Abstract
Sprouting angiogenesis is a highly coordinately process controlled by vascular endothelial growth factor receptor (VEGFR)-Notch signaling. Here we investigated whether Tripartite motif-containing 28 (TRIM28), which is an epigenetic modifier implicated in gene transcription and cell differentiation, is essential to mediate sprouting angiogenesis. We observed that knockdown of TRIM28 ortholog in zebrafish resulted in developmental vascular defect with disorganized and reduced vasculatures. Consistently, TRIM28 knockdown inhibited angiogenic sprouting of cultured endothelial cells (ECs), which exhibited increased mRNA levels of VEGFR1, Delta-like (DLL) 3, and Notch2 but reduced levels of VEGFR2, DLL1, DLL4, Notch1, Notch3, and Notch4.The regulative effects of TRIM28 on these angiogenic factors were partially mediated by hypoxia-inducible factor 1 α (HIF-1α) and recombination signal-binding protein for immunoglobulin kappa J region (RBPJκ). In vitro DNA-binding assay showed that TRIM28 knockdown increased the association of RBPJκ with DNA sequences containing HIF-1α-binding sites. Moreover, the phosphorylation of TRIM28 was controlled by VEGF and Notch1 through a mechanism involving RBPJκ-dual-specificity phosphatase (DUSP)-p38 MAPK, indicating a negative feedback mechanism. These findings established TRIM28 as a crucial regulator of VEGFR-Notch signaling circuit through HIF-1α and RBPJκ in EC sprouting angiogenesis.
Keywords: DLL4, sprouting angiogenesis, Tip cell, TRIM28, VEGFR
1 ∣. INTRODUCTION
Angiogenesis plays crucial roles in physiological conditions including development and wound healing, as well as pathogenesis of serious diseases, such as, myocardial infarction, stroke, arteriosclerosis, arthritis, cancer, and ocular disease, etc.1,2 At the onset of angiogenesis, the new blood vessel sprouts from pre-existing vessels, which is coordinated by the selection of a leading tip cell and the trailing stalk cells.3,4Specifically, it is shown that tip cells are selected from the endothelial cells (ECs) of existing vessels to invade surrounding tissue and guide new vessel sprouts. Trailing the tip ECs, stalk ECs proliferate and establish tight junctions to allow the vessel to grow in length and ensure the integrity of new sprouts.3
Recent studies show that ECs can dynamically compete with each other for tip-stalk cell selection and shuffling, which are coordinately modulated by several key factors, including vascular endothelial growth factor (VEGF), delta-like ligand (DLL), Jagged-1, Fringe, platelet-derived growth factor B (PDGFB), etc.3 VEGF receptor (VEGFR)-mediated signaling cascades stimulate ECs to become the tip cells, which express abundant DLL4, a ligand activating Notch of neighbor ECs.5 Notch signaling activation subsequently reduces VEGFR2 and increases the VEGFR1 expressions as well as the expressions of different Notch target genes to induce the stalk cell phenotype.3,6 This process of lateral inhibition between tip-stalk ECs plays an important role in correcting sprouting and branching patterns.
TRIM family is involved in several cellular processes such as gene transcription, cell growth and differentiation, genome stability, immunity, development, and carcinogenesis.7-10 TRIM28, TRIM22, and TRIM27 were found to be the three most expressed transcripts of TRIM family in cultured ECs, whereas little is known about their biological functions in cardiovascular system. We previously reported that ablation of TRIM28 expression decreased the expressions of tumor necrosis factor receptors and VEGFR2 in ECs and subsequently inhibit endothelial inflammation and angiogenic activity.11 Previous studies have reported that TRIM28 decreases the transcriptional activity of hypoxia inducible factor 1 α (HIF-1α), a physiological regulator of VEGF expression, in A549 and HEK-293 cells.12 Follow-up studies show that TRIM28 regulates the transcriptional activity of HIF-1α through decreasing its association with recombination signal-binding protein for immunoglobulin kappa J region (RBPJα),13 another key transcriptional regulator of angiogenic growth factors.14
In this study, we demonstrated that Tripartite motif 28 (TRIM28) controls VEGF-Notch signaling and sprouting angiogenesis. We observed that TRIM28 knockdown reduced the developmental vascularization in zebrafish and sprouting of cultured human ECs. Mechanistic analysis indicates that TRIM28 maintains the expression of VEGFR/DLL/Notch partially through RBPJκ and HIF-1α. Additionally, a negative feedback mechanism that VEGF-Notch controls the phosphorylation of TRIM28 was identified. These results identify TRIM28 as a new regulator of sprouting angiogenesis and provide insights into the regulation of EC behavior and sprouting angiogenesis.
2 ∣. MATERIALS AND METHODS
2.1 ∣. Cell culture and reagents
Human umbilical vein ECs (HUVECs) were purchased from ScienCell company (Carlsbad, CA, USA) and cultured in EC medium containing EC growth supplement (ScienCell company), 2% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 U/mL streptomycin (Invitrogen, Carlsbad, CA, USA) in 5% CO2 at 37°C. The ECs at passages 3-5 were used for all experiments. HEK-293 cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% FBS, 100 U/mL penicillin, and 100 U/mL streptomycin in 5% CO2 at 37°C. Recombinant human VEGF165 were purchased from R&D Company (Minneapolis, MN, USA). LY294002, SB203580, and PD98059 were purchased from Selleck (Houston, TX, USA). Lipofectamine RNAi MAX and lipofectamine 2000 were purchased from Invitrogen. Antibody against TRIM28 was purchased from Santa Cruz (Dallas, TX, USA). Antibody against phospho-TRIM28 (Ser473) and CD31 were obtained from Abcam (Cambridge, UK). Antibodies against Akt, phospho-Akt, ERK, phospho-ERK, p38MAPK, phospho-p38MAPK, MAPKAPK2, phospho-MAPKAPK2,VEGFR1, VEGFR2, DLL1, DLL3, DLL4, Notch1, Notch4, HIF-1α, RBPJκ, and horseradish peroxidase-conjugated goat anti-rabbit or anti-mouse secondary antibodies were obtained from Cell Signaling Technology (Beverly, MA, USA). Fluorescein isothiocyanate-conjugated secondary antibody was purchased from Proteintech Biotechnology (Chicago, IL, USA).Please see the Major Resources Table in the Supplemental Table 2.
2.2 ∣. Zebrafish strains and morpholino injections
Zebrafish were used and maintained according to Institutional Animal Care and Use Committee guidelines at Mayo Clinic. An initial 5-dose screening of trim33l morpholino (1.5 nL of 100, 200, 500, 750 μM, respectively), was performed to identify its optimal dose, which generated sufficient phenotypic penetrance (ie, more than 50% of injected embryos showed a specific phenotype) but not cause too much morpholino toxicity such as lethality of injected embryos. Then, one-cell stage zebrafish embryos Tg (fli1:EGFP) were arrayed in an agarose microinjection template and TRIM33, like (trim33l) morpholinos (MOs) (1.5 nL of 500 μM) or nonspecific control MOs (1.5 nL of 500 μM) was micro-injected into the yolk/cell interface of the one cell embryo. Trim33l (5′-CGGGCCAGCGGTAAACATCTGTTAC-3′) MOs and control MOs (5′-CATCATATTCAGGGTAGTCGAAGTT-3′) were designed and purchased from Gene Tools, LLC (Philomath, USA). At 48 hours post-fertilization (hpf), embryos were anesthetized with 0.015% tricaine methanesulfonate (tricaine) from Western Chemical, Inc USA, and mounted in 1.2% low melting agarose and imaged using a ZEISS LSM 880 confocal microscope using standard EGFP filter sets as we previously described.15 O-dianisidine staining was used to detect the presence of hemoglobin positive erythrocytes within intact zebrafish embryos as previously described,16 briefly, MO-injected embryos at 48 hpf were dechorionated and stained for 15 minute in dark in staining buffer containing 0.6 mg/mL o-dianisidine (Sigma, MO, USA), 10 mM sodium acetate (pH 4.5), 0.65% hydrogen peroxide, and 40% (v/v) ethanol. Images were acquired with Olympus bright field microscope using 4.5 X objective.
2.3 ∣. Zebrafish in situ hybridization
A total of 590 bases of the open reading frame of zebrafish TRIM28 ortholog, trim33l, were amplified from zebrafish cDNA and cloned into pCR4-TOPO vector (Invitrogen). Trim33l cloning primers used were 5′-TGACCCGTGACCATGAAGTC-3′ and 5′-GGACTCAGGGGTGCATGTTT-3′. Digoxigenin (DIG)-labeled antisense probes were generated using SP6 polymerase (New England BioLabs) and the DIG RNA labeling mix (Sigma), after the DNA plasmid was linearized with Sac1 (New England BioLabs). In situ hybridization was then performed as previously described.16
2.4 ∣. Injection of trim33l mRNA
Synthetic mRNA encoding the trim33l protein from the targeted locus was synthesized as described.15,17 Briefly, the full-length cDNA was amplified using forward primer 5′-ATCTATCTCCATCGATGCCCAAGCTAAAAAAAGTTC-3′ and reverse primer 5′ CCCTTGCTCACCATGGCTCCGCCACTGCCACCATGTTTAGCAGCTGTAGTGT-3′ and cloned into the pt3ts expression vector18 (a generous gift from Dr Stephen C. Ekker at Mayo Clinic). ATG MO targeting sequences were modified by introducing 5′-Cla1- CGATGCCCAAGCTAAAAAAAGTTCTAGGAAGAAACTGCTTATTGTACGGCTTACCGTTAGATCAACGGGTCGAATTTCCCTAACGACCAAATATATGTAAACGTTACGCCTTTGTTTACATATTCTTTTTTTTTTGCCGCCACCATGTTCACTGCGGGTCCAACAGGGCGTTCAGCGTCGCCCCGT-3′ and 5′-BspE1-CCGGACGGGGCGACGCTGAACGCCCTGTTGGACCCGCAGTGAACATGGTGGCGGCAAAAAAAAAAGAATATGTAAACAAAGGCGTAACGTTTACATATATTTGGTCGTTAGGGAAATTCGACCCGTTGATCTAACGGTAAGCCGTACAATAAGCAGTTTCTTCCTAGAACTTTTTTTAGCTTGGGCAT-3′. The caped mRNA was generated in vitro using the mMessage mMachine T3 transcription Kit (Thermo Fisher Scientific), aliquoted, concentration, and quality determined, and stored at −80°C. A typical dose range of 100pg was delivered in 2 nL solutions per embryo.
2.5 ∣. Endothelial sprouting assay
Fibrin gel bead assay for the study of angiogenic sprouting was performed as described previously.19 In brief, Cytodex-3 beads (Amersham, Buckinghamshire, UK) were hydrated in phosphate buffered saline (PBS) and sterilized by autoclaving. ECs were trypsinized and mixed with beads at concentration of ~400 ECs per bead in 1 mL of warm EC growth medium in a fluorescence-activated cell sorting tube. The mixture was incubated for 4 hours at 37°C, with shaking the tube every 20 minutes. After that, the coated beads were transferred to a 6-well culture plate in 2 mL of EC growth medium and leave for 2 hours. After washed with EC growth medium, the coated beads were mixed with 2.0 mg/mL fibrinogen (Sigma-Aldrich, Munich, Germany) solution containing 0.15 U/mL of aprotinin (Sigma-Aldrich) and 0.625 U/mL of thrombin (Sigma-Aldrich) and then, added to each well of a 24-well plate. The plate was left for 5 minutes in the hood, and then, placed in the 37°C incubator for 10 minutes to generate a clot. Fibroblasts were then seeded on top of fibrin gel at a concentration of 20,000 cells per well. A total of 1 mL of EC growth medium per well was finally added. The media was changed every other day.
2.6 ∣. Tip cell competition assay
The scrambled or TRIM28 siRNA transfected ECs were infected with adenovirus expressing either red fluorescent protein (Ad-RFP) or green fluorescent protein (Ad-GFP), respectively. Two thousands of Ad-RFP or Ad-GFP infected ECs were mixed 1:1 and cultured in 96-well plates pre-coated with 0.8% agarose for 16 hours for cellular spheroid formation. The spheroids were then collected and embedded in fibrin gels.20 EC growth medium was added. Forty-eight hours later, the spheroids were analyzed with confocal microscopy (Zeiss, Leusden, The Netherlands).
2.7 ∣. Aortic ring angiogenesis
All investigations were conducted conforming to the Guide for the Care and Use of Laboratory Animals published by the US National Institute of Health, 8th Edition (2011) and approved by the Animal Care and Use Committee of Putuo Hospital affiliated to Shanghai University of Traditional Chinese Medicine. Male Sprague Dawley rats (weighing about 200 g) were sacrificed by 5% isoflurane exposure. Immediately after confirming the death of animals, the thoracic aortas were dissected, collected, stripped of adventitial tissue, and cut into 0.5-mm segments. The aortic segments were then exposed to lentivirus in a 96-well culture plate for 6 hours. After that, the segments were placed in Matrigel and cultured with EC growth medium.
2.8 ∣. Gene silencing and quantitative PCR
Small-interfering RNAs (siRNAs) targeting human TRIM28, HIF-1α, RBPJκ, Nocth1, p38MAPK, and STAT3 were synthesized and transfected into cells using Lipofectamine RNAi MAX. The scrambled siRNA was used as a control. RNAiso reagent (Invitrogen) was used for total RNA isolation. cDNA was synthesized from the total RNA with the use of cDNA synthesis kits (Takara, Beijing, China). qPCR was performed with SYBR Green Master Mix (Clontech, Mountain View, CA, USA). The 18s rRNA was used as an internal control. The primer and siRNA sequences were shown in the Supplementary Table 1.
2.9 ∣. Western blotting analysis
Total cellular protein was extracted by RIPA lysis buffer (Thermo Fisher) supplemented with proteinase inhibitor cocktail (Sigma-Aldrich). The cellular proteins were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and immunoblotted with primary antibodies followed by secondary antibodies conjugated with horseradish peroxidase. The signals were detected with enhanced chemiluminescence plus (Amersham Pharmacia Biotech, Buckinghamshire, UK). The relative expressions of the target proteins were quantified by ImageJ software. The quantitative results were shown in the supplementary Data 7.
2.10 ∣. In vitro DNA-binding affinity assay
In vitro DNA-binding assay of RBPJκ was performed as described previously.13 Briefly, two complementary 5′-biotinylated oligonucleotides with the HIF-1α-binding site were annealed to be double-stranded DNA and coupled to streptavidin-conjugated Dynabeads (Thermo Fisher). Cell nuclear extracts were incubated with DNA-coupled Dynabeads in lysis buffer at a total volume of 300 μL overnight at 4°C. The precipitates were washed three times with lysis and boiled in SDS sample buffer and analyzed by western blotting assay.
2.11 ∣. Plasmid and adenovirus construction
The cDNA fragment encoding NICD with 2× His-tag was subcloned to pAdenoX-DsRed-Express vector using In-Fusion Dry-Down PCR Cloning Kit (Clontech, CA, USA). Plasmids were purified using the NucleoBond Xtra Midi Kit, linearized by PacI enzyme, and then, transfected into HEK-293 cells to produce Ad-NICD-His recombinant adenovirus. The adenoviruses were amplified in HEK293 cells and then, purified using the cesium chloride method.
2.12 ∣. Immunofluorescent assay
Cells or aortic vessels were fixed in 4% paraformaldehyde and rinsed in 0.01 M PBS. After that, the samples were permeabilized with Triton X-100 and blocked with 3% bovine serum albumins in PBS for 1 hours, following incubation with primary antibodies at 4°C overnight. After washing away unbound antibody, fluorescent-conjugated secondary antibodies in 3% bovine serum albumins were incubated for 1 hours before nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI). The images were analyzed with confocal microscopy.
2.13 ∣. Statistical analysis
Values were expressed as mean ± SEM. Statistical differences were determined to be significant at P < .05. Statistical significance was calculated by one-way ANOVA and Tukey’s multiple comparison test. All the experiments were routinely repeated at least three times.
3 ∣. RESULTS
3.1 ∣. TRIM28 ortholog is required for vascularization of zebrafish in vivo
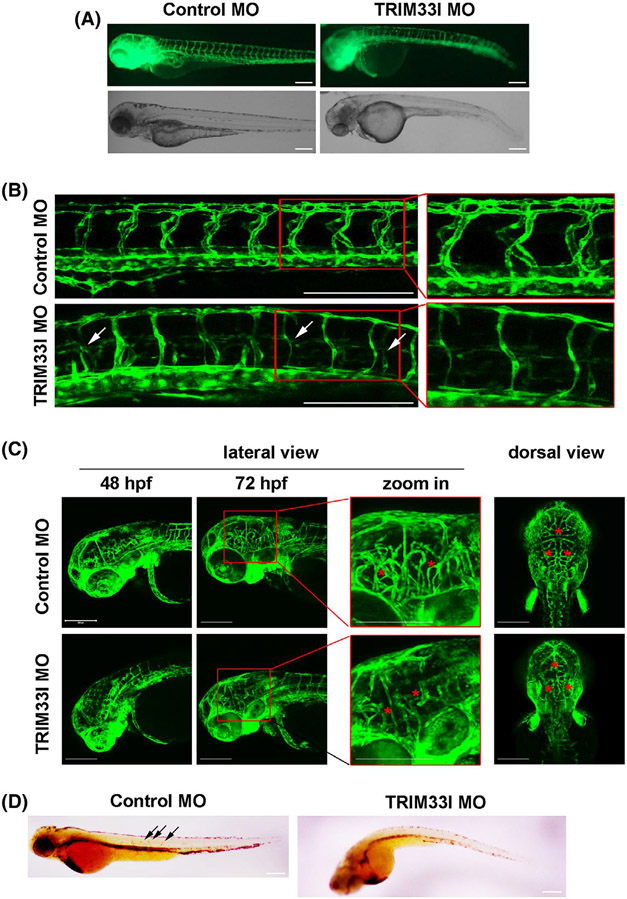
Zebrafish (Danio rerio) is widely used in angiogenesis studies due to its rapid development, optical transparency, and high number of offspring.21The expression pattern of TRIM28 ortholog, trim33l, in the developing zebrafish embryos was analyzed using whole-mount in situ hybridization. Extensive expression was shown in the head region at all the developmental stages at 24, 28, 48, and 72 hpf. Trim33l was also detected in several tissues including heart, gut endoderm, and pectoral fin buds, etc Importantly, enriched expression of trim33l was observed in the blood vessels of trunk and tails at 24, 28, and 48 hpf, and ISVs at 72 hfp (Supplementary DATA 1). To define the role of TRIM28 in angiogenesis in vivo, we knockdown the expression of, trim33l in zebrafish Tg (fli1:EGFP), which stably express EGFP within vascular ECs.17 Nonspecific control and trim33l MOs were injected into one-cell stage embryos of transgenic zebrafish Tg (fli1:EGFP), and the knockdown efficiency was confirmed with reduced fluorescent signal of trim33l morphants injected with trim33l mRNA-eGFP fusion (Supplementary Data 2). At 48 hpf, while 94% control embryos showed normal morphology, 64% trim33l morphants exhibited shorter and/or curved tails, with smaller head sizes (Figure 1A). The trim33l morphants had defected and misshapen intersegmental vessels (ISV) which either had single axial vessels or did not prolong beyond the midline (Figure 1B).Meanwhile, confocal microscopy revealed that compared to the well-organized cerebral vessels of control zebrafish, trim33l morphants exhibited disorganized and reduced cranial vasculatures (Figure 1C), as shown by the decreased total central artery length (Supplementary Data 3G, H), indicative of impaired angiogenesis. Consequently, o-dianisidine staining showed less hemoglobin accumulation in the trim33l morphants (Figure 1D). These results suggest that the zebrafish ortholog of TRIM28, trim33l, is required for development of both cranial and peripheral vasculatures. To examine the specificity of the effects of trim33l MOs, trim33l MOs were injected together with the synthetic trim33l mRNA which does not encode the morpholino target sequence. As shown in the Supplementary data 3A-H, Trim33l mRNA reversed the phenotype of reduced cranial vasculature growth and misshapen ISV, supporting the specific effect of Trim33l MOs. These results suggested that TRIM28 ortholog is required for vascularization in zebrafish.
FIGURE 1.
TRIM28 ortholog regulates vascular development in zebrafish. One-cell stage zebrafish embryos Tg (fli1:EGFP) were injected with control (N = 221) or Trim33l MOs (N = 208). A, Images were acquired at 48 hpf to show the morphology development. Abnormal zebrafish embryos which have delayed growth and/or curved tails were manually counted and compared. Scale bar, 200 μm. B, C, Lateral and dorsal views of cerebral vasculatures (C) and the intersegmental vessels (B) were acquired. Arrows annotate impaired intersegmental vessels. Asterisks annotate reduced and impaired vasculatures in the brain. Scale bar, 200 μm. D, O-dianisidine staining was performed at 48 hpf and lateral views of zebrafish embryos were imaged. Scale bar, 200 μm
3.2 ∣. TRIM28 is required for endothelial sprouting
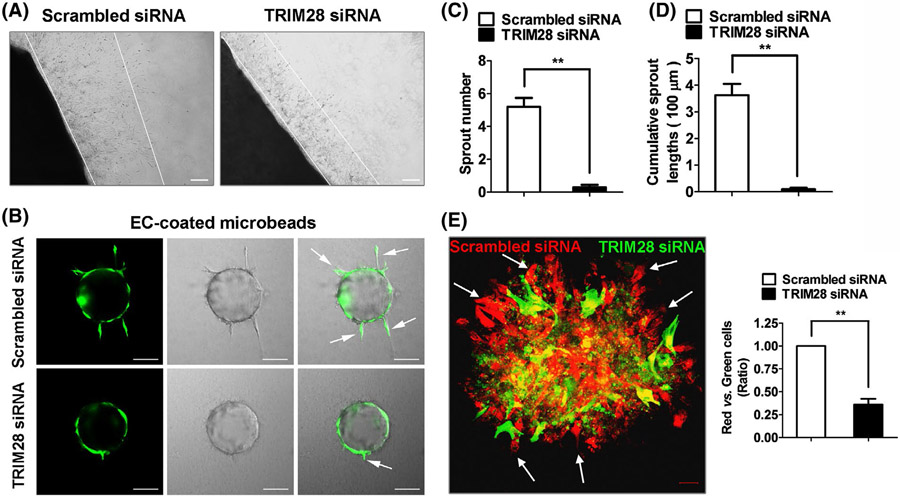
To further define the role of TRIM28 in angiogenesis, we next performed an ex vivo aorta ring assay, and observed that TRIM28 knockdown reduced the sprouting microvessels of rat aorta (Figure 2A, Supplementary Data 4). The effect of TRIM28 on EC sprouting was further validated in human ECs using a 3D fibrin gel bead assay. As shown in Figure 2B-D, knockdown of TRIM28 dramatically inhibited EC sprouting, including sprouting numbers as well as cumulative sprout lengths. These results collectively indicate that TRIM28 is uniformly required for EC sprouting of different species, including human, rat, and zebrafish. We next performed an EC spheroid-sprouting assay and observed that TRIM28 knockdown ECs were under-represented on the edge of cell spheroid (Figure 2E). These results indicated that TRIM28 is required for sprouting angiogensis.
FIGURE 2.
The effect of TRIM28 on endothelial angiogenic sprouting. A, Aortic tissues were infected with Lenti-shTRIM28 and Lenti-shControl and then, embedded in Matrigel to induce vascular sprouting for 5 days. n = 3. Scale bar, 200 μm. B, The scrambled- or TRIM28 siRNA transfected ECs were infected with Ad-GFP, coated onto Cytodex microcarriers and then, embedded into a fibrin gel. The angiogenic sprouting was monitored using confocal microscopy. Scale bar, 100 μm. C, D, Quantification of the sprout number (C) and cumulative sprout length (D) per bead. Data from three independent experiments were included and analyzed. **P < .01. E, The scrambled- and TRIM28 siRNA transfected ECs were infected with Ad-RFP and Ad-GFP, respectively, and then, mixed for 3D spheroid sprouting assay. The arrows indicate ECs on the edge of cell spheroid. Scale bar, 20 μm. n = 3. Statistical significance was calculated referring to the biological replicates by unpaired Student’s t test
3.3 ∣. TRIM28 determines VEGFR and Notch/Notch ligand expression in ECs
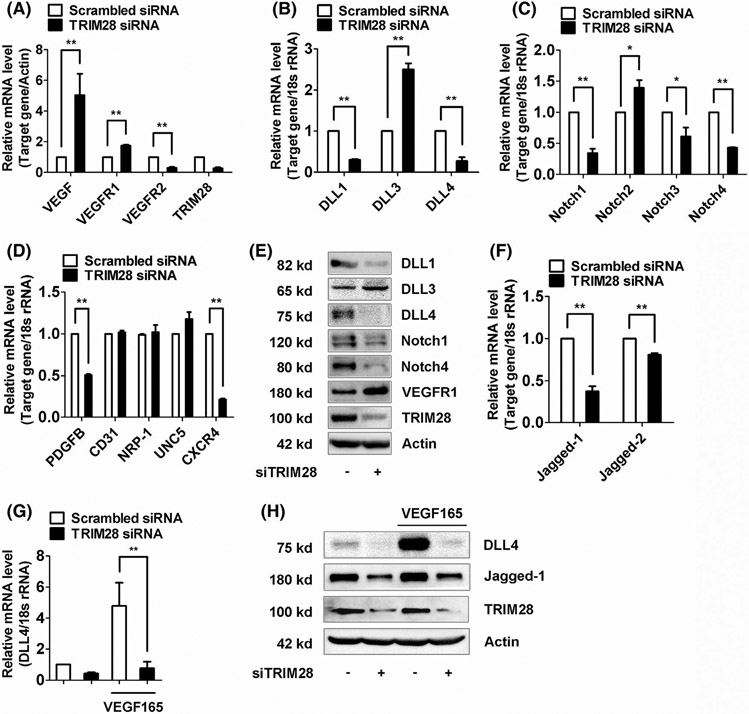
Tip cells distinguish from stalk cells by their distinct gene expression profiles, thus we sought to examine how TRIM28 controls the expression of these genes. It was shown that tip cells displayed strong expressions of VEGFR2, VEGFR3, PDGFB, DLL4, neuropilin 1, Unc-5 netrin receptor B (UNC5B), and C-X-C motif chemokine receptor 4 (CXCR4), whereas stalk cells were enriched in transcripts of VEGFR1, Hey1, Hes1, and Jagged-1.Although TRIM28 knockdown did not alter the transcript levels of neuropilin-1, UNC5B, or CD31, the EC marker, it induced greater mRNA levels of VEGFR1 but reduced the mRNA levels of VEGFR2, PDGFB, andCXCR4 (Figure 3A,D). Stalk and tip cells are known to have distinct levels of DLL-Notch, so we next investigated the regulation of TRIM28 on DLL-Notch signaling pathway. Our results show that TRIM28 knockdown decreased the mRNA expressions of DLL1, DLL4, Notch1, Notch3, and Notch4, but increased the mRNA expressions of DLL3 and Notch2 in ECs (Figure 3B, C). The altered expression of these genes, including VEGFR1, DLL1, DLL3, DLL4, Notch1, and Notch4, was further confirmed by analysis their protein levels with western blotting (Figure 3E, Supplementary Data 7A). Furthermore, TRIM28 knockdown decreased the expressions of Jagged-1 and Jagged-2, two of Notch transmembrane ligands (Figure 3F). Additionally, TRIM28 knockdown abolished VEGF165-stimulated upregulation of DLL4 expression (Figure 3G, H, Supplementary Data 7B). These results suggested that TRIM28 is required for maintaining the expression levels of VEGFR-DLL-Notch of ECs.
FIGURE 3.
TRIM28 determines expression levels of VEGFRs, Notch ligands, and receptors in ECs. A-D, ECs were transfected with scrambled or TRIM28 siRNA for 48 hours. The mRNA levels of VEGF, VEGFR1, VEGFR2 A, DLL1, DLL3, DLL4 B, Notch1, Notch2, Notch3, Notch4 C, and PDGFB, CD31,UNC5, CXCR4, and Neuropilin 1 D, were determined using qRT-PCR method. n = 3. *P < .05, **P < .01. E, The protein expressions of DLL1, DLL3, DLL4, Notch1, Notch4, and VEGFR1 in control and TRIM28 silenced ECs. n = 3. The representative images were shown. F, The mRNA expressions of Jagged-1 and Jagged-2 in TRIM28 knockdown ECs. n = 3. **P < .01. G, H, TRIM28 silenced ECs were treated with VEGF165 (20 ng/mL) for 16 hours. The qPCR (G) and immunoblot analysis (H) was performed to examine expressions of DLL4, Jagged-1, and TRIM28. n = 3. **P < .01. Statistical significance was calculated referring to the biological replicates by unpaired Student’s t test
3.4 ∣. HIF-1α partially mediates TRIM28-mediated transcriptional regulation of sprouting signal
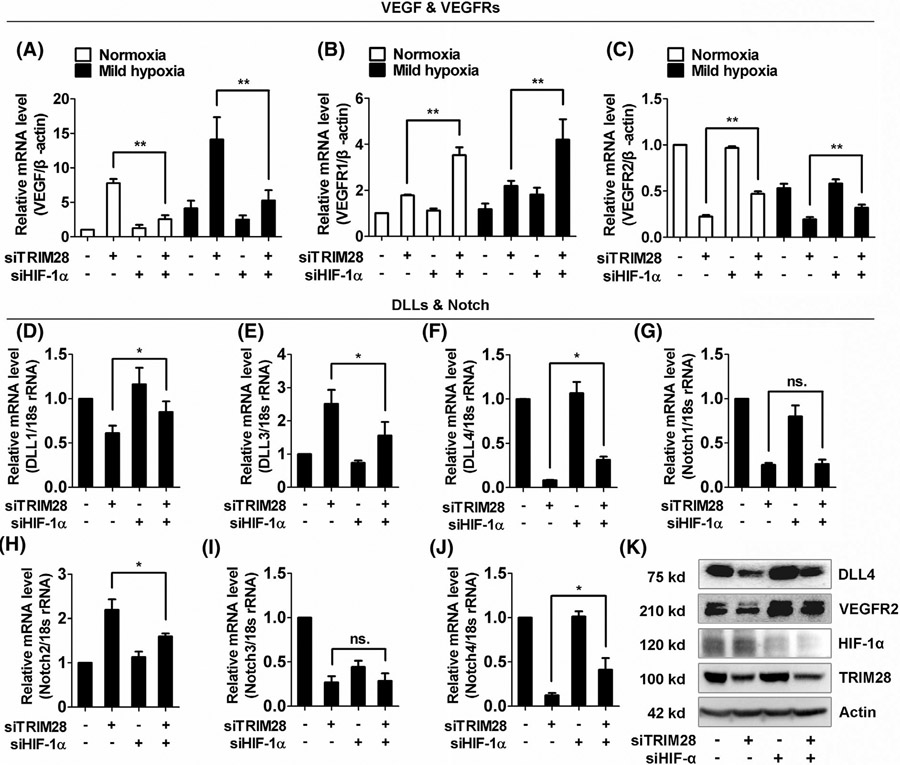
Previous study reported that TRIM28 repressed the transcriptional activity of HIF-1α, a hypoxia-induced master regulator of angiogenesis. Supported by our results showing the same effect of TRIM28 knockdown on expressions of VEGFA, VEGFR1, and VEGFR2 in normoxia and hypoxia (Figure 3A and Supplementary Data 5A), we next investigate whether HIF-1α participated in TRIM28-mediated transcriptional regulation of sprouting signal. Our data showed that the HIF-1α knockdown partially abolished the suppressive effect of TRIM28 silencing on VEGFR2 mRNA level. Meanwhile, HIF-1α knockdown showed a synergistic effect with TRIM28 siRNA on the expression of VEGFR1 expression (Figure 4A-C and Supplementary Data 5B, C). Furthermore, depletion of HIF-1α also reversed the effect of TRIM28 siRNA on DLL1, DLL3, DLL4, Notch2, and Notch4 in ECs (Figure 4D-F, H,J). Interestingly, HIF-1α siRNA did not significantly affect the expressions of Notch1 and Notch3 in TRIM28 knockdown ECs, suggesting other mechanism downstream of TRIM28 is involved (Figure 4G&I).
FIGURE 4.
HIF-1α is involved in the regulation of TRIM28 on DLL, Notch and VEGFR expression. A-C, The ECs were transfected with scrambled or TRIM28 siRNA alone or co-transfected with HIF-1α siRNA for 48 hours and then, cultured for 16 hours under normoxia or mild hypoxia (2% O2) in Tri-gas CO2 Incubators. The mRNA expressions of VEGF (A), VEGFR1 (B) and VEGFR2 (C) were quantified using qRT-PCR method. n = 3. **P < .01. D-K, ECs were transfected with either scrambled or TRIM28 siRNA alone, or co-transfected with HIF-1α siRNA for 48 hours. The mRNA expressions of DLL1 (D), DLL3 (E), DLL4 (F), and Notch1 (G), Notch2 (H), Notch3 (I), Notch4 (J) were analyzed using qPCR method. n = 4. *P < .05, ns., no significance. Total proteins in control or TRIM28 silenced cells were collected for western blotting assay (K). n = 3. The representative images were shown. Statistical significance was calculated by Tukey’s multiple comparison test
Finally, the protein levels of VEGFR2 and DLL4, two of the most important molecules in regulation of angiogenesis, in HIF-1α and/or TRIM28 knockdown ECs were confirmed using western blot (Figure 4K, Supplementary Data 7C). This result suggests that HIF-1α partially mediates the regulative effect of TRIM28 on VEGFR2-Notch signaling pathway.
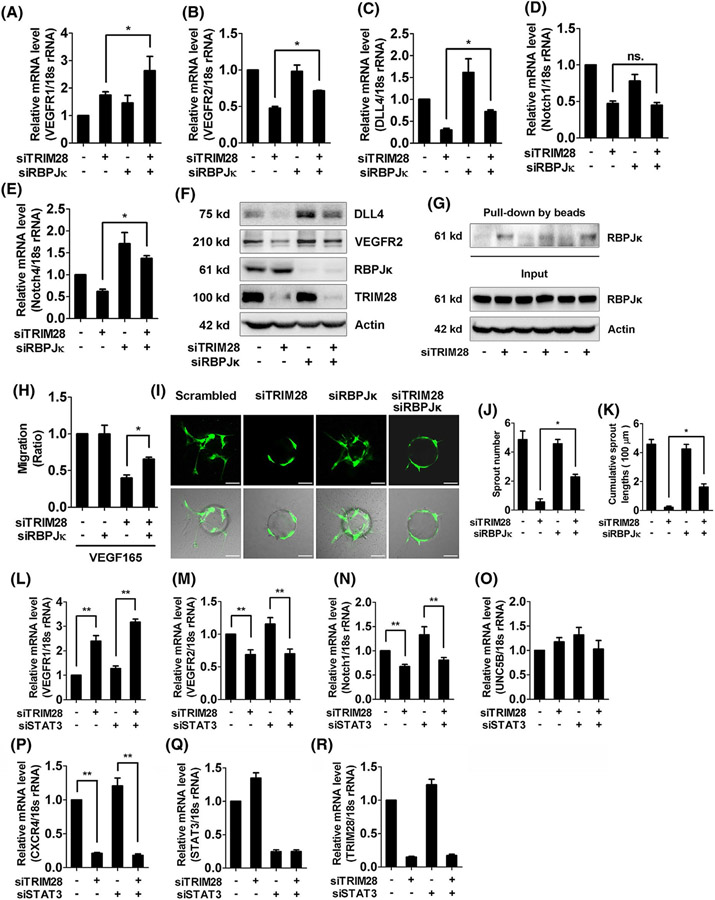
3.5 ∣. RBPJκ coordinates with HIF-1αto mediate the regulation of angiogenic sprouting in TRIM28 knockdown ECs
It was shown that TRIM28 can regulate the association between RBPJκ and HIF-1α in B lymphoma cells.13 We, therefore, examined whether RBPJκ also mediates TRIM28-regulated expressions of sprouting signals. RBPJκ siRNA showed similar effect with HIF-1α knockdown that RBPJκ siRNA and TRIM28 siRNA synergistically upregulated the VEGFR1 expression (Figure 5A). In addition, the silence of RBPJκ reversed the expressions of VEGFR2, DLL4, and Notch4 but not Notch1 in TRIM28 knockdown ECs (Figure 5B-F, Supplementary Data 7D), suggesting that RBPJκ mediates TRIM28 knockdown-mediated transcriptional regulation of angiogenic signals. As previous studies suggest that the complex of RBPJκ and HIF-1α controls the gene transcription through binding to hypoxia response element (HRE), the sequences recognized by HIF-1α. We next examined the effect of TRIM28 on the association of RBPJκ with oligonucleotides containing HRE, and observed that TRIM28 ablation enhanced the association of RBPJκ with HRE in ECs (Figure 5G). Consequently, RBPJκ knockdown reversed both migration (Figure 5H) and endothelial angiogenic sprouting (Figure 5I-K) in TRIM28 knockdown cells. These data demonstrated that RBPJκ and HIF-1α coordinately mediate the regulation of angiogenic sprouting in TRIM28 knockdown ECs. Whereas, STAT3, a potential TRIM28 target22 and one of the major transcription factors that regulate angiogenesis, was not involved in the regulation of TRIM28 on Notch and VEGFR expression (Figure 5L-R).
FIGURE 5.
TRIM28 regulates the interaction between HIF-1α and RBPJκ in ECs. A-F, ECs were transfected with the different siRNA fragments as indicated and harvested at 48 hours, and mRNA expressions of VEGFR1 (A), VEGFR2 (B), DLL4 (C), Notch1 (D), and Notch4 (E) were subjected to qRT-PCR. n = 3. *P < .05, ns., no significance. The protein expressions of DLL4, VEGFR2, RBPJκ, and TRIM28 were examined using western blot (F). n = 3. The representative images were shown. G, Nuclear extracts from control or TRIM28 knockdown ECs were incubated with oligonucleotides containing HIF-1α-binding site. The precipitations were checked using western blot with a RBPJκ antibody. n = 3. H, ECs were transfected with scrambled, TRIM28, RBPJκ siRNA alone or TRIM28 combined with RBPJκ siRNA for 48 hours, then exposed to VEGF165 (20 ng/mL), and the scratch migration assay was performed. n = 3. *P < .05. I-K,ECs were transfected with scrambled or TRIM28 siRNA or TRIM28 siRNA combined with RBPJκ siRNA and then, subjected to angiogenic sprouting assay. The representative images were shown (I). Scale bar, 100 μm. The sprout number (J) and cumulative sprout length (K) per bead were quantified. Data from three independent experiments were included and analyzed.**P < .01. L-R, ECs were transfected with either scrambled or TRIM28 siRNA alone, or co-transfected with STAT3 siRNA for 48 hours. The mRNA expressions of VEGFR1 (L), VEGFR2 (M), Notch1 (N), UNC5B (O), CXCR4 (P), STAT3 (Q), and TRIM28 (R) were analyzed using qPCR method. n = 3. **P < .01.Statistical significance was calculated by Tukey’s multiple comparison test
3.6 ∣. VEGF induces phosphorylation of TRIM28 in ECs
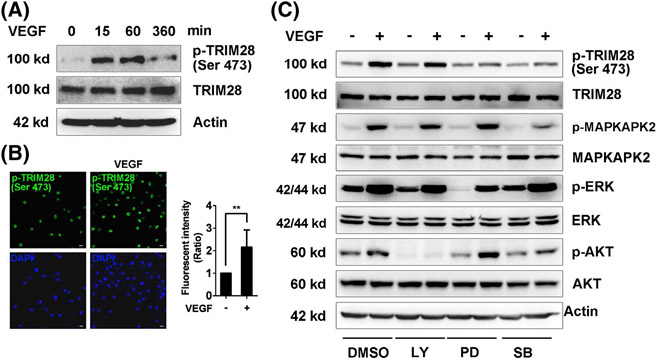
VEGF is a major inducer of angiogenesis, so we next examined whether TRIM28 is a downstream target of VEGF. Immunohistochemistry and western blotting revealed that the phosphorylation of TRIM28 at Ser473 was induced by recombinant VEGF165 in ECs (Figure 6A, B, Supplementary Data 7E). Furthermore, administration of the PD98059 and SB203580 reduced the effect of VEGF-mediated phosphorylation of TRIM28 at Ser 473, indicating that VEGF165-induced phosphorylation of TRIM28 through p42/44- and p38MAPK-dependent mechanism in ECs (Figure 6C, Supplementary Data 7F). This data indicated that TRIM28 is a downstream target of VEGF in ECs.
FIGURE 6.
VEGFA induces phosphorylation of TRIM28. ECs were starved in low-serum (1% FBS) medium overnight and then, treated with VEGF165 (20 ng/mL) for 15 min. Immunoblot (A) and immunofluorescent (B) analysis were performed to examine the phosphorylation of TRIM28 (Ser473). Scale bar, 10 μm. n = 3. **P < .01. Statistical significance was calculated referring to the biological replicates by unpaired Student’s t test. C, The serum-starved ECs were incubated with DMSO, LY294002 (10μM), PD98059 (10 μM), or SB203580 (10 μM) for 10 minutes, and then, stimulated with VEGF165 for another 15 minutes. Phosphorylated levels and total TRIM28, ERK, MAPKAPK-2, and AKT were examined using western blot. n = 3. The representative images were shown
3.7 ∣. Notch signaling suppresses TRIM28 expression in ECs through a feedback mechanism
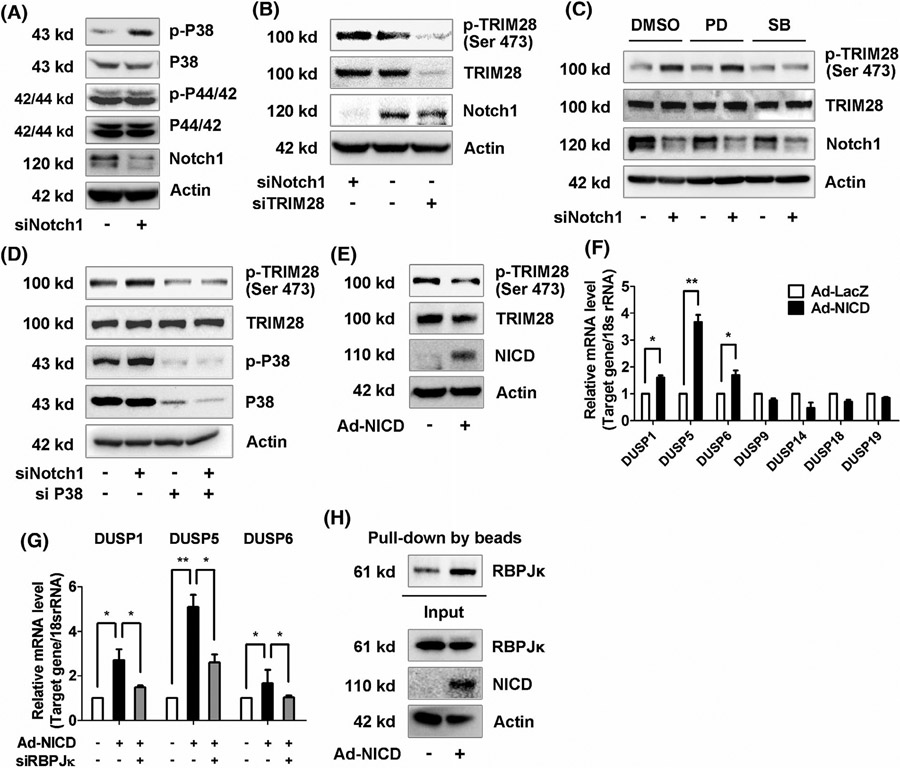
As we showed above, TRIM28 is a downstream of p38MAPK (Figure 6C). Previously, it was reported that Notch inactivates p38MAPK.23 Therefore, we proposed that Notch can regulate the phosphorylation of TRIM28 in ECs through a feedback mechanism. To test this hypothesis, we first checked the activation of p38MAPK in Notch1 silenced ECs and observed that silence of Notch1, the most predominant Notch receptor in ECs (Supplementary Data 6A), induced phosphorylation of p38- but not p42/44-MAPK (Figure 7A, Supplementary data 7G). Accordingly, phosphorylation of TRIM28 at Ser 473 was increased in Notch1 silenced ECs (Figure 7B, Supplementary Data 7H). Furthermore, inhibitors of p38- or p42/44-MAPK were administrated and showed that blockade of p38- but not p42/44-MAPK inhibited Notch1 siRNA-induced TRIM28 phosphorylation (Figure 7C, Supplementary data 7I). Meanwhile, knockdown of p38MAPK also inhibited the effect of Notch1 knockdown on the phosphorylation of TRIM28 in ECs (Figure 7D).
FIGURE 7.
The feedback loop between Notch1 and TRIM28 in ECs. A, ECs were transfected with scrambled or Notch1 siRNA for 48 hours. MAPK activation were analyzed using western blot. n = 3. B, The protein expressions of Notch1, phosphorylated, and total TRIM28 in Notch1 or TRIM28 knockdown ECs were analyzed using western blot. n = 3. C, The control or Notch1 knockdown ECs were incubated with DMSO, PD98059 (10 μM), or SB203580 (10 μM) for 16 hours. Cells were lysed and analyzed using western blot. n = 3. D, Phosphorylation of TRIM28 and p38 MAPK in cells transfected with Notch1 siRNA alone or Notch1 combined with p38 MAPK siRNA. n = 3. E-F,ECs were infected with Ad-GFP or Ad-NICD for 48 hours. Cell lysates were subjected to immunoblotting as indicated (E). n = 3. Expressions of DUSP family were checked using qRT-PCR assay (F). n = 3. * P < .05, **P < .01. Statistical significance was calculated referring to the biological replicates by unpaired Student’s t test. G, The control or RBPJκ knockdown ECs were infected with Ad-NICD for 48 hours. The mRNA expressions of DUSP1, DUSP5, and DUSP6 were analyzed using qRT-PCR method. n = 3. * P < .05, ** P < .01. Statistical significance was calculated by Tukey’s multiple comparison test. H, Nuclear extracts were isolated from Ad-GFP or Ad-NICD infected ECs and subjected to in vitro DNA-binding affinity assay. n = 3
Conversely, overexpression of NICD, an inducer of stalk cell signatures (Supplementary data 6B), inhibited phosphorylation and expression of TRIM28 (Figure 7E). Previous studies reported that Notch induces dephosphorylation of MAPKs through RBPJκ-mediated upregulation of dual-specificity phosphatase (DUSP). Indeed, we observed that NICD overexpression increased the mRNA levels of DUSP 1, 5, and 6 (Figure 7F), the three most abundant phosphatases in ECs (Supplementary Data 6C), and the regulative effect was reversed by RBPJκ siRNA (Figure 7G). Therefore, it is likely that Notch1 controls the phosphorylation of TRIM28 through a mechanism involving RBPĴDUSP-p38 MAPK in ECs. Consistent with our results showing that TRIM28 decreased the interaction of RBPJκ with HIF-1α-binding sites (Figure 5G), NICD overexpression exhibited the similar effect that overexpression of NICD enhanced the association of RBPJκ with HIF-1α-binding sites in the DNA-binding affinity analysis (Figure 7H). These results suggest that a feedback loop is present between TRIM28 and Notch-RBPJκ pathways in ECs.
4 ∣. DISCUSSION
In this study, we identified TRIM28 as a novel regulator of sprouting angiogenesis as shown by the impaired angiogenesis in TRIM28 knockdown zebrafish embryos, rat aorta, and human ECs. We demonstrated that TRIM28 ablation decreased the expressions of VEGFR2 level and DLL-Notch pathway, whereas increased VEGFR1 level. We identified that HIF-1α and RBPJκ coordinately mediated the effects of TRIM28 on expressions of VEGFR2-DLL/Notch and sprouting angiogenesis. A feedback regulation of TRIM28 by Notch1 was further identified. Taken together, our results strongly indicate that TRIM28 is required for the tight control of VEGFR2-DLL/Notch signaling pathway during angiogenic sprouting.
VEGFR1 and VEGFR2 are two high affinity receptors for VEGF, both of which are required for normal angiogenesis. During the initiation of sprouting angiogenesis, the endothelial tip cell potential was determined by VEGFR1 and VEGFR2 levels. The ECs with higher VEGFR2 and lower VEGFR1 levels preferentially stand a better chance to take and maintain the leading position.24 Furthermore, ECs can dynamically compete for the tip cell position through relative levels of VEGFR1 and VEGFR2.6We previously reported that TRIM28 is essential to maintain VEGFR2 expression in ECs,11 this follow-up study further defines the role of TRIM28 in sprouting angiogenesis and control on VEGFR/DLL/Notch pathway in sprouting angiogenesis. We observed that TRIM28 knockdown cells exhibited increased levels of several stalk cell markers, including VEGFR1, DLL3, and Notch2, but reduced levels of tip cell markers, such as VEGFR2, DLL1, DLL4, Notch1, Notch3, and Notch4. Consequently, TRIM28 knockdown ECs showed reduced sprouting in both spheroid-sprouting assay and aorta ring assay. These results suggest that TRIM28 potentially determines the stalk and tip cell selection during sprouting angiogenesis, however, the gene expression changes on the single cell level during angiogenic sprouting will need to be validated in the future study.
A functional Notch system downstream of VEGFRs is also required to control tip cell selection.6 In this study, we systemically test the effect of TRIM28 on the expressions of five Notch ligands: DLL1, DLL3, DLL4, Jagged-1, and Jagged-2. It is well-known that VEGFA activates VEGFR2 to induce the DLL4 expression in tip cells,25 which activates the Notch signaling in adjacent stalk cells, consequently suppressing the tip cell phenotype.26 However, both DLL4 upregulation and knockout can inhibit VEGF-induced EC function indicating an appropriate expression level of DLL4 is important for sprouting angiogenesis.27 DLL4 was found to be highly expressed in tip cells but expressed at lower levels in stalk cells. We observed herein that TRIM28 knockdown inhibited the basal and VEGFA-induced DLL4 expression in ECs, which is associated with reduced expression levels of VEGFR2. It is thus possible that TRIM28 controls the DLL expression by inhibiting the expression of VEGFR2. In addition to DLL4, two other canonical Notch ligands, DLL1 and Jagged-1, are also expressed abundantly in ECs for the selection of tip and stalk cells. The DLL1 has also been reported to maintain arterial identity and be involved in pathological angiogenesis.28Whereas Jagged-1 can antagonize DLL4-Notch signaling to maintain an activated stalk cell phenotype,29 promoting angiogenesis. Our data showed that TRIM28 knockdown inhibited expressions of DLL1 and Jagged-1. Of note, DLL3 was increased in TRIM28 knockdown ECs, which lacks an extracellular delta domain as well as lysine residues in the intracellular domain, can inhibit Notch signaling.30 Thus, these data indicate that TRIM28 has diverse effect on the expression of individual Notch ligand to coordinately regulate sprouting angiogenesis.
In mammals, there are four Notch receptors (Notch1-4). DLL-Notch crosstalk between neighboring cells could potentially generate distinct responses. Studies have shown that loss of Notch1 resulted in embryonic lethality due to defects in sprouting angiogenesis and arterial/venous specification.31 However, ectopic expression of Notch1 or Notch4 within the endothelium also resulted in early embryonic lethality, suggesting that appropriate levels of Notch signaling are necessary for proper patterning of the vasculature. While the expression of Notch 3 is relatively low in ECs,32 activation of Notch2, a pro-apoptotic receptor,33 was found to inhibit angiogenesis. We revealed that TRIM28 knockdown decreased expressions of Notch1, Notch3, and Notch4, but increased expression of Notch2. These results demonstrated that TRIM28 regulates the expressions of not only Notch ligands but also Notch receptors in ECs to control sprouting angiogenesis.
Consistent with previous studies,34 our results showed that mild hypoxia can increase the VEGFR1 expression level whereas deplete the VEGFR2 expression. The VEGFR expression pattern of mild hypoxic ECs was similar to that of TRIM28 knockdown ECs, indicating that TRIM28 knockdown can induce hypoxia-like response in ECs. TRIM28 was shown to participate in von Hippel-Lindau tumor suppressor-mediated transcriptional repression of HIF-1α in COS-7 cells.12 We herein demonstrated that TRIM28 knockdown regulated expressions of VEGFR2 through HIF-1α in ECs. Interestingly, a synergic effect on VEGFR1 was observed in ECs transfected with TRIM28 siRNA and HIF-1α siRNA, indicating that TRIM28 regulated the expression of VEGFR1 through a HIF-1α-independent mechanism. Meanwhile, we observed that HIF-1α siRNA can rescue expressions of DLL1, DLL3, DLL4, Nocth2, and Notch4 but not Notch1 and Notch3 in the TRIM28 knockdown cells. This data supports that HIF-1α partially mediates the regulation of TRIM28 on DLL-Notch signaling pathway.
Endothelial RBPJκ is required for maintaining proper artery, capillary, and vein organization in brain.35 It can control the angiogenic factor gene expression by antagonizing the activity of HIF-1α.36 However, RBPJκ also can coordinate with HIF-1α to positively drive the expression of replication and transcriptional activator in normoxia or hypoxia. This effect was greatly enhanced by inhibition of TRIM28.13 In this study, we observed that RBPJκ can partly reverse the regulation effects of TRIM28 on expressions of VEGFR2, DLL4, and Notch4. Given that TRIM28 knockdown increased interaction between RBPJκ and HIF-1α-binding elements in ECs, the activation of HIF-1α-RBPJκ complex is likely responsible for the TRIM28-regulated angiogenic factor gene expression. RBPJκ knockdown alone can alter several angiogenic gene expressions in ECs, indicating that RBPJκ mediates some basal level of activation on target genes. The association of RBPJκ to DNA is highly dynamic. Upon other cellular signaling activation, some cofactors can interact with RBPJκ off the DNA and subsequently is recruited to the inducible target sites to activate the gene expression. So, our data indicated that TRIM28 may regulate dynamic RBPJκ binding to target genes. We noticed that VEGFR2 protein was increased by siHIF-1α and siRBPJk but mRNA was not changed, indicating HIF-1α and RBPJk potentially regulate the expression of VEGFR2 through a posttranscriptional mechanism. This is consistent with previous studies showing that the upregulation of VEGFR2 by hypoxia is mediated by posttranscriptional regulation.2,37 While HIF-1α is known to mediate VEGFR2 upregulation through an indirect mechanism involving VEGF autocrine loop under hypoxia,1 our results suggest that HIF-1αplays a distinct role in VEGFR2 expression in the normoxic conditions. Meanwhile, Notch-DLL signaling is known to limit VEGFR2 level in ECs, but it is still controversial whether Notch-DLL directly controls the transcription of VEGFR2.26,32,38,39 Our results raise possibilities that RBPJκ, the major transcriptional effector of Notch signaling, is involved in the posttranscriptional regulation of VEGFR2 through an unknown mechanism.
We also revealed that TRIM28 is a novel component of the feedback loop of VEGF-Notch signaling pathway in EC sprouting angiogenesis. It is well-known that VEGF signals through VEGFR2 to increase the DLL4 expression in tip cells, which further engage Notch receptors on adjacent stalk cells to decrease the expressions of VEGFR2 and VEGFR3 but increase the levels of VEGFR1, leading to reduced sensitivity to VEGF.22,35,39 Our results show that the phosphorylation of TRIM28 is enhanced by VEGF and Notch1 siRNA but reduced by overexpression of Notch NICD through an RBPJκ-DUSP-p38-dependent manner. Two known TRIM28 phosphorylation sites, including Ser 473 and Ser 824, have been reported. Phosphorylation of Ser 473 is known to regulate its corepressor activity, while phosphorylation of Ser 824 is induced during DNA damage and mostly involved in DNA repair.40,41 Previous studies have shown that the phosphorlaytion of Ser473 is stimulated by protein kinase C,42 which is a potential target for the treatment of angiogenic diseases.43,44 Other studies have reported that phosphorylation of TRIM28 Ser473 can also be induced by metabolic stresses through ROS and p38, both of which play important roles in VEGF-induced angiogenesis. Given the important roles of TRIM28 in sprouting angiogenesis and its regulation of VEGFR and DLL-Notch, our results suggest that TRIM28 integrates with VEGF-Notch signaling pathway to control EC sprouting angiogenesis.
In this study, we identified the novel role of TRIM28 ortholog, trim33l, in angiogenesis of developing zebrafish embryos. Our results show that trim33l morphants exhibited defects of both cranial vasculatures and misshapen ISVs. Notably, although the misshapen ISV phenotype in trim33l morphants is not compatible with the angiogenic sprouting defect as observed in VEGF deficient embryos previously,45,46 reduced sprouting of cranial vasculatures of trim33l was observed, supporting angiogenic sprouting defect. Given that trim33l mRNA was predominantly expressed in the head region shown by in situ hybridization, our results suggest a spatial-dependent manner is involved in trim33l-mediated controls angiogenesis in zebrafish model. Additionally, although the in situ hybridization results of enriched expression of trim33l in zebrafish vessels supports that an cell automous effect of trim33l in ECs is likely involved in the observed angiogenetic defects, the EC specific, trim33l knockout zebrafish model will need to be developed to address how cell autonomous effect of trim33l contributes to the impaired angiogenesis in the future. Because trim33l antibody is not available now, the trim33l mRNA-eGFP fusion which combined the morpholino target sequences with GFP was injected in trim33l morphants. Although decreased fluorescent signals were observed, it is not an ideal tool to validate knockdown efficiency due to the potential different expression levels between eGFP reporter and endogenous mRNA as well as nonspecific response of eGFP reporter to MOs, etc.5 Although our results show that the effect of trim33l MOs on developmental vascular defect was rescued by trim33l mRNA, it is still essential to generate stable trim33l mutant lines to validate its roles in vascular development. According to the published guidelines of MO use in zebrafish, MOs may generate a more severe phenotypes by blocking maternally provided wild-type mRNAs, off-target effects, and lack of genetic compensation, etc,35 thus, trim33l mutant zebrafish lines will be required for precisely analyzing its role in vascular development in the follow-up studies, which is a potential limitation of the current study.
In summary, we demonstrated that TRIM28-VEGFR-DLL4-Notch pathway contributes to endothelial sprouting angiogenesis. Our results in zebrafish embryos show that knockdown of TRIM28 impaired both cranial and peripheral angiogenesis, suggesting that TRIM28 is uniformly required for embryonic angiogenesis. Although TRIM28 knockout mice are embryonic lethal,47 mice deficient of TRIM28 in adulthood are viable,48 which makes TRIM28 a promising target to modulate angiogenesis in disease conditions. We anticipate that our findings will have broad implications for development as well as disease conditions associated with aberrant angiogenesis.
Supplementary Material
Highlights.
TRIM28 sustains VEGFR2 and DLL4, whereas decreases VEGFR1 expressions inendothelial cells.
The activation of HIF-1α-RBPJκ complex is responsible for the TRIM28-regulated angiogenic factor gene expression.
TRIM28-VEGFR-DLL4-Notch feedback loop contributes to sprouting angiogenesis.
ACKNOWLEDGMENTS
We thank Drs. Stephen C. Ekker and Ankit Sabharwal at Mayo Clinic for providing the pt3ts expression vector and helpful suggestion on the mRNA-eGFP fusion generation, respectively.
Funding information
This work was supported by the National Natural Science Foundation of China (81770443 to PZ, 81870327 to YFW), NHLBI (#HL140411 to DM), American Heart Association (#19CDA34700013 to YW), and Mayo Clinic CCaTS (Ted and Loretta Rogers Cardiovascular Career Development Award Honoring Hugh C. Smith to YW), Clinical Superior Discipline Development Fund of Shanghai Putuo District(#ptkwws201901 and #2019ysxk01 to PZ).
Abbreviations:
- DLL
delta-like ligand
- DUSP
dual-specificity phosphatase
- ECs
endothelial cells
- HIF-1α
hypoxia inducible factor 1 α
- MAPKs
mitogen-activated protein kinases
- PDGFB
platelet-derived growth factor B
- RBPJk
recombination signal-binding protein for immunoglobulin kappa J region
- TRIM28
tripartite motif-containing 28
- VEGFR
vascular endothelial growth factor receptor
Footnotes
CONFLICT OF INTEREST
The authors have declared that no conflict of interest exists.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section.
REFERENCES
- 1.Tang N, Wang L, Esko J, et al. Loss of HIF-1alpha in endothelial cells disrupts a hypoxia-driven VEGF autocrine loop necessary for tumorigenesis. Cancer Cells. 2004;6:485–495. [DOI] [PubMed] [Google Scholar]
- 2.Gerber HP, Condorelli F, Park J, Ferrara N. Differential transcriptional regulation of the two vascular endothelial growth factor receptor genes. Flt-1, but not Flk-1/KDR, is up-regulated by hypoxia. J Biol Chem. 1997;272:23659–23667. [DOI] [PubMed] [Google Scholar]
- 3.Blanco R, Gerhardt H. VEGF and Notch in tip and stalk cell selection. Cold Spring Harb Perspect Med. 2013;3:a006569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hellstrom M, Phng LK, Gerhardt H. VEGF and Notch signaling: the yin and yang of angiogenic sprouting. Cell Adhesion Migration. 2007;1:133–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suchting S, Eichmann A. Jagged gives endothelial tip cells an edge. Cell. 2009;137:988–990. [DOI] [PubMed] [Google Scholar]
- 6.Jakobsson L, Franco CA, Bentley K, et al. Endothelial cells dynamically compete for the tip cell position during angiogenic sprouting. Nature Cell Biol. 2010;12:943–953. [DOI] [PubMed] [Google Scholar]
- 7.Santoni de Sio FR, Barde I, Offner S, et al. KAP1 regulates gene networks controlling T-cell development and responsiveness. FASEB J. 2012;26:4561–4575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iyengar S, Farnham PJ. KAP1 protein: an enigmatic master regulator of the genome. J Biol Chem. 2011;286:26267–26276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hatakeyama S. TRIM proteins and cancer. Nat Rev Cancer. 2011;11:792–804. [DOI] [PubMed] [Google Scholar]
- 10.Messerschmidt DM, de Vries W, Ito M, Solter D, Ferguson-Smith A, Knowles BB. Trim28 is required for epigenetic stability during mouse oocyte to embryo transition. Science. 2012;335:1499–1502. [DOI] [PubMed] [Google Scholar]
- 11.Wang Y, Li J, Huang Y, et al. Tripartite motif-containing 28 bridges endothelial inflammation and angiogenic activity by retaining expression of TNFR-1 and −2 and VEGFR2 in endothelial cells. FASEB J. 2017;31:2026–2036. [DOI] [PubMed] [Google Scholar]
- 12.Li Z, Wang D, Na X, Schoen SR, Messing EM, Wu G. The VHL protein recruits a novel KRAB-A domain protein to repress HIF-1alpha transcriptional activity. The EMBO J. 2003;22:1857–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang L, Zhu C, Guo Y, et al. Inhibition of KAP1 enhances hypoxia-induced Kaposi's sarcoma-associated herpesvirus reactivation through RBP-Jkappa. Journal of Virol. 2014;88:6873–6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castel D, Mourikis P, Bartels SJ, Brinkman AB, Tajbakhsh S, Stunnenberg HG. Dynamic binding of RBPJ is determined by Notch signaling status. Genes Develop. 2013;27:1059–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ekker SC, Ungar AR, Greenstein P, et al. Patterning activities of vertebrate hedgehog proteins in the developing eye and brain. Curr Biol: CB. 1995;5:944–955. [DOI] [PubMed] [Google Scholar]
- 16.Thisse C, Thisse B. High-resolution in situ hybridization to whole-mount zebrafish embryos. Nature Protoco. 2008; 3:59–69. [DOI] [PubMed] [Google Scholar]
- 17.Layden MJ, Rottinger E, Wolenski FS, Gilmore TD, Martindale MQ. Microinjection of mRNA or morpholinos for reverse genetic analysis in the starlet sea anemone, Nematostella vectensis. Nature Protoco. 2013;8:924–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hyatt TM, Ekker SC. Vectors and techniques for ectopic gene expression in zebrafish. Methods Cell Biol. 1999;59:117–126. [DOI] [PubMed] [Google Scholar]
- 19.Nakatsu MN, Davis J, Hughes CC. Optimized fibrin gel bead assay for the study of angiogenesis. J Vis Exp. 2007;186. 10.3791/186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng W, Tammela T, Yamamoto M, et al. Notch restricts lymphatic vessel sprouting induced by vascular endothelial growth factor. Blood. 2011;118:1154–1162. [DOI] [PubMed] [Google Scholar]
- 21.Chitramuthu BP, Bennett HP. High resolution whole mount in situ hybridization within zebrafish embryos to study gene expression and function. J Vis Exp. 2013;e50644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.King CA. Kaposi's sarcoma-associated herpesvirus kaposin B induces unique monophosphorylation of STAT3 at serine 727 and MK2-mediated inactivation of the STAT3 transcriptional repressor TRIM28. J Virol. 2013;87:8779–8791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kondoh K, Sunadome K, Nishida E. Notch signaling suppresses p38 MAPK activity via induction of MKP-1 in myogenesis. J Biological Chem. 2007;282:3058–3065. [DOI] [PubMed] [Google Scholar]
- 24.Gerhardt H, Golding M, Fruttiger M, et al. VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J Cell Biol. 2003;161:1163–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang C, Guo Y, Jadlowiec CC, et al. Vascular endothelial growth factor-A inhibits EphB4 and stimulates delta-like ligand 4 expression in adult endothelial cells. J Surgical Res. 2013;183:478–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lobov IB, Renard RA, Papadopoulos N, et al. Delta-like ligand 4 (Dll4) is induced by VEGF as a negative regulator of angiogenic sprouting. Proc Natl Acad Sci USA. 2007;104:3219–3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Trindade A, Djokovic D, Gigante J, Mendonca L, Duarte A. Endothelial Dll4 overexpression reduces vascular response and inhibits tumor growth and metastasization in vivo. BMC Cancer. 2017;17:189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gessler M. Dll1 and Dll4: similar, but not the same. Blood. 2009;113:5375–5376. [DOI] [PubMed] [Google Scholar]
- 29.Benedito R, Roca C, Sorensen I, et al. The notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell. 2009;137:1124–1135. [DOI] [PubMed] [Google Scholar]
- 30.Ladi E, Nichols JT, Ge W, et al. The divergent DSL ligand Dll3 does not activate Notch signaling but cell autonomously attenuates signaling induced by other DSL ligands. J Cell Biol. 2005;170:983–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Andersson ER, Sandberg R, Lendahl U. Notch signaling: simplicity in design, versatility in function. Development. 2011;138:3593–3612. [DOI] [PubMed] [Google Scholar]
- 32.Hellstrom M, Phng LK, Hofmann JJ, et al. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature. 2007;445:776–780. [DOI] [PubMed] [Google Scholar]
- 33.Ho R-Y, Meyer RD, Chandler KB, et al. MINAR1 is a Notch2-binding protein that inhibits angiogenesis and breast cancer growth. J Mol Cell Biol. 2018;10:195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ulyatt C, Walker J, Ponnambalam S. Hypoxia differentially regulates VEGFR1 and VEGFR2 levels and alters intracellular signaling and cell migration in endothelial cells. Biochem Biophys Res Commun. 2011;404:774–779. [DOI] [PubMed] [Google Scholar]
- 35.Stainier DYR, Raz E, Lawson ND, et al. Guidelines for morpholino use in zebrafish. PLoS Genet. 2017;13:e1007000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Diaz-Trelles R, Scimia MC, Bushway P, et al. Notch-independent RBPJ controls angiogenesis in the adult heart. Nat Commun. 2016;7:12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Waltenberger J, Mayr U, Pentz S, Hombach V. Functional upregulation of the vascular endothelial growth factor receptor KDR by hypoxia. Circulation. 1996;94:1647–1654. [DOI] [PubMed] [Google Scholar]
- 38.Leslie JD, Ariza-McNaughton L, Bermange AL, McAdow R, Johnson SL, Lewis J. Endothelial signalling by the Notch ligand Delta-like 4 restricts angiogenesis. Development. 2007;134:839–844. [DOI] [PubMed] [Google Scholar]
- 39.Siekmann AF, Lawson ND. Notch signalling limits angiogenic cell behaviour in developing zebrafish arteries. Nature. 2007;445:781–784. [DOI] [PubMed] [Google Scholar]
- 40.Bunch H, Lawney BP, Lin YF, et al. Transcriptional elongation requires DNA break-induced signalling. Nat Commun. 2015;6:10191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Noon AT, Shibata A, Rief N, et al. 53BP1-dependent robust localized KAP-1 phosphorylation is essential for heterochromatic DNA double-strand break repair. Nature Cell Biol. 2010;12:177–184. [DOI] [PubMed] [Google Scholar]
- 42.Chang CW, Chou HY, Lin YS, et al. Phosphorylation at Ser473 regulates heterochromatin protein 1 binding and corepressor function of TIF1beta/KAP1. BMC Mol Biol. 2008;9:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Moriya J, Ferrara N. Inhibition of protein kinase C enhances angiogenesis induced by platelet-derived growth factor C in hyperglycemic endothelial cells. Cardiovasc Diabetol. 2015;14:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harrington EO, Loffler J, Nelson PR, Kent KC, Simons M, Ware JA. Enhancement of migration by protein kinase Calpha and inhibition of proliferation and cell cycle progression by protein kinase Cdelta in capillary endothelial cells. J Biol Chem. 1997;272:7390–7397. [DOI] [PubMed] [Google Scholar]
- 45.Childs S, Chen JN, Garrity DM, Fishman MC. Patterning of angiogenesis in the zebrafish embryo. Development. 2002;129:973–982. [DOI] [PubMed] [Google Scholar]
- 46.Habeck H, Odenthal J, Walderich B, Maischein H, Schulte-Merker S. Analysis of a zebrafish VEGF receptor mutant reveals specific disruption of angiogenesis. Curr Biol: CB. 2002;12:1405–1412. [DOI] [PubMed] [Google Scholar]
- 47.Cammas F, Mark M, Dolle P, Dierich A, Chambon P, Losson R. Mice lacking the transcriptional corepressor TIF1beta are defective in early postimplantation development. Development. 2000;127:2955–2963. [DOI] [PubMed] [Google Scholar]
- 48.Rousseaux MW, Revelli JP, Vazquez-Velez GE, et al. Depleting Trim28 in adult mice is well tolerated and reduces levels of alpha-synuclein and tau. eLife. 2018;7. 10.7554/eLife.36768 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.