Abstract
Background and Objectives
The University of Pennsylvania Smell Identification Test (UPSIT) is commonly used to assess olfaction and screen for early detection of disorders including Parkinson (PD) and Alzheimer disease. Our objective was to develop updated percentiles, based on substantially larger samples than previous norms, to more finely discriminate age- and sex-specific UPSIT performance among ≥50-year-old adults who may be candidates for studies of prodromal neurodegenerative diseases.
Methods
The UPSIT was administered cross-sectionally to participants recruited between 2007–2010 and 2013–2015 for the Parkinson Associated Risk Syndrome (PARS) and Parkinson's Progression Markers Initiative (PPMI) cohort studies, respectively. Exclusion criteria included age <50 years and a confirmed or suspected PD diagnosis. Demographics, family history, and prodromal features of PD including self-reported hyposmia were collected. Normative data including mean, SDs, and percentiles were derived by age and sex.
Results
The analytic sample included 9,396 individuals (5,336 female and 4,060 male), aged 50–95 years, who were predominantly White, non-Hispanic US residents. UPSIT percentiles were derived and are provided across 7 age categories (50–54, 55–59, 60–64, 65–69, 70–74, 75–79, and ≥80 years) for female and male participants separately; relative to existing norms, subgroups included between 2.4 and 20 times as many participants. Olfactory function declined with age and was better among women than men; accordingly, the percentile corresponding to a given raw score varied markedly by age and sex. UPSIT performance was comparable among individuals with vs without first-degree family history of PD. Comparisons of self-reported hyposmia vs UPSIT percentiles indicated a strong association (χ2 p < 0.0001) but minimal agreement (Cohen simple kappa [95% CI]: = 0.32 [0.28–0.36] for female participants; 0.34 [0.30–0.38] for male participants).
Discussion
Updated age/sex-specific UPSIT percentiles are provided for ≥50-year-old adults who reflect a population likely to be recruited into studies of prodromal neurodegenerative diseases. Our findings highlight the potential advantages of evaluating olfaction relative to age and sex instead of in absolute terms (i.e., based on raw UPSIT scores) or based on subjective (i.e., self-reported) measures. This information addresses the need to support studies of disorders including PD and Alzheimer disease by providing updated normative data from a larger sample of older adults.
Trial Registration Information
NCT00387075 and NCT01141023.
Detection of olfactory dysfunction can be an accessible indicator for medical conditions that feature smell loss, including several important neurodegenerative conditions. For example, olfactory dysfunction has been demonstrated to have predictive utility for the early detection of Parkinson disease (PD).1,2 Similar findings have been shown for Alzheimer disease.3,4 More recently, abnormal olfaction has been identified as a diagnostic feature of COVID-19, caused by the SARS-CoV-2 coronavirus.5 Thus, simple and scalable olfactory testing has substantial utility across medicine, including for public health considerations.
The University of Pennsylvania Smell Identification Test (UPSIT) is frequently used to assess olfaction and is suitable to widespread distribution to the general public.6 Use of the UPSIT is particularly convenient due to the ease of administration; it can be self-administered by the individual, and test materials can be sent through the mail to the recipient and back to the researcher or clinician.7 Results from at-home UPSIT collection have previously been shown to be comparable to UPSIT data collected in the clinic.8
Originally characterized in 1984, the UPSIT is a 40-item scratch-and-sniff test developed following the recognition that a standardized method of assessment could be widely useful in the medical field.6 From the early experiments to develop and validate this assessment tool, the effect of demographic factors on UPSIT scores was recognized, with multiple regression analyses revealing relationships between both age and sex.6 More specifically, there is an age-related decline in olfactory function, not attributable to cognition, that becomes evident above approximately 60 years of age, and female individuals have been found to reliably, on average, have better olfactory function compared with male individuals.6
Previously reported guidelines use threshold values to assign a person's UPSIT score to an olfactory diagnosis; the classifications for adults include normosmia (UPSIT ≥34 in male vs ≥ 35 in female adults), mild microsmia (30–33 in male vs 31–34 in female adults), moderate microsmia (26–29 in male vs 26–30 in female adults), severe microsmia (19–25), and total anosmia (UPSIT ≤18).7,9 Notably, these classifications describe olfactory dysfunction in an absolute sense and, accordingly, make minimal adjustments for sex and none for age (among adults). Depending on the context, this approach presents difficulty of interpretation. For example, an UPSIT score of 25 is classified as severe microsmia both for an 80-year-old male individual and a 50-year-old female individual,9 yet, relative to age and sex, the former is far closer to normal, whereas the latter is considerably less common and may have different clinical implications, for example, in the setting of a screening test for prodromal neurodegenerative disease. This context is not represented by threshold values alone. When exploring olfactory dysfunction as an indicator of disease, such as PD, the ability to finely discriminate within the lower range of scores becomes important. This is unattainable when relying on threshold values and olfactory diagnostic categories only. A valuable alternative strategy is to use normative data expressed as percentiles.
The availability of normative data enables interpretation of a given test result in the context of the broader population. However, there are some limitations to the previously published norms9 when considering the utility of the UPSIT in the study of neurologic conditions that typically have an onset later in life. It is known that age significantly affects olfaction, but the older age categories are underrepresented in the cohorts used to generate existing norms, with sex-specific percentiles for every age category ≥50 years derived from fewer than 100 individuals. This relatively small sample size resulted in a lack of precision, particularly at the lower tail of the distribution (e.g., ≤10th percentile).9 In addition, these norms were reported over 25 years ago, and in that time, some key health behaviors affecting smell have likely changed. Specifically, smoking behavior has been consistently reported to affect olfaction starting from the original description of the UPSIT nearly 4 decades ago.6 Importantly, the prevalence of cigarette smoking within the US population has continued to decrease over this time period.10
Thus, to interpret UPSIT scores more precisely from individuals with, or at risk for, neurologic conditions such as PD, it is important to generate updated normative data from a sample that is larger in size and more closely reflects the demographic characteristics of PD patients. As mentioned above, because the UPSIT is easily adapted to distribution through the mail and self-administration at home, it is very well suited to large-scale studies and has been used in the large Parkinson Associated Risk Syndrome (PARS) and Parkinson's Progression Markers Initiative (PPMI) studies described here. The present study was designed to provide normative data for the UPSIT by age and sex using percentiles based on analyses of these 2, large, prospectively collected cohorts.
Methods
Participants
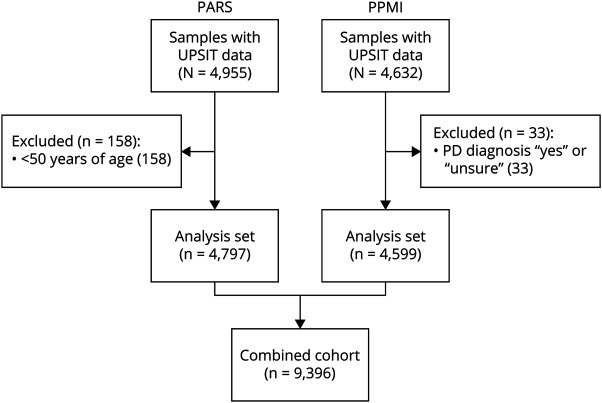
The study sample comprised participants from 2 distinct cohorts: PARS2 and PPMI (ppmi-info.org).11 The PARS study recruited participants using a community-based strategy that combined purchased mailing lists (including homeowners, nurses, and veterans from the Northeastern US), postings on PD-related websites (e.g., PARS study website, National Parkinson Foundation, and PatientsLikeMe) and direct recruitment of first-degree relatives by PD patients at 16 US-based movement disorder specialty clinics. Participants fell into 2 groups: one with a first-degree family history of PD and a second with no family history of PD. To be eligible, participants were required to not have any diagnosis of parkinsonism, other neurodegenerative disorder, or condition affecting olfaction (e.g., sinusitis). Of the 4,955 PARS participants who completed an UPSIT, a subset of 4,797 who were ≥50 years of age were selected for the analyses described herein; UPSIT scores from these participants were collected between May 2007 and June 2010.
Similarly, within PPMI, UPSIT data were collected from a community population of 4,632 individuals. Recruitment methods have been described previously.12 Briefly, strategies included centralized efforts coordinated by The Michael J. Fox Foundation (e.g., targeted social media ads, emails, events, and veterans mailing lists) and local outreach (e.g., local media ads and direct distribution of surveys at PPMI site outpatient clinics to friends/family of PD patients). Participants were aged 60 years or older and completed a prescreening survey to rule out diagnoses of parkinsonism or other neurodegenerative disorders, previous trauma to the nose or sinuses, or other sinus conditions affecting olfaction. From this sample, 33 participants were excluded because they responded either yes or unsure to a postscreening question asking if they had a diagnosis of parkinsonism. UPSIT scores were collected between March 2013 and September 2015 predominantly from throughout the United States (∼3% of participants were from outside of the United States, including Germany and Italy). Combining the PPMI participants (n = 4,599) with the selected PARS subset (n = 4,797) yielded a total analytic sample of 9,396 individuals.
Standard Protocol Approvals, Registrations, and Patient Consents
All study participants provided written informed consent to participate in the associated studies (i.e., PARS or PPMI). Both studies were conducted in accordance with the Declaration of Helsinki and the Good Clinical Practice guidelines after approval of the local ethics committees of the participating sites.
Olfactory Testing
Olfactory function was assessed in all study participants using the UPSIT, a standardized, forced-choice assessment of odor identification in which each participant is exposed sequentially to 40 odorants. For each odorant, the participant is asked to select among 4 choices to identify the odorant presented. Scoring of the UPSIT is based on the number of odorants that are correctly identified. Therefore, a lower score reflects worse olfactory function.6 The UPSIT was sent to all participants via mail to be completed at home; the test is amenable to self-administration outside of a health care setting due to the inclusion of easy-to-follow instructions. All participants completed the test as instructed and returned the results to the investigators by mail.
Self-Report Questionnaires
All study participants were provided self-report questionnaires to evaluate demographics and risk factors for PD. The PARS and PPMI studies administered different questionnaires; however, several items overlapped between protocols. For instance, participants in both studies self-reported age, sex assigned at birth, race, ethnicity, family history of PD or parkinsonism, bowel movement frequency, laxative use, and REM sleep behavior disorder (RBD) symptoms (e.g., violent movements during sleep). In addition, participants were asked to subjectively assess whether they had noticed a decrease in their sense of smell. Response options included yes, no, and unsure.
Statistical Analysis
Normative data were computed using the UNIVARIATE procedure in SAS v9.4 (SAS Institute Inc., Cary, NC; sas.com; RRID:SCR_008567). Descriptive statistics (mean, SDs, and percentiles) were derived separately by sex and across 7 age categories (50–54, 55–59, 60–64, 65–69, 70–74, 75–79, and ≥80 years). To calculate percentiles, the default method (based on an empirical distribution function with averaging) was applied. Several rules were implemented to address ambiguous scenarios. First, if a raw value corresponded to multiple percentile values, the median percentile value was selected. Second, if a given raw score was not assigned a percentile but fell in between 2 raw scores that were assigned percentiles, the upper and lower bordering percentiles were averaged and then rounded up to the nearest integer value. Finally, if the 100th percentile corresponded to a raw value below 40, all higher raw scores were also assigned to the 100th percentile, and, conversely, if a raw value fell below the one that corresponded to the 1st percentile, it was also assigned to the 1st percentile. Demographics were compared between cohorts, separately for male and female participants, using χ2 and t tests. Separately for female and male participants, the association between self-reported hyposmia and UPSIT percentile subgroup (≤10, 11–25, 26–50, >50) was measured using χ2 tests, and the agreement between self-reported hyposmia and UPSIT percentile (≤10 vs >10) was evaluated using Cohen simple kappa.13
Data Availability
PPMI is an open access data set; data used in the preparation of this manuscript and documentation of the self-report questionnaire were downloaded from the PPMI database (ppmi-info.org/access-data-specimens/download-data) on June 30, 2020. Study protocol and manuals are available at ppmi-info.org/study-design. For PARS, the protocol, self-report questionnaire, and a subset of the deidentified data may be shared at the request of any qualified investigator for purposes of replicating procedures and results.
Results
Characteristics of Study Participants
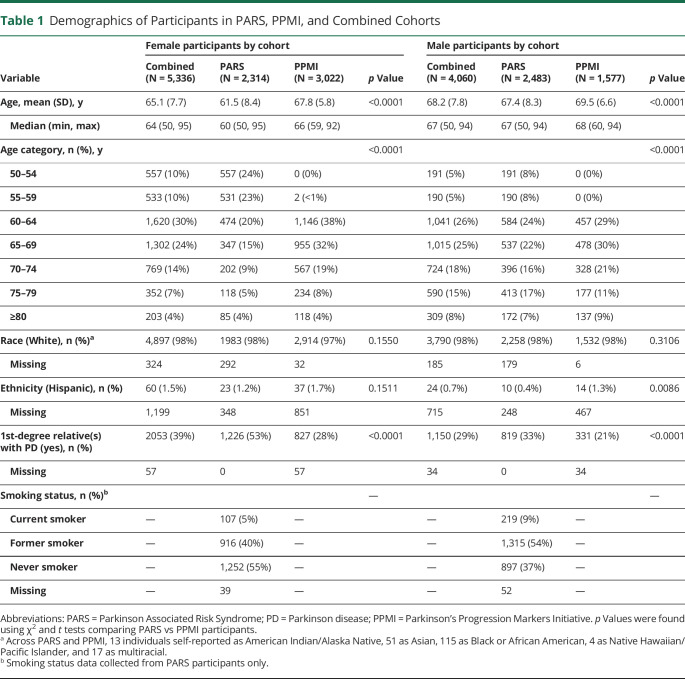
The inclusion of study participants from PARS and PPMI is characterized in Figure 1. Demographics are summarized in Table 1. The mean age was younger among PARS participants, both for female (61.5 in PARS vs 67.8 in PPMI) and male (67.4 vs 69.5) participants. Nearly all participants in the 50–59-year-old age range came from PARS. The cohorts were comparable with respect to race and ethnicity. Overall, participants were predominantly White (98%) and non-Hispanic (99%). A first-degree family history of PD was more common in the PARS cohort, both among female (53% in PARS vs 28% in PPMI) and male (33% vs 21%) participants. Smoking status data (collected in PARS only) indicated that just 5% of female participants and 9% of male participants were current smokers, although an additional 40% of female participants and 54% of male participants were former smokers. Regarding other prodromal features of PD (data not shown), 9.4% of female vs 6.5% of male participants self-reported regular (≥3 months) use of laxatives; 21% of female vs 13% of male participants endorsed constipation (operationalized as < 1 bowel movement per day); and 7.9% of female vs 20% of male participants reported RBD symptoms, as defined by a history of either violent, purposeful movements during sleep including grabbing, arm flailing, punching, kicking, sitting, jumping out of bed, crawling, or running (PARS question) or acting out one's dream during sleep as evidenced by punching, flailing your arms in the air, making running movements, etc. (PPMI question).
Figure 1. Flow Diagram of PARS and PPMI Participants Included in the Analytic Sample.
PARS = Parkinson Associated Risk Syndrome; PD = Parkinson disease; PPMI = Parkinson's Progression Markers Initiative; UPSIT = University of Pennsylvania Smell Identification Test.
Table 1.
Demographics of Participants in PARS, PPMI, and Combined Cohorts
UPSIT Percentiles
Percentiles derived from this data set are presented in Tables 2 and 3 for female and male participants, respectively. Percentiles for UPSIT scores are provided across 7 different age categories (50–54, 55–59, 60–64, 65–69, 70–74, 75–79, and ≥80 years). The corresponding percentile value for any UPSIT score can be identified from these tables using knowledge of a person's age and sex (eAppendix 1, links.lww.com/WNL/C662, includes a data file containing a separate row for every combination of age category, sex, and UPSIT score—which can be used to compute percentile values programmatically). Tables 2 and 3 also include mean (SD) raw score values for each age and sex category. In this total cohort of 4,060 men and 5,336 women, women had better olfactory function than men and olfactory function declined with age.
Table 2.
Age-Specific UPSIT Percentile Values for Female Participants
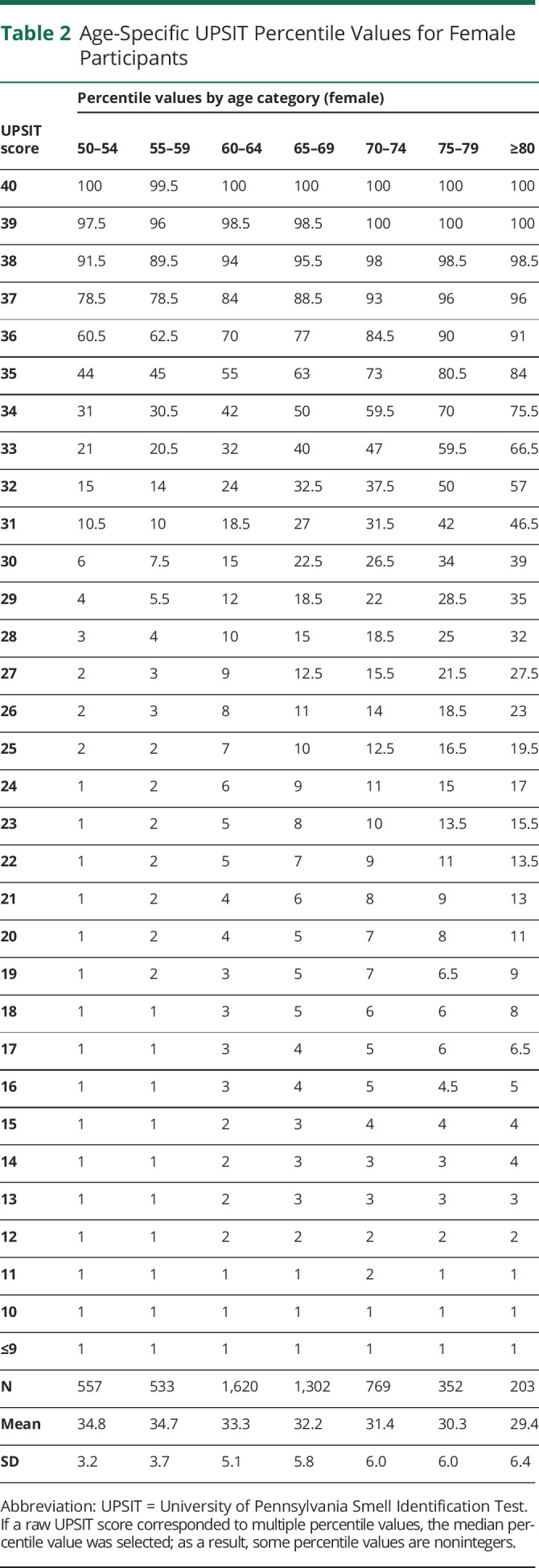
Table 3.
Age-Specific UPSIT Percentile Values for Male Participants
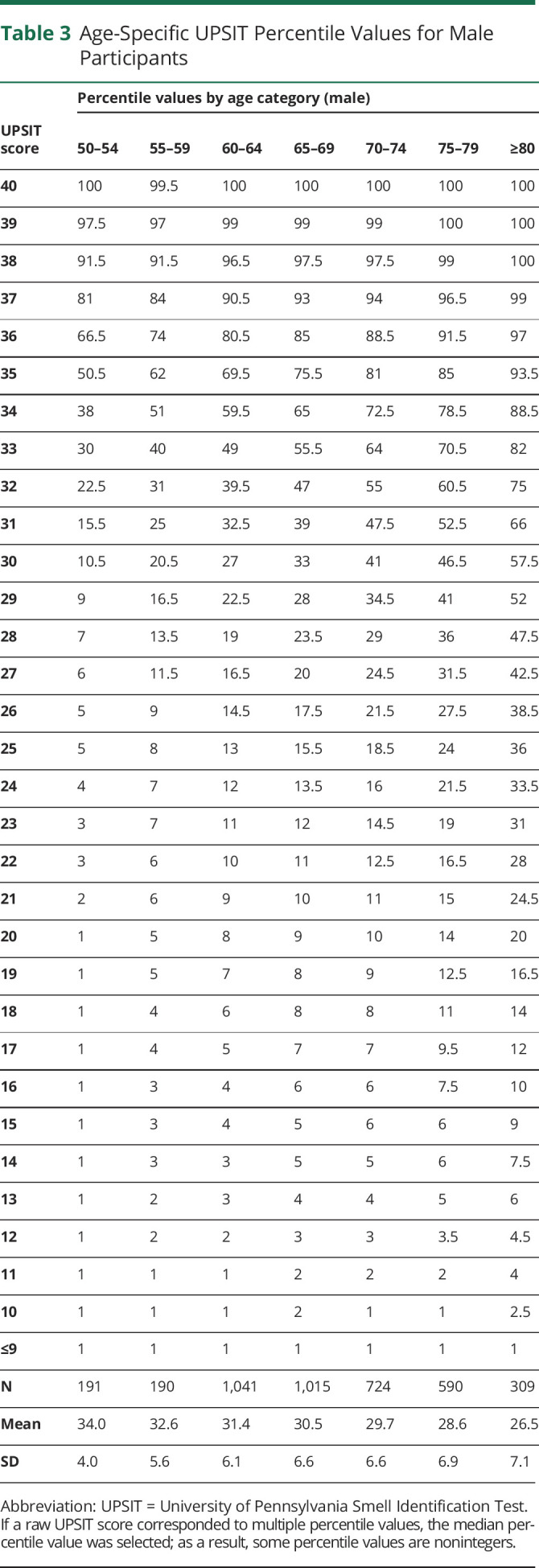
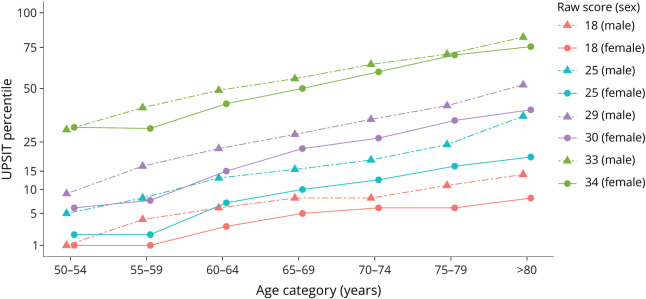
Figure 2 depicts the UPSIT percentile corresponding to selected raw scores, separately for female and male participants, within each age category. The raw values chosen (18, 25, 30, and 34 for female participants; 18, 25, 29, and 33 for male participants) reflect the upper thresholds of the score ranges defining olfactory diagnoses of total anosmia, severe microsmia, moderate microsmia, and mild microsmia, respectively.9 This visualization of the data from Tables 2 and 3 illustrates several points. If olfactory function did not differ by age or sex, the percentiles corresponding to each given threshold value would be flat across the age categories (i.e., parallel with the x-axis) and congruent among male vs female participants (e.g., a score of 29 would be at the same percentile across all age by sex categories rather than varying from the 4th percentile in 50–54-year-old female participants to the 52nd percentile among ≥80-year-old male participants). In fact, the percentiles increased with age and were higher for male participants than female participants. That is, a given threshold value tended to correspond to a better percentile for older (relative to younger) and male (relative to female) participants. It is also apparent that a given percentile aligns with highly variable raw scores depending on an individual's age and sex. For instance, an 80-year-old male participant with a raw score of 18 and a 60-year-old female participant with a raw score of 30—2 individuals whose olfactory function differs markedly in an absolute sense—are both near the 15th percentile relative to age and sex.
Figure 2. UPSIT Percentile by Raw Score, Age Category, and Sex.
Selected raw scores correspond to upper cutoffs for olfactory diagnoses of total anosmia (18), severe microsmia (25), moderate microsmia (29 for male and 30 for female participants), and mild microsmia (33 for male and 34 for female participants). UPSIT = University of Pennsylvania Smell Identification Test.
Self-Reported Sense of Smell
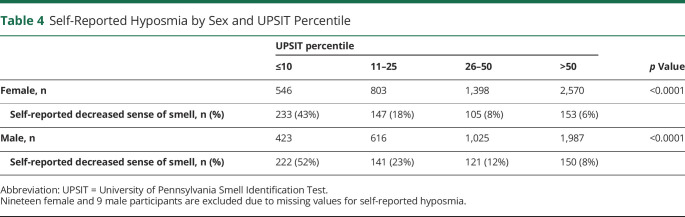
An impaired sense of smell was reported by 12% of female participants and 16% of male participants. As shown in Table 4, there was a significant association (χ2 p < 0.0001) between UPSIT performance and self-reported olfactory dysfunction, both for female and male participants, with participants in worse UPSIT percentile categories being more likely to subjectively endorse hyposmia. However, discordance between self-reported olfactory impairment and UPSIT was observed. For example, among those whose UPSIT score fell at or below the 10th percentile, only 43% of female and 52% of male participants self-reported an impairment in their sense of smell. Also, Cohen's simple kappa analyses of UPSIT percentile (dichotomized as ≤10th vs >10th) by self-reported hyposmia (yes vs no or unsure) yielded estimates of 0.32 (95% CI 0.28–0.36) for female participants and 0.34 (95% CI 0.30–0.38) for male participants, indicating a minimal level of agreement.13
Table 4.
Self-Reported Hyposmia by Sex and UPSIT Percentile
Family History of Parkinson Disease
As per Table 1, 34% (3,203/9,396) of the combined PARS/PPMI cohort reported a first-degree relative with PD. Given that first-degree family history is a risk marker for PD,14,15 we derived a second set of percentiles that excluded these participants (data not shown). Comparisons were limited by a small number of nonrelatives among male participants aged 50–54 (n = 22) and 55–59 (n = 56) years. Across the remaining age by sex categories, the results were comparable, with mean raw scores differing by no more than 0.3 points depending on whether first-degree relatives were included or excluded, and the raw score that delineated being at or below the 15th percentile matching in 6 cases and differing by 1 in the other 6 cases. Of note, where differences were evident, they tended to be in the direction of nonrelatives performing slightly worse than those with a family history of PD.
Discussion
We report on an analysis combining 2 large cohorts of community-dwelling volunteers who completed the UPSIT for the purpose of screening for hyposmia related to PD. Because the vast majority of respondents did not have incipient neurodegeneration but may carry an elevated risk for PD based on their participation in PARS or PPMI, the responses from these cohorts may be viewed as a very large sample of typical UPSIT results across a range of ages for both men and women who are believed to be representative of individuals likely to be recruited into studies of prodromal neurodegenerative disease. We have used these data sets to derive normative data for the UPSIT, reporting percentile values for older men and women with diverse presentation of prodromal features of PD. In addition, we have created lookup tables by age and sex for this population. This information addresses the need to support studies of disorders including PD and Alzheimer disease by providing updated normative data from a larger sample of older adults who would typically participate in this type of clinical research.
Our data represent a substantial methodological advance over existing normative data. Previously published norms were derived from nearly 4,000 participants; however, that sample included only 1,221 participants (544 male and 677 female) aged 50 years or above, and percentiles for all applicable age categories (50–54, 55–59 years, etc.) were derived from subgroups of less than 100 individuals (range: 57–98).9 In comparison, among overlapping age categories (i.e., 50–54, 55–59, 60–64, 65–69, 70–74, and 75–79 years), the percentiles described herein were derived from between 2.4 times (50–54-year-old male participants) and 20 times (65–69-year-old female participants) as many participants. These larger sample sizes enable a greater degree of precision, especially at the lower end of the distribution (e.g., ≤10th percentile). To illustrate this point, we cite a specific example. The original percentile values for 60–64-year-old male participants were derived from a sample of 68 individuals; here, a raw score of 17 corresponded to the 9th percentile, a score of 16 to the 5th percentile, and a score below 16 to the <5th percentile range.7,16 In the context of screening for incipient neurodegeneration, it is a limitation to only be able to partition this range in a small number of ways. By contrast, our percentiles were derived from a considerably larger cohort of 60–64-year-old male participants (n = 1,041; Table 3); here, the ≤10th percentile range can be subdivided into 10 categories (i.e., 1st–10th). A novel aspect of our work is this ability to finely discriminate within the range of percentiles that is of greatest interest clinically in neurodegenerative research.
Our analysis also addresses possible changes in response to the UPSIT that may have occurred since the time that normative data for this test were first developed.6 Importantly, the prevalence of cigarette smoking, which is known to affect olfactory performance,6,17,18 has changed substantially over time.10 Between 1985 and 2015, the estimated prevalence of current cigarette smoking in the US roughly halved, dropping from 33% to 17% among male individuals, and from 28% to 14% among female individuals.19,20 In our cohort, current smoking rates (available for PARS participants only) were 9% for male participants and 5% for female participants. Notably, an additional 54% of male and 40% of female participants in PARS reported former smoking; however, a 2017 meta-analysis reported that only current (and not former) smoking was associated with olfactory dysfunction.18
Furthermore, our results highlight the potential advantages of using age- and sex-specific UPSIT percentile values instead of thresholding methods based primarily on raw scores. The latter approach has been reported extensively in the literature. For instance, previous PPMI analyses have adapted existing olfactory diagnosis guidelines9 to classify participants into 3 categories: normosmia (UPSIT ≥34/35 male/female), hyposmia (19–33/34), and anosmia (≤18).12,21,22 However, our findings reported here extend that prior work by applying the updated norms expressed in the percentile lookup tables, which suggest that classifying smell dysfunction using olfactory diagnoses alone may be biased toward overcounting older (particularly male) individuals and undercounting younger (particularly female) individuals.
A recent study that used our percentiles to assign a cohort of 162 LRRK2 G2019S PD participants to 3 clusters of olfactory performance reported that the worst-performing UPSIT subgroup (mean [SD] percentile: 4.8 [3.2]) had an earlier age at PD onset and more rapid motor progression than those with better olfactory performance.23 Notably, a previous cross-sectional analysis of 126 individuals from the same LRRK2 G2019S PD cohort, which defined olfactory clusters using raw UPSIT scores, did not observe a significant association with age at onset.24 In tandem, these results suggest that stratification based on percentiles may yield greater discriminatory value than methods using raw scores alone.
The UPSIT percentiles reported herein are not only an advancement beyond the threshold values previously used, but also provide an advantage over self-reported loss of sense of smell. To illustrate, among those who fell at or below the 10th percentile on the UPSIT, only approximately half of respondents self-reported impaired smell. The discordance observed between self-report and the UPSIT result supports the need for use of standardized, validated assessment tools in the clinic.
Although this study benefits from a very large study population from which the percentiles were derived, some limitations must be noted. First, a large majority of the population is from the United States (100% of PARS and ∼97% of PPMI), White (98%), and non-Hispanic (99%). Therefore, it is possible that the data presented here are not generalizable to populations outside of the United States or to non-White or Hispanic populations. For instance, previous studies have reported cross-cultural differences in odorant recognition25-28 and differences by race and ethnicity in the prevalence of olfactory dysfunction in the United States.29,30 An important focus of future research would be to learn more about olfaction in the broader population by recruiting a more culturally, racially, and ethnically diverse sample. Second, the study population was a combination of 2 population-based studies focused on investigating risk factors for PD, recruitment strategies included outreach to the wider PD community (e.g., postings on PD-related websites and distribution of surveys to friends/family of PD patients), and participants reported the presence of several prodromal features at various frequencies. For these reasons, it must be acknowledged that this cohort cannot be assumed to be a purely unselected population. As reported in Table 1, 39% of female participants and 29% of male participants in the combined cohort reported first-degree relatives with PD. In a sensitivity analysis, percentiles were also derived after exclusion of study participants who reported a first-degree relative with PD; importantly, this exercise yielded only a small effect on the percentile rankings. In addition, of the nearly 5,000 PARS participants who completed UPSITs, a subgroup of 203/669 individuals with hyposmia (defined as ≤15th percentile) consented to a longitudinal clinical imaging protocol,8 185/203 subsequently completed at least 1 follow-up visit, and 26/185 clinically converted to manifest disease (25 to PD and 1 to dementia with Lewy bodies) over a mean (SD) follow-up period of 6.3 (2.2) years.31 In the case of PPMI, similar longitudinal data are currently being collected on an enriched subgroup of 26 participants with hyposmia (of the 4,632 who completed UPSITs).12 However, in certain contexts (e.g., studies aimed at identifying at-risk individuals for neurodegenerative diseases), it may be desirable to have a reference population that includes individuals with various prodromal features. Third, the UPSIT was revised in 2020 to modify some of the odorants and response distractors, whereas the scores and percentiles collected in this study reflect the originally described UPSIT.6 Because the revised UPSIT is still relatively new and not yet in widespread use (given limitations to translation availability), the majority of data sets that would be accessible to investigate the role of olfaction in identifying risk for PD would have used the original UPSIT. Many important ongoing longitudinal cohort studies have used and/or continue to use the original version, including PPMI, the LRRK2 Cohort Consortium, the NINDS Parkinson's Disease Biomarkers Program, and PREDICT-PD.24,32,33 These all reflect rich data sets that will continue to be investigated for quite some time to come, making our development of percentile lookup tables based on the original instrument still valuable today and continuing into the foreseeable future. In addition, new research is underway by the authors to determine percentiles for the 2020 revised UPSIT and to compare data for the same individuals with both versions of the UPSIT to evaluate performance differences. It should also be noted that although smell identification, such as with the UPSIT, may be the most commonly used assessment of olfaction in the clinic, there are also other methods of olfactory assessment such as threshold testing or smell discrimination that are used in research and clinical settings that have not been explored in this study.7
The potential for nonresponse bias should also be noted. In PARS, 53% of eligible participants completed and returned an UPSIT, and relative to nonresponders, completers were more likely to be younger, female, White, have a family member with PD, and not report a decreased sense of smell.2 In PPMI, approximately 60% of those eligible returned an UPSIT; however, comparable comparisons between responders and nonresponders could not be performed. Finally, our conclusions regarding the prevalence of smoking in this combined cohort are limited by these data being available for PARS participants only. This also hinders our ability to evaluate whether smoking, which is associated with an increased risk of olfactory dysfunction but a decreased risk of PD,14,15 could be an important modifier in our results. An interesting area of future research would be to investigate more fully the interactions between smoking behavior, olfaction, and PD risk.
The UPSIT is currently being deployed in the ongoing PPMI study with the goal of reaching up to 100,000 respondents (ppmi-info.org). For this effort, and consistent with the PARS study,2 hyposmia is being operationally defined by an UPSIT percentile at or below the 15th percentile for age and sex. Evaluation of alternative cutoffs could be an area of future research.
The work reported herein provides information to further support the interpretation of this convenient olfactory assessment tool in PD, as well as in various other conditions affecting people sharing the demographics of the combined cohort described in this study. It is the hope that the lookup tables provided within this article will enable researchers to perform refined investigations of existing datasets to more deeply explore olfactory function and its connection to neurodegenerative disease.
Acknowledgment
The authors are grateful to the many individuals who sacrificed their time and gave of themselves to be participants in the PARS and PPMI studies. PARS Investigators and Coordinators: David Russell, MD, Abby Fiocco, Institute for Neurodegenerative Disorders, New Haven, CT; Kapil Sethi, MD, Paula Jackson, Medical College of Georgia, Augusta, GA; Samuel Frank, MD, Anna Hohler, MD, Cathi A. Thomas MS, RN, Raymond C. James, Boston University Medical Center, Boston, MA; Tanya Simuni, MD, Emily Borushko, MPH, Northwestern University, Chicago, IL; Matt Stern, MD, Jacqueline Rick, PhD, University of Pennsylvania PDMDC, Philadelphia, PA; Robert Hauser, MD, Leyla Khavarian, University of South Florida, Tampa, FL; Irene Richard, MD, Cheryl Deely, University of Rochester, Rochester, NY; Grace S. Liang, MD, Liza Reys, The Parkinson's Institute, Sunnyvale, CA; Charles H. Adler, MD, PhD, Amy K. Duffy, Mayo Clinic Arizona, Scottsdale, AZ; Rachel Saunders-Pullman, MD, MPH, Beth Israel Medical Center, New York, NY; Marian L. Evatt, MD, Becky McMurray, RN, Linda McGinn, RN, Emory University, Atlanta, GA; Eugene Lai, MD, Shawna Johnson RN, BSN, Michael E. DeBakey, Department of Veteran's Affairs Medical Center, Houston, TX; Indu Subramanian, MD, Angelina Gratiano, UCLA Medical Center, Los Angeles, CA; Kathryn Chung, MD, Brenna Lobb, Susan O'Conner, Portland VA Medical Center, Portland, OR. PPMI Executive Steering Committee: Kenneth Marek, MD (Principal Investigator), Institute for Neurodegenerative Disorders, New Haven, CT; Caroline Tanner, MD, PhD, University of California, San Francisco, CA; Tanya Simuni, MD, Northwestern University, Chicago, IL; Andrew Siderowf, MD, MSCE, University of Pennsylvania, Philadelphia, PA; Douglas Galasko, MD, University of California, San Diego, CA; Lana Chahine, MD, University of Pittsburgh, Pittsburgh, PA; Christopher Coffey, PhD, University of Iowa, Iowa City, IA; Kalpana Merchant, PhD, TransThera Consulting; Kathleen Poston, MD, Stanford University, Stanford, CA; Roseanne Dobkin, PhD, Rutgers University, Piscataway, NJ; Tatiana Foroud, PhD, Indiana University, Indianapolis, IN; Brit Mollenhauer, MD, Paracelsus-Elena Klinik, Kassel, Germany; Dan Weintraub, MD, University of Pennsylvania, Philadelphia, PA; Ethan Brown, MD, University of California, San Francisco, CA; Karl Kieburtz, MD, MPH, University of Rochester, Rochester, NY. PPMI Steering Committee: Duygu Tosun-Turgut, PhD, University of California, San Francisco, CA; Werner Poewe, MD, Innsbruck Medical University, Innsbruck, Austria; Susan Bressman, MD, Mount Sinai Beth Israel, New York, NY; Jan Hamer, Indiana University, Indianapolis, IN; Raymond James, RN, Boston University, Boston, MA; Ekemini Riley, PhD, Center for Strategy Philanthropy at Milken Institute, Washington D.C.; John Seibyl, MD, Institute for Neurodegenerative Disorders, New Haven, CT; Leslie Shaw, PhD, University of Pennsylvania, Philadelphia, PA; David Standaert, MD, PhD, University of Alabama at Birmingham, Birmingham, AL; Sneha Mantri, MD, MS, Duke University, Durham, NC; Nabila Dahodwala, MD, University of Pennsylvania, Philadelphia, PA; Michael Schwarzschild, MD, PhD, Massachusetts General Hospital, Boston, MA; Connie Marras, MD, PhD, FRCP(C), Toronto Western Hospital, Toronto, Canada; Hubert Fernandez, MD, Cleveland Clinic, Cleveland, OH; Ira Shoulson, MD, University of Rochester, Rochester, NY; Helen Rowbotham, 23andMe; Lucy Norcliffe-Kaufmann, 23andMe; Claudia Trenkwalder, MD, Paracelsus-Elena Klinik, Kassel, Germany. Michael J. Fox Foundation (Sponsor): Todd Sherer, PhD; Sohini Chowdhury; Mark Frasier, PhD; Jamie Eberling, PhD; Katie Kopil, PhD; Alyssa O'Grady; James Gibaldi, MSc; Maggie McGuire Kuhl; Leslie Kirsch, EdD. PPMI Site Investigators and Coordinators: Ruth Schneider, MD, Anisha Singh, BS, University of Rochester, Rochester, NY; Kelvin Chou, MD, Angela Stovall, BS, University of Michigan, Ann Arbor, MI; David Russell, MD, PhD, Julie Festa, BA, Lianne Ramia, BS, Institute for Neurodegenerative Disorders, New Haven, CT; Stewart Factor, DO, Barbara Sommerfeld MSN, RN, CNRN, Emory University of Medicine, Atlanta, GA; Penelope Hogarth, MD, Katrina Wakeman, BS, Oregon Health and Science University, Portland, OR; Robert Hauser, MD, MBA, Fnu Madhuri, MS, University of South Florida, Tampa, FL; Nabila Dahodwala, MD, MSc, Ashwini Ramachandran, MSc, University of Pennsylvania, Philadelphia, PA; Marie H Saint-Hilaire, MD, FRCPC, FAAN, Raymond James, BS, RN, Boston University, Boston, MA; David Shprecher, DO, Kelly Clark, Banner Sun Health Research Institute, Sun City, AZ; Hubert Fernandez, MD, Jennifer Mule, BS, Cleveland Clinic, Cleveland, OH; Kathrin Brockmann, MD, Ella Hilt, University of Tuebingen, Tuebingen, Germany; Yen Tai, MD, PhD, Raquel Lopes, BSN, MS, Imperial College of London, London, UK; Paolo Barone, MD, PhD, Susan Ainscough, BA, University of Salerno, Salerno, Italy; Stuart Isaacson, MD, Lisbeth Pennente, BA, Parkinson's Disease and Movement Disorders Center, Boca Raton, FL; Alberto Espay, MD, MSc, FAAN, FANA, Julia Brown, BS, Christina Gruenwald, BS, CCRP, University of Cincinnati, Cincinnati, OH; Maria Jose Martí, MD, PhD, Eduardo Tolosa, MD, PhD, Alicia Garrido, MD, Hospital Clinic of Barcelona, Barcelona, Spain; Shu-Ching Hu, MD, PhD, Krista Specketer, BS, University of Washington, Seattle, WA; Douglas Galasko, MD, Shawnees Peacock, BS, University of California, San Diego, CA; Emile Moukheiber, MD, Kori Ribb, RN, BSN, CNRN, Johns Hopkins University, Baltimore, MD; Jean-Christophe Corvol, MD, Stephanie Carvalho, University Hospitals Pitié Salpêtrière, Paris, France; Nir Giladi, MD, Shira Paz, BS, Tel Aviv Sourasky Medical Center, Tel Aviv, Israel; Javier Ruiz Martinez, MD, PhD, Ioana Croitoru, Hospital Universitario Donostia, San Sebastian, Spain; Jan O. Aasly, MD, Anne Grete Kristiansen, St. Olav's University Hospital, Trondheim, Norway; Leonidas Stefanis, MD, PhD, Christos Koros, MD, PhD, National and Kapodistrian University of Athens, Athens, Greece; Karen Marder, MD, MPH, Helen Mejia Santana, MA, Columbia University Irving Medical Center, New York, NY; Arjun Tarakad, MD, Anjana Singh, BS, Baylor College of Medicine, Houston, TX; Connie Marras, MD, PhD, FRCP(C), Danica Nogo, BS, Toronto Western Hospital, Toronto, Canada; Tiago Mestre, MD, PhD, Shawna Reddie, BA, The Ottawa Hospital, Ottawa, Canada; Aleksandar Videnovic, MD, MSc, Samantha Murphy, BS, Massachusetts General Hospital, Boston, MA; Rajesh Pahwa, MD, Lauren O'Brien, University of Kansas Medical Center, Kansas City, KS; Mark Lew, MD, Daniel Freire, MS, University of Southern California, Los Angeles, CA; Holly Shill, MD, Farah Ismail, MBChB, Barrow Neurological Institute, Phoenix, AZ; Amy Amara, MD, PhD; Courtney Blair, MA, University of Alabama at Birmingham, Birmingham, AL; Charles Adler, MD, PhD, Tom Osgood, BA, CCRP, Mayo Clinic Arizona, Scottsdale, AZ; Caroline Tanner, MD, PhD, Farah Kausar, PhD, University of California, San Francisco, CA; Susan Bressman, MD, Deborah Raymond, MS, CGC, Mount Sinai Beth Israel, New York, NY; Tanya Simuni, MD, Karen Williams, BA, CCRP, Heidi Friedeck, BS, Northwestern University, Chicago, IL; Maureen Leehey, MD, Jenny Frisendahl, BS, Ying Liu, MD, University of Colorado, Aurora, CO; Giulietta Riboldi, MD, Caitlin Romano, BA, NYU Langone Medical Center, New York, NY; Nikolaus McFarland, MD, PhD, FAAN, Kyle Rizer, BA, University of Florida, Gainesville, FL; Lana Chahine, MD, Sherri Mosovsky, MPH, University of Pittsburgh, Pittsburgh, PA; Ron Postuma, MD, FRCPC, Farah Sulaiman, MPH, Montreal Neurological Institute and Hospital/McGill, Montreal, QC, Canada; Brit Mollenhauer, MD, Diana Willeke, Paracelsus-Elena Klinik, Kassel, Germany; Werner Poewe, MD, Dora Valent, MS, Innsbruck Medical University, Innsbruck, Austria; Zoltan Mari, MD, Michelle Torreliza, AS, Cleveland Clinic-Las Vegas Lou Ruvo Center for Brain Health, Las Vegas, NV; Nicola Pavese, MD, PhD, Victoria Kate Foster, Clinical Ageing Research Unit, Newcastle, UK; Michele Hu, MD, PhD, Jamil Razzaque, MS, John Radcliffe Hospital Oxford and Oxford University, Oxford, UK; Norbert Brüggemann, MD, Christine Klein, MD, FEAN, Madita Grümmer, Universität Lübeck, Luebeck, Germany; Bastiaan Bloem, MD, PhD, Myrthe Burgler, MA, Sabine van Zundert, MS, Radboud University, Nijmegen, Netherlands.
Appendix. Authors

Study Funding
Support for the Parkinson Associated Risk Syndrome (PARS) study is provided by the Department of Defense award number W81XWH-06-067. PPMI—a public-private partnership—is funded by the Michael J. Fox Foundation for Parkinson's Research funding partners 4D Pharma, AbbVie, Acurex Therapeutics, Allergan, Amathus Therapeutics, Aligning Science Across Parkinson's, Avid Radiopharmaceuticals, Bial Biotech, Biogen, BioLegend, Bristol-Myers Squibb, Calico, Celgene, DaCapo Brainscience, Denali, The Edmond J. Safra Foundation, GE Healthcare, Genentech, GlaxoSmithKline, Golub Capital, Handl Therapeutics, Insitro, Janssen Neuroscience, Lilly, Lundbeck, Merck, Meso Scale Discovery, Neurocrine Biosciences, Pfizer, Piramal, Prevail, Roche, Sanofi Genzyme, Servier, Takeda, Teva, UCB, Verily, and Voyager Therapeutics.
Disclosure
The authors report no relevant disclosures. Go to Neurology.org/N for full disclosures.
References
- 1.Ross GW, Petrovitch H, Abbott RD, et al. Association of olfactory dysfunction with risk for future Parkinson's disease. Ann Neurol. 2008;63(2):167-173. doi: 10.1002/ana.21291 [DOI] [PubMed] [Google Scholar]
- 2.Siderowf A, Jennings D, Eberly S, et al. Impaired olfaction and other prodromal features in the Parkinson At-Risk Syndrome Study. Mov Disord. 2012;27(3):406-412. doi: 10.1002/mds.24892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Devanand DP, Lee S, Manly J, et al. Olfactory deficits predict cognitive decline and Alzheimer dementia in an urban community. Neurology 2015;84(2):182-189. doi: 10.1212/wnl.0000000000001132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woodward MR, Amrutkar CV, Shah HC, et al. Validation of olfactory deficit as a biomarker of Alzheimer disease. Neurol Clin Pract. 2017;7(1):5-14. doi: 10.1212/cpj.0000000000000293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whitcroft KL, Hummel T. Olfactory dysfunction in COVID-19: diagnosis and management. JAMA 2020;323(24):2512-2514. doi: 10.1001/jama.2020.8391 [DOI] [PubMed] [Google Scholar]
- 6.Doty RL, Shaman P, Dann M. Development of the University of Pennsylvania Smell Identification Test: a standardized microencapsulated test of olfactory function. Physiol Behav. 1984;32(3):489-502. doi: 10.1016/0031-9384(84)90269-5 [DOI] [PubMed] [Google Scholar]
- 7.Doty RL. Psychophysical testing of smell and taste function. Handb Clin Neurol. 2019;164:229-246. doi: 10.1016/B978-0-444-63855-7.00015-0 [DOI] [PubMed] [Google Scholar]
- 8.Jennings D, Siderowf A, Stern M, et al. Imaging prodromal Parkinson disease: the Parkinson associated risk Syndrome study. Neurology 2014;83(19):1739-1746. doi: 10.1212/wnl.0000000000000960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Doty RL. The Smell Identification Test: Administration Manual, 3rd ed. Sensonics, Inc.; 1995. [Google Scholar]
- 10.United States Public Health Service Office of the Surgeon G, National Center for Chronic Disease P, Health Promotion Office on S, Health. Publications and Reports of the Surgeon General. Smoking Cessation: A Report of the Surgeon General: US Department of Health and Human Services; 2020. [Google Scholar]
- 11.Marek K, Chowdhury S, Siderowf A, et al. The Parkinson's progression markers initiative (PPMI) - establishing a PD biomarker cohort. Ann Clin Transl Neurol. 2018;5(12):1460-1477. doi: 10.1002/acn3.644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chahine LM, Urbe L, Caspell-Garcia C, et al. Cognition among individuals along a spectrum of increased risk for Parkinson's disease. PLoS One. 2018;13(8):e0201964. doi: 10.1371/journal.pone.0201964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McHugh ML. Interrater reliability: the kappa statistic. Biochem Med (Zagreb). 2012;22(3):276-282. doi: 10.11613/bm.2012.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berg D, Postuma RB, Adler CH, et al. MDS research criteria for prodromal Parkinson's disease. Mov Disord. 2015;30(12):1600-1611. doi: 10.1002/mds.26431 [DOI] [PubMed] [Google Scholar]
- 15.Heinzel S, Berg D, Gasser T, Chen H, Yao C, Postuma RB. Update of the MDS research criteria for prodromal Parkinson's disease. Mov Disord. 2019;34(10):1464-1470. doi: 10.1002/mds.27802 [DOI] [PubMed] [Google Scholar]
- 16.Doty RL, Bromley SM, Stern MB. Olfactory testing as an aid in the diagnosis of Parkinson's disease: development of optimal discrimination criteria. Neurodegeneration 1995;4(1):93-97. doi: 10.1006/neur.1995.0011 [DOI] [PubMed] [Google Scholar]
- 17.Frye RE, Schwartz BS, Doty RL. Dose-related effects of cigarette smoking on olfactory function. JAMA 1990;263(9):1233-1236. doi: 10.1001/jama.1990.03440090067028 [DOI] [PubMed] [Google Scholar]
- 18.Ajmani GS, Suh HH, Wroblewski KE, Pinto JM. Smoking and olfactory dysfunction: a systematic literature review and meta-analysis. Laryngoscope 2017;127(8):1753-1761. doi: 10.1002/lary.26558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Centers for Disease Control. Current Trends Smoking and Health: A National Status Report. MMWR Morb Mortal Wkly Rep. 1986;35(46):709-711. [PubMed] [Google Scholar]
- 20.Phillips E, Wang TW, Husten CG, et al. Tobacco product use among adults - United States, 2015. MMWR Morb Mortal Wkly Rep. 2017;66(44):1209-1215. doi: 10.15585/mmwr.mm6644a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simuni T, Caspell-Garcia C, Coffey CS, et al. Baseline prevalence and longitudinal evolution of non-motor symptoms in early Parkinson's disease: the PPMI cohort. J Neurol Neurosurg Psychiatry. 2018;89(1):78-88. doi: 10.1136/jnnp-2017-316213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simuni T, Uribe L, Cho HR, et al. Clinical and dopamine transporter imaging characteristics of non-manifest LRRK2 and GBA mutation carriers in the Parkinson's Progression Markers Initiative (PPMI): a cross-sectional study. Lancet Neurol. 2020;19(1):71-80. doi: 10.1016/S1474-4422(19)30319-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saunders-Pullman R, Ortega RA, Wang C, et al. Association of olfactory performance with motor decline and age at onset in people with Parkinson disease and the LRRK2 G2019S variant. Neurology 2022;99(8):e814–e823. doi: 10.1212/wnl.0000000000200737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saunders-Pullman R, Mirelman A, Wang C, et al. Olfactory identification in LRRK2 G2019S mutation carriers: a relevant marker?. Ann Clin Transl Neurol. 2014;1(9):670-678. doi: 10.1002/acn3.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yucepur C, Ozucer B, Degirmenci N, Yildirim Y, Veyseller B, Ozturan O. University of Pennsylvania smell identification test: application to Turkish population. Kulak Burun Bogaz Ihtis Derg. 2012;22(2):77-80. doi: 10.5606/kbbihtisas.2012.014 [DOI] [PubMed] [Google Scholar]
- 26.Fornazieri MA, Doty RL, dos Santos CA, de Rezende Pinna F, Bezerra TFP, Voegels RL. A new cultural adaptation of the university of Pennsylvania smell identification test. Clinics (Sao Paulo). 2013;68(1):65-68. doi: 10.6061/clinics/2013(01)oa10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muirhead N, Benjamin E, Saleh H. Is the university of Pennsylvania smell identification test (UPSIT) valid for the UK population. Otorhinolaryngologist 2013;6:99-103. [Google Scholar]
- 28.Rodriguez-Violante M, Gonzalez-Latapi P, Camacho-Ordonez A, Martinez-Ramirez D, Morales-Briceno H, Cervantes-Arriaga A. Comparing the accuracy of different smell identification tests in Parkinson's disease: relevance of cultural aspects. Clin Neurol Neurosurg. 2014;123:9-14. doi: 10.1016/j.clineuro.2014.04.030 [DOI] [PubMed] [Google Scholar]
- 29.Pinto JM, Schumm LP, Wroblewski KE, Kern DW, McClintock MK. Racial disparities in olfactory loss among older adults in the United States. Journals Gerontol Ser A. 2014;69A(3):323-329. doi: 10.1093/gerona/glt063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu G, Zong G, Doty RL, Sun Q. Prevalence and risk factors of taste and smell impairment in a nationwide representative sample of the US population: a cross-sectional study. BMJ Open. 2016;6(11):e013246. doi: 10.1136/bmjopen-2016-013246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Siderowf A, Jennings D, Stern M, et al. Clinical and imaging progression in the PARS cohort: long-term follow-up. Mov Disord. 2020;35(9):1550-1557. doi: 10.1002/mds.28139 [DOI] [PubMed] [Google Scholar]
- 32.Noyce AJ, Bestwick JP, Silveira-Moriyama L, et al. PREDICT-PD: identifying risk of Parkinson's disease in the community: methods and baseline results. J Neurol Neurosurg Psychiatry. 2014;85(1):31-37. doi: 10.1136/jnnp-2013-305420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosenthal LS, Drake D, Alcalay RN, et al. The NINDS Parkinson's disease biomarkers program. Mov Disord. 2016;31(6):915-923. doi: 10.1002/mds.26438 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
PPMI is an open access data set; data used in the preparation of this manuscript and documentation of the self-report questionnaire were downloaded from the PPMI database (ppmi-info.org/access-data-specimens/download-data) on June 30, 2020. Study protocol and manuals are available at ppmi-info.org/study-design. For PARS, the protocol, self-report questionnaire, and a subset of the deidentified data may be shared at the request of any qualified investigator for purposes of replicating procedures and results.