Abstract
In everyday life, information from different cognitive domains—such as visuospatial attention, alertness and inhibition—needs to be integrated between different brain regions. Early models suggested that completely segregated brain networks control these three cognitive domains. However, more recent accounts, mainly based on neuroimaging data in healthy participants, indicate that different tasks lead to specific patterns of activation within the same, higher-order and ‘multiple-demand’ network. If so, then a lesion to critical substrates of this common network should determine a concomitant impairment in all three cognitive domains. The aim of the present study was to critically investigate this hypothesis, i.e. to identify focal stroke lesions within the network that can concomitantly affect visuospatial attention, alertness and inhibition.
We studied an unselected sample of 60 first-ever right-hemispheric, subacute stroke patients using a data-driven, bottom-up approach. Patients performed 12 standardized neuropsychological and oculomotor tests, four per cognitive domain. A principal component analysis revealed a strong relationship between all three cognitive domains: 10 of 12 tests loaded on a first, common component. Analysis of the neuroanatomical lesion correlates using different approaches (i.e. voxel-based and tractwise lesion-symptom mapping, disconnectome maps) provided convergent evidence on the association between severe impairment of this common component and lesions at the intersection of superior longitudinal fasciculus II and III, frontal aslant tract and, to a lesser extent, the putamen and inferior fronto-occipital fasciculus. Moreover, patients with a lesion involving this region were significantly more impaired in daily living cognition, which provides an ecological validation of our results. A probabilistic functional atlas of the multiple-demand network was performed to confirm the potential relationship between patients’ lesion substrates and observed cognitive impairments as a function of the multiple-demand network connectivity disruption.
These findings show, for the first time, that a lesion to a specific white matter crossroad can determine a concurrent breakdown in all three considered cognitive domains. Our results support the multiple-demand network model, proposing that different cognitive operations depend on specific collaborators and their interaction, within the same underlying neural network. Our findings also extend this hypothesis by showing (i) the contribution of superior longitudinal fasciculus and frontal aslant tract to the multiple-demand network; and (ii) a critical neuroanatomical intersection, crossed by a vast amount of long-range white matter tracts, many of which interconnect cortical areas of the multiple-demand network. The vulnerability of this crossroad to stroke has specific cognitive and clinical consequences; this has the potential to influence future rehabilitative approaches.
Keywords: visuospatial attention, alertness, inhibition, multiple-demand network, right-hemispheric stroke
By studying 60 right-hemispheric stroke patients, Kaufmann et al. show that lesions at a strategic intersection of branches II and III of the superior longitudinal fasciculus and frontal aslant tract can lead to a breakdown of the multiple-demand network, causing impaired cognitive performance on tests as well as in everyday life.
See Weiller and Rijntes (https://doi.org/10.1093/brain/awad081) for a scientific commentary on this article.
See Weiller and Rijntes (https://doi.org/10.1093/brain/awad081) for a scientific commentary on this article.
Introduction
A dynamic interaction between different cognitive functions forms the basis of everyday behaviour. Cognitive functions are recruited depending on the situation's requests and alternate in action on environmental changes. Hereby, information from various cognitive domains needed for this complex behaviour, such as alertness (e.g. the preparedness to respond to stimuli from the environment1), visuospatial attention (e.g. the voluntary or automatic orientation of attention towards visual targets across space2) and inhibition (e.g. the ability to withhold a response that is not suitable given the changing environmental information3) needs to be shared and integrated between different brain areas.
Initial concepts have suggested that the cognitive domains of visuospatial attention, alertness and inhibition are controlled by distributed, separate neural networks. For instance, a ventral and dorsal visual attention network,4 a vigilant attention network5 and an inhibitory control network6 have been described.
In other accounts, the functional connections between visuospatial attention, alertness and inhibition have led to the assumption that these cognitive domains depend on at least partially shared neural networks, as described in the fronto-parietal control network,7 the superordinate cognitive control network8,9 or, more recently, in the higher-order, ‘multiple-demand’ (MD) network.10–12 These network models encompass similar cortical regions, such as the lateral frontal surface, the dorsomedial frontal cortex (including presupplementary motor area and dorsal anterior cingulate), areas in and around the anterior insula, the intraparietal sulcus and often also a region at the occipitotemporal border.7,8,10
The close functional and anatomical relationship between visuospatial attention, alertness and inhibition has mainly been described in healthy subjects.10–13 This leads to the hypothesis that these cognitive functions should often be concomitantly impaired in patients with brain lesions, for example after stroke. This hypothesis is in line with results from observational studies in patients with right-hemispheric lesions and signs of spatial neglect, in whom visuospatial attention towards the contralesional space is typically impaired, and a concomitant decrease in alertness and inhibition seems to be often associated.14–17
In the present study, we aimed to investigate whether and how strongly the three considered cognitive domains (i.e. visuospatial attention, alertness and inhibition) relate to each other, behaviourally and at the neuroanatomical level, in stroke patients. More precisely, we aimed to investigate whether impairments in these three cognitive domains co-occur in stroke patients and whether this co-occurrence can be explained by a lesion to a common neural substrate.
These three cognitive domains were chosen on the basis of their importance for successfully performing activities of daily living.18,19 Previous studies have separately shown that these three cognitive domains are often impaired after right-hemispheric brain lesions.5,20–22 Hence, we applied a data-driven, bottom-up approach in a sample of 60 first-ever, right-hemispheric, subacute stroke patients. For each patient, four standardized and commonly used neuropsychological and oculomotor tests were administered to comprehensively assess each of the three cognitive domains: visuospatial attention, alertness and inhibition (resulting in a total of 12 tests). A principal component analysis (PCA) assessed the patients’ common patterns of performance across the 12 tests. Three lesion analysis techniques (voxel-based lesion-symptom mapping (VLSM), tractwise lesion-symptom mapping (TLSM) and disconnectome maps) determined the lesion and network correlates of the performance components. Finally, cognition during daily living23 was assessed by independent therapists, who were blind with respect to the study aims, to compare and ecologically validate the measures obtained on a test level with measures of cognitive performance in everyday life. Conclusively, an analysis using a probabilistic functional atlas of the MD network24,25 was performed to confirm the potential relationship between the patients’ lesion substrates and the observed cognitive impairments as a function of the MD network connectivity disruption.
Materials and methods
Patients
Sixty patients with a first-ever subacute right-hemispheric stroke were included in this prospective study: 25 female; mean age = 74.400 years (SD = 10.081, range 50–90); days since stroke mean = 19.783 (SD = 10.441, range 5–65); years of education mean = 11.183 (SD = 2.633, range 6–16); 91.667% right handed (two left-handed, one ambidexter, two originally left-handed but retrained to right); 43 ischaemic, 17 haemorrhagic stroke), an overlay plot of the lesions of all 60 patients is shown in Supplementary Fig. 1. All patients were admitted to the Neurocenter of the Cantonal Hospital in Lucerne, Switzerland, to receive multidisciplinary inpatient neurorehabilitation, and were consecutively enrolled in the study after giving informed consent between January 2018 and March 2020.
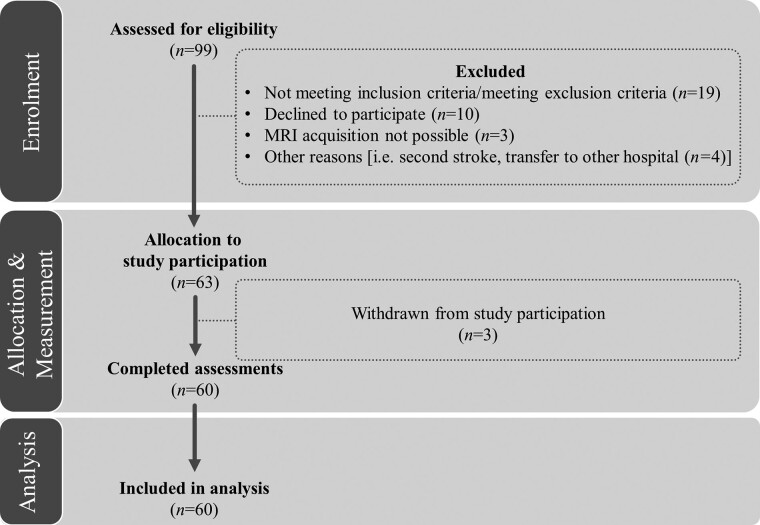
Apart from a history of first-ever right-hemispheric stroke, the main inclusion criteria were age >18 years, normal or corrected-to-normal visual acuity and being able to undergo an MRI scan. Exclusion criteria were the presence of other neurological diseases (e.g. epilepsy, multiple sclerosis, tumour etc.), major psychiatric disorders and alcohol/drug abuse (Fig. 1). By excluding left-hemispheric stroke patients, who often show aphasia and/or other language disorders,26 we aimed to ensure not to confound our results with difficulties in understanding the task instructions.
Figure 1.
Consort flow diagram. Patients’ inclusion flow-chart based on the CONSORT 2010 guidelines: 99 patients were assessed for eligibility. Apart from a history of first-ever right-hemispheric stroke, the main inclusion criteria were age >18 years, normal or corrected-to-normal visual acuity, and being able to undergo an MRI scan. Exclusion criteria were other neurological diseases, major psychiatric diagnoses and alcohol/drug abuse. 63 patients were allocated to study participation, from which three patients withdrew for personal reasons. In the end, 60 patients completed the assessments and were included in the final analyses.
The study followed the STROBE guidelines for reporting observational studies27 and was conducted in accordance with the principles laid down in the Declaration of Helsinki (WHO, 2013). The study was approved by the local Ethics Committee (Ethics Committee Nordwest and Zentralschweiz, Switzerland).
Behavioural data acquisition and analysis
For each patient, four standardized and commonly used neuropsychological and oculomotor tests were administered to comprehensively assess each of the three cognitive domains visuospatial attention, alertness and inhibition (resulting in a total of 12 tests, summarized in Table 1). To assess visuospatial attention, the Letter Cancellation Test [centre of cancellation (CoC) of cancelled items],28 the Line Bisection Test (mean relative deviation from actual midline),29 the Five-Point Test (CoC of drawn designs)16 and video-oculography during free visual exploration (FVE; mean gaze position35,45) were performed. Alertness was assessed by means of two subtests of a computerized, validated attention test battery (median reaction time in tonic and phasic alertness of the Testbatterie für die Aufmerksamkeitsprüfung, TAP30) and two outcome variables of the FVE paradigm (mean fixation duration,39 and peak saccade velocity46). To investigate inhibition, three neuropsychological measures (perseverative errors in the Five-Point Test,33 number of errors in a Go-NoGo task,31,32 number of errors in the Stroop interference task34,47) and one video-oculographic measure (false responses in the antisaccade task38,40) were used.
Table 1.
Overview of neuropsychological tests and oculography paradigms included in the study
| Visuospatial attention | Alertness | Inhibition | |
|---|---|---|---|
| Neuropsychological tests | Letter Cancellation Test: spatial distribution (CoC) of cancelled items28 Line Bisection Test: mean relative deviation from actual midline29 Five-Point Test: spatial distribution (CoC) of drawn designs16 |
TAP tonic alertness: median reaction time30 TAP phasic alertness: median reaction time30 |
FAB Go-NoGo: number of errors31,32 Five-Point Test: number of perseverative errors33 Stroop34: Number of errors in the interference test |
| Oculography paradigms | FVE: Mean gaze position35 | FVE: Mean fixation duration36 FVE: Mean peak saccadic velocity37 |
Antisaccade task: Number of errors38 |
CoC = Center of Cancellation; FAB = Frontal Assessment Battery; FVE = Free Visual Exploration; TAP = Test of Attentional Performance.
For a detailed description of the 12 neuropsychological tests and the respective outcome variables, as well as of the video-oculography paradigms and apparatus, please see the Supplementary material.
Statistics
To allow a direct comparison between variables, all outcome variables were z-transformed, based on the normative values of the respective healthy control groups.16,30,32,34,36–38,41,44
Descriptive statistics
The results of all outcome variables were plotted by means of violin wrapping box-and-whisker plots, to qualitatively evaluate the overall variability of the outcome variables included.
Furthermore, for each patient, the severity of deficits within each cognitive domain was plotted by means of the number of clinically significant test results [i.e. how many out of the four tests per cognitive domain (i.e. 12 in total) had standardized scores of z < −1.5], as defined with respect to the performance of healthy controls48; see Table 1 for references to the respective normative datasets) using box-and-whisker plots.
Principal component analysis
A PCA was performed to explore potential common factors underlying the three cognitive domains (visuospatial attention, alertness and inhibition) investigated in our data sample.
As a part of the PCA, Pearson's correlations coefficients were computed and tested for significance (one-tailed) between all pairs of outcome variables. Then, the PCA was conducted on the 12 outcome variables (as described in Table 1) without rotation. The Kaiser–Meyer–Olkin measure was used to verify the sampling adequacy for the analysis.42 The Bartlett’s test of sphericity was used to investigate whether the correlations between the 12 outcome variables were sufficiently large for a PCA. Kaiser’s criterion was used to define the number of components that were retained in the final analysis.
In the PCA, missing data were replaced by the function ‘mean’, as implemented in SPSS v.27 (number of missing data replaced: FVE n = 3; Five-Point Test n = 2; antisaccade task n = 6; TAP tonic/phasic alertness n = 2; Go-NoGo n = 3; STROOP n = 6). All outcome variables with a factor loading of ≥0.40 on a given component were considered as relevant.42,49 The patients’ individual factor values per component were then computed and used as predictors for the VLSM analysis, as described in the following.
For all analyses, a P-value of <0.05 was considered as statistically significant.
Neuroanatomical data and analysis
MRI acquisition and lesion mapping
High-resolution MRI were acquired in all patients, using two sequences: (i) a fluid-attenuated inversion-recovery sequence (TR/TE = 5000/389 ms, slice thickness = 0.9 mm, voxel size = 0.4 × 0.4 × 0.9 mm), which was used for identification and demarcation of lesions; (ii) a magnetization prepared rapid acquisition gradient echo sequence (TR/TE = 2240/3.72 ms, slice thickness = 0.9 mm, voxel size = 0.9 × 0.9 × 0.9 mm), which was used to enhance the quality of normalization. Lesion mapping was performed as outlined in Karnath et al.50 In short, lesions were manually delineated on the patients’ individual MRI images using the MRIcron software (https://www.nitrc.org/projects/mricron). Images were then normalized into the Montreal Neurological Institute (MNI) space using the Clinical Toolbox for SPM12 (Rordon et al.43; https://www.nitrc.org/projects/clinicaltbx/), applying enantiomorphic normalization51 (SPM12, http://www.fil.ion.ucl.ac.uk/spm; MATLAB, MathWorks Inc., Natick, MA, USA).
We used VLSM to establish causal inferences between behaviour and underlying neuroanatomical structures.52–54 Combined lesion analysis and (dis)connectome analysis, using TLSM and disconnectome maps, were further used to determine whether lesions at different sites causing similar symptoms were located within the same neural network.52,55
Voxel-based lesion-symptom mapping
Previous studies showed that 3D MRI scans are highly valuable in assessing the relationship between disconnected areas and the patients’ neuropsychological performance.55 To establish the potential brain-function relationship between lesion location and PCA results, standard VLSM analyses were conducted using the open source NPM software (https://www.nitrc.org/projects/mricron). VLSM was conducted using the Brunner–Munzel test for continuous behavioural data,56 using the individual factor values for each component as derived from the PCA, as described previously. Only voxels that were lesioned in ≥20% of the patients were included in the analysis, and multiple comparisons were controlled for using a permutation-based threshold, applying 4000 iterations.57,58 The significance threshold was adjusted by means of a false discovery rate approach (criterion of 0.05) to control for type I errors.
Tractwise lesion-symptom mapping
The significant lesion clusters predicting PCA factor values, as identified by the VLSM analyses, were located within the cerebral white matter (see VLSM in ‘Results’ section). Therefore, Tractotron (a part of the BCBtoolkit,55http://www.toolkit.bcblab.com/) was used to compute the probability that specific tracts would be affected by the lesions, as well as to calculate the damaged proportion of the respective tracts for each patient. Among the 68 white matter tracts available in the BCBtoolkit library, we selected the tracts that showed an overlap with the significant lesion clusters predicting PCA factor values in the VLSM analyses. These tracts were: the frontal aslant tract (FAT), the superior longitudinal fasciculus II (SLF II), the superior longitudinal fasciculus III (SLF III) and to a lesser extent the inferior fronto-occipital fasciculus (IFOF).
On the basis of the VLSM results, we assumed that white matter tract disconnections would result in a decline of cognitive performance, as reflected by the factor values in the respective PCA component. Therefore, one-tailed Pearson's correlations (Bonferroni-corrected for multiple comparisons) were calculated between PCA factor values and disconnection probabilities, as well as the damaged tract proportions, for each white matter tract.
Disconnectome maps
To account for potential effects beyond focal lesions, Disconnectome maps were calculated using the BCBtoolkit.55 The toolkit includes healthy control subjects’ diffusion weighted imaging datasets,59 which are used to estimate the fibres passing through each lesion. For each of the 60 patients included in the present study, tractography was estimated as described by Thiebaut de Schotten and colleagues.2 In brief, each patient’s lesion was registered to native space of the healthy control group, using affine and diffeomorphic deformations,60,61 and subsequently used as seed for the tractography in Trackvis (http://trackvis.org/). Tractographies from the lesions were then transformed in visitation maps,2 binarized and brought to MNI space. The corresponding percentage overlap map was computed by summing the normalized visitation map of each healthy control subject at each point in MNI space. Hence, in the resulting disconnectome map of each individual patient, the value in each voxel considers the interindividual variability of tract reconstructions in the healthy control group. The value for each voxel indicates the probability of disconnection, ranging from 0 to 100%, for a lesion in each individual patient.62
To establish the potential relationships between white matter tract disconnections (as reflected by disconnectome maps) and behavioural correlates (as reflected by PCA results), a standard VLSM analysis for continuous data was conducted on the disconnectome maps, with the same procedures described before (VLSM). For this purpose, we used a region of interest approach. The region of interest was defined as the total, summed extension of the tracts that intersected a significant lesion cluster predicting PCA factor values, as identified in the first series of VLSM analyses. These tracts were the FAT, SLF II, SLF III and IFOF.2,59 To define the region of interest, the probability of voxels belonging to a given tract was set at >50%).
Test-level cognition and cognition during daily living
First, to establish the potential relationship between PCA results and cognitive performance, PCA loadings were correlated with the number of tests showing clinically relevant impairment (z < −1.5) in the three considered cognitive domains (visuospatial attention, alertness and inhibition).
Second, to investigate the relationship between cognitive performance as reflected by test results and as observed in daily living, the Lucerne ICF-Based Multidisciplinary Observation Scale (LIMOS) was used.23,63 The LIMOS is a sensitive, reliable and valid scale for the multidisciplinary observation of stroke patients’ ability to perform activities of daily living,23 which includes four subscales: motor, cognition, communication and domestic life. Thereby, the LIMOS cognition subscale consists of 15 items observing cognitive functions in daily living, such as planning tasks, solving simple problems and making decisions.23 Each item is scored from 1 (‘patient is not able to fulfil a task or needs assistance up to 75%’) to 5 (‘patient is able to fulfil tasks independently’), leading to a score ranging from 15 to 75.23 The LIMOS was rated by independent therapists, who were blind with respect to the study aims. To investigate the potential relationship between PCA factor values and cognition during daily living (as reflected by the LIMOS cognition subscale), Pearson’s correlations were calculated (two-tailed). Additionally, to investigate whether cognition during daily living is influenced by the volume of the affected brain area, a partial correlation was calculated between test-level cognition (as represented by PCA values) and LIMOS cognition subscale scores, while controlling for lesion volume (two-tailed).
Third, to confirm the potential relationship between lesions to critical cerebral substrates and cognitive performance in daily living, we compared the LIMOS cognition subscale scores between patients with versus without a lesion including the significant lesion clusters predicting PCA factor values (i.e. SLFII, SLF III, FAT intersection and putamen/IFOF, as identified in the VLSM analysis). The scores were statistically compared by means of an independent-samples t-test.
Functional connectivity
To confirm the potential relationship between the patients’ lesion substrates and the observed cognitive impairments (test-level cognition and cognition during daily living) as a function of the MD-network connectivity disruption, we used a probabilistic functional atlas of the MD network25 and calculated MD-weighted lesion volumes.24 In short, the probabilistic functional atlas contains the probability of belonging to the MD network for any given location in the brain. Hauptman et al.25 constructed the probabilistic functional atlas using data from 691 healthy participants. The atlas includes voxels with a network probability range of 0.001 to 0.75, representing the ‘proportion of participants for whom that voxel belongs to the top 10% of localizer-responsive voxels’. Each of our patient’s lesion was weighted with respect to the probabilistic functional atlas,24,25 and the corresponding MD-weighted lesion volume was correlated with test-level cognition (represented by PCA factor values) as well as with cognition during daily living (as reflected by the LIMOS cognition subscale scores; a two-tailed, non-parametric correlation was applied after visual inspection of the distribution of the MD-weighted lesion volume).
Data availability
The conditions of our ethics approval do not permit the public archiving of the data supporting the conclusions of this study. On the basis of the Swiss Human Research Act, the HRA (Humanforschungsgesetz) in Switzerland, readers seeking access to the data and the study materials must therefore complete a formal data sharing agreement to obtain the data. Interested readers should contact the corresponding author for more information and help.
Results
Descriptive statistics
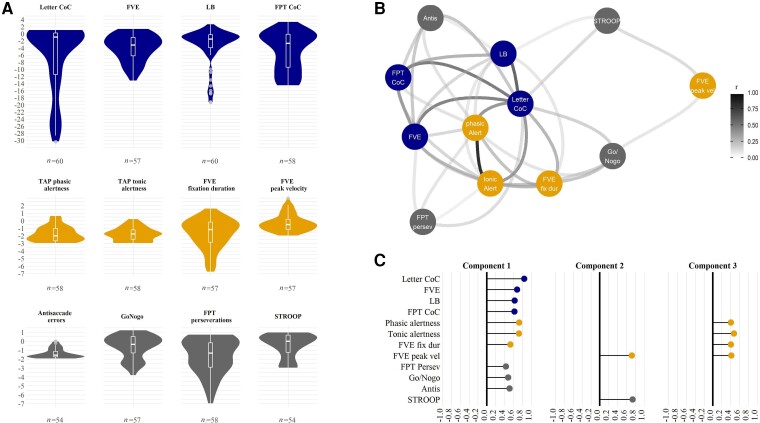
The violin wrapping box-and-whisker plots, depicting the patients’ individual severity of deficits in all considered cognitive domains and variables, revealed a broad variability across patients (Fig. 2A). Heterogeneous distributions were found in all three cognitive domains: visuospatial attention, alertness and inhibition. A similar pattern was observable in the box-and-whisker plots (Supplementary Fig. 2) depicting the number of tests per cognitive domain in which individual patients showed a clinically relevant impairment (i.e. z < −1.5).
Figure 2.
Behavioural analyses for the three cognitive domains. (A) The violin wrapping box-and-whisker plots of all z-transformed outcome variables included in the study. The width of the violins represents the proportion of patients with an equivalent z-value. The overall median z-values are indicated by the horizontal white line in each box-and-whisker plot. Each box represents the lower (Q1) to the upper (Q3) quartiles, with whiskers extending from the minimum to the maximum of 1.5 times the interquartile range. The number of available patient datasets for each variable is depicted at the bottom of each violin. Outliers are depicted by grey circles. Blue represents outcome variables typically measuring visuospatial attention (Letter Cancellation Tests = Letter CoC; Line Bisection Test = LB; CoC in the Five-Point Test = FPT CoC; mean gaze position during FVE), yellow represents outcome variables typically measuring alertness (TAP phasic alertness = phasic Alert; TAP tonic alertness = tonic Alert; mean fixation duration during FVE = FVE fix dur; peak saccade velocity during FVE = FVE peak vel), grey represents outcome variables typically measuring inhibition (percentage of perseverative errors in the Five-Point Test = FPT Persev; Go-NoGo paradigm of the FAB = Go-NoGo; errors in the Stroop Interference condition = STROOP; antisaccade errors = Antis). (B) The significant correlations between all 12 variables included in the PCA. The lines between variables represent significant correlations and their strength: the darker the shade, the stronger the correlation, as represented by the legend on the right-hand side of the panel. (C) The principal components extracted from outcome variables of the cognitive domains of visuospatial attention, alertness and inhibition, with factor loadings >0.40. The length of the bars represents the loading of each outcome variable onto the extracted factor components. The components were named as follows: Component 1 = common component; Component 2 = inhibition/alertness component; Component 3 = alertness component. The figure was illustrated using the R package ggplot2.159,160
Principal component analysis
First, to investigate whether patients with higher impairment in one cognitive domain also presented with increased deficits in other cognitive domains, Pearson's correlations were computed. Pearson’s correlations between all pairs of outcome variables showed significant results for several variable combinations (Fig. 2B). Hereby, outcome variables of the visuospatial attention domain correlated with each other, but also with some of the outcome variables associated with the alertness and the inhibition domain.
These results indicate, at least for some of the considered variables, the existence of common underpinnings for all three cognitive domains. Hence, to explore in a more systematic way the common underlying components of the considered cognitive domains, a PCA was computed.
For the PCA including all 12 outcome variables, the Kaiser–Meyer–Olkin measure (KMO = 0.720, to be interpreted as ‘good’42) supported the sampling adequacy for the analysis. Bartlett’s test of sphericity [χ²(66) = 187.863, P < 0.001] indicated that the correlations between outcome variables were sufficiently large for the PCA to be performed. An initial analysis was run to obtain eigenvalues for each component in the data. Three components had eigenvalues above Kaiser’s criterion of 1, and in combination explained 56.341% of the total variance (component 1 explaining 34.175%, component 2 explaining 12.093% and component 3 explaining 10.073% of the variance, respectively; Fig. 2C).
Ten out of the 12 variables showed medium to strong loadings on the first component (all factor loadings ≥0.431; Fig. 2C). Four out of these 10 variables belonged to the visuospatial attention domain (Letter CoC,41 mean gaze position during FVE,35 Line Bisection,64 CoC in the Five-Point Test16), three to the alertness domain (phasic alertness,30 tonic alertness,30 Fixation Duration65) and three to the inhibition domain (Perseverations in the Five-Point Test,16 Go-Nogo31 and Antisaccade Errors38). Therefore, this component was named the common component.
The outcome variables that clustered on the second component (all factor loadings ≥0.722) belonged to the alertness (FVE peak saccade velocity46) and the inhibition (Stroop34,47) domains, and were therefore named the inhibition/alertness component. A third component consisted of all four variables belonging to the alertness domain, namely phasic alertness, tonic alertness, fixation duration and FVE peak saccade velocity (all factor loadings ≥0.408), and was therefore named the alertness component.
Neuroanatomical data
Voxel-based lesion-symptom mapping
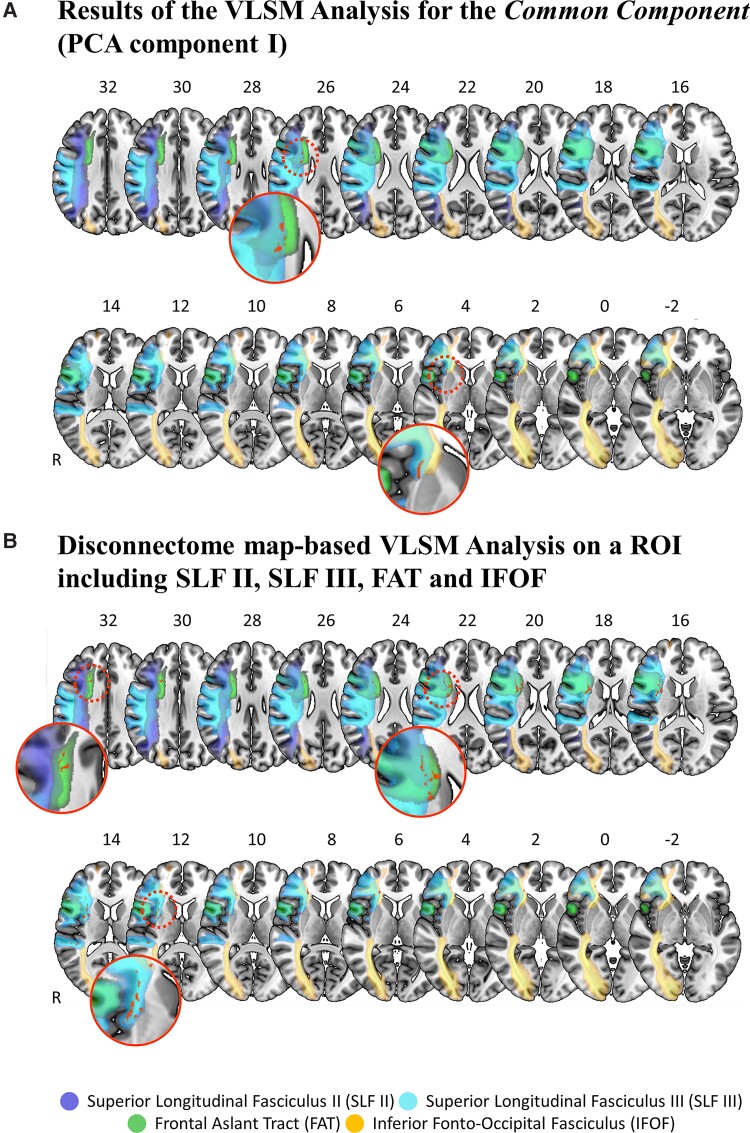
To ascertain whether the three PCA components would rely onto discrete anatomical substrates, VLSM analyses were performed. For the common component (i.e. the first PCA component), the analysis revealed a total of 325 significant voxels (0.33 cm3 in total). A larger cluster was located in the FAT, the SLF II and the SLF III [MNI coordinates of the centre of mass of the cluster: 28, −2, 26, located at the intersection of the right SLF II (probability of the significant voxels to belong to SLF II of 90%2), the right SLF III (probability of 100%2) and the right FAT (probability of 100%2; Fig. 3A, top row)]. It is of note that a comparison of the location of the critical lesion cluster lying within the SLFII/III/FAT intersection and the location of the maximum lesion overlap (Supplementary Fig. 3) shows that these two locations do not match. This speaks against a simple bias in terms of a non-specifically higher frequency of lesions in the area of the identified critical cluster.
Figure 3.
Neuroanatomical analysis. (A) The results of the VLSM analysis using the PCA factor values of the common component (i.e. PCA Component I) as predictive values. The results show two significant lesion clusters (red, with a total volume of 325 voxels). The first and larger cluster (top row) is located within the second branch of the SLF (SLF II, dark blue), the third branch of the SLF (SLF III, light blue) and the FAT (green). The second and smaller cluster (bottom row) is located within the SLF III, the anterior part of the putamen and the IFOF (yellow). Patients with right-hemispheric stroke presenting with a lesion within these clusters were significantly more likely to show an impairment in overall cognitive performance in all three considered cognitive domains, as reflected by the lower factor values in the common component (PCA Component I). (B) The results of the disconnectome map analysis for a ROI including all four white matter tracts identified as affected by the previous VLMS analysis, i.e. the SLF II, the SLF III, the FAT and the IFOF. Patients with right-hemispheric stroke presenting with a lesion within these clusters were significantly more likely to show an impairment in overall cognitive performance in all three considered cognitive domains, as reflected by the lower factor values in the common component. For both panels, lesion voxels that were a significant predictor for the common component factor values are depicted in red (significance level P < 0.05, based on the Brunner–Munzel test, false discovery rate-corrected, 4000 permutations). Lesion clusters and white matter tracts are displayed on the MNI152 template in MNI space, as available in MRIcroGL (https://www.nitrc.org/projects/mricrogl/). The axial slices are oriented according to the neurological convention. The position of each slice in MNI space is indicated by numbers at the top of the respective slices. White matter tracts are depicted according to published probabilistic diffusion tensor imaging atlases2,59 (the probability of voxels belonging to the SLF II (in dark blue), SLF III (in light blue), the FAT (in green) and the IFOF (in yellow) was set at ≥50%).
A smaller cluster was found in the putamen and the IFOF: MNI coordinates of the centre of mass of the cluster were 30, 16 and 2, involving the right SLF III (probability of 100%2), the right IFOF; probability of 96%2) and the putamen (probability of 84%, according to the MNI structural atlas66,67; Fig. 3A, bottom row).
VLSM analyses for the second and the third PCA component, i.e. the inhibition/alertness component and the alertness component, did not yield any significant results.
Because only the common component showed significant lesion correlates, further neuroanatomical lesion analyses were performed only on this component.
Tractwise lesion-symptom mapping
For the common component, the Bonferroni-corrected Pearson's correlations (i.e. corrected considering four comparisons, corresponding to the main intersection found in the VLSM analysis, including the three tracts SLF II, SLF III and FAT, as well as the IFOF from the second, smaller VLSM cluster; resulting in a corrected critical P-value of ≤0.0125) revealed that impaired cognitive performance (represented by lower factor values) significantly correlated with a higher disconnection probability, as well as with an increased damaged tract proportion, within all the four considered white matter tracts (disconnection probability: FAT r = −0.322, P = 0.012; SLF II r = −0.299, P = 0.020; SLF III r = −0.373, P = 0.003; IFOF r = −0.378, P = 0.003. Damage proportion: FAT r = −0.442, P < 0.001; SLF II r = −0.369, P = 0.004; SLF III r = −0.390, P = 0.002; IFOF r = −0.397, P = 0.002).
Disconnectome maps
The region of interest-restricted Brunner–Munzel test including the PCA factor values of the common component revealed that patients presenting with more severe cognitive impairment (represented by lower factor values) were more likely to show a lesion at the intersection of SLF II, SLF III and FAT white matter tracts, as well as along the FAT, the SLF III and the IFOF (significant lesion cluster with a total volume of 1640 voxels,2 1.64 cm3; Fig. 3B). The largest cluster was found to belong to the FAT (702 voxels, 1.455% of the FAT tract), followed by SLF III (937 voxels, 0.982% of the tract), IFOF (328 voxels, 0.436% of the tract) and SLF II (164 voxels, 0.175% of the tract).
Test-level cognition and cognition during daily living
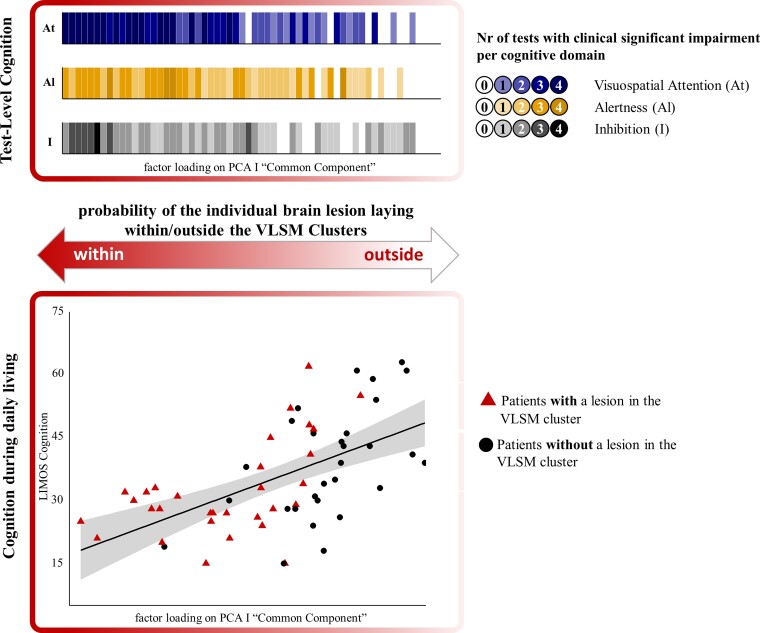
The factor values of the common component were significantly correlated with the number of tests showing clinically relevant impairment (i.e. z < −1.5) in the three cognitive domains, respectively: visuospatial attention (r = −0.783, P < 0.001), alertness (r = −0.687, P < 0.001) and inhibition (r = −0.661, P < 0.001). To illustrate these results, we plotted the number of tests showing a clinically relevant impairment in each considered cognitive domain against the factor values of the common component (Fig. 4, top). These plots showed that patients with smaller PCA values (y-axis) also showed a higher number of clinically relevant deficits in all three cognitive domains [as represented by darker colour shades; visuospatial attention (blue), alertness (yellow) and inhibition in (grey)].
Figure 4.
Clinical relevance. The relationship between factor values of the common component (i.e. PCA Component I), the number of tests with clinical significant impairments (top) and cognition during daily living (bottom) is shown. Top shows a clinically significant impaired behaviour in a larger number of tests measuring visuospatial attention (At, blue), Alertness (Al, yellow) and inhibition (I, grey) was accompanied by lower PCA values. Darker colours indicate a higher number of tests with clinically significant impairment (z < −1.5) in the respective cognitive domain. Bottom shows the individual PCA factor values on the common component (PCA Component I) significantly correlated with measures of cognitive performance in daily living (LIMOS cognition; P < 0.001, r = 0.575). Patients with a lesion involving the intersection of SLF II/III and FAT as well as Putamen/IFOF (red triangles) showed a more severe impairment in cognition during daily living than patients with a lesion not involving the aforementioned VLSM clusters [black circles; t(58) = −2.507, P = 0.015]. The probability of an individual brain lesion being in or outside the VLSM clusters is further depicted by the double-headed arrow. The figure was illustrated using the R package ggplot2.159,160
In a next step, we aimed to ecologically validate the results, ascertaining whether they would extend beyond the clinical assessments level, i.e. whether patients with more severe deficits on a test level (represented by the factor values of the common component) and a brain lesion involving the intersection of the SLF/FAT and the putamen/IFOF (as suggested by the VLSM analysis) would also show more severe cognitive deficits in the activities of daily living. Hence, to further move towards such real-world scenarios, cognition during daily living was assessed by independent therapists, and a potential transfer effect from the level of clinical test scores (i.e. the common component) to the level of cognition in everyday behaviour (i.e. LIMOS cognition) was investigated using Pearson’s correlation. Our results showed that patients with more severely impaired test-level cognition (as represented by lower PCA values) also presented with more severely impaired cognition during daily living (r = 0.575, P < 0.001; Fig. 4, bottom; a partial correlation controlling for lesion volume revealed the same significant result, r = 0.548, P < 0.001, showing that lesion volume was not a major determinant for this relationship).
Finally, we compared LIMOS cognition scores between patients with versus without lesion in the SLFII/SLFIII/FAT and putamen/IFOF Cluster (as indicated by the neuroanatomical analysis presented above), and found that LIMOS cognition was significantly more severely impaired in patients whose lesion laid within the SLFII/SLFIII/FAT and the putamen/IFOF Cluster [t(58)=−2.101, P = 0.040; n = 31 with a lesion within the cluster, represented by red triangles in Fig. 4, bottom, mean = 32.23, SD = 11.40; n = 29 without a lesion within the cluster, represented by black circles in Fig. 4, bottom, mean = 38.93, SD = 13.30].
Functional connectivity
To confirm the potential relationship between the critical cerebral substrates of the MD-network we used a probabilistic functional atlas25 to calculate the MD-weighted lesion volume.24 The corresponding MD-weighted lesion volume significantly correlated with test-level cognition (represented PCA factor values; Spearman’s rho = −0.295, P = 0.022) as well as cognition during daily living (as reflected by the LIMOS cognition subscale; Spearman’s rho = −0.312, P = 0.015). This supports our previous neuroanatomical and behavioural results suggesting that a lesion involving the MD network is associated with impaired cognition on a test level as well as during daily living.
Discussion
The aim of the present data-driven study was to investigate how the three cognitive domains of visuospatial attention, alertness and inhibition relate to each other, both on a behavioural and neuroanatomical level. To this end, each cognitive domain was comprehensively assessed by means of four standardized and commonly used neuropsychological and oculomotor tests, within a large, heterogeneous sample of first-ever right-hemispheric subacute stroke patients. The PCA demonstrated that the three cognitive domains are strongly related to each other: 10 out of the 12 tests loaded on a first common component. Besides, two tests loaded on a second, inhibition/alertness component and four tests loaded on a third, alertness component.
Next, we assessed the neuroanatomy of the lesions underlying these three components. VLSM analyses using factor loadings of the inhibition/alertness component or the alertness component did not reveal any significant effects. However, the VLSM analysis using the factor loadings of the common component revealed that right-hemispheric stroke patients presenting with more severe cognitive impairment were significantly more likely to show a lesion involving the intersection of the SLF II, SLF III and FAT (larger cluster), as well as the right SLF III and some voxels in the anterior part of the putamen and the IFOF (smaller cluster).
In line with these results, further analyses with TLSM and disconnectome maps revealed that impaired cognitive performance in the common component significantly correlated with a higher disconnection probability, as well as an increased damaged tract proportion, within the SLFII, SLFIII, FAT and putamen/IFOF.
To ecologically validate these findings, we next asked how a lesion involving the intersection of SLFII/SLFIII/FAT and the putamen/IFOF would influence cognition during daily living. To this end, independent clinicians, who were blind to the study aims, evaluated the cognitive part of the LIMOS.23,63 Our results revealed that patients with more severely impaired test-level cognition (represented by the factor values of the common component) and a brain lesion involving the intersection of the SLFII/SLFIII/FAT and putamen/IFOF also presented with significantly more severely impaired cognition during daily living.
A further analysis using a probabilistic functional atlas of the MD network24,25 indicated a relationship between the patients’ lesion substrates and the observed cognitive impairments as a function of the MD-network connectivity disruption.
Cognitive tests
On a behavioural level, the present study revealed a heterogeneous distribution of cognitive test results in our unselected patient sample. This pattern is typical for studies investigating cognition after stroke, reflecting the heterogeneity of deficits and their severity on an individual level, which are partially associated with specific lesion locations, as shown in previous studies.68
Crucially, however, our analyses were able to identify within this heterogeneity a common ground between cognitive impairments in visuospatial attention, inhibition and alertness. Indeed, correlational analyses revealed significant results not only between outcome variables within the same cognitive domain, but also between outcome variables belonging to different cognitive domains. Moreover, our PCA revealed a meaningful and coherent behavioural component (the common component) that entailed outcome variables of all three cognitive domains and on which 10 out of the 12 outcome variables loaded.
All four visuospatial attention outcome variables and three out of four alertness outcome variables loaded on the common component. Our bottom-up analyses in a large and unselected patient group thus supports the view of a strong interplay between visuospatial attention and alertness, proposed by previous studies both in healthy subjects and in stroke patients.69–72 As a novel and important finding, in addition three typical inhibition outcome variables (false responses in the antisaccade task, errors in the Go-NoGo task, perseverative errors in the Five-Point Test) also loaded on the same PCA component. These three tests involve reactive inhibition to a stimulus, and a component of proactive inhibition when anticipating the possibility of cancelling a prepared action.73 More precisely, the patient must intentionally supress an action, such as looking at an appearing target in the antisaccade task, imitating a hand movement in the go-no go test or repeating a design in the Five-Point Test. It is therefore plausible to postulate that this kind of inhibition, which is intentional, controlled and effortful, is supported by visuospatial attention and alertness. Furthermore, focused visuospatial attention, combined with high alertness and resistance to distraction, are important aspects common to many cognitive tasks.11,74–76
Although proactive, goal-directed inhibition is also an important component of the Stroop task,73 additional functions, such as response selection under competition, are necessary to accomplish this task. Moreover, to suppress a prepotent response (reading the word) and name the colour of the ink in which the word is written instead, conflict resolving is mandatory.77 These might be possible reasons as to why the Stroop task did not load together with all the other outcome variables, but instead, together with the alertness measure of peak saccade velocity, loaded on a second, independent component (i.e. inhibition/alertness component). Hereby, fatigue, reflected in a decrease in saccade velocity,37,78,79 may result in more frequent inhibition failures, which is also a well-known phenomenon.80 Also, peak saccade velocity has been discussed in the context of cognitive control. For example, in case of an already initiated saccade, a lower peak velocity may reflect an effort to inhibit the error as it is being executed.81 In line with this suggestion, the present results revealed that patients who were not able to inhibit cognitive interference (as reflected in an increase in errors in the Stroop task) also showed a reduced ability to sustain saccadic performance (as represented in an increase in peak velocity).
Finally, all four alertness tests loaded on a third alertness component, which demonstrates the strong link between different aspects of alertness.30,39,46 Indeed, the TAP phasic and tonic alertness test is widely used in adult clinical neuropsychology, and previous studies showed a close connection between the two measures.71 Also, mean visual fixation duration82 and peak saccade velocity83 are known to be a sensitive index of the degree of alertness.
Brain networks
To investigate the neuroanatomical landmarks related to the PCA common component, three conclusive analyses were performed: VLSM, TLSM and disconnectome map analyses. All three analysis approaches consistently showed that patients presenting with more severe cognitive impairments in the common component were also significantly more likely to show a lesion involving the intersection of the SLF II, SLF III and FAT, as well as the putamen/IFOF. The VLSM analysis further revealed that most of the significant voxels was associated with the FAT, the SLF II and the SLF III.
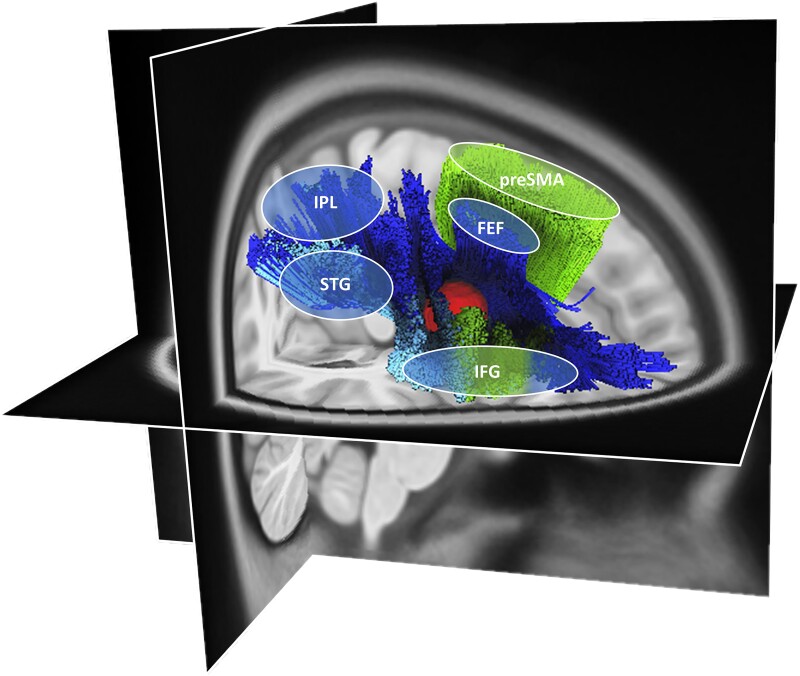
The SLF II, SLF III and FAT interconnect the inferior parietal lobule (IPL), the superior temporal gyrus (STG), the frontal eye field (FEF), the inferior frontal gyrus (IFG) and the presupplementary motor area (pre-SMA2,4,69,84–91). This extended network of interconnected cortical areas is thus compatible with the idea of their relevance not only to a single, but rather to several cognitive domains. Indeed, the IPL, STG, FEF, IFG and pre-SMA are all nodes of the cortical network subserving visuospatial attention.4,92–101 Furthermore, alertness has been shown to be regulated by the identical cortical areas (IPL,22,102 STG,103,104 FEF,105 IFG,71,106 pre-SMA107). Finally, the IPL,108,109 STG,110–112 FEF,113,114 IFG20,115–117 and pre-SMA86,118–120 have also been associated with inhibition control.
Supplementary analyses (Supplementary Fig. 4A–F and Supplementary Table 1) confirmed this notion: separate VLSM analyses, one for each outcome variable, led to significant clusters in different areas, but crucially also to a common FAT/SLFII/SLFIII intersection over all three cognitive domains.
Some of the parietal and frontal areas are also anatomically connected to the putamen. For instance, a study using fibre tractography121 has evidenced connectivity between the putamen and the IFG, as well as with rostral parietal areas, such as supramarginal gyrus at the border of STG.121 The putamen has been shown to be involved in visuospatial attention,95,121–123 and has also been suggested as a central component of the frontal-subcortical circuit involved in inhibitory processes of executive control.73,124 In this context, previous studies described the putamen as a hub connecting the networks subtending the control of visual attention and inhibition.16,121,125 Finally, the putamen has also been reported to be involved in alertness.126,127
The IFOF, which has been attributed to the ventral pathway of the human brain,128 mainly connects the frontal (IFG, MFG, dlPFC129) with the occipital lobe.59 However, the anatomic dissection of the IFOF130 and probabilistic tract-to-region connectome matrices131 further identified terminations in the IPL and the posterior part of the temporo-basal area. The IFOF has also been shown to contribute to visuospatial attention,132–134 as well as inhibition135 and alertness.136
Taken together, our findings show that visuospatial attention, alertness and inhibition are tightly connected not only on a behavioural, but also on a neuroanatomical level. A lesion to a discrete white matter location, coinciding with the crossroad of specific white matter tracts, seems to be able to determine a concurrent functional breakdown in all three domains. We therefore argue that beyond the historical segregated networks for alertness, visuospatial attention and inhibition, a common component/network is involved in these cognitive domains. This novel and intriguing finding is strongly reminiscent of the MD network model. Indeed, cognitive operations have been suggested to depend on different collaborators and their interaction, combining an underlying MD activity with more specialized systems.12,137,138 Also, on a neuroanatomical level, studies in humans139 and primates140,141 revealed common activation patterns in MD regions encoding information across very different tasks.10 Furthermore, cortical areas commonly reported as belonging to the MD network, reflecting the co-recruitment by multiple task demands in fMRI studies10,12 are identical to the cortical regions connected by the SLF II, SLIII and FAT, the critical white matter tracts identified in our study. This assumption is further supported by our additional analysis using a probabilistic functional atlas based on the fMRI data of >600 participants.24,25 Our results revealed that MD-weighted lesions are associated with test-level cognition of our patients, as well as their performance in cognition during daily living.
Rich inter- and intra-hemispheric connections are thought to be of crucial importance for the functioning of the extensive MD network, allowing information to be rapidly exchanged and integrated,10 and providing the brain with a mechanism to orchestrate cognition and constantly adapt behaviour to the ongoing conditions.142 Correspondingly, a recent diffusion tensor imaging study in healthy subjects postulated that the SLF and FAT white matter connections might be of central importance for the functioning of the MD network.143 Our findings confirm and extend this hypothesis in two ways. First, they show that a disconnection of right-hemispheric SLFII/III/FAT in a lesion model in stroke patients leads to impairments in several cognitive domains, as predicted by the notion of a MD network. Second, they highlight a critical and discrete neuroanatomical locus, i.e. the intersection between SLF II/SLFIII/FAT, as a particularly vulnerable spot within the network.
The role of the IFOF, the white matter tract affected by the second, smaller lesion cluster, is less straightforward to define within the concept of the MD network. So far, IFOF white matter connections seem not be discussed as typical ones in the relatively young literature on the MD-network structural connectivity. This opens at least two interim, speculative interpretations. First, considering—on the one hand—the previously mentioned anatomic dissection studies of the IFOF130 and very recent connectome matrices studies,131 which show terminations of this tract also in parietal and temporal areas, and—on the other hand—the present results, one may speculate that the IFOF has more to do with the MD network than previously assumed. Second, and mutually not exclusive, the MD network may conceptually and functionally share features with the ventral attention pathways, which have been shown to interconnect several multifunctional areas, and within which the IFOF plays an important connectivity role.128
Clinical relevance
Importantly, impaired cognitive performance was not only measurable on a test level, but also in the activities of daily living, which were rated by therapists who were blind to the study goals. The ecological validation of our results in daily living showed that patients with a lesion involving the intersection of SLF II/SLFIII/FAT and putamen/IFOF had significantly more severely impaired cognition during daily living than patients without a lesion in this region. More precisely, patients showed more severe difficulties in carrying out simple or complex actions, which are relevant to manage and complete the requirements of daily living.23,63 This extends earlier findings from dementia and traumatic brain injury patients, namely that the interaction of visuospatial attention, alertness and inhibition is an important determinant to successfully perform the activities of daily living.18,19
More generally, our findings confirm that white matter lesions can lead to a breakdown of large-scale brain networks, resulting in several associated cognitive deficits, whereas focal cortical damage provokes more circumscribed patterns of clinical impairment.26,144–148 Critically, here we show how damage to a strategic crossroad of white matter pathways can provoke even larger-scale disruptions of activity in a higher-order MD network, with consequent multiple deficits in different cognitive domains.
Towards an integrative model
We showed that the intersection between SLF II/SLFIII/FAT is particularly crucial. A frontal lesion (Fig. 5 in red) at this strategical intersection of fronto-frontal tracts (FAT connects the posterior part of the IFG and the pre-SMA86–89; Fig. 5 in green) and fronto-parietal tracts (SLF II connects the IPL with the FEF2,90,91 and SLF III connects the IPL and the STG with the IFG2,4,69,84,85; Fig. 5 in blue), may cause a widespread breakdown of connectivity through the whole right-hemispheric MD network, leading to simultaneous impairment in several cognitive domains such as visuospatial attention, alertness and inhibition, both on a test level as well as in cognition during daily living.
Figure 5.
A putative neuroanatomical model. The putative neuroanatomical model explains how a frontal lesion (red volume), located at the strategical intersection of fronto-frontal and fronto-parietal tracts, can disrupt multiple tracts interconnecting cortical areas within the MD network.10 In particular, the affected white matter fibre tracts are the SLF II (dark blue84,161), the SLF III (light blue84,161) and the FAT (green162). The SLF II and III are generally known to connect parieto-temporal areas to frontal areas (SLF II connects the IPL with the frontal eye field2,90,91 and SLF III connects the IPL and the STG with the IFG2,4,69,84,85). The FAT connects the posterior part of the IFG and the pre-SMA.86–89 The illustration was created using the HCP1065.2 mm template and the implemented automated fibre tracking tool, visualized on the respective T1-image implemented in DSIstudio (v.2021.12.03; available at http://dsi-studio.labsolver.org/).
Taken together, previous results and our current findings, which point towards the central importance of disconnection between cortical areas associated with the MD network, strongly suggest that a MD-network disorder is an important contributor to the occurrence and persistence of neglect signs. Neglect has indeed been conceptualized as a multicomponent syndrome,149–151 and many neglect patients do not only suffer from visual attention deficits and impaired alertness, but also present deficits in response inhibition (see for example Kaufmann et al.,16 Sieroff et al.,152 and Bartolomeo153). In addition, compensation of neglect signs may critically depend on inhibitory processes,153,154 which, as suggested by our results, are no longer available after a lesion at the SLFII/SLFIII and FAT intersection. Among other authors, we have indeed previously shown that inhibition failure, with ensuing repetitive behaviour, is influenced by a visual attentional gradient in neglect patients,16,155,156 and that inhibition failure can even increase neglect severity.16 Furthermore, rehabilitation studies showed that not only exercising visuospatial attention, but also training alertness and inhibition has a positive effect on neglect recovery.154,157,158
Limitations
Our study has some limitations. In our data-driven analysis, the cognitive domains of visuospatial attention, alertness and inhibition as outcome variables were chosen because of their common link with the activities of daily living18,19 and their association with right-hemispheric brain lesion.5,20–22 However, other cognitive domains (e.g. working memory) were not explored. Furthermore, as with any vascular lesion study, stroke lesions are dictated by the vascular architecture of the brain, wherefore critical regions with conserved vascular supply are likely to be underrepresented. Also, we did not include left-hemispheric stroke patients with language deficits such as aphasia. Therefore, future studies may want to investigate the importance of the observed lesion intersection, and its association with deficits in an even broader range of cognitive domains in both hemispheres. Finally, future studies are needed to characterize the potential impact of MD-network lesions on the therapeutic effects of conventional therapy approaches, e.g. by investigating the effects of total and partial white matter disconnection (SLF, FAT, IFOF) between MD-related brain areas on therapy outcome.
Conclusion
In conclusion, the present study highlights that visuospatial attention, alertness and inhibition share common grounds on the behavioural as well as the neuroanatomical level. Correlational analyses revealed significant results not only between behavioural outcome variables of the same cognitive domains, but also between outcome variables of different cognitive domains. Fittingly, lesions critically involving the intersection of white matter connections between parieto-frontal areas (typically SLF II/III, to a lesser extent IFOF) and between pre-SMA/IFG (FAT), were shown to determine a concurrent functional breakdown in all three domains: visuospatial attention, alertness and inhibition. Furthermore, patients with more severely impaired test-level cognition and a brain lesions involving the intersection of the previously mentioned tracts also presented with significantly more severely impaired cognition during daily living.
This novel and intriguing finding is reminiscent of the MD-network model, suggesting that different cognitive operations depend on different collaborators and their interaction, within the same underlying, high-order brain network. Hence, a lesion involving the corresponding intersection would cause a widespread breakdown of connectivity throughout the whole higher-order network, with dramatic consequences on cognitive performance and daily living activities. Such anatomical and cognitive findings, should their influence on clinical outcome be confirmed in longitudinal designs, have the potential to influence rehabilitation approaches.
Supplementary Material
Acknowledgements
This work was supported by the Swiss National Science Foundation (SNSF).
We would like to thank Professor Fedorenko for her suggestions on the analysis of the MD-weighted lesion volume. and for sharing the probabilistic functional atlas. We are grateful to all the patients who took part in our study. We would also like to thank the clinical team at the Kantonsspital Luzern for their assistance and support.
Contributor Information
Brigitte C Kaufmann, Sorbonne Université, Institut du Cerveau—Paris Brain Institute—ICM, Inserm, CNRS, Paris, France; Neurocenter, Luzerner Kantonsspital, 6000 Lucerne, Switzerland.
Dario Cazzoli, Neurocenter, Luzerner Kantonsspital, 6000 Lucerne, Switzerland; ARTORG Center for Biomedical Engineering Research, Gerontechnology and Rehabilitation, University of Bern, 3008 Bern, Switzerland; Department of Psychology, University of Bern, Bern, Switzerland.
Manuela Pastore-Wapp, Neurocenter, Luzerner Kantonsspital, 6000 Lucerne, Switzerland; ARTORG Center for Biomedical Engineering Research, Gerontechnology and Rehabilitation, University of Bern, 3008 Bern, Switzerland.
Tim Vanbellingen, Neurocenter, Luzerner Kantonsspital, 6000 Lucerne, Switzerland; ARTORG Center for Biomedical Engineering Research, Gerontechnology and Rehabilitation, University of Bern, 3008 Bern, Switzerland.
Tobias Pflugshaupt, Neurocenter, Luzerner Kantonsspital, 6000 Lucerne, Switzerland.
Daniel Bauer, Neurocenter, Luzerner Kantonsspital, 6000 Lucerne, Switzerland.
René M Müri, ARTORG Center for Biomedical Engineering Research, Gerontechnology and Rehabilitation, University of Bern, 3008 Bern, Switzerland; Department of Neurology, Inselspital, University Hospital, University of Bern, 3010 Bern, Switzerland.
Tobias Nef, ARTORG Center for Biomedical Engineering Research, Gerontechnology and Rehabilitation, University of Bern, 3008 Bern, Switzerland.
Paolo Bartolomeo, Sorbonne Université, Institut du Cerveau—Paris Brain Institute—ICM, Inserm, CNRS, Paris, France.
Thomas Nyffeler, Neurocenter, Luzerner Kantonsspital, 6000 Lucerne, Switzerland; ARTORG Center for Biomedical Engineering Research, Gerontechnology and Rehabilitation, University of Bern, 3008 Bern, Switzerland; Department of Neurology, Inselspital, University Hospital, University of Bern, 3010 Bern, Switzerland.
Funding
This work was supported by SNSF Grant No. P2BEP3_195283 to B.K., SNSF Grant No. Z00P3_154714/1 to D.C., as well as 320030_169789 and 32003b_196915 to T.Ny. and by Agence Nationale de la Recherche through ANR-16-CE37-0005 and ANR-10-IAIHU-06 to P.B.
Competing interests
The authors report no competing interests.
Supplementary material
Supplementary material is available at Brain online.
References
- 1. Sturm W, Willmes K. On the functional neuroanatomy of intrinsic and phasic alertness. NeuroImage. 2001;14:76–84. [DOI] [PubMed] [Google Scholar]
- 2. Thiebaut de Schotten M, Dell’Acqua F, Forkel S, et al. A lateralized brain network for visuospatial attention. Nat Preced. 2011;14:1245–1246. [DOI] [PubMed] [Google Scholar]
- 3. Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex ‘frontal lobe’ tasks: A latent variable analysis. Cogn Psychol. 2000;41:49–100. [DOI] [PubMed] [Google Scholar]
- 4. Corbetta M, Shulman G. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–205. [DOI] [PubMed] [Google Scholar]
- 5. Langner R, Eickhoff S. Sustaining attention to simple tasks: A meta-analytic review of the neural mechanisms of vigilant attention. Psychol Bull. 2013;139:870–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cieslik E, Mueller V, Eickhoff C, Langner R, Eickhoff S. Three key regions for supervisory attentional control: Evidence from neuroimaging meta-analyses. Neurosci Biobehav Rev. 2015;48:22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vincent J, Kahn I, Snyder A, Raichle M, Buckner R. Evidence for a frontoparietal control system revealed by intrinsic functional connectivity. J Neurophysiol. 2008;100:3328–3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Niendam T, Laird A, Ray K, Dean M, Glahn D, Carter C. Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn Affect Behav Neurosci. 2012;12:241–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cole MW, Schneider W. The cognitive control network: Integrated cortical regions with dissociable functions. Neuroimage. 2007;37:343–360. [DOI] [PubMed] [Google Scholar]
- 10. Assem M, Glasser M, Van Essen D, Duncan J. A domain-general cognitive core defined in multimodally parcellated human cortex. Cerebral Cortex. 2020;30:4361–4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fedorenko E, Duncan J, Kanwisher N. Broad domain generality in focal regions of frontal and parietal cortex. Proc Natl Acad Sci U S A. 2013;110:16616–16621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Duncan J, Assem M, Shashidhara S. Integrated intelligence from distributed brain activity. Trends Cog Sci. 2020;24(10):838–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang X, Gao Z, Smallwood J, Jefferies E. Both default and multiple-demand regions represent semantic goal information. J Neurosci. 2020;41:3679–3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Manly T, Dobler V, Dodds C, George M. Rightward shift in spatial awareness with declining alertness. Neuropsychologia. 2005;43:1721–1728. [DOI] [PubMed] [Google Scholar]
- 15. Coulthard E, Rudd A, Husain M. Motor neglect associated with loss of action inhibition. J Neurol Neurosurg Psychiatry. 2008;79:1401–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kaufmann B, Frey J, Pflugshaupt T, et al. The spatial distribution of perseverations in neglect patients during a nonverbal fluency task depends on the integrity of the right putamen. Neuropsychologia. 2018;115:42–50. [DOI] [PubMed] [Google Scholar]
- 17. Paladini R, Wyss P, Kaufmann B, et al. Re-fixation and perseveration patterns in neglect patients during free visual exploration. Eur J Neurosci. 2019;49:1244–1252. [DOI] [PubMed] [Google Scholar]
- 18. Bronnick K, Ehrt U, Emre M, et al. Attentional deficits affect activities of daily living in dementia-associated with Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2006;77:1136–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Robertson I, Manly T, Andrade J, Baddeley B, Yiend J. “Oops”; performance correlates of everyday attentional failures in traumatic brain injured and normal subjects. Neuropsychologia. 1997;24:747–758. [DOI] [PubMed] [Google Scholar]
- 20. Aron A, Robbins T, Poldrack R. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8(4):170–177. [DOI] [PubMed] [Google Scholar]
- 21. Bartolomeo P, de Schotten M T, Chica A. Brain networks of visuospatial attention and their disruption in visual neglect. Front Hum Neurosci. 2012;6:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sturm W, Willmes K. On the functional neuroanatomy of intrinsic and phasic alertness. Neuroimage. 2001;14:76–84. [DOI] [PubMed] [Google Scholar]
- 23. Ottiger B, Vanbellingen T, Gabriel C, et al. Correction: Validation of the new lucerne ICF based multidisciplinary observation scale (LIMOS) for stroke patients. PLoS ONE. 2015;10:e0134186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Woolgar A, Duncan J, Manes F, Fedorenko E. The multiple-demand system but not the language system supports fluid intelligence. Nat Hum Behav. 2019;2:200–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hauptman M, Blank I, Fedorenko E. Non-literal language processing is jointly supported by the language and theory of mind networks: Evidence from a novel meta-analytic fMRI approach. bioRxiv. [Preprint]. doi: 10.1101/2022.03.08.481056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Griffis J, Nenert R, Allendorfer J, Szaflarski J. Damage to white matter bottlenecks contributes to language impairments after left hemispheric stroke. Neuroimage Clin. 2017;14:552–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. von Elm E, Altman D, Egger M, Pocock S, Gotzsche P, Vandenbroucke J. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: Guidelines for reporting observational studies. Bull World Health Organ. 2007;85:867–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Weintraub S, Mesulam M. Visual hemispatial inattention: Stimulus parameters and exploratory strategies. J Neurol, Neurosurg Psychiatry. 1988;51:1481–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wilson BA, Cockburn J, Halligan P. Development of a behavioral test of visuospatial neglect. Arch Phys Med Rehabil. 1987;68: 98–102. [PubMed] [Google Scholar]
- 30. Zimmermann P, Fimm B. Testbatterie zur Aufmerksamkeitsprüfung (TAP). Psytest Verlag; 1993. [Google Scholar]
- 31. Dubois B, Slachevsky A, Litvan I, Pillon B. The FAB: A frontal assessment battery at bedside. Neurology. 2000;55:1621–1626. [DOI] [PubMed] [Google Scholar]
- 32. Benke T, Karner E, Delazer M. FAB-D: German version of the frontal assessment battery. J Neurol. 2013;360:3066–2072. [DOI] [PubMed] [Google Scholar]
- 33. Regard M, Strauss E, Knapp P. Children's production on verbal and non-verbal fluency tasks. Percept Mot Skills. 1982;55:839–844. [DOI] [PubMed] [Google Scholar]
- 34. Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System (D-KEFS). Pearson; 2001. doi: 10.1037/t15082-000 [DOI] [PubMed] [Google Scholar]
- 35. Kaufmann B, Cazzoli D, Pflugshaupt T, et al. Eyetracking during free visual exploration detects neglect more reliably than paper-pencil tests. Cortex. 2020;129:223–235. [DOI] [PubMed] [Google Scholar]
- 36. Kaufmann B, Knobel S, Nef T, Müri R, Cazzoli D, Nyffeler T. Visual exploration area in neglect: A new analysis method for video-oculography data based on foveal vision. Front Neurosci. 2020;13:1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cazzoli D, Antoniades C, Kennard C, Nyffeler T, Bassetti C, Müri R. Eye movements discriminate fatigue due to chronotypical factors and time spent on task --A double dissociation. PLoS ONE. 2013;9(1):e87146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mosimann U, Müri R, Burn D, Felblinger J, O'Brien J, McKeith I. Saccadic eye movement changes in Parkinson's disease dementia and dementia with Lewy bodies. Brain. 2005;128:1267–1276. [DOI] [PubMed] [Google Scholar]
- 39. Salthouse T, Ellis C. Determinants of eye-fixation duration. Am J Psychol. 1980;93:207–234. [PubMed] [Google Scholar]
- 40. Jamadar S, Fielding J, Egan G. Quantitative meta-analysis of fMRI and PET studies reveals consistent activation in fronto-striatal-parietal regions and cerebellum during antisaccades and prosaccades. Front Psychol. 2013;4:749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rorden C, Karnath H. A simple measure of neglect severity. Neuropsychologia. 2010;48:2758–2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Field A. Discovering statistics using SPSS. Sage; 2009. [Google Scholar]
- 43. Rorden C, Bonilha L, Fridriksson J, Bender B, Karnath H. Age-specific CT and MRI templates for spatial normalization. Neuroimage. 2012;61:957–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Binder J, Marshall R, Lazar R, Benjamin J, Mohr J. Distinct syndromes of hemineglect. Arch Neurol. 1992;49:1187–1194. [DOI] [PubMed] [Google Scholar]
- 45. Delazer M, Sojer M, Ellmerer P, Boehme B, Benke T. Eye-tracking provides a sensitive measure of exploration deficits after acute right MCA stroke. Front Neurol. 2018;9:359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Findlay J, Gilchrist I. Active vision the psychology of looking and seeing. Oxford University Press; 2003. [Google Scholar]
- 47. Stroop J. Studies of interferences in serial verbal reaction. J Exp Psychol. 1935;18:643–662. [Google Scholar]
- 48. Ribeiro F, de Mendonça A, Guerreiro M. Mild cognitive impairment: Deficits in cognitive domains other than memory. Dement Geriatr Cogn Disord. 2006;1:284–290. [DOI] [PubMed] [Google Scholar]
- 49. Stevens JP. Applied multivariate statistics for the social sciences. Taylor & Francis Group; 2009. [Google Scholar]
- 50. Karnath H, Rennig J, Johannsen L, Rorden C. The anatomy underlying acute versus chronic spatial neglect: A longitudinal study. Brain. 2011;134:903–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nachev P, Coulthard E, Jäger H, Kennard C, Husain M. Enantiomorphic normalization of focally lesioned brains. Neuroimage. 2008;39:1215–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fox M. Mapping symptoms to brain networks with the human connectome. N Engl J Med. 2018;379:2237–2245. [DOI] [PubMed] [Google Scholar]
- 53. Corp D, Joutsa J, Darby R, et al. Network localization of cervical dystonia based on causal brain lesions. Brain. 2019;142:1660–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Adolphs R. Human lesion studies in the 21st century. Neuron. 2016;90:1151–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Foulon C, Cerliani L, Kinkingnéhun S, et al. Advanced lesion symptom mapping analyses and implementation as BCBtoolkit. GigaScience. 2018;7(3):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Rorden C, Karnath H, Bonilha L. Improving lesion–symptom mapping. J Cogn Neurosci. 2007;19:1081–1088. [DOI] [PubMed] [Google Scholar]
- 57. Kimberg D, Coslett H, Schwartz M. Power in voxel-based lesion-symptom mapping. J CognNeurosci. 2007;19:1067–1080. [DOI] [PubMed] [Google Scholar]
- 58. Medina J, Kimberg D, Chatterjee A, Coslett H. Inappropriate usage of the Brunner–Munzel test in recent voxel-based lesion-symptom mapping studies. Neuropsychologia. 2010;48:341–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rojkova K, Volle E, Urbanski M, Humbert F, Dell’Acqua F, Thiebaut de Schotten MT. Atlasing the frontal lobe connections and their variability due to age and education: A spherical deconvolution tractography study. Brain Structure and Function. 2016;221:1751–1766. [DOI] [PubMed] [Google Scholar]
- 60. Avants B, Tustison N, Song G, Cook P, Klein A, Gee J. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage. 2011;54:2033–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Klein A, Andersson J, Ardekani B, et al. Evaluation of 14 nonlinear deformation algorithms applied to human brain MRI registration. Neuroimage. 2009;1:786–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Thiebaut de Schotten M, Dell’Acqua F, Ratiu P, et al. From Phineas Gage and Monsieur Leborgne to H.M.: Revisiting disconnection syndromes. Cereb Cortex. 2015;25:4812–4827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Vanbellingen T, Ottiger B, Pflugshaupt T, et al. The responsiveness of the lucerne ICF-based multidisciplinary observation scale: A comparison with the functional independence measure and the Barthel index. Front Neurol. 2016;7:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ishiai S, Koyama Y, Seki K, Nakayama T. What is line bisection in unilateral spatial neglect? Analysis of perceptual and motor aspects in line bisection tasks. Brain Cogn. 1998;36:239–252. [DOI] [PubMed] [Google Scholar]
- 65. Mapstone M, Weintraub S, Nowinski C, Kaptanoglu G, Gitelman D, Mesulam M. Cerebral hemispheric specialization for spatial attention: Spatial distribution of search-related eye fixations in the absence of neglect. Neuropsychologia. 2003;41:1396–1409. [DOI] [PubMed] [Google Scholar]
- 66. Mazziotta J, Toga A, Evans A, et al. A probabilistic atlas and reference system for the human brain: International consortium for brain mapping (ICBM). Phil Trans Royal Soc B Biol Sci. 2001;356:1293–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Collins D, Holmes C, Peters T, Evans A. Automatic 3-D model-based neuroanatomical segmentation. Hum Brain Mapp. 1995;3:190–208. [Google Scholar]
- 68. Saj A, Verdon V, Vocat R, Vuilleumier P. Letter to the editors: ‘The anatomy underlying acute versus chronic spatial neglect’ also depends on clinical tests. Brain. 2012;135:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Vossel S, Geng J, Fink G. Dorsal and ventral attention systems: Distinct neural circuits but collaborative roles. Neuroscientist. 2014;20(2):150–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Chandrakumar D, Keage H, Gutteridge D, Dorrian J, Banks S, Loetscher T. Interactions between spatial attention and alertness in healthy adults: A meta-analysis. Cortex. 2019;119:61–73. [DOI] [PubMed] [Google Scholar]
- 71. Cazzoli D, Kaufmann B, Paladini R, Müri R, Nef T, Nyffeler T. Anterior insula and inferior frontal gyrus: Where ventral and dorsal visual attention systems meet. Brain Commun. 2020;3:fcaa220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Bartolomeo P, Chokron S. Left unilateral neglect or right hyperattention. Neurology. 1999;53:2023–2027. [DOI] [PubMed] [Google Scholar]
- 73. Jahanshahi M, Obeso I, Rothwell J, Obeso J. A fronto-striato-subthalamic-pallidal network for goal-directed and habitual inhibition. Nat Rev Neurosci. 2015;16:719–732. [DOI] [PubMed] [Google Scholar]
- 74. Duncan J. The multiple-demand (MD) system of the primate brain: Mental programs for intelligent behaviour. Trends Cogn Sci. 2010;14:172–179. A [DOI] [PubMed] [Google Scholar]
- 75. Farooqui A, Mitchell D, Thompson R, Duncan J. Hierarchical organization of cognition reflected in distributed frontoparietal activity. J Neurosci. 2012;32:17373–17381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Woolgar A, Thompson R, Bor D, Duncan J. Multi-voxel coding of stimuli, rules, and responses in human frontoparietal cortex. NeuroImage. 2011;56:744–752. [DOI] [PubMed] [Google Scholar]
- 77. Brittain J, Watkins K, Joundi R, et al. A role for the subthalamic nucleus in response inhibition during conflict. Neurosci. 2012;32:13396–13401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Hirvonen K, Puttonen S, Gould K, Korpela J, Koefoed V, Müller K. Improving the saccade peak velocity measurement for detecting fatigue. J Neurosci Methods. 2010;187:199–206. [DOI] [PubMed] [Google Scholar]
- 79. Schmidt D, Abel L, Dell’Osso L, Daroff R. Saccadic velocity characteristics: Intrinsic variability and fatigue. Aviat Space Environ Med. 1979;50:393–395. [PubMed] [Google Scholar]
- 80. van der Linden D, Frese M, Meijman T. Mental fatigue and the control of cognitive processes: Effects on perseveration and planning. Acta Psychol. 2003;113:45–65. [DOI] [PubMed] [Google Scholar]
- 81. Jazbec S, McClure E, Hardin M, Pine D, Ernst M. Cognitive control under contingencies in anxious and depressed adolescents: An antisaccade task. Biol Psychiatry. 2005;58:632–639. [DOI] [PubMed] [Google Scholar]
- 82. Paladini R, Muri R, Meichtry J, et al. The influence of alertness on the spatial deployment of visual attention is mediated by the excitability of the posterior parietal cortices. Cereb Cortex. 2017;27:233–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Glue P, White E, Wilson S, Ball D, Nutt D. Pharmacology of saccadic eye movements in man. Psychopharmacology (Berl). 1991;105:368–373. [DOI] [PubMed] [Google Scholar]
- 84. Baker C, Burks J, Briggs R, et al. A connectomic atlas of the human cerebrum—Chapter 7: The lateral parietal lobe. Oper Neurosurg. 2018;15(6):S295–S349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Bartolomeo P. Attention disorders after right brain damage: Living in halved worlds. Springer; 2014. [Google Scholar]
- 86. Aron A, Behrens T, Smith S, Frank M, Poldrack R. Triangulating a cognitive control network using diffusion-weighted magnetic resonance imaging (MRI) and functional MRI. J Neurosci. 2007;27:3743–3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Briggs RG, Conner AK, Rahimi M, et al. A connectomic atlas of the human cerebrum—Chapter 14: Tractographic description of the frontal aslant tract. Oper Neurosurg (Hagerstown). 2018;15(6):S444–S449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Rutten G, Landers M, De Baene W, Meijerink T, van der Hek S, Verheul J. Executive functional deficits during electrical stimulation of the right frontal aslant tract. Brain Imaging Behav. 2021;15:2731–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Thiebaut de Schotten M, Dell’Acqua F, Valabregue R, Catani M. Monkey to human comparative anatomy of the frontal lobe association tracts. Cortex. 2012;48:82–96. [DOI] [PubMed] [Google Scholar]
- 90. Bartolomeo P, Seidel-Malkinson T. Hemispheric lateralization of attention processes in the human brain. Curr Opin Psychol. 2019;29:90–96. [DOI] [PubMed] [Google Scholar]
- 91. Corbetta M, Shulman G. Spatial neglect and attention networks. Annu Rev Neurosci. 2011;34:569–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Bushara K, Weeks R, Ishii K, et al. Modality-specific frontal and parietal areas for auditory and visual spatial localization in humans. Nat Neurosci. 1999;2:759–766. [DOI] [PubMed] [Google Scholar]
- 93. Doricchi F, Thiebaut de Schotten M, Tomaiuolo F, Bartolomeo P. White matter (dis)connections and gray matter (dys)functions in visual neglect: Gaining insights into the brain networks of spatial awareness. Cortex. 2008;44:983–995. [DOI] [PubMed] [Google Scholar]
- 94. Giesbrecht B, Woldorff M, Song A, Mangun G. Neural mechanisms of top-down control during spatial and feature attention. NeuroImage. 2003;19:496–512. [DOI] [PubMed] [Google Scholar]
- 95. Karnath H, Rorden C. The anatomy of spatial neglect. Neuropsychologia. 2012;50:1010–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Schall J. On the role of frontal eye field in guiding attention and saccades. Vision Res. 2004;44:1453–1467. [DOI] [PubMed] [Google Scholar]
- 97. Shapiro K, Hillstrom A, Husain M. Control of visuotemporal attention by inferior parietal and superior temporal cortex. Curr Biol. 2002;12:1320–1325. [DOI] [PubMed] [Google Scholar]
- 98. Singh-Curry V, Husain M. The functional role of the inferior parietal lobe in the dorsal and ventral stream dichotomy. Neuropsychologia. 2009;47:1434–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Thiebaut de Schotten M, Ffytche D, Bizzi A, et al. Atlasing location, asymmetry and inter-subject variability of white matter tracts in the human brain with MR diffusion tractography. NeuroImage. 2011;54:45–59. [DOI] [PubMed] [Google Scholar]
- 100. Thompson K, Biscoe K, Sato T. Neuronal basis of covert spatial attention in the frontal eye field. J Neurosci. 2005;25:9479–9487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Vernet M, Quentin R, Chanes L, Mitsumasu A, Valero-Cabré A. Frontal eye field, where art thou? Anatomy, function, and non-invasive manipulation of frontal regions involved in eye movements and associated cognitive operations. Front Integr Neurosci. 2014;8:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Gao Y, Zheng J, Li Y, et al. Decreased functional connectivity and structural deficit in alertness network with right-sided temporal lobe epilepsy. Medicine. 2018;97:e0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Weinbach N, Henik A. Phasic alertness can modulate executive control by enhancing global processing of visual stimuli. Cognition. 2011;121:454–458. [DOI] [PubMed] [Google Scholar]
- 104. Thiel C, Fink G. Visual and auditory alertness: Modality-specific and supramodal neural mechanisms and their modulation by nicotine. J Neurophysiol. 2007;97:2758–2768. [DOI] [PubMed] [Google Scholar]
- 105. Dücker F, Dormisano R, Sack A. Hemispheric differences in the voluntary control of spatial attention: Direct evidence for a right-hemispheric dominance within frontal cortex. J Cogn Neurosci. 2013;25:1332–1342. [DOI] [PubMed] [Google Scholar]
- 106. Langner R, Kellermann T, Eickhoff S, et al. Staying responsive to the world: Modality-specific and -nonspecific contributions to speeded auditory, tactile, and visual stimulus detection. Hum Brain Mapp. 2012;33:398–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Yanaka H, Saito D, Uchiyama Y, Sadato N. Neural substrates of phasic alertness: A functional magnetic resonance imaging study. Neurosci Res. 2010;68:51–58. [DOI] [PubMed] [Google Scholar]
- 108. Wang J, Zhang J, Rong M, et al. Functional topography of the right inferior parietal lobule structured by anatomical connectivity profiles. Hum Brain Mapp. 2016;37:4316–4332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Desmurget M, Sirigu A. Conscious motor intention emerges in the inferior parietal lobule. Curr Opin Neurobiol. 2012;22:1004–1011. [DOI] [PubMed] [Google Scholar]
- 110. Horn N, Dolan M, Elliott R, Deakin J, Woodruff P. Response inhibition and impulsivity: An fMRI study. Neuropsychologia. 2003;41:1959–1966. [DOI] [PubMed] [Google Scholar]
- 111. Liddle P, Kiehl K, Smith A. Event-related fMRI study of response inhibition. Hum Brain Mapp. 2001;12:100–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Zheng D, Oka T, Bokura H, Yamaguchi S. The key locus of common response inhibition network for no-go and stop signals. J Cogn Neurosci. 2008;20:1434–1442. [DOI] [PubMed] [Google Scholar]
- 113. Walker R, Techawachirakul P, Haggard P. Frontal eye field stimulation modulates the balance of salience between target and distractors. Brain Res. 2009;1270:54–63. [DOI] [PubMed] [Google Scholar]
- 114. Muggleton N, Chen C, Tzeng O, Hung D, Juan C. Inhibitory control and the frontal eye fields. J Cogn Neurosci. 2010;22:2804–2812. [DOI] [PubMed] [Google Scholar]
- 115. Aron A, Fletcher P, Bullmore E, Sahakian B, Robbins T. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nature. 2003;6:115–116. [DOI] [PubMed] [Google Scholar]
- 116. Menon V, Adleman N, White C, Glover G, Reiss A. Error-related brain activation during a Go/NoGo response inhibition task. Hum Brain Mapp. 2001;12:131–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Adleman N, Menon V, Blasey C, et al. A developmental fMRI study of the Stroop color-word task. NeuroImage. 2002;16:61–75. [DOI] [PubMed] [Google Scholar]
- 118. Ricci H, Aron A. Towards real-world generalizability of a circuit for action-stopping. Nat Rev Neurosci. 2021;22:538–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Forstmann B, Jahfari S, Scholte H, Wolfensteller U, van den Wildenberg W, Ridderinkhof K. Function and structure of the right inferior frontal cortex predict individual differences in response inhibition: A model-based approach. J Neurosci. 2008;28:9790–9796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Sharp D, Bonnelle V, De Boissezon X, et al. Distinct frontal systems for response inhibition, attentional capture, and error processing. Proc Natl Acad Sci U S A. 2010;107:6106–6111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Jarbo K, Verstynen T. Converging structural and functional connectivity of orbitofrontal, dorsolateral prefrontal, and posterior parietal cortex in the human striatum. J Neurosci. 2015;35:3865–3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Vallar G, Perani D. The anatomy of spatial neglect in humans. Adv Psychol. 1987;45:235–258. [Google Scholar]
- 123. Karnath H, Himmelbach M, Rorden C. The subcortical anatomy of human spatial neglect: Putamen, caudate nucleus and pulvinar. Brain. 2002;125:350–360. [DOI] [PubMed] [Google Scholar]
- 124. van Belle J, Vink M, Durston S, Zanbelt BB. Common and unique neural networks for proactive and reactive response inhibition revealed by independent component analysis of functional MRI data. NeuroImage. 2014;103:65–74. [DOI] [PubMed] [Google Scholar]
- 125. Govindarajan S, Pan R, Krupp L, Charvet L, Duong T. Gray matter morphometry correlates with attentional efficiency in young-adult multiple sclerosis. Brain Sci. 2021;11:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Thomas M, Sing H, Belenky G, et al. Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation on waking human regional brain activity. J Sleep Res. 2000;9:335–352. [DOI] [PubMed] [Google Scholar]
- 127. Zhou M, Jiang W, Zhong D, Zheng J. Resting-state brain entropy in right temporal lobe epilepsy and its relationship with alertness. Brain Behav. 2019;9:e01446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Weiller C, Reisert M, Peto I, et al. The ventral pathway of the human brain: A continuous association tract. NeuroImage. 2021;234:117977. [DOI] [PubMed] [Google Scholar]
- 129. Sarubbo S, DeBenedictis A, Maldonado I, Basso G, Duffau H. Frontal terminations for the inferior fronto-occipital fascicle: Anatomical dissection, DTI study and functional considerations on a multi-component bundle. Brain Struct Funct. 2013;218:21–37. [DOI] [PubMed] [Google Scholar]
- 130. Martino J, Brogna C, Robles S, Vergani F, Duffau H. Anatomic dissection of the inferior fronto-occipital fasciculus revisited in the lights of brain stimulation data. Cortex. 2010;46:691–699. [DOI] [PubMed] [Google Scholar]
- 131. Yeh F. Population-based tract-to-region connectome of the human brain and its hierarchical topology. Nat Commun. 2022;13:4933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Urbanski M, Thiebaut de Schotten M, Rodrigo S, et al. DTI-MR tractography of white matter damage in stroke patients with neglect. Exp Brain Res. 2011;208:491–505. [DOI] [PubMed] [Google Scholar]
- 133. Urbanski M, Thiebaut de Schotten M, Rodrigo S, et al. Brain networks of spatial awareness: Evidence from diffusion tensor imaging tractography. J Neurol Neurosurg Psychiatry. 2007;79:598–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Umarova R, Saur D, Schnell S, et al. Structural connectivity for visuospatial attention: Significance of ventral pathways. Cerebral Cortex. 2010;20:121–129. [DOI] [PubMed] [Google Scholar]
- 135. Forstmann B, Jahfari S, Scholte HS, et al. Function and structure of the right inferior frontal cortex predict individual differences in response inhibition: A model-based approach. J Neurosci. 2008;29:9790–9796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Leng Y, Shi Y, Yu Q, et al. Phenotypic and genetic correlations between the lobar segments of the inferior frontooccipital fasciculus and attention. Sci Rep. 2016;6:33015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Power J, Schlaggar B, Lessov-Schlaggar C, Petersen S. Evidence for hubs in human functional brain networks. Neuron. 2013;79:798–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Cole M, Reynolds J, Power J, Repovs G, Anticevic A, Braver T. Multi-task connectivity reveals flexible hubs for adaptive task control. Nat Neurosci. 2013;16(9):1348–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Woolgar A, Jackson J, Duncan J. Coding of visual, auditory, rule, and response information in the brain: 10 years of multivoxel pattern analysis. J Cogn Neurosci. 2016;28:1433–1454. [DOI] [PubMed] [Google Scholar]
- 140. Duncan J. An adaptive coding model of neural function in prefrontal cortex. Nature. 2001;2:820–829. [DOI] [PubMed] [Google Scholar]
- 141. Miller E, Cohen J. An integrative theory of prefrontal cortex function. Annu Rev Neurosci. 2001;24:167–202. [DOI] [PubMed] [Google Scholar]
- 142. Duncan J. The structure of cognition: Attentional episodes in mind and brain. Neuron. 2013;80:35–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Chen P, Chen C, Hsu Y, Cam-CAN TW. Fluid intelligence is associated with cortical volume and white matter tract integrity within multiple-demand system across adult lifespan. NeuroImage. 2020;212:116576. [DOI] [PubMed] [Google Scholar]
- 144. Verdon V, Schwartz S, Lovblad K, Hauert K, Vuilleumier P. Neuroanatomy of hemispatial neglect and its functional components: A study using voxel-based lesion-symptom mapping. Brain. 2010;133:880–894. [DOI] [PubMed] [Google Scholar]
- 145. Coulthard E, Parton A, Husain M. The modular architecture of the neglect syndrome: Implications for action control in visual neglect. Neuropsychologia. 2007;45:1982–1984. [Google Scholar]
- 146. Corbetta M, Ramsey L, Callejas A, et al. Common behavioral clusters and subcortical anatomy in stroke. Neuron. 2015;85:927–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Toba MN, Migliaccio R, Batrancourt B, et al. Common brain networks for distinct deficits in visual neglect. A combined structural and tractography MRI approach. Neuropsychologia. 2018;115:167–178. [DOI] [PubMed] [Google Scholar]
- 148. Bartolomeo P, Thiebaut de Schotten M, Doricchi F. Left unilateral neglect as a disconnection syndrome. Cereb Cortex. 2007;17:2479–2490. [DOI] [PubMed] [Google Scholar]
- 149. Husain M. Visual attention: What inattention reveals about the brain. Curr Biol. 2019;29:R262–R264. [DOI] [PubMed] [Google Scholar]
- 150. Gainotti G, D’Erme P, Bartolomeo P. Early orientation of attention toward the half space ipsilateral to the lesion in patients with unilateral brain damage. J Neurol Neurosurg Psychiatry. 1991;54:1082–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Bartolomeo P. Visual neglect. Curr Opin Neurol. 2007;20:381–386. [DOI] [PubMed] [Google Scholar]
- 152. Sieroff E, Decaix C, Chokron S, Bartolomeo P. Impaired orienting of attention in left unilateral neglect: A componential analysis. Neuropsychology. 2007;21:94–113. [DOI] [PubMed] [Google Scholar]
- 153. Bartolomeo P. Inhibitory processes and spatial bias after right hemisphere damage. Neuropsychol Rehabil. 2000;10:511–526. [Google Scholar]
- 154. Robertson I. Do we need the “lateral” in unilateral neglect? Spatially nonselective attention deficits in unilateral neglect and their implications for rehabilitation. NeuroImage. 2001;14:85–90. [DOI] [PubMed] [Google Scholar]
- 155. Kleinman J, DuBois J, Newhart M, Hillis A. Disentangling the neuroanatomical correlates of perseveration from unilateral spatial neglect. Behav Neurol. 2013;26(1–2):131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Nys G, van Zandvoort M, van der Worp H, Kappelle L, de Haan E. Neuropsychological and neuroanatomical correlates of perseverative responses in subacute stroke. Brain. 2006;129:2148–2157. [DOI] [PubMed] [Google Scholar]
- 157. DeGutis J, Van Vleet T. Tonic and phasic alertness training: A novel behavioral therapy to improve spatial and non-spatial attention in patients with hemispatial neglect. Front Hum Neurosci. 2010;4:60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Robertson I, Tegner R, Tham K, Lo A. Sustained attention training for unilateral neglect: Theoretical and rehabilitation implications. J Clin Exp Neuropsychol. 1995;17:416–430. [DOI] [PubMed] [Google Scholar]
- 159. Wickham H, ggplot2: Elegant graphics for data analysis. Springer; 2010. [Google Scholar]
- 160. R Core Team , R: A language and environment for statistical computing. R Foundation for Statistical Computing; 2017. https://www.R-project.org/ [Google Scholar]
- 161. Baker C, Burks J, Briggs R, et al. A connectomic atlas of the human cerebrum—Chapter 2: The lateral frontal lobe. Oper Neurosurg. 2018;15(6):S10–S74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Dick A, Garic D, Graziano P, Tremblay P. The frontal aslant tract (FAT) and its role in speech, language and executive function. Cortex. 2019;111:148–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The conditions of our ethics approval do not permit the public archiving of the data supporting the conclusions of this study. On the basis of the Swiss Human Research Act, the HRA (Humanforschungsgesetz) in Switzerland, readers seeking access to the data and the study materials must therefore complete a formal data sharing agreement to obtain the data. Interested readers should contact the corresponding author for more information and help.