ratios: HRV (LF vs. HF), depression (somatic vs. cognitive), and coping (fighting spirit vs. acceptance) for each level of injury group for early and late duration.
Results: Individuals with SCI with high HRV had lower odds of depression (OR = 0.14, CI = 0.03–0.78) than individuals with SCI with low HRV in the early duration group. Individuals with SCI with high acceptance had lower odds of depression (OR = 0.19, CI = 0.44–0.79) than individuals with SCI with low acceptance in the later duration group. In the later duration, HRV ratio negatively correlated with coping ratio in individuals with low paraplegia and depression ratio in individuals with high paraplegia.
Conclusion: The aftermath of spinal cord injury might reflect a close association between the physiological response of autonomic variability and psychological response of coping and depression with implications for the level of injury and post-injury duration.
Keywords: Coping, HRV, Spinal cord injury, Depression
Introduction
Spinal cord injury (SCI) is an injury to the nervous system and the spinal cord due to traumatic or non-traumatic reasons.1 Along with a physical disability, impairment of cardiac autonomic regulation, individuals with SCI may experience psychological disorders like depression.1,2
Coping is the ability to respond to stress.3 After the injury, considerable variability in the coping responses is observed in individuals with spinal cord injury. The primary ways of coping observed in spinal cord injury are acceptance of injury, maintaining a fighting spirit, and relying on social support.3 Coping with the injury is an essential mediator of post-injury life adjustment such that mal-adjustment contributes to the risk of depression among individuals with SCI.4–7 Apart from reflecting initial reaction to the injury, coping also reflects an adjustment to the injury and the situational demands. Compared to the coping strategy of maintaining a fighting-sprit, acceptance-based coping predicts life satisfaction, quality of life, and emotional wellbeing.3,8 Therefore, acceptance-based coping in the early phase of injury lessens the chances of depression.7
A recent meta-analysis documented that the risk of depression is high in individuals with SCI; however, the prevalence of depression showed considerable variability across studies, and the source of variability in the risk of developing depression is largely unknown.9 Apart from heterogeneity in coping response, variability in depression in individuals with SCI might be due to the complex nature of depression. Depression has somatic and cognitive manifestations, and the somatic manifestation is marked by physical symptoms such as sleeplessness, fatigue, and loss of appetite. In contrast, the cognitive manifestation is thoughts characterized by sadness, pessimism, and the prevalence of negative moods.10–12 It is possible that people with spinal cord injury cope differently with the injury and that the psychological impact of spinal cord injury in the form of depression differs between individuals with SCI.3,13
The literature indicates that impaired autonomic regulation14,15 contributes to depression; it remains unknown whether autonomic regulation is associated with depression in spinal cord injury. Heart rate variability (HRV) is defined as variability between two successive heartbeats and is a measure of autonomic regulation16,17 associated with depression.14,15,18 Specifically, autonomic regulation denotes the action of the sympathetic and parasympathetic nervous system with sympathetic branch triggering response (e.g. fight/flight) and the parasympathetic branch inhibiting response (tend/befriend).16,17 As the injury to the spinal cord impairs the interaction between the autonomic and the central nervous system, failure of autonomic regulation might contribute to depression.19,20 In depression studies, HRV measures autonomic regulation in terms of time and frequency domains.16 The frequency domain of HRV has a low frequency (LF) and high frequency (HF) components. The HF represents the parasympathetic influence on the heart; the LF represents sympathetic and parasympathetic systems influence. The HRV (LF/HF) ratio indicates a balance of sympathetic – parasympathetic nervous systems.16,17 The frequency-domain components (LF, HF, and LF/HF ratio) are associated with depression.14,15 Meta-analysis indicates that depression is associated with an imbalanced ratio reflected as sympathetic system dominance over parasympathetic activity.14,19–21 Literature indicates that the link between the HRV measure is more prominent for the somatic, compared to the cognitive manifestation of depression, indicating that the somatic manifestation of depression might be associated with imbalanced sympathetic and parasympathetic activity.22,23 It is possible that psychological response of coping and autonomic regulation of HRV after the injury are related to the somatic and cognitive manifestations of depression in individuals with SCI.
Further, the level at which the spinal cord is injured and the post-injury duration (early vs. late) might be two additional factors that might influence depression and coping trajectory in individuals with SCI. The level of injury reflects the extent of autonomic impairment. Higher impairment is observed in tetraplegia and high paraplegia (injury till the T-6 vertebrae) where the sympathetic system is affected, and this autonomic imbalance1 is reflected in the form of low HRV in the spinal cord injury.1,24,25 Since the frequency domain of HRV is considered a reliable indicator of autonomic function in spinal cord injury; low HRV might reflect impaired autonomic regulation.26 The level of injury reflects the functional ability in individuals with SCI, which is an important correlate of depression in spinal cord injury27,28 Post-injury duration(early vs. late) influences depression and coping trajectory such that the highest depression risk lies in the first six to eight months post-injury.2,29 Acceptance-based coping is critical for post-injury adjustments, especially in the first year of injury.6,30 It is possible that injury level and post-injury duration are critical aspects of understanding psychophysiological response and depression risk in spinal cord injury.
The study aimed to find the association between HRV, coping, and depression in spinal cord injury across different injury levels and the duration of the injury. Further, we explored the post-injury balance between the somatic and cognitive components of autonomic systems (LF/HF ratio), coping (fighting/acceptance), and depression (somatic/cognitive).
Material and methods
Participants
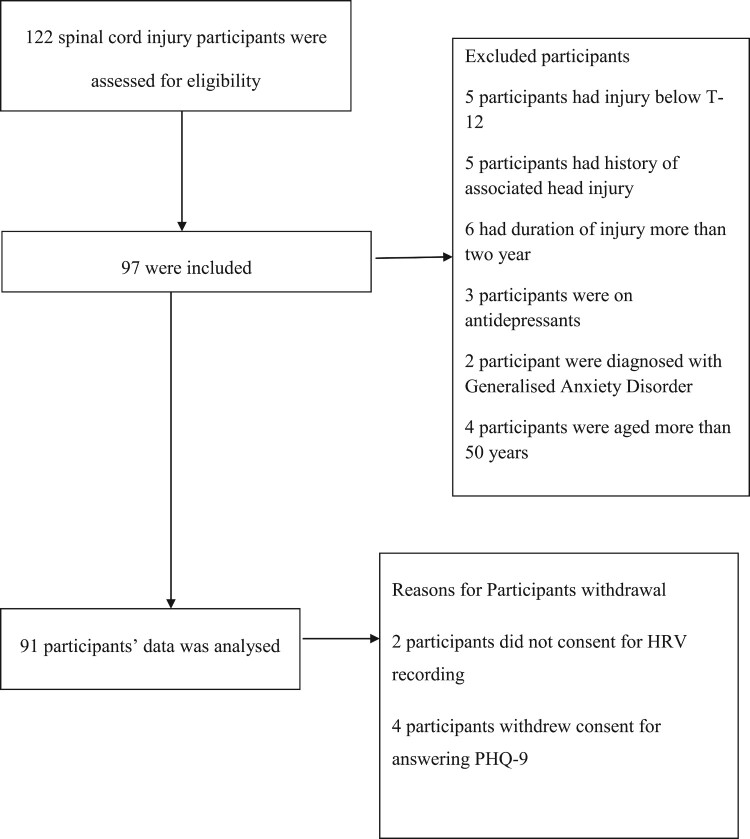
All traumatic spinal cord injury participants admitted to the center from 2017 to 2018 were approached for the study. A total of 122 participants agreed to participate in the study. Ninety-seven participants participated in the current work according to the inclusion and exclusion criteria. Out of the ninety-seven recruited participants, ninety-one completed all the test procedures. Ninety-one traumatic spinal cord injury participants participated in the study. Inclusion criteria: 1) aged between 18 and 50 years 2) can understand English and Hindi 3) traumatic spinal cord injury from C-4 to T-12 with AIS level A, B, and C. All participants with psychiatric disorders or neurological conditions other than spinal cord injury and non-traumatic spinal cord injury were excluded as per medical records (Figure 1). All participants had given a written consent form after reading and understanding the patient information sheet encompassing information on the measures of the study.
Figure 1.
Consort flowchart of the participants.
Research ethics
The data was a part of the corresponding author's doctoral thesis work. The Institutional Ethical Committee approved the doctoral thesis (ISIC/IIRS/RP/2015/081).
Study design and procedure
The current work has an observational study design. Polar heart rate monitor RS 800 CX recorded the HRV data for five minutes. We instructed the participants to do normal breathing and restrain from any activities during the recording procedure, thus forming the baseline resting recording.31 Following the recording, the participants filled the PHQ 9 and Spinal Cord Lesion Coping Strategies Questionnaire (SCL-CSQ). The signal processing was done by Kubios software standard version 2.1.
Study measures
Depression-PHQ-9
PHQ- 932,33 questionnaire screened depression in spinal cord injury. Patient Health questionnaire – 9 is a self-administered nine-item depression questionnaire devised to screen depression. The questions are marked by how persistent the symptoms have been in the past two weeks: 0 – not at all, 1 – several days, 2 – more than half of the days, 3 – nearly every day. The total score of the questionnaire ranges from 0 to 27. The reported internal consistency of PHQ-9 in spinal cord injury was 0.87.33 The internal consistency for the current study is 0.93.
Coping: spinal cord lesion coping strategies questionnaire
Spinal Cord Lesion Coping Strategies Questionnaire (SCL-CSQ)3 has been used to study coping for the current study. The scale was initially developed in the Swedish language3,34 and then translated to English.35 We used the English version of the scale with the original factor structure for the current work.3,30 The scale has the following factors: acceptance (four items), fighting spirit (five items), and social reliance (three items). The Cronbach alpha for the factors was acceptance (0.79), fighting spirit (0.72), and social reliance (.73).3 In the current study, Cronbach's alpha for the factors was acceptance (0.71), fighting spirit (0.79), and social reliance (.56). In the current study, we did not use the item “I refuse to let the lesion rule my life” in the current study, as being negatively worded would have been misinterpreted. After removing the item, the Cronbach alpha of the fighting spirit sub-scale was 0.82.
Heart rate variability
Polar RS 800 CX and Kubios Heart rate variability software (standard version 2.1) calculated the heart rate variability. As per norms of recording of HRV,16 we did a five-minute recording at a sampling rate of 1000 Hz. As used by others, the software used the Fast Fourier transformation method to calculate frequency domains for obtaining the LF, HF, and LF/HF ratio in the Kubios heart rate variability software.36 The limits for these bands are 0.04–0.15 Hz (LF) and 0.15–0.40 Hz (HF).16 For the study, absolute values of LF and HF in millisecond2(ms2) were used, similar to previous work in spinal cord injury.37,38 Since the frequency domain has been associated with depression14,15 and is highly reproducible in spinal cord injury,26 we calculated HRV frequency domains,16 specifically, the frequency-domain components of LF, HF, and LF/HF ratio.
Data analysis
We analyzed the data in the following sequence. We obtained participants’ demographic details regarding age, sex, duration of injury, level of injury, marital status, and educational qualification for the study. Next, the Shapiro Wilk test tested the normality of HRV parameters, PHQ-9 score, and SCL-CSQ. All variables had p≤ 0.05; thus, the variables did not have a normal distribution. The variables were changed into categories to understand the association of depression with HRV and coping. A cut-off score of PHQ-9 = 10 grouped the participants into probable depressed and non-depressed groups.39 The odds ratio estimated the risk of having probable depression as a function of HRV and coping in the early and late duration group.
Next, we derived the depression and coping ratios to reflect the LF/HF ratio indicating the dominance of the sympathetic nervous system over the parasympathetic nervous system.21 Acceptance-based coping is associated with the parasympathetic nervous system; we derived a ratio of fighting spirit and acceptance-based coping, denoting a ratio of somatic and cognitive coping. Similarly, the somatic symptoms of depression are associated with low parasympathetic activation, and therefore a ratio of somatic and cognitive components of depression was taken. To the variables that contained a value of ‘0’, we uniformly added a ‘1’ to ensure a non-zero denominator in the ratio. Spearman rho tested the association between the balance of autonomic functioning, coping strategies, and somatic-cognitive symptoms of depression across each level of injury for each duration group (early vs. late). Data were analyzed by SPSS 21.
Results
Demographics of the participants
A total of ninety-one (N = 91) participants with traumatic spinal cord injury with a mean age of 26.82(±6.84) participated in the study. The mean duration of injury was 7.64 (±3.46) months. Out of 91 participants, there were thirty-six (n = 36) individuals with tetraplegia, twenty-nine (n = 29) with high- paraplegia, and twenty-six (n = 26) with low -paraplegia. The participants were divided into two groups (Early and Late post-injury duration), using the median split method based on the injury duration. The early post-injury group (Group1) represents participants with less than eight months of injury, and the late post-injury group (Group 2) had participants with eight or more than eight months of injury till 17 months of injury (Table 1).
Table 1.
Demographic details of the participants.
| Group 1a(N= 44) | Group 2b(N= 47) | ||||
|---|---|---|---|---|---|
| Parametrs | N (%) | Mean (SD) | N (%) | Mean (SD) | |
| Age(years) | 25.77(5.51) | 27.81(7.82) | |||
| Sex | Male | 42 (95.45) | 45(95.74) | ||
| Female | 2 (4.55) | 2 (4.26) | |||
| Duration of injury(months) | 4.68(1.68) | 10.40(2.14) | |||
| Level of Injury | Tetraplegia | 17(38.63) | 19(40.43) | ||
| High paraplegia | 16(36.63) | 13(27.66) | |||
| Low paraplegia | 11(24.74) | 15(31.91) | |||
| AIS level | A | 22(50.00) | 18 (38.30) | ||
| B | 13(29.55) | 16 (34.04) | |||
| C | 9(20.45) | 13 (27.66) | |||
| Probable depressed | 33(75) | 30(63.80) | |||
| Non- Depressed | 11(25) | 17(36.20) | |||
| Marital status | Married | 23 (57.27) | 29 (61.20) | ||
| Unmarried | 21(42.73) | 18 (38.80) | |||
| Education qualification | Below Matriculation | 5(11.36) | 2(4.26) | ||
| matriculation | 9(20.45) | 12(25.53) | |||
| High School | 12(27.27) | 11(23.40) | |||
| Graduate & above | 18(40.90) | 22(46.81) | |||
Early duration group
Late duration group
Association of HRV, coping and depression
For analysis, participants were grouped into non-depressed and probable depression using the PHQ-9 cut-off score of 10.39 HRV was dichotomized as high and low HRV, using the median split method HF-HRV as the variable.40 The median split method dichotomized acceptance (high vs. low) and fighting spirit (high vs. low).41 We computed the Chi-square test and odds ratio with duration group (early vs. late) as layer variable.
Early duration group
Depression and HRV reached significant negative association with χ2 = 5.94 (1, N = 44), P = 0.02 and Phi = - 0.36. The high HRV group had lower odds of having probable depression than the low HRV group (OR = 0.14, C.I. = 0.03–0.78). Since the OR <1 and the C.I. did not span 1, high HRV group has low risk for probable depression.42 Depression lacked significant association with acceptance with χ2 = 3.13 (1, N = 44), P = 0.07 and Phi = - 0.27. Similarly depression lacked significant association with fighting spirit with χ2 = 1.12 (1, N = 44), P = 0.29 and Phi = - 0.16 (Table 2).
Table 2.
Association of depression (probable depression& non-depressed) with HRV (high and Low), Acceptance (High & Low), and Fighting Spirit (High & Low).
| Probable depression | Non-depressed | χ2 | P | |
|---|---|---|---|---|
| Group1a | ||||
| High HRV | 13 | 9 | 5.94 | 0.02* |
| Low HRV | 20 | 2 | ||
| High Acceptance | 17 | 9 | 3.13 | 0.07 |
| Low Acceptance | 16 | 2 | ||
| High Fighting Spirit | 18 | 8 | 1.13 | 0.29 |
| Low Fighting Spirit | 15 | 3 | ||
| Group 2b | ||||
| High HRV | 12 | 10 | 2.12 | 0.15 |
| Low HRV | 18 | 7 | ||
| High Acceptance | 14 | 14 | 5.74 | 0.02* |
| Low Acceptance | 16 | 3 | ||
| High Fighting Spirit | 14 | 9 | 0.17 | 0.68 |
| Low Fighting Spirit | 16 | 8 | ||
Group 1- Early duration
Group 2- Late duration
*Level of significance α ≤ 0.05
Late duration group
Depression lacked significant association with HRV with χ2 = 2.12 (1, N = 47), P = 0.15 and Phi = - 0.21. Depression had significant negative association with acceptance with χ2 = 5.74 (1, N = 47), P = 0.02 and Phi = - 0.35. The individuals with SCI with high acceptance had lower odds of having probable depression than low acceptance (OR = 0.19, C.I. = 0.44–0.79). Since OR <1and C.I. did not span 1, the risk of having probable depression in individuals with SCI with high acceptance is low.42 The fighting spirit lacked association with probable depression with χ2 = 1.13 (1, N = 47), P = 0.68 and Phi = - 0.06 (Table 2).
Psychophysiological variability: HRV, coping, and depression
Although post-injury duration groups were informative for understanding the association between HRV, coping, and depression, it is unclear how variability in autonomic functioning and coping and depression might be interrelated.
Results of the correlation between the HRV ratio (LF/HF), coping ratio (fighting spirit/ acceptance), and depression ratio (somatic/cognitive) indicated no significant correlation between the ratios in the tetraplegia group in early and in late injury duration. No significant correlations were observed in the high paraplegia group in the early duration group. A significant negative correlation was present in the late duration group in high paraplegia between the HRV ratio and depression ratio (r = −0.67, P < 0.05, 95% C.I. = −.90 to −.12).
Similarly, no significant correlation was present in low paraplegia in the early duration, whereas, in the late injury duration, a significant negative correlation was present between HRV ratio and coping ratio (r = −0.69, P < 0.01, 95% C.I. = −.90 to −.21) (Table 3).
Table 3.
Correlation between depression, coping, and HRV ratio.
| Group 1a | Group 2b | |||||
|---|---|---|---|---|---|---|
| Tetraplegia | High paraplegia | Low paraplegia | Tetraplegia | High paraplegia | Low paraplegia | |
| N = 17 | N = 16 | N = 11 | N = 19 | N = 13 | N = 15 | |
| HRV ratio | HRV ratio | |||||
| Depression ratio | 0.15 | −0.41 | 0.06 | 0.04 | −0.67* | 0.18 |
| Coping ratio | 0.05 | −0.08 | 0.49 | 0.23 | 0.43 | −0.69** |
early post-injury duration group
late post-injury duration group
*Correlation is significant at the 0.05 level (2-tailed)
**Correlation is significant at the 0.01 level (2-tailed).
Discussion
As expected, results indicated that depression might be associated with low HRV in individuals with SCI.15,20,43 The results align with our earlier work that individuals with SCI with probable depression showed low HF-HRV than the non-depressed.44 In the present work, we first explored the association of HRV, coping, and depression across groups where post-injury time duration varied from early to late. We observed that the odds of having probable depression were lower in individuals with SCI having high HRV compared to those having low HRV (OR = 0.14, C.I. = 0.03–0.78), especially in the early phase of injury, indicating the risk of probable depression in the early period of adjustment might be lower in individuals with SCIs with high HRV. The results align with the literature15,43 and indicate that depression is associated with a low parasympathetic activity. In contrast, depression lacked association with HRV in the late injury phase (χ2 = 2.12, P = 0.15). This aligns with others who documented the risk of depression in individuals with SCI was higher in the first 6–8 months of spinal cord injury;2,29 results support the association of depression with low autonomic regulation being critical in the early phase post-injury adjustment.
The odds of having probable depression in individuals with SCI with acceptance as a predominant coping strategy was lower than those who showed low acceptance in the late injury phase (OR = 0.19, C.I. = 0.44–0.79), indicating that high acceptance as a coping strategy might be critical for lowering the risk of depression. In the individuals with SCI of early duration group, depression and acceptance as coping strategy lacked significance. The results align with others who have suggested that post-injury acceptance is a critical factor in the risk of depression in individuals with SCI.7,45 Acceptance-based coping response to the injury might explain the ‘Disability Paradox’ in spinal cord injury,46 i.e. counter-intuitively, a considerable number of individuals with SCI tend to report good quality of life despite the injury. The association between fighting spirit as a coping strategy and depression lacked significance in the early phase [χ2 = 1.12 (1, N = 44), P = 0.29] as well as in the later phase [χ2 = 1.13 (1, N = 47), P = 0.68]. Aligned with the literature, acceptance-based coping was an important coping strategy post-injury for depression, however contrary to some studies3,13 our results showed that fighting spirit-based coping might not be associated with depression.
The results help us understand the nature and extent of the psychophysiological balance in individuals with SCI, that is, the post-injury physiological balance of sympathetic – parasympathetic cardiac regulation (LF-HF ratio), the balance of psychological coping response (fighting and acceptance ratio), and the balance of somatic and cognitive components of depression (somatic – cognitive subscale ratio) might be interlinked. Sympathetic dominance (LF-HF ratio) was negatively associated with somatic-cognitive depression symptoms (r = −0.67, P ≤0.05) in the high paraplegic group, indicating that high sympathetic dominance might be linked with lower cognitive manifestation of depression. The level of injury might be critical for understanding the psychophysiological balance between HRV, coping, and depression because their correlation was not significant in the case of an individual with higher severity of injury (tetraplegia). These results can be explained by observations of others, specifically compared to other injury levels; autonomic activity is relatively less affected in individuals with high paraplegia than tetraplegic1 thus, indicating residual autonomic function.47 Similarly, sympathetic dominance was negatively associated with fighting-to-acceptance coping (r = −0.69, P≤0.01) in low paraplegics, indicating that higher sympathetic dominance might be linked with low acceptance-based coping in low paraplegics. A possible reason may be that functional ability is better in low paraplegics,48 relatively intact functional ability might fuel and enable fighting spirit in low paraplegics. Preliminary evidence favors the hypothesized relationship between the three ratios that reflect a synergistic psychophysiological balance between the somatic and cognitive components of autonomic variability (LF-HF HRV ratio), coping (fighting-acceptance ratio), and depression (cognitive–affective ratio). To our knowledge, this is the first study that helps us understand the psychophysiological impact of spinal cord injury, incorporating post-injury autonomic regulation as a psychophysiological response and coping and depression as a psychological response to spinal cord injury.
Conclusion
Injury to the spinal cord causes irreversible life changes; if the early period is marked with maladjustment, it might increase the risk of depression. On the other hand, the results indicate that the ‘Disability Paradox,’ which is a good quality of life and absence or reduced mental health problems despite the disability, might be explained with our results where variability in coping was associated with variability in psychophysiological response to the injury. An autonomic measure such as HRV might be less affected by factors associated with the social desirability of survey reporting and stigmatization of depression. Although preliminary, the results also carry potential clinical relevance of using HRV at hospital discharge for monitoring the risk of depression in spinal cord injury for the critical period of early post-injury adjustments.
Limitations
The study is cross-sectional, and further follow-up research will help understand how coping influences the longitudinal risk of depression. Unequal (levels) and small sample size across the three injury levels were limitations for computing regression analysis and other statistical analysis. Although the literature supports PHQ-9 for measuring depression, it is a primary-level screener for depression, and DSM criteria diagnose depression. The spinal cord lesion coping strategy questionnaire is a condition-specific questionnaire, but cross-cultural validation in the Indian population is needed.
Funding Statement
The current work is non-funded.
Data availability
As the data concerns the participants’ mental health, the manuscript data is available on request from the corresponding author
Conflict of interest
No potential conflict of interest was reported by the author(s).
References
- 1.Biering-sørensen F, Biering-sørensen T, Liu N, Malmqvist L, Maria J, Krassioukov A.. Autonomic neuroscience : basic and clinical alterations in cardiac autonomic control in spinal cord injury. Auton Neurosci Basic Clin [Internet] 2017; doi: 10.1016/j.autneu.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 2.Craig A, Tran Y, Middleton J.. Psychological morbidity and spinal cord injury: A systematic review. Spinal Cord 2009;47(2):108–14. [DOI] [PubMed] [Google Scholar]
- 3.Elfström ML, Rydén A, Kreuter M, Persson LO, Sullivan M.. Linkages between coping and psychological outcome in the spinal cord lesioned: Development of SCL-related measures. Spinal Cord 2002;40(1):23–9. [DOI] [PubMed] [Google Scholar]
- 4.Chevalier Z, Kennedy P, Sherlock O.. Spinal cord injury, coping and psychological adjustment: A literature review. Spinal Cord 2009;47(11):778–82. [DOI] [PubMed] [Google Scholar]
- 5.Galvin LR, Godfrey HP.. The impact of coping on emotional adjustment to spinal cord injury (SCI): review of the literature and application of a stress appraisal and coping formulation. Spinal cord Off J Int Med Soc Paraplegia 2001;39(12):615–27. [DOI] [PubMed] [Google Scholar]
- 6.Kennedy P, Marsh N, Lowe R, Grey N, Short E, Rogers B.. A longitudinal analysis of psychological impact and coping strategies following spinal cord injury. Br J Health Psychol 2000;5(2):157–72. [Google Scholar]
- 7.Aaby A, Ravn SL, Kasch H, Andersen TE.. The associations of acceptance with quality of life and mental health following spinal cord injury: a systematic review. Spinal Cord [Internet] 2019;58(2):130–48. doi: 10.1038/s41393-019-0379-9. [DOI] [PubMed] [Google Scholar]
- 8.Elfström ML, Kreuter M, Rydén A, Persson LO, Sullivan M.. Effects of coping on psychological outcome when controlling for background variables: A study of traumatically spinal cord lesioned persons. Spinal Cord 2002;40(8):408–15. [DOI] [PubMed] [Google Scholar]
- 9.Williams R, Murray A.. Prevalence of depression after spinal cord injury: A meta-analysis. Arch Phys Med Rehabil [Internet] 2015;96(1):133–40. doi: 10.1016/j.apmr.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 10.Krause JS, Reed KS, McArdle JJ.. Prediction of somatic and non-somatic depressive symptoms between inpatient rehabilitation and follow-up. Spinal Cord [Internet] 2010 Mar;48(3):239–44. Available from: https://pubmed.ncbi.nlm.nih.gov/19736559/. [DOI] [PubMed] [Google Scholar]
- 11.Krause JS, Reed KS, McArdle JJ.. Factor structure and predictive validity of somatic and nonsomatic symptoms from the patient health questionnaire-9: A longitudinal study after spinal cord injury. Arch Phys Med Rehabil [Internet] 2010;91(8):1218–24. Available from: https://pubmed.ncbi.nlm.nih.gov/20684902/. [DOI] [PubMed] [Google Scholar]
- 12.Aikat R, Singh DV.. Identification of mood and body mass index as modifiable factors for health improvement in spinal cord injury. Arch Rehabil Res Clin Transl [Internet] 2021;100174; doi: 10.1016/j.arrct.2021.100174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kennedy P, Evans M, Sandhu N.. Psychological adjustment to spinal cord injury: The contribution of coping, hope and cognitive appraisals. Psychol Heal Med 2009;14(1):17–33. [DOI] [PubMed] [Google Scholar]
- 14.Koch C, Wilhelm M, Salzmann S, Rief W, Euteneuer F.. A meta-Analysis of heart rate variability in major depression. Psychol Med 2019 Sep 1;49(12):1948–57. [DOI] [PubMed] [Google Scholar]
- 15.Kemp AH, Quintana DS, Gray MA, Felmingham KL, Brown K, Gatt JM.. Impact of Depression and Antidepressant Treatment on Heart Rate Variability: A Review and Meta-Analysis. Biol Psychiatry [Internet] 2010;67(11):1067–74. doi: 10.1016/j.biopsych.2009.12.012. [DOI] [PubMed] [Google Scholar]
- 16.Marek Malik J, Thomas Bigger A, Camm J, Kleiger RE, Malliani A, Arthur J, Moss PJS.. Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart rate variability standards of measurement, physiological interpretation, and clinical use. Eur. Eur Heart J 1996;17:354–81. [PubMed] [Google Scholar]
- 17.Shaffer F, Ginsberg JP.. An Overview of Heart Rate Variability Metrics and Norms. Front Public Heal 2017;5(September):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kemp AH, Quintana DS, Felmingham KL, Matthews S, Jelinek HF.. Depression, comorbid anxiety disorders, and heart rate variability in physically healthy, unmedicated patients: Implications for cardiovascular risk. PLoS One 2012;7(2):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thayer JF, Lane RD.. A model of neurovisceral integration in emotion regulation and dysregulation. J Affect Disord 2000;61(3):201–16. [DOI] [PubMed] [Google Scholar]
- 20.Thayer JF, Lane RD.. Claude Bernard and the heart-brain connection: Further elaboration of a model of neurovisceral integration. Neurosci Biobehav Rev 2009;33(2):81–8. [DOI] [PubMed] [Google Scholar]
- 21.Thayer JF. Heart Rate Variability : A Neurovisceral Integration Model. In: Encyclopedia of Neuroscience [Internet]. 2009. p. 1041–7. Available from: http://www.sciencedirect.com/science/article/B98GH-4TVBCX5-1G1/2/126d18db912eb6e07fddf799c70f10b8.
- 22.De Jonge P, Mangano D, Whooley MA.. Differential association of cognitive and somatic depressive symptoms with heart rate variability in patients with stable coronary heart disease: Findings from the heart and soul study. Psychosom Med 2007;69(8):735–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benvenuti SM, Buodo G, Mennella R, Palomba D.. Somatic, but not cognitive – affective, symptoms are associated with reduced heart rate variability in individuals with dysphoria. Front Psychol 2015;6(May):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malmqvist L, Bartholdy K, Krassioukov A, Welling K, Svendsen JH, Kruse A, et al. Assessment of autonomic function after acute spinal cord injury using heart rate variability analyses. Spinal Cord 2015;53(November 2014):54–8. [DOI] [PubMed] [Google Scholar]
- 25.El-Kotob R, Craven BC, Mathur S, Ditor DS, Oh P, Miyatani M, et al. Assessing Heart Rate Variability As a Surrogate Measure of Cardiac Autonomic Function in Chronic Traumatic Spinal Cord Injury. Top Spinal Cord Inj Rehabil 2017;24(1):28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ditor DS, Kamath M V, MacDonald MJ, Bugaresti J, McCartney N, Hicks AL.. Reproducibility of heart rate variability and blood pressure variability in individuals with spinal cord injury. Clin Auton Res 2005;15(6):387–93. [DOI] [PubMed] [Google Scholar]
- 27.Hartoonian N, Hoffman JM, Kalpakjian CZ, Taylor HB, Krause JK, Bombardier CH.. Evaluating a spinal cord injury-specific model of depression and quality of life. Arch Phys Med Rehabil [Internet] 2014;95(3):455–65. doi: 10.1016/j.apmr.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 28.Kennedy P, Rogers BA.. Anxiety and depression after spinal cord injury: A longitudinal analysis. Arch Phys Med Rehabil 2000;81(7):932–7. [DOI] [PubMed] [Google Scholar]
- 29.Shin JC, Goo HR, Yu SJ, Kim DH, Yoon SY.. Depression and quality of life in patients within the first 6 months after the spinal cord injury. Ann Rehabil Med 2012;36(1):119–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elfström ML, Kennedy P, Lude P, Taylor N.. Condition-related coping strategies in persons with spinal cord lesion: A cross-national validation of the spinal cord lesion-related coping strategies questionnaire in four community samples. Spinal Cord 2007;45(6):420–8. [DOI] [PubMed] [Google Scholar]
- 31.Serra-Añó P, Montesinos LL, Morales J, López-Bueno L, Gomis M, García-Massó X, et al. Heart rate variability in individuals with thoracic spinal cord injury. Spinal Cord 2015;53(1):59–63. [DOI] [PubMed] [Google Scholar]
- 32.Kalpakjian CZ, Toussaint LL, Albright KJ, Bombardier CH, Krause JK, Tate DG.. Patient health questionnaire-9 in spinal cord injury: An examination of factor structure as related to gender. J Spinal Cord Med 2009;32(2):147–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bombardier CH, Richards JS, Krause JS, Tulsky D, Tate DG.. Symptoms of major depression in people with spinal cord injury: Implications for screening. Arch Phys Med Rehabil 2004;85(11):1749–56. [DOI] [PubMed] [Google Scholar]
- 34.Elfström ML, Kreuter M, Persson LO, Sullivan M.. General and condition-specific measures of coping strategies in persons with spinal cord lesion. Psychol Heal Med 2005;10(3):231–42. [Google Scholar]
- 35.Migliorini CE, Elfström ML, Tonge BJ.. Translation and Australian validation of the spinal cord lesion-related coping strategies and emotional wellbeing questionnaires. Spinal Cord 2008;46(10):690–5. [DOI] [PubMed] [Google Scholar]
- 36.Tarvainen MP, Niskanen JP, Lipponen JA, Ranta-aho PO, Karjalainen PA.. Kubios HRV - Heart rate variability analysis software. Comput Methods Programs Biomed [Internet] 2014;113(1):210–20. doi: 10.1016/j.cmpb.2013.07.024. [DOI] [PubMed] [Google Scholar]
- 37.Craig A, Rodrigues D, Tran Y, Guest R, Middleton J.. Daytime sleepiness and its relationships to fatigue and autonomic dysfunction in adults with spinal cord injury. J Psychosom Res 2018;112(May):90–8. [DOI] [PubMed] [Google Scholar]
- 38.Rodrigues D, Tran Y, Guest R, Middleton J, Craig A.. Influence of neurological lesion level on heart rate variability and fatigue in adults with spinal cord injury. Spinal Cord 2016;54(4):292–7. [DOI] [PubMed] [Google Scholar]
- 39.Kroenke K, Spitzer RL, Williams JBW.. The PHQ-9: Validity of a brief depression severity measure. J Gen Intern Med 2001;16(9):606–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hansen AL, Johnsen BH, Thayer JF.. Relationship between heart rate variability and cognitive function during threat of shock. Anxiety. Stress Coping 2009;22(1):77–89. [DOI] [PubMed] [Google Scholar]
- 41.DeCoster J, Gallucci M, Iselin A-MR.. Best Practices for Using Median Splits, Artificial Categorization, and their Continuous Alternatives. J Exp Psychopathol 2011;2(2):197–209. [Google Scholar]
- 42.Sedgwick P, Marston L.. Statistical question: Odds ratios. Br Med J 2010;341(7769):407. [Google Scholar]
- 43.Koch C, Wilhelm M, Salzmann S, Rief W, Euteneuer F.. A meta-analysis of heart rate variability in major depression. Psychol Med 2019. [DOI] [PubMed] [Google Scholar]
- 44.Mitra S, Singh V.. Autonomic dysregulation and low heart rate variability in spinal cord injury(SCI): A marker for depression. Int J Physiother 2020 Jul 10;7(3):108–13. [Google Scholar]
- 45.Pollard C, Kennedy P.. A longitudinal analysis of emotional impact, coping strategies and post-traumatic psychological growth following spinal cord injury: A 10-year review. Br J Health Psychol 2007;12(3):347–62. [DOI] [PubMed] [Google Scholar]
- 46.Migliorini C, Tonge B.. Reflecting on subjective well-being and spinal cord injury. J Rehabil Med 2009;41(6):445–50. [DOI] [PubMed] [Google Scholar]
- 47.Alexander M, Biering-Sorensen F, Bodner D, Brackett N, Cardenas D, Charlifue S, et al. International standards to document remaining autonomic function after spinal cord injury. Spinal Cord [Internet] 2009;47:36–43. Available from: www.nature.com/sc. [DOI] [PubMed] [Google Scholar]
- 48.Kirshblum SC, Waring W, Biering-Sorensen F, Burns SP, Johansen M, Schmidt-Read M, et al. Reference for the 2011 revision of the International Standards for Neurological Classification of Spinal Cord Injury. J Spinal Cord Med 2011;34(6):547–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
As the data concerns the participants’ mental health, the manuscript data is available on request from the corresponding author