Abstract
The use of endovascular stents has become widely established in maintaining both arterial and venous patency in congenital heart disease. Stent implantation is now applied to pulmonary arterial stenoses, coarctation, pulmonary and systemic venous obstruction, and obstructed homografts and conduits, in both the pediatric and adult populations. The purpose of this report is to describe 3 new applications of stent technology: 1) double pulmonary artery stent implantation with simultaneous balloon dilation of a previously placed stent; 2) a new technique for traversing tight pulmonary arterial corners for stent delivery using the “sheath-within-sheath” method; and 3) a new technique for recannulation and stent implantation in unilateral femoral venous occlusion.
Key words: Balloon dilatation; femoral vein; heart defects, congenital; pulmonary artery; stents
The rapidity of development in stent technology over the last decade has been equaled only by the rapidity of development in stent applications. Nowhere is this more obvious than in the application of endovascular stent implantations in congenital heart disease, both in pediatric and adult patients. Stent implants have been shown to be effective in the treatment of pulmonary arterial stenoses, 1 coarctation of the aorta, 2 systemic and central venous obstructions, 3 and such postoperative conditions as cavopulmonary communications and obstructed conduits and homografts. 4 Stent implantation has proved to be a safe procedure with minimal complications. There has also been a low restenosis rate, and restenoses are amenable to redilation with excellent results. 5 Only pulmonary venous stenoses, as a group, have not maintained long-term patency after stent implantation. 6
The purpose of this report is to describe 3 new applications of stent technology: 1) double pulmonary artery stent implantation with simultaneous balloon dilation of a previously implanted stent (3 simultaneous stent dilations); 2) a new technique of traversing tight pulmonary arterial corners for stent delivery “sheath within sheath;” and 3) a new technique to enable recannulation and stent implantation in unilateral complete femoral venous obstruction.
Case Report
A 26-year-old man had been diagnosed with tetralogy of Fallot in the newborn period and had undergone a Waterston anastomosis in the 2nd week of life. At 2-½ years of age, he underwent total surgical repair, with closure of the ventricular septal defect, resection of the right ventricular outflow tract obstruction, and take-down of the Waterston shunt.
Previous Pulmonary Artery Stent Implantations.
In April 1998, 23 years following surgical repair, this patient was referred for cardiac catheterization because of decreasing exercise tolerance. At that time, there were right upper lobe (RUL) and right lower lobe (RLL) pulmonary artery branch stenoses, with a 20-mmHg gradient detected across both branches. Two 10-F transeptal sheaths (TSS) (Cook Inc.; Bloomington, Ind) were placed in the left and right femoral veins, and two 0.035-inch Superstiff® exchange wires (Boston Scientific; Quincy, Mass) were placed into the RUL and RLL branches of the pulmonary artery. Two P-308 Palmaz stents (Johnson and Johnson; Piscataway, NJ) were mounted on two UDT 9-2-5.8-120 balloons (Boston Scientific), which were advanced through the 10-F sheaths in the left and right femoral veins. When the stents were in appropriate position, the balloons were inflated simultaneously, which achieved satisfactory overlapping of the stents. The stents were further dilated using two XXL 12-4-5.8-120 balloons (Boston Scientific). The gradient across the RLL and RUL branches was reduced to 10 mmHg. The diameter of the distal right main pulmonary artery increased from 5.4 to 17.9 mm, that of the RUL branch from 5.1 to 10.3 mm, and that of the right middle lobe (RML) branch from 2.3 to 11.7 mm; the right ventricle-to-femoral artery (RV:FA) ratio decreased from 0.48 to 0.37. The procedure was uncomplicated.
Three Simultaneous Stent Dilations and “Sheath-within-Sheath” Technique
One year later, the patient returned for cardiac catheterization. Two sheaths (Cordis; Miami, Fla), 8-F and 7-F, were placed in the left femoral vein (LFV), and a 7-F sheath was placed in the distal right femoral vein (RFV), which was almost completely occluded above the access site. The RFV was recannulated (this technique is described later), because 3 sheaths were needed to enable simultaneous double-stent implantation and balloon dilation of one of the previously placed stents. Angiography revealed stenosis of the RML and RLL pulmonary artery branches (Fig. 1). Three 0.038-inch Superstiff wires were placed across the appropriate right pulmonary artery branches. An XXL 12-2-5.8-120 balloon (Boston Scientific) was advanced into the 8-F sheath in the left femoral vein over a 0.035-inch Superstiff wire, and into the RUL stent. A P-204 stent (Johnson and Johnson) was mounted on a UTG 3-2-5-120 balloon (Boston Scientific) and advanced into the RML branch, over a 2nd 0.035-inch Superstiff wire and through an 8-F transeptal sheath (TSS USCI) (Medtronic-AVE; Shoreview, Minn) placed in the left femoral vein. A P-308 stent mounted on a Marshall 10-3-5.8-135 balloon (Boston Scientific) was advanced through the 9-F TSS in the RLL branch over the 3rd 0.035-inch Superstiff wire. However, the balloon and stent could not reach the RLL pulmonary artery because of an acute angle and were removed from the 9-F TSS.
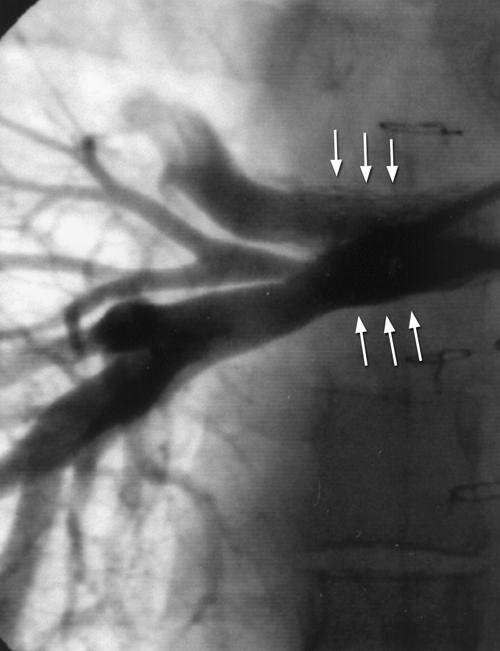
Fig. 1 Angiogram in the right pulmonary artery reveals RML and RLL branch pulmonary artery stenosis with 2 stents (arrows) implanted in the RUL and RML. RLL = right lower lobe; RML = right middle lobe; RUL = right upper lobe
The 9-F TSS was exchanged over a 0.035-inch Superstiff wire for a 10-F TSS (Cook, Inc.). The Marshall balloon and P-308 stent were then loaded into an 8-85 TSS (Cook, Inc.), which was advanced distally into the RLL branch through the 10-F sheath. The balloon and stent were then advanced through both sheaths until they were positioned directly at the site of stenosis (Fig. 2). Angiography through the 8-85 TSS was performed to confirm the position. Balloon dilation of the RUL stent was performed simultaneously with balloon dilation and stent implantation in the RML and RLL branches (12 atm, UDT balloon; 8 atm, XXL balloon; and 10 atm, Marshall balloon), each for 8 seconds (Fig. 3). Repeat balloon inflations were performed simultaneously at the same pressures for 4 seconds and then for 6 seconds. The RUL balloon “milked” proximally during the 1st inflation. All 3 balloons were reinflated at lower pressure (3 atm for 14 sec each), in order to keep them in place. Angiography performed in the main pulmonary artery after stent implantation confirmed excellent positioning of the stents, with an increase in luminal diameter from 8 to 10.7 mm, 3.2 to 6.3 mm, and 7.5 to 10.3 mm in the upper, middle, and lower lobe branches, respectively (Fig. 4). The mean gradient across the right pulmonary artery decreased from 20 to 8 mmHg, and the RV:FA ratio decreased from 0.45 to 0.38. A prophylactic antibiotic (intravenous cefazolin, 1 g) was administered after the repeat stent implantation and redilation.
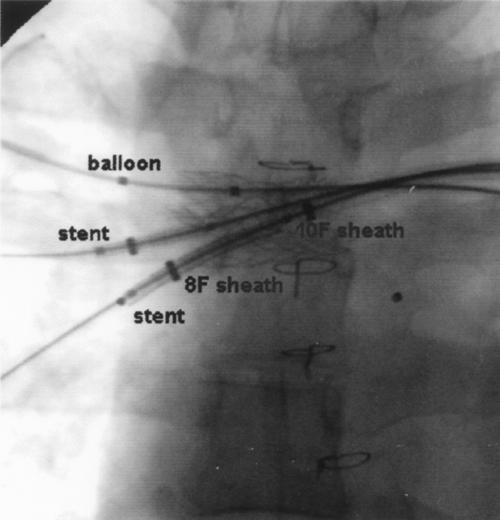
Fig. 2 Both mounted stents are seen over the areas of stenosis in the RML and RLL, before balloon dilation. Note simultaneous balloon placement in the RUL. Also demonstrated is the “sheath-within-sheath” technique for access to right lower pulmonary artery. RLL = right lower lobe; RML = right middle lobe; RUL = right upper lobe
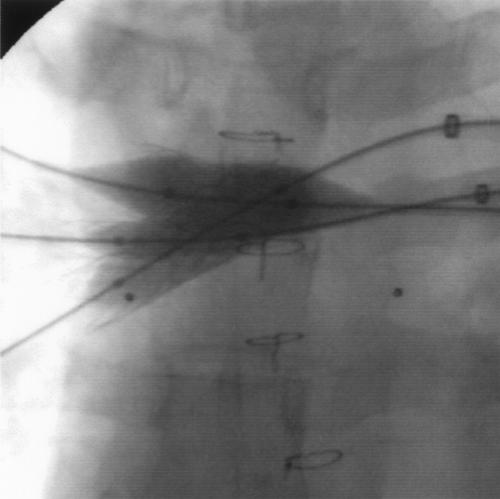
Fig. 3 Simultaneous dilation of both new stents and of the previously implanted RUL stent. RUL = right upper lobe
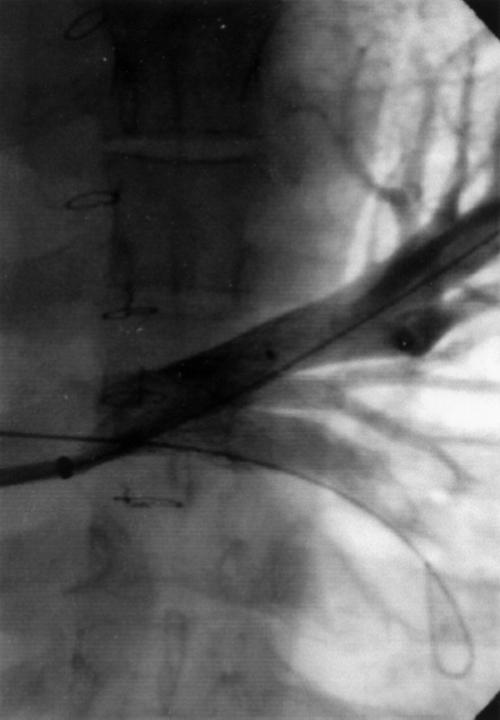
Fig. 4 Angiogram following balloon dilation and double-stent implantation.
Technique of Right Femoral Vein Recannulation
Angiography documented right femoral vein occlusion. An 8-100 Glide® catheter (Boston Scientific) was advanced into the left femoral vein and deflected as far distal as possible into the obstructed right femoral vein (Fig. 5A). A 0.021-inch AMC needle (Maxxim/Argon-Athens; Athens, Tex) was introduced through the right groin and, under fluoroscopic guidance, was introduced into the lumen of the Glide catheter (Fig. 5B). An 0.018-inch Microvena® wire (Microvena Corp.; Whitebear, Minn) was introduced through the AMC needle into the Glide catheter and then pulled through the catheter, out of the LFV (Fig. 5C). The AMC needle was removed. A 6-F dilator was placed over the wire (Fig. 5D), and the Glide catheter was gradually withdrawn. The wire and Glide catheter were removed. Contrast medium injected through the dilator revealed the area of stenosis. A 0.035-inch Superstiff wire was placed through the dilator, and a 10-F short TSS was exchanged over the wire. A UDT 10-4-5.8-120 balloon was mounted with a Palmaz PS-564 stent and advanced over the 0.035-inch wire into the 10-F TSS placed in the right femoral vein. At the site of venous obstruction, we inflated the balloon to 12 atmospheres for 14 seconds (Fig. 6A). There was some residual venous obstruction distal to the stent, so a 2nd UDT 10-4-5.8-120 balloon was mounted with a Palmaz PS-564 stent and was inflated distal to the 1st stent, with approximately 0.5 cm of stent overlap.
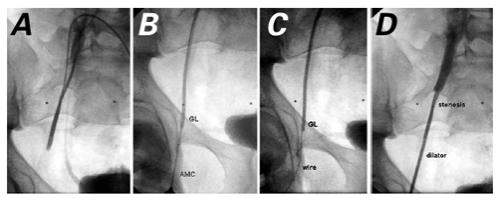
Fig. 5 A) Contrast injection through the Glide® catheter reveals right femoral vein occlusion. B) AMC needle is being placed into the Glide catheter under fluoroscopic guidance. C) An 0.018-inch Microvena® wire is passed into the Glide catheter. D) A 6-F dilator is passed over the Microvena wire.
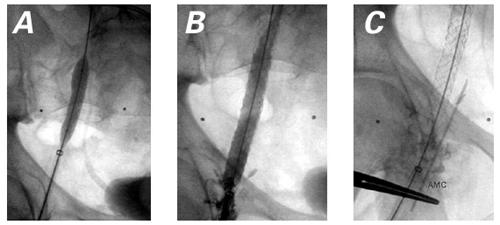
Fig. 6 A) Balloon dilation of stent in the RFV. B) Contrast injection in the RFV following stent implantation demonstrates widely patent RFV with mild contrast extravasation. C) Cine of the AMC needle site for reference, for future percutaneous procedure. RFV = right femoral vein
Angiography after stenting showed satisfactory positioning of the stents, together with a widely patent femoral vein (approximately 7 to 8 mm in diameter) and some mild extravasation of contrast medium into the right groin (Fig. 6B). A cineangiogram was performed with the needle outside the body, placed in the position where an AMC needle would, if necessary, be introduced to obtain access in future catheterizations (Fig. 6C).
Discussion
Over the last decade, stent implantation has become a widely accepted, effective therapy for the treatment of pulmonary arterial stenoses. 1,7,8 To our knowledge, this is the 1st report of double pulmonary artery stent implantation with simultaneous balloon redilation of a previously placed stent. The “sheath-within-sheath” technique to gain stent-and-balloon access to one of the branches has also not been described. Given this patient's previous intervention, simultaneous inflation of all 3 stents was mandatory to avoid collapse or proximal distortion of the previous overlapping right middle and upper lobe stents, and to avoid perforation of one of the balloons. In certain cases, the pulmonary artery branch has an acute angle that makes access for stent implantation difficult. The use of the sheath-within-sheath technique enabled the operator to traverse the acute angle of the arterial branch without compromising the integrity of the balloon or stent.
Repeated cardiac catheterization is often associated with unilateral or bilateral femoral venous occlusion. 9–11 In such cases, operators might need to use other routes of vascular access, such as jugular venous, transhepatic, 9 and, more recently, translumbar 12 vascular access. Although repeated success has been reported with each of these routes, each possesses the potential for specific sequelae not encountered with femoral venous cannulation. These include pneumo-thorax (jugular venous), 10 intraperitoneal hemorrhage, hepatic injury (transhepatic), 11 and injury to the kidney and bowel (translumbar). 12 We advocate ensuring unilateral femoral venous access with femoral venous stenting in patients who are likely to require further catheterizations, because the development of bilateral femoral obstruction commits the operator to potentially more complicated alternative routes. Application of the principle of needle-guided sheath-and-catheter access proximal to the femoral venous obstruction was uncomplicated in this patient and enabled successful femoral venous stent implantation, together with preservation of bilateral femoral access.
Footnotes
Address for reprints: Colin J. McMahon, MB, BAO, BCh, MRCPI, Division of Pediatric Cardiology, Texas Children's Hospital, 6621 Fannin, MC 2-2280, Houston, TX 77030
References
- 1.Mullins CE, O'Laughlin MP, Vick GW, Mayer DC, Myers TJ, Kearney DL, et al. Implantation of balloon-expandable intravascular grafts by catheterization in pulmonary arteries and systemic veins. Circulation 1988;77:188–99. [DOI] [PubMed]
- 2.Magee AG, Brzezinska-Rajszys G, Qureshi SA, Rosenthal E, Zubrzycka M, Ksiazyk J, Tynan M. Stent implantation for aortic coarctation and recoarctation. Heart 1999;82:600–6. [DOI] [PMC free article] [PubMed]
- 3.Ward CJ, Mullins CE, Nihill MR, Grifka RG, Vick GW. Use of intravascular stents in systemic venous and systemic venous baffle obstructions. Short-term follow-up results. Circulation 1995;91:2948–54. [DOI] [PubMed]
- 4.Powell AJ, Lock JE, Keane JF, Perry SB. Prolongation of RV-PA conduit life span by percutaneous stent implantation. Intermediate-term results. Circulation 1995;92:3282–8. [DOI] [PubMed]
- 5.Ing FF, Grifka RG, Nihill MR, Mullins CE. Repeat dilation of intravascular stents in congenital heart defects. Circulation 1995;92:893–7. [DOI] [PubMed]
- 6.Cullen S, Ho SY, Shore D, Lincoln C, Redington A. Congenital stenosis of the pulmonary veins—failure to modify natural history by intraoperative placement of stents. Cardiol Young 1994;4:395–8.
- 7.Schaffer KM, Mullins CE, Grifka RG, O'Laughlin MP, McMahon W, Ing FF, Nihill MR. Intravascular stents in congenital heart disease: short-and long-term results from a large single-center experience. J Am Coll Cardiol 1998;31:661–7. [DOI] [PubMed]
- 8.Redington AN, Weil J, Somerville J. Self expanding stents in congenital heart disease. Br Heart J 1994;72:378–83. [DOI] [PMC free article] [PubMed]
- 9.Shim D, Lloyd TR, Cho KJ, Moorehead CP, Beekman RH. Transhepatic cardiac catheterization in children. Evaluation of efficacy and safety. Circulation 1995;92:1526–30. [DOI] [PubMed]
- 10.Anderson JL, Marshall HW. The femoral venous approach to endomyocardial biopsy: comparison with internal jugular and transarterial approaches. Am J Cardiol 1984;53:833–7. [DOI] [PubMed]
- 11.Erenberg FG, Shim D, Beekman RH. Intraperitoneal hemorrhage associated with transhepatic cardiac catheterization: a report of two cases. Cathet Cardiovasc Diagn 1998;43:177–8. [DOI] [PubMed]
- 12.Cheatham JP, McCowan TC, Fletcher SE. Percutaneous translumbar cardiac catheterization and central venous line insertion: an alternative approach in children with congenital heart disease. Catheter Cardiovasc Interv 1999;46:187–92. [DOI] [PubMed]


