Abstract
Objectives
The objective of this study was to develop clinical scores to predict the risk of intensive care unit (ICU) admission in patients with COVID-19 and end stage kidney disease (ESKD).
Methods
This was a prospective study in which 100 patients with ESKD were enrolled and divided into two groups: the ICU group and the non-ICU group. We utilized univariate logistic regression and nonparametric statistics to analyze the clinical characteristics and liver function changes of both groups. By plotting receiver operating characteristic curves, we identified clinical scores that could predict the risk of ICU admission.
Results
Out of the 100 patients with Omicron infection, 12 patients were transferred to the ICU due to disease aggravation, with an average of 9.08 days from hospitalization to ICU transfer. Patients transferred to the ICU more commonly experienced shortness of breath, orthopnea, and gastrointestinal bleeding. The peak liver function and changes from baseline in the ICU group were significantly higher, with p values <.05. We found that the baseline platelet-albumin-bilirubin score (PALBI) and neutrophil-to-lymphocyte ratio (NLR) were good predictors of ICU admission risk, with area under curve values of 0.713 and 0.770, respectively. These scores were comparable to the classic Acute Physiology and Chronic Health Evaluation II (APACHE-II) score (p > .05).
Conclusion
Patients with ESKD and Omicron infection who are transferred to the ICU are more likely to have abnormal liver function. The baseline PALBI and NLR scores can better predict the risk of clinical deterioration and early transfer to the ICU for treatment.
Keywords: Omicron infection, end stage kidney disease, abnormal liver function, ICU, platelet-albumin-bilirubin score, neutrophil-to-lymphocyte ratio, inflammation
Introduction
Coronavirus disease 2019 (COVID-19) is a respiratory illness caused by the SARS-CoV-2 virus [1]. The COVID-19 pandemic has had significant impacts on human life and society, making it one of the most significant threats to human health in recent times. Since its appearance in December 2019, there have been over 625 million confirmed cases of COVID-19 globally, resulting in more than 6.5 million deaths as of this writing [2].
Currently, the Omicron variant of COVID-19 has become the dominant variant globally due to hypermutation of the spike protein, resulting in widespread virus escape and rapid spread [3,4]. The emerging Omicron variant has been spreading in Shanghai since March 2022 and is now under control. According to Shen’s report, there were over 42,000 confirmed cases and 0.37 million asymptomatic cases reported in Shanghai from 26 February 2022 to 23 April 2022, of which 0.4% were severe or critically ill, and 0.1% resulted in death [5]. In contrast to the Wuhan outbreak in 2020, where there were 50,234 confirmed cases, 63% of which were critical cases, and 7.7% mortality was reported, the severity and mortality ratio for COVID-19 patients in Shanghai during this period was much lower [6]. Similar phenomena have been observed in other cities or countries. Omicron has been responsible for the vast majority of new cases in Houston between 11 November 2021 and 5 January 2022, with significantly lower oxygen requirements than those caused by alpha or delta variants [3].
The global prevalence of chronic kidney disease (CKD) is estimated to be 9% to 12% [7]. Immunity declines in patients with advanced CKD, and the risk of all-cause mortality increases with increased exposure to infections during advanced CKD [8]. According to a meta-analysis by Chung et al. [9], the incidence of COVID-19 may be higher in populations requiring renal replacement therapy than in patients with advanced CKD or kidney transplant recipients who do not require renal replacement therapy. The mortality rate of CKD cases with COVID-19 may be higher than that of CKD cases without COVID-19 [9]. A report by Council et al. indicates that dialysis patients, CKD patients, and organ transplant recipients are among the four comorbidities with the highest COVID-19 mortality rate [10].
End stage kidney disease (ESKD) and COVID-19 infection are associated with increased inflammatory burden [11]. Patients with COVID-19 often develop concurrent liver injury. Evaluating the clinical features of patients with ESKD is essential during the Omicron variant epidemic. We hypothesize that the inflammatory burden and liver injury may contribute to clinical deterioration. Therefore, we selected clinical scores related to liver disease and systemic inflammation to predict the risk of intensive care unit (ICU) admission in ESKD patients with COVID-19. These scores include the following: Aminotransferase-to-platelet ratio index (APRI), albumin-bilirubin score (ALBI), aminotransferase-to-alanine aminotransferase ratio (AAR), platelet-albumin-bilirubin score (PALBI), monocyte-to-lymphocyte ratio (MLR), neutrophil-to-lymphocyte ratio (NLR), and disseminated intravascular coagulation score (DIC score). Acute Physiology and Chronic Health Evaluation II (APACHE-II) is a commonly used clinical score to assess the critical condition of ICU patients. In this study, we compared the above clinical scores with APACHE-II [12]. Therefore, the primary objective of this study was to analyze the clinical features and liver function changes in patients with ESKD and Omicron infection and develop clinical scores to predict the risk of ICU admission.
Methods
Study design
This study included adult patients with ESKD who had Omicron variant infection and were admitted to Ruijin Hospital Affiliated to Shanghai Jiaotong University School of Medicine from April 2022 to June 2022. All patients had previously undergone regular hemodialysis or peritoneal dialysis. Omicron variant infection was confirmed in all admitted patients using polymerase chain reaction (PCR) testing. The patients were classified, diagnosed, and treated in accordance with the COVID-19 diagnosis and treatment guidelines in China [13]. The primary outcome was the need for ICU admission based on disease progression, and the enrolled patients were divided into the ICU group and the non-ICU group. This was a prospective study approved by the Ethics Committee of Ruijin Hospital, Shanghai Jiaotong University School of Medicine (2022-078). All participants provided informed consent. The study only collected the patients’ clinical data, did not affect the formulation of the patients’ treatment plan, and did not pose any risks to the patients. The researchers made every effort to protect the security of patients’ information and ensured that personal privacy was not disclosed.
Data
Data were collected from our hospital’s electronic medical record database, and all patients were carefully evaluated for eligibility. The variables analyzed included patient demographic data such as age, gender, height, weight, body mass index (BMI), etc., as well as the days of nucleic acid turning negative after COVID-19 infection, clinical deterioration time, results of CT scans, past medical history, vaccination history, the number of days since the last dialysis treatment, manifestations, consciousness, and laboratory test results at admission. Laboratory tests included C-reactive protein (CRP), white blood cell (WBC) count, lymphocyte count (LYM), neutrophil count (NEU), monocyte count (MONO), hemoglobin (HGB), hematocrit (HCT), platelet count (PLT), activated partial thromboplastin time (APTT), international normalized ratio (INR), prothrombin time (PT), fibrinogen (Fg), D-dimer, fibrin degradation products (FDP), aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma-glutamyl transpeptidase (GGT), alkaline phosphatase (ALP), direct bilirubin (DBIL), total bilirubin (TBIL), albumin (ALB), blood urea nitrogen (BUN), uric acid (URIC), serum creatinine (CREA), estimated glomerular filtration rate (eGFR), potassium (K), sodium (Na), chlorine (CL), magnesium (MG), phosphorus (PHOS), and calcium (Ca). Aminotransferase-to-platelet ratio index (APRI), albumin-bilirubin score (ALBI), aminotransferase-to-alanine aminotransferase ratio (AAR), platelet-albumin-bilirubin score (PALBI), monocyte-to-lymphocyte ratio (MLR), neutrophil-to-lymphocyte ratio (NLR), and disseminated intravascular coagulation score (DIC score) were calculated based on the laboratory data at admission. Peak values referred to the highest value obtained during hospitalization, and the peak data were subtracted from the baseline laboratory value to obtain the change in the variable. Patients with COVID-19 often developed concurrent liver injury, which also occurred in the patients included in this study. Thus, the study further analyzed whether the liver injury was related to the degree of deterioration of the patient’s condition. Acute liver injury (ALI) was defined as patients who developed coagulopathy without any change in their level of consciousness [14]. The formulas and components of the five scoring systems for calculating the predictive scores are as follows: APRI = [(AST/ULN) × 100]/PLT; ALBI = (Log10TB × 0.66) − 0.085 × ALB; AAR = AST/ALT; PALBI = (2.02 × Log10TB) − 0.37 × (Log10TB) ^ 2 − 0.04 × ALB − 3.48 × Log10PLT + 1.01 × (Log10PLT) ^ 2; MLR = monocyte count/lymphocyte count and NLR = neutrophil count/lymphocyte count. The calculation method of the DIC score is shown in Supplemental Table 1 [15].
Statistical analysis
Categorical variables were presented as frequencies and percentages. Normally distributed continuous variables were expressed as mean ± standard deviation, while non-normally distributed continuous variables were presented as median and range. Univariate logistic regression analysis was employed to identify the risk factors associated with ICU admission. The odds ratios (ORs), 95% confidence intervals (95% CIs), and p values were calculated for each predictor. Pearson’s chi-square test was used to compare categorical variables between the ICU and non-ICU groups, and the Wilcoxon rank-sum test was used to compare continuous variables between the two groups. Receiver operating characteristic (ROC) curves of NLR and PALBI were plotted to examine their discriminative power, and the area under the curve (AUC), odds ratios (ORs), 95% confidence intervals (95% CIs), and p values were calculated. We used paired t-tests to assess the statistical differences between PALBI and NLR in predicting the risk of ICU deterioration. All statistical analyses were performed using R Studio software version 1.3.1093 (©2016 The R Foundation).
Results
Demographic and clinical characteristics
In this study, we enrolled 100 patients with ESKD and Omicron infection, of which 41% were females, with an average age of 65.77 ± 12.75 years. Ninety-nine patients received hemodialysis, and one patient received peritoneal dialysis treatment. The patients had moderate azotemia, with a mean BUN of 35–50 mmol/L. This is likely due to factors such as reduced food intake and excessive water load. Three patients had viral hepatitis, and two patients had polycystic liver disease. The initial symptoms were primarily respiratory symptoms (such as cough, expectoration, and chest tightness), followed by gastrointestinal symptoms (nausea, vomiting, anorexia, etc.). The patients were divided into two groups based on whether they were transferred to the ICU. Twelve cases were transferred to the ICU due to clinical deterioration, with an average of 9.08 days from hospitalization to ICU transfer. In our study, six patients died within 30 days. Dyspnea, orthopnea, and hematemesis were more common in the ICU group (p < .05). Among the non-ICU group cases, 88 cases were mild COVID-19 infections. Among the ICU group cases, 11 cases were severe COVID-19 infections, and one case was critically severe. Five patients were in a somnolent state, and two were in a coma. All coma patients were in the ICU group. There were no significant differences between the ICU and non-ICU groups in gender, age, height, weight, BMI, vaccination status, history of hypertension, diabetes, HBV, and HCV. The mean time of PCR positivity (before first negative PCR) for all patients was 16.56 ± 8.39 days. A slightly longer duration was observed in the ICU group (20.25 ± 11.83 days) compared to the non-ICU group (16.06 ± 7.76 days). However, the difference was not statistically significant (p = .54). Table 1 shows the baseline data.
Table 1.
Baseline data of enrolled patients.
| All patients | Not admitted to the ICU | Admitted to the ICU | ||
|---|---|---|---|---|
| Variable | N = 100 | N = 88 | N = 12 | p Value |
| Sex (male, n(%)) | 59.00 (59.00%) | 49.00 (55.68%) | 10.00 (83.33%) | .12 |
| Age (years, mean ± SD) | 65.77 ± 12.75 | 65.03 ± 12.87 | 71.17 ± 10.84 | .16 |
| BMI (kg/m², mean ± SD) | 21.98 ± 3.19 | 22.09 ± 3.17 | 21.18 ± 3.35 | .50 |
| Height (cm, mean ± SD) | 166.14 ± 8.24 | 165.90 ± 8.38 | 167.92 ± 7.13 | .41 |
| Weight (kg, mean ± SD) | 61.10 ± 12.17 | 61.25 ± 12.29 | 60.04 ± 11.72 | .92 |
| turn-NE-day (day, mean ± SD) | 16.56 ± 8.39 | 16.06 ± 7.76 | 20.25 ± 11.83 | .54 |
| Exacerbation time (day) | / | / | 9.08 | / |
| COVID-19 class | <.01 | |||
| Mild disease | 88.00 (88.00%) | 88.00 (100%) | 0.00 (0.00%) | |
| Severe disease | 11.00 (11.00%) | 0.00 (0.00%) | 11.00 (91.67%) | |
| Critical disease | 1.00 (1.00%) | 0.00 (0.00%) | 1 (8.33%) | |
| Vaccinated, n(%) | 6 (6%) | 5 (5.68%) | 1.00 (8.33%) | .58 |
| HBP, n(%) | 86.00 (86.00%) | 76.00 (86.36%) | 10.00 (83.33%) | .67 |
| DM, n(%) | 33.00 (33.00%) | 26.00 (29.55%) | 7.00 (58.33%) | .06 |
| Polycysticliver, n(%) | 7.00 (7.00%) | 6.00 (6.82%) | 1.00 (8.33%) | .85 |
| HBV, n(%) | 3.00 (3.00%) | 3.00 (3.41%) | 0.00 (0.00%) | .52 |
| HCV, n(%) | 2.00 (2.00%) | 2.00 (2.27%) | 0.00 (0.00%) | .60 |
| Liver cirrhosis, n(%) | 3.00 (3.00%) | 2.00 (2.27%) | 1.00 (8.33%) | .25 |
| Manifestation | ||||
| Fever, n(%) | 14.00 (14.00%) | 12.00 (13.64%) | 2.00 (16.67%) | .78 |
| Throatpain, n(%) | 16.00 (16.00%) | 13.00 (14.77%) | 3.00 (25.00%) | .36 |
| Cough, n(%) | 45.00 (45.00%) | 40.00 (45.45%) | 5.00 (41.67%) | .8 |
| Expectoration, n(%) | 27.00 (27.00%) | 22.00 (25.00%) | 5.00 (41.67%) | .22 |
| Dyspnea, n(%) | 36.00 (36.00%) | 27.00 (30.68%) | 9.00 (75.00%) | <.01 |
| Orthopnea, n(%) | 5.00 (5.00%) | 2.00 (2.27%) | 3.00 (25.00%) | .01 |
| Edema, n(%) | 8.00 (8.00%) | 6.00 (6.82%) | 2.00 (16.67%) | .24 |
| Nausea, n(%) | 13.00 (13.00%) | 11.00 (12.50%) | 2.00 (16.67%) | .69 |
| Vomit, n(%) | 7.00 (7.00%) | 6.00 (6.82%) | 1.00 (8.33%) | .85 |
| Anorexia, n(%) | 3.00 (3.00%) | 2.00 (2.27%) | 1.00 (8.33%) | .25 |
| Diarrhea, n(%) | 2.00 (2.00%) | 2.00 (2.27%) | 0.00 (0.00%) | .6 |
| Hematemesis, n(%) | 3.00 (3.00%) | 0.00 (0.00%) | 3.00 (25.00%) | <.01 |
| Conscious | <.01 | |||
| Clear-headed state, n(%) | 93.00 (93.00%) | 86.00 (97.73%) | 7.00 (58.33%) | |
| Somnolence state, n(%) | 5.00 (5.00%) | 2.00 (2.27%) | 3.00 (25.00%) | |
| Coma, n(%) | 2.00 (2.00%) | 0.00 (0.00%) | 2.00 (16.67%) |
Note: ICU: Intensive care unit; SD: standard deviation; BMI: body mass index; turn-NE-day: days of nucleic acid turning negative after COVID-19 infection; HBP: hypertension; DM: diabetes mellitus; HBV: hepatitis B virus; HCV: hepatitis C virus.
The boldface represent that the p value of these variables was less than 0.05, indicating that there was a statistical difference between the two groups.
Laboratory data discrepancies at admission
To determine whether laboratory data at admission could serve as a prognostic indicator of COVID-19 severity, we conducted a univariate logistic regression analysis on cases in the ICU and non-ICU groups. The results showed that in the baseline laboratory data, white blood cell count (p = .01, OR = 1.302, 95% CI 1.056–1.622), neutrophil count (p < .01, OR = 1.369, 95% CI 1.105–1.727), platelet count (p = .03, OR = 1.006, 95% CI 0.996–1.015), C-reactive protein (p < .01, OR = 1.018, 95% CI 1.004–1.035), prothrombin time (p = .04, OR = 1.401, 95% CI 0.966–2.016), international normalized ratio (p = .04, OR = 50.577, 95% CI 0.671–3489.613), fibrin degradation products (p < .01, OR = 1.130, 95% CI 0.987–1.293), and D-dimer (p = .048, OR = 1.590, 95% CI 0.550–3.760) were significantly higher in the ICU group than in the non-ICU group. In contrast, the two groups did not differ significantly in baseline liver function and renal function (p > .5, as shown in Table 2).
Table 2.
Laboratory data discrepancies at admission between the ICU group and the non-ICU group.
| Not admitted to the ICU | Admitted to the ICU | |||||
|---|---|---|---|---|---|---|
| Variable | N = 88, mean ± SD or median (range) | N = 12, mean ± SD or median (range) | p Value | OR | 95% LCI | 95% UCI |
| WBC(×103/μL) | 5.09 ± 2.36 | 7.15 ± 2.93 | .01 | 1.302 | 1.056 | 1.622 |
| NEU(×103/μL) | 3.65 ± 2.10 | 5.94 ± 3.00 | <.01 | 1.369 | 1.105 | 1.727 |
| LYM(×103/μL) | 0.87 ± 0.49 | 0.65 ± 0.35 | .10 | |||
| MONO(×103/μL) | 0.46 ± 0.23 | 0.51 ± 0.40 | .73 | |||
| HGB(g/L) | 102.40 ± 17.87 | 97.75 ± 18.75 | .33 | |||
| HCT(mean ± SD) | 0.31 ± 0.05 | 0.30 ± 0.06 | .29 | |||
| PLT(×103/μL) | 136.88 ± 61.47 | 161.25 ± 51.82 | .03 | 1.006 | 0.996 | 1.015 |
| CRP(mg/L)a | 5 (1.00–200.00) | 28 (5.00–190.00) | <.01 | 1.018 | 1.004 | 1.035 |
| APTT(s) | 33.80 ± 5.03 | 39.59 ± 13.59 | .12 | |||
| PT(s) | 10.78 ± 1.25 | 11.62 ± 1.84 | .04 | 1.401 | 0.966 | 2.016 |
| INR(mean ± SD) | 0.96 ± 0.11 | 1.03 ± 0.16 | .04 | 50.577 | 0.671 | 3489.613 |
| Fg(g/L) | 3.65 ± 0.71 | 3.71 ± 0.71 | .60 | |||
| FDP(μg/ml)a | 1.98 (0.50–21.16) | 4.30 (1.83–13.52) | <.01 | 1.130 | 0.987 | 1.293 |
| D-dimer(μg/ml)a | 0.24 (0.05–3.12) | 0.46 (0.15–1.86) | .048 | 1.590 | 0.550 | 3.760 |
| ALT(U/L) | 14.31 ± 7.73 | 24.08 ± 18.29 | .05 | |||
| AST(U/L) | 15.59 ± 8.07 | 29.17 ± 25.39 | .06 | |||
| ALP(U/L) | 95.59 ± 41.22 | 104.67 ± 38.98 | .29 | |||
| GGT(U/L) | 30.38 ± 33.92 | 38.17 ± 25.56 | .05 | |||
| TBIL(μmol/L) | 9.17 ± 2.35 | 9.70 ± 1.93 | .28 | |||
| DBIL(μmol/L) | 1.61 ± 0.80 | 1.88 ± 1.02 | .33 | |||
| ALB(g/L) | 37.01 ± 3.88 | 34.67 ± 5.68 | .19 | |||
| BUN(mmol/L) | 36.35 ± 11.14 | 47.80 ± 21.98 | .06 | |||
| CREA(μmol/L) | 1222.78 ± 464.90 | 1087.50 ± 432.84 | .51 | |||
| URIC(μmol/L) | 550.59 ± 162.93 | 521.73 ± 187.89 | .63 | |||
| EGFR(ml/min/1.73m²) | 4.08 ± 2.11 | 5.64 ± 4.57 | .24 | |||
| Xb | 3.51 ± 1.70 | 3.67 ± 2.10 | .6 | |||
| Na(mmol/L) | 136.33 ± 4.15 | 136.33 ± 3.80 | .95 | |||
| K(mmol/L) | 4.98 ± 0.92 | 5.40 ± 1.18 | .24 | |||
| CL(mmol/L) | 100.69 ± 5.30 | 100.33 ± 2.23 | .62 | |||
| Ca(mmol/L) | 2.17 ± 0.21 | 2.13 ± 0.27 | .80 | |||
| PHOS(mmol/L) | 2.40 ± 1.08 | 2.42 ± 1.16 | .93 | |||
| MG(mmol/L) | 1.09 ± 0.19 | 1.11 ± 0.17 | .67 |
Note: ICU: intensive care unit; OR: odds ratio; LCI: lower confidence interval; UCI: upper confidence interval; SD: standard deviation; WBC: white blood cell; NEU: neutrophils; LYM: lymphocytes; MONO: monocyte; HGB: hemoglobin; Hct: hematocrit; PLT: platelet; CRP: C-Reactive protein; APTT: activated partial thromboplastin time; PT: prothrombin time; INR: international normalized ratio; Fg: fibrinogen; FDP: fibrin degradation products; ALT: alanine aminotransferase; AST: aspartate aminotransferase; ALP: alkaline phosphatase; GGT: gamma glutamyltranspeptidase; TBIL: total bilirubin; DBIL: direct bilirubin; ALB: albumin; BUN:blood urea nitrogen; CREA: serum creatinine; URIC: uric acid; EGFR: estimated glomerular filtration rate; Na: sodium; K: potassium; CL: chlorine; Ca: calcium; PHOS: phosphorus; MG: magnesium.
aThese results will be expressed as median and range.
bThe number of days since the last dialysis treatment.
The boldface represent that the p value of these variables was less than 0.05, indicating that there was a statistical difference between the two groups.
Changes in liver function
Although liver function did not differ significantly between the ICU and non-ICU groups upon admission, abnormal liver function was more prevalent in the ICU group. To determine the correlation between abnormal liver function and COVID-19 severity, we compared changes in liver function between the two groups. The changes in ALT, AST, GGT, ALP, TB, DB, and INR were higher in the ICU group than the non-ICU group. The non-ICU group had lower peak values for these parameters, which were ALT (7.82 ± 10.43 vs 392.42 ± 784.15, p < 0.01), AST (18.73 ± 10.26 vs 986.00 ± 2, 115.94, p < .01), ALP (102.64 ± 46.28 vs 135.00 ± 46.21, p < .01), GGT (33.95 ± 38.34 vs 82.08 ± 45.17, p < .01), TBIL (10.65 ± 2.91 vs 40.10 ± 44.43, p < .01), DBIL (2.12 ± 1.32 vs 19.99 ± 29.40, p < .01), and INR (1.03 ± 0.25 vs 1.42 ± 0.69, p < .01)The patients in the ICU group were more prone to liver damage, with three developing acute liver injury (Table 3).
Table 3.
Differences of liver function between ICU and non-ICU groups in change value and peak value.
| Not admitted to the ICU | Admitted to the ICU | ||
|---|---|---|---|
| Variable | N = 88, mean ± SD | N = 12, mean ± SD | p Value |
| ALT (admission, U/L) | 14.31 ± 7.73 | 24.08 ± 18.29 | .05 |
| AST (admission, U/L) | 15.59 ± 8.07 | 29.17 ± 25.39 | .06 |
| ALP (admission, U/L) | 95.59 ± 41.22 | 104.67 ± 38.98 | .29 |
| GGT (admission, U/L) | 30.38 ± 33.92 | 38.17 ± 25.56 | .05 |
| TBIL (admission, μmol/L) | 9.17 ± 2.35 | 9.70 ± 1.93 | .28 |
| DBIL (admission, μmol/L) | 1.61 ± 0.80 | 1.88 ± 1.02 | .33 |
| INR (mean ± SD) | 0.96 ± 0.11 | 1.03 ± 0.16 | .04 |
| ALT (peak, U/L) | 17.82 ± 10.43 | 392.42 ± 784.15 | <.01 |
| AST (peak, U/L) | 18.73 ± 10.26 | 986.00 ± 2115.94 | <.01 |
| ALP (peak, U/L) | 102.64 ± 46.28 | 135.00 ± 46.21 | <.01 |
| GGT (peak, U/L) | 33.95 ± 38.34 | 82.08 ± 45.17 | <.01 |
| TBIL (peak, μmol/L) | 10.65 ± 2.91 | 40.10 ± 44.43 | <.01 |
| DBIL (peak, μmol/L) | 2.12 ± 1.32 | 19.99 ± 29.40 | <.01 |
| INR (peak, μmol/L) | 1.03 ± 0.25 | 1.42 ± 0.69 | <.01 |
| ALT (change, U/L) | 3.51 ± 7.18 | 368.33 ± 787.33 | .01 |
| AST (change, U/L) | 3.14 ± 6.63 | 956.83 ± 2119.87 | <.01 |
| ALP (change, U/L) | 7.05 ± 16.03 | 30.33 ± 35.69 | .01 |
| GGT (change, U/L) | 3.58 ± 8.66 | 43.92 ± 51.67 | <.01 |
| TBIL (change, μmol/L) | 1.48 ± 2.01 | 30.40 ± 43.54 | <.01 |
| DBIL (change, μmol/L) | 0.51 ± 0.93 | 18.12 ± 29.03 | <.01 |
| INR (change, μmol/L) | 0.06 ± 0.17 | 0.39 ± 0.71 | .02 |
Note: ICU: Intensive care unit; SD: standard deviation; ALT: Alanine aminotransferase; AST: aspartate aminotransferase; ALP: alkaline phosphatase; GGT: gamma glutamyltranspeptidase; TBIL: total bilirubin; DBIL: direct bilirubin.
The boldface represent that the p value of these variables was less than 0.05, indicating that there was a statistical difference between the two groups.
Differences in clinical scores
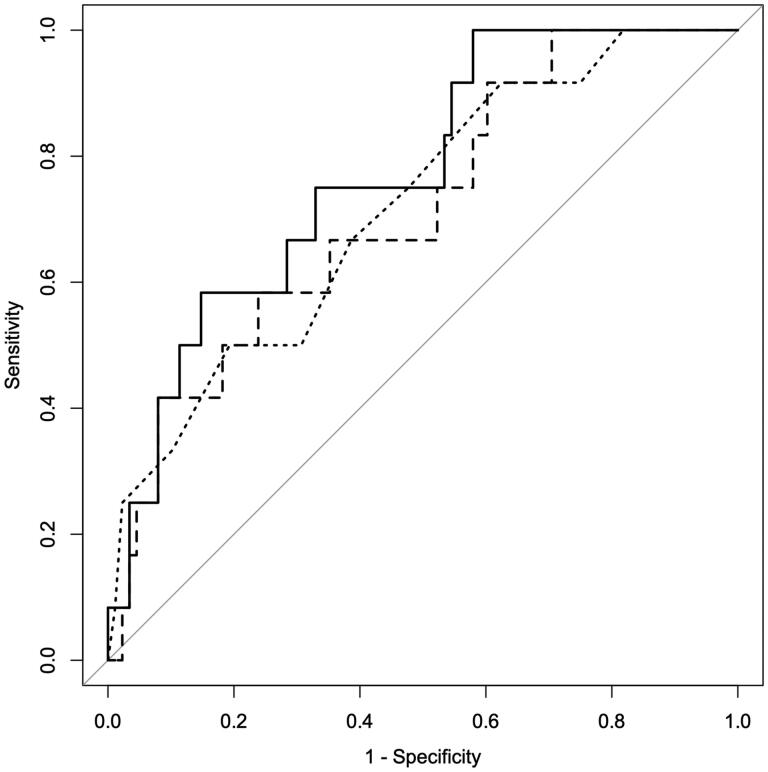
To further clarify whether clinical scores could predict the risk of clinical deterioration in patients with ESKD complicated by omicron infection, we selected APRI, AAR, ALBI, PALBI, NLR, MLR, and DICS to predict risk and compared to APACHE-II score. PALBI (p = .02, OR = 14.165, 95% CI 1.675–138.509) and NLR (p < .01, OR = 1.057, 95% CI 1.010–1.132) were screened out as the most effective scores for predicting the risk of ICU transfer (Table 4). The AUC value of PALBI was 0.713 (95% CI 0.559–0.867), and NLR was 0.770 (95% CI 0.636–0.904), indicating that both scores can be used to predict the risk of clinical deterioration in patients. Additionally, we plotted ROC curves and set the cutoff points for each score to determine their specificity and sensitivity. The results showed that PALBI had a specificity of 76.1% and sensitivity of 58.3% when the cutoff was set to −2.574, while NLR had a specificity of 85.2% and sensitivity of 58.3% when the cutoff was set to 8.442. The AUC value of APACHE-II was 0.716 (95% CI 0.561–0.871), and when the cutoff was set to 20.5, the specificity was 80.7%, and the sensitivity was 50.0%. ROC curves were plotted (Figure 1). There was no significant difference in the efficacy of PALBI and NLR in judging patients’ risk of clinical deterioration compared with APACHE-II (p = .975, .650, respectively, Table 5).
Table 4.
Differences in clinical scores between the two groups.
| Not admitted to the ICU | Admitted to the ICU | |||||
|---|---|---|---|---|---|---|
| Variable | N = 88, mean ± SD | N = 12, mean ± SD | p Value | OR | 95% LCI | 95% UCI |
| APRI | 0.33 ± 0.23 | 0.67 ± 0.85 | .30 | |||
| AAR | 1.18 ± 0.51 | 1.34 ± 0.70 | .52 | |||
| ALBI | −2.52 ± 0.33 | −2.30 ± 0.50 | .19 | |||
| PALBI | −2.72 ± 0.25 | −2.52 ± 0.29 | .02 | 14.17 | 1.68 | 138.51 |
| NLR | 5.85 ± 6.69 | 17.60 ± 29.60 | <.01 | 1.06 | 1.01 | 1.13 |
| MLR | 0.71 ± 0.65 | 0.95 ± 0.72 | .20 | |||
| DIC score | 1.41 ± 0.72 | 1.33 ± 0.78 | .76 | |||
| APACHE II | 17.47 ± 3.15 | 19.92 ± 2.84 | .02 | 1.34 | 1.73 | 1.07 |
Note: ICU: intensive care unit; OR: odds ratio; LCI: lower confidence interval; UCI: upper confidence interval; SD: standard deviation; APRI: aminotransferase-to-platelet ratio index; AAR: aminotransferase-to-alanine aminotransferase ratio; ALBI: albumin- bilirubin score; PALBI: platelet-albumin-bilirubin score; NLR: neutrophil to lymphocyte ratio; MLR: monocyte to lymphocyte ratio; DIC score: disseminated intravascular coagulation score. APACHE II: Acute Physiology and Chronic Health Evaluation II.
Figure 1.
ROC curve of PALBI (line type:dashed), NLR (line type:solid) and APACHE II (line type:dotted) scores. ROC curve: receiver operator characteristic curve; PALBI: platelet-albumin-bilirubin score; NLR: neutrophil to lymphocyte ratio; APACHE II: Acute Physiology and Chronic Health Evaluation II.
Table 5.
ROC curve characteristics of PALBI, NLR and APACHE II scores.
| AUC | 95%CI | Cutoff | Specificity | Sensitivity | p Value vs APACHE II | |
|---|---|---|---|---|---|---|
| PALBI | 0.713 | 0.559–0.867 | −2.574 | 0.761 | 0.583 | .975 |
| NLR | 0.77 | 0.636–0.904 | 8.442 | 0.852 | 0.583 | .650 |
| APACHE II | 0.716 | 0.561–0.871 | 20.5 | 0.807 | 0.50 |
Note: ROC curve: receiver operator characteristic curve; AUC: area under curve; CI: confidence interval; PALBI: platelet-albumin-bilirubin score; NLR: neutrophil to lymphocyte ratio. APACHE II: Acute Physiology and Chronic Health Evaluation II.
Discussion
Since the emergence of the COVID-19 pandemic in 2019, the virus has continued to mutate, resulting in significant changes in the disease’s transmission, symptoms, and severity. The Omicron variant is a recent mutation of SARS-CoV-2, which can be transmitted to vaccinated individuals without any symptoms. Common symptoms of this variant include cough, body aches, and fatigue. In this study, we aimed to investigate the clinical features and risk factors for disease severity among ESKD patients infected with the Omicron variant in Shanghai.
The study included 100 ESKD patients, among whom 59 were males (59%), with an average age of 65.77 ± 12.75 years old. The first symptoms experienced by the patients were primarily respiratory, followed by gastrointestinal symptoms, which is consistent with the findings of previous studies [16,17]. Some studies have reported complications, such as retroperitoneal hemorrhage [18]. While fever and fatigue were the most common symptoms of early COVID-19 patients [19], ESKD patients have less fever, which may be due to their immunological aging state [20].
In all patients, the average time for the nucleic acid to turn negative after Omicron infection was 16.56 ± 8.39 days, with a slightly longer duration observed in the ICU group (20.25 ± 11.83 days) compared to the non-ICU group (16.06 ± 7.76 days). Although this difference was not statistically significant (p = .54), the average duration of positive PCR results in ESKD patients may be slightly longer than that of the general population, especially for severe cases. Kojima et al. reported that the average duration of positive PCR results for 734 Omicron-infected patients was 14.3 ± 7 days [21]. The average number of days that the nucleic acid turned negative for ESKD patients may be slightly longer than that of the general population, especially severe patients. Flythe et al. reported that dialysis patients had a shorter time from symptom onset to ICU admission (median of four days for maintenance dialysis patients). Compared to patients without preexisting CKD, dialysis patients had a higher risk of 28-day in-hospital mortality [22]. However, their article did not suggest what kind of infection the COVID-19 variant was. Meanwhile, the Omicron variant had not been reported worldwide when Flythe’s study was published. In our study, 12 patients were transferred to the ICU due to clinical deterioration, with an average time of 9.08 days from hospitalization to ICU transfer. These results suggest that the severity of Omicron infection may be less than that of previous variants.
To determine the prognostic factors for disease severity, we performed univariate logistic regression analyses of clinical features and laboratory data. After screening seven clinical scores, we found that PALBI (p = .02, OR = 14.165, 95% CI 1.675–138.509) and NLR (p < .01, OR = 1.057, 95% CI 1.010–1.132) were predictive of patient transfer to the ICU. The ROC analysis revealed that PALBI and NLR had comparable discriminative ability to APACHE-II score (p = .975, .650, respectively), indicating their potential use as prognostic indicators for ESKD patients infected with the Omicron variant.
In the baseline laboratory data of this study, the ICU group had higher levels of leukocyte count, neutrophil count, platelet count, CRP, prothrombin time, international normalized ratio, FDP, and D-dimer. Similar to previous studies, lymphopenia was also observed, but there was no significant difference between the two groups [17,23].
Liver injury in COVID-19 patients has been widely reported in the literature [24–27]. Wagner et al. suggested that hypoalbuminemia and elevated transaminases in COVID-19 are predictors of disease severity, and proposed the MELD-na score to assess the severity of COVID-19 patients [24,25]. Zou et al. found that abnormal liver function was more common in patients with COVID-19 and chronic HBV co-infection, and was associated with a higher risk of severe disease, higher mortality, and more complications [28]. Abnormal ALT and AST incidences in previous studies ranged from 15% to 39% and 15% to 58.4%, respectively [29,30]. Notably, the ICU group in this study had more severe liver dysfunction compared to the non-ICU group.
From the seven clinical scores, we identified PALBI and NLR as predictors of patient transfer to the ICU. The platelet-albumin-bilirubin (PALBI) score, which is adapted from the albumin-bilirubin (ALBI) score used in prognosis assessment for hepatocellular carcinoma, pancreatic cancer, acute ischemic stroke, and liver cirrhosis [31–34], was found to predict the risk of patient transfer to the ICU. In contrast to other viral infections, severe COVID-19 patients are characterized by lymphopenia and elevated neutrophil counts, and the neutrophil-to-lymphocyte ratio (NLR) correlates with disease severity [35]. NLR has been used to determine the prognosis in various conditions, including cancer, sepsis, and COVID-19, and is independently associated with mortality [36].
The study’s results are in agreement with previous studies that showed the utility of APACHE II for predicting disease severity in ICU patients. We compared PALBI and NLR with APACHE-II in this study and found no significant difference in their efficacy in predicting clinical deterioration risk.
However, there are some limitations in the study. Firstly, it did not analyze the underlying cause of ESKD, which may affect the disease course. Secondly, the small sample size might lead to selection bias, and the low number of patients transferred to the ICU (n = 12 which is extremely small) decreases the confidence in any findings. Thirdly, the low mortality rate within 30 days limits the ability to evaluate the value of the parameters in predicting mortality, which is a more important outcome than ICU admission. Finally, as a single-center study, its findings may not generalize to other settings. Therefore, further validation of PALBI and NLR in large-scale multi-center studies is needed.
In conclusion, our study showed that Omicron-infected ESKD patients are more likely to have abnormal liver function in the ICU. PALBI and NLR can be used to predict the risk of ICU admission in Omicron-infected ESKD patients.
Supplementary Material
Acknowledgements
We want to thank the patients who used the records in this study and all the co-authors of this study.
Funding Statement
This research did not receive any specific grant from public, commercial, or not-for-profit funding agencies.
Disclosure statement
No potential conflict of interest was reported by the author(s).
References
- 1.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020; 382(18):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.https://covid19.who.int/. WHO website. 2022. [Google Scholar]
- 3.Christensen PA, Olsen RJ, Long SW, et al. Signals of significantly increased vaccine breakthrough, decreased hospitalization rates, and less severe disease in patients with coronavirus disease 2019 caused by the omicron variant of severe acute respiratory syndrome coronavirus 2 in Houston, Texas. Am J Pathol. 2022;192(4):642–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dejnirattisai W, Huo J, Zhou D, et al. SARS-CoV-2 Omicron-B.1.1.529 leads to widespread escape from neutralizing antibody responses. Cell. 2022;185(3):467.e15–484.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shen Y, Ai J, Lin N, et al. An open, prospective cohort study of VV116 in Chinese participants infected with SARS-CoV-2 omicron variants. Emerg Microbes Infect. 2022;11(1):1518–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.GBDCKD Collaboration. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet. 2020;395(10225):709–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chang C-H, Fan P-C, Kuo G, et al. Infection in advanced chronic kidney disease and subsequent adverse outcomes after dialysis initiation: a nationwide cohort study. Sci Rep. 2020;10(1):2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chung EYM, Palmer SC, Natale P, et al. Incidence and outcomes of COVID-19 in people with CKD: a systematic review and meta-analysis. Am J Kidney Dis. 2021;78(6):804–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Council E-E, Group EW.. Chronic kidney disease is a key risk factor for severe COVID-19: a call to action by the ERA-EDTA. Nephrol Dial Transplant. 2021;36:87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nalbant A, Kaya T, Varim C, et al. Can the neutrophil/lymphocyte ratio (NLR) have a role in the diagnosis of coronavirus 2019 disease (COVID-19)? Rev Assoc Med Bras (1992). 2020;66(6):746–751. [DOI] [PubMed] [Google Scholar]
- 12.Sungono V, Hariyanto H, Soesilo TEB, et al. Cohort study of the APACHE II score and mortality for different types of intensive care unit patients. Postgrad Med J. 2022;98(1166):914–918. [DOI] [PubMed] [Google Scholar]
- 13.Xu Y, Chen Y, Tang X.. Guidelines for the diagnosis and treatment of coronavirus disease 2019 (COVID-19) in China. Glob Health Med. 2020;2(2):66–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.European Association for the Study of the Liver. EASL clinical practical guidelines on the management of acute (fulminant) liver failure. J Hepatol. 2017;66(5):1047–1081. [DOI] [PubMed] [Google Scholar]
- 15.Zheng R, Zhou J, Song B, et al. COVID-19-associated coagulopathy: thromboembolism prophylaxis and poor prognosis in ICU. Exp Hematol Oncol. 2021;10(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y, Hu M, Ye G, et al. Clinical characteristics of patients with uremia undergoing maintenance hemodialysis complicated with COVID-19. Medicine. 2020;99(32):e21547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xiong F, Tang H, Liu L, et al. Clinical characteristics of and medical interventions for COVID-19 in hemodialysis patients in Wuhan, China. J Am Soc Nephrol. 2020;31(7):1387–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oka H, Homma Y, Nishino Y, et al. Retroperitoneal hemorrhage in patients with COVID-19 undergoing hemodialysis: three case reports. Intern Med. 2022;61(12):1869–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. J Am Med Assoc. 2020;323(11):1061–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Betjes MGH. Uremia-Associated immunological aging and severity of COVID-19 infection. Front Med. 2021;8:675573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kojima N, Roshani A, Klausner JD.. Duration of COVID-19 PCR positivity for omicron vs earlier variants. J Clin Virol Plus. 2022;2(3):100085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flythe JE, Assimon MM, Tugman MJ, et al. Characteristics and outcomes of individuals with pre-existing kidney disease and COVID-19 admitted to intensive care units in the United States. Am J Kidney Dis. 2021;77(2):190.e1–203.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andhika R, Makmun A, Hartantri Y, et al. Challenge in diagnosis of COVID-19 in hemodialysis patient: a case report and brief review of the literature. CEN Case Rep. 2021;10(3):341–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wagner J, Garcia-Rodriguez V, Yu A, et al. Elevated transaminases and hypoalbuminemia in Covid-19 are prognostic factors for disease severity. Sci Rep. 2021;11(1):10308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wagner J, Garcia-Rodriguez V, Yu A, et al. The model for end-stage liver disease-sodium score at admission is prognostic of covid-19 disease severity. SN Compr Clin Med. 2020;2(11):1978–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harapan H, Fajar JK, Supriono S, et al. The prevalence, predictors and outcomes of acute liver injury among patients with COVID-19: a systematic review and meta-analysis. Rev Med Virol. 2022;32(3):e2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gracia-Ramos AE, Jaquez-Quintana JO, Contreras-Omaña R, et al. Liver dysfunction and SARS-CoV-2 infection. World J Gastroenterol. 2021;27(26):3951–3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zou X, Fang M, Li S, et al. Characteristics of liver function in patients with SARS-CoV-2 and chronic HBV coinfection. Clin Gastroenterol Hepatol. 2021;19(3):597–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McGrowder DA, Miller F, Anderson Cross M, et al. Abnormal liver biochemistry tests and acute liver injury in COVID-19 patients: current evidence and potential pathogenesis. Diseases. 2021;9(3):50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in wuhan, China. Lancet. 2020;395(10223):497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hou D, Wang C, Luo Y, et al. Systemic immune-inflammation index (SII) but not platelet-albumin-bilirubin (PALBI) grade is associated with severity of acute ischemic stroke (AIS). Int J Neurosci. 2021;131(12):1203–1208. [DOI] [PubMed] [Google Scholar]
- 32.Wong WG, Perez Holguin RA, Tarren AY, et al. Albumin-bilirubin score is superior to platelet-albumin-bilirubin score and model for end-state liver disease sodium for predicting posthepatectomy liver failure. J Surg Oncol. 2022;126(4):667–679. [DOI] [PubMed] [Google Scholar]
- 33.Han R, Tian Z, Jiang Y, et al. Prognostic significance of systemic immune-inflammation index and platelet-albumin-bilirubin grade in patients with pancreatic cancer undergoing radical surgery. Gland Surg. 2022;11(3):576–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Faisal MS, Singh T, Amin H, et al. Role of platelet-albumin-bilirubin score in predicting re-bleeding after band ligation for acute variceal hemorrhage. World J Hepatol. 2020;12(10):880–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McKenna E, Wubben R, Isaza-Correa JM, et al. Neutrophils in COVID-19: not innocent bystanders. Front Immunol. 2022;13:864387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buonacera A, Stancanelli B, Colaci M, et al. Neutrophil to lymphocyte ratio: an emerging marker of the relationships between the immune system and diseases. Int J Mol Sci. 2022;23(7):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.