Abstract
Leber hereditary optic neuropathy (LHON) is an important example of mitochondrial blindness with the m.11778G>A mutation in the MT-ND4 gene being the most common disease-causing mtDNA variant worldwide.
The REFLECT phase 3 pivotal study is a randomized, double-masked, placebo-controlled trial investigating the efficacy and safety of bilateral intravitreal injection of lenadogene nolparvovec in patients with a confirmed m.11778G>A mutation, using a recombinant adeno-associated virus vector 2, serotype 2 (rAAV2/2-ND4). The first-affected eye received gene therapy; the fellow (affected/not-yet-affected) eye was randomly injected with gene therapy or placebo. The primary end point was the difference in change from baseline of best-corrected visual acuity (BCVA) in second-affected/not-yet-affected eyes treated with lenadogene nolparvovec versus placebo at 1.5 years post-treatment, expressed in logarithm of the minimal angle of resolution (LogMAR).
Forty-eight patients were treated bilaterally and 50 unilaterally. At 1.5 years, the change from baseline in BCVA was not statistically different between second-affected/not-yet-affected eyes receiving lenadogene nolparvovec and placebo (primary end point). A statistically significant improvement in BCVA was reported from baseline to 1.5 years in lenadogene nolparvovec-treated eyes: −0.23 LogMAR for the first-affected eyes of bilaterally treated patients (P < 0.01); and −0.15 LogMAR for second-affected/not-yet-affected eyes of bilaterally treated patients and the first-affected eyes of unilaterally treated patients (P < 0.05). The mean improvement in BCVA from nadir to 1.5 years was −0.38 (0.052) LogMAR and −0.33 (0.052) LogMAR in first-affected and second-affected/not-yet-affected eyes treated with lenadogene nolparvovec, respectively (bilateral treatment group). A mean improvement of −0.33 (0.051) LogMAR and −0.26 (0.051) LogMAR was observed in first-affected lenadogene nolparvovec-treated eyes and second-affected/not-yet-affected placebo-treated eyes, respectively (unilateral treatment group). The proportion of patients with one or both eyes on-chart at 1.5 years was 85.4% and 72.0% for bilaterally and unilaterally treated patients, respectively. The gene therapy was well tolerated, with no systemic issues. Intraocular inflammation, which was mostly mild and well controlled with topical corticosteroids, occurred in 70.7% of lenadogene nolparvovec-treated eyes versus 10.2% of placebo-treated eyes. Among eyes treated with lenadogene nolparvovec, there was no difference in the incidence of intraocular inflammation between bilaterally and unilaterally treated patients.
Overall, the REFLECT trial demonstrated an improvement of BCVA in LHON eyes carrying the m.11778G>A mtDNA mutation treated with lenadogene nolparvovec or placebo to a degree not reported in natural history studies and supports an improved benefit/risk profile for bilateral injections of lenadogene nolparvovec relative to unilateral injections.
Keywords: lenadogene nolparvovec, leber hereditary optic neuropathy, mitochondrial DNA, NADH dehydrogenase 4, recombinant adeno-associated virus vector 2
Newman et al. report that bilateral injection of lenadogene nolparvovec improves vision in patients carrying the m.11778G>A MT-ND4 mutation causing Leber hereditary optic neuropathy, with a larger treatment effect in patients who received bilateral treatment and an excellent safety profile.
Introduction
Leber hereditary optic neuropathy (LHON) is a blinding maternally-inherited mitochondrial genetic disease. Retinal ganglion cells (RGCs), whose axons form the optic nerve, are the major cellular target affected by the resultant mitochondrial dysfunction.1 LHON classically manifests as acute to sub-acute, bilateral, painless central vision loss, often with sequential onset. There is a male predominance of about 80% and patients typically become affected between age 15 and 35 years.2,3
Approximately 90% of all LHON cases are caused by one of three point mutations in the mtDNA: m.3460G>A in MT-ND1, m.11778G>A in MT-ND4 and m.14484T>C in MT-ND6.4 A primary mtDNA mutation is necessary, but not sufficient to cause vision loss, as there is well-documented incomplete penetrance with 20–50% of male and 4–20% of female carriers manifesting the clinical disease during their lifetime.5
The m.11778G>A mutation is the most common LHON mutation, accounting for about 70% of LHON in North America and Europe.1,3,4 Genotype is the most significant prognostic factor of visual outcome, followed by the age at onset of vision loss. The m.11778G>A mutation causes a severe clinical form of LHON, with multiple natural history studies in MT-ND4 LHON patients confirming rare and poor recovery, although younger age at onset, especially onset at less than 12 years, portends a relatively better visual prognosis.2,3,6
Over the past decade, substantial progress has been made in the application of gene therapy to monogenic blinding diseases, with the first treatment approved by both American and European regulatory agencies for an inherited retinal degenerative disorder, Leber congenital amaurosis caused by biallelic RPE65 mutations.7 Gene therapy in mitochondrial disorders is challenging, as the wild-type protein needs to reach the mitochondrial matrix compartment by crossing both the mitochondrial outer and inner membranes, and then assemble within mature complex I, competing with the mutant ND4 protein that is still synthesized locally by mitoribosomes. The allotopic expression strategy involves the nuclear expression of the wild-type mitochondrial gene engineered with an additional mitochondrial targeting sequence (MTS) and results in mRNA translation and co-translocation of the protein into mitochondria.8 This strategy has been successfully applied in cell models and safely translated in induced rodent LHON models with preservation of RGCs and visual function.9–12
Lenadogene nolparvovec is a replication-defective single-stranded DNA recombinant adeno-associated virus vector 2, serotype 2 (rAAV2/2), containing a codon-modified complementary DNA (cDNA) that encodes for the human wild-type ND4 protein, under the control of the cytomegalovirus (CMV) immediate early promoter in an intron-containing expression cassette (beta globin intron, HBB2), flanked by the AAV inverted terminal repeats. The construct includes the cis-acting elements of the human cytochrome c oxidase 10 (COX10) mtRNA (MTS in 5′ of the cDNA, and 3′ UTR at the 3′ end of the cDNA) ensuring the efficient delivery of the corresponding hybrid mRNA to the mitochondrial surface.12,13 Expression of the transgene results in the synthesis of a functional human ND4 protein that translocates into the mitochondrial compartment. Serotype 2 (rAAV2/2) was chosen because it results in highly efficient transduction of the inner retinal layers, including RGCs which are the major target cells affected in LHON.14 Lenadogene nolparvovec gene therapy proposes to permanently correct the underlying mtDNA mutation, based on the allotopic nuclear expression of the wild-type ND4 subunit of complex I targeted to mitochondria. Lenadogene nolparvovec is delivered via an intravitreal injection (IVT) for the optimal transduction of RGCs.
The clinical development of lenadogene nolparvovec consists of one phase 1/2 study, REVEAL,15 three phase 3 pivotal studies, REVERSE,16–18 RESCUE17–19 and REFLECT, and a long-term follow-up study, RESTORE.18,20 RESCUE and REVERSE assessed the efficacy and safety of an unilateral IVT of lenadogene nolparvovec in patients carrying the m.11778G>A mutation with vision loss ≤1 year and followed for 2 years after treatment.16,19 Patients who completed the RESCUE and REVERSE studies are being followed in the ongoing extension study, RESTORE, and results at 3 years post-treatment have been reported.20
In the previously published studies, lenadogene nolparvovec was exclusively administered as a unilateral IVT injection. Given that LHON is a bilateral disease that rapidly affects both eyes of a patient, REFLECT was conducted to assess the efficacy and safety of bilateral IVT of lenadogene nolparvovec. Here, we report the results of the primary analyses for REFLECT at 1.5 years post-treatment.
Materials and methods
Study design
REFLECT (ClinicalTrials.gov NCT03293524) is a phase 3, pivotal, randomized, double-masked, placebo-controlled study that was conducted in 13 centres across seven countries (Belgium, France, Italy, Spain, Taiwan and UK each had one centre and the USA had seven centres). The study included LHON patients with vision loss ≤1 year in one or both eyes caused by the m.11778G>A MT-ND4 mutation. Patients were randomized to one of two treatment arms. In both treatment arms, the first-affected eye received an IVT of lenadogene nolparvovec. The second-affected/not-yet-affected eye was randomized to treatment with either an IVT of lenadogene nolparvovec or placebo (balanced salt solution). In patients reporting simultaneous onset of vision loss, the second-affected eye was randomly selected. Patients and study personnel were unaware whether they were receiving unilateral or bilateral active drug, or which eye was treated in the unilateral cases.
This study was designed and conducted in accordance with the ethical principles established in the Declaration of Helsinki (7th revision, 2013), with the principles of Good Clinical Practice according to the International Council for Harmonization guideline [ICH E6(R2), 2016], as well as with applicable regulatory requirements. The protocol was reviewed and approved by independent ethics committees at all centres. Written informed consent at the Screening Visit (Visit 1) was obtained prior to the patient entering the study and before initiation of any study-related procedure.
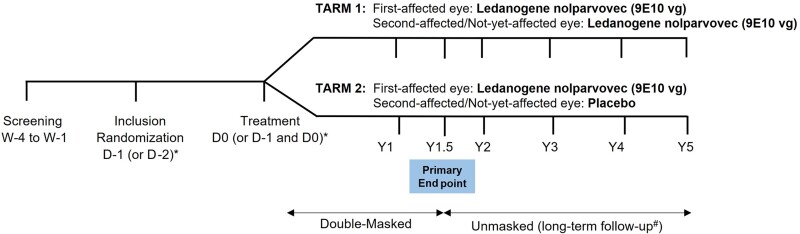
An interactive web response system was used to enroll and randomize the patients in the study. Upon confirmation of eligibility for inclusion, patients were randomized following simple randomization procedures (computerized random numbers) to treatment arm 1 (bilateral treatment, TARM1) or treatment arm 2 (unilateral treatment, TARM2) in a 1:1 allocation according to a predefined central randomization scheme (Fig. 1). Bilaterally lenadogene nolparvovec-treated patients received lenadogene nolparvovec IVT in both eyes at a dose of 9 × 1010 vector genomes (vg) in 90 µl for each eye. Unilaterally lenadogene nolparvovec-treated patients received lenadogene nolparvovec IVT in their first-affected eye (9 × 1010 vg in 90 µl) and placebo IVT (90 µl) in their second-affected/not-yet-affected eye. The dose of lenadogene nolparvovec selected in REFLECT (9 × 1010 vg/eye) was the maximal tolerated dose determined in the previous phase 1/2a REVEAL study.15,21 This dose was also selected in the phase 3 studies RESCUE and REVERSE.16,19
Figure 1.
REFLECT study design. Asterisk indicates the allocated study treatment was administered on the same day (Day 0) or on two consecutive days (Day −1 and Day 0) at the investigator’s discretion. In all cases, the initial treatment administration had to be performed the day following the Inclusion visit; thus, the Inclusion visit was performed on Day −1 if eyes were treated on the same day, or on Day −2 if eyes were treated on 2 consecutive days. #Participation in the long-term follow-up phase of the study up to Year 5 was/will be sought at the Year 2 visit with a separate informed consent. For patients who did not consent to participate in the long-term follow-up period, the end-of-study visit was the Year 2 visit. D = day; W = week; Y = year.
Each eye underwent administration of the allocated treatment as a single IVT. Pre-IVT procedures included pupil dilation, topical antisepsis and anaesthesia. Administration of an intraocular pressure (IOP) lowering agent of the investigator’s choice preceded all IVTs. In addition, all patients received a peri-treatment, systemic immune modulating corticoid regimen (a 28-day oral corticosteroid therapy starting 2 days before the first IVT) to ameliorate the potential ocular inflammation related to the IVT of lenadogene nolparvovec.
Additional details on the study design are presented in the Supplementary material.
Outcome measures
Ocular and vision evaluations included assessment of best-corrected visual acuity (BCVA) using the Early Treatment Diabetic Retinopathy Study (ETDRS) chart at 1 or 4 m, slit lamp biomicroscopy, applanation tonometry, fundoscopy, contrast sensitivity (CS) assessed with the Pelli-Robson Low Vision Contrast Sensitivity chart,22 Humphrey visual field (HVF) perimetry, spectral domain optical coherence tomography (SD-OCT), and colour fundus photographs. Quality of life assessments were conducted using the National Eye Institute Visual Functioning Questionnaire 25 (VFQ-25).23
Additional details on the study outcome measures are provided in the Supplementary material.
Statistical analyses
Statistical analyses were conducted using SAS® software v9.4 (SAS Institute, Cary, NC).
The primary efficacy end point was the difference of change from baseline to 1.5 years post-treatment in BCVA between second-affected/not-yet-affected lenadogene nolparvovec- and placebo-treated eyes in ND4 LHON patients. Logarithm of the minimal angle of resolution (LogMAR) BCVA was used to represent BCVA. A difference of −0.3 LogMAR (15 ETDRS letters equivalent) was considered clinically significant based on US Food and Drug Administration recommendations.
Additional details on the statistical methods are presented in the Supplementary material.
Data availability
The data supporting the results of this study and the trial protocol are available upon reasonable request to the corresponding author.
Results
Patient disposition and follow-up
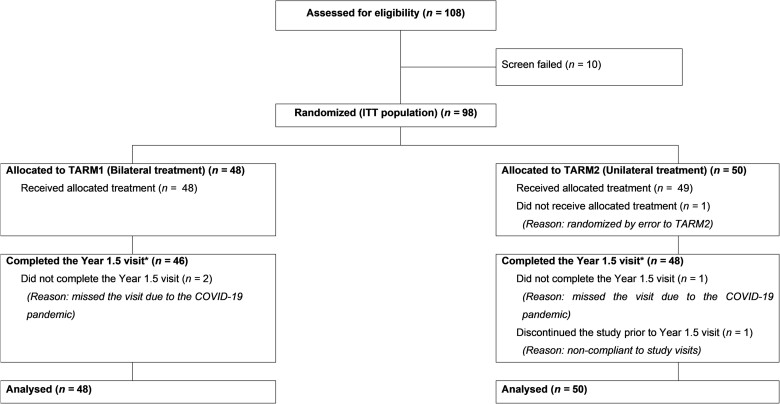
From March 2018 to June 2019, a total of 108 patients were screened for study eligibility and 98 were randomized to either the bilateral-treated group (48 patients) or unilateral-treated group (50 patients). The main reasons for non-inclusion were the presence of eye diseases, excluding LHON, which may interfere with ocular assessments, patient decision not to stop idebenone, or inability to comply with all protocol requirements. All 98 randomized patients received the study treatment. One bilaterally treated patient was randomized by error to the unilateral group [included in the unilateral Group for Intent-to-treat (ITT)/Efficacy analysis]. At the data cut-off date for this interim analysis (April 13, 2021), 94 (95.9%) of the 98 randomized patients had completed the Year 1.5 visit, and three patients were still ongoing but had not completed the Year 1.5 visit. These three patients missed their Year 1.5 visit due to the COVID-19 pandemic and were considered as having major protocol deviation. One patient was withdrawn before the Year 1.5 visit due to lack of compliance with study visits (Fig. 2).
Figure 2.
Participants flow at the 1.5 year interim analysis. TARM1: First-affected eye and second-affected/not-yet-affected eye administered lenadogene nolparvovec. TARM2: First-affected eye administered lenadogene nolparvovec, second-affected/not-yet-affected eye administered placebo. *Taking in account reassigned visit at Year 1.5 as per the rules described in the ‘Statistical analyses’ section.
Demographic and baseline disease characteristics of the study population
Demographic and baseline disease characteristics for the ITT population were comparable between the treatment arms (Table 1). The overall mean age of patients was 31.5 years at onset of vision loss (range: 14–73 years) and 32.1 years at screening (age 15–74 years). Most patients were male (79.6%) and were randomized at a study site located in the USA (57.1%). The paediatric population (aged 15–17 years at screening) comprised 10 patients.
Table 1.
Baseline demographics and disease characteristics (ITT population)
| Statistic | TARM1 Bilateral treatment |
TARM2 Unilateral treatment |
Total | |
|---|---|---|---|---|
| (N = 48) | (N = 50) | (N = 98) | ||
| Age, years | Mean (SD) | 32.4 (14.4) | 31.9 (13.4) | 32.1 (13.8) |
| Median | 27.0 | 29.5 | 28.5 | |
| Min, Max | 15, 74 | 15, 65 | 15, 74 | |
| Gender | ||||
| Male | n (%) | 37 (77.1) | 41 (82.0) | 78 (79.6) |
| Female | n (%) | 11 (22.9) | 9 (18.0) | 20 (20.4) |
| Region | ||||
| Asia | n (%) | 8 (16.7) | 7 (14.0) | 15 (15.3) |
| Europe | n (%) | 11 (22.9) | 16 (32.0) | 27 (27.6) |
| USA | n (%) | 29 (60.4) | 27 (54.0) | 56 (57.1) |
| Affected eye status a | ||||
| Bilateral | n (%) | 47 (97.9) | 50 (100.0) | 97 (99.0) |
| Unilateral | n (%) | 1 (2.1) | 0 | 1 (1.0) |
| Duration of disease, months | n | 48 | 50 | 98 |
| Mean (SD) | 8.33 (3.36) | 8.27 (3.09) | 8.30 (3.20) | |
| Median | 8.85 | 8.85 | 8.85 | |
| Min, Max | 1.7, 11.9 | 2.4, 11.9 | 1.7, 11.9 | |
| Time interval of vision loss between first and second-affected eyes, days | n | 48 | 50 | 98 |
| Mean (SD) | 56.85 (66.34) | 61.88 (54.08) | 59.42 (60.14) | |
| Median | 33.50 | 59.50 | 46.00 | |
| Min, Max | 0.0; 266 | 0.0; 197 | 0.0; 266 | |
| Baseline LogMAR BCVA of first-affected eye | n | 48 | 50 | 98 |
| Mean (SD) | 1.59 (0.47) | 1.68 (0.43) | 1.64 (0.45) | |
| Median | 1.50 | 1.60 | 1.60 | |
| Min, Max | 0.6; 2.3 | 0.8; 2.3 | 0.6; 2.3 | |
| Baseline LogMAR BCVA of second/not-yet-affected eye | n | 48 | 50 | 98 |
| Mean (SD) | 1.44 (0.51) | 1.50 (0.46) | 1.47 (0.48) | |
| Median | 1.40 | 1.50 | 1.40 | |
| Min, Max | 0.0; 2.3 | 0.7; 2.3 | 0.0; 2.3 |
TARM1: First-affected eye and second-affected/not-yet-affected eye administered lenadogene nolparvovec. TARM2: First-affected eye administered lenadogene nolparvovec, second-affected/not-yet-affected eye administered placebo. N = number of patients in the ITT population; n = number of patients; % = percentage of patients calculated relative to N.
Bilateral status is defined as LogMAR > 0 for both eyes on the day of injection. Unilateral status is defined as LogMAR = 0 for one of the two eyes on the day of injection.
All but one patient had bilateral vision loss at baseline. Mean [standard deviation (SD)] duration of disease (i.e. duration of vision loss for the first-affected eye) was 8.3 (3.2) months (range: 1.7–11.9 months), in line with the inclusion criteria requiring vision loss duration ≤1 year for study eligibility. The mean (SD) time interval of vision loss between the first- and second-affected eye was 2.0 (2.0) months (range: 0.0–8.7 months). Mean (SD) baseline values for LogMAR BCVA were 1.64 (0.45) for first-affected eyes, and 1.47 (0.48) for second/not-yet-affected eyes.
Efficacy data
Best-corrected visual acuity
The mean (SD) changes in BCVA from baseline to 1.5 years was −0.09 (0.072) and −0.04 (0.071) LogMAR for the second/not-yet-affected lenadogene nolparvovec- and second/not-yet-affected placebo-treated eyes, respectively (Table 2. The least squares (LS) mean difference in the change of BCVA between these two treatment arms at 1.5 years was −0.05 LogMAR [P = 0.6080, analysis of covariance (ANCOVA)]. The primary end point, defined as a difference of at least −0.3 LogMAR, was therefore not met.
Table 2.
First-affected and second-affected/not-yet-affected eyes: change from baseline to 1.5 years in LogMAR BCVA—observed data and ANCOVA analysis (ITT population)
| TARM1 (Bilateral treatment) | TARM2 (Unilateral treatment) | TARM1 (Bilateral treatment) | TARM2 (Unilateral treatment) | ||
|---|---|---|---|---|---|
| First-affected eye | Second/not-yet-affected eye | ||||
| Statistic | Lenadogene nolparvovec (N = 48) |
Lenadogene nolparvovec (N = 50) |
Lenadogene nolparvovec (N = 48) |
Placebo (N = 50) |
|
| Baseline | n | 48 | 50 | 48 | 50 |
| Mean (SD) | 1.59 (0.470) | 1.68 (0.432) | 1.44 (0.512) | 1.50 (0.455) | |
| Year 1.5 (Visit 12) | n a | 48 | 50 | 48 | 50 |
| Observed | Mean (SD) | 1.34 (0.527) | 1.46 (0.587) | 1.35 (0.585) | 1.45 (0.604) |
| Change from baseline (observed) | Mean (SD) | −0.25 (0.468) | −0.22 (0.412) | −0.08 (0.568) | −0.05 (0.467) |
| Estimates from ANCOVAb | LS mean (SE) [95% CI] |
−0.26 (0.063) [−0.38, −0.13] |
−0.21 (0.061) [−0.33, −0.09] |
−0.09 (0.072) [−0.24, 0.05] |
−0.04 (0.071) [−0.18, 0.10] |
|
P-value H0: LS mean = 0 |
<0.0001 | 0.0008 | 0.1955 | 0.5536 | |
| Lenadogene nolparvovec versus placebo | LS mean difference (SE) [95% CI] | − |
−0.05 (0.101) [−0.25, 0.15] |
||
| P-value | − | 0.6080 | |||
TARM1: First-affected eye and second-affected/not-yet-affected eye administered lenadogene nolparvovec. TARM2: First-affected eye administered lenadogene nolparvovec, second-affected/not-yet-affected eye administered placebo. For patients whose LogMAR evaluation date was out of the visit 12 window (518–578 days post-treatment), the first LogMAR value after visit 12 (578 days post-treatment) was used. CI = confidence interval; N = number of patients in the ITT population; n = number of patients; SE = standard error.
Missing data were imputed using the last observation carried Fforward method.
An ANCOVA model was used to model change from baseline to 1.5 years (visit 12) for LogMAR BCVA, using baseline LogMAR as covariate and treatment as fixed effect.
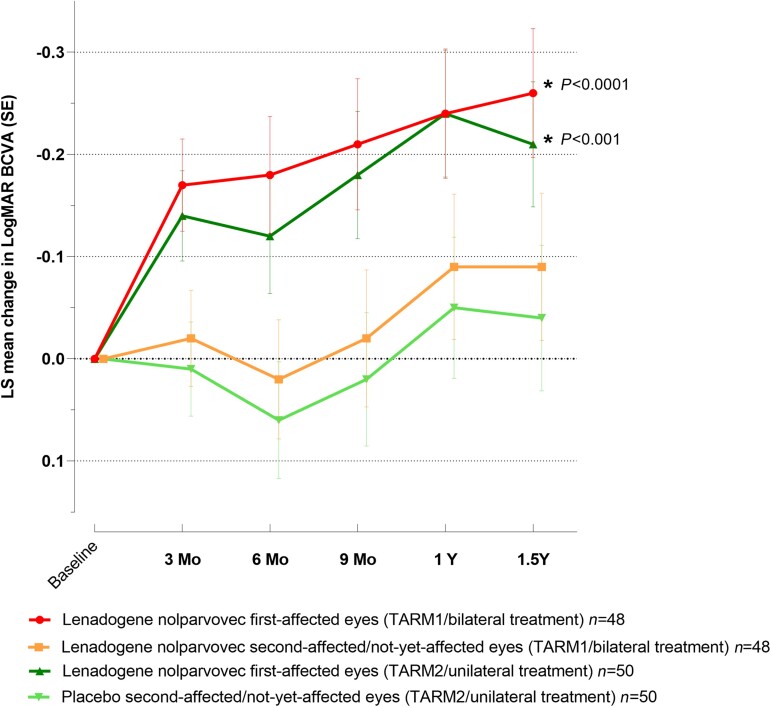
Using the ANCOVA model, the change in mean BCVA from baseline to 1.5 years of the first-affected lenadogene nolparvovec-treated eyes was −0.26 (0.063) LogMAR (+13 ETDRS letters equivalent) for bilaterally treated patients and −0.21 (0.061) LogMAR (+11 ETDRS letters equivalent) for unilaterally treated patients (P < 0.0001 and P < 0.001, respectively), whereas the improvement from baseline of the second/not-yet-affected lenadogene nolparvovec- and second/not-yet-affected placebo-treated eyes was not statistically significant (Table 2). The time course of the mean change in LogMAR BCVA estimated by ANCOVA model is shown in Fig. 3. When applying a linear mixed model which was a pre-defined sensitivity analysis, all eye groups showed an improvement in LS mean BCVA at 1.5 years versus baseline, which was statistically significant for the three groups of eyes treated with lenadogene nolparvovec, but not for the placebo-treated eyes. In the first-affected eyes of patients treated bilaterally with lenadogene nolparvovec, the difference in the change between baseline and 1.5 years was −0.23 (0.070) LogMAR (+12 ETDRS letters equivalent) (P < 0.01) (Supplementary Table 1). The change in mean BCVA from baseline to 1.5 years between second-affected/not-yet-affected eyes treated bilaterally, and first-affected eyes of patients treated unilaterally with lenadogene nolparvovec was −0.15 (0.070) LogMAR and −0.15 (0.069) LogMAR (+8 ETDRS letters equivalent) (P < 0.05), respectively (Supplementary Table 1). A mean difference of −0.08 (0.069) LogMAR (+4 ETDRS letters equivalent) was observed in second-affected/not-yet-affected placebo eyes of patients treated unilaterally with lenadogene nolparvovec. The time course of the mean change in LogMAR BCVA estimated by linear mixed model is shown in Supplementary Fig. 1.
Figure 3.
Time course of LS mean change in LogMAR BCVA from baseline to 1.5 years for first-affected and second-affected/not-yet-affected eyes—estimated by ANCOVA model (ITT population). An ANCOVA model (considering both eyes of each patient) was used to model the change from baseline to time point of interest for LogMAR BCVA, using baseline LogMAR as covariate and treatment as fixed effect. Data are shown as LS mean.
A nadir BCVA value was obtained for each eye between baseline and 1.5 years. The mean time from baseline to nadir in BCVA was 8.2 weeks for first-affected eyes (pooled eyes treated with lenadogene nolparvovec), 11.8 weeks for second/not-yet-affected eyes treated with lenadogene nolparvovec and 11.2 weeks for placebo-treated eyes. The mean nadir was LogMAR +1.71 and LogMAR +1.66 for first-affected and second-affected/not-yet-affected eyes treated with lenadogene nolparvovec, respectively (TARM1). The mean nadir was LogMAR +1.82 and LogMAR +1.70 for first-affected lenadogene nolparvovec- and second-affected/not-yet-affected placebo-treated eyes, respectively (TARM2). All eye groups showed a statistically significant and clinically relevant24 improvement in LS mean LogMAR BCVA at 1.5 years versus nadir by ANCOVA and linear mixed models (P < 0.0001) (Tables 3 and 4). Using the ANCOVA model, the mean improvement in BCVA from nadir to 1.5 years was −0.38 (0.050) LogMAR and −0.31 (0.042) LogMAR (+19 ETDRS and +16 ETDRS letters equivalent) in first-affected and second-affected/not-yet-affected eyes treated with lenadogene nolparvovec, respectively (TARM1). A mean improvement of −0.36 (0.049) LogMAR and −0.25 (0.041) LogMAR (+18 ETDRS and +13 ETDRS letters equivalent) was observed in first-affected lenadogene nolparvovec- and second-affected/not-yet-affected placebo-treated eyes, respectively (TARM2) (Table 3). Using the linear mixed model, the mean improvement in BCVA from nadir to 1.5 years was −0.38 (0.052) LogMAR (+19 ETDRS letters equivalent) and −0.33 (0.052) LogMAR (+17 ETDRS letters equivalent) in first-affected and second-affected/not-yet-affected eyes treated with lenadogene nolparvovec, respectively (TARM1). A mean improvement of −0.33 (0.051) LogMAR (+17 ETDRS letters equivalent) and −0.26 (0.051) LogMAR (+13 ETDRS letters equivalent) was observed in first-affected lenadogene nolparvovec-treated eyes and second-affected/not-yet-affected placebo-treated eyes, respectively (TARM2) (Table 4).
Table 3.
First-affected and second-affected/not-yet-affected eyes: change from nadir to 1.5 years in LogMAR BCVA—observed data and ANCOVA analysis (ITT population)
| TARM1 (Bilateral treatment) | TARM2 (Unilateral treatment) | ||||
|---|---|---|---|---|---|
| Statistic | First-affected eye | Second/not-yet-affected eye | First-affected eye | Second/not-yet-affected eye | |
| Lenadogene nolparvovec (N = 48) |
Lenadogene nolparvovec (N = 48) |
Lenadogene nolparvovec (N = 50) |
Placebo (N = 50) |
||
| Nadir | n | 48 | 48 | 50 | 50 |
| Mean (SD) | 1.71 (0.46) | 1.66 (0.46) | 1.82 (0.53) | 1.70 (0.44) | |
| Year 1.5 (Visit 12) | n a | 48 | 48 | 50 | 50 |
| Observed | Mean (SD) | 1.34 (0.53) | 1.35 (0.59) | 1.46 (0.59) | 1.45 (0.60) |
| Change from nadir (Observed) | Mean (SD) | −0.37 (0.29) | −0.31 (0.32) | −0.37 (0.40) | −0.25 (0.28) |
| Estimates from ANCOVAb | LS mean (SE) [95% CI] |
−0.38 (0.050) [−0.48, −0.28] |
−0.31 (0.042) [−0.39, −0.23] |
−0.36 (0.049) [−0.46, −0.26] |
−0.25 (0.041) [−0.34, −0.17] |
|
P-value H0: LS mean = 0 |
<0.0001 | <0.0001 | <0.0001 | <0.0001 | |
TARM1: First-affected eye and second-affected/not-yet-affected eye administered lenadogene nolparvovec. TARM2: First-affected eye administered lenadogene nolparvovec, second-affected/not-yet-affected eye administered placebo. For patients whose LogMAR evaluation date was out of the visit 12 window (518–578 days post-treatment), the first LogMAR value after visit 12 (578 days post-treatment) was used. The nadir was defined as the worst value observed from baseline to the post-treatment time point of interest, including baseline and the post-treatment time point values. CI = confidence interval; N = number of patients in the ITT population; n = number of patients; SE = standard error.
Missing data were imputed using the last observation carried forward method.
An ANCOVA model was used to model change from nadir to 1.5 years (visit 12) for LogMAR BCVA, using nadir LogMAR as covariate and treatment as fixed effect.
Table 4.
First-affected and second-affected/not-yet-affected eyes: change from nadir to 1.5 years in LogMAR BCVA–estimated by linear mixed model (ITT population)
| TARM1 (Bilateral treatment) | TARM2 (Unilateral treatment) | ||||
|---|---|---|---|---|---|
| Statistic | First-affected eye | Second/Not-yet-affected eye | First-affected eye | Second/Not-yet-affected eye | |
| Lenadogene nolparvovec (N = 48) |
Lenadogene nolparvovec (N = 48) |
Lenadogene nolparvovec (N = 50) |
Placebo (N = 50) |
||
| Nadir | n | 48 | 48 | 50 | 50 |
| Mean (SD) | 1.71 (0.46) | 1.66 (0.46) | 1.82 (0.53) | 1.70 (0.44) | |
| Year 1.5 (Visit 12) | n a | 48 | 48 | 50 | 50 |
| Estimates from linear mixed modelb | LS mean (SE) [95% CI] |
−0.38 (0.052) [−0.48, −0.27] |
−0.33 (0.052) [−0.44, −0.23] |
−0.33 (0.051) [−0.44, −0.23] |
−0.26 (0.051) [−0.36, −0.16] |
|
P-value H0: LS mean = 0 |
<0.0001 | <0.0001 | <0.0001 | <0.0001 | |
TARM1: First-affected eye and second-affected/not-yet-affected eye administered lenadogene nolparvovec. TARM2: First-affected eye administered lenadogene nolparvovec, second-affected/not-yet-affected eye administered placebo. The nadir was defined as the worst value observed from baseline to the post-treatment time point of interest, including baseline and the post-treatment time point values. N = number of patients in the ITT population; n = number of patients; SE = standard error.
Missing data were imputed using the last observation carried forward method.
A linear mixed model (considering both eyes of each patient) was used to model the change from baseline to 1.5 years (visit 12) for LogMAR BCVA, using treatment and baseline value as fixed effects, and intercept per patient as random effect.
Shifts from off-chart BCVA (LogMAR >1.6) at baseline to on-chart (LogMAR ≤1.6) BCVA at 1.5 years are illustrated in Supplementary Figure 2. Patients treated bilaterally with lenadogene nolparvovec demonstrated a shift of 68.4% in first-affected eyes versus 53.3% in second/not-yet-affected eyes (TARM1). A shift of 42.9% in first-affected lenadogene nolparvovec eyes versus 15.4% in second/not-yet-affected placebo eyes was reported in patients treated unilaterally with lenadogene nolparvovec (TARM2). The shift was statistically significant in first-affected eyes of bilaterally treated patients only (TARM1, P = 0.0124, McNemar test).
In bilaterally treated patients, the proportion of eyes that were on-chart at 1.5 years was 81.3% and 72.9% in first- and second-affected lenadogene nolparvovec-treated eyes, respectively. In unilaterally treated patients, the proportion was 68.0% for both lenadogene nolparvovec- and placebo-treated eyes. The proportion of patients with one or both eyes on-chart at 1.5 years was 85.4% and 72.0% for bilaterally and unilaterally treated patients, respectively, with an odds ratio (OR) of 2.30 in favour of bilateral treatment compared to unilateral treatment [OR = 2.30 (95% confidence interval = 0.78–6.76), P = 0.1305 by logistic regression model using treatment as factor and baseline LogMAR as covariate].
The responder rate for clinically relevant response (CRR) at 1.5 years reached 68.8% and 62.0% for bilaterally and unilaterally treated patients, respectively (Table 5). The improvement of at least −0.3 LogMAR (equivalent to a gain of ≥15 letters) from nadir to 1.5 years showed a responder rate of 68.8% and 64.0% for bilaterally and unilaterally treated patients, respectively (Table 5).
Table 5.
BCVA responder analyses versus nadir at 1.5 years (ITT population)
| First-affected eye | Second/Not-yet-affected eye | Patienta | |||||
|---|---|---|---|---|---|---|---|
| TARM1 (Bilateral treatment) |
TARM2 (Unilateral treatment) |
TARM1 (Bilateral treatment) |
TARM2 (Unilateral treatment) |
TARM1 (Bilateral treatment) (N = 48) |
TARM2 (Unilateral treatment) (N = 50) |
||
| Statistic | Lenadogene nolparvovec (N = 48) |
Lenadogene nolparvovec (N = 50) |
Lenadogene nolparvovec (N = 48) |
Placebo (N = 50) |
|||
| Improved by ≥0.3 LogMAR from nadir at 1.5 years | |||||||
| Responder | n | 30 | 27 | 26 | 23 | 33 | 32 |
| % | 62.5% | 54.0% | 54.2% | 46.0% | 68.8% | 64.0% | |
| TARM1 versus TARM2 | OR [95% CI] | 1.50 [0.66, 3.40] | 1.37 [0.62, 3.05] | 1.29 [0.55, 3.03] | |||
| P-valueb | 0.3302 | 0.4339 | 0.5526 | ||||
| Clinically relevant recovery from nadir at 1.5 years | |||||||
| Responder | n | 30 | 29 | 26 | 25 | 33 | 31 |
| % | 62.5% | 58.0% | 54.2% | 50.0% | 68.8% | 62.0% | |
| TARM1 versus TARM2 | OR [95% CI] | 1.07 [0.46, 2.50] | 1.15 [0.49, 2.69] | 1.25 [0.50, 3.16] | |||
| P-valueb | 0.8757 | 0.7464 | 0.6364 | ||||
TARM1: First-affected eye and second-affected/not-yet-affected eye administered lenadogene nolparvovec. TARM2: First-affected eye administered lenadogene nolparvovec, second-affected/not-yet-affected eye administered placebo. Clinically relevant recovery was defined as: (i) for eyes on-chart at nadir, a decrease (i.e. improvement) of ≥0.2 LogMAR from nadir; and (ii) for eyes off-chart at nadir, eyes which became on chart (i.e. BCVA ≤1.6 LogMAR). The nadir was defined as the worst value observed from baseline to the post-treatment time point of interest, including baseline and the post-treatment time point values. Missing data were imputed using the last observation carried forward method. N = number of eyes (at eye level) or number of patients (at patient level); n = number of eyes (at eye level) or number of patients (at patient level); OR = odds ratio; % = percentage of eyes or patients calculated relative to N.
For analyses at the patient level, response in one or both eyes.
A logistic regression model was used to model the proportion of responders using treatment as factor, and nadir LogMAR as covariate.
Secondary end points
The switch from off-chart at baseline to on-chart at 1.5 years in LogCS varied from 33.3% to 55.2% in each eye group. Overall, for patients with both eyes off-chart at baseline, 64.3% of bilaterally treated patients and 55.6% of unilaterally treated patients moved to on-chart LogCS in one or both eyes at 1.5 years. The mean time from baseline to nadir in LogCS was 4.5 weeks for first-affected eyes (pooled eyes treated with lenadogene nolparvovec), 9.9 weeks for second/not-yet-affected eyes treated with lenadogene nolparvovec, and 5.0 weeks for placebo-treated eyes. The improvement of at least −0.3 LogCS from nadir to 1.5 years showed a responder rate of 50.0% and 48.0% for bilaterally and unilaterally treated patients, respectively.
Overall, the HVF parameters were stable with mean deviation (MD) ranging on average from +1.44 dB improvement to −1.29 dB worsening in each eye group. Subgroup analyses were performed to investigate the relationship between HVF MD and final BCVA at 1.5 years post-treatment. Four subgroups of eyes were defined based on final LogMAR ranges, with each subgroup including a comparable number of treated eyes: Group 1: ≤ 1 LogMAR; Group 2: >1 to ≤1.3 LogMAR; Group 3: >1.3 to ≤1.6 LogMAR; Group 4: >1.6 LogMAR. The relationship between change from baseline of HVF MD and final BCVA subgroups is shown in Supplementary Fig. 3.
Overall, the ganglion cell layer (GCL) macular volume as measured on optical coherence tomography (OCT) showed thinning from baseline to 1.5 years of on average −0.069 mm3 and −0.092 mm3 for first and second eyes of bilaterally treated patients, respectively. For unilaterally treated patients, the reduction was larger for placebo-treated eyes (−0.117 mm3) than for lenadogene nolparvovec-treated eyes (−0.019 mm3). Similarly, as for the HVF MD, subgroup analyses were performed to investigate the relationship between GCL macular volume and final BCVA at 1.5 years post-treatment. The relationship between change from baseline of HVF MD and final BCVA subgroups is shown in Supplementary Fig. 4.
Quality of life
All patients in the REFLECT trial were treated with active product either bilaterally or unilaterally and the change in VFQ 25 questionnaire from baseline to 1.5 years represents the change of the VFQ 25 from untreated to treated status. REFLECT patients showed a clinically meaningful improvement in their vision-related quality of life at 1.5 years when compared to baseline, with a mean increase in the composite score by +6.4 and +6.3 points for bilaterally and unilaterally treated patients, respectively. The largest improvements were observed for the following vision-targeted subscale scores (expressed as mean increase from baseline to 1.5 years in bilaterally and unilaterally treated patients, respectively): mental health (+14.2 and +12.7); role difficulties (+10.8 and +13.2); dependency (+13.5 and +5.1); and general vision (+7.6 and +11.1) (Supplementary Table 2).
Safety data at 1.5 years
Most patients (66.3%) experienced at least one systemic adverse event (AE) up to 1.5 years. There was no systemic AE leading to study discontinuation, no systemic life-threatening AE, and no systemic AE leading to death in any patient.
The proportion of patients experiencing at least one systemic AE was 71.4% for bilaterally treated patients and 61.2% for unilaterally treated patients. Most systemic AEs were of mild intensity, whether overall (135/179 events, 75.4%) or in each treatment arm for bilaterally treated patients [68/88 events (77.3%)], and unilaterally treated patients [67/91 events (73.6%)]. Most systemic AEs were considered unrelated to study treatment or to study procedure. Overall, 2 (2.0%) patients reported a total of five systemic AEs considered related to the study treatment: one patient experienced toothache and headache on the day of IVT (counted as four separate events in total); and another patient reported a nasopharyngitis on Day 22 after the IVT. All five treatment-related systemic events were also considered related to the study procedure. Overall, 14 (14.3%) patients reported a total of 19 systemic AEs considered related to the study procedure. The most frequently reported systemic AEs related to the study procedure (patients overall) were headache (4.1%), rash (3.1%), and nasopharyngitis (2.0%).
A total of three severe systemic AEs were reported in three patients, all considered unrelated to the study treatment or to the study procedure. In all, five (5.1%) patients experienced one serious systemic AE. None of the serious systemic AEs were considered related to the study treatment or to the study procedure.
Overall, the most frequent systemic AEs reported by patients were headaches (18.4%), nasopharyngitis (12.2%), and insomnia (5.1%). There was no indication that systemic AEs occurred more frequently in bilaterally treated patients compared to unilaterally treated patients for each AE category. During the study, two patients developed multiple sclerosis after treatment administration, both reported as a serious AE of moderate intensity: one patient (bilateral treatment) developed symptoms of multiple sclerosis approximately 7 months after the IVT, and the other patient (unilateral treatment) developed multiple sclerosis symptoms approximately 2 years after the IVT injection. Neither of these two events were considered to be related to the study treatment or study procedure.
Most eyes (77.6%) experienced at least one ocular AE. The proportion of eyes experiencing at least one ocular AE was higher in eyes treated with lenadogene nolparvovec (ranging from 77.6% to 89.8%) versus eyes treated with placebo (55.1%). There were no ocular AEs leading to study discontinuation and no serious ocular AEs in any patient.
Most ocular AEs were of mild intensity, whether overall (433/479 events, 90.4%) or in each eye group: 262/292 events (89.7%) of mild intensity for lenadogene nolparvovec eyes of bilaterally treated patients, 123/137 (89.8%) for lenadogene nolparvovec eyes of unilaterally treated patients, and 48/50 (96.0%) for placebo eyes. Only two eyes (1.0%) experienced a total of four severe ocular AEs, which all occurred in the same bilaterally treated patient: two eye pruritus (one in each eye) considered unrelated to study treatment or procedure, and two punctate keratitis (one in each eye) considered unlikely related to study treatment and probably related to study procedure.
In eyes treated with lenadogene nolparvovec, the majority of ocular AEs were considered related to study treatment: 174/292 (59.6%) events for bilaterally treated patients and 89/137 (65.0%) events for unilaterally treated patients. The most common treatment-related ocular AEs were intraocular inflammation: vitritis (approximately 50% of eyes); iridocyclitis (approximately 25%); keratic precipitates (approximately 23%); and iritis (approximately 15%).
The proportion of ocular AEs considered related to study procedure was low, with 82/292 (28.1%) events for bilaterally treated patients and 40/137 (29.2%) events for unilaterally treated patients. The most common procedure-related ocular AEs were events of superficial punctate keratitis, which were reported by approximately 17% of bilaterally treated eyes and 6% of unilaterally treated eyes.
The proportion of placebo-treated eyes experiencing at least one related ocular AE was 18.4% and 30.6% for AEs related to study treatment and study procedure, respectively. Punctate keratitis was reported as procedure-related AEs in 8% of eyes of patients injected with placebo.
Overall, 109 eyes (55.6%) experienced at least one AE of intraocular inflammation. The incidence of intraocular inflammation was higher in eyes treated with lenadogene nolparvovec versus eyes treated with placebo (approximately 70% versus 10%). Among eyes treated with lenadogene nolparvovec, there was no difference in the incidence of intraocular inflammation between bilaterally and unilaterally treated patients.
All events of intraocular inflammation were of mild or moderate intensity. Nearly all events of intraocular inflammation occurred in the anterior chamber (mainly iridocyclitis, keratic precipitates and iritis) and in the intermediate chamber (mainly vitritis). There were only three events of posterior inflammation, all occurring in eyes of bilaterally treated patients (retinal vasculitis). All three events were mild or moderate in intensity, were considered related to the study treatment and resolved after treatment without sequelae. The proportion of eyes with intraocular inflammation not treated by corticosteroids was higher in patients treated unilaterally (44.9%) than bilaterally (36.7%). About half of intraocular inflammation events were managed with a topical corticosteroid alone (105/229 events, 46%), about 15% (35/229) with both topical and systemic treatments, and about 6% (14/229) with a systemic corticosteroid alone. The use of corticosteroids for the management of intraocular inflammation mainly occurred during the first 6 months post-IVT.
There was no inflammation at baseline in any groups of eyes (scores of 0 in all eyes). The mean of all global inflammation scores measured during AEs of intraocular inflammation was approximately 0.8/16 in eyes treated with lenadogene nolparvovec (i.e. low grade inflammation), and 0.3/16 in eyes treated with placebo. The highest mean sub-score was the vitreous cell score for lenadogene nolparvovec eyes (around 0.4/4) and the anterior chamber cell score for placebo eyes (around 0.2/4). In lenadogene nolparvovec eyes, mean inflammation scores measured were globally comparable between eyes of bilaterally and unilaterally treated patients.
Overall, 27 eyes (13.8%) experienced an AE of an increase of IOP. The incidence of IOP increase was higher in eyes treated with lenadogene nolparvovec versus eyes treated with placebo (approximately 18% versus 2%). All events of IOP increase were of mild or moderate intensity. In a minority of cases, the increase in IOP was considered related to study treatment (5/29, 17.2%) and/or procedure (9/29, 31.0%). Four events of IOP increase occurred either on the day or on the following day of the IVT, all being considered related to the study procedure, and one additionally considered to be related to study treatment. Most events of IOP increase were successfully managed with a topical IOP-lowering agent (21/29 events, 72%), and mainly occurred during the first 6 months post-IVT.
Regarding the humoral immune response, 29 patients had no (defined as titre <10) or low titres (defined as titre <100) of neutralizing antibodies (NAbs). In the remaining 69 patients with NAbs >100, NAbs tended to peak between 14 and 56 days following treatment, and then to decrease. No relevant differences were observed between patients treated bilaterally and unilaterally.
Regarding the cellular immune response, 68 patients had no observable response. Among patients with positive response in at least one measurement (n = 30), cellular immune response tended to occur between 14 and 56 days after treatment. Again, no relevant differences were observed between patients treated bilaterally and patients treated unilaterally.
Biodissemination of lenadogene nolparvovec in blood was assessed at baseline and Days 14 and 28 post-treatment. At Day 14, lenadogene nolparvovec was detected at quantifiable levels in only 2 out of the 97 tested blood samples (2%), with amounts close to the lower limit of quantification. None of the 98 tested samples were positive for lenadogene nolparvovec at Day 28.
Discussion
The clinical development of lenadogene nolparvovec consists of one phase 1/2 study, REVEAL,21 three phase 3 pivotal studies, REVERSE,16–18 RESCUE17–19 and REFLECT, and a long-term follow-up of REVERSE and RESCUE patients in the RESTORE study.18,20 The REFLECT study is the third phase 3 study evaluating the efficacy and safety of lenadogene nolparvovec in LHON patients with the m.11778G>A mutation. It is also the first clinical study assessing the efficacy of a bilateral IVT of lenadogene nolparvovec, while in the three previous clinical studies REVEAL,21 REVERSE16–18 and RESCUE,17–19 lenadogene nolparvovec was exclusively administered as unilateral IVT. With the completion of REFLECT, lenadogene nolparvovec has been administered to 174 patients across three phase 3 studies, with 126 patients having received a unilateral injection and 48 bilateral injections.
Patient demographic characteristics in REFLECT were typical of the LHON disease population and consistent across all phase 3 clinical studies, with a majority of males enrolled (80%), and a mean age of 32 years. The mean duration of vision loss at the time of lenadogene nolparvovec administration in the REFLECT study was 8.3 months, more similar to the timing of REVERSE enrollment (restricted to vision loss for 6–12 months) than that of RESCUE (vision loss within 6 months) (Supplementary Table 3). Consistent with the duration of vision loss, the mean BCVA before treatment in REFLECT patients [+1.5 (Snellen 20/600) to +1.6 (Snellen 20/800)] was more aligned with that of REVERSE [+1.6 (Snellen 20/800)] than that of RESCUE [+1.3 (Snellen 20/400)] (Supplementary Table 3).
In REFLECT, all eye groups, including placebo eyes, showed a statistically significant and clinically relevant improvement24 in mean LogMAR BCVA at 1.5 years versus nadir, similar to the bilateral response seen in both treated and sham eyes in REVERSE and RESCUE, and to a degree not reported in natural history studies of LHON patients of corresponding age and genotype.18 Further evidence of a contralateral effect among unilaterally treated REFLECT patients can be found in the time course of mean LogMAR BCVA from baseline to 1.5 years. This contralateral effect is consistent across the three phase 3 clinical studies, being maintained in the long-term follow-up study RESTORE at 3 years post-IVT, and even demonstrated in the phase 1/2 study REVEAL. In the REFLECT study, the improvement in BCVA of placebo eyes (contralateral therapeutic effect) observed in unilaterally treated patients likely accounts for the study’s failure to meet its primary end point, as the placebo eye in a patient who receives unilateral treatment does not constitute a relevant control.
Two similar gene therapy programmes led by groups in the USA25,26 and China,27–30 have targeted the m.11778G>A mutation in MT-ND4, also using a viral vector containing a cDNA coding the human wild-type mitochondrial ND4 protein. For all trials, the route of administration was intravitreal and nearly always unilaterally. Most of these gene therapy trials have shown comparable bilateral improvements after unilateral IVT of gene therapy (AAV2-ND4) in MT-ND4 LHON patients. Indeed, the recent full results of the phase 1/2 study conducted by Lam et al.31 confirmed bilateral improvement in both injected and fellow eyes, although, as the authors themselves emphasize, the low number of patients provided limited power to assess efficacy of the gene therapy and inconclusive results when compared to their equally limited prospective natural history study.32
The physiologic basis of the contralateral effect of unilateral lenadogene nolparvovec IVT has been investigated in a mechanistic study conducted in non-human primates.16,33 This study suggested there is some transfer of viral vector DNA from the injected eyes to the uninjected contralateral eyes after unilateral lenadogene IVT. Because lenadogene nolparvovec DNA was detected and quantified in the optic chiasm, it was speculated that the anatomic route taken by the viral vector DNA from the treated eye to the non-treated eye may be via the optic nerve and chiasm (through anterograde and subsequent retrograde transport along the optic projections). While a systemic transfer of lenadogene nolparvovec cannot be excluded, it is unlikely given that the results of biodissemination studies have shown limited and only transient presence of vector genetic material in blood. Other mechanisms, such as the transfer of mitochondria or active mediators between eyes, could also provide a basis for this bilateral effect.16,33
Interestingly, bilaterally treated patients showed better improvement of BCVA and responder rates, as compared to unilaterally treated patients. The uniformity of this finding across all measurements of BCVA efficacy is striking, with placebo-injected eyes consistently demonstrating inferior visual outcomes. These same trends were observed for LogCS measurements.
Remarkably, first-affected eyes in all REFLECT patients consistently had better BCVA outcomes than their second-affected eyes, even when the second-affected eyes received lenadogene nolparvovec, albeit placebo eyes fared the worst. This seemingly counterintuitive better response in eyes receiving gene therapy later in the course of their visual decline (i.e. between 6 and 12 months from onset) was also demonstrated in the prior RESCUE and REVERSE studies. It has been suggested that acutely metabolically swollen RGCs and their axons may impart a relative barrier to RGC transfection by lenadogene nolparvovec.19
Regarding secondary outcomes, there was a meaningful relationship between the change in HVF MD (improvement/stabilization/deterioration) and the final BCVA subgroups: the better the evolution of HVF MD was versus baseline, the better was the final BCVA. This relationship was also demonstrated in both RESCUE and REVERSE and further illustrates the remarkable coherence in efficacy results for all three studies.15,18
OCT has proven to be an important tool in evaluating the clinical course of LHON.4,34–38 In our analyses, among the parameters measured by OCT, the GCL macular volume has been shown to be the most sensitive measure to characterize anatomical changes. Moster et al.39 showed that GCL thickness has a stronger relationship to BCVA than other OCT measurements. In this current study, similar to the visual field assessment, a subgroup analysis investigating the relationship between GCL measurements and final BCVA at 1.5 years post-treatment showed a correspondence between the absolute change in the GCL macular volume on OCT and the final BCVA groups, with less thinning of GCL macular volume in the group with final best BCVA in comparison with the other final BCVA groups. The GCL macular volume measurements displayed a similar relationship with final BCVA groups in RESCUE and REVERSE.15,18
Across all phase 3 clinical studies, patient reported outcome measures, assessed using the validated VFQ-25 questionnaire, supported a treatment benefit with lenadogene nolparvovec treatment. Mean improvement in composite score was approximately +6 points at Year 1.5 in REFLECT, +4 points at Week 96 in REVERSE and RESCUE, and +7 points at Year 3 in RESTORE. Patients showed a clinically meaningful improvement in vision-related quality of life measures post-treatment when compared to baseline in most sub-scores across all these phase 3 studies.
Overall, lenadogene nolparvovec has an excellent systemic safety profile. There were no systemic AEs leading to study discontinuation, no systemic life-threatening AEs, and no systemic AE leading to death in any patient in REFLECT. The absence of systemic issues related to lenadogene nolparvovec treatment is supported by the limited biodissemination of the product, which is negligible in the blood of REFLECT patients, similar to what has been observed in previous clinical studies.16,19 Furthermore, the general humoral and cellular immunologic response was limited, consistent with the local ocular nature of the immune response with intraocular inflammation. Importantly, the favourable safety profile of lenadogene nolparvovec was comparable for bilaterally and unilaterally treated patients.
LHON can be associated with a number of neurological conditions such as multiple sclerosis (Harding’s syndrome).40 The multiple sclerosis-like illness diagnosed in two participants of the REFLECT study is in keeping with the frequency of this condition in previous studies on larger cohorts of LHON patients.40
Most ocular AEs were of mild intensity, with no ocular AE leading to study discontinuation and no serious ocular AE in any patient. Intraocular inflammation, the main ocular AE, was invariably deemed related to study treatment. Despite a 28-day oral corticosteroid therapy started 2 days before the first IVT, a regimen similar to that provided in several other clinical studies using IVT or sub-retinal AAV-based gene therapies,41–45 70.7% of lenadogene nolparvovec-treated eyes developed some degree of intraocular inflammation versus 10.2% of placebo-treated eyes. Intraocular inflammation occurred almost exclusively in the anterior chamber and vitreous compartment, with posterior segment inflammation being rare. Intraocular inflammation was treated and controlled with local (topical) corticosteroid alone and only rarely required the addition of oral corticosteroids. The characteristics of the intraocular inflammation of unilaterally (REVEAL, REVERSE, RESCUE and REFLECT unilateral arm) and bilaterally (REFLECT bilateral arm) treated patients were comparable. Data on bilateral use of lenadogene nolparvovec did not show an increased severity or frequency of ocular AEs as compared with unilateral injection.
The most commonly reported ocular AEs related to the study procedure were punctate keratitis, conjunctival haemorrhage and conjunctival hyperemia, which were observed at a similar frequency in lenadogene nolparvovec-treated eyes and uninjected/placebo-treated eyes (with an incidence of about 20, 7 and 6%, respectively). In clinical practice, these minor local reactions are frequently observed with IVT and are treated symptomatically. IOP increases were rarely considered related to the study treatment, with few events occurring at the time of the IVT or immediately post-IVT, confirming that the injection of a 90 µl volume was well tolerated. In summary, lenadogene nolparvovec has an overall good safety profile with excellent systemic tolerability and a good ocular tolerability, characterized by mostly mild ocular side effects, responsive to conventional ophthalmologic treatments.
As with any clinical trial, REFLECT has several limitations. The contralateral effect in unilaterally treated patients has been observed across all lenadogene nolparvovec studies, demonstrating that contralateral placebo/sham eyes are not appropriate controls. The lack of a patient placebo-controlled arm in REFLECT and previous studies necessitates the indirect comparison of outcomes with published natural history data. Although a large meta-analysis of natural history MT-ND4 LHON patients has shown that spontaneous visual recovery is rare and limited (11%),3 and below the responder rates seen in lenadogene nolparvovec-treated patients with comparable demographic characteristics, these natural history studies were not prospective and suffer from lack of consistent data acquisition. It is also not possible to fully exclude that some of the visual gains are due to better use of eccentric fixation as has been documented in patients with central scotomas from macular disease.46
Another limitation in the design of REFLECT is the use of the change of BCVA from baseline as a primary end point. The range of disease onset time (any time within 12 months) makes comparison to baseline BCVA problematic given that it is inhomogeneous, encompassing eyes that are at various stages of BCVA decline. The comparison to the nadir (i.e. worst BCVA) may be a more clinically relevant reference for evaluating the treatment effect on BCVA at 1.5 years than baseline BCVA, although, by definition, eyes can only improve from nadir. The change in BCVA from the nadir enables determination of the treatment effect in a more standardized way for first-affected eyes (earlier affected) and second/not-yet-affected eyes (later affected or even not affected).
In conclusion, LHON is a devastating blinding disease with an unmet medical need for treatment. Lenadogene nolparvovec is a gene therapy specifically developed to treat patients carrying the m.11778G>A mtDNA mutation, targeting the root cause of the disease by allotopic expression of a replacement wild-type MT-ND4. The efficacy results of the REFLECT study showed an improvement of BCVA in LHON patients treated with lenadogene nolparvovec to a degree not demonstrated in natural history studies. REFLECT also demonstrated a contralateral therapeutic effect in placebo eyes of unilaterally treated patients, consistent with the contralateral therapeutic effect observed for sham eyes in the previous phase 3 studies, REVERSE and RESCUE. A larger treatment effect was observed in patients who received bilateral treatment as compared to those receiving unilateral treatment. Lenadogene nolparvovec has an overall good safety profile with favourable systemic and ocular tolerability, which is comparable for bilaterally and unilaterally treated patients. The REFLECT study, therefore, supports an improved benefit/risk profile for bilateral injection of lenadogene nolparvovec relative to unilateral injection.
Supplementary Material
Acknowledgements
We would like to sincerely thank the patients and their families for participating in this research.
Appendix 1
LHON REFLECT Study Group list
Full details are provided in the Supplementary material.
Amore Giulia, Anand Shweta, Banik Rudrani, Barboni Piero, Biousse Valérie, Boston Hayley, Burale Asma, Carbonelli Michele, Carelli Valerio, Chen Celia, Cheng Hui-Chen, Cho Steve, Chwalisz Bart K., Contin Manuela, D’Agati Pietro, DeBusk Adam A., De Zaeytijd Julie, Dobbs Jannah, Donahue Sean P., DuBois Lindreth, Esposti Simona, Fernandes Filho Alcides, Fortin Elizabeth, Gangaputra Sapna, Gibbs Deborah, Girmens Jean François, Hage Rabih, Haller Julia A., Heilweil Gad, Hubbard III George Baker, Hwang Jeong-Min, Jaumendreu Urquijo Laia, Jurkute Neringa, Karanjia Rustum, Khemliche Wahiba, La Morgia Chiara, Leroy Bart P., Massini Maria, Mathias Marc, Memon Muhammad A, Mohamed Susan, Moster Mark L., Muñoz Negrete Francisco J., Newman Nancy J., O’Keefe Ghazala, Patel Shriji, Pecen Paula, Peragallo Jason H., Plaine Lise, Preston Mary, Rebolleda Fernández Gema, Romagnoli Martina, Sadun Alfredo A., Sahel José-Alain., SantaMaria Melissa, Sergott Robert C., Subramanian Prem S., Sun Chuanbin, Tai Katy, Tollis Heather, Tsui Irena, Tucker William R., Vignal-Clermont Catherine, Wang An-Guor, Wilkins Saige and Yu-Wai-Man Patrick.
Contributor Information
Nancy J Newman, Departments of Ophthalmology, Neurology and Neurological Surgery, Emory University School of Medicine, Atlanta, GA, USA.
Patrick Yu-Wai-Man, Cambridge Centre for Brain Repair and MRC Mitochondrial Biology Unit, Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK; Cambridge Eye Unit, Addenbrooke’s Hospital, Cambridge University Hospitals, Cambridge, UK; Moorfields Eye Hospital, London, UK; UCL Institute of Ophthalmology, University College London, London, UK.
Prem S Subramanian, Sue Anschutz-Rodgers University of Colorado Eye Center, University of Colorado School of Medicine, Aurora, CO, USA.
Mark L Moster, Departments of Neurology and Ophthalmology, Wills Eye Hospital and Thomas Jefferson University, Philadelphia, PA, USA.
An-Guor Wang, Department of Ophthalmology, Taipei Veterans General Hospital, National Yang Ming Chiao Tung University, Taipei, Taiwan.
Sean P Donahue, Department of Ophthalmology, Neurology, and Pediatrics, Vanderbilt University, and Vanderbilt Eye Institute, Vanderbilt University Medical Center, Nashville, TN, USA.
Bart P Leroy, Department of Ophthalmology and Center for Medical Genetics, Ghent University Hospital, and Department of Head & Skin, Ghent University, Ghent, Belgium.
Valerio Carelli, IRCCS Istituto delle Scienze Neurologiche di Bologna, Programma di Neurogenetica, Bologna, Italy; Unit of Neurology, Department of Biomedical and Neuromotor Sciences, University of Bologna, Bologna, Italy.
Valerie Biousse, Departments of Ophthalmology, Neurology and Neurological Surgery, Emory University School of Medicine, Atlanta, GA, USA.
Catherine Vignal-Clermont, Department of Neuro Ophthalmology and Emergencies, Rothschild Foundation Hospital, Paris, France; Department of Ophthalmology, Centre Hospitalier National D’Ophtalmologie des Quinze Vingts, Paris, France.
Robert C Sergott, Departments of Neurology and Ophthalmology, Wills Eye Hospital and Thomas Jefferson University, Philadelphia, PA, USA.
Alfredo A Sadun, Doheny Eye Institute, UCLA School of Medicine, Los Angeles, CA, USA.
Gema Rebolleda Fernández, Department of Ophthalmology, Alcala University, Madrid, Spain.
Bart K Chwalisz, Department of Ophthalmology, Massachusetts Eye & Ear, Harvard Medical School, Boston, MA, USA; Department of Neurology, Massachusetts General Hospital, Harvard Medical School, Boston, MA, USA.
Rudrani Banik, Department of Ophthalmology, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Fabienne Bazin, eXYSTAT, Data Management and Statistics, Paris, France.
Michel Roux, GenSight Biologics, Paris, France.
Eric D Cox, GenSight Biologics, Paris, France.
Magali Taiel, GenSight Biologics, Paris, France.
José-Alain Sahel, Sorbonne Université, INSERM, CNRS, Institut de la Vision, Paris, France; Fondation Ophtalmologique A. de Rothschild, Paris, France; Department of Ophthalmology, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA; CHNO des Quinze-Vingts, Institut Hospitalo-Universitaire FOReSIGHT, INSERM-DGOS CIC, Paris, France.
the LHON REFLECT Study Group:
Amore Giulia, Anand Shweta, Banik Rudrani, Barboni Piero, Biousse Valérie, Boston Hayley, Burale Asma, Carbonelli Michele, Carelli Valerio, Chen Celia, Cheng Hui-Chen, Cho Steve, Bart K Chwalisz, Contin Manuela, D’Agati Pietro, Adam A DeBusk, De Zaeytijd Julie, Dobbs Jannah, Sean P Donahue, DuBois Lindreth, Esposti Simona, Fernandes Filho Alcides, Fortin Elizabeth, Gangaputra Sapna, Gibbs Deborah, Girmens Jean François, Hage Rabih, Julia A Haller, Heilweil Gad, Hubbard III George Baker, Hwang Jeong-Min, Jaumendreu Urquijo Laia, Jurkute Neringa, Karanjia Rustum, Khemliche Wahiba, Morgia La Chiara, Bart P Leroy, Massini Maria, Mathias Marc, Muhammad A Memon, Mohamed Susan, Mark L Moster, Francisco J Muñoz Negrete, Nancy J Newman, O’Keefe Ghazala, Patel Shriji, Pecen Paula, Jason H Peragallo, Plaine Lise, Preston Mary, Rebolleda Fernández Gema, Romagnoli Martina, Alfredo A Sadun, Sahel José-Alain, SantaMaria Melissa, Robert C Sergott, Prem S Subramanian, Sun Chuanbin, Tai Katy, Tollis Heather, Tsui Irena, William R Tucker, Vignal-Clermont Catherine, Wang An-Guor, Wilkins Saige, and Yu-Wai-Man Patrick
Funding
This study was funded by GenSight Biologics. N.J.N. and V.B. are supported in part by NIH/NEI core grant P30-EY06360 (Department of Ophthalmology, Emory University School of Medicine), and by NIH/NINDS (RO1NSO89694). P.Y-.W-.M. is supported by an Advanced Fellowship Award (NIHR301696) from the UK National Institute of Health Research (NIHR) and a Clinician Scientist Fellowship Award (G1002570) from the UK Medical Research Council (MRC). P.Y-.W-.M. also receives funding from Fight for Sight (UK), the Isaac Newton Trust (UK), Moorfields Eye Charity (GR001376), the Addenbrooke’s Charitable Trust, the National Eye Research Centre (UK), the International Foundation for Optic Nerve Disease (IFOND), the NIHR as part of the Rare Diseases Translational Research Collaboration, the NIHR Cambridge Biomedical Research Centre (BRC-1215-20014), and the NIHR Biomedical Research Centre based at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. V.C. is supported by grants from the Italian Ministry of Health (RF-2018-12366703), the Italian Ministry of University and Research (20172T2MHH), and Telethon-Italy (GGP20115). V.C. is also supported by patients’’ organizations MITOCON and IFOND, and patients’’ donations. B.P.L. is supported by grants from the Research Foundation - Flanders, Belgium (Senior Clinical Investigator 1803821N) and the Concerted Research Action of the Special Research Fund Ghent University (BOF20/GOA/023).
Competing interests
N.J.N. is a consultant for GenSight Biologics, Santhera Pharmaceuticals, and Stealth BioTherapeutics; has received research support from GenSight Biologics and Santhera Pharmaceuticals; served on the Data Safety Monitoring Board for Quark NAION study; and is a medical legal consultant. P.Y.W.M. is a consultant for GenSight Biologics and Stealth BioTherapeutics and has received research support from GenSight Biologics and Santhera Pharmaceuticals. P.S.S. is a consultant for GenSight Biologics, Horizon Therapeutics, Invex Therapeutics, Viridian Therapeutics, and Kriya Therapeutics, and has received research support from Santhera Pharmaceuticals, GenSight Biologics, and Horizon Therapeutics; and is a medical legal consultant. M.L.M. is a consultant for GenSight Biologics and has received research support from GenSight. S.P.D. has been a fee-for-service consultant for GenSight Biologics. B.P.L. is a consultant for GenSight Biologics, 4DMT, AAVantgardeBio, Akouos, Asthena Therapeutics, Bayer, Biogen, GenSight Biologics, IVERIC Bio, MeiraGTx-Jansen Pharmaceuticals, LookoutGTx, Novartis, Opus Genetics, Oxurion, ProQR Therapeutics, Santen, Spark Therapeutics, REGENXBIO, Vedere Bio, ViGeneron, and has received research support from GenSight Biologics, MeiraGTx-Jansen Pharmaceuticals, Novartis and ProQR Therapeutics. V.C. is a consultant for GenSight Biologics, Santhera Pharmaceuticals, and Stealth BioTherapeutics, and has received research support from Santhera Pharmaceuticals and Stealth BioTherapeutics. V.B. is a consultant for GenSight Biologics. C.V.-C. is a consultant for Santhera Pharmaceuticals and GenSight Biologics. R.C.S. is a consultant for GenSight Biologics. A.A.S. is a consultant for Stealth BioTherapeutics. R.B. is a consultant for Horizon Therapeutics, Healthy Directions and Guardion Health Sciences and has received research support from Santhera Pharmaceuticals, Regenera and Quark Pharmaceuticals. E.D.C., M.R. and M.T. are employed by GenSight Biologics, the sponsor of these studies. J-.A.S. is the cofounder and shareholder of GenSight Biologics and the patent coauthor on allotopic transport.
Supplementary material
Supplementary material is available at Brain online.
References
- 1. Carelli V, Carbonelli M, de Coo IF, et al. International consensus statement on the clinical and therapeutic management of Leber hereditary optic neuropathy. J Neuroophthalmol. 2017;37:371–381. [DOI] [PubMed] [Google Scholar]
- 2. Newman NJ, Yu-Wai-Man P, Biousse V, Carelli V. Understanding the molecular basis and pathogenesis of hereditary optic neuropathies: Towards improved diagnosis and management. Lancet Neurology. 2023;22:172–188. [DOI] [PubMed] [Google Scholar]
- 3. Newman NJ, Carelli V, Taiel M, Yu-Wai-Man P. Visual outcomes in Leber hereditary optic neuropathy patients with the m.11778G>A (MTND4) mitochondrial DNA mutation. J Neuro-Ophthalmol. 2020;40:547–557. [DOI] [PubMed] [Google Scholar]
- 4. Yu-Wai-Man P, Votruba M, Burté F, La Morgia C, Barboni P, Carelli V. A neurodegenerative perspective on mitochondrial optic neuropathies. Acta Neuropathol (Berl). 2016;132:789–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yu-Wai-Man P, Griffiths PG, Chinnery PF. Mitochondrial optic neuropathies—Disease mechanisms and therapeutic strategies. Prog Retin Eye Res. 2011;30:81–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wallace DC, Singh G, Lott MT, et al. Mitochondrial DNA mutation associated with Leber’s hereditary optic neuropathy. Science. 1988;242:1427–1430. [DOI] [PubMed] [Google Scholar]
- 7. Russell S, Bennett J, Wellman JA, et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: A randomised, controlled, open-label, phase 3 trial. Lancet Lond Engl. 2017;390:849–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gray RE, Law RHP, Devenish RJ, Nagley P. Allotopic expression of mitochondrial ATP synthase genes in nucleus of Saccharomyces cerevisiae. Methods Enzymol. 1996;264:369–389. [DOI] [PubMed] [Google Scholar]
- 9. Koilkonda R, Yu H, Talla V, et al. LHON Gene therapy vector prevents visual loss and optic neuropathy induced by G11778A mutant mitochondrial DNA: Biodistribution and toxicology profile. Invest Ophthalmol Vis Sci. 2014;55:7739–7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Koilkonda RD, Chou TH, Porciatti V, Hauswirth WW, Guy J. Induction of rapid and highly efficient expression of the human ND4 complex I subunit in the mouse visual system by self-complementary adeno-associated virus. Arch Ophthalmol. 2010;128:876–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Cwerman-Thibault H, Augustin S, Lechauve C, et al. Nuclear expression of mitochondrial ND4 leads to the protein assembling in complex I and prevents optic atrophy and visual loss. Mol Ther Methods Clin Dev. 2015;2:15003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bonnet C, Augustin S, Ellouze S, et al. The optimized allotopic expression of ND1 or ND4 genes restores respiratory chain complex I activity in fibroblasts harboring mutations in these genes. Biochim Biophys Acta. 2008;1783:1707–1717. [DOI] [PubMed] [Google Scholar]
- 13. Bonnet C, Kaltimbacher V, Ellouze S, et al. Allotopic mRNA localization to the mitochondrial surface rescues respiratory chain defects in fibroblasts harboring mitochondrial DNA mutations affecting complex I or v subunits. Rejuvenation Res. 2007;10:127–144. [DOI] [PubMed] [Google Scholar]
- 14. Hellström M, Ruitenberg MJ, Pollett MA, et al. Cellular tropism and transduction properties of seven adeno-associated viral vector serotypes in adult retina after intravitreal injection. Gene Ther. 2009;16:521–532. [DOI] [PubMed] [Google Scholar]
- 15. Vignal C, Uretsky S, Fitoussi S, et al. Safety of rAAV2/2-ND4 gene therapy for Leber hereditary optic neuropathy. Ophthalmology. 2018;125:945–947. [DOI] [PubMed] [Google Scholar]
- 16. Yu-Wai-Man P, Newman NJ, Carelli V, et al. Bilateral visual improvement with unilateral gene therapy injection for Leber hereditary optic neuropathy. Sci Transl Med. 2020;12:eaaz7423. [DOI] [PubMed] [Google Scholar]
- 17. Moster ML, Sergott RC, Newman NJ, et al. Cross-sectional analysis of baseline visual parameters in subjects recruited into the RESCUE and REVERSE ND4-LHON gene therapy studies. J Neuroophthalmol. 2021;41:298–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Newman NJ, Yu-Wai-Man P, Carelli V, et al. Intravitreal gene therapy versus. Natural history in patients with Leber hereditary optic neuropathy carrying the m.11778G>A ND4 mutation: Systematic review and indirect comparison. Front Neurol. 2021; 12:662838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Newman NJ, Yu-Wai-Man P, Carelli V, et al. Efficacy and safety of intravitreal gene therapy for Leber hereditary optic neuropathy treated within 6 months of disease onset. Ophthalmology. 2021;128:649–660. [DOI] [PubMed] [Google Scholar]
- 20. Biousse V, Newman NJ, Yu-Wai-Man P, et al. Long-term follow-up after unilateral intravitreal gene therapy for Leber hereditary optic neuropathy: The RESTORE study. J Neuroophthalmol. 2021;41:309–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vignal-Clermont C, Girmens JF, Audo I, et al. Safety of intravitreal gene therapy for treatment of subjects with Leber hereditary optic neuropathy due to mutations in the mitochondrial ND4 gene: The REVEAL study. Biodrugs. 2021;35:201–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pelli DG, Robson JG, Wilkins AJ. The design of a new letter chart for measuring contrast sensitivity. Clin Vis Sci. 1988;2:187–199. [Google Scholar]
- 23. Mangione CM, Lee PP, Gutierrez PR, et al. Development of the 25-item national eye institute visual function questionnaire. Arch Ophthalmol. 2001;119:1050–1058. [DOI] [PubMed] [Google Scholar]
- 24. Csaky K, Ferris F, Chew EY, Nair P, Cheetham JK, Duncan JL. Report from the NEI/FDA endpoints workshop on age-related macular degeneration and inherited retinal diseases. Invest Ophthalmol Vis Sci. 2018;58:3456–3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Feuer WJ, Schiffman JC, Davis JL, et al. Gene therapy for Leber hereditary optic neuropathy: Initial results. Ophthalmology. 2016;123:558–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Guy J, Feuer WJ, Davis JL, et al. Gene therapy for Leber hereditary optic neuropathy: Low- and medium-dose visual results. Ophthalmology. 2017;124:1621–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wan X, Pei H, Zhao MJ, et al. Efficacy and safety of rAAV2-ND4 treatment for Leber’s hereditary optic neuropathy. Sci Rep. 2016;6:21587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yang S, Ma SQ, Wan X, et al. Long-term outcomes of gene therapy for the treatment of Leber’s hereditary optic neuropathy. EBioMedicine. 2016;10:258–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Liu HL, Yuan JJ, Zhang Y, et al. Factors associated with rapid improvement in visual acuity in patients with Leber’s hereditary optic neuropathy after gene therapy. Acta Ophthalmol (Copenh). 2020;98:e730–e733. [DOI] [PubMed] [Google Scholar]
- 30. Yuan JJ, Zhang Y, Wang LL, et al. Visual field variability after gene therapy for Leber’s hereditary optic neuropathy. Ophthalmic Res. 2018;60:176–184. [DOI] [PubMed] [Google Scholar]
- 31. Lam BL, Feuer WJ, Davis JL, et al. Leber hereditary optic neuropathy gene therapy: Adverse events and visual acuity results of all patient groups. Am J Ophthalmol. 2022;241:262–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lam BL, Feuer WJ, Schiffman JC, et al. Trial end points and natural history in patients with G11778A Leber hereditary optic neuropathy. JAMA Ophthalmol. 2014;132:428–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Calkins DJ, Yu-Wai-Man P, Newman NJ, et al. Biodistribution of intravitreal lenadogene nolparvovec gene therapy in nonhuman primates. Mol Ther Methods Clin Dev. 2021;23:307–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Barboni P, Carbonelli M, Savini G, et al. Natural history of Leber’s hereditary optic neuropathy: Longitudinal analysis of the retinal nerve fiber layer by optical coherence tomography. Ophthalmology. 2010;117:623–627. [DOI] [PubMed] [Google Scholar]
- 35. Pan BX, Ross-Cisneros F, Carelli V, et al. Mathematically modeling the involvement of axons in Leber’s hereditary optic neuropathy. Invest Ophthalmol Vis Sci. 2012;53:7608–7617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Balducci N, Savini G, Cascavilla ML, et al. Macular nerve fibre and ganglion cell layer changes in acute Leber’s hereditary optic neuropathy. Br J Ophthalmol. 2016;100:1232–1237. [DOI] [PubMed] [Google Scholar]
- 37. Savini G, Barboni P, Valentino ML, et al. Retinal nerve fiber layer evaluation by optical coherence tomography in unaffected carriers with Leber’s hereditary optic neuropathy mutations. Ophthalmology. 2005;112:127–131. [DOI] [PubMed] [Google Scholar]
- 38. Barboni P, Savini G, Valentino ML, et al. Retinal nerve fiber layer evaluation by optical coherence tomography in Leber’s hereditary optic neuropathy. Ophthalmology. 2005;112:120–126. [DOI] [PubMed] [Google Scholar]
- 39. Moster SJ, Moster ML, Scannell Bryan M, Sergott RC. Retinal ganglion cell and inner plexiform layer loss correlate with visual acuity loss in LHON: A longitudinal, segmentation OCT analysis. Invest Ophthalmol Vis Sci. 2016;57:3872–3883. [DOI] [PubMed] [Google Scholar]
- 40. Pfeffer G, Burke A, Yu-Wai-Man P, Compston DAS, Chinnery PF. Clinical features of MS associated with Leber hereditary optic neuropathy mtDNA mutations. Neurology. 2013;81:2073–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bainbridge JWB, Smith AJ, Barker SS, et al. Effect of gene therapy on visual function in Leber’s congenital amaurosis. N Engl J Med. 2008;358:2231–2239. [DOI] [PubMed] [Google Scholar]
- 42. Maguire AM, Simonelli F, Pierce EA, et al. Safety and efficacy of gene transfer for Leber’s congenital amaurosis. N Engl J Med. 2008;358:2240–2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bennett J, Ashtari M, Wellman J, et al. AAV2 Gene therapy readministration in three adults with congenital blindness. Sci Transl Med. 2012;4:120ra15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. MacLaren RE, Groppe M, Barnard AR, et al. Retinal gene therapy in patients with choroideremia: Initial findings from a phase 1/2 clinical trial. Lancet. 2014:383:1129–1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Bainbridge JWB, Mehat MS, Sundaram V, et al. Long-term effect of gene therapy on Leber’s congenital amaurosis. N Engl J Med. 2015;372:1887–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ratra D, Gopalakrishnan S, Dalan D, Ratra V, Damkondwar D, Laxmi G. Visual rehabilitation using microperimetric acoustic biofeedback training in individuals with central scotoma. Clin Exp Optom. 2019;102:172–179. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the results of this study and the trial protocol are available upon reasonable request to the corresponding author.