Abstract
Background & Aims:
This study aimed to analyse the association of sex hormone levels with liver enzyme levels and non-alcoholic fatty liver disease (NAFLD) in a nationally representative sample of men.
Methods:
A total of 919 men from the US National Health and Nutrition Examination Study (NHANES) III were included in this cross-sectional analysis of data from 1988 to 1991. We used existing data on serum total and free testosterone, total and free estradiol, androstanediol glucuronide (AAG) and sex steroid-binding globulin (SHBG), and estimated their associations with aspartate aminotransferase (AST), and alanine aminotransferase (ALT) and NAFLD, as determined using ultrasound, after adjusting for possible confounders including age, race, smoking, alcohol, physical activity, waist circumference and steroid hormones.
Results:
Lower total testosterone (TT) and higher free estradiol were associated with higher odds of NAFLD after adjusting for confounders including the other sex hormones. Lower TT was associated with higher odds of elevated AST, but not ALT. Free testosterone, total estradiol, SHBG and AAG were not associated with NAFLD or liver enzymes.
Conclusions:
This study supports an inverse association between TT concentration and NAFLD in men independent of other sex hormones (SHBG, AAG and estradiol) and known risk factors, such as obesity, age and lifestyle. Exploration of whether TT might be a non-invasive marker for NAFLD diagnosis is warranted.
Keywords: estradiol, hepatic steatosis, non-alcoholic fatty liver disease, sex hormone-binding globulin, testosterone
1 |. INTRODUCTION
Non-alcoholic fatty liver disease (NAFLD) is the most common cause of liver disease worldwide. It has a prevalence in the United States of at least 21%,1 which is increasing in parallel with the obesity and type 2 diabetes mellitus pandemic.2 NAFLD was first described by Ludwig in 1980 on examination of histopathological liver biopsy specimens,3 and was divided into two categories; (a) non-alcoholic fatty liver (NAFL) with hepatic steatosis ≥5% and mild non-specific inflammation (70%−75% of cases) and (b) non-alcoholic steatohepatitis (NASH) with histopathological features of hepatocellular injury (20%−25% of cases). NASH is described as the progressive type of NAFLD, with a reported 20% of patients going on to develop cirrhosis.4 Since liver biopsy is invasive and not readily available, NAFLD diagnosis is usually made based on clinical history and non-invasive imaging suggesting the presence of hepatic steatosis, or elevated liver enzymes (alanine aminotransferase [ALT] and aspartate aminotransferase [AST]), once that other causes of hepatic steatosis including excessive alcohol intake or specific medications, are excluded.5 Ultrasound scan is able to detect the presence of fatty infiltration with high sensitivity when steatosis is greater than 30%.4
Mechanisms leading to hepatic steatosis are multifactorial.6 An alteration in the balance between influx or synthesis of hepatic lipids and their export or oxidation leads to hepatic triglyceride accumulation. This leaves the liver susceptible to secondary insults leading to hepatocellular inflammation and fibrosis. In particular, hyperinsulinaemia drives hepatic triglyceride production by increasing serum free fatty acid levels, and promotes de novo hepatic lipogenesis through up-regulation of lipogenic transcription factors.
Since insulin resistance is a major risk factor for NAFLD, it is considered to be the hepatic manifestation of the metabolic syndrome. Obesity, male gender, Hispanic ethnicity and type 2 diabetes mellitus are all independent risk factors for NAFLD.1 In addition, patients with NAFLD have higher mortality compared with similarly aged persons without NAFLD, with the most common cause of death being cardiovascular disease even after controlling for other metabolic risk factors.7
NAFLD is more common in men than in women,8 and gender-related differences in liver disease can depend on circulating levels of sex hormones and hepatic expression of sex hormone receptors.9 In a meta-analysis of five trials (n = 4715 men), higher total testosterone (TT) in men was associated with reduced odds of NAFLD, whereas the opposite relationship was seen in women (three trials, n = 1581 most postmenopausal women). Furthermore, higher sex hormone-binding globulin (SHBG) was associated with reduced odds of NAFLD in both men and women.10 The relationship between other sex hormones and NAFLD is less well explored.
1.1 |. Case report
We present the case of a 68-year-old white man with and prostate cancer with rising PSA levels and who was receiving androgen deprivation therapy (ADT) for 5 years. He had a history of diabetes (metformin), normal baseline HDL and LDL cholesterol, elevated triglycerides and social alcohol use. Weight was 105.8 kg and BMI was 30.8 kg/m2. The patient was enrolled in a clinical trial (NCT01084759), in which he received cyclic treatment with FDA-approved, high-dose testosterone (testosterone cypionate 400 mg intramuscularly every 28 days). Baseline CT scan was performed prior to starting testosterone and showed signs of a fatty liver compatible with NAFLD. After 3 months of treatment, a CT scan showed complete resolution of the fatty liver disease. Weight was 104.2 kg with BMI 30.3 kg/m2. After cessation of testosterone, the patient resumed ADT and had a follow-up CT scan after 3 months to assess disease response which showed that fatty liver had returned. After 2 years on ADT, the patient was re-exposed to a high-dose testosterone therapy for 3 months. A CT scan performed to assess disease response demonstrated prompt disappearance of fatty liver immediately after the start of the therapy (Figure 1).
FIGURE 1.
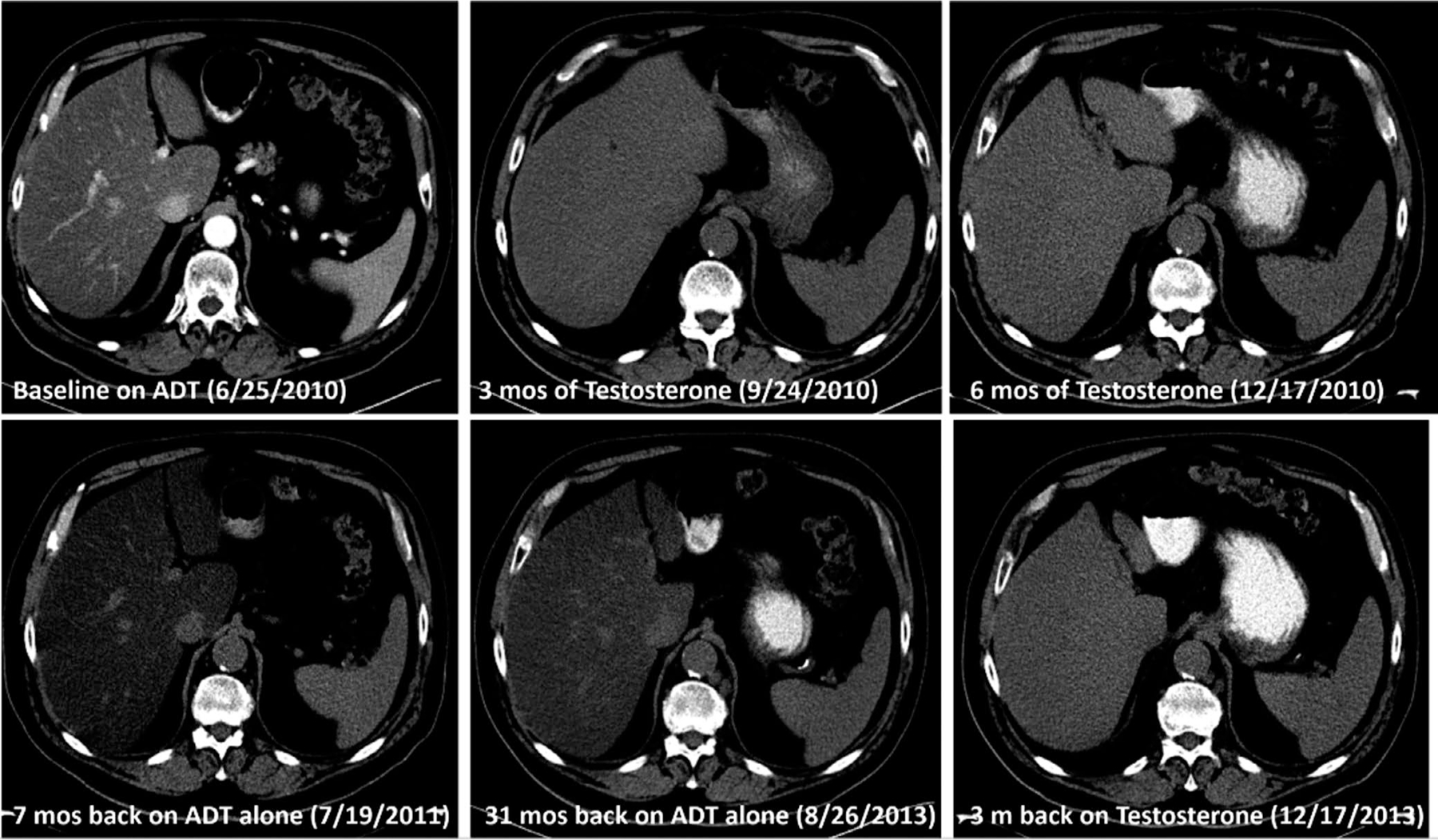
CT scans of a 68-y-old white man with a history of prostate cancer with rising PSA levels
Based on the results of this case report, we aimed to evaluate the association between circulating sex hormone levels and hepatic steatosis and elevated liver enzymes, three manifestations of NAFLD, in a nationally representative sample of non-institutionalized men from the Third National Health and Nutrition Examination Survey (NHANES III).
2 |. MATERIAL S AND METHODS
2.1 |. Subjects and study design
NHANES III is a cross-sectional study of 33 199 participants (14 894 males) and was conducted by the National Center for Health Statistics between 1988 and 1994.11 It was designed as a multistage stratified, clustered probability sample of the US civilian non-institutionalized population at least 2 months old and consisted of an interview, a physical examination and laboratory data. Mexican-Americans, non-Hispanic blacks and the elderly were over-sampled to generate more precise estimates for these subgroups of the US population.11 NHANES III was approved by the Centers for Disease Control and Prevention Institutional Review Board and all participants provided written consent to participate.
In total, 33 944 individuals participated in NHANES III; 14 781 of them were males with a physical examination. Of those, 7772 were at least 20 years old, of whom 1998 participated in the morning session of phase I. NHANES III was conducted in two phases (1988–1991 and 1991–1994), and unbiased national estimates of health and nutrition characteristics can be independently produced for each phase. Within each phase, subjects were randomly assigned to participate in either the morning or afternoon/evening examination session. Morning sample participants were chosen for this hormone study to reduce extraneous variation because of the diurnal production of hormones. Data on hormone levels were available for 1450 men. For this analysis, the following exclusion criteria were applied: alcohol consumption of three or more standard alcoholic drinks per day, diabetes mellitus, active hepatitis B or C infection, fasting of less than 9 hours before blood tests, incomplete data regarding smoking, physical activity, body fat measurements and ultrasound of the liver. After applying those exclusion criteria, 919 men constituted the analytical sample of this study.
2.2 |. Measurement of hepatic steatosis and NAFLD definition
For this population-based study, NAFLD was defined as sonographic evidence of moderate to severe hepatic steatosis in the absence of other causes for fatty liver disease, such as hepatitis C virus infection, diabetes mellitus and alcohol consumption of three or more standard drinks per day. The Hepatic Steatosis Ultrasound-Examination was conducted to assess the presence of fat within the hepatic parenchyma by reviewing archived Gallbladder Ultrasound-Examination videotapes that were originally obtained in NHANES III. In brief, the following information was recorded: (a) the presence of liver-to-kidney contrast (yes/no/not assessed), (b) the degree of the brightness of the liver parenchyma (none/intermediate/moderate/severe), (c) the presence of posterior deep beam attenuation (yes/no/not assessed), (d) the presence of echogenic walls in the small intrahepatic vessels (yes/no/not assessed) and (e) the definition of the gallbladder walls (clear/intermediate/obliterated/not assessed). A standardized algorithm was developed such that an overall primary finding of hepatic steatosis was based on the presence or absence of each of the parameters listed above. The liver was graded as normal, mild, moderate or severe steatosis, and dichotomized as present (moderate or severe) or absent (normal or mild). The intra- and inter-rater reliability of the presence of hepatic steatosis were 0.77 (95% CI: 0.73–0.82) and 0.70 (95% CI: 0.64–0.76) respectively.12 The raters were unaware of the participant health characteristics when grading the ultrasounds. The full protocol can be found elsewhere.12
2.3 |. Measurement of alanine aminotransferase (ALT) and aspartate aminotransferase (AST)
Blood was drawn during an examination session at the Mobile Examination Center11 and standard blood parameters were measured for all participants. Levels of ALT and AST were considered elevated when exceeding the NHANES III’s laboratory’s upper limit of normal (ALT > 40 U/L, AST > 37 U/L).13
2.4 |. Measurement of Sex Steroid Hormones and SHBG
Blood samples of participants in this study were drawn after an overnight fast at the same timepoint as the ultrasound scan was conducted. Surplus specimens were separated into their components and stored at −70°C. Competitive electrochemiluminescence immunoassays on the 2010 Elecsys autoanalyzer (Roche Diagnostics) were used to quantify serum testosterone, estradiol and SHBG concentrations. Androstanediol glucuronide (AAG) was measured by an enzyme immunoassay (Diagnostic Systems Laboratories). All samples were measured at the Children’s Hospital Boston, MA. The samples were randomly ordered for testing and the laboratory technicians were blinded to the men’s identities and ages. The lowest detection limits of the assays were: testosterone 0.07 nmol/L, estradiol 18.4 pmol/L, AAG 0.70 nmol/L and SHBG 3 nmol/L. The coefficients of variation for quality control specimens included during the analyses of the NHANES III specimens were: testosterone 5.9% and 5.8% at 8.6 and 19.1 nmol/L respectively; estradiol 6.5% and 6.7% at 0.38 nmol/L and 1.7 nmol/L respectively; AAG 9.5% and 5.0% at 6.2 and 21.6 nmol/L respectively; and SHBG 5.3% and 5.9% at 5.3 and 16.6 nmol/L respectively. In a separate run, we tested the quality control samples with a mean estradiol concentration of 144.6 pmol/L, which is in the range of typical adult male estradiol concentration; the CV% was 2.5%. Free testosterone concentration was estimated from measured testosterone, SHBG and albumin (already available in the NHANES III public use database).14,15 Free estradiol was calculated from total estradiol, SHBG and albumin.14,16
2.5 |. Definition of covariates
Race/ethnicity information was self-reported during the interview and men were categorized as non-Hispanic black, non-Hispanic white, Mexican-American or other. Based on the interview, men were classified according to their smoking habit into current (1–34, or ≥35 cigarettes/day), former or never smokers. Current body weight and height as well as waist circumference were measured during a physical examination that was part of NHANES III. BMI was categorized as <25.0 kg/m2 (normal), 25.0 to <30 kg/m2 (overweight) and ≥30.0 kg/m2 (obese). Waist circumference of greater than 102 cm was an indicator of abdominal obesity. The consumption frequency of alcoholic beverages (beer, wine and liquor) during the past month was assessed using a food-frequency questionnaire during the interview. Men with alcohol consumption of three or more drinks per day were excluded from the analysis. Men with a consumption of up to two alcoholic drinks per day were defined as moderate alcohol consumers. Leisure-time physical activity (LTPA) was assessed by a physical activity questionnaire and classified by type of activity (eg running, swimming and biking), frequency during the past month (‘times per week’ using the conversion factor 4.3 weeks per month) and intensity according to the standardized classification criteria of metabolic equivalent (MET) level by the Compendium of Physical Activities.17 For more detailed description, see Rohrmann et al 2005.18 LTPA was categorized in an ordinal scale integrating intensity and frequency of moderate and vigorous activity to achieve a total scale of LTPA. ‘No LTPA’ was defined as performing no vigorous and no moderate physical activity. ‘Irregular LTPA’ was defined as participating in moderate activity up to four times or in vigorous activity up to two times per week. ‘Regular LTPA’ was defined as engaging in moderate activity more than four times or in vigorous activity more than twice per week.
2.6 |. Statistical analysis
The participants’ baseline characteristics, liver enzymes (ALT and AST) and hormone levels were compared by the presence or absence of fatty liver. Logistic regression models were used to examine the associations of serum concentrations of total and estimated free testosterone and estradiol, respectively, AAG and SHBG with odds of hepatic steatosis, elevated ALT and elevated AST, separately. Model 1 was adjusted for age and race; model 2 was additionally adjusted for smoking, alcohol and physical activity; model 3 was additionally adjusted for waist circumference; and model 4 was additionally mutually adjusted for the steroid hormones. The fourth model was used because SHBG is the main binding protein of both testosterone and estradiol. Hence, mutual adjustment of testosterone, estradiol and SHBG takes into account the competition for the binding protein. We also mutually adjusted free testosterone and free estradiol. Tests for trend were calculated to evaluate the presence of dose-response relationships between quartiles of hormones and the above-mentioned outcomes. Subanalyses were conducted among men with BMI ≥ 25 kg/m2 and men older than 40 years of age. Other factors may influence the development of hepatic steatosis. Therefore, we conducted several sensitivity analyses to see if results change when we (a) exclude participants who take certain types of medication (amiodarone, nucleoside reverse transcriptase inhibitors, perhexiline maleate, tamoxifen, methotrexate, fluorouracil, irinotecan, glucocorticoids and zidovudine) or (b) excluded men with hypercholesterolaemia (defined as self-reported physician-based diagnosis, self-reported use of medication or total cholesterol concentration >240 mg/dL). All analyses were performed taking sampling weights into account.
3 |. RESULTS
In the study population of 919 men, hepatic steatosis was observed in 188 (20.5%). Mean age, waist circumference, concentrations of triglycerides, ALT, AST and triglycerides-to-high density lipoprotein ratio were higher among men with NAFLD than among men without NAFLD (Table 1). The prevalence of obesity, defined by both BMI (overall obesity) and waist circumference (abdominal obesity), was significantly higher among men with NAFLD than men without NAFLD. Subjects of Mexican-American ethnicity had a higher prevalence of NAFLD compared with non-NAFLD, whereas the reverse was observed for non-Hispanic whites and non-Hispanic blacks; however, the differences were not statistically significant. Men without NAFLD were more frequently current smokers and moderate alcohol consumers; they were also more physically active than men with NAFLD.
TABLE 1.
Baseline characteristics of study participants by non-alcoholic fatty liver disease (NAFLD) status; men in the Third National Health and Nutrition Examination Survey (1988–1991)
| No NAFLD |
NAFLD |
||||
|---|---|---|---|---|---|
| N = 731b | N = 188b | P valuea | |||
| Age, y (mean, SE) | 38.8 | 0.8 | 46.6 | 1.4 | <.001 |
| Race/ethnicity (%, SE) | |||||
| Non-Hispanic white | 86.4 | 1.8 | 85.1 | 2.5 | .11 |
| Non-Hispanic black | 8.6 | 1.4 | 6.9 | 1.5 | |
| Mexican-American | 5.1 | 0.9 | 8.1 | 1.5 | |
| Body mass index, kg/m2 (%, SE) | |||||
| <18.5 | 1.2 | 0.6 | 0.0 | <.001 | |
| 18.5–24.9 | 49.2 | 2.5 | 16.3 | 4.5 | |
| 25–29.9 | 38.0 | 2.3 | 35.3 | 6.1 | |
| 30–34.9 | 9.2 | 1.5 | 35.6 | 6.2 | |
| ≥35 | 2.4 | 1.2 | 12.8 | 3.1 | |
| High waist circumference >102 cm (%, SE) | 15.0 | 1.9 | 65.8 | 4.9 | <.001 |
| Regular leisure-time physical activity (%, SE) | 59.8 | 3.7 | 38.6 | 6.2 | .001 |
| Current smoking (%, SE) | 33.9 | 2.7 | 25.0 | 5.0 | .07 |
| Moderate alcohol consumption (%, SE) | 48.1 | 3.6 | 36.9 | 6.7 | .028 |
| Total cholesterol mg/dL (mean, SE) | 201.7 | 1.8 | 219.8 | 5.9 | .016 |
| High density lipoprotein cholesterol mg/dL (mean, SE) | 54.2 | 7.0 | 45.2 | 2.0 | .260 |
| Triglycerides mg/dL (mean, SE) | 123.7 | 4.3 | 236.3 | 42.7 | .015 |
| Triglycerides: high density lipoprotein cholesterol ratio (mean, SE) | 3.0 | 0.1 | 6.1 | 1.0 | .004 |
| Alanine aminotransferase, U/L (mean, SE) | 16.7 | 0.3 | 23.6 | 1.4 | <.001 |
| Aspartate aminotransferase, U/L (mean, SE) | 21.3 | 0.5 | 25.1 | 1.1 | .008 |
| Sex steroid hormone concentrations (geometric mean, 95% CI) | |||||
| Total testosterone, ng/mL | 5.4 | (5.2–5.7) | 4.0 | (3.8–4.3) | <.001 |
| Free testosterone, ng/mL | 0.108 | (0.103–0.114) | 0.085 | (0.076–0.095) | .001 |
| Total estradiol, pg/mL | 35.8 | (34.2–37.5) | 35.4 | (33.1–37.8) | .69 |
| Free estradiol, pg/mL | 0.905 | (0.861–0.951) | 0.949 | (0.884–1.019) | .026 |
| SHBG nmol/L | 35.1 | (33.5–36.7) | 29.2 | (26.2–32.5) | .005 |
| AAG, ng/mL | 12.3 | (11.5–13.2) | 11.3 | (9.7–13.2) | .38 |
Abbreviations: AAG, androstanediol glucuronide; CI, confidence interval; SHBG, sex hormone-binding globulin.
t-test for independent samples or Pearson χ2 test.
Unweighted N.
After adjusting for age and race, the prevalence of hepatic steatosis decreased across quartiles of TT, free testosterone and SHBG (Table 2). The prevalence of hepatic steatosis did not consistently change across quratiles of total estradiol and AAG. The prevalence of hepatic steatosis increased across quartiles of free estradiol quartiles.
TABLE 2.
Age- and race-standardizeda prevalence of non-alcoholic fatty liver disease by quartiles of sex steroid hormone concentrations in men, NHANES III
| Hormone | Quartile | Range | Prevalence (%) |
|---|---|---|---|
| Total testosterone (ng/mL) | 1 | 0.05–4.08 | 31.9 |
| 2 | 4.09–5.26 | 28.6 | |
| 3 | 5.27–6.63 | 11.8 | |
| 4 | 6.65–13.82 | 7.5 | |
| Free testosterone (ng/mL) | 1 | 0.001–0.081 | 27.0 |
| 2 | 0.081–0.106 | 23.4 | |
| 3 | 0.106–0.135 | 17.1 | |
| 4 | 0.135–0.317 | 17.7 | |
| Total estradiol (pg/mL) | 1 | 8.75–29.51 | 21.5 |
| 2 | 29.53–35.56 | 19.9 | |
| 3 | 35.58–43.62 | 19.8 | |
| 4 | 43.68–175.8 | 19.8 | |
| Free estradiol (pg/mL) | 1 | 0.239–0.741 | 17.0 |
| 2 | 0.743–0.913 | 18.2 | |
| 3 | 0.914–0.122 | 18.8 | |
| 4 | 1.124–3.738 | 26.4 | |
| SHBG (nmol/l) | 1 | 9.82–25.35 | 38.7 |
| 2 | 25.42–34.31 | 23.1 | |
| 3 | 34.42–47.04 | 17.1 | |
| 4 | 47.13–163.2 | 8.2 | |
| AAG (ng/mL) | 1 | 1.46–8.01 | 21.9 |
| 2 | 8.02–11.49 | 21.1 | |
| 3 | 11.49–16.75 | 18.7 | |
| 4 | 16.76–182.5 | 21.5 |
Abbreviations: AAG, androstanediol glucuronide; SHBG, sex hormone-binding globulin.
Weights constructed from our subpopulation.
When only adjusting for age and race/ethnicity (model 1), the odds of hepatic steatosis decreased with increasing TT and SHBG concentrations, whereas the odds increased with increasing free E2 concentrations (Table 3). No statistically significant associations were observed between increasing levels of free testosterone, total estradiol, AAG and the odds of hepatic steatosis. These associations were similar in model 2. However, in model 3, only the association between TT and hepatic steatosis remained statistically significant. In model 4, after mutually adjusting for the hormones and SHBG, the association for TT was still statistically significant as was the association for free estradiol. Excluding men taking certain medication or with a history of hypercholesterolaemia did not materially affect our results. Besides the results for TT, none of the associations changed the level of significance. The association between TT and hepatic steatosis became statistically significant comparing quartile 4 with quartile 1 (excluding men with hypercholesterolaemia, OR: 0.39, 95% CI: 0.17–0.92, n = 673 men; excluding men taking certain medication, OR: 0.42, 95% CI: 0.18–0.99, n = 859 men).
TABLE 3.
Association of sex steroid hormone concentrations with non-alcoholic fatty liver disease in men, NHANES III
| Model 1a |
Model 2 |
Model 3 |
Model 4 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Hormone | Quartilesb | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI |
| Total testosterone | 1 | 1 | 1 | 1 | 1 | ||||
| 2 | 0.64 | [0.38,1.08] | 0.70 | [0.42,1.18] | 0.78 | [0.46,1.32] | 0.82 | [0.46,1.46] | |
| 3 | 0.24 | [0.11,0.53] | 0.23 | [0.10,0.53] | 0.41 | [0.17,0.98] | 0.44 | [0.17,1.12] | |
| 4 | 0.17 | [0.06,0.49] | 0.17 | [0.06,0.47] | 0.41 | [0.18,0.95] | 0.43 | [0.18,1.00] | |
| P-trend | .002 | .001 | .036 | .044 | |||||
| Free testosterone | 1 | 1 | 1 | 1 | 1 | ||||
| 2 | 1.42 | [0.76,2.65] | 1.35 | [0.70,2.62] | 1.43 | [0.66,3.07] | 1.25 | [0.60,2.62] | |
| 3 | 0.55 | [0.24,1.22] | 0.55 | [0.23,1.30] | 0.69 | [0.29,1.66] | 0.56 | [0.25,1.22] | |
| 4 | 0.48 | [0.18,1.29] | 0.46 | [0.15,1.41] | 0.88 | [0.32,2.44] | 0.55 | [0.21,1.46] | |
| P-trend | .06 | .08 | .48 | .09 | |||||
| Total estradiol | 1 | 1 | 1 | 1 | 1 | ||||
| 2 | 1.05 | [0.63,1.74] | 1.03 | [0.63,1.68] | 1.11 | [0.71,1.73] | 1.17 | [0.76,1.82] | |
| 3 | 0.88 | [0.50,1.53] | 0.88 | [0.49,1.60] | 0.68 | [0.36,1.28] | 0.84 | [0.44,1.61] | |
| 4 | 1.08 | [0.59,1.98] | 1.20 | [0.61,2.38] | 1.20 | [0.55,2.58] | 1.66 | [0.82,3.38] | |
| P-trend | .96 | .71 | .97 | .30 | |||||
| Free estradiol | 1 | 1 | 1 | 1 | 1 | ||||
| 2 | 1.72 | [0.88,3.37] | 1.62 | [0.81,3.22] | 1.59 | [0.92,2.74] | 1.59 | [0.96,2.62] | |
| 3 | 1.58 | [0.89,2.81] | 1.71 | [0.91,3.21] | 1.24 | [0.67,2.29] | 1.45 | [0.85,2.48] | |
| 4 | 2.79 | [1.58,4.91] | 2.92 | [1.50,5.69] | 2.00 | [0.98,4.10] | 2.58 | [1.28,5.24] | |
| P-trend | .002 | .003 | .10 | .0128 | |||||
| SHBG | 1 | 1 | 1 | 1 | 1 | ||||
| 2 | 0.47 | [0.21,1.05] | 0.52 | [0.22,1.22] | 0.78 | [0.30,2.02] | 0.90 | [0.32,2.49] | |
| 3 | 0.43 | [0.20,0.92] | 0.46 | [0.22,0.99] | 0.73 | [0.30,1.78] | 0.89 | [0.33,2.43] | |
| 4 | 0.17 | [0.07,0.43] | 0.20 | [0.08,0.51] | 0.44 | [0.15,1.28] | 0.64 | [0.18,2.19] | |
| P-trend | <.001 | .002 | .11 | .46 | |||||
| AAG | 1 | 1 | 1 | 1 | |||||
| 2 | 0.86 | [0.36,2.04] | 0.88 | [0.37,2.07] | 0.77 | [0.27,2.22] | — | ||
| 3 | 0.95 | [0.44,2.07] | 1.03 | [0.48,2.20] | 0.80 | [0.35,1.84] | |||
| 4 | 0.86 | [0.36,2.08] | 0.90 | [0.37,2.20] | 0.90 | [0.33,2.46] | |||
| P-trend | .78 | .90 | .83 | ||||||
Abbreviations: AAG, androstanediol glucuronide; SHBG, sex hormone-binding globulin.
Model 1 adjusted for age and race.
Model 2 additionally adjusted for smoking, alcohol and physical activity. Model 3 additionally adjusted for waist circumference.
Model 4 as model 3, but mutual adjustment for hormones.
See Table 2 for hormone concentrations per quartile.
The results of a sub-analysis in men 40+ years old were similar to the results in the overall study population (Table S1).
In overweight and obese men, increasing TT levels and SHBG were associated with significantly lower odds of hepatic steatosis (models 1–3; Table S2). The association for TT remained statistically significant in model 4, but not the association for SHBG. There were no statistically significant associations between increasing levels of free testosterone, total estradiol, free estradiol and AAG with the odds of hepatic steatosis in men with BMI ≥ 25 kg/m2.
Increasing concentrations of sex steroid hormone were not statistically significantly associated with the odds of high ALT level in any of the four models (Table 4). In models 1–4, increasing TT concentrations were inversely associated with high AST level (all P-trend < .05). Similar results were observed for free testosterone, but none of the other hormones was associated with high AST level (Table 5).
TABLE 4.
Association of sex steroid hormone concentrations with high ALT concentrations (>40 U/L) in men, NHANES III
| Model 1a |
Model 2 |
Model 3 |
Model 4 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Hormone | Quartilesb | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI |
| Total testosterone | 1 | 1 | 1 | 1 | 1 | ||||
| 2 | 0.70 | [0.21,2.29] | 0.80 | [0.24,2.62] | 1.02 | [0.30,3.51] | 0.98 | [0.29,3.34] | |
| 3 | 0.38 | [0.11,1.32] | 0.35 | [0.12,1.04] | 0.63 | [0.18,2.16] | 0.55 | [0.13,2.30] | |
| 4 | 0.48 | [0.07,3.13] | 0.40 | [0.05,3.00] | 0.93 | [0.09,9.53] | 0.65 | [0.08,5.04] | |
| P-Trend | .24 | .18 | .83 | .50 | |||||
| Free testosterone | 1 | 1 | 1 | 1 | 1 | ||||
| 2 | 0.43 | [0.12,1.59] | 0.40 | [0.10,1.50] | 0.40 | [0.09,1.72] | 0.43 | [0.10,1.86] | |
| 3 | 0.28 | [0.05,1.69] | 0.29 | [0.05,1.69] | 0.38 | [0.06,2.18] | 0.47 | [0.08,2.88] | |
| 4 | 0.69 | [0.19,2.56] | 0.49 | [0.12,2.02] | 0.82 | [0.22,3.08] | 1.16 | [0.22,6.04] | |
| P-Trend | .48 | .29 | .76 | .84 | |||||
| Total estradiol | 1 | 1 | 1 | 1 | 1 | ||||
| 2 | 0.69 | [0.27,1.78] | 0.60 | [0.21,1.69] | 0.60 | [0.21,1.74] | 0.64 | [0.21,1.95] | |
| 3 | 0.79 | [0.19,3.34] | 0.67 | [0.15,3.04] | 0.49 | [0.12,2.05] | 0.49 | [0.13,1.83] | |
| 4 | 1.25 | [0.49,3.20] | 0.83 | [0.29,2.41] | 0.76 | [0.27,2.11] | 0.87 | [0.36,2.12] | |
| P-Trend | .62 | .81 | .54 | .61 | |||||
| Free estradiol | 1 | 1 | 1 | 1 | 1 | ||||
| 2 | 1.12 | [0.37,3.40] | 0.94 | [0.30,2.92] | 0.85 | [0.27,2.60] | 0.88 | [0.30,2.59] | |
| 3 | 0.80 | [0.19,3.38] | 0.73 | [0.20,2.74] | 0.50 | [0.14,1.82] | 0.48 | [0.16,1.49] | |
| 4 | 1.93 | [0.64,5.84] | 1.31 | [0.39,4.34] | 0.79 | [0.33,1.89] | 0.60 | [0.17,2.17] | |
| P-Trend | .31 | .74 | .30 | .23 | |||||
| SHBG | 1 | 1 | 1 | 1 | 1 | ||||
| 2 | 0.20 | [0.04,0.98] | 0.22 | [0.04,1.25] | 0.33 | [0.06,1.88] | 0.36 | [0.07,1.91] | |
| 3 | 0.83 | [0.26,2.62] | 0.90 | [0.30,2.69] | 1.42 | [0.43,4.68] | 1.53 | [0.59,3.99] | |
| 4 | 0.61 | [0.13,2.90] | 0.79 | [0.17,3.76] | 1.86 | [0.27,12.86] | 2.43 | [0.48,12.21] | |
| P-Trend | .98 | .78 | .29 | .13 | |||||
| AAG | 1 | 1 | 1 | 1 | |||||
| 2 | 0.53 | [0.13,2.12] | 0.55 | [0.14,2.27] | 0.55 | [0.12,2.45] | — | ||
| 3 | 1.34 | [0.40,4.45] | 1.64 | [0.46,5.78] | 1.23 | [0.38,3.97] | |||
| 4 | 1.26 | [0.41,3.84] | 1.34 | [0.40,4.51] | 1.41 | [0.37,5.41] | |||
| P-Trend | .39 | .35 | .41 | ||||||
Abbreviations: AAG, androstanediol glucuronide; SHBG, sex hormone-binding globulin.
Model 1 adjusted for age and race.
Model 2 additionally adjusted for smoking, alcohol and physical activity. Model 3 additionally adjusted for waist circumference.
Model 4 as model 3, but mutual adjustment for hormones.
See Table 2 for hormone concentrations per quartile.
TABLE 5.
Association of sex steroid hormone concentrations with high AST concentrations (>37 U/L) in men, NHANES III
| Model 1a |
Model 2 |
Model 3 |
Model 4 |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Hormone | Quartilesb | OR | 95% CI | OR | 95% CI | OR | 95% CI | OR | 95% CI |
| Total testosterone | 1 | 1 | 1 | 1 | 1 | ||||
| 2 | 1.04 | [0.57,1.90] | 0.93 | [0.46,1.87] | 0.91 | [0.43,1.94] | 0.75 | [0.32,1.77] | |
| 3 | 0.23 | [0.02,2.49] | 0.19 | [0.02,2.09] | 0.22 | [0.02,2.71] | 0.16 | [0.02,1.49] | |
| 4 | 0.42 | [0.17,1.07] | 0.32 | [0.13,0.79] | 0.42 | [0.16,1.07] | 0.23 | [0.04,1.20] | |
| P-Trend | .040 | .007 | .038 | .009 | |||||
| Free testosterone | 1 | 1 | 1 | 1 | 1 | ||||
| 2 | 0.32 | [0.10,1.06] | 0.30 | [0.09,1.01] | 0.28 | [0.08,1.02] | 0.22 | [0.07,0.74] | |
| 3 | 0.73 | [0.33,1.63] | 0.67 | [0.29,1.55] | 0.68 | [0.27,1.76] | 0.53 | [0.20,1.43] | |
| 4 | 0.27 | [0.08,0.87] | 0.22 | [0.07,0.64] | 0.26 | [0.09,0.75] | 0.13 | [0.03,0.55] | |
| P-Trend | .05 | .014 | .031 | .017 | |||||
| Total estradiol | 1 | 1 | 1 | 1 | 1 | ||||
| 2 | 0.82 | [0.13,4.99] | 0.90 | [0.13,5.99] | 0.90 | [0.14,6.02] | 1.01 | [0.11,8.97] | |
| 3 | 1.09 | [0.21,5.64] | 1.33 | [0.27,6.58] | 1.11 | [0.24,5.21] | 1.50 | [0.31,7.24] | |
| 4 | 1.75 | [0.67,4.60] | 2.00 | [0.57,7.07] | 1.94 | [0.56,6.67] | 2.98 | [0.68,13.01] | |
| P-Trend | .17 | .16 | .19 | .08 | |||||
| Free estradiol | 1 | 1 | 1 | 1 | 1 | ||||
| 2 | 1.19 | [0.21,6.76] | 1.33 | [0.23,7.60] | 1.22 | [0.21,7.02] | 1.71 | [0.33,8.76] | |
| 3 | 1.30 | [0.21,7.83] | 1.44 | [0.24,8.74] | 1.19 | [0.21,6.68] | 1.65 | [0.30,8.95] | |
| 4 | 1.92 | [0.74,4.97] | 2.19 | [0.79,6.06] | 1.74 | [0.65,4.71] | 3.93 | [1.03,14.93] | |
| P-Trend | .16 | .12 | .28 | .05 | |||||
| SHBG | 1 | 1 | 1 | 1 | 1 | ||||
| 2 | 0.08 | [0.02,0.43] | 0.07 | [0.01,0.40] | 0.09 | [0.01,0.58] | 0.11 | [0.01,0.85] | |
| 3 | 0.59 | [0.11,3.09] | 0.53 | [0.11,2.58] | 0.69 | [0.12,4.05] | 1.00 | [0.09,11.10] | |
| 4 | 0.39 | [0.10,1.52] | 0.35 | [0.08,1.47] | 0.51 | [0.09,3.00] | 0.71 | [0.07,7.62] | |
| P-Trend | .16 | .58 | .99 | .75 | |||||
| AAG | 1 | 1 | 1 | 1 | |||||
| 2 | 0.78 | [0.33,1.84] | 0.78 | [0.32,1.94] | 0.77 | [0.28,2.13] | |||
| 3 | 0.61 | [0.20,1.87] | 0.62 | [0.18,2.09] | 0.53 | [0.15,1.88] | |||
| 4 | 0.24 | [0.05,1.24] | 0.22 | [0.04,1.16] | 0.21 | [0.04,1.26] | |||
| P-Trend | .68 | .07 | .07 | ||||||
Abbreviations: AAG, androstanediol glucuronide; SHBG, sex hormone-binding globulin.
Model 1 adjusted for age and race.
Model 2 additionally adjusted for smoking, alcohol and physical activity. Model 3 additionally adjusted for waist circumference.
Model 4 as model 3, but mutual adjustment for hormones.
See Table 2 for hormone concentrations per quartile.
4 |. DISCUSSION
This study supports the findings of a recently published meta-analysis,10 showing that in male participants of a cross-sectional, US nationally representative study, higher TT was associated with lower odds of prevalent NAFLD. This finding remained significant after adjusting for potential confounders including age, race, smoking, alcohol, physical activity, waist circumference and other sex hormones, and was consistent in the different subpopulations explored including BMI ≥ 25 kg/m2 and participants > 40 years of age. Higher SHBG was associated with reduced odds of developing NAFLD, but this was no longer statistically significant after adjusting for waist circumference and sex hormones (ie testosterone and estradiol) in the overall population.
While other studies have also observed that higher testosterone concentrations are associated with lower odds of NAFLD, a particular strength of our study is that we measured a number of sex hormones (testosterone, estradiol, SHBG and AAG) and adjusted for these in the model, which was not the case in other analyses.10,19–23 Hence, the findings further support the independent nature of the association between testosterone and NAFLD. Another strength of our study is that it is representative of the US population and that none of the assessments (hormones, AST, ALT or NAFLD) was done for clinical reasons. However, because it was a cross-sectional study, we cannot confirm whether this finding reflects a causal association, and it is possible that testosterone plays both a protective role in preventing fatty liver infiltration, and that in patients with NAFLD TT declines as a consequence of the disease. The former is supported by the case report presented; in a man with prostate cancer, androgen replacement therapy led to a radiological improvement in fatty liver infiltration as demonstrated by repeated CT scans. In addition, a recently published cohort study found an independent association between lower free and bioavailable testosterone and the presence and severity of NASH in patients with NAFLD.24
Mechanistically, this prevention of fatty liver infiltration could be explained by the fact that testosterone normally inhibits adipogenic differentiation and reduces lipoprotein lipase activity in adipose tissue. Therefore, in the absence of testosterone, there may be higher propensity for the development of visceral obesity, favouring systemic insulin resistance, a critical factor in the development of NAFLD.21 In addition, androgens, such as testosterone, could directly modulate liver fatty acid β-oxidation and de novo lipid synthesis.21 However, in another study with observational follow-up of Korean men undergoing a health promotion program (n = 1944), serum testosterone was not associated with the development or regression of NAFLD after adjusting for waist circumference, indicating that visceral obesity may be a more important factor for NAFLD pathogenesis.22 In a small study of 27 patients with chronic spinal cord injury, an increased risk of NAFLD was found to be associated with decreasing total and free testosterone after adjusting for age, BMI, HOMA-IR, triglycerides and coexisting diabetes.21 Further prospective studies are needed to better understand if testosterone plays a causal role in NAFLD, and whether androgen replacement therapy is a valuable treatment option in men.
SHBG is involved in transporting testosterone to target tissues and modulating its biological activity. Other studies have also observed an inverse association between SHBG and NAFLD, although this association was stronger in women than men.10 Similar to Lazo et al,19 our data suggest that this association may be partly dependent upon visceral obesity (waist circumference), a marker of insulin sensitivity. The diminishing relationship after adjusting for sex hormones indicates that it could additionally be mediated through hormones, especially TT.
We observed that higher free E2 was associated with a higher odds of NAFLD. This was consistent in the subpopulation of participants older than 40 years of age, but not in those with BMI ≥ 25 kg/m2. This is in contrast with several studies that have shown that oestrogens may play a protective role in NAFLD.25 For example, in mouse models of impaired oestrogen synthesis or mode of action, there is an increase in visceral adipose tissue and an increase in liver lipid droplets.26 A male patient with aromatase deficiency and a low oestrogen level exhibited features of metabolic syndrome, including insulin resistance and steatohepatitis. When treated with oestrogen these features improved.27 However, it is possible that the association between estradiol and NAFLD that we found in men in this study is directionally opposite in women, and it would be interesting to explore whether this is the case in a separate study. In addition, free E2 is known to be positively associated with body fat in men.28 Therefore, our findings could also be explained if free E2 is capturing the measurement error of body fat by BMI and waist circumference.
Although ALT and AST are indicative of liver inflammation, it has been suggested that ALT is a hallmark of NAFLD since it is more specific to liver injury than AST.29 In the Dionysos Nutrition and Liver Study conducted in Italian patients, ALT did not independently predict the odds of NAFLD compared with normal liver and only 54% of patients with NAFLD had elevated ALT.30 In our study, both ALT and AST were significantly higher in participants with NAFLD compared with those without NAFLD. Furthermore, we observed that lower TT concentrations were associated with higher odds of elevated AST levels, and although the odds ratio for ALT was in the same direction as for AST, it did not reach statistical significance. There was no significant association between the other sex hormones measured and AST or ALT. To our knowledge, this has not previously been investigated.
Sex steroid hormone concentrations were measured using immunoassays. Mass spectrometry produces more accurate quantitation of sex steroid hormones and is considered to be the gold standard. However, when the analyses were done, immunoassays were FDA-approved and widely used in clinical practice. These analytes were measured several years after blood collection. However, the markers are stable over time and also when exposed to multiple freeze-thaw cycles.31
5 |. CONCLUSION
Given the rising prevalence of obesity, type 2 diabetes mellitus and NAFLD, there is a critical need to modify the Western lifestyle and diet to prevent the onset and progression of these conditions. The statistically significant association between TT levels and lower odds of elevated liver enzymes and NAFLD fits our clinical experience documented in the above case report. With a recently documented trend towards increased prescription and use of testosterone replacement,32 future studies on the direct clinical relevance of ADT-induced or testosterone-diminished NAFLD are desirable. However, based on the case report presented, no firm conclusions on the therapeutic utility of testosterone replacement can be made. Taking all findings together, this study shows that there is a possible role for TT as a biomarker in the diagnosis of NAFLD.
Supplementary Material
Keypoints.
What is current knowledge
Non-alcoholic fatty liver disease (NAFLD) is the most common and increasing cause of chronic liver disease and a leading cause of liver transplant in US adults and many other countries.
Established NASH risk factors are obesity, hypertension, dyslipidaemia, type 2 diabetes and metabolic syndrome.
The increase in NAFLD prevalence is in parallel but disproportionate to the increase in prevalence of obesity and type 2 diabetes. People with NAFLD have a greater chance of developing cardiovascular disease.
In a recently published large systematic review and meta-analysis, it was shown that men are at higher risk of NAFLD than women which could be because of the differences in sex hormones, but women are more likely to have non-alcoholic steatohepatitis (NASH) and progressive disease.
In men, higher levels of both testosterone and sex steroid-binding globulin (SHBG) are associated with reduced risk for fatty liver, whereas higher levels of estradiol are associated with fatty liver in both sexes.
Testosterone replacement therapy is increasingly being prescribed in clinical practice and its effects on NAFLD is therefore of interest as a potential therapeutic agent.
What is new here
We present a case report of a 68-year-old male with prostate cancer undergoing androgen deprivation therapy followed by supraphysiologic androgen replacement as part of a novel prostate cancer treatment, with serial changes in fatty liver infiltration by computed tomography (CT) scan. Subsequently, a cross-sectional analysis of 919 US males was conducted, which is the first to explore the association of sex hormones (total testosterone, free testosterone, estradiol, androstanediol glucuronide [AAG] and SHBG) with liver enzymes (AST and ALT) and NAFLD in a nationally representative study of men in the US population. Taken together, the findings indicate that the increasingly common use of testosterone replacement therapy for conditions such as prostate cancer should be further explored as a therapeutic agent in NAFLD.
Acknowledgments
This study is part of the Hormone Demonstration Program funded by the Maryland Cigarette Restitution Fund at Johns Hopkins (Nelson).
Funding information
Maryland Cigarette Restitution Fund at Johns Hopkins; Johns Hopkins
Abbreviations:
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- NAFL
Non-alcoholic fatty liver
- NAFLD
non-alcoholic fatty liver disease
- NASH
non-alcoholic steatohepatitis
- NHANES III
Third National Health and Nutrition Examination Survey
Footnotes
ETHICS APPROVAL
We used public use data. In NHANES, informed consent for data collection and storage was obtained from all participants.
CONFLICT OF INTEREST
The authors do not have any disclosures to report.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
REFERENCES
- 1.Lazo M, Hernaez R, Eberhardt MS, et al. Prevalence of nonalcoholic fatty liver disease in the United States: the Third National Health and Nutrition Examination Survey, 1988–1994. Am J Epidemiol 2013;178(1):38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ruhl CE, Everhart JE. Fatty liver indices in the multiethnic United States National Health and Nutrition Examination Survey. Aliment Pharmacol Ther 2015;41(1):65–76. [DOI] [PubMed] [Google Scholar]
- 3.Brown GT, Kleiner DE. Histopathology of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Metabolism 2016;65(8):1080–1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rinella ME. Nonalcoholic fatty liver disease: a systematic review. JAMA 2015;313(22):2263–2273. [DOI] [PubMed] [Google Scholar]
- 5.Lazo M, Clark JM. The epidemiology of nonalcoholic fatty liver disease: a global perspective. Semin Liver Dis 2008;28(4): 339–350. [DOI] [PubMed] [Google Scholar]
- 6.Adams LA, Angulo P, Lindor KD. Nonalcoholic fatty liver disease. CMAJ 2005;172(7):899–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018;67(1):328–357. [DOI] [PubMed] [Google Scholar]
- 8.Balakrishnan M, et al. Women have lower risk of nonalcoholic fatty liver disease but higher risk of progression vs men: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2020. [DOI] [PMC free article] [PubMed]
- 9.Lonardo A, Carani C, Carulli N, et al. ‘Endocrine NAFLD’ a hormonocentric perspective of nonalcoholic fatty liver disease pathogenesis. J Hepatol 2006;44(6):1196–1207. [DOI] [PubMed] [Google Scholar]
- 10.Jaruvongvanich V, Sanguankeo A, Riangwiwat T, et al. Testosterone, sex hormone-binding globulin and nonalcoholic fatty liver disease: a systematic review and meta-analysis. Ann Hepatol 2017;16(3):382–394. [DOI] [PubMed] [Google Scholar]
- 11.National Center for Health Statistics. Plan and operation of the Third National Health and Nutrition Examination Survey, 1988–94. Series 1: programs and collection procedures. Vital Health Stat 1, 1994(32): 1–407. [PubMed] [Google Scholar]
- 12.National Center for Health Statistics. Third National Health and Nutrition Survey: Hepatic Steatosis Assessment Procedure Manual Hyattsville, MD: National Center for Health Statistics; 2010. [Google Scholar]
- 13.Giovannucci E, et al. Dinucleotide repeat in the insulin-like growth factor-I gene is not related to risk of colorectal adenoma. Cancer Epidemiol Biomarkers Prev 2002;11(11):1509–1510. [PubMed] [Google Scholar]
- 14.Södergard R, et al. Calculation of free and bound fractions of testosterone and estradiol-17[beta] to human plasma proteins at body temperature. J Steroid Biochem 1982;16(6):801–810. [DOI] [PubMed] [Google Scholar]
- 15.Vermeulen A, Verdonck L, Kaufman JM. A Critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 1999;84(10):3666–3672. [DOI] [PubMed] [Google Scholar]
- 16.Rinaldi S, et al. Validity of free testosterone and free estradiol determinations in serum samples from postmenopausal women by theoretical calculations. Cancer Epidemiol Biomarkers Prev 2002;11(10 Pt 1):1065–1071. [PubMed] [Google Scholar]
- 17.Ainsworth BE, Haskell WL, Leon AS, et al. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc 1993;25(1):71–80. [DOI] [PubMed] [Google Scholar]
- 18.Rohrmann S, Crespo CJ, Weber JR, et al. Association of cigarette smoking, alcohol consumption and physical activity with lower urinary tract symptoms in older American men: findings from the third National Health And Nutrition Examination Survey. BJU Int 2005;96(1):77–82. [DOI] [PubMed] [Google Scholar]
- 19.Lazo M, Zeb I, Nasir K, et al. Association between endogenous sex hormones and liver fat in a multiethnic study of atherosclerosis. Clin Gastroenterol Hepatol 2015;13(9):1686–1693.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim S, et al. A low level of serum total testosterone is independently associated with nonalcoholic fatty liver disease. BMC Gastroenterol 2012;12:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barbonetti A, Caterina Vassallo MR, Cotugno M, et al. Low testosterone and non-alcoholic fatty liver disease: evidence for their independent association in men with chronic spinal cord injury. J Spinal Cord Med 2016;39(4):443–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seo NK, Koo HS, Haam J-H, et al. Prediction of prevalent but not incident non-alcoholic fatty liver disease by levels of serum testosterone. J Gastroenterol Hepatol 2015;30(7):1211–1216. [DOI] [PubMed] [Google Scholar]
- 23.Li Y, Liu L, Wang B, et al. Nonalcoholic fatty liver disease and alteration in semen quality and reproductive hormones. Eur J Gastroenterol Hepatol 2015;27(9):1069–1073. [DOI] [PubMed] [Google Scholar]
- 24.Sarkar M, Yates K, Suzuki A, et al. Low testosterone is associated with nonalcoholic steatohepatitis (NASH) and severity of NASH fibrosis in men with NAFLD. Clin Gastroenterol Hepatol 2019. [DOI] [PMC free article] [PubMed]
- 25.Marino L, Jornayvaz FR. Endocrine causes of nonalcoholic fatty liver disease. World J Gastroenterol 2015;21(39):11053–11076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones MEE, Thorburn AW, Britt KL, et al. Aromatase-deficient (ArKO) mice have a phenotype of increased adiposity. Proc Natl Acad Sci USA 2000;97(23):12735–12740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maffei L, Murata Y, Rochira V, et al. Dysmetabolic syndrome in a man with a novel mutation of the aromatase gene: effects of testosterone, alendronate, and estradiol treatment. J Clin Endocrinol Metab 2004;89(1):61–70. [DOI] [PubMed] [Google Scholar]
- 28.Rohrmann S, Shiels MS, Lopez DS, et al. Body fatness and sex steroid hormone concentrations in US men: results from NHANES III. Cancer Causes Control 2011;22(8):1141–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hall P, Cash J. What is the real function of the liver ‘function’ tests? Ulster Med J 2012;81(1):30–36. [PMC free article] [PubMed] [Google Scholar]
- 30.Bedogni G, Miglioli L, Masutti F, et al. Prevalence of and risk factors for nonalcoholic fatty liver disease: the Dionysos nutrition and liver study. Hepatology 2005;42(1):44–52. [DOI] [PubMed] [Google Scholar]
- 31.Comstock GW, Burke AE, Norkus EP, et al. Effects of repeated freeze-thaw cycles on concentrations of cholesterol, micronutrients, and hormones in human plasma and serum. Clin Chem 2001;47(1):139–142. [PubMed] [Google Scholar]
- 32.Rao PK, Boulet SL, Mehta A, et al. Trends in testosterone replacement therapy use from 2003 to 2013 among reproductive-age men in the United States. J Urol 2017;197(4):1121–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


