PURPOSE
CheckMate 651 (ClinicalTrials.gov identifier: NCT02741570) evaluated first-line nivolumab plus ipilimumab versus EXTREME (cetuximab plus cisplatin/carboplatin plus fluorouracil ≤ six cycles, then cetuximab maintenance) in recurrent/metastatic squamous cell carcinoma of the head and neck (R/M SCCHN).
METHODS
Patients without prior systemic therapy for R/M SCCHN were randomly assigned 1:1 to nivolumab plus ipilimumab or EXTREME. Primary end points were overall survival (OS) in the all randomly assigned and programmed death-ligand 1 combined positive score (CPS) ≥ 20 populations. Secondary end points included OS in the programmed death-ligand 1 CPS ≥ 1 population, and progression-free survival, objective response rate, and duration of response in the all randomly assigned and CPS ≥ 20 populations.
RESULTS
Among 947 patients randomly assigned, 38.3% had CPS ≥ 20. There were no statistically significant differences in OS with nivolumab plus ipilimumab versus EXTREME in the all randomly assigned (median: 13.9 v 13.5 months; hazard ratio [HR], 0.95; 97.9% CI, 0.80 to 1.13; P = .4951) and CPS ≥ 20 (median: 17.6 v 14.6 months; HR, 0.78; 97.51% CI, 0.59 to 1.03; P = .0469) populations. In patients with CPS ≥ 1, the median OS was 15.7 versus 13.2 months (HR, 0.82; 95% CI, 0.69 to 0.97). Among patients with CPS ≥ 20, the median progression-free survival was 5.4 months (nivolumab plus ipilimumab) versus 7.0 months (EXTREME), objective response rate was 34.1% versus 36.0%, and median duration of response was 32.6 versus 7.0 months. Grade 3/4 treatment-related adverse events occurred in 28.2% of patients treated with nivolumab plus ipilimumab versus 70.7% treated with EXTREME.
CONCLUSION
CheckMate 651 did not meet its primary end points of OS in the all randomly assigned or CPS ≥ 20 populations. Nivolumab plus ipilimumab showed a favorable safety profile compared with EXTREME. There continues to be a need for new therapies in patients with R/M SCCHN.
INTRODUCTION
Squamous cell carcinomas of the head and neck (SCCHN) are common worldwide, with most patients presenting with advanced disease.1,2 Key risk factors are heavy consumption of alcohol and tobacco and human papillomavirus (HPV) infection, which, when detected in tumors, is associated with a favorable prognosis for oropharyngeal SCCHN.3 Overall, > 50% of patients with locally advanced SCCHN treated with multimodal approaches develop recurrence or metastases within 3 years of curative-intent treatment completion.4,5 Recurrent/metastatic (R/M) SCCHN is associated with poor prognosis, high levels of morbidity, and deterioration in quality of life.1,2,5
CONTEXT
Key Objective
Recurrent/metastatic squamous cell carcinoma of the head and neck (SCCHN) is associated with poor prognosis and notable morbidity. The phase III CheckMate 651 study evaluated dual immunotherapy with nivolumab plus ipilimumab versus the EXTREME (cetuximab plus cisplatin/carboplatin plus fluorouracil ≤ six cycles, then cetuximab maintenance) regimen as first-line treatment for patients with recurrent/metastatic SCCHN.
Knowledge Generated
First-line nivolumab plus ipilimumab did not result in a significant improvement in overall survival versus EXTREME in all randomly assigned or programmed death-ligand 1 combined positive score ≥ 20 populations. Nivolumab plus ipilimumab had a favorable safety profile versus EXTREME.
Relevance (G.K. Schwartz)
Use of immunotherapy in the treatment of SCCHN is still evolving, with a continued unmet need for first-line regimens that provide durable clinical benefit with tolerable safety. Further research is needed to determine the utility of dual immunotherapy as a treatment option for SCCHN and identify novel biomarkers to predict benefit with immunotherapy.*
*Relevance section written by JCO Associate Editor Gary K. Schwartz, MD.
First-line systemic therapy for R/M SCCHN previously relied on agents such as platinum, taxanes, and antimetabolites.3,5 In a phase III trial, the EXTREME (cetuximab plus cisplatin/carboplatin plus fluorouracil ≤ six cycles, then cetuximab maintenance) regimen significantly improved overall survival (OS) versus chemotherapy alone (median OS, 10.1 v 7.4 months).6,7 However, responses with EXTREME were not durable, and treatment was not generally well tolerated, with an increased incidence of grade 3/4 skin reactions, sepsis, hypomagnesemia, and anorexia. The TPEx regimen (cetuximab combined with docetaxel and cisplatin) showed no OS benefit versus EXTREME (median OS, 14.5 v 13.4 months) despite improved compliance and favorable safety.8
Interventions targeting programmed death-1 (PD-1) have shifted the standard of care to immunotherapy in both first- and second-line settings for R/M SCCHN.3 Pembrolizumab monotherapy improved OS versus chemotherapy-based regimens in platinum-refractory R/M SCCHN and in the first-line setting for programmed death-ligand 1 (PD-L1)–positive platinum-eligible R/M SCCHN; first-line pembrolizumab plus chemotherapy improved OS in platinum-eligible R/M SCCHN.9,10 In CheckMate 141, nivolumab monotherapy improved OS versus investigator's choice of chemotherapy in patients with platinum-refractory R/M SCCHN and in a subgroup of patients who progressed ≤ 6 months of platinum-based chemotherapy for locally advanced disease (LAD) in the adjuvant or primary setting.11-13 These immunotherapy-based regimens are recommended by the National Comprehensive Cancer Network Clinical Practice Guidelines in Oncology (NCCN Guidelines) and European Society for Medical Oncology (ESMO) Guidelines as treatment options for appropriate patients with R/M SCCHN.4,14 Despite advances in the treatment of R/M SCCHN, there persists an unmet need to improve clinical outcomes.
The immune checkpoint inhibitors nivolumab (a fully human anti–PD-1 antibody) and ipilimumab (a fully human anticytotoxic T-lymphocyte–associated antigen 4 antibody) have distinct but complementary mechanisms of action15 and have shown OS benefit and durable responses in several solid tumors, including non–small-cell lung cancer, malignant pleural mesothelioma, melanoma, renal cell carcinoma, and esophageal squamous cell carcinoma.16-21 Here, we report the results from CheckMate 651 (ClinicalTrials.gov identifier: NCT02741570), a randomized, open-label, phase III trial that evaluated first-line nivolumab plus ipilimumab versus EXTREME in platinum-eligible R/M SCCHN.
METHODS
Patients
Eligible patients were age 18 years or older with histologically confirmed R/M SCCHN of the oral cavity, oropharynx, hypopharynx, or larynx not amenable to curative therapy, measurable disease per RECIST v1.1,22 documented tumor PD-L1 and HPV (determined by p16 for oropharyngeal cancer [OPC]) status, Eastern Cooperative Oncology Group performance status 0-1, no prior systemic therapy in the R/M setting, and no prior treatment with epidermal growth factor receptor inhibitors. Patients who received prior chemotherapy as part of multimodal therapy for LAD were eligible if disease progression did not occur ≤ 6 months of definitive treatment.
Study Design and Treatment
Patients were randomly assigned 1:1 to nivolumab (3 mg/kg intravenously once every 2 weeks) plus ipilimumab (1 mg/kg intravenously once every 6 weeks) or EXTREME.6 Stratification factors were tumor PD-L1 expression (< 1% v ≥ 1%), p16 status (OPC p16-positive v p16-negative/non-OPC), and prior chemotherapy for LAD (yes v no). Treatment continued until disease progression, unacceptable toxicity, withdrawal of consent, or for ≤ 2 years on immunotherapy. Crossover between treatment arms was not permitted. Additional details on study design, treatment, assessments, and statistical analyses are included in the Data Supplement (online only).
CheckMate 651 was conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice guidelines. Institutional review boards or independent ethics committees approved the study Protocol (online only) and patient consent form at each site before study initiation. All patients provided written informed consent.
End Points and Assessments
The primary end points were OS in the all randomly assigned and PD-L1 combined positive score (CPS) ≥ 20 populations. PD-L1 staining was performed on tumor tissue using the Dako PD-L1 immunohistochemistry 28-8 pharmDx assay.23 Tumor PD-L1 expression was defined as the percentage of tumor cells exhibiting plasma membrane staining at any intensity; CPS was calculated as the number of PD-L1–staining cells, including tumor cells, lymphocytes, and macrophages, divided by the total number of viable tumor cells × 100. A key secondary end point was OS in patients with CPS ≥ 1. Other secondary end points were blinded independent central review–assessed progression-free survival (PFS), objective response rate (ORR), and duration of response (DOR; assessed in patients with complete/partial responses) in the all randomly assigned and CPS ≥ 20 populations.
Exploratory end points included OS subgroup analysis, blinded independent central review–assessed PFS, ORR, and DOR in patients with CPS ≥ 1, safety, and tolerability. Tumor progression and response were assessed by RECIST v1.1 using computed tomography or magnetic resonance imaging. Safety was assessed in all patients who received ≥ 1 dose of any treatment component. Adverse events (AEs) were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events v4.0. Patient-reported outcomes were assessed as exploratory end points in the all randomly assigned and CPS ≥ 20 populations. Symptom deterioration in the Functional Assessment of Cancer Therapy – Head and Neck (FACT-H&N) was assessed using a 10-item Symptom Index (FHNSI-10), and overall self-reported health status was evaluated using the 3-level version of the EQ-5D (EQ-5D-3L) 100-point visual analog scale.24,25
Statistical Analyses
OS, PFS, and DOR were estimated by the Kaplan-Meier method. OS in the all randomly assigned and CPS ≥ 20 populations (primary end points) were tested in parallel, with equal overall two-sided α = .025 (incorporating the O'Brien-Fleming α spending function) using stratified log-rank test. A statistical testing hierarchy was used for OS assessment in the all randomly assigned, CPS ≥ 20, and CPS ≥ 1 populations. OS in CPS ≥ 1 (secondary end point) was to be tested at the same α level as CPS ≥ 20 only if OS in CPS ≥ 20 was statistically significant. If OS in CPS ≥ 20 was not statistically significant, OS in CPS ≥ 1 was to be analyzed using descriptive statistics. Hazard ratio (HR) and the corresponding two-sided 100 × (1 – adjusted α)% CI were estimated using a stratified Cox proportional hazards model. PFS was analyzed using a stratified Cox proportional hazards model. ORR and associated CIs were computed using the Clopper and Pearson method. FHNSI-10 and EQ-5D-3L analyses included patients with baseline and ≥ 1 postbaseline on-treatment assessments.
A protocol-defined sensitivity analysis was conducted to assess the impact of nonproportional hazards on OS in the event of curve crossing. In addition, a post hoc sensitivity analysis was conducted to evaluate the effect of subsequent immunotherapy (second-line or later) on OS.
RESULTS
Patients and Treatment
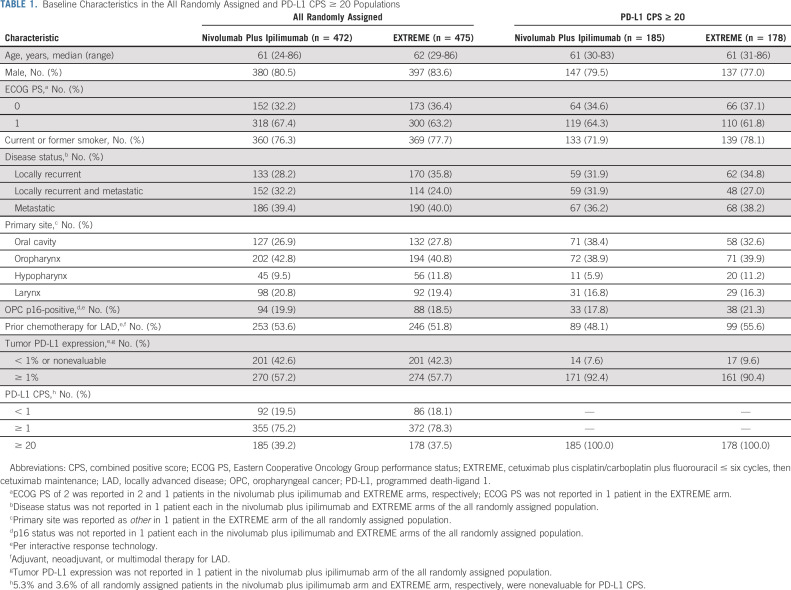
In CheckMate 651, 947 patients were randomly assigned to nivolumab plus ipilimumab (n = 472) or EXTREME (n = 475); 468 and 441 patients received ≥ 1 dose of treatment, respectively. The median age (range) was 61 years (24-86) in the nivolumab plus ipilimumab arm and 62 years (29-86) in the EXTREME arm; 80.5% and 83.6% were male, 76.3% and 77.7% were current/former smokers, 39.2% and 37.5% had CPS ≥ 20, and 57.2% and 57.7% had tumor PD-L1 expression ≥ 1%, respectively. HPV status was OPC p16-positive in 19.9% and 18.5%, and 53.6% and 51.8% had received prior chemotherapy for LAD, respectively. Similar distributions were reported in the CPS ≥ 20 population (Table 1).
TABLE 1.
Baseline Characteristics in the All Randomly Assigned and PD-L1 CPS ≥ 20 Populations
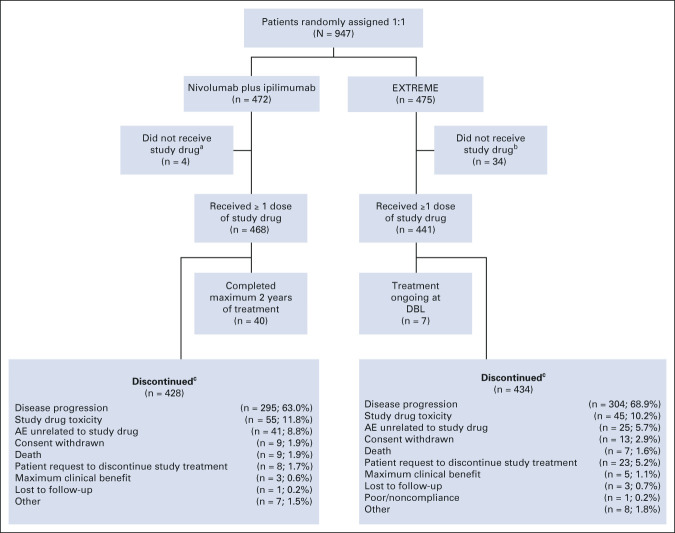
At database lock (June 21, 2021), the minimum and median follow-up was 27.3 and 39.1 months, respectively. In the nivolumab plus ipilimumab arm, no patients remained on treatment, with 8.5% completing the full 2 years of treatment; in the EXTREME arm, 1.6% remained on treatment (Fig 1). In all randomly assigned patients, the median duration of therapy was 3.8 months (range < 0.1-24.0) with nivolumab plus ipilimumab versus 5.0 months (range < 0.1-50.7) with EXTREME. A median of eight doses (range, 1-53) of nivolumab and three doses (range, 1-18) of ipilimumab were administered. In the EXTREME arm, 34% of patients received cisplatin, 54% received carboplatin, and 11% received cisplatin and carboplatin during treatment. A median of 4.0 doses (range, 1-6) of cisplatin and 5.0 doses (range, 1-6) of carboplatin were administered. Overall, 52.8% of patients received cetuximab maintenance therapy. Subsequent systemic therapy was administered in 49.2% (nivolumab plus ipilimumab) and 60.2% (EXTREME) of patients with 8.5% and 46.3%, respectively, receiving subsequent immunotherapy (mostly nivolumab), 42.2% and 16.2% receiving platinum-based chemotherapy, and 27.3% and 12.8% receiving cetuximab. A similar proportion of patients received subsequent therapy in the CPS ≥ 20 population (Data Supplement).
FIG 1.
CONSORT diagram of patient disposition in all randomly assigned patients. Minimum follow-up: 27.3 months; database lock: June 21, 2021. aPatients who were randomly assigned and did not receive nivolumab plus ipilimumab treatment owing to patient no longer meeting study criteria (n = 3) and AEs unrelated to study drug (n = 1). bPatients who were randomly assigned and did not receive EXTREME treatment owing to consent withdrawal (n = 18), patient no longer meeting study criteria (n = 6), loss to follow-up (n = 2), patient request (n = 2), disease progression (n = 1), death (n = 1), poor compliance or noncompliance (n = 1), and other (n = 3). cData are reported as the number of patients discontinued per reason/number of treated patients in each arm (%). AE, adverse event; DBL, database lock; EXTREME, cetuximab plus cisplatin/carboplatin plus fluorouracil ≤ six cycles, then cetuximab maintenance.
Efficacy
The median OS was 13.9 months (95% CI, 12.1 to 15.8) with nivolumab plus ipilimumab versus 13.5 months (95% CI, 12.6 to 15.2) with EXTREME in all randomly assigned patients and 17.6 months (95% CI, 13.8 to 22.0) versus 14.6 months (95% CI, 12.3 to 16.0) in the CPS ≥ 20 population. The primary end points of OS with nivolumab plus ipilimumab versus EXTREME were not met in the all randomly assigned (HR, 0.95; 97.9% CI, 0.80 to 1.13; P = .4951; Fig 2A) or CPS ≥ 20 (HR, 0.78; 97.51% CI, 0.59 to 1.03; P = .0469; Fig 2B) populations. OS with nivolumab plus ipilimumab versus EXTREME was similar, regardless of cisplatin or carboplatin administration, in both the all randomly assigned (HR, 1.04; 95% CI, 0.85 to 1.26, and 0.94; 95% CI, 0.79 to 1.11) and CPS ≥ 20 (HR, 0.84; 95% CI, 0.60 to 1.19, and 0.82; 95% CI, 0.62 to 1.10) populations. In the CPS ≥ 1 population, the median OS was 15.7 months (95% CI, 13.7 to 18.8) with nivolumab plus ipilimumab versus 13.2 months (95% CI, 11.1 to 14.6) with EXTREME (HR, 0.82; 95% CI, 0.69 to 0.97; Fig 2C); P value was not calculated on the basis of the protocol-specified testing hierarchy.
FIG 2.
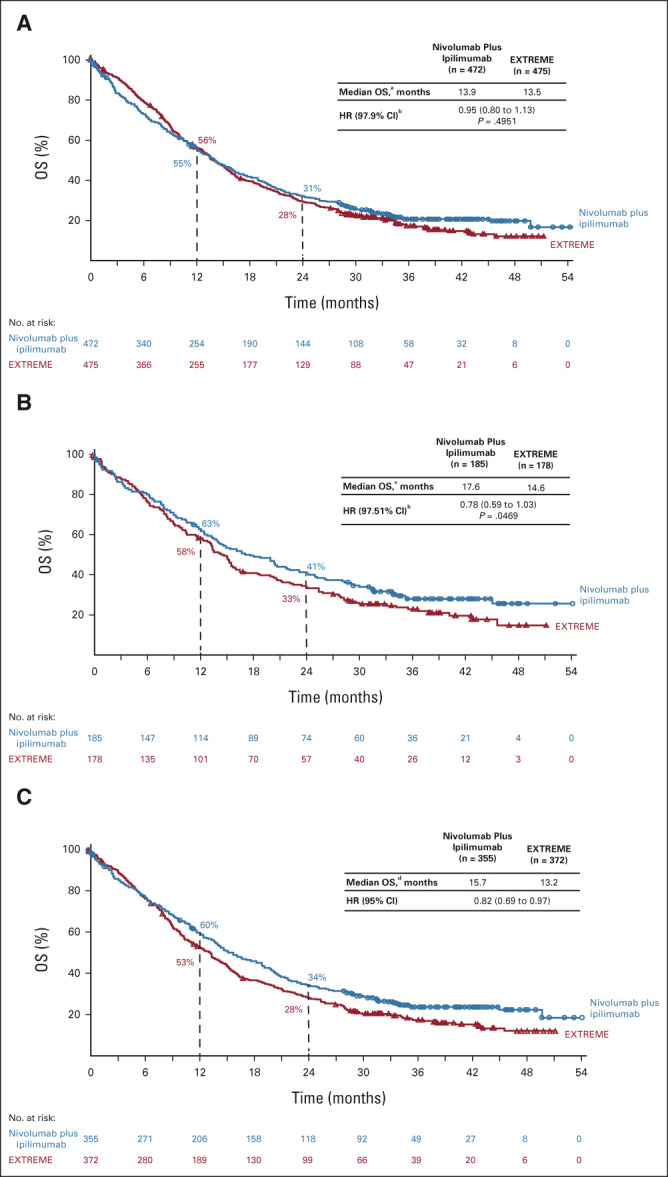
OS in the (A) all randomly assigned population, (B) PD-L1 CPS ≥ 20 population, and (C) PD-L1 CPS ≥ 1 population. Minimum follow-up: 27.3 months. a95% CI, 12.1 to 15.8 (nivolumab plus ipilimumab) and 12.6 to 15.2 (EXTREME). bCIs are adjusted on the basis of the final α levels for each primary end point. c95% CI, 13.8 to 22.0 (nivolumab plus ipilimumab) and 12.3 to 16.0 (EXTREME). d95% CI, 13.7 to 18.8 (nivolumab plus ipilimumab) and 11.1 to 14.6 (EXTREME). CPS, combined positive score; EXTREME, cetuximab plus cisplatin/carboplatin plus fluorouracil ≤ six cycles, then cetuximab maintenance; HR, hazard ratio; OS, overall survival; PD-L1, programmed death-ligand 1.
As late benefit is often observed with immunotherapy versus chemotherapy-based regimens, the impact of nonproportional hazards on OS was analyzed; the results showed no statistically significant benefit with nivolumab plus ipilimumab versus EXTREME in the all randomly assigned or CPS ≥ 20 populations (Data Supplement).
Given the larger proportion of patients in the EXTREME arm receiving subsequent immunotherapy (46% v 9% in the nivolumab plus ipilimumab arm), an ad hoc analysis was performed to adjust for its impact on the primary outcome. Adjusting both treatment arms, the analysis showed a median OS of 12.4 months with nivolumab plus ipilimumab versus 10.8 months with EXTREME (HR, 0.80; 95% CI, 0.68 to 0.92; Data Supplement) in the all randomly assigned population and 14.1 versus 11.7 months (HR, 0.71; 95% CI, 0.55 to 0.91; Data Supplement) in the CPS ≥ 20 population.
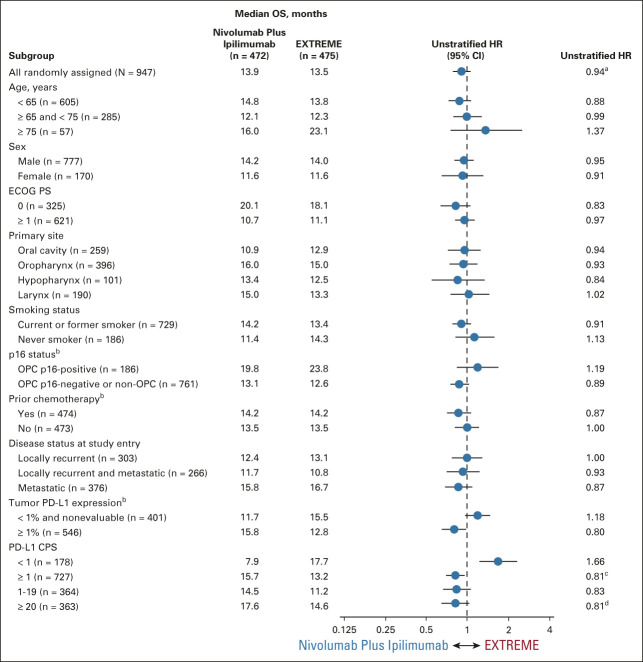
Exploratory analyses by baseline characteristics showed no notable difference in median OS across treatment arms in most subgroups in the all randomly assigned population (Fig 3). Generally, similar results were noted across most subgroups in the CPS ≥ 20 population (Data Supplement).
FIG 3.
OS subgroup analyses in the all randomly assigned population. aStratified HR, 0.95. bPer interactive response technology. cStratified HR, 0.82. dStratified HR, 0.78. CPS, combined positive score; ECOG PS, Eastern Cooperative Oncology Group performance status; EXTREME, cetuximab plus cisplatin/carboplatin plus fluorouracil ≤ six cycles, then cetuximab maintenance; HR, hazard ratio; OPC, oropharyngeal cancer; OS, overall survival; PD-L1, programmed death-ligand 1.
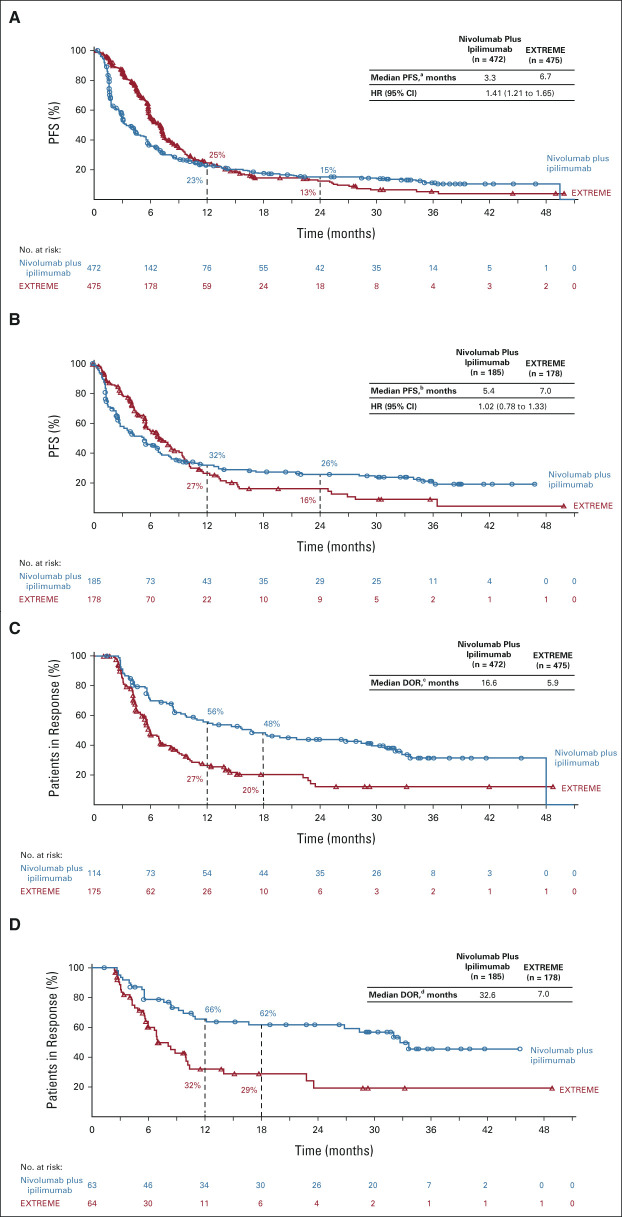
Median PFS was shorter with nivolumab plus ipilimumab versus EXTREME in the all randomly assigned (median, 3.3 months [95% CI, 2.8 to 4.2] v 6.7 months [95% CI, 5.8 to 7.0]; HR, 1.41 [95% CI, 1.21 to 1.65]; Fig 4A), CPS ≥ 20 (median [95% CI]: 5.4 [3.1 to 6.9] v 7.0 [5.6 to 8.7] months; HR, 1.02 [95% CI, 0.78 to 1.33]; Fig 4B), and CPS ≥ 1 (median, 4.2 months [95% CI, 2.9 to 5.4] v 6.1 months [95% CI, 5.6 to 7.0]; HR, 1.23 [95% CI, 1.03 to 1.47]; Data Supplement) populations. The ORR was 24.2% (95% CI, 20.4 to 28.3) with nivolumab plus ipilimumab versus 36.8% (95% CI, 32.5 to 41.4) with EXTREME in all randomly assigned patients and 34.1% (95% CI, 27.3 to 41.4) versus 36.0% (95% CI, 28.9 to 43.5) in the CPS ≥ 20 population (Data Supplement); complete response rates were 7.2% versus 4.6% and 12.4% versus 7.3%, respectively. The median DOR was 16.6 months with nivolumab plus ipilimumab versus 5.9 months with EXTREME (all randomly assigned; Fig 4C) and 32.6 versus 7.0 months (CPS ≥ 20; Fig 4D). Tumor response data for the CPS ≥ 1 population are summarized in the Data Supplement.
FIG 4.
PFS by BICR in the (A) all randomly assigned and (B) PD-L1 CPS ≥ 20 populations; DOR (in patients with complete or partial responses) by BICR in the (C) all randomly assigned and (D) PD-L1 CPS ≥ 20 populations. Minimum follow-up: 27.3 months. a95% CI, 2.8 to 4.2 (nivolumab plus ipilimumab) and 5.8 to 7.0 (EXTREME). b95% CI, 3.1 to 6.9 (nivolumab plus ipilimumab) and 5.6 to 8.7 (EXTREME). c95% CI, 9.7 to 29.4 (nivolumab plus ipilimumab) and 5.4 to 7.0 (EXTREME). d95% CI, 12.1 to NR (nivolumab plus ipilimumab) and 5.6 to 10.1 (EXTREME). BICR, blinded independent central review; CPS, combined positive score; DOR, duration of response; EXTREME, cetuximab plus cisplatin/carboplatin plus fluorouracil ≤ six cycles, then cetuximab maintenance; HR, hazard ratio; NR, not reached; PD-L1, programmed death-ligand 1; PFS, progression-free survival.
Safety
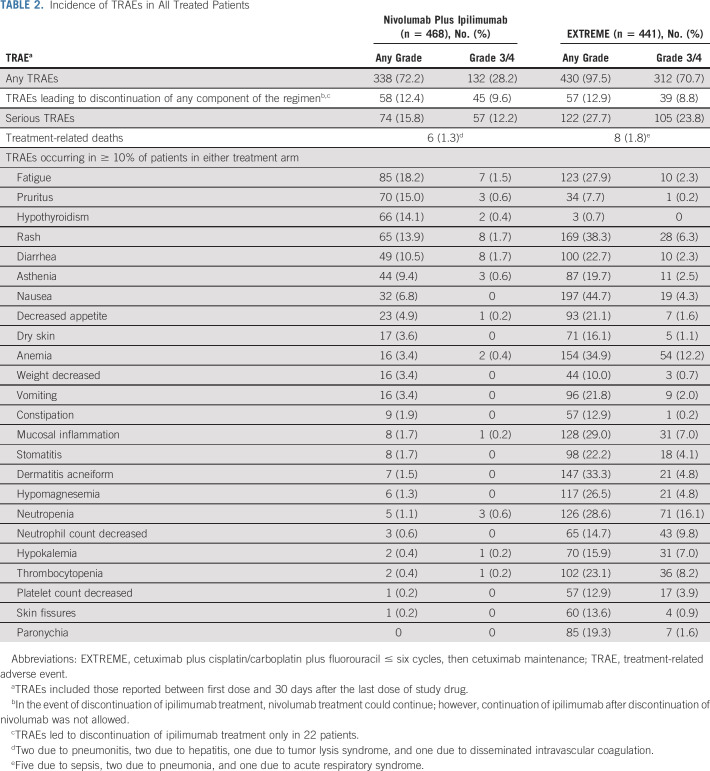
Any-grade and grade 3/4 treatment-related AEs (TRAEs) were reported in 72.2% and 28.2% (nivolumab plus ipilimumab) versus 97.5% and 70.7% (EXTREME) of treated patients (Table 2). Any-grade and grade 3/4 TRAEs leading to discontinuation of any component of the regimen were reported in 12.4% and 9.6% versus 12.9% and 8.8% of patients, respectively. The most common any-grade TRAEs were fatigue (18.2%), pruritus (15.0%), and hypothyroidism (14.1%) with nivolumab plus ipilimumab and nausea (44.7%), rash (38.3%), and anemia (34.9%) with EXTREME (Table 2). Any-grade and grade 3/4 serious TRAEs were reported in 15.8% and 12.2% (nivolumab plus ipilimumab) versus 27.7% and 23.8% (EXTREME); treatment-related deaths were reported in 1.3% versus 1.8% of patients, respectively.
TABLE 2.
Incidence of TRAEs in All Treated Patients
The most common any-grade immune-mediated AEs (IMAEs) with nivolumab plus ipilimumab were hypothyroidism/thyroiditis (16.0%), rash (12.6%), and hyperthyroidism (6.8%); grade 3/4 events were uncommon (Data Supplement). Most events occurred within the first 5 months of treatment (Data Supplement). The median duration of corticosteroid use for IMAEs ranged from 0.3 (hypophysitis) to 6.0 (hypothyroidism/thyroiditis) weeks; most nonendocrine IMAEs resolved with corticosteroids ≥ 40 mg once daily. Times to onset and resolution of IMAEs, including treatment with immune-modulating medications, are summarized in the Data Supplement.
Patient-Reported Outcomes
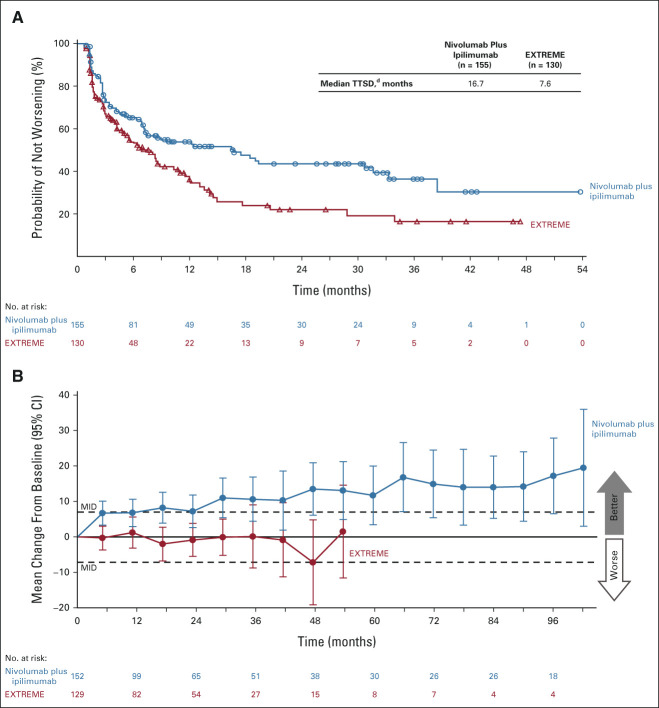
Completion rates for patient-reported outcome assessments were > 80% at baseline. In the CPS ≥ 20 population, nivolumab plus ipilimumab versus EXTREME tended to delay symptom deterioration (per FHNSI-10, median time to symptom deterioration: 16.7 v 7.6 months, respectively; Fig 5A) and resulted in clinically meaningful improvement in overall self-rated health status (per EQ-5D-3L visual analog scale, mean changes in scores from baseline exceeded the minimally important difference after week 24; Fig 5B). The results were similar, but less pronounced, in the all randomly assigned population (Data Supplement).
FIG 5.
(A) Time to symptom deterioration (FHNSI-10)a and (B) overall self-rated health status (EQ-5D-3L VAS)b,c in the PD-L1 CPS ≥ 20 population. Minimum follow-up: 27.3 months. aTime to symptom deterioration is defined as the time from random assignment to first clinically meaningful decline (reduction of ≥ 3 points) from baseline in FHNSI-10 score.26 bMean (95% CI) change from baseline; horizontal reference line indicates MID = 7-point change on EQ-5D-3L VAS.27 cOnly on-treatment time points with data for ≥ 10 patients in either treatment group are shown, not adjusted for multiplicity. d95% CI, 7.4 to 31.6 (nivolumab plus ipilimumab) and 4.3 to 10.9 (EXTREME). CPS, combined positive score; EQ-5D-3L VAS, EQ-5D 3-level version visual analog scale; EXTREME, cetuximab plus cisplatin/carboplatin plus fluorouracil ≤ six cycles, then cetuximab maintenance; FHNSI-10, Functional Assessment of Cancer Therapy Head and Neck Cancer Symptom 10-Item Index; MID, minimally important difference; PD-L1, programmed death-ligand 1; TTSD, time to symptom deterioration.
DISCUSSION
CheckMate 651 was a large phase III study designed to evaluate first-line nivolumab plus ipilimumab versus EXTREME in platinum-eligible R/M SCCHN. This study did not meet its primary end points of OS in the all randomly assigned or PD-L1 CPS ≥ 20 populations. The median OS was 13.9 months (95% CI, 12.1 to 15.8) with nivolumab plus ipilimumab versus 13.5 months (95% CI, 12.6 to 15.2) with EXTREME in the all randomly assigned population and 17.6 months (95% CI, 13.8 to 22.0) versus 14.6 months (95% CI, 12.3 to 16.0) in the CPS ≥ 20 population and did not reach statistical significance in either population. Notably, the number of patients with CPS ≥ 20 (n = 363) was smaller than the number of events (n = 372) required to maintain planned statistical power for the primary end point, resulting in a loss of statistical power to demonstrate a difference. ORR was higher with EXTREME in the all randomly assigned population but similar in both treatment arms of the CPS ≥ 20 population (with higher proportions of complete responses with nivolumab plus ipilimumab versus EXTREME in both populations). The median DOR was 32.6 months (nivolumab plus ipilimumab) versus 7.0 months (EXTREME) in the CPS ≥ 20 population.
Notably, median OS with EXTREME in the all randomly assigned population of CheckMate 651 was higher (13.5 months [95% CI, 12.6 to 15.2]) than the historically reported range of 10.1 (95% CI, 8.6 to 11.2) to 10.7 (95% CI, 9.3 to 11.7) months for first-line R/M SCCHN6,9; a similar result was reported in the TPEx study in which all patients received a cisplatin-based regimen (median OS with EXTREME, 13.4 months [95% CI, 12.2 to 15.4]).8 Although these OS outcomes across studies may be due to differences in patient characteristics, such as disease burden, or differences in study designs, a notable change from previous studies was the increasing availability of subsequent immunotherapy after study discontinuation. With the increasing use of second-line immunotherapy because of regulatory approvals of nivolumab in multiple countries,28-30 the results in the EXTREME arm of CheckMate 651 may better reflect contemporary clinical practice versus earlier studies such as KEYNOTE-048 in which fewer patients (25%) in the EXTREME arm received subsequent immunotherapy.9 Post hoc sensitivity analyses, conducted in the all randomly assigned and CPS ≥ 20 populations to investigate the effect of subsequent immunotherapy on OS, yielded lower median OS versus the primary analyses in both treatment arms. However, the extent of reduction in median OS was greater in the EXTREME arm with a notable reduction in the HRs for OS, indicating that the higher proportion of patients who received subsequent immunotherapy, mostly nivolumab, in the EXTREME arm (46.3% v 8.5% in the nivolumab plus ipilimumab arm) may have potentially contributed to the higher-than-expected median OS with EXTREME.
Unlike OS, PFS is not affected by postrandom assignment variables such as subsequent therapy. Median PFS was shorter with nivolumab plus ipilimumab versus EXTREME in the all randomly assigned, CPS ≥ 20, or CPS ≥ 1 populations. Importantly, median PFS with EXTREME in the all randomly assigned population (6.7 months [95% CI, 5.8 to 7.0]) of CheckMate 651 was higher than that reported previously (ranging from 5.1 months [95% CI, 4.9 to 6.0] to 5.6 months [95% CI, 5.0 to 6.0]), which may be related to differences in the patient populations between the studies.6,9 Delayed PFS benefit with nivolumab plus ipilimumab was seen in the CPS ≥ 20 population of CheckMate 651, with 26% of patients remaining progression free at 2 years versus 16% with EXTREME. A similar effect was reported in the CPS ≥ 20 population of KEYNOTE-048, in which the median PFS was 3.4 months (pembrolizumab) versus 5.0 months (EXTREME), with 1-year PFS rates of 23% and 12%, respectively.9
Immunotherapies targeting PD-1, such as nivolumab and pembrolizumab, have demonstrated OS benefits in patients with platinum-refractory or platinum-eligible R/M SCCHN. In CheckMate 141, a post hoc subgroup analysis of nivolumab in patients with R/M SCCHN who experienced disease progression on or ≤ 6 months of platinum-based chemotherapy for LAD in the adjuvant or primary setting showed improved OS versus chemotherapy; benefit was maintained at 2-year follow-up.31 In KEYNOTE-048, first-line pembrolizumab alone or with chemotherapy demonstrated long-term OS benefit versus EXTREME in patients with platinum-eligible disease with CPS ≥ 20 or ≥ 19,32; the median OS was 14.9 months (pembrolizumab) and 14.7 months (pembrolizumab plus chemotherapy) in the CPS ≥ 20 population.10 Although cross-trial comparisons should be approached with caution because of differences in study design and patient populations, the median OS of 17.6 months with nivolumab plus ipilimumab in patients with CPS ≥ 20 in CheckMate 651 is the longest reported in this patient population at this time.
Of previous studies evaluating dual immunotherapy in R/M SCCHN, phase III trials KESTREL and EAGLE assessing durvalumab alone or in combination with tremelimumab failed to demonstrate clinical benefit versus chemotherapy in the first- and second-line settings, respectively.33,34 In the phase II CheckMate 714 trial (ClinicalTrials.gov identifier: NCT02823574), which compared first-line nivolumab plus ipilimumab versus nivolumab in platinum-refractory or platinum-eligible R/M SCCHN, the primary end point of ORR in the platinum-refractory population was not met (13.2% [95% CI, 8.4 to 19.5] with nivolumab plus ipilimumab v 18.3% [95% CI, 10.6 to 28.4] with nivolumab). ORR in the platinum-eligible population was 20.3% (95% CI, 13.6 to 28.5) versus 29.5% (95% CI, 18.5 to 42.6).35-37 Lack of clinical benefit with dual immunotherapy compared with single-agent immunotherapy underscores the need for further research to understand the role of the components of dual immunotherapy with anti–PD-(L)1 and anticytotoxic T-lymphocyte–associated antigen 4 inhibition in hard-to-treat SCCHN.
The use of immunotherapy in SCCHN is mainly driven by CPS.9,38 For patients with CPS ≥ 20, pembrolizumab monotherapy is recommended as a first-line treatment option on the basis of high-level evidence. This is also an option for certain patients with CPS ≥ 1; regardless of CPS status, patients may receive pembrolizumab plus chemotherapy.3,14 Effective treatment options for patients who progress on or are refractory to first-line immunotherapy remain a substantial unmet need that warrants exploration, including novel immunotherapy-based combinations or treatment sequencing in R/M SCCHN.
The nivolumab plus ipilimumab dosing regimen in CheckMate 651 was informed by results from the phase I CheckMate 012 study in advanced non–small-cell lung cancer, in which nivolumab 3 mg/kg once every 2 weeks plus ipilimumab 1 mg/kg once every 6 weeks resulted in tolerable safety and promising efficacy.39 In CheckMate 651, nivolumab plus ipilimumab demonstrated a favorable safety profile versus EXTREME with a lower frequency of any- and serious-grade 3/4 TRAEs and no unexpected IMAEs. Nivolumab plus ipilimumab improved health-related quality of life with delayed time to symptom deterioration versus EXTREME.
In summary, first-line nivolumab plus ipilimumab did not result in a statistically significant improvement in OS versus EXTREME in platinum-eligible R/M SCCHN in the all randomly assigned or CPS ≥ 20 populations. Safety with nivolumab plus ipilimumab was favorable compared with EXTREME.
ACKNOWLEDGMENT
The authors thank the patients, their families, and the clinical study teams for making this study possible. We thank Dako for collaborative development of the PD-L1 IHC 28-8 pharmDx assay, and Bristol Myers Squibb (Princeton, NJ) and Ono Pharmaceutical Company Ltd (Osaka, Japan). The authors wish to acknowledge Bryan Bennett and Anagha Gogate for the PRO analysis. Writing and editorial assistance were provided by Meenakshi Subramanian, PhD, CMPP, of Evidence Scientific Solutions, Inc, funded by Bristol Myers Squibb.
The list of CheckMate 651 investigators is listed in Appendix 1 (online only).
APPENDIX 1. AUTHOR CONSORTIUM: LIST OF CHECKMATE 651 INVESTIGATORS
Australia: Muhammed Alamgeer, Venessa Chin, Joanna Dewar, Bo Gao, Alexander Guminski, Rohit Joshi, Craig Kukard, Margaret McGrath, Zulfiquer Otty, Danny Rischin; Austria: Martin Burian, Thorsten Fuereder; Brazil: Sergio Azevedo, Fábio Franke, Otavio Gampel, Gustavo Girotto, Milena Mak, Andre Murad, Gustavo Dix Junqueira Pinto, Fernanda Pruski Ramos; France: Bertrand Baujat, Christian Borel, Amandine Bruyas, Alexandre Coutte, Amaury Daste, Caroline Even, Jerome Fayette, Christophe Le Tourneau, Gautier Lefebvre, Julien Pavillet, Esma Saada-Bouzid, Sebastien Salas; Germany: Juergen Alt, Peter Brossart, Andreas Dietz, Simon Heidegger, Philipp Ivanyi, Juergen Krauss, Simon Laban, Urs Müller-Richter, Justyna Rawluk, Phillippe Schafhausen; Greece: Gerasimos Aravantinos, Athanassios Argiris, Pavlos Papakotoulas; Ireland: Cliona Grant; Israel: Salem Billan, Iris Gluck, Orit Gutfeld, Amichay Meirovitz, Salomon Stemmer; Italy: Mario Airoldi, Federica Bertolini, Francesco Caponigro, Alessandra Cassano, Verena De Angelis, Francesco de Rosa, Nerina Denaro, Lisa Licitra, Vincenzo Montesarchio; Japan: Masahiro Goto, Hiroki Hara, Hidetoshi Hayashi, Sadakatsu Ikeda, Shigemichi Iwae, Shigenori Kadowaki, Naomi Kiyota, Torahiko Nakashima, Kenji Okami, Nobuhiko Oridate, Yasushi Shimizu, Makoto Tahara, Masahiro Takahashi, Shunji Takahashi, Akihito Tsuji, Tomoko Yamazaki, Tomoya Yokota, Seiichi Yoshimoto; Mexico: Miguel Alvarez Avitia, Jose Gonzalez Trujillo, Carlos Hernandez-Hernandez, Alejandro Juarez Ramiro, Joaquin Reinoso, Leticia Vasquez Cortes; Poland: Andrzej Kawecki, Piotr Koralewski, Katarzyna Matuszewska, Tomasz Rutkowski, Bogdan Zurawski; South Korea: Jin-Hyoung Kang, Sung-Bae Kim, Keunchil Park; Spain: Irene Brana, Maria Jose Flor Oncala, Lara Iglesias Docampo, Ricard Mesia Nin, Miguel Pastor Borgonon, Ainara Soria; Switzerland: Tamara Rordorf, Sacha Rothschild; Taiwan: Yi-Chun Liu, Pei-Jen Lou; United Kingdom: Bernadette Foran, James Good, Kevin Harrington, Charles Kelly, Robert Metcalf, Shanmugasundaram Ramkumar; United States: Mohammed Almubarak, Julie Bauman, Eric Bernicker, George Blumenschein, Marcelo Bonomi, Christine Chung, Alexander Colevas, John Deeken, Robert Ferris, Elizabeth Gaughan, Troy Guthrie, Robert Haddad, Randall Hughes, Jennifer Johnson, Erminia Massarelli, Robert Reilly, Nabil Saba, Ashley Sumrall, Francis Worden, Everett Vokes
See accompanying editorial on page 2134
PRIOR PRESENTATION
Presented at the ESMO Congress 2021, Paris, France, September 16-21, 2021 (abstr LBA36).
SUPPORT
This study was sponsored by Bristol Myers Squibb (Princeton, NJ) in collaboration with Ono Pharmaceutical Company Ltd (Osaka, Japan).
CLINICAL TRIAL INFORMATION
R.I.H. and A.A. contributed equally to this work.
DATA SHARING STATEMENT
Data are available upon reasonable request. Bristol Myers Squibb policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.
AUTHOR CONTRIBUTIONS
Conception and design: Robert I. Haddad, Kevin Harrington, Robert L. Ferris, Maura Gillison, Urs Müller-Richter, Mustimbo Roberts, Karen Miller-Moslin, Li Wei, Athanassios Argiris
Provision of study materials or patients: Kevin Harrington, Robert L. Ferris, Maura Gillison, Jerome Fayette, Amaury Daste, Piotr Koralewski, Bogdan Zurawski, Andrzej Kawecki, Gustavo Girotto, Miguel Angel Alvarez Avitia, Caroline Even, Joaquin Gabriel Reinoso Toledo, Alexander Guminski, Urs Müller-Richter, Athanassios Argiris
Collection and assembly of data: Robert I. Haddad, Kevin Harrington, Robert L. Ferris, Maura Gillison, Jerome Fayette, Piotr Koralewski, Bogdan Zurawski, Miren Taberna, Nabil F. Saba, Milena Mak, Andrzej Kawecki, Gustavo Girotto, Miguel Angel Alvarez Avitia, Caroline Even, Joaquin Gabriel Reinoso Toledo, Alexander Guminski, Urs Müller-Richter, Tariq Aziz Khan, Karen Miller-Moslin, Athanassios Argiris
Data analysis and interpretation: Robert I. Haddad, Kevin Harrington, Makoto Tahara, Robert L. Ferris, Jerome Fayette, Amaury Daste, Miren Taberna, Nabil F. Saba, Gustavo Girotto, Caroline Even, Urs Müller-Richter, Naomi Kiyota, Mustimbo Roberts, Tariq Aziz Khan, Karen Miller-Moslin, Li Wei, Athanassios Argiris
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Nivolumab Plus Ipilimumab Versus EXTREME Regimen as First-Line Treatment for Recurrent/Metastatic Squamous Cell Carcinoma of the Head and Neck: The Final Results of CheckMate 651
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Robert I. Haddad
Employment: Dana-Farber Cancer Institute
Leadership: NCCN
Consulting or Advisory Role: Celgene, Merck, Eisai, Bristol Myers Squibb, AstraZeneca, Pfizer, Loxo, Genentech, Immunomic Therapeutics, GlaxoSmithKline, Gilead Sciences, Vaccinex, EMD Serono, BioNTech, Achilles Therapeutics, Bayer, Coherus Biosciences, Boehringer Ingelheim, Mirati Therapeutics
Research Funding: Boehringer Ingelheim (Inst), Merck (Inst), Bristol Myers Squibb (Inst), Celgene (Inst), AstraZeneca (Inst), Genentech (Inst), Pfizer (Inst), Kura Oncology (Inst)
Patents, Royalties, Other Intellectual Property: UpToDate
Other Relationship: Nanobiotix, ISA Pharmaceuticals
Kevin Harrington
This author is a member of the Journal of Clinical Oncology Editorial Board. Journal policy recused the author from having any role in the peer review of this manuscript.
Honoraria: Arch Oncology (Inst), AstraZeneca (Inst), BMS (Inst), Boehringer Ingelheim (Inst), Merck Serono (Inst), MSD (Inst), Oncolys BioPharma (Inst), Pfizer (Inst), Replimune (Inst), Inzen Therapeutics (Inst), Codiak Biosciences (Inst), Scenic Biotech
Consulting or Advisory Role: Arch Oncology (Inst), AstraZeneca (Inst), BMS (Inst), Boehringer Ingelheim (Inst), Merck Serono (Inst), MSD (Inst), Oncolys BioPharma (Inst), Replimune (Inst), Inzen Therapeutics (Inst)
Speakers' Bureau: BMS (Inst), Merck Serono (Inst), MSD (Inst)
Research Funding: AstraZeneca (Inst), Replimune (Inst), Boehringer Ingelheim (Inst)
Makoto Tahara
Honoraria: Bristol Myers Squibb, Eisai, Ono Pharmaceutical, MSD, Lilly, Bayer, Merck Serono
Consulting or Advisory Role: Ono Pharmaceutical, MSD, Pfizer, Bristol Myers Squibb, Rakuten Medical, Bayer, Lilly, Eisai, Boehringer Ingelheim, Genmab, Nektar, Janssen, Nanobiotix, Astellas Pharma
Research Funding: Merck Sharp & Dohme (Inst), AstraZeneca (Inst), Ono Pharmaceutical (Inst), Novartis (Inst), Pfizer (Inst), Bristol Myers Squibb (Inst), Rakuten Medical (Inst), Bayer (Inst), GlaxoSmithKline (Inst), Lilly (Inst), Merck Serono (Inst)
Robert L. Ferris
Stock and Other Ownership Interests: Novasenta
Consulting or Advisory Role: Merck, Pfizer, Numab, Macrogenics, Novasenta, Sanofi, Zymeworks, Bristol Myers Squibb, Aduro Biotech, Achilles Therapeutics, Bicara Therapeutics, Everest Clinical Research, F. Hoffmann LaRoche, Genocea Biosciences, Hookipa Pharma, Instil Bio, Kowa Research Institute, Lifescience Dynamics, Mirati Therapeutics, OncoCyte, PPD, Rakuten Medical, Seattle Genetics, VIR Biotechnology, MeiraGTx, LLC, Adagene Incorporated, Brooklyn Immunotherapeutics LLC, Cantenion, Coherus BioSciences Inc, Mirror Biologics Inc, Nanabiotix, Novartis, SIRPant Immunotherapies
Research Funding: Bristol Myers Squibb, AstraZeneca/MedImmune, Merck, Tesaro, Novasenta
Maura Gillison
This author is an Associate Editor for Journal of Clinical Oncology. Journal policy recused the author from having any role in the peer review of this manuscript.
Consulting or Advisory Role: Bristol Myers Squibb, Merck, EMD Serono, BioNTech, Shattuck Labs, Bayer, Debiopharm Group, Ipsen, Gilead Sciences, Bicara Therapeutics, Nektar, Istari, LLX Solutions, OncLive, Seattle Genetics, Kura Oncology, Mirati Therapeutics, Sensei Biotherapeutics, Eisai
Research Funding: Bristol Myers Squibb (Inst), Genocea Biosciences (Inst), Cullinan Oncology (Inst), Genentech (Inst), Agenus (Inst), Kura Oncology (Inst)
Jerome Fayette
Honoraria: AstraZeneca, Bristol Myers Squibb, Merck Sharp & Dohme, Merck Serono, Innate Pharma, Roche
Consulting or Advisory Role: AstraZeneca, Bristol Myers Squibb, Merck Sharp & Dohme, Merck Serono, Innate Pharma, Roche
Research Funding: Bristol Myers Squibb (Inst)
Travel, Accommodations, Expenses: Bristol Myers Squibb, AstraZeneca, Merck Sharp & Dohme
Amaury Daste
Consulting or Advisory Role: Merck, MSD, BMS
Travel, Accommodations, Expenses: BMS, Merck
Bogdan Zurawski
Honoraria: BMS, MSD, Merck, GSK, Janssen, Astellas, Exelixis, Syneos, Roche, AstraZeneca
Research Funding: BMS, MSD, Merck, GSK, Janssen, Astellas, Exelixis, Syneos, Roche, AstraZeneca
Expert Testimony: Janssen, Exelixis, BMS, MSD, Merck, GSK, Astellas
Miren Taberna
Employment: Savana
Leadership: Savana
Consulting or Advisory Role: Nanobiotix, Merck, MSD Oncology
Speakers' Bureau: Merck, AstraZeneca Spain, Bristol Myers Squibb, MSD Oncology
Travel, Accommodations, Expenses: AstraZeneca Spain, Merck, MSD Oncology
Nabil F. Saba
Honoraria: Merck, CUE Biopharma, BioNTech, EMD Serono, AstraZeneca, ReachMD, vaccinex, WebMD, Medscape, Clinical Care Options, Kura Oncology, Aduro Biotech
Consulting or Advisory Role: GlaxoSmithKline, Mirati Therapeutics, Eisai, Philips Electronics
Research Funding: Bristol Myers Squibb, Exelixis
Patents, Royalties, Other Intellectual Property: Uptodate chapter writing and editing, Springer textbook Royalty
Travel, Accommodations, Expenses: Merck, Pfizer, GlaxoSmithKline, Blueprint Medicines
Milena Mak
Honoraria: Bayer, Pfizer, Merck Serono, Takeda, Amgen
Consulting or Advisory Role: AstraZeneca
Andrzej Kawecki
Honoraria: Bristol Myers Squibb/Celgene, MSD, Merck Serono
Consulting or Advisory Role: MSD, Bristol Myers Squibb/Celgene, Merck Serono
Research Funding: Bristol Myers Squibb/Celgene, MSD, GlaxoSmithKline, Merck Serono, Sanofi, Roche, AstraZeneca, Macrogenics
Gustavo Girotto
Honoraria: MSD Oncology
Speakers' Bureau: MSD Oncology, Lilly
Research Funding: MSD Oncology (Inst), BMS Brazil (Inst)
Caroline Even
Consulting or Advisory Role: Innate Pharma, Bristol Myers Squibb, MSD Oncology, Merck Serono
Travel, Accommodations, Expenses: MSD Oncology
Joaquin Gabriel Reinoso Toledo
Employment: BMS
Alexander Guminski
Consulting or Advisory Role: Bristol Myers Squibb, Merck, Pfizer, Regeneron, Sun Pharma, MSD Oncology, Sanofi
Research Funding: Sun Pharma (Inst)
Travel, Accommodations, Expenses: Merck KGaA, Sun Pharma
Urs Müller-Richter
Stock and Other Ownership Interests: BioNTech SE
Consulting or Advisory Role: MSD Oncology, BMS GmbH & Co. KG, Sanofi Aventis GmbH
Speakers' Bureau: BMS GmbH & Co KG, MSD Oncology
Naomi Kiyota
Honoraria: Ono Pharmaceutical, Bristol Myers Squibb Japan, Bayer, Chugai Pharma, Merck Serono, MSD, Eisai, AstraZeneca
Consulting or Advisory Role: Shift Zero, Ono Pharmaceutical, Adlai Nortye
Speakers' Bureau: Ono Pharmaceutical, Bristol Myers Squibb Japan, Merck Serono, Eisai, Bayer, MSD, Chugai Pharma
Research Funding: Ono Pharmaceutical (Inst), Bristol Myers Squibb (Inst), Pfizer (Inst), Roche (Inst), Rakuten Medical (Inst), Adlai Nortye (Inst)
Mustimbo Roberts
Employment: Bristol Myers Squibb
Stock and Other Ownership Interests: Bristol Myers Squibb
Travel, Accommodations, Expenses: Bristol Myers Squibb
Tariq Aziz Khan
Employment: Bristol Myers Squibb/Celgene
Stock and Other Ownership Interests: Bristol Myers Squibb/Celgene
Travel, Accommodations, Expenses: Bristol Myers Squibb/Celgene
Karen Miller-Moslin
Employment: Bristol Myers Squibb, Bristol Myers Squibb
Stock and Other Ownership Interests: Bristol Myers Squibb, Bristol Myers Squibb
Li Wei
Employment: Bristol Myers Squibb
Stock and Other Ownership Interests: Bristol Myers Squibb
Athanassios Argiris
Consulting or Advisory Role: Merck Serono, Bristol Myers Squibb
Speakers' Bureau: Merck Serono, Bristol Myers Squibb, Debiopharm Group, AstraZeneca
Research Funding: Genentech/Roche, Bristol Myers Squibb
Travel, Accommodations, Expenses: Bristol Myers Squibb, Merck Serono
No other potential conflicts of interest were reported.
REFERENCES
- 1.Sung H, Ferlay J, Siegel RL, et al. : Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209-249, 2021 [DOI] [PubMed] [Google Scholar]
- 2.Johnson DE, Burtness B, Leemans CR, et al. : Head and neck squamous cell carcinoma. Nat Rev Dis Primers 6:92, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chow LQM: Head and neck cancer. N Engl J Med 382:60-72, 2020 [DOI] [PubMed] [Google Scholar]
- 4.Machiels JP, Rene Leemans C, Golusinski W, et al. : Squamous cell carcinoma of the oral cavity, larynx, oropharynx and hypopharynx: EHNS-ESMO-ESTRO clinical practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 31:1462-1475, 2020 [DOI] [PubMed] [Google Scholar]
- 5.Argiris A, Karamouzis MV, Raben D, et al. : Head and neck cancer. Lancet 371:1695-1709, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vermorken JB, Mesia R, Rivera F, et al. : Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med 359:1116-1127, 2008 [DOI] [PubMed] [Google Scholar]
- 7.ClinicalTrials.gov : Cetuximab (Erbitux) in Combination With Cisplatin or Carboplatin and 5-Fluorouracil in the First Line Treatment of Subjects With Recurrent and/or Metastatic Squamous Cell Carcinoma of the Head and Neck (EXTREME). https://www.clinicaltrials.gov/ct2/show/NCT00122460 [Google Scholar]
- 8.Guigay J, Aupérin A, Fayette J, et al. : Cetuximab, docetaxel, and cisplatin versus platinum, fluorouracil, and cetuximab as first-line treatment in patients with recurrent or metastatic head and neck squamous-cell carcinoma (GORTEC 2014-01 TPExtreme): A multicentre, open-label, randomised, phase 2 trial. Lancet Oncol 22:463-475, 2021 [DOI] [PubMed] [Google Scholar]
- 9.Burtness B, Harrington KJ, Greil R, et al. : Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet 394:1915-1928, 2019 [DOI] [PubMed] [Google Scholar]
- 10.Harrington KJ, Burtness B, Greil R, et al. : Pembrolizumab with or without chemotherapy in recurrent or metastatic head and neck squamous cell carcinoma: Updated results of the phase III KEYNOTE-048 study. J Clin Oncol 41:790-802, 2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferris RL, Blumenschein G, Jr, Fayette J, et al. : Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med 375:1856-1867, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gillison ML, Blumenschein G, Jr, Fayette J, et al. : CheckMate 141: 1-Year update and subgroup analysis of nivolumab as first-line therapy in patients with recurrent/metastatic head and neck cancer. Oncologist 23:1079-1082, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferris RL, Blumenschein G, Jr, Fayette J, et al. : Nivolumab vs investigator's choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of CheckMate 141 with analyses by tumor PD-L1 expression. Oral Oncol 81:45-51, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Head and neck cancers Version 2.2022 — April 26, 2022. https://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf [DOI] [PubMed] [Google Scholar]
- 15.Pardoll DM: The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 12:252-264, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Albiges L, Tannir NM, Burotto M, et al. : Nivolumab plus ipilimumab versus sunitinib for first-line treatment of advanced renal cell carcinoma: Extended 4-year follow-up of the phase III CheckMate 214 trial. ESMO Open 5:e001079, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. : Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 381:1535-1546, 2019 [DOI] [PubMed] [Google Scholar]
- 18.Motzer RJ, Rini BI, McDermott DF, et al. : Nivolumab plus ipilimumab versus sunitinib in first-line treatment for advanced renal cell carcinoma: Extended follow-up of efficacy and safety results from a randomised, controlled, phase 3 trial. Lancet Oncol 20:1370-1385, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baas P, Scherpereel A, Nowak AK, et al. : First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (CheckMate 743): A multicentre, randomised, open-label, phase 3 trial. Lancet 397:375-386, 2021 [DOI] [PubMed] [Google Scholar]
- 20.Chau I, Doki Y, Ajani JA, et al. : Nivolumab (NIVO) plus ipilimumab (IPI) or NIVO plus chemotherapy (chemo) versus chemo as first-line (1L) treatment for advanced esophageal squamous cell carcinoma (ESCC): First results of the CheckMate 648 study. J Clin Oncol 39, 2021. (suppl 18; abstr LBA4001) [Google Scholar]
- 21.Paz-Ares LG, Ramalingam SS, Ciuleanu TE, et al. : First-line nivolumab plus ipilimumab in advanced non-small cell lung cancer: 4-Year outcomes from the randomized, open-label, phase 3 CheckMate 227 Part 1 trial. J Thorac Oncol 17:289-308, 2022 [DOI] [PubMed] [Google Scholar]
- 22.Eisenhauer EA, Therasse P, Bogaerts J, et al. : New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 45:228-247, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Dako : PD-L1 IHC 28-8 pharmDx. https://www.accessdata.fda.gov/cdrh_docs/pdf15/P150027c.pdf
- 24.FACIT : Functional Assessment of Cancer Therapy Head & Neck Cancer Symptom Index—10 Item Version (FHNSI-10). https://www.facit.org/measures/FHNSI [Google Scholar]
- 25.EuroQoL : 3-level version of the EQ-5D (EQ-5D-3L). https://euroqol.org/eq-5d-instruments/eq-5d-3l-about/
- 26.Yount S, List M, Du H, et al. : A randomized validation study comparing embedded versus extracted FACT Head and Neck Symptom Index scores. Qual Life Res 16:1615-1626, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Pickard AS, Neary MP, Cella D: Estimation of minimally important differences in EQ-5D utility and VAS scores in cancer. Health Qual Life Outcomes 5:70, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.US Food and Drug Administration : Nivolumab for SCCHN. https://www.fda.gov/drugs/resources-information-approved-drugs/nivolumab-scchn
- 29.European Medicines Agency : Opdivo (nivolumab). https://www.ema.europa.eu/en/medicines/human/EPAR/opdivo
- 30.Pharmaceuticals and Medical Devices Agency : Nivolumab Review Report. https://www.pmda.go.jp/files/000223201.pdf [Google Scholar]
- 31.Gillison MI, Blumenschein G, Jr, Fayette J, et al. : Long-term outcomes with nivolumab as first-line treatment in recurrent or metastatic head and neck cancer: Subgroup Analysis of CheckMate 141.Oncologist 27:e194-e198, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Greil R, Rischin D, Harrington KJ, et al. : Long-term outcomes from KEYNOTE-048: Pembrolizumab (pembro) alone or with chemotherapy (pembro+C) vs EXTREME (E) as first-line (1L) therapy for recurrent/metastatic (R/M) head and neck squamous cell carcinoma (HNSCC) (abstr 915MO). Ann Oncol 31:S660-S661 [Google Scholar]
- 33.Ferris RL, Haddad R, Even C, et al. : Durvalumab with or without tremelimumab in patients with recurrent or metastatic head and neck squamous cell carcinoma: EAGLE, a randomized, open-label phase III study. Ann Oncol 31:942-950, 2020 [DOI] [PubMed] [Google Scholar]
- 34.ClinicalTrials.gov : Phase III Open Label Study of MEDI 4736 With/Without Tremelimumab Versus Standard of Care (SOC) in Recurrent/Metastatic Head and Neck Cancer (KESTREL). https://clinicaltrials.gov/ct2/show/results/NCT02551159 [Google Scholar]
- 35.ClinicalTrials.gov : Study of Nivolumab in Combination With Ipilimumab Versus Nivolumab in Combination With Ipilimumab Placebo in Patients With Recurrent or Metastatic Squamous Cell Carcinoma of the Head and Neck (CheckMate 714). https://clinicaltrials.gov/ct2/show/results/NCT02823574 [Google Scholar]
- 36.Bristol-Myers Squibb Reports First Quarter Financial Results. https://news.bms.com/news/details/2019/Bristol-Myers-Squibb-Reports-First-Quarter-Financial-Results/default.aspx [Google Scholar]
- 37.Haddad R, Gillison M, Ferris RL, et al. : CheckMate 714: A double-blind, two-arm, phase 2 study of nivolumab in combination with ipilimumab vs nivolumab and ipilimumab-placebo as first-line therapy in patients with recurrent or metastatic squamous cell carcinoma of the head and neck. Presented at the European Society for Medical Oncology 41st Congress, Copenhagen, Denmark, October 7-11, 2016 (abstr 1017TiP)
- 38.Emancipator K, Huang L, Aurora-Garg D, et al. : Comparing programmed death ligand 1 scores for predicting pembrolizumab efficacy in head and neck cancer. Mod Pathol 34:532-541, 2021 [DOI] [PubMed] [Google Scholar]
- 39.Hellmann MD, Rizvi NA, Goldman JW, et al. : Nivolumab plus ipilimumab as first-line treatment for advanced non-small-cell lung cancer (CheckMate 012): Results of an open-label, phase 1, multicohort study. Lancet Oncol 18:31-41, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon reasonable request. Bristol Myers Squibb policy on data sharing may be found at https://www.bms.com/researchers-and-partners/independent-research/data-sharing-request-process.html.