Abstract
A COVID-19 patient often presents with multiple comorbidities and is associated with adverse outcomes. A comprehensive assessment of the prevalence of comorbidities in patients with COVID-19 is essential. This study aimed to assess the prevalence of comorbidities, severity and mortality with regard to geographic region, age, gender and smoking status in patients with COVID-19. A systematic review and multistage meta-analyses were reported using PRISMA guidelines. PubMed/MEDLINE, SCOPUS, Google Scholar and EMBASE were searched from January 2020 to October 2022. Cross-sectional studies, cohort studies, case series studies, and case–control studies on comorbidities reporting among the COVID-19 populations that were published in English were included. The pooled prevalence of various medical conditions in COVID-19 patients was calculated based on regional population size weights. Stratified analyses were performed to understand the variations in the medical conditions based on age, gender, and geographic region. A total of 190 studies comprising 105 million COVID-19 patients were included. Statistical analyses were performed using STATA software, version 16 MP (StataCorp, College Station, TX). Meta-analysis of proportion was performed to obtain pooled values of the prevalence of medical comorbidities: hypertension (39%, 95% CI 36–42, n = 170 studies), obesity (27%, 95% CI 25–30%, n = 169 studies), diabetes (27%, 95% CI 25–30%, n = 175), and asthma (8%, 95% CI 7–9%, n = 112). Moreover, the prevalence of hospitalization was 35% (95% CI 29–41%, n = 61), intensive care admissions 17% (95% CI 14–21, n = 106), and mortality 18% (95% CI 16–21%, n = 145). The prevalence of hypertension was highest in Europe at 44% (95% CI 39–47%, n = 68), obesity and diabetes at 30% (95% CI, 26–34, n = 79) and 27% (95%CI, 24–30, n = 80) in North America, and asthma in Europe at 9% (95% CI 8–11, n = 41). Obesity was high among the ≥ 50 years (30%, n = 112) age group, diabetes among Men (26%, n = 124) and observational studies reported higher mortality than case–control studies (19% vs. 14%). Random effects meta-regression found a significant association between age and diabetes (p < 0.001), hypertension (p < 0.001), asthma (p < 0.05), ICU admission (p < 0.05) and mortality (p < 0.001). Overall, a higher global prevalence of hypertension (39%) and a lower prevalence of asthma (8%), and 18% of mortality were found in patients with COVID-19. Hence, geographical regions with respective chronic medical comorbidities should accelerate regular booster dose vaccination, preferably to those patients with chronic comorbidities, to prevent and lower the severity and mortality of COVID-19 disease with novel SARS-CoV-2 variants of concern (VOC).
Subject terms: Diseases, Health care, Medical research, Risk factors
Introduction
Novel coronavirus disease 2019 (nCOVID-19) is a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) announced as a global pandemic that affected more than 60 million population with more than 6 million deaths to date1. At present, the number of Covid-19 cases is still alarming around the world with frequent mutations in the structure of the SARS-COV-2 Virus; hence there is an increasing global concern about this outbreak. Clinical characteristics of COVID-19 manifest as asymptomatic or mild infections in children and young adults, while in older adults, it manifests as severe to critical presentations with acute respiratory distress syndrome (ARDS) and even death2. Ppatients with COVID-19 manifest several clinical symptoms as mild, moderate, severe, and critical illness. Initially, COVID-19 present with flu-like symptoms such as high-grade fever, dry cough, and fatigue, followed by dyspnoea2.
A spate of recent studies has shown that patients who have pre-existing comorbidities are at increased risk of COVID-19 severity, hospitalization, admission to an intensive care unit, intubation, and mortality compared with those without any comorbidity3. In addition, few studies have explored the effect of various comorbidities on the severity of COVID-19 disease that concomitant medical comorbidities such as diabetes, hypertension, chronic kidney diseases, obesity, respiratory diseases and malignancy and age > 50 years were associated with an increased risk of COVID-19 severity, morbidity and mortality3–8.
However, as the pandemic progressed, more and more clinical data from all around the world became available to synthesize the updated evidence, to better understand insights into evolving COVID-19 disease severity and to develop strategies for better management of SARS-CoV-2 infected patients. Nonetheless, there is a dearth of data on the prevalence of the most common medical comorbidities that aare associated with increased severity and mortality of the disease such as hypertension, diabetes, obesity, asthma and smoking in patients with COVID-19 among different populations of various continents2. Therefore, to address these gaps in the research, the present study was conducted to estimate the prevalence rates and geographical distribution of prior mentioned comorbidities, COVID-19 disease severity and mortality and to evaluate the association between age, gender and smoking status characteristics on hospitalization, ICU admission, and mortality by geographic region, study design among SARS-CoV-2 infected patients from the real-world clinical studies data which will helps to effectively allocate healthcare resources, endorse appropriate preventive and containment measures, and guide emerging treatment protocols .
Methods
The present systematic review and multistage meta-analyses were reported according to the PRISMA (Preferred Reporting Items for the Systematic Review and Meta-analysis) guidelines.
Search strategy
A literature search of PubMed/MEDLINE, SCOPUS, Google Scholar and EMBASE was performed from January 2020 to October 2022, using the MESH terms and /or keywords “(Corona Virus Disease-2019) OR (COVID-19) OR (Severe acute respiratory syndrome corona virus 2) OR (SARS-Cov-2) AND (Comorbidities)” with filters for the cross-sectional studies, cohort studies, case series studies, and case–control studies on comorbidities reporting among the COVID-19 populations that were published in English were included. In addition, we searched the reference lists of the relevant publications, reviews and meta-analyses to identify additional potentially relevant studies. Studies with similar authors, the study duration, and the location of the study were strictly matched to further identify any duplicated study. All the duplicates were omitted from the analyses. The search was independently screened by two researchers (SC and VS) and discrepancies were resolved by discussion with a third researcher (SP).
Study selection
The titles and/or abstracts were reviewed qualitatively by two different authors (CS/MR) reviewed separately to identify studies that evaluated the effect of comorbidities on COVID-19 severity and mortality among hospitalized patients, performed duplicate removal, full-text assessment and discrepancies were resolved through discussion with a third researcher (SP).
Eligibility criteria
Studies were eligible for inclusion in our systematic review metaanalysis and metaregression study if they met the following criteria: (1) originally published in the English language (2) included confirmed diagnosis of COVID-19 through RTPCR laboratory diagnosis test; (3) provided information about comorbidities; (5) contained information on the disease outcomes: severity or mortality within comorbidity; and (6) published as an original investigation. Studies without diagnostic information, studies that included but did not report comorbidities were excluded from the analysis. When studies did not have available data, we emailed the corresponding authors for information. We excluded studies only if data were not provided at the time of meta-analysis.
Data extraction
Demographic study characteristics which included first author’s last name, publication year, country and continent (North America, Europe, Asia, Africa, South America) where the research was conducted in, study design, study description or name, study period, the average age in years with standard deviation or interquartile range; status of the comorbidities (number of subjects without any comorbidity, number of subjects with one comorbidity), the type of comorbidity that included hypertension, diabetes mellitus, obesity, asthma, and smoking status, sample size with the number of hospitalizations, ICU admissions and mortality within each comorbid condition. The detail information on the inclusion of comorbidities, outcomes including the criteria for COVID-19 severity assessment, and comparing variables is provided in an additional (Annexure 1). Data were extracted by CS and SP and extractions were checked for accuracy by MC.
Data analysis
The estimates of obesity, diabetes, hypertension, asthma, smoking, hospitalization rate, ICU admission rate, and mortality rates were expressed as proportions (%) with corresponding 95% confidence intervals (CI). The pooled prevalence estimates of outcome variables were calculated using regional population size weights. The magnitude of heterogeneity between the studies was assessed using the I2 statistic (% residual variation due to heterogeneity), and Tau2 (method of moments estimate of between-study variance) was used for each of the pooled estimates. I2 values range between 0 and 100% and are considered low for I2 < 25%, modest for 25–50%, and large for > 50%9. As differences between the studies were very high (95–99% inconsistency), a random effect DerSimonian-Laird model was used in all analyses9. In case of substantial heterogeneity, the source of heterogeneity was investigated using subgroup analyses based on the study-level characteristics, such as geographical region-wise, study design type, mean age, and women-to-men ratio. The association between the subgroups of each factor was assessed using Cochran’s Q test, degree of freedom(df), and p-value resulting from Cochran’s Q test. A p value of < 0.10 was considered statistically significant for Cochran’s Q test (Huedo-Medina et al., 2006). Meta-regression analysis was also performed to find out the strength of the association between age as a moderator and different health conditions. The risk of publication bias was inspected by using the symmetry of funnel plots, and Egger’s and Begg’s tests were also used. Statistical analyses were performed using STATA software, version 16 MP (StataCorp, College Station, TX).
Results
Characteristics of all included studies
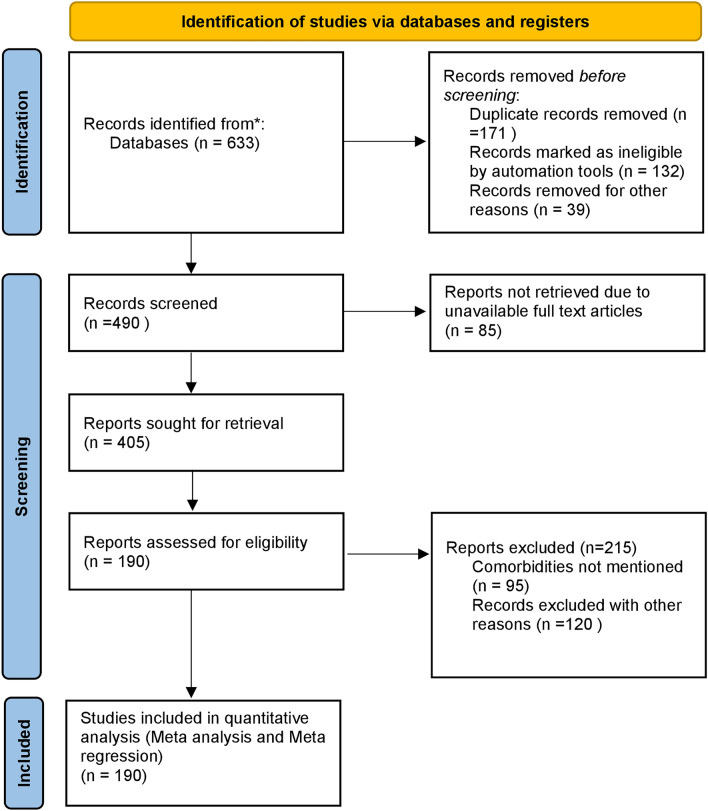
All the studies included in the present study were published between January 2020 and 30th October 2022 2021. A total of 647 references were initially identified through electronic databases. After removing duplicates, a total of 490 titles and abstracts were screened to determine if they met the inclusion criteria, as described in the methodology section. Full-text assessment of 405 potentially relevant articles resulted in 190 eligible studies as shown in Fig. 1. Sample size varied on a regional basis from 22 to 55, 86,521, making a total of 1, 05,98, 010 patients. All the studies included both women and men. However, one hundred and thirty-four studies included more men than women. The average age of the study population ranged between 17 and 81 years. Among the included studies, seven were case–control designs and one hundred and eighty-three studies were cohort studies. The majority of the studies were conducted in North America, Seventy-seven in Europe, twenty-three in Asia, six in South America, and one in Africa10–191. The characteristics of the included studies are summarized in Annexure 1.
Figure 1.
The PRISMA flow diagram for study selection.
Prevalence of obesity
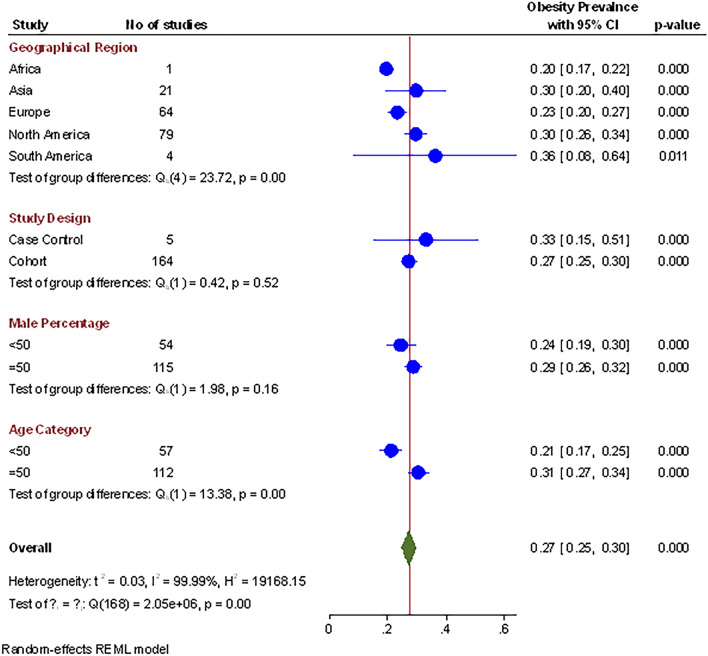
Out of 190 publications, sixty-nine studies, comprising 99, 57,215 participants, reported a prevalence of obesity among covid-19 patients. The pooled prevalence of obesity, after weighing the geographical population size, was 27% (n = 19, 70,472, 95% CI 0.25–0.30, I2 = 99.9%, p < 0.01, τ2 = 0.03), which indicated substantial heterogeneity, as shown in Table 1. Comparison of obesity proportions across the globe showed significant differences (Q = 12.8, df = 4; p < 0.01). South America, North America, and Asia demonstrated a relatively higher pooled prevalence of 36% (95% CI 8–64, p < 0.01), 30%( 95% CI 26–34, p < 0.01), and 29% (95% CI 19–39, p < 0.01) respectively, while Europe had a lower pooled prevalence of 23% (95% CI 20–26, p < 0.01). The variations in the pooled prevalence of obesity are further illustrated in the forest plot in Fig. 2.
Table 1.
Stratified meta-analysis of the prevalence of different conditions of COVID-19 patients.
| Subgroups | No of studies | Prevalence (95% CI) | Test for heterogeneity | Between subgroup differences | ||||
|---|---|---|---|---|---|---|---|---|
| Tau2 | I2 (%) | P value | Q | df | P value | |||
| Obesity | ||||||||
| Overall | 169 | 0.27 (0.25 to 0.30) | 0.03 | 99.9 | < 0.01 | |||
| Geographical Region | ||||||||
| Africa | 1 | 0.20 (0.14 to 0.25 | 0.001 | – | – | 12.8 | 4 | 0.01 |
| Asia | 21 | 0.29 (0.19 to 0.39) | 0.05 | 95.8 | < 0.01 | |||
| Europe | 64 | 0.23 (0.20 to 0.26) | 0.02 | 99.7 | < 0.01 | |||
| North America | 79 | 0.30 (0.26 to 0.34) | 0.03 | 99.9 | < 0.01 | |||
| South America | 4 | 0.36 (0.08 to 0.64) | 0.08 | 99.2 | < 0.01 | |||
| Study design | ||||||||
| Case–Control | 5 | 0.31 (0.16 to 0.46) | 0.03 | 99.3 | < 0.01 | 0.3 | 1 | 0.57 |
| Cohort | 164 | 0.27 (0.24 to 0.29) | 0.03 | 99.9 | < 0.01 | |||
| Age (years) | ||||||||
| < 50 | 57 | 0.21 (0.17 to 0.24) | 0.01 | 99.9 | < 0.01 | 14.7 | 1 | 0.00 |
| ≥ 50 | 112 | 0.30 (0.27 to 0.33) | 0.03 | 99.7 | < 0.01 | |||
| Male (%) | ||||||||
| < 50 | 54 | 0.24 (0.19 to 0.29) | 0.03 | 99.9 | < 0.01 | 1.8 | 1 | 0.18 |
| ≥ 50 | 115 | 0.28 (0.25 to 0.31) | 0.02 | 99.7 | < 0.01 | |||
| Diabetes | ||||||||
| Overall | 175 | 0.27 (0.25 to 0.30) | 0.03 | 99.9 | < 0.01 | |||
| Geographical Region | ||||||||
| Africa | 1 | 0.20 (0.17 to 0.23) | 0.00 | – | – | 18.23 | 4 | 0.00 |
| Asia | 21 | 0.29 (0.19 to 0.39) | 0.01 | 80.85 | < 0.01 | |||
| Europe | 68 | 0.20 (0.17 to 0.23) | 0.01 | 99.58 | < 0.01 | |||
| North America | 80 | 0.27 (0.24 to 0.30) | 0.02 | 99.90 | < 0.01 | |||
| South America | 5 | 0.29 (0.16 to 0.41) | 0.02 | 96.53 | < 0.01 | |||
| Study design | ||||||||
| Case–control | 7 | 0.22 (0.16 to 0.46) | 0.01 | 96.10 | < 0.01 | 0.11 | 1 | 0.74 |
| Cohort | 175 | 0.23 (0.21 to 0.25) | 0.02 | 99.84 | < 0.01 | |||
| Age (years) | ||||||||
| < 50 | 50 | 0.16 (0.13 to 0.20) | 0.02 | 99.92 | < 0.01 | 23.61 | 1 | 0.00 |
| ≥ 50 | 123 | 0.26(0.24 to 0.29) | 0.01 | 99.45 | < 0.01 | |||
| Male (%) | ||||||||
| < 50 | 49 | 0.17 (0.13 to 0.21) | 0.02 | 99.92 | < 0.01 | 13.12 | 1 | 0.00 |
| ≥ 50 | 124 | 0.26(0.24 to 0.28) | 0.01 | 99.51 | < 0.01 | |||
| Hypertension | ||||||||
| Overall | 170 | 0.39 (0.36 to 0.42) | 0.04 | 99.92 | < 0.01 | |||
| Geographical Region | ||||||||
| Africa | 1 | 0.41 (0.36 to 0.46) | 0.00 | – | – | 39.88 | 4 | 0.00 |
| Asia | 20 | 0.21 (0.15 to 0.27) | 0.01 | 87.34 | < 0.01 | |||
| Europe | 68 | 0.43 (0.39 to0.47) | 0.03 | 99.80 | < 0.01 | |||
| North America | 74 | 0.40 (0.35 to 0.45) | 0.04 | 99.96 | < 0.01 | |||
| South America | 6 | 0.44 (0.24 to 0.63) | 0.05 | 98.77 | < 0.01 | |||
| Study design | ||||||||
| Case control | 7 | 0.34 (0.22 to 0.45) | 0.02 | 98.94 | < 0.01 | 0.90 | 1 | 0.34 |
| Observational | 162 | 0.40 (0.36 to 0.43) | 0.04 | 99.93 | < 0.01 | |||
| Age (years) | ||||||||
| < 50 | 49 | 0.23 (0.18 to 0.27) | 0.02 | 99.94 | < 0.01 | 75.96 | 1 | 0.00 |
| ≥ 50 | 118 | 0.47 (0.43 to 0.50) | 0.03 | 99.69 | < 0.01 | |||
| Male (%) | ||||||||
| < 50 | 50 | 0.32 (0.26 to 0.38) | 0.05 | 99.96 | < 0.01 | 8.31 | 1 | 0.00 |
| ≥ 50 | 119 | 0.42 (0.39 to 0.46) | 0.03 | 99.79 | < 0.01 | |||
| Asthma | ||||||||
| Overall | 112 | 0.08 (0.07 to 0.09) | 0.00 | 98.33 | < 0.01 | |||
| Geographical Region | ||||||||
| Africa | 1 | 0.05 (− 0.00 to 0.10) | 0.00 | – | – | 58.76 | 4 | 0.00 |
| Asia | 11 | 0.07 (0.03 to 0.11) | 0.00 | 57.41 | 0.01 | |||
| Europe | 41 | 0.09 (0.08 to 0.11) | 0.00 | 97.47 | < 0.01 | |||
| North America | 55 | 0.08 (0.06 to 0.10) | 0.00 | 98.78 | < 0.01 | |||
| South America | 4 | 0.02 (0.00 to 0.03) | 0.00 | 0.31 | 0.50 | |||
| Study design | ||||||||
| Case control | 4 | 0.05 (0.04 to 0.06) | 0.00 | 0.04 | 0.82 | 24.86 | 1 | 0.00 |
| Observational | 108 | 0.08 (0.07 to 0.10) | 0.00 | 98.39 | < 0.01 | |||
| Age (years) | ||||||||
| < 50 | 43 | 0.07 (0.05 to 0.09) | 0.00 | 99.21 | < 0.01 | 4.46 | 1 | 0.03 |
| ≥ 50 | 69 | 0.09 (0.08 to 0.11) | 0.00 | 93.89 | < 0.01 | |||
| Male (%) | ||||||||
| < 50 | 35 | 0.09 (0.06 to 0.11) | 0.00 | 97.92 | < 0.01 | 0.11 | 1 | 0.74 |
| ≥ 50 | 77 | 0.08 (0.07 to 0.09) | 0.00 | 98.23 | < 0.01 | |||
| Smoking | ||||||||
| Overall | 99 | 0.15 (0.12 to 0.18) | 0.02 | 99.83 | < 0.01 | |||
| Geographical Region | ||||||||
| Africa | 1 | 0.07 (0.02 to 0.12) | 0.00 | – | – | 7.27 | 3 | 0.06 |
| Asia | 12 | 0.16 (0.07 to 0.24) | 0.02 | 83.00 | < 0.01 | |||
| Europe | 38 | 0.16 (0.11 to 0.22) | 0.03 | 99.88 | < 0.01 | |||
| North America | 48 | 0.14 (0.10 to 0.18) | 0.01 | 99.80 | < 0.01 | |||
| Study design | ||||||||
| Case control | 6 | 0.13 (0.08 to 0.18) | 0.00 | 92.42 | < 0.01 | 0.69 | 1 | 0.41 |
| Observational | 93 | 0.15 (0.12 to 0.18) | 0.02 | 99.85 | < 0.01 | |||
| Age (years) | ||||||||
| < 50 | 35 | 0.11 (0.07 to 0.16) | 0.02 | 99.87 | < 0.01 | 4.25 | 1 | 0.04 |
| ≥ 50 | 64 | 0.17 (0.14 to 0.21) | 0.02 | 99.70 | < 0.01 | |||
| Male (%) | ||||||||
| < 50 | 29 | 0.12 (0.08 to 0.16) | 0.01 | 99.73 | < 0.01 | 2.38 | 1 | 0.12 |
| ≥ 50 | 70 | 0.16 (0.12 to 0.20) | 0.02 | 99.82 | < 0.01 | |||
| Hospitalized | ||||||||
| Overall | 61 | 0.35 (0.29 to 0.41) | 0.00 | 99.96 | < 0.01 | |||
| Geographical Region | ||||||||
| Africa | 1 | 0.47 (0.42 to 0.53) | 0.00 | – | – | 69.94 | 4 | 0.00 |
| Asia | 2 | 0.31 (0.13 to 0.49) | 0.02 | 90.58 | < 0.01 | |||
| Europe | 17 | 0.35 (0.18 to 0.510 | 0.12 | 99.96 | < 0.01 | |||
| North America | 40 | 0.35 (0.29 to 0.41) | 0.04 | 99.97 | < 0.01 | |||
| South America | 1 | 0.57(0.55 to 0.58) | 0.00 | – | – | |||
| Study design | ||||||||
| Case control | 3 | 0.42 (0.19 to 0.65) | 0.04 | 99.76 | < 0.01 | 0.36 | 1 | 0.00 |
| Observational | 58 | 0.35 (0.29 to 0.41) | 0.06 | 99.98 | < 0.01 | |||
| Age (years) | ||||||||
| < 50 | 36 | 0.31 (0.25 to 0.37) | 0.03 | 99.97 | < 0.01 | 2.53 | 1 | 0.11 |
| ≥ 50 | 25 | 0.41 (0.30 to 0.53) | 0.08 | 99.97 | < 0.01 | |||
| Male (%) | ||||||||
| < 50 | 32 | 0.31 (0.23 to 0.39) | 0.06 | 99.98 | < 0.01 | 2.22 | 1 | 0.00 |
| ≥ 50 | 29 | 0.40 (0.32 to 0.48) | 0.05 | 99.96 | < 0.01 | |||
| ICU Admission | ||||||||
| Overall | 106 | 0.17 (0.14 to 0.21) | 0.03 | 99.85 | < 0.01 | |||
| Geographical Region | ||||||||
| Africa | 1 | 0.06 (0.01 to 0.11) | 0.00 | – | – | 27.57 | 4 | 0.00 |
| Asia | 15 | 0.18 (0.08 to 0.28) | 0.03 | 95.01 | < 0.01 | |||
| Europe | 37 | 0.18 (0.12 to 0.25) | 0.04 | 99.78 | < 0.01 | |||
| North America | 52 | 0.16 (0.12 to 0.20) | 0.02 | 99.85 | < 0.01 | |||
| South America | 1 | 0.20 (0.19 to 0.22) | 0.00 | – | – | |||
| Study design | ||||||||
| Case control | 5 | 0.13 (0.04 to 0.22) | 0.01 | 98.35 | < 0.01 | 0.95 | 1 | 0.33 |
| Observational | 101 | 0.18 (0.14 to 0.21) | 0.03 | 99.86 | < 0.01 | |||
| Age (years) | ||||||||
| < 50 | 45 | 0.13 (0.08) | 0.02 | 99.90 | < 0.01 | 5.12 | 1 | 0.02 |
| ≥ 50 | 61 | 0.20 (0.16) | 0.03 | 99.53 | < 0.01 | |||
| Male (%) | ||||||||
| < 50 | 37 | 0.14 (0.09 to 0.19) | 0.02 | 99.60 | < 0.01 | 2.16 | 1 | 0.14 |
| ≥ 50 | 69 | 0.19 (0.15 to 0.23) | 0.03 | 99.88 | < 0.01 | |||
| Mortality | ||||||||
| Overall | 145 | 0.18 (0.16 to 0.21) | 0.04 | 98.65 | < 0.01 | |||
| Geographical Region | ||||||||
| Africa | 1 | 0.11 (0.06 to 0.16) | 0.00 | – | – | 19.04 | 4 | 0.00 |
| Asia | 9 | 0.08 (0.01 to 0.14) | 0.01 | 84.63 | < 0.01 | |||
| Europe | 61 | 0.20 (0.16 to 0.23) | 0.02 | 99.58 | < 0.01 | |||
| North America | 72 | 0.18 (0.14 to 0.21) | 0.02 | 99.93 | < 0.01 | |||
| South America | 4 | 0.37 (0.17 to 0.56) | 0.04 | 98.65 | < 0.01 | |||
| Study design | ||||||||
| Case control | 6 | 0.14 (0.07 to 0.21) | 0.01 | 96.80 | < 0.01 | 1.40 | 1 | 0.00 |
| Observational | 141 | 0.19 (0.16 to 0.21) | 0.02 | 99.89 | < 0.01 | |||
| Age (years) | ||||||||
| < 50 | 36 | 0.09 (0.06 to 0.13) | 0.01 | 99.91 | < 0.01 | 28.84 | 1 | 0.00 |
| ≥ 50 | 111 | 0.22 (0.19 to 0.24) | 0.02 | 99.58 | < 0.01 | |||
| Male (%) | ||||||||
| < 50 | 44 | 0.12 (0.08 to 0.16) | 0.02 | 99.87 | < 0.01 | 15.12 | 1 | 0.00 |
| ≥ 50 | 101 | 0.21 (0.18 to 0.24) | 0.02 | 99.77 | < 0.01 | |||
Figure 2.
Prevalence of obesity in patients with Covid-19 across various potential variables.
Prevalence of diabetes
One hundred and seventy-five studies investigated the prevalence of diabetes among Covid-19 patients. The pooled estimate of diabetes was 27% (n = 9, 56,475, 95% CI 0.25–0.30, I2 = 99.9%, p < 0.01, τ2 = 0.03), which indicated substantial heterogeneity, as shown in Table 1. Comparison of diabetes prevalence across the world showed significant differences (Q = 18.2, df = 4; p < 0.01). South America and Asia have shown a higher pooled prevalence of 29% each, followed by North America with 27% (95% CI 0.24–0.30, I2 = 99.9%). Whereas Europe has demonstrated a relatively lower pooled prevalence of 20% (95% CI 17–23, p < 0.01).
Prevalence of hypertension
Among included studies, one hundred and seventy studies provided hypertension estimates among Covid-19 patients. The pooled prevalence of hypertension was found to be 39% by using the random effects model (n = 17, 68,567, 95% CI 0.36–0.42, I2 = 99.9%, p < 0.01, τ2 = 0.04), which indicates considerable heterogeneity as shown in Table 1. Comparison of hypertension proportions across the globe showed significant differences (Q = 39.88, df = 4; p < 0.01). South America, Europe and North America demonstrated a relatively higher pooled prevalence of 44% (95% CI 24–63, p < 0.01),43% (95% CI 39–47, p < 0.01), and 40% (95% CI 35–45, p < 0.01)respectively, while the Asiatic region had a lower pooled prevalence of 21%( 95% CI 15–27, p < 0.01).
Prevalence of asthma
Out of 190 publications, a total of 112 studies reported the prevalence of asthma in patients with Covid-19. The pooled prevalence of asthma, after weighing the geographical population size, was 8% (n = 1, 75,177, 95% CI 0.7–0.9, I2 = 98.3%, p < 0.01, τ2 = 0.00), which indicated substantial heterogeneity, as shown in Table 1. Comparison of asthma proportions across the globe showed significant differences (Q = 58.7, df = 4; p < 0.01). Europe, North America, and Asia demonstrated a relatively higher pooled prevalence of 9% (95% CI 8–11, p < 0.01), 8% (95% CI 6–10, p < 0.01), and 7% (95% CI 3–11, p < 0.01)respectively, while South America had a lower pooled prevalence of 2% (95% CI 0.0–03, p = 0.05).
Prevalence of smoking
Ninety-nine studies investigated the prevalence of smoking in patients with Covid-19. The pooled estimate of smoking was 15% (n = 4, 41, 809, 95% CI 0.12–0.18, I2 = 99.9%, p < 0.01, τ2 = 0.03), which indicated substantial heterogeneity, as shown in Table 1. Comparison of smoking prevalence across the world showed non-significant differences (Q = 7.2, df = 3; p = 0.06). Europe and Asia have shown a higher pooled prevalence of 16% (95% CI 11–22, p < 0.01) and 16% (95% CI 07–24, p < 0.01). While North America with 14% (95% CI 10–18, p < 0.01).
Prevalence of hospitalization
Out of 195 publications, a total of 61 studies reported the prevalence of hospitalization in patients with Covid-19. The pooled prevalence of hospitalization, after weighing the geographical population size, was 35% (n = 7, 48,526, 95% CI 0.29–0.41, I2 = 99.9%, p < 0.01, τ2 = 0.00), which indicated substantially heterogeneity, as shown in Table 1. Comparison of hospitalization proportions across the globe showed significant differences (Q = 69.9, df = 4; p < 0.01). South America demonstrated a relatively higher pooled prevalence of 57% (95% CI 0.55–0.58, p < 0.01), while Asia had pooled prevalence of hospitalization of 31% (95% CI 13–49, p < 0.01).
Prevalence of ICU admissions
One hundred and six studies investigated the prevalence of ICU admissions among Covid-19 patients. The pooled estimate of ICU admissions was 17% (n = 1, 93,980, 95% CI 0.14–021, I2 = 99.8%, p < 0.01, τ2 = 0.03), which indicated substantial heterogeneity, as shown in Table 1. Comparison of ICU admission prevalence across the world showed significant differences (Q = 27.5, df = 4; p < 0.01). South America has showed a higher pooled prevalence of 20% (95% CI 0.19–0.22), followed by Asia and Europe with 18% (95% CI 0.08—0.28), 18% (95% CI (0.12–0.25) of each. Whereas North America has shown a lower pooled prevalence of 16% (95% CI 0.12–0.20).
Prevalence of mortality
Out of 195 publications, a total of 145 studies reported the prevalence of mortality rate in patients with Covid-19. The pooled prevalence of mortality, after weighing the geographical population size, was 18% (n = 4, 45,854, 95% CI 0.16–0.21, I2 = 98.3%, p < 0.01, τ2 = 0.00), which indicated substantially heterogeneity, as shown in Table 1. Comparison of mortality proportions across the globe showed significant differences (Q = 19.04, df = 4; p < 0.01). South America, Europe, and North America demonstrated a relatively higher pooled prevalence of 37% (95% CI 0.17–56, p < 0.01), 20% (95% CI 0.16–0.23, p < 0.01), and 18% (95% CI 0.14–0.21, p < 0.01)respectively, while Asia had a lower pooled prevalence of 8% (95% CI 0.01- 0.14).
Subgroup analysis
Subgroup analysis by geographic region, study design, age category, and male percentage did not influence the prevalence estimates of obesity, diabetes, hypertension, asthma, smoking, hospitalization, ICU admissions, and mortality rate as shown in Table 1. However, the prevalence of mortality (12%), ICU admission rate (14%), and hospitalization rate (31%) was low among patients with ages < 50 years. The prevalence of each condition was high in the > 50 years age male population as compared to the < 50 years age male population, except in Asthma.
Meta-regression analysis
Meta-regression suggested a statistically significant relationship between the prevalence of hospitalization and smoking (p = 0.04, asthma (p = 0.03), obesity (p = 0.03), and hypertension (p = 0.02) (Table 2). However, the prevalence of mortality showed no relationship between all the comorbidity conditions, smoking and hospitalization (Table 3). Meta-regression suggested a statistically significant relationship between age and diabetes (p < 0.001, Fig. 3), hypertension (p < 0.001), asthma (p < 0.05), ICU admission rate (p < 0.05), and mortality (p < 0.001). However, there was no evidence of a relationship between age and obesity (p = 0.07), smoking (p = 0.11), and hospitalization (p = 0.057) as shown in Table 4.
Table 2.
Meta-regression between prevalence of hospitalization and different conditions as moderator.
| Condition | Coefficient | SE | Z | 95% CI | P value |
|---|---|---|---|---|---|
| Obesity | − 4.94 | 2.29 | − 2.16 | − 9.42 to − 4.53 | 0.03 |
| Diabetes | − 1.25 | 6.92 | − 1.81 | − 2.61 to 1.03 | 0.07 |
| Hypertension | − 7.29 | 3.23 | − 2.26 | − 1.36 to − 9.60 | 0.02 |
| Asthma | − 8.41 | 3.92 | − 2.07 | − 0.0001 to − 4.72 | 0.03 |
| Smoking | − 2.67 | 1.34 | − 2.00 | − 5.29 to − 4.72 | 0.04 |
| ICU Admission | − 4.59 | 6.07 | − 0.08 | − 0.0001 to 0.0001 | 0.94 |
| Mortality | − 3.27 | 2.45 | − 1.33 | − 8.07 to 1.54 | 0.18 |
SE standard error, CI confidence interval.
Table 3.
Meta-regression between prevalence of mortality and different conditions as moderator.
| Condition | Coefficient | SE | Z | 95% CI | P value |
|---|---|---|---|---|---|
| Obesity | − 6.45 | 6.91 | − 0.93 | − 2.00 to 7.10 | 0.35 |
| Diabetes | − 5.80 | 1.95 | − 0.30 | − 4.41 to 3.25 | 0.76 |
| Hypertension | − 7.34 | 9.23 | − 0.80 | − 2.54 to 1.07 | 0.42 |
| Asthma | 1.48 | 1.63 | 0.91 | − 1.71 to 4.68 | 0.36 |
| Smoking | 7.66 | 8.07 | 0.95 | − 8.15 to 2.35 | 0.34 |
| ICU Admission | − 9.17 | 1.27 | 0.72 | − 1.57 to 3.40 | 0.46 |
| Hospitalization | 2.92 | 3.46 | 5.69 | − 3.86 to 9.71 | 0.39 |
SE standard error, CI confidence intervel.
Figure 3.
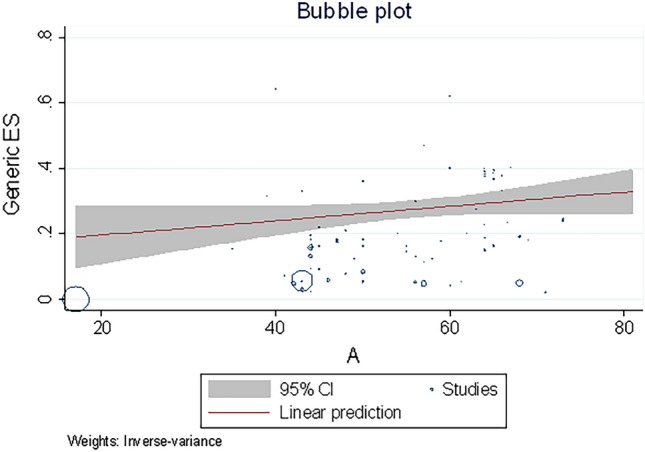
Regression for the prevalence of diabetes according to the age in patients with Covid-19.
Table 4.
Meta-regression between different conditions and age as moderator.
| Condition | Coefficient | SE | Z | 95% CI | P value |
|---|---|---|---|---|---|
| Obesity | 0.002 | 0.001 | 1.79 | − 0.0002 to 0.004 | 0.07 |
| Diabetes | 0.005 | 0.0009 | 5.77 | 0.003 to 0.007 | 0.000 |
| Hypertension | 0.012 | 0.001 | 11.04 | 0.10 to 0.146 | 0.000 |
| Asthma | 0.001 | 0.0004 | 2.40 | 0.0002 to 0.002 | 0.016 |
| Smoking | 0.002 | 0.001 | 1.58 | − 0.005 to 0.004 | 0.113 |
| Hospitalised | 0.006 | 0.003 | 1.90 | − 0.0001 to 0.012 | 0.057 |
| ICU Admission | 0.003 | 0.001 | 2.08 | 0.0001 to 0.006 | 0.037 |
| Mortality | 0.006 | 0.001 | 5.22 | 0.0039 to 0.008 | 0.000 |
SE standard error; CI confidence.
Publication bias assessment
The Egger’s and Begg’s tests indicated statistically significant publication bias for the estimates of obesity(Egger test: p < 0.0001 & Begg’s test: p < 0.0001, Fig. 4), diabetes (Egger test: p < 0.0001&Begg’s test: p < 0.0001), hypertension (Egger test: p < 0.0001&Begg’s test: p < 0.0001), asthma (Egger test: p < 0.005 & Begg’s test: p < 0.07), smoking (Egger test: p < 0.005 & Begg’s test: p < 0.0001), hospitalization (Egger test: p = 0.12 & Begg’s test: p < 0.0001), ICU admissions (Egger test: p < 0.0001 & Begg’s test: p < 0.0001), and mortality rate (Egger test: p < 0.0001 & Begg’s test: p < 0.0001). Visual examination of the funnel plots showed symmetry and suggested no publication bias, as shown in Fig. 4.
Figure 4.
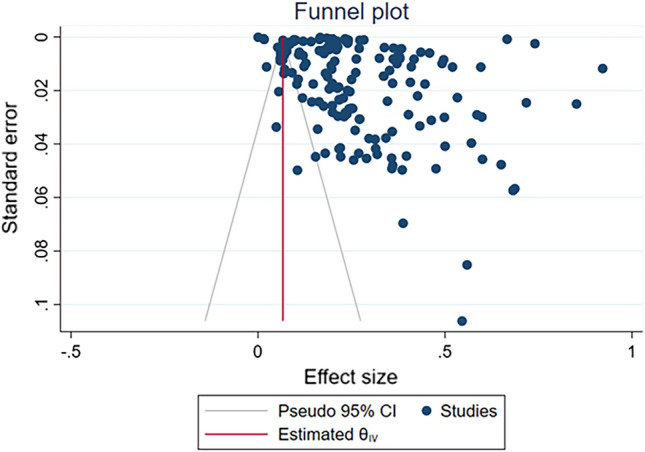
Funnel plot of the effect size on prevalence of obesity in patients with Covid-19.
Discussion
To our knowledge, the present study is one of the largest meta-analyses of the global prevalence of the most common comorbidities such as diabetes, hypertension, obesity, asthma, and meta-regression of the association between age, gender, smoking status and hospitalization, ICU admissions, and mortality in patients with COVID-19. In addition to estimating the prevalence of common comorbidities, the present study results also revealed some new insights into novel corona disease 2019 in the current era of the ongoing pandemic. The present study estimated the highest and lowest proportions of the four most common comorbidities in patients with COVID-19 from different geographic regions from real-world studies. The prevalence of obesity was higher in South America, followed by Europe and Asia, Diabetes in South America, North America and Europe, Hypertension in South America, Europe, Africa and North America, Asthma in Europe, North America and Asia, Smoking in Europe, Asia and North America. Although the prevalence of obesity, diabetes, and hypertension was higher in South America, it is not possible to comment on the prevalence of all three comorbidities in South America due to a wide 95% confidence interval (due to a smaller number of studies), making this a wise decision. Overall, the prevalence of various comorbid conditions in patients with COVID-19 was highest in North America, Europe, and Asia, while both South America and Africa had a lower prevalence of all four major comorbidities. The most common reason for an increased prevalence of these comorbidities in North America, Europe and Africa might be due to the large number of studies published in South America and Africa. Findings of the prevalence rate of hospitalization among patients with COVID-19 had shown a significant difference across the globe (p < 0.01). Both North America and Europe have demonstrated a relatively higher prevalence of hospitalization, followed by Asia, South America and Africa. The prevalence rate of ICU admission was higher with severe COVID-19, was higher in Europe, North America and Asia. The prevalence of mortality among COVID-19 patients was higher in Europe, North America and Asia. Overall Prevalence of each comorbidity was more among the > 50 years age group population than < 50 years and in males, as compared to the < 50 years age group and female population, except for Asthma. In our present study, the prevalence of hospitalization, ICU admission and mortality rate were lower in patients < 50 years of age, than in patients > 50 years of age group, with a higher prevalence of concomitant comorbidities. The prevalence of comorbidities might be the cause of increased mortality among patients in the > 50 years of age group than the < 50 years of age group. Globally, the relationship between age and comorbidities diabetes, hypertension, asthma, ICU admission rate, and mortality has been shown as significant relation.
A spate of previous meta-analysis studies has shown that pre-existing diabetes, hypertension, obesity and smoking were associated with higher mortality associated with COVID-19 a total of nearly 30%192–196. In patients with diabetes mellitus, hyperglycaemia-associated causes modify immunological and inflammatory processes, predisposing individuals to severe, potentially fatal COVID-19196. Obesity is associated with significant changes in the distribution and number of immune cells in the adipose tissues, with fewer Treg cells, Th2 cells, and M2 macrophages, which will cause cells to decrease in quantity, especially M1 macrophages and CD8 + T cells increases, in similar with autoimmune diseases196,197. Therefore, obesity affects the immune defence and T cell activity196,197. Overall, comorbidities such as Hypertension, diabetes mellitus, obesity and smoking are significantly associated with vascular endothelial injury, dysfunctional haemostatic system, and pro-inflammatory or chronic inflammation state, leading to cytokine storm, multi-organ failure (MOF) and acute respiratory distress syndrome (ARDS) 197–199. This relationship was further supported in a recent study, which showed that the male gender and elderly ages were associated with higher morbidity or mortality due to COVID-19200. While former smokers appear to be at increased risk of hospitalization, increased disease severity and mortality from COVID-19 than never smokers and current smokers201. However, this relationship was further supported in a recent study, which showed that asthma as co-morbidity doesn’t have a significant risk of SARS-CoV-2 infection, severity and mortality with COVID-19201–204.
There are several limitations in the present systematic review and meta-analysis. First, most of the included studies had observational (prospective and retrospective) study design heterogeneity of studies was observed in the analyses of continuous variables. In addition, only studies in the English language were included in the present study. Moreover, there were a smaller number of studies found in the geographic regions of Africa and South America, whereas the majority of studies were from North America, Europe, and Asia which further increases the possibility of publication bias.
Conclusion
In this systematic review, metaanalysis and metaregression study, an overall higher prevalence of hypertension (39%), diabetes(27%), obesity (27%), and 18% of mortality among hospitalized patients with COVID-19 across the world. Geographic regions with a higher pooled prevalence of comorbidities, specifically, North America, and Europe, had shown a high prevalence estimates of all the major comorbid conditions and mortality followed by South America, Asia and Africa. The present meta-analysis and meta-regression will help to make an appropriate decisions by administrators, stakeholders and health care providers to take a clinical decision among patients with comorbidities and to be vigilant over disease severity and mortality in relation to smoking status, age and gender wise. We suggest for regular booster dose vaccination preferably for those patients with chronic comorbidities and to follow regular preventive measures to contain the spread of highly infectious novel variants of SARS-CoV-2 omicron variants and to prevent the severety, mortality of COVID-19 disease.
Supplementary Information
Author contributions
S.C., K.V., S.P. conducted the literature search and data extraction and drafted the manuscript, S.S. and M.R.C., M.M., P.R., A.S.B., M.C., R.G., K.C.A., M.G. and S.M. revised the manuscript. All authors reviewed and approved the final version of the manuscript.
Funding
Authors did not receive any funding support.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-33314-9.
References
- 1.Johns Hopkins University. Coronavirus Resource Center. https://coronavirus.jhu.edu/map.html. Accessed 10 Dec 2021.
- 2.Khan MMA, et al. Effects of underlying morbidities on the occurrence of deaths in COVID-19 patients: A systematic review and meta-analysis. J. Glob. Health. 2020;10(2):020503. doi: 10.7189/jogh.10.020503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Santenna C, Vidyasagar K, Amarneni KC, Ghanta SN, Sadasivam B, Pathan S, Padmavathi R. The safety, tolerability and mortality reduction efficacy of remdesivir; based on randomized clinical trials, observational and case studies reported safety outcomes: An updated systematic review and meta-analysis. Ther. Adv. Drug Saf. 2021 doi: 10.1177/20420986211042517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ssentongo, P., et al. Association of cardiovascular disease and 10 other pre-existing comorbidities with COVID-19 mortality: A systematic review and meta-analysis. PLoS One. e0238215 (2020). [DOI] [PMC free article] [PubMed]
- 5.Luo L, et al. The potential association between common comorbidities and severity and mortality of coronavirus disease 2019: A pooled analysis. Obes. Res. Clin. Pract. 2020;43(12):1478–1493. doi: 10.1002/clc.23465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hussain A, Mahawar K, Xia Z, Yang W, El-Hasani S. Obesity and mortality of COVID-19. Metaanalysis. Obes. Res. Clin. Pract. 2020;14:295–300. doi: 10.1016/j.orcp.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 7.de Almeida-Pititto B, Dualib PM, Zajdenverg L, Dantas JR, de Souza FD, Rodacki M, et al. Severity and mortality of COVID 19 in patients with diabetes, hypertension and cardiovascular disease: A meta-analysis. DiabetolMetabSyndr. 2020;12:75. doi: 10.1186/s13098-020-00586-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barek, M. A., Aziz, M. A. & Islam, M. S. Impact of age, sex, comorbidities and clinical symptoms on the severity of COVID-19 cases: A meta-analysis with 55 studies and 10014 cases. Heliyon e05684 (2020). [DOI] [PMC free article] [PubMed]
- 9.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khawaja, A. P., et al. Associations with covid‐19 hospitalization amongst 406,793 adults: The UK Biobank prospective cohort study. medRxiv (2020): 2020.05.06.20092957.
- 11.Giannouchos, T. V., Sussman, R., Mier, J. M., Poulas, K. & Farsalinos, K. Characteristics and risk factors for COVID‐19 diagnosis and adverse outcomes in Mexico: An analysis of 89,756 laboratory‐confirmed COVID‐19 cases. medRxiv (2020). [DOI] [PMC free article] [PubMed]
- 12.Terada, M., et al. Risk factors for severity on admission and the disease progression during hospitalisation in a large cohort of patients with COVID-19 in Japan. BMJ Open e047007 (2021). [DOI] [PMC free article] [PubMed]
- 13.Bello‐Chavolla, O. Y., et al. Predicting mortality due to SARS‐CoV‐2: A mechanistic score relating obesity and diabetes to COVID‐19 outcomes in Mexico. medRxiv (2020): 2020.04.20.20072223. [DOI] [PMC free article] [PubMed]
- 14.Antonio‐Villa, N. E., et al. Health‐care workers with COVID‐19 living in Mexico City: Clinical characterization and related outcomes. medRxiv (2020).
- 15.Denova-Gutiérrez E, et al. The association between obesity, type 2 diabetes, and hypertension with severe COVID‐19 on admission among Mexicans. Obesity. 2020;28(10):1826–1832. doi: 10.1002/oby.22946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ho, F. K., et al. Modifiable and non-modifiable risk factors for COVID-19, and comparison to risk factors for influenza and pneumonia: Results from a UK Biobank prospective cohort study. BMJ Open e040402 (2020). [DOI] [PMC free article] [PubMed]
- 17.Gao, M., et al. Associations between body-mass index and COVID-19 severity in 6·9 million people in England: A prospective, community-based, cohort study. Lancet Diabetes Endocrinol. 350–359 (2021). [DOI] [PMC free article] [PubMed]
- 18.Goyal, P., et al. Clinical characteristics of Covid-19 in New York City. N. Engl. J. Med. 2372–2374 (2020). [DOI] [PMC free article] [PubMed]
- 19.Dana, R., et al. Obesity and mortality in critically ill COVID-19 patients with respiratory failure. Int. J. Obes. 1–10 (2021). [DOI] [PMC free article] [PubMed]
- 20.Hernández‐Garduño E, et al. Obesity is the comorbidity more strongly associated for Covid‐19 in Mexico. A case‐control study. Obes. Res. Clin Pract. 2020;14(4):375–379. doi: 10.1016/j.orcp.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.COVID-ICU Group on behalf of the REVA Network and the COVID-ICU Investigators. Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: A prospective cohort study. Intensive Care Med.47, 60–73 (2021). [DOI] [PMC free article] [PubMed]
- 22.Krishnamoorthy G, Arsene C, Jena N, Mogulla SM, Coakley R, Khine J, et al. Racial disparities in COVID-19 hospitalizations do not lead to disparities in outcomes. Public Health. 2021;190:93–98. doi: 10.1016/j.puhe.2020.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Price-Haywood, E. G., Burton, J., Fort, D. & Seoane, L. Hospitalization and mortality among black patients and white patients with Covid-19. N. Engl. J. Med. 2534–2543 (2020). [DOI] [PMC free article] [PubMed]
- 24.Chen, Q., et al. Clinical characteristics of 145 patients with corona virus disease 2019 (COVID-19) in Taizhou, Zhejiang, China. Infection 543–551 (2020). [DOI] [PMC free article] [PubMed]
- 25.Al-Sabah, S., Al-Haddad, M., Al-Youha, S., Jamal, M. & Almazeedi, S. COVID-19: Impact of obesity and diabetes on disease severity. Clin. Obes. e12414 (2020). [DOI] [PMC free article] [PubMed]
- 26.Chima, M., Williams, D., Thomas, N. J. & Krawiec, C. COVID-19-associated pulmonary embolism in pediatric patients. Hosp. Pediatr. e90–e94 (2021). [DOI] [PMC free article] [PubMed]
- 27.Carrillo, G., et al. Clinical course and severity outcome 530 indicators among COVID-19 hospitalized patients in relation to comorbidities distribution: 531 Mexican cohort. medRxiv. (2020): 2007.2031.20165480.
- 28.Louapre, C., et al. Clinical characteristics and outcomes in patients with coronavirus disease 2019 and multiple sclerosis. JAMA Neurol. 1079–1088 (2020). [DOI] [PMC free article] [PubMed]
- 29.Rao, X., et al. The importance of overweight in COVID-19: A retrospective analysis in a single center of Wuhan, China. Medicine e22766 (2020). [DOI] [PMC free article] [PubMed]
- 30.Gao, F., et al. Obesity is a risk factor for greater COVID‐19 severity. Diabetes Care (2020). [DOI] [PubMed]
- 31.Berumen, J., et al. Risk of infection and hospitalization by Covid‐19 in Mexico: A case‐control study. medRxiv (2020): 2020.05.24.20104414.
- 32.Jayanama K, Srichatrapimuk S, Thammavaranucupt K, Kirdlarp S, Suppadungsuk S, Wongsinin T, et al. The association between body mass index and severity of Coronavirus Disease 2019 (COVID-19): A cohort study. PLoS ONE. 2021;16:e0247023. doi: 10.1371/journal.pone.0247023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giacomelli A, Ridolfo AL, Milazzo L, Oreni L, Bernacchia D, Siano M, et al. 30-day mortality in patients hospitalized with COVID-19 during the first wave of the Italian epidemic: A prospective cohort study. Pharmacol. Res. 2020;158:104931. doi: 10.1016/j.phrs.2020.104931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Al Heialy, S., et al. Combination of obesity and co-morbidities leads to unfavorable outcomes in COVID-19 patients. Saudi J. Biol. Sci. 1445–1450 (2021). [DOI] [PMC free article] [PubMed]
- 35.Hendren NS, de Lemos JA, Ayers C, Das SR, Rao A, Carter S, et al. Association of body mass index and age with morbidity and mortality in patients hospitalized With COVID-19: Results From the American Heart Association COVID-19 Cardiovascular Disease Registry. Circulation. 2021;143:135–144. doi: 10.1161/CIRCULATIONAHA.120.051936. [DOI] [PubMed] [Google Scholar]
- 36.Wu X, Li C, Chen S, Zhang X, Wang F, Shi T, et al. Association of body mass index with severity and mortality of COVID-19 pneumonia: A two-center, retrospective cohort study from Wuhan, China. Aging. 2021;13:7767–7780. doi: 10.18632/aging.202813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Babar I, Ekenna O, Clarkson MR, Boudreaux D, Bennett W, Roth R. COVID-19 in a Mississippi Community Hospital. South Med J. 2021;114:305–310. doi: 10.14423/SMJ.0000000000001245. [DOI] [PubMed] [Google Scholar]
- 38.Ruíz-Quiñonez, J. A., et al. Features of patients that died for COVID-19 in a hospital in the south of Mexico: A observational cohort study. PLoS One e0245394 (2021). [DOI] [PMC free article] [PubMed]
- 39.Vera-Zertuche JM, Mancilla-Galindo J, Tlalpa-Prisco M, Aguilar-Alonso P, Aguirre-García MM, Segura-Badilla O, et al. Obesity is a strong risk factor for short-term mortality and adverse outcomes in Mexican patients with COVID-19: A national observational study. Epidemiol. Infect. 2021;149:e109. doi: 10.1017/S0950268821001023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rottoli, M., et al. How important is obesity as a risk factor for respiratory failure, intensive care admission and death in hospitalised COVID-19 patients? Results from a single Italian centre. Eur. J. Endocrinol. 389–397 (2020). [DOI] [PMC free article] [PubMed]
- 41.Lucar J, Wingler MJB, Cretella DA, Ward LM, SimsGomillia CE, Chamberlain N, et al. Epidemiology, clinical features, and outcomes of hospitalized adults with COVID-19: Early experience from an Academic Medical Center in Mississippi. South Med J. 2021;114:144–149. doi: 10.14423/SMJ.0000000000001222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Azoulay, E., et al. Increased mortality in patients with severe SARS-CoV-2 infection admitted within seven days of disease onset. Intensive Care Med. 1714–1722 (2020). [DOI] [PMC free article] [PubMed]
- 43.Lighter J, Phillips M, Hochman S, et al. Obesity in patients younger than 60 years is a risk factor for Covid-19 hospital admission. Clin. Infect Dis. 2020;71:896–897. doi: 10.1093/cid/ciaa415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mehta, H. B., Li, S. & Goodwin, J. S. Risk factors associated With SARS-CoV-2 infections, hospitalization, and mortality among US nursing home residents. JAMA Netw. Open e216315 (2021). [DOI] [PMC free article] [PubMed]
- 45.Petrilli CM, Jones SA, Yang J, Rajagopalan H, O'Donnell L, Chernyak Y, et al. Factors associated with hospital admission and critical illness among 5279 people with coronavirus disease 2019 in New York City: Prospective cohort study. BMJ. 2020;369:m1966. doi: 10.1136/bmj.m1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goncalves TJM, Gonçalves SEAB, Guarnieri A, Risegato RC, Guimarães MP, de Freitas DC, et al. Association between low zinc levels and severity of acute respiratory distress syndrome by new coronavirus SARS-CoV-2. Nutr. Clin. Pract. 2021;36:186–191. doi: 10.1002/ncp.10612. [DOI] [PubMed] [Google Scholar]
- 47.Tartof, S. Y., et al. Obesity and mortality among patients diagnosed With COVID-19: Results from an integrated health care organization. Ann. Intern. Med. 773–781 (2020). [DOI] [PMC free article] [PubMed]
- 48.Cai, Q., et al. Obesity and COVID-19 severity in a designated hospital in Shenzhen, China. Diabetes Care 1392–1398 (2020). [DOI] [PubMed]
- 49.Perez-Cruz, E., Castañón-González, J. A., Ortiz-Gutiérrez, S., Garduño-López, J. & Luna-Camacho, Y. Impact of obesity and diabetes mellitus in critically ill patients with SARS-CoV-2. Obes. Res. Clin. Pract. (2021). [DOI] [PMC free article] [PubMed]
- 50.Mani VR, Kalabin A, Valdivieso SC, Murray-Ramcharan M, Donaldson B. New York Inner City Hospital COVID-19 experience and current data: Retrospective analysis at the epicenter of the American coronavirus outbreak. J. Med. Internet Res. 2020;22:e20548. doi: 10.2196/20548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Palaiodimos L, Kokkinidis DG, Li W, Karamanis D, Ognibene J, Arora S, et al. Severe obesity, increasing age and male sex are independently associated with worse in-hospital outcomes, and higher in-hospital mortality, in a cohort of patients with COVID-19 in the Bronx, New York. Metabolism. 2020;108:154262. doi: 10.1016/j.metabol.2020.154262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Simonnet, A., et al. Intensive Care COVID-19 and Obesity study group. High prevalence of obesity in severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) requiring invasive mechanical ventilation. Obesity (2020). [DOI] [PMC free article] [PubMed]
- 53.Smati, S., et al. Relationship between obesity and severe COVID-19 outcomes in patients with type 2 diabetes: Results from the CORONADO study. Diabetes ObesMetab. 391–403 (2021). [DOI] [PMC free article] [PubMed]
- 54.Kim, T. S., Roslin, M., Wang, J. J., Kane, J., Hirsch, J. S., Kim, E. J.; Northwell Health COVID-19 Research Consortium. BMI as a risk factor for clinical outcomes in patients hospitalized with COVID-19 in New York. Obesity29, 279–284 (2021). [DOI] [PMC free article] [PubMed]
- 55.García-Posada M, Aruachan-Vesga S, Mestra D, Humánez K, Serrano-Coll H, Cabrales H, et al. Clinical outcomes of patients hospitalized for COVID-19 and evidence-based on the pharmacological management reduce mortality in a region of the Colombian Caribbean. J. Infect. Public Health. 2021;14:696–701. doi: 10.1016/j.jiph.2021.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nakeshbandi, M., et al. The impact of obesity on COVID-19 complications: A retrospective cohort study. Int. J. Obes. 1832–1827 (2020). [DOI] [PMC free article] [PubMed]
- 57.Cottini, M., Lombardi, C., Berti, A.; Primary Care Physicians, ATS Province of Bergamo, Italy. Obesity is a major risk factor for hospitalization in community-managed COVID-19 pneumonia. Mayo Clin. Proc. 921–931 (2021). [DOI] [PMC free article] [PubMed]
- 58.Olivas-Martínez M, et al. In-hospital mortality from severe COVID-19 in a tertiary care center in Mexico City; causes of death, risk factors and the impact of hospital saturation. PLoS One. 2021;16(2):e0245772. doi: 10.1371/journal.pone.0245772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang, J., et al. The association between obesity and severity in patients with coronavirus disease 2019: A retrospective, single-center study, Wuhan. Int. J. Med. Sci. 1768–1777 (2021). [DOI] [PMC free article] [PubMed]
- 60.Hajifathalian, K., et al. Obesity is associated with worse outcomes in COVID-19: Analysis of early data from New York City. Obesity 1606–1612 (2020). [DOI] [PMC free article] [PubMed]
- 61.Caussy, C., Wallet, F., Laville, M. & Disse, E. Obesity is associated with severe forms of COVID-19. Obesity 1993 (2020). [DOI] [PMC free article] [PubMed]
- 62.Kalligeros, M., et al. Association of obesity with disease severity among patients with COVID-19. Obesity 1200-04 (2020). [DOI] [PMC free article] [PubMed]
- 63.Huang, R., et al. Clinical findings of patients with coronavirus disease 2019 in Jiangsu province, China: A retrospective, multi-center study. PLoSNegl Trop Dis. (2020). [DOI] [PMC free article] [PubMed]
- 64.Kompaniyets L, Goodman AB, Belay B, Freedman DS, Sucosky MS, Lange SJ, et al. Body mass index and risk for COVID-19-related hospitalization, intensive care unit admission, invasive mechanical ventilation, and death—United States, March-December. MMWR Morb. Mortal. Wkly. Rep. 2020;2021:355–361. doi: 10.15585/mmwr.mm7010e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Guerson-Gil, A., et al. Sex-specific impact of severe obesity in the outcomes of hospitalized patients with COVID-19: A large retrospective study from the Bronx, New York. Eur. J. Clin. Microbiol. Infect. Dis. 1–12 (2021). [DOI] [PMC free article] [PubMed]
- 66.Hur K, Price CPE, Gray EL, et al. Factors associated with intubation and prolonged intubation in hospitalized patients With COVID-19. Otolaryngology-Head Neck Surg. 2020;163:170–178. doi: 10.1177/0194599820929640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Halasz G, Leoni ML, Villani GQ, Nolli M, Villani M. Obesity, overweight and survival in critically ill patients with SARS-CoV-2 pneumonia: Is there an obesity paradox? Preliminary results from Italy. Eur J PrevCardiol. 2020; 1–3. [DOI] [PMC free article] [PubMed]
- 68.Anderson MR, Geleris J, Anderson DR, Zucker J, Nobel YR, Freedberg D, et al. Body mass index and risk for intubation or death in SARS-CoV-2 infection: A retrospective cohort study. Ann. Intern. Med. 2020;173:782–790. doi: 10.7326/M20-3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ong, S. W. X., Young, B. E., Leo, Y. S. & Lye, D. C. Association of higher body mass index (BMI) with severe coronavirus disease 2019 (COVID-19) in younger patients. Clin. Infect. Dis. 2300-02 (2020). [DOI] [PMC free article] [PubMed]
- 70.Ortiz-Brizuela, E., et al. Clinical and epidemiological characteristics of patients diagnosed with COVID-19 in a tertiary care centre in mexicocity: A prospective cohort study. Rev. Invest. Clin. 165–177 (2020). [DOI] [PubMed]
- 71.Gupta S, Hayek SS, Wang W, Chan L, Mathews KS, Melamed ML, et al. Factors associated with death in critically ill patients with coronavirus disease 2019 in the US. JAMA Intern. Med. 2020;180:1–12. doi: 10.1001/jamainternmed.2020.3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Steinberg, E. & Kushner, B. In young adults with COVID-19, obesity is associated with adverse outcomes. West. J. Emerg. Med. 752–755 (2010). [DOI] [PMC free article] [PubMed]
- 73.Rossi, A. P., et al. Obesity as a risk factor for unfavourable outcomes in critically ill patients affected by Covid19. Nutr. Metab. Cardiovasc. Dis. 762–768 (2021). [DOI] [PMC free article] [PubMed]
- 74.Suresh, S., et al. Association of obesity with illness severity in hospitalized patients with COVID-19: A retrospective cohort study. Obes. Res. Clin. Pract. 172–176 (2021). [DOI] [PMC free article] [PubMed]
- 75.Suleyman G, Fadel RA, Malette KM, Hammond C, Abdulla H, Entz A, Characteristics C, Disease MAWC, et al. In a series of patients in metropolitan detroit. JAMA Netw. Open. 2019;2020:e2012270. doi: 10.1001/jamanetworkopen.2020.12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Moriconi, D. et al. Obesity prolongs the hospital stay in patients affected by COVID-19, and may impact on SARS-COV-2 shedding. Obes. Res. Clin. Pract. 205–309 (2020). [DOI] [PMC free article] [PubMed]
- 77.Dicker, D., et al. A time frame for testing negative for SARS-COV2 in people with obesity. Obes. Facts 1–6 (2020). [DOI] [PMC free article] [PubMed]
- 78.Wolf M, et al. Obesity and critical illness in COVID-19: Respiratory pathophysiology. Obesity. 2021;29:870–878. doi: 10.1002/oby.23142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chetboun, M., et al. BMI and pneumonia outcomes in critically ill COVID-19 patients: An international multicenter study. Obesity (2021). [DOI] [PMC free article] [PubMed]
- 80.Yordanov, Y., et al. AP-HP/Universities/Inserm COVID-19 research collaboration. Clinical characteristics and factors associated with hospital admission or death in 43 103 adult outpatients with coronavirus disease 2019 managed with the Covidomtelesurveillance solution: A prospective cohort study. Clin. Microbiol. Infect. (2021). [DOI] [PMC free article] [PubMed]
- 81.Mayer, M. A., et al. Clinical characterization of patients with COVID-19 in primary care in Catalonia: Retrospective observational study. JMIR Public Health Surveill. e25452 (2021). [DOI] [PMC free article] [PubMed]
- 82.Singh S, Bilal M, Pakhchanian H, Raiker R, Kochhar GS, Thompson CC, et al. Impact of obesity on outcomes of patients with coronavirus disease 2019 in the United States: A multicenter electronic health records network study. Gastroenterology. 2020;159:2221–2225. doi: 10.1053/j.gastro.2020.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Palmieri L, Vanacore N, Donfrancesco C, Lo Noce C, Canevelli M, Punzo O, et al. Italian National Institute of Health COVID-19 Mortality Group. Clinical characteristics of hospitalized individuals dying with COVID-19 by age group in Italy. J. Gerontol. A Biol. Sci. Med. Sci. 2020;75:1796–1800. doi: 10.1093/gerona/glaa146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Pietri L, Giorgi R, Bégu A, Lojou M, Koubi M, Cauchois R, Grangeot R, Dubois N, Kaplanski G, Valéro R, Béliard S. Excess body weight is an independent risk factor for severe forms of COVID-19. Metabolism. 2021;117:154703. doi: 10.1016/j.metabol.2021.154703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Thomson, R. J., et al. Clinical characteristics and outcomes of critically ill patients with COVID-19 admitted to an intensive care unit in London: A prospective observational cohort study. PLoS One e0243710 (2020). [DOI] [PMC free article] [PubMed]
- 86.Iaccarino G, Grassi G, Borghi C, Carugo S, Fallo F, Ferri C, et al. SARS-RAS Investigators. Gender differences in predictors of intensive care units admission among COVID-19 patients: The results of the SARS-RAS study of the Italian Society of Hypertension. PLoS ONE. 2020;15:e0237297. doi: 10.1371/journal.pone.0237297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kass DA, Duggal P, Cingolani O. Obesity could shift severe COVID-19 disease to younger ages. Lancet. 2020;395(10236):1544–1545. doi: 10.1016/S0140-6736(20)31024-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bello-Chavolla, O. Y., et al. Predicting mortality due to SARS-Cov-2: A mechanistic score relation obesity and diabetes to COVID-19 outcomes in Mexico. J. Clin. Endocrinol. Metab. 105 (2020). [DOI] [PMC free article] [PubMed]
- 89.Cummings MJ, Baldwin MR, Abrams D, Jacobson SD, Meyer BJ, Balough EM, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: A prospective cohort study. Lancet. 2020;395:1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Docherty, A. B., et al. Features of 20133 UK patients in hospital with covid-19 using the ISARIC WHO clinical characterisation protocol: A prospective observational cohort study.BMJ 369 (2020). [DOI] [PMC free article] [PubMed]
- 91.Hamer, M., Gale, C. R., Kivimäki, M. & Batty, G. D. Overweight, obesity, and risk of hospitalization for COVID-19: A community-based cohort study of adults in the United Kingdom. Proc. Natl. Acad. Sci. U. S. A. 21011–21013 (2020). [DOI] [PMC free article] [PubMed]
- 92.Parra-Bracamonte GM, Lopez-Villalobos N, Parra-Bracamonte FE. Clinical characteristics and risk factors for mortality of patients with COVID-19 in a large data set from Mexico. Ann. Epidemiol. 2020;52:93–98.e2. doi: 10.1016/j.annepidem.2020.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Peters SAE, MacMahon S, Woodward M. Obesity as a risk factor for COVID-19 mortality in women and men in the UK biobank: Comparisons with influenza/pneumonia and coronary heart disease. Diabetes ObesMetab. 2021;23:258–262. doi: 10.1111/dom.14199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Argenziano MG, Bruce SL, Slater CL, Tiao JR, Baldwin MR, Barr RG, et al. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: Retrospective case series. BMJ. 2020;369:m1996. doi: 10.1136/bmj.m1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Auld, S. C., Caridi-Scheible, M., Robichaux, C., Coopersmith, C. M. & Murphy, D. J.; Emory COVID-19 Quality and Clinical Research Collaborative. Declines in Mortality over time for critically Ill adults with coronavirus disease 2019. Crit Care Med. e1382–e1384 (2020). [DOI] [PMC free article] [PubMed]
- 96.Zheng, K. I., et al. Obesity as a risk factor for greater severity of COVID-19 in patients with metabolic associated fatty liver disease. Metabolism. 108 (2020). [DOI] [PMC free article] [PubMed]
- 97.Klang E, Kassim G, Soffer S, Freeman R, Levin MA, Reich DL. Severe obesity as an independent risk factor for COVID-19 mortality in hospitalized patients younger than 50. Obesity (Silver Spring) 2020;28:1595–1599. doi: 10.1002/oby.22913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shah P, Owens J, Franklin J, Mehta A, Heymann W, Sewell W, et al. Demographics, comorbidities and outcomes in hospitalized Covid-19 patients in rural southwest Georgia. Ann Med. 2020;52:354–360. doi: 10.1080/07853890.2020.1791356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sapey, E., et al.; All clinicians and students at University Hospitals Birmingham NHS Foundation Trust. Ethnicity and risk of death in patients hospitalised for COVID-19 infection in the UK: An observational cohort study in an urban catchment area. BMJ Open Respir. Res. e000644 (2020). [DOI] [PMC free article] [PubMed]
- 100.Favà, A., et al. Clinical characteristics and risk factors for severe COVID-19 in hospitalized kidney transplant recipients: A multicentric cohort study. Am. J. Transpl. 3030–3041 (2020). [DOI] [PMC free article] [PubMed]
- 101.Bellan, M., et al. Fatality rate and predictors of mortality in a large Italian cohort of hospitalized COVID-19 patients 2020. Sci. Rep. 20731 (2020). [DOI] [PMC free article] [PubMed]
- 102.Murillo-Zamora E, Mendoza-Cano O, Cárdenas-Rojas MI, Hernandez-Suarez CM, Guzmán-Esquivel J. Survival in adult pneumonia inpatients fulfilling suspected COVID-19 criteria and baseline negative RT-qPCR. Public Health. 2021;195:123–125. doi: 10.1016/j.puhe.2021.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Reyes Gil M, Gonzalez-Lugo JD, Rahman S, Barouqa M, Szymanski J, Ikemura K, et al. Correlation of coagulation parameters with clinical outcomes during the coronavirus-19 Surge in New York: Observational cohort. Front Physiol. 2021;23(12):618929. doi: 10.3389/fphys.2021.618929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rentsch, C. T., et al. Covid-19 testing, hospital admission, and intensive care among 2,026,227 United States Veterans aged 54–75 years. medRxiv [Preprint] (2020).
- 105.Burn, E., et al. The natural history of symptomatic COVID-19 during the first wave in Catalonia. Nat. Commun. 777 (2021). [DOI] [PMC free article] [PubMed]
- 106.Borobia, A. M., et al. On Behalf Of the Covid Hulp Working Group. A cohort of patients with COVID-19 in a major teaching hospital in Europe. J. Clin. Med. 1733 (2020). [DOI] [PMC free article] [PubMed]
- 107.de Roquetaillade, C., et al. Timing and causes of death in severe COVID-19 patients. Crit. Care 224 (2021). [DOI] [PMC free article] [PubMed]
- 108.Ferrando, C., et al. Patient characteristics, clinical course and factors associated to ICU mortality in critically ill patients infected with SARS-CoV-2 in Spain: A prospective, cohort, multicentre study. Rev. EspAnestesiolReanim. 425–437 (2020). [DOI] [PMC free article] [PubMed]
- 109.Bonnet, G., et al. Characteristics and outcomes of patients hospitalized for COVID-19 in France: The critical COVID-19 France (CCF) study. Arch. Cardiovasc. Dis. 352–363 (2021). [DOI] [PMC free article] [PubMed]
- 110.Ouellet, J. A., et al.. An observational case series of targeted virtual geriatric medicine and palliative care consults for hospitalized older adults with COVID-19. Ann. Palliat. Med. 6297–306 (2021). [DOI] [PubMed]
- 111.lvarez-Arroyo, L., et al. Descriptive study of a cohort of COVID-19 hospitalized patients in Spain. Gac. Med. Mex. 76–83 (2021). [DOI] [PubMed]
- 112.Álvarez-Maldonado, P., et al. Characteristics and mortality of Mexican patients with COVID-19 and mechanical ventilation. Gac. Med. Mex. 97–101 (2021). [DOI] [PubMed]
- 113.Schreyer, K. E., et al. Rate of decompensation of normoxic emergency department patients with SARS-CoV-2. West J. Emerg. Med. 580–586 (2021). [DOI] [PMC free article] [PubMed]
- 114.Saib, A., et al. Lack of efficacy of hydroxychloroquine and azithromycin in patients hospitalized for COVID-19 pneumonia: A retrospective study. PLoS One e0252388 (2021). [DOI] [PMC free article] [PubMed]
- 115.Pouwels S, Ramnarain D, Aupers E, Rutjes-Weurding L, van Oers J. Obesity may not be associated with 28-day mortality, duration of invasive mechanical ventilation and length of intensive care unit and hospital stay in critically Ill patients with severe acute respiratory syndrome coronavirus-2: A retrospective cohort study. Medicina. 2021;57:674. doi: 10.3390/medicina57070674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ramos-Martínez, A., et al. Frequency, risk factors, and outcomes of hospital readmissions of COVID-19 patients. Sci. Rep. 13733 (2021). [DOI] [PMC free article] [PubMed]
- 117.Gupta R, Agrawal R, Bukhari Z, et al. Higher comorbidities and early death in hospitalized African-American patients with Covid-19. BMC Infect. Dis. 2021;21:78. doi: 10.1186/s12879-021-05782-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Prado-Galbarro FJ, Sanchez-Piedra C, Gamiño-Arroyo AE, Cruz-Cruz C. Determinants of survival after severe acute respiratory syndrome coronavirus 2 infection in Mexican outpatients and hospitalised patients. Public Health. 2020;189:66–72. doi: 10.1016/j.puhe.2020.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shi, R., et al. COVID-19 ARDS is characterized by higher extravascular lung water than non-COVID-19 ARDS: The PiCCOVID study. Crit Care. 186 (2021). [DOI] [PMC free article] [PubMed]
- 120.Thoreau B, et al. D-dimer level and neutrophils count as predictive and prognostic factors of pulmonary embolism in severe non-ICU COVID-19 patients. Viruses. 2020;13(5):758. doi: 10.3390/v13050758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Guisado-Clavero, M., et al. Clinical characteristics of SARS-CoV-2 pneumonia diagnosed in a primary care practice in Madrid. BMC Fam. Pract. 83 (2021). [DOI] [PMC free article] [PubMed]
- 122.AntunezMuiños, P. J., et al. The COVID-19 lab score: An accurate dynamic tool to predict in-hospital outcomes in COVID-19 patients. Sci. Rep. 9361 (2021). [DOI] [PMC free article] [PubMed]
- 123.Marín-Corral, J., et al. Metabolic signatures associated with severity in hospitalized COVID-19 patients. Int. J. Mol. Sci. 4794 (2021). [DOI] [PMC free article] [PubMed]
- 124.Yacobitti, A., et al. Clinical characteristics of vulnerable populations hospitalized and diagnosed with COVID-19 in Buenos Aires, Argentina. Sci. Rep. 9679 (2021). [DOI] [PMC free article] [PubMed]
- 125.De Lorenzo, R., et al. Residual clinical damage after COVID-19: A retrospective and prospective observational cohort study. PLoS One e0239570 (2020). [DOI] [PMC free article] [PubMed]
- 126.Mikami, T., et al. Risk factors for mortality in patients with COVID-19 in New York City. J. Gen. Intern. Med. 17–26 (2021). [DOI] [PMC free article] [PubMed]
- 127.Piroth, L., et al. Comparison of the characteristics, morbidity, and mortality of COVID-19 and seasonal influenza: A nationwide, population-based retrospective cohort study. Lancet Respir. Med. 251–259 (2021). [DOI] [PMC free article] [PubMed]
- 128.Garibaldi, B. T., et al. Patient trajectories among persons hospitalized for COVID-19: A cohort study. Ann. Intern. Med. 33–41 (2021). [DOI] [PMC free article] [PubMed]
- 129.Roedl, K., et al. Mechanical ventilation and mortality among 223 critically ill patients with coronavirus disease 2019: A multicentric study in Germany. Aust. Crit Care 167–175 (2021). [DOI] [PMC free article] [PubMed]
- 130.Svensson, P., et al. Association between cardiometabolic disease and severe COVID-19: A nationwide case-control study of patients requiring invasive mechanical ventilation. BMJ Open. e044486 (2021). [DOI] [PMC free article] [PubMed]
- 131.Awad, N., Schiller, D. S., Fulman, M. & Chak, A. Impact of hydroxychloroquine on disease progression and ICU admissions in patients with SARS-CoV-2 infection. Am. J. Health Syst. Pharm. 689–696 (2021). [DOI] [PMC free article] [PubMed]
- 132.Pepe, M., et al. Clinical presentation, therapeutic approach, and outcome of young patients admitted for COVID-19, with respect to the elderly counterpart. Clin. Exp. Med. 249–268 (2021). [DOI] [PMC free article] [PubMed]
- 133.Mash, R. J., et al. Evaluation of patient characteristics, management and outcomes for COVID-19 at district hospitals in the Western Cape, South Africa: Descriptive observational study. BMJ Open e047016 (2021). [DOI] [PMC free article] [PubMed]
- 134.De Lorenzo, A., et al. Fat mass affects nutritional status of ICU COVID-19 patients. J. Transl. Med. 299 (2020). [DOI] [PMC free article] [PubMed]
- 135.Nascimento, J. H. P., et al. COVID-19 and Myocardial Injury in a Brazilian ICU: High Incidence and Higher Risk of In-Hospital Mortality. Arq. Bras. Cardiol. 275–282 (2021). [DOI] [PMC free article] [PubMed]
- 136.Apea, V. J., et al. Ethnicity and outcomes in patients hospitalised with COVID-19 infection in East London: An observational cohort study. BMJ Open e042140 (2021). [DOI] [PMC free article] [PubMed]
- 137.Lu, X., et al. Glycemic status affects the severity of coronavirus disease 2019 in patients with diabetes mellitus: An observational study of CT radiological manifestations using an artificial intelligence algorithm. Acta Diabetol. 575–586 (2021). [DOI] [PMC free article] [PubMed]
- 138.Guler, S. A., et al. Pulmonary function and radiological features 4 months after COVID-19: First results from the national prospective observational Swiss COVID-19 lung study. Eur Respir J. 2003690 (2021). [DOI] [PMC free article] [PubMed]
- 139.Rodilla E, et al. SEMI-COVID-19 Network. Impact of arterial stiffness on all-cause mortality in patients hospitalized with COVID-19 in Spain. Hypertension. 2021;77:856–867. doi: 10.1161/HYPERTENSIONAHA.120.16563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pablos, J. L., et al.; RIER Investigators Group; RIER investigators group. Clinical outcomes of hospitalised patients with COVID-19 and chronic inflammatory and autoimmune rheumatic diseases: A multicentric matched cohort study. Ann. Rheum Dis. 1544–1549 (2020). [DOI] [PubMed]
- 141.Fond, G., et al. Increased in-hospital mortality from COVID-19 in patients with schizophrenia. Encephale 89–95 (2021). [DOI] [PMC free article] [PubMed]
- 142.Fteiha, B., et al. QTc prolongation among hydroxychloroquine sulphate-treated COVID-19 patients: An observational study. Int. J. Clin. Pract. e13767 (2021). [DOI] [PMC free article] [PubMed]
- 143.Dennis JM, Mateen BA, Sonabend R, et al. Type 2 diabetes and COVID-19-related mortality in the critical care setting: A national cohort study in England, March-July 2020. Diabetes Care. 2021;44:50–57. doi: 10.2337/dc20-1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Conte, C., et al. Epicardial adipose tissue characteristics, obesity and clinical outcomes in COVID-19: A post-hoc analysis of a prospective cohort study. NutrMetab. Cardiovasc. Dis. 2156–2164 (2021). [DOI] [PMC free article] [PubMed]
- 145.Grodecki K, Lin A, Razipour A, Cadet S, McElhinney PA, Chan C, Pressman BD, et al. Epicardial adipose tissue is associated with extent of pneumonia and adverse outcomes in patients with COVID-19. Metabolism. 2021;115:154436. doi: 10.1016/j.metabol.2020.154436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Yates, T., et al. Obesity, ethnicity, and risk of critical care, mechanical ventilation, and mortality in patients admitted to hospital with COVID-19: Analysis of the ISARIC CCP-UK cohort. Obesity 1223–1230 (2021). [DOI] [PMC free article] [PubMed]
- 147.Eriksson, K. E., Campoccia, J. F., Rysz, S. & Rimes-Stigare, C. Continuous renal replacement therapy in intensive care patients with COVID-19; survival and renal recovery. J. Crit Care. 125–130 (2021). [DOI] [PMC free article] [PubMed]
- 148.Castilla, J., Guevara M, Miqueleiz A, Baigorria F, Ibero-Esparza C, NavascuésA, et al; The Working Group For The Study Of Covid-In Navarra. Risk factors of infection, hospitalization and death from SARS-CoV-2: A population-based cohort study. J. Clin Med. 2608 (2021). [DOI] [PMC free article] [PubMed]
- 149.Mather, J. F., Seip, R. L. & McKay, R. G. Impact of famotidine use on clinical outcomes of hospitalized patients with COVID-19. Am. J. Gastroenterol. 1617–1623 (2020). [DOI] [PMC free article] [PubMed]
- 150.Suardi, L. R., et al. Risk factors for non-invasive/invasive ventilatory support in patients with COVID-19 pneumonia: A retrospective study within a multidisciplinary approach. Int. J. Infect. Dis. 258–263 (2020). [DOI] [PMC free article] [PubMed]
- 151.Bennett, T. D., et al. National COVID Cohort Collaborative (N3C) Consortium. Clinical characterization and prediction of clinical severity of SARS-CoV-2 infection among US adults using data from the US National COVID Cohort Collaborative. JAMA Netw. Open. e2116901 (2021). [DOI] [PMC free article] [PubMed]
- 152.Posso, M., et al. Comorbidities and mortality in patients with COVID-19 aged 60 years and older in a University Hospital in Spain. Arch. Bronconeumol. (Engl. Ed.) 756–758 (2020). [DOI] [PubMed]
- 153.Ferrando-Vivas, P., et al. Prognostic factors for 30-day mortality in critically Ill patients with coronavirus disease 2019: An observational cohort study. Crit. Care Med. 102-111 (2021). [DOI] [PMC free article] [PubMed]
- 154.Estenssoro E, Loudet CI, Ríos FG, KanooreEdul VS, Plotnikow G, Andrian M, et al. Clinical characteristics and outcomes of invasively ventilated patients with COVID-19 in Argentina (SATICOVID): A prospective, multicentre cohort study. Lancet Respir. Med. 2021;S2213–2600(21):00229. doi: 10.1016/S2213-2600(21)00229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ferreira, J. C., Ho, Y. L., Besen, B. A. M. P., Malbouisson, L. M. S., Taniguchi, L. U., Mendes, P. V., et al; EPICCoV Study Group. Protective ventilation and outcomes of critically ill patients with COVID-19: A cohort study. Ann. Intens. Care11, 92 (2021). [DOI] [PMC free article] [PubMed]
- 156.Ciceri F, Castagna A, Rovere-Querini P, De Cobelli F, Ruggeri A, Galli L, et al. Early predictors of clinical outcomes of COVID-19 outbreak in Milan. Italy. Clin Immunol. 2020;217:108509. doi: 10.1016/j.clim.2020.108509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Arshad, S., et al. Henry Ford COVID-19 Task Force. Treatment with hydroxychloroquine, azithromycin, and combination in patients hospitalized with COVID-19. Int. J. Infect. Dis. 396–403 (2020). [DOI] [PMC free article] [PubMed]
- 158.Bode, B., et al. Glycemic characteristics and clinical outcomes of COVID-19 patients hospitalized in the United States. J. Diabetes Sci. Technol. 813–821 (2020). [DOI] [PMC free article] [PubMed]
- 159.Cai, S. H., et al. Association between obesity and clinical prognosis in patients infected with SARS-CoV-2. Infect. Dis. Poverty. 80 (2020). [DOI] [PMC free article] [PubMed]
- 160.Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wu, J., et al. Early antiviral treatment contributes to alleviate the severity and improve the prognosis of patients with novel coronavirus disease (COVID-19). J. Intern. Med. 128–38 (2020). [DOI] [PubMed]
- 162.Yanover, C., et al. What factors increase the risk of complications in SARS-CoV-2-infected patients? A cohort study in a Nationwide Israeli Health Organization. JMIR Public Health Surveill e20872 (2020). [DOI] [PMC free article] [PubMed]
- 163.Reilev, M., et al. Characteristics and predictors of hospitalization and death in the first 11 122 cases with a positive RT-PCR test for SARS-CoV-2 in Denmark: A nationwide cohort. Int. J. Epidemiol. 1468–1481 (2020). [DOI] [PMC free article] [PubMed]
- 164.Mendy, A., Apewokin, S., Wells, A. A. & Morrow, A. L. Factors associated with hospitalization and disease severity in a racially and ethnically diverse population of COVID-19 patients. medRxiv (2020).
- 165.Murillo-Zamora E, Hernandez-Suarez CM. Survival in adult in patients with COVID-19. Public Health. 2021;190:1–3. doi: 10.1016/j.puhe.2020.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Leal, F. E., et al. Clinical features and natural history of the first 2073 suspected COVID-19 cases in the Corona São Caetano primary care programme: A prospective cohort study. BMJ Open e042745 (2021). [DOI] [PMC free article] [PubMed]
- 167.Kebisek J, Forrest L, Maule A, Steelman R, Ambrose J. Special report: Prevalence of selected underlying health conditions among active component Army service members with coronavirus disease 2019, 11 February–6 April 2020. MSMR. 2020;27:50–54. [PubMed] [Google Scholar]
- 168.Baqui, P., Bica, I., Marra, V., Ercole, A. & van der Schaar, M. Ethnic and regional variations in hospital mortality from COVID-19 in Brazil: A cross-sectional observational study. Lancet Glob Health e1018–e1026 (2020). [DOI] [PMC free article] [PubMed]
- 169.Kaeuffer, C., Le Hyaric, C., Fabacher, T., Mootien, J., Dervieux, B., Ruch, Y., et al.; COVID Alsace Study Group. Clinical characteristics and risk factors associated with severe COVID-19: Prospective analysis of 1,045 hospitalised cases in North-Eastern France, March 2020. Euro Surveill. 2000895 (2020). [DOI] [PMC free article] [PubMed]
- 170.Aveyard, P., et al. Association between pre-existing respiratory disease and its treatment, and severe COVID-19: A population cohort study. Lancet Respir Med. (2021). [DOI] [PMC free article] [PubMed]
- 171.Carrillo-Vega, M. F., Salinas-Escudero, G., García-Peña, C., Gutiérrez-Robledo, L. M. & Parra-Rodríguez, L. Early estimation of the risk factors for hospitalization and mortality by COVID-19 in Mexico. PLoS One e0238905 (2020). [DOI] [PMC free article] [PubMed]
- 172.Blair, J. E., et al. Mild to moderate COVID-19 illness in adult outpatients: Characteristics, symptoms, and outcomes in the first 4 weeks of illness. Medicine e26371 (2021). [DOI] [PMC free article] [PubMed]
- 173.Sharif, N., et al. Prevalence and impact of diabetes and cardiovascular disease on clinical outcome among patients with COVID-19 in Bangladesh. Diabetes MetabSyndr. 1009–1016 (2021). [DOI] [PMC free article] [PubMed]
- 174.Woolcott, O. O. & Castilla-Bancayán, J. P. The effect of age on the association between diabetes and mortality in adult patients with COVID-19 in Mexico. Sci. Rep. 8386 (2021). [DOI] [PMC free article] [PubMed]
- 175.Abdalkader, M., et al. Cerebral venous sinus thrombosis in COVID-19 patients: A multicenter study and review of literature. J. Stroke Cerebrovasc. Dis. (2021). [DOI] [PMC free article] [PubMed]
- 176.Knight, S. R., Ho, A., Pius, R., Buchan, I., Carson, G., Drake, T. M., et al.; ISARIC4C investigators. Risk stratification of patients admitted to hospital with covid-19 using the ISARIC WHO Clinical Characterisation Protocol: Development and validation of the 4C Mortality Score. BMJ (2020). [DOI] [PMC free article] [PubMed]
- 177.Chao JY, Derespina KR, Herold BC, Goldman DL, Aldrich M, Weingarten J, et al. Clinical characteristics and outcomes of hospitalized and critically Ill children and adolescents with coronavirus disease 2019 at a tertiary care medical center in New York City. J. Pediatr. 2020;223:14–9.e2. doi: 10.1016/j.jpeds.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Girardin, J. L., et al. Contribution of pulmonary diseases to COVID-19 mortality in a diverse urban community of New York. Chron. Respir. Dis. (2021). [DOI] [PMC free article] [PubMed]
- 179.Laake, J. H., et al. Characteristics, management and survival of ICU patients with coronavirus disease-19 in Norway, March–June 2020. A prospective observational study. Acta Anaesthesiol. Scand. 618–628 (2021). [DOI] [PMC free article] [PubMed]
- 180.Chow, D. S., et al. Development and external validation of a prognostic tool for COVID-19 critical disease. PLoS One e0242953 (2020). [DOI] [PMC free article] [PubMed]
- 181.Sentilhes L, De Marcillac F, Jouffrieau C, Kuhn P, Thuet V, Hansmann Y, Coronavirus disease,, et al. in pregnancy was associated with maternal morbidity and preterm birth. Am J Obstet Gynecol. 2019;2020:914.e1–914.e15. doi: 10.1016/j.ajog.2020.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Deng, M., et al. Obesity as a potential predictor of disease severity in young COVID-19 patients: A retrospective study. Obesity 1815–1825 (2020). [DOI] [PMC free article] [PubMed]
- 183.Monteiro, A. C., et al. Obesity and smoking as risk factors for invasive mechanical ventilation in COVID-19: A retrospective, observational cohort study. PLoS One e0238552 (2020). [DOI] [PMC free article] [PubMed]
- 184.Munblit, D., et al. Stop COVID cohort: An observational study of 3480 patients admitted to the Sechenov University Hospital Network in Moscow City for Suspected Coronavirus Disease 2019 (COVID-19) Infection. Clin. Infect. Dis. 1–11 (2021). [DOI] [PMC free article] [PubMed]
- 185.Alali, A. S., et al. Demographics, comorbidities, and outcomes among young and middle-aged COVID-19 patients in Saudi Arabia. Saudi Pharm J. (2021). [DOI] [PMC free article] [PubMed]
- 186.Alharthy, A., et al. Clinical characteristics and predictors of 28-day mortality in 352 critically Ill patients with COVID-19: A retrospective study. J. Epidemiol. Glob. Health 98–104 (2021). [DOI] [PMC free article] [PubMed]
- 187.O'Malley, G., et al. COVID-19 hospitalization in adults with type 1 diabetes: Results from the T1D exchange multicenter surveillance study. J. Clin. Endocrinol. Metab. e936–e42 (2021). [DOI] [PMC free article] [PubMed]
- 188.Cordero-Franco HF, De La Garza-Salinas LH, Gomez-Garcia S, Moreno-Cuevas JE, Vargas-Villarreal J. Risk factors for SARS-CoV-2 infection, pneumonia, intubation, and death in Northeast Mexico. Front Public Health. 2021;9:645739. doi: 10.3389/fpubh.2021.645739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Martos-Benítez FD, Soler-Morejón CD, García-Del BD. Chronic comorbidities and clinical outcomes in patients with and without COVID-19: A large population-based study using national administrative healthcare open data of Mexico. Intern. Emerg. Med. 2021;16:1507–1517. doi: 10.1007/s11739-020-02597-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Duanmu Y, Brown IP, Gibb WR, et al. Characteristics of emergency department patients with COVID-19 at a single site in Northern California: Clinical observations and public health implications. Acad. Emerg. Med. 2020;27:505–509. doi: 10.1111/acem.14003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Nachega JB, Ishoso DK, Otokoye JO, Hermans MP, Machekano RN, Sam-Agudu NA, et al. Clinical characteristics and outcomes of patients hospitalized for COVID-19 in Africa: Early insights from the democratic Republic of the Congo. Am. J. Trop. Med Hyg. 2020;103:2419–2428. doi: 10.4269/ajtmh.20-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Mahamat-Saleh, Y., et al. Diabetes, hypertension, body mass index, smoking and COVID-19-related mortality: A systematic review and meta-analysis of observational studies. BMJ Open e052777 (2021). [DOI] [PMC free article] [PubMed]
- 193.Xu, J., et al. A meta-analysis on the risk factors adjusted association between cardiovascular disease and COVID-19 severity. BMC Public Health 1533 (2021). [DOI] [PMC free article] [PubMed]
- 194.Cai Z, Yang Y, Zhang J. Obesity is associated with severe disease and mortality in patients with coronavirus disease 2019 (COVID-19): A meta-analysis. BMC Public Health. 2021;21(1):1505. doi: 10.1186/s12889-021-11546-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Poly, T. N., et al. Obesity and mortality among patients diagnosed with COVID-19: A systematic review and meta-analysis. Front. Med. (Lausanne) 620044 (2021). [DOI] [PMC free article] [PubMed]
- 196.Yang J, Zheng Y, Gou X, Pu K, Chen Z, Guo Q, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: A systematic review and meta-analysis. Int. J. Infect. Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Badawi A, Ryoo SG. Prevalence of comorbidities in the Middle East respiratory syndrome coronavirus (MERS-CoV): A systematic review and meta-analysis. Int. J. Infect. Dis. 2016;49:129–133. doi: 10.1016/j.ijid.2016.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Behrens, E. M. & Koretzky, G. A. Review: Cytokine storm syndrome: Looking toward the precision medicine era. Arthritis Rheum 1135–1143 (2017). [DOI] [PubMed]
- 199.Xu, Z., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 420–422 (2020). [DOI] [PMC free article] [PubMed]
- 200.Hou, H., Xu, J., Li, Y., Wang, Y. & Yang H. The Association of Asthma With COVID-19 Mortality: An updated meta-analysis based on adjusted effect estimates. J. Allergy Clin. Immunol. Pract. 3944–3968 (2021). [DOI] [PMC free article] [PubMed]
- 201.Halaji M, Heiat M, Faraji N, Ranjbar R. Epidemiology of COVID-19: An updated review. J. Res. Med. Sci. 2021;30(26):82. doi: 10.4103/jrms.JRMS_506_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Simons, D., Shahab, L., Brown, J. & Perski, O. The association of smoking status with SARS-CoV-2 infection, hospitalization and mortality from COVID-19: A living rapid evidence review with Bayesian meta-analyses (version 7). Addiction 1319–1368 (2021). [DOI] [PMC free article] [PubMed]
- 203.Sunjaya AP, Allida SM, Di Tanna GL, Jenkins CR. Asthma and Coronavirus Disease 2019 Risk: A systematic review and meta-analysis. Eur. Respir. J. 2021;24:2101209. doi: 10.1183/13993003.01209-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Han, X., Xu, J., Hou, H., Yang, H. & Wang Y. Impact of asthma on COVID-19 mortality in the United States: Evidence based on a meta-analysis. Int. Immunopharmacol. 108390 (2022). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.