ABSTRACT
MF59-adjuvanted H5N1, cell culture-derived inactivated influenza vaccine (aH5N1c, AUDENZ®, Seqirus) is available for persons 6 months of age and older. During a pandemic, lack of preexisting immunity to novel influenza strains increases morbidity and mortality. This study examined the potential for an adjuvanted vaccine to provide cross-protection to novel viruses. Two similarly designed studies involving separate cohorts aged 18–64 and ≥65 y assessed immune responses to five heterologous H5N1 influenza strains elicited by two 7.5 μg doses of aH5N1c given 3 weeks apart. Geometric mean titers (GMT) on Days 1 and 43 and Day 43/Day 1 geometric mean ratios (GMRs) were determined with hemagglutination inhibition (HI) and microneutralization (MN). Rates of seroconversion (SC) and percentages of subjects with HI and MN ≥ 1:40 were determined. Significant increases in GMTs were observed on Day 43 after vaccination for all 5 heterologous strains in all ages tested. SC rates were 28–55% and 17–46% among those aged 18–64 and ≥65 y, respectively. MN ≥ 1:40 was observed in 38–100% of younger and 37–97% of older subjects, and HI ≥ 1:40 was achieved by 28–64% of subjects aged 18–64 y and by 17–57% of subjects aged ≥65 y. A SC rate ≥40% (97.5% CI) was met for two heterologous strains tested in adults aged 18–64 y. In adults aged 18–64 and ≥65 y, two 7.5 μg doses of aH5N1c demonstrated increased immunogenicity from baseline against five heterologous H5N1 strains, illustrating the potential for aH5N1c to provide cross-protection against other H5N1 strains.
KEYWORDS: Cell culture-derived vaccine, H5N1 influenza, pandemic, adults, older adults
Introduction
The COVID-19 pandemic has highlighted the tremendous burden pandemics can place on individuals and society. Likewise, influenza A pandemics involving novel influenza A viruses can dramatically increase deaths and hospitalizations.1–3 The potential for morbidity and mortality is higher with pandemic than seasonal influenza because the population has little or no preexisting immunity. During a non-pandemic flu season, children and older adults (i.e., those >65 y of age) tend to be most vulnerable to complications associated with influenza. However, during an influenza pandemic, the risk of severe influenza-associated complications for healthy adults may be similar to that of young children and older adults.3 Vaccines, the main prophylactic measure against pandemic influenza, thus have an important role in pandemic preparedness plans worldwide.2–4 Pandemic influenza strains result from an antigenic shift and may include hemagglutinin (HA) subtypes of either avian origin such as H5, H7, and H9 or swine variants of HA subtypes H1, H2, and H3 that have further acquired adaptive mutations to become infectious from human to human.5,6 Among these subtypes, the highly pathogenic avian influenza (HPAI) H5N1 subtype and its genetic reassortants (including H5N2, H5N5, H5N6, and H5N8) are a global threat not only to poultry and wildlife but also to poultry workers and the general population because these viruses can cross the animal-human barrier and the human population lacks preexisting immunity to H5 viruses. Most human cases reported to date have resulted from exposure to poultry or to environments contaminated by poultry-borne viruses.7–9 Based on the latest World Health Organization (WHO) report of human infection with avian influenza H5 virus, from January 2003 to 25 November 2022, there have been 868 cases of human infection with avian influenza A(H5N1) virus reported from 21 countries. Of these 868 cases, 457 were fatal (case fatality rate of 53%).10
According to the US Department of Human and Health Services (DHHS) and the Department of Homeland Security, a vaccine specific to an emerging pandemic influenza strain should be available to vaccinate the entire US population within 6 months of a declaration of a pandemic.2,4 However, time is a limiting factor in the process to develop, approve, and produce a pandemic-specific vaccine in large quantities, especially when using egg-based production methods, which require very large numbers of fertilized eggs. Hence, these US agencies prioritize maintenance of pandemic influenza vaccine stockpiles, which can be used to vaccinate front-line personnel, including healthcare workers, first responders, and the military.4 Given the unpredictable nature of pandemic viruses, a stockpiled vaccine that can provide broad heterologous coverage would represent a major asset to pandemic control efforts. Such a vaccine would offer enhanced protection until an antigenically matched vaccine could be manufactured and distributed.11,12
The adjuvant MF59 (Seqirus Inc., Boston, MA, USA) is an oil-in-water emulsion stabilized by Tween 80 and Span 85 that enhances differentiation of immune cells into antigen-presenting cells and also promotes macrophage recruitment, antigen uptake, lymph node migration, T-cell activation, and B-cell expansion.13–20 MF59 adjuvant is present in egg propagated trivalent and quadrivalent vaccines licensed for immunization against seasonal influenza in adults ≥65 y, a recently licensed cell culture-derived H5N1 vaccine, and two versions of egg propagated H1N1 vaccine licensed during the 2009 pandemic, all for persons 6 months of age or older. MF59 promotes production of cross-reactive antibodies against antigenically different influenza strains and has a well-established safety profile.21–25 In a recent pair of studies with identical designs, aH5N1c demonstrated a robust immune response to the homologous virus strain in separate adult populations aged 18–64 y and ≥65 y.26 This report examines the immunogenicity of aH5N1c against heterologous strains of H5N1 in the same two study populations.
Materials and methods
Study design
Two phase 2, randomized, observer-blind, multicenter studies involving adults 18–64 y (NCT01776541) and older adults aged ≥65 y (NCT01766921) were conducted in Australia, New Zealand, the United States, and Thailand. The design, objectives, and endpoints were identical for both studies, as previously described.26 The studies were conducted in compliance with Good Clinical Practices guidelines and the Declaration of Helsinki, and study protocols were approved by the Ethics Review Committees of the participating centers. Written informed consent was obtained from subjects before enrollment.
In a pre-defined exploratory analysis, hemagglutination inhibition (HI) and microneutralization (MN) antibody responses against five H5N1 heterologous influenza strains were evaluated in a randomly selected subset of subjects from the full-dose treatment arm from these two phase 2 trials.
Vaccine administration
The aH5N1c vaccine contained A/turkey/Turkey/1/05 (H5N1)-like strain (NIBRG-23) antigen (Seqirus Inc., NC, USA; f/k/a Novartis Influenza Vaccines GmbH, Marburg, Germany). Subjects were randomized at a 1:1 ratio to receive two vaccinations with either full-dose (7.5 μg of hemagglutinin antigen per dose, 0.25 mL MF59) or a half-dose (3.75 μg of hemagglutinin antigen per dose, 0.125 mL MF59). Vaccines were administered on Day 1 and Day 22 as single intramuscular injections in the non-dominant arm. Only subjects who received the 7.5 μg antigen dose of aH5N1c were included in the heterologous analysis.
Participants
As described previously, adults aged 18–64 y and those aged ≥65 y were enrolled in separate studies. The main exclusion criteria were the presence of serious chronic or progressive disease; pregnancy or breastfeeding; prior receipt of any H5N1 vaccine; receipt of any other influenza vaccines within 60 d prior to enrollment; a body mass index ≥35 kg/m2, body temperature ≥38.0°C, and/or any acute illness within 3 d of receiving study vaccines. The present analysis of heterologous responses includes a prespecified subset of subjects receiving the full dose of study vaccine; heterologous responses to the half dose were not evaluated.
Endpoints
Antibody responses against the homologous H5N1 strain A/turkey/Turkey/1/2005 and the following heterologous strains were measured by HI and MN assays: A/Anhui/2005 CC Ab (Clade 2.3.4), A/Egypt/2010 CC Ab (Clade 2.2.1), A/Hubei/2010 CC Ab (Clade 2.3.2.1), A/Indonesia/2005 CC Ab (Clade 2.1.3), and A/Vietnam/1203/2004 CC Ab (Clade 1), selected to represent various genetic clades and subclades from WHO-identified H5N1 viruses of concern. Measures of immunogenicity included geometric mean titers (GMTs) on Days 1 and 43, Day 43/Day 1 geometric mean ratio (GMR) of HI and MN titers, the percentage of subjects with HI ≥ 1:40 or MN ≥ 1:40 on Days 1 and 43, and percentage of subjects achieving seroconversion (defined as HI ≥ 1:40 for subjects negative at baseline [HI < 1:10] or a minimum 4-fold increase in HI titer for subjects positive at baseline [HI ≥ 1:10]) or a ≥ 4-fold rise in MN titers on Day 43.
Statistical methods
Statistical methodology of the primary studies has been previously described.26 The percentages of subjects with HI titers ≥1:40 and achieving seroconversion were calculated along with the associated 97.5% Clopper-Pearson confidence intervals (CIs). Although the Center for Biologics Evaluation and Research (CBER) licensure criteria define lower limits of 95% CIs, 97.5% CIs were calculated because two vaccine formulations were assessed, and the 0.05 alpha was distributed across tests. GMTs, GMRs, and the associated 2-sided 95% CIs were calculated using analysis of covariance (ANCOVA) with factors for baseline titer and study center applied on log10-transformed values. For the homologous strain, similar methods were used.
Results
Study population
Of subjects enrolled to receive the full dose aH5N1c in the two primary studies, 488 were 18–64 y of age (mean age 39 y) and 700 were ≥65 y (mean age 71 y).26 Among both younger and older adults, the majority were white and female. More older than younger adults had an influenza vaccination within the prior 12 months, and baseline characteristics were similar between the age groups (Table 1). HI antibody responses, for subjects who had blood drawn/serum availability, to the homologous strain were tested in 478 adults aged 18–64 y and 693 adults aged ≥65 y in the primary studies,26 and MN titers against the homologous strain and HI and MN titers against heterologous strains were measured in 69 and 35 younger and older adults, respectively, in this exploratory analysis. The sample size for the heterologous strain assays was based on historical precedents for influenza descriptive statistics.
Table 1.
Study population demographics.
| Age 18–64 y (n = 488) | Age ≥65 y (n = 700) | |
|---|---|---|
| Age, mean ± SD (y) | 39.0 ± 13.7 | 71.2 ± 5.1 |
| Male, n (%) | 203 (42) | 293 (42) |
| Weight, mean ± SD (kg) | 74.1 ± 15.1 | 71.0 ± 15.9 |
| Height, mean ± SD (cm) | 167.8 ± 10.5 | 164.1 ± 10.7 |
| BMI, mean ± SD (kg/m2) | 26.2 ± 4.3 | 26.1 ± 4.0 |
| Previous influenza vaccination, n (%) | 118 (24) | 429 (61) |
| Influenza vaccination within 12 months prior to study, n (%) | 98 (20) | 388 (55) |
| Race, n (%) | ||
| White | 291 (60) | 445 (64) |
| Black | 97 (20) | 10 (1) |
| Asian | 93 (19) | 240 (34) |
| Native American | 2 (<1) | 2 (<1) |
| Other | 5 (1) | 3 (<1) |
| Hispanic ethnicity, n (%) | 107 (22) | 17 (2) |
Immunogenicity
As shown in Table 2, on Day 43 (3 weeks after the second vaccination), HI GMT increased 41-fold vs Day 1 for the homologous strain and by 2- to 12-fold against all five heterologous strains in subjects aged 18–64 y. As measured with MN in the same population, homologous GMT was 61 times higher and heterologous GMTs were 5–34 times higher on Day 43 vs Day 1. In adults aged ≥65 y, homologous GMTs increased 16- and 23-fold as measured with HI and MN assays, respectively. Heterologous HI GMTs increased by 2- to 5-fold and MN GMTs by 4- to 12-fold.
Table 2.
HI and MN antibody responses (GMT and GMR) against H5N1 at Day 1 and Day 43 by age group.
| HI |
MN |
|||||
|---|---|---|---|---|---|---|
| GMT Day 1 (95% Cl) | GMT Day 43 (95% Cl) | GMR Day 43/Day 1 (95% Cl) | GMT Day 1 (95% Cl) | GMT Day 43 (95% Cl) | GMR Day 43/Day 1 (95% Cl) | |
| Age 18–64 y | ||||||
| Homologous | n = 478 | n = 451 | n = 451 | n = 69 | ||
| Turkey/2005 | 6.11 (5.78–6.46)a | 250 (208–302)a | 41 (34–49)a | 6.7 (5.62–7.98) | 410 (313–537) | 61 (44–86) |
| Heterologous | n = 69 | n = 69 | ||||
| Anhui/2005 | 5.02 (4.87–5.18) | 11 (6.64–17) | 2.09 (1.29–3.37) | 16 (12–21) | 156 (131–186) | 9.93 (7.47–13) |
| Egypt/2010 | 5.87 (4.51–7.63) | 39 (22–71) | 6.52 (3.62–12) | 15 (11–22) | 522 (398–685) | 34 (23–49) |
| Hubei/2010 | 7.12 (5.01–10) | 56 (31–102) | 7.3 (4.01–13) | 7.2 (6–8.63) | 51 (41–63) | 7.07 (5.56–8.98) |
| Indonesia/2005 | 5.02 (4.87–5.18) | 16 (9.17–28) | 3.12 (1.81–5.39) | 8.79 (7–11) | 114 (89–146) | 13 (9.42–18) |
| Vietnam/1203/2004 | 5.97 (4.41–8.06) | 44 (24–81) | 6.99 (3.84–13) | 5.59 (4.98–6.27) | 27 (21–34) | 4.76 (3.74–6.06) |
| Age ≥65 y | ||||||
| Homologous | n = 693 | n = 673 | n = 673 | n = 35 | ||
| Turkey/2005 | 8.29 (7.57–9.08)a | 129 (110–152)a | 16 (13–19)a | 7.49 (5.87–9.56) | 169 (107–268) | 23 (13–39) |
| Heterologous | n = 35 | n = 35 | ||||
| Anhui/2005 | 5 (5–5) | 8.53 (5.92–12) | 1.71 (1.18–2.46) | 28 (20–41) | 128 (99–165) | 4.52 (2.93–6.97) |
| Egypt/2010 | 6.86 (4.94–9.53) | 27 (15–47) | 3.88 (2.27–6.64) | 25 (15–41) | 303 (206–447) | 12 (6.69–22) |
| Hubei/2010 | 7.43 (5.28–10) | 36 (20–64) | 4.83 (2.8–8.32) | 11 (7.98–16) | 42 (31–58) | 3.75 (2.6–5.4) |
| Indonesia/2005 | 5 (5–5) | 12 (8.02–18) | 2.39 (1.6–3.56) | 18 (13–27) | 87 (63–120) | 4.77 (3.1–7.34) |
| Vietnam/1203/2004 | 7.81 (5.24–12) | 34 (19–63) | 4.41 (2.57–7.57) | 7.32 (5.39–9.95) | 27 (19–39) | 3.68 (2.62–5.17) |
Abbreviations: CI, confidence interval; GMR, geometric mean ratio; GMT, geometric mean titer; HI, hemagglutination inhibition; MN, microneutralization.
a97.5% CI.
HI ≥ 1:40 was achieved by 28% to 64% of subjects aged 18–64 y and by 17% to 57% of subjects aged ≥65 y (Figure 1). MN ≥ 1:40 was observed in 38% to 100% of younger and 37% to 97% of older subjects (Figure 2).
Figure 1.
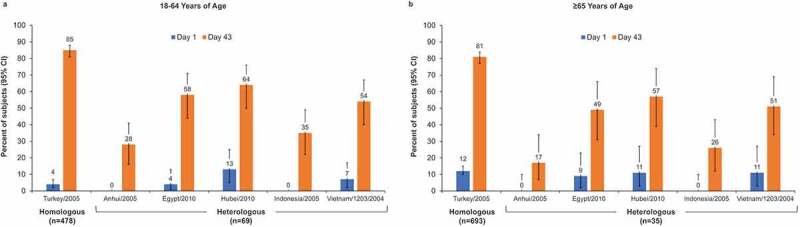
Percentage of subjects with hemagglutination inhibition (HI) titers ≥1:40 in adults aged 18–64 y (a) and aged ≥65 y (b). Error bars represent the 95% confidence interval (CI) for heterologous strains and the 97.5% CI for the homologous strain.
Figure 2.
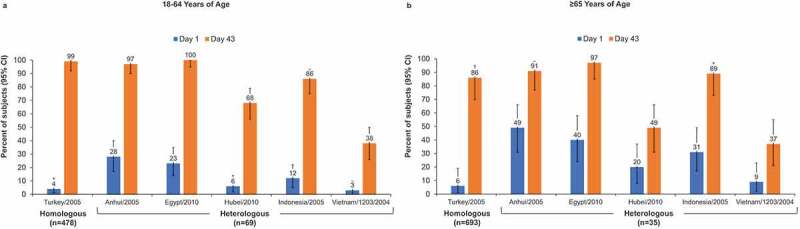
Percentage of subjects with microneutralization (MN) titers ≥1:40 in adults aged 18–64 y (a) and aged ≥65 y (b). Error bars represent the 95% confidence interval (CI) for heterologous strains and the 97.5% CI for the homologous strain.
HI seroconversion rates against heterologous strains ranged from 28% to 55% among adults 18–64 y and 17% to 46% among those ≥65 y of age. The lower bound of the 97.5% CI of the seroconversion rate in adults aged 18–64 y was ≥40% for the Egypt and Hubei heterologous strains (Figure 3(a,b)). The percentage of subjects with a ≥ 4-fold increase in MN titers against heterologous strains ranged from 32% to 88% in subjects aged 18–64 y and 26% to 74% for older adults. Most younger adults achieved a ≥ 4-fold increase in MN against all but the Vietnam strain, whereas among older adults, the majority achieved this threshold only against the Egypt strain (Figure 3(c,d)).
Figure 3.
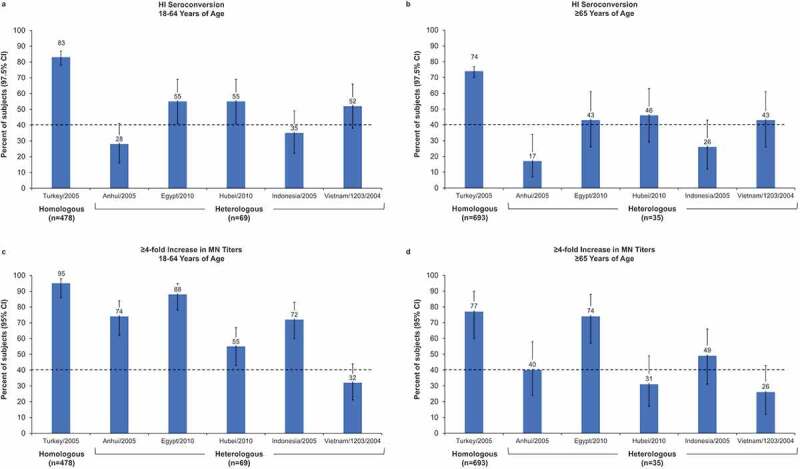
Percentage of subjects with seroconversion (defined as hemagglutination inhibition [HI] ≥1:40 for subjects negative at baseline [HI <1:10] or a minimum 4-fold increase in HI titer for subjects positive at baseline [HI ≥ 1:10]) on Day 43) or a ≥4-fold increase in microneutralization (MN) titers. (a, b) Seroconversion in adults aged 18–64 y (a) and ≥65 y (b). Error bars represent the 97.5% confidence interval (CI). (c, d) at least 4-fold increase in MN titers in adults aged 18–64 y (c) and ≥65 y (d). Error bars represent the 95% CI.
Discussion
This exploratory analysis confirmed that aH5N1c not only elicits a robust immune response against the homologous strain but also against heterologous strains of the H5N1 pandemic influenza virus in adults younger and older than 65 y. CBER criteria (97.5% CI of the seroconversion rate ≥40%) were met for the Egypt/2010 and Hubei/2010 strains in adults aged 18–64 y. Thus, this vaccine could provide essential interim coverage to a population – particularly those 10–40 y of age where H5N1 morbidity and mortality is highest – threatened by an H5N1 pandemic while strain-specific vaccine was being developed and manufactured.
Influenza pandemics have historically been characterized by shifts in the virus subtype, successive pandemic waves, higher transmissibility compared with seasonal influenza, and variable impact in different geographic regions, as well as increased morbidity and mortality in older children and adults younger than 65 y – the population typically most resistant to seasonal influenza complications.3 The vulnerability of younger adults to pandemic influenza strains is of special concern, as this age group is most likely to be on the front lines of the pandemic response, providing health care and other essential services.4 The cross-reactivity against five antigenic variants shown by aH5N1c in this analysis suggests this vaccine could provide these essential workers with cross-protection during the period between the start of a pandemic and distribution of an antigenically matched vaccine.
Millions of fertilized eggs are needed to produce a vaccine, and efficiency of this method is often low, requiring one or more eggs for each dose of the vaccine produced. These constraints could pose challenges during a highly pathogenic avian influenza outbreak or pandemic if either egg quantity or quality become compromised. Cell culture manufacturing methods offer efficient vaccine production that minimizes dependency on the egg supply and may provide increased efficacy relative to egg-derived vaccines.27–32 In addition, enhanced immunogenicity with MF59 adjuvant against heterologous strains, when added to both seasonal and pandemic influenza vaccines, is well documented in children and adults.33–37
Observed differences between MN and HI titers in this study may be due to amino acid substitutions found in the receptor-binding site of the HA molecule of influenza A strains, which can alter the results of HI assays when cell culture-derived vaccines are evaluated.38–40 The MN assay is not affected by HI mutations because MN measures the concentration of antibodies needed to prevent infection of a eukaryotic cell.41,42 It is possible that the MN assay may represent a more mechanistically relevant estimation of antibody-mediated protection than HI.43,44 The choice of erythrocyte species can influence the HI titer, which may also explain the lack of correlation between HI and MN for some results in this study.45 The discrepancy in sample size between the HI and MN analysis groups could also have contributed to differences in titers. However, overall, the trends were consistent between the two assays (i.e., when HI increased, so did MN).
This study was limited by the small number of sera samples tested from the subgroup in which heterologous responses were assessed. Nevertheless, responses were robust, with CBER criteria being met for two of five strains despite the small sample size. The five heterologous strains tested represented a broad spectrum of possible H5N1 variants, although these were previously identified strains, and it cannot be predicted whether the responses to new strains would be similar. A limitation of the studies included in this analysis is that antibodies with H5-specific Fc-mediated effector functions were not determined. Vaccination with adjuvanted H7N9 and H5N1 vaccines has been shown to induce antibody-dependent cellular cytotoxicity (ADCC)–mediating antibodies. Protection from influenza may require Fc-mediated antibody functions in addition to neutralizing antibodies. Additional studies are necessary to understand how and to what extend ADCC- and antibody-dependent phagocytosis (ADP)–mediating antibodies contribute to protection from influenza, as these antibodies may also have an immunopathological role.
In conclusion, in adults younger than 65 y and those 65 y and older, two 7.5 μg doses of aH5N1c, administered three weeks apart, demonstrated increased immunogenicity from baseline against multiple heterologous H5N1 strains, of five separate genetic clades or subclades. These findings illustrate the potential for the aH5N1c vaccine to provide cross-protection against other H5N1 strains during a pandemic and provide support for future research in this area.
Acknowledgments
We thank the study subjects and their families as well as the investigators and study staff. Medical consultants C. Gordon Beck and Amanda M. Justice provided medical writing and editorial support, which was funded by Seqirus, Inc.
Funding Statement
This study was funded by CSL Seqirus, Inc. The sponsor had primary responsibility for study design and study vaccines, protocol development, study monitoring, data management, and statistical analyses. This project has been funded in whole or in part with federal funds by the Biomedical Advanced Research and Development Authority (BARDA) under Contract Number HHS0100200700030C.
Disclosure statement
S.S.F. received study fees paid to her Institution by CSL Seqirus. E.V., E.V.T., and M.H. are employees of CSL Seqirus.
Author contributions
All authors participated in either the conception and design of the study, acquisition of data and/or analysis and interpretation of data. In addition, all authors participated in drafting and critically revising the article for important intellectual content, and all authors provided final approval of the submitted version.
References
- 1.Centers for Disease Control and Prevention . Influenza historic timeline; 2019. Jan 30 [accessed 2022 July 27]. https://www.cdc.gov/flu/pandemic-resources/pandemic-timeline-1930-and-beyond.htm.
- 2.Holloway R, Rasmussen SA, Zaza S, Cox NJ, Jernigan DB.. Updated preparedness and response framework for influenza pandemics. MMWR Recomm Rep. 2014;63:1–7. PMID: 25254666. [PubMed] [Google Scholar]
- 3.Miller MA, Viboud C, Balinska M, Simonsen L.. The signature features of influenza pandemics–implications for policy. N Engl J Med. 2009;360:2595–8. doi: 10.1056/NEJMp0903906. PMID: 19423872. [DOI] [PubMed] [Google Scholar]
- 4.Homeland Security Council . National strategy for pandemic influenza. Washington (DC): Department of Homeland Security; 2005. [Google Scholar]
- 5.Taubenberger JK, Kash JC. Influenza virus evolution, host adaptation, and pandemic formation. Cell Host & Microbe. 2010;7:440–51. doi: 10.1016/j.chom.2010.05.009. PMID: 20542248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bouvier NM, Palese P. The biology of influenza viruses. Vaccine. 2008;26(Suppl 4):D49–53. doi: 10.1016/j.vaccine.2008.07.039. PMID: 19230160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Kerkhove MD, Mumford E, Mounts AW, Bresee J, Ly S, Bridges CB, Otte J. Highly pathogenic avian influenza (H5N1): pathways of exposure at the animal-human interface, a systematic review. PLos One. 2011;6:e14582. doi: 10.1371/journal.pone.0014582. PMID: 21283678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rabinowitz P, Perdue M, Mumford E. Contact variables for exposure to avian influenza H5N1 virus at the human-animal interface. Zoonoses Public Health. 2010;57:227–38. doi: 10.1111/j.1863-2378.2008.01223.x. PMID: 19486500. [DOI] [PubMed] [Google Scholar]
- 9.Tajudeen YA, Bamigboye NTA, Oladunjoye IO. Emerging strain (H5N8) of highly pathogenic avian influenza virus: an impending pandemic threat. J Infect Dis Epidemiol. 2021;7:217. doi: 10.23937/2474-3658/1510217. [DOI] [Google Scholar]
- 10.World Health Organization . Cumulative number of confirmed human cases for avian influenza A(H5N1) reported to WHO, 2003-2023; 2023. Jan 5 [accessed 2023 Feb 23]. https://www.who.int/publications/m/item/cumulative-number-of-confirmed-human-cases-for-avian-influenza-ah5n1-reported-to-who-2003-2022-5-jan-2023.
- 11.Fedson DS. Pandemic influenza and the global vaccine supply. Clin Infect Dis. 2003;36:1552–61. doi: 10.1086/375056. PMID: 12802755. [DOI] [PubMed] [Google Scholar]
- 12.Stephenson I, Bugarini R, Nicholson KG, Podda A, Wood JM, Zambon MC, Katz JM. Cross-reactivity to highly pathogenic avian influenza H5N1 viruses after vaccination with nonadjuvanted and MF59-adjuvanted influenza A/Duck/Singapore/97 (H5N3) vaccine: a potential priming strategy. J Infect Dis. 2005;191:1210–15. doi: 10.1086/428948. PMID: 15776364. [DOI] [PubMed] [Google Scholar]
- 13.Seubert A, Calabro S, Santini L, Galli B, Genovese A, Valentini S, Aprea S, Colaprico A, D’oro U, Giuliani MM, et al. Adjuvanticity of the oil-in-water emulsion MF59 is independent of Nlrp3 inflammasome but requires the adaptor protein MyD88. Proc Natl Acad Sci USA. 2011;108:11169–74. doi: 10.1073/pnas.1107941108. PMID: 21690334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seubert A, Monaci E, Pizza M, O’hagan DT, Wack A. The adjuvants aluminum hydroxide and MF59 induce monocyte and granulocyte chemoattractants and enhance monocyte differentiation toward dendritic cells. J Immunol. 2008;180:5402–12. doi: 10.4049/jimmunol.180.8.5402. PMID: 18390722. [DOI] [PubMed] [Google Scholar]
- 15.Schultze V, D’agosto V, Wack A, Novicki D, Zorn J, Hennig R. Safety of MF59 adjuvant. Vaccine. 2008;26:3209–22. doi: 10.1016/j.vaccine.2008.03.093. PMID: 18462843. [DOI] [PubMed] [Google Scholar]
- 16.Khurana S, Chearwae W, Castellino F, Manischewitz J, King LR, Honorkiewicz A, Rock MT, Edwards KM, Del Giudice G, Rappuoli R, et al. Vaccines with MF59 adjuvant expand the antibody repertoire to target protective sites of pandemic avian H5N1 influenza virus. Sci Transl Med. 2010;2:15ra15. doi: 10.1126/scitranslmed.3000624. PMID: 20371470. [DOI] [PubMed] [Google Scholar]
- 17.Calabro S, Tortoli M, Baudner BC, Pacitto A, Cortese M, O’hagan DT, De Gregorio E, Seubert A, Wack A. Vaccine adjuvants alum and MF59 induce rapid recruitment of neutrophils and monocytes that participate in antigen transport to draining lymph nodes. Vaccine. 2011;29:1812–23. doi: 10.1016/j.vaccine.2010.12.090. PMID: 21215831. [DOI] [PubMed] [Google Scholar]
- 18.Vono M, Taccone M, Caccin P, Gallotta M, Donvito G, Falzoni S, Palmieri E, Pallaoro M, Rappuoli R, Di Virgilio F, et al. The adjuvant MF59 induces ATP release from muscle that potentiates response to vaccination. Proc Natl Acad Sci USA. 2013;110:21095–100. doi: 10.1073/pnas.1319784110. PMID: 24324152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’hagan DT, Ott GS, De Gregorio E, Seubert A. The mechanism of action of MF59—an innately attractive adjuvant formulation. Vaccine. 2012;30:4341–8. doi: 10.1016/j.vaccine.2011.09.061. PMID: 22682289. [DOI] [PubMed] [Google Scholar]
- 20.Khurana S, Verma N, Yewdell JW, Hilbert AK, Castellino F, Lattanzi M, Del Giudice G, Rappuoli R, Golding H. MF59 adjuvant enhances diversity and affinity of antibody-mediated immune response to pandemic influenza vaccines. Sci Transl Med. 2011;3:85ra48. doi: 10.1126/scitranslmed.3002336. PMID: 21632986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Banzhoff A, Gasparini R, Laghi-Pasini F, Staniscia T, Durando P, Montomoli E, Capecchi PL, di Giovanni P, Sticchi L, Gentile C, et al. MF59®-adjuvanted H5N1 vaccine induces immunologic memory and heterotypic antibody responses in non-elderly and elderly adults. PLoS One. 2009;4:e4384. doi: 10.1371/journal.pone.0004384. PMID: 19197383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Galli G, Hancock K, Hoschler K, DeVos J, Praus M, Bardelli M, Malzone C, Castellino F, Gentile C, McNally T, et al. Fast rise of broadly cross-reactive antibodies after boosting long-lived human memory B cells primed by an MF59 adjuvanted prepandemic vaccine. Proc Natl Acad Sci USA. 2009;106:7962–7. doi: 10.1073/pnas.0903181106. PMID: 19416838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’hagan DT. MF59 is a safe and potent vaccine adjuvant that enhances protection against influenza virus infection. Exp Rev Vaccines. 2007;6:699–710. doi: 10.1586/14760584.6.5.699. PMID: 17931151. [DOI] [PubMed] [Google Scholar]
- 24.Knuf M, Leroux-Roels G, Rumke HC, Abarca K, Rivera L, Lattanzi M, Pedotti P, Arora A, Kieninger-Baum D, Della Cioppa G. Safety and immunogenicity of an MF59-adjuvanted A/H1N1 pandemic influenza vaccine in children from three to seventeen years of age. Vaccine. 2015;33:174–81. doi: 10.1016/j.vaccine.2014.10.085. PMID: 25444803. [DOI] [PubMed] [Google Scholar]
- 25.Pellegrini M, Nicolay U, Lindert K, Groth N, Della Cioppa G. MF59-adjuvanted versus non-adjuvanted influenza vaccines: integrated analysis from a large safety database. Vaccine. 2009;27:6959–65. doi: 10.1016/j.vaccine.2009.08.101. PMID: 19751689. [DOI] [PubMed] [Google Scholar]
- 26.Frey SE, Shakib S, Chanthavanich P, Richmond P, Smith T, Tantawichien T, Kittel C, Jaehnig P, Mojares Z, Verma B, et al. Safety and immunogenicity of MF59-adjuvanted cell culture–Derived A/H5N1 subunit influenza virus vaccine: dose-finding clinical trials in adults and the elderly. Open Forum Infect Dis. 2019;6:ofz107. doi: 10.1093/ofid/ofz107. PMID: 30968056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hatz C, Cramer JP, Vertruyen A, Schwarz TF, von Sonnenburg F, Borkowski A, Lattanzi M, Hilbert AK, Cioppa GD, Leroux-Roels G. A randomised, single-blind, dose-range study to assess the immunogenicity and safety of a cell-culture-derived A/H1N1 influenza vaccine in adult and elderly populations. Vaccine. 2012;30:4820–7. doi: 10.1016/j.vaccine.2012.05.013. PMID: 22626675. [DOI] [PubMed] [Google Scholar]
- 28.Keitel W, Groth N, Lattanzi M, Praus M, Hilbert AK, Borkowski A, Tsai TF. Dose ranging of adjuvant and antigen in a cell culture H5N1 influenza vaccine: safety and immunogenicity of a phase 1/2 clinical trial. Vaccine. 2010;28:840–8. doi: 10.1016/j.vaccine.2009.10.019. PMID: 19835829. [DOI] [PubMed] [Google Scholar]
- 29.Izurieta HS, Chillarige Y, Kelman J, Wei Y, Lu Y, Xu W, Lu M, Pratt D, Chu S, Wernecke M, et al. Relative effectiveness of cell-cultured and egg-based influenza vaccines among elderly persons in the United States, 2017–2018. J Infect Dis. 2019;220:1255–64. doi: 10.1093/infdis/jiy716. PMID: 30561688. [DOI] [PubMed] [Google Scholar]
- 30.Eick-Cost A, Hu Z. Relative effectiveness of cell-based influenza vaccines compared to egg-based influenza vaccines, active component U.S. Service members, 2017-18 season. International Conference on Emerging Infectious Diseases; 2018; Atlanta (GA). 54. Abstr. 129. [Google Scholar]
- 31.Klein NP, Fireman B, Goddard K, Zerbo O, Asher J, Zhou J, King J, Lewis N. Vaccine effectiveness of Flucelvax relative to inactivated influenza vaccine during the 2017-18 influenza season in Northern California [abstract]. Open Forum Infect Dis. 2018;5(Supplement 1):S764. doi: 10.1093/ofid/ofy229.2189. PMID: 629442678. [DOI] [Google Scholar]
- 32.Barr IG, Donis RO, Katz JM, McCauley JW, Odagiri T, Trusheim H, Tsai TF, Wentworth DE. Cell culture-derived influenza vaccines in the severe 2017-2018 epidemic season: a step towards improved influenza vaccine effectiveness. NPJ Vaccines. 2018;3:44. doi: 10.1038/s41541-018-0079-z. PMID: 30323955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ansaldi F, Bacilieri S, Durando P, Sticchi L, Valle L, Montomoli E, Icardi G, Gasparini R, Crovari P. Cross-protection by MF59-adjuvanted influenza vaccine: neutralizing and haemagglutination-inhibiting antibody activity against A(H3N2) drifted influenza viruses. Vaccine. 2008;26:1525–9. doi: 10.1016/j.vaccine.2008.01.019. PMID: 18294741. [DOI] [PubMed] [Google Scholar]
- 34.Ansaldi F, Zancolli M, Durando P, Montomoli E, Sticchi L, Del Giudice G, Icardi G. Antibody response against heterogeneous circulating influenza virus strains elicited by MF59- and non-adjuvanted vaccines during seasons with good or partial matching between vaccine strain and clinical isolates. Vaccine. 2010;28:4123–9. doi: 10.1016/j.vaccine.2010.04.030. PMID: 20433807. [DOI] [PubMed] [Google Scholar]
- 35.Nicolay U, Heijnen E, Nacci P, Patriarca PA, Leav B. Immunogenicity of aIIV3, MF59-adjuvanted seasonal trivalent influenza vaccine, in older adults ≥65 years of age: meta-analysis of cumulative clinical experience. Int J Infect Dis. 2019;85:S1–9. doi: 10.1016/j.ijid.2019.03.026. PMID: 30926542. [DOI] [PubMed] [Google Scholar]
- 36.Vesikari T, Knuf M, Wutzler P, Karvonen A, Kieninger-Baum D, Schmitt HJ, Baehner F, Borkowski A, Tsai TF, Clemens R. Oil-in-water emulsion adjuvant with influenza vaccine in young children. N Engl J Med. 2011;365:1406–16. doi: 10.1056/NEJMoa1010331. PMID: 21995388. [DOI] [PubMed] [Google Scholar]
- 37.Vesikari T, Kirstein J, Go GD, Leav B, Ruzycky ME, Isakov L, de Bruijn M, Oberye J, Heijnen E. Efficacy, immunogenicity, and safety evaluation of an MF59-adjuvanted quadrivalent influenza virus vaccine compared with non-adjuvanted influenza vaccine in children: a multicentre, randomised controlled, observer-blinded, phase 3 trial. Lancet Respir Med. 2018;6:345–56. doi: 10.1016/s2213-2600(18)30108-5. PMID: 29631857. [DOI] [PubMed] [Google Scholar]
- 38.van Baalen CA, Els C, Sprong L, van Beek R, van der Vries E, Osterhaus AD, Rimmelzwaan GF. Detection of nonhemagglutinating influenza a(h3) viruses by enzyme-linked immunosorbent assay in quantitative influenza virus culture. J Clin Microbiol. 2014;52:1672–7. doi: 10.1128/jcm.03575-13. PMID: 24622097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nakowitsch S, Waltenberger AM, Wressnigg N, Ferstl N, Triendl A, Kiefmann B, Montomoli E, Lapini G, Sergeeva M, Muster T, et al. Egg- or cell culture-derived hemagglutinin mutations impair virus stability and antigen content of inactivated influenza vaccines. Biotechnol J. 2014;9:405–14. doi: 10.1002/biot.201300225. PMID: 24323790. [DOI] [PubMed] [Google Scholar]
- 40.Hegde NR. Cell culture-based influenza vaccines: a necessary and indispensable investment for the future. Human Vaccin Immunother. 2015;11:1223–34. doi: 10.1080/21645515.2015.1016666. PMID: 25875691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van Baalen CA, Jeeninga RE, Penders GH, van Gent B, van Beek R, Koopmans MP, Rimmelzwaan GF. ViroSpot microneutralization assay for antigenic characterization of human influenza viruses. Vaccine. 2017;35:46–52. doi: 10.1016/j.vaccine.2016.11.060. PMID: 27899226. [DOI] [PubMed] [Google Scholar]
- 42.Trombetta CM, Perini D, Mather S, Temperton N, Montomoli E. Overview of serological techniques for influenza vaccine evaluation: past, present and future. Vaccines (Basel). 2014;2:707–34. doi: 10.3390/vaccines2040707. PMID: 26344888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heeringa M, Leav B, Smolenov I, Palladino G, Isakov L, Matassa V. Comparability of titers of antibodies against seasonal influenza virus strains as determined by hemagglutination inhibition and microneutralization assays. J Clin Microbiol. 2020;58:e00750–00720. doi: 10.1128/JCM.00750-20. PMID: 32493784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Verschoor CP, Singh P, Russell ML, Bowdish DM, Brewer A, Cyr L, Ward BJ, Loeb M. Microneutralization assay titres correlate with protection against seasonal influenza H1N1 and H3N2 in children. Plos One. 2015;10:e0131531. doi: 10.1371/journal.pone.0131531. PMID: 26107625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trombetta CM, Ulivieri C, Cox RJ, Remarque EJ, Centi C, Perini D, Piccini G, Rossi S, Marchi S, Montomoli E. Impact of erythrocyte species on assays for influenza serology. J Prev Med Hyg. 2018;59:E1–e7. doi: 10.15167/2421-4248/jpmh2018.59.1.870. PMID: 29938233. [DOI] [PMC free article] [PubMed] [Google Scholar]


