The superior colliculus (SC) is an evolutionary conserved sensorimotor structure that is specialized for detecting, localizing, and orienting toward environmental events and has a critical role in orienting motor responses, visuospatial attention, and perceptual decision-making.1-8 The SC consists of a superficial layer that receives visual inputs and intermediate and deep motor layers that initiate saccades and other orienting movements toward the contralateral hemispace. Modern molecular and genetic methods combined with behavioral assessments have provided further understanding of the cell types, circuits, and functions of the SC (reviewed in ref. 9,10). The SC is affected by α-synuclein and tau neuropathology in Lewy body dementia (LBD),11 shows abnormal responses to visual stimuli in Parkinson disease (PD),12,13 may contribute to impaired saccades in progressive supranuclear palsy (PSP),14 and may be involved in the pathophysiology of cervical dystonia.15,16 This brief review will focus on recent concepts on the functional organization and connectivity of the SC that are relevant to understand the pathophysiology of these and other neurologic disorders.
Laminar Organization and Connections
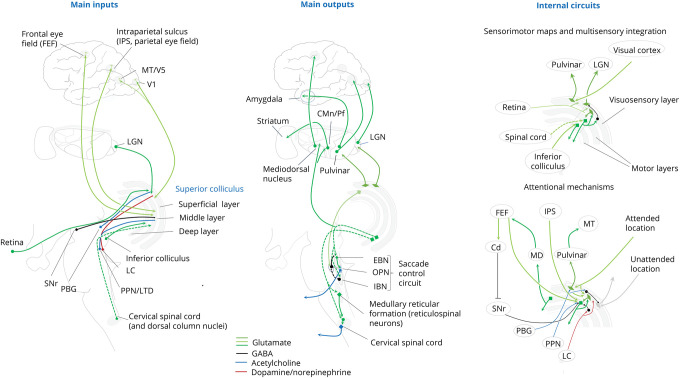
The SC has a distinct laminar structure consisting of alternating layers of neurons and fibers. It can be divided into 3 major gray matter lamina or strata, superficial (stratum griseum superficiale), intermediate (stratum griseum intermediate), and deep (stratum griseum profundum), interspersed with predominantly fiber-rich layers3,17 (Figure). The superficial or visuosensory layer receives retinotopically organized afferents from the retina (primarily direction-sensitive ganglion cells), lateral geniculate nucleus (LGN), primary visual cortex (V1), and medial temporal visual area (MT) representing the contralateral visual field.18 The intermediate and deep layers receive inputs from virtually the entire neuraxis.19,20 Cortical excitatory inputs originate from the frontal eye field (FEF)21,22 and the intraparietal sulcus (parietal eye field, lateral intraparietal area in monkeys).23,24 The deep and middle layers also receive excitatory auditory input from the inferior colliculus19 and somatosensory input from the contralateral spinal cord and dorsal column nuclei.25 By contrast, the substantia nigra pars reticulata (SNr) provides a tonic GABAergic inhibitory input to the SC.26,27 The SC also receives modulatory inputs from several brainstem areas, including the parabrachial nucleus,28 parabigeminal nucleus,29,30 pedunculopontine nucleus,31 locus ceruleus,32 and dorsal raphe.33
Figure. Organization, Extrinsic Connections, and Internal Circuits in the Superior Colliculus.
The superior colliculus (SC) has a laminar structure consisting of superficial, middle, and deep layers. The superficial (visuosensory) layer receives afferents from the retina, lateral geniculate nucleus (LGN), primary visual cortex (V1), and medial temporal visual area (MT/V5). The intermediate and deep layers receive inputs from the frontal eye field (FEF), intraparietal sulcus (IPS, parietal eye field), substantia nigra pars reticulata (SNr), inferior colliculus, and contralateral spinal cord. Brainstem modulatory inputs to the SC originate from the parabigeminal nucleus (PBG), pedunculopontine nucleus (PPN), laterodorsal tegmental nucleus (LTD), locus ceruleus (LC), and dorsal raphe (not shown). Superficial layer neurons project to the medial subnucleus of the inferior pulvinar, which in turn targets area MT/V5, and to the dorsal LGN, which targets V1. Neurons in the intermediate and deep layers provide a crossed descending projection (dotted lines) to innervate the saccadic control circuit, which includes excitatory burst neurons (EBNs) and inhibitory burst neurons (IBNs) targeted by the caudal pole of the SC and omnipause neurons (OPN) targeted by the rostral pole. The crossed descending axons from the SC descend areas of the reticular formation containing reticulospinal neurons and then reach the cervical spinal cord targeting primarily interneurons. Ascending projections from the middle and deep layers include collateral projections from saccadic neurons to the mediodorsal (MD) nucleus of the thalamus, which in turn projects to the FEF; projections to the centromedian/parafascicular (CMn/Pf) nuclei, which target the caudate nucleus (Cd); and projections to the medial pulvinar, which projects to the amygdala. The SC also sends inputs to the substantia nigra pars compacta, ventral tegmental area, subthalamic nucleus, PPN/LDT, and periaqueductal gray (not shown). Local circuits in the SC are critical for multisensory integration for orienting responses, visuospatial attention, and perceptual decision-making. Neurons in the intermediate and deep layers receive inputs from the visuosensory neurons of the superficial layer, are organized into a motor map in register with the visual map, and integrate visual with auditory inputs from the inferior colliculus and somatosensory inputs from the cervical spinal cord and dorsal column nuclei. Recurrent collaterals from the intermediate to the superficial layer may either excite superficial layer neurons projecting to the deep layers or, through GABAergic interneurons, suppress the activity of superficial layer neurons projecting to the pulvinar and dLGN. The SC receives input from the FEF and IPS, which are components of the dorsal attention network involved in goal-oriented visuospatial attention. These inputs trigger voluntary and reflex saccades. The FEF also projects to the Cd, which inhibits GABAergic neurons of the SNr projecting to the SC. Reciprocally, saccadic neurons of the SC send signals to the FEF through the MD. A signal from the deep layers of the SC modulates the sensory responsiveness of neurons in the superficial layers. Cholinergic input from the PBG and PPN/LDT may create focal excitation and global surround inhibition mediated by local GABAergic neurons within the SC. Dopaminergic projections from the LC modulate visual threat processing through GABAergic neurons at the intermediate layers of the SC.
The outputs of the SC target widespread areas of the neuraxis.17 The superficial layer projects to thalamic nuclei that project to cortical visual areas. A group of superficial layer neurons that receive primarily input from the visual cortex projects to the medial subnucleus of the inferior pulvinar.34,35 This portion of the pulvinar, in turn, projects to the medial temporal visual area (area V5/MT), which is a component of the dorsal visual stream.36 Tectopulvinar neurons respond to moving visual stimuli and provide a major source of motion information for visuospatial attention that bypasses the pathway from the retina to the dorsal LGN (dLGN) and then V1. A second group of superficial layer SC neurons that receive primarily retinal input projects to the dLGN. The superficial layer of the SC also projects to the pretectal nucleus, which projects back to the SC.17
Neurons in the intermediate and deep layers provide crossed descending projections to the brainstem reticular formation and spinal cord and uncrossed projections to the midbrain and thalamus. The crossed descending projections innervate brainstem centers controlling saccadic eye movements.17,37,38 The brainstem saccade control circuit targeted by the SC includes excitatory burst neurons (EBNs) and inhibitory burst neurons (IBNs) located within the paramedian pontine reticular formation for horizontal saccades and in the interstitial nucleus of the medial longitudinal fasciculus for vertical saccades and omnipause neurons (OPNs) located in the nucleus raphe interpositus.39,40 The rostral pole of the SC contains a map of the contralateral fovea and harbors fixation neurons that send a monosynaptic excitatory input to OPNs that tonically inhibit the saccadic burst neurons.37,38 The caudal portion of the SC harbors neurons that discharge before both reflex and voluntary saccades and project to burst neurons that in turn activate EBNs and IBNs. Projections from these neurons to ocular motor nuclei mediate the visual grasp reflex, which is a component of an orienting response to salient stimuli.8 Inhibitory burst neurons also inhibit contralateral OPNs, thereby providing a disynaptic pathway that triggers saccades.37,38 Topographic connections involving excitatory and inhibitory commissural neurons in the SC may have an important role in conjugate upward and downward vertical saccades.41 Neurons in the intermediate layer of the SC also discharge during orienting responses linked to movements of the head42 and upper limb.43 The middle and deep layers of the SC provide crossed descending projections to areas of the reticular formation such as the gigantocellular nucleus harboring reticulospinal neurons44 and to the upper cervical spinal cord (tectospinal tract) where they target primarily interneurons to control coordinated head-eye movements.45-48
The ascending projections from the intermediate and deep layers of the SC are denser than the descending projections and contribute to higher aspects of visual function, such as attention and perceptual decision-making.6 For example, SC neurons projecting to the brainstem saccade generator send collateral projections to the mediodorsal nucleus of the thalamus, which in turn targets the FEF, thus providing a feedforward information (corollary discharge) about impending eye movements.49 Outputs of the SC to the thalamus also affect basal ganglia and limbic circuits.50 The SC projects to the centromedian and parafascicular nuclei, which provide output to the caudate nucleus,50 thus affecting goal-driven actions. The SC also projects to areas of the pulvinar that target the basolateral amygdala, thus providing a subcortical circuit for orienting responses to threat stimuli.51,52 The SC also sends direct excitatory projection to dopaminergic neurons of the substantia nigra pars compacta,53 to the subthalamic nucleus,54 and to cholinergic neurons in the pedunculopontine and laterodorsal tegmental nucleus,55 which are involved in attention and action selection.
Local Circuits
The SC contains maps of the sensory space and actions that are topographically arranged and in register with each other through a direct link between the visuosensory superficial layer and the motor intermediate and deep layers56-62 (Figure). The retinal map of the space serves as a template for aligning sensory and motor maps.63 The superficial layer projects to the motor layers and provides a disynaptic excitatory pathway from the retina to neurons generating saccades and other orienting movements.64-67 The map of saccadic eye movements is organized according to the spatial motor error computed from the position of the eyes relative to the position of the visual target of interest.68,69 Neurons in the deep layers of the SC also receive auditory and somatosensory inputs that match the retinotopy.19,25 This indicates that these neurons constitute multisensory functional units,70-72 which are able to generate amplified responses to spatiotemporally concordant visual and auditory stimuli.73
Local GABAergic neurons in the superficial layer allow visuosensory neurons to function as salience detectors controlling bursting activity in neurons in the intermediate layer.74 Neurons in the intermediate layer provide 2 recurrent collateral pathways of the superficial layer6; one of them excites neurons in the superficial layers projecting to the deep layers enhancing their response to visual stimuli,68 whereas the other terminates on local GABAergic interneurons that suppress the activity of superficial layer neurons projecting the pulvinar and dLGN and contributes to suppression of retinal motion perception during eye movements.75 The disynaptic intralaminar pathway from the retina to the middle through the superficial layer of the LC triggers short latency express saccades,76 whereas the cortical input from the FEF and intraparietal sulcus to the middle layer is necessary for conventional voluntary and reflex saccades.6 The FEF, through inputs to the caudate nucleus, also promotes saccadic eye movements by releasing the SC from the tonic GABAergic inhibition by the SNr.27 This nigrotectal connection not only regulates burst initiation but also modulates the spatiotemporal properties of premotor neurons through connections to local GABAergic neurons.77
Role in Visuospatial Attention and Perceptual Decision-Making
Spatial attention enhances sensory signals to facilitate the processing of sensory information for goal-oriented behaviors.8,78 Studies in monkeys indicate that the SC has an important role in visuospatial attention.9 Saccadic neurons of the SC use a population vector average for single targets and use a winner-takes-all code for multiple possible targets according to their behavioral relevance.1,79 The SC receives inputs from the FEF and intraparietal sulcus,21-24 which are core components of the dorsal attention network involved in goal-oriented visuospatial attention.80-82 This circuit can modulate the gain of incoming retinal signals reaching cortical and subcortical structures and thus affect visual processing.83-87 Most of the FEF projections to the SC signal cognitive operations preceding the onset of saccades,88 whereas input from the intraparietal sulcus provides online visuospatial information for triggering of reflexive saccades.89 Attention modulates the influence of sensory inputs to the SC in the selection of saccades90; motor commands, in turn, influence the sensory responses in the SC independently of the execution of the eye movements.68,91 This indicates that the SC is involved in the shift in the focus of attention to the target location.92-94 Although the SC, through the pulvinar, connects to cortical visual areas involved in spatial attention such as area MT and superior temporal sulcus,95,96 inactivation studies show that the SC can modulate spatial attention independently of the cerebral cortex,97 for example, through the basal ganglia.27 Brainstem cholinergic and GABAergic inputs, through interactions with forebrain inputs, may create a priority map within the SC by a mechanism of focal excitation and global surround inhibition; this establishes the spatial location of interest and drives an orienting movement toward the selected target.98,99 Cholinergic inputs from the parabigeminal nucleus29,30 to the superficial layers of the SC may enhance the visual responsiveness and spatial selectivity of visuosensory neurons through nicotinic receptors in retinal axon terminals and local GABAergic neurons, whereas GABAergic inputs from this nucleus may suppress activity to unattended regions in the SC map.99-102 Cholinergic inputs from the pedunculopontine nucleus may contribute to selective attention and generation of saccades through nicotinic receptor activation of neurons in intermediate and deep layers of the SC projecting the brainstem.103-105 The SC is also activated during REM sleep; cholinergic and GABAergic modulation of the SC may thus also affect REM sleep and may be associated with visuospatial learning and memory consolidation.106
Role in Emotional Responses
Studies in rodents show that the SC influences innate or reflexive actions through 2 segregated pathways originating from its intermediate layer, a crossed tectobulbar pathway that targets the contralateral brainstem and spinal cord and mediates approach behaviors and an uncrossed tectopontine pathway that targets the ipsilateral pons and mediates avoidance behaviors.64,107 Neurons in the superficial layers project to the amygdala both through the pulvinar and through the parabigeminal nucleus. The deep layers of the SC provide excitatory projection areas required for the initiation of threat-evoked escaped responses, including the dorsal periaqueductal gray108 and ventral tegmental area.109 Stress enhances escape behavior through projections of the locus ceruleus to the SC.110 These projections provide dopaminergic inputs that modulate visual threat processing through D2 receptors in GABAergic neurons at the intermediate and deep layers.111 The context-dependent innate behaviors initiated by SC neurons are accompanied by stereotypical cardiovascular and respiratory responses, which are mediated by projections of the SC to neurons in the medullary gigantocellular reticular nucleus projecting to the spinal cord.112
Clinical Correlations
Functional MRI neuroimaging studies have allowed the detection of SC activation in response to visual stimuli in both normal and pathologic conditions. One such study showed that the activity of the SC increases linearly with increasing luminance contrast in normal individuals and that luminance contrast processing in the SC is affected by normal aging.113 These studies also show that the SC-pulvinar-amygdala pathway is activated on exposure to threat and may underlie the ability of humans with blindsight to recognize fearful emotion in facial expressions.114-117 Diffusion tensor imaging confirmed the presence of a pathway connecting the FEF to the superior colliculus in humans.22 Consistent with studies in monkeys,83,92-94 transcranial magnetic stimulation studies show that the preparatory activity of the FEF before onset of a saccade toward a target modulates the visual detection threshold in humans.118,119
Involvement of the SC may contribute to impaired visuospatial attention and orienting responses in neurodegenerative disorders such as DLB. A neuropathologic study showed substantial reductions in neuronal density and accumulation of α-synuclein and tau inclusions primarily involving the intermediate and deep layers of the SC.11 As these layers receive inputs from brainstem areas vulnerable to α-synuclein and tau neuropathology,120 these findings can be interpreted as consistent with a prion-like disease propagation as occurs in neurodegenerative disorders.11 The intermediate and deep layers of the SC are also vulnerable to tau pathology in chronic traumatic encephalopathy.121 The neuropathologic changes in the SC may contribute to some of the clinical manifestations of neurodegenerative disorders such as LBD. Dysfunction of the dorsal attention network connected with the SC has been implicated in visual hallucinations.122 Neuropathologic studies showed that the degree of neuronal loss and tau accumulation (but not the brunt of α-synuclein pathology) in the SC was related to the severity and frequency of visual hallucinations in LBD.11 The SC is also affected in PD. An fMRI study showed a lack of modulation of SC activation in response to luminance contrast in de novo patients with PD compared with controls, suggesting that abnormal visual processing in the SC occurs early in the disease course.12 Patients with PD may show short-latency saccades and difficult to inhibit reflexive saccades toward visual stimuli.123,124 These findings have been interpreted as in part reflecting neuroadaptation in the SC to compensate for excessive inhibition from the SNr, as shown in experimental models of PD.125 It has been suggested that levodopa may modulate the balance between voluntary and reflex saccades in PD by facilitating planning movements in the FEF and suppressing the release of reflexive saccades originating in the SC.123 The superior colliculus is also involved by tau pathology in PSP.14 In patients with this disorder, the progressively reduced accuracy of horizontal saccades suggests a brainstem oculomotor pathology that includes the SC and/or paramedian pontine reticular formation, whereas the functioning of the oculomotor system above the brainstem was found to be similar between patients with PSP and PD.126 In PSP, saccade disturbances reflect paucity in burst generation by EBNs and imprecise timing and premature discharge of IBNs, reflecting maladaptive SC activity leading to a change in the intended trajectory of the ongoing saccade.127 Functional MRI studies also suggest SC dysfunction in cervical dystonia.15 One such study showed slower SC activation in response to both luminant and chromatic visual stimuli in these patients compared with controls.16 It has been proposed that cervical dystonia may reflect an alteration in the SC circuitry resulting from impaired GABAergic activity and manifested by abnormal temporal discrimination in visuosensory neurons of the superficial layer and disinhibited burst activity of the tectospinal neurons in deeper layers.128 An fMRI study using causal connectivity modeling showed that the modulation of the strength of functional connection from the striatum to the SC in response to a looming visual stimulus was greater in patients with cervical dystonia than that in controls.129
Perspective
The evidence briefly discussed in this review indicates that the SC, like the thalamus, striatum, and brainstem, should be considered as an important contributor to network dysfunction underlying cognitive and motor abnormalities in neurodegenerative disorders. Unfortunately, although SC activation can be identified on fMRI, the SC is too small to be identified with fluorodeoxyglucose PET (FDG-PET). Higher sensitivity imaging procedures and neuropathologic studies with careful clinical correlations may provide further insight on the contribution of the SC in these and other disorders.
Glossary
- dGLN
dorsal LGN
- EBNs
excitatory burst neurons
- FDG-PET
fluorodeoxyglucose PET
- FEF
frontal eye field
- fMRI
functional MRI
- IBNs
inhibitory burst neurons
- LGN
lateral geniculate nucleus
- LBD
Lewy body dementia
- OPNs
omnipause neurons
- PD
Parkinson disease
- PSP
progressive supranuclear palsy
- V1
primary visual cortex
- V5/MT
medial temporal visual area
- SC
superior colliculus
- MT
medial temporal visual area
- SNr
substantia nigra pars reticulata
Appendix. Author
Study Funding
The author reports no targeted funding.
Disclosure
E. Benarroch reports no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Wurtz RH, Albano JE. Visual-motor function of the primate superior colliculus. Annu Rev Neurosci. 1980;3(1):189-226. doi. 10.1146/annurev.ne.03.030180.001201 [DOI] [PubMed] [Google Scholar]
- 2.Sparks DL. Conceptual issues related to the role of the superior colliculus in the control of gaze. Curr Opin Neurobiol. 1999;9(6):698-707. doi. 10.1016/s0959-4388(99)00039-2 [DOI] [PubMed] [Google Scholar]
- 3.Krauzlis RJ, Lovejoy LP, Zenon A. Superior colliculus and visual spatial attention. Annu Rev Neurosci. 2013;36(1):165-182. doi. 10.1146/annurev-neuro-062012-170249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Katyal S, Zughni S, Greene C, Ress D. Topography of covert visual attention in human superior colliculus. J Neurophysiol. 2010;104(6):3074-3083. doi. 10.1152/jn.00283.2010 [DOI] [PubMed] [Google Scholar]
- 5.Mizzi R, Michael GA. The role of the collicular pathway in the salience-based progression of visual attention. Behav Brain Res. 2014;270:330-338. doi. 10.1016/j.bbr.2014.05.043 [DOI] [PubMed] [Google Scholar]
- 6.Basso MA, May PJ. Circuits for action and cognition: a view from the superior colliculus. Annu Rev Vis Sci. 2017;3(1):197-226. doi. 10.1146/annurev-vision-102016-061234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crapse TB, Lau H, Basso MA. A role for the superior colliculus in decision criteria. Neuron. 2018;97(1):181-194.e6. doi. 10.1016/j.neuron.2017.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corneil BD, Munoz DP. Overt responses during covert orienting. Neuron. 2014;82(6):1230-1243. doi. 10.1016/j.neuron.2014.05.040 [DOI] [PubMed] [Google Scholar]
- 9.Basso MA, Bickford ME, Cang J. Unraveling circuits of visual perception and cognition through the superior colliculus. Neuron. 2021;109(6):918-937. doi. 10.1016/j.neuron.2021.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu X, Huang H, Snutch TP, Cao P, Wang L, Wang F. The superior colliculus: cell types, connectivity, and behavior. Neurosci Bull. 2022;38(12):1519-1540. doi. 10.1007/s12264-022-00858-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Erskine D, Thomas AJ, Taylor JP, et al. Neuronal loss and alpha-synuclein pathology in the superior colliculus and its relationship to visual hallucinations in dementia with Lewy bodies. Am J Geriatr Psychiatry. 2017;25(6):595-604. doi. 10.1016/j.jagp.2017.01.005 [DOI] [PubMed] [Google Scholar]
- 12.Moro E, Bellot E, Meoni S, et al. Visual dysfunction of the superior colliculus in de novo parkinsonian patients. Ann Neurol. 2020;87(4):533-546. doi. 10.1002/ana.25696 [DOI] [PubMed] [Google Scholar]
- 13.Bellot E, Kauffmann L, Coizet V, Meoni S, Moro E, Dojat M. Effective connectivity in subcortical visual structures in de novo Patients with Parkinson's Disease. Neuroimage Clin. 2022;33:102906. doi. 10.1016/j.nicl.2021.102906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dugger BN, Tu M, Murray ME, Dickson DW. Disease specificity and pathologic progression of tau pathology in brainstem nuclei of Alzheimer's disease and progressive supranuclear palsy. Neurosci Lett. 2011;491(2):122-126. doi. 10.1016/j.neulet.2011.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mc Govern EM, Killian O, Narasimham S, et al. Disrupted superior collicular activity may reveal cervical dystonia disease pathomechanisms. Sci Rep. 2017;7(1):16753. doi. 10.1038/s41598-017-17074-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Williams L, Butler JS, Thirkettle M, et al. Slowed luminance reaction times in cervical dystonia: disordered superior colliculus processing. Mov Disord. 2020;35(5):877-880. doi. 10.1002/mds.27975 [DOI] [PubMed] [Google Scholar]
- 17.May PJ. The mammalian superior colliculus: laminar structure and connections. Prog Brain Res. 2006;151:321-378. doi. 10.1016/S0079-6123(05)51011-2 [DOI] [PubMed] [Google Scholar]
- 18.Perry VH, Cowey A. Retinal ganglion cells that project to the superior colliculus and pretectum in the macaque monkey. Neuroscience. 1984;12(4):1125-1137. doi. 10.1016/0306-4522(84)90007-1 [DOI] [PubMed] [Google Scholar]
- 19.Edwards SB, Ginsburgh CL, Henkel CK, Stein BE. Sources of subcortical projections to the superior colliculus in the cat. J Comp Neurol. 1979;184(2):309-329. doi. 10.1002/cne.901840207 [DOI] [PubMed] [Google Scholar]
- 20.Sparks DL, Hartwich-Young R. The deep layers of the superior colliculus. Rev Oculomot Res. 1989;3:213-255. [PubMed] [Google Scholar]
- 21.Lynch JC, Hoover JE, Strick PL. Input to the primate frontal eye field from the substantia nigra, superior colliculus, and dentate nucleus demonstrated by transneuronal transport. Exp Brain Res. 1994;100(1):181-186. doi. 10.1007/bf00227293 [DOI] [PubMed] [Google Scholar]
- 22.Quentin R, Chanes L, Migliaccio R, Valabregue R, Valero-Cabre A. Fronto-tectal white matter connectivity mediates facilitatory effects of non-invasive neurostimulation on visual detection. Neuroimage. 2013;82:344-354. doi. 10.1016/j.neuroimage.2013.05.083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lynch JC, Graybiel AM, Lobeck LJ. The differential projection of two cytoarchitectonic subregions of the inferior parietal lobule of macaque upon the deep layers of the superior colliculus. J Comp Neurol. 1985;235(2):241-254. doi. 10.1002/cne.902350207 [DOI] [PubMed] [Google Scholar]
- 24.Rushworth MFS, Behrens TEJ, Johansen-Berg H. Connection patterns distinguish 3 regions of human parietal cortex. Cereb Cortex. 2006;16(10):1418-1430. doi. 10.1093/cercor/bhj079 [DOI] [PubMed] [Google Scholar]
- 25.Antonetty CM, Webster KE. The organisation of the spinotectal projection. An experimental study in the rat. J Comp Neurol. 1975;163(4):449-465. doi. 10.1002/cne.901630405 [DOI] [PubMed] [Google Scholar]
- 26.Hopkins DA, Niessen LW. Substantia nigra projections to the reticular formation, superior colliculus and central gray in the rat, cat and monkey. Neurosci Lett. 1976;2(5):253-259. doi. 10.1016/0304-3940(76)90156-7 [DOI] [PubMed] [Google Scholar]
- 27.Hikosaka O, Kim HF, Amita H, et al. Direct and indirect pathways for choosing objects and actions. Eur J Neurosci. 2019;49(5):637-645. doi. 10.1111/ejn.13876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang D, Grady FS, Peltekian L, Geerling JC. Efferent projections of Vglut2, Foxp2, and Pdyn parabrachial neurons in mice. J Comp Neurol. 2021;529(4):657-693. doi. 10.1002/cne.24975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Graybiel AM. A satellite system of the superior colliculus: the parabigeminal nucleus and its projections to the superficial collicular layers. Brain Res. 1978;145(2):365-374. doi. 10.1016/0006-8993(78)90870-3 [DOI] [PubMed] [Google Scholar]
- 30.Knudsen EI. Evolution of neural processing for visual perception in vertebrates. J Comp Neurol. 2020;528(17):2888-2901. doi. 10.1002/cne.24871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hall WC, Fitzpatrick D, Klatt LL, Raczkowski D. Cholinergic innervation of the superior colliculus in the cat. J Comp Neurol. 1989;287(4):495-514. doi. 10.1002/cne.902870408 [DOI] [PubMed] [Google Scholar]
- 32.Li L, Wang L. Modulation of innate defensive responses by locus coeruleus-superior colliculus circuit. J Exp Neurosci. 2018;12:117906951879203. doi. 10.1177/1179069518792035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Villar MJ, Vitale ML, Hokfelt T, Verhofstad AAJ. Dorsal raphe serotoninergic branching neurons projecting both to the lateral geniculate body and superior colliculus: a combined retrograde tracing-immunohistochemical study in the rat. J Comp Neurol. 1988;277(1):126-140. doi. 10.1002/cne.902770109 [DOI] [PubMed] [Google Scholar]
- 34.Zhou NA, Maire PS, Masterson SP, Bickford ME. The mouse pulvinar nucleus: organization of the tectorecipient zones. Vis Neurosci. 2017;34:E011. doi. 10.1017/s0952523817000050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berman RA, Wurtz RH. Functional identification of a pulvinar path from superior colliculus to cortical area MT. J Neurosci. 2010;30(18):6342-6354. doi. 10.1523/jneurosci.6176-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stepniewska I, Qi HX, Kaas JH. Do superior colliculus projection zones in the inferior pulvinar project to MT in primates? Eur J Neurosci. 1999;11(2):469-480. doi. 10.1046/j.1460-9568.1999.00461.x [DOI] [PubMed] [Google Scholar]
- 37.Shinoda Y, Takahashi M, Sugiuchi Y. Brainstem neural circuits for fixation and generation of saccadic eye movements. Prog Brain Res. 2019;249:95-104. doi. 10.1016/bs.pbr.2019.04.007 [DOI] [PubMed] [Google Scholar]
- 38.Takahashi M, Sugiuchi Y, Na J, Shinoda Y. Brainstem circuits triggering saccades and fixation. J Neurosci. 2022;42(5):789-803. doi. 10.1523/jneurosci.1731-21.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zee DS. Brain stem and cerebellar deficits in eye movement control. Trans Ophthalmol Soc U K (1962). 1986;105(Pt 5):599-605. [PubMed] [Google Scholar]
- 40.Ramat S, Leigh RJ, Zee DS, Optican LM. Ocular oscillations generated by coupling of brainstem excitatory and inhibitory saccadic burst neurons. Exp Brain Res. 2005;160(1):89-106. doi. 10.1007/s00221-004-1989-8 [DOI] [PubMed] [Google Scholar]
- 41.Takahashi M, Sugiuchi Y, Shinoda Y. Topographic organization of excitatory and inhibitory commissural connections in the superior colliculi and their functional roles in saccade generation. J Neurophysiol. 2010;104(6):3146-3167. doi. 10.1152/jn.00554.2010 [DOI] [PubMed] [Google Scholar]
- 42.Walton MMG, Bechara B, Gandhi NJ. Role of the primate superior colliculus in the control of head movements. J Neurophysiol. 2007;98(4):2022-2037. doi. 10.1152/jn.00258.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Werner W, Dannenberg S, Hoffmann KP. Arm-movement-related neurons in the primate superior colliculus and underlying reticular formation: comparison of neuronal activity with EMGs of muscles of the shoulder, arm and trunk during reaching. Exp Brain Res. 1997;115(2):191-205. doi. 10.1007/pl00005690 [DOI] [PubMed] [Google Scholar]
- 44.Isa T, Sasaki S. Brainstem control of head movements during orienting; organization of the premotor circuits. Prog Neurobiol. 2002;66(4):205-241. doi. 10.1016/s0301-0082(02)00006-0 [DOI] [PubMed] [Google Scholar]
- 45.Grantyn A, Grantyn R. Axonal patterns and sites of termination of cat superior colliculus neurons projecting in the tecto-bulbo-spinal tract. Exp Brain Res. 1982;46(2):243-256. doi. 10.1007/bf00237182 [DOI] [PubMed] [Google Scholar]
- 46.Grantyn A, Berthoz A. Reticulo-spinal neurons participating in the control of synergic eye and head movements during orienting in the cat. I. Behavioral properties. Exp Brain Res. 1987;66(2):339-354. doi. 10.1007/bf00243309 [DOI] [PubMed] [Google Scholar]
- 47.Rose PK, Abrahams VC. Tectospinal and tectoreticular cells: their distribution and afferent connections. Can J Physiol Pharmacol. 1978;56(4):650-658. doi. 10.1139/y78-103 [DOI] [PubMed] [Google Scholar]
- 48.Rose PK, MacDonald J, Abrahams VC. Projections of the tectospinal tract to the upper cervical spinal cord of the cat: a study with the anterograde tracer PHA-L. J Comp Neurol. 1991;314(1):91-105. doi. 10.1002/cne.903140109 [DOI] [PubMed] [Google Scholar]
- 49.Sommer MA, Wurtz RH. What the brain stem tells the frontal cortex. I. Oculomotor signals sent from superior colliculus to frontal eye field via mediodorsal thalamus. J Neurophysiol. 2004;91(3):1381-1402. doi. 10.1152/jn.00738.2003 [DOI] [PubMed] [Google Scholar]
- 50.Ichinohe N, Shoumura K. A di-synaptic projection from the superior colliculus to the head of the caudate nucleus via the centromedian-parafascicular complex in the cat: an anterograde and retrograde labeling study. Neurosci Res. 1998;32(4):295-303. doi. 10.1016/s0168-0102(98)00095-9 [DOI] [PubMed] [Google Scholar]
- 51.Wei P, Liu N, Zhang Z, et al. Processing of visually evoked innate fear by a non-canonical thalamic pathway. Nat Commun. 2015;6(1):6756. doi. 10.1038/ncomms7756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McFadyen J. Investigating the subcortical route to the amygdala across species and in disordered fear responses. J Exp Neurosci. 2019;13:117906951984644. doi. 10.1177/1179069519846445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Comoli E, Coizet V, Boyes J, et al. A direct projection from superior colliculus to substantia nigra for detecting salient visual events. Nat Neurosci. 2003;6(9):974-980. doi. 10.1038/nn1113 [DOI] [PubMed] [Google Scholar]
- 54.Coizet V, Graham JH, Moss J, et al. Short-latency visual input to the subthalamic nucleus is provided by the midbrain superior colliculus. J Neurosci. 2009;29(17):5701-5709. doi. 10.1523/jneurosci.0247-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Huerta-Ocampo I, Dautan D, Gut NK, Khan B, Mena-Segovia J. Whole-brain mapping of monosynaptic inputs to midbrain cholinergic neurons. Sci Rep. 2021;11(1):9055. doi. 10.1038/s41598-021-88374-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Helms MC, Ozen G, Hall WC. Organization of the intermediate gray layer of the superior colliculus. I. Intrinsic vertical connections. J Neurophysiol. 2004;91(4):1706-1715. doi. 10.1152/jn.00705.2003 [DOI] [PubMed] [Google Scholar]
- 57.Vokoun CR, Jackson MB, Basso MA. Circuit dynamics of the superior colliculus revealed by in vitro voltage imaging. Ann N Y Acad Sci. 2011;1233(1):41-47. doi. 10.1111/j.1749-6632.2011.06166.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yamagata M, Weiner JA, Dulac C, Roth KA, Sanes JR. Labeled lines in the retinotectal system: markers for retinorecipient sublaminae and the retinal ganglion cell subsets that innervate them. Mol Cell Neurosci. 2006;33(3):296-310. doi. 10.1016/j.mcn.2006.08.001 [DOI] [PubMed] [Google Scholar]
- 59.Reinhard K, Li C, Do Q, Burke EG, Heynderickx S, Farrow K. A projection specific logic to sampling visual inputs in mouse superior colliculus. Elife. 2019;8:e50697. doi. 10.7554/elife.50697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Barchini J, Shi X, Chen H, Cang J. Bidirectional encoding of motion contrast in the mouse superior colliculus. Elife. 2018;7:e35261. doi. 10.7554/elife.35261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McIlwain JT. Distributed spatial coding in the superior colliculus: a review. Vis Neurosci. 1991;6(1):3-13. doi. 10.1017/s0952523800000857 [DOI] [PubMed] [Google Scholar]
- 62.Gandhi NJ, Katnani HA. Motor functions of the superior colliculus. Annu Rev Neurosci. 2011;34(1):205-231. doi. 10.1146/annurev-neuro-061010-113728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang L, Liu M, Segraves MA, Cang J. Visual experience is required for the development of eye movement maps in the mouse superior colliculus. J Neurosci. 2015;35:12281-12286. doi. 10.1523/jneurosci.0117-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Isa K, Sooksawate T, Kobayashi K, Kobayashi K, Redgrave P, Isa T. Dissecting the tectal output channels for orienting and defense responses. eNeuro. 2020;7(5):ENEURO.0271-20.2020. doi. 10.1523/eneuro.0271-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Isa T. Intrinsic processing in the mammalian superior colliculus. Curr Opin Neurobiol. 2002;12(6):668-677. doi. 10.1016/s0959-4388(02)00387-2 [DOI] [PubMed] [Google Scholar]
- 66.Robinson DA. Eye movements evoked by collicular stimulation in the alert monkey. Vis Res. 1972;12(11):1795-1808. doi. 10.1016/0042-6989(72)90070-3 [DOI] [PubMed] [Google Scholar]
- 67.Savjani RR, Katyal S, Halfen E, Kim JH, Ress D. Polar-angle representation of saccadic eye movements in human superior colliculus. Neuroimage. 2018;171:199-208. doi. 10.1016/j.neuroimage.2017.12.080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ghitani N, Bayguinov PO, Vokoun CR, McMahon S, Jackson MB, Basso MA. Excitatory synaptic feedback from the motor layer to the sensory layers of the superior colliculus. J Neurosci. 2014;34(20):6822-6833. doi. 10.1523/jneurosci.3137-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mays LE, Sparks DL. Dissociation of visual and saccade-related responses in superior colliculus neurons. J Neurophysiol. 1980;43(1):207-232. doi. 10.1152/jn.1980.43.1.207 [DOI] [PubMed] [Google Scholar]
- 70.Meredith MA, Wallace MT, Stein BE. Visual, auditory and somatosensory convergence in output neurons of the cat superior colliculus: multisensory properties of the tecto-reticulo-spinal projection. Exp Brain Res. 1992;88(1):181-186. doi. 10.1007/bf02259139 [DOI] [PubMed] [Google Scholar]
- 71.Wallace MT, Meredith MA, Stein BE. Converging influences from visual, auditory, and somatosensory cortices onto output neurons of the superior colliculus. J Neurophysiol. 1993;69(6):1797-1809. doi. 10.1152/jn.1993.69.6.1797 [DOI] [PubMed] [Google Scholar]
- 72.Ghose D, Maier A, Nidiffer A, Wallace MT. Multisensory response modulation in the superficial layers of the superior colliculus. J Neurosci. 2014;34(12):4332-4344. doi. 10.1523/jneurosci.3004-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rowland BA, Quessy S, Stanford TR, Stein BE. Multisensory integration shortens physiological response latencies. J Neurosci. 2007;27(22):5879-5884. doi. 10.1523/jneurosci.4986-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kaneda K, Isa T. GABAergic mechanisms for shaping transient visual responses in the mouse superior colliculus. Neuroscience. 2013;235:129-140. doi. 10.1016/j.neuroscience.2012.12.061 [DOI] [PubMed] [Google Scholar]
- 75.Lee KH, Tran A, Turan Z, Meister M. The sifting of visual information in the superior colliculus. Elife. 2020;9:e50678. doi. 10.7554/elife.50678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Marino RA, Levy R, Munoz DP. Linking express saccade occurance to stimulus properties and sensorimotor integration in the superior colliculus. J Neurophysiol. 2015;114(2):879-892. doi. 10.1152/jn.00047.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kaneda K, Isa K, Yanagawa Y, Isa T. Nigral inhibition of GABAergic neurons in mouse superior colliculus. J Neurosci. 2008;28(43):11071-11078. doi. 10.1523/jneurosci.3263-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shen K, Pare M. Predictive saccade target selection in superior colliculus during visual search. J Neurosci. 2014;34(16):5640-5648. doi. 10.1523/jneurosci.3880-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Basso MA, Wurtz RH. Modulation of neuronal activity in superior colliculus by changes in target probability. J Neurosci. 1998;18:7519-7534. doi. 10.1523/jneurosci.18-18-07519.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Meehan TP, Bressler SL, Tang W, et al. Top-down cortical interactions in visuospatial attention. Brain Struct Funct. 2017;222(7):3127-3145. doi. 10.1007/s00429-017-1390-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Thiebaut de Schotten M, Dell'Acqua F, Valabregue R, Catani M. Monkey to human comparative anatomy of the frontal lobe association tracts. Cortex. 2012;48(1):82-96. doi. 10.1016/j.cortex.2011.10.001 [DOI] [PubMed] [Google Scholar]
- 82.Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201-215. doi. 10.1038/nrn755 [DOI] [PubMed] [Google Scholar]
- 83.Reynolds JH, Chelazzi L. Attentional modulation of visual processing. Annu Rev Neurosci. 2004;27(1):611-647. doi. 10.1146/annurev.neuro.26.041002.131039 [DOI] [PubMed] [Google Scholar]
- 84.O'Connor DH, Fukui MM, Pinsk MA, Kastner S. Attention modulates responses in the human lateral geniculate nucleus. Nat Neurosci. 2002;5(11):1203-1209. doi. 10.1038/nn957 [DOI] [PubMed] [Google Scholar]
- 85.Schneider KA, Kastner S. Effects of sustained spatial attention in the human lateral geniculate nucleus and superior colliculus. J Neurosci. 2009;29(6):1784-1795. doi. 10.1523/jneurosci.4452-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schlag-Rey M, Schlag J, Dassonville P. How the frontal eye field can impose a saccade goal on superior colliculus neurons. J Neurophysiol. 1992;67(4):1003-1005. doi. 10.1152/jn.1992.67.4.1003 [DOI] [PubMed] [Google Scholar]
- 87.Shipp S. The brain circuitry of attention. Trends Cogn Sci. 2004;8(5):223-230. doi. 10.1016/j.tics.2004.03.004 [DOI] [PubMed] [Google Scholar]
- 88.Sommer MA, Wurtz RH. Composition and topographic organization of signals sent from the frontal eye field to the superior colliculus. J Neurophysiol. 2000;83(4):1979-2001. doi. 10.1152/jn.2000.83.4.1979 [DOI] [PubMed] [Google Scholar]
- 89.Gaymard B, Lynch J, Ploner CJ, Condy C, Rivaud-Pechoux S. The parieto-collicular pathway: anatomical location and contribution to saccade generation. Eur J Neurosci. 2003;17(7):1518-1526. doi. 10.1046/j.1460-9568.2003.02570.x [DOI] [PubMed] [Google Scholar]
- 90.Li X, Basso MA. Competitive stimulus interactions within single response fields of superior colliculus neurons. J Neurosci. 2005;25(49):11357-11373. doi. 10.1523/jneurosci.3825-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lovejoy LP, Krauzlis RJ. Changes in perceptual sensitivity related to spatial cues depends on subcortical activity. Proc Natl Acad Sci U S A. 2017;114(23):6122-6126. doi. 10.1073/pnas.1609711114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Goldberg ME, Wurtz RH. Activity of superior colliculus in behaving monkey. II. Effect of attention on neuronal responses. J Neurophysiol. 1972;35(4):560-574. doi. 10.1152/jn.1972.35.4.560 [DOI] [PubMed] [Google Scholar]
- 93.Gattass R, Desimone R. Responses of cells in the superior colliculus during performance of a spatial attention task in the macaque. Rev Bras Biol. 1996;56(Su 1 Pt 2):257-279. [PubMed] [Google Scholar]
- 94.Maunsell JHR. Neuronal mechanisms of visual attention. Annu Rev Vis Sci. 2015;1:373-391. doi. 10.1146/annurev-vision-082114-035431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gattass R, Soares JGM, Lima B. The role of the pulvinar in spatial visual attention. Adv Anat Embryol Cel Biol. 2018;225:57-60. doi. 10.1007/978-3-319-70046-5_12 [DOI] [PubMed] [Google Scholar]
- 96.Bogadhi AR, Katz LN, Bollimunta A, Leopold DA, Krauzlis RJ. Midbrain activity shapes high-level visual properties in the primate temporal cortex. Neuron. 2021;109(4):690-699.e5. doi. 10.1016/j.neuron.2020.11.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zenon A, Krauzlis RJ. Attention deficits without cortical neuronal deficits. Nature. 2012;489(7416):434-437. doi. 10.1038/nature11497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mysore SP, Knudsen EI. The role of a midbrain network in competitive stimulus selection. Curr Opin Neurobiol. 2011;21(4):653-660. doi. 10.1016/j.conb.2011.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Koch C, Ullman S. Shifts in selective visual attention: towards the underlying neural circuitry. Hum Neurobiol. 1985;4:219-227. [PubMed] [Google Scholar]
- 100.King WM, Schmidt JT. A cholinergic circuit intrinsic to optic tectum modulates retinotectal transmission via presynaptic nicotinic receptors. Ann N Y Acad Sci. 1991;627(1 Activity-Driv):363-367. doi. 10.1111/j.1749-6632.1991.tb25940.x [DOI] [PubMed] [Google Scholar]
- 101.Lee PH, Schmidt M, Hall WC. Excitatory and inhibitory circuitry in the superficial gray layer of the superior colliculus. J Neurosci. 2001;21(20):8145-8153. doi. 10.1523/jneurosci.21-20-08145.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Endo T, Yanagawa Y, Obata K, Isa T. Nicotinic acetylcholine receptor subtypes involved in facilitation of GABAergic inhibition in mouse superficial superior colliculus. J Neurophysiol. 2005;94(6):3893-3902. doi. 10.1152/jn.00211.2005 [DOI] [PubMed] [Google Scholar]
- 103.Aizawa H, Kobayashi Y, Yamamoto M, Isa T. Injection of nicotine into the superior colliculus facilitates occurrence of express saccades in monkeys. J Neurophysiol. 1999;82(3):1642-1646. doi. 10.1152/jn.1999.82.3.1642 [DOI] [PubMed] [Google Scholar]
- 104.Watanabe M, Kobayashi Y, Inoue Y, Isa T. Effects of local nicotinic activation of the superior colliculus on saccades in monkeys. J Neurophysiol. 2005;93(1):519-534. doi. 10.1152/jn.00558.2004 [DOI] [PubMed] [Google Scholar]
- 105.Stubblefield EA, Thompson JA, Felsen G. Optogenetic cholinergic modulation of the mouse superior colliculus in vivo. J Neurophysiol. 2015;114(2):978-988. doi. 10.1152/jn.00917.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Mishra Y, Mallick BN. Modulation of cholinergic, GABA-ergic and glutamatergic components of superior colliculus affect REM sleep in rats. Behav Brain Res. 2023;438:114177. doi. 10.1016/j.bbr.2022.114177 [DOI] [PubMed] [Google Scholar]
- 107.Comoli E, Das Neves Favaro P, Vautrelle N, Leriche M, Overton PG, Redgrave P. Segregated anatomical input to sub-regions of the rodent superior colliculus associated with approach and defense. Front Neuroanat. 2012;6:9. doi. 10.3389/fnana.2012.00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Evans DA, Stempel AV, Vale R, Ruehle S, Lefler Y, Branco T. A synaptic threshold mechanism for computing escape decisions. Nature. 2018;558(7711):590-594. doi. 10.1038/s41586-018-0244-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhou Z, Liu X, Chen S, et al. A VTA GABAergic neural circuit mediates visually evoked innate defensive responses. Neuron. 2019;103(3):473-488.e6. doi. 10.1016/j.neuron.2019.05.027 [DOI] [PubMed] [Google Scholar]
- 110.Li L, Feng X, Zhou Z, et al. Stress accelerates defensive responses to looming in mice and involves a locus coeruleus-superior colliculus projection. Curr Biol. 2018;28(6):859-871.e5. doi. 10.1016/j.cub.2018.02.005 [DOI] [PubMed] [Google Scholar]
- 111.Montardy Q, Zhou Z, Li L, et al. Dopamine modulates visual threat processing in the superior colliculus via D2 receptors. iScience. 2022;25(6):104388. doi. 10.1016/j.isci.2022.104388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lynch E, Dempsey B, Saleeba C, et al. Descending pathways from the superior colliculus mediating autonomic and respiratory effects associated with orienting behaviour. J Physiol. 2022;600(24):5311-5332. doi. 10.1113/jp283789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bellot E, Coizet V, Warnking J, Knoblauch K, Moro E, Dojat M. Effects of aging on low luminance contrast processing in humans. Neuroimage. 2016;139:415-426. doi. 10.1016/j.neuroimage.2016.06.051 [DOI] [PubMed] [Google Scholar]
- 114.Koller K, Rafal RD, Platt A, Mitchell ND. Orienting toward threat: contributions of a subcortical pathway transmitting retinal afferents to the amygdala via the superior colliculus and pulvinar. Neuropsychologia. 2019;128:78-86. doi. 10.1016/j.neuropsychologia.2018.01.027 [DOI] [PubMed] [Google Scholar]
- 115.Gerbella M, Caruana F, Rizzolatti G. Pathways for smiling, disgust and fear recognition in blindsight patients. Neuropsychologia. 2019;128:6-13. doi. 10.1016/j.neuropsychologia.2017.08.028 [DOI] [PubMed] [Google Scholar]
- 116.Ajina S, Pollard M, Bridge H. The superior colliculus and amygdala support evaluation of face trait in blindsight. Front Neurol. 2020;11:769. doi. 10.3389/fneur.2020.00769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kragel PA, Ceko M, Theriault J, et al. A human colliculus-pulvinar-amygdala pathway encodes negative emotion. Neuron. 2021;109(15):2404-2412.e5. doi. 10.1016/j.neuron.2021.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chanes L, Quentin R, Vernet M, Valero-Cabre A. Arrhythmic activity in the left frontal eye field facilitates conscious visual perception in humans. Cortex. 2015;71:240-247. doi. 10.1016/j.cortex.2015.05.016 [DOI] [PubMed] [Google Scholar]
- 119.Vernet M, Quentin R, Chanes L, Mitsumasu A, Valero-Cabré A. Frontal eye field, where art thou? Anatomy, function, and non-invasive manipulation of frontal regions involved in eye movements and associated cognitive operations. Front Integr Neurosci. 2014;8:66. doi. 10.3389/fnint.2014.00066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Dugger BN, Murray ME, Boeve BF, et al. Neuropathological analysis of brainstem cholinergic and catecholaminergic nuclei in relation to rapid eye movement (REM) sleep behaviour disorder. Neuropathol Appl Neurobiol. 2012;38(2):142-152. doi. 10.1111/j.1365-2990.2011.01203.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Armstrong RA, McKee AC, Cairns NJ. Pathology of the superior colliculus in chronic traumatic encephalopathy. Optom Vis Sci. 2017;94(1):33-42. doi. 10.1097/opx.0000000000000911 [DOI] [PubMed] [Google Scholar]
- 122.Shine JM, O'Callaghan C, Halliday GM, Lewis SJ. Tricks of the mind: visual hallucinations as disorders of attention. Prog Neurobiol. 2014;116:58-65. doi. 10.1016/j.pneurobio.2014.01.004 [DOI] [PubMed] [Google Scholar]
- 123.Terao Y, Fukuda H, Ugawa Y, Hikosaka O. New perspectives on the pathophysiology of Parkinson's disease as assessed by saccade performance: a clinical review. Clin Neurophysiol. 2013;124(8):1491-1506. doi. 10.1016/j.clinph.2013.01.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.van Stockum S, Macaskill MR, Myall D, Anderson TJ. A perceptual discrimination task abnormally facilitates reflexive saccades in Parkinson's disease. Eur J Neurosci. 2011;33(11):2091-2100. doi. 10.1111/j.1460-9568.2011.07697.x [DOI] [PubMed] [Google Scholar]
- 125.Rolland M, Carcenac C, Overton PG, Savasta M, Coizet V. Enhanced visual responses in the superior colliculus and subthalamic nucleus in an animal model of Parkinson's disease. Neuroscience. 2013;252:277-288. doi. 10.1016/j.neuroscience.2013.07.047 [DOI] [PubMed] [Google Scholar]
- 126.Terao Y, Fukuda H, Shirota Y, et al. Deterioration of horizontal saccades in progressive supranuclear palsy. Clin Neurophysiol. 2013;124(2):354-363. doi. 10.1016/j.clinph.2012.07.008 [DOI] [PubMed] [Google Scholar]
- 127.Shaikh AG, Factor SA, Juncos JL. Saccades in progressive supranuclear palsy - maladapted, irregular, curved, and slow. Mov Disord Clin Pract. 2017;4(5):671-681. doi. 10.1002/mdc3.12491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hutchinson M, Isa T, Molloy A, et al. Cervical dystonia: a disorder of the midbrain network for covert attentional orienting. Front Neurol. 2014;5:54. doi. 10.3389/fneur.2014.00054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Duggan O, Narasimham S, Govern EM, et al. A study of the midbrain network for covert attentional orienting in cervical dystonia patients using dynamic causal modelling. Annu Int Conf IEEE Eng Med Biol Soc. 2019;2019:3519-3522. doi. 10.1109/EMBC.2019.8857152 [DOI] [PubMed] [Google Scholar]